Abstract
Pathogenic Leptospira species are the etiological agents of leptospirosis, a widespread disease of human and veterinary concern. In this study, we report that Leptospira species are capable of binding plasminogen (PLG) in vitro. The binding to the leptospiral surface was demonstrated by indirect immunofluorescence confocal microscopy with living bacteria. The PLG binding to the bacteria seems to occur via lysine residues because the ligation is inhibited by addition of the lysine analog 6-aminocaproic acid. Exogenously provided urokinase-type PLG activator (uPA) converts surface-bound PLG into enzymatically active plasmin, as evaluated by the reaction with the chromogenic plasmin substrate d-Val-Leu-Lys 4-nitroanilide dihydrochloridein. The PLG activation system on the surface of Leptospira is PLG dose dependent and does not cause injury to the organism, as cellular growth in culture was not impaired. The generation of active plasmin within Leptospira was observed with several nonvirulent high-passage strains and with the nonpathogenic saprophytic organism Leptospira biflexa. Statistically significant higher activation of plasmin was detected with a low-passage infectious strain of Leptospira. Plasmin-coated virulent Leptospira interrogans bacteria were capable of degrading purified extracellular matrix fibronectin. The breakdown of fibronectin was not observed with untreated bacteria. Our data provide for the first time in vitro evidence for the generation of active plasmin on the surface of Leptospira, a step that may contribute to leptospiral invasiveness.
The spirochete Leptospira interrogans is a highly invasive pathogen and the causal agent of leptospirosis, one of the most widespread zoonoses of human and veterinary concern (7, 20, 25, 39, 76). The disease occurs mainly in peripheral metropolitan regions lacking adequate sanitary conditions during activities that involve direct contact with contaminated water, soil, or animals (25, 36, 76). Humans are accidental and terminal hosts in the transmission process of leptospirosis (20, 65). The leptospires enter the body via abrasions on skin or actively through mucosa, spreading to any tissue, but particularly colonizing kidneys and liver (39).
Despite its importance and the genomic sequencing of five strains of Leptospira, four pathogenic (9, 57, 66) and one saprophytic (64), molecular aspects of the pathogenesis, virulence, and invasion processes by which the leptospires infect the hosts and initiate tissue colonization are poorly characterized. To date, few virulence factors contributing to the pathogenesis of the disease have been identified (3, 48, 67).
It is known that one characteristic of leptospiral infection is the rapid dissemination within the host and colonization of renal tubules that constitute immunologically safe environments (20). The ability of the leptospires to adhere to extracellular matrix (ECM) macromolecules has been shown (4), and to date a few adhesins, ECM-binding proteins, have been identified (4, 11, 29, 30, 72). After adherence, the next step must be to overcome the barriers imposed by epithelial tissues and ECMs. For this, the proteolytic activity achieved by subversion of host proteases by pathogens, such as plasmin, has been demonstrated to be important during several bacterial infections (37).
Plasmin is a broad-spectrum serine protease component of the fibrinolytic system, which has plasminogen (PLG) as the main component. It has been shown that several pathogens, including the spirochete Borrelia burgdorferi, bind PLG on the surface and convert it to plasmin by host activators (6, 13, 16, 19, 22, 31, 34, 37, 38, 60, 68, 73); this binding promotes degradation of ECM components and is essential for dissemination of the bacteria through the host tissues, suggesting its role during infection and pathogenesis (12, 14, 15, 28, 37, 58).
Based on these assertions, we were prompted to investigate the ability of pathogenic L. interrogans to bind PLG. We show in this work by in vitro assays that leptospires are capable of capturing PLG in its outer surface, that the conversion to enzymatically active plasmin could be achieved by an exogenous source, and that the active plasmin generated on the surface of Leptospira can degrade the fibronectin ECM component. Outer membrane proteins (OMPs) are involved with PLG acquisition, but aqueous soluble proteins also contribute to the binding. Neither temperature shift to the mammalian body, under normal and febrile conditions, nor physiologic osmolarity affected plasmin generation by leptospires. We also demonstrate a significant difference in the plasminogen activation system (PAS) between infectious and noninfectious leptospires, suggesting that this feature might have a role in leptospiral virulence.
MATERIALS AND METHODS
Bacterial isolates and culture conditions.
Virulent L. interrogans serovar Copenhageni strain Fiocruz L1-130 is routinely cultured at Faculdade de Medicina Veterinária da Universidade de São Paulo by iterative passages in Golden Syrian hamsters for maintenance of virulence. Recently weaned hamsters were intraperitoneally infected with 500 μl containing ∼1.0 × 104 virulent leptospires (50% lethal dose [LD50] of ∼103 leptospires/animal). The animals were sacrificed after appearance of symptoms such as loss of weight and mobility (∼5 days postinfection). Kidney and liver were removed and macerated. The organ-derived leptospires were cultured at 28°C in semisolid modified Elinghausen-McCullough-Johnson-Harris (EMJH) medium supplemented with 10% rabbit sera. On the other hand, nonvirulent attenuated leptospires are equally routinely cultured by maintenance in culture in liquid modified EMJH medium supplemented with 10% rabbit sera. Animals infected with these high-passage attenuated-leptospire-derived strains do not show any sign of the disease. The nonvirulent Leptospira strains used were: L. interrogans serovar Canicola strain Hound Utrech IV, L. interrogans serovar Copenhageni strain M 20, L. interrogans serovar Icterohaemorrhagiae strain RGA, L. interrogans serovar Pomona strain Pomona, Leptospira borgpetersenii serovar Castelonis strain Castellon 3, L. borgpetersenii serovar Whitcombi strain Whitcomb, Leptospira kirshneri serovar Cynoptery strain 3522 C, L. kirshneri serovar Grippotyphosa strain Moskva V, Leptospira santarosai serovar Shermani strain 1342 K, and Leptospira biflexa serovar Patoc strain Patoc.
For the experiments with PLG binding, to exclude PLG interference from the rabbit serum supplementing the culture medium, serum-free leptospires were obtained by three passages in liquid modified EMJH medium supplemented with 10% Leptospira enrichment EMJH medium (BD, Difco), cultured at 28°C. For the temperature shift experiments, the bacteria were incubated for 24 h at 37 or 39°C. For the osmolarity shift analysis, 120 mM NaCl was added to the EMJH medium supplemented with 10% Leptospira enrichment EMJH medium, followed by incubation at 28°C for 24 h.
Labeling of leptospires with plasmin.
A total of 7.0 × 109 leptospires were centrifuged at 6,000 × g for 10 min at 25°C, resuspended in 1.4 ml of EMJH culture medium supplemented with 10% Leptospira enrichment EMJH medium, divided into seven aliquots of 0.2 ml each (1.0 × 109 leptospires) in 2-ml microcentrifuge tubes, and recentrifuged. The seven tubes containing the leptospires received different treatments: (i) 30% plasma and 3 U urokinase-type uroplasminogen activator (uPA) (urokinase; Sigma) in 100 μl low-salt phosphate-buffered saline (lsPBS; with 50 mM NaCl), (ii) 5 μg PLG (native PLG purified from human plasma was from Merck), and 3 U uPA in 100 μl lsPBS, (iii) 2 μg PLG and 3 U uPA in 100 μl lsPBS, (iv) 0.5 μg PLG and 3 U uPA in 100 μl lsPBS, (v) 5 μg PLG in 100 μl lsPBS, (vi) 3 U uPA in 100 μl lsPBS, and (vii) 100 μl lsPBS. All of the preparations were incubated for 1 h at 37°C, under gentle shaking, prior to the addition of the uPA, and followed by 1 h of incubation at 37°C. Leptospires were then centrifuged and washed three times with 0.7 ml lsPBS.
Measurement of enzymatic activity of plasmin-coated leptospires.
The treated leptospires (1.0 × 109 per sample) were resuspended in 300 μl lsPBS and divided into three aliquots. Each aliquot received 100 μl of 0.5 mg/ml of the chromogenic substrate d-Val-Leu-Lys 4-nitroanilide dihydrochloridein (Sigma) in lsPBS, to a final substrate concentration of 0.25 mg/ml. The suspensions were incubated for 1.5 h at 37°C under gentle shaking and then centrifuged at 6,000 × g for 10 min at 25°C. The supernatants (150 μl) were transferred to 96-well microplates, and the cleavage of the specific plasmin substrate was quantified with a microplate reader set at a wavelength of 405 nm.
ACA binding-inhibition assay.
Low-passage virulent L. interrogans serovar Copenhageni strain Fiocruz L1-130 leptospires (1.0 × 109 leptospires/sample) were treated with PLG and uPA, as described above, except for the addition of increasing concentrations of 6-aminocaproic acid (ACA; Sigma) ranging from 0 to 1,000 mM, added together with the PLG. The PLG quantity was set at 5 μg and that of uPA was set at 3 U. Aliquots treated with only PLG, uPA, or ACA and those treated with just lsPBS were used as controls. The enzymatic plasmin activity was measured as described before.
Isolation of leptospiral OMPs and cytoplasmatic fractions by TX-114 partitioning.
Leptospires cultured as outlined before were washed in PBS-5 mM MgCl2 and then extracted in the presence of 2% Triton X-114 (TX-114; Sigma-Aldrich), 150 mM NaCl, 10 mM Tris-HCl (pH 7.4), and 1 mM phenylmethylsulfonyl fluoride on ice for 4 h (24). The insoluble material was removed by centrifugation at 17,000 × g for 10 min at 4°C. After centrifugation, the TX-114 soluble fraction was stored at −20°C overnight and then thawed on ice. Phase separation was performed by warming the supernatant at 37°C and subjecting it to centrifugation for 10 min at 1,000 × g. Three distinct fractions became apparent: the aqueous phase (AP), the TX-114 phase (detergent phase [DP]), and the insoluble pellet. The DP was washed with 0.06% TX-114, 150 mM NaCl, and 10 mM Tris-HCl (pH 7.4) by incubation for 10 min on ice followed by 37°C incubation and centrifugation. The washing step was performed one more time, and after incubation for 10 min on ice, the solution was centrifuged at 17,000 × g, and the upper phase (TX-114) was incubated at 37°C for 10 min. After centrifugation, the DP was precipitated with acetone. All protein fractions were subjected to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) for analysis of the extraction.
PLG binding to extracted proteins.
The binding of the proteins contained in the AP and DP or whole-cell lysate to PLG was evaluated by a modified enzyme-linked immunosorbent assay (ELISA), as follows. Ninety-six-well plates (Costar high binding; Corning) were coated overnight in PBS at 4°C with 0.15 μg/well of proteins or bovine serum albumin (BSA), as a negative control. Plates were washed once with PBS supplemented with 0.05% (vol/vol) Tween 20 (PBS-T) and blocked for 2 h at 37°C with PBS with 10% (mass/vol) nonfat dry milk. The blocking solution was discarded, and 100 μl of 10 μg/ml of PLG in PBS was incubated for 2 h at 37°C. Wells were washed four times with PBS-T and incubated for 1 h at 37°C with mouse anti-human PLG (Sigma-Aldrich) (1:4,000 in PBS). Plates were washed again and incubated with horseradish peroxidase-conjugated anti-mouse immunoglobulin G (IgG), diluted 1:5,000 in PBS. After three washings, 1 mg/ml o-phenylenediamine (OPD) plus 1 μl/ml H2O2 in citrate phosphate buffer (pH 5.0) was added at 100 μl/well. The reactions were carried out for 5 min and stopped by addition of 50 μl/well of 2 N H2SO4. Readings were taken at 492 nm.
Growth monitoring of plasmin-coated leptospires in culture.
Virulent L. interrogans serovar Copenhageni strain Fiocruz L1-130 (1.0 × 109 bacteria/sample) were treated with the PAS as described above; after the treatment, the bacteria were centrifuged and the cell pellets were resuspended in 4 ml of fresh EMJH medium supplemented with 10% Leptospira enrichment EMJH medium. The bacterial cultures were incubated under aerobic conditions at 28°C. The growth was monitored by optical density measurements at 600 nm (OD600), where an OD600 of 0.2 corresponds to ∼9.6 × 108 leptospires), at the 2nd, 6th, 10th, and 14th days. All of the solutions used were filter sterilized prior to use.
L-IFA.
For the liquid-phase immunofluorescence assay (L-IFA), live bacterial suspensions (2.5 × 109) were harvested at 12,800 × g for 15 min, washed twice with lsPBS, resuspended in 200 μl lsPBS containing 8 μg of human PLG, and incubated for 45 min at 37°C. After the incubation, 6 μg/ml of propidium iodide (Sigma) was added to stain the nuclei, and the suspensions were incubated for another 45 min at 37°C. After this time, the leptospires were gently washed three times with lsPBS and incubated for 45 min at 37°C with mouse-produced antiserum against human PLG at a 1:50 dilution. The leptospires were washed three times and incubated with goat anti-mouse IgG antibodies conjugated to fluorescein isothiocyanate (FITC; Sigma) at a dilution of 1:50 for 45 min at 37°C. After this incubation, the leptospires were washed twice and resuspended in lsPBS-antifading solution (ProLong Gold; Molecular Probes). The immunofluorescence-labeled leptospires were examined by use of a confocal LSM 510 META immunofluorescence microscope (Zeiss, Germany). As a control for cell integrity, we used antibodies against recombinant Leptospira protein LipL32 or GroEL, following all of the previously mentioned procedures for PLG.
SDS-PAGE and affinity blotting.
Total leptospiral protein extracts for SDS-PAGE were prepared from 10 ml of ∼109 bacteria in EMJH serum-free cultures. The cells were harvested by centrifugation, washed three times with 5 mM MgCl2 in lsPBS, and resuspended in 100 μl PBS. The proteins were loaded for 10% SDS-PAGE and transferred to nitrocellulose membranes (Hybond-ECL; GE Healthcare) in semidry equipment. The membranes were blocked for 2 h at 37°C with 5% BSA, washed three times (10 min for each wash) with PBS-T solution, and incubated overnight with 3 μg/ml PLG or 3 μg/ml PLG plus 100 mM ACA at 4°C, followed by a 2-h incubation at room temperature. Then, the membranes were washed three times and incubated with mouse anti-human PLG (1:750) for 3 h at room temperature, followed by more three washings and 1 h of incubation at room temperature with anti-mouse IgG (1:5,000). The membranes were washed, and the protein's reactivity was revealed by the ECL enhanced chemiluminescence reagent (GE Healthcare) with subsequent exposition to X-ray films.
Assay for the degradation of fibronectin.
Ninety-six-well plates were coated overnight at 37°C with 0.5 μg/well of cellular fibronectin (Sigma), washed four times at 200 μl per wash with PBS-T, and blocked with 2% BSA in PBS-T for 2 h at 37°C, followed by two washings. The spirochetes (1.0 × 108 leptospires per sample) were treated as described above with 10 μg PLG and 3 U uPA or lsPBS (untreated). Bacteria were washed, resuspended in 100 μl lsPBS, and transferred to the plate′s previously coated wells. The plates were centrifuged at 180 × g for 15 min to ensure the contact of the leptospires with the immobilized ECM component. The plates were incubated at 37°C for 20 h and washed five times to remove the bacteria. The degradation of fibronectin was detected by reduction in absorbance followed by incubation with anti-fibronectin IgG antibodies (1:5,000 dilution in 100 μl PBS-T for 45 min at 37°C), anti-IgG peroxidase-conjugated antibodies (1:5,000 dilution in 100 μl PBS-T for 45 min at 37°C) and 100 μl/well of 1 mg/ml OPD plus 1 μl/ml H2O2. The reaction was stopped with 50 μl/well 4 N H2SO4, and the absorbance was measured at 492 nm. Percentage degradation was calculated by the formula (A − B)/A(100), where A is the mean PBS sample absorbance (positive control group for fibronectin reactivity) and B is the mean experimental group absorbance (untreated or plasmin).
Statistics.
For the data that were tested for statistical significance, Student's two-tailed test was applied, considering the minimum significance at P < 0.05.
RESULTS
Binding of human PLG by L. interrogans cells.
The ability of live L. interrogans serovar Copenhageni strain Fiocruz L1-130 cells to bind human PLG was examined via L-IFA. Leptospires were visualized by propidium iodide staining (Fig. 1A) (PLG1, PLG2, PLG3, LipL32, GroEL, and PBS) followed by protein detection with polyclonal mouse antiserum against each protein in the presence of anti-mouse IgG antibodies conjugated to FITC. In Fig. 1B, green fluorescence could be observed for PLG (PLG1, PLG2, and PLG3) and LipL32, an outer membrane protein used as a surface-positive control (56), but not with GroEL, a protoplasmic cylinder marker used as a negative control (26). The localization of the protein green light within the leptospires was achieved by superimposing both fields, and the results obtained are shown in Fig. 1C for PLG1, PLG2, PLG3, LipL32, GroEL, and PBS.
FIG. 1.
Recognition of PLG binding to Leptospira by L-IFA. Live virulent L. interrogans serovar Copenhageni isolates were treated with PLG, and the recognition was assessed through polyclonal anti-PLG antibodies under a confocal immunofluorescence microscope (PLG1 to -3). PLG-treated leptospires were stained for the protein LipL32 (outer surface protein marker) or GroEL (a protoplasmic cylinder marker); untreated leptospires (PBS) are shown as controls. (A) DNA propidium iodide-stained DNA; (B) FITC-stained DNA; (C) composite images of panel A plus B.
Activation and enzymatic activity of PLG-bound Leptospira.
To analyze the Leptospira PLG binding and quantify the enzymatic activity after conversion to plasmin, we assayed the treated leptospires with a specific plasmin chromogenic substrate. As shown in Fig. 2, both the virulent and the nonvirulent strains of L. interrogans serovar Copenhageni tested were capable of capturing PLG and the bound PLG was converted to enzymatically active plasmin only by the addition of the exogenous activator uPA. The binding and consequently the plasmin enzymatic activity occurred in a dose-dependent manner with the PLG concentration range studied (Fig. 2). In addition to the purified PLG, low-passage virulent leptospires were also capable of binding PLG in 30% human plasma (∼6 μg/ml of PLG). No proteolytic activity was detected with either intact Leptospira (Fig. 2) or cell lysate (not shown) or when the bacteria were treated with PLG or uPA alone.
FIG. 2.
Cleavage of the plasmin-specific chromogenic substrate by plasmin-bound leptospires. Live low-passage virulent and high-passage nonvirulent L. interrogans serovar Copenhageni cells received the following treatments: PBS only (PBS), uPA alone (uPA), 5 μg PLG alone (PLG), PLG at 0.5, 2, and 5 μg together with uPA; and 30% human plasma together with uPA (30% plasma). Bars represent mean absorbance as a measure of relative substrate degradation ± the standard deviation of three replicates for each experimental group and are representative of three independent experiments. *, virulent leptospire experiments statistically significant (P < 0.0001) in comparison to the PBS control; **, nonvirulent leptospire experiments statistically significant (P < 0.01) in comparison to the PBS control. The 30% plasma (as the PLG source) and all of the PLG-uPA samples of the virulent leptospires were statistically significant (P < 0.001) in comparison to the same samples of nonvirulent bacteria tested.
Analysis of L. interrogans culture growth after PLG labeling.
To analyze if the PLG binding to the surface of L. interrogans serovar Copenhageni strain Fiocruz L1-130 and its conversion to enzymatically active plasmin could cause any impairment in the growth of the leptospire culture, the bacteria were treated with PLG; activated by the addition of uPA; reinoculated into a fresh, serum-free EMJH medium; and monitored for the growth rate for 14 days. Cell division was not inhibited in plasmin-coated leptospires since bacterial growth was similar to that of untreated (PBS) or PLG- or PLG-uPA-treated Leptospira cells (data not shown).
Inhibition of proteolytic activity acquired by L. interrogans by ACA.
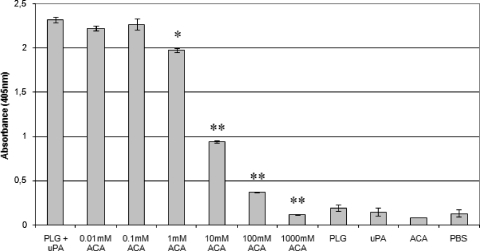
It is known that PLG kringle domains frequently mediate interactions with lysine residues of the cellular receptors (37). To evaluate the participation of these domains in the binding of PLG with L. interrogans serovar Copenhageni strain L1-130, a binding assay was carried out in the presence of ACA, a derivative and analogue of lysine amino acid. As shown in Fig. 3, ACA reduced the proteolytic activity of virulent L. interrogans serovar Copenhageni cells incubated with PLG in a dose-dependent manner, statistically significant from 1 mM ACA to almost total inhibition at 1,000 mM ACA, as a consequence of decreased PLG binding.
FIG. 3.
Inhibition of PLG binding to L. interrogans by ACA. Low-passage virulent L. interrogans serovar Copenhageni cells were treated with the following: PLG together with uPA (PLG + uPA), PLG together with uPA with the addition of crescent concentrations of ACA (0.01 to 1,000 mM ACA), PLG alone (PLG), uPA alone (uPA), ACA alone (ACA), and no additions (PBS). The cleavage of the plasmin-specific substrate d-Val-Leu-Lys 4-nitroanilide dihydrochloridein by the treated spirochetes was measured by absorbance readings at 405 nm. Bars represent mean absorbance ± the standard deviation of three replicates for each experimental group and are representative of three independent experiments. Statistically significant substrate degradation inhibition results in comparison to the positive control (PLG + uPA) are depicted: P < 0.01 (*) and P < 0.0001 (**), as assessed by Student's two-tailed t test.
Involvement of leptospiral OMPs on PLG binding.
OMPs that are surface exposed are expected to be relevant in pathogenesis. We thus examined the binding of proteins after treatment/extraction of leptospires with the nonionic detergent TX-114, as previously described (24). Experiments were performed with the virulent low-passage L. interrogans serovar Copenhageni strain Fiocruz L1-130. The binding assay was performed with proteins from the soluble AP and DP and with whole-cell lysate. PLG acquisition, as evaluated by a modified ELISA, shows that OMPs partitioned in the DP contribute to PLG binding (Fig. 4), but a higher number of proteins contained in the soluble AP appear to be involved in the PLG acquisition by Leptospira (Fig. 4).
FIG. 4.
PLG binding to extracted fractions of leptospira. Low-passage virulent L. interrogans serovar Copenhageni proteins were fractioned by TX-114 extraction. Whole-cell lysate (WCL), DP, and AP were coated onto 96-well plates and allowed to bind to PLG. The interaction was quantified by specific anti-human PLG antibodies. The results are representative of two independent TX-114 partitioning and binding experiments. The bars represent the mean absorbance at 492 nm ± the standard deviation of four replicates.
Effect of environmental factors on plasmin generation by Leptospira.
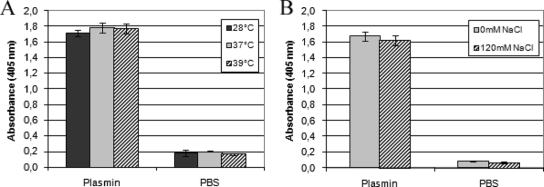
Because some virulence factors are induced by salt and temperature (43, 49, 55), we decided to examine whether osmolarity and temperature could influence PLG binding and activation by leptospires. Assays were performed with the virulent low-passage L. interrogans serovar Copenhageni strain Fiocruz L1-130. The transition of temperatures from ambient to mammalian body (37 and later 39°C) has been correlated with changes in the expression of virulence determinants in many pathogens (53). Therefore, we compared plasmin generation in leptospires of cultures grown at 28°C, 37°C, and 39°C, reflecting growth under laboratory conditions and in the mammalian host under normal and febrile temperatures, respectively. Plasmin generation by Leptospira showed no difference under the temperatures tested (Fig. 5A). PLG binding/activation by osmolarity was assessed by addition of 120 mM NaCl to the EMJH medium supplemented with 10% Leptospira enrichment EMJH medium and incubation at 28°C. The addition of 120 mM NaCl to the medium mimics physiological conditions (∼300 mosmol/liter) encountered by leptospires upon entry into the host (49). Cultures were incubated for 24 h and examined for PLG binding. As shown in Fig. 5B, PLG binding/activation is not affected by osmolarity because similar plasmin generation was detected upon incubation in normal growth medium and with additional NaCl.
FIG. 5.
Influence of temperature and osmolarity on the binding of PLG to leptospires. Low-passage virulent L. interrogans serovar Copenhageni cells cultured at 28°C in EHJM medium supplemented with 10% Leptospira enrichment EMJH medium were shifted to 37 or 39°C for 24 h (A) or maintained at 28°C but supplemented with 120 mM NaCl (B). The cells were then treated with 5 μg PLG together with uPA or only with PBS. Bars represent the mean absorbance as a measure of relative substrate degradation ± the standard deviation of four replicates for each experimental group.
Analysis of PLG binding to proteins of L. interrogans.
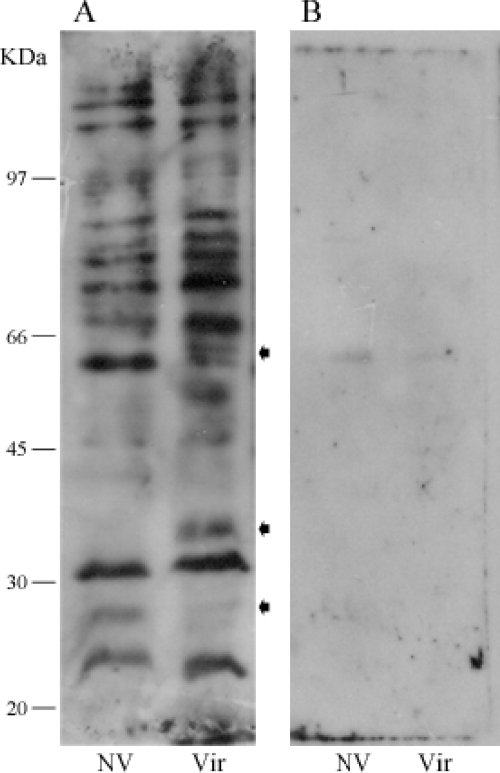
The binding of leptospiral protein receptors to PLG was visualized by affinity blotting using low-passage virulent and high-passage nonvirulent bacterial protein extracts. Blotted membranes were probed with PLG followed by immune detection. Similar results were obtained from three independent experiments, and one representative affinity blotting is depicted in Fig. 6A. It is possible to observe at least 10 leptospiral proteins bind to PLG under the conditions employed with both the virulent and nonvirulent strains. The three arrows point out the existence of three distinct reactive protein regions between the protein extracts of the virulent and the nonvirulent attenuated L. interrogans strains. The protein reactive bands were almost totally abolished when the lysine analog ACA was added to both leptospiral protein extracts (Fig. 6B).
FIG. 6.
Binding of human PLG to L. interrogans proteins by affinity blotting. Total protein extracts were resolved by 10% SDS-PAGE and electroblotted into nitrocellulose membranes. The membranes were incubated with PLG (A) or PLG plus ACA (B), and the binding was detected by anti-PLG antibodies and peroxidase-conjugated antibodies. The reactivity was revealed by ECL (GE Healthcare) and exposure to X-ray films. NV, high-passage nonvirulent L. interrogans serovar Copenhageni strain M 20; Vir, low-passage virulent L. interrogans serovar Copenhageni strain Fiocruz L1-130. The arrows indicate reactivity protein regions seen in the virulent bacterial extract but absent in the nonvirulent bacterial extract. Positions of protein molecular mass markers are shown on the left.
PAS among different species of Leptospira.
In order to evaluate if the PLG binding and activation are extended to other Leptospira species, we performed experiments with different pathogenic high-passage nonvirulent strains of Leptospira (L. interrogans serovars Canicola, Interohaemorrhagiae, and Pomona; L. borgpetersenii serovars Castelonis and Whitcombi; L. santarosai serovar Shermani; and L. kirshneri serovars Cynoptery and Grippotyphosa) and one saprophytic strain (L. biflexa serovar Patoc). Nonvirulent L. interrogans serovar Copenhageni was added in these experiments for comparison. As shown in Fig. 7, our results indicate that all of the strains tested are capable of binding PLG and generating active plasmin in the presence of uPA at the surface of bacteria, including, to some extent, the nonpathogenic saprophytic species L. biflexa.
FIG. 7.
Acquisition of plasmin activity by different strains of Leptospira. Live high-passage pathogenic L. interrogans serovar Canicola, L. interrogans serovar Interohaemorrhagiae, L. interrogans serovar Pomona, L. borgpetersenii serovar Castelonis, L. borgpetersenii serovar Whitcombi, L. santarosai serovar Shermani, L. kirshneri serovar Cynoptery, L. kirshneri serovar Grippotyphosa, and saprophytic L. biflexa serovar Patoc strains were treated with the following: no additions (PBS), uPA alone (uPA), 5 μg PLG alone (PLG), and 5 μg PLG together with uPA (plasmin). Bars represent the mean absorbance as a measure of relative substrate degradation ± the standard deviation of three replicates for each experimental group and are representative of two independent experiments.
Plasmin-coated Leptospira degrades immobilized fibronectin.
Binding and activation of PLG to generate the proteolytic enzyme plasmin have been correlated with the invasive potential of certain bacteria (77). In order to determine whether plasmin-coated leptospires were able to degrade the ECM component fibronectin, as previously demonstrated with B. burgdorferi (15), purified soluble human fibronectin was adhered onto 96-well plates. Degradation was evaluated by exposing the ECM proteins to low-passage virulent L. interrogans cells pretreated with PLG and/or uPA. The substrate breakdown was measured with polyclonal antibodies. Degradation of fibronectin was calculated by decreased absorbance compared to that of the positive control without bacteria. As shown in Fig. 8, the plasmin-coated leptospires were able to significantly break down the immobilized fibronectin compared to the untreated bacteria.
FIG. 8.
Fibronectin degradation by plasmin-coated leptospires. Spirochetes were incubated in lsPBS with no additions (UT) and with addition of PLG and uPA (plasmin). ELISA plate wells coated with fibronectin (three replicates) were incubated for 20 h with the spirochetes from each experimental group. Undegraded substrate was detected, and percent degradation (with a reduction in absorbance value interpreted as substrate degradation) was calculated as described in Materials and Methods. Bars represent the mean percent substrate degradation relative to the positive control (0% degradation) ± the standard deviation of three replicates. The experiment was independently performed three times, and significance was assessed by Student′s two-tailed t test. *, P < 0.005.
DISCUSSION
Several invasive gram-positive and gram-negative bacteria have shown the ability to interact with the host PLG system (6, 13, 19, 37, 73), a phenomenon that has been extended to viruses (22, 38, 60) and parasites (68), either by expression of PLG receptors or by stimulation of host activators. It has been demonstrated by in vitro and/or in vivo animal model of pathogenesis and infection that the PAS has a critical function for invasiveness and establishment of the infection for several microorganisms such as Streptococcus spp., Leishmania mexicana, and Staphylococcus aureus (23, 41, 46, 47, 69, 74). In the case of spirochetes, the PAS was studied with several species of Borrelia and with Treponema denticola and was suggested to have an important role during infectiveness (14, 16, 21, 35, 58).
L. interrogans serovar Copenhageni is capable of binding PLG in its outer surface as visualized by indirect immunofluorescence with living leptospires. The bound PLG is activated to plasmin by addition of human activator uPA, promoting a surface-associated plasmin activity within the bacteria. Plasmin bound to the surface of L. interrogans is enzymatically active, as measured by the degradation of chromogenic specific plasmin substrate. Small amounts of PLG were required for the detection of statistically significant plasmin activity compared to that of the controls. The fluorescence observed from the binding of leptospires with PLG suggests that the bacteria have receptors along their surfaces. This is comparable to, although less intense than, the binding pattern observed with LipL32, but it is in contrast with our previous data with two proteins, MPL17 and MPL21 (61), that presented a dot pattern of fluorescence. The leptospiral membrane probably remains intact as treatment with mouse antibody to GroEL, the protoplasmic-cylinder heat shock protein, and goat anti-mouse FITC-conjugated antibody did not exhibit any fluorescence within the bacteria. In addition, we have observed that the bacterial cells preserved the ordinary rotational movement after the treatment. As reported for the spirochetes Borrelia and Treponema (37), Leptospira, either as whole cells or lysate, is dependent on the host PLG activation system because the labeling with PLG alone did not generate proteolytic activity, in contrast to some pathogens that present a PLG endogenous activation system (40-42, 52, 59, 70, 71, 79).
PLG is present in plasma at a concentration of ∼20.8 ± 1.9 mg/100 ml (17). Our data show that virulent L. interrogans, but not the attenuated strain, has the ability to bind PLG from human plasma (∼6 μg of PLG contained in 30% of human plasma) under the conditions assayed. The plasmin activity measured is lower than that with a similar concentration of added purified PLG (5 μg), probably due to the presence of a complex mixture of proteins in human fluid that could compete for the binding reaction. In any event, once in the host, the leptospires can acquire PLG when they reach the blood circulation. Despite the proteolytic activity associated with the outer surface conferred by bound PLG activated to plasmin, L. interrogans was probably not damaged because cellular growth was not impaired.
The process for binding to bacterial receptors on the cell surface has been shown for several pathogens to be mediated by the PLG kringle domains through the contained lysine-binding sites (2, 10, 16, 32, 33, 46, 63, 68, 77, 78). Likewise, Leptospira probably interacts with PLG through the lysine residues because the binding and generation of active plasmin on the bacterial surface were inhibited by the presence of the lysine analogue ACA in the reaction. The inhibition was dose dependent on the ACA concentration,with the plasmin activity almost totally abolished by the addition of 100 mM ACA.
The involvement of OMPs in the acquisition of PLG by Leptospira, as expected, is clearly shown in the experiments with DP partitioning proteins. Surprisingly, our data shows that the contribution of aqueous soluble proteins in the binding process is higher than with proteins in the detergent fraction (OMPs). It has been shown that the major leptospiral antigens LipL32 and LipL48 are digested, probably by an endogenous protease, during TX-114 solubilization (18). Moreover, some subsurface proteins, such as penicillin-binding proteins, did not solubilize in the TX-114 phase (27). These events may account for the differences observed with proteins from the DP and AP. In any event, our data suggest that there are several proteins that may act as a PLG receptor in Leptospira (Vieira et al., unpublished data). It has been demonstrated that GAPDH (glyceraldehyde-3-phosphate dehydrogenase) is a major surface protein on group A streptococci with multiple binding activities, including plasmin receptor (8, 44, 45, 62). GAPDH or members of the GAPDH-related family are conserved among several bacteria, including spirochetes, and it has been suggested that borrelial GAPDH homolog may contribute to plasmin(ogen) acquisition (1). In Leptospira, there is one gene coding for GAPDH that is predicted to be located at the cytoplasm of the bacteria, according to the PSORT program (54). Thus, GAPDH may be one of the proteins involved in PLG acquisition observed with AP proteins.
Regulation of virulence by environmental cues, such as temperature and osmolarity, has been reported in a variety of pathogens (53). In Leptospira, several genes that are differentially expressed under different temperature conditions were identified by whole-genome microarrays (43). Moreover, 6% of the leptospiral genes were susceptible to osmoregulation (49). Intriguingly, plasmin generation was not affected when Leptospira cultivated at 28°C was shifted to temperatures found in the mammalian host under normal and febrile conditions. Likewise, the shift to physiologic osmolarity had no influence on PLG binding and plasmin generation by leptospires. Thus, virulence factors identified in Leptospira, such as LigA/LigB (50, 51) and Lsa21 (3), and known to be upregulated by temperature and osmolarity are probably not involved in PLG acquisition by leptospires.
The phenomenon of binding to PLG was observed with pathogenic high-passage nonvirulent strains from different species of the genus Leptospira and was also extended to the nonpathogenic saprophytic organism L. biflexa. However, as observed with the spirochete B. burgdorferi (12), the infectious low-passage L. interrogans serovar Copenhageni strain Fiocruz L1-130 was more efficient in capturing PLG (P < 0.001) on its surface than the noninfectious high-passage L. interrogans serovar Copenhageni strain M 20, a result that suggests an alteration of PLG protein receptors during the attenuation process.
The affinity blotting experiments using leptospire whole-cell lysates corroborate with these data, although the conditions assayed do not simulate the ones from the PLG binding with the intact bacteria. Comparable to the spirochete B. burgdorferi (16), the affinity blotting was inhibited by the presence of the lysine analog ACA. It is noteworthy that with the whole-cell lysates of the virulent strain, a cluster of proteins are involved in the PLG binding different from the profile of the attenuated strain. Whether the binding observed with the virulent strain of Leptospira is due to the expression of diverse protein receptors, a higher number of the same receptors, or both, deserves to be further studied.
L. interrogans is a highly invasive pathogen. The bacteria are thought to penetrate the skin or a break in the skin to initiate infection and then rapidly disseminate via the bloodstream to cause multisystem infection, targeting the liver and kidney (5, 20). ECM-binding proteins such LenA (72), formerly LfhA/Lsa24 (4, 75), LigA/LigB (11), and LipL32 (29, 30) have been identified, but thus far, ECM degradation by leptospires has not been described. The spirochete B. burgdorferi was demonstrated to be capable of degrading ECM macromolecules when bound to plasmin (15), and this activity has been suggested to contribute to bacterial invasion (12, 15, 16). Plasmin-coated leptospires were also capable of employing this proteolytic activity to in vitro degrade the immobilized ECM component fibronectin.
Although more studies are required to identify leptospiral protein receptors involved in plasmin activation as well as to fully investigate the degradation of ECM components, our data show for the first time that Leptospira can borrow the PAS from the host and that this activity might be employed for the invasion process. We trust that the description of this new feature of Leptospira presented in this study would help to identify proteins of importance in pathogenesis and hence potential antigens for the development of an efficient vaccine against leptospirosis.
Acknowledgments
This work has benefited from grants from FAPESP, CNPq, and Fundação Butantan. M.L.V. has a Ph.D. fellowship from FAPESP (Brazil).
Editor: A. Camilli
Footnotes
Published ahead of print on 6 July 2009.
REFERENCES
- 1.Anda, P., J. A. Gebbia, P. B. Backenson, J. L. Coleman, and J. L. Benach. 1996. A glyceraldehyde-3-phosphate dehydrogenase homolog in Borrelia burgdorferi and Borrelia hermsii. Infect. Immun. 64262-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attali, C., C. Durmort, T. Vernet, and A. M. Di Guilmi. 2008. The interaction of Streptococcus pneumoniae with plasmin mediates transmigration across endothelial and epithelial monolayers by intercellular junction cleavage. Infect. Immun. 765350-5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atzingen, M. V., A. S. Barbosa, T. De Brito, S. A. Vasconcellos, Z. M. de Morais, D. M. Lima, P. A. Abreu, and A. L. Nascimento. 2008. Lsa21, a novel leptospiral protein binding adhesive matrix molecules and present during human infection. BMC Microbiol. 870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbosa, A. S., P. A. E. Abreu, F. O. Neves, M. V. Atzingen, M. M. Watanabe, M. L. Vieira, Z. M. Morais, S. A. Vasconcellos, and A. L. T. O. Nascimento. 2006. A newly identified leptospiral adhesin mediates attachment to laminin. Infect. Immun. 746356-6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barocchi, M. A., A. I. Ko, M. G. Reis, K. L. McDonald, and L. W. Riley. 2002. Rapid translocation of polarized MDCK cell monolayers by Leptospira interrogans, an invasive but nonintracellular pathogen. Infect. Immun. 706926-6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergmann, S., and S. Hammerschmidt. 2007. Fibrinolysis and host response in bacterial infections. Thromb. Haemos. 98512-520. [PubMed] [Google Scholar]
- 7.Bharti, A. R., J. E. Nally, J. N. Ricaldi, M. A. Matthias, M. M. Diaz, M. A. Lovett, P. N. Levett, R. H. Gilman, M. R. Willig, E. Gotuzzo, and J. M. Vinetz. 2003. Leptospirosis: a zoonotic disease of global importance. Lancet Infect. Dis. 3757-771. [DOI] [PubMed] [Google Scholar]
- 8.Border, W. A., S. Okuda, T. Nakamura, L. R. Languino, and E. Ruoslahti. 1991. Role of TGF-beta 1 in experimental glomerulonephritis. Ciba Found. Symp. 157178-193. [PubMed] [Google Scholar]
- 9.Bulach, D. M., R. L. Zuerner, P. Wilson, T. Seemann, A. McGrath, P. A. Cullen, J. Davis, M. Johnson, E. Kuczek, D. P. Alt, B. Peterson-Burch, R. L. Coppel, J. I. Rood, J. K. Davies, and B. Adler. 2006. Genome reduction in Leptospira borgpetersenii reflects limited transmission potential. Proc. Natl. Acad. Sci. USA 10314560-14565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Candela, M., G. Miccoli, S. Bergmann, S. Turroni, B. Vitali, S. Hammerschmidt, and P. Brigidi. 2008. Plasminogen-dependent proteolytic activity in Bifidobacterium lactis. Microbiology 1542457-2462. [DOI] [PubMed] [Google Scholar]
- 11.Choy, H. A., M. M. Kelley, T. L. Chen, A. K. Møller, J. Matsunaga, and D. A. Haake. 2007. Physiological osmotic induction of Leptospira interrogans adhesion: LigA and LigB bind extracellular matrix proteins and fibrinogen. Infect. Immun. 752441-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coleman, J. L., and J. L. Benach. 2000. The generation of enzymatically active plasmin on the surface of spirochetes. Methods 21133-141. [DOI] [PubMed] [Google Scholar]
- 13.Coleman, J. L., and J. L. Benach. 1999. Use of the plasminogen activation system by microorganisms. J. Lab. Clin. Med. 134567-576. [DOI] [PubMed] [Google Scholar]
- 14.Coleman, J. L., J. A. Gebbia, J. Piesman, J. L. Degen, T. H. Bugge, and J. L. Benach. 1997. Plasminogen is required for efficient dissemination of B. burgdorferi in ticks and for enhancement of spirochetemia in mice. Cell 891111-1119. [DOI] [PubMed] [Google Scholar]
- 15.Coleman, J. L., E. J. Roemer, and J. L. Benach. 1999. Plasmin-coated Borrelia burgdorferi degrades soluble and insoluble components of the mammalian extracellular matrix. Infect. Immun. 673929-3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coleman, J. L., T. J. Sellati, J. E. Testa, R. R. Kew, M. B. Furie, and J. L. Benach. 1995. Borrelia burgdorferi binds plasminogen, resulting in enhanced penetration of endothelial monolayers. Infect. Immun. 632478-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collen, D., G. Tytgat, H. Claeys, M. Verstraete, and P. Wallen. 1972. Metabolism of plasminogen in healthy subjects: effect of tranexamic acid. J. Clin. Investig. 511310-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cullen, P. A., D. A. Haake, and B. Adler. 2004. Outer membrane proteins of pathogenic spirochetes. FEMS Microbiol. Rev. 28291-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Degen, J. L., T. H. Bugge, and J. D. Goguen. 2007. Fibrin and fibrinolysis in infection and host defense. J. Thromb. Haemost. 5(Suppl 1)24-31. [DOI] [PubMed] [Google Scholar]
- 20.Faine, S., B. Adler, C. Bolin, and P. Perolat. 1999. Leptospira and leptospirosis, 2nd ed, p. 259. MediSci, Melbourne, Australia.
- 21.Fenno, J. C., M. Tamura, P. M. Hannam, G. W. K. Wong, R. A. Chan, and B. C. McBride. 2000. Identification of a Treponema denticola OppA homologue that binds host proteins present in the subgingival environment. Infect. Immun. 681884-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goto, H., and Y. Kawaoka. 1998. A novel mechanism for the acquisition of virulence by a human influenza A virus. Proc. Natl. Acad. Sci. USA 9510224-10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo, Y., J. Li, E. Hagstrom, and T. Ny. 2008. Protective effects of plasmin(ogen) in a mouse model of Staphylococcus aureus-induced arthritis. Arthritis Rheum. 58764-772. [DOI] [PubMed] [Google Scholar]
- 24.Haake, D. A., G. Chao, R. L. Zuerner, J. K. Barnett, D. Barnett, M. Mazel, J. Matsunaga, P. N. Levett, and C. A. Bolin. 2000. The leptospiral major outer membrane protein LipL32 is a lipoprotein expressed during mammalian infection. Infect. Immun. 682276-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haake, D. A., M. Dundoo, R. Cader, B. M. Kubak, R. A. Hartskeerl, J. J. Sejvar, and D. A. Ashford. 2002. Leptospirosis, water sports, and chemoprophylaxis. Clin. Infect. Dis. 34e40-e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haake, D. A., and J. Matsunaga. 2002. Characterization of the leptospiral outer membrane and description of three novel leptospiral membrane proteins. Infect. Immun. 704936-4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haake, D. A., E. M. Walker, D. R. Blanco, C. A. Bolin, M. N. Miller, and M. A. Lovett. 1991. Changes in the surface of Leptospira interrogans serovar grippotyphosa during in vitro cultivation. Infect. Immun. 591131-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haile, W. B., J. L. Coleman, and J. L. Benach. 2006. Reciprocal upregulation of urokinase plasminogen activator and its inhibitor, PAI-2, by Borrelia burgdorferi affects bacterial penetration and host-inflammatory response. Cell. Microbiol. 81349-1360. [DOI] [PubMed] [Google Scholar]
- 29.Hauk, P., F. Macedo, E. C. Romero, S. A. Vasconcellos, Z. M. de Morais, A. S. Barbosa, and P. L. Ho. 2008. In LipL32, the major leptospiral lipoprotein, the C terminus is the primary immunogenic domain and mediates interaction with collagen IV and plasma fibronectin. Infect. Immun. 762642-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoke, D. E., S. Egan, P. A. Cullen, and B. Adler. 2008. LipL32 is an extracellular matrix-interacting protein of Leptospira spp. and Pseudoalteromonas tunicata. Infect. Immun. 762063-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu, L. T., G. Perides, R. Noring, and M. S. Klempner. 1995. Binding of human plasminogen to Borrelia burgdorferi. Infect. Immun. 633491-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jobin, M. C., J. Brassard, S. Quessy, M. Gottschalk, and D. Grenier. 2004. Acquisition of host plasmin activity by the swine pathogen Streptococcus suis serotype 2. Infect. Immun. 72606-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kinnby, B., N. A. Booth, and G. Svensater. 2008. Plasminogen binding by oral streptococci from dental plaque and inflammatory lesions. Microbiology 154924-931. [DOI] [PubMed] [Google Scholar]
- 34.Klempner, M. S., R. Noring, M. P. Epstein, B. McCloud, R. Hu, S. A. Limentani, and R. A. Rogers. 1995. Binding of human plasminogen and urokinase-type plasminogen activator to the Lyme disease spirochete, Borrelia burgdorferi. J. Infect. Dis. 1711258-1265. [DOI] [PubMed] [Google Scholar]
- 35.Klempner, M. S., R. Noring, M. P. Epstein, B. McCloud, and R. A. Rogers. 1996. Binding of human urokinase type plasminogen activator and plasminogen to Borrelia species. J. Infect. Dis. 17497-104. [DOI] [PubMed] [Google Scholar]
- 36.Ko, A. I., M. Galvao Reis, C. M. Ribeiro Dourado, W. D. Johnson, Jr., L. W. Riley, et al. 1999. Urban epidemic of severe leptospirosis in Brazil. Lancet 354820-825. [DOI] [PubMed] [Google Scholar]
- 37.Lahteenmaki, K., P. Kuusela, and T. K. Korhonen. 2001. Bacterial plasminogen activators and receptors. FEMS Microbiol. Rev. 25531-552. [DOI] [PubMed] [Google Scholar]
- 38.LeBouder, F., E. Morello, G. F. Rimmelzwaan, F. Bosse, C. Péchoux, B. Delmas, and B. Riteau. 2008. Annexin II incorporated into influenza virus particles supports virus replication by converting plasminogen into plasmin. J. Virol. 826820-6828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levett, P. N. 2001. Leptospirosis. Clin. Microbiol. Rev. 14296-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leytus, S. P., L. K. Bowles, J. Konisky, and W. F. Mangel. 1981. Activation of plasminogen to plasmin by a protease associated with the outer membrane of Escherichia coli. Proc. Natl. Acad. Sci. USA 781485-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li, Z., V. A. Ploplis, E. L. French, and M. D. Boyle. 1999. Interaction between group A streptococci and the plasmin(ogen) system promotes virulence in a mouse skin infection model. J. Infect. Dis. 179907-914. [DOI] [PubMed] [Google Scholar]
- 42.Lijnen, H. R., F. De Cock, B. Van Hoef, B. Schlott, and D. Collen. 1994. Characterization of the interaction between plasminogen and staphylokinase. Eur. J. Biochem. 224143-149. [DOI] [PubMed] [Google Scholar]
- 43.Lo, M., D. M. Bulach, D. R. Powell, D. A. Haake, J. Matsunaga, M. L. Paustian, R. L. Zuerner, and B. Adler. 2006. Effects of temperature on gene expression patterns in Leptospira interrogans serovar Lai as assessed by whole-genome microarrays. Infect. Immun. 745848-5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lottenberg, R., C. C. Broder, and M. D. P. Boyle. 1987. Identification of a specific receptor for plasmin on a group A streptococcus. Infect. Immun. 551914-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lottenberg, R., C. C. Broder, M. D. P. Boyle, S. J. Kain, B. L. Schroeder, and R. Curtiss III. 1992. Cloning, sequence analysis, and expression in Escherichia coli of a streptococcal plasmin receptor. J. Bacteriol. 1745204-5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magalhaes, V., I. Veiga-Malta, M. R. Almeida, M. Baptista, A. Ribeiro, P. Trieu-Cuot, and P. Ferreira. 2007. Interaction with human plasminogen system turns on proteolytic activity in Streptococcus agalactiae and enhances its virulence in a mouse model. Microbes Infect. 91276-1284. [DOI] [PubMed] [Google Scholar]
- 47.Maldonado, J., C. Marina, J. Puig, Z. Maizo, and L. Avilan. 2006. A study of cutaneous lesions caused by Leishmania mexicana in plasminogen-deficient mice. Exp. Mol. Pathol. 80289-294. [DOI] [PubMed] [Google Scholar]
- 48.Matsunaga, J., M. A. Barocchi, J. Croda, T. A. Young, Y. Sanchez, I. Siqueira, C. A. Bolin, M. G. Reis, L. W. Riley, D. A. Haake, and A. I. Ko. 2003. Pathogenic Leptospira species express surface-exposed proteins belonging to the bacterial immunoglobulin superfamily. Mol. Microbiol. 49929-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsunaga, J., M. Lo, D. M. Bulach, R. L. Zuerner, B. Adler, and D. A. Haake. 2007. Response of Leptospira interrogans to physiologic osmolarity: relevance in signaling the environment-to-host transition. Infect. Immun. 752864-2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsunaga, J., M. A. Medeiros, Y. Sanchez, K. F. Werneid, and A. I. Ko. 2007. Osmotic regulation of expression of two extracellular matrix-binding proteins and a haemolysin of Leptospira interrogans: differential effects on LigA and Sph2 extracellular release. Microbiology 1533390-3398. [DOI] [PubMed] [Google Scholar]
- 51.Matsunaga, J., Y. Sanchez, X. Xu, and D. A. Haake. 2005. Osmolarity, a key environmental signal controlling expression of leptospiral proteins LigA and LigB and the extracellular release of LigA. Infect. Immun. 7370-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCoy, H. E., C. C. Broder, and R. Lottenberg. 1991. Streptokinases produced by pathogenic group C streptococci demonstrate species-specific plasminogen activation. J. Infect. Dis. 164515-521. [DOI] [PubMed] [Google Scholar]
- 53.Mekalanos, J. J. 1992. Environmental signals controlling expression of virulence determinants in bacteria. J. Bacteriol. 1741-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakai, K., and M. Kanehisa. 1991. Expert system for predicting protein localization sites in gram-negative bacteria. Proteins 1195-110. [DOI] [PubMed] [Google Scholar]
- 55.Nally, J. E., J. F. Timoney, and B. Stevenson. 2001. Temperature-regulated protein synthesis by Leptospira interrogans. Infect. Immun. 69400-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nally, J. E., J. P. Whitelegge, S. Bassilian, D. R. Blanco, and M. A. Lovett. 2007. Characterization of the outer membrane proteome of Leptospira interrogans expressed during acute lethal infection. Infect. Immun. 75766-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nascimento, A. L., S. Verjovski-Almeida, M. A. Van Sluys, C. B. Monteiro-Vitorello, L. E. Camargo, L. A. Digiampietri, R. A. Harstkeerl, P. L. Ho, M. V. Marques, M. C. Oliveira, J. C. Setubal, D. A. Haake, and E. A. Martins. 2004. Genome features of Leptospira interrogans serovar Copenhageni. Braz. J. Med. Biol. Res. 37459-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nordstrand, A., A. Shamaei-Tousi, A. Ny, and S. Bergstrom. 2001. Delayed invasion of the kidney and brain by Borrelia crocidurae in plasminogen-deficient mice. Infect. Immun. 695832-5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Okada, K., S. Ueshima, T. Takaishi, H. Yuasa, H. Fukao, and O. Matsuo. 1996. Effects of fibrin and alpha2-antiplasmin on plasminogen activation by staphylokinase. Am. J. Hematol. 53151-157. [DOI] [PubMed] [Google Scholar]
- 60.Okumura, Y., M. Yano, M. Murakami, S. Mori, T. Towatari, and H. Kido. 1999. The extracellular processing of HIV-1 envelope glycoprotein gp160 by human plasmin. FEBS Lett. 44239-42. [DOI] [PubMed] [Google Scholar]
- 61.Oliveira, T. R., M. T. Longhi, Z. M. de Morais, E. C. Romero, R. M. Blanco, K. Kirchgatter, S. A. Vasconcellos, and A. L. Nascimento. 2008. Evaluation of leptospiral recombinant antigens MPL17 and MPL21 for serological diagnosis of leptospirosis by enzyme-linked immunosorbent assays. Clin. Vaccine Immunol. 151715-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pancholi, V., and V. A. Fischetti. 1992. A major surface protein on group A streptococci is a glyceraldehyde-3-phosphate-dehydrogenase with multiple binding activity. J. Exp. Med. 176415-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pantzar, M., Å. Ljungh, and T. Wadström. 1998. Plasminogen binding and activation at the surface of Helicobacter pylori CCUG 17874. Infect. Immun. 664976-4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Picardeau, M., D. M. Bulach, C. Bouchier, R. L. Zuerner, N. Zidane, P. J. Wilson, S. Creno, E. S. Kuczek, S. Bommezzadri, J. C. Davis, A. McGrath, M. J. Johnson, C. Boursaux-Eude, T. Seemann, Z. Rouy, R. L. Coppel, J. I. Rood, A. Lajus, J. K. Davies, C. Medigue, and B. Adler. 2008. Genome sequence of the saprophyte Leptospira biflexa provides insights into the evolution of Leptospira and the pathogenesis of leptospirosis. PLoS ONE 3e1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Plank, R., and D. Dean. 2000. Overview of the epidemiology, microbiology, and pathogenesis of Leptospira spp. in humans. Microbes Infect. 21265-1276. [DOI] [PubMed] [Google Scholar]
- 66.Ren, S. X., G. Fu, X. G. Jiang, R. Zeng, Y. G. Miao, H. Xu, Y. X. Zhang, H. Xiong, G. Lu, L. F. Lu, H. Q. Jiang, J. Jia, Y. F. Tu, J. X. Jiang, W. Y. Gu, Y. Q. Zhang, Z. Cai, H. H. Sheng, H. F. Yin, Y. Zhang, G. F. Zhu, M. Wan, H. L. Huang, Z. Qian, S. Y. Wang, W. Ma, Z. J. Yao, Y. Shen, B. Q. Qiang, Q. C. Xia, X. K. Guo, A. Danchin, I. Saint Girons, R. L. Somerville, Y. M. Wen, M. H. Shi, Z. Chen, J. G. Xu, and G. P. Zhao. 2003. Unique physiological and pathogenic features of Leptospira interrogans revealed by whole-genome sequencing. Nature 422888-893. [DOI] [PubMed] [Google Scholar]
- 67.Ristow, P., P. Bourhy, F. W. da Cruz McBride, C. P. Figueira, M. Huerre, P. Ave, I. S. Girons, A. I. Ko, and M. Picardeau. 2007. The OmpA-like protein Loa22 is essential for leptospiral virulence. PLoS Pathog. 3e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rojas, M., I. Labrador, J. L. Concepcion, E. Aldana, and L. Avilan. 2008. Characteristics of plasminogen binding to Trypanosoma cruzi epimastigotes. Acta Trop. 10754-58. [DOI] [PubMed] [Google Scholar]
- 69.Sanderson-Smith, M. L., K. Dinkla, J. N. Cole, A. J. Cork, P. G. Maamary, J. D. McArthur, G. S. Chhatwal, and M. J. Walker. 2008. M protein-mediated plasminogen binding is essential for the virulence of an invasive Streptococcus pyogenes isolate. FASEB J. 222715-2722. [DOI] [PubMed] [Google Scholar]
- 70.Schlott, B., K. H. Guhrs, M. Hartmann, A. Rocker, and D. Collen. 1998. NH2-terminal structural motifs in staphylokinase required for plasminogen activation. J. Biol. Chem. 27322346-22350. [DOI] [PubMed] [Google Scholar]
- 71.Sodeinde, O. A., Y. V. Subrahmanyam, K. Stark, T. Quan, Y. Bao, and J. D. Goguen. 1992. A surface protease and the invasive character of plague. Science 2581004-1007. [DOI] [PubMed] [Google Scholar]
- 72.Stevenson, B., H. A. Choy, M. Pinne, M. L. Rotondi, M. C. Miller, E. Demoll, P. Kraiczy, A. E. Cooley, T. P. Creamer, M. A. Suchard, C. A. Brissette, A. Verma, and D. A. Haake. 2007. Leptospira interrogans endostatin-like outer membrane proteins bind host fibronectin, laminin and regulators of complement. PLoS ONE 2e1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun, H. 2006. The interaction between pathogens and the host coagulation system. Physiology 21281-288. [DOI] [PubMed] [Google Scholar]
- 74.Svensson, M. D., U. Sjobring, F. Luo, and D. E. Bessen. 2002. Roles of the plasminogen activator streptokinase and the plasminogen-associated M protein in an experimental model for streptococcal impetigo. Microbiology 1483933-3945. [DOI] [PubMed] [Google Scholar]
- 75.Verma, A., J. Hellwage, S. Artiushin, P. F. Zipfel, P. Kraiczy, J. F. Timoney, and B. Stevenson. 2006. LfhA, a novel factor H-binding protein of Leptospira interrogans. Infect. Immun. 742659-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vinetz, J. M. 2001. Leptospirosis. Curr. Opin. Infect. Dis. 14527-538. [DOI] [PubMed] [Google Scholar]
- 77.Xolalpa, W., A. J. Vallecillo, M. Lara, G. Mendoza-Hernandez, M. Comini, R. Spallek, M. Singh, and C. Espitia. 2007. Identification of novel bacterial plasminogen-binding proteins in the human pathogen Mycobacterium tuberculosis. Proteomics 73332-3341. [DOI] [PubMed] [Google Scholar]
- 78.Yavlovich, A., A. A.-R. Higazi, and S. Rottem. 2001. Plasminogen binding and activation by Mycoplasma fermentans. Infect. Immun. 691977-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Young, K. C., G. Y. Shi, D. H. Wu, L. C. Chang, B. I. Chang, C. P. Ou, and H. L. Wu. 1998. Plasminogen activation by streptokinase via a unique mechanism. J. Biol. Chem. 2733110-3116. [DOI] [PubMed] [Google Scholar]