Abstract
The aim of this study was to investigate the significance of multiple mutations in the rpoB gene as well as predominant nucleotide changes and their correlation with high levels of resistance to rifampin (rifampicin) in Mycobacterium tuberculosis isolates that were randomly collected from the sputa of 46 patients with primary and secondary cases of active pulmonary tuberculosis from the southern region (Afghanistan border) of Iran where tuberculosis is endemic. Drug susceptibility testing was performed using the CDC standard conventional proportional method. DNA extraction, rpoB gene amplification, and DNA sequencing analysis were performed. Thirty-five (76.09%) isolates were found to have multiple mutations (two to four) in the rpoB (β-subunit) gene. Furthermore, we demonstrate that the combination of mutations with more prevalent nucleotide changes were observed in codons 523, 526, and 531, indicating higher frequencies of mutations among patients with secondary infection. In this study, 76.08% (n = 35) of all isolates found to have mutation combinations involving nucleotide changes in codons 523 (GGG→GCG), 531 (TCG→TTG or TTC), and 526 (CAC→CGC, TTC, AAC, or CAA) demonstrated an association with higher levels of resistance to rifampin (MIC, ≥100 μg/ml).
In bacterial populations, the generation of antibiotic resistance depends on the rate of emergence of resistant mutants (1, 19, 23). Correlations between high mutation rates, the geographic distribution of mutations, antibiotic resistance, and virulence in bacteria have been reported in several studies (9, 20, 33, 37). Knowledge of geographic variations is important for monitoring rifampin (rifampicin) resistance within a defined population of patients infected with Mycobacterium tuberculosis, as the prevalence of the mutations studied so far varies for M. tuberculosis strains isolated from different countries (24, 26, 29, 33, 36). In 2004, the prevalence of tuberculosis in Iran was reported to be 17 per 100,000, and at the southern border of Iran (Zabol province) where tuberculosis is endemic, the prevalence was 141 per 100,000 (20). Rifampin resistance is of particular epidemiologic importance, since it represents a valuable surrogate marker for multidrug-resistant (MDR) tuberculosis strains, and the prevalence of MDR strains is a significant obstacle to tuberculosis therapy (4, 21, 26). DNA sequencing studies indicate that more than 95% of rifampin-resistant M. tuberculosis strains have mutations within the 81-bp hot-spot region (codons 507 to 533) of the RNA polymerase β-subunit (rpoB) gene (4, 19, 32). Over the last 15 years, Kapur et al. and Telenti et al. have identified the molecular basis of rifampin resistance in M. tuberculosis (9, 29). Thus, it is important to determine the molecular bases of mutations and their distribution at the level of each country prior to molecular testing introduction for routine diagnostics (9, 11, 13, 15, 16, 23).
In this study, we investigated the significance of multiple mutations in the rpoB gene and their correlation with highly prevalent nucleotide changes in codons 523, 531, and 526 and also demonstrated the highly prevalent nucleotide changes observed in the last nine codons of the β-subunit (523 to 531) that are associated with higher levels of resistance to rifampin (MIC, ≥100 μg/ml) in patients bearing secondary M. tuberculosis infection.
MATERIALS AND METHODS
Mycobacterial strains.
A total of 286 M. tuberculosis strains were isolated from the sputa of patients with active pulmonary tuberculosis and collected from the southern border of Iran (Zabol province, Afghanistan border) where tuberculosis is endemic from March 2005 to May 2006 as part of a routine tuberculosis surveillance. In this study, 46 rifampin-resistant isolates were randomly selected from patients with proven WHO-defined clinical symptom radiography, and tuberculin skin test results (35) were recorded before collection of the clinical specimens. All isolates were cultured on Löwenstein-Jensen solid medium and grown colonies were identified to the species level using TCH (2-thiophene carboxylic acid) and PN99B (paranitrobenzoic acid) selective media using Centers for Disease Control and Prevention (CDC) standard biochemical procedures (10). M. tuberculosis reference strains CDC1551 and H37Rv and four susceptible isolates of M. tuberculosis were used from the banked strain collection of the Pasteur Institute of Iran. M. tuberculosis strain 210 is a susceptible reference isolate from the M. tuberculosis bank at the Belarusian Research Institute of Epidemiology and Clinical Microbiology and was also used as an internal negative control.
Susceptibility testing.
Antimicrobial drug susceptibility testing (AMST) was performed using the CDC standard conventional proportional method: rifampin (40 μg/ml), isoniazid (2 μg/ml), ethambutol (2 μg/ml), ethionamide (20 μg/ml), streptomycin (4 μg/ml), and kanamycin (20 μg/ml) were used in slants and in addition to breakpoint concentrations for isoniazid (0.1 μg/ml) and rifampin (2.0 μg/ml) in the Bactec system (10). Four sensitive M. tuberculosis isolates and an H37Rv strain were used as negative controls. Mutations in the rpoB gene were identified from 41 rifampin-resistant isolates by sequencing methods (see below), and AMST was performed following sequencing to confirm resistance using different concentrations of rifampin (50, 75, and 100 μg/ml) through the slant proportional method (10).
Standard PCR identification and rpoB gene amplification.
DNA was extracted and purified using the Fermentas DNA extraction procedure (K512). M. tuberculosis reference strains CDC1551 and H37Rv and four sensitive isolates of M. tuberculosis from the banked strain collection of the Pasteur Institute of Iran were used as negative controls. A 411-bp segment of the rpoB gene was amplified by PCR using the following synthetic oligonucleotide primers rpoB-F (5-TACGGTCGGCGAGCTGATCC-3) and rpoB-R (5-TACGGCGTTTCGATGAAC-3) (4). PCR was carried out in a reaction mixture containing 50 μl KCl, 10 μl Tris (pH 8.0), 1.5 μl MgCl2, 5 μl of deoxynucleoside triphosphates, 1 U Taq polymerase, 20 pmol of each set of primers, and 6 μl of chromosomal DNA. The following thermocycling parameters were applied: initial denaturation at 94°C for 5 min, 33 cycles of denaturation at 94°C for 1 min, primer annealing at 57°C for 1 min, extension at 72°C for 1 min, and a final extension at 72°C for 5 min. The PCR product was amplified, purified again, and controlled on the electrophoresis gel. The final purified mycobacterial DNA was used for sequencing.
DNA sequencing and analysis.
The 411-bp fragments of the rpoB gene were amplified by PCR using the forward or reverse primers mentioned above using the following conditions: 33 cycles of denaturation at 94°C for 30 s, primer annealing at 57°C for 30 s, and extension at 72°C for 90 s. The rpoB gene fragments were sequenced by using the protocol of the DYEnamic ET dye terminator kit (MegaBACE) and a MegaBACE 4000 DNA sequencer (Amersham Biosciences [currently GE Healthcare], Piscataway, NJ). Alignment of the DNA fragments (rpoB) was carried out using MEGA and DNAMAN software and was compared with the standard M. tuberculosis reference strains CDC1551, H37Rv, and 210. The Blast2 sequencing program (NCBI) was used for DNA sequence comparisons (http://www.ncbi.nlm.nih.gov/BLAST/). Alignment of the DNA fragments (rpoB) was carried out with MEGA 3.1 software (www.megasoftware.net/), and data were analyzed and edited with DNAMAN software (Lynnonon Biosoft, Quebec, Canada).
In this study, all smear microscopy, acid-fast bacilli (AFB) culture, susceptibility testing, and PCR assays were performed at the mycobacteriology department of the Pasteur Institute of Iran, and the DNA sequencing analysis was conducted at the Belarusian Research Institute of Epidemiology and Microbiology, Clinical Microbiology Department.
Definitions.
In this study, primary cases (never-treated patients) refer to patients who did not have a previous history of tuberculosis disease or medical treatment. Secondary cases (previously treated patients) demonstrated a previous history of tuberculosis disease in their medical records.
RESULTS
Mycobacterial strains and susceptibilities.
In this study, we had 286 available M. tuberculosis isolates, of which 29 were resistant to drugs not including rifampin or isoniazid, 37 were MDR, 41 were rifampin resistant in combination with other drugs, and 179 were susceptible. Of the 78 rifampin-resistant isolates, 46 randomly selected isolates were chosen based on the WHO-defined clinical symptom definition (35). Of these 46 rifampin-resistant isolates, 22 (47.82%) were isolated from the sputa of patients with primary infections and 19 (41.30%) were from that of patients bearing secondary cases.
Of the 179 susceptible isolates, 5 were randomly selected, and no mutations were detected for the 5 isolates with rifampin resistance in the 411-bp regions of the rpoB gene (control isolates). No mutations were also found in the three reference strains (CDC1551, H37Rv, and 210). In addition, five rifampin-resistant isolates (10.86%) demonstrated no mutation in the 411-bp fragment of the rpoB gene. Mutations were observed in affected codons 507, 508, 511, 516, 519, 520, 523, 526, 527, and 531 in the 411-bp rpoB fragments. In 26 of 46 rifampin-resistant isolates, two types of mutations were identified in codon 523: GGG→GCG (n = 25; 54.34%) and GGG →GG_ (n = 1; 2.17%). Two types of mutations were found in codon 531: TCG→TTG (n = 7; 15.21%) and TCG→TTC (n = 5; 10.86%). In 21 (45.62%) isolates, five types of mutations were demonstrated in codon 526: CAC→TAC (n = 5; 10.86%), CAC→AAC (n = 3; 6.52%), CAC→CGC (n = 8; 17.39%), CAC→CAA (n = 3; 6.52%), and CAC→TTC (n = 2; 4.34%).
Prevalent nucleotide changes were observed in 25 isolates in codon 523 (GGG→GCG), of which 11 (44%) were identified from secondary and 14 (56%) from primary cases. Additional mutations were found in codon 531 (TCG→TTG [n = 7] and TCG→TTC [n = 5]) and codon 526 (CAC→CGC [n = 3], CAC→TTC [n = 2], CAC→CAA [n = 3], and CAC→TAC [n = 2]) identified from secondary cases and in codon 526 (CAC→CGC [n = 5], CAC→AAC [n = 3], and CAC→TAC [n = 3]) identified from primary cases (see Table 2).
TABLE 2.
Correlation between prevalent nucleotide and amino acid changes among isolates with high levels of rifampin resistance collected from active primary and secondary tuberculosis patients at the Afghanistan border of Iran (Zabol province)
| Codon(s)d | Change of nucleotide(s) | Change of amino acid(s) | No. of isolatesa | No. of primary cases (MIC [μg/ml]) | No. of secondary cases (MIC [μg/ml]) |
|---|---|---|---|---|---|
| 523 | GGG→GCG | Gly→Ala | 25 | 14 | 11 |
| 526 | CAC→AAC | His→Asn | 3 | 3 | 0 |
| Com. 523, 526 | GGG→GCG, CAC→AAC | Gly→Ala, His→Asn | 3 | 3 (≥100) | 0 |
| 523 | GGG→GCG | Gly→Ala | 25 | 14 | 11 |
| 531 | TCG→TTG | Ser→Leu | 7 | 0 | 7 |
| Com. 523, 531 | GGG→GCG, TCG→TTG | Gly→Ala, Ser→Leu | 5 | 0 | 5 (≥100) |
| 511 | CTG→GTG | Leu→Val | 5 | 2 | 3 |
| 523 | GGG→GCG | Gly→Ala | 25 | 14 | 11 |
| Com. 511, 523 | CTG→GTG, GGG→GCG | Leu→Val, Gly→Ala | 3 | 0 | 3 (≥100) |
| 511 | CTG→CCG | Leu→Pro | 3 | 3 | 0 |
| 523 | GGG→GCG | Gly→Ala | 25 | 14 | 11 |
| Com. 511, 523 | CTG→CCG, GGG→GCG | Leu→Pro, Gly→Ala | 3 | 3 (≥50) | 0 |
| 507 | GGC→AGC | Gly→Ser | 1 | 0 | 1 |
| 508 | ACC→CCC | Thr→Pro | 4 | 3 | 1 |
| Com. 507, 508 | GGC→AGC, ACC→CCC | Gly→Ser, Thr→Pro | 1 | 0 | 1 (∼50) |
| 507 | GGC→GGT | Gly→Glyc | 9 | 3 | 6 |
| 523 | GGG→GCG | Gly→Ala | 25 | 14 | 11 |
| Com. 507, 523 | GGC→GGT, GGG→GCG | Gly→Gly,c Gly→Ala | 3 | 3 (∼50) | 0 |
| 508 | ACC→GCC | Thr→Ala | 2 | 2 | 0 |
| 523 | GGG→GCG | Gly→Ala | 25 | 14 | 11 |
| Com. 508, 523 | ACC→GCC, GGG→GCG | Thr→Ala, Gly→Ala | 2 | 2 (∼50) | 0 |
| 508 | ACC→CCC | Thr→Pro | 4 | 3 | 1 |
| 516 | GAC→CAC | Asp→His | 3 | 3 | 0 |
| 526 | CAC→CGC | His→Arg | 8 | 5 | 3 |
| Com. 508, 516, 526 | ACC→CCC, GAC→CAC, CAC→CGC | Thr→Pro, Asp→His, His→Arg | 3 | 3 (∼50-75) | 0 |
| 507 | GGC→GGT | Gly→Glyc | 9 | 3 | 6 |
| 508 | ACC→CACb | Thr→His | 6 | 0 | 6 |
| 523 | GGG→GCG | Gln→Ala | 25 | 14 | 11 |
| 526 | CAC→CGC | His→Arg | 8 | 5 | 3 |
| Com. 507, 508, 523, 526 | GGC→GGT, ACC→CAC,b GGG→GCG, CAC→CGC | Gly→Gly,c Thr→His, Gln→Ala, His→Arg | 3 | 0 | 3 (≥100 μg/ml) |
| 507 | GGC→GGT | Gly→Glyc | 9 | 3 | 6 |
| 508 | ACC→CACb | Thr→His | 6 | 0 | 6 |
| 526 | CAC→CAA | His→Gln | 3 | 0 | 3 |
| 531 | TCG→TTCb | Ser→Phe | 5 | 0 | 5 |
| Com. 507, 508, 526, 531 | GGC→GGT, ACC→CAC,b CAC→CAA, TCG→TTCb | Gly→Gly,c Thr→His, His→Gln, Ser→Phe | 3 | 0 | 3 (≥100) |
| 507 | GGC→GATb | Gly→Asp | 2 | 0 | 2 |
| 513 | CAA→TAA | Gln→STOP | 2 | 0 | 2 |
| 526 | CAC→TAC | His→Tyr | 5 | 3 | 2 |
| 531 | TCG→TTCb | Ser→Phe | 5 | 0 | 5 |
| Com. 507, 513, 526, 531 | GGC→GAT,b CAA→TAA, CAC→TAC, TCG→TTCb | Gly→Asp, Gln→STOP, His→Tyr, Ser→Phe | 2 | 0 | 2 (≥100) |
| 511 | CTG→GTG | Leu→Val | 5 | 2 | 3 |
| 513 | CAA→GAA | Gln→Glu | 2 | 2 | 0 |
| 519 | AAC→AAG | Asn→Lys | 2 | 2 | 0 |
| 526 | CAC→CGC | His→Arg | 8 | 5 | 3 |
| Com. 511, 513, 519, 526 | CTG→GTG, CAA→GAA, AAC→AAG, CAC→CGC | Leu→Val, Gln→Glu, Asn→Lys, His→Arg | 2 | 2 (≤50) | 0 |
| 512 | AGC→GGC | Ser→Thr | 2 | 0 | 2 |
| 513 | CAA→AATb | Gln→Asn | 2 | 0 | 2 |
| 526 | CAC→TTCb | His→Phe | 2 | 0 | 2 |
| 531 | TCG→TTG | Ser→Leu | 7 | 0 | 7 |
| Com. 512, 513, 526, 531 | AGC→GGC, CAA→AAT,b CAC→TTC,b TCG→TTG | Ser→Thr, Gln→Asn, His→Phe, Ser→Leu | 2 | 0 | 2 (≥100) |
The number of isolates is not mutually exclusive.
Novel double mutation.
Silent mutation.
Com., combination.
Of the 131 mutations identified among the 41 rifampin-resistant isolates, prevalent nucleotide and amino acid changes were seen in codon 523 (GGG→GCG [Gly→Ala]; 19.08% [n = 25]), codon 531 (TCG→TTG or TTC [Ser→Leu or Phe]; 9.16% [n = 12]), and codon 526 (CAC→AAC, CGC, CAA, TTC, or TAC [His→Asn, Arg, Gln, Phe, or Tyr]; 16.3% [n = 21]).
Thirty-five (76.09%) of the 46 rifampin-resistant isolates demonstrated combinations of multiple mutations in at least two, three, and four codons (507, 508, 511, 513, 523, 526, and 531). Thirty-three isolates (71.74%) from secondary cases demonstrated higher nucleotide combinations and frequencies in codon 507, GGC→GGT (n = 6; 18.18%); codon 508, ACC→CAC (n = 6; 18.18%); codon 511, CTG→GTG (n = 5; 15.15%); codon 513, CAA→TAA or AAT (n = 4; 12.12%); codon 523, GGG→GCG (n = 11; 33.33%); codon 526, CAC→CGC, CAA, TTC, or TAC (n = 10; 30.30%); and codon 531, TCG→TTG or TTC (n = 12; 36.36%). All nucleotide changes observed in codons 531 were identified among isolates from secondary cases, and none were found in isolates from primary cases. Twenty-five isolates (54.35%) indicating higher frequencies of nucleotide combinations were identified in codon 523, GGG→GCG (n = 14; 56%), and codon 526, CAC→TAC, AAC, or CGC (n = 11; 44%), from primary cases.
Different types of mutations were observed in codon 507 (GGC→AGC, GGT, or GAT; n = 9; 19.57%), codon 508 (ACC→CCC or CAC; n = 7; 15.22%), 511 (CTG→GTG; n = 3; 6.52%), and codon 513 (CAA→TAA or AAT; n = 4; 8.70%), which were recognized among isolates obtained from secondary cases. Isolates possessing single (n = 6; 14.63%), double (n = 20; 48.78%), triple (n = 3; 7.31%), or quadruple (n = 12; 29.26%) mutations were observed in the rifampin-resistant determining region among 46 rifampin-resistant isolates (Table 1).
TABLE 1.
Frequency of amino acid and nucleotide changes of different codons in the rpoB gene of 41 rifampin-resistant strains of M. tuberculosis collected from active primary and secondary tuberculosis patients in the southern region (Afghanistan border) of Iran (Zabol province) where tuberculosis is endemic
| No. of mutations (no. [%] of isolates) | Codon(s) | Change of nucleotide(s) | Change of amino acid(s) | MIC (μg/ml) | Frequency of changes [no. (%)] |
|---|---|---|---|---|---|
| 1 (6 [14.63%]) | 526 | CAC→TAC | His→Tyr | ≤50 | 3 (7.31) |
| 523 | GGG→GCG | Gly→Ala | ≤50 | 3 (7.31) | |
| 2 (20 [48.78%]) | 523, 526 | GGG→GCG, CAC→AAC | Gly→Ala, His→Asn | ≥100 | 3 (7.31) |
| 508, 523 | ACC→GCC, GGG→GCG | Thr→Ala, Gly→Ala | ≤75 | 2 (4.87) | |
| 523, 531 | GGG→GCG, TCG→TTG | Gly→Ala, Ser→Leu | ≥100 | 5 (12.19) | |
| 507, 508 | GGC→AGC, ACC→CCC | Gly→Ser, Thr→Pro | ≤50 | 1 (2.43) | |
| 507, 523 | GGC→GGT, GGG→GCG | Gly→Gly(silent), Gly→Ala | ≤75 | 3 (7.31) | |
| 511, 523 | CTG→GTG, GGG→GCG | Leu→Val, Gly→Ala | ≥100 | 3 (7.31) | |
| 511, 523 | CTG→CCG, GGG→GCG | Leu→Pro, Gly→Ala | ∼50-75 | 3 (7.31) | |
| 3 (3 [7.31%]) | 508, 516, 526 | ACC→CCC, GAC→CAC, CAC→CGC | Thr→Pro, Asp→His, His→Arg | ∼50-75 | 3 (7.31) |
| 4 (12 [29.26%]) | 507, 508, 526, 531 | GGC→GGT, ACC→CAC, CAC→CAA, TCG→TTC | Gly→Gly, Thr→His, His→Gln, Ser→Phe | ≥100 | 3 (7.31) |
| 512, 513, 526, 531 | AGC→GGC, CAA→AAT, CAC→TTC, TCG→TTG | Sre→Thr, Gly→Asn, His→Phe, Ser→Leu | ≥100 | 2 (4.87) | |
| 507, 508, 523, 526 | GGC→GGT, ACC→CAC, GGG→GCG, CAC→CGC | Gly→Gly, Thr→His, Gln→Ala, His→Arg | ≥100 | 3 (7.31) | |
| 507, 513 526, 531 | GGC→GAT, CAA→TAA, CAC→TAC, TCG→TTC | Gly→Asp, Gln→Stop, His→Tyr, Ser→Phe | ≥100 | 2 (4.87) | |
| 511, 513 519, 526 | CTG→GTG, CAA→GAA, AAC→AAG, CAC→CGC | Leu→Val, Gln→Glu, Asn→Lys, His→Arg | ∼50 | 2 (4.87) |
Six isolates with single mutations in codons 526 (n = 3) and 523 (n = 3) demonstrated resistance to rifampin with MICs of ≤50 μg/ml. Isolates with double mutations (n = 20) in combinations composed of codons 523 and 526 (n = 3) demonstrated resistance to rifampin with MICs of ≥100 μg/ml. Of three isolates with triple mutations, the combinations of codons 508, 516, and 526 demonstrated resistance to rifampin at MICs of ∼50 to 75 μg/ml and were found in primary cases. The combination of codon 523 with any one of the nucleotide changes located in β-subunit segment 507 to 531 demonstrated an MIC level of ≥100 μg/ml in secondary cases. Isolates with quadruple codon mutations in combination with codons 507, 508, 511, 512, 513, 516, 519, 523, 526, and 531 also demonstrated MIC levels of ≥100 μg/ml (n = 12) in secondary cases (Tables 1 and 2). All mutations were nonsynonymous, except the nine mutations found in codon 507 showing nucleotide changes GGC to GGT (Gly→Gly), of which six were found in secondary and three in primary cases (Table 2).
DISCUSSION
Rifampin resistance is a surrogate marker for MDR M. tuberculosis (rifampin and isoniazid resistance) and is the result of mutations within certain regions of the rpoB gene which encodes the β-subunit of RNA polymerase. It is important to understand the correlation of the clinical states of patients with tuberculosis to mutations and high resistance levels to rifampin to determine whether the patients are initially infected with the MDR M. tuberculosis strain or whether the emergence of MDR M. tuberculosis was due to inadequate or inappropriate antibiotic treatment that resulted in the acquisition of mutations and antibiotic resistance. Specific amino acid substitutions within the regions of the rpoB gene are strongly associated with resistance to other classes of drugs, most notably isoniazid (9, 12, 18, 23, 29). Codons 511, 516, 526, and 531 have been reported as the rpoB sites with the most frequent mutations worldwide (1, 9, 18, 20, 26, 29), although variations in the relative frequencies of the mutations in these codons have been described for M. tuberculosis isolates from different geographic locations (8, 18, 27, 34). These differences reflect the complex and crucial interactions between rifampin and drug targets at the molecular level, where the positions of the affected nucleotide changes seem variable. Other authors have reported different levels of high and low resistance associated with specific nucleotide replacements (2, 7, 17, 23). This study demonstrates that higher combinations of nucleotide changes in multiple-mutated isolates (total, 35) (in codon 523, GGG→GCG [n = 25; 71.43%], codon 531, TCG→TTG or TTC [n = 12; 34.29%], and codon 526, CAC→CGC, CAA, TTC, or TAC [n = 10; 28.57%]) are most commonly observed in patients identified with secondary M. tuberculosis infection and also conferred a high rifampin resistance level (MIC, ≥100 μg/ml) (Tables 1 and 2).
Sequencing analyses of the rpoB gene from 46 rifampin-resistant isolates demonstrated a total of 131 mutations. Other investigators have reported point mutations in codon 531, TCG→TTG (Ser→Leu), for seven strains demonstrating highly resistant phenotypes, requiring MICs of ≥64 μg/ml (22). However, in our data, other point mutations were observed which have not been previously reported. Mutations in codon 526 (CAC→CGC, CAA, TTC, or TAC; n = 21; 16.03%), codon 531 (TCG→TTG or TTC; n = 12; 9.16%), and codon 523 (GGG→GCG; n = 25; 19.08%) were most frequently observed in patients with secondary infections conferring high levels of rifampin resistance (MIC, ≥100 μg/ml).
We observed most often in secondary cases (P = 0.007) and not in agreement with the observations reported by other investigators (13, 16, 22, 25) nucleotide stop mutations in codon 513 (CAA→TAA [Gln→STOP] [n = 2]); silent synonymous mutations in codon 507 (GGC→GGT [Gly→Gly] [n = 9]); deletions in codon 520 (CCG→___ [Pro→deletion] [n = 3]), codon 523 (GGG→GG_ [Gly→deletion] [n = 5]), and codon 527 (AAG→deletion [n = 1]); and double mutations in single codons (n = 17) (codon 507, GGC→GAT [Gly→Asp] [n = 2]; codon 508, ACC→CAC [Thr→His] [n = 6]; codon 513, CAA→AAT [Gly→Asp] [n = 2]; codon 526, CAC→TTC [His→Phe] [n = 2]; and codon 531, TCG→TTC [Ser→Phe] [n = 5]), which indicate changes of amino acid to Phe, Asp, and His (Table 2 and Fig. 1). Since rpoB encodes the RNA polymerase which is essential for the survival of the cell, the single-nucleotide deletion, stop codons, and double mutations result in conformational changes in β-subunit protein and ultimately alter the rifampin binding site and/or its affinity for the drug, which results in resistance to rifampin. Although all isolates were resubcultured and resequenced, we have no explanation for what type of protein might have been produced due to mutations in codons 507, 523, 526, 527, and 531 that were consistently isolated from secondary cases bearing high rifampin resistance levels (MIC, ≥100 μg/ml). The higher frequencies of mutation-bearing sites found in our data in codons 531, 526 and 523 can be partially compared with those isolates reported from other countries (6, 9, 13, 20, 24). Other authors have reported additional variations of nucleotide changes in codon 531 (in India, TCG→TGG or TTG; in Russia, TCG→TGG, CAG, or TGT; in China, TCG→TTG; in Japan, TCG→TTG; in Korea, TCG→TTG; and in Taiwan, TCG→TTG) and in codon 526 (in India, CAC→CTC, TAC, GAC, CGC, or ACC; in Russia, CAC→CTC, GAC, CAA, CAG, TGC, AAC, CGC, or CCC; in China, CAC→TAC; in Japan, CAC→TAC; in Taiwan, CAC→TAC and CGC; in Korea, CAC→TAC; and in Brazil, TCG→TTG) (6, 7, 13, 17, 20, 30, 31).
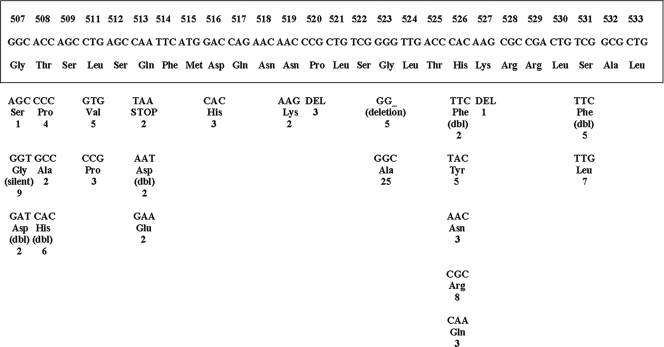
FIG. 1.
Mutations in the rifampin-resistant determining region of the rpoB gene of M. tuberculosis (Afghanistan border) isolates. The bottom panel shows the mutated codons with corresponding amino acids. The original sequence is shown boxed (28). The numbers below the amino acid designations indicate the numbers of the isolates showing the mutation. dbl, double mutation; DEL, codon deletion; deletion, amino acid deletion; silent, silent mutation; STOP, stop codon.
In this study, we observed two nucleotide polymorphism changes in codon 531 among 12 isolates: 7 isolates (15.22%) changed from TCG→TTG (Ser→Leu), and 5 changed from TCG→TTC (Ser→Phe). In our study, 10.87% of the mutations found have not been previously reported, and these notably observed polymorphisms were among multiple-mutated isolates bearing triple and quadruple mutations predominantly differentiated from secondary infection cases with high rifampin resistance levels (MIC, ≥100 μg/ml) (Table 2). Additionally, prevalent nucleotide changes in codon 526, CAC→TTC (His→Phe), CAA (His→Gln), CGC (His→Arg), and TAC (His→Tyr) (n = 10; 21.74%), were also found to be more prevalent in patient isolates bearing secondary cases with high-level resistance to rifampin (MIC, ≥100 μg/ml). Nucleotide changes from CAC→CGC (His→Arg), AAC (His→Asn), and TAC (His→Tyr) were found most often among primary cases with MICs of ≤50 μg/ml. However, our data significantly differs from previously reported mutation frequencies for codon 526 (CAC to GAC) among Italian isolates (40.1%) (23) and Greek isolates (CAC to GAC; 17.6%) (15), and the CAC to TAC mutation was reported to be more prevalent among American isolates (27.9%) (9). Our data reveal a mutation frequency (60%) similar to what others have observed in Asia; however, the mutation frequency of 40.1% for His526Asp reported among the Italian and Greek isolates was not found among any of our isolates (n = 50) (4, 9, 17, 20, 23, 32). A total of 131 mutations were found among the 41 rifampin-resistant isolates. Of these, 39 (29.77%) and 92 (70.23%) mutations were found among primary and secondary cases, respectively. Among the isolates from primary and secondary cases, 15 (38%) and 51 (56%) of the total mutations were observed in the small narrow segment of the β-subunit region of the rpoB gene located between codons 523 and 531, respectively. Multiple mutations in the rpoB gene associated with secondary cases of tuberculosis were found in 33 of 46 (71.74%) of the rifampin-resistant isolates.
Of the 131 mutations found in our study, the high proportion of double mutations (12.98%) in various single codons (507, 508, 513, 526, and 531) (Fig. 1) demonstrates a consistent correlation with high rifampin resistance levels (MIC, ≥100 μg/ml) in the isolates collected from secondary cases which has not been reported previously and clearly exceeds the findings of other studies (5, 7, 9, 13, 20, 23, 26). Other investigators have shown that mutants isolated more frequently from clinical practice demonstrate higher robustness and that the prevalence of each mutant type depends on its ability to survive (3). This could possibly be a reason for the higher occurrence of mutations we observed in this unique region of endemicity. In addition, we observed a high frequency of triple (2.29%) and quadruple (9.16%) mutations in different codons which has not been previously reported (Table 1).
The combinations of mutations and highly prevalent nucleotide changes were observed with higher frequencies in the multiple-mutated codons (total, n = 35; 76.09%) 523 (n = 25; 54.35%), 531 (n = 12; 26.09%), and 526 (n = 21; 45.65%), conferring double (n = 20; 43.48%), triple (n = 3; 6.52%), and quadruple (n = 12; 26.09%) mutations among 46 rifampin resistance isolates, and this clearly differs from other reported studies (7, 9, 20, 22, 23, 26). Of the 131 mutations, a high proportion of multiple mutations (n = 35, 26.72%) was found in a narrow segment of the rpoB gene (β-subunit, codons 507 to 531) and was consistently isolated from patients with secondary infection cases bearing high levels of rifampin resistance (MIC, ≥100 μg/ml). Therefore, we have resequenced, resubcultured, and repeated AMST for all 46 isolates in order to ensure rifampin resistance and reproducibility.
In this study, we also found five rifampin-resistant strains revealing no mutations. This finding is in agreement with other reports indicating geographic variations in nature, including mutations outside the 81-bp segment of rpoB or additional molecular mechanisms that may be involved in the rifampin resistance of M. tuberculosis, suggesting that conferred resistance might be due to mutations occurring elsewhere in the rpoB gene (4, 23). The high level of mutations conferring resistance in this unique geographic area where tuberculosis is endemic could be explained by the poor border control, population movement due to economic and tribal communications, lack of rapid identification methods for MDR M. tuberculosis, and inadequate chemotherapy. Another explanation for these findings is the possibility that a subset of the mutations that are naturally occurring polymorphisms or silent synonymous mutations that do not change the amino acid sequence is present in isolates circulating in this region of endemicity. Thus, these naturally occurring polymorphisms may not correlate with rifampin resistance. While it is unusual, other investigators have reported deletions in different codons of the rpoB gene, but the science behind stop codons in the rpoB gene is lacking for prokaryotes. In our strain set, we observed this unusual stop codon in only two isolates, and we have no explanation of how the mRNA reads the codon and transcribes the message to a protein. One possible reason, as described for eukaryotes (identified in GenBank), is that the TAA stop codon is completed by the addition of the 3′ A residues to the mRNA. An additional explanation may be the presence of a compensatory repair mechanism that reads the stop codon frame by selecting hypermutable (mutator) alleles based on an alteration in a DNA repair gene. The presence of several copies of multiT gene and the transcription of the multiT gene(s) to proteins may remove the oxidized guanine nucleotide, thus counteracting replication or transcription errors.
In conclusion, this study demonstrates a correlation between the multiple mutations of the rpoB gene and the prevalent nucleotide changes in codons 526, 523, and 531. Notably, in multiple isolates bearing double, triple, and quadruple mutations predominantly identified to be from secondary infection cases, high levels of rifampin resistance (MIC, ≥100 μg/ml) were seen. Specific nucleotide changes in codon 523 (GGG→GCG [Gly→Ala]), codon 526 (CAC→TTC [His→Phe], CAA [His→Gln], CGC [His→Arg], or TAC [His→Tyr]), and codon 531 (TCG→TTG or TTC [Ser→Leu or Phe]) and double mutations in single codons were more prevalently observed for secondary cases and demonstrated a trend toward associations with high-level rifampin resistance (MIC, ≥100 μg/ml; P = 0.06). These data illustrate the need for further investigations to develop a more rapid and specific assay for the detection of MDR M. tuberculosis to be used as a screening method in areas where tuberculosis is highly endemic.
Acknowledgments
We thank colleagues of the Mycobacteriology Department of the Pasteur Institute of Iran, especially A. Karimi, N. Ebrahimzadeh, S. Khanipour, A. Masoumi, and A. Noor-Nematolahi for specimen processing, culture, AMST, PCR, and clinical strains. We especially appreciate S. Zaker and laboratory personnel for performing the DNA sequencing analyses of our strains at the Belarusian Research Institute of Epidemiology and Microbiology, Clinical Microbiology. We also thank G. Bagherzadeh for her administrative help.
This work was supported by a research grant from the Pasteur Institute of Iran.
Footnotes
Published ahead of print on 20 May 2009.
REFERENCES
- 1.Bakonyte, D., A. Baranauskaite, J. Cicenaite, A. Sosnovskaja, and P. Stakenas. 2005. Mutations in the rpoB gene of rifampin-resistant Mycobacterium tuberculosis clinical isolates from Lithuania. Int. J. Tuberc. Lung Dis. 9936-938. [PubMed] [Google Scholar]
- 2.Barfai, Z., A. Somoskovi, C. Kodmon, N. Szabo, E. Puskas, L. Kosztolanyi, E. Frago, J. Mester, L. M. Parsons, and M. Salfinger. 2001. Molecular characterization of rifampin-resistant isolates of Mycobacterium tuberculosis from Hungary by DNA sequencing and the line probe assay. J. Clin. Microbiol. 393736-3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Billington, O. J., T. D. McHugh, and S. H. Gillespie. 1999. Physiological cost of rifampin resistance induced in vitro in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 431866-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bobadilla-del-Valle, M., A. Ponce-de-Leon, C. Arenas-Huertero, G. Vargas-Alarcon, M. Kato-Maeda, P. M. Small, P. Couary, G. M. Ruiz-Palacios, and J. Sifuentes-Osornio. 2001. rpoB gene mutations in rifampin-resistant Mycobacterium tuberculosis identified by polymerase chain reaction single-stranded conformational polymorphism. Emerg. Infect. Dis. 71010-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan, X. Y., Z. Y. Hu, F. H. Xu, Z. Q. Yan, S. Q. Guo, and Z. M. Li. 2003. Rapid detection of rpoB gene mutations in rifampin-resistant Mycobacterium tuberculosis isolates in Shanghai by using the amplification refractory mutation system. J. Clin. Microbiol. 41993-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirano, K., C. Abe, and M. Takahashi. 1999. Mutations in the rpoB gene of rifampin-resistant Mycobacterium tuberculosis strains isolated mostly in Asian countries and their rapid detection by line probe assay. J. Clin. Microbiol. 372663-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang, H., Q. W. Jin, Y. Ma, X. Chen, and Y. Zhuangz. 2002. Characterization of rpoB mutations in rifampin-resistant Mycobacterium tuberculosis isolated in China. Tuberculosis 8279-83. [DOI] [PubMed] [Google Scholar]
- 8.Isfahani, B. N., A. Tavakoli, M. Salehi, and M. Tazhibi. 2006. Detection of rifampin resistance patterns in Mycobacterium tuberculosis strains isolated in Iran by polymerase chain reaction-single-strand conformation polymorphism and direct sequencing methods. Mem. Inst. Oswaldo Cruz 101597-602. [DOI] [PubMed] [Google Scholar]
- 9.Kapur, V., L.-L. Li, S. Iordanescu, M. R. Hamrick, A. Wagner, B. N. Kreiswirth, and J. M. Musser. 1994. Characterization by automated DNA sequencing of mutations in the gene (rpoB) encoding the RNA polymerase β subunit in rifampin-resistant Mycobacterium tuberculosis strains from New York and Texas. J. Clin. Microbiol. 321095-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kent, P. T., and G. P. Kubica. 1985. Public health mycobacteriology, a guide for the level III laboratory, p. 21-30. Publication no. (CDC) 86-216546. Centers for Disease Control, U.S. Department of Health and Human Services, Atlanta, GA.
- 11.Kim, B. J., S. K. Hong, K. H. Lee, Y. J. Yun, E. C. Kim, Y. G. Park, G. H. Bai, and Y. H. Kook. 2004. Differential identification of Mycobacterium tuberculosis complex and nontuberculous mycobacteria by duplex PCR assay using the RNA polymerase gene (rpoB). J. Clin. Microbiol. 421308-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin, S. Y. G., W. Probert, M. Lo, and E. Desmond. 2004. Rapid detection of isoniazid and rifampin resistance mutations in Mycobacterium tuberculosis complex from cultures or smear-positive sputa by use of molecular beacons. J. Clin. Microbiol. 424204-4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mani, C., N. Selvakumar, S. Narayanan, and P. R. Narayanan. 2001. Mutations in the rpoB gene of multidrug-resistant Mycobacterium tuberculosis clinical isolates from India. J. Clin. Microbiol. 392987-2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marin, M., D. G. de Viedma, M. J. Ruiz-Serrano, and E. Bouza. 2004. Rapid direct detection of multiple rifampin and isoniazid resistance mutations in Mycobacterium tuberculosis in respiratory samples by real-time PCR. Antimicrob. Agents Chemother. 484293-4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsiota-Bernard, P., G. Vrioni, and E. Marinis. 1998. Characterization of rpoB mutations in rifampin-resistant clinical Mycobacterium tuberculosis isolates from Greece. J. Clin. Microbiol. 3620-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCammon, M. T., J. S. Gillette, D. P. Thomas, S. V. Ramaswamy, E. A. Graviss, B. N. Kreiswirth, J. Vijg, and T. N. Quitugua. 2005. Detection of rpoB mutation associated with rifampin resistance in Mycobacterium tuberculosis using denaturing gradient gel electrophoresis. Antimicrob. Agents Chemother. 492200-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mikhailovich, V., S. Lapa, D. Gryadunov, A. Sobolev, B. Strizhkov, N. Chernyh, O. Skotnikova, O. Irtuganova, A. Moroz, V. Litvinov, M. Vladimirovskii, M. Perelman, L. Chernousova, V. Erokhin, A. Zasedatelev, and A. Mirzabekov. 2001. Identification of rifampin-resistant Mycobacterium tuberculosis strains by hybridization, PCR, and ligase detection reaction on oligonucleotide microchips. J. Clin. Microbiol. 392531-2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohammad, H. N., A. Sadeghian, M. Naderinasab, and M. Ziaee. 2006. Prevalence of primary drug resistant Mycobacterium tuberculosis in Mashhad, Iran. Indian J. Med. Res. 12477-80. [PubMed] [Google Scholar]
- 19.Mokrousov, I. 2004. Multiple rpoB mutants of Mycobacterium tuberculosis and second-order selection. Emerg. Infect. Dis. 101337-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mokrousov, I., T. Otten, B. Vyshnevskiy, and O. Narvskaya. 2003. Allele-specific rpoB PCR assays for detection of rifampin-resistant Mycobacterium tuberculosis in sputum smears. Antimicrob. Agents Chemother. 472231-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Musser, J. M. 1995. Antimicrobial agent resistance in mycobacteria: molecular genetic insights. Clin. Microbiol. Rev. 8496-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohno, H., H. Koga, S. Kohno, T. Tashiro, and K. Hara. 1996. Relationship between rifampin MICs for and rpoB mutations of Mycobacterium tuberculosis strains isolated in Japan. Antimicrob. Agents Chemother. 401053-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pozzi, G., M. Meloni, E. Iona, G. Orro, O. F. Thorensen, M. L. Ricci, M. R. Oggioni, L. Fattorini, and G. Orefici. 1999. rpoB mutations in multidrug resistant strains of Mycobacterium tuberculosis isolated in Italy. J. Clin. Microbiol. 371197-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qian, L., C. Abe, T. P. Lin, M. Yu, S. Cho, S. Wang, and J. T. Douglas. 2002. rpoB genotypes of Mycobacterium tuberculosis Beijing family isolates from east Asian countries. J. Clin. Microbiol. 401091-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruiz, M., M. J. Torres, A. C. Llanos, A. Arroyo, and J. C. Palomares. 2004. Direct detection of rifampin- and isoniazid-resistant Mycobacterium tuberculosis in auramine-rhodamine-positive sputum specimens by real-time PCR. J. Clin. Microbiol. 421585-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sajduda, A., A. Brzostek, M. Popławska, E. Augustynowicz-Kopeć, Z. Zwolska, S. Niemann, J. Dziadek, and D. Hillemann. 2004. Molecular characterization of rifampin- and isoniazid-resistant Mycobacterium tuberculosis strains isolated in Poland. J. Clin. Microbiol. 422425-2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spindola de Miranda, S., A. L. Kritski, I. Filliol, C. Mabilat, and D. E. Panteix. 2001. Mutation in the rpoB gene of rifampin-resistant Mycobacterium tuberculosis strains isolated in Brazil and France. Mem. Inst. Oswaldo Cruz 96247-250. [DOI] [PubMed] [Google Scholar]
- 28.Telenti, A., P. Imboden, F. Marchesi, D. Lowrie, S. Cole, M. J. Colston, L. Matter, K. Schopfer, and T. Bodmer. 1993. Detection of rifampin-resistance mutations in Mycobacterium tuberculosis. Lancet 341647-650. [DOI] [PubMed] [Google Scholar]
- 29.Telenti, A., N. Honore, C. Brenasconi, J. March, H. E. Takiff, and S. T. Cole. 1997. Genotyping assessment of isoniazid and rifampin resistance in Mycobacterium tuberculosis: a blind study at reference laboratory level. J. Clin. Microbiol. 35719-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Titov, L. P., S. B. Zaker, V. Slizen, L. K. Surkova, M. Taghikhani, and A. R. Bahrmand. 2006. Molecular characterization of rpoB gene mutations in rifampin-resistant Mycobacterium tuberculosis isolates from tuberculosis patients in Belarus. Biotechnol. J. 241447-1452. [DOI] [PubMed] [Google Scholar]
- 31.Valim, A. R., M. L. Rosetti, M. O. Ribeiro, and A. Zaha. 2000. Mutations in the rpoB gene of multidrug-resistant Mycobacterium tuberculosis isolates from Brazil. J. Clin. Microbiol. 383119-3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Zanden, A. G. M., E. M. Te Koppele-Vije, N. Vijaya Bhanu, D. van Soolingen, and L. M. Schouls. 2003. Use of DNA extracts from Ziehl-Neelsen-stained slides for molecular detection of rifampin resistance and spoligotyping of Mycobacterium tuberculosis. J. Clin. Microbiol. 411101-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams, D. L., I. Spring, I. Collins, L. P. Miller, L. B. Heifets, P. R. J. Gangadharam, and T. P. Gillis. 1998. Contribution of rpoB mutations to development of rifampin cross-resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 421853-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams, D. L., C. Wanguespack, K. Eisanach, J. T. Crawford, F. Porteals, M. Salfinger, C. M. Nolan, C. Abe, V. Sticht-Groh, and T. P. Gillis. 1994. Characterization of rifampin resistance in pathogenic Mycobacteria. Antimicrob. Agents Chemother. 382380-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization. 2002. An expanded DOTS framework for effective tuberculosis control. Document WHO/CDS/TB.297. World Health Organization, Geneva, Switzerland.
- 36.Yun, Y. J., K. H. Lee, L. Haihua, Y. J. Ryu, B. J. Kim, Y. H. Lee, G. H. Baek, H. J. Kim, M. S. Chung, M. C. Lee, S. H. Lee, I. H. Choi, T. J. Cho, B. S. Chang, and Y. H. Kook. 2005. Detection and identification of Mycobacterium tuberculosis in joint biopsy specimens by rpoB PCR cloning and sequencing. J. Clin. Microbiol. 43174-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zaker, S. B., L. P. Titov, A. R. Bahrmand, and M. Tagikhani. 2006. Mutability of stability genes to isoniazid and rifampin in M. tuberculosis of patients. Dokl. Natl. Acad. Sci. Belarus 5084-89. [Google Scholar]