Abstract
In this report, we present a clinical case of chronic aortic valve endocarditis caused by Enterococcus faecalis small-colony variants (SCVs), with ensuing characterization of the SCV phenotype in comparison to the clonally related normal phenotype with respect to alterations in microscopic and ultrastructural morphology, growth behavior, and metabolic pathways. In contrast to the normal phenotype, light and electron microscopy of the Enterococcus SCVs demonstrated the presence of heterogeneous cells of different sizes with aberrant shapes. Furthermore, SCVs showed excessive production of an intercellular substance and alterations in cell division displayed by a thick, coarse cell wall and incomplete, branched, and multiple cross walls without obvious cell separation. In addition, empty “ghost” cells were visible. In growth experiments, SCVs displayed an extended lag phase with delayed entrance into the stationary phase. Interestingly, SCV cells growing under aerobic conditions did not attain the growth and viability of the normal phenotype or those of SCVs growing under microaerobic conditions, suggesting impaired growth behavior and enhanced vulnerability in the presence of oxygen. By metabolite analysis, SCVs failed to produce significant amounts of acetate or lactate under aerobic growth conditions but were able to produce lactate under microaerobic growth conditions, implicating the induction of a fermentative metabolism. In conclusion, the observed structural alterations and changes in the cellular growth and metabolic pathways facilitated the survival of Enterococcus SCVs under microaerobic conditions in vitro and thus presumably in vivo during endocarditis.
Small-colony variants (SCVs) constitute a slow-growing subpopulation of bacteria with distinctive phenotypic and pathogenic characteristics. Since their first description in 1910, SCVs have been described in a wide range of gram-positive and gram-negative bacterial species, including Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli, Salmonella enterica, Pseudomonas aeruginosa, and Burkholderia cepacia (23). Generally, SCVs display a small colony size, a low growth rate, and atypical colony morphology (16). Biochemical characteristics of SCVs include a deficiency in electron transport or in thymidine biosynthesis (4, 23). SCVs are specialized for intracellular persistence in host cells and have been shown to be associated with persistent and recurrent infections, such as osteomyelitis, arthritis, abscesses, and respiratory infections, in humans (23). Reduced susceptibility to antimicrobial substances relative to that of their normal counterparts (26), which are often isolated simultaneously, restrict the therapeutic options for SCV infections. While SCVs of staphylococci, especially those of S. aureus, are intensively studied for their morphological, ultrastructural, metabolic, and growth characteristics (3, 4, 15-17, 22), little is known about the SCVs of Enterococcus species. Their occurrence in a patient with endocarditis (13) and in chickens with amyloid arthropathy (20) has only been briefly mentioned, without further characterization. In chickens, Enterococcus faecalis SCVs appeared to be more virulent in an in vivo infection model than the normal phenotype, but the underlying mechanisms were not investigated (20).
In this report, we present a clinical case of persistent aortic valve endocarditis caused by E. faecalis SCVs. Furthermore, we characterize the Enterococcus SCVs in comparison to the normal phenotype with respect to microscopic and ultrastructural morphology, growth behavior, metabolite production, and antimicrobial susceptibility. Our observations, defining the specific characteristics of Enterococcus SCVs, will contribute to a deeper understanding of the pathogenesis of these SCVs.
CASE REPORT
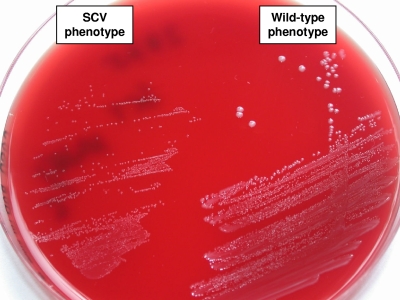
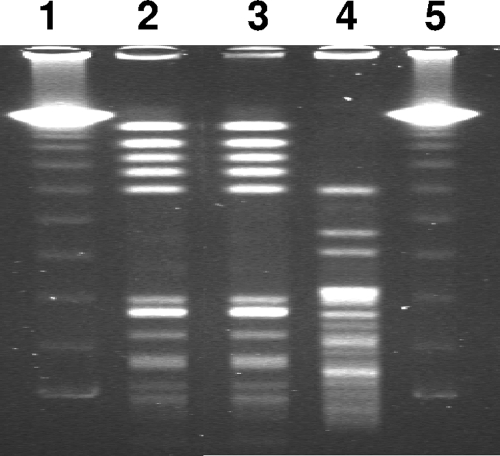
A 74-year-old female patient was admitted to the emergency department with persistent fatigue for several weeks. Her medical history included aortic valve replacement with a bovine bioprosthesis 3 years earlier due to pronounced aortic valve stenosis. She had not received any antibiotics in the previous 6 months. On admission, the patient was afebrile, and her C-reactive protein level was 56 mg/liter (reference value, <5 mg/liter). Transesophageal echocardiography showed 3- to 4-mm-long floating vegetations on the prosthetic aortic valve. Two sets of blood cultures taken on admission grew gram-positive diplococci within 24 h of incubation. Cocci were identified as Enterococcus faecalis by biochemical and molecular methods. Noticeably, on casein soy peptone (CASO) sheep blood agar and Columbia blood agar, enterococci grew with two different phenotypes: a normal phenotype and an SCV phenotype with much smaller colonies (Fig. 1). Pulsed-field gel electrophoresis (PFGE) confirmed the clonal identity of the two phenotypes (Fig. 2). Antimicrobial susceptibility testing of normal E. faecalis and SCVs was possible only on Mueller-Hinton agar containing blood, because SCVs did not grow on Mueller-Hinton agar alone. The same susceptibility results were obtained for the two phenotypes with levofloxacin, vancomycin, rifampin (rifampicin), and trimethoprim-sulfamethoxazole, while the results differed slightly for penicillin, ampicillin, and imipinem (within a twofold dilution range) but were clearly different for gentamicin by Etest, with a threefold increase in the MIC for SCVs (Table 1). The patient was allergic to penicillin and was therefore treated with imipenem (500 mg) four times daily plus rifampin (300 mg) three times daily for 6 weeks. Three weeks after finishing antimicrobial therapy, the patient was readmitted due to fatigue and suspicion of relapse of endocarditis. Transesophageal echocardiography was performed and revealed large (8- by 5-mm) vegetations on the prosthetic aortic valve. Blood cultures were not taken. The patient was treated with a combination of imipenem (500 mg) and gentamicin (80 mg) three times daily and was scheduled for an aortic valve replacement. Seven days after readmission, the artificial aortic valve was replaced with a bovine prosthetic valve (Perimount). On day 13, imipenem was changed to vancomycin (1 g) twice daily. Therapy with vancomycin and gentamicin was continued for 4 weeks. Finally, the patient was discharged in good health.
FIG. 1.
Colony morphologies of E. faecalis wild-type and SCV phenotypes on CASO blood agar after 24 h of incubation.
FIG. 2.
PFGE of E. faecalis wild-type and SCV clinical isolates after SmaI restriction. Lanes 1 and 5, 48.5- to 970-kb molecular weight marker (lambda λc1857Sam7; Bio-Rad, Germany); lane 2, wild-type phenotype; lane 3, SCV phenotype; lane 4, E. faecalis reference strain ATCC 29212.
TABLE 1.
Antimicrobial susceptibility testing of the wild-type and SCV phenotypes
| Drug | MIC by the indicated test
|
||
|---|---|---|---|
| Normal phenotype
|
SCV (Etest ) | ||
| CLSI broth microdilutiona | Etest | ||
| Penicillin | 2 (R) | 1.5 | 3 |
| Ampicillin | 0.5 (S) | 0.38 | 1 |
| Imipenem | 0.5 (S) | 1.5 | 2 |
| Levofloxacin | 1 (S) | 0.75 | 0.75 |
| Vancomycin | 2 (S) | 1 | 1 |
| Rifampin | 4 (I) | 4 | 4 |
| Gentamicin | 16 (R) | 4 | 12 |
| Linezolid | 1 (S) | 1.5 | 1.5 |
| Trimethoprim-sulfamethoxazole | ≤8 (Rb) | >32 | >32 |
Interpretations of MICs are given in parentheses. R, resistant; S, susceptible.
According to CLSI standard M100-S15, MICs should not be interpreted as indicating susceptibility to trimethoprim-sulfamethoxazole.
MATERIALS AND METHODS
Morphological and biochemical identification of E. faecalis.
E. faecalis was biochemically identified by a negative catalase-reaction, a positive PYR test (Murex Diagnostika, Burgwedel, Germany), and the API 20 Strep system (BioMérieux, Nürtingen, Germany). Growth of the isolates was evaluated on CASO sheep blood agar, Columbia blood agar, bile-esculin agar, and Mueller-Hinton agar with and without blood (all from Heipha, Heidelberg, Germany).
Molecular identification of Enterococcus faecalis.
The complete 16S rRNA gene was sequenced using primers 16Sfor and 16Srev as described previously (11). Sequence analysis was performed on a model 310 genetic analyzer (ABI Prism Biosystems, England) with the help of the Dye Terminator cycle sequencing ready reaction kit (ABI Prism). The sequences were compared to sequences available in the GenBank database by applying the BLAST algorithm. In addition, the manganese-dependent superoxide dismutase gene sodA was sequenced using primers d1 and d2 as described by Poyart et al. (21).
Antimicrobial susceptibility testing.
MICs were determined by broth microdilution according to CLSI guidelines (5) on the Micronaut system (Merlin, Bornheim-Hersel, Germany). Since the SCV phenotype did not grow in Mueller-Hinton broth without blood, susceptibility testing of the SCV and normal phenotypes was also performed on Mueller-Hinton agar with sheep blood by Etest (AB Biodisk, Stockholm, Sweden). MICs were interpreted according to CLSI criteria as suggested by the manufacturer (5).
PFGE.
PFGE was carried out with CHEF DRIII equipment (Bio-Rad, Munich, Germany) in 1% agarose at 14°C and a voltage of 200 V with a pulse-rate of 5 to 30 s, using the restriction enzyme SmaI. For interpretation of banding patterns, the recommendations of Tenover et al. were followed (27).
Electron microscopy.
For scanning electron microscopy (SEM) and transmission electron microscopy (TEM), some colonies of an overnight culture on CASO blood agar were fixed in 0.1 M phosphate buffer containing 2.5% glutaraldehyde and 1% sucrose, pH 7.3. SEM was performed with a Hitachi S-5200 electron microscope. Ultrathin sections for TEM were counterstained with 2% osmium tetroxide for 1 h, with 2% uranyl acetate in 100% ethanol for 30 min at 37°C, and with 0.3% lead citrate for 1 min; then they were examined with a Philips EM 400T electron microscope.
Auxotrophy testing.
Auxotrophy testing was carried out as described recently (14). Briefly, auxotrophy for hemin and NAD+ (Unipath, Basingstoke, United Kingdom) was tested using standard disks, and auxotrophy for thymidine (Fluka Chemie, Buchs, Switzerland) and menadione (Sigma Aldrich Chemie, Deisendorf, Germany) was tested by impregnating disks with 15 μl of thymidine at 100 μl/ml or menadione at 10 μl/ml. To determine single auxotrophism, test isolates were inoculated on chemically defined medium (CDM) agar (28). Compounds were from Merck and from Boehringer Ingelheim, Heidelberg, Germany. Impregnated disks were placed on the agar surface. Auxotrophism was determined as enhanced growth surrounding the impregnated disk after 24 h and 48 h of incubation. Likewise, for determination of double and triple auxotrophy, disks were tested on CDM agar supplemented with the different substrates (100 μg of thymidine/ml, 10 μg of menadione/ml, and 1 μg of hemin/ml) singly or in combination.
Growth and survival assays.
For investigation of growth characteristics, all bacterial cultures were inoculated from overnight cultures and diluted to an optical density at 600 nm (OD600) of 0.1 in Todd-Hewitt broth with 1% yeast extract (THY broth) (Oxoid, Wesel, Germany). For generation of aerobic growth conditions, culture flasks were incubated with shaking (200 rpm) at 36°C, with a flask-to-medium ratio of 10:1. For generation of microaerobic growth conditions, culture flasks were incubated under room air (without shaking), with a flask-to-medium ratio of 2:1. Aliquots were taken aseptically at different time intervals (up to 48 h) for determination of OD600 values and for CFU counts by plating on CASO sheep blood agar (Heipha, Heidelberg, Germany) in duplicate after serial dilution of the samples.
Determination of levels of glucose and other metabolites (acetate, lactate, ammonia) in culture supernatants.
Assays were performed as described previously (2). Briefly, aliquots of bacteria (1 ml) were centrifuged for 10 min at 21,000 × g and 4°C at the indicated time points (4 h, 8 h, 24 h, and 48 h). The culture supernatants were removed and adjusted to pH 7 to 8 with 5 M KOH, if required. Glucose, acetate, lactate, and ammonia concentrations were determined with commercial kits (R-Biopharm AG, Darmstadt, Germany) as required by the manufacturer.
Internalization assays in MonoMac-6 cells.
MonoMac-6 cells (MM6 cells; Deutsche Sammlung für Mikroorganismen und Zellkulturen) were grown in RPMI medium (RPMI 1640; Gibco Invitrogen, Karlsruhe, Germany) supplemented with 20 ml/liter AA-Mix (Promo-Cell, Heidelberg, Germany), 1 mM sodium pyruvate (Sigma, Steinheim, Germany), 2 mM l-glutamine (Biochrom, Berlin, Germany), 10 μg/ml insulin (Sigma), 100 IU penicillin and 100 μg/ml streptomycin (Biochrom), and 10% fetal calf serum (PAA Laboratories, Pasching, Austria). For the internalization experiments, 5 × 106 MM6 cells were seeded on the day of the experiment in invasion medium (RPMI 1640 without antibiotics or fetal calf serum) in 6-well plates (Nunclon, Roskilde, Denmark). Bacteria of an overnight culture in THY broth were washed once with phosphate-buffered saline (PAA Laboratories) and diluted in invasion medium. Directly before infection of MM6 cells, bacteria were separated for 30 s in an ultrasound bath. A total of 5 × 107 CFU of bacteria was added to 5 × 106 cells (multiplicity of infection [MOI], 1:10), centrifuged for 10 min (1,200 rpm, 37°C), and incubated for another 110 min at 37°C under room air with 5% CO2. Thereafter, 20 μg/ml ampicillin and 50 mg/ml gentamicin were added (Gibco), and plates were incubated for another 3 h. The supernatant was plated undiluted and in 1:10 and 1:100 dilutions on CASO blood agar for controls. In all experiments, no viable bacteria were detectable in the supernatant after the addition of antibiotics. The infected cells were lysed with sterile distilled water and pressed eight times through a 0.27-gauge needle (Braun, Melsungen, Germany) for additional mechanical disruption. Cell suspensions were serially diluted and plated on CASO blood agar in duplicate for enumeration of intracellular bacteria. CFU were counted after overnight culture at 37°C under room air with 5% CO2. For determination of intracellular survival and replication, MM6 cells were infected as described above but were incubated only 50 min before the addition of antibiotics. Thereafter, cells were incubated at 37°C and then lysed after 2, 4, 8, or 24 h.
Statistical analysis.
Levels of significance were determined by the t test and the Wilcoxon signed-rank test.
RESULTS
Identification and clonal relation of SCVs.
Smaller colonies of the SCV phenotype were observed not only on CASO blood agar but also on Columbia agar and Mueller-Hinton agar with blood incubated under room air with 5% CO2. On Mueller-Hinton agar without blood, Enterococcus SCVs did not grow. On bile-esculin agar, SCVs grew, displaying black colonies, as observed for the normal phenotype. While the normal and the SCV phenotype were isolated together from both blood cultures of the patient, the SCV phenotype was very stable and did not revert to the normal phenotype after 33 passages on CASO blood agar. Also, the normal phenotype did not switch to the SCV phenotype. The PYR test was positive for both the normal and the SCV phenotype. In addition, the API profiles (code 5143711) of the two organisms were identical. Sequencing of the 16S rRNA gene showed a homology of 100% to E. faecalis strains (for instance, GenBank accession numbers AY692453 and AF515223), and sequencing of the sodA gene confirmed the identification of the isolate as E. faecalis (99.3% homology to strain CIP 105042). PFGE typing revealed the clonal identity of the normal and SCV phenotypes (Fig. 2).
Microscopic and ultrastructural morphology.
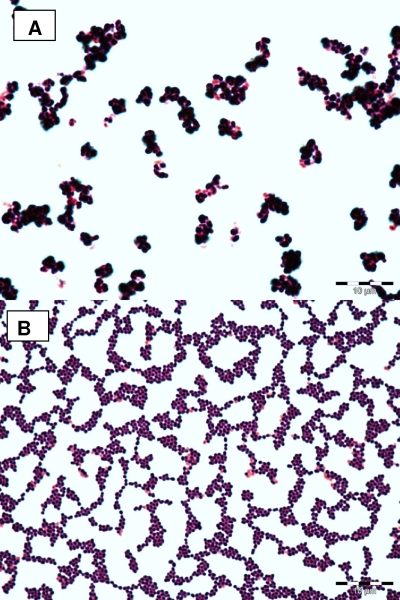
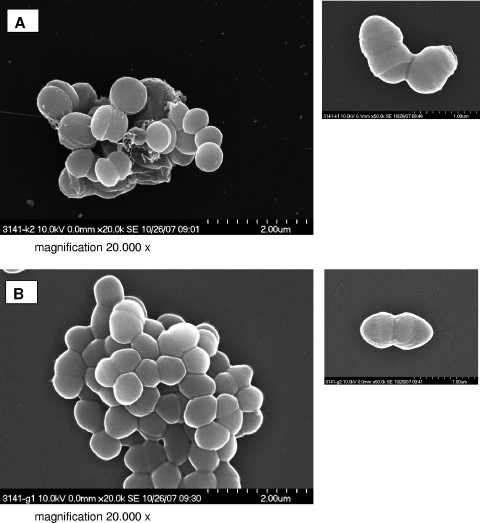
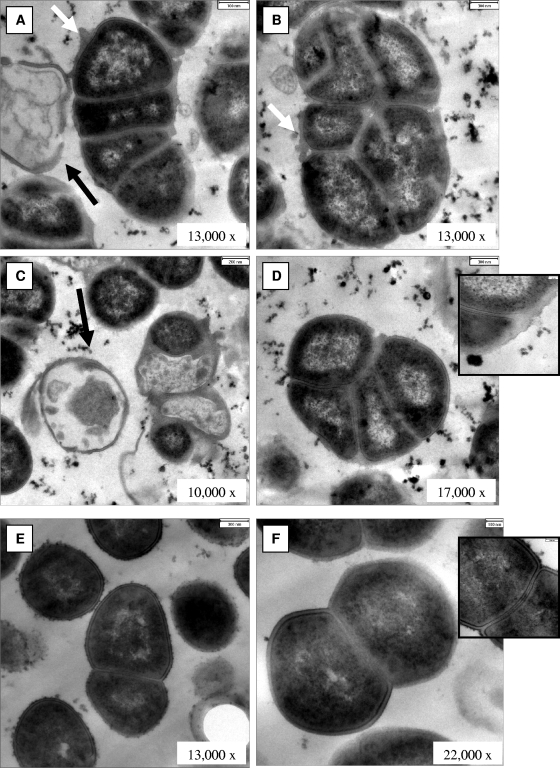
In order to investigate whether the colony morphology of the SCV is associated with structural changes of the isolate, we performed Gram staining and electron microscopy. By Gram staining, SCVs displayed cocci with very heterogeneous sizes and shapes, including small fragments as well as conglomerates of large cocci up to 2 μm in diameter (Fig. 3A). In contrast, the normal phenotype was homogeneous, with regular small cocci (Fig. 3B). SEM of SCVs revealed heterogeneous bacteria of different sizes and aberrant shapes (Fig. 4A). Cells were covered with debris and seemed to stick together by means of an intercellular substance (Fig. 4A). In contrast, the normal phenotype was homogeneous in size, without any debris (Fig. 4B). Some SCVs were up to eight times larger than normal cells. TEM revealed large cells with irregular cell division, visible by incomplete, branched, and multiple cross walls without obvious cell separation (Fig. 5A to D). The surfaces of SCVs appeared coarse, possibly due to enhanced production of an intercellular substance (Fig. 5A and B). The cell walls of SCVs were thicker than those of normal cells and lacked the bilayer structure (insets in Fig. 5D and F). In addition, “ghost” (empty) cells with defects in the cell walls were observed in SCVs (Fig. 5A and C). In contrast, the clonally related normal cells demonstrated regular cell division with a single cross wall in dividing cells, a smooth cellular surface, and the absence of empty cells (Fig. 5E and F).
FIG. 3.
Gram staining of SCV (A) and wild-type (B) cells after 24 h of incubation on CASO blood agar.
FIG. 4.
SEM of SCV (A) and wild-type (B) cells. Magnification, ×20,000.
FIG. 5.
TEM of SCV (A to D) and wild-type (E and F) cells. White arrows point to coarse surfaces of SCV cells; black arrows indicate “ghost,” or empty, cells. Insets in panels D and F show details of cell wall structure at the same magnification (bars, 100 nm).
Auxotrophism testing.
Since the SCV phenotype in other gram-positive cocci, such as S. aureus, is associated with alterations in thymidine or menadione metabolism, we performed auxotrophy testing on CDM agar for Enterococcus SCVs. The normal phenotype was able to grow on CDM agar without any supplementation. In contrast, SCVs failed to grow on CDM agar alone. After 24 h, SCVs grew only on CDM agar supplemented with hemin. After 48 h, additional enhancement of growth around the hemin disk was visible. These results suggest that this Enterococcus SCV is hemin dependent.
Enhanced susceptibility of growth and survival of E. faecalis SCVs under aerobic growth conditions.
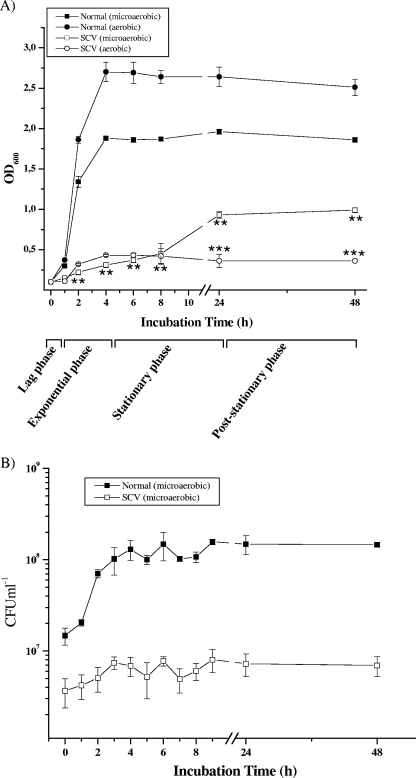
In order to determine whether the structural abnormalities of enterococcal SCVs are also associated with growth behavior different from that of the normal Enterococcus phenotype, long-term growth assays were performed in THY broth under aerobic and microaerobic conditions up tol 48 h. The normal strain exhibited a short lag phase (to 1 h), followed by a rapid increase in cell density (OD600) during the exponential-growth phase (2 to 4 h), and finally reached a constant optical density at the stationary (5 to 24 h) and poststationary (up to 48 h) phases (Fig. 6A). In sharp contrast, SCVs displayed an extended lag phase throughout the exponential-growth phase of the normal strain and reached the stationary phase at around 10 h (Fig. 6A). The overall growth patterns for the normal strain under aerobic and microaerobic conditions were similar except that a comparatively higher optical density was attained under aerobic conditions. Interestingly, SCVs growing under aerobic growth conditions demonstrated a significantly lower optical density than those under microaerobic growth conditions during the late-growth phase (24 h to 48 h), indicating a sensitivity to oxygen.
FIG. 6.
(A) Growth curves (OD600) of E. faecalis normal and SCV strains were determined in THY medium under aerobic and microaerobic conditions. Data are means ± standard errors of the means of values obtained in three independent experiments. **, P ≤ 0.005 for comparison to normal cells growing under microaerobic conditions; ***, P < 0.005 for comparison to SCV cells growing under microaerobic conditions (t test). (B) Viability assay for determination of the CFU counts (CFU ml−1) of E. faecalis normal and SCV strains. At different intervals, aliquots were removed, and CFU ml−1 was determined in duplicate. Data are means ± standard deviations of values obtained in two independent experiments.
SCVs displayed lower viable bacterial counts than cells with the normal phenotype throughout the viability assay (approximately 14-fold to 21-fold fewer CFU ml−1 for the SCV than for the normal phenotype between 2 h and 48 h) (Fig. 6B). Interestingly, microaerobically growing SCVs (SCVmicroaerobic) demonstrated greater viability at 8 h (SCVmicroaerobic, 5.9 × 106 CFU ml−1; SCVaerobic, 2.45 × 106 CFU ml−1), 24 h (SCVmicroaerobic, 8.2 × 106 CFU ml−1; SCVaerobic, 2.25 × 106 CFU ml−1), and 48 h (SCVmicroaerobic, 7.65 × 106 CFU ml−1; SCVaerobic, 1.5 × 106 CFU ml−1) than aerobically growing SCVs (twofold, fourfold, and fivefold enhancements of viability, respectively [means of two independent experiments]), suggesting increased susceptibility of SCVs growing in the presence of oxygen and protection under microaerobic conditions.
E. faecalis SCV utilizes fermentative pathways under microaerobic growth conditions.
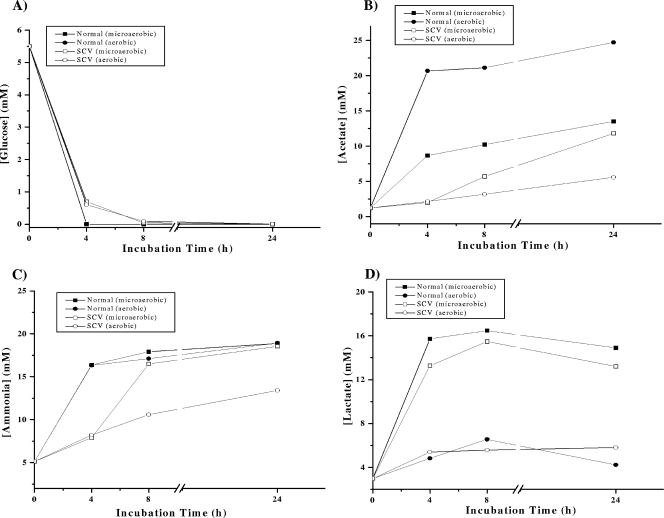
In addition to the alterations in the growth and survival characteristics of SCVs, changes in the metabolism of SCVs of different pathogenic bacteria, including S. aureus, have been repeatedly documented (3, 17). To test if this is also true for Enterococcus SCVs, we determined the glucose, acetate, lactate, and ammonia concentrations in supernatants of Enterococcus normal and SCV cultures. In accordance with the literature (7), glucose was completely consumed in normal and SCV cultures by 4 h (Fig. 7A) under both aerobic and microaerobic growth conditions. Glucose catabolization yields ATP and pyruvate through intermediary carbohydrate metabolism. Pyruvate is ultimately catabolized by enterococci to CO2 and acetate under aerobic growth conditions and to CO2 and lactate under microaerobic growth conditions (19, 25). Accordingly, on depletion of glucose during the exponential-growth phase (4 h), we observed a rapid increase in acetate concentrations (Fig. 7B) and very small amounts of lactate (Fig. 7D) in the normal strain under aerobic growth conditions, while lactate was the major end product, along with minor quantities of acetate, under microaerobic growth conditions (Fig. 7D). In striking contrast, SCVs totally failed to produce significant amounts of acetate or lactate under aerobic growth conditions up to 24 h (Fig. 7B and D), suggesting that Enterococcus SCVs were unable to produce acetate and/or lactate in the presence of oxygen. However, under microaerobic conditions, lactate was detected in SCV cultures (Fig. 7D), implicating a preference for the induction of a fermentative metabolism. Production of acetate was also observed, although at a delayed time point (≥8 h) (Fig. 7B).
FIG. 7.
Determination of glucose concentrations and levels of metabolites (acetate, ammonia, and lactate) in culture supernatants under aerobic and microaerobic growth conditions. At different times, supernatants of E. faecalis normal and SCV strains, cultivated in THY medium, were analyzed for concentrations of glucose (A), acetate (B), ammonia (C), and lactate (D). Values are representative results of at least two independent experiments.
In addition to determining the concentrations of the metabolites, we also measured the pHs of the culture supernatants of normal and SCV strains, since a decrease in pH (an effect of glycolysis) is an indicator of acid production during normal growth in most microorganisms (7, 10). During the exponential-growth phase (2 h), the pH of the supernatant decreased slightly, to 7.3 (the pH of THY medium is 7.8), for both strains under both aerobic and microaerobic growth conditions, followed by an increase during late-exponential phase, and reached 7.9 during stationary phase (24 h) (a consequence of the generation of ammonia by amino acid catabolism [7]). Interestingly, there was a delay in ammonia production in Enterococcus SCVs during the exponential-growth phase (4 h) under both growth conditions, indicating delayed amino acid catabolism (Fig. 7C).
Invasion and intracellular persistence in human mononuclear cells.
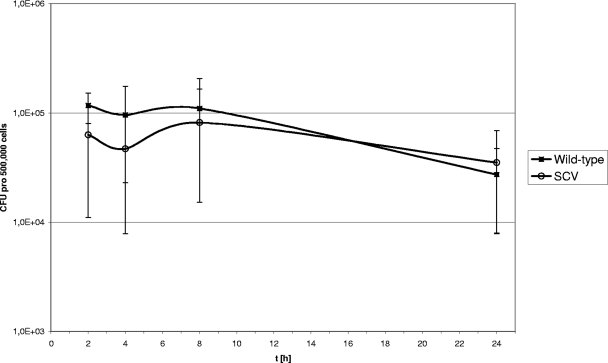
Since S. aureus SCVs differ from the S. aureus normal phenotype in their ability to invade and persist in mammalian cells, we conducted internalization assays with the human mononuclear cell line MM6. After 2 h of incubation at an MOI of 1:10, the percentages of bacteria that were internalized in MM6 cells differed between the normal and the SCV phenotype (mean percentages of bacteria internalized ± standard deviations [SD], 10.3% ± 13.2% for the normal phenotype and 6.5% ± 6.0% for the SCV phenotype) without reaching statistical significance (P = 0.47) (results of six experiments). Also, the persistence of internalized bacteria in MM6 cells after 24 h differed for the normal versus the SCV phenotype: 50% of the SCV bacteria persisted, in contrast to 20% of the normal bacteria (results of four experiments) (P = 0.068) (Fig. 8).
FIG. 8.
Intracellular replication of SCV and wild-type bacteria in MM6 cells. MM6 cells were infected at an MOI of 1:10, and cells were lysed 2, 4, 8, and 24 h after infection. The mean, minimal, and maximal values for four independent experiments are presented.
DISCUSSION
SCVs present a slow-growing subpopulation of bacteria that are specifically adapted to persist in mammalian hosts. Clinically, SCVs mainly cause chronic and persistent infections, such as osteomyelitis, abscesses, or respiratory infection, for instance, in cystic fibrosis patients. There is only limited information about the occurrence of SCVs in the background of endocarditis. To date, only two cases of endocarditis caused by SCVs had been reported; the first involved a case of prosthetic valve endocarditis caused by S. epidermidis SCVs, and the second, presented in a meeting report, concerned a case of prosthetic mitral valve endocarditis caused by E. faecalis SCVs (1, 13). To our knowledge, this is the first comprehensive characterization of clinical E. faecalis SCVs, which were isolated from a patient with endocarditis, including morphological, ultrastructural, metabolic, and growth phase analyses and antimicrobial susceptibility testing.
Antimicrobial therapy of infections with SCVs is challenging, since the SCV phenotype is often associated with higher antimicrobial resistance than that of the normal phenotype (22). Our E. faecalis SCV was specifically more resistant to gentamicin. With β-lactam antibiotics, only a slightly increased MIC was detected. This is somewhat unexpected, since structural changes of the bacterial cell wall are supposed to be associated with increased resistance to β-lactam antibiotics in SCVs of other bacteria, such as S. aureus. However, growth of SCVs was possible only on blood-supplemented agar, which might have led to partial reversion of SCVs and might thus have produced wrong results for susceptibility testing. Due to a penicillin allergy, the patient was treated with imipenem, and instead of aminoglycosides, rifampin was added. Therefore, the observed recurrence of the endocarditis is difficult to evaluate. The endocarditis was finally cured after replacement of the prosthetic aortic valve and appropriate antimicrobial therapy for 6 weeks.
Among gram-positive bacteria, S. aureus SCVs have been most extensively investigated, and much is known about the biochemical and pathophysiological alterations in SCVs relative to their normal phenotypes (3, 4, 15, 17, 23). Like S. aureus SCVs and those of other bacterial species, the Enterococcus SCVs described here formed smaller colonies than the clonally related normal strain on universal culture media and failed to grow on simple media such as Mueller-Hinton-agar and broth without the addition of blood. Interestingly, the SCV phenotype was remarkably stable, in contrast to the findings of a previous report (13). In comparison to the normal phenotype, Enterococcus SCVs lacked an exponential-growth phase and reached significantly reduced growth (optical density) and viability (CFU). Furthermore, our data demonstrated that SCV cells were sensitive to oxidative stress, undergoing an additional reduction in growth and viability under aerobic growth conditions. This could be due to regulation of key enzymes involved in the production/utilization of pyruvate, which might act as metabolic branch points between the normal and SCV phenotypes under oxidative growth conditions. Some of these enzymes, for example, PflB/EF1613 (pyruvate-formate lyase, oxygen sensitive), PDH (pyruvate dehydrogenase complex, regulated by the NADH/NAD+ ratio) (19, 25), and SdhA/EF0098 (l-serine dehydratase, oxygen sensitive due to the presence of an iron-sulfur cluster protein) (8), are interesting candidates, and their involvement in the regulation of the E. faecalis SCV phenotype will be investigated in the future.
Since the growth and physiology of a microorganism is the outcome of its metabolic adaptations, we observed important alterations in the metabolism of the Enterococcus SCVs. E. faecalis cells do not synthesize heme (9), and genes for known porphyrin biosynthetic enzymes are not found in the genome sequence of E. faecalis strain V583 (6). Thus, enterococci lack the enzymes required for oxidative energy-linked metabolism, especially the tricarboxylic acid cycle (oxidative-level phosphorylation) (12). Instead, enterococci depend mainly on substrate-level phosphorylation (glycolysis) for energy production. In accordance with this, we observed the catabolism of glucose through glycolysis and the resulting accumulation of acetate/lactate during substrate-level phosphorylation in the normal strain (post-exponential-growth phase at 4 h), which coincided with its rapid exponential growth. Ideally, as glucose is depleted, it is stoichiometrially converted into acetate/lactate (1 glucose molecule = 2 acetate molecules). However, the lack of accumulation of acetate/lactate in the SCV culture medium, the reduced formation of ammonia, and the reduced growth and viability of the bacteria under aerobic growth conditions suggested that glucose carbons were being diverted from substrate-level phosphorylation to another metabolic pathway or other cellular processes. Interestingly, Enterococcus SCVs preferably utilized a fermentative pathway under microaerobic growth conditions, as seen in S. aureus hemin-dependent (hemB mutant) SCVs (17). This was also demonstrated by the auxotrophism tests. In contrast, the alterations in survival and metabolism in the aerobically grown Enterococcus SCVs were different from those of the S. aureus thymidine-dependent SCVs grown under similar conditions (16).
In addition to the metabolic and physiologic modifications, we could also demonstrate gross morphological alterations in the individual SCV cells. SCVs formed heterogeneously sized, abnormal, large cocci, morphologies comparable those to reported for S. aureus SCVs (16). Electron microscopy confirmed alterations in the building of cross walls and cell division that led to irregular and very large cells. Similar ultrastructural alterations have recently been described for S. aureus thymidine-dependent SCVs and were reversible by thymidine supplementation (16). In our E. faecalis SCV, however, as in the previously reported E. faecalis SCV (13), thymidine dependency was not detectable. Although enterococci cannot synthesize hemin, as mentioned above, the normal Enterococcus cells were able to grow on a minimal medium, such as CDM agar, without any supplementation, and the addition of disks impregnated with hemin did not enhance the growth of the normal phenotype. However, Enterococcus SCVs failed to grow on CDM agar alone and were able to grow only in the presence of hemin. These results show that the normal Enterococcus phenotype was independent of external hemin, while the SCVs depended on hemin supplementation. Further investigation will be performed to determine which pathways are responsible for this differential response to hemin.
In addition, the surfaces of the SCVs appeared coarse, possibly due to enhanced production of an intercellular substance. Although relatively little is known about the virulence determinants of E. faecalis, an aggregation substance is assumed to be an important virulence factor. The E. faecalis aggregation substance is a surface protein that primarily promotes the conjugative transfer of sex pheromone plasmids by the formation of mating aggregates between donor and acceptor cells (18, 29). The aggregation substance is encoded on the low-copy-number 60-kb plasmid paD1, which also encodes a virulence-related cytolytic exotoxin (18, 29). Cellular adherence of E. faecalis, internalization into mammalian cells, and intracellular survival in human macrophages have been found to be promoted by the aggregation substance (24, 26). One may thus speculate that the SCV phenotype is associated with alterations in the aggregation substance.
Taken together, Enterococcus SCVs showed higher levels of antibiotic resistance than the normal phenotype. The detailed analysis of these SCVs revealed gross structural alterations and changes in cellular growth and in metabolic pathways, which partially reverted under microaerobic conditions, thereby allowing SCVs to survive under conditions such as those present in endocarditis.
Acknowledgments
We gratefully thank Paul Walther and Eberhard Schmid, Department of Electron Microscopy, University of Ulm, for assistance in performing TEM and SEM; Angelika Moericke for performing PFGE; Stefanie Mauerer for sequencing the sodA gene; and Susanne Stoecker for performing susceptibility testing.
The contribution of I.C. to this article was supported by a grant from the Deutsche Forschungsgemeinschaft (He1850/8-1).
Footnotes
Published ahead of print on 15 July 2009.
REFERENCES
- 1.Baddour, L. M., and G. D. Christensen. 1987. Prosthetic valve endocarditis due to small-colony staphylococcal variants. Rev. Infect. Dis. 91168-1174. [DOI] [PubMed] [Google Scholar]
- 2.Chatterjee, I., P. Becker, M. Grundmeier, M. Bischoff, G. A. Somerville, G. Peters, B. Sinha, N. Harraghy, R. A. Proctor, and M. Herrmann. 2005. Staphylococcus aureus ClpC is required for stress resistance, aconitase activity, growth recovery, and death. J. Bacteriol. 1874488-4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chatterjee, I., M. Herrmann, R. A. Proctor, G. Peters, and B. C. Kahl. 2007. Enhanced post-stationary-phase survival of a clinical thymidine-dependent small-colony variant of Staphylococcus aureus results from lack of a functional tricarboxylic acid cycle. J. Bacteriol. 1892936-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatterjee, I., A. Kriegeskorte, A. Fischer, S. Deiwick, N. Theimann, R. A. Proctor, G. Peters, M. Herrmann, and B. C. Kahl. 2008. In vivo mutations of thymidylate synthase (encoded by thyA) are responsible for thymidine dependency in clinical small-colony variants of Staphylococcus aureus. J. Bacteriol. 190834-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial susceptibility testing. M100-S15. Clinical and Laboratory Standards Institute, Wayne, PA.
- 6.Frankenberg, L., M. Brugna, and L. Hederstedt. 2002. Enterococcus faecalis heme-dependent catalase. J. Bacteriol. 1846351-6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gardner, J. F., and J. Lascelles. 1962. The requirement for acetate of a streptomycin-resistant strain of Staphylococcus aureus. J. Gen. Microbiol. 29157-164. [DOI] [PubMed] [Google Scholar]
- 8.Giard, J. C., J. M. Laplace, A. Rince, V. Pichereau, A. Benachour, C. Leboeuf, S. Flahaut, Y. Auffray, and A. Hartke. 2001. The stress proteome of Enterococcus faecalis. Electrophoresis 222947-2954. [DOI] [PubMed] [Google Scholar]
- 9.Gibson, C. M., T. C. Mallett, A. Claiborne, and M. G. Caparon. 2000. Contribution of NADH oxidase to aerobic metabolism of Streptococcus pyogenes. J. Bacteriol. 182448-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldschmidt, M. C., and D. M. Powelson. 1953. Effect of the culture medium on the oxidation of acetate by Micrococcus pyogenes var. aureus. Arch. Biochem. Biophys. 46154-163. [DOI] [PubMed] [Google Scholar]
- 11.Hiraishi, A. 1992. Direct automated sequencing of 16S rDNA amplified by polymerase chain reaction from bacterial cultures without DNA purification. Lett. Appl. Microbiol. 15210-213. [DOI] [PubMed] [Google Scholar]
- 12.Huycke, M. M., D. Moore, W. Joyce, P. Wise, L. Shepard, Y. Kotake, and M. S. Gilmore. 2001. Extracellular superoxide production by Enterococcus faecalis requires demethylmenaquinone and is attenuated by functional terminal quinol oxidases. Mol. Microbiol. 42729-740. [DOI] [PubMed] [Google Scholar]
- 13.Kaase, M., A. Anders, and S. G. Gatermann. 2004. First description of small-colony variants of Enterococcus faecalis isolated from an endocarditis patient. Int. J. Med. Microbiol. 294146. [Google Scholar]
- 14.Kahl, B., M. Herrmann, A. Schulze Everding, H. G. Koch, K. Becker, E. Harms, R. A. Proctor, and G. Peters. 1998. Persistent infection with small colony variant strains of Staphylococcus aureus in patients with cystic fibrosis. J. Infect. Dis. 1771023-1029. [DOI] [PubMed] [Google Scholar]
- 15.Kahl, B. C., G. Belling, P. Becker, I. Chatterjee, K. Wardecki, K. Hilgert, A. L. Cheung, G. Peters, and M. Herrmann. 2005. Thymidine-dependent Staphylococcus aureus small-colony variants are associated with extensive alterations in regulator and virulence gene expression profiles. Infect. Immun. 734119-4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahl, B. C., G. Belling, R. Reichelt, M. Herrmann, R. A. Proctor, and G. Peters. 2003. Thymidine-dependent small-colony variants of Staphylococcus aureus exhibit gross morphological and ultrastructural changes consistent with impaired cell separation. J. Clin. Microbiol. 41410-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohler, C., C. von Eiff, G. Peters, R. A. Proctor, M. Hecker, and S. Engelmann. 2003. Physiological characterization of a heme-deficient mutant of Staphylococcus aureus by a proteomic approach. J. Bacteriol. 1856928-6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muscholl-Silberhorn, A., E. Rozdzinski, and R. Wirth. 2000. Aggregation substance of Enterococcus faecalis: a multifunctional adhesin. Adv. Exp. Med. Biol. 48575-83. [DOI] [PubMed] [Google Scholar]
- 19.Neijssel, O. M., J. L. Snoep, and M. J. Teixeira de Mattos. 1997. Regulation of energy source metabolism in streptococci. Soc. Appl. Bacteriol. Symp. Ser. 2612S-19S. [PubMed] [Google Scholar]
- 20.Petersen, A., M. S. Chadfield, J. P. Christensen, H. Christensen, and M. Bisgaard. 2008. Characterization of small colony variants of Enterococcus faecalis isolated from chickens with amyloid arthropathy. J. Clin. Microbiol. 462686-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poyart, C., G. Quesne, S. Coulon, P. Berche, and P. Trieu-Cuot. 1998. Identification of streptococci to species level by sequencing the gene encoding the manganese-dependent superoxide dismutase. J. Clin. Microbiol. 3641-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Proctor, R. A., B. Kahl, C. von Eiff, P. E. Vaudaux, D. P. Lew, and G. Peters. 1998. Staphylococcal small colony variants have novel mechanisms for antibiotic resistance. Clin. Infect. Dis. 27(Suppl. 1)S68-S74. [DOI] [PubMed] [Google Scholar]
- 23.Proctor, R. A., C. von Eiff, B. C. Kahl, K. Becker, P. McNamara, M. Herrmann, and G. Peters. 2006. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat. Rev. Microbiol. 4295-305. [DOI] [PubMed] [Google Scholar]
- 24.Sartingen, S., E. Rozdzinski, A. Muscholl-Silberhorn, and R. Marre. 2000. Aggregation substance increases adherence and internalization, but not translocation, of Enterococcus faecalis through different intestinal epithelial cells in vitro. Infect. Immun. 686044-6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snoep, J. L., M. J. Teixeira de Mattos, and O. M. Neijssel. 1991. Effect of the energy source on the NADH/NAD ratio and on pyruvate catabolism in anaerobic chemostat cultures of Enterococcus faecalis NCTC 775. FEMS Microbiol. Lett. 8163-66. [Google Scholar]
- 26.Süssmuth, S. D., A. Muscholl-Silberhorn, R. Wirth, M. Susa, R. Marre, and E. Rozdzinski. 2000. Aggregation substance promotes adherence, phagocytosis, and intracellular survival of Enterococcus faecalis within human macrophages and suppresses respiratory burst. Infect. Immun. 684900-4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 332233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van de Rijn, I., and R. E. Kessler. 1980. Growth characteristics of group A streptococci in a new chemically defined medium. Infect. Immun. 27444-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wirth, R. 1994. The sex pheromone system of Enterococcus faecalis. More than just a plasmid-collection mechanism? Eur. J. Biochem. 222235-246. [DOI] [PubMed] [Google Scholar]