Abstract
We performed a cost analysis study using decision tree modeling to determine whether the use of multiplex PCR testing for respiratory viruses (xTAG RVP test) is a more or less costly strategy than the status quo testing methods used for the diagnosis of respiratory virus infections in pediatric patients. The decision tree model was constructed by using four testing strategies for respiratory virus detection, viz., direct fluorescent-antibody staining (DFA) alone, DFA plus shell vial culture (SVC), the xTAG RVP test alone, or DFA plus the xTAG RVP test. A review of the charts of 661 pediatric patients was used to determine the length of hospital stay, the number of days in isolation, antibiotic usage, and all other medical procedures performed. The cost of hospitalization by diagnostic status was determined on the basis of the average cost per patient and the number of patients in each arm of the decision tree. The cost per case was the highest for DFA plus SVC at $3,914 (in Canadian dollars), and the lowest was for the xTAG RVP test alone at $3,623, while the costs of DFA alone ($3,911) and DFA plus RVP ($3,849) were intermediate. When all four diagnostic strategies were compared, the least costly strategy was the xTAG RVP test alone when the prevalence of infection was 11% or higher and DFA alone when the prevalence was under 11%. These data indicate a savings of $291 per case investigated if the strategy of using the xTAG RVP test alone was used to replace the status quo test of DFA plus SVC, resulting in a savings of $529,620 per year in direct costs for the four Hamilton, Ontario, Canada, hospitals on the basis of the testing of specimens from 1,820 pediatric inpatients. We conclude that the use of the xTAG RVP test is the least costly strategy for the diagnosis of respiratory virus infections in children and would generate a significant savings for hospitals.
Clinical virology laboratories have historically used traditional methods, such as culture, direct fluorescent-antibody staining (DFA), and enzyme immunoassay, for the diagnosis of respiratory tract infections (3). DFA offers a rapid turnaround time for results but is labor-intensive and subjective and requires specific monoclonal antibodies and trained technologists. Both DFA and shell vial culture (SVC) are limited by the availability of monoclonal antibodies, precluding their use for the detection of newly discovered viruses. DFA has a low sensitivity for the detection of some viruses, especially adenovirus, and many laboratories reflex DFA-negative specimens into SVCs to improve the detection rates. For traditional methods such as DFA and SVC, turnaround times for results can be slow for laboratories handling large volumes of specimens. Rapid enzyme immunoassays have been used for the detection of influenza virus and respiratory syncytial virus (RSV), but these tests are only 50 to 70% sensitive (5, 15), which limits their use to specific point-of-care settings at times when the prevalence of infection is high.
Over the past 10 years, nucleic acid amplification tests have been developed for an increasing number of respiratory viruses. Nucleic acid amplification tests, including PCR and nucleic acid-sequence based amplification, have shown enhanced sensitivity compared with the sensitivities of DFA and culture for the detection of a number of respiratory viruses (8). The emergence of five new respiratory viruses since 2000, including human metapneumovirus, the sudden acute respiratory syndrome-associated coronavirus, avian influenza virus H5N1, coronaviruses NL63 and HKU1, and human bocavirus, has presented new challenges for clinical laboratories. The absence of commercially available tests for the detection of these emerging viruses often leaves laboratories without the ability to diagnose these important virus infections. Multiplex PCR assays for the detection of multiple respiratory viruses have recently been introduced (for a review, see reference 8). These multiplex assays have heralded a new era in the molecular diagnostics of respiratory virus infections. Some of these tests are now commercially available and can detect up to 18 different respiratory viruses (6). A multiplex PCR test for respiratory viruses (the xTAG RVP test) is the first multiplex PCR to be cleared by the U.S. Food and Drug Administration and has been approved for use for the detection of 12 different respiratory viruses (16). The xTAG RVP test detects 30 to 40% more virus infections than DFA and culture, in part because it is more sensitive for the detection of traditional respiratory viruses, but it also detects nine additional viruses not detected by DFA and SVC (6, 7, 9, 10, 14). These newer multiplex tests are often costly, and the clinical and economic impacts of their implementation in routine hospital laboratories have not been evaluated. We therefore conducted a cost analysis study to compare the costs of the xTAG RVP test to those of conventional tests for the diagnosis of respiratory virus infections in hospitalized patients.
MATERIALS AND METHODS
Detection of respiratory viruses.
The Regional Virology Laboratory at St. Joseph's Healthcare Hamilton, Hamilton, Ontario, Canada, serves a population of 1 million and processes specimens from approximately 4,000 patients annually for the diagnosis of respiratory virus infections. Approximately two-thirds of these are inpatients and one-third are outpatients. At the time that the study was performed, the respiratory virus testing algorithm included DFA and SVC (3). Nasopharyngeal swab specimens were tested by DFA by centrifuging 2.0 ml of the specimen at 2,000 rpm and resuspending the pellet in a minimal volume of phosphate-buffered saline. Cells were spotted onto microscope slides; fixed in cold acetone; and stained with seven monoclonal antibodies to influenza viruses A and B; RSV; parainfluenza virus types 1, 2, and 3; adenovirus; and metapneumovirus (Diagnostic Hybrids Inc., Athens, OH). Specimens that were negative by DFA staining were inoculated the same day into an R-Mix SVC (Diagnostic Hybrids Inc. Athens OH), centrifuged at 2,800 rpm for 45 min, and stained at 48 h with the same monoclonal antibodies used for DFA (3). The xTAG RVP test is a multiplex PCR from Luminex Molecular Diagnostics that detects 18 different respiratory virus types and subtypes using a microfluidic array which is read on a Luminex 100 instrument (4, 6). The test was performed according to the instructions in the package insert. The turnaround times for DFA and DFA plus SVC were 4 h and 48 h, respectively. The turnaround time for both RVP alone and DFA plus RVP was 24 h.
Decision analytic model.
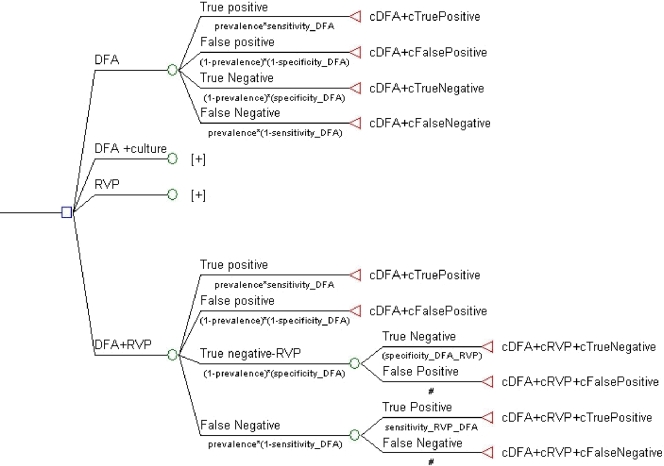
We used a decision analytic model to determine the comparative costs of four diagnostic approaches for the detection of respiratory viruses. These strategies were DFA alone, DFA plus SVC, the xTAG RVP test alone, or DFA plus the xTAG RVP. The cost for each strategy included not only the cost of the viral test(s) but also the cost of the entire inpatient stay. A graphical representation of the decision tree structure showing all four diagnostic testing strategies represented as four nodes is presented in Fig. 1. For each testing strategy, branches were constructed to include the proportion of patients with each diagnostic status, viz., true positive, false positive, true negative, and false negative, defined as prevalence × sensitivity, (1 − prevalence) × (1 − specificity), (1 − prevalence) × specificity, and prevalence × (1 − sensitivity), respectively, on the basis of the outcomes of the laboratory tests (Fig. 1). The tree structure is the same that for the strategy for DFA alone and for the strategy for the xTAG RVP test alone. For the other two test strategies (DFA plus SVC, DFA plus the xTAG RVP test), in which two tests are involved, the structure of the model includes additional nodes for the true-negative and the false-negative results because a second diagnostic test (culture or the xTAG RVP test) was performed for patients initially testing negative by DFA. Therefore, the true diagnostic status of patients receiving a second test depends on the accuracies of both the first DFA for virus and the second test.
FIG. 1.
Graphical representation of the decision tree model showing four nodes representing the four diagnostic strategies. The model was constructed on the basis of a comparison of the costs (c) for the four diagnostic strategies. The model shows four nodes representing the four diagnostic strategies, and each of those nodes is expanded to show each of the four true diagnostic statuses for each diagnostic strategy and the costs associated with each diagnostic status. The number of patients with each diagnostic status, viz., true positive, false positive, true negative, and false negative, was determined for each of the four diagnostic strategies from the chart review. The costs for each testing arm was then determined by using laboratory test costs and all hospital-associated costs.
Model inputs.
A number of different inputs were required for the model. These included the prevalence of virus infection in the population, the sensitivity and the specificity of each laboratory test, the costs of the laboratory tests, and the cost of hospitalization by infection status or diagnosis (true positive, false positive, true negative, false negative). In the model, we used a prevalence of 63.2% of respiratory virus infection, as this was the observed overall prevalence in pediatric cases over the 2 years of the study (9, 10). The performance characteristics (sensitivity, specificity) for each laboratory test were determined from data published in the literature and are shown in Table 1. The sensitivity of 70% for DFA was based on studies described in the literature in which a minimum of three different tests, usually DFA, SVC, and a molecular test, were used; and the sensitivity and specificity of each test were determined by using a combined reference standard of positivity by two or more tests (2). This sensitivity is consistent with data generated from clinical evaluations of the xTAG RVP test, which detected 30 to 35% additional positive specimens, including rhinoviruses and coronaviruses that were not detected by DFA plus culture (6, 9). The sensitivity of 72% for DFA plus SVC was used since a review of all positive results by DFA and SVC over the 2-year period (2005 to 2007) showed that SVC picked up an additional 2% positive specimens, slightly lower than the 5% that we have averaged over the past 10 years. The sensitivity of the xTAG RVP test is based on the overall average sensitivity of detection of the 12 viruses for which the test is approved (4). The specificity of each test was assumed to be 98%.
TABLE 1.
Sensitivity and specificity of the diagnostic tests used in the modela
| Test | Sensitivity (%) | Specificity (%) |
|---|---|---|
| DFA | 70 | 98 |
| DFA + culture | 72 | 98 |
| xTAG RVP | 94 | 98 |
The sensitivity of DFA was taken from the literature (6, 13, 17), where DFA was compared to at least one other test, including at least one molecular test, and where test performance was calculated by using a combined reference standard for positivity. The sensitivity of DFA plus SVC was set at 72%, which showed a 2% increase in the positive detection rate obtained in our laboratory during the study period when SVC was used. The sensitivity of the xTAG RVP test reflects the median sensitivity for 12 respiratory viruses detected by the xTAG RVP test (4). The specificity of each test was set at 98%.
The cost of performing the laboratory tests for respiratory virus detection was based on unit labor costs and the hands-on time, calculated in minutes, required to run a batch of 20 specimens. The actual reagent costs (reagents plus plasticware; all monetary values in this report are in Canadian dollars, unless indicated otherwise) were $13.80 for DFA, $23.20 for SVC, and $80.00 for the xTAG RVP test and were based on list prices; and these costs were used with the mode labor cost of $28.00/h plus 30% fringe benefits for a medical laboratory technologist with 10 years of experience. A chart review of 661 pediatric inpatients (representing a stratified random sample) who were investigated for respiratory virus infections at two tertiary-care hospitals in Hamilton was undertaken to estimate the hospitalization costs for the model. These inpatient episodes occurred between January 2006 and December 2007 at two Hamilton hospitals (St. Josephs Healthcare Hamilton and McMaster University Medical Centre). By using the chart review data, the average cost of a single case by diagnostic status (true positive, false positive, true negative, false negative) was determined. Details of the chart review are provided in the following section.
Chart review.
During the study, patients with suspected respiratory virus infections were tested by DFA or DFA plus SVC and the xTAG RVP test. Since we had over 2,300 inpatients who were tested by all three tests between November 2005 and October 2007, we used a stratified sampling to select a subset of 661 cases for the chart review. Age, gender, admission details, admitting diagnosis, medical history, presenting symptoms and physical findings, management, and discharge diagnosis and medications were abstracted from the charts by using a standardized case report form. The conditions considered in the patient's medical history included allergies, lung disease, asthma, cardiac disease, or cancer. Presenting symptoms included cough, fever, rhinorrhea, shortness of breathe, anorexia, apnea, vomiting, diarrhea, and lethargy. Physical findings included nasal flaring, tracheal tug, grunting, indrawing, wheezing, crackles, and decreased gas exchange efficiency. Laboratory findings included heart rate, respiratory rate, temperature, weight, oxygen saturation, pH, white blood cell count, absolute neutrophil count, and percent bands. Admission details included length of stay, number of days in the intensive care unit, and the number of days in isolation (for droplet containment) were collected. The admission diagnoses included bronchitis, pneumonia, urinary tract infection, fever, otitis media, sepsis, asthma exacerbation, croup, gastroenteritis, or dehydration. The medical procedures included chest X ray; bacterial culture; placement of an endotracheal tube or aspiration; and the provision of supplemental oxygen, mechanical ventilation, antibiotics, antiviral agents, bronchodilators, and steroids; and these were recorded. Discharge diagnosis, medications, and length of days on medications were also recorded. The protocol was approved by the Research Ethics Board at St. Joseph's Healthcare Hamilton and Hamilton Health Sciences, McMaster University.
Hospital-associated costs.
The health care resource use data collected from the chart review were used to calculate the average hospital cost for patients by diagnostic status (true positive, false positive, true negative, false negative). Health care resource use data included the length of hospital stay, the frequency of selected tests (complete blood count, chest X ray, arterial blood gas, bacterial culture, lumbar puncture), and antibiotic use. The costs per day in a pediatric ward ($690.72) and the pediatric intensive care unit ($1,548) were provided by a hospital participating in the Ontario Case Costing Initiative (11). A review of monthly infection control summaries over a 6-month period indicated a rate of isolation of 3.994/1,000 hospital days during the study period. We therefore made the assumption that for patients with a positive diagnosis (a true-positive or a false-positive result), 4 of every 1,000 hospital days would involve isolation for droplet containment precautions. We used a cost of $1,665.12 per day for isolation, the current cost of isolation in Hamilton hospitals. The costs of a complete blood count ($4), chest X ray ($43), arterial blood gas ($10), bacterial culture ($24), and lumbar puncture ($96) were derived from the Ontario Hospital Association rates book (11). The cost of antibiotics was based on the costs for the Ontario drug benefit formulary (12). The average hospital cost for patients with true-positive results was assumed to be equal to the average cost for the 52 patients in the chart review who tested positive by both DFA and the xTAG RVP test. The average hospital cost for patients with false-negative results was assumed to be equal to the average cost for the 115 patients in the chart review who tested negative by DFA but positive by the xTAG RVP test. The average hospital cost for patients with true-negative results was assumed to be equal to the average cost for the 231 patients in the chart review who tested negative by both DFA and SVC. There were not a sufficient number of patients with false-positive results in the chart review for the estimation of mean costs. Therefore, we assumed that the hospital cost for patients with false-positive results was the same as the cost for patients with true-negative results. The cost of isolation days was added to the hospital cost for patients with false-positive results.
RESULTS
When the costs per case were analyzed by test outcome, the results show that the cost of a true-positive result was the lowest at $2,413, while the cost of a false-positive result was the highest at $5,248 (Table 2). The cost for a false-negative result was $4,756, while the cost for a true-negative result was $5,228. These data clearly show the cost to a hospital of a missed diagnosis and failure to detect a respiratory virus infection.
TABLE 2.
Breakdown of cost per case by diagnostic status
| Diagnostic status | Cost ($)
|
|||
|---|---|---|---|---|
| Hospitalization | Tests and investigations | Antibiotics | Total | |
| True positive | 2,347 | 60 | 6 | 2,413 |
| False negative | 4,697 | 53 | 6 | 4,756 |
| True negative | 5,166 | 55 | 6 | 5,228 |
| False positive | 5,186 | 55 | 6 | 5,248 |
Table 3 provides the details used to calculate the average weighted cost per case from the decision tree for each of the four testing strategies. The cost for each strategy was calculated by first multiplying the proportion of patients with each diagnostic status (true positive, false positive, true negative, false negative) by the costs associated with each diagnostic status. The resulting weighted costs for each diagnostic status were then summed to estimate the weighted cost for the testing strategy. For example, the proportions of results for patients tested by the xTAG RVP test that were true positive, false positive, true negative, and false negative were estimated to be 0.592, 0.007, 0.363, and 0.038, respectively. These proportions were based upon the true prevalence of viral infection, along with the specificity and the sensitivity of the xTAG RVP diagnostic test. The costs associated with each diagnostic status for xTAG RVP test were $2,493, $5,327, $5,307, and $4,836 for patients with true-positive, false-positive, true-negative, and false-negative results, respectively. These costs comprised the hospital costs shown in Table 2 plus the cost of the viral test. As shown in Table 3, the weighted cost for the xTAG RVP test strategy is $3,623.
TABLE 3.
Calculation of weighted costs for each diagnostic strategy by using proportions and actual costs
| Test | Viral test result | Diagnostic status | Proportion | Cost ($) | Weighted cost ($)a |
|---|---|---|---|---|---|
| RVPb | Positive | True positive | 0.592 | 2,493.00 | 1,476 |
| Positive | False positive | 0.007 | 5,327.00 | 39 | |
| Negative | True negative | 0.363 | 5,307.00 | 1,926 | |
| Negative | False negative | 0.038 | 4,836.00 | 61 | |
| Total | 1.000 | 3,623 | |||
| DFA | Positive | True positive | 0.441 | 2,426.80 | 1,070 |
| Positive | False positive | 0.007 | 5,260.80 | 39 | |
| Negative | True negative | 0.363 | 5,240.80 | 1,900 | |
| Negative | False negative | 0.189 | 4,769.80 | 901 | |
| Total | 1.000 | 3,911 | |||
| DFA + culture | DFA positive | True positive | 0.441 | 2,426.80 | 1,070 |
| DFA positive | False positive | 0.007 | 5,260.80 | 37 | |
| DFA negative, culture positive | True positive | 0.004 | 2,447.45 | 9 | |
| DFA negative, culture positive | False positive | 0.007 | 5,281.45 | 38 | |
| DFA negative, culture negative | True negative | 0.356 | 5,261.45 | 1,872 | |
| DFA negative, culture negative | False negative | 0.185 | 4,790.45 | 887 | |
| Total | 1.000 | 3,914 | |||
| DFA + RVP | DFA positive | True positive | 0.441 | 2,426.80 | 1,070 |
| DFA positive | False positive | 0.007 | 5,260.80 | 37 | |
| DFA negative, RVP positive | True positive | 0.045 | 2,506.80 | 114 | |
| DFA negative, RVP positive | False positive | 0.007 | 5,340.80 | 39 | |
| DFA negative, RVP negative | True negative | 0.356 | 5,320.80 | 1,893 | |
| DFA negative, RVP negative | False negative | 0.144 | 4,849.80 | 697 | |
| Total | 1.000 | 3,849 |
The weighted cost is proportion × cost.
RVP, xTAG RVP test.
Total weighted costs broken down by resource type for each strategy are shown in Table 4. The cost per case was the highest for DFA plus SVC at $3,914 per case and was the lowest for the xTAG RVP test alone at $3,623. The costs per case for DFA alone and DFA plus the xTAG RVP test were intermediate at $3,911 and $3,849, respectively. The cost per case investigated by the xTAG RVP test alone ($3,623) was $291 lower than the cost per case investigated by DFA plus SVC ($3,914). In order to determine where the savings are accrued, the cost per case was broken down into its component parts, including test cost, cost for hospital stay, in-hospital medical costs, and cost of antibiotics. Almost all of the savings associated with the xTAG RVP test compared to the cost of DFA plus SVC were costs associated with the length of stay in hospital: $3,479 for the xTAG RVP test alone versus $3,826 for DFA plus SVC.
TABLE 4.
Component costs used to calculate the weighted cost per case for the four testing strategiesa
| Test | Cost ($)
|
||||
|---|---|---|---|---|---|
| Viral diagnostic tests | Hospitalization | Other tests and procedures | Antibiotics | Total | |
| xTAG RVP | $80 | 3,479 | 58 | 6 | 3,623 |
| DFA | 14 | 3,834 | 57 | 6 | 3,911 |
| DFA + SVC | 25 | 3,826 | 57 | 6 | 3,914 |
| DFA + xTAG RVP | 58 | 3,728 | 57 | 6 | 3,849 |
Since the prevalence of respiratory virus infections may vary by season and geographical area, a sensitivity analysis was undertaken to estimate which testing strategy was the least costly according to the true prevalence rates. At a prevalence rate of 11%, the costs estimated for the xTAG RVP test, DFA, DFA plus culture, and DFA plus xTAG RVP test are $5,009, $5,013, $5,026, and $5,063, respectively. At a prevalence rate of 8%, the costs estimated for the xTAG RVP test, DFA, DFA plus culture, and DFA plus the xTAG RVP test are $5,072, $5,093, $5,091, and $5,133, respectively. By comparison of all four testing strategies, RVP alone was the least costly strategy for a prevalence of 11% or higher, while DFA was the least costly strategy for a prevalence of less than 11%. When the cost of DFA plus SVC was compared to that of the xTAG RVP test alone, the xTAG RVP test alone was the least costly strategy at a prevalence of 8% or higher, while DFA plus SVC was the least costly strategy at a prevalence rate under 8%.
DISCUSSION
We used decision tree analytic modeling techniques to compare the costs of four strategies for the diagnosis of respiratory virus infections. We determined the average cost per case investigated using four different testing strategies and showed that testing by the xTAG RVP test alone was the least costly approach for the diagnosis of respiratory tract infections. The xTAG RVP test was less costly than the testing algorithm of DFA followed by SVC testing of DFA-negative specimens, a strategy which is used in many clinical virology laboratories in North America. The cost savings achieved by use of the xTAG RVP test were $291 per case investigated, and these savings would be achieved for laboratory positivity rates of 11% or higher. In our study, almost all of the savings were due to a shortened length of stay in hospital.
Our study, which compared the cost of multiplex PCR testing to the cost of DFA plus culture, is the first cost analysis study involving multiplex nucleic acid amplification testing for the detection of infectious diseases. The fact that multiplex PCR is the least costly diagnostic strategy for the detection of respiratory viruses was surprising and significant for a number of reasons. The introduction of new technology is usually associated with increased costs, as new technology is often more expensive and is deployed as an add-on test which increases costs. In our study, we demonstrated a cost per case savings when the xTAG RVP test was used as a replacement test for the status quo form of testing of DFA plus SVC, which may have contributed to the cost savings since it was not an add-on test. Our finding that the xTAG RVP test was the least costly strategy at an infection prevalence of 11% or higher is particularly important, as this suggests that the savings associated with the use of the xTAG RVP test will apply all 12 months of the year, including the months with a lower prevalence of respiratory virus infections in the community. In a recent 24-month epidemiological study of respiratory virus infections in which we tested 2,307 specimens (100 specimens per month for 24 consecutive months by use of a stratified random sampling), we had an overall positivity rate of 63% for children, and the range was from a monthly low of 36% to a monthly high of 87% (7, 10). This suggests that the xTAG RVP test would be the least costly testing strategy across the entire year, including the summer months, when respiratory virus infections are less prevalent than in the winter months in North America.
Three cost-benefit studies of tests for the detection of respiratory virus have been conducted over the past 10 years. Two of those studies dealt with the introduction of DFA and the savings associated with the introduction of rapid viral testing for the detection of respiratory viruses (1, 17), while a third dealt with real-time PCR (13). The first two studies demonstrated clinical and financial benefits associated with the introduction of rapid testing by DFA. In the first study, Woo et al. studied 214 pediatric patients at Queen Mary Hospital in Hong Kong over a 2-year period and showed that rapid testing reduced the length of hospital stay by 1.3 days (17). In that before-and-after study in which culture was used in year 1 and DFA was used in year 2, there was a reduction in both the length of hospital stay and the rate of antibiotic usage, resulting in a net savings of HK$391,000 per year after accounting for the cost of performing DFA. In a second before-and-after study with 38 adult patients seen over 2 years in a community teaching hospital in Springfield, IL, Barenfanger et al. (1) showed that the introduction of rapid testing by DFA was associated with a faster turnaround time for the reporting of results (4.5 days to 0.9 days), a shortened hospital stay (10.6 days to 5.3 days), and reduced patient care costs (US$7,893 to US$2,177), resulting in a savings of US$144,332 per year. In that study, costs were based on the cost of a positive case, while in our study, we calculated the cost per case investigated; so direct comparisons cannot be made. One randomized controlled trial was conducted to evaluate the clinical and economic impacts of real-time PCR (13). In that study of 107 hospitalized adult patients with lower respiratory tract infections, the PCR results were made available to the physicians within 48 h of specimen receipt for the intervention group, while for the control group, the PCR results were withheld from the physicians. Although real-time PCR increased the yield of viruses and bacteria by 22% compared with the yield achieved by culture, the overall rates of antibiotic usage were similar in the both groups, and more importantly, the use of real-time PCR increased the treatment and diagnostic costs by 318.17 euros per patient. With our model, we were unable to show a reduction in the rate of antibiotic usage, perhaps because we were unable to track antibiotic usage following discharge from the hospital and use of the xTAG RVP test was associated with a shorter hospital stay by 3 days, which accounted for over 90% of the savings. In our study, we showed that multiplex PCR testing was the least costly diagnostic strategy and that when the cost of that strategy was compared with the cost of DFA plus culture, it would result in a savings of $291 per case investigated. This would translate into a savings of $529,620 per year on the basis of investigations of 1,820 pediatric inpatients with suspected viral respiratory infections. We have not calculated the costs for adult patients in our model, but the costs for adult patients should be similar to or slightly higher than those for pediatric patients, as hospitalizations are often longer for adults. If the costs for adult patients are included in the model, then the savings for the four hospitals in Hamilton would be at least $756,600 per year on the basis of investigations for a total of 2,700 inpatients with respiratory virus infections.
The limitations of our study are, for the most part, intrinsic to the decision tree modeling methodology and the use of certain assumptions. We used test performance parameters, i.e., sensitivity and specificity, that were based on data published in the literature, and while they are as accurate as possible, they may not be perfect. For example, we used 70% sensitivity for DFA, which is based on data reported in the literature from studies in which multiple tests were used as the comparator. Many evaluations and comparisons reported in the literature do not use discordant analysis and a combined reference standard with a minimum of three tests to determine true sensitivity and specificity and therefore could not be used. We used a sensitivity of 72% for DFA plus SVC, since a review of data from our laboratory collected over a 2-year period showed that during the study period DFA plus SVC picked up an additional 2% positive specimens that DFA missed. Some laboratories have reported an additional pick up of 5 to 10% more positive results by the use of SVC; indeed, and over the past 10 years, we have seen a variable contribution of SVC that averages about 5% for most years. The SVC-positive results that are missed by DFA usually have low cell counts, and this may be due to the presence of an insufficient number of cells in the specimen or an inexperienced technologist reading weakly positive samples. The six technologists in the Regional Virology Laboratory, St. Joseph's Healthcare, Hamilton, Ontario, have an average of 16 years of experience and a combined 96 years of experience performing DFA. They handle an average of 4,000 respiratory specimens per year, which may account for our low number of SVC-positive results. We assumed a specificity of 98% for all the diagnostic tests due to imprecise data in the literature on the specificity of DFA and culture, as culture is usually performed only with DFA-negative specimens and not all specimens, thus precluding an accurate determination of specificity. We used a sensitivity of 94% for the xTAG RVP test since this is the average sensitivity for the 12 viruses for which this test is currently approved for use for testing (4). These included sensitivities of detection of 100% for RSV types A and B, parainfluenza virus types 1 and 2, and rhinovirus; 96.4% for influenza virus A; 91.5% for influenza virus B; 84.2% for parainfluenza virus type 3; 96% for metapneumovirus; and 78% for adenovirus. The sensitivity of the xTAG RVP test may, in fact, be a little higher since the 78% sensitivity for the detection of adenovirus is not accurate. We have recently reevaluated the sensitivity of the xTAG RVP test for the detection of adenovirus using over 3,200 specimens and 250 positive specimens and found a sensitivity of 92.4% (J. B. Mahony, M. Echavarria, C. Robinson, G. Gray, S. Chong, and C. Ginocchio, submitted for publication). Arguments could be made for the use of different values for sensitivity and specificity used in the model; however, the use of values slightly different from the ones that we used would have only a minimal effect on the overall outcome. For example, the model predicts that a 1-percentage-point increase in the overall sensitivity of the xTAG RVP test (assuming a sensitivity of 92% for the detection of adenovirus) would equate to an additional savings of about $15 per case, which would minimally raise the savings per case from $291 to $306. The cost of tests and reagents also vary for different settings. We used list price costs for reagents, including those for the xTAG RVP test. Cost reductions for volume or discount pricing should have a minimal effect on model outcomes since the majority of the costs and, hence, savings are due to hospital stay costs. In the model, we attempted to include all associated costs for hospitalized patients to derive the most accurate costs. The resources that we included in the estimation of hospital costs were somewhat selective; and patients may have received tests, medical procedures, or medications other than those collected from the chart review. As mentioned above, we were not able to capture antibiotic usage for discharged patients, which could in part explain the failure to show reduced antibiotic usage in the xTAG RVP test arm. The major limitation of the model is the lack of generalizability of the output, which is dependent on the input parameters and which will likely vary tremendously from country to country and state to state.
In Ontario, laboratory diagnostic testing is funded on a global basis and third-party reimbursement for individual tests is essentially nonexistent (it is permitted for only a very small number of specialty tests). In our setting, testing by the xTAG RVP test therefore represents an increase in the cost to the laboratory; however, despite the additional cost of $40 for an xTAG RVP test, we saved $331 in direct hospital costs for a net savings of $291 per case investigated. In some health management organizations or health care systems that allow reimbursement for testing by the xTAG RVP test, the savings per case should be higher than that which we obtained in Hamilton. In both types of health care systems, the xTAG RVP test is highly cost-effective, and its use results in better patient outcomes (shorter hospital stays) at lower costs.
Footnotes
Published ahead of print on 1 July 2009.
REFERENCES
- 1.Barenfanger, J., C. Drake, N. Leon, T. Mueller, and T. Troutt. 2000. Clinical and financial benefits of rapid detection of respiratory viruses: an outcomes study. J. Clin. Microbiol. 382824-2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chernesky, M. A., D. Jang, J. Sellors, K. Luinstra, S. Chong, S. Castriciano, and J. B. Mahony. 1997. Urinary inhibitors of polymerase chain reaction and ligase chain reaction and testing of multiple specimens may contribute to lower assay sensitivities for diagnosing Chlamydia trachomatis infected women. Mol. Cell. Probes 11243-249. [DOI] [PubMed] [Google Scholar]
- 3.Ginocchio, C. C. 2007. Detection of respiratory viruses using non-molecular based methods. J. Clin. Virol. 40(Suppl. 1)S11-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krunic, N., T. D. Yager, D. Himsworth, F. Merante, S. Yaghoubian, and R. Janeczko. 2007. xTAG RVP assay: analytical and clinical performance. J. Clin. Virol. 40(Suppl.)S39-S46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liao, R. S., L. L. Tomalty, A. Majury, and D. E. Zoutman. 2009. Comparison of viral isolation and multiplex real-time reverse transcription-PCR for confirmation of respiratory syncytial virus and influenza virus detection by antigen immunoassays. J. Clin. Microbiol. 47527-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahony, J., S. Chong, F. Merante, S. Yaghoubian, T. Sinha, C. Lisle, and R. Janeczko. 2007. Development of a respiratory virus panel test for detection of twenty human respiratory viruses by use of multiplex PCR and a fluid microbead-based assay. J. Clin. Microbiol. 452965-2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahony, J., S. Chong, M. Smieja, A. Petrich, S. Buracond, and J. Babwah. 2007. Establishing the epidemiology of respiratory virus infections using molecular technology, abstr. C-070. Abstr. 107th Gen. Meet. Am. Soc. Microbiol. American Society for Microbiology, Washington, DC.
- 8.Mahony, J. B. 2008. Detection of respiratory viruses by molecular methods Clin. Microbiol. Rev. 21716-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahony, J. B., and S. Chong. 2007. Clinical evaluation of the xTAG™ RVP assay for respiratory viruses, abstr. O-348. Abstr. Annu. Meet. of CACMID/AMMI.
- 10.Mahony J. B., S. Chong, M. Smieja, A. Petrich, S. Buracond, and J. Babwah. 2008. Abstr. 18th Annu. Meet. ECCMID, abstr. P1605.
- 11.Ontario Case Costing Initiative and the Ontario Case Cost Project. 2006. Ontario Case Cost Program Database. 1995-96. Ontario guide to case costing. Ontario Ministry of Health and Long-Term Care, Toronto, Ontario, Canada. Accessed March 5 2009.
- 12.Ontario Ministry of Health and Long-Term Care. 2007. e-Formulary. Ontario drug benefit formulary/comparative drug index: electronic version, version 1.4. Queen's Printer for Ontario, Toronto, Ontario, Canada. https://www.healthinfo.moh.gov.on.ca/formulary/index.jsp.
- 13.Oosterheert, J. J., A. M. van Loon, R. Schuurman, A. I. M. Hoepelman, E. Hak, S. Thijsen, G. Nossent, M. M. E. Schneider, W. M. N. Hustinx, and M. J. M. Bonten. 2005. Impact of rapid detection of viral and atypical bacterial pathogens by real-time polymerase chain reaction for patients with lower respiratory tract infection. Clin. Infect. Dis. 411438-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pabbaraju, K., K. L. Tokaruk, S. Wong, and J. D. Fox. 2008. Comparison of the Luminex xTAG respiratory viral panel with in-house nucleic acid amplification tests for diagnosis of respiratory virus infections. J. Clin. Microbiol. 463056-3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smit, M., K. A. Beynon, D. R. Murdoch, and L. C. Jennings. 2007. Comparison of the NOW Influenza A & B, NOW Flu A, NOW Flu B, and Directigen Flu A+B assays, and immunofluorescence with viral culture for the detection of influenza A and B viruses. Diagn. Microbiol. Infect. Dis. 5767-70. [DOI] [PubMed] [Google Scholar]
- 16.U.S. FDA. 2008. U.S. FDA review, decision summary, database updated 6 March 2008, p. 1-39. U.S. FDA, Washington, DC. www.accessdata.fda.gov.scripts.cdrh/.
- 17.Woo, P. C., S. S. Chiu, W.-E. Seto, and M. Peiris. 1997. Cost-effectiveness of rapid diagnosis of viral respiratory tract infections in pediatric patients. J. Clin. Microbiol. 351579-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]