Abstract
Summary: The small DNA tumor viruses have provided a very long-lived source of insights into many aspects of the life cycle of eukaryotic cells. In recent years, the emphasis has been on cancer-related signaling. Here we review murine polyomavirus middle T antigen, its mechanisms, and its downstream pathways of transformation. We concentrate on the MMTV-PyMT transgenic mouse, one of the most studied models of breast cancer, which permits the examination of in situ tumor progression from hyperplasia to metastasis.
INTRODUCTION
Polyomaviruses have proven to be invaluable models. Much of our understanding of eukaryotic transcription and replication mechanisms has come from studies of their lytic cycles. Insights into basic mechanisms that regulate cell growth and the impact of their deregulation have emerged from transformation and tumorigenesis studies. Since the discovery of murine polyomavirus (PyV) in the 1950s (123, 279), this virus family has called attention to, among other things, tyrosine phosphorylation, phosphoinositide 3-kinase (PI3K), and p53. It is a testament to the importance of these issues that they have already generated over 100,000 papers.
PyV can cause a wide variety of tumors in different types of cells (78, 96, 105). The three early proteins studied for their contributions to neoplastic transformation are named large, middle, and small tumor antigen (LT, MT, and ST, respectively), because they were discovered using antibodies from tumor-bearing animals. These viral oncoproteins are produced by differential splicing of the viral early region (296) (Fig. 1). A fourth spliced product of unknown function, tiny T (238), has been observed in some situations. The pattern of splicing is such that all T antigens share a common 79-amino-acid J domain connecting the T antigens to molecular chaperone (27, 157, 238, 265, 278, 281, 283). MT and ST share an additional 112 amino acids. LT, MT, and ST antigens each have a unique C-terminal sequence of 706, 230, and 4 amino acids, respectively. This arrangement of gene products is, depending on the point of view, either a quirk or a stroke of evolutionary genius. PyV and hamster polyomavirus (65, 80, 122) are the only two family members that have MT. Other polyomaviruses (for example, simian virus 40 [SV40] and the human viruses BK and JC) lack it. All three T antigens are involved in both virus replication and in cell transformation. The genomic arrangement has sometimes made it hard to decipher individual roles of each protein in virus experiments.
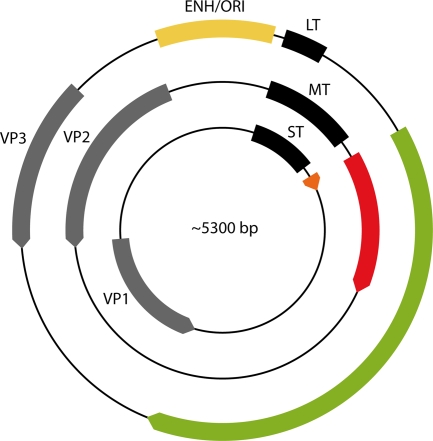
FIG. 1.
The polyomavirus genome. Early transcription of the T antigens proceeds in a clockwise direction. Late transcription of the capsid proteins (VP1, VP2, and VP3) is counterclockwise. Replication and transcription are controlled by the enhancer (ENH) and the origin of viral DNA replication (ORI). T-antigen coding sequences are in different colors to emphasize reading frame differences.
Although LT and ST also have independent abilities to regulate cell growth and survival, MT is the most important PyV transforming protein. As we discuss in detail below, MT is necessary, and in many cases sufficient, for transformation in tissue culture. It is also critical for tumorigenesis after viral infection. Expressed from a transgene, MT causes tumors in a variety of tissues. In vivo tumorigenesis is important because signal transduction hypotheses developed in the laboratory can be tested in animals. The importance of tyrosine phosphorylation and PI3K were first appreciated by studying MT. As inhibitors of PI3K now enter the clinic, there is real hope that this basic research will have important practical outcomes. While its importance in transformation is evident, it should be noted that MT also plays a significant role in PyV infection. This is perhaps not surprising. The goal of the virus is to produce a cellular environment that is hospitable for virus replication.
To place our understanding of MT in the proper context, a brief mention of some aspects of PyV biology is worthwhile. Early genetic studies identified host-range transformation-defective hr-t mutants that could grow in some PyV-transformed cells but not in normal cells (see reference 14 for a review). This suggested that the hr-t “gene,” which we now know from sequence analysis (79, 274, 296) represents ST and MT, has a function(s) in the lytic cycle.
Most tumor studies have been carried out following infections of neonatal mice, because tumor induction decreases as a function of age at the time of infection (77, 242). A role for immune responses has been implicated in the resistance of adult mice to tumor development, since experimental or genetic immunoincompetence partially restores the tumor induction potential (2, 183, 190, 305). Nonimmune factors are also involved, since the replication potential declines significantly with the age of the mouse, regardless of its immune status (334). In mice infected as adults, only three organs—mammary gland, skin, and bone—show a major involvement. The fact that these tissues maintain cellular replication at the adult stage is likely to be a factor, suggesting that PyV infection requires cell cycling. Exogenous stimulation of cell replication, as demonstrated in adult kidney (9), resulted in replication. This is in contrast with infection of cultured G0-arrested NIH 3T3 mouse fibroblasts, which are as sensitive to PyV infection as cycling cells and in which viruses stimulate replication of their DNA (41). Analysis of a model of polycystic kidney disease suggests that differentiation could even be more important than replication (10). These varying results indicate that the ability of PyV gene products, including MT, to regulate the state of the cell can be limited by the cellular context.
Our goal here is to examine in some detail MT's role in signaling, transformation, and tumorigenesis. One emphasis is on applying knowledge gained from examining MT's role in the lytic cycle. MT's primary structure, diagrammed in Fig. 2, shows that it associates with many proto-oncogenes involved in cellular signaling. It has sometimes been considered a mimic of a constitutively activated receptor tyrosine kinase (RTK) (85). Like an RTK, MT uses tyrosine kinase activity to generate binding sites for a number of key signal transducers. Unlike receptors for insulin or platelet-derived growth factor, MT has no intrinsic tyrosine kinase activity but rather recruits tyrosine kinases of the Src family. First, we broadly examine MT function. Next, we examine the anatomy of MT and its signaling networks. Finally, we turn our attention to models of MT transformation and tumorigenesis. We pay particular attention to transgenic models. The mouse mammary tumor virus (MMTV)-PyMT mammary tumor system continues to be extensively employed to examine virtually every issue that is relevant to breast cancer. The reader is also referred to earlier reviews dealing with MT (84, 85, 121, 144, 251, 258).
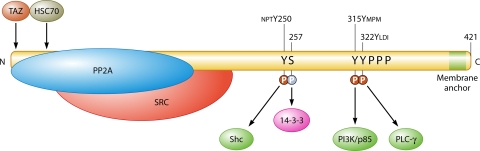
FIG. 2.
MT and its direct interactions with cellular proteins. SRC, Src family tyrosine kinase (Src, Yes, Fyn). The binding proteins Shc, PI3K, phosphoinositide 3-kinase, and PLCγ1, which bind phosphotyrosine sequences, are shown in dark green. The sequences near the tyrosine phosphorylation sites are shown above the sequence. 14-3-3, which binds pSer257, is shown in magenta. PPP represents the proline rich region. The light green area represents the hydrophobic membrane attachment site. The C-terminal sequence is AHSM384QRHLRRLGR393TLLLVTFLAALLGICLMLFILIKRSRHF421 (underlining indicates the hydrophobic stretch). Proteins such as Grb2 that associate with MT partners are not shown here.
OVERVIEW OF MT FUNCTION
MT is essential for PyV transformation and tumorigenesis. Mutations that affect MT, but not ST or LT, abrogate the transforming activity of the virus in vitro (30, 288). The role of MT in PyV tumor induction is equally clear (8, 24, 71, 108, 109, 341, 342). Unlike mutations of single tyrosine phosphorylation sites on growth factor receptors, MT mutations affecting particular associations with host proteins have generally had clear phenotypes (for example, see references 71 and 108).
MT can be sufficient for transformation of cells in vitro and for the induction of tumors in animals. Treisman and colleagues (297) originally showed MT to be sufficient for transformation of cells in tissue culture. When it is not sufficient, other oncogenes can act together with MT to cause transformation. It appears that there may be more than one kind of complementing pathway. A number of nuclear oncogenes, such as those encoding PyLT (167), E1A (244), and Myc (167), complement. Mutant p53 (303) and ST (194, 216), which acts on the p53 pathway, complement as well. MT can also be sufficient to cause tumors in animals when introduced through a viral vector or a transgene (1, 12, 92, 126, 170, 234, 286). In some cases, complementation of the sort seen between PyV gene products in transformation assays has been seen in experimental tumor models as well (7, 8).
As expected, MT is also important for productive viral infection (109, 114, 290). Since there is no evidence for transformed cells acting as a reservoir for reactivation of latent virus, MT function in transformation and tumorigenesis seems almost to be a bystander effect of its requirement for replication. MT's effects on productive infection are exerted at different levels. MT is a major regulator of both viral DNA replication and early and late RNA transcription (41-43). Its target is the PyV enhancer (Fig. 1), which contains both Pea3 (Ets family) and Ap-1 binding sites and which controls both viral gene expression and DNA replication (82, 195, 299, 312). MT leads to the activation of c-jun and c-fos promoters (262, 301), phosphorylation of c-Jun (277), and activation of Pea3 (321). (As a practical matter, there is a partial overlap of these lytic functions between MT and ST, such that defects are masked when MT mutations are studied in the context of a functional ST.) MT also appears to be important for virus maturation. hr-t mutants lacking MT show an assembly defect (113). This defect is associated with altered phosphorylation of the major capsid protein VP1 (172). The replication-associated functions of MT play an indirect role in tumor formation. In most mouse strains, there is a long delay between infection and tumor induction. Thus, tumors arise long past the acute viremic stage of infection, during the persistence stage. Since the viral genome is often not integrated into the host genome in tumors, genome persistence is a requirement for oncogenesis. As is the case for replication, MT function is required for viral persistence, with an even more stringent dosage requirement (X. Wang and M. Fluck, unpublished data).
MT ANATOMY
There are 421 amino acids in murine MT. Comparison of the MT sequences from the murine and hamster proteins shows strong sequence similarity through approximately the first 190 amino acids, i.e., those in common with ST. This is likely to represent a conserved structure. The C-terminal sequences, except for a membrane anchor and some of the tyrosine phosphorylation sites, seem quite different between the two. This may suggest that these residues provide simple peptide motif docking sites rather than an organized structure.
MT Is Associated with Membranes
MT is a protein tightly associated with membranes (6, 11, 87, 146, 147, 256, 269, 349). Only the stretch of amino acids near the C terminus is sufficiently long and hydrophobic to span a lipid bilayer (Fig. 2). Hamster MT also has such a sequence. Association with membranes is critical to function. Truncations completely eliminating the hydrophobic sequence (Py1387T [30]) and those extending six amino acids into the C-terminal side of the hydrophobic stretch render MT nontransforming (211).
Genetic analysis has probed the role of the hydrophobic sequences and the residues surrounding it. In the case of bovine papillomavirus E5 oncoprotein, the transmembrane domain is specifically important for transforming ability (88). A unique hydrophobic sequence does not appear to be required for MT transformation. Its replacement with a CAAX box (the C-terminal lipid modification sequence from H-Ras) to provide an alternative mechanism for membrane localization supported transformation (99). On the other hand, its role is not entirely nonspecific. Mutations within the hydrophobic domain could render MT nontransforming (185). Substitution of other membrane localization motifs, such as those from vesicular stomatitis virus G protein (291) or cytochrome b5 (160), resulted in MTs that failed to transform. The residues adjacent to the hydrophobic sequence have also been examined. The MT C-terminal hexapeptide (KRSRHF) can be deleted without affecting transformation of F111 rat embryo fibroblasts (73). Residues in the basic region N-terminal to the hydrophobic sequence seem more important. E392G is cold sensitive for transformation (287). R393E, just N-terminal to the hydrophobic sequence, is transformation defective, as tested by anchorage-independent growth (73).
MT is associated with both plasma and intracellular membranes. The apparent fraction of MT in different membranes differs depending on how the experiment is done. Immunofluorescence and immunoelectron microscopy emphasize the connection with intracellular membranes, particularly the endoplasmic reticulum (87, 291, 350), whereas biochemical fractionation finds a greater fraction associated with the plasma membrane (146, 256). While the orientation of the hexapeptide C-terminal sequence is not clear, most of MT is oriented toward the inside of the cell (256).
MT also appears to be in contact with membrane skeleton and cytoskeletal elements (6, 164, 256). Elliot and colleagues (99) have presented evidence that the basic residues N-terminal to the hydrophobic sequence are important for these associations. Such sublocalization is likely to be functionally important, because MT-protein tyrosine kinase (PTK) complexes are enriched there (256).
It seems possible that an important element remains missing from the MT-membrane story. It was originally hypothesized that MT, like cytochrome b5, might spontaneously associate with membranes. However, model binding experiments (160) suggest that MT carboxy-terminal sequences do not spontaneously insert into membranes but rather associate only electrostatically. This result may indicate a more complex process for membrane insertion.
MT-Associated Proteins
As mentioned above, MT has no known catalytic activity but rather functions as a scaffold on which cellular signaling proteins are assembled (Fig. 2). In addition to the proteins mentioned here, several laboratories are using proteomics to uncover new MT binding proteins, such as lipin 1 and 2 (B. Schaffhausen, S. Andrabi, and J. Hwang, unpublished data). We begin by describing the assembly of the key signal generators.
PP2A.
Protein phosphatase 2A (PP2A) is arguably the most important cellular serine/threonine protein phosphatase (97, 148, 149, 191, 198). PP2A is found as a trimeric ABC complex (Fig. 3). A catalytic subunit, C, is brought together with a regulatory subunit, B, on a scaffolding A subunit. A and C each have two isoforms, while there are a large number of B subunits, which are divided into at least four families.
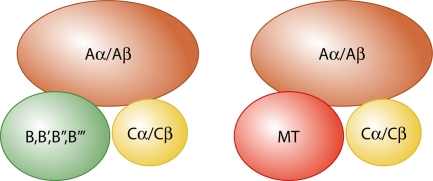
FIG. 3.
PP2A and MT-PP2A complexes. For PP2A, two different A subunit scaffolds, α and β, bind one of two different catalytic C subunits and one of very many different B family regulatory subunits. MT replaces B subunits in the complex.
MT and ST bind PP2A (219, 317). MT and ST bind to the A and C subunits (219, 317) in a way that either displaces B subunits or prevents them from binding. Structural analysis of SV40 ST shows that it binds the A subunit in an area involved in B binding (47, 55). This would explain the absence of B subunits in MT-PP2A complexes. Genetic analysis of MT indicates that sequences from near the N terminus through residues around amino acid 190 are important for the association (23, 26, 51, 116, 117, 187, 269). Some residues identified as important are in cysteine (CXCXXC) motifs. Based on SV40 ST structures (47, 55), these are expected to be involved in zinc binding (241). Although SV40 ST binds only the A alpha form of PP2A, MT binds both A alpha and A beta (348). The importance of targeting A beta is not certain. However, mutations in PP2A A beta have been associated with lung and colon cancer (243, 319). The wild type, but not cancer- associated mutants, of A beta regulates the RalA GTPase (245).
The binding of PP2A is critical for the assembly of MT signaling complexes. The association of MT with the Src family PTKs depends on this interaction (23, 26, 213). As a result, mutants that fail to bind PP2A are unable to transform cells. It is likely that PP2A contributes to a structural scaffold that binds the PTKs. Its phosphatase activity is not required for MT to recruit PTKs (212). PP2A may play a role in subcellular localization of MT, since a PP2A− mutant is mislocalized (23). However, this conclusion could reflect the fact that such mutations allow Hsc70 to bind (26); Hsc70 binding might well affect intracellular localization.
The effects of MT on PP2A catalytic activity have not been examined in detail. This is unfortunate, because PP2A is involved in most cellular processes. More than 50 protein kinases are regulated by PP2A (191). PP2A is clearly relevant in the context of cancer, where it seems to function as a tumor suppressor (149, 324). For example, changes in activity of PP2A toward Myc have been implicated in head and neck carcinoma and colon cancer (153). This raises the question of how MT changes the activity of PP2A. There are at least two possible scenarios. One is that the MT complex uses PP2A catalytic activity to carry out some functions. Earlier PP2A inhibition experiments (118) and a report suggesting that PP2A might be able to dephosphorylate phosphotyrosine (37) might suggest a catalytic role for PP2A in activation of PTK in the MT complex. There is also a report that MT uses PP2A to activate c-Jun kinase (197). A related notion is that MT directs PP2A toward specific substrates. Lipin, a target of insulin signaling, is dephosphorylated as a result of its interaction with MT (Schaffhausen et al., unpublished). The idea that MT might direct PP2A toward substrates is supported by the observations that SV40 ST increases the activity of PP2A for substrates such as histone H1 (339) and the androgen receptor (337). In a second scenario, MT might perturb the activity of PP2A complexes in cells, either in terms of inhibiting activity or by changing the range of B-subunit associations. SV40 ST provides a paradigm here. It is able to inhibit PP2A activity toward a number of substrates (259, 339). The effect of SV40 ST in transformation can be partially mimicked by knockdown of specific B subunits, while overexpression of B subunits reverses some of the effect of SV40 ST (45). While there cannot be enough MT to titrate the cell's PP2A, it could create a situation similar to haploinsufficiency, which is known to affect the spectrum of PP2A complexes (44).
MT and the Src family tyrosine kinases.
Walter Eckhart, Mary Anne Hutchinson, and Tony Hunter demonstrated tyrosine phosphorylation in MT immunoprecipitates (95), opening a new research area. For a period, the source of the activity was unclear, since the evidence argued that it was not MT itself (256). Sarah Courtneidge and Alan Smith eventually found that c-Src associating with MT could provide tyrosine kinase activity (63). MT was subsequently shown to bind two other Src family PTKs, c-Yes and c-Fyn (50, 137, 163). In Fisher rat cells, 50 to 75% of the activity is from Src complexes and the remainder is from the other family members (21). Some members of the Src family, such as Lck (177) and Hck (93), fail to bind MT.
The tyrosine kinase activity coming from association with these kinases is required for transformation (31, 95, 128, 253, 273). Using an antisense strategy to manipulate Src levels altered the degree of transformation (5). From the other direction, regulating MT levels to change the amount of MT-PTK activity also affected the transformed phenotype (233). There may be differences in the relative importance of MT association with particular family members in different tissue backgrounds. For induction of mammary tumors in a transgenic model, c-Src is very important (127). For hemangiomas induced by MT, Yes knockouts respond much more weakly to MT, with fewer tumors of longer latencies, than knockouts of Src or Fyn (159, 292).
There is a substantial amount of information available on the nature of the MT-PTK interaction. Mutations of MT have defined the sequences necessary for the interaction with PTKs. The primary binding site for PTKs on MT is between residues 185 and 210. It has two basic motifs followed by an S or T that contribute significantly (23, 118). However, other sequences also contribute to the assembly of the MT-PTK complex. Mutations near the N terminus of MT (59, 116, 289), in the J domain (325), and in the PP2A binding site (26, 253) also prevent association of MT with PTKs. Most of these mutants fail to bind PP2A as well, supporting PP2A's role discussed above in forming MT complexes. There are two unexplained exceptions. The PTK-defective Q37A mutant (325) has been reported to bind PP2A, and L5E (116) binds Src but apparently binds a phosphatase different from PP2A.
c-Src is a multidomain protein anchored in membranes by an N-terminal myristylation sequence. After the myristylation signal, there is a unique domain, followed in turn by an SH3 domain and an SH2 domain that binds tyrosine-phosphorylated sequences. Last is the catalytic domain, followed by a C-terminal sequence that contains a regulatory phosphorylation site at Y527. When Src is phosphorylated at 527, its activity is restricted by the binding of the phosphorylated tyrosine (pY527) to the SH2 domain. The catalytic domain and C-terminal sequences are sufficient for association of Src with MT (93). Sequences very near the C terminus are important for binding. Truncation of Src at 516 and mutations in the C-terminal region abolish MT binding; v-Src that has an altered C terminus also fails to bind (34, 52). Src sequences further N-terminal are apparently also involved, since a Src-Lck fusion binds MT even though the C terminus is from Lck, which does not bind MT (177). Studies on the Src family member Hck have suggested that MT fails to bind to the closed configuration of this PTK but could associate with an open form (93). The observation that a form of Src that has the SH2 domain deleted, which would prevent closure, showed enhanced MT binding supported this idea.
There remain unexplained aspects of the association of MT with the Src family PTKs. The interaction between MT and Src requires hours to complete (56). It is unclear why this should be so. It is equally unclear why only small fractions (∼10%) of MT and Src are associated (21), even when either partner is overexpressed (60, 226, 255). These observations suggest that the interaction is somehow gated.
Once associated with MT, Src shows a substantial increase in the Vmax of kinase activity (22, 64). MT has been important in understanding the role of C-terminal 527 phosphorylation in restricting Src activity (34, 62, 161, 227). Src associated with MT is not phosphorylated at the C-terminal Y527 (35, 64). On the other hand, phosphorylation of Src Y416, which is known to be activating, is observed in MT complexes (35). There are also MT-associated tyrosine phosphorylations in the more N-terminal region of Src (35, 345). The significance of these phosphorylations is unknown.
How far the effects of increased Src activity in the MT complexes extend is an open question. Unlike v-Src transformation, which causes a broad increase in phosphotyrosine (142), MT transformation causes no large increase in cellular tyrosine phosphorylation (263). Phosphotyrosine turnover may increase after MT expression, because treatment of cells with the tyrosine phosphate inhibitor vanadate did lead to a significant increase in phosphotyrosine (344). Some specific Src substrates (Stat3 [115]) but not others (p34 [53]) were phosphorylated in response to MT. As discussed below, signal-transducing proteins, such as PI3K or Shc, that become associated with the MT complex do become tyrosine phosphorylated.
There is no question that MT itself is a key PTK substrate. MT is phosphorylated on tyrosine residues at 250, 315, and 322 (128, 141, 252). Phosphorylation is also observed at 297 and possibly 258 (141; B. Schaffhausen, unpublished data). The phosphorylation of MT connects it to signaling pathways that are discussed in the next sections.
(i) Tyrosine 250.
MT is phosphorylated at tyrosine 250 in the MT-Src family complexes (141). Mutation of tyrosine 250 (185, 214, 303) has a dramatic effect on transforming ability in many systems. When the MT gene is expressed as a transgene in the mammary gland, there is a dramatic decrease in tumorigenesis compared to that seen with the wild type (322). Studies with animals infected with virus bearing a Y250F mutation have also confirmed a role for the 250 site in polyoma tumorigenesis (24, 342). For instance, mutation of Y250 results in a drastic decrease in the number of kidney tumors.
Comparisons of the Y250 mutant and the wild type point out the importance of context to signaling. In human mammary epithelial cells and fibroblasts, mutation of Y250 to F has no effect on transformation (303). Even in a single cell type, rat F111 fibroblasts, different clones respond differently to Y250F mutations (214). In superficial conflict to the transgenic experiments in the mammary gland described above, virus infection with the Y250S mutant showed little, if any, difference from the wild type in the frequency of mammary tumors (24, 342). (There did appear to be differences in the morphology of tumors [342].) However, this is something of an “apples-to-pears” comparison, since virus infection, unlike the transgenic MT, comes with the expression of LT and ST. Intriguingly, the Y250S mutation resulted in virus that was more active than wild type in some regards. Y250S mutants produced hair follicle tumors that were much larger than those produced by the wild type and induced otherwise rare penile papillomas in two-thirds of the mice (24).
Phosphorylation of Y250 generates a binding site for adaptors of the ShcA family (28, 86). Figure 4 shows somewhat simplified versions of the signaling pathways. As a result of MT binding, Shc becomes phosphorylated on tyrosine residues. There are three overlapping ShcA isoforms: p46, p52, and p66 (46, 52, and 66 kDa, respectively) (224, 236). Expression of p66 is more restricted than that of p46 and p52 (313). ShcAs have an N-terminal phosphotyrosine binding (PTB) domain and a C-terminal SH2 domain that could both bind phosphotyrosine sequences (260) as well as a central proline-rich CH1 region. The PTB domain is clearly involved in binding MT. The ShcA PTB recognition motif is ΨXNPXpY (304, 307). This kind of motif distinguishes it from SH2 domains that also bind phosphotyrosine sequences but use residues C terminal to the phosphotyrosine to provide specificity. Mutation of MT residues immediately N terminal to the pY250 clearly alters both the transforming properties of MT and its ability to bind and affect Shc phosphorylation (28, 89, 90). In addition, when overexpressed in NIH 3T3 cells, the Shc PTB domain inhibits MT transformation (20). It is less clear whether the SH2 domain of Shc is also involved in MT binding and function. MT can bind the Shc SH2 in vitro (86). Also, MT P248H, which should be defective only in PTB binding, but not Y250F, which should be defective in both PTB and SH2 interactions, induces some Shc tyrosine phosphorylation in vivo (28). It remains possible that the SH2 interaction could contribute to MT function.
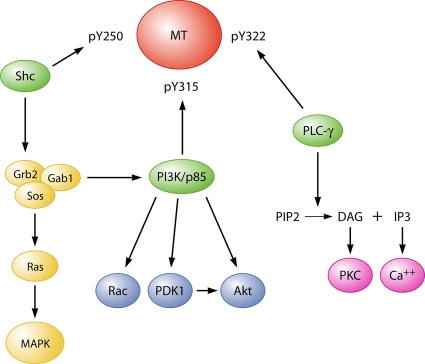
FIG. 4.
Simplified diagram of signaling pathways downstream of MT phosphotyrosines.
Shc can be phosphorylated on at least three tyrosines, Y317 (Y313 in mouse) (246) and a double Y239/Y240 site (306). Blotting with phosphospecific antibodies shows that MT affects all three sites (T. Jiang, Y. Zhu, and B. S. Schaffhausen, unpublished) A large fraction of p52 ShcA is phosphorylated in MT-transformed cells, even though the fraction of p52 associated with MT is small (28). This indicates that the ShcA in MT complexes may turn over. Differing roles for individual phosphorylations have been suggested (for example, see reference 120). Expression of Y239F/Y240F Shc, but not Y317F Shc, affects the size of agar colonies of MT-transformed cells (20).
Shc is an adaptor molecule that binds Grb2 (236). The association of Shc and Grb2 is driven by MT (28, 86), and Grb2 is also found in MT complexes (207). Grb2 function is important for MT tumorigenesis. Knockout of Grb2 reduced MT tumorigenesis in the mammary cell model (48), while overexpression of Grb2 (or Shc) reduced tumor latency of Y250F-expressing mice (235).
The roles of ShcA and Grb2 have been probed by sequence substitutions around MT residue 250 or insertions of binding sequences between residues 281 and 284, a region not thought to be important for MT transformation. When the sequence around residue 250 of MT is replaced with ShcA binding sites known to bind Grb2 (the Y317 [Y313] ShcA site [246] and the Y239/Y240 site [306]), MT bearing the 239/240 sequence was better able to transform Rat-2 cells than MT bearing the 313 sequence (207). However, these mutants, which allow binding of amounts of Grb2 matching the wild type, were still less active than MT. This may suggest that Shc has additional functions beyond binding Grb2. On the other hand, hemangiomas could be produced with latency similar to that of the wild type, when Grb2 SH2 binding sequences (YVNQ or YYND) were substituted into MT (214).
The binding of Grb2 allows the recruitment of additional proteins such as the Ras exchange factor SOS (207). This is consistent with observations that MT activates Ras (277). Ras activation should cause Raf, Mek, and Erk activation and then phosphorylation of transcription factors such as c-Fos and Elk (a member of the Ets family). These pathways have not been much explored for MT, except to show changes in Erk in HL60 cells undergoing differentiation (340). Grb2 also allows recruitment of Gab1 (207, 214). The adaptor Gab1 is involved in signaling for some growth factor and cytokine receptors (210). Gab1 is expected to bind PI3K, SHP-2 tyrosine phosphatase, and the Crkl adaptor. Except for PI3K, which is discussed in the next section, the significance of MT signaling specifically downstream of Gab1 is not known.
(ii) Tyrosine 315 and PI3K.
Tyrosine 315 was discovered early on to be a major MT phosphorylation site (252, 254). It was also the first phosphorylation site tested by site-directed mutagenesis. Curiously, comparisons between infection of wild-type and Y315F viruses in F111 cells showed the mutant to be highly defective in transformation (31), while when plasmid constructs expressing only MT were used, a Y315F mutant was almost as effective in Rat-1 cell transformation as the wild type (215). This was likely an early example emphasizing the importance of context to the requirements for transformation. Experiments in animals confirmed the importance of Y315. Virus-induced tumors at some sites (kidney, salivary gland, mammary gland, tooth, and adrenal gland) were highly reduced when Y315F was used (108). On the other hand, tumors in the thymus, hair follicles, and subcutaneous connective tissue were not much affected. Surprisingly, Y315F caused even more bone or lung tumors than the wild type. Whether this is a secondary consequence of the mutant-infected animals surviving longer or of Y315 in restricting tumor growth in some backgrounds is unknown. The MT model for mammary carcinogenesis also point to the importance of the Y315 site, as mice expressing Y315F/Y322F mutant MT showed a T50 (time for 50% of the mice to develop tumors) of 123 days, compared to 53 days for mice expressing the wild type (322).
The mechanism by which Y315 contributes to MT transformation became clear when phosphatidylinositol kinase (PIK) activity was discovered in MT immunoprecipitates (330). MTs defective in transformation, including deletions or point mutations of Y315, were defective in or lacked PIK activity (156). Later, it became clear the MT-bound PIK activity possessed unique biochemical properties, designated type I PIK, which distinguished it from the bulk of the PIK in the cell (329). Following this lead, the type I PIK was shown to produce phosphatidylinositol 3-phosphate, making it a PI3K (328). The presence of PI3K activity in MT immunoprecipitates closely matched the presence of an 85-kDa polypeptide (66, 155). Importantly, both PI3K activity and p85 were also associated with a variety of activated RTKs (101, 155, 309). Genetic analysis argued that PI3K activity was essential for the biological functions of both RTKs and oncoproteins. Purified PI3K associated with MT and RTKs consisted of two subunits: a p85 adaptor plus a catalytic subunit of 110 kDa (32).
The family of PI3Ks has grown with purification and cloning (133, 139; for reviews, see references 110 and 308). It has been divided into three groups: class I PI3Ks, which can use PI, PI(4)P, and PI(4,5)P2 as substrates in vitro; class II PI3Ks, which use PI and PI(4)P; and class III PI3Ks, which use only PI. In vivo, the class I enzymes use PI(4,5)P2 as a substrate. Class I has been further divided into the three class IA enzymes, each consisting of a p85 adaptor subunit complexed with a 110-kDa catalytic subunit, and class IB, which is found primarily in leukocytes. MT associates with the class IA enzymes.
The class IA enzymes have three catalytic subunits (α, β, and δ). The oncogenic potential of PI3K was demonstrated by showing that p110α causes tumors in the context of avian retrovirus (40). Its relevance to cancer was reinforced with the observation that the PIK3CA gene, encoding p110α, was frequently mutated in different cancers, such as those of the colon, breast, prostate, liver, and brain (249). The mutations found in p110α in human tumors have been shown to be activating (145, 154, 248, 347). Knockout of p110α is sufficient to block transformation in standard mouse embryo fibroblasts systems (303). Conditional knockout of p110α (346) or heterozygous knock-in of a kinase dead allele of p110α (107) shows that p110α is also critical to RTK signaling. The PIK3CB gene, encoding p110β, seems to be different. It is amplified in several tumor types. However, knockout of p110β has little or no effect on RTK signaling (151) but rather is important for heterotrimeric G-protein-coupled receptor signaling (124, 151).
Cloning of the gene for the p85 regulatory subunit showed it to be an SH2-containing protein (272). It is designed to couple the class IA PI3K to activated RTKs and oncoproteins such as MT via its interactions with specific phosphotyrosine motifs (101, 138, 343). In the case of MT, this is the sequence phospho-YMPM starting at Tyr315 on MT. The interaction with MT has two effects. One is to recruit the enzyme to the membranes where MT is located. The second is activation of enzymatic activity by the binding of tyrosine-phosphorylated peptides such as the MT 315 sequence to the enzyme (33). It turned out that, like the catalytic subunits, there are highly similar isoforms of p85, p85α, and p85β, to which MT binds (56). Knockout studies have indicated that the p85α and p85β have distinct roles in development (110), but there is no information on isoform-specific function in MT.
Apart from the biological insights coming from MT-PI3K studies, the interactions have been useful for more fundamental studies as well. The association between MT and the N-terminal SH2 of p85 led to experiments defining the specificity of SH2 domains (275). The same interaction has also been used to illustrate how NMR can identify ligand-induced conformation intermediates during high-affinity binding (125, 192).
The PIP3 product of the class IA enzyme reaction is able to recruit proteins containing particular pleckstrin homology domains to the membrane via direct binding. Among the key targets are the Ser/Thr kinase PDK1 and the Ser/Thr kinase PKB/Akt, which is activated by PDK1. As expected, MT activates Akt (72, 189, 284). Activation of Akt by MT also blocks apoptosis (72, 189, 284). There are three Akt isoforms (Akt1, Akt2, and Akt3). In breast tumors driven by transgenic PyMT, knockout of Akt1 dramatically inhibited tumor induction, while knockout of Akt2 enhanced tumorigenesis (186). Since single-isoform knockouts are viable but double knockouts of Akt1 and Akt2 are not, it seems that the isoforms may have some overlapping functions. However, there are also significant differences in isoform function in cell motility (188) or in controlling differentiation (131). Akt would be expected to phosphorylate many targets, such as GSK3, TSC1/2, MDM2, and IKK, that participate in a wide range of cell processes (94). So far, how these respond to MT has not been reported.
The small GTPase Rac is also activated as a result of PI3K activation of pleckstrin homology domain-containing nucleotide exchange factor (for a review, see reference 25). MT does activate Rac, increasing the fraction in the GTP-bound state, in a manner that is dependent on PI3K activation (D. Denis and B. S. Schaffhausen, unpublished data). Dominant-negative Rac (or Cdc42) blocks MT transformation of both fibroblasts (302) and endothelial cells (58). In the case of endothelial cells, S6 kinase and PI3 kinase may be the relevant Rac effectors. Other experiments suggest that MT uses Rac activation to activate c-Fos (46, 302).
(iii) PLCγ1 binding to tyrosine 322.
Tyrosine 322 is also phosphorylated in MT immunoprecipitates (128, 257). Su and colleagues recognized that the YDLI motif at 322 resembled a binding motif for the phospholipase Cγ1 (PLCγ1) SH2 domains (282). Experiments then showed binding to MT and enhanced phosphorylation of PLCγ1. Mutating Y322 on MT did not appear to have a significant effect on transformation under normal serum conditions (257, 282). However, an effect was seen in low serum (282). Inositol trisphosphate (IP3), a second messenger downstream of PLCγ1, is increased by wild-type MT (119, 300), although it has not been connected to Y322 genetically. However, in Jurkat cells, MT amplified stimuli that normally activate calcium signaling and NFAT in a manner dependent on Y322 (158). It may also be that Y322 signaling could be involved in more distal phenomena not easily seen in culture. Although the primary tumors in the MMTV-PyMT634 breast tumor model were not affected, lung metastases were markedly decreased in PLCγ knockout mice (266).
(iv) Minor tyrosines: Y257, Y288, and Y297.
In an examination of the role of MT in induction of viral transcription and replication, it was observed that Y3F MT that lost the three major tyrosines (Y250F/Y315F/Y322F) retained substantial activity. In contrast, a Y6F sextuply mutated MT lacking all six carboxy-terminal tyrosines lost all function, revealing a role for Y258, Y288, and/or Y297 (42). This function(s) could be novel. Since mutation of these three tyrosines had little effect when the 250, 315, and 322 residues were wild type, their function could also overlap that of Y250, Y315, or Y322.
Other MT binding proteins. (i) TAZ.
The very N terminus of MT binds TAZ (294). TAZ regulates mesenchymal stem cell differentiation (136), promotes cell proliferation (168), and functions in tumorigenesis of breast cancer cells (39). It is a regulator of transcription (136) and protein degradation through a SCFβ-Trcp E3 ligase complex (293). MT amino acid residues 2 to 4 bind the WW domain of TAZ (294). Tian et al. mentioned that a TAZ− mutant virus is unable to transform or induce tumors. Since LT and ST of such a mutant also would not bind TAZ, interpretation of this observation is uncertain.
(ii) Heat shock 70 family proteins.
The N terminus common to MT, LT, and ST resemble a classic DnaJ domain in structure (18) and in the ability to activate the Hsp70/Hsc70 family of DnaK cellular heat shock proteins (238). The binding of Hsc70 by LT is key to the ability to activate E2F-containing promoters (265). The function of the J-domain region in MT is much less clear. Deletion of the HPDK loop amino acid sequence that is critical for molecular chaperone binding has no effect on MT transformation (26, 116). Further, PP2A and Hsc70 compete for binding to MT. PP2A wins that competition. Only when MT is expressed at high levels (218), or when mutation prevents PP2A binding (26, 316), are Hsp/Hsc70 proteins immunoprecipitated in significant quantities with MT. It is worth reemphasizing that the J domain sequences are important for MT even if not for their “J'ness.” As discussed previously, they are important for the recruitment of PP2A, Src, and TAZ.
(iii) 14-3-3 proteins.
MT is phosphorylated on serine 257 (71). This phosphorylation allows MT to bind 14-3-3 proteins (71, 217). Consonant with a common role for 14-3-3 proteins, it has been suggested that this permits multimerization of MT (264). However, not all workers have observed this (49). The 14-3-3 interaction seems unimportant for transformation in vitro or for tumor induction at most sites. However, virus bearing a mutation that prevents 14-3-3 binding fails to induce salivary gland tumors and is deficient in causing fibrosarcomas (71). This is another example of the role of tissue context in the importance of signaling. The precise molecular role of the 14-3-3 interaction is unknown, but it does affect MT function in some transcription reporter assays (71).
The proline-rich region.
There is a proline-rich region from residue 332 to 347 in the C-terminal region of MT (Fig. 2). The mutant dl1015, which lacks residues 339 to 347, is quite defective in transformation (181, 182). Deletion of prolines 336 to 338 causes a defect in inducing tumors in animals (341). Differences in tumor type with Δ336-338 were quite striking. The mutant induced no kidney, salivary gland, or thymic tumors. Effects at other sites were smaller. Although the frequency of breast tumors was decreased somewhat, the tumors themselves appeared to be histologically similar to those induced by the wild type. The basis for the defect in transformation is unclear. Dl1015 has a normal tyrosine kinase associated with it (254). The behavior of the E349K point mutant, which mimics the defect of dl1015 (Denis and Schaffhausen, unpublished), raises the possibility that PI3 kinase signaling is blocked between recruiting the enzyme to membranes and PI3K signaling to downstream targets such as Akt.
MT MODELS OF TUMORIGENESIS
The ability of MT to produce tumors in animals has been tested by three basic approaches—PyV infection, delivery of MT as cDNA or in a retroviral vector, and expression of the MT gene as a transgene. Each method has advantages and drawbacks (104, 105). In the case of PyV infection, the ability of MT to drive tumor formation in a broad variety of tissues can be tested simultaneously. However, since the experiment is carried out in the context of viral infection, there are also contributions from LT and ST to be considered. Furthermore, the induction of tumors is the result of multiple steps involving numerous rounds of replication, virus spread, and persistence, each of which is dependent upon MT (as well as ST and LT). However, the mutation of single tyrosines (e.g., Y250F) has a much more dramatic impact on the tumor outcome than on the replication and persistence pattern (Wang and Fluck, unpublished), so that in this case the effect of a single tyrosine MT mutation on tumor induction can be separated from its effect on persistence. Thus, the traditional method for examining the role of MT in transformation and tumorigenesis, i.e., comparing wild-type and mutant viruses, remains fruitful.
MT can be delivered either as cDNA or using retroviral vectors. Recent experiments have employed vectors that allow delivery of MT to specific tissues in mice that have been engineered to express retroviral receptors in particular tissues. In this system, tumorigenesis can be initiated in a background of normal cells. The recent development of lentivirus vectors offers the prospect of being able to infect nonproliferating cells.
Transgenic animals have been used in a variety of systems. Here, patterns of expression are easier to predict and determine. Very strikingly, rather synchronous results are obtained. However, how the expression of MT might affect the development of the tissues is unknown. These problems can be solved by the use of conditional expression. In principle, there is the possibility that the outcome might also be affected by the global expression of MT in the tissue. Real-world tumors mostly begin in a background of normal cells.
Polyomavirus Infection
Newborn mice are generally used in infection experiments, but adult immunodeficient animals have also been employed (16, 129, 240). Even before ST and MT were identified, it was apparent that they played a key role in viral tumorigenesis (268). In the most favorable circumstances, it is possible to reconstruct a virus with a mutant MT with little or no change in LT or ST. For example, PyV 1387T, in which MT is truncated after M384, is virtually unable to induce tumors (109). This is not surprising, since PyV 1387T, which does not activate Src (30), would be defective in its downstream signaling (109). However, the simultaneous effect on persistence also contributes to the lack of tumors (Wang and Fluck, unpublished). In contrast to the absence of tumors with PyV 1387T, mutation of the 14-3-3 binding site (S257C) had relatively little effect on the tumor profile apart from a striking deficiency in induction of salivary gland tumors (71). As in several instances noted above, this tissue-specific response is part of a general picture showing that signaling through a particular pathway may have different consequences in different contexts. Mutations of tyrosines Y250 and Y315 provide other examples of this. It is also worth reemphasizing that, in the case of virus infection, LT and ST can modulate or enhance MT signaling.
Introduction of MT as DNA or Using Viral Vectors
When cloned T antigens became available, the effect of introducing MT without using PyV was tested. MT-expressing DNA was sufficient to cause tumors in hamsters with longer latency, but in rats, complementation by PyV LT or ST or by SV40 ST or LT was needed (7, 8). The introduction of MT using an avian retrovirus was shown to cause hemangiomas or hemangiosarcomas in chickens (162).
A more recent approach allows the targeting of MT to specific tissues using retrovirus vectors in mice engineered to express retrovirus receptors in a tissue-specific way. The RCAS-TVA system, developed by Varmus and colleagues (103), uses mice derived to express the ALSV-A (TVA) avian retrovirus receptors in specific tissues. When these mice are infected with RCAS (replication-competent ALV long terminal repeat with a splice acceptor) viruses, only cells expressing the receptor are infected. For MT, mammary tumors (91, 92), liver tumors (170), and pancreatic tumors (171) have been examined in this way. The approach has now been applied to lentivirus constructs to allow efficient infection of nondividing cells (270). When applied to targeting progenitor cells, MT was able to cause hemangiomas (250).
Transgenic Expression of MT
Transgenic expression of MT has been used to test its ability to cause tumors. When expressed this way, MT can cause tumors in a broad range of tissues (1, 13, 38, 126, 234, 286, 331). For example, MT can induce fatal prostate cancer (286). In endothelial cells, MT induces hemangiomas. Intriguingly, the transformed endothelial cells recruit nontransformed endothelial cells into the tumors (229, 332). As noted above, one issue with the traditional transgenic approach is the timing of the delivery of the oncogenic signal. One way to approach this problem has been to use conditional expression of Cre (38).
Along with other genetically engineered mouse models of breast cancer, the MMTV-MT transgenic model permits studies in a nonimmunocompromised host (29). Metastatic carcinomas are induced (126) when MT is expressed in mammary glands under the control of the MMTV promoter, a result not seen in most MMTV-dependent oncogene-based models. Another of the MT model's advantages is the focal induction of tumors, in contrast to the equally metastatic ErbB2/Neu models, in which the whole mammary gland coalesces into a large tumor. The MT system has been intensively studied by a wide variety of investigators interested in different aspects of tumorigenesis. It provides a relatively good model for the human disease (174). There are, of course, some differences between these and human tumors. Although 50% of human tumors are estrogen receptor alpha (ERα) positive, the MT tumors are largely ERα negative (similarly to other MMTV transgenic models). The MT tumors metastasize to the lung, whereas human metastases are more broadly distributed. It is worth discussing the picture that emerges from these studies in some detail.
The widely used transgenic MMTV-PyMT634 model of mammary carcinogenesis.
Mice designated FVB/N Tg(MMTV/PyMT634Mul) (MMTV-PyMT634 herein) have been extensively studied for their development of mammary tumors. Surprisingly, this model is often described as a model of “spontaneous” breast oncogenesis in the literature. MMTV-PyMT634 is one of seven independent founder lines isolated following microinjections using the mammary gland-targeting, steroid-regulated MMTV promoter to express MT in one-cell embryos of FVB mice. Five founders passed the transgene to their progeny. High levels of the transgene in the female mammary glands lead to altered mammary functions, such as an inability to lactate and/or nurse. In addition to the mammary gland, MT was expressed, albeit at much lower levels, in female and male salivary glands, ovaries, epididymis, and seminal vesicles, as seen with other MMTV-dependent transgenes. No tumors developed in these tissues, a result which perhaps correlated to low MT expression and/or cellular differentiation state. However, an examination of the parotid gland showed the formation of parotid hyperplasias in 100% of transgenic female mice (98). It is worth noting that the founder lines had varying characteristics. Three lines developed mammary tumors with latencies of 94, 155, and 175 days, following at least two cycles of pregnancy. This pregnancy dependence recapitulates that seen for tumors induced by MMTV virus in some mouse strains (e.g., GR) that carry an endogenous MMTV virus. It reflects a prolactin-dependent regulation of the MMTV promoter (199). In these three lines, tumors appeared rapidly at a time of intense cell growth following the onset of high-level MT expression. Males did not express MT and did not get tumors. In two founder lines, virtually all female virgin mice developed palpable tumors with quasisynchronous short latencies (34 days in MMTV-PyMT634 mice and 36 days in line 668). Furthermore, tumors were also induced in male mice with an ∼50-day delay. Accelerated, pregnancy-independent expression, as seen in line 634 (and 668), has also been observed with other MMTV-transgenic models. For the 634 mice, crosses with prolactin knockout mice had little effect on PyMT expression and induced only a small delay (9 days) in tumor formation (315). Overall, the difference in tumor latency in females in different lines is likely to be related to the kinetics and level of MT expression, which are presumably influenced by the characteristics of the integration site. Lung metastases were induced in both prolactin-independent and -dependent lines. In the 634 line, 94% of females had metastatic lung disease by 3 months of age (126). The unusual rapidity of tumor growth and enhanced metastatic potential observed in MT—in contrast to other transgenic models—have made induced tumors in MMTV-PyMT634 a favorite model.
In MMTV-PyMT634 mice, the earliest hyperplastic lesions arise at a very early age and are located very close to the nipple and thereby easy to track. The early lesion involves prepubertal, nipple-proximal buds (end buds) at the ends of growing immature ducts surrounding the main collecting duct (Fig. 5). The hyperplasia usually consists of a single focus with a cluster of densely packed lobules (126, 174, 180). Following prepubertal growth, the ducts elongate further under hormonal influence, and new lesions arise on the distal end buds (Fig. 5). The most distal foci arise at a stage of ER-dependent growth. At all ages, the distribution of focal atypical lesions along the ducts displays a gradient, with more numerous and larger lesions closer to the nipple than the other end of the ducts (180). At late stages, the nipple-proximal tumor fuses with numerous distal foci.
FIG. 5.
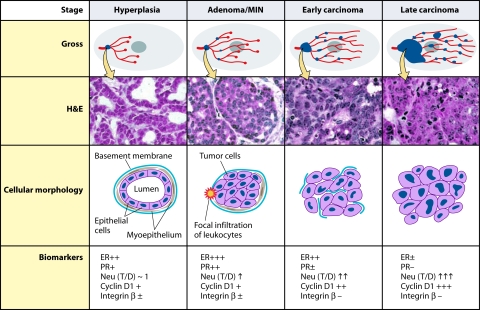
Summary of tumor progression and biomarker expression in the MMTV-PyMT634 mouse model of breast cancer. Four tumor stages are observed in transgenic MMTV-PyMT634 mice at different ages and in ducts that are at different stages of development. At the earliest stage (2 to 4 weeks after birth), growth of the short, underdeveloped prepubertal ducts takes place from the bulbous end buds under the influence of growth factors. In sexually mature mice (from 6 weeks on, when adenomas are observed), ducts continue to grow from the end buds under the control of estrogen and growth factors until they reach the confines of the gland. (The figure and legend [see below] are adapted from reference 174 with permission from the American Society for Investigative Pathology.) The Gross panel displays the overall development of lesions in mammary glands of PyMT mice. Tumor lesions are indicated by blue dots. The hematoxylin-and-eosin panel displays the corresponding histology of primary lesions at different stages of tumor progression. The cellular morphology panel schematically illustrates changes in the cytology of the cells as well as the integrity of the basement membrane and the presence or absence of myoepithelial and focal inflammation. Moreover, the changes in biomarkers during tumor progression are summarized in the panel of biomarkers. T/D, the ratio of Neu expression between lesions and normal ducts in age-matched mammary glands.
The progression of the earliest nipple-proximal lesion, which is destined to become the main tumor, was tracked in situ by Lin and colleagues (174), and four stages with similarities to human tumors were identified. These stages—hyperplasia, adenoma/mammary intraepithelial neoplasia (MIN), carcinoma in situ, and invasive carcinoma—and their timing, associated properties, and markers are described in Fig. 5 (174). The preneoplastic MIN lesions are characterized by a spectrum of intraluminal proliferation with cytological atypia (nuclear morphological abnormality) (29). Points of interest in the development of the earliest 634 tumor are early breakage of the basement membrane accompanied by leukocyte and/or macrophage infiltration, disappearance of myoepithelial cells, and changes in the expression of the classical breast tumor markers, ER and progesterone (PR) receptors. A marked increase in their expression is observed in the epithelial cells at the adenoma/MIN stage, with a more mosaic distribution as the tumor progresses, followed by expansion of ER-negative cells. PR expression lags behind ER expression, reaching 70% of cells by early carcinoma in about 30% of mice and, similarly to ER expression, essentially completely disappears at the late carcinoma stage. In parallel with these changes, progressive overexpression of ErbB2 takes place up to the late carcinoma stage. Cyclin D1 undergoes a redistribution as well as an increase in expression; changes are also observed in β1 integrin expression, initiating at the earliest stages and then declining. While the p53 gene sequence is wild type (208, 230), no or very little p53 expression is detected (174, 208).
An important question is whether MT itself is sufficient to cause the tumor progression (in a “one-hit” process) or whether MT initiates a series of events that lead to other genetic changes required for tumor formation. The near 100% frequency and short latency of the tumors, the similarity of gene expression patterns of independently derived tumors (132), and the absence of major chromosomal aberrations (see below) might seem to argue that MT is all that is needed. The same argument has been made for the RCAS model of MT mammary tumorigenesis (91).
To address the issue of tumor heterogeneity, some work was devoted to examining the state of the genome in tumors from the transgenic mice. An early report examined loss of heterozygocity and concluded that MT may be sufficient to drive tumorigenesis and metastasis (239). However, examination of aberrations in gene copy number has shown alterations that could perhaps affect cellular behavior. A later report showed high-level copy number gains in chromosome 11, associated with an increase in septin 9 expression in cultures of cells derived from tumors (193). In contrast, Hodgson and colleagues reported low-level copy number amplification on distal chromosome 11 when tumors were examined directly (134). There is also some evidence that MT can increase the frequency of frameshift mutations (280). Altogether, there is not a strong case for a role of extensive chromosomal changes in the rapid MMTV-PyMT634 tumors derived in situ with wild-type MT, although the possibility of important but subtle changes cannot be excluded.
The ability of MIN preneoplastic lesions to produce tumors (29) was tested by a stringent transplantation criterion. In such experiments, two outcomes were obtained, suggesting heterogeneity in the lesions. A small number of MINs taken from the nipple-proximal region of 4-week-old mice gave rise to invasive tumors following subcutaneous injections (180). Such MINs appear to have already acquired a ‘latent’ malignant phenotype and establish PyMT as an oncogene with a one-hit potential. In contrast, the outgrowths of some MINs, including ones isolated from the periphery of the mammary gland of 8-week-old mice, did not yield tumors at ectopic sites and displayed normal growth patterns in fat pad transplantation. They were immortalized, overexpressed the telomerase gene, and had stem cell properties. They eventually developed focal areas of invasive cells after variable latencies usually longer than those observed for in situ tumors (179). Furthermore, individual preneoplastic MINs showed distinct intrinsic characteristics in terms of morphology, gene expression, and genetic aberrations, which were maintained in the tumors arising from them (203, 204). Their gene expression patterns were distinct from each other as well as from the characteristic pattern of the in situ 634 tumors. By all criteria, their resulting tumors could be distinguished from the more uniform in situ tumors. These truly preneoplastic MINs have served as a valuable model of tumor progression (74).
The existence of two types of MT-expressing MINs, both derived from ductal epithelial cells, provides another indication that PyMT's potency as an oncogene is strongly dependent upon cellular context. It is possible that these differences in malignant potential in different MINs are related to the characteristics of the cell type progenitors in which initiating events took place. End bud growth in 4-week-old mice is under growth factor control (57), whereas distal lesions arising at 8 weeks are dependent upon ER for growth. As noted above, dramatic age-related differences in sensitivity to mammary tumor induction have also been observed following viral infection (240). A difficulty in the interpretation of these kinds of experiments is that the transformed phenotype varies with the amount of T-antigen expression (233). The precise level of MT signaling complexes in different lesions is difficult to ascertain. Furthermore, it should be noted that transplantation of MMTV-PyMT634-derived MINs or tumors into syngeneic naive FVB/N mice may result in some immune response, since MT is not an endogenous protein and as such is capable of inducing transplantation rejection (271). This effect may be increased in the case of transplanted MMTV-PyMT634 tumors, as the response to endogenous MT is likely diminished by superantigen Sag7, encoded by the MMTV long terminal repeat. This protein causes the deletion of Vβ6+ T cells, which recognize an MT immunodominant sequence (178). Indeed, the Sag7 gene is overexpressed in MMTV-PyMT634 tumors (232).
(i) Metastasis: a recent putative paradigm shift?
One of the facets that distinguishes MMTV-PyMT634 from human breast cancer is the almost exclusive distribution of the metastases to the lung, with some metastatic foci also detected in the lymphatics. This was assumed to reflect the trapping of tumor emboli in the fine capillary beds of the lungs. However, recent experiments have detected tumor cells and micrometastases in the bone marrow (143, 196). These studies used MMTV-PyMT634 tumors to reexamine the timing of tumor cell migration. In MMTV-PyMT634 mice, mammary cells were detected in the bone marrow (femur) in mice as early as 4 to 6 weeks of age, when in situ lesions are at a hyperplastic stage, i.e., long preceding lung metastases, which were detected from 14 weeks of age. These data indicate that premalignant cells are capable of migration. Whereas crossing of the basement membrane by preneoplastic cells was detected by electron microscope observations, this phenomenon was undetectable in standard histological analyses (143). In a different system (228), involving doxycycline-inducible MT, uninduced mammary cells were introduced intravenously into a recipient mouse, so that the cells would go to the lung. Recipient mice were then treated with the inducer at various times postinjection. Untreated transplanted mammary cells continued to grow normally in the lungs, while treated mice developed “metastases” 2 weeks after treatment with inducer and as late as 17 weeks after the transplant. These results suggest that normal nonmalignant cells have inherent properties formerly associated with metastatic cells, namely, the capacity to survive in the circulation, to extravasate, and to establish and grow at ectopic sites.
(ii) MT genetic requirements for MMTV-PyMT634 tumorigenesis.
For comparison to the wild type, founders were also subsequently obtained with transgenic mice that express MT mutants or mice that lack specific MT binding proteins. For example, crossing MT mice with c-Src knockout mice demonstrates that c-Src is a key target for the formation of mammary tumors (127). The requirements for the MT connection to its downstream targets, Shc and PI3K, were studied with two mutants: eight founders were derived with Y250F MT, which fails to activate Shc, and seven founders with the double mutant Y315/322F MT, which lacks the connections to PI3K and PLCγ1. Comparisons were carried out between MMTV-PyMT634 and mutant founders that express high levels of MT and that, like 634, are not pregnancy dependent.
These genetic experiments show the importance of Y250, the connector to Shc, in causing tumors in transgenic animals. In Y250F mice, histological examination of mammary glands in virgin mice at 12 weeks of age showed extensive hyperplasia that involved alveolar growth. However, tumors developed after a long delay (T50, 143 days) compared to WT-derived (T50, 53 days), and their induction was focal. This suggests that there could be differences in the differentiation state at the time of tumor initiation in wild-type and mutant MT animals. In addition, a proportion of the tumors showed that MT had been altered to regenerate an Shc binding site. While metastasis was also observed with the mutant, these metastatic cells were more likely to have the reverted MT. Tumors that lacked the MT reversion showed elevated ErbB2/ErbB3 expression that was not related to altered levels of MT expression, suggesting a functional complementation between MT and ErbB overexpression. Consistent with this idea, overexpression of Shc or Grb2 accelerated tumor formation in the mutant mice with an associated increase in epidermal growth factor (EGF) receptor family expression (235). There remains a clear, unexplained discrepancy between the need for Y250 in the transgenic model and its relative unimportance in the ability of PyV to cause mammary tumors. The easiest explanation would be if LT and/or ST replaced the need to activate Shc.
Similar genetic experiments show an important role for Y315/Y322. Again, hyperplasia was observed at 12 weeks, but in Y315F/Y322F mice the hyperplasia was ductal and highly apoptotic, resulting in cystic ducts. Eventually, after a long, founder-specific delay (latency between 107 and 255 days), focal tumors developed. The MT-Y315F/Y322F tumors showed a marked decrease in angiogenic blood supply as well as a 10-fold decrease in metastatic potential (54). Eighty percent of the Y315F/Y322F tumors overexpressed the ERBB2 and ERBB3 genes to very high levels, perhaps providing receptor-mediated activation of PI3K to complement the MT defect. This case provides an example of a secondary “hit,” more likely observed in tumors with longer latencies. While it is likely that most of the phenotype of Y315F/Y322F comes from the PI3K rather than from the PLCγ defect, this has not been directly confirmed.
(iii) What can gene expression studies tell us about MT tumorigenesis?
Array analysis has been extensively used to examine independently derived MMTV-PyMT634 tumors (81, 132, 232). As noted above, different tumors show homogeneous patterns of gene expression. MMTV-PyMT634 tumors display a large number of deregulated genes, including many implicated in breast cancer. These define a number of upregulated pathways: the glycolytic pathway; elongation translation factors plus structural RNAs; cell cycle regulators; signaling receptors and their effectors, including G proteins; and downstream transcription factors (81). Interestingly, many of the same pathways are deregulated in mammary tumors derived with MMTV-c-myc, MMTV-Ha-ras, MMTV-neu, and WAP-SV40T, so their induction arises from diverse events. More detailed analyses, using different procedures and arrays, show that expression patterns in different tumor models can be distinguished. MT tumors most closely resemble those that express mutant Ras (232) or overexpress Her2/Neu (132). For example, in contrast to c-myc and SV40T, genes active at the G2/M transition are not involved in the Neu-Ras-PyMT group. Rather, induction of cyclin D1, Cdk2, and E2F was noted, i.e., indicators of mitogenic stimulation and Ras pathway activation. The close clustering between MMTV-PyMT634 and MMTV-Neu further validates the relevance of PyMT as a model for breast cancer. Hierarchical clustering analyses of the MMTV-PyMT634 tumors along with classified human tumors suggest the placement of MT tumors in a luminal group. This group is characterized by expression of the luminal keratin K8/18 and tight junction structural components and by particularly high expression of the transcription factors PDEF/SPDEF (Sam pointed domain, an Ets-family factor), GATA3, and XBP1. In the human case, the luminal group generally expressed ER and ER-dependent genes (225). This was not true of MMTV-PyMT634 tumors. However, a luminal subgroup, subgroup B, that expresses only low levels of ER and overexpresses ErbB2, has been defined. This matches better the status of the MT tumors. Luminal B tumors have a poor prognosis (276). Interestingly, in other situations, ER status is important for the development of breast tumors induced by MT. ERα (and PR) is expressed in hyperplastic lesions from MMTV-PyMT634 mice from which the major tumor emerges. Indeed, outgrowth from transplanted MINs is sensitive to the presence of selective ER receptor modulators (205). Ovarian hormone dependence has been observed in the induction of mammary tumors following infection with PyV; this dependence is strongly age-linked, with a total loss of mammary gland tumors in 10-week-old mice following ovariectomy, in contrast to skin and bone tumors; these observations further support the importance of differences in tumor target cells at the time of initiation (240). Furthermore, hormone dependence for the maintenance of the viral tumors has also been observed (J. Wirth and M. Fluck, unpublished data).
(iv) Linking MT transcriptional activation function to MT-dependent oncogenesis.
Gene expression changes in tumor cells point to the key role of some transcription factors. In the introduction, the role of Pea3/Ets and Ap-1 transcription factors in PyV infection was highlighted. These same factors have a well-established connection to breast cancer. Overexpression of members of the Ets family (e.g., ESX/Ese1 [15]) has long been associated with a poor prognosis (reviewed in reference 165). Furthermore, expression of dominant negative mutants of both Ets and Ap-1 reverses the transformed phenotype and tumorigenicity of various breast tumor cells (111, 267). A systematic quantitative RT-PCR comparison of the expression of 25 ets family members, including many genes not present in early arrays, was carried out for MMTV-PyMT634 tumors. Comparisons to normal mammary cell lines, primary mammary cells, and MMTV-Neu tumors revealed that many ets genes are expressed in the mammary tissues, and a subset of nine genes is highly overexpressed in MT and Neu tumors (112). The highest relative expression, a 36-fold increase in MMTV-PyMT634 tumors, was found for pdef, a member of the luminal B tumor signature (132). The current view suggests that there is great redundancy in promoter usage by the various Ets/Pea3 proteins (135). Combined extensive overexpression of ets genes could affect a very large number of Ets/Pea3 targets. The pattern of increase of Ets family gene expression as a function of progression has not been examined except for pea3. In this case, upregulation is already manifest in hyperplastic lesions (165); high induction is seen in tumors and metastases (298). Also in support of the connection of MT to the Ets/Pea3 family, upregulation of ets genes was reduced in tumors induced by Y315/Y322F mutant MT. Data showing that dominant negative Pea3 blocks Neu tumorigenesis strongly suggest an equivalent role for MT (267). Consistent with the role of Ets transcription factors mentioned above, disruption of coactivators such as AIB1 (231) and Src1 (318) that can be connected to ets family members has the effect of reducing metastasis in MT mammary carcinoma. This signaling uses IRS-2, which is downstream of PI3K and Shc signaling pathways (202, 220).
As stated earlier, studies on the role of MT in the polyomavirus lytic cycle demonstrate that Ap-1 and Ets/Pea3 are key MT targets for replication and transcription. A bipartite Ap-1+Ets binding site in the PyV enhancer is of particular importance. Similar bipartite sites are found upstream of a large number of cellular genes that play a role in cancer progression, including genes known to be upregulated by MT and others, such as cyclin D1, the matrix metalloproteases (MMPs), urokinase plasminogen activator, cyclooxygenase 2, and vascular EGF (VEGF). Further support for the contribution of bipartite sites to transcription and malignancy comes from studies on stromelysin. A single nucleotide polymorphism in tumor tissue results in the creation of an additional bipartite site, which leads to increased stromelysin expression and is associated with a more invasive tumor phenotype (295).
How would synergy between Ap-1 and Ets work? For many Ets proteins there is a well-documented DNA binding inhibition. There is also an Ap-1 problem. In invasive tumors, the expression of c-Fos, a strong activator, is switched to Fra-1, which lacks the transactivation domain, resulting in a weak, even repressive, Ap-1. This switch arises from increases in MAP kinase, increases in c-Fos leading to induction of fra-1, and then Fra-1-dependent repression of c-fos (17, 36, 61, 130). The end point is a switch in Ap-1, from Fos/Jun to Fra-1/Jun heterodimers. Met cells derived from MMTV-PyMT634 tumors contain very high levels of Fra-1, with an almost total absence of c-Fos (E. Miller and M. Fluck, unpublished data). The switch from Fos to Fra-1 may repress some tumor suppressor genes involved in cell movement (152). Bipartite sites solve both the Ets/Pea3 and Ap-1 problems. By mediating an interaction between Ap-1 and Ets proteins, a binary complex has a strong transcriptional activator (Ets) and an efficient mediator (Ap-1) to target it to DNA.
The observed changes in Ap-1 and Ets/Pea3 expression in progressing MMTV-PyMT634 tumors are likely to arise from changes in signaling occurring during progression. These can involve autocrine factors whose downstream targets involve Ap-1 and Ets and/or paracrine factors leading to association with stromal cells and cross-signaling that also yields increases in Ap-1/Ets activity (see below). The transcriptional changes that are observed can themselves be effected by products induced by MT. Certainly MT mutant 315F/322F tumors, which are resistant to progression, show decreased levels of Ets proteins (112), and fail to switch from c-Fos to Fra-1 (Miller and Fluck, unpublished). One specific example of an MT-induced diffusible product is osteopontin, encoded by an Ap-1- and Pea3/Ets-dependent gene (100). Its overexpression has been demonstrated in invasive nMTV-PyMT634 tumors and cell lines derived from them, as well as in tissue culture cells transformed by MT (325). Osteopontin is in turn able to induce Ap-1- plus Ets-mediated secretion of a urokinase-type plasminogen activator through EGF activation (75). This effectively creates a positive feedback loop. Indeed, the silencing of osteopontin in MT-transformed and tumor cell lines reduced their migration and metastatic potential (150, 326). Other examples of known autocrine mammary growth factors whose expression increases gradually during MT tumor progression are amphiregulin and Cripto (209). In addition, the possibility that hormonal or signaling changes affect MT expression during progression has not been eliminated.
(v) Stromal-cell-epithelial-cell interactions play a key role in tumor progression.
The kinds of effects just described also impact interactions of tumor cells with stromal cells. The importance of such interactions is well known. For example, in the case of MMTV-PyMT634, transplantation of tumor cells into genetically modified mice with altered ETS2 expression strongly affects tumor development (184, 206). The polyoma MMTV-PyMT634 model has been used to examine stroma-carcinoma interactions, especially with respect to the function of macrophages, which represent a major fraction of the leukocytes that infiltrate tumors. Many aspects of the mutual epithelial cell-macrophage interactions have been validated or established in this model system.
Epithelial cells and macrophages associate for their mutual benefit. Epithelial cells respond to EGF and produce colony-stimulating factor 1 (CSF-1). CSF-1 is a chemoattractant and a main growth factor for macrophages which express the CSF-1 receptor, with functions similar to those of the platelet-derived growth factor or Met receptors. CSF-1 is overexpressed in many breast tumors and is considered a marker for poor prognosis. On their side, macrophages have multiple functions which play a role in progression: they are potent producers of (i) proteases (e.g., MMP-12 or plasminogen activator arising from being regulated by CSF-1), which contribute to epithelial cell invasion, (ii) Ang-1 and VEGF, which promote vascularization, and (iii) EGF, which stimulates epithelial cell movement in addition to tumor growth. Interestingly, most of these players are controlled by Ap-1 plus Ets.
Higher concentrations of macrophages were located at the surface and close to perivascular regions of 634 tumor cells (320, 336). Because the absence of CSF-1 results in the relative absence of macrophages, the behavior of MMTV-PyMT634 has been tested in CSF-1-null mice. While the development of the primary MMTV-PyMT634 lesion to carcinoma in situ is not affected, the tumors do not invade effectively and there is both a dramatic delay and decrease in lung metastases (176). Conversely, overexpression of CSF-1 in the mammary gland accelerates tumor progression to invasive carcinoma. It is evident that macrophages have multiple functions. Intravasation is assisted by macrophages (336). Differential labeling of macrophages and tumor cells to allow the monitoring of in situ behavior showed that intravasation always occurred within 20 μm of a macrophage. Furthermore, the number of tumor cells reaching the bloodstream depends on both EGF and CSF. Although intravasation may not depend on local angiogenesis (336), an angiogenic switch also takes place between adenoma or MIN and early carcinoma, as gauged by the increase in blood vessel networks. The interplay between tumor-generated CSF-1 and macrophage-produced EGF is important in these processes. High-speed migration of invasive cells toward and into needles that contain EGF or CSF as a chemoattractant was demonstrated, along with the synergistic comigration of macrophages and tumor cells toward either chemoattractant. Inhibition of either CSF or EGF signals decreased migration of both cell types (335). Compared to the main tumor population, chemoattracted tumor cells were more invasive, demonstrated less apoptosis, contained a smaller proportion of dead cells, and displayed a reduced proliferative index. VEGF is clearly important in these effects. Overexpression of VEGF overcomes the defects in tumorigenesis seen in macrophage-depleted CSF-1-null deletion mice (175). Further evidence for the complex role of macrophages was obtained by studying the role of macrophage-stimulating protein (MSP) (323). MSP is a serum protein, activated by a macrophage protease (MT-SP1 or matripase) that promotes macrophage as well as epithelial cell chemotaxis and is often overexpressed in breast tumors. MMTV-PyMT634 tumor cells transduced with MSP, as well as tumors generated by a stem cell retrovirus expressing MT, had accelerated tumor growth and showed an increase in the frequency of metastasis at all sites (lung, lymph node, spleen, and liver). Unlike the case of parental MMTV-PyMT634 tumors, metastasis was also induced in bone and accompanied by osteolytic lesions, as seen in aggressive breast cancer.
MMTV-PyVMT634 mammary tumors are enriched in proteases, including the MMPs, which are important for tissue remodeling (3, 223, 285). Proteases are involved in the process of metastasis, since metastases can be reduced by treatment with the general metalloproteinase inhibitor galardin (4) or knockout of urokinase (223). Most proteases seem to come from the stroma (223). Of the proteases examined, only cathepsin B (310, 311) and targeted MT2-MMP (285) seem to come from the epithelial cells.
(vi) Effect of the mouse background on MT tumorigenesis.
It has been known for a long time that the extent of polyoma tumorigenesis depends on the mouse strain used for study. In the case of virus-induced tumors, the immune response is one major part of the story. It is also abundantly clear that the genetic background of the host affects the ability of transgenic MT to produce both breast tumors and metastases (see reference 333 for a more detailed review) in ways not directly related to the immune system. The original MMTV-PyMT transgenics were produced in FVB/N animals (126). In an early study, MMTV-PyMT634 mice were bred to females from a series of 27 other inbred mouse strains (173). Some strains, such as I/LnJ, showed significant acceleration in tumor development (21 days faster) compared to FVB/N, while others, such as ST/J, showed significantly increased latency (20 days slower). Backcross analysis followed by a bioinformatics approach identified cdc25A and c-myc as genes that modified latency (67, 169). There is practical value to using different strains. By using C57Bl/6J, which has a dramatically increased latency, it was possible to discern a role for inducible nitric oxide synthase in lung metastasis (76). (The lung remains the destination for metastases in different mouse strains.) Most strains showed a lower metastatic index than FVB/N, with C58/J showing a 25-fold reduction. Backcrossing implicated sequences on mouse chromosomes 5, 6, 9, 17, and 19 (140, 166). Further analysis identified the Sipa1 gene, on chromosome 19, as a likely candidate gene (221, 222). Sipa1 is a Rap-GAP, for which polymorphisms have been associated with poor outcomes in breast cancer (70). Sipa1 associates with Brd4 (102), a chromatin-bound protein involved regulating cell cycle progression at G2/M (83). Recent work indicates that Brd4, which maps to chromosome 17, can modify both tumor growth and pulmonary metastasis, concomitant with regulation of extracellular matrix genes (68). Sipa1 has also been shown to associate with Rrp1b (RNA processing 1 homolog B) (69). The highly metastatic AKR/J strain was found to have a less active Rrp1b promoter than the poorly metastatic DBA/2J strain. As with BrD4, Rrp1b expression was associated with extracellular matrix protein expression and with survival in human breast cancer.
A more specific means of testing the effect of mouse background is to carry out crosses with mice lacking specific gene products. Many of these, such as SRC, GRB2, AKT, and CSF1 knockouts, have already been described. However, there are others worthy of mention in the context of a tumor growth or metastasis focus. Targeted disruption of β1 integrin, for example, causes a dramatic reduction of MT tumorigenesis (327). On the other hand, overexpression of VEGF accelerates tumor formation (261). The Src substrate SAM68 seems to be important, because even haploinsufficiency delayed metastasis and tumor growth in a cell-autonomous fashion (237). Knockout of G-protein-coupled protease-activated receptor 2 (PAR2), but not PAR1, showed its importance in the development of breast tumors, including a role in chemokine production (314). Modulating transforming growth factor β (TGF-β) signaling has given a somewhat varying picture. Early results suggested a positive contribution. Blockade of TGF-β signaling reduced tumor cell viability and migration potential (200), while overexpression of TGF-β1 accelerated metastases (201). More recent work suggests that TGF-β inhibits tumor formation. Knockout of the type II receptor showed reduced tumor latency and enhanced metastasis (19, 106). Very recent work suggests that this metastasis is enhanced by recruitment of specific myeloid cells in this situation (338). In general, this approach will continue to be fruitful for examining the role of particular pathways and targets.
EPILOGUE
The decision of microbiologists of the 1950s to concentrate on a few model systems led to rapid progress in understanding microbial physiology. The effort put into the extensive analysis of the MT model has similarly provided repeated insights into multiple aspects of tumorigenesis. It continues to be a fruitful model from several perspectives. One is that there are aspects of MT signaling that offer the prospect of novel regulatory mechanisms. A second is the ability to combine MT studies with the ability to manipulate the genetic background of the murine host. A particularly interesting issue is the differential responses of cells (e.g., mammary ductal cells) at different stages of development.
Acknowledgments
This work was supported in part by Public Health Service NIH grants R01-CA29270 and R01-CA58763 (M.M.F.) and grants PO1-CA50661 and RO1-CA34722 (B.S.S.).
Biography
 Michele Fluck received her education at the University of Geneva, Switzerland, starting in nuclear physics (M.S.) and switching to molecular biology. Her Ph.D. thesis on the influence of the context on the suppression of nonsense mutations was carried out under the guidance of Dick Epstein. An introduction to tumor viruses, taught by Howard Temin and Tom Benjamin at Cold Spring Harbor, triggered her interest in the field. She joined Tom Benjamin's laboratory for her postdoctoral studies at Harvard Medical School (1972 to 1979), initiating her studies of polyomavirus and overlapping with Brian Schaffhausen for five years. In 1979, she moved to the Department of Microbiology and Molecular Genetics at Michigan State University, where she currently holds the title of Distinguished University Professor. Her major interest has been deciphering the role of the middle T oncogene in the virus life cycle to gain novel insights into the oncogenesis process.
Michele Fluck received her education at the University of Geneva, Switzerland, starting in nuclear physics (M.S.) and switching to molecular biology. Her Ph.D. thesis on the influence of the context on the suppression of nonsense mutations was carried out under the guidance of Dick Epstein. An introduction to tumor viruses, taught by Howard Temin and Tom Benjamin at Cold Spring Harbor, triggered her interest in the field. She joined Tom Benjamin's laboratory for her postdoctoral studies at Harvard Medical School (1972 to 1979), initiating her studies of polyomavirus and overlapping with Brian Schaffhausen for five years. In 1979, she moved to the Department of Microbiology and Molecular Genetics at Michigan State University, where she currently holds the title of Distinguished University Professor. Her major interest has been deciphering the role of the middle T oncogene in the virus life cycle to gain novel insights into the oncogenesis process.
 Brian Schaffhausen first became interested in polyomavirus as a graduate student in Biochemistry at Brandeis University. There he worked with Bill Murakami on the structure of the polyoma virion. He then moved to the Department of Pathology at Harvard, where he started working on the polyomavirus T antigens. Subsequently he moved to the Department of Biochemistry at the Tufts University School of Medicine, where he is currently Professor and Chair. His lab works on all of the T antigens and their interrelationships.
Brian Schaffhausen first became interested in polyomavirus as a graduate student in Biochemistry at Brandeis University. There he worked with Bill Murakami on the structure of the polyoma virion. He then moved to the Department of Pathology at Harvard, where he started working on the polyomavirus T antigens. Subsequently he moved to the Department of Biochemistry at the Tufts University School of Medicine, where he is currently Professor and Chair. His lab works on all of the T antigens and their interrelationships.
REFERENCES
- 1.Aguzzi, A., E. F. Wagner, R. L. Williams, and S. A. Courtneidge. 1990. Sympathetic hyperplasia and neuroblastomas in transgenic mice expressing polyoma middle T antigen. New Biol. 2533-543. [PubMed] [Google Scholar]
- 2.Allison, A. C., J. N. Monga, and V. Hammond. 1974. Increased susceptibility to virus oncogenesis of congenitally thymus-deprived nude mice. Nature 252746-747. [DOI] [PubMed] [Google Scholar]
- 3.Almholt, K., K. A. Green, A. Juncker-Jensen, B. S. Nielsen, L. R. Lund, and J. Romer. 2007. Extracellular proteolysis in transgenic mouse models of breast cancer. J. Mammary Gland Biol. Neoplasia 128383-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almholt, K., A. Juncker-Jensen, O. D. Laerum, K. Dano, M. Johnsen, L. R. Lund, and J. Romer. 2008. Metastasis is strongly reduced by the matrix metalloproteinase inhibitor Galardin in the MMTV-PymT transgenic breast cancer model. Mol. Cancer Ther. 72758-2767. [DOI] [PubMed] [Google Scholar]
- 5.Amini, S., V. DeSeau, S. Reddy, D. Shalloway, and J. B. Bolen. 1986. Regulation of pp60c-src synthesis by inducible RNA complementary to c-src mRNA in polyomavirus-transformed rat cells. Mol. Cell. Biol. 62305-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrews, D. W., J. Gupta, and G. Abisdris. 1993. Evidence that the middle T antigen of polyomavirus interacts with the membrane skeleton. Mol. Cell. Biol. 134703-4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asselin, C., C. Gelinas, and M. Bastin. 1983. Role of the three polyoma virus early proteins in tumorigenesis. Mol. Cell. Biol. 31451-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asselin, C., C. Gelinas, P. Branton, and M. Bastin. 1984. Polyoma middle T antigen requires cooperation from another gene to express the malignant phenotype in vivo. Mol. Cell. Biol. 4755-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atencio, I. A., F. F. Shadan, X. J. Zhou, N. D. Vaziri, and L. P. Villarreal. 1993. Adult mouse kidneys become permissive to acute polyomavirus infection and reactivate persistent infections in response to cellular damage and regeneration. J. Virol. 671424-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atencio, I. A., and L. P. Villarreal. 1994. Polyomavirus replicates in differentiating but not in proliferating tubules of adult mouse polycystic kidneys. Virology 20126-35. [DOI] [PubMed] [Google Scholar]
- 11.Ballmer-Hofer, K., and T. L. Benjamin. 1985. Phosphorylation of polyoma middle T antigen and cellular proteins in purified plasma membranes of polyoma virus-infected cells. EMBO J. 42321-2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bautch, V. L. 1989. Effects of polyoma virus oncogenes in transgenic mice. Mol. Biol. Med. 6309-317. [PubMed] [Google Scholar]
- 13.Bautch, V. L., S. Toda, J. A. Hassell, and D. Hanahan. 1987. Endothelial cell tumors develop in transgenic mice carrying polyoma virus middle T oncogene. Cell 51529-537. [DOI] [PubMed] [Google Scholar]
- 14.Benjamin, T. L. 1982. The hr-t gene of polyoma virus. Biochim. Biophys. Acta 69569-95. [DOI] [PubMed] [Google Scholar]
- 15.Benz, C. C., R. C. O'Hagan, B. Richter, G. K. Scott, C. H. Chang, X. Xiong, K. Chew, B. M. Ljung, S. Edgerton, A. Thor, and J. A. Hassell. 1997. HER2/Neu and the Ets transcription activator PEA3 are coordinately upregulated in human breast cancer. Oncogene 151513-1525. [DOI] [PubMed] [Google Scholar]
- 16.Berebbi, M., L. Dandolo, J. Hassoun, A. M. Bernard, and D. Blangy. 1988. Specific tissue targeting of polyoma virus oncogenicity in athymic nude mice. Oncogene 2149-156. [PubMed] [Google Scholar]
- 17.Bergers, G., P. Graninger, S. Braselmann, C. Wrighton, and M. Busslinger. 1995. Transcriptional activation of the fra-1 gene by AP-1 is mediated by regulatory sequences in the first intron. Mol. Cell. Biol. 153748-3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berjanskii, M. V., M. I. Riley, A. Xie, V. Semenchenko, W. R. Folk, and S. R. Van Doren. 2000. NMR structure of the N-terminal J. domain of murine polyomavirus T antigens. Implications for DnaJ-like domains and for mutations of T antigens. J. Biol. Chem. 27536094-36103. [DOI] [PubMed] [Google Scholar]
- 19.Bierie, B., D. G. Stover, T. W. Abel, A. Chytil, A. E. Gorska, M. Aakre, E. Forrester, L. Yang, K. U. Wagner, and H. L. Moses. 2008. Transforming growth factor-beta regulates mammary carcinoma cell survival and interaction with the adjacent microenvironment. Cancer Res. 681809-1819. [DOI] [PubMed] [Google Scholar]
- 20.Blaikie, P. A., E. Fournier, S. M. Dilworth, D. Birnbaum, J. P. Borg, and B. Margolis. 1997. The role of the Shc phosphotyrosine interaction/phosphotyrosine binding domain and tyrosine phosphorylation sites in polyoma middle T antigen-mediated cell transformation. J. Biol. Chem. 27220671-20677. [DOI] [PubMed] [Google Scholar]
- 21.Bolen, J. B., V. Deseau, J. O'Shaughnessy, and S. Amini. 1987. Analysis of middle tumor antigen and pp60c-src interactions in polyoma virus-transformed rat cells J. Virol. 613299-3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bolen, J. B., C. J. Thiele, M. A. Israel, W. Yonemoto, L. A. Lipsich, and J. S. Brugge. 1984. Enhancement of cellular src gene product associated tyrosyl-kinase activity following polyoma virus infection and transformation. Cell 38767-777. [DOI] [PubMed] [Google Scholar]
- 23.Brewster, C. E., H. R. Glover, and S. M. Dilworth. 1997. pp60c-src binding to polyomavirus middle T-antigen (MT) requires residues 185 to 210 of the MT sequence. J. Virol. 715512-5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bronson, R., C. Dawe, J. Carroll, and T. Benjamin. 1997. Tumor induction by a transformation-defective polyoma virus mutant blocked in signaling through shc. Proc. Natl. Acad. Sci. USA 947954-7958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bustelo, X. R., V. Sauzeau, and I. M. Berenjeno. 2007. GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. Bioessays 29356-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell, K. S., K. R. Auger, B. A. Hemmings, T. M. Roberts, and D. C. Pallas. 1995. Identification of regions in polyomavirus middle T and small t antigens important for association with protein phosphatase 2A. J. Virol. 693721-3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell, K. S., K. P. Mullane, I. A. Aksoy, H. Stubdal, J. Zalvide, J. M. Pipas, P. A. Silver, T. M. Roberts, B. S. Schaffhausen, and J. A. DeCaprio. 1997. DnaJ/hsp40 chaperone domain of SV40 large T antigen promotes efficient viral DNA replication. Genes Dev. 111098-1110. [DOI] [PubMed] [Google Scholar]
- 28.Campbell, K. S., E. Ogris, B. Burke, W. Su, K. R. Auger, B. J. Druker, B. S. Schaffhausen, T. M. Roberts, and D. C. Pallas. 1994. Polyoma middle T antigen interacts with SHC via the NPTY motif in middle T. Proc. Natl. Acad. Sci. USA 916344-6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cardiff, R. D., M. R. Anver, B. A. Gusterson, L. Hennighausen, R. A. Jensen, M. J. Merino, S. Rehm, J. Russo, F. A. Tavassoli, L. M. Wakefield, J. M. Ward, and J. E. Green. 2000. The mammary pathology of genetically engineered mice: the consensus report and recommendations from the Annapolis meeting. Oncogene 19968-988. [DOI] [PubMed] [Google Scholar]
- 30.Carmichael, G., B. Schaffhausen, D. Dorsky, D. Oliver, and T. Benjamin. 1982. The role of the carboxy terminus of middle T antigen of polyoma virus. Proc. Natl. Acad. Sci. USA 793579-3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carmichael, G., B. S. Schaffhausen, G. Mandel, T. J. Liang, and T. L. Benjamin. 1984. Transformation by polyoma virus is drastically reduced by substitution of phenylalanine for tyrosine at residue 315 of middle-sized tumor antigen. Proc. Natl. Acad. Sci. USA 81679-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carpenter, C., B. Duckworth, K. Auger, B. Cohen, B. S. Schaffhausen, and L. C. Cantley. 1990. Purification and characterization of phosphoinositide 3-kinase from rat liver. J. Biol. Chem. 26519704-19711. [PubMed] [Google Scholar]
- 33.Carpenter, C. L., K. R. Auger, M. Chanudhuri, M. Yoakim, B. Schaffhausen, S. Shoelson, and L. C. Cantley. 1993. Phosphoinositide 3-kinase is activated by phosphopeptides that bind to the SH2 domains of the 85-kDa subunit. J. Biol. Chem. 2689478-9483. [PubMed] [Google Scholar]
- 34.Cartwright, C. A., W. Eckhart, S. Simon, and P. L. Kaplan. 1987. Cell transformation by pp60c-src mutated in the carboxy-terminal regulatory domain. Cell 498383-91. [DOI] [PubMed] [Google Scholar]
- 35.Cartwright, C. A., P. L. Kaplan, J. A. Cooper, T. Hunter, and W. Eckhart. 1986. Altered sites of tyrosine phosphorylation in pp60c-src associated with polyoma middle tumor antigen. Mol. Cell. Biol. 61562-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casalino, L., D. De Cesare, and P. Verde. 2003. Accumulation of Fra-1 in ras-transformed cells depends on both transcriptional autoregulation and MEK-dependent posttranslational stabilization. Mol. Cell. Biol. 234401-4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cayla, X., K. Ballmer-Hofer, W. Merlevede, and J. Goris. 1993. Phosphatase 2A associated with polyomavirus small-T or middle-T antigen is an okadaic acid-sensitive tyrosyl phosphatase. Eur. J. Biochem. 214281-286. [DOI] [PubMed] [Google Scholar]
- 38.Cecena, G., F. Wen, R. D. Cardiff, and R. G. Oshima. 2006. Differential sensitivity of mouse epithelial tissues to the polyomavirus middle T oncogene. Am. J. Pathol. 168310-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan, S. W., C. J. Lim, K. Guo, C. P. Ng, I. Lee, W. Hunziker, Q. Zeng, and W. Hong. 2008. A role for TAZ in migration, invasion, and tumorigenesis of breast cancer cells. Cancer Res. 682592-2598. [DOI] [PubMed] [Google Scholar]
- 40.Chang, H. W., M. Aoki, D. Fruman, K. R. Auger, A. Bellacosa, P. N. Tsichlis, L. C. Cantley, T. M. Roberts, and P. K. Vogt. 1997. Transformation of chicken cells by the gene encoding the catalytic subunit of PI 3-kinase. Science 2761848-1850. [DOI] [PubMed] [Google Scholar]
- 41.Chen, L., and M. M. Fluck. 2001. Role of middle T-small T in the lytic cycle of polyomavirus: control of the early-to-late transcriptional switch and viral DNA replication. J. Virol. 758380-8389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen, L., X. Wang, and M. M. Fluck. 2006. Independent contributions of polyomavirus middle T and small T to the regulation of early and late gene expression and DNA replication. J. Virol. 807295-7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen, M., D. Redenius, F. Osati-Ashtiani, and M. Fluck. 1995. Enhancer-mediated role for polyomavirus middle T/small T in DNA replication. J. Virol. 69326-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen, W., J. D. Arroyo, J. C. Timmons, R. Possemato, and W. C. Hahn. 2005. Cancer-associated PP2A Aα subunits induce functional haploinsufficiency and tumorigenicity. Cancer Res. 658183-8192. [DOI] [PubMed] [Google Scholar]
- 45.Chen, W., R. Possemato, K. T. Campbell, C. A. Plattner, D. C. Pallas, and W. C. Hahn. 2004. Identification of specific PP2A complexes involved in human cell transformation. Cancer Cell 5127-136. [DOI] [PubMed] [Google Scholar]
- 46.Chen, Y., R. Freund, M. Listerud, Z. Wang, and D. A. Talmage. 1999. Retinoic acid inhibits transformation by preventing phosphatidylinositol 3-kinase dependent activation of the c-fos promoter. Oncogene 18139-148. [DOI] [PubMed] [Google Scholar]
- 47.Chen, Y., Y. Xu, Q. Bao, Y. Xing, Z. Li, Z. Lin, J. B. Stock, P. D. Jeffrey, and Y. Shi. 2007. Structural and biochemical insights into the regulation of protein phosphatase 2A by small t antigen of SV40. Nat. Struct. Mol. Biol. 14527-534. [DOI] [PubMed] [Google Scholar]
- 48.Cheng, A. M., T. M. Saxton, R. Sakai, S. Kulkarni, G. Mbamalu, W. Vogel, C. G. Tortorice, R. D. Cardiff, J. C. Cross, W. J. Muller, and T. Pawson. 1998. Mammalian Grb2 regulates multiple steps in embryonic development and malignant transformation. Cell 95793-803. [DOI] [PubMed] [Google Scholar]
- 49.Cheng, S. H., P. C. Espino, J. Marshall, R. Harvey, and A. E. Smith. 1990. Stoichiometry of cellular and viral components in the polyomavirus middle-T antigen-tyrosine kinase complex. Mol. Cell. Biol. 105569-5574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng, S. H., R. Harvey, P. C. Espino, K. Semba, T. Yamamoto, T. K., and A. E. Smith. 1988. Peptide antibodies to the human c-fyn gene product demonstrate pp59c-fyn is capable of complex formation with the middle-T antigen of polyomavirus. EMBO J. 73845-3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng, S. H., W. Markland, A. F. Markham, and A. E. Smith. 1986. Mutations around the NG59 lesion indicate an active association of polyoma virus middle-T antigen with pp60c-src is required for cell transformation. EMBO J. 5325-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng, S. H., W. H. Piwnica, R. W. Harvey, T. M. Roberts, and A. E. Smith. 1988. The carboxy terminus of pp60c-src is a regulatory domain and is involved in complex formation with the middle-T antigen of polyomavirus. Mol. Cell. Biol. 81736-1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng, Y. S., and L. B. Chen. 1981. Detection of phosphotyrosine-containing 34,000-dalton protein in the framework of cells transformed with Rous sarcoma virus. Proc. Natl. Acad. Sci. USA 782388-2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheung, A., L. Young, P. Chen, C. Chao, A. Ndoye, P. Barry, W. Muller, and R. Cardiff. 1997. Microcirculation and metastasis in a new mouse mammary tumor model system. Int. J. Oncol. 1169-77. [PubMed] [Google Scholar]
- 55.Cho, U. S., S. Morrone, A. A. Sablina, J. D. Arroyo, W. C. Hahn, and W. Xu. 2007. Structural basis of PP2A inhibition by small t antigen. PLoS Biol. 5e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cohen, B., Y. X. Liu, B. Druker, T. M. Roberts, and B. S. Schaffhausen. 1990. Characterization of pp85, a target of oncogenes and growth factor receptors. Mol. Cell. Biol. 102909-2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coleman, S., G. B. Silberstein, and C. W. Daniel. 1988. Ductal morphogenesis in the mouse mammary gland: evidence supporting a role for epidermal growth factor. Dev. Biol. 127304-315. [DOI] [PubMed] [Google Scholar]
- 58.Connolly, J. O., N. Soga, X. L. Guo, U. Alvarez, and K. A. Hruska. 2000. Rac is essential in the transformation of endothelial cells by polyoma middle T. Cell Adhes. Commun. 7409-422. [DOI] [PubMed] [Google Scholar]
- 59.Cook, D. N., and J. A. Hassell. 1990. The amino terminus of polyomavirus middle T antigen is required for transformation. J. Virol. 641879-1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cook, D. N., N. Pavloff, and J. A. Hassell. 1990. Simultaneous overexpression of avian pp60c-src and polyomavirus middle T antigen in mammalian cells. J. Virol. 642392-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cook, S. J., N. Aziz, and M. McMahon. 1999. The repertoire of Fos and Jun proteins expressed during the G1 phase of the cell cycle is determined by the duration of mitogen-activated protein kinase activation. Mol. Cell. Biol. 19330-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cooper, J. A., K. L. Gould, C. A. Cartwright, and T. Hunter. 1986. Tyr527 is phosphorylated in pp60c-src: implications for regulation. Science 2311431-1434. [DOI] [PubMed] [Google Scholar]
- 63.Courtneidge, S., and A. E. Smith. 1983. Polyoma virus transforming protein associates with the product of the c-src cellular gene. Nature 303435-439. [DOI] [PubMed] [Google Scholar]
- 64.Courtneidge, S. A. 1985. Activation of the pp60c-src kinase by middle T antigen binding or by dephosphorylation. EMBO J. 41471-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Courtneidge, S. A., L. Goutebroze, A. Cartwright, A. Heber, S. Scherneck, and J. Feunteun. 1991. Identification and characterization of the hamster polyomavirus middle T antigen. J. Virol. 653301-3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Courtneidge, S. A., and A. Heber. 1987. An 81kd protein complexed with middle T antigen and pp60c-src: a possible phosphatidylinositol kinase. Cell 501031-1037. [DOI] [PubMed] [Google Scholar]
- 67.Cozma, D., L. Lukes, J. Rouse, T. H. Qiu, E. T. Liu, and K. W. Hunter. 2002. A bioinformatics-based strategy identifies c-Myc and Cdc25A as candidates for the Apmt mammary tumor latency modifiers. Genome Res. 12969-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crawford, N. P., J. Alsarraj, L. Lukes, R. C. Walker, J. S. Officewala, H. H. Yang, M. P. Lee, K. Ozato, and K. W. Hunter. 2008. Bromodomain 4 activation predicts breast cancer survival. Proc. Natl. Acad. Sci. USA 1056380-6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Crawford, N. P., X. Qian, A. Ziogas, A. G. Papageorge, B. J. Boersma, R. C. Walker, L. Lukes, W. L. Rowe, J. Zhang, S. Ambs, D. R. Lowy, H. Anton-Culver, and K. W. Hunter. 2007. Rrp1b, a new candidate susceptibility gene for breast cancer progression and metastasis. PLoS Genet. 3e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Crawford, N. P., A. Ziogas, D. J. Peel, J. Hess, H. Anton-Culver, and K. W. Hunter. 2006. Germline polymorphisms in SIPA1 are associated with metastasis and other indicators of poor prognosis in breast cancer. Breast Cancer Res. 8R16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cullere, X., P. Rose, U. Thathamangalam, A. Chatterjee, K. P. Mullane, D. C. Pallas, T. L. Benjamin, T. M. Roberts, and B. S. Schaffhausen. 1998. Serine 257 phosphorylation regulates association of polyomavirus middle T antigen with 14-3-3 proteins. J. Virol. 72558-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dahl, J., A. Jurczak, L. Cheng, D. Baker, and T. Benjamin. 1998. Evidence of a role for phosphatidylinositol 3-kinase activation in the blocking of apoptosis by polyomavirus middle T antigen. J. Virol. 723221-3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dahl, J., U. Thathamangalam, R. Freund, and T. L. Benjamin. 1992. Functional asymmetry of the regions juxtaposed to the membrane-binding sequence of polyomavirus middle T antigen. Mol. Cell. Biol. 125050-5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Damonte, P., J. G. Hodgson, J. Q. Chen, L. J. Young, R. D. Cardiff, and A. D. Borowsky. 2008. Mammary carcinoma behavior is programmed in the precancer stem cell. Breast Cancer Res. 10R50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Das, R., G. H. Mahabeleshwar, and G. C. Kundu. 2004. Osteopontin induces AP-1-mediated secretion of urokinase-type plasminogen activator through c-Src-dependent epidermal growth factor receptor transactivation in breast cancer cells. J. Biol. Chem. 27911051-11064. [DOI] [PubMed] [Google Scholar]
- 76.Davie, S. A., J. E. Maglione, C. K. Manner, D. Young, R. D. Cardiff, C. L. MacLeod, and L. G. Ellies. 2007. Effects of FVB/NJ and C57Bl/6J strain backgrounds on mammary tumor phenotype in inducible nitric oxide synthase deficient mice. Transgenic Res. 16193-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dawe, C. J. 1960. Cell sensitivity and specificity of response to polyoma virus. Natl. Cancer Inst. Monogr. 467-128. [PubMed] [Google Scholar]
- 78.Dawe, C. J., R. Freund, G. Mandel, K. Ballmer-Hofer, D. A. Talmage, and T. L. Benjamin. 1987. Variations in polyoma virus genotype in relation to tumor induction in mice. Characterization of wild type strains with widely differing tumor profiles. Am. J. Pathol. 127243-261. [PMC free article] [PubMed] [Google Scholar]
- 79.Deininger, P. L., A. Esty, P. LaPorte, H. Hsu, and T. Friedmann. 1980. The nucleotide sequence and restriction enzyme sites of the polyoma genome. Nucleic Acids Res. 8855. [PMC free article] [PubMed] [Google Scholar]
- 80.Delmas, V., C. Bastien, S. Scherneck, and J. Feunteun. 1985. A new member of the polyomavirus family: the hamster papovavirus. Complete nucleotide sequence and transformation properties. EMBO J. 41279-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Desai, K. V., N. Xiao, W. Wang, L. Gangi, J. Greene, J. I. Powell, R. Dickson, P. Furth, K. Hunter, R. Kucherlapati, R. Simon, E. T. Liu, and J. E. Green. 2002. Initiating oncogenic event determines gene-expression patterns of human breast cancer models. Proc. Natl. Acad. Sci. USA 996967-6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.de Villiers, J., W. Schaffner, C. Tyndall, S. Lupton, and R. Kamen. 1984. Polyoma virus DNA replication requires an enhancer. Nature 312242-246. [DOI] [PubMed] [Google Scholar]
- 83.Dey, A., J. Ellenberg, A. Farina, A. E. Coleman, T. Maruyama, S. Sciortino, J. Lippincott-Schwartz, and K. Ozato. 2000. A bromodomain protein, MCAP, associates with mitotic chromosomes and affects G2-to-M transition. Mol. Cell. Biol. 206537-6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dilworth, S. M. 2002. Polyoma virus middle T antigen and its role in identifying cancer-related molecules. Nat. Rev. Cancer. 2951-956. [DOI] [PubMed] [Google Scholar]
- 85.Dilworth, S. M. 1995. Polyoma virus middle T antigen: meddler or mimic? Trends Microbiol. 331-35. [DOI] [PubMed] [Google Scholar]
- 86.Dilworth, S. M., C. E. Brewster, M. D. Jones, L. Lanfrancone, G. Pelicci, and P. G. Pelicci. 1994. Transformation by polyoma virus middle T-antigen involves the binding and tyrosine phosphorylation of Shc. Nature 36787-90. [DOI] [PubMed] [Google Scholar]
- 87.Dilworth, S. M., H. A. Hansson, C. Darnfors, G. Bjursell, C. H. Streuli, and B. E. Griffin. 1986. Subcellular localisation of the middle and large T-antigens of polyoma. EMBO J. 5491-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.DiMaio, D., and D. Mattoon. 2001. Mechanisms of cell transformation by papillomavirus E5 proteins. Oncogene 207866-7873. [DOI] [PubMed] [Google Scholar]
- 89.Druker, B. J., L. E. Ling, B. Cohen, T. M. Roberts, and B. S. Schaffhausen. 1990. A completely transformation-defective point mutant of polyomavirus middle T antigen which retains full associated phosphatidylinositol kinase activity. J. Virol. 644454-4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Druker, B. J., L. Sibert, and T. M. Roberts. 1992. Polyomavirus middle T-antigen NPTY mutants. J. Virol. 665770-5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Du, Z., and Y. Li. 2007. RCAS-TVA in the mammary gland: an in vivo oncogene screen and a high fidelity model for breast transformation? Cell Cycle 6823-826. [DOI] [PubMed] [Google Scholar]
- 92.Du, Z., K. Podsypanina, S. Huang, A. McGrath, M. J. Toneff, E. Bogoslovskaia, X. Zhang, R. C. Moraes, M. Fluck, D. C. Allred, M. T. Lewis, H. E. Varmus, and Y. Li. 2006. Introduction of oncogenes into mammary glands in vivo with an avian retroviral vector initiates and promotes carcinogenesis in mouse models. Proc. Natl. Acad. Sci. USA 10317396-17401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dunant, N. M., M. Senften, and K. Ballmer-Hofer. 1996. Polyomavirus middle-T antigen associates with the kinase domain of Src-related tyrosine kinases. J. Virol. 701323-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Duronio, V. 2008. The life of a cell: apoptosis regulation by the PI3K/PKB pathway. Biochem. J. 415333-344. [DOI] [PubMed] [Google Scholar]
- 95.Eckhart, W., M. A. Hutchinson, and T. Hunter. 1979. An activity phosphorylating tyrosine in polyoma T antigen immunoprecipitates. Cell 18925. [DOI] [PubMed] [Google Scholar]
- 96.Eddy, B. 1969. Polyoma virus. Virol. Monogr. 71-114. [Google Scholar]
- 97.Eichhorn, P. J., M. P. Creyghton, and R. Bernards. 2009. Protein phosphatase 2A regulatory subunits and cancer. Biochim. Biophys. Acta 17951-15. [DOI] [PubMed] [Google Scholar]
- 98.Ellies, L. G. 2003. PyV-mT-induced parotid gland hyperplasia as detected by altered lectin reactivity is not modulated by inducible nitric oxide deficiency. Arch. Oral Biol. 48415-422. [DOI] [PubMed] [Google Scholar]
- 99.Elliott, J., M. D. Jones, B. E. Griffin, and N. Krauzewicz. 1998. Regulation of cytoskeletal association by a basic amino acid motif in polyoma virus middle T antigen. Oncogene 171797-1806. [DOI] [PubMed] [Google Scholar]
- 100.El-Tanani, M. K., F. C. Campbell, V. Kurisetty, D. Jin, M. McCann, and P. S. Rudland. 2006. The regulation and role of osteopontin in malignant transformation and cancer. Cytokine Growth Factor Rev. 17463-474. [DOI] [PubMed] [Google Scholar]
- 101.Escobedo, J. A., D. R. Kaplan, W. M. Kavanaugh, C. W. Turck, and L. T. Williams. 1991. A phosphatidylinositol-3 kinase binds to platelet-derived growth factor receptors through a specific receptor sequence containing phosphotyrosine. Mol. Cell. Biol. 111125-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Farina, A., M. Hattori, J. Qin, Y. Nakatani, N. Minato, and K. Ozato. 2004. Bromodomain protein Brd4 binds to GTPase-activating SPA-1, modulating its activity and subcellular localization. Mol. Cell. Biol. 249059-9069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fisher, G. H., S. Orsulic, E. Holland, W. P. Hively, Y. Li, B. C. Lewis, B. O. Williams, and H. E. Varmus. 1999. Development of a flexible and specific gene delivery system for production of murine tumor models. Oncogene 185253-5260. [DOI] [PubMed] [Google Scholar]
- 104.Fluck, M. 1997. Tumor studies with polyomaviruses, p. 1926-1939. In J. R. Bertino (ed.), Encyclopedia of cancer, vol. III. Academic Press, San Diego, CA.
- 105.Fluck, M. M., and S. Z. Haslam. 1996. Mammary tumors induced by polyomavirus. Breast Cancer Res. Treat. 3945-56. [DOI] [PubMed] [Google Scholar]
- 106.Forrester, E., A. Chytil, B. Bierie, M. Aakre, A. E. Gorska, A. R. Sharif-Afshar, W. J. Muller, and H. L. Moses. 2005. Effect of conditional knockout of the type II TGF-beta receptor gene in mammary epithelia on mammary gland development and polyomavirus middle T antigen induced tumor formation and metastasis. Cancer Res. 652296-2302. [DOI] [PubMed] [Google Scholar]
- 107.Foukas, L. C., M. Claret, W. Pearce, K. Okkenhaug, S. Meek, E. Peskett, S. Sancho, A. J. Smith, D. J. Withers, and B. Vanhaesebroeck. 2006. Critical role for the p110α phosphoinositide-3-OH kinase in growth and metabolic regulation. Nature 441366-370. [DOI] [PubMed] [Google Scholar]
- 108.Freund, R., C. J. Dawe, J. P. Carroll, and T. L. Benjamin. 1992. Changes in frequency, morphology, and behavior of tumors induced in mice by a polyoma virus mutant with a specifically altered oncogene. Am. J. Pathol. 1411409-1425. [PMC free article] [PubMed] [Google Scholar]
- 109.Freund, R., A. Sotnikov, R. T. Bronson, and T. L. Benjamin. 1992. Polyoma virus middle T is essential for virus replication and persistence as well as for tumor induction in mice. Virology 191716-723. [DOI] [PubMed] [Google Scholar]
- 110.Fruman, D. A., R. E. Meyers, and L. C. Cantley. 1998. Phosphoinositide kinases. Annu. Rev. Biochem. 67481-507. [DOI] [PubMed] [Google Scholar]
- 111.Galang, C. K., J. Garcia-Ramirez, P. A. Solski, J. K. Westwick, C. J. Der, N. N. Neznanov, R. G. Oshima, and C. A. Hauser. 1996. Oncogenic Neu/ErbB-2 increases ets, AP-1, and NF-κB-dependent gene expression, and inhibiting ets activation blocks Neu-mediated cellular transformation. J. Biol. Chem. 2717992-7998. [DOI] [PubMed] [Google Scholar]
- 112.Galang, C. K., W. J. Muller, G. Foos, R. G. Oshima, and C. A. Hauser. 2004. Changes in the expression of many Ets family transcription factors and of potential target genes in normal mammary tissue and tumors. J. Biol. Chem. 27911281-11292. [DOI] [PubMed] [Google Scholar]
- 113.Garcea, R., and T. Benjamin. 1983. Host range transforming gene of polyomavirus plays a role in virus assembly. Proc. Natl. Acad. Sci. USA 803413-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Garcea, R. L., D. A. Talmage, A. Harmatz, R. Freund, and T. L. Benjamin. 1989. Separation of host range from transformation functions of the hr-t gene of polyomavirus. Virology 168312-319. [DOI] [PubMed] [Google Scholar]
- 115.Garcia, R., C. L. Yu, A. Hudnall, R. Catlett, K. L. Nelson, T. Smithgall, D. J. Fujita, S. P. Ethier, and R. Jove. 1997. Constitutive activation of Stat3 in fibroblasts transformed by diverse oncoproteins and in breast carcinoma cells. Cell Growth Differ. 81267-1276. [PubMed] [Google Scholar]
- 116.Glenn, G. M., and W. Eckhart. 1995. Amino-terminal regions of polyomavirus middle T antigen are required for interactions with protein phosphatase 2A. J. Virol. 693729-3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Glenn, G. M., and W. Eckhart. 1993. Mutation of a cysteine residue in polyomavirus middle T antigen abolishes interactions with protein phosphatase 2A, pp60c-src, and phosphatidylinositol-3 kinase, activation of c-fos expression, and cellular transformation. J. Virol. 671945-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Glover, H. R., C. E. Brewster, and S. M. Dilworth. 1999. Association between src-kinases and the polyoma virus oncogene middle T-antigen requires PP2A and a specific sequence motif. Oncogene 184364-4370. [DOI] [PubMed] [Google Scholar]
- 119.Gorga, F. R., C. E. Riney, and T. L. Benjamin. 1990. Inositol trisphosphate levels in cells expressing wild-type and mutant polyomavirus middle T antigens: evidence for activation of phospholipase C via activation of pp60c-src. J. Virol. 64105-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gotoh, N., A. Tojo, and M. Shibuya. 1996. A novel pathway from phosphorylation of tyrosine residues 239/240 of Shc, contributing to suppress apoptosis by IL-3. EMBO J. 156197-6204. [PMC free article] [PubMed] [Google Scholar]
- 121.Gottlieb, K. A., and L. P. Villarreal. 2001. Natural biology of polyomavirus middle T antigen. Microbiol. Mol. Biol. Rev. 65288-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Goutebroze, L., N. M. Dunant, K. Ballmer-Hofer, and J. Feunteun. 1997. The N terminus of hamster polyomavirus middle T antigen carries a determinant for specific activation of p59c-Fyn. J. Virol. 711436-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gross, L. 1953. A filterable agent, recovered from AK leukemic extracts, causing salivary gland carcinomas in C3H mice. Proc. Soc. Exp. Biol. Med. 83414. [DOI] [PubMed] [Google Scholar]
- 124.Guillermet-Guibert, J., K. Bjorklof, A. Salpekar, C. Gonella, F. Ramadani, A. Bilancio, S. Meek, A. J. Smith, K. Okkenhaug, and B. Vanhaesebroeck. 2008. The p110β isoform of phosphoinositide 3-kinase signals downstream of G protein-coupled receptors and is functionally redundant with p110gamma. Proc. Natl. Acad. Sci. USA 1058292-8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gunther, U., T. Mittag, and B. Schaffhausen. 2002. Probing Src homology 2 domain ligand interactions by differential line broadening. Biochemistry 4111658-11669. [DOI] [PubMed] [Google Scholar]
- 126.Guy, C. T., R. D. Cardiff, and W. J. Muller. 1992. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol. Cell. Biol. 12954-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Guy, C. T., S. K. Muthuswamy, R. D. Cardiff, P. Soriano, and W. J. Muller. 1994. Activation of the c-Src tyrosine kinase is required for the induction of mammary tumors in transgenic mice. Genes Dev. 823-32. [DOI] [PubMed] [Google Scholar]
- 128.Harvey, R., G. L. Oostra, G. J. Belsham, P. Gillett, and A. E. Smith. 1984. An antibody to a synthetic peptide recognizes polyoma virus middle T antigen and reveals multiple in vitro tyrosine phosphorylation sites. Mol. Cell. Biol. 41334-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Haslam, S. Z., J. J. Wirth, L. J. Counterman, and M. M. Fluck. 1992. Characterization of the mammary hyperplasia, dysplasia and neoplasia induced in athymic female adult mice by polyomavirus. Oncogene 71295-1303. [PubMed] [Google Scholar]
- 130.Hazzalin, C. A., and L. C. Mahadevan. 2002. MAPK-regulated transcription: a continuously variable gene switch? Nat. Rev. Mol. Cell Biol. 330-40. [DOI] [PubMed] [Google Scholar]
- 131.Heron-Milhavet, L., D. Mamaeva, A. Rochat, N. J. Lamb, and A. Fernandez. 2008. Akt2 is implicated in skeletal muscle differentiation and specifically binds prohibitin2/REA. J. Cell Physiol. 214158-165. [DOI] [PubMed] [Google Scholar]
- 132.Herschkowitz, J. I., K. Simin, V. J. Weigman, I. Mikaelian, J. Usary, Z. Hu, K. E. Rasmussen, L. P. Jones, S. Assefnia, S. Chandrasekharan, M. G. Backlund, Y. Yin, A. I. Khramtsov, R. Bastein, J. Quackenbush, R. I. Glazer, P. H. Brown, J. E. Green, L. Kopelovich, P. A. Furth, J. P. Palazzo, O. I. Olopade, P. S. Bernard, G. A. Churchill, T. Van Dyke, and C. M. Perou. 2007. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 8R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hiles, I. D., M. Otsu, S. Volinia, M. J. Fry, I. Gout, R. Dhand, G. Panayotou, F. Ruiz-Larrea, A. Thompson, N. Totty, J. Hsuan, S. Courtneidge, P. Parker, and M. Waterfield. 1992. Phosphatidylinositol 3-kinase: structure and expression of the 110 kd catalytic subunit. Cell 70419-429. [DOI] [PubMed] [Google Scholar]
- 134.Hodgson, J. G., T. Malek, S. Bornstein, S. Hariono, D. G. Ginzinger, W. J. Muller, and J. W. Gray. 2005. Copy number aberrations in mouse breast tumors reveal loci and genes important in tumorigenic receptor tyrosine kinase signaling. Cancer Res. 659695-9704. [DOI] [PubMed] [Google Scholar]
- 135.Hollenhorst, P. C., A. A. Shah, C. Hopkins, and B. J. Graves. 2007. Genome-wide analyses reveal properties of redundant and specific promoter occupancy within the ETS gene family. Genes Dev. 211882-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hong, J. H., E. S. Hwang, M. T. McManus, A. Amsterdam, Y. Tian, R. Kalmukova, E. Mueller, T. Benjamin, B. M. Spiegelman, P. A. Sharp, N. Hopkins, and M. B. Yaffe. 2005. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science 3091074-1078. [DOI] [PubMed] [Google Scholar]
- 137.Horak, I. D., T. Kawakami, F. Gregory, K. C. Robbins, and J. B. Bolen. 1989. Association of p60fyn with middle tumor antigen in murine polyomavirus-transformed rat cells. J. Virol. 632343-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hu, P., B. Margolis, E. Y. Skolnik, R. Lammers, A. Ullrich, and J. Schlessinger. 1992. Interaction of phosphatidylinositol 3-kinase-associated p85 with epidermal growth factor and platelet-derived growth factor receptors. Mol. Cell. Biol. 12981-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hu, P., A. Mondino, E. Y. Skolnik, and J. Schlessinger. 1993. Cloning of a novel, ubiquitously expressed human phosphatidylinositol 3-kinase and identification of its binding site on p85. Mol. Cell. Biol. 137677-7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hunter, K. W., K. W. Broman, T. L. Voyer, L. Lukes, D. Cozma, M. T. Debies, J. Rouse, and D. R. Welch. 2001. Predisposition to efficient mammary tumor metastatic progression is linked to the breast cancer metastasis suppressor gene Brms1. Cancer Res. 618866-8872. [PubMed] [Google Scholar]
- 141.Hunter, T., M. A. Hutchinson, and W. Eckhart. 1984. Polyoma middle-T antigen can be phosphorylated on tyrosine at multiple sites in vitro. EMBO J. 373-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hunter, T., and B. Sefton. 1980. The transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc. Natl. Acad. Sci. USA 771311-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Husemann, Y., J. B. Geigl, F. Schubert, P. Musiani, M. Meyer, E. Burghart, G. Forni, R. Eils, T. Fehm, G. Riethmuller, and C. A. Klein. 2008. Systemic spread is an early step in breast cancer. Cancer Cell 1358-68. [DOI] [PubMed] [Google Scholar]
- 144.Ichaso, N., and S. M. Dilworth. 2001. Cell transformation by the middle T-antigen of polyoma virus. Oncogene 207908-7916. [DOI] [PubMed] [Google Scholar]
- 145.Isakoff, S. J., J. A. Engelman, H. Y. Irie, J. Luo, S. M. Brachmann, R. V. Pearline, L. C. Cantley, and J. S. Brugge. 2005. Breast cancer-associated PIK3CA mutations are oncogenic in mammary epithelial cells. Cancer Res. 6510992-11000. [DOI] [PubMed] [Google Scholar]
- 146.Ito, Y. 1979. Polyoma virus-specific 55K protein isolated from plasma membrane of productively infected cells is virus-coded and important cell transformation. Virology 98261-266. [DOI] [PubMed] [Google Scholar]
- 147.Ito, Y., J. R. Brocklehurst, and R. Dulbecco. 1977. Virus-specific proteins in the plasma membrane of cells lytically infected or transformed by polyoma virus. Proc. Natl. Acad. Sci. USA 744666-4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Janssens, V., and J. Goris. 2001. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem. J. 353417-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Janssens, V., J. Goris, and C. Van Hoof. 2005. PP2A: the expected tumor suppressor. Curr. Opin. Genet. Dev. 1534-41. [DOI] [PubMed] [Google Scholar]
- 150.Jessen, K. A., S. Y. Liu, C. G. Tepper, J. Karrim, E. T. McGoldrick, A. Rosner, R. J. Munn, L. J. Young, A. D. Borowsky, R. D. Cardiff, and J. P. Gregg. 2004. Molecular analysis of metastasis in a polyomavirus middle T mouse model: the role of osteopontin. Breast Cancer Res. 6R157-R169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jia, S., Z. Liu, S. Zhang, P. Liu, L. Zhang, S. H. Lee, J. Zhang, S. Signoretti, M. Loda, T. M. Roberts, and J. J. Zhao. 2008. Essential roles of PI(3)K-p110β in cell growth, metabolism and tumorigenesis. Nature 454776-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Johnston, I. M., H. J. Spence, J. N. Winnie, L. McGarry, J. K. Vass, L. Meagher, G. Stapleton, and B. W. Ozanne. 2000. Regulation of a multigenic invasion programme by the transcription factor, AP-1: re-expression of a down-regulated gene, TSC-36, inhibits invasion. Oncogene 195348-5358. [DOI] [PubMed] [Google Scholar]
- 153.Junttila, M. R., P. Puustinen, M. Niemela, R. Ahola, H. Arnold, T. Bottzauw, R. Ala-aho, C. Nielsen, J. Ivaska, Y. Taya, S. L. Lu, S. Lin, E. K. Chan, X. J. Wang, R. Grenman, J. Kast, T. Kallunki, R. Sears, V. M. Kahari, and J. Westermarck. 2007. CIP2A inhibits PP2A in human malignancies. Cell 13051-62. [DOI] [PubMed] [Google Scholar]
- 154.Kang, S., A. G. Bader, and P. K. Vogt. 2005. Phosphatidylinositol 3-kinase mutations identified in human cancer are oncogenic. Proc. Natl. Acad. Sci. USA 102802-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kaplan, D. R., M. Whitman, B. Schaffhausen, D. C. Pallas, M. White, L. Cantley, and T. M. Roberts. 1987. Common elements in growth factor stimulation and oncogenic transformation: 85 kd phosphoprotein and phosphatidylinositol kinase activity. Cell 501021-1029. [DOI] [PubMed] [Google Scholar]
- 156.Kaplan, D. R., M. Whitman, B. Schaffhausen, L. Raptis, R. L. Garcea, D. Pallas, T. M. Roberts, and L. Cantley. 1986. Phosphatidylinositol metabolism and polyoma-mediated transformation. Proc. Natl. Acad. Sci. USA 833624-3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kelley, W. L., and C. Georgopoulos. 1997. The T/t common exon of simian virus 40, JC, and BK polyomavirus T antigens can functionally replace the J.-domain of the Escherichia coli DnaJ molecular chaperone. Proc. Natl. Acad. Sci. USA 943679-3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kennedy, A. P., A. Sekulic, B. J. Irvin, A. E. Nilson, S. M. Dilworth, and R. T. Abraham. 1998. Polyomavirus middle T antigen as a probe for T cell antigen receptor-coupled signaling pathways. J. Biol. Chem. 27311505-11513. [DOI] [PubMed] [Google Scholar]
- 159.Kiefer, F., I. Anhauser, P. Soriano, A. Aguzzi, S. A. Courtneidge, and E. F. Wagner. 1994. Endothelial cell transformation by polyomavirus middle T antigen in mice lacking Src-related kinases. Curr. Biol. 4100-109. [DOI] [PubMed] [Google Scholar]
- 160.Kim, P. K., F. Janiak-Spens, W. S. Trimble, B. Leber, and D. W. Andrews. 1997. Evidence for multiple mechanisms for membrane binding and integration via carboxyl-terminal insertion sequences. Biochemistry 368873-8882. [DOI] [PubMed] [Google Scholar]
- 161.Kmiecik, T. E., and D. Shalloway. 1987. Activation and suppression of pp60c-src transforming ability by mutation of its primary sites of tyrosine phosphorylation. Cell 4965-73. [DOI] [PubMed] [Google Scholar]
- 162.Kornbluth, S., F. R. Cross, M. Harbison, and H. Hanafusa. 1986. Transformation of chicken embryo fibroblasts and tumor induction by the middle T antigen of polyomavirus carried in an avian retroviral vector. Mol. Cell. Biol. 61545-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kornbluth, S., M. Sudol, and H. Hanafusa. 1987. Association of the polyomavirus middle-T antigen with c-yes protein. Nature 325171-173. [DOI] [PubMed] [Google Scholar]
- 164.Krauzewicz, N., J. Elliott, and B. E. Griffin. 1994. Cell fractionation in non-ionic detergent distinguishes sub-populations of polyoma virus middle T antigen and reveals a novel form. Oncogene 92283-2291. [PubMed] [Google Scholar]
- 165.Kurpios, N. A., N. A. Sabolic, T. G. Shepherd, G. M. Fidalgo, and J. A. Hassell. 2003. Function of PEA3 Ets transcription factors in mammary gland development and oncogenesis. J. Mammary Gland Biol. Neoplasia. 8177-190. [DOI] [PubMed] [Google Scholar]
- 166.Lancaster, M., J. Rouse, and K. W. Hunter. 2005. Modifiers of mammary tumor progression and metastasis on mouse chromosomes 7, 9, and 17. Mamm. Genome 16120-126. [DOI] [PubMed] [Google Scholar]
- 167.Land, H., L. F. Parada, and R. A. Weinberg. 1983. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature 304596-602. [DOI] [PubMed] [Google Scholar]
- 168.Lei, Q. Y., H. Zhang, B. Zhao, Z. Y. Zha, F. Bai, X. H. Pei, S. Zhao, Y. Xiong, and K. L. Guan. 2008. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol. Cell. Biol. 282426-2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Le Voyer, T., Z. Lu, J. Babb, T. Lifsted, M. Williams, and K. Hunter. 2000. An epistatic interaction controls the latency of a transgene-induced mammary tumor. Mamm. Genome 11883-889. [DOI] [PubMed] [Google Scholar]
- 170.Lewis, B. C., D. S. Klimstra, N. D. Socci, S. Xu, J. A. Koutcher, and H. E. Varmus. 2005. The absence of p53 promotes metastasis in a novel somatic mouse model for hepatocellular carcinoma. Mol. Cell. Biol. 251228-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lewis, B. C., D. S. Klimstra, and H. E. Varmus. 2003. The c-myc and PyMT oncogenes induce different tumor types in a somatic mouse model for pancreatic cancer. Genes Dev. 173127-3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Li, M., and R. L. Garcea. 1994. Identification of the threonine phosphorylation sites on the polyomavirus major capsid protein VP1: relationship to the activity of middle T antigen. J. Virol. 68320-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lifsted, T., T. Le Voyer, M. Williams, W. Muller, A. Klein-Szanto, K. H. Buetow, and K. W. Hunter. 1998. Identification of inbred mouse strains harboring genetic modifiers of mammary tumor age of onset and metastatic progression. Int. J. Cancer 77640-644. [DOI] [PubMed] [Google Scholar]
- 174.Lin, E. Y., J. G. Jones, P. Li, L. Zhu, K. D. Whitney, W. J. Muller, and J. W. Pollard. 2003. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am. J. Pathol. 1632113-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lin, E. Y., J. F. Li, G. Bricard, W. Wang, Y. Deng, R. Sellers, S. A. Porcelli, and J. W. Pollard. 2007. VEGF restores delayed tumor progression in tumors depleted of macrophages. Mol. Oncol. 1288-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lin, E. Y., A. V. Nguyen, R. G. Russell, and J. W. Pollard. 2001. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J. Exp. Med. 193727-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Louie, R. R., C. S. King, A. MacAuley, J. D. Marth, R. M. Perlmutter, W. Eckhart, and J. A. Cooper. 1988. p56lck protein-tyrosine kinase is cytoskeletal and does not bind to polyomavirus middle T antigen. J. Virol. 624673-4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lukacher, A., Y. Ma, J. Carroll, S. Abromson-Leeman, J. Laning, M. Dorf, and T. Benjamin. 1995. Susceptibility to tumors induced by polyoma virus is conferred by an endogenous mouse mammary tumor virus superantigen. J. Exp. Med. 1811683-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Maglione, J. E., E. T. McGoldrick, L. J. Young, R. Namba, J. P. Gregg, L. Liu, D. Moghanaki, L. G. Ellies, A. D. Borowsky, R. D. Cardiff, and C. L. MacLeod. 2004. Polyomavirus middle T-induced mammary intraepithelial neoplasia outgrowths: single origin, divergent evolution, and multiple outcomes. Mol. Cancer Ther. 3941-953. [PubMed] [Google Scholar]
- 180.Maglione, J. E., D. Moghanaki, L. J. Young, C. K. Manner, L. G. Ellies, S. O. Joseph, B. Nicholson, R. D. Cardiff, and C. L. MacLeod. 2001. Transgenic Polyoma middle-T mice model premalignant mammary disease. Cancer Res. 618298-8305. [PubMed] [Google Scholar]
- 181.Magnusson, G., and P. Berg. 1979. Construction and analysis of viable deletion mutants of polyoma virus. J. Virol. 32523-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Magnusson, G., M. Nilsson, S. Dilworth, and N. Smolar. 1981. Characterization of polyoma mutants with altered middle T and large T antigens. J. Virol. 39673-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Malmgren, R. A., A. S. Rabson, and P. G. Carney. 1964. Immunity and viral carcinogenesis. effect of thymectomy on polyoma virus carcinogenesis in mice. J. Natl. Cancer Inst. 33101-104. [PubMed] [Google Scholar]
- 184.Man, A. K., L. J. Young, J. A. Tynan, J. Lesperance, M. Egeblad, Z. Werb, C. A. Hauser, W. J. Muller, R. D. Cardiff, and R. G. Oshima. 2003. Ets2-dependent stromal regulation of mouse mammary tumors. Mol. Cell. Biol. 238614-8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Markland, W., C. S. H., B. A. Oostra, and A. E. Smith. 1986. In vitro mutagenesis of the putative membrane-binding domain of polyomavirus middle-T antigen. J. Virol. 5982-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Maroulakou, I. G., W. Oemler, S. P. Naber, and P. N. Tsichlis. 2007. Akt1 ablation inhibits, whereas Akt2 ablation accelerates, the development of mammary adenocarcinomas in mouse mammary tumor virus (MMTV)-ErbB2/neu and MMTV-polyoma middle T transgenic mice. Cancer Res. 67167-177. [DOI] [PubMed] [Google Scholar]
- 187.Martens, I., S. A. Nilsson, S. Linder, and G. Magnusson. 1989. Mutational analysis of polyomavirus small-T-antigen functions in productive infection and in transformation. J. Virol. 632126-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.McKenna, L. B., G. L. Zhou, and J. Field. 2007. Isoform-specific functions of Akt in cell motility. Cell Mol. Life Sci. 642723-2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Meili, R., P. Cron, B. Hemmings, and K. Ballmer-Hofer. 1998. Protein kinase B/Akt is activated by polyomavirus middle-T antigen via a phosphatidylinositol 3-kinase-dependent mechanism. Oncogene 16903-907. [DOI] [PubMed] [Google Scholar]
- 190.Miller, J. F. 1964. Influence of thymectomy on tumor induction by polyoma virus in C57bl mice. Proc. Soc. Exp. Biol. Med. 116323-327. [DOI] [PubMed] [Google Scholar]
- 191.Millward, T. A., S. Zolnierowicz, and B. A. Hemmings. 1999. Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem. Sci. 24186-191. [DOI] [PubMed] [Google Scholar]
- 192.Mittag, T., B. Schaffhausen, and U. L. Gunther. 2004. Tracing kinetic intermediates during ligand binding. J. Am. Chem. Soc. 1269017-9023. [DOI] [PubMed] [Google Scholar]
- 193.Montagna, C., M. S. Lyu, K. Hunter, L. Lukes, W. Lowther, T. Reppert, B. Hissong, Z. Weaver, and T. Ried. 2003. The septin 9 (MSF) gene is amplified and overexpressed in mouse mammary gland adenocarcinomas and human breast cancer cell lines. Cancer Res. 632179-2187. [PubMed] [Google Scholar]
- 194.Moule, M. G., C. H. Collins, F. McCormick, and M. Fried. 2004. Role for PP2A in ARF signaling to p53. Proc. Natl. Acad. Sci. USA 10114063-14066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mueller, C., W. Muller, and J. Hassell. 1988. The polyomavirus enhancer comprises multiple functional elements. J. Virol. 621667-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mukherjee, P., C. S. Madsen, A. R. Ginardi, T. L. Tinder, F. Jacobs, J. Parker, B. Agrawal, B. M. Longenecker, and S. J. Gendler. 2003. Mucin 1-specific immunotherapy in a mouse model of spontaneous breast cancer. J. Immunother. 2647-62. [DOI] [PubMed] [Google Scholar]
- 197.Mullane, K. P., M. Ratnofsky, X. Cullere, and B. Schaffhausen. 1998. Signaling from polyomavirus middle T and small T defines different roles for protein phosphatase 2A. Mol. Cell. Biol. 187556-7565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mumby, M. 2007. PP2A: unveiling a reluctant tumor suppressor. Cell 13021-24. [DOI] [PubMed] [Google Scholar]
- 199.Munoz, B., and F. F. Bolander, Jr. 1989. Prolactin regulation of mouse mammary tumor virus (MMTV) expression in normal mouse mammary epithelium. Mol. Cell. Endocrinol. 6223-29. [DOI] [PubMed] [Google Scholar]
- 200.Muraoka, R. S., N. Dumont, C. A. Ritter, T. C. Dugger, D. M. Brantley, J. Chen, E. Easterly, L. R. Roebuck, S. Ryan, P. J. Gotwals, V. Koteliansky, and C. L. Arteaga. 2002. Blockade of TGF-beta inhibits mammary tumor cell viability, migration, and metastases. J. Clin. Invest. 1091551-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Muraoka-Cook, R. S., H. Kurokawa, Y. Koh, J. T. Forbes, L. R. Roebuck, M. H. Barcellos-Hoff, S. E. Moody, L. A. Chodosh, and C. L. Arteaga. 2004. Conditional overexpression of active transforming growth factor β1 in vivo accelerates metastases of transgenic mammary tumors. Cancer Res. 649002-9011. [DOI] [PubMed] [Google Scholar]
- 202.Nagle, J. A., Z. Ma, M. A. Byrne, M. F. White, and L. M. Shaw. 2004. Involvement of insulin receptor substrate 2 in mammary tumor metastasis. Mol. Cell. Biol. 249726-9735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Namba, R., J. E. Maglione, R. R. Davis, C. A. Baron, S. Liu, C. E. Carmack, L. J. Young, A. D. Borowsky, R. D. Cardiff, and J. P. Gregg. 2006. Heterogeneity of mammary lesions represent molecular differences. BMC Cancer 6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Namba, R., J. E. Maglione, L. J. Young, A. D. Borowsky, R. D. Cardiff, C. L. MacLeod, and J. P. Gregg. 2004. Molecular characterization of the transition to malignancy in a genetically engineered mouse-based model of ductal carcinoma in situ. Mol. Cancer Res. 2453-463. [PubMed] [Google Scholar]
- 205.Namba, R., L. J. Young, J. E. Maglione, E. T. McGoldrick, S. Liu, G. T. Wurz, M. W. DeGregorio, A. D. Borowsky, C. L. MacLeod, R. D. Cardiff, and J. P. Gregg. 2005. Selective estrogen receptor modulators inhibit growth and progression of premalignant lesions in a mouse model of ductal carcinoma in situ. Breast Cancer Res. 7R881-R889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Neznanov, N., A. K. Man, H. Yamamoto, C. A. Hauser, R. D. Cardiff, and R. G. Oshima. 1999. A single targeted Ets2 allele restricts development of mammary tumors in transgenic mice. Cancer Res. 594242-4246. [PubMed] [Google Scholar]
- 207.Nicholson, P. R., S. Empereur, H. R. Glover, and S. M. Dilworth. 2001. ShcA tyrosine phosphorylation sites can replace ShcA binding in signalling by middle T-antigen. EMBO J. 206337-6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Nielsen, L. L., M. Gurnani, B. Shi, G. Terracina, R. C. Johnson, J. Carroll, J. M. Mathis, and G. Hajian. 2000. Derivation and initial characterization of a mouse mammary tumor cell line carrying the polyomavirus middle T antigen: utility in the development of novel cancer therapeutics. Cancer Res. 607066-7074. [PubMed] [Google Scholar]
- 209.Niemeyer, C. C., B. Spencer-Dene, J. X. Wu, and E. D. Adamson. 1999. Preneoplastic mammary tumor markers: Cripto and Amphiregulin are overexpressed in hyperplastic stages of tumor progression in transgenic mice. Int. J. Cancer 81588-591. [DOI] [PubMed] [Google Scholar]
- 210.Nishida, K., and T. Hirano. 2003. The role of Gab family scaffolding adapter proteins in the signal transduction of cytokine and growth factor receptors. Cancer Sci. 941029-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Novak, U., and B. E. Griffin. 1981. Requirement for the C-terminal region of middle-T antigen in cellular transformation by polyoma virus. Nucleic Acids Res. 92055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ogris, E., D. M. Gibson, and D. C. Pallas. 1997. Protein phosphatase 2A subunit assembly: the catalytic subunit carboxy terminus is important for binding cellular B subunit but not polyomavirus middle tumor antigen. Oncogene 15911-917. [DOI] [PubMed] [Google Scholar]
- 213.Ogris, E., I. Mudrak, E. Mak, D. Gibson, and D. C. Pallas. 1999. Catalytically inactive protein phosphatase 2A can bind to polyomavirus middle tumor antigen and support complex formation with pp60c-src. J. Virol. 737390-7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ong, S. H., S. Dilworth, I. Hauck-Schmalenberger, T. Pawson, and F. Kiefer. 2001. ShcA and Grb2 mediate polyoma middle T antigen-induced endothelial transformation and Gab1 tyrosine phosphorylation. EMBO J. 206327-6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Oostra, B., R. Harvey, B. Ely, A. Markham, and A. Smith. 1983. Transforming activity of polyoma virus middle-T antigen probed by site-directed mutagenesis. Nature (London) 304456-459. [DOI] [PubMed] [Google Scholar]
- 216.O'Shea, C. C., and M. Fried. 2005. Modulation of the ARF-p53 pathway by the small DNA tumor viruses. Cell Cycle 4449-452. [DOI] [PubMed] [Google Scholar]
- 217.Pallas, D. C., H. Fu, L. C. Haehnel, W. Weller, R. J. Collier, and T. M. Roberts. 1994. Association of polyomavirus middle tumor antigen with 14-3-3 proteins. Science 265535-537. [DOI] [PubMed] [Google Scholar]
- 218.Pallas, D. C., W. Morgan, and T. M. Roberts. 1989. The cellular proteins which can associate specifically with polyomavirus middle T antigen in human 293 cells include the major human 70-kilodalton heat shock proteins. J. Virol. 634533-4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Pallas, D. C., L. K. Shahrik, B. L. Martin, S. Jaspers, T. B. Miller, D. L. Brautigan, and T. M. Roberts. 1990. Polyoma small and middle T antigens and SV40 small t antigen form stable complexes with protein phosphatase 2A. Cell 60167-176. [DOI] [PubMed] [Google Scholar]
- 220.Pankratz, S. L., E. Y. Tan, Y. Fine, A. M. Mercurio, and L. M. Shaw. 2008. Insulin receptor substrate-2 regulates aerobic glycolysis in mouse mammary tumor cells via glucose transporter 1. J. Biol. Chem. 2842031-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Park, Y. G., R. Clifford, K. H. Buetow, and K. W. Hunter. 2003. Multiple cross and inbred strain haplotype mapping of complex-trait candidate genes. Genome Res. 13118-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Park, Y. G., X. Zhao, F. Lesueur, D. R. Lowy, M. Lancaster, P. Pharoah, X. Qian, and K. W. Hunter. 2005. Sipa1 is a candidate for underlying the metastasis efficiency modifier locus Mtes1. Nat. Genet. 371055-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Pedersen, T. X., C. J. Pennington, K. Almholt, I. J. Christensen, B. S. Nielsen, D. R. Edwards, J. Romer, K. Dano, and M. Johnsen. 2005. Extracellular protease mRNAs are predominantly expressed in the stromal areas of microdissected mouse breast carcinomas. Carcinogenesis 261233-1240. [DOI] [PubMed] [Google Scholar]
- 224.Pelicci, G., L. Lanfrancone, F. Grignani, J. McGlade, F. Cavallo, G. Forni, I. Nicoletti, F. Grignani, T. Pawson, and P. G. Pelicci. 1992. A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction. Cell 7093-104. [DOI] [PubMed] [Google Scholar]
- 225.Perou, C. M., T. Sorlie, M. B. Eisen, M. van de Rijn, S. S. Jeffrey, C. A. Rees, J. R. Pollack, D. T. Ross, H. Johnsen, L. A. Akslen, O. Fluge, A. Pergamenschikov, C. Williams, S. X. Zhu, P. E. Lonning, A. L. Borresen-Dale, P. O. Brown, and D. Botstein. 2000. Molecular portraits of human breast tumours. Nature 406747-752. [DOI] [PubMed] [Google Scholar]
- 226.Piwnica-Worms, H., D. R. Kaplan, M. Whitman, and T. M. Roberts. 1986. Retrovirus shuttle vector for study of kinase activities of pp60c-src synthesized in vitro and overproduced in vivo. Mol. Cell. Biol. 62033-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Piwnica-Worms, H., K. B. Saunders, T. M. Roberts, A. E. Smith, and S. H. Cheng. 1987. Tyrosine phosphorylation regulates the biochemical properties of pp60c-src. Cell 4975-82. [DOI] [PubMed] [Google Scholar]
- 228.Podsypanina, K., Y. C. Du, M. Jechlinger, L. J. Beverly, D. Hambardzumyan, and H. Varmus. 2008. Seeding and propagation of untransformed mouse mammary cells in the lung. Science 3211841-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Primo, L., C. Roca, C. Ferrandi, L. Lanfrancone, and F. Bussolino. 2000. Human endothelial cells expressing polyoma middle T induce tumors. Oncogene 193632-3641. [DOI] [PubMed] [Google Scholar]
- 230.Putzer, B. M., J. L. Bramson, C. L. Addison, M. Hitt, P. M. Siegel, W. J. Muller, and F. L. Graham. 1998. Combination therapy with interleukin-2 and wild-type p53 expressed by adenoviral vectors potentiates tumor regression in a murine model of breast cancer. Hum. Gene Ther. 9707-718. [DOI] [PubMed] [Google Scholar]
- 231.Qin, L., L. Liao, A. Redmond, L. Young, Y. Yuan, H. Chen, B. W. O'Malley, and J. Xu. 2008. The AIB1 oncogene promotes breast cancer metastasis by activation of PEA3-mediated matrix metalloproteinase 2 (MMP2) and MMP9 expression. Mol. Cell. Biol. 285937-5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Qiu, T. H., G. V. Chandramouli, K. W. Hunter, N. W. Alkharouf, J. E. Green, and E. T. Liu. 2004. Global expression profiling identifies signatures of tumor virulence in MMTV-PyMT-transgenic mice: correlation to human disease. Cancer Res. 645973-5981. [DOI] [PubMed] [Google Scholar]
- 233.Raptis, L., H. Lamfrom, and T. L. Benjamin. 1985. Regulation of cellular phenotype and expression of polyoma virus middle T antigen in rat fibroblasts. Mol. Cell. Biol. 52476-2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Rassoulzadegan, M., S. A. Courtneidge, R. Loubiere, P. el Baze and F. Cuzin. 1990. A variety of tumours induced by the middle T antigen of polyoma virus in a transgenic mouse family. Oncogene 51507-1510. [PubMed] [Google Scholar]
- 235.Rauh, M. J., V. Blackmore, E. R. Andrechek, C. G. Tortorice, R. Daly, V. K. Lai, T. Pawson, R. D. Cardiff, P. M. Siegel, and W. J. Muller. 1999. Accelerated mammary tumor development in mutant polyomavirus middle T transgenic mice expressing elevated levels of either the Shc or Grb2 adapter protein. Mol. Cell. Biol. 198169-8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ravichandran, K. S. 2001. Signaling via Shc family adapter proteins. Oncogene 206322-6330. [DOI] [PubMed] [Google Scholar]
- 237.Richard, S., G. Vogel, M. E. Huot, T. Guo, W. J. Muller, and K. E. Lukong. 2008. Sam68 haploinsufficiency delays onset of mammary tumorigenesis and metastasis. Oncogene 27548-556. [DOI] [PubMed] [Google Scholar]
- 238.Riley, M. I., W. Yoo, N. Y. Mda, and W. R. Folk. 1997. Tiny T antigen: an autonomous polyomavirus T antigen amino-terminal domain. J. Virol. 716068-6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Ritland, S. R., G. J. Rowse, Y. Chang, and S. J. Gendler. 1997. Loss of heterozygosity analysis in primary mammary tumors and lung metastases of MMTV-MTAg and MMTV-neu transgenic mice. Cancer Res. 573520-3525. [PubMed] [Google Scholar]
- 240.Rondinelli, R. H., S. Z. Haslam, and M. M. Fluck. 1995. The role of ovarian hormones, age and mammary gland development in polyomavirus mammary tumorigenesis. Oncogene 111817-1827. [PubMed] [Google Scholar]
- 241.Rose, P., and Schaffhausen. 1995. Zinc-binding and protein-protein interaction mediated by the polyomavirus large T zinc finger. J. Virol. 692842-2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Rowe, W. P. 1961. The epidemiology of mouse polyoma virus infection. Bacteriol. Rev. 2518-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Ruediger, R., H. T. Pham, and G. Walter. 2001. Alterations in protein phosphatase 2A subunit interaction in human carcinomas of the lung and colon with mutations in the A beta subunit gene. Oncogene 201892-1899. [DOI] [PubMed] [Google Scholar]
- 244.Ruley, H. E. 1983. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature 304602-606. [DOI] [PubMed] [Google Scholar]
- 245.Sablina, A. A., W. Chen, J. D. Arroyo, L. Corral, M. Hector, S. E. Bulmer, J. A. DeCaprio, and W. C. Hahn. 2007. The tumor suppressor PP2A Aβ regulates the RalA GTPase. Cell 129969-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Salcini, A. E., J. McGlade, G. Pelicci, I. Nicoletti, T. Pawson, and P. Peilicci. 1994. Formation of Shc-Grb2 complexes is necessary to induce neoplastic transformation by overexpression of Shc proteins. Oncogene 92827-2836. [PubMed] [Google Scholar]
- 247.Reference deleted.
- 248.Samuels, Y., L. A. Diaz, Jr., O. Schmidt-Kittler, J. M. Cummins, L. Delong, I. Cheong, C. Rago, D. L. Huso, C. Lengauer, K. W. Kinzler, B. Vogelstein, and V. E. Velculescu. 2005. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell 7561-573. [DOI] [PubMed] [Google Scholar]
- 249.Samuels, Y., and V. E. Velculescu. 2004. Oncogenic mutations of PIK3CA in human cancers. Cell Cycle 31221-1224. [DOI] [PubMed] [Google Scholar]
- 250.Sausville, J., A. A. Molinolo, X. Cheng, J. Frampton, N. Takebe, J. S. Gutkind, and R. A. Feldman. 2008. RCAS/SCL-TVA animal model allows targeted delivery of polyoma middle T oncogene to vascular endothelial progenitors in vivo and results in hemangioma development. Clin. Cancer Res. 143948-3955. [DOI] [PubMed] [Google Scholar]
- 251.Schaffhausen, B. 1982. Transforming genes and gene products of polyoma and SV40. CRC Crit. Rev. Biochem. 13215-286. [DOI] [PubMed] [Google Scholar]
- 252.Schaffhausen, B. S., and T. Benjamin. 1981. Two polyoma middle T antigen species differing in in vivo phosphorylation and in vitro kinase activity. J. Virol. 40184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Schaffhausen, B. S., and T. L. Benjamin. 1979. Phosphorylation of polyoma T antigens. Cell 18935-946. [DOI] [PubMed] [Google Scholar]
- 254.Schaffhausen, B. S., and T. L. Benjamin. 1981. Polyoma virus middle T antigen associated protein kinase, p. 1281-1300. In O. M. Rosen and E. G. Krebs (ed.), Protein phosphorylation, vol. 8. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- 255.Schaffhausen, B. S., B. J. Bockus, K. L. Berkner, D. Kaplan, and T. M. Roberts. 1987. Characterization of middle T antigen expressed by using an adenovirus expression system. J. Virol. 611221-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Schaffhausen, B. S., H. Dorai, G. Arakere, and T. L. Benjamin. 1982. Polyoma virus middle T antigen: relationship to cell membranes and apparent lack of ATP-binding activity. Mol. Cell. Biol. 21187-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Schaffhausen, B. S., T. J. Liang, G. G. Carmichael, and T. L. Benjamin. 1985. Residual transforming activity of PY1178T, a mutant lacking the principal in vitro phosphorylation site is not affected by removal of the secondary tyrosine phosphorylation site at residue 322. Virology 143671-675. [DOI] [PubMed] [Google Scholar]
- 258.Schaffhausen, B. S., and T. M. Roberts. 2009. Lessons from polyoma middle T antigen on signaling and transformation: a DNA tumor virus contribution to the war on cancer. Virology 384304-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Scheidtmann, K. H., M. C. Mumby, K. Rundell, and G. Walter. 1991. Dephosphorylation of simian virus 40 large-T antigen and p53 protein by protein phosphatase 2A: inhibition by small-t antigen. Mol. Cell. Biol. 111996-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Schlessinger, J., and M. A. Lemmon. 2003. SH2 and PTB domains in tyrosine kinase signaling. Sci. STKE 2003RE12. [DOI] [PubMed] [Google Scholar]
- 261.Schoeffner, D. J., S. L. Matheny, T. Akahane, V. Factor, A. Berry, G. Merlino, and U. P. Thorgeirsson. 2005. VEGF contributes to mammary tumor growth in transgenic mice through paracrine and autocrine mechanisms. Lab. Invest. 85608-623. [DOI] [PubMed] [Google Scholar]
- 262.Schonthal, A., S. Srinivas, and W. Eckhart. 1992. Induction of c-jun protooncogene expression and transcription factor AP-1 activity by the polyoma virus middle-sized tumor antigen. Proc. Natl. Acad. Sci. USA. 894972-4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Sefton, B. M., T. Hunter, K. Beemon, and W. Eckhart. 1980. Evidence that the phosphorylation of tyrosine is essential for cellular transformation by Rous sarcoma virus. Cell 20807-816. [DOI] [PubMed] [Google Scholar]
- 264.Senften, M., S. Dilworth, and K. Ballmer-Hofer. 1997. Multimerization of polyomavirus middle-T antigen. J. Virol. 716990-6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Sheng, Q., D. Denis, M. Ratnofsky, T. Roberts, J. DeCaprio, and B. Schaffhausen. 1997. The DnaJ domain of polyoma Large T is required to regulate Rb family tumor tuppressor function. J. Virol. 71.9410-9416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Shepard, C. R., J. Kassis, D. L. Whaley, H. G. Kim, and A. Wells. 2007. PLC gamma contributes to metastasis of in situ-occurring mammary and prostate tumors. Oncogene 263020-3026. [DOI] [PubMed] [Google Scholar]
- 267.Shepherd, T., and J. A. Hassell. 2001. Role of Ets transcription factors in mammary gland development and oncogenesis. J. Mammary Gland Biol. Neoplasia 6129-140. [DOI] [PubMed] [Google Scholar]
- 268.Siegler, R., and T. L. Benjamin. 1975. Oncogenicity of wild-type and nontransforming mutants of polyoma virus. Proc. Am. Assoc. Cancer Res. 1699. [Google Scholar]
- 269.Silver, J., B. Schaffhausen, and T. Benjamin. 1978. Tumor antigens induced by nontransforming mutants of polyoma virus. Cell 15485-496. [DOI] [PubMed] [Google Scholar]
- 270.Siwko, S. K., W. Bu, C. Gutierrez, B. Lewis, M. Jechlinger, B. Schaffhausen, and Y. Li. 2008. Lentivirus-mediated oncogene introduction into mammary cells in vivo induces tumors. Neoplasia 10653-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Sjoegren, H. O. 1964. Studies on specific transplantation resistance to polyoma-virus-induced tumors. IV. Stability of the polyoma cell antigen. J. Natl. Cancer Inst. 32661-666. [PubMed] [Google Scholar]
- 272.Skolnik, E. Y., B. Margolis, M. Mohammadi, E. Lowenstein, R. Fischer, A. Drepps, A. Ullrich, and J. Schlessinger. 1991. Cloning of PI3 kinase-associated p85 utilizing a novel method for expression/cloning of target proteins for receptor tyrosine kinases. Cell 6583-90. [DOI] [PubMed] [Google Scholar]
- 273.Smith, A. E., R. Smith, B. Griffin, and M. Fried. 1979. Protein kinase activity associated with polyoma virus middle T antigen in vitro. Cell 18915-924. [DOI] [PubMed] [Google Scholar]
- 274.Soeda, E., J. R. Arrand, N. Smolar, and B. E. Griffin. 1979. Sequence from early region of polyoma virus DNA containing viral replication origin and encoding small, middle and (part of) large T antigens. Cell 17357-370. [DOI] [PubMed] [Google Scholar]
- 275.Songyang, Z., S. Shoelson, M. Chauduri, G. Gish, T. Pawson, W. Haser, F. King, T. Roberts, S. Ratnofsky, R. Lechleider, B. Neel, R. Birge, J. Fajardo, M. Chou, H. Hanafusa, B. Schaffhausen, and L. Cantley. 1993. SH2 domains recognize specific phosphopeptide sequences. Cell 72767-778. [DOI] [PubMed] [Google Scholar]
- 276.Sorlie, T., R. Tibshirani, J. Parker, T. Hastie, J. S. Marron, A. Nobel, S. Deng, H. Johnsen, R. Pesich, S. Geisler, J. Demeter, C. M. Perou, P. E. Lonning, P. O. Brown, A. L. Borresen-Dale, and D. Botstein. 2003. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl. Acad. Sci. USA 1008418-8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Srinivas, S., A. Schonthal, and W. Eckhart. 1994. Polyoma virus middle-sized tumor antigen modulates c-jun phosphorylation and transcriptional activity. Proc. Natl. Acad. Sci. USA 9110064-10068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Srinivasan, A., A. J. McClellan, J. Vartikar, I. Marks, P. Cantalupo, Y. Li, P. Whyte, K. Rundell, J. L. Brodsky, and J. M. Pipas. 1997. The amino-terminal transforming region of simian virus 40 large T and small t antigens functions as a J. domain. Mol. Cell. Biol. 174761-4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Stewart, S. E., B. E. Eddy, A. M. Gochenour, G. Borgese, and G. E. Grubbs. 1957. The induction of neoplasms with a substance released from mouse tumors by tissue culture. Virol. 3380-400. [DOI] [PubMed] [Google Scholar]
- 280.Stringer, J. R., J. S. Larson, J. M. Fischer, M. Medvedovic, M. N. Hersh, G. P. Boivin, and S. L. Stringer. 2005. Modeling variation in tumors in vivo. Proc. Natl. Acad. Sci. USA 1022408-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Stubdal, H., J. Zalvide, K. S. Campbell, C. Schweitzer, T. M. Roberts, and J. A. DeCaprio. 1997. Inactivation of pRB-related proteins p130 and p107 mediated by the J. domain of simian virus 40 large T antigen. Mol. Cell. Biol. 174979-4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Su, W., W. Liu, B. Schaffhausen, and T. Roberts. 1995. Association of polyomavirus middle tumor antigen with phospholipase C-gamma 1. J. Biol. Chem. 27012331-12335. [DOI] [PubMed] [Google Scholar]
- 283.Sullivan, C. S., and J. M. Pipas. 2002. T antigens of simian virus 40: molecular chaperones for viral replication and tumorigenesis. Microbiol. Mol. Biol. Rev. 66179-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Summers, S. A., L. Lipfert, and M. J. Birnbaum. 1998. Polyoma middle T antigen activates the Ser/Thr kinase Akt in a PI3-kinase-dependent manner. Biochem. Biophys. Res. Commun. 24676-81. [DOI] [PubMed] [Google Scholar]
- 285.Szabova, L., S. S. Yamada, H. Birkedal-Hansen, and K. Holmbeck. 2005. Expression pattern of four membrane-type matrix metalloproteinases in the normal and diseased mouse mammary gland. J. Cell Physiol. 205123-132. [DOI] [PubMed] [Google Scholar]
- 286.Tehranian, A., D. W. Morris, B. H. Min, D. J. Bird, R. D. Cardiff, and P. A. Barry. 1996. Neoplastic transformation of prostatic and urogenital epithelium by the polyoma virus middle T gene. Am. J. Pathol. 1491177-1191. [PMC free article] [PubMed] [Google Scholar]
- 287.Templeton, D., and W. Eckhart. 1984. Characterization of viable mutants of polyomavirus cold sensitive for maintenance of cell transformation. J. Virol. 49799-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Templeton, D., and W. Eckhart. 1982. Mutation causing premature termination of the polyoma virus middle T antigen blocks cell transformation. J. Virol. 411014-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Templeton, D., and W. Eckhart. 1984. N-terminal amino acid sequences of the polyoma middle-size T antigen are important for protein kinase activity and cell transformation. Mol. Cell. Biol. 4817-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Templeton, D., S. Simon, and W. Eckhart. 1986. Truncated forms of the polyomavirus middle T antigen can substitute for the small T antigen in lytic infection. J. Virol. 57367-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Templeton, D., A. Voronova, and W. Eckhart. 1984. Construction and expression of a recombinant DNA gene encoding a polyoma middle-size tumor antigen with the carboxyl terminus of the VSV glycoprotein G. Mol. Cell. Biol. 4282-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Thomas, J. E., A. Aguzzi, P. Soriano, E. F. Wagner, and J. S. Brugge. 1993. Induction of tumor formation and cell transformation by polyoma middle T antigen in the absence of Src. Oncogene 82521-2529. [PubMed] [Google Scholar]
- 293.Tian, Y., R. Kolb, J. H. Hong, J. Carroll, D. Li, J. You, R. Bronson, M. B. Yaffe, J. Zhou, and T. Benjamin. 2007. TAZ promotes PC2 degradation through a SCFβ-Trcp E3 ligase complex. Mol. Cell. Biol. 276383-6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Tian, Y., D. Li, J. Dahl, J. You, and T. Benjamin. 2004. Identification of TAZ as a binding partner of the polyomavirus T antigens. J. Virol. 7812657-12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Tower, G. B., C. I. Coon, and C. E. Brinckerhoff. 2003. The 2G single nucleotide polymorphism (SNP) in the MMP-1 promoter contributes to high levels of MMP-1 transcription in MCF-7/ADR breast cancer cells. Breast Cancer Res. Treatment 8275-82. [DOI] [PubMed] [Google Scholar]
- 296.Treisman, R., A. Cowie, J. Favaloro, P. Jat, and R. Kamen. 1981. The structures of spliced mRNAs encoding polyoma virus early region proteins. J. Mol. Appl. Gen. 183-92. [PubMed] [Google Scholar]
- 297.Treisman, R., U. Novak, J. Favaloro, and R. Kamen. 1981. Transformation of rat cells by an altered polyoma virus genome expressing only the middle T protein. Nature 292595-600. [DOI] [PubMed] [Google Scholar]
- 298.Trimble, M. S., J. H. Xin, C. T. Guy, W. J. Muller, and J. A. Hassell. 1993. PEA3 is overexpressed in mouse metastatic mammary adenocarcinomas. Oncogene 83037-3042. [PubMed] [Google Scholar]
- 299.Tyndall, C., G. La Mantia, C. M. Thacker, J. Favaloro, and R. Kamen. 1981. A region of the polyoma virus genome between the replication origin and late protein coding sequences is required in cis for both early gene expression and viral DNA replication. Nucleic Acids Res. 96231-6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Ulug, E. T., P. T. Hawkins, M. R. Hanley, and S. A. Courtneidge. 1990. Phosphatidylinositol metabolism in cells transformed by polyomavirus middle T antigen. J. Virol. 643895-3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Urich, M., M. Y. el Shemerly, D. Besser, Y. Nagamine, and K. Ballmer-Hofer. 1995. Activation and nuclear translocation of mitogen-activated protein kinases by polyomavirus middle-T or serum depend on phosphatidylinositol 3-kinase. J. Biol. Chem. 27029286-29292. [DOI] [PubMed] [Google Scholar]
- 302.Urich, M., M. Senften, P. E. Shaw, and K. Ballmer-Hofer. 1997. A role for the small GTPase Rac in polyomavirus middle-T antigen-mediated activation of the serum response element and in cell transformation. Oncogene 141235-1241. [DOI] [PubMed] [Google Scholar]
- 303.Utermark, T., B. S. Schaffhausen, T. M. Roberts, and J. J. Zhao. 2007. The p110alpha isoform of phosphatidylinositol 3-kinase is essential for polyomavirus middle T antigen-mediated transformation. J. Virol. 817069-7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.van der Geer, P., S. Wiley, G. D. Gish, V. K. Lai, R. Stephens, M. F. White, D. Kaplan, and T. Pawson. 1996. Identification of residues that control specific binding of the Shc phosphotyrosine-binding domain to phosphotyrosine sites. Proc. Natl. Acad. Sci. USA 93963-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Vandeputte, M., H. Eyssen, H. Sobis, and P. de Somer. 1974. Induction of polyoma tumors in athymic nude mice. Int. J. Cancer 14445-450. [DOI] [PubMed] [Google Scholar]
- 306.van der Geer, P., S. Wiley, G. D. Gish, and T. Pawson. 1996. The Shc adaptor protein is highly phosphorylated at conserved, twin tyrosine residues (Y239/240) that mediate protein-protein interactions. Curr. Biol. 61435-1444. [DOI] [PubMed] [Google Scholar]
- 307.van der Geer, P., S. Wiley, V. K. Lai, J. P. Olivier, G. D. Gish, R. Stephens, D. Kaplan, S. Shoelson, and T. Pawson. 1995. A conserved amino-terminal Shc domain binds to phosphotyrosine motifs in activated receptors and phosphopeptides. Curr. Biol. 5404-412. [DOI] [PubMed] [Google Scholar]
- 308.Vanhaesebroeck, B., and M. D. Waterfield. 1999. Signaling by distinct classes of phosphoinositide 3-kinases. Exp. Cell Res. 253239-254. [DOI] [PubMed] [Google Scholar]
- 309.Varticovski, L., B. Druker, D. Morrison, L. Cantley, and T. Roberts. 1989. The colony stimulating factor-1 receptor associates with and activates phosphatidylinositol-3 kinase. Nature 342699-702. [DOI] [PubMed] [Google Scholar]
- 310.Vasiljeva, O., M. Korovin, M. Gajda, H. Brodoefel, L. Bojic, A. Kruger, U. Schurigt, L. Sevenich, B. Turk, C. Peters, and T. Reinheckel. 2008. Reduced tumour cell proliferation and delayed development of high-grade mammary carcinomas in cathepsin B-deficient mice. Oncogene 274191-4199. [DOI] [PubMed] [Google Scholar]
- 311.Vasiljeva, O., A. Papazoglou, A. Kruger, H. Brodoefel, M. Korovin, J. Deussing, N. Augustin, B. S. Nielsen, K. Almholt, M. Bogyo, C. Peters, and T. Reinheckel. 2006. Tumor cell-derived and macrophage-derived cathepsin B promotes progression and lung metastasis of mammary cancer. Cancer Res. 665242-5250. [DOI] [PubMed] [Google Scholar]
- 312.Veldman, G., S. Lupton, and R. Kamen. 1985. Polyomavirus enhancer contains multiple redundant sequence elements that activate both DNA replication and gene expression. Mol. Cell. Biol. 5649-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Ventura, A., L. Luzi, S. Pacini, C. T. Baldari, and P. G. Pelicci. 2002. The p66Shc longevity gene is silenced through epigenetic modifications of an alternative promoter. J. Biol. Chem. 27722370-22376. [DOI] [PubMed] [Google Scholar]
- 314.Versteeg, H. H., F. Schaffner, M. Kerver, L. G. Ellies, P. Andrade-Gordon, B. M. Mueller, and W. Ruf. 2008. Protease-activated receptor (PAR) 2, but not PAR1, signaling promotes the development of mammary adenocarcinoma in polyoma middle T mice. Cancer Res. 687219-7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Vomachka, A. J., S. L. Pratt, J. A. Lockefeer, and N. D. Horseman. 2000. Prolactin gene-disruption arrests mammary gland development and retards T-antigen-induced tumor growth. Oncogene 191077-1084. [DOI] [PubMed] [Google Scholar]
- 316.Walter, G., A. Carbone, and W. J. Welch. 1987. Medium tumor antigen of polyomavirus transformation-defective mutant NG59 is associated with 73-kilodalton heat shock protein. J. Virol. 61405-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Walter, G., R. Ruediger, C. Slaughter, and M. Mumby. 1990. Association of protein phosphatase 2A with polyoma virus medium tumor antigen. Proc. Natl. Acad. Sci. USA 872521-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Wang, S., Y. Yuan, L. Liao, S. Q. Kuang, J. C. Tien, B. W. O'Malley, and J. Xu. 2009. Disruption of the SRC-1 gene in mice suppresses breast cancer metastasis without affecting primary tumor formation. Proc. Natl. Acad. Sci. USA 106151-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Wang, S. S., E. D. Esplin, J. L. Li, L. Huang, A. Gazdar, J. Minna, and G. A. Evans. 1998. Alterations of the PPP2R1B gene in human lung and colon cancer. Science 282284-287. [DOI] [PubMed] [Google Scholar]
- 320.Wang, W., J. B. Wyckoff, S. Goswami, Y. Wang, M. Sidani, J. E. Segall, and J. S. Condeelis. 2007. Coordinated regulation of pathways for enhanced cell motility and chemotaxis is conserved in rat and mouse mammary tumors. Cancer Res. 673505-3511. [DOI] [PubMed] [Google Scholar]
- 321.Wasylyk, C., P. Flores, A. Gutman, and B. Wasylyk. 1989. PEA3 is a nuclear target for transcription activation by non-nuclear oncogenes. EMBO J. 83371-3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Webster, M., J. Hutchinson, M. Rauh, S. Muthuswamy, M. Anton, C. Tortorice, R. Cardiff, F. Graham, J. Hassell, and W. Muller. 1998. Requirement for both Shc and phosphatidylinositol 3′ kinase signaling pathways in polyomavirus middle T-mediated mammary tumorigenesis. Mol. Cell. Biol. 182344-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Welm, A. L., J. B. Sneddon, C. Taylor, D. S. Nuyten, M. J. van de Vijver, B. H. Hasegawa, and J. M. Bishop. 2007. The macrophage-stimulating protein pathway promotes metastasis in a mouse model for breast cancer and predicts poor prognosis in humans. Proc. Natl. Acad. Sci. USA 1047570-7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Westermarck, J., and W. C. Hahn. 2008. Multiple pathways regulated by the tumor suppressor PP2A in transformation. Trends Mol. Med. 14152-160. [DOI] [PubMed] [Google Scholar]
- 325.Whalen, K. A., R. de Jesus, J. A. Kean, and B. S. Schaffhausen. 2005. Genetic analysis of the polyomavirus DnaJ domain. J. Virol. 799982-9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Whalen, K. A., G. F. Weber, T. L. Benjamin, and B. S. Schaffhausen. 2008. Polyomavirus middle T antigen induces the transcription of osteopontin, a gene important for the migration of transformed cells. J. Virol. 824946-4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.White, D. E., N. A. Kurpios, D. Zuo, J. A. Hassell, S. Blaess, U. Mueller, and W. J. Muller. 2004. Targeted disruption of β1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell 6159-170. [DOI] [PubMed] [Google Scholar]
- 328.Whitman, M., C. P. Downes, M. Keeler, T. Keller, and L. Cantley. 1988. Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate. Nature 332644-646. [DOI] [PubMed] [Google Scholar]
- 329.Whitman, M., D. Kaplan, T. Roberts, and L. Cantley. 1987. Evidence for two distinct phosphatidylinositol kinases in fibroblasts. Biochem. J. 247165-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Whitman, M., D. R. Kaplan, B. Schaffhausen, L. Cantley, and T. M. Roberts. 1985. Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature 315239-242. [DOI] [PubMed] [Google Scholar]
- 331.Williams, R. L., S. A. Courtneidge, and E. F. Wagner. 1988. Embryonic lethalities and endothelial tumors in chimeric mice expressing polyoma virus middle T oncogene. Cell 52121-131. [DOI] [PubMed] [Google Scholar]
- 332.Williams, R. L., W. Risau, H. G. Zerwes, H. Drexler, A. Aguzzi, and E. F. Wagner. 1989. Endothelioma cells expressing the polyoma middle T oncogene induce hemangiomas by host cell recruitment. Cell 571053-1063. [DOI] [PubMed] [Google Scholar]
- 333.Winter, S. F., and K. W. Hunter. 2008. Mouse modifier genes in mammary tumorigenesis and metastasis. J. Mammary Gland Biol. Neoplasia 13337-342. [DOI] [PubMed] [Google Scholar]
- 334.Wirth, J. J., A. Amalfitano, R. Gross, M. B. Oldstone, and M. M. Fluck. 1992. Organ- and age-specific replication of polyomavirus in mice. J. Virol. 663278-3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Wyckoff, J., W. Wang, E. Y. Lin, Y. Wang, F. Pixley, E. R. Stanley, T. Graf, J. W. Pollard, J. Segall, and J. Condeelis. 2004. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 647022-7029. [DOI] [PubMed] [Google Scholar]
- 336.Wyckoff, J. B., Y. Wang, E. Y. Lin, J. F. Li, S. Goswami, E. R. Stanley, J. E. Segall, J. W. Pollard, and J. Condeelis. 2007. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 672649-2656. [DOI] [PubMed] [Google Scholar]
- 337.Yang, C. S., M. J. Vitto, S. A. Busby, B. A. Garcia, C. T. Kesler, D. Gioeli, J. Shabanowitz, D. F. Hunt, K. Rundell, D. L. Brautigan, and B. M. Paschal. 2005. Simian virus 40 small t antigen mediates conformation-dependent transfer of protein phosphatase 2A onto the androgen receptor. Mol. Cell. Biol. 251298-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Yang, L., J. Huang, X. Ren, A. E. Gorska, A. Chytil, M. Aakre, D. P. Carbone, L. M. Matrisian, A. Richmond, P. C. Lin, and H. L. Moses. 2008. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell 1323-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Yang, S. I., R. L. Lickteig, R. Estes, K. Rundell, G. Walter, and M. C. Mumby. 1991. Control of protein phosphatase 2A by simian virus 40 small-t antigen. Mol. Cell. Biol. 111988-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Yen, A., V. Cherington, B. Schaffhausen, K. Marks, and S. Varvayanis. 1999. Transformation-defective polyoma middle T antigen mutants defective in PLCγ, PI-3, or src kinase activation enhance ERK2 activation and promote retinoic acid-induced, cell differentiation like wild-type middle T. Exp. Cell Res. 248538-551. [DOI] [PubMed] [Google Scholar]
- 341.Yi, X., and R. Freund. 1998. Deletion of proline-rich domain in polyomavirus T antigens results in virus partially defective in transformation and tumorigenesis. Virology 248420-431. [DOI] [PubMed] [Google Scholar]
- 342.Yi, X., J. Peterson, and R. Freund. 1997. Transformation and tumorigenic properties of a mutant polyoma virus containing a middle T antigen defective in shc binding. J. Virol. 716279-6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Yoakim, M., W. Hou, Y. Liu, C. L. Carpenter, R. Kapeller, and B. S. Schaffhausen. 1992. Interactions of polyomavirus middle T with the SH2 domains of the pp85 subunit of phosphatidylinositol-3-kinase. J. Virol. 665485-5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Yonemoto, W., A. J. Filson, A. E. Queral-Lustig, J. Y. Wang, and J. S. Brugge. 1987. Detection of phosphotyrosine-containing proteins in polyomavirus middle tumor antigen-transformed cells after treatment with a phosphotyrosine phosphatase inhibitor. Mol. Cell. Biol. 7905-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Yonemoto, W., M. Jarvis-Morar, J. S. Brugge, J. B. Bolen, and M. A. Israel. 1985. Tyrosine phosphorylation within the amino-terminal domain of pp60c-src molecules associated with polyoma virus middle-sized tumor antigen. Proc. Natl. Acad. Sci. USA 824568-4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Zhao, J. J., H. Cheng, S. Jia, L. Wang, O. V. Gjoerup, A. Mikami, and T. M. Roberts. 2006. The p110α isoform of PI3K is essential for proper growth factor signaling and oncogenic transformation. Proc. Natl. Acad. Sci. USA 10316296-16300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Zhao, J. J., Z. Liu, L. Wang, E. Shin, M. F. Loda, and T. M. Roberts. 2005. The oncogenic properties of mutant p110α and p110β phosphatidylinositol 3-kinases in human mammary epithelial cells. Proc. Natl. Acad. Sci. USA 10218443-18448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Zhou, J., H. T. Pham, R. Ruediger, and G. Walter. 2003. Characterization of the Aα and Aβ subunit isoforms of protein phosphatase 2A: differences in expression, subunit interaction, and evolution. Biochem. J. 369387-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Zhu, W., A. Eicher, B. Leber, and D. W. Andrews. 1998. At the onset of transformation polyomavirus middle-T recruits shc and src to a perinuclear compartment coincident with condensation of endosomes. Oncogene 17565-576. [DOI] [PubMed] [Google Scholar]
- 350.Zhu, Z. Y., G. M. Veldman, A. Cowie, A. Carr, B. Schaffhausen, and K. R. 1984. Construction and functional characterization of polyomavirus genomes that separately encode the three early proteins. J. Virol. 51170. [DOI] [PMC free article] [PubMed] [Google Scholar]