Abstract
We present descriptions of two human Mycobacterium microti infections: one of a patient with pulmonary disease and one of a patient with osteomyelitis of the hip. Both patients had acid-fast bacilli and a positive Mycobacterium tuberculosis complex PCR from clinical specimens, but mycobacterial cultures remained negative. The microbiological diagnosis was established by molecular methods.
CASE REPORTS
Patient 1 was a 60-year-old human immunodeficiency virus-negative man who was admitted for a diagnostic pulmonary biopsy. One year prior to admission, the patient suffered from a 1-month period of weight loss, high fever, and night sweats. At that time, a chest X ray showed an infiltrate in his right lung and he was treated with a 6-month course of amoxicillin for a presumed pulmonary actinomycosis, which was diagnosed by cytological examination of a bronchoalveolar lavage sample. The infiltrate decreased during therapy and cleared 6 months after therapy. During follow-up, 6 months after cessation of amoxicillin, he developed a new lesion in his left upper lobe suspected of malignancy, with increased 18-fluordeoxyglucose uptake on a positron emission tomography scan. The lesion was resected by thoracotomy for diagnostic purposes. Histopathologic examination revealed granulomatous necrotic inflammatory tissue with acid-fast bacilli (AFB); malignant cells were not found. A Mycobacterium tuberculosis (MTB) complex PCR targeting the IS6110 sequence was positive (10). The lung tissue specimen was cultured in liquid medium (Bactec MGIT; Becton Dickinson [BD], Sparks, MD) and on agar-based media (Middlebrook and Cohn 7H10; BD) and egg-based media (Coletsos plus ossein agar; Bio-Rad, Marnes-la-Coquette, France). All cultures were incubated at 37°C for 8 weeks. After these cultures remained negative, a second attempt at mycobacterial culture was performed on tissue specimens stored at −20°C using the same media and 9 weeks of incubation. Again, mycobacterial cultures remained negative. Following the positive AFB staining and PCR results from the lung tissue specimens, sputum samples were collected on three separate occasions that were negative for AFB and mycobacterial culture. The patient was treated with isoniazid, rifampin, and ethambutol for a total of 6 months and pyrazinamide for the initial 2 months. One month after completion of therapy, the patient was well and without symptoms.
Patient 2 was a 58-year-old man who was admitted for a total hip prosthesis because of avascular necrosis of his right hip. Nineteen months earlier, corticosteroid treatment was started for polymyalgia rheumatica. He developed pain in his right hip and night sweats 7 months before admission and 3 months after cessation of corticosteroids. He reported 30 kg of weight loss since the onset of pain in the hip. He had no pulmonary complaints and no abnormalities on chest X-ray examination.
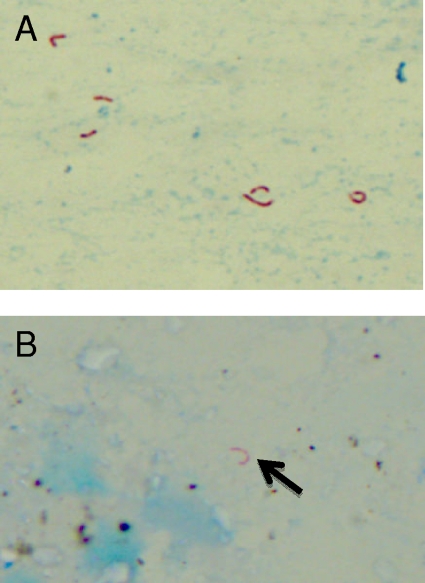
At surgery, a femoral head resection was performed, but no prosthesis was implanted since an infection was suspected intraoperatively. A Girdlestone situation was created. Synovial fluid and surgical tissue specimens showed sharply curved AFB (Fig. 1A). The synovial fluid was positive for MTB complex by the IS6110 PCR. Histopathologic examination showed a granulomatous necrotic osteomyelitis and synovitis with AFB.
FIG. 1.
Representative photographs of the Ziehl-Neelsen stains of pus from the hip from patient 2 (A) and the lung biopsy specimen from patient 1 (B). Magnification, ×630.
Synovial fluid and the four surgical tissue specimens were treated with 1.5% NaOH and were routinely cultured in liquid medium (Bactec MGIT) at 37°C for 8 weeks. After these cultures remained negative, all specimens, which were stored at −20°C, were cultured a second time without NaOH pretreatment in liquid medium and on agar-based and egg-based media at 37°C. A repeat culture of the synovial fluid was also performed on agar-based and egg-based media at 30°C, 37°C, and 42°C. Finally, the synovial fluid was inoculated in liquid medium (MYCO/F-lytic medium; BD) using the Bactec 9120 automated culture system (BD). These cultures were incubated for 22 weeks. All cultures remained negative. Three sputum specimens collected after surgery were negative for AFB and mycobacterial culture. Treatment with isoniazid, ethambutol, levofloxacin, and amikacin was initiated because of the presence of liver test abnormalities of unknown etiology. After 3 weeks, the liver test abnormalities improved and amikacin and levofloxacin were stopped and replaced with rifampin and moxifloxacin. Treatment was continued for a total of 9 months. Postoperatively, wound healing was delayed and complicated by Staphylococcus aureus infections for which the patient underwent surgical debridement and antimicrobial treatment. Mycobacteria were not detected in the surgical specimens.
In both cases, Mycobacterium microti infection was suspected when all culture attempts remained negative and review of the microscopy showed typically curved mycobacteria (Fig. 1). Therefore, the GenoType MTBC reverse hybridization assay (Hain Lifescience GmbH, Nehren, Germany) was performed directly on the lung biopsy specimen and the pus from the hip. The hybridization patterns were consistent with M. microti infection.
Patient histories were reevaluated with respect to possible exposure to MTB. Both patients had no recent contacts with known tuberculosis patients, and they had not traveled to countries with a high prevalence of tuberculosis. There was no risk of occupational exposure to tuberculosis patients: patient 1 worked as a construction supervisor and patient 2 as a sales manager. Patient 2 did have contact with a cousin suffering from active tuberculosis 40 years ago. The patients were unaware of any recent or past contacts with or occupational exposure to rodents. In both cases, no potential source of infection was identified by the public health authorities. Close contacts who were screened for tuberculosis had no signs of active tuberculosis, and the purified protein derivative skin tests were negative.
We describe a patient with a pulmonary infection and the first case of human osteomyelitis caused by M. microti. Most likely both patients presented with reactivation of a latent infection, considering their clinical presentation. No human source of these infections could be established, and no contacts with small rodents were reported. Sputum samples from both patients were negative for AFB, and no secondary cases were identified in the direct environment.
M. microti is a pathogen of rodents causing disease in voles, wood mice, and shrews, with a prevalence ranging from 5 to 50% in the United Kingdom (1). M. microti has long been considered not to be a human pathogen (12). However, several case reports have now been published describing M. microti infections in immunocompromised and immunocompetent patients. Most patients suffered from pulmonary infection, but disseminated disease and peritonitis in a patient on peritoneal dialysis have also been described (2-5, 7, 12, 13). In most cases, animal reservoirs have been implicated as the likely source of human M. microti infections, but the possibility of human-to-human transmission has been suggested (12).
M. microti is a member of the MTB complex, which is composed of the genetically closely related species M. tuberculosis, M. bovis, M. canetti, M. africanum, M. microti, and M. pinnipedii. M. microti has a typical sickle-shaped, spiral or S-like morphology in acid-fast stains of fresh material, but this property may be lost upon subculture (8). It is an extremely slow-growing Mycobacterium, and in most reported cases, cultures did not turn positive within the incubation time of 8 weeks that is routinely used in most clinical microbiology laboratories. In vitro, growth can be best obtained using pyruvate-supplemented media, which can be explained by the intrinsic lack of pyruvate kinase activity (6). Biochemically, M. microti is difficult to separate from other members of the MTB complex, and the widely used IS6110 PCR for identification of M. tuberculosis, which is positive with all members of the MTB complex, cannot make this distinction (8, 10). As already suggested by others, human M. microti infection has probably been underestimated, considering the need for extended culture procedures and the difficulty to identify the different species within the MTB complex by routine microbiology methods (12). M. microti has only recently been described as a human pathogen after the introduction of novel molecular methods for identification and direct detection of mycobacteria in clinical specimens (12).
The clinical specimens of both patients were positive for AFB and the IS6110 PCR. The AFB appeared typically curved, but this was only noticed after reexamination of the slides when cultures remained negative despite multiple attempts, use of pyruvate-supplemented media, and prolonged incubation. Infection with M. microti was then suspected, and specimens from both patients were sent to the Dutch national reference laboratory for mycobacterial cultures (National Institute of Public Health and the Environment [RIVM]) for further analysis by the GenoType MTBC reverse hybridization assay. This assay was developed to distinguish the members of the MTB complex and is based on detection of an MTB complex-specific 23S rRNA gene fragment, gyrB DNA sequence polymorphisms, and RD1 (region of difference 1) deletion of M. bovis BCG by PCR and reverse hybridization (9). The method has also been evaluated for use on AFB-positive clinical specimens (11). The specimens from both patients showed the specific hybridization pattern for M. microti.
In conclusion, molecular methods used directly on clinical specimens may readily establish the microbiological diagnosis of M. microti infection. M. microti should be suspected in case of AFB-positive specimens with positive PCR results for the MTB complex, when either cultures remain negative in the absence of tuberculostatic drugs or typical curved AFB are present. These recent molecular techniques will facilitate the investigation of the true incidence and epidemiology of human M. microti infections.
Footnotes
Published ahead of print on 1 July 2009.
REFERENCES
- 1.Burthe, S., M. Bennett, A. Kipar, X. Lambin, A. Smith, S. Telfer, and M. Begon. 2008. Tuberculosis (Mycobacterium microti) in wild field vole populations. Parasitology 135309-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foudraine, N. A., D. van Soolingen, G. T. Noordhoek, and P. Reiss. 1998. Pulmonary tuberculosis due to Mycobacterium microti in a human immunodeficiency virus-infected patient. Clin. Infect. Dis. 271543-1544. [DOI] [PubMed] [Google Scholar]
- 3.Geiss, H. K., R. Feldhues, S. Niemann, O. Nolte, and R. Rieker. 2005. Landouzy septicemia (sepsis tuberculosa acutissima) due to Mycobacterium microti in an immunocompetent man. Infection 33393-396. [DOI] [PubMed] [Google Scholar]
- 4.Horstkotte, M. A., I. Sobottka, C. K. Schewe, P. Schäfer, R. Laufs, S. Rüsch-Gerdes, and S. Niemann. 2001. Mycobacterium microti llama-type infection presenting as pulmonary tuberculosis in a human immunodeficiency virus-positive patient. J. Clin. Microbiol. 39406-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jenkins, N. E., M. B. Beadsworth, J. J. Anson, F. J. Nye, V. J. Martlew, and N. J. Beeching. 2006. Immune restoration disease in HIV patient. Emerg. Infect. Dis. 12689-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keating, L. A., P. R. Wheeler, H. Mansoor, J. K. Inwald, J. Dale, R. G. Hewinson, and S. V. Gordon. 2005. The pyruvate requirement of some members of the Mycobacterium tuberculosis complex is due to an inactive pyruvate kinase: implications for in vivo growth. Mol. Microbiol. 56163-174. [DOI] [PubMed] [Google Scholar]
- 7.Niemann, S., E. Richter, H. Dalügge-Tamm, H. Schlesinger, D. Graupner, B. Konigstein, G. Gurath, U. Greinert, and S. Rusch-Gerdes. 2000. Two cases of Mycobacterium microti derived tuberculosis in HIV-negative immunocompetent patients. Emerg. Infect. Dis. 6539-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pattyn, S. R., F. Portaels, L. Spanoghe, and J. Magos. 1970. Further studies on African strains of Mycobacterium tuberculosis: comparison with M. bovis and M. microti. Ann. Soc. Belges Med. Trop. Parasitol. Mycol. 50211-227. [PubMed] [Google Scholar]
- 9.Richter, E., M. Weizenegger, S. Rüsch-Gerdes, and S. Niemann. 2003. Evaluation of Genotype MTBC assay for differentiation of clinical Mycobacterium tuberculosis complex isolates. J. Clin. Microbiol. 412672-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savelkoul, P. H., A. Catsburg, S. Mulder, L. Oostendorp, J. Schirm, H. Wilke, A. G. van der Zanden, and G. T. Noordhoek. 2006. Detection of Mycobacterium tuberculosis complex with real time PCR: comparison of different primer-probe sets based on the IS6110 element. J. Microbiol. Methods 66177-180. [DOI] [PubMed] [Google Scholar]
- 11.Somoskovi, A., J. Dormandy, J. Rivenburg, M. Pedrosa, M. McBride, and M. Salfinger. 2008. Direct comparison of the GenoType MTBC and genomic deletion assays in terms of ability to distinguish between members of the Mycobacterium tuberculosis complex in clinical isolates and in clinical specimens. J. Clin. Microbiol. 461854-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Soolingen, D., A. G. M. van der Zanden, P. E. W. de Haas, G. T. Noordhoek, A. Kiers, N. A. Foudraine, F. Portaels, A. H. J. Kolk, K. Kremer, and J. D. A. van Embden. 1998. Diagnosis of Mycobacterium microti infections among humans by using novel genetic markers. J. Clin. Microbiol. 361840-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xavier, E. F., A. L. Seagar, C. Doig, A. Rayner, P. Claxton, and I. Laurenson. 2007. Human and animal infections with Mycobacterium microti, Scotland. Emerg. Infect. Dis. 131924-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]