Abstract
We report the first case of granulomatous mastitis due to Corynebacterium kroppenstedtii linked to strongly impaired neutrophil responses to Nod2 agonist and a single nucleotide polymorphism within the NOD2 gene (SNP13 [Leu1007fsinsC]) in a heterozygous state. These findings provided the first demonstration of impaired Nod2 function associated with corynebacterial infection.
CASE REPORT
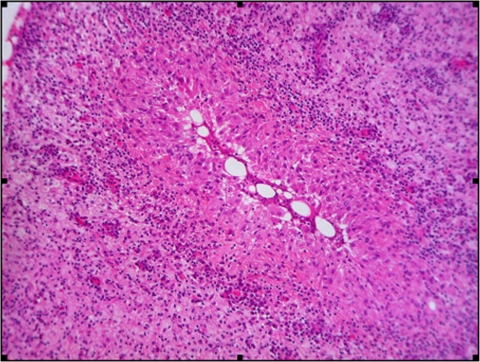
The patient, a 41-year-old Caucasian woman, presented in 2006 with a single inflammation mass of the breast. Her medical history was unremarkable. Histological findings from percutaneous drainage of the breast abscess evidenced noncaseating granulomatous inflammation (Fig. 1), establishing the diagnosis of granulomatous mastitis (GM) (13, 15), a rare nonmalignant inflammatory breast lesion. Microscopic examination after Gram, Ziehl-Neelsen, periodic acid-Schiff, and Grocott staining were negative. Two pus samples from the breast abscess taken 12 days apart were sent to the Department of Microbiology at Lariboisiere Hospital. The cultures remained negative for mycobacteria but evidenced a Corynebacterium sp. both on aerobic Columbia sheep blood agar and in BacTAlert culture bottles within 2 days. The cells were gram-positive, non-spore-forming, and nonmotile, and the colonies were nonpigmented, small, smooth, convex, and nonhemolytic on blood agar. The API Coryne System v3.0 (bioMerieux) showed a doubtful profile of Propionibacterium acnes (API code number 5620344). Finally, using the forward primer A2 (5′AGAGTTTGATCATGGCTCAG) and the reverse primer S15 (5′GGGCGGTGTGTACAAGGCC), a large region (1,285 bp) of the rrs gene coding for 16S rRNA was amplified by PCR and sequenced. Therefore, genetic methods identified up to 99% identity with Corynebacterium kroppenstedtii VA1719_2000 and BK5689_2003 (GenBank accession nos. AY524774 and AY524774). Antimicrobial susceptibility of the Corynebacterium kroppenstendtii strains was determined by disk diffusion method on Mueller-Hinton agar supplemented with 5% sheep blood, indicating a high susceptibility to most of the tested antibiotics, especially to β-lactams. Treatment required multiple incision and drainage procedures associated with a long course of amoxicillin (amoxicilline) (3 g/day) for 1 year, leading to complete recovery. Two years later, she was admitted for typical bacterial pneumonia in the left lower lobe associated with minor pleural effusion. Laboratory data included a white blood cell count of 17,400/μl, with 90.4% neutrophils, 9% lymphocytes, and 0.3% eosinophils. Bacteriological investigations including blood cultures remained negative. Due to the lack of bacterial isolation, oral amoxicillin-clavulanic acid (1 g three times daily) was administered and led to complete recovery.
FIG. 1.
Histopathology sections revealed noncaseating inflammation with a major presence of neutrophils (hematoxylin and eosin stain; original magnification, ×100).
Further immunological explorations were performed to understand this reinfection. Standard immunological studies gave normal results (for lymphocyte subsets and complement system) except for hypogammaglobulinemia (4.3 g/liter; normal range, 6.2 to 15 g/liter) associated with low immunoglobulin G (IgG) serum level (3.53 g/liter; normal range, 6.59 to 12.23 g/liter), while IgA and IgM were within normal limits. No monoclonal antibody peak was identified. Standard polymorphonuclear neutrophil (PMN) functional testing evidenced normal PMN migration (tested using the under-agarose method) and normal PMN chemiluminescence, ruling out leukocyte adhesion deficiency and chronic granulomatous disease, respectively.
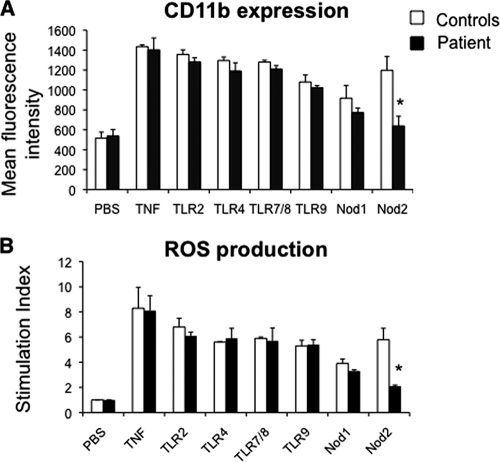
We thus studied PMN responses elicited by Toll-like receptors (TLRs) and Nod stimulation as previously described (7) by analyzing the β2-integrin CD11b/CD18, which plays a major role in the high-affinity phase of the interaction between PMNs and endothelial cells and reactive oxygen species (ROS) production under priming conditions, the latter being critical for bacterial killing. In our patient, tumor necrosis factor (TNF) (used as a positive control), TLR agonists, and Nod1 agonist induced increases in CD11b expression at the PMN surface that were similar to those of the controls. In contrast, after treating our patient's sample with Nod2 agonist (muramyl dipeptide [MDP]), no significant increase in CD11b expression was observed (Fig. 2A). Similarly, pretreatment of whole blood with TLR agonists or Nod1 agonist and with TNF followed by stimulation with N-formyl-methionyl-leucyl-phenylalanyl (fMLP), a structural analog of bacterial metabolic products, strongly increased the production of ROS (Fig. 2B). In contrast, incubation of whole blood with MDP did not stimulate ROS production by PMNs from the patient.
FIG. 2.
Impaired PMN functions after Nod2 stimulation in a patient with granulomatous mastitis due to C. kroppenstedtii. (A) CD11b expression at the PMN surface. Whole-blood samples were incubated at 37°C for 45 min with phosphate-buffered saline (PBS), TNF (100 U/ml), TLR agonist macrophage-activating lipopeptide 2 (10 ng/ml) (TLR2/6), lipopolysaccharide (10 ng/ml) (TLR4), R-848 (10 μg/ml) (TLR7/8), or CpG DNA (100 μg/ml) (TLR9), the Nod1 agonist C12-iE-DAP (lauroyl-γ-d-glutamyl-meso-diaminopimelic acid) (50 μg/ml), or the Nod2 agonist MDP (10 μM). Samples were then stained with phycoerythrin-conjugated anti-CD11b monoclonal antibody at 4°C for 30 min. Results are expressed as mean fluorescence intensity. (B) PMN oxidative burst. Whole-blood samples were pretreated with hydroethidine (1,500 ng/ml) for 15 min at 37°C and then incubated for 45 min with PBS, TNF, or TLR and Nod agonists as described above, followed by fMLP stimulation (10−6 M, 5 min). Results are expressed as a stimulation index (ratio of the mean fluorescence intensity of stimulated cells to that of unstimulated cells). Values that are significantly different (P < 0.05) from those of the controls are indicated by an asterisk. Data are reported as means plus standard errors of the means (error bars). Comparisons were based on analysis of variance and Tukey's posthoc test, using Prism 3.0 software.
These data suggest that the Nod2-dependent activity of PMNs is severely altered in the patient. Therefore, genetic analysis of the NOD2 gene (data available upon request) from the patient was performed after written informed consent was obtained. We found a single nucleotide polymorphism (SNP) in the coding region of the NOD2 gene (SNP13 Leu1007fsinsC) in an heterozygous state, which is one of the three major polymorphisms genetically associated with Crohn's disease (5). We studied the cDNA associated with the NOD2 gene, and we also found that Leu1007fs was in an heterozygous state, ruling out the hypothesis of specific expression of the mutated allele.
Corynebacterium species have been detected in various habitats, including soils, food, animals, and humans. The application of chemotaxonomic methods and molecular biology-based approaches, especially 16S rRNA gene sequencing, has resulted in a much-improved taxonomy of the genus Corynebacterium. The genus currently comprises over 70 recognized species, many of which have been described during the last decade; most of the newly described Corynebacterium species have been isolated from clinical human or animal sources. Some species of the genus Corynebacterium belong to normal flora, while other species were isolated from specific cutaneous lesions (4). Recently, it has been suggested that GM can be associated with a corynebacterial infection, particularly C. kroppenstedtii (15). GM is a nonmalignant breast disease mimicking breast cancer and therefore is frequently mistaken with malignancy or tuberculosis and classified on the basis of histological criteria as granulomatous lobulitis of “uncertain etiology.” As Corynebacterium species are found in normal flora, they are usually considered contaminants from skin by microbiologists, and surgical resection often remains the only treatment option. C. kroppenstedtii, first isolated from a sputum sample from a patient in Sweden in 1998, is a lipophilic extracellular species whose growth depends on the supplementation of synthetic media with appropriate lipids (14). This novel species show a similarity ranging from 91.1% to 93.6% within the genus Corynebacterium based on the 16S rRNA sequence (3). Considering its characteristics related to the lack of mycolic acids and the presence of only one gene encoding a virulence factor (neuraminidase gene), this bacteria seems to be nonvirulent. However, the ability of C. kroppenstedtii to grow in fat globules obviously links its lipophilic metabolism with pathogenicity by providing access to exogenous fatty acids for growth.
We reported, herein, the case of a patient with consecutive GM related to C. kroppenstedtii and severe bacterial pneumonia. We observed altered PMN functions in response to MDP, a minimal motif common to peptidoglycans from all classes of bacteria, recognized by the Nod2 protein (8, 10). In addition, genetic analysis found an SNP located in the coding region of the NOD2 gene (SNP13 Leu1007fsinsC) which results in a frameshift mutation removing the last 33 amino acids of the Nod2 polypeptide; the corresponding mutant was shown to be functionally impaired (5). We cannot exclude an exonic deletion of NOD2, missed by classical sequencing analysis. In spite of the presumed expression of the remaining wild-type allele, we strongly suggest that the impairment of Nod2 was due to the presence of SNP13 associated to other as yet unknown genetic factors. Indeed, human monocytes harboring the Leu1007fsinsC SNP or macrophages from NOD2-deficient mice were reported to exhibit decreased MDP sensing and decreased synergy with TLRs (9, 12). Few studies have explored the association between an SNP in NOD2 and bacterial infection. Three SNPs, i.e., Pro268Ser, Arg702Trp, and Ala725Gly, demonstrated significant association with tuberculosis in African American population (1). However, the same observation could not be extended to Mycobacterium avium subsp. paratuberculosis in a cohort of patients with Crohn's disease (2). In addition, a recent study conducted in an intensive care unit showed that patients with at least one NOD2 variant had reduced phagocytosis by macrophages (P = 0.03) and higher risk of bacteremia than patients with the wild-type NOD2 gene (6).
Finally, it has been recently reported that Nod-mediated inflammatory stimulation in response to peptidoglycans triggers an increase in the ability of PMNs to phagocytose opsonized pneumococci, leading to engulfment and killing (11). During the phagocytosis process, IgG (IgG1 and IgG3) expressed at the microorganism surface are involved in bacterial recognition by binding to Fc-γ receptors at the PMN surface. Defective PMN responses to Nod2 stimulation associated with decreased opsonophagocytosis related to hypogammaglobulinemia may be involved at least in part in the occurrence of GM. Nevertheless, we could not exclude other genetic determinants in other genes which have not yet been identified, in agreement with the hypothesis of a polygenic disease.
In conclusion, the patient presented here provided the first description of impaired neutrophil response elicited to Nod2 stimulation associated with GM due to corynebacteria. It should be therefore proposed to analyze Nod2 function and the NOD2 gene in patients with a clinical history of GM due to Corynebacterium species. In addition, several Corynebacterium species have been implicated in disease in humans. Further studies are needed to emphasize the involvement of the Nod2 pathway in PMN control of these and other unusual pathogens in apparently otherwise healthy patients who present with unexpected or unexpectedly severe bacterial infection.
Nucleotide sequence accession number.
The nucleotide sequence of the rrs gene has been submitted to the GenBank nucleotide sequence database (accession no. GQ244303).
Acknowledgments
We are grateful to Rachid Kaci for his technical assistance and Jean-François Bergmann for his assistance in reviewing the final version of the manuscript.
Footnotes
Published ahead of print on 29 July 2009.
REFERENCES
- 1.Austin, C. M., X. Ma, and E. A. Graviss. 2008. Common nonsynonymous polymorphisms in the NOD2 gene are associated with resistance or susceptibility to tuberculosis disease in African Americans. J. Infect. Dis. 1971713-1716. [DOI] [PubMed] [Google Scholar]
- 2.Bentley, R. W., J. I. Keenan, R. B. Gearry, M. A. Kennedy, M. L. Barclay, and R. L. Roberts. 2008. Incidence of Mycobacterium avium subspecies paratuberculosis in a population-based cohort of patients with Crohn's disease and control subjects. Am. J. Gastroenterol. 1031168-1172. [DOI] [PubMed] [Google Scholar]
- 3.Collins, M. D., E. Falsen, and E. Akervall. 1998. Corynebacterium kroppenstedtii sp. nov., a novel corynebacterium that does not contain mycolic acids. Int. J. Syst. Bacteriol. 481449-1454. [DOI] [PubMed] [Google Scholar]
- 4.Funke, G., A. von Graevenitz, J. E. Clarridge, and K. A. Bernard. 1997. Clinical microbiology of coryneform bacteria. Clin. Microbiol. Rev. 10125-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gasche, C., M. Nemeth, P. Grundtner, C. Willheim-Polli, P. Ferenci, and R. Schwarzenbacher. 2008. Evolution of Crohn's disease-associated Nod2 mutations. Immunogenetics 60115-120. [DOI] [PubMed] [Google Scholar]
- 6.Henckaerts, L., K. R. Nielsen, R. Steffensen, K. Van Steen, C. Mathieu, A. Giulietti, P. J. Wouters, I. Milants, I. Vanhorebeek, L. Langouche, S. Vermeire, P. Rutgeerts, S. Thiel, A. Wilmer, T. K. Hansen, and G. Van den Berghe. 2009. Polymorphisms in innate immunity genes predispose to bacteremia and death in the medical intensive care unit. Crit. Care Med. 37192-201. [DOI] [PubMed] [Google Scholar]
- 7.Hoarau, C., B. Gérard, E. Lescanne, D. Henry, S. François, J. J. Lacapère, J. El Benna, P. M. C. Dang, B. Grandchamp, Y. Lebranchu, M. A. Gougerot-Pocidalo, and C. Elbim. 2007. TLR9 activation induces normal neutrophil responses in a child with IRAK-4 deficiency: involvement of the direct PI3K pathway. J. Immunol. 1794754-4765. [DOI] [PubMed] [Google Scholar]
- 8.Inohara, N., Y. Ogura, and A. Fontalba. 2003. Host recognition of bacterial muramyl dipeptide mediated through NOD2. J. Biol. Chem. 2785509-5512. [DOI] [PubMed] [Google Scholar]
- 9.Inohara, N., M. Chamaillard, C. McDonald, and G. Nuñez. 2005. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu. Rev. Biochem. 74355-383. [DOI] [PubMed] [Google Scholar]
- 10.Kanneganti, T. D., M. Lamkanfi, and G. Nuñez. 2007. Intracellular NOD-like receptors in host defense and disease. Immunity 27549-559. [DOI] [PubMed] [Google Scholar]
- 11.Lysenko, E. S., T. B. Clarke, M. Shchepetov, A. J. Ratner, D. I. Roper, C. G. Dowson, and J. N. Weiser. 2007. Nod1 signaling overcomes resistance of S. pneumoniae to opsonophagocytic killing. PLoS Pathog. 31073-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogura, Y., N. Inohara, A. Benito, F. F. Chen, S. Yamaoka, and G. Nunez. 2001. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J. Biol. Chem. 2764812-4818. [DOI] [PubMed] [Google Scholar]
- 13.Paviour, S., S. Musaad, S. Roberts, G. Taylor, S. Taylor, K. Shore, S. Lang, and D. Holland. 2002. Corynebacterium species isolated from patients with mastitis. Clin. Infect. Dis. 351434-1440. [DOI] [PubMed] [Google Scholar]
- 14.Riegel, P., P. Liégeois, M. P. Chenard, C. Mathelin, and H. Monteil. 2004. Isolations of Corynebacterium kroppenstedtii from a breast abscess. Int. J. Med. Microbiol. 294413-416. [DOI] [PubMed] [Google Scholar]
- 15.Taylor, G. B., S. D. Paviour, S. Musaad, W. O. Jones, and D. J. Holland. 2003. A clinicopathological review of 34 cases of inflammatory breast disease showing an association between corynebacteria infection and granulomatous mastitis. Pathology 35109-119. [PubMed] [Google Scholar]