Abstract
The clinical impact of severe infections with yeasts and yeast-like fungi has increased, especially in immunocompromised hosts. In recent years, new antifungal agents with different and partially species-specific activity patterns have become available. Therefore, rapid and reliable species identification is essential for antifungal treatment; however, conventional biochemical methods are time-consuming and require considerable expertise. We evaluated matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) for the rapid routine identification of clinical yeast isolates. A total of 18 type collection strains and 267 recent clinical isolates of Candida (n = 250), Cryptococcus, Saccharomyces, Trichosporon, Geotrichum, Pichia, and Blastoschizomyces spp. were identified by MALDI-TOF MS. The results were compared with those obtained by conventional phenotyping and biochemical tests, including the API ID 32C system (bioMérieux, Nürtingen, Germany). Starting with cells from single colonies, accurate species identification by MALDI-TOF MS was achieved for 247 of the clinical isolates (92.5%). The remaining 20 isolates required complementation of the reference database with spectra for the appropriate reference strains which were obtained from type culture collections or identified by 26S rRNA gene sequencing. The absence of a suitable reference strain from the MALDI-TOF MS database was clearly indicated by log(score) values too low for the respective clinical isolates; i.e., no false-positive identifications occurred. After complementation of the database, all isolates were unambiguously identified. The established API ID 32C biochemical diagnostic system identified 244 isolates in the first round. Overall, MALDI-TOF MS proved a most rapid and reliable tool for the identification of yeasts and yeast-like fungi, with the method providing a combination of the lowest expenditure of consumables, easy interpretation of results, and a fast turnaround time.
Pathogenic fungi such as Candida, Cryptococcus, and Aspergillus spp. and species of other genera commonly cause life-threatening infections of immunocompromised hosts, including patients receiving immunosuppressive therapy. In the United States, Candida species are the fourth leading cause of nosocomial bloodstream infections. With an estimated total of 72.8 million opportunistic Candida infections per year worldwide, the case/fatality rate due to Candida species is in the range of 33.9% (32). Although Candida albicans remains the leading and most important pathogenic yeast species, the incidence rates of non-C. albicans yeast infections have been increasing in recent years. Moreover, outbreaks in perinatal intensive care and oncohematological units have been described (23). Recently, infections caused by less common yeasts and yeast-like species such as Pichia, Rhodotorula, Saccharomyces, and Trichosporon species and other rarely encountered species have been reported (17).
At present, more than 100 yeast and yeast-like species are known to be human pathogens and have been isolated from virtually all body sites. Identification of the increasing diversity of fungal pathogens by conventional phenotypic methods is difficult and time-consuming, and the findings may sometimes be inconclusive, especially for unusual yeasts. For treatment with the appropriate antifungal agents, particularly with drugs belonging to the azole group, fast and reliable yeast identification can be time-saving because of the species-specific susceptibility patterns. Commercially available chromogenic agar media (17) and biochemical or enzymatic panels, e.g., the API ID 20C, API ID 32C, and Vitek ID YST systems (bioMérieux, Nürtingen, Germany), are widely used, although they are occasionally disadvantageous due to limited databases, and misidentifications have been reported (8, 27).
Since the introduction of gentle ionization techniques, mass spectrometry (MS) has been developed into a standard method in the field of protein analysis (2, 3). Time of flight (TOF) instruments are state of the art for applications in the field of proteomics, particularly in combination with matrix-assisted laser desorption ionization (MALDI) procedures. MALDI-TOF MS is also used to analyze the protein compositions of complex mixtures, e.g., biological fluids or tissues, as well as microbial cells or cell components (13, 26).
The convenient and rapid identification of microbial species by the extraction of cells from single colonies and determination of species-specific patterns of peptides and protein masses have been reported (7, 18, 20, 21). Moreover, the characterization and differentiation of bacteria to the subspecies level have been described (4, 11, 13).
In clinical and applied laboratory settings, MALDI-TOF MS has been shown to be suitable for the fast and secure identification of pathogenic microorganisms, particularly slowly growing microorganisms, whose identification by current automated biochemical tests is problematic. These microorganisms include, e.g., nonfermenting bacteria (10, 28), some gram-positive bacteria (4, 14, 30), and fungi (34, 36).
In the study described here, we demonstrate the suitability of identification of clinical yeasts and yeast-like fungi by MALDI-TOF MS. For the first time, the technology was used for the identification of more than 250 clinical isolates, with part of the study being run under routine diagnostic conditions. The study was controlled by conventional cultural, morphological, and biochemical criteria as well as by 26S rRNA sequencing of isolates with discrepant results.
MATERIALS AND METHODS
Strains and culture conditions.
The study included 52 archived and 215 fresh nonrepetitive fungal isolates subcultured from the clinical diagnostic laboratory of the Institute of Medical Microbiology, Immunology, and Parasitology of the University of Bonn, Bonn, Germany, between May 2007 and July 2008. The isolates were obtained predominantly from clinical materials, such as blood, cerebrospinal fluid, bronchoalveolar lavage fluid, intra-abdominal fluid, and intraoperative wound and tissue specimens. All isolates were cultured on diagnostic fungal media, such as Columbia agar with 5% sheep blood (Becton Dickinson, Heidelberg, Germany), Sabouraud 2% glucose agar (Oxoid, Wesel, Germany), and Candi4 select agar (Bio-Rad, Munich, Germany), followed by morphological identification, including determination of growth characteristics on CHROMagar Candida (Becton Dickinson, Heidelberg, Germany) and germ tube or chlamydospore formation (17).
Commercially available strains were obtained from the American Type Culture Collection (ATCC) and from the German Collection of Microorganisms and Cell Cultures (Deutsche Sammlung von Mikroorganismen und Zellkulturen [DSMZ]) and included Candida albicans ATCC 11949, ATCC 24433, ATCC 44373, ATCC 44374, and ATCC 90028; Candida glabrata ATCC 90030 and DSMZ 11950; Candida kefyr DSMZ 11954; Candida parapsilosis ATCC 22019, ATCC 90018, and DSMZ 11955; Candida tropicalis ATCC 750 and ATCC 90874; Issatchenkia orientalis (Candida krusei) ATCC 6258, ATCC 90878, and DSMZ 11957; Cryptococcus neoformans ATCC 62066; and Saccharomyces cerevisiae ATCC 9763.
Biochemical identification.
For the biochemical identification of the strains, the API ID 32C identification system for yeasts (bioMérieux) was used according to the manufacturer's instructions, and the recommended additional tests were employed when necessary (17). Reading and the interpretation of the results were performed after incubation times of 24 and 48 h.
Sequence data.
The 26S rRNA genes of strains that yielded discrepant results were sequenced by Scanbec GmbH (Halle/Saale, Germany) (22).
MALDI-TOF MS.
After incubation of the test strains for 48 h at 30°C on Sabouraud agar, cells from up to five representative single colonies were transferred into a 1.5-ml screw-cap extraction tube (Eppendorf, Germany) with a pipette tip and mixed thoroughly in 0.3 ml double-distilled water. Absolute ethanol (0.9 ml) was added, the contents of the tube were carefully mixed, and the tubes were then centrifuged at 20,000 × g (2K15 centrifuge; Sigma, Germany) for 2 min; the supernatant was discarded, and the pellet was air dried. Approximately 10 μl of the pellet was mixed thoroughly with 50 μl of formic acid (70%), before addition of an equivalent volume of acetonitrile. The mixture was centrifuged at 20,000 × g for 2 min, and 1 μl of the supernatant was placed onto a ground steel MALDI target plate and allowed to dry at room temperature. Subsequently, each sample was overlaid with 2 μl of matrix, which consisted of a saturated solution of α-cyano-4-hydroxy-cinnamic acid in 50% acetonitrile-2.5% trifluoroacetic acid, and air dried at room temperature.
The MALDI-TOF MS analyses of all strains were performed in parallel on a Microflex LT spectrometer in the Bruker application laboratory in Leipzig, Germany, and on a Biflex III spectrometer in the microbiological diagnostic laboratory of the Institute of Medical Microbiology, Immunology, and Parasitology in Bonn (both apparatuses were from Bruker Daltonik GmbH, Bremen, Germany). The spectra were recorded in the linear positive mode at a laser frequency of 20 Hz within a mass range from 2,000 to 20,000 Da. The instrument parameter settings were ion source 1 at 20 kV, ion source 2 at 18.5 kV, lens at 8.5 kV, pulsed ion extraction of 250 ns, and no gating for the Microflex LT spectrometer and IS1 at 19 kV, IS2 at 16.5 kV, lens at 8 kV, PIE of 200 ns, and maximum gating for the Biflex III spectrometer. For each spectrum, 240 laser shots in 30-shot steps from different positions of the target spot were collected and analyzed. The spectra were externally calibrated by using Escherichia coli ribosomal proteins.
Data analysis.
FlexAnalysis (version 2.0) software (Bruker Daltonik GmbH) was used for visual inspection of the mass spectra. For automated data analysis, the raw spectra for unknown fungi were processed by using version 1.1 (Bonn) and version 2.0 (Leipzig) of MALDI Biotyper software (Bruker Daltonik GmbH) with the default settings. The software performs smoothing, normalization, baseline subtraction, and peak picking, thereby creating a list of the most significant peaks (m/z values) of the spectrum.
As an integrated part of the MALDI Biotyper software, the main spectra were used as reference libraries that contained information about the average masses, the average intensities, and the relative abundances in the measurements for all characteristic peaks. To identify unknown fungal isolates, the raw spectra were imported into the MALDI Biotyper software and analyzed by use of the standard pattern-matching algorithm, which compared the raw spectra with the spectra in the library without any user intervention by using the standard settings. For the pattern matchings, the fingerprints of unknown samples were compared to the fingerprints for all entries in the database (main spectra), and the results were listed in a ranking table. The results of the pattern-matching process were expressed as log(score) values, which ranged from 0 to 3. Log(score) values of >1.7 generally indicated relationships at the genus level, and log(score) values of >2.0 generally indicated relationships at the species level. The highest log(score) of a match against the score in the database was used for species identification. The spectra for unequivocally identified isolates were added to the reference database in the course of the study, resulting in a total of 241 fungal strains representing 131 species.
RESULTS
Yeast collection strains.
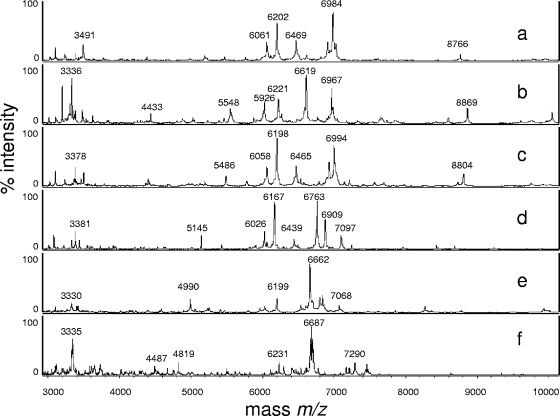
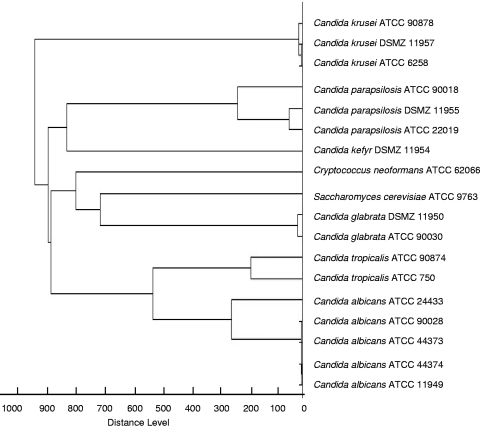
The cell extracts of 18 type culture collection strains representing the majority of clinically relevant yeast species gave suitable spectra and were included in the MALDI-TOF MS reference database. Table 1 shows the results of all log(score) values obtained in both laboratories on the Microflex LT and Biflex III spectrometers. In general, the log(score) values were above 2.0; only the values for C. neoformans and S. cerevisiae (Microflex LT spectrometer) and one of the C. krusei isolates (Biflex III spectrometer) were slightly lower. Figure 1 shows representative MS spectra for type strains measured with the Biflex III spectrometer. The score-oriented dendrogram (Fig. 2) shows the spectral similarities of the type strains.
TABLE 1.
MALDI-TOF log(score) values for ATCC and DSMZ type strains of Candida species and yeast-like organisms obtained with Microflex LT and Biflex III spectrometersa
| Species | Strain no. | MALDI-TOF log(score) value
|
|
|---|---|---|---|
| Microflex LT spectrometer (Leipzig) | Biflex III spectrometer (Bonn) | ||
| Candida albicans | ATCC 11949 | 2.561 | 2.156 |
| Candida albicans | ATCC 24433 | 2.488 | 2.322 |
| Candida albicans | ATCC 44373 | 2.451 | 2.401 |
| Candida albicans | ATCC 44374 | 2.508 | 2.497 |
| Candida albicans | ATCC 90028 | 2.451 | 2.268 |
| Candida glabrata | ATCC 90030 | 2.492 | 2.347 |
| Candida glabrata | DSMZ 11950 | 2.469 | 2.433 |
| Candida kefyr | DSMZ 11954 | 2.107 | 2.130 |
| Candida parapsilosis | ATCC 22019 | 2.367 | 2.550 |
| Candida parapsilosis | ATCC 90018 | 2.202 | 2.183 |
| Candida parapsilosis | DSMZ 11955 | 2.495 | 2.301 |
| Candida tropicalis | ATCC 750 | 2.484 | 2.302 |
| Candida tropicalis | ATCC 90874 | 2.183 | 2.256 |
| Candida krusei | ATCC 6258 | 2.384 | 2.011 |
| Candida krusei | ATCC 90878 | 2.412 | 2.061 |
| Candida krusei | DSMZ 11957 | 2.369 | 1.980 |
| Cryptococcus neoformans | ATCC 62066 | 1.965 | 2.392 |
| Saccharomyces cerevisiae | ATCC 9763 | 1.974 | 2.140 |
Eighteen isolates were tested.
FIG. 1.
MALDI-TOF mass spectra (m/z 3,000 to 10,000) of Candida albicans (a), Candida glabrata (b), Candida tropicalis (c) Candida krusei (d), Candida parapsilosis (e), and Cryptococcus neoformans (f) revealing differences among the species.
FIG. 2.
Score-oriented dendrogram of ATCC and DSMZ type strains of Candida species and yeast-like fungi (n = 18) obtained by the Microflex LT spectrometer.
Clinical isolates of Candida species.
Among the clinical isolates, 250 Candida species and 17 yeast-like fungi were tested. MALDI-TOF MS yielded the correct species identification for 240 clinical Candida isolates in the first attempt. Strains of Candida norvegensis, Candida rugosa, Candida dubliniensis, and Candida ciferrii could not be identified since spectra for appropriate reference strains had not been included in the database.
Inappropriate species or genus assignments were clearly indicated by low log(scores), generally below 1.7. With a log(score) of 1.72, the Candida dubliniensis isolate was first identified as Candida to the genus level and with a best hit of C. albicans. Introduction of the spectra for the respective type strains into the database and subsequent comparison with the unidentified spectra resulted in the unequivocal identification of all 250 Candida isolates.
Biochemical analysis with the API ID 32C system correctly identified 234 (93.6%) of the Candida isolates (Table 2, first run). When the findings of MALDI-TOF MS and biochemical identification disagreed, the API test was repeated, starting with single colonies from pure culture, which increased the number of correctly identified Candida isolates to 245 (98%) (Table 2). The remaining strains that yielded discrepant results were subjected to 26S rRNA sequencing (Scanbec GmbH), resulting in the identification of Pichia kluyveri (99% nucleic acid sequence identity) and Pichia fabianii (100% nucleic acid sequence identity) rather than Candida krusei or Candida pelliculosa. Also, Debaryomyces etchellsii/D. carsonii (API ID 32C system, n = 3) was identified as Candida parapsilosis (n = 2) and Candida lusitaniae by 26S rRNA sequencing with 100% nucleic acid sequence identity (Table 3). One isolate, misidentified as Candida utilis by use of the API ID 32C system, was also examined by 26S rRNA sequencing and was identified as Pichia fabianii or Pichia veronae, which both share 100% nucleic acid sequence identity. Table 3 presents a compilation of the metabolic profiles and incorrect designations of isolates by use of the API ID 32C system compared to the results of the reference methods.
TABLE 2.
Identification of clinical yeast isolates by MALDI-TOF MS and conventional tests
| Species | Total no. of isolates | No. of isolates identified with API ID 32C system
|
No. of isolates identified by MALDI-TOF MSb | |
|---|---|---|---|---|
| First run | Second runa | |||
| Candida albicans | 87 | 85 | 87 | 87 |
| Candida glabrata | 52 | 49 | 52 | 52 |
| Candida tropicalis | 35 | 33 | 35 | 35 |
| Candida parapsilosis | 28 | 26 | 26 | 28 |
| Candida metapsilosis | 1 | 0 | 0 | 1 |
| Candida krusei | 18 | 18 | 18 | 18 |
| Candida kefyr | 9 | 7 | 9 | 9 |
| Candida (Clavispora) lusitaniae | 7 | 4 | 6 | 7 |
| Candida norvegensis | 6 | 6 | 6 | 0 → 6 |
| Candida rugosa | 1 | 1 | 1 | 0 → 1 |
| Candida dubliniensis | 2 | 1 | 1 | 0 → 2 |
| Candida lipolytica | 1 | 1 | 1 | 1 |
| Candida ciferrii | 1 | 1 | 1 | 0 → 1 |
| Candida pelliculosa | 1 | 1 | 1 | 1 |
| Candida guilliermondii | 1 | 1 | 1 | 1 |
| Subtotal | 250 | 234 | 245 | 240 → 250 |
| Cryptococcus neoformans | 3 | 3 | 3 | 3 |
| Saccharomyces cerevisiae | 2 | 1 | 2 | 2 |
| Trichosporon cutaneum | 3 | 3 | 3 | 0 → 3 |
| Magnusiomyces/Blastoschizomyces capitatus | 2 | 2 | 2 | 2 |
| Galactomyces geotrichum/Geotrichum silvicola | 1 | 0 | 0 | 0 → 1 |
| Pichia kluyveri | 1 | 0 | 0 | 0 → 1 |
| Pichia fabianii/Pichia veronae | 2 | 0 | 0 | 0 → 2 |
| Pichia (Kodamaea) ohmeri | 1 | 1 | 1 | 0 → 1 |
| Cryptococcus curvatus | 1 | 0 | 0 | 0 → 1 |
| Pichia rhodanensis | 1 | 0 | 0 | 0 → 1 |
| Subtotal | 17 | 10 | 11 | 7 → 17 |
| Total | 267 | 244 | 256 | 247 → 267 |
After repeating the test with the API ID 32C system for isolates with discrepant results.
MALDI-TOF MS identification was performed in parallel on a Microflex LT spectrometer (Leipzig) and a Biflex III spectrometer (Bonn), and there were no discrepancies in the results. →, identified after integration into the database.
TABLE 3.
Phenotypic identities of clinical yeast isolates differing from those determined by MALDI-TOF MS and/or 26S rRNA sequencing
| API ID 32C system
|
MALDI-TOF MS26S rRNA sequencing
|
|||
|---|---|---|---|---|
| Organism identified | Identification profile | % Identity | Organism identified | % NASIa |
| Candida pelliculosa | 4274 3501 | 91.6 | Pichia fabianii | 100 |
| Candida utilis | 4271 3501 | 95.6 | Pichia fabianii/Pichia veronae | 100 |
| Candida parapsilosis | 7147 3507 | 99.9 | Candida metapsilosis | |
| Candida albicans | 7347 1400 | 99.8 | Candida dubliniensis | 100 |
| Candida krusei | 0300 0100 | 88.6 | Pichia kluyveri | 99 |
| Debaryomyces etchellsii/D. carsonii | 5546 3501 | 87 | Candida parapsilosis | 100 |
| Debaryomyces etchellsii/D. carsonii | 5546 3501 | 91.4 | Candida parapsilosis | 100 |
| Debaryomyces etchellsii/D. carsonii | 5547 1501 | 91.4 | Candida lusitaniae | 100 |
| Geotrichum species | 3000 3100 | 99.3 | Galactomyces geotrichum/Geotrichum silvicola | 99 |
| No identification possible | Cryptococcus curvatus | 100 | ||
| No identification possible | Pichia rhodanensis | 100 | ||
NASI, nucleic acid sequence identity.
In one case concerning a DNA sequencing-confirmed Candida dubliniensis isolate, MALDI-TOF MS yielded the correct identification with a log(score) value of 2.365, whereas the API ID 32C system identified this isolate as Candida albicans (Table 3). Another isolate which had been unambiguously identified as C. parapsilosis by the API ID 32C system was identified as Candida metapsilosis with a log(score) value of 2.103. Candida metapsilosis was recently described as a new species which cannot be identified by biochemical tests (38) but which can be identified unequivocally by MALDI-TOF MS (15). The spectra for all newly identified isolates and isolates whose identities were confirmed by 26S rRNA sequencing were included in the database.
Clinical isolates of yeast-like fungi.
For 7 of 17 clinical isolates of yeast-like fungi, MALDI-TOF MS, biochemical testing, and morphological analysis yielded congruent results in the first round of identification; one Cryptococcus neoformans isolate required subculturing on Sabouraud agar for accurate identification. The identification of the remaining 10 strains by MALDI-TOF-MS required the addition of the spectra for the respective reference strains to the database (Table 2).
One strain identified as a Geotrichum sp. by use of the API ID 32C system was identified as Galactomyces geotrichum or Geotrichum silvicola (33) by 26S rRNA sequencing with 99% nucleic acid sequence identity (Table 3). Two isolates could not be identified by use of the API ID 32C system but were determined by 26S rRNA sequencing to be Cryptococcus curvatus (100% sequence identity) and Pichia rhodanensis (100% sequence identity) (Table 3).
DISCUSSION
The correct and fast identification of fungal pathogens from clinical specimens and from patients' environments, especially in outbreak situations, is of major concern for optimal patient management and the implementation of effective measures for disease control. Molecular approaches are currently being developed to provide methods for the identification of yeasts that are more rapid and reliable than the classical phenotypic methods. However, high-resolution DNA-based molecular techniques, such as 26S rRNA or internal transcribed spacer DNA sequencing (6, 29, 36) and real-time PCR assays (35), are expensive and time-consuming.
In the present study MALDI-TOF MS proved to be a rapid and reliable procedure for the accurate identification of pathogenic Candida strains and required minimal hands-on time or time for the interpretation of the results. The test procedure was generally completed within 10 min per isolate and within 3 h for 96 samples, starting from single yeast colonies on the agar plate. In contrast, the identification of germ tube-negative Candida species by phenotypic methods requires incubation periods of up to 72 h. Overall, MALDI-TOF MS did not produce any misidentifications, provided that spectra for the appropriate reference strains were present in the database (Table 2). Incorrect species assignments as best hits, due to the absence of reference strains, were clearly indicated by log(score) values that were too low, according to the thresholds of the MALDI Biotyper software.
The discrepancies between the results of MALDI-TOF MS after database complementation and the phenotypic identification (Table 2, first run) might have been due to accidentally mixed or contaminated cultures; incorrect phenotypic identifications based on subtle alterations in yeast carbohydrate assimilation and biochemical characteristics and the subjective interpretation of the results of morphological methods may also occur.
Due to the close phylogenetic relationship, Candida dubliniensis is often misidentified as Candida albicans (6, 23), and in addition to state-of-the-art molecular biology methods, large-scale techniques such as fatty acid analysis were applied for correct diagnosis, until recently (31). Candida parapsilosis proved to be a heterogeneous group of yeasts with distinct DNA sequence patterns (38) and different antifungal susceptibilities (25). Therefore, in addition to C. parapsilosis, two new species, Candida metapsilosis and Candida orthopsilosis, have recently been proposed (38), but these two species cannot be separated by phenotypic tests.
Molecular methods for the detection and differentiation of yeasts are also able to distinguish the closely related new species Candida nivariensis (1) and Candida bracarensis (9) that were previously misidentified as Candida glabrata by conventional chemotaxonomy. Recently, in a global collection of 1,598 C. glabrata strains received from 28 countries, Lockhart et al. found the prevalence of these cryptic species to be 0.2% (24). As our C. glabrata isolates (n = 52) were identified with log(score) values greater than 2.0, reference strains of these new species have so far not been introduced into the database.
Uncommon yeast species are emerging as human pathogens, and their identification may pose a challenge when the type strains have not yet been included in the diagnostic databases of any identification system, leading to their misidentification as related species. Interestingly, and in agreement with our observation (Table 3), in the endocarditis case reported by Hamal et al. (16), the isolates were initially identified biochemically as Candida pelliculosa; however, on the basis of direct sequencing of the internal transcribed spacer region of rRNA, they were subsequently reidentified as Pichia fabianii with strong biofilm formation and voriconazole resistance. Pichia fabianii is an example of a yeast species which requires molecular techniques for correct identification (5).
Two questionable strains from our study which could not be identified by standard routine methods yielded perfect DNA sequence matches for Cryptococcus curvatus and Pichia rhodanensis (Table 3). Cryptococcus curvatus can be detected by molecular methods in submarine and terrestrial environments (37) and is known to be a rare opportunistic pathogen of animals, including humans (12). Pichia rhodanensis has been reported to be involved in the spontaneous process of fermentation of untreated green Arbequina table olives in minor quantities (19).
The present study clearly demonstrates that MALDI-TOF MS can be used as a fast technique for the reliable identification of fungi. Moreover, the technology has been shown to be broadly applicable in different fields of clinical bacteriology. With state-of-the-art instruments and the adoption of user-friendly software, MA specialists are not needed and laboratory technicians are able to run the system after a few days of training. The investment in and maintenance of the instrument are balanced by the low costs of the consumables. Thus, MALDI-TOF MS-based microorganism identification is ready to be used not only by specialized research facilities but also by clinical diagnostic laboratories, provided that appropriate standard operating procedures and comprehensive quality-controlled databases with the spectra for human pathogens are available.
Footnotes
Published ahead of print on 1 July 2009.
REFERENCES
- 1.Alcoba-Flórez, J., S. Méndez-Álvarez, J. Cano, J. Guarro, E. Pérez-Roth, and M. del Pilar Arévalo. 2005. Phenotypic and molecular characterization of Candida nivariensis sp. nov., a possible new opportunistic fungus. J. Clin. Microbiol. 434107-4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amiri-Eliasi, B., and C. Fenselau. 2001. Characterization of protein biomarkers desorbed by MALDI from whole fungal cells. Anal. Chem. 735228-5231. [DOI] [PubMed] [Google Scholar]
- 3.Arnold, R. J., and J. P. Reilly. 1998. Fingerprint matching of E. coli strains with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry of whole cells using a modified correlation approach. Rapid Commun. Mass Spectrom. 12630-636. [DOI] [PubMed] [Google Scholar]
- 4.Barbuddhe, S. B., T. Maier, G. Schwarz, M. Kostrzewa, H. Hof, E. Domann, T. Chakraborty, and T. Hain. 2008. Rapid identification and typing of Listeria species by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Appl. Environ. Microbiol. 745402-5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhally, H. S., S. Jain, C. Shields, N. Halsey, E. Cristofalo, and W. G. Merz. 2006. Infection in a neonate caused by Pichia fabianii: importance of molecular identification. Med. Mycol. 44185-187. [DOI] [PubMed] [Google Scholar]
- 6.Boyanton, B. L., Jr., R. A. Luna, L. R. Fasciano, K. G. Menne, and J. Versalovic. 2008. DNA pyrosequencing-based identification of pathogenic Candida species by using the internal transcribed spacer 2 region. Arch. Pathol. Lab. Med. 132667-674. [DOI] [PubMed] [Google Scholar]
- 7.Claydon, M. A., S. N. Davey, V. Edwards-Jones, and D. B. Gordon. 1996. The rapid identification of intact microorganisms using mass spectrometry. Nat. Biotechnol. 141584-1586. [DOI] [PubMed] [Google Scholar]
- 8.Coignard, C., S. F. Hurst, L. E. Benjamin, M. E. Brandt, D. W. Warnock, and C. J. Morrisson. 2004. Resolution of discrepant results for Candida species identification by using DNA probes. J. Clin. Microbiol. 42858-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Correia, A., P. Sampaio, S. James, and C. Pais. 2006. Candida bracarensis sp. nov., a novel anamorphic yeast species phenotypically similar to Candida glabrata. Int. J. Syst. Evol. Microbiol. 56313-317. [DOI] [PubMed] [Google Scholar]
- 10.Degand, N., E. Carbonnelle, B. Dauphin, J. L. Beretti. M. Le Bourgeois, Semet-Gaudelus, C. Segonds, P. Berche, X. Nassif, and A. Ferroni. 2008. Matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of nonfermenting gram-negative bacilli isolated from cystic fibrosis patients. J. Clin. Microbiol. 463361-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dieckmann, R., R. Helmuth, M. Erhard, and B. Malorny. 2008. Rapid classification and identification of salmonellae at the species and subspecies levels by whole-cell matrix-assisted laser desorption ionization-time of flight mass spectrometry. Appl. Environ. Microbiol. 747767-7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dromer, F., A. Moulignier, B. Dupont, E. Gueho, M. Baudrimont, L. Improvisi, F. Provost, and G. Gonzalez-Canali. 1995. Myeloradiculitis due to Cryptococcus curvatus in AIDS. AIDS 9395-396. [PubMed] [Google Scholar]
- 13.Fenselau, C., and P. A. Demirev. 2001. Characterization of intact microorganisms by MALDI mass spectrometry. Mass Spectrom. Rev. 20157-171. [DOI] [PubMed] [Google Scholar]
- 14.Grosse-Herrenthey, A., T. Maier, F. Gessler, R. Schaumann, H. Böhnel, M. Kostrzewa, and M. Krüger. 2008. Challenging the problems of clostridial identification with matrix-assisted laser desorption and ionization-time-of-flight mass spectrometry (MALDI-TOF MS). Anaerobe 14242-249. [DOI] [PubMed] [Google Scholar]
- 15.Haase, G., T. Maier, K. Ritter, and M. Kostrzewa. 2008. Differentiation of Candida metapsilosis, Candida orthopsilosis, and Candida parapsilosis using MALDI-TOF mass spectral fingerprinting. Int. J. Med. Microbiol. 298S216, abstr. DVV08. schaft für Hygiene und Mikrobiologie. [Google Scholar]
- 16.Hamal, P., J. Ostransky, M. Dendis, R. Horváth, F. Ruzicka, V. Buchta, M. Vejsova, P. Sauer, P. Hejnar, and V. Raclavsky. 2008. A case of endocarditis caused by the yeast Pichia fabianii with biofilm production and developed in vitro resistance to azoles in the course of antifungal treatment. Med. Mycol. 46601-605. [DOI] [PubMed] [Google Scholar]
- 17.Hazen, K. C., and S. A. Howell. 2007. Candida, Cryptococcus, and other yeasts of medical importance, p. 1762-1788. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. L. Landry, and M. A. Pfaller (ed.) Manual of clinical microbiology, vol. 9. ASM Press, Washington, DC. [Google Scholar]
- 18.Holland, R. D., J. G. Wilkes, F. Rafii, J. B. Sutherland, C. C. Persons, K. J. Voorhee, and J. O. J. Lay. 1996. Rapid identification of intact whole bacteria based on spectral patterns using matrix-assisted laser desorption/ionization with time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 101227-1232. [DOI] [PubMed] [Google Scholar]
- 19.Hurtado, A., C. Reguant, B. Esteve-Zarzoso, A. Bordons, and N. Rozès. 2008. Microbial population dynamics during the processing of Arbequina table olives. Food Res. Int. 41738-744. [Google Scholar]
- 20.Jarman, K. H., D. S. Daly, C. E. Petersen, A. J. Saenz, N. B. Valentine, and K. L. Wahl. 1999. Extracting and visualizing matrix-assisted laser desorption/ionization time-of-flight mass spectral fingerprints. Rapid Commun. Mass Spectrom. 131586-1594. [DOI] [PubMed] [Google Scholar]
- 21.Krishnamurthy, T., P. L. Ross, and U. Rajamani. 1996. Detection of pathogenic and nonpathogenic bacteria by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 10883-888. [DOI] [PubMed] [Google Scholar]
- 22.Kurzmann, C. P., and C. J. Robnett. 1998. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie van Leeuwenhoek 73331-371. [DOI] [PubMed] [Google Scholar]
- 23.Leaw, S. N., H. C. Chang, H. F. Sun, R. Barton, J. P. Bouchara, and T. C. Chang. 2006. Identification of medically important yeast species by sequence analysis of the internal transcribed spacer regions. J. Clin. Microbiol. 44693-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lockhart, S. R., S. A. Messer, M. Gherna, J. A. Bishop, W. A. Merz, M. A. Pfaller, and D. J. Diekema. 2009. Identification of Candida nivariensis and Candida bracarensis in a large global collection of Candida glabrata isolates: comparison to the literature. J. Clin. Microbiol. 471216-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lockhart, S. R., S. A. Messer, M. A. Pfaller, and D. J. Diekema. 2008. Geographic distribution and antifungal susceptibility of the newly described species Candida orthopsilosis and Candida metapsilosis in comparison to the closely related species Candida parapsilosis. J. Clin. Microbiol. 462659-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maier, T., and M. Kostrzewa. 2007. Fast and reliable MALDI-TOF MS-based microorganism identification. Chem. Today 2568-71. [Google Scholar]
- 27.Massonet, C., J. V. Eldere, M. Vaneechoutte, T. DeBaere, J. Verhaegen, and K. Lagrou. 2004. Comparison of VITEK 2 with ITS2-fragment length polymorphism analysis for identification of yeast species. J. Clin. Microbiol. 422209-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mellmann, A., J. Cloud, T. Maier, U. Keckevoet, I. Ramminger, P. Iwen, J. Dunn, G. Hall, D. Wilson, P. LaSala, M. Kostrzewa, and D. Harmsen. 2008. Evaluation of matrix-assisted laser desorption ionization-time-of-flight mass spectrometry in comparison to 16S rRNA gene sequencing for species identification of nonfermenting bacteria. J. Clin. Microbiol. 461946-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montero, C. I., Y. R. Shea, P. A. Jones, S. M. Harrington, N. E. Tooke, F. G. Witebsky, and P. R. Murray. 2008. Evaluation of pyrosequencing (R) technology for the identification of clinically relevant non-dematiaceous yeasts and related species. Eur. J. Clin. Microbiol. Infect. Dis. 27821-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moura, H., A. R. Woolfitt, M. G. Calvalho, A. Pavlopulos, L. M. Teixeira, G. A. Satten, and J. R. Barr. 2008. MALDI-TOF mass spectrometry as a tool for differentiation of invasive and noninvasive Streptococcus pyogenes isolates. FEMS Immunol. Med. Microbiol. 53333-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peltroche-Llacsahuanga, H., S. Schmidt, M. Seibold, R. Lütticken, and G. Haase. 2000. Differentiation between Candida dubliniensis and Candida albicans by fatty acid methyl ester analysis using gas-liquid chromatography. J. Clin. Microbiol. 383696-3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pfaller, M. A., and D. J. Diekema. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20133-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pimenta, R. S., P. D. D. Alves, A. Corrêa, Jr., M.-A. Lachance, G. S. Prasad, Rajaram, B. R. Sinha, and C. A. Rosa. 2005. Geotrichum silvicola sp. nov., a novel asexual arthroconidial yeast species related to the genus Galactomyces. Int. J. Syst. Evol. Microbiol. 55497-501. [DOI] [PubMed] [Google Scholar]
- 34.Qian, J., J. E. Cutler, R. B. Cole, and Y. Cai. 2008. MALDI-TOF mass signatures for differentiation of yeast species, strain grouping and monitoring of morphogenesis markers. Anal. Bioanal. Chem. 392439-449. [DOI] [PubMed] [Google Scholar]
- 35.Schabereiter-Gurtner, C., B. Selitsch, M. L. Rotter, A. M. Hirschl, and B. Willinger. 2007. Development of novel real-time PCR assays for detection and differentiation of eleven medically important Aspergillus and Candida species in clinical specimens. J. Clin. Microbiol. 45906-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seyfarth, F., M. Ziemer, H. G. Sayer, A. Burmester, M. Erhard, M. Welker, S. Schliemann, E. Straube, and U. C. Hipler. 2008. The use of ITS DNA sequence analysis and MALDI-TOF mass spectrometry in diagnosing an infection with Fusarium proliferatum. Exp. Dermatol. 17965-971. [DOI] [PubMed] [Google Scholar]
- 37.Takishita, K., M. Tsuchiya, J. D. Reimer, and T. Maruyama. 2006. Molecular evidence demonstrating the basidiomycetous fungus Cryptococcus curvatus is the dominant microbial eukaryote in sediment at the Kuroshima Knoll methane seep. Extremophiles 10165-169. [DOI] [PubMed] [Google Scholar]
- 38.Tavanti, A., A. D. Davidson, N. A. R. Gow, M. C. J. Maiden, and F. C. Odds. 2005. Candida orthopsilosis and Candida metapsilosis sp. nov. to replace Candida parapsilosis groups II and III. J. Clin. Microbiol. 43284-292. [DOI] [PMC free article] [PubMed] [Google Scholar]