Abstract
Noroviruses (NoV) are a major cause of epidemic nonbacterial gastroenteritis and affect all age groups worldwide. Three of five NoV genogroups, namely, genogroup I (GI), GII, and GIV, are associated with human disease. Unfortunately, these genogroups demonstrate a high degree of sequence diversity, complicating the design of pan-NoV diagnostic PCR tests. To decrease the risk of false-negative test results, we have developed a new one-step real-time TaqMan reverse transcription-PCR protocol. This protocol detects all human NoV genogroups in one reaction with a sensitivity of 400 virus genome equivalents/reaction for both GI and GII. The use of in vitro-transcribed NoV RNA as an external standard allows (semi)quantification of viral loads in samples. In a retrospective analysis of 206 stool samples from 77 patient episodes, the duration of NoV excretion and the amount of virus excreted were determined. Twenty (26.0%) of these episodes lasted longer than 10 days. Univariate risk factor analysis revealed the patient status after organ transplantation (odds ratio [OR], 7.49 [95% confidence interval, 2.06 to 28.32]; P < 0.001), immunosuppression (OR, 9.19 [95% confidence interval, 2.50 to 35.39]; P < 0.001), and age of less than 10 years (OR, 4.58 [95% confidence interval, 1.36 to 15.77]; P = 0.004) as risk factors for a NoV excretion period of more than 10 days. These findings were confirmed by time-dependent Kaplan-Meier analyses, whereas multivariate Cox regression analyses identified immunosuppression as the sole risk factor. Surprisingly, in contrast to the excretion periods, the viral loads in stools did not increase in connection with age or immunosuppressive status. This fact may be one important piece in the pattern of high-level NoV transmissibility and may have an impact on the development of transmission prevention strategies.
Noroviruses (NoV) are now recognized to be one of the most important causative agents of outbreaks of gastroenteritis throughout the world (4). Transmitted by several routes, such as oral ingestion of contaminated food or water, person-to-person contact, and inhalation of aerosols developed during vomiting, they affect patients of all age groups (4, 11, 14). NoV are plus-sense, single-stranded, nonenveloped RNA viruses belonging to the family Caliciviridae. Three of five NoV genogroups, namely, genogroup I (GI), GII, and GIV, are pathogenic to humans (31). Characteristic of these genogroups is a high degree of sequence diversity, leading to a risk of false-negative results from diagnostic assays such as NoV antigen detection assays and, to a lesser extent, PCR assays (6). To date, several PCR assays to increase the rate of detection of NoV have been developed (8, 9, 19, 25, 27, 30). The new, one-step PCR protocol described herein combines reverse transcription (RT) and DNA amplification in a single, closed reaction tube. It detects all human genogroups by using a multiplex primer set and convenient real-time detection of specific amplicons.
Enteric viruses can cause severe symptoms, as well as prolonged viral excretion, in immunocompromised hosts. Examples of such viruses are members of the Astroviridae and Picornaviridae in immunosuppressed children (2, 13) and Reoviridae and Adenoviridae in bone marrow transplant recipients (28). Prolonged infection with NoV, which is associated with chronic diarrhea and virus shedding, has so far been reported to occur in patients after intestinal, heart, and bone marrow transplantation and in children with malignancies (5, 10, 15, 16, 18). Here, we report the characteristics of NoV excretion in stools and analyze risk factors for a prolonged NoV excretion period in a retrospective cohort study.
MATERIALS AND METHODS
Primer and probe design.
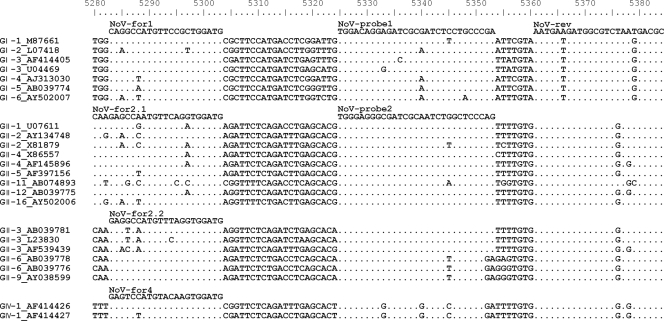
A multiple-sequence alignment was created using the Clustal W algorithm of BioEdit (7). The newly designed primers (Fig. 1) produce a 96-bp amplicon of the orf1-orf2 junction, nucleotides 5280 to 5376 of the reference sequence with accession number M87661. Melting temperatures were determined using MeltCalc99free (24). All oligonucleotides were synthesized by Eurogentec (Seraing, Belgium). Phylogenetic analyses were conducted by using Molecular Evolutionary Genetics Analysis software (MEGA version 4.0) (12).
FIG. 1.
Excerpt from the Clustal alignment depicting all primers and probes and the most variable sequences. Associated genogroups and accession numbers are indicated to the left. Dots indicate identity to the direct primer/probe above. Positions refer to the reference Norwalk virus sequence (accession number M87661).
RNA extraction from stool specimens.
An approximately 10% (vol/vol) stool suspension was prepared with sterile 1× Dulbecco's phosphate-buffered saline (Cytogen, Sinn, Germany) and clarified by centrifugation at 7,000 × g for 10 min at 4°C. Viral RNA was extracted from 140 μl of this stool suspension with the QIAamp viral RNA kit according to the instructions of the manufacturer (Qiagen, Hilden, Germany). RNA was eluted with 50 μl of heated RNase-free water and kept at −80°C.
One-step RT-PCR assay.
Apart from the oligonucleotides, all reaction components were included in the RNA UltraSense one-step quantitative RT-PCR system (Invitrogen, Carlsbad, CA). Forward primers (NoV-for1, CAGGCCATGTTCCGCTGGATG; NoV-for2.1, CAAGAGCCAATGTTCAGGTGGATG; NoV-for2.2, GAGGCCATGTTTAGGTGGATG; and NoV-for4, GAGTCCATGTACAAGTGGATG) and probes (NoV-probe-1, hexachloro-6-carboxyfluorescein-TCGGGCAGGAGATCGCGATCTCCTGTCCA-6-carboxytetramethylrhodamine, and NoV-probe-2, 6-carboxyfluorescein-CTGGGAGCCAGATTGCGATCGCCCTCCCA-6-carboxytetramethylrhodamine) were added to a final concentration of 0.2 μM, and the reverse primer (NoV-rev, GCGTCATTAGACGCCATCTTCATT) was added to a final concentration of 0.4 μM. RT and PCR amplification were performed with 5 μl of eluted RNA by using an ABI 7500 real-time PCR thermocycler under the following conditions: after RT at 50°C for 20 min and denaturation at 95°C for 2 min, amplification was performed in 45 cycles with an annealing temperature of 54°C for 30 s, an elongation step at 60°C for 60 s, and denaturation at 95°C for 30 s. Amplification data were analyzed with Sequence Detection software, version 1.4.
Concentrations of GI and GII NoV RNAs were determined independently by serial dilution of an in vitro-transcribed NoV RNA template as an external quantification standard. Standards were produced by cloning a DNA product from the above-described PCR into the pGEM-T Easy vector (Promega, Madison, WI). Orientation of the insert was checked by sequencing, the vector was linearized, and RNA was transcribed using T7 polymerase (Promega, Madison, WI). The NoV RNA concentration was determined photometrically.
Retrospective cohort study and data inclusion criteria.
In order to determine the duration of NoV excretion and the loads excreted over time, all NoV-positive stool samples from hospitalized patients tested between 1 January 2005 and 30 June 2008 were included in a retrospective analysis if at least one additional stool specimen collected from the same patient at least 2 days later was available. Samples were originally obtained for NoV detection purposes. The time point of the first positive test result was defined as time point 1, although clinical symptoms may have started earlier. The duration of NoV excretion was calculated by subtracting the day of the first positive PCR result from the day of the last positive PCR result. Prolonged NoV excretion was defined as a period of virus excretion exceeding 10 days. A second outcome, extremely prolonged NoV excretion, was defined as a period of virus excretion longer than 28 days. In total, 77 patient episodes among 75 patients were included. Two patients had two episodes within a year's time and were therefore counted as having two patient episodes each. The following potential risk factors for prolonged NoV excretion were analyzed: age of ≤3 years, age of ≤10 years, gender, status after transplantation, and intensive immunosuppression. Intensive immunosuppression was defined as the result of receiving high-level immunosuppressive drug therapy with calcineurin inhibitors. Patients given a low dosage of azathioprine or cortisone were subsumed in the variable no/mild immunosuppression.
Statistical analysis.
SPSS v15.0 and EpiInfo v3.3.2 software was used for all uni- and multivariate analyses. Categorical variables were compared using a chi-square test or Fisher's exact test. Variables were evaluated by multiple logistic regression analysis with stepwise variable selection for inclusion in the final logistic regression model. The duration of NoV excretion was analyzed by Kaplan-Meier and Cox regression analyses in which the first negative PCR result was defined as an event. If the last available test was positive for NoV, the patient episode was censored. Linear regression lines were calculated for each individual patient who had at least two positive samples with quantifiable loads.
Nucleotide sequence accession number.
A sequence typical of those detected in 15 samples from the 2006-2007 NoV infection season, which were initially positive for NoV only by a research PCR protocol and confirmed to be positive by our multiplex assay and sequencing, was uploaded into the GenBank database under accession number FM210353.
RESULTS
Development of a one-step real-time RT-PCR assay.
One hundred fifty-one well-characterized NoV sequences (9, 31) were analyzed in a multiple-sequence alignment using the Clustal W algorithm (representative sequences, including primer and probe sequences, are depicted in Fig. 1). Consensus primer sequences were designed to balance the maximum number of mismatches by using the MeltCalc software (24) to calculate the melting temperatures for the interaction of all primers with each NoV sequence in the alignment. In total, four forward primers and one reverse primer were included in the reaction to allow generic amplification of all human pathogenic NoV genogroups (GI, GII, and GIV) in one reaction. The use of two differently labeled probes allowed discrimination between GI and GII/IV in each reaction.
Analytical sensitivity as defined by the European Agency for the Evaluation of Medicinal Products (i.e., the 95% detection limit) was evaluated in five repeated runs with 10 replicates of each dilution of an in vitro-transcribed RNA template. Four hundred genome equivalents (copies) per reaction for GI as well as for GII/IV were detected. Thus, considering the dilution factor (1:70) for RNA extraction, the sensitivity corresponded to approximately 2.8 × 104 copies per ml of stool suspension.
Specificity was tested with a panel of routine surveillance stool samples obtained from recent bone marrow transplant recipients during the direct posttransplantation phase. The collection periods included the summer months of 2007 and 2008. Negative results for 55 of 55 negative samples demonstrated a high specificity of 100.0%.
Even though qualitative results for stool or vomit samples are sufficient for the clinical diagnosis of NoV infection, the new RT-PCR protocol was evaluated as a quantitative tool for use in research studies. The linearity and dynamic range of the real-time RT assay were determined by testing serial dilutions of in vitro-transcribed RNA. Amplification efficiencies (efficiency = 10−1/slope) were high, with mean values ± standard deviations of 1.93 ± 0.05 for GII and 1.90 ± 0.07 for GI, although there are two mismatches in the probe binding region between the NoV GI standard and the corresponding probe. Interassay variability was low, with maximum crossing-point variation coefficients of 16.82% for GI and 12.12% for GII. The dynamic range of linear quantification comprised at least 7 logs (9.1 × 104 to 8 × 1011 copies/ml of stool suspension).
Clinical evaluation.
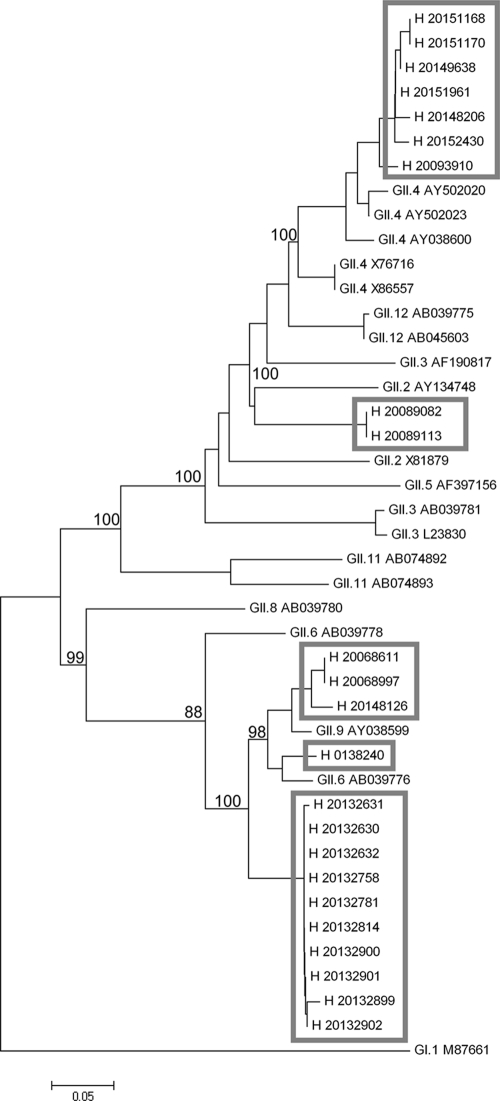
During a 12-month period (July 2007 to July 2008), 963 stool and vomit specimens were tested with the newly designed PCR, giving 276 (28.7%) positive results. Samples were mainly from patients with diarrhea in whom the presence of NoV as etiologic agents should be confirmed. As expected for the mainly hospital-based patient collective, GII/IV was the predominant genogroup, with only two GI-positive specimens found. A GII/IV-GI double infection was not discovered. However, GI NoV RNA was detected in eight of nine samples in a Swedish panel known to be positive for NoV GI. A comparison with an older, established nested PCR protocol (23) revealed concordance in 100 (98.0%) of 102 results for test samples from the 2005-2006 and 2007-2008 seasons. Moreover, 15 samples from the 2006-2007 NoV infection season, which were positive only by a research PCR protocol and failed to be detected as positive by the nested PCR assay, were detected as positive by this multiplex assay. The true positivity of these samples was confirmed by sequencing. A typical sequence from these samples showed only 93% identity to the sequence of its closest relative, GII.6 (31). Other sequenced variants belonged to the following clusters: GII.2, GII.4, GII.6, and GII.9. These clusters differ greatly at the nucleotide level, as shown in phylogenetic analyses (Fig. 2), but are nevertheless covered by the new PCR assay.
FIG. 2.
Phylogenetic dendrogram of NoV GII. The phylogenetic tree is based on a 280-bp fragment within NoV orf1 and was calculated with the neighbor-joining algorithm. Twenty-three Hannoverian sequences from the years 2005 to 2008 were compared to 20 well-classified sequence database entries. The GI.1 Norwalk virus sequence was used as the out-group. Hannoverian sequences are indicated by gray boxes. Bootstrap values indicate percentages of 1,000-fold replicates.
Patient characteristics.
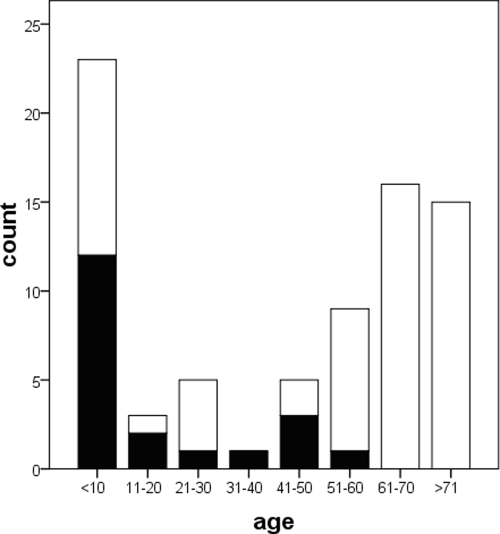
In order to determine the duration of NoV excretion from patients and the viral loads, 206 stool samples from 77 patient episodes among 75 patients from whom at least two sequential specimens could be obtained were included in a retrospective cohort study. Patient ages ranged from 14 days to 89 years (Fig. 3), and the age distribution closely resembled the notification statistics of the German Federal Ministry of Health (11; http://www3.rki.de/SurvStat), with a preponderance of the very young and very old. The median age was 64 years, and the female/male ratio was 1:1.02. Twenty (26.0%) patients, mainly transplant recipients, were treated with intensive immunosuppressive therapy (Fig. 3). Up to seven samples per patient episode, collected during an observation period of 2 to 81 days (median, 3 days), were analyzed.
FIG. 3.
Distribution of 77 NoV excretion episodes among patients with respect to age (in years) and immunosuppressive status. Episodes in patients with intense immunosuppression are indicated in black, and those in patients with no or mild immunosuppression are indicated in white.
Analysis of risk factors for prolonged NoV excretion.
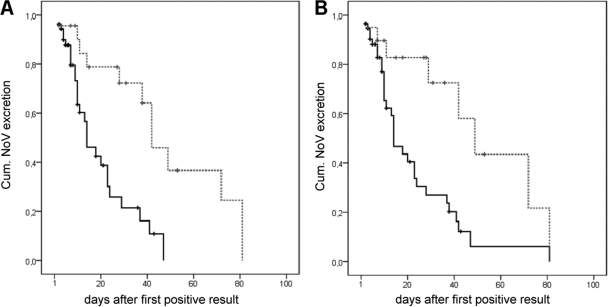
The median duration of viral excretion was 4 days (range, 1 to 61 days), increasing to 9 days (range, 1 to 61 days) for children younger than 10 years of age and 16.5 days (range, 1 to 53 days) for intensively immunosuppressed patients. In children receiving intensive immunosuppressive therapy, the median duration further increased to 28 days (range, 1 to 53 days). In 20 patient episodes, the period of NoV excretion exceeded 10 days, and in 10 of these cases the period was longer than 28 days. Univariate risk factor analysis identified status after organ transplantation, intensive immunosuppression, and young age (less than 3 years and less than 10 years) as risk factors for prolonged virus excretion (Table 1). Multivariate analysis revealed intensive immunosuppressive therapy as the sole risk factor for a prolonged excretion period of ≥10 days, with an adjusted odds ratio (OR) of 9.19 (95% confidence interval [CI95], 2.86 to 29.47). In a multivariate analysis of an extremely long virus excretion period of ≥28 days, intensive immunosuppression and age under 10 years were independently associated with prolonged viral shedding. These data could be confirmed by log rank testing in Kaplan-Meier analyses, which again indicated young age and immunosuppression as risk factors (Fig. 4). Multivariate Cox regression resulted in a significantly increased hazard ratio for young age (P = 0.001; hazard ratio 3.89 [CI95, 1.71 to 8.85]); however, intensive immunosuppression failed to achieve the level of significance in this test (P = 0.105).
TABLE 1.
Uni- and multivariate analyses of risk factors for prolonged NoV excretiona
| Potential risk factor | No. of episodes with excretion period of ≥10 days/no. in patients with risk factor | OR (CI95) | P value (χ2 test) | Multivariate OR (CI95) | No. of episodes with excretion period of ≥28 days/no. in patients with risk factor | OR (CI95) | P value (Fisher two-tailed test) | Multivariate OR (CI95) |
|---|---|---|---|---|---|---|---|---|
| Gender | 1.67 (0.53-5.32) | 0.331 | NS | 2.55 (0.53-13.78) | 0.310 | NS | ||
| Male | 12/39 | 7/39 | ||||||
| Female | 8/38 | 3/38 | ||||||
| Immunosuppression | 9.19 (2.50-35.39) | <0.001 | 9.19 (2.86-29.47) | 9.69 (1.87-56.08) | 0.002 | 5.02 (1.00-25.08) | ||
| Intense | 12/20 | 7/20 | ||||||
| None/mild | 8/57 | 3/57 | ||||||
| Transplant recipient | 7.49 (2.06-28.32) | <0.001 | NS | 6.23 (1.29-31.77) | 0.012 | NS | ||
| Yes | 11/19 | 6/19 | ||||||
| No | 9/58 | 4/58 | ||||||
| Age of ≤3 yr | 4.36 (1.23-15.85) | 0.008 | NS | 6.88 (1.41-35.52) | 0.009 | NS | ||
| ≤3 yr | 9/18 | 6/18 | ||||||
| >3 yr | 11/59 | 4/59 | ||||||
| Age of ≤10 yr | 4.58 (1.36-15.77) | 0.004 | NS | 13.87 (2.32-107.01) | 0.001 | 8.34 (1.46-47.63) | ||
| ≤10 yr | 11/23 | 8/23 | ||||||
| >10 yr | 9/54 | 2/54 |
Data are from 77 patient episodes among 75 patients. NS, not significant.
FIG. 4.
Kaplan-Meier survival plots for age (A) and intense immunosuppression (B). The y axes indicate the probability of NoV excretion x days after the first positive sample was taken. Crosses indicate censored cases. Cum., cumulative. (A) Black continuous line, age greater than 10 years; gray dashed line, age less than 10 years; log rank, P < 0.001. (B) Black continuous line, no/mild immunosuppression; gray dashed line, intense immunosuppression; log rank, P = 0.003.
Characteristics of NoV loads in 77 patient episodes.
NoV load values ranged from the detection limit (2.8 × 104 copies/ml) up to 6.5 × 1010 copies/ml of stool suspension (Fig. 5). The median for all positive samples with quantifiable loads was 2.9 × 107 copies/ml of stool suspension.
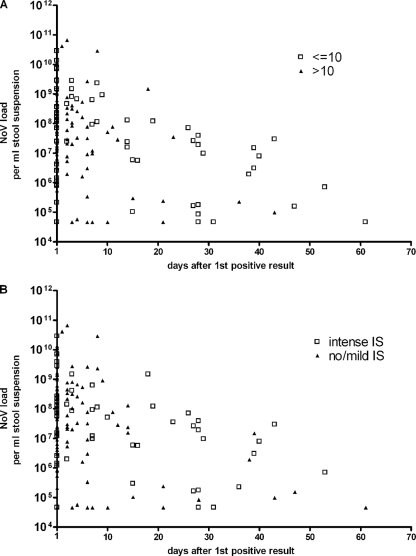
FIG. 5.
Plots of viral loads against the number of days after the first positive result, stratified by the ages (in years) (A) or immunosuppressive statuses (B) of patients. Due to log scaling, negative samples were excluded and values for samples that were positive but had viral loads below the quantification limit were calculated as half the quantification limit, i.e., 45,500 copies/ml of stool suspension. IS, immunosuppression.
Comparing the NoV loads in stool specimens over time revealed that neither the initial NoV load nor the viral concentration during the course of infection differed among the patient groups, i.e., patients younger and older than 10 years (Fig. 5A) and those with different immunosuppressive statuses (Fig. 5B). In addition, there was no difference in the median slopes which were calculated from linear regression curves for NoV loads in each patient (Mann-Whitney test: P, 0.717 for age and 0.467 for immunosuppressive status).
DISCUSSION
Recently, several new real-time PCR systems for NoV detection in stool and environmental samples have been established (8, 9, 19, 25, 27, 30), often involving two PCRs to cover both prevalent, but highly divergent, genogroups, GI and GII. Some PCR assays use a two-step protocol, with RT of the RNA followed by an amplification step. This procedure increases the hands-on time and goes along with a higher contamination risk. SYBR green real-time protocols, as favored by others, may be limited by low specificity, as nonspecific PCR by-products which are likely to be built by amplification from stool samples also cause a positive signal.
The protocol described herein uses a multiplex one-step real-time RT-PCR system with specific TaqMan probes, which proved to rapidly detect human pathogenic genogroups with good sensitivity and specificity in a single reaction. Due to the multiplex approach, differentiation between GI and GII/IV as well as the detection of double infection was possible. The one-step protocol accelerated testing, as no hands-on time for setting up the RT reaction was needed. In addition, the reduction of the open handling of PCR products decreases the contamination risk. These arguments outweigh the discrete loss of sensitivity of our protocol compared to those of more contamination-prone nested two-step protocols. Published sensitivity values range from 5 × 103 copies/g of stool (8, 23) to 2.0 × 104 copies/g of stool (9). Thus, the analytical sensitivity of 2.8 × 104 copies/ml of stool suspension for the herein-described protocol lies in the upper range of published values. Furthermore, our protocol was evaluated for diagnostic samples like stool and vomit specimens, which are expected to have high viral loads.
As established on the basis of an extensive alignment of 151 highly divergent, well-characterized NoV sequences (9, 31), a high number of divergent NoV strains were detected by this assay. In particular, 15 NoV-positive samples from the 2006-2007 season which had failed to be detected by an older nested PCR system (23) were detected by the new system. Whether or not the sequences from these samples, which show only 93% identity to the most closely related sequence, represent a new strain or a variant of a known strand cannot be totally determined, as assignments are evaluated based on larger sequence fragments, such as the entire VP1 sequence (31).
Recently, anecdotal reports of prolonged NoV excretion in immunosuppressed patients have been published (5, 10, 15). Even an extremely long period of NoV excretion, over 2 years, has been reported (18). Here, we analyzed 206 samples from 77 NoV patient episodes among 75 patients in a retrospective cohort study. Intensive immunosuppression and young age were independent risk factors for prolonged viral excretion (Table 1) in the multivariate analysis.
In our cohort comprising all age groups, the longest excretion period was 61 days, in a 4-month-old boy with terminal polycystic renal disease. This result is in accordance with the findings of Murata et al. (17), who documented extremely prolonged (42- to 47-day) viral excretion periods in otherwise healthy children aged 3 to 6 months. The median duration of viral shedding in the previous study was 16 days (range, 5 to 47 days), which is distinctly longer than that in the study presented here. This difference may be due to the very strict calculation of the duration of NoV excretion in this study, which underestimates real duration since only the period of proven positivity, from the time of the first positive PCR result to the time of the last positive result, is considered. The excretion period may have started much earlier, as observed by Ludwig et al. (15), who reported that the first NoV testing of their cancer patient cohort was performed a median of 7 days after the onset of symptoms.
In our study, children regardless of their immunosuppressive status shed the virus in a median of 9 days (range, 1 to 61 days), which is distinctly longer than the median excretion period of 3 days (range, 1 to 43 days) for the comparable adult cohort. Additional intensive immunosuppressive treatment of children increased the median period of viral shedding to 28 days (range, 1 to 53 days). Thus, young age and intense immunosuppression synergistically contribute to prolonged NoV excretion from immunocompromised children. This finding may also explain the median NoV excretion period of 46 days (range, 22 to 433 days) determined previously for a cohort of nine pediatric cancer patients with sustained immunosuppression (15).
NoV shedding over a long period of time seems to be more common than previously recognized, as demonstrated by the median NoV excretion period of 28 days for healthy adults who were experimentally infected with Norwalk virus (1). In this line, Rockx et al. (21) may have underestimated the duration of NoV excretion when designing their prospective cohort study by ending sample collection on day 22. Nearly 30% of their cohort still shed virus on this day, although the median duration of symptoms was only 5 days. Long-term shedding was not associated with increased severity of disease or with prolonged duration of symptoms. Unfortunately, a possible correlation of prolonged NoV shedding with persisting symptoms in our cohort could not be investigated due to a lack of detailed patient charts. Prolonged duration of NoV infection symptoms seems to be more common in diseased patients than in otherwise healthy patients, as documented by several studies. In a comparison of hospitalized patients and inhabitants of aged-care settings, Lopman et al. (14) observed prolonged NoV symptoms in the hospitalized patients, whom they presumed to be more diseased. Also, Mattner et al. (16) reported longer durations of symptoms after renal transplantation, and Siebenga and colleagues found prolonged illness in a series of eight patients with long periods of NoV shedding of up to 182 days (26). Interestingly, in the last study, all patients either were younger than 29 months or were immunosuppressed.
Although not needed in routine testing, quantification over at least 7 log10 steps by the real-time PCR approach was essential for investigating patterns of prolonged shedding. Only few data about viral loads in stools are available. Reported values range from 102 to 1010 copies/0.1 g of stool, with median/mean values between 107 and 108 copies/g of stool or stool suspension (1, 3, 8, 15, 29). Our positive results ranged from viral loads below the quantification limit of 9.1 × 104 copies/ml to a load of 6.5 × 1010 copies/ml of stool suspension, with a median of 2.9 × 107 copies/ml of stool suspension, and were comparable to the reported values. However, NoV concentrations in different stool samples collected from the same patient on the same day differed by up to 2 log (data not shown). Thus, the measured viral load may be only a momentary view of the pathophysiology in the gut. This possibility argues against an association between disease severity and viral load.
Strikingly, patients who excreted NoV for a long period of time did so at comparatively high viral loads (in the range of 5 × 107 copies/ml of stool suspension), irrespective of age or the extent of immunosuppression. Consequently, young age and intense immunosuppression are risk factors only for prolonged NoV excretion. Therefore, our data suggested that adaptive immunity is responsible for the termination of virus shedding. Impaired adaptive immunity, such as that in patients who are immunosuppressed after transplantation, may be the reason for prolonged NoV excretion. In contrast, innate immunity (e.g., interferon or cytokine induction) may influence the amount of viral load in stools. As this branch of the immune system is supposed to be unaffected in little children and transplant recipients, no significant difference in the viral load was seen.
The high NoV loads are in line with the results from a study of Norwalk virus-infected healthy volunteers (1), who also continued to excrete Norwalk virus in stools at high loads in the range of 107 to 108 copies/g of stool for at least 14 days. Taking into account the low infective dose of 10 to 100 virions, it is astonishing that the infectiousness of immunocompetent persons, e.g., health care workers in a hospital outbreak (22), is considered to be low or nonexistent 48 to 72 h after symptoms stop. Whether herd immunity in a completely infected ward, virion neutralization with immunoglobulin A antibodies, or increased attention to hand hygiene and environmental disinfection plays a role in this setting remains to be elucidated.
In conclusion, NoV infection can no longer be considered to cause a relatively mild gastroenteritic infection of short duration (20). Our data indicate that highly immunosuppressed patients as well as young children fail to eliminate NoV infection and shed the virus at high loads for a long period of time. In addition, the prolonged excretion of NoV in stools from hospitalized young children may have an impact on the isolation time needed to prevent nosocomial transmission or even outbreaks.
Acknowledgments
We thank A. Matussek, Department of Clinical Microbiology, Jönköping, Sweden, and D. Hofmann, Institute of Virology, Munich, Germany, for providing NoV-positive material for validation.
Footnotes
Published ahead of print on 22 July 2009.
REFERENCES
- 1.Atmar, R. L., A. R. Opekun, M. A. Gilger, M. K. Estes, S. E. Crawford, F. H. Neill, and D. Y. Graham. 2008. Norwalk virus shedding after experimental human infection. Emerg. Infect. Dis. 141553-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellmunt, A., G. May, R. Zell, P. Pring-Akerblom, W. Verhagen, and A. Heim. 1999. Evolution of poliovirus type I during 5.5 years of prolonged enteral replication in an immunodeficient patient. Virology 265178-184. [DOI] [PubMed] [Google Scholar]
- 3.Chan, M. C., J. J. Sung, R. K. Lam, P. K. Chan, N. L. Lee, R. W. Lai, and W. K. Leung. 2006. Fecal viral load and norovirus-associated gastroenteritis. Emerg. Infect. Dis. 121278-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Estes, M. K., B. V. Prasad, and R. L. Atmar. 2006. Noroviruses everywhere: has something changed? Curr. Opin. Infect. Dis. 19467-474. [DOI] [PubMed] [Google Scholar]
- 5.Gallimore, C. I., D. Lewis, C. Taylor, A. Cant, A. Gennery, and J. J. Gray. 2004. Chronic excretion of a norovirus in a child with cartilage hair hypoplasia (CHH). J. Clin. Virol. 30196-204. [DOI] [PubMed] [Google Scholar]
- 6.Gray, J. J., E. Kohli, F. M. Ruggeri, H. Vennema, A. Sanchez-Fauquier, E. Schreier, C. I. Gallimore, M. Iturriza-Gomara, H. Giraudon, P. Pothier, I. Di Bartolo, N. Inglese, E. de Bruin, B. van der Veer, S. Moreno, V. Montero, M. C. de Llano, M. Hohne, and S. M. Diedrich. 2007. European multicenter evaluation of commercial enzyme immunoassays for detecting norovirus antigen in fecal samples. Clin. Vaccine Immunol. 141349-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall, T. A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 4195-99. [Google Scholar]
- 8.Hohne, M., and E. Schreier. 2004. Detection and characterization of norovirus outbreaks in Germany: application of a one-tube RT-PCR using a fluorogenic real-time detection system. J. Med. Virol. 72312-319. [DOI] [PubMed] [Google Scholar]
- 9.Kageyama, T., S. Kojima, M. Shinohara, K. Uchida, S. Fukushi, F. B. Hoshino, N. Takeda, and K. Katayama. 2003. Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J. Clin. Microbiol. 411548-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaufman, S. S., N. K. Chatterjee, M. E. Fuschino, D. L. Morse, R. A. Morotti, M. S. Magid, G. E. Gondolesi, S. S. Florman, and T. M. Fishbein. 2005. Characteristics of human calicivirus enteritis in intestinal transplant recipients. J. Pediatr. Gastroenterol. Nutr. 40328-333. [DOI] [PubMed] [Google Scholar]
- 11.Koch, J., T. Schneider, K. Stark, and E. Schreier. 2006. Norovirus infections in Germany. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 49296-309. (In German.) [DOI] [PubMed] [Google Scholar]
- 12.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 5150-163. [DOI] [PubMed] [Google Scholar]
- 13.Liste, M. B., I. Natera, J. A. Suarez, F. H. Pujol, F. Liprandi, and J. E. Ludert. 2000. Enteric virus infections and diarrhea in healthy and human immunodeficiency virus-infected children. J. Clin. Microbiol. 382873-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopman, B. A., M. H. Reacher, I. B. Vipond, J. Sarangi, and D. W. Brown. 2004. Clinical manifestation of norovirus gastroenteritis in health care settings. Clin. Infect. Dis. 39318-324. [DOI] [PubMed] [Google Scholar]
- 15.Ludwig, A., O. Adams, H. J. Laws, H. Schroten, and T. Tenenbaum. 2008. Quantitative detection of norovirus excretion in pediatric patients with cancer and prolonged gastroenteritis and shedding of norovirus. J. Med. Virol. 801461-1467. [DOI] [PubMed] [Google Scholar]
- 16.Mattner, F., D. Sohr, A. Heim, P. Gastmeier, H. Vennema, and M. Koopmans. 2006. Risk groups for clinical complications of norovirus infections: an outbreak investigation. Clin. Microbiol. Infect. 1269-74. [DOI] [PubMed] [Google Scholar]
- 17.Murata, T., N. Katsushima, K. Mizuta, Y. Muraki, S. Hongo, and Y. Matsuzaki. 2007. Prolonged norovirus shedding in infants <=6 months of age with gastroenteritis. Pediatr. Infect. Dis. J. 2646-49. [DOI] [PubMed] [Google Scholar]
- 18.Nilsson, M., K. O. Hedlund, M. Thorhagen, G. Larson, K. Johansen, A. Ekspong, and L. Svensson. 2003. Evolution of human calicivirus RNA in vivo: accumulation of mutations in the protruding P2 domain of the capsid leads to structural changes and possibly a new phenotype. J. Virol. 7713117-13124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pang, X. L., J. K. Preiksaitis, and B. Lee. 2005. Multiplex real time RT-PCR for the detection and quantitation of norovirus genogroups I and II in patients with acute gastroenteritis. J. Clin. Virol. 33168-171. [DOI] [PubMed] [Google Scholar]
- 20.Parrino, T. A., D. S. Schreiber, J. S. Trier, A. Z. Kapikian, and N. R. Blacklow. 1977. Clinical immunity in acute gastroenteritis caused by Norwalk agent. N. Engl. J. Med. 29786-89. [DOI] [PubMed] [Google Scholar]
- 21.Rockx, B., M. De Wit, H. Vennema, J. Vinje, E. De Bruin, Y. Van Duynhoven, and M. Koopmans. 2002. Natural history of human calicivirus infection: a prospective cohort study. Clin. Infect. Dis. 35246-253. [DOI] [PubMed] [Google Scholar]
- 22.Said, M. A., T. M. Perl, and C. L. Sears. 2008. Healthcare epidemiology: gastrointestinal flu: norovirus in health care and long-term care facilities. Clin. Infect. Dis. 471202-1208. [DOI] [PubMed] [Google Scholar]
- 23.Schreier, E., F. Doring, and U. Kunkel. 2000. Molecular epidemiology of outbreaks of gastroenteritis associated with small round structured viruses in Germany in 1997/98. Arch. Virol. 145443-453. [DOI] [PubMed] [Google Scholar]
- 24.Schutz, E., and N. von Ahsen. 1999. Spreadsheet software for thermodynamic melting point prediction of oligonucleotide hybridization with and without mismatches. BioTechniques 271218-1222, 1224. [DOI] [PubMed] [Google Scholar]
- 25.Scipioni, A., A. Mauroy, D. Ziant, C. Saegerman, and E. Thiry. 2008. A SYBR Green RT-PCR assay in single tube to detect human and bovine noroviruses and control for inhibition. Virol. J. 594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siebenga, J. J., M. F. Beersma, H. Vennema, P. van Biezen, N. J. Hartwig, and M. Koopmans. 2008. High prevalence of prolonged norovirus shedding and illness among hospitalized patients: a model for in vivo molecular evolution. J. Infect. Dis. 198994-1001. [DOI] [PubMed] [Google Scholar]
- 27.Tajiri-Utagawa, E., M. Hara, K. Takahashi, M. Watanabe, and T. Wakita. 2009. Development of a rapid high-throughput method for high-resolution melting analysis for routine detection and genotyping of noroviruses. J. Clin. Microbiol. 47435-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Troussard, X., F. Bauduer, E. Gallet, F. Freymuth, P. Boutard, J. J. Ballet, O. Reman, and M. Leporrier. 1993. Virus recovery from stools of patients undergoing bone marrow transplantation. Bone Marrow Transplant. 12573-576. [PubMed] [Google Scholar]
- 29.Tu, E. T., R. A. Bull, M. J. Kim, C. J. McIver, L. Heron, W. D. Rawlinson, and P. A. White. 2008. Norovirus excretion in an aged-care setting. J. Clin. Microbiol. 462119-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolf, S., W. M. Williamson, J. Hewitt, M. Rivera-Aban, S. Lin, A. Ball, P. Scholes, and G. E. Greening. 2007. Sensitive multiplex real-time reverse transcription-PCR assay for the detection of human and animal noroviruses in clinical and environmental samples. Appl. Environ. Microbiol. 735464-5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng, D. P., T. Ando, R. L. Fankhauser, R. S. Beard, R. I. Glass, and S. S. Monroe. 2006. Norovirus classification and proposed strain nomenclature. Virology 346312-323. [DOI] [PubMed] [Google Scholar]