Abstract
Cholera, caused by Vibrio cholerae, is a noninvasive dehydrating enteric disease with a high mortality rate if untreated. Infection with V. cholerae elicits long-term protection against subsequent disease in countries where the disease is endemic. Although the mechanism of this protective immunity is unknown, it has been hypothesized that a protective mucosal response to V. cholerae infection may be mediated by anamnestic responses of memory B cells in the gut-associated lymphoid tissue. To characterize memory B-cell responses to cholera, we enrolled a cohort of 39 hospitalized patients with culture-confirmed cholera and evaluated their immunologic responses at frequent intervals over the subsequent 1 year. Memory B cells to cholera antigens, including lipopolysaccharide (LPS), and the protein antigens cholera toxin B subunit (CTB) and toxin-coregulated pilus major subunit A (TcpA) were enumerated using a method of polyclonal stimulation of peripheral blood mononuclear cells followed by a standard enzyme-linked immunospot procedure. All patients demonstrated CTB, TcpA, and LPS-specific immunoglobulin G (IgG)and IgA memory responses by day 90. In addition, these memory B-cell responses persisted up to 1 year, substantially longer than other traditional immunologic markers of infection with V. cholerae. While the magnitude of the LPS-specific IgG memory B-cell response waned at 1 year, CTB- and TcpA-specific IgG memory B cells remained significantly elevated at 1 year after infection, suggesting that T-cell help may result in a more durable memory B-cell response to V. cholerae protein antigens. Such memory B cells could mediate anamnestic responses on reexposure to V. cholerae.
Vibrio cholerae, the etiologic agent of cholera, causes an estimated 3 to 5 million cases of secretory diarrhea, resulting in over 100,000 deaths annually (24). Strains of V. cholerae can be differentiated serologically by the O side chain of the lipopolysaccharide (LPS) component of the outer membrane. Although more than 200 different serogroups have been isolated from the environment, the vast majority of strains that produce cholera belong to serogroup O1 or O139, both of which consist of noninvasive pathogens that colonize the mucosal surface of the small intestine (19). V. cholerae O1 biotype El Tor is currently the predominant cause of cholera globally and in Bangladesh.
The mechanisms of protective immunity to cholera are not known. Volunteer and epidemiologic studies demonstrate that clinically apparent infection with V. cholerae confers long-term protection of at least 3 years against subsequent disease (7, 12, 13). The best-studied marker of protective immunity is the vibriocidal antibody, a complement-dependent bactericidal antibody; however, there is no vibriocidal antibody titer at which complete protection is achieved (20). Furthermore, the vibriocidal response wanes rapidly, and it is hypothesized that the vibriocidal antibody may reflect other longer-lasting, protective immune responses occurring at the mucosal surface (3).
Patients with cholera develop additional humoral immune responses to several antigens including cholera toxin subunit B (CTB), toxin-coregulated pilus major subunit A (TcpA), and LPS (1). We have recently shown that serum anti-CTB immunoglobulin A (IgA) antibody levels are also associated with protective immunity independent of the vibriocidal antibody on exposure to cholera, but serum IgA levels also wane rapidly after infection (10). Although levels of serum anti-LPS and anti-CTB IgG antibodies increase considerably after infection, these have not been shown to correlate with protection from V. cholerae infection in humans (8, 10).
Cholera patients develop substantial mucosal immune responses after infection. These can be measured by the transient increase of antigen-specific IgA antibody-secreting cells (ASC) in the circulation. The ASC assay quantifies lymphocytes that are activated in the gut-associated lymphoid tissue (GALT) when they transiently circulate in blood before rehoming to mucosal effector sites (6, 16, 17). These predominantly gut-homing ASC peak in the circulation between 5 and 10 days after onset of illness but are no longer detected during late convalescence as they return to populate the GALT (1, 11). Because V. cholerae is a noninvasive pathogen, it is hypothesized that protective immunity is derived from the activity of the secretory IgA system of the GALT (14, 22, 23). Volunteer studies of subjects receiving CTB orally demonstrate local and systemic generation of anti-CTB IgA antibodies that peak at 7 days following ingestion but decline to baseline by 15 months; however, these volunteers mount anamnestic responses with a rapid return to peak mucosal antibody titers in as few as 3 days after subsequent challenge with oral CTB (22, 23). It is thus hypothesized that protection from cholera may be mediated by rapid anamnestic responses of memory B cells in the GALT to V. cholerae antigens.
In this study, we examined the memory B-cell immune responses to V. cholerae infection, using a polyclonal stimulation method to enhance the detection of memory B cells in the circulation by inducing their proliferation and differentiation into antibody-secreting plasmablasts (4, 5). A standardized two-color enzyme-linked immunospot (ELISPOT) assay allows for the quantification of small numbers of circulating V. cholerae antigen-specific memory B cells as a proportion of total memory B cells (2, 4, 5, 21). Using this system, we have previously shown that cholera patients develop CTB-specific IgG memory B-cell responses that persist for at least 3 months after infection (11). The present study further characterizes memory B-cell responses to CTB, TcpA, and LPS for both IgA and IgG isotypes for a period of 1 year following acute infection and examines differences between the memory B-cell responses to the T-cell-dependent protein antigens CTB and TcpA and the T-cell-independent antigen LPS.
MATERIALS AND METHODS
Study subjects.
Prior to enrollment and initiation of the study protocol, we obtained approval by institutional review boards of the International Centre for Diarrheal Disease Research, Bangladesh (ICDDR,B), and Massachusetts General Hospital. Written informed consent for the study was obtained from 39 patients admitted to the ICDDR,B with severe acute watery diarrhea and positive stool cultures for V. cholerae O1 between December 2006 and May 2008. All patients were treated with intravenous fluid resuscitation and with azithromycin. Blood samples were obtained during acute infection (the second day of hospitalization) and again on days 7, 30, 90, 180, 270, and 360 following onset of illness. For each time point, we performed assays for vibriocidal antibodies; for serum IgG and IgA antibodies to CTB, TcpA, and the homologous serotype of LPS (V. cholerae-specific Inaba or Ogawa); and for circulating antigen-specific IgG and IgA ASC. Antigen-specific IgG and IgA memory B-cell ELISPOT assays were performed on study days 2, 30, 90, 180, 270, and 360.
Isolation of PBMC.
Heparinized blood was diluted in phosphate-buffered saline. After centrifugation on Ficoll-Isopaque (Pharmacia, Piscataway, NJ), the peripheral blood mononuclear cells (PBMC) and plasma were isolated. Plasma specimens were frozen at −70°C prior to use in immunologic assays. Isolated PBMC were resuspended at a concentration of 1 × 106 cells/ml in RPMI complete medium (Gibco, Carlsbad, CA) with 10% heat-inactivated fetal bovine serum (HyClone, Logan, UT). Resuspended cells were used immediately for detecting circulating antigen-specific IgG and IgA ASC by ELISPOT assay or placed in appropriate culture media for the memory B-cell assay.
Vibriocidal antibody assay.
Vibriocidal antibody assays were performed as previously described, using guinea pig complement and the homologous serotype of V. cholerae O1 Ogawa (X-25049) or Inaba (19479) as the target organism (18). The vibriocidal titer was defined as the reciprocal of the highest serum dilution resulting in >50% reduction of the optical density compared to that of the control wells without serum.
ELISAs for IgG and IgA antibodies in serum specific for CTB, TcpA, and LPS.
The CTB-, TcpA-, and LPS-specific IgG and IgA responses in plasma were quantified using standardized enzyme-linked immunosorbent assay (ELISA) protocols (15, 18). For anti-CTB, ELISA plates were coated with ganglioside GM1 (0.3 nM/ml) followed by recombinant CTB (2.5 μg/ml) (gifts of A. M Svennerholm, Göteborg University). For anti-LPS, ELISA plates were coated with LPS (2.5 μg/ml) (15). For anti-TcpA, ELISA plates were coated with TcpA (1 μg/ml) (1). For each antigen, 100 μl/well of serum (diluted 1:200 in 0.1% bovine serum albumin in phosphate-buffered saline-Tween) was added. Horseradish peroxidase-conjugated secondary antibodies to human IgG or IgA were applied in separate wells. After overnight incubation at 4°C, the plates were washed and developed with ortho-phenylene diamine (Sigma, St. Louis, MO) in 0.1 M sodium citrate buffer and 0.1% hydrogen peroxide. Plates were read kinetically at 450 nm for 5 minutes. The maximal rate of optical density change was expressed as milli-absorbance units per minute, and ELISA units were normalized by calculating the ratio of the test sample to a standard of pooled convalescent-phase sera from patients recovered from cholera run as a positive control on each plate.
Quantification of circulating IgG and IgA ASC using the two-color ELISPOT assay.
Nitrocellulose-bottom plates (Mahan-4550; Millipore, Bedford, MA) were coated with GM1 ganglioside (3 nM/ml), TcpA (5 μg/ml), LPS (25 μg/ml), or affinity-purified goat anti-human Ig (total Ig) (Jackson Immunology Research, West Grove, PA) (5 μg/ml) (18). Plates were incubated overnight at 4°C. Prior to blocking, recombinant CTB was applied to the GM1-coated plates. All plates were blocked for 2 h at 37°C prior to use with RPMI 1640 containing 10% fetal bovine serum. A total of 4 × 105 PBMC/well were added to the CTB-, TcpA-, and LPS-coated plates, while 1 × 105 PBMC were added per starting well to the total Ig-coated plates and serially diluted. Following a 3-hour incubation, plates were washed and IgG ASC were detected using alkaline phosphatase-conjugated mouse anti-human IgG (Southern Biotech, Birmingham, AL) diluted 1:500, and IgA ASC were detected using horseradish peroxidase-conjugated mouse anti-human IgA (Southern Biotech, Birmingham, AL) diluted 1:1,000. After overnight incubation, IgG-conjugated plates were developed with 5-bromo-4-chloro-3-indolyl-phosphate-nitroblue tetrazolium and IgA-conjugated plates with 3-amino-9-ethylcarbazole. The IgG ASC were visualized as blue spots, and IgA ASC were visualized as red spots on the same nitrocellulose membranes. The ASC per well were quantified under a stereomicroscope independently by two individuals, and the data were averaged. The numbers of antigen-specific IgG and IgA ASC were expressed as the percentage of total circulating ASC of the same isotype.
Memory B-cell culture and two-color ELISPOT assay.
Memory B-cell assays were performed on days 2, 30, 90, 180, 270, and 360, based on previously described methods (4, 5, 11). For this assay, 5 × 105 PBMC/well were placed in 24-well cell culture plates (BD Biosciences, San Jose, CA) containing culture medium optimized to stimulate antigen-independent proliferation and differentiation of memory B cells into ASC. The stimulation medium consisted of RPMI 1640, 10% fetal bovine serum, 200 units/ml penicillin, 200 μg/ml streptomycin, 200 mM l-glutamine, 50 mM β-mercaptoethanol, and a mixture of three B-cell mitogens: 6 μg/ml of CpG oligonucleotide (Operon, Huntsville, AL), a 1/100,000 dilution of crude pokeweed mitogen extract, and a 1/10,000 dilution of fixed Staphylococcus aureus Cowan (Sigma, St. Louis, MO). As a negative control, PBMC were placed in RPMI medium alone. Plates were incubated at 37°C in 5% CO2 for 5 to 6 days, after which cells were harvested and washed, and antigen-specific (CTB, TcpA, and LPS) as well as total IgG and total IgA ELISPOT assays were performed as described above. From each culture well, 20% of the cells were used for detecting total IgG and IgA ASC, while 80% were used for detecting antigen-specific IgG and IgA ASC. ELISPOT counts were expressed as the percentage of antigen-specific memory B cells out of the total IgG or IgA memory B cells. Wells coated with keyhole limpet hemocyanin (KLH) (Pierce Biotechnology, Rockford, IL) (2.5 μg/ml) were used as negative controls. We defined appropriate stimulation of PBMC in our assay as a ≥4-fold increase in the number of total Ig memory cells following stimulation compared to the unstimulated cells. This definition included approximately 90% of all stimulated cultures for both IgG and IgA memory cells. We excluded data from the analysis for the following reasons: (i) the averaged total Ig samples for each patient sample did not have appropriate stimulation, (ii) patient samples had four or more antigen-specific ASC spots prior to stimulation (complicating interpretation of the memory B-cell assay), or (iii) patient samples had three or more ASC spots to the negative control antigen KLH.
Statistical analyses.
Comparisons of immunologic responses were tested for significance using the Mann-Whitney U test. All reported P values are two tailed, with a cutoff P value of ≤0.05 considered a threshold for statistical significance. Analyses were performed with GraphPad Prism 4.0 and SPSS 14.0.
RESULTS
Study population.
Demographic, microbiologic, and clinical characteristics of the patients are presented in Table 1. Thirty-nine patients were initially enrolled in the study at the time of acute infection (day 2).
TABLE 1.
Demographic, serologic, and clinical characteristics of patients
| Characteristic | Value |
|---|---|
| Follow-up days completed, no. of patients | |
| 2 | 39 |
| 7 | 38 |
| 30 | 37 |
| 90 | 37 |
| 180 | 36 |
| 270 | 33 |
| 360 | 29 |
| Sex, no. of patients | |
| Male | 21 |
| Female | 18 |
| Vibrio cholerae O1 serotype, no. of patients | |
| Inaba | 13 |
| Ogawa | 26 |
| Median age (range), yr | 24 (5-59) |
| Mean (SD) duration of diarrhea prior to | |
| hospitalization, h | 17.89 (16.08) |
| Mean (SD) duration of stay in hospital, h | 38.32 (23.51) |
Vibriocidal responses.
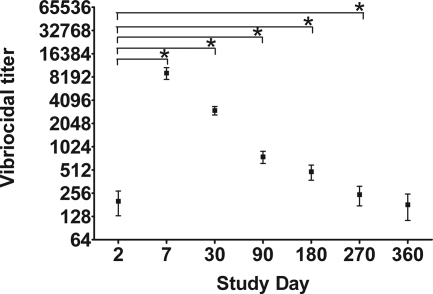
V. cholerae O1 Inaba or Ogawa serotype-specific vibriocidal antibody responses were measured on study days 2, 7, 30, 90, 180, 270, and 360 (Fig. 1). The geometric mean (GM) vibriocidal titer on day 2 was 74 (95% confidence interval [CI], 46 to 118). All patients mounted strong vibriocidal responses and seroconversion, represented by a fourfold or greater rise in the vibriocidal titer. The vibriocidal antibody titer peaked on day 7 (GM, 6,006; 95% CI, 4,303 to 8,383; P < 0.001) and remained significantly elevated through day 270, before declining to baseline levels by day 360 (GM, 97; 95% CI, 56 to 169; P = 0.48).
FIG. 1.
Mean serum vibriocidal antibody responses with standard error bars. An asterisk denotes a statistically significant difference (P < 0.05) from the baseline (day 2) titer.
CTB-, TcpA-, and LPS-specific antibody responses.
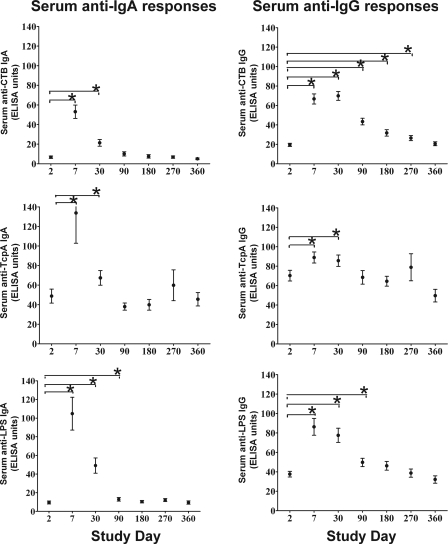
CTB-, TcpA-, and LPS-specific IgG and IgA responses are shown in Fig. 2. Peak TcpA and LPS IgG levels were seen at day 7, and CTB IgG peaked on day 30. All IgA responses peaked on day 7. At day 90, anti-CTB IgA returned to baseline, while anti-CTB IgG returned to statistical baseline by day 360. Serum IgG and IgA responses to TcpA were of lower magnitude than those to CTB and had returned to baseline by day 90. LPS-specific IgG and IgA responses were of a higher initial magnitude and returned to baseline by day 180.
FIG. 2.
Mean normalized serum antigen-specific IgA and IgG antibody responses with standard error bars. An asterisk denotes a statistically significant difference (P < 0.05) from baseline (day 2) levels.
CTB-, TcpA-, and LPS-specific IgA and IgG ASC responses.
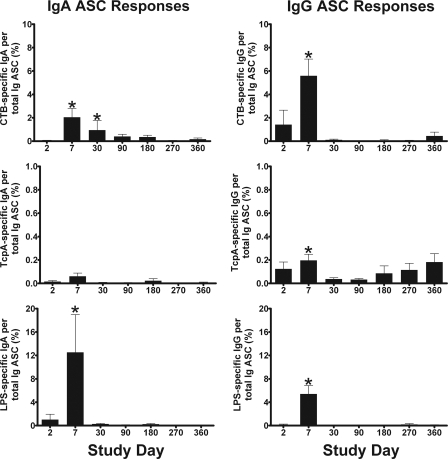
Antigen-specific IgA and IgG ASC ELISPOT assay results are shown in Fig. 3. Although most patients had no detectable antigen-specific IgG or IgA ASC at day 2, some patients had detectable ASC, which could represent an early primary or an anamnestic response to infection (11). As expected, ASC responses peaked on day 7 for all three antigens and both isotypes and returned to baseline by day 30 after the onset of disease, except for CTB-specific IgA ASC, which returned to baseline at day 90. IgG and IgA ASC specific for TcpA were detected at a lower frequency than those for the other two antigens, and the day 7 levels were not significantly higher compared to day 2 for IgA (P = 0.196). Occasionally, individuals had episodic elevations of antigen-specific ASC beyond day 30 during the year of follow-up; although we did not observe a fourfold or greater increase in the vibriocidal antibody titer in these patients, it is possible that these responses could represent an anamnestic response to reexposure.
FIG. 3.
Mean circulating antigen-specific IgA and IgG ASC responses with standard error bars. An asterisk denotes a statistically significant difference (P < 0.05) from baseline (day 2).
Stimulation of IgG and IgA memory B cells into ASC.
Previous studies using this method of memory B-cell detection have defined adequate stimulation with a cutoff value of approximately 5,000 total IgG ASC after 5 to 6 days of culture with mitogens (4, 11, 21). Since we also examined IgA memory B-cell responses in this study, we assessed the adequacy of polyclonal stimulation of IgA memory B cells by comparing the ratio of stimulated total Ig to unstimulated total Ig ASC spots for IgG and IgA. Stimulation resulted in median fold increases of 27 for the number of IgG ASC detected and 25 for the number of IgA ASC detected, which suggests that these conditions elicited a proliferative response that was comparable for IgA and IgG memory B cells. Using a cutoff of greater than a fourfold increase of stimulated compared to unstimulated total IgG and IgA ASC, we found that >90% and >87% of polyclonal stimulations reached this threshold, respectively, resulting in medians of 16,250 IgG ASC and 3,050 IgA ASC and limits of detection of approximately 0.001% for IgG and 0.004% for IgA antigen-specific memory B cells per 5 × 105 PBMC after 6 days of stimulation. In general, patients with acute cholera (day 2) had a diminished response to polyclonal stimulation, i.e., 5,400 median total IgG ASC versus 17,300 for later days (P < 0.001) and 900 median total IgA ASC versus 3,000 for days 30 and after (P < 0.001); thus, more individuals were excluded from the analysis of their memory B-cell responses on day 2 than on subsequent days because of inadequate overall stimulation.
Antigen-specific IgG memory B-cell responses.
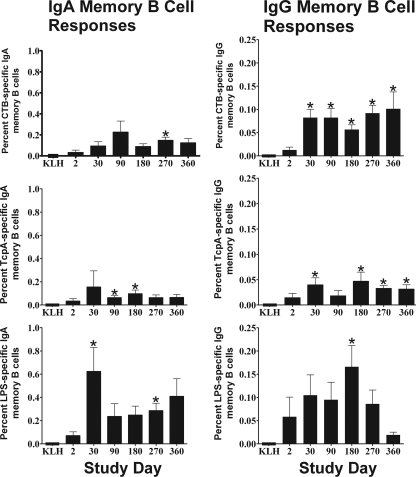
Antigen-specific IgG and IgA memory B-cell responses are shown in Fig. 4. Of the patients with adequate IgG stimulation, detectable CTB-, TcpA-, and LPS-specific memory B cells were found in 31%, 38%, and 78% of patients, respectively, at day 2. In contrast, most patients had detectable antigen-specific IgG memory B cells to CTB (100%), TcpA (82%), and LPS (100%) by day 30. This increase in CTB- and TcpA-specific memory B cells persisted at a significant level for the entire year of follow-up and remained detectable in 95% and 89%, respectively, of the patients for both antigens.
FIG. 4.
Mean antigen-specific and KLH-specific IgA and IgG memory B-cell kinetics with standard error bars. An asterisk denotes a statistically significant difference (P < 0.05) from baseline (day 2).
Although the magnitude of the LPS-specific IgG memory B-cell response was highest, it also lasted the shortest time and had returned to baseline levels by day 360, suggesting that immunologic memory for LPS may not persist as long as it does for CTB and TcpA. TcpA-specific IgG memory B-cell responses were much lower than those to the other two antigens but still persisted out to day 360 of follow-up at levels that were significantly higher than at day 2 (P = 0.029).
Memory B-cell responses to the negative control antigen, KLH, were seen in 7 out of 422 patient samples (1.7% of total samples tested). For these seven individual patient time points, all corresponding V. cholerae antigen-specific data were excluded from analysis. The remaining patients did not have a measurable memory B-cell response to the negative control antigen.
Antigen-specific IgA memory B cells.
Of the patients with adequate IgA stimulation on day 2, detectable CTB-, TcpA-, and LPS-specific memory B cells were found in 33%, 21%, and 50% of patients, respectively. TcpA- and LPS-specific IgA memory B-cell responses peaked on day 30 following infection, while CTB-specific IgA memory B-cell responses peaked on day 90 (Fig. 4). These responses persisted during the subsequent follow-up out to day 360 following acute infection, where 74%, 56%, and 86% of patients had detectable CTB-, TcpA-, and LPS-specific IgA memory B-cell responses, respectively. While all of the mean proportions of CTB-, TcpA-, and LPS-specific IgA memory B cells increased on day 30 and remained elevated compared to the lower frequency of V. cholerae antigen-specific B cells seen on day 2, values did not reach statistical significance all time points.
DISCUSSION
Protective immunity to cholera persists after circulating antibody levels return to baseline levels, and it has been hypothesized that protection may be mediated by anamnestic mucosal immune responses to V. cholerae antigens (23). While preexisting antibody is produced by long-lived plasma cells, memory B cells represent a distinct population that respond rapidly to antigenic stimulation by differentiating into ASC. Unlike naive B cells, memory B cells are responsible for rapid anamnestic responses to infection (4, 9). If it is true that anamnestic mucosal responses mediate protection against cholera, then it is likely that V. cholerae-specific memory B cells play a key role in protective immunity.
We demonstrate here that cholera patients develop significant memory B-cell responses to V. cholerae antigens CTB, LPS, and TcpA. In the majority of patients, V. cholerae antigen-specific memory B cells remained detectable in the circulation for at least 1 year following acute infection. These memory B cells are of both the IgA and IgG classes and remained significantly elevated after V. cholerae antigen-specific ASC were no longer detected and serum antibody titers had declined to baseline levels. These findings demonstrate that the measurement of circulating V. cholerae antigen-specific memory B cells may provide a useful long-term marker of the immune response to cholera, and they support a model in which protective immunity to cholera could be mediated by anamnestic responses of memory B cells, rather than by preformed antibody.
The robustness of the peripheral memory B-cell response to cholera was most evident in the measurement of IgG memory B-cell responses to the V. cholerae protein antigens CTB and TcpA. Memory B-cell responses to these antigens remained significantly elevated through the entire period of follow-up and were increased >9- and >2.5-fold, respectively, after 1 year postinfection compared to a baseline during acute illness. In contrast, the LPS IgG memory B-cell response lasted the shortest time and returned to below baseline levels by day 360. The reason for this difference is unknown. While B-cell responses to LPS likely occur through a T-cell-independent pathway, B-cell responses to CTB and TcpA are T-cell dependent. Thus, these findings suggest an important role for T-cell help in the development and/or maintenance of memory B cells to T-cell dependent protein antigens. Furthermore, even though V. cholerae is a noninvasive pathogen, these findings raise the possibility that T-cell help may play a key role in protective immunity against cholera.
We also observed persistent cholera antigen-specific IgA memory B-cell responses up to 1 year. Although measurements of these antigen-specific IgA memory B cells were higher than baseline for all time points from 1 month to 1 year, they were only significantly higher at various time points for each antigen, perhaps reflecting our sample size. Although our limit of detection of both IgG and IgA memory B cells was sufficient to detect V. cholerae antigen-specific memory cells of both isotypes in the majority of patients, the lower numbers of circulating IgA cells may have diminished our capacity to characterize significant differences in the proportion of antigen-specific IgA memory B cells between different time points.
V. cholerae is a noninvasive mucosal pathogen. It remains unknown whether the circulating memory B-cell populations measured in this study reflect the population of gut lymphocytes that are likely involved in mediating protective anamnestic immune responses to cholera. For this reason, future studies will focus on examining the relationship between the proportion of circulating memory B cells and the numbers of antigen-specific memory B cells in mucosal biopsy specimens in patients following cholera. In addition, to assess the hypothesis that anamnestic responses of V. cholerae antigen-specific memory B cells may mediate protective immunity, future studies will examine exposed household contacts of cholera patients, who are at high risk of developing infection, and look for a direct correlation between circulating memory B cells on exposure and the subsequent risk of illness.
Acknowledgments
This research was supported by ICDDR,B, Centre for Health and Population Research, and by the following grants: U01 AI058935 (S.B.C.), RO3 AI063079 (F.Q.), U01 AI077883 (E.T.R.), International Research Scientist Development Award KO1 TW07144 (R.C.L.), International Research Scientist Development Award KO1 TW07409 (J.B.H.); and A. Fogarty International Center Global Infectious Disease Research Training Program Award in Vaccine Development D43 TW05572 (M.S.B.). A. M. Harris, F. Chowdhury, E. A. Kendall, and A. I. Khan are recipients of a Fogarty/Ellison Fellowship in Global Health awarded by the Fogarty International Center at the National Institutes of Health (D43 TW005572 and R24 TW007988).
We thank the study participants as well as the dedicated field and laboratory workers at the ICDDR,B.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 15 June 2009.
REFERENCES
- 1.Asaduzzaman, M., E. T. Ryan, M. John, L. Hang, A. I. Khan, A. S. Faruque, R. K. Taylor, S. B. Calderwood, and F. Qadri. 2004. The major subunit of the toxin-coregulated pilus TcpA induces mucosal and systemic immunoglobulin A immune responses in patients with cholera caused by Vibrio cholerae O1 and O139. Infect. Immun. 724448-4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernasconi, N. L., E. Traggiai, and A. Lanzavecchia. 2002. Maintenance of serological memory by polyclonal activation of human memory B cells. Science 2982199-2202. [DOI] [PubMed] [Google Scholar]
- 3.Clements, M. L., M. M. Levine, C. R. Young, R. E. Black, Y. L. Lim, R. M. Robins-Browne, and J. P. Craig. 1982. Magnitude, kinetics, and duration of vibriocidal antibody responses in North Americans after ingestion of Vibrio cholerae. J. Infect. Dis. 145465-473. [DOI] [PubMed] [Google Scholar]
- 4.Crotty, S., R. D. Aubert, J. Glidewell, and R. Ahmed. 2004. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J. Immunol. Methods 286111-122. [DOI] [PubMed] [Google Scholar]
- 5.Crotty, S., P. Felgner, H. Davies, J. Glidewell, L. Villarreal, and R. Ahmed. 2003. Long-term B cell memory in humans after smallpox vaccination. J. Immunol. 1714969-4973. [DOI] [PubMed] [Google Scholar]
- 6.Forrest, B. D. 1992. Indirect measurement of intestinal immune responses to an orally administered attenuated bacterial vaccine. Infect. Immun. 602023-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glass, R. I., S. Becker, M. I. Huq, B. J. Stoll, M. U. Khan, M. H. Merson, J. V. Lee, and R. E. Black. 1982. Endemic cholera in rural Bangladesh, 1966-1980. Am. J. Epidemiol. 116959-970. [DOI] [PubMed] [Google Scholar]
- 8.Glass, R. I., A. M. Svennerholm, M. R. Khan, S. Huda, M. I. Huq, and J. Holmgren. 1985. Seroepidemiological studies of El Tor cholera in Bangladesh: association of serum antibody levels with protection. J. Infect. Dis. 151236-242. [DOI] [PubMed] [Google Scholar]
- 9.Gourley, T. S., E. J. Wherry, D. Masopust, and R. Ahmed. 2004. Generation and maintenance of immunological memory. Semin. Immunol. 16323-333. [DOI] [PubMed] [Google Scholar]
- 10.Harris, J. B., R. C. Larocque, F. Chowdhury, A. I. Khan, T. Logvinenko, A. S. Faruque, E. T. Ryan, F. Qadri, and S. B. Calderwood. 2008. Susceptibility to Vibrio cholerae infection in a cohort of household contacts of patients with cholera in Bangladesh. PLoS Negl. Trop. Dis. 2e221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jayasekera, C. R., J. B. Harris, S. Bhuiyan, F. Chowdhury, A. I. Khan, A. S. Faruque, R. C. Larocque, E. T. Ryan, R. Ahmed, F. Qadri, and S. B. Calderwood. 2008. Cholera toxin-specific memory B cell responses are induced in patients with dehydrating diarrhea caused by Vibrio cholerae O1. J. Infect. Dis. 1981055-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koelle, K., X. Rodo, M. Pascual, M. Yunus, and G. Mostafa. 2005. Refractory periods and climate forcing in cholera dynamics. Nature 436696-700. [DOI] [PubMed] [Google Scholar]
- 13.Levine, M. M., R. E. Black, M. L. Clements, L. Cisneros, D. R. Nalin, and C. R. Young. 1981. Duration of infection-derived immunity to cholera. J. Infect. Dis. 143818-820. [DOI] [PubMed] [Google Scholar]
- 14.Levine, M. M., J. B. Kaper, R. E. Black, and M. L. Clements. 1983. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol. Rev. 47510-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qadri, F., F. Ahmed, M. M. Karim, C. Wenneras, Y. A. Begum, M. Abdus Salam, M. J. Albert, and J. R. McGhee. 1999. Lipopolysaccharide- and cholera toxin-specific subclass distribution of B-cell responses in cholera. Clin. Diagn. Lab. Immunol. 6812-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qadri, F., G. Jonson, Y. A. Begum, C. Wenneras, M. J. Albert, M. A. Salam, and A. M. Svennerholm. 1997. Immune response to the mannose-sensitive hemagglutinin in patients with cholera due to Vibrio cholerae O1 and O0139. Clin. Diagn. Lab. Immunol. 4429-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qadri, F., E. T. Ryan, A. S. Faruque, F. Ahmed, A. I. Khan, M. M. Islam, S. M. Akramuzzaman, D. A. Sack, and S. B. Calderwood. 2003. Antigen-specific immunoglobulin A antibodies secreted from circulating B cells are an effective marker for recent local immune responses in patients with cholera: comparison to antibody-secreting cell responses and other immunological markers. Infect. Immun. 714808-4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qadri, F., C. Wenneras, M. J. Albert, J. Hossain, K. Mannoor, Y. A. Begum, G. Mohi, M. A. Salam, R. B. Sack, and A. M. Svennerholm. 1997. Comparison of immune responses in patients infected with Vibrio cholerae O139 and O1. Infect. Immun. 653571-3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sack, D. A., R. B. Sack, G. B. Nair, and A. K. Siddique. 2004. Cholera. Lancet 363223-233. [DOI] [PubMed] [Google Scholar]
- 20.Saha, D., R. C. LaRocque, A. I. Khan, J. B. Harris, Y. A. Begum, S. M. Akramuzzaman, A. S. Faruque, E. T. Ryan, F. Qadri, and S. B. Calderwood. 2004. Incomplete correlation of serum vibriocidal antibody titer with protection from Vibrio cholerae infection in urban Bangladesh. J. Infect. Dis. 1892318-2322. [DOI] [PubMed] [Google Scholar]
- 21.Sasaki, S., M. C. Jaimes, T. H. Holmes, C. L. Dekker, K. Mahmood, G. W. Kemble, A. M. Arvin, and H. B. Greenberg. 2007. Comparison of the influenza virus-specific effector and memory B-cell responses to immunization of children and adults with live attenuated or inactivated influenza virus vaccines. J. Virol. 81215-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Svennerholm, A. M., L. Gothefors, D. A. Sack, P. K. Bardhan, and J. Holmgren. 1984. Local and systemic antibody responses and immunological memory in humans after immunization with cholera B subunit by different routes. Bull. W. H. O. 62909-918. [PMC free article] [PubMed] [Google Scholar]
- 23.Svennerholm, A. M., M. Jertborn, L. Gothefors, A. M. Karim, D. A. Sack, and J. Holmgren. 1984. Mucosal antitoxic and antibacterial immunity after cholera disease and after immunization with a combined B subunit-whole cell vaccine. J. Infect. Dis. 149884-893. [DOI] [PubMed] [Google Scholar]
- 24.Zuckerman, J. N., L. Rombo, and A. Fisch. 2007. The true burden and risk of cholera: implications for prevention and control. Lancet Infect. Dis. 7521-530. [DOI] [PubMed] [Google Scholar]