Abstract
A new rapid human metapneumovirus (hMPV) detection kit using immunochromatography (SAS hMPV test) was compared to real-time PCR for 224 nasal swab specimens, 96.4% of which were obtained from children of <15 years of age. The overall sensitivity and specificity were 82.3% and 93.8%, respectively, suggesting that this test is useful for pediatricians to diagnose hMPV infection in a clinical setting.
Human metapneumovirus (hMPV) was first isolated from nasopharyngeal specimens from children with acute respiratory infection (ARI) in 2001, and hMPV has been categorized as a member of the genus Metapneumovirus of the subfamily Pneumovirinae of the family Paramyxoviridae (21). It has been recognized as a common cause of respiratory infections, ranging from upper respiratory tract infections to severe lower respiratory tract infections, in individuals of all ages, though particularly in young children, around the globe (1, 3, 4, 19, 21, 23, 24). Serological studies have indicated that most individuals have been exposed to hMPV by the age of 5 years (15, 18, 21).
hMPV is difficult to detect by cell culture due to its slow growth and weak cytopathic effect. Therefore, reverse transcription-PCR including real-time PCR has been used widely for the detection and laboratory diagnosis of hMPV (22, 23). In view of the clinical importance of hMPV, there is a need for a rapid and simple diagnostic method, such as an immunochromatography (IC) test, that can be completed simply in approximately 15 min without any special requirements. At present, IC tests for influenza virus, respiratory syncytial virus (RSV), and adenovirus are widely used for the management of infectious diseases. In this paper, we evaluate the usefulness of a newly developed IC antigen detection kit (SAS hMPV test; SA Scientific, San Antonio, TX) in comparison with the real-time PCR method for the diagnosis of hMPV infection.
A total of 224 nasopharyngeal swab specimens were obtained from patients suspected of having an hMPV infection who visited any of three pediatric clinics in Yamagata and Sendai, Japan, between December 2007 and July 2008. Specimens were collected from patients showing symptoms of ARI with fever, cough, and/or rhinorrhea. Specimens that had previously tested positive by rapid antigen test for RSV, influenza A and B viruses, or adenovirus were excluded. Among the 244 specimens, 216 (96.4%) were from children under 15 years of age, with the following overall age distribution: 49 specimens were from children aged under 1 year, 52 were from children aged 1 year, 24 were from children aged 2 years, 21 were from children aged 3 years, 20 were from children aged 4 years, 24 were from children aged 5 years, 19 were from children aged between 6 and 9 years, 7 were from children aged between 10 and 15 years, and 8 were from patients of >15 years. Specimens were collected from 117 males and 107 females. Two specimens were collected from each patient; one was used for the IC test at the respective clinic, and the other was placed immediately in a tube containing 3 ml of transport medium, consisting of Eagle's minimum essential medium (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) with 0.5% gelatin, 100 units of penicillin G (Meiji Seika, Ltd., Tokyo, Japan), and 100 μg of streptomycin (Meiji Seika, Ltd., Tokyo, Japan) (19), for application to real-time PCR assay. The specimens were sent to the Department of Microbiology, Yamagata Prefectural Institute of Public Health, frozen at −80°C within 3 days, and stored until testing.
Viral RNA was extracted from 140 μl of specimen with a QIAamp Viral RNA Mini kit (Qiagen, Hilden, Germany) according to the manufacturer's protocol. Reverse transcription was performed in a 20-μl reaction mixture containing 10 μl of extracted RNA and random primers, using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). Real-time PCR was carried out in a 10-μl reaction mixture containing 1 μl of cDNA, 0.9 μM (each) of sense and antisense primers, 0.25 μM of TaqMan probe, and 5 μl of TaqMan Fast universal PCR master mix (Applied Biosystems), using an ABI Prism 7500 Fast real-time PCR system. Primers and a probe targeting a 163-bp fragment of the hMPV N gene were designed based on the reports of Maertzdorf et al. (16) and Bonroy et al. (5). The sense primer sequence was 5′-CATAYAARCATGCTATATTAAAAGAGTCTCA-3′, the antisense primer sequence was 5′-CCTATYTCWGCAGCATATTTGTARTCAG-3′, and the probe sequence was 5′-FAM (6-carboxyfluorescein)-CAACHGCAGTRACACCYTCATCATTRCA-TAMRA (6-carboxytetramethylrhodamine)-3′ (where Y is a mix of C and T, R is a mix of A and G, W is a mix of A and T, and H is a mix of A, C, and T). The PCR conditions comprised an initial activation at 95°C for 20 s and 45 cycles of 3 s at 95°C and 30 s at 60°C. The quantitation of hMPV RNA was performed using a standard curve generated by the threshold cycle values obtained from serial 10-fold dilutions of in vitro transcripts containing 102 to 109 copies of the N gene. Each sample was analyzed in triplicate, and the average copy number was calculated.
The SAS hMPV test is an IC test using a gold colloid-conjugated hMPV antibody. A 150-μl portion of each specimen was applied to the testing device, and the hMPV antigen-antibody complex formed a test line that became visible within 15 min. The evaluation of cross-reactivity of the SAS hMPV test preceded this study. Propagated viruses, including influenza A, B, and C viruses, RSV, parainfluenza virus, adenovirus, coxsackievirus, and rhinovirus, were tested for cross-reactivity by the SAS hMPV test, and the results obtained for all viruses tested were negative.
Of the 224 specimens analyzed, 61 (27.2%) tested positive by SAS hMPV test and 62 (27.7%) tested positive by real-time PCR. Fifty-one of the 62 real-time PCR-positive specimens and 10 of the 162 real-time PCR-negative specimens were found to be positive by the SAS hMPV test (Table 1). The latter 10 specimens had reactions that were so weak that they were considered to have been false-positive results for the SAS hMPV test. Therefore, taking the real-time PCR results as the reference standard, the sensitivity and specificity were found to be 82.3% and 93.8%, respectively, for the SAS hMPV test. There was 90.6% agreement between the results of the SAS hMPV test and those of real-time PCR.
TABLE 1.
Comparison of SAS hMPV test with real-time PCR for hMPV detection
| Time (days) from onset of fever to specimen collection | Total no. of specimens | No. of specimens with resulta
|
Sensitivity (%) | Specificity (%) | |||
|---|---|---|---|---|---|---|---|
| P+, T+ | P+, T− | P−, T+ | P−, T− | ||||
| All specimens | 224 | 51 | 11 | 10 | 152 | 82.3 | 93.8 |
| 1 | 48 | 13 | 2 | 3 | 30 | 86.7 | 90.9 |
| 2 | 77 | 19 | 2 | 5 | 51 | 90.5 | 91.1 |
| 3 | 38 | 8 | 0 | 0 | 30 | 100 | 100 |
| 4 | 25 | 5 | 1 | 1 | 18 | 83.3 | 94.7 |
| >4 | 36 | 6 | 6 | 1 | 23 | 50.0 | 95.8 |
Abbreviations: P, real-time PCR; T, SAS hMPV test.
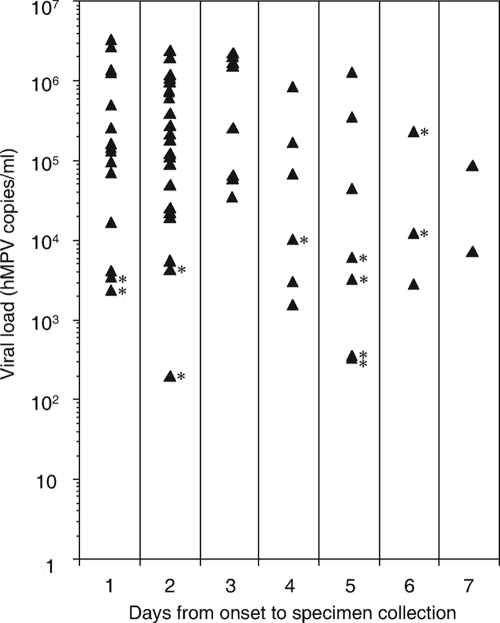
Viral loads of the 62 specimens that tested positive by real-time PCR and the number of days from onset of fever to specimen collection for each were plotted in Fig. 1. The mean number of hMPV copies/ml was 7.22 × 105 on the first day after the onset of symptoms (n = 15), 5.10 × 105 on day 2 (n = 21), 1.03 × 106 on day 3 (n = 8), 1.90 × 105 on day 4 (n = 6), 2.55 × 105 on day 5 (n = 7), 8.35 × 104 on day 6 (n = 3), and 4.85 × 104 on day 7 (n = 2). Of the 62 real-time PCR-positive specimens, all specimens having more than 1.26 × 104 hMPV copies/ml were found to be positive by SAS hMPV test, though one specimen with 2.35 × 105 copies/ml, collected on day 6 after the onset of symptoms, was found to be negative by SAS hMPV test. The sensitivity and specificity of the SAS hMPV test for specimens obtained 1, 2, 3, 4, and more than 4 days after the onset of symptoms are also shown in Table 1. The sensitivity of the SAS hMPV test for specimens obtained within 4 days after the onset of symptoms was statistically higher than that for specimens obtained more than 4 days after the onset of symptoms (90.0% versus 50.0%; P = 0.0042 by Fisher's exact probability test). Therefore, specimens should be collected within 4 days after the onset of symptoms. Since a previous study reported that the duration of fever is about 4 days for hMPV-infected children (18), the SAS hMPV test would be useful for the diagnosis of hMPV infections with associated fever.
FIG. 1.
Viral loads of real-time PCR-positive specimens and the number of days from onset of fever to specimen collection. Solid triangles indicate the 62 specimens that tested positive for hMPV by real-time PCR assay. Specimens testing negative by the SAS hMPV test are marked with asterisks.
Previous reports have described higher sensitivities of the IC test with specimens from young children than with those from older children (6, 9). In the present study, 96.4% of patients were under 15 years of age, with 55.8% of patients being less than 3 years old. When the specimens obtained within 4 days after the onset of symptoms were analyzed by age group, the SAS hMPV test was found to have greater sensitivity with specimens from children of less than 3 years of age (20 of 21 patients [95.2%]) than with those from children between 3 and 15 years of age (22 of 26 patients [84.6%]). The mean viral loads in specimens collected from children under 3 years of age and from children between 3 and 15 years of age were 8.02 × 105 copies/ml and 4.64 × 105 copies/ml, respectively. Therefore, the higher sensitivity of this test with specimens from young children appears to be associated with the larger viral loads observed in such children. However, the performance of this test for young adults and elderly people needs further evaluation.
The specificity of the SAS hMPV test was 93.8%, which is slightly lower than those for other hMPV antigen tests using immunofluorescent antibodies, which range from 94.1% to 100% (2, 7, 8, 14, 17, 20). The intensities of the 10 specimens that gave false-positive results by SAS hMPV test were very weak compared with those of true positive specimens. Of the 51 true positive specimens, the test line intensity was strong for 38 specimens and weak for the remaining 13 specimens, with the viral loads of 10 of the 13 specimens that reacted weakly being less than 105 copies/ml. Since it is likely that there is an association between viral load in specimens and the intensity of the test line, diagnosis will become more difficult as the viral load in the specimen decreases.
Rapid IC assays are widely used for the management of patients with infections caused by respiratory viruses such as influenza virus, RSV, and adenoviruses in a clinical setting. However, there is no available IC assay for hMPV and parainfluenza virus infections, despite the fact that the clinical diagnosis of ARI caused by these viruses remains difficult. Recently, an IC assay for hMPV detection was reported, but the sensitivity of this assay was not more than 70.6% (11). In general, the most rapid antigen test with the highest sensitivity is requested, and the sensitivity and specificity of the SAS hMPV test are equivalent to those of the IC assay for influenza A and B viruses and RSV (2, 9, 10, 12, 13). The SAS hMPV test is a readily available, one-step method that can be completed within 15 min, thereby helping pediatricians to clarify the causal agent of ARI in a clinical setting.
Acknowledgments
This work was supported in part by Ohkura Pharmaceutical Co., Ltd.
Footnotes
Published ahead of print on 1 July 2009.
REFERENCES
- 1.Abiko, C., K. Mizuta, T. Itagaki, N. Katsushima, S. Ito, Y. Matsuzaki, M. Okamoto, H. Nishimura, Y. Aoki, T. Murata, H. Hoshina, S. Hongo, and K. Ootani. 2007. Outbreak of human metapneumovirus detected by use of the Vero E6 cell line in isolates collected in Yamagata, Japan, in 2004 and 2005. J. Clin. Microbiol. 451912-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aslanzadeh, J., X. Zheng, H. Li, J. Tetreault, I. Ratkiewicz, S. Meng, P. Hamilton, and Y. Tang. 2008. Prospective evaluation of rapid antigen tests for diagnosis of respiratory syncytial virus and human metapneumovirus infections. J. Clin. Microbiol. 461682-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bastien, N., D. Ward, P. van Caeseele, K. Brandt, S. H. S. Lee, G. McNabb, B. Klisko, E. Chan, and Y. Li. 2003. Human metapneumovirus infection in the Canadian population. J. Clin. Microbiol. 414642-4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boivin, G., Y. Abed, G. Pelletier, L. Ruel, D. Moisan, S. Cote, T. C. Peret, D. D. Erdman, and L. J. Anderson. 2002. Virological features and clinical manifestations associated with human metapneumovirus: a new paramyxovirus responsible for acute respiratory-tract infections in all age groups. J. Infect. Dis. 1861330-1334. [DOI] [PubMed] [Google Scholar]
- 5.Bonroy, C., A. Vankeerberghen, A. Boel, and H. De Beenhouwer. 2007. Use of a multiplex real-time PCR to study the incidence of human metapneumovirus and human respiratory syncytial virus infections during two winter seasons in a Belgian paediatric hospital. Clin. Microbiol. Infect. 13504-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cazacu, A. C., S. E. Chung, J. Greer, and G. J. Demmler. 2004. Comparison of the Directigen Flu A+B membrane enzyme immunoassay with viral culture for rapid detection of influenza A and B viruses in respiratory specimens. J. Clin. Microbiol. 423707-3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ebihara, T., R. Endo, X. Ma, N. Ishiguro, and H. Kikuta. 2005. Detection of human metapneumovirus antigens in nasopharyngeal secretions by an immunofluorescent antibody test. J. Clin. Microbiol. 431138-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fenwick, F., B. Young, R. McGuckin, M. J. Robinson, Y. Taha, C. E. Taylor, and G. L. Toms. 2007. Diagnosis of human metapneumovirus by immunofluorescence staining with monoclonal antibodies in the North-East of England. J. Clin. Virol. 40193-196. [DOI] [PubMed] [Google Scholar]
- 9.Hurt, A. C., R. Alexander, J. Hibbert, N. Deed, and I. G. Barr. 2007. Performance of six influenza rapid tests in detecting human influenza in clinical specimens. J. Clin. Virol. 39132-135. [DOI] [PubMed] [Google Scholar]
- 10.Ito, M., M. Watanabe, N. Nakagawa, T. Ihara, and Y. Okuno. 2006. Rapid detection and typing of influenza A and B by loop-mediated isothermal amplification: comparison with immunochromatography and virus isolation. J. Virol. Methods 135272-275. [DOI] [PubMed] [Google Scholar]
- 11.Kikuta, H., C. Sakata, R. Gamo, A. Ishizaka, Y. Koga, M. Konno, Y. Ogasawara, H. Sawada, Y. Taguchi, Y. Takahashi, K. Yasuda, N. Ishiguro, A. Hayashi, H. Ishiko, and K. Kobayashi. 2008. Comparison of a lateral-flow immunochromatography assay with real-time reverse transcription-PCR for detection of human metapneumovirus. J. Clin. Microbiol. 46928-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuroiwa, Y., K. Nagai, L. Okita, S. Ukae, T. Mori, T. Hotsubo, and H. Tsutsumi. 2004. Comparison of an immunochromatography test with multiplex reverse transcription-PCR for rapid diagnosis of respiratory syncytial virus infections. J. Clin. Microbiol. 424812-4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landry, M. L., S. Cohen, and D. Ferguson. 2004. Comparison of Binax NOW and Directigen for rapid detection of influenza A and B. J. Clin. Virol. 31113-115. [DOI] [PubMed] [Google Scholar]
- 14.Landry, M. L., S. Cohen, and D. Ferguson. 2008. Prospective study of human metapneumovirus detection in clinical samples by use of light diagnostics direct immunofluorescence reagent and real-time PCR. J. Clin. Microbiol. 461098-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leung, J., F. Esper, C. Weibel, and J. S. Kahn. 2005. Seroepidemiology of human metapneumovirus (hMPV) on the basis of a novel enzyme-linked immunosorbent assay utilizing hMPV fusion protein expressed in recombinant vesicular stomatitis virus. J. Clin. Microbiol. 431213-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maertzdorf, J., C. K. Wang, J. B. Brown, J. D. Quinto, M. Chu, M. de Graaf, B. G. van den Hoogen, R. Spaete, A. D. Osterhaus, and R. A. Fouchier. 2004. Real-time reverse transcriptase PCR assay for detection of human metapneumoviruses from all known genetic lineages. J. Clin. Microbiol. 42981-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manoha, C., J. B. Bour, C. Pitoiset, M. Darniot, S. Aho, and P. Pothier. 2008. Rapid and sensitive detection of metapneumovirus in clinical specimens by indirect fluorescence assay using a monoclonal antibody. J. Med. Virol. 80154-158. [DOI] [PubMed] [Google Scholar]
- 18.Matsuzaki, Y., T. Itagaki, C. Abiko, Y. Aoki, A. Suto, and K. Mizuta. 2008. Clinical impact of human metapneumovirus genotypes and genotype-specific seroprevalence in Yamagata, Japan. J. Med. Virol. 801084-1089. [DOI] [PubMed] [Google Scholar]
- 19.Mizuta, K., C. Abiko, Y. Aoki, A. Suto, H. Hoshina, T. Itagaki, N. Katsushima, Y. Matsuzaki, S. Hongo, M. Noda, H. Kimura, and K. Ootani. 2008. Analysis on monthly isolation of respiratory viruses from children by cell culture using a microplate method: a two-year study from 2004 to 2005 in Yamagata, Japan. Jpn. J. Infect. Dis. 61196-201. [PubMed] [Google Scholar]
- 20.Percivalle, E., A. Sarasini, L. Visai, M. G. Revello, and G. Gerna. 2005. Rapid detection of human metapneumovirus strains in nasopharyngeal aspirates and shell vial cultures by monoclonal antibodies. J. Clin. Microbiol. 433443-3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van den Hoogen, B. G., J. C. de Jong, J. Groen, T. Kuiken, R. de Groot, R. A. Fouchier, and A. D. Osterhaus. 2001. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat. Med. 7719-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van den Hoogen, B. G., A. D. Osterhaus, and R. A. Fouchier. 2004. Clinical impact and diagnosis of human metapneumovirus infection. Pediatr. Infect. Dis. J. 23S25-S32. [DOI] [PubMed] [Google Scholar]
- 23.Williams, J. V., P. A. Harris, S. J. Tollefson, L. L. Halburnt-Rush, J. M. Pingsterhaus, K. M. Edwards, P. F. Wright, and J. E. Crowe, Jr. 2004. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N. Engl. J. Med. 350443-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams, J. V., C. K. Wang, C. Yang, S. J. Tollefson, F. S. House, J. M. Heck, M. Chu, J. B. Brown, L. D. Lintao, J. D. Quinto, D. Chu, R. R. Spaete, K. M. Edwards, P. F. Wright, and J. E. Crowe, Jr. 2006. The role of human metapneumovirus in upper respiratory tract infections in children: a 20-year experience. J. Infect. Dis. 193387-395. [DOI] [PMC free article] [PubMed] [Google Scholar]