Abstract
Summary: Streptococci readily colonize mucosal tissues in the nasopharynx; the respiratory, gastrointestinal, and genitourinary tracts; and the skin. Each ecological niche presents a series of challenges to successful colonization with which streptococci have to contend. Some species exist in equilibrium with their host, neither stimulating nor submitting to immune defenses mounted against them. Most are either opportunistic or true pathogens responsible for diseases such as pharyngitis, tooth decay, necrotizing fasciitis, infective endocarditis, and meningitis. Part of the success of streptococci as colonizers is attributable to the spectrum of proteins expressed on their surfaces. Adhesins enable interactions with salivary, serum, and extracellular matrix components; host cells; and other microbes. This is the essential first step to colonization, the development of complex communities, and possible invasion of host tissues. The majority of streptococcal adhesins are anchored to the cell wall via a C-terminal LPxTz motif. Other proteins may be surface anchored through N-terminal lipid modifications, while the mechanism of cell wall associations for others remains unclear. Collectively, these surface-bound proteins provide Streptococcus species with a “coat of many colors,” enabling multiple intimate contacts and interplays between the bacterial cell and the host. In vitro and in vivo studies have demonstrated direct roles for many streptococcal adhesins as colonization or virulence factors, making them attractive targets for therapeutic and preventive strategies against streptococcal infections. There is, therefore, much focus on applying increasingly advanced molecular techniques to determine the precise structures and functions of these proteins, and their regulatory pathways, so that more targeted approaches can be developed.
INTRODUCTION
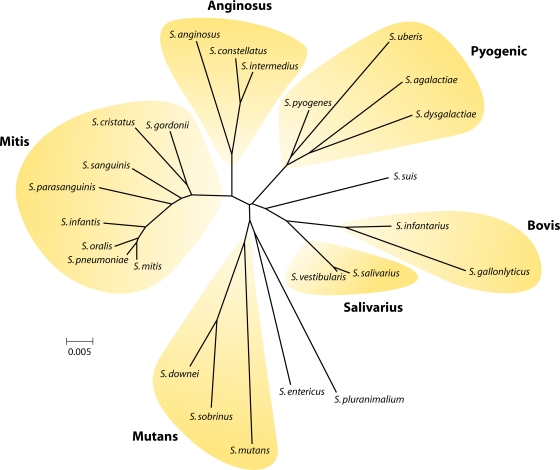
The Streptococcus, although not named, was first recorded in 1683 in van Leeuwenhoek's drawings of microscope images of the material removed from between his teeth. The main entry of streptococci into history was in 1879, when Louis Pasteur was studying puerperal fever. This was causing high mortality rates in maternity wards. Within the bodies of diseased women, he found rounded granules (microorganisms) arranged in the form of chains or strings of beads. He was convinced, and it was later proven, that this was the cause of infections in women after childbirth. Thus, streptococci were one of the first microbes to be identified as causing contagious disease, and their existence led to the introduction of hygiene and aseptic practices into hospital wards. Streptococcus comes from the Greek strepto (twisted) and coccus (spherical). There are now over 100 recognized species of Streptococcus. Historically, the classification of streptococci was based on the Lancefield scheme, which groups streptococcal strains according to the carbohydrate composition of cell wall antigens (331). Such antigens, known as group-specific antigens or C substances, are either polysaccharides (as in groups A, B, C, E, F, and G), teichoic acids (as in groups D and N), or lipoteichoic acid (as in group H) (505). This approach has proved successful for the more pathogenic streptococci, but its widespread application is hindered by the fact that group-specific antigens for other species may be absent or shared between distinct taxa. The streptococci may also be organized into six groupings (Fig. 1) based on 16S rRNA gene sequences (295). The pyogenic group includes Streptococcus pyogenes (Lancefield group A), S. agalactiae and S. uberis (group B), and S. dysgalactiae (group C, G, or L) (Fig. 1). S. equi (group C) and S. iniae are also in this group but are not shown in Fig. 1. These organisms are involved mainly in the colonization of humans and other mammals (although S. iniae colonizes fish). They are associated with a range of diseases including tonsillitis, pharyngitis, impetigo, mastitis, and sequelae such as rheumatic fever, glomerulonephritis (S. pyogenes), and neonatal sepsis (S. agalactiae). S. dysgalactiae is a major organism associated with bovine mastitis, while S. equi causes strangles in horses. The mitis group (Fig. 1) comprise species almost all of which may be isolated from the human oral cavity or nasopharynx. S. oralis, S. mitis, S. gordonii, and S. pneumoniae are highly related, and because of extensive horizontal gene transfer, the delineation of strains into these species is often blurred. S. pneumoniae is a major pathogen associated with otitis media, bronchitis, sinusitis, meningitis, and pneumonia. Colonization by S. pneumoniae will not be addressed in detail in this article, and the reader is referred to recent excellent reviews of adherence and virulence factors (43, 284). Other groupings include the anginosus and salivarius groups, which contain mainly human and animal oral cavity microbes, and the bovis group (Fig. 1). Mutans group streptococci comprise the least related organisms. They include a range of bacteria colonizing the oral cavities of humans (S. mutans and S. sobrinus), macaques (S. downei), rats (S. ratti), and hamsters (S. criceti) that are all associated with the development of dental caries (tooth decay). Oral cavity microbes are often cited as viridans group streptococci because colonies cause greening of blood agar. This is referred to as alpha-hemolysis and is indicative of hydrogen peroxide production. The pyogenic group of organisms is beta-hemolytic, producing true hemolysis on blood agar.
FIG. 1.
Taxonomic relationship tree for Streptococcus based on 16S rRNA gene sequence comparisons showing positions of selected species. A number of species are not included to simplify the figure, and a full description may be found in a review by Kilian (295). (Courtesy of Mogens Kilian, Aarhus University, Denmark, reproduced with permission.)
Colonization Attributes
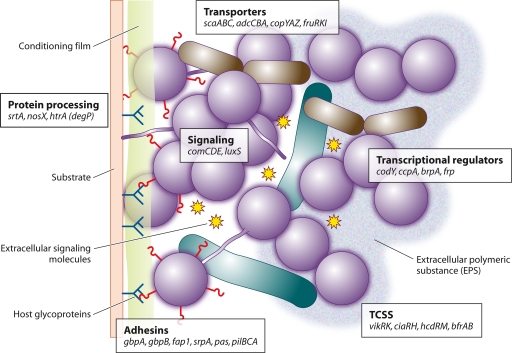
This article focuses on the adherence and colonization properties of selected members of the genus Streptococcus, particularly from the perspective of community development. Bacteria in the natural environment often grow upon surfaces, and it is thought that many species of streptococci that colonize mammals exist naturally within communities of bacteria growing as biofilms. Different streptococci vary in their propensities to form biofilm communities, but in all cases, biofilm formation depends first upon the adherence of cells to a surface. Cell division and multiplication then occur to produce a society (clonal), and the integration of other microorganisms within the society leads to the formation of a community (mixed species) (Fig. 2). Environmental conditions such as pH, temperature, oxygen availability, and organic metabolites, etc., influence the development of these communities, and signaling molecules for cell-cell communication are integral to population control (Fig. 2). The coverage of this review is summarized diagrammatically in Fig. 3. Surface and secreted proteins are mediators of adherence and virulence, membrane transporters are central to the import of nutrients and the export of small signaling molecules, and two-component signal transduction systems (TCSS) enable environmental sensing, sampling, and cellular responses. All of these surface components contribute to colonization processes, biofilm formation, and microbial community development.
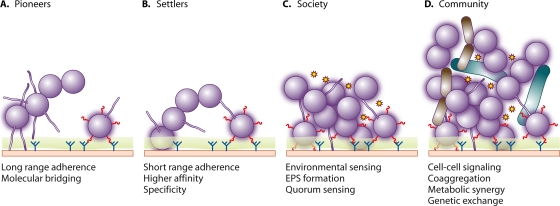
FIG. 2.
Temporal sequence of adherence and colonization by streptococci. (A) Pioneer streptococcal species associate with a conditioned surface (green), utilizing longer-range interactions, e.g., pili, which can penetrate mucus, or shorter-range interactions. (B) Some of the pioneers form stronger bonds with the surface molecules (blue) engaging multiple adhesins (red). (C) Nutritional adaptation, intermicrobial signaling (stars), and extracellular polymeric substance (EPS) production result in the formation of societies. (D) Incorporation of other microorganisms, including intergeneric coaggregation and cell-cell signaling, leads to the development of complex communities. These communities contain specific microbial associations within metabolic networks, ensuring more efficient utilization of nutrients and reduced susceptibility to antibiotics and immune surveillance.
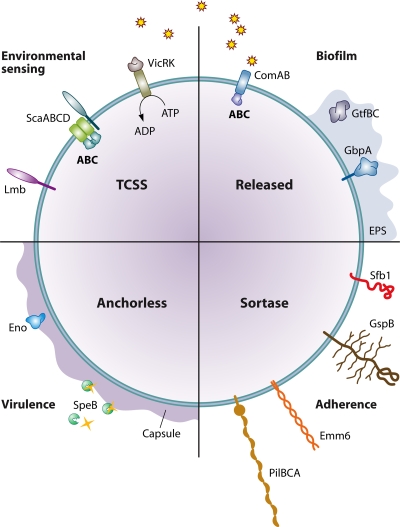
FIG. 3.
Streptococcus colonization depends upon adherence, signaling, nutritional adaptation, and host modulation. Adhesins include cell wall-anchored polypeptides, e.g., SfbI, and anchorless proteins, e.g., Eno, which mediate attachment and possibly also host cell modulation. Secreted polypeptides may be synthetic, e.g., GtfBC producing polysaccharides, or degrade host proteins, e.g., SpeB, and supply additional nutrients. Extracellular polymeric substance (EPS) (blue shading) and capsular material (purple shading) contribute to a developing ECM. Secreted peptides, and possibly other signaling molecules, e.g., AI-2 (stars), and environmental stimuli, e.g., pH, may be sensed by TCSS, with an ensuing modulation of transcription. ABC transporters, e.g., ScaABCD, ensure nutritional homeostasis as well as a possible involvement in regulating adherence, directly or indirectly. The quadrants are labeled to indicate the processes of adherence, environmental sensing, biofilm formation, and virulence that may be orchestrated by the expression of surface-bound or secreted proteins. These processes are not, however, exclusive to those molecules in each quadrant. For example, cell wall-linked proteins (southeast quadrant) may also contribute to virulence, while transporters (northwest quadrant) may contribute to adherence.
Studies of the structure and function of proteins in streptococci have been crucial to an understanding of pathogenesis ever since the S. pyogenes M protein was first purified from the group A Streptococcus (GAS) cell surface (121). Cell surface proteins are responsible for initial interactions of streptococci with the host. These interactions may include adherence to host cells or tissues, e.g., salivary pellicle on teeth; binding of soluble factors, e.g., lactoferrin at mucosal surfaces, or of plasma proteins, e.g., immunoglobulins (Igs); and stimulation of host responses, e.g., cytokine production. Similarities between cell surface proteins produced by various species of streptococci enable the delineation of proteins into families in which sequences and overall structures are relatively well conserved. Horizontal gene transfer between strains of streptococci and duplication of genes encoding surface proteins have generated mosaics of structure and function. Despite high genetic identities of strains within species, it is clear that different isolates may express different complements of cell surface proteins. This results in considerable phenotypic heterogeneity between strains with respect to their abilities to adhere to surfaces, develop biofilms, and colonize the host.
From a single cell deposited onto a surface in the human body, a mixed-species community may be developed within 24 h (Fig. 2). The processes involved in colonization have been difficult to study in vivo. However, the advancement of molecular methods to determine the composition and spatial arrangement of microorganisms within communities has led to studies of oral biofilm development in situ (89). Also, with the introduction of live-animal imaging, it is possible to monitor the temporal and spatial course of colonization and infection (561). Adherence and colonization attributes have of course been studied mainly in the laboratory. Here, new technologies involving the use of flow cells to generate biofilms (58, 110) and multilayer differentiated host cell systems will enable fundamental studies of the role of cell surface proteins in adherence and host cell interactions. Some of these proteins may turn out to be candidates or provide epitopes for incorporation into new vaccines. In addition, since biofilm formation is in many instances associated directly with the development of infections and disease, new agents that interfere with or disrupt biofilms may be important for therapy or prevention.
STREPTOCOCCUS ADHERENCE
Cell Surface Components
Adherence studies have featured throughout the research life of Streptococcus. From the early days of dental research, it was acknowledged that the attachment of S. mutans to oral surfaces, which are usually coated with salivary molecules, was an initial event in the development of dental caries. Likewise, it was observed that S. pyogenes cell surface molecules, including proteins and lipoteichoic acid (LTA), were important for the adherence of these organisms to cultured human cells (34) and to extracellular matrix (ECM) proteins such as fibronectin (Fn). One of the first oral streptococcal surface protein antigens shown to be an adhesin was antigen I/II (AgI/II) (515). The nomenclature for this protein family is confusing and arose because two cell surface antigens, designated AgI and AgII, were initially identified and thought to be the products of distinct genes. However, AgII was found to be a breakdown product of the larger AgI (291). The breakdown of streptococcal surface proteins into discrete fragments is a typical property and continually hinders functional analysis in the laboratory. As interest in the molecular biology of Streptococcus extended, it became established that adherence was mediated principally by surface proteins. LTA plays a part in streptococcal interactions with human tissue components (116, 239) and indeed triggers Toll-like receptor 2 (232, 532, 536), but the precise mechanism by which LTA might be involved in adherence is still not entirely clear. In S. mutans, the production of insoluble extracellular polysaccharides is essential for biofilm formation. However, glucan polysaccharides do not mediate the initial adherence to the tooth surface unless glucan-binding proteins (GBPs) are present within the salivary pellicle (26).
Adhesin-Receptor Interactions
Streptococci colonize different sites in the human body because they express multiple adhesins. These are usually proteins that recognize specific receptors, often sugars or oligosaccharides, expressed at various body sites. The keratinized epithelial cells at the buccal mucosal surface display different receptors from, for example, those present within the salivary pellicle formed on the tooth surface. This provides selectivity for the adherence of different streptococcal species (272). Following initial adherence processes (Fig. 2), bacteria will grow and survive only if the physical and chemical environment, e.g., pH, oxygen levels, and redox potential, is conducive. It is sometimes forgotten that adherence is a dynamic process. It may be advantageous for bacteria to detach from a surface if the growth conditions become unfavorable. As a result, streptococci have evolved methods for detachment as well as attachment.
In the development of a community, the initial adherence of single cells gives rise to microcolonies (Fig. 2). This results in the formation of what has been termed a linking film (80). Cell division leads to the generation of a small society with the concomitant incorporation and accumulation of other bacterial cells to establish a community (Fig. 2). In a dental plaque community, there may be over 100 different kinds of bacteria present (1). Multiple adhesive and metabolic interactions occur between bacterial cells in these communities. Appendages such as fimbriae (or pili), made up of multiple protein subunits (polymeric), are present on the surfaces of some streptococcal cells (see “Pili” below). These extended structures allow long-range adhesion to occur, sometimes across 5 μm, and may confer the ability to penetrate mucus or slime layers present on the surfaces of tissues. Shorter-range adherence processes occur through interactions of protein monomers or oligomers attached to the bacterial cell wall or membrane with host glycoproteins, e.g., integrins, or ECM proteins, e.g., Fn (431). Very close interactions between microbial cells and surfaces are mediated by hydrophobic or electrostatic forces. Collectively these can be quite strong, but generally they have low affinity (431).
Adhesins may be proteins that are linked directly to the cell surface or components of surface structures, e.g., pili projected away from the confines of the cell wall. The protein subunits of pili may themselves mediate adherence, or they may carry the adhesins along their lengths or at their tips. The specificity of microbial adherence is often associated with protein-carbohydrate (lectin-like) reactions. Common sugars (saccharides) that are recognized include galactose (Gal), N-acetyl-galactosamine (GalNAc), sialic acid (neuraminic acid [NeuNAc]), fucose (Fuc), N-acetyl-glucosamine (GlcNAc), and glucose (Glu). The recognition of Gal-containing carbohydrate chains or oligosaccharides is a common feature in oral microbial community development (306). On the other hand, the cell wall-anchored Hsa and GspB polypeptides in S. gordonii recognize NeuNAc residues on human cells (590), while the GBPs expressed by S. mutans attach to dextrans (26). Adherence processes may lead to one or more outcomes: commensal bacterial colonization, superficial infection of tissues, or intracellular invasion by pathogens and systemic spread.
Adherence Forces
It is thought that the streptococcal cell surface presents a vast spectrum of proteins to the host environment. Complex arrays of molecules are present upon host surfaces, e.g., salivary pellicle and epithelia, etc., with which streptococcal cells interact. Therefore, adhesin-receptor interactions between Streptococcus and the host will be multiply mediated. Interactions may be lectin-like (as described above), but they may also involve protein-protein or carbohydrate-carbohydrate recognition events. Adherence may involve ionic or Coulombic interactions, hydrogen bonding, the hydrophobic effect, or coordination involving divalent metal ions. These interactions differ quite considerably in their ranges and intermolecular forces. Van der Waals forces can play important roles in protein-protein recognition when complementary shapes are involved. This is the case for adhesin-receptor recognition, where a lock-and-key fit yields extensive Van der Waals attractions. Hydrophobic side chains of proteins can closely associate and are shielded from interactions with solvent H2O. The force generating a hydrophobic bond is approximately 20 pN (488), while the forces required to maintain stereospecific lectin-CHO binding range from 50 to 120 pN (608). With the development of force spectroscopy, forces between cells or purified adhesins and individual molecules can be reasonably accurately determined (609). This methodology provides a new means for comparative analyses of adhesin-receptor interactions. Measurements of forces between microbial cells and their receptors, or of bacterial cell softness or viscoelasticity, provide novel biomechanical information on bacterial adhesins and cellular functions (619).
A model for initial attachment envisages first a weak association of the microbial cell with substratum. For S. pyogenes, it has been suggested that LTA may counteract the electrostatic repulsion between bacteria and host surfaces. Alternatively, longer-distance first-attachment events (Fig. 2) may be mediated through surface appendages such as fimbriae (or pili). The initial attachment events are dynamic in that they demonstrate an on-off kinetic effect. They may be rather weak electrostatic or ionic forces or strong hydrophobic interactions and involve multiple molecular forces (117). A second stage might then involve more specific and irreversible interactions occurring, e.g., lectin-carbohydrate or protein-protein interactions, which could be of higher affinity or high complexity and involve multiple adhesins. A two-step model has been proposed for the interaction of GAS with human cells (223). The first step would involve a weak hydrophobic interaction between LTA and potential binding domains on the host cell surface, while the second step might involve protein adhesins such as M protein, Fn-binding proteins, and laminin-binding proteins, etc., depending upon the receptors available. In general, the adherence mechanisms for streptococci follow the general paradigms for adherence in other bacteria quite closely. However, it should be emphasized that streptococci, like other gram-positive bacteria, present a potentially more vast and complex surface proteome than gram-negative bacteria. This highly advanced surface protein repertoire enables multiple interactions with different host components, and so streptococci have versatility when it comes to occupying adherence sites and evading immune recognition. This property of streptococci to engage multiple adhesin-receptor interactions with various affinities is one reason for the difficulties that have been encountered in the characterization of adhesins. For example, single-gene knockouts may reveal very little about adherence mechanisms, and antibodies generated to specific surface proteins may have little or no effect on streptococcal adherence.
Surface Fibrillar Structures
When viewed under an electron microscope, there are at least two types of filamentous structures that may be seen extending away from the streptococcal cell surface: fibrils and pili (otherwise known as fimbriae). Fibrils were first visualized in the 1970s and appeared as thin flexible rods of various lengths from 40 nm to about 400 nm. These structures have been best described for oral streptococci, including S. sanguinis and S. salivarius, and exhibit either a peritrichous (hair-like, all around the surface) distribution or localized distribution as tufts (214, 215). Clearly, the surface structures are important for growth and survival in the oral cavity because all freshly isolated streptococci show some form of surface structure (215). However, the composition of these structures remained unknown for many years, and precisely what controls their surface topography is still not explained. Recent evidence supporting a concept that there may be specific sites for the secretion of proteins through the cell membrane of streptococci (490, 506) might provide a future basis for understanding the lateral distribution of fibril tufts, composed of glycoproteins, observed in some strains of S. cristatus and S. mitis (265). Surface fibrils have been implicated in mediating adhesion to host cells and to ECM and salivary components (217), although direct evidence has been lacking because mutants have been hard to generate and because fibrillar proteins have been difficult to purify. Fibrils have also been implicated in facilitating interbacterial coaggregation (215, 217, 333, 650), but the composition and assembly of most of these fibrillar structures remain unknown. However, the CshA polypeptide (259 kDa) produced by S. gordonii has been identified as the structural and functional component of one fibril type (394) (Table 1). The fibrils composed of CshA mediate attachment to immobilized Fn and to other oral bacteria. CshA-like proteins have also been found on the surfaces of S. oralis and S. sanguinis (148), on which they possibly function in a similar capacity. Also, serine-rich repeat (Srr) proteins have been characterized for several Streptococcus species (S. gordonii, S. sanguinis, S. parasanguinis, S. cristatus, and S. pneumoniae) (Table 1). The Hsa (Srr) polypeptide forms surface fibrils in S. gordonii (588), while SrpA has been shown to comprise long (400-nm) fibrils in S. cristatus and to confer coaggregation capabilities (216). Because these fibrillar structures presumably confer a selective advantage to streptococci colonizing the host, perhaps initially by mediating adherence interactions, they are important to consider as potential targets for inhibitors or vaccines. Unfortunately, it has repeatedly proved technically challenging to identify the genes necessary for the production of these structures, except for the few instances described above. However, sequencing of Streptococcus genomes has thrown up a considerable number of surprises, not the least being the identification of pilus islands (PI) in the pyogenic group and recently in S. sanguinis. Studies of pili have been more fulfilling and provide much promise for the development of new protein vaccines.
TABLE 1.
Cell wall-anchored or surface-associated adhesins
| Protein group | Protein(s) | Species | Cell surface linkageb | Function(s) and/or substrate(s) | Reference(s) |
|---|---|---|---|---|---|
| AgI/II familya | SpaP/P1/PAc/Sr/AgB | S. mutans | LPxTz | Coaggregation; multiple substrates | 291 |
| SpaA/PAg | S. sobrinus | LPxTz | 335 | ||
| SspA, SspB | S. gordonii | LPxTz | 240, 271, 328, 329 | ||
| Pas | S. intermedius | LPxTz | 271 | ||
| Spy1325 | S. pyogenes | LPxTz | 667 | ||
| PAaA, PAaB | S. criceti | LPxTz | 596, 598 | ||
| PAh | S. downei | LPxTz | 597 | ||
| Ssp-5 | S. agalactiae | LPxTz | 604 | ||
| Serine-rich repeat familya | GspB, Hsa | S. gordonii | LPxTz | Multiple substrates | 39, 41, 587 |
| Fap1 | S. parasanguinis | LPxTz | 652, 653 | ||
| SrpA | S. sanguinis | LPxTz | 460 | ||
| SrpA | S. cristatus | LPxTz | 216 | ||
| Srr-1, Srr-2 | S. agalactiae | LPxTz | 520, 539 | ||
| PsrP | S. pneumoniae | LPxTz | 509 | ||
| Alp familya | α Protein | S. agalactiae | LPxTz | Host glycosaminoglycans | 49 |
| Rib/R4 | S. agalactiae | LPxTz | 556, 572 | ||
| R28/Alp3 | S. pyogenes, S. agalactiae | LPxTz | 332, 571 | ||
| Alp2 | S. agalactiae | LPxTz | 326 | ||
| R proteins (R1-R4) | S. pyogenes, S. agalactiae, S. dysgalactiae | LPxTz | 167, 649 | ||
| Pili/fimbriae/fibrilsa | PI-1, PI-2a, PI-2b | S. agalactiae | LPxTz | Coaggregation, biofilm formation, phagocyte resistance; multiple substrates | 336, 512 |
| PI-1, PI-2 | S. pneumoniae | LPxTz | 23, 342 | ||
| PI | S. pyogenes | LPxTz | 157, 412 | ||
| Fap1 | S. parasanguinis | LPxTz | 652, 653 | ||
| SrpA | S. cristatus | LPxTz | 216 | ||
| HAF | S. salivarius | Unknown | 213 | ||
| AgB, AgC | S. salivarius | Unknown | 639, 640 | ||
| CspB | S. salivarius | LPxTz | 347 | ||
| CshA-like | S. oralis | LPxTz | 148 | ||
| CshA-like | S. sanguinis | LPxTz | 148, 213 | ||
| CshA | S. gordonii | LPxTz | 240, 394 | ||
| GspB, Hsa | S. gordonii | LPxTz | 39, 41, 587 | ||
| Uncharacterized | S. intermedius, S. mutans, S. mitis, S. constellatus | 170, 346, 650, 658 | |||
| Saliva-binding proteins | AgI/II familya | S. mutans, S. sobrinus, S. gordonii, S. oralis, S. intermedius | LPxTz | Salivary components (gp340, salivary glycoproteins, proline-rich proteins) | 261, 271, 274, 516, 597 |
| Pili, fimbriae, fibrilsa | S. pyogenes, S. parasanguinis, S. intermedius, S. salivarius, S. gordonii | LPxTz | gp340 | 145, 153, 217, 489, 641 | |
| SsaB | S. sanguinis | LXXC/XXGC | Salivary components | 176, 178 | |
| FimA | S. parasanguinis | LXXC/XXGC | Salivary components | 436 | |
| AbpA, AbpB | S. gordonii | LPxTz (AbpA) | α-Amylase | 92, 138, 351, 501 | |
| Uncharacterized | S. mitis, S. salivarius, S. cristatus, S. anginosus, S. parasanguinis | α-Amylase | 69, 529 | ||
| EP-GP binding protein | S. salivarius | Unknown | Extra parotid glycoprotein | 531 | |
| Fn-binding proteins | SfbI/PrtF1a | S. pyogenes | LPxTz | Fn | 219, 443, 592 |
| FbaAa | S. pyogenes | LPxTz | Fn | 601 | |
| FbaB/PFBP/PrtF2 | S. pyogenes | LPxTz | Fn | 257, 480, 603 | |
| SOF/SfbIIa | S. pyogenes | LPxTz | Fn | 311, 314, 479 | |
| SfbX | S. pyogenes | LPxTz | Fn | 269 | |
| Fbp54a | S. pyogenes | Anchorless | Fn | 114 | |
| ScpA (C5a peptidase)a | S. pyogenes | LPxTz | Fn | 94, 96, 106 | |
| GAPDH/SDHa | S. pyogenes | Anchorless | Fn | 447 | |
| M proteinsa | S. pyogenes | LPxTz | Fn | 120 | |
| M-like proteinsa | S. pyogenes | LPxTz | Fn | 120 | |
| Shra | S. pyogenes | Anchorless | Fn | 165 | |
| FnbA | S. dysgalactiae | LPxTz | Fn | 358, 359 | |
| FnbB | S. dysgalactiae | LPxTz | Fn | 358, 359 | |
| GfbA | S. dysgalactiae | LPxTz | Fn | 300 | |
| SmFnB | S. mutans | Anchorless | Fn | 406 | |
| FBP-130a | S. mutans | Unknown | Fn | 99 | |
| PavA-likea | S. mutans | Anchorless | Fn | 407 | |
| ScpB (C5a peptidase)a | S. agalactiae | LPxTz | Fn | 96, 100 | |
| FbsA, FbsBa | S. agalactiae | LPxTz (FbsA) | Fn | 207, 535 | |
| PavA-like/GBS1263a | S. agalactiae | Anchorless | Fn | 407 | |
| PavAa | S. pneumoniae | Anchorless | Fn | 241 | |
| FbpA | S. gordonii | Anchorless | Fn | 102 | |
| CshA | S. gordonii | LPxTz | Fn | 396 | |
| CshA-likea | S. sanguinis | LPxTz | Fn | 530 | |
| AgI/II familya | S. mutans, S. intermedius | LPxTz | Fn | 36, 455, 456 | |
| Glucan-binding proteins | GbpABCD | S. mutans | LPxTz (GbpC) | Dextran-dependent aggregation; glucan | 26, 517, 527, 544, 558 |
| Gbp2-Gbp5, Dei | S. sobrinus | Unknown | 26, 559, 580 | ||
| Collagen-binding proteins | Cpa | S. pyogenes | LPxTz | Collagen | 313 |
| WapA | S. mutans | LPxTz | Collagen | 162, 210 | |
| Cnm | S. mutans | LPxTz | Collagen | 525 | |
| AgI/II familya | S. mutans, S. gordonii | LPxTz | Collagen | 272, 368 | |
| Plasminogen-binding proteins | Enolase | S. pyogenes, S. pneumoniae, S. oralis, S. anginosus, S. mutans, S. salivarius, S. sanguinis | Anchorless | Plasminogen, plasmin | 44, 76, 180, 298, 302, 448, 450, 648 |
| M proteina | S. pyogenes | LPxTz | Plasminogen | 500 | |
| PAM | S. pyogenes | LPxTz | Plasminogen | 42, 651 | |
| GAPDH/Plra | S. oralis, S. anginosus, S. agalactiae, S. pyogenes, S. pneumoniae, S. gordonii, S. dysgalactiae | Anchorless | Plasminogen, plasmin | 45, 179, 277, 366, 540 | |
| CbpE | S. pneumoniae | CBD | Plasminogen | 22 | |
| Streptokinase (Ska) | S. pyogenes | None | Plasminogen | 28 | |
| Laminin-binding proteins | Lbpa | S. pyogenes | LXXC/XXGC | Laminin | 602 |
| Lsp | S. pyogenes | LXXC/XXGC | Laminin | 149 | |
| SpeB | S. pyogenes | Anchorless/none | Laminin | 251 | |
| Lmba | S. agalactiae, S. pyogenes | LXXC/XXGC | Laminin | 567, 631 | |
| AgI/II familya | S. mutans, S. intermedius | LPxTz | Laminin | 81, 272, 456 | |
| PLBP | S. anginosus | LXXC/XXGC | Laminin | 10 | |
| Cnm | S. mutans | LPxTz | Laminin | 525 | |
| 145-kDa protein | S. gordonii | Unknown | Laminin | 563 | |
| Uncharacterized | S. mitis | Laminin | 584 | ||
| Fibrinogen-binding proteins | M proteina | S. pyogenes | LPxTz | Fibrinogen | 245 |
| SOF/SfbIIa | S. pyogenes | LPxTz | Fibrinogen | 113 | |
| Fbp54a | S. pyogenes | Anchorless | Fibrinogen | 114 | |
| Mrpa | S. pyogenes | LPxTz | Fibrinogen | 290 | |
| DemA/Emma | S. dysgalactiae | LPxTz | Fibrinogen | 183, 623 | |
| FbsA, FbsBa | S. agalactiae | LPxTz (FbsA) | Fibrinogen | 207, 535 | |
| CspA | S. agalactiae | LPxTz | Fibrinogen (cleavage) | 221 | |
| SpaP/P1/PAc/Sr/AgB | S. mutans | LPxTz | Fibrinogen | 36 | |
| Uncharacterized | S. gordonii, S. sanguinis, S. mitis, S. oralis | Fibrinogen | 338 | ||
| Ig-binding proteins | SfbI/PrtF1a | S. pyogenes | LPxTz | IgG | 401 |
| Sib35 | S. pyogenes | Anchorless | IgG | 289 | |
| SibA | S. pyogenes | None | IgG, IgA, IgM | 154 | |
| Protein H | S. pyogenes | LPxTz | IgG | 8, 196 | |
| Arp | S. pyogenes | LPxTz | IgA | 48, 576, 577, 606 | |
| Sir | S. pyogenes | LPxTz | IgA, IgG | 577 | |
| Enn protein | S. pyogenes | LPxTz | IgA | 47 | |
| M proteina | S. pyogenes | LPxTz | IgG, IgA | 120 | |
| M-like proteinsa | S. pyogenes | LPxTz | IgG, IgA | 120 | |
| Mrpa | S. pyogenes | LPxTz | IgG | 61, 105, 576 | |
| FcRA | S. pyogenes | LPxTz | IgG | 227 | |
| Lzp | S. pyogenes, S. agalactiae | Anchorless | IgG, IgA, IgM | 434 | |
| Bac/β antigena | S. agalactiae | LPxTz | IgA | 229, 275, 276 | |
| Protein B | S. agalactiae | Unknown | IgA | 158 | |
| Protein G/Spga | S. dysgalactiae | LPxTz | IgG | 155, 205, 438 | |
| FOG | S. dysgalactiae | LPxTz | IgG | 425 | |
| MAG, MIGa | S. dysgalactiae | LPxTz | IgG | 282, 283 | |
| DemA/Emma | S. dysgalactiae | LPxTz | IgG | 623 | |
| CbpA/SpsA/PspC/PbcA/Hica | S. pneumoniae | CBD | IgA | 209 | |
| Platelet-binding proteins | GspB, Hsa | S. gordonii | LPxTz | Platelets (adhesion) | 41, 589 |
| SspA, SspB | S. gordonii | LPxTz | Platelets (aggregation) | 261 | |
| SrpA | S. sanguinis | LPxTz | Platelets | 460 | |
| PAAP | S. sanguinis | Unknown | Platelets | 233 | |
| PblA, PblB, PblT | S. mitis | Unknown | Platelets | 40 | |
| Complement-binding proteins | M proteina | S. pyogenes | LPxTz | Factor H, factor H-like protein 1 | 243, 308 |
| M-like proteinsa | S. pyogenes | LPxTz | Factor H, factor H-like protein 1 | 294 | |
| FbaAa | S. pyogenes | LPxTz | Factor H, factor H-like protein 1 | 451 | |
| CbpA/SpsA/PspC/PbcA/Hica | S. pneumoniae | CBD | Factor H | 266 | |
| CbpA/SpsA/PspC/PbcA/Hica | S. pneumoniae | CBD | Complement C3 | 95 | |
| ScpBa | S. agalactiae | LPxTz | Complement C5a | 106 | |
| Bac/β antigena | S. agalactiae | LPxTz | Factor H | 19 | |
| PspA | S. agalactiae | CBD | Inhibits complement C3 deposition; lactoferrin | 407, 613 | |
| Host cell-binding proteins | BibAa | S. agalactiae | LPxTz | C4-binding protein | 524 |
| ScpAa | S. pyogenes | LPxTz | Complement C5a | 94, 645 | |
| BibAa | S. agalactiae | LPxTz | Epithelial cells | 524 | |
| FbsAa | S. agalactiae | LPxTz | Epithelial/endothelial cells | 534 | |
| ScpBa | S. agalactiae | LPxTz | Epithelial cells | 96 | |
| Lmba | S. agalactiae | LXXC/XXGC | Endothelial cells | 600 | |
| Spb1 | S. agalactiae | LPxTz | Epithelial cell invasion | 4 | |
| 6PGDc | S. pneumoniae | Anchorless | Epithelial cells | 127 | |
| PsaAa | S. pneumoniae | LXXC/XXGC | Epithelial cells | 504 | |
| PsrP | S. pneumoniae | LPxTz | Epithelial cells | 509 | |
| RrgA | S. pneumoniae | LPxTz | Epithelial cells | 422 | |
| PavAa | S. pneumoniae | Anchorless | Epithelial/endothelial cells | 465 | |
| CbpA/SpsA/PspC/PbcA/Hica | S. pneumoniae | CBD | Epithelial cells | 510 | |
| PclA | S. pneumoniae | LPxTz | Epithelial cells | 452 | |
| Shra | S. pyogenes | Anchorless | Epithelial cells | 165 | |
| ScpAa | S. pyogenes | LPxTz | Epithelial cells | 471 | |
| Lbpa | S. pyogenes | LXXC/XXGC | Epithelial cells | 602 | |
| R28 | S. pyogenes | LPxTz | Epithelial cells | 571 | |
| M proteina | S. pyogenes | LPxTz | Epithelial/endothelial cells (α5β1 integrin, Fn bridge) | 118, 147 | |
| SfbI/PrtF1a | S. pyogenes | LPxTz | Epithelial/endothelial cells (α5β1 integrin, Fn bridge) | 219, 442, 591 | |
| SclA, SclB (Scl1, Scl2) | S. pyogenes | LPxTz | Epithelial cells (α2β1, α11β1 integrins) | 86, 248, 369, 483, 484 | |
| FBP-130a | S. mutans | Unknown | Endothelial cells | 99 | |
| GtfG | S. gordonii | None | Endothelial cells | 618 | |
| HAFa | S. salivarius | Unknown | 213 | ||
| Alp familya | S. agalactiae | LPxTz | Epithelial cells | 30, 60, 571 | |
| Pili, fimbriae, fibrilsa | S. agalactiae, S. pyogenes, S. pneumoniae, S. salivarius | LPxTz | Epithelial/endothelial cells | 2, 23, 139, 217, 377, 459 | |
| AgI/II familya | S. mutans, S. gordonii | LPxTz | Epithelial/endothelial cells (α5β1 integrin) | 12, 427 | |
| Enzymes | HtrA | S. mutans, S. pneumoniae, S. pyogenes | Anchorless | Serine protease | 131, 371, 537 |
| GAPDH, α-enolase, PGK, PGM, TPIa,c | S. pyogenes, S. oralis, S. anginosus | Anchorless | Glycolytic enzymes | 298, 447 | |
| GAPDH/SDHa | S. pyogenes | Anchorless | Lysozyme, cytoskeletal proteins, CD87/uPAR | 277, 447 | |
| PulA | S. pyogenes | LPxTz | Pullulanase | 250 | |
| SAP | S. agalactiae | LPxTz | Pullulanase | 523 | |
| SpuA | S. pneumoniae | LPxTz | Pullulanase | 621 | |
| Neuraminidase (NanA, NanB) | S. pneumoniae | LPxTz (NanA) | Breaks down N-acetyl neuraminic acid | 247, 381 | |
| Hyal1 | S. pneumoniae | LPxTz | Hyaluronidase | 46 | |
| StrH | S. pneumoniae | LPxTz | β-N-Acetylhexoseaminidase | 104 | |
| GlnA | S. agalactiae | LPxTz | Glutamine synthetase | 583 | |
| FruA | S. mutans | LPxTz | β-d-Fructosidase | 77 | |
| Dex, DexA | S. mutans, S. sobrinus | LPxTz | Dextranase | 254, 632 | |
| Other adhesins | CbpA/SpsA/PspC/PbcA/Hica | S. pneumoniae | CBD | Polymeric Ig receptor | 665 |
| PsaAa | S. pneumoniae | LXXC/XXGC | E-cadherin | 16 | |
| Srr-1 | S. agalactiae | LPxTz | Keratin | 520 | |
| ScaA | S. gordonii | LXXC/XXGC | Coaggregation | 15, 305 | |
| GRAB | S. pyogenes | LPxTz | α2-Macroglobulin; protects against proteolysis | 485 | |
| Protein G/Spga | S. dysgalactiae | LPxTz | α2-Macroglobulin, serum albumin | 7, 413 | |
| MAG, MIGa | S. dysgalactiae | LPxTz | α2-Macroglobulin, serum albumin (MAG) | 281-283 | |
| PhtA, PhtB, PhtD, PhtE | S. pneumoniae | LXXC/XXGC | Unknown | 3 | |
| Sip | S. agalactiae | Anchorless | Unknown | 67 | |
| BPS | S. agalactiae | LPxTz | Unknown | 152 |
Protein appears more than once in the table due to multiple binding specificities.
LPxTz, sortase motif; LXXC/XXGC, lipoprotein consensus sequence; CBD, choline binding domain.
PGK, phosphoglycerate kinase; PGM, phosphoglycerate mutase; TPI, triosephosphate isomerase; 6PGD, 6-phosphogluconate dehydrogenase.
Pili
Pili are thicker (3 to 10 nm in diameter) and longer appendages than fibrils, typically extending 1 to 3 μm from the bacterial cell surface (Fig. 4A). Interestingly, while they have been reported for just two species of oral streptococci, S. parasanguinis and S. salivarius (166, 214), they have been found in the three major streptococcal human pathogens, namely, S. agalactiae (336), S. pyogenes (412), and S. pneumoniae (29) (Table 1). The precise composition of S. salivarius pili remains to be defined, but the structural component of S. parasanguinis pili has been identified as a serine-rich repeat protein, designated Fap1 (652, 653). The genes encoding pili of the pathogenic streptococci are found in discrete loci, termed PI. To date, three PI have been identified for S. agalactiae (512), two have been identified for S. pneumoniae (23), and nine have been identified for S. pyogenes (157), with the latter occurring within the Fn-binding, collagen-binding, T-antigen (FCT) region that forms part of the Lancefield T-serotyping system. While the exact composition varies, each PI comprises genes that encode LPxTz family proteins, which are linked to cell wall peptidoglycan precursors via sortase cleavage between T and z (see below). These form the physical structure of the pilus and can be divided into the “backbone” subunit, which forms the shaft of the pilus, and one or two “ancillary” subunits, which appear intermittently. There are also genes encoding transpeptidase enzymes of the sortase C subfamily that function to polymerize the protein subunits (see reference 469 for a detailed model of pilus assembly). Much attention has recently been given to such pili, as the protein subunits have been shown to elicit protective immunity against the corresponding pathogen in mouse models of infection, making them potential vaccine candidates (187, 376, 412). Such strategies have particular promise for S. agalactiae, where pilus protein conservation across a large number of clinical isolates has been shown to be relatively high (383).
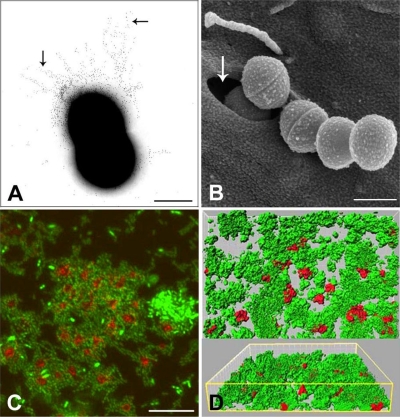
FIG. 4.
Streptococcus-host interactions. (A) Pili (arrows) of GBS immunogold labeled with antibody generated to the backbone subunit of PI-2a. Bar, 0.5 μm. (B) Internalization of GAS by cultured epithelial cells showing formation of caveolae (arrow) containing a streptococcal cell being engulfed. Bar, 1 μm. (Image courtesy of Manfred Rohde, GBF-German Research Centre for Biotechnology, Braunschweig, Germany, reproduced with permission.) (C) Aggregation of human platelets (red) (phalloidin stained) by S. sanguinis (green) (fluorescein isothiocyanate stained). Bar, 20 μm. (Image courtesy of Steve Kerrigan, Royal College of Surgeons in Ireland, reproduced with permission.) (D) Flow cell biofilm (24 h) showing xy perspective and three-dimensional projection by confocal imaging of S. gordonii (green) and Veillonella atypica (red) growing in human saliva. Under salivary flow conditions, V. atypica is unable to form monospecies biofilms, but it is able to form mixed-species biofilms with S. gordonii. (Image courtesy of Rob Palmer, National Institute of Dental and Craniofacial Research, NIH, Bethesda, MD, reproduced with permission.)
Pili have also been implicated as putative virulence factors. Using recombinant protein subunits or whole bacterial cells, pili have been associated with mediating adhesion to a wide variety of host epithelia including cells derived from the lungs, cervix, nasopharynx, tonsils, and intestine (2, 23, 139, 459). In addition to mediating cell attachment, pili of S. agalactiae (group B Streptococcus [GBS]) have been shown to promote the invasion of human endothelial cells (377) and may facilitate paracellular translocation across the epithelial barrier (459). Immunomodulatory capabilities have also been associated with pili. Those of S. pneumoniae have been shown to promote inflammatory cytokine release (29), while S. agalactiae pili confer resistance to phagocytic killing (378). The pili of S. pyogenes promote bacterial aggregation via binding to the salivary component gp340, a process that may lead to increased bacterial clearance (145). Similarly, pili of S. parasanguinis were shown to bind salivary molecules adsorbed onto the surface of hydroxyapatite, an in vitro model of the tooth surface (153, 653).
Because of the inherent difficulties associated with their dissociation and purification, relatively little is known about the actual structures of pili. There have, however, been some recent breakthroughs. Advanced microscopy techniques, including atomic force and cryo-electron microscopy, have been utilized to visualize pneumococcal pili (156, 237). From these data, a model has been proposed in which protofilaments of backbone protein organize into coiled-coil structures to which “clusters” of ancillary proteins are attached intermittently (237). An ancillary protein component of S. agalactiae pili was crystallized (320) and was shown to have an IgG-rev fold. Such a conformation has been found in microbial surface components recognizing adhesive matrix molecules (MSCRAMMs) of other gram-positive bacteria and contributes to the adhesive capabilities of these proteins. The crystal structure of a backbone subunit from S. pyogenes has also been resolved (287). From these data, backbone subunits were shown to organize into filamentous structures supported by sortase-catalyzed intermolecular bonds and by self-generated intramolecular isopeptide bonds. This organization has been proposed to stabilize the thin pilus structures, enabling them to withstand the tensile forces associated with adhesion to host tissues. Interestingly, sequence comparisons have identified similar intramolecular isopeptide bonds in other surface adhesins, including PFBP of S. pyogenes, FnbB of S. dysgalactiae, and Cna of Staphylococcus aureus (287).
Backbone and ancillary subunits from each PI have been shown to elicit protective immunity against GBS in a neonatal mouse model of immunization (376, 383, 512), and a recent analysis of pilus distribution and conservation found that 94% of 289 clinical isolates expressed pili on their surface. Those studies also showed that a vaccine comprising just three pilus protein subunits could confer protection against 94% of GBS strains currently found in the United States and Italy (383). A pilus-based vaccine, therefore, has great potential for development as an effective universal vaccine against GBS disease.
Sortases
Many streptococcal surface proteins are attached to the bacterial cell wall by membrane-associated transpeptidases of the sortase family (comprehensively reviewed in reference 384). These enzymes function by cleaving target proteins at a C-terminal cell wall sorting signal (CWSS), typically LPxTz, to form an acyl enzyme intermediate. This is then resolved by the nucleophilic attack of amino groups, often provided by the lipid II precursor of peptidoglycan, which is subsequently incorporated into the cell envelope via the transglycosylation and transpeptidation reactions of cell wall synthesis. Target proteins of sortases are initially expressed in a precursor form bearing at least two topogenic sequences: an N-terminal signal peptide and the C-terminal CWSS. The signal sequence directs the protein for translocation across the plasma membrane, typically via the Sec secretion system, until the CWSS is reached. At this point, the protein is held in the membrane by a stretch of hydrophobic amino acids immediately downstream of the CWSS. The CWSS is then available for cleavage by the membrane-bound sortase, resulting in a protein that is exposed on the bacterial surface while securely embedded within the cell envelope.
Sortases have been found in virtually all gram-positive bacterial genomes available to date. Based on phylogenetic analyses, two recent studies have proposed their classification into either four (subfamilies A to D) or five (SrtA, SrtB, and families 3 to 5) subfamilies (109, 140). The sortase A (SrtA) subgroup contains the archetypal SrtA from Staphylococcus aureus, and virtually all gram-positive bacteria analyzed to date possess a single srtA gene. Often referred to as the “housekeeping” sortase, this enzyme typically anchors the majority of surface proteins with a CWSS expressed by a given bacterium. As such, SrtA target proteins are abundant and functionally diverse. The genes encoding SrtA-type enzymes are never proximal to their substrates, and SrtA enzymes show a preference for an LPxTG CWSS motif. Members of the sortase B (SrtB) subgroup can be found in a small number of gram-positive bacilli and cocci. The prototype SrtB is from S. aureus and acts upon IsdC, a protein involved in heme-iron acquisition. Similarly, the genes encoding other SrtB members occur in the same operon as their target proteins and are involved in iron uptake. SrtB enzymes possess three amino acid regions that are not present in SrtA, and the CWSS motifs recognized by SrtB include NPQTN (S. aureus), NPKSS (Listeria species), and NPKTG (Bacillus anthracis). The sortase C (SrtC) subgroup (or subfamily 3) has the highest number of members. Multiple copies of genes encoding SrtC are often found per genome, and they are frequently located adjacent to their substrates. As for SrtA, SrtC enzymes recognize the LPxTG CWSS motif, often followed by a second G residue, but act upon a much smaller group of proteins than SrtA. Furthermore, while SrtA has an N-terminal stretch of hydrophobic amino acids, similar to type I membrane proteins, SrtC enzymes have a hydrophobic C terminus that could act as the membrane anchor, as for type II membrane proteins. Members of this subgroup have recently been found in pathogenic streptococci including S. pyogenes, S. pneumoniae, and S. agalactiae. In these genomes, the srtC genes occur within loci that encode pili, putative virulence factors, and function to covalently link pilus protein subunits to form the filamentous pilus structure. Subfamily D sortases (or subgroups 4 and 5) have been found in bacilli, clostridia, and actinomycetales. Their target proteins often have enzymatic functions and are characterized by atypical CWSS motifs, including LPxTA and LAxTG.
Despite extensive knowledge of the biochemical reactions that result in the covalent linkage of secreted polypeptides to the cell wall peptidoglycan, the control mechanisms operating on sortase-catalyzed linkage and on surface protein localization are not well understood. There is evidence for a membrane microdomain in S. pyogenes, enriched in anionic phospholipids, within which proteins such as SpeB (cysteine protease) are secreted and folded (507). This microdomain, termed the ExPortal, was suggested to be the primary cellular site for protein secretion, accumulating high concentrations of translocons of the general secretion (Sec) pathway in addition to accessory proteins, e.g., HtrA, necessary for postsecretion folding (506). Since, however, the localization patterns of surface proteins are quite distinct, there must be additional signals that play a role in targeting polypeptides to discrete sites. One such signal appears to be the leader peptide (signal sequence), which, in S. pyogenes, directs the secretion of proteins to different subcellular regions. The signal sequence of M protein directs secretion at the division septum, whereas that of PrtF preferentially promotes the secretion of this polypeptide at the old pole (85). In those studies, SecA was shown to be distributed throughout the periphery of S. pyogenes, thus arguing against evidence for a single ExPortal. More recently, evidence has been obtained to show that sortase is distributed to the new division septum, colocalizing with areas of M protein anchoring (490). This supports the notion that the sorting process is dynamic and linked closely with cell division. The control of sortase activity is also of interest, and it was suggested that the C-terminal peptide that remains as a result of sortase-catalyzed cleavage may play a subsequent role in modulating the expression or secretion of other proteins (J. Kreth, A. Khammanivong, Y. Lei, Y. Zhang, and M. C. Herzberg, presented at the International Association for Dental Research 85th General Session, New Orleans, LA, 21 to 24 March 2007). Overall, this area of study is of significant interest since the regulation of surface protein expression is closely linked to the ability of streptococci to colonize and invade tissues.
STREPTOCOCCUS ADHESINS
There are three ways in which streptococcal proteins may be held at the cell surface. First, they may be covalently anchored through the C terminus to the cell wall peptidoglycan. Second, they may be tethered to the cell membrane through N-terminal modifications with lipid (lipoproteins). Third, they may be retained on the cell surface, or bound back to the cell surface, through noncovalent interactions with cell surface components such as other proteins or polysaccharides. Many of the polypeptides listed in Table 1 belong to the superfamily of gram-positive bacterial proteins that are linked to the cell wall. These contain a C-terminal motif, the consensus for which is LPxTz, followed by a hydrophobic region and a charged tail. The motif is recognized by sortase A, for which there is one gene on the streptococcal chromosome. Since it is thought that all C-terminal protein linkage to the cell wall peptidoglycan occurs through the activity of SrtA (see above), there must be strong evolutionary selection against mutation within srtA. Streptococci appear to differ considerably in the numbers of LPxTz proteins produced on their cell surfaces. Genomic analyses show that whereas there are 33 genes encoding potential cell wall-anchored proteins in S. sanguinis SK36, S. mutans UA159 has only 6, S. pyogenes M1 has 13, S. gordonii CH1 has 20, and S. agalactiae NEM316 has 21 (161, 194, 657). However, there is always some redundancy in cell wall protein genes, and within the 33 genes in S. sanguinis, there are 9 paralogous genes across three families (657). These observations underscore the notion that streptococci utilize different complements of adhesins in order to successfully colonize the host.
Cell Wall-Anchored Polypeptides
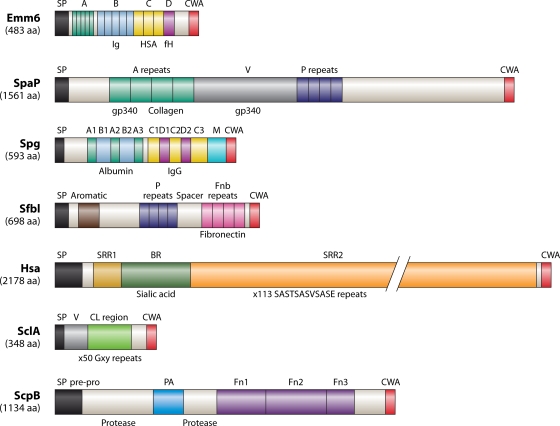
Most of the adhesins listed in Table 1 are of the LPxTz superfamily. These adhesins come in many sizes, designs, and potential conformations (e.g., α-helical, coiled coil, and β-sheet). Within the superfamily, there are proteins that seem to be assembled, at least in part, from a relatively defined range of modules that are related in primary sequence and in adherence functions. An overriding feature of these polypeptides is that they contain amino acid repeat blocks or motifs. These may be short sequences, for example, the five blocks of 14 amino acid (aa) residues making up the A repeats of the M6 protein (Fig. 5), or highly repeated motifs, such as the 113 repeats of SASTSASVSASE in the S. gordonii Hsa protein (Fig. 5). Alternatively, they may comprise much larger repeats, for example, the 13 repeat blocks of 101 aa residues within S. gordonii CshA (398). The genes encoding these polypeptides are highly subject to intragenic recombination, intergenic recombination, gene duplication, and point mutation as a result of host selective pressures. For example, 34 distinct alleles of the Fn-binding protein SfbI (Fig. 5) have been found in 54 strains of S. pyogenes (610) as a result of horizontal gene transfer. The N-terminal aromatic-amino-acid-rich domain has a high degree of sequence variability, while the deletion or duplication of repeat units has resulted in variable numbers of proline-rich (P) repeats (1 to 11 repeats) and Fn-binding (Fnb) repeats (1 to 5 repeats) (Fig. 5). This generates antigenic variation and also variable functional capabilities. Different lengths of repeat blocks also elicit different antibody repertoires (199), so lower-repeat-number variants with fewer epitopes might be able to escape antibodies generated to higher-repeat-number variants.
FIG. 5.
Structural features of seven streptococcal cell surface proteins that function in adherence and colonization. Emm6, S. pyogenes; SpaP, S. mutans; Spg, S. dysgalactiae; SfbI, S. pyogenes; Hsa, S. gordonii; SclA, S. pyogenes; ScpB, S. agalactiae. Precursor polypeptides are drawn N terminal (left) to C terminal (right), and all of the proteins are thought to be held at the cell surface through covalent cell wall anchorage (CWA) via a specialized C-terminal motif (see text). Leader (signal) peptides (SP) are cleaved at conventional sites by signal peptidase I. Specific structural features and amino acid residue repeat block regions are indicated (see descriptions in the text). Like-shaded regions across the different proteins indicate only similarities in amino acid composition or predicted secondary structure, e.g., α-helical coiled coil, and not sequence homologies. Conversely, amino acid residue repeat blocks within a polypeptide, e.g., SpaP, are highly conserved. Some of the substrates bound by the polypeptides, and the approximate locations of the binding sites, are indicated below each structure. fH, factor H; gp340, cysteine-rich scavenger protein (salivary agglutinin).
The structures of seven streptococcal cell wall-anchored proteins are depicted in Fig. 5. They have been selected to cover many of the different design features of streptococcal proteins. Each of the precursor proteins carries a leader (signal) peptide, usually about 30 to 40 aa residues in length but much longer (up to 90 aa residues) for some polypeptides, e.g., Hsa (Fig. 5). These leader sequences direct the respective proteins to the primary Sec secretion apparatus or to an accessory secretion system (Sec2) dedicated to exporting specific subsets of proteins (499, 670).
M proteins.
GAS species are classified into 180 emm types based on the sequence of the hypervariable (N-terminal) region of the M protein. M protein typically consists of four repeat regions (Fig. 5), the A repeats of which are hypervariable and the B repeats of which are semivariable. Physicochemical studies suggested that M proteins form entirely α-helical coiled-coil dimers and are in an extended conformation on the cell surface as hair-like projections (424). Secondary structure predictions indicated that the N-terminal regions are stabilized by antiparallel interactions, so this may contribute to cell-cell aggregation (174). Many of the hypervariable repeat regions from different M proteins bind human C4b-binding protein (C4BP), a plasma protein that inhibits complement activation (17), plasminogen, IgA, IgG, and factor H (389). The B repeats bind fibrinogen, human serum albumin (HSA), and IgG, while the C repeats bind factor H, HSA, and the membrane cofactor protein CD46 (188, 433). Different M proteins have the ability to interact only with various subsets of the human plasma proteins specified, and A and B repeats vary in most of the known GAS serotypes. Strong sequence similarities to the C repeats are found in Arp polypeptides (M-like proteins), but the so-called B repeats in Arp are not similar to those in M6 or other serotypes (Table 1). Structural irregularities and instabilities throughout the coiled-coil structure of the M1 protein enhance fibrinogen binding, thus promoting proinflammatory and antiphagocytic activities (399). Soluble M1 protein released from S. pyogenes is a potent inducer of T-cell proliferation and the release of Th1-type cytokines. This leads to the suggestion that it is also a novel streptococcal superantigen contributing to excessive T-cell activation and inflammatory responses during invasive streptococcal infections (445). The pathological inflammatory response is believed to be enhanced by IgG antibodies to the C repeats (Fig. 5), complexing with fibrinogen and activating FcgII receptors on neutrophils to release heparin-binding protein, resulting in vascular leakage (285).
AgI/II polypeptides.
Most indigenous streptococci found in the oral cavity express cell surface polypeptides of the AgI/II family. These have been shown to interact with multiple host environmental receptors such as collagen, Fn, laminin, and other oral microorganisms, e.g., Actinomyces naeslundii, S. oralis, Porphyromonas gingivalis, and Candida albicans (271). It was suggested, therefore, that these proteins contribute to initial adherence as well as to microbial community development. A major receptor for AgI/II family proteins is gp340, a highly glycosylated (∼25% carbohydrate) innate defense molecule produced at mucosal surfaces (466) and involved in the regulation of cellular immune responses (59) and epithelial differentiation (288). In the fluid phase, gp340 promotes bacterial aggregation and clearance, but when surface immobilized, it provides a receptor for streptococcal adherence (62). AgI/II polypeptides interact with gp340 oligosaccharides and with peptide backbone sequences (52, 263). The AgI/II polypeptides from oral streptococci contain between 1,310 and 1,653 aa residues, while the genes present in some strains of S. pyogenes and in S. agalactiae encode polypeptides in the range of 863 to 1,352 aa residues. The overall structure of AgI/II polypeptides from different streptococci is conserved, with ligand-binding domains assigned to alanine-rich (A) and central (V) regions and to a C-terminal region downstream of the proline-rich (P) region (Fig. 5). The N-terminal region including the A repeats is predicted to form α-helical coiled-coil structures similar to M protein. The V region (approximately 370 aa residues in SpaP) is predicted to carry a lectin-like trench for carbohydrate binding (611). The P region carries repeats of 39 aa residues rich in proline and is essential for the secretion and stability of AgI/II (541). Adhesive and immunodominant epitopes of AgI/II have been identified and enabled the development of pilot synthetic peptide inhibitors of bacterial adherence. One of these, a synthetic 20-aa-residue peptide designated p1025, comprising 1,025 to 1,044 aa of AgI/II from S. mutans, blocks the binding of streptococci to gp340 (292). Epitopes within the A and P regions are in close proximity in native AgI/II (622), and so it is believed that the A and P regions interact to orientate the V region to bind oligosaccharides. Ag I/II-binding domains for P. gingivalis are discussed below (see Community Development).
Ig-binding proteins.
A number of Ig-binding proteins (Table 1) contribute to the ability of GAS to escape detection by the immune system. The proteins include M protein; the M-like proteins Enn, Mrp, and protein H; IgA receptor protein (Arp); streptococcal Ig receptor (Sir); streptococcal Ig-binding protein from GAS (SibA); and SfbI. All of these proteins contain leucine zipper motifs that consist of leucine residues spaced 6 or 7 aa residues apart. The Lzp protein is found in GAS and GBS and is a 66-kDa protein with 24 leucine zipper motifs and a signal peptide but no LPxTz motif. It reacts with light chains of human IgG and IgA and heavy chains of IgG and IgM. Protein H, SfbI, and Mrp bind only IgG, while Arp, Sir, and SibA bind IgA and IgG. Protein H has the highest affinity for IgG (1.6 × 10−9 M), while the affinity of Lzp for IgG is 5.5 × 10−8 M. Lzp binds several isotypic IgGs with high affinity and also binds to itself and forms multimers on the cell surface. Like protein H (173), Lzp binds a wide range of proteins (434). Protein G (Spg) (Fig. 5) (57) and FOG (425) contribute to the accumulation of IgG on the surfaces of group C and group G streptococci. FOG has no homology to the IgG-binding sequences of protein G. Protein G binds all four IgG subclasses, while FOG does not bind IgG3. The interaction with protein G ablates the ability of IgG to bind C1q, thus preventing the activation of the classical pathway of complement. The alanine-rich E region of Spg (Fig. 5) binds α2-macroglobulin, which is a protease inhibitor and immunoregulator. This occurs independently of IgG binding, and of HSA binding, to the three-α-helix-containing B1B2 region (Fig. 5). The IgG-binding region of Spg is similar in sequence to the IgG-binding regions of the MIG and MAG proteins, but there is no homology toward the N terminus.
The S. equi M protein is known as fibrinogen binding protein and binds to the interdomain region of IgG Fc (350). The same regions are recognized by staphylococcal protein A and by protein G. The unrelated IgA-binding proteins Sir22 (GAS), beta-protein (GBS), and SSL27 from S. aureus all bind the Fc domain interface in human IgA (481).
Fn-binding proteins.
Fn-binding proteins are expressed by all streptococci (Table 1), but the proteins differ in their binding affinities. Some are able to bind soluble Fn with high affinities in the nM range, while others appear not to be able to bind soluble Fn and attach only to Fn immobilized onto a surface (396). Most of the Fn-binding proteins are anchored to the cell wall peptidoglycan through the LPxTz motif (Table 1), although some are classed as anchorless adhesins (see below). The Fn-binding proteins attach bacteria to the ECM, which acts as a bridge between streptococci and host cells. There are at least 11 Fn-binding proteins in S. pyogenes (310), including SfbI (PrtF1) (Fig. 5), protein F2 (PrtF2/FbaB/PFBP), serum opacity factor (SOF/SfbII), FbaA (601), and several different M proteins. The expression of Fn-binding proteins is highly regulated in response to environmental factors (312). The binding of Fn by these proteins is associated mainly with sequences within the amino acid repeat blocks toward the C-terminal end of the polypeptide (Fig. 5). The numbers and sizes of these blocks vary between different proteins. SfbI contains four blocks of 37 aa residues, and they each contain the core consensus sequence ED(T/S)(X,7-10)GG(X,4)(I/V)(D/E)(F/I/T) for binding Fn, which is also found in many of the other proteins with Fn-binding repeats (Table 1). However, regions upstream of the repeat blocks have been shown to be involved secondarily in binding Fn, and in SfbI, the upstream spacer region (Fig. 5) is able to bind Fn when expressed as a separate polypeptide (593). The N-terminal aromatic domain of SfbI (Fig. 5) may provide some specificity in the recognition of glycosylated receptors or carbohydrate repeat structures. The region has homology to an aromatic sequence comprising YG repeat blocks of approximately 20 aa residues found within the C-terminal repeating unit of glucosyltransferases (GTFs) (190). The YG repeats are also found in S. mutans GBPs (25), Clostridium difficile toxin, and the S. pneumoniae autolysin LytA. Polypeptides such as S. gordonii CshA, which bind only to immobilized Fn, do not contain the core consensus sequence (398). In addition, C5a peptidases of S. pyogenes (ScpA) and S. agalactiae (ScpB) (Fig. 5) are able to bind Fn with high affinity (96, 595), but this is not associated with the protease cleavage site or with Fn-binding repeats (the repeats shown in Fig. 5 are Fn type III domains). There is therefore still much to be learned about the molecular mechanisms involved in interactions of cell surface proteins with Fn and other ECM molecules.
Serine-rich repeat polypeptides.
Serine-rich repeat (Srr) glycoproteins have been found in oral streptococci, pathogenic streptococci, staphylococci, and lactobacilli (Table 1). These are characterized by containing multiple serine-rich repeats running through approximately 75% of the polypeptide. A unique N-terminal region is thought to be held out from the cell surface via a stalk-like structure generated by the O-glycosylated repeats (587). The Hsa polypeptide from S. gordonii DL1 (Fig. 5) is one of two alleles encoding Srr proteins in this species, the other being GspB (41). Like all Srr proteins, Hsa contains two repeat regions, with the smaller repeat region at the N terminus being flanked by two nonrepeated regions. The latter are diverse, and the nonrepeat II region may be basic (Fig. 5) or acidic (pI < 6.0). The pI value may be important in directing this region to binding host receptors. The B region in Hsa, GspB, and SrpA directs the recognition of sialic acid oligosaccharides (39, 460) found on epithelial cells, platelets, and salivary glycoproteins. Isogenic mutants in Srr protein genes are correspondingly deficient in adherence to host cells (261) and the salivary pellicle (653). The Srr protein genes are found at loci containing additional genes encoding glycosylation enzymes and an alternative secretion system. There is a core region of seven genes that are conserved in every genome that contains an Srr protein gene (670). The rest of the locus contains genes encoding glycosyltransferases, but these genes may also be at other sites on the chromosome. The diversity of glycosyltransferases produced across streptococci ensures that glycolytic modifications to these proteins are structurally and antigenically distinct. The presence of an alternative secretion system (SecA2) has also sparked interest in novel export functions, with evidence that the glycosylation of Fap1 in S. parasanguinis is linked to secretion and that the Sec2 system in Listeria monocytogenes is associated with the secretion of virulence factors and of proteins like superoxide dismutase that do not possess conventional signal sequences (18, 499). Interestingly, while genes encoding Srr proteins have been identified for a wide range of streptococci (Table 1), they have not yet been found for S. pyogenes. Clearly, the selection pressures for the retention of a locus of approximately 20 kb encoding a surface protein and a dedicated secretion and modification system must be high, suggesting that the expression of Srr polypeptides by oral streptococci and GBS must be critical to their successful colonization.
Collagen-like proteins.
Collagen-like proteins are characterized by carrying a conserved collagen-like region and a hypervariable N-terminal portion (484). There are two genes encoding collagen-like proteins in S. pyogenes, designated SclA (Scl1) and SclB (Scl2) (Table 1). The transcription of sclA is upregulated by the transcriptional regulator Mga, while sclB is downregulated. In addition, slipped-strand mispairing at sites containing pentanucleotide noncoding repeats leads to phase variation of SclB (483). Orthologs of these genes have not been found in any of the Streptococcus genomes sequenced to date, except S. equi (closely related to S. pyogenes). The collagen-like sequences are comprised of Gxy triplet amino acid residue motifs, and there are 50 of these contiguous repeats in SclA (Fig. 5) (369). The noncollagenous globular domain of SclA binds factor H (87) and apolipoprotein B, a component of low-density lipoprotein (210). The collagen-like region has the functional properties of human collagen, interacting with α2β1 integrin and activating intracellular signaling pathways (248). Interestingly, proteins containing the collagen-like sequences (Gxy triplet motif) are found in S. pneumoniae, Clostridium perfringens, and Haemophilus species. This suggests that this is a well-conserved sequence distributed widely across bacteria, possibly providing extension, flexibility, and additional functionality to surface protein modules.
Peptidases.
A number of hydrolytic enzymes such as glycanases, glycosidases, proteinases, and peptidases are secreted by streptococci. Some of these carry the LPxTz motif for covalent cell wall anchorage, and some do not and are released into the environment (Tables 1 and 2). A dipeptidase from S. gordonii is secreted and released but carries no signal peptide (195). Clearly, there is still a limited understanding of the molecular mechanisms of protein secretion in streptococci. Also, it is apparent from genomic sequencing data that there are numerous genes encoding LPxTz polypeptides with potential proteolytic activities based on homology searches, which are yet to be characterized. However, GAS and GBS both produce a C5a peptidase (Scp), a cell wall-anchored LPxTz protein that specifically inactivates complement factor C5a (Fig. 5). This factor is normally a chemotactic peptide that attracts polymorphonuclear neutrophils, which are critical for phagocytosis. Scp is a subtilisin-like protease with a 68-aa-residue prosequence that must be removed to produce active Scp (Fig. 5). The PA domain is shared by other proteases such as the Lactococcus cell wall-associated protease, and there are three Fn type III repeats in the C-terminal region. It is thought that the Pro- and Gly-rich sequences within these repeats are α-helical and could push the protein and active-site region out from the cell surface. Two RGD (integrin recognition) sequences could interact with cellular receptors and increase Scp activity through conformational changes (70).
TABLE 2.
Secreted factors and transporters
| Protein group | Protein(s) | Species | Function(s) and/or substrate(s) | Reference(s) |
|---|---|---|---|---|
| Glucosyltransferases (secreted) | GtfB, GtfC, GtfD | S. mutans | Glucan synthesis | 26, 533, 659 |
| GtfB, GtfP | S. sanguinis | 244, 657 | ||
| GtfG | S. gordonii | 628 | ||
| GtfI, GtfS, GtfT, GtfU | S. sobrinus | 192, 211, 212, 420, 519 | ||
| GtfR | S. oralis | 175 | ||
| GtfJ, GtfK, GtfL, GtfM | S. salivarius | 189, 191, 553 | ||
| Fructosyltransferases (secreted) | SacB/Ftf | S. mutans | Fructan synthesis | 6 |
| Ftf | S. salivarius | 487 | ||
| Hemolysins (secreted) | Hemolysin III | S. mutans | Erythrocytes | 6, 407 |
| CylE | S. agalactiae | 468 | ||
| SLO, SLS | S. pyogenes, S. dysgalactiae | 13, 51, 575 | ||
| Other degradative enzymes (secreted) | DNase A-DNase D | S. pyogenes | DNA | 13 |
| Hyaluronidase | S. pyogenes, S. pneumoniae | Hyaluronic acid | 236, 482 | |
| EndoS | S. pyogenes | Endoglycosidase, IgG | 108 | |
| SpeB | S. pyogenes | IgG (cleavage) | 108 | |
| Toxins (secreted) | SMEZ, SMEZ-2, SMEZ-3 | S. pyogenes | Superantigens | 182, 286, 470 |
| SSA | S. pyogenes | 14, 410 | ||
| SpeA, SpeC, SpeG, SpeH, SpeI, SpeJ, SpeK, SpeL, SpeM | S. pyogenes | 14, 407, 470 | ||
| NADase | S. pyogenes | NAD+-glycohydrolase; promotes survival and proliferation | 66 | |
| Pneumolysin | S. pneumoniae | Cytolysis | 107 | |
| Other secreted factors | Streptokinase (Ska) | S. pyogenes, S. dysgalactiae | Plasminogen | 379, 407 |
| Mac | S. pyogenes | Inhibits opsonophagocytosis; CD11b, CR3 | 339, 407 | |
| SIC | S. pyogenes | Inhibits complement-mediated lysis | 9 | |
| Sg-xPDPP | S. gordonii | X-prolyl dipeptidyl-peptidase | 195 | |
| Transporters | PiuBCDA/PiaABCD | S. pneumoniae | Fe | 71, 73 |
| FhuBDGC | S. pneumoniae | Fe | 467 | |
| PitADBC | S. pneumoniae | Fe | 72 | |
| FtsABCD | S. pyogenes | Fe | 218 | |
| SiaABC/HtsABC | S. pyogenes | Fe | 32, 340 | |
| SiuADBG | S. pyogenes | Fe | 411 | |
| SsaCBA | S. sanguinis | Fe | 177, 270 | |
| PsaABCD | S. pneumoniae | Mn | 522 | |
| MtsABC | S. pyogenes, S. agalactiae | Mn, Fe, Zn, Cu | 63, 267, 268 | |
| ScaCBA | S. gordonii | Mn | 303, 304, 647 | |
| FimCBA | S. parasanguinis | Mn | 160, 270, 430 | |
| SloABCR | S. mutans | Mn, Fe | 299, 446 | |
| AdcRCBA | S. pneumoniae, S. gordonii, S. pyogenes, S. agalactiae, S. mitis, S. mutans | Mn, Zn | 134, 365 | |
| CopYAZ | S. gordonii, S. mutans, S. pneumoniae, S. agalactiae, S. pyogenes, S. sobrinus, S. mitis | Cu | 408, 494, 624 | |
| PstSCAB | S. pneumoniae | Phosphate | 429 | |
| FruRKI | S. mutans, S. gordonii, S. pneumoniae, S. pyogenes, S. agalactiae, S. mitis | Fructose | 38, 364 | |
| FxpABC | S. mutans, S. pyogenes, S. pneumoniae | Fructose, xylitol | 38 | |
| LacABCDFE | S. mutans | Lactose | 511 | |
| ScbCBA | S. cristatus | Unknown | 111 | |
| BfrCD, BfrEFG | S. gordonii | Biofilm formation | 669 | |
| BfrCD, BfrXY | S. sanguinis | Biofilm formation | 669 | |
| SilDE | S. pyogenes | Virulence | 151, 235 | |
| OppABCDF | S. pyogenes | Virulence | 461, 637 | |
| HppABCDF | S. gordonii | Adhesion, coaggregation | 397 |
Anchorless Adhesins
The majority of streptococcal surface proteins anchor through their C termini via an LPxTz motif. There are, however, a small group of proteins that bind to the cell surface but possess no recognized anchor. Such proteins also frequently lack an N-terminal signal sequence. The mechanism by which these so-called “anchorless adhesins” are exported from the cytoplasm to the cell surface is not understood. However, many can be removed by chaotropic agents (129), implying that they are likely bound to the cell surface through less-defined charge or hydrophobic interactions.
The anchorless adhesins have not been grouped together as a family in Table 1 because they are structurally and functionally diverse and have a wide range of adherence properties. A large proportion of the anchorless adhesin family comprises proteins with enzymatic functions. Five of the streptococcal anchorless adhesins identified to date are glycolytic enzymes typically found in the cytosol, namely, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), α-enolase, phosphoglycerate kinase, phosphoglycerate mutase, and triosephosphate isomerase (298, 447). Of these, GAPDH and α-enolase are the best characterized. α-Enolase is the major plasmin- and plasminogen-binding protein of streptococci (Table 1), although it has also been associated with binding to mucin MG2 for S. mutans (180). Plasminogen can be converted following cleavage by plasminogen activators to the serine protease plasmin. This in turn can degrade ECM proteins. Thus, surface-associated plasmin has been proposed to facilitate bacterial invasion and dissemination through epithelial barriers.
GAPDH (variously named SDH and Plr in GAS) has been described for several species of Streptococcus (Table 1) and exhibits binding to a broad spectrum of extracellular targets, including the matrix proteins plasmin, plasminogen, Fn, and fibrinogen. Given its multiple binding specificities, GAPDH is therefore likely to make a significant contribution to the colonization capabilities of streptococci. Furthermore, GAPDH has been shown to interact with the pharyngeal cell cytoskeletal proteins actin and myosin (447, 540) and with urokinase plasminogen activator receptor (277), factors that may contribute to the modulatory effects of GAPDH in triggering host cell internalization by GAS (as described elsewhere). In addition to their adhesive and glycolytic functions, another interesting property of the five anchorless enzymes described above is that they function as a complex in the production of ATP (164). This has important implications for host modulatory effects, as extracellular ATP is able to bind P2X7 receptors on the surface of immune and epithelial cells and induce apoptosis (135, 660). Thus, an ability to produce extracellular ATP on the cell surface provides an opportunity for streptococci to manipulate host cell behavior and, potentially, the progression of infection.
Other enzymes within the anchorless adhesin family include GtfG, SpeB, and HtrA. GtfG of S. gordonii is a GTF responsible for the synthesis of α-1,3- and α-1,6-linked glucans. Similar to GTFs of other streptococci (Table 2), GtfG is secreted, but a proportion of this protein has been shown to bind back to the cell surface via its C terminus (626). The regulator Rgg (see below) controls gtfG transcription, and levels of cell-associated GtfG have been shown to influence S. gordonii adhesion to host surfaces and dispersal (626). SpeB is a potent cysteine protease known to play a significant role in GAS virulence, being able to degrade host proteins and activate interleukin-1β (IL-1β). Originally identified as a secreted product, SpeB was also found to become associated with the cell surface, possibly via its propeptide moiety (251). In this form, SpeB mediates adhesion to glycoproteins and the ECM component laminin, functions that may promote the colonization capabilities of GAS. HtrA is also a protease and has been found in a number of streptococci (Table 1). Unlike SpeB, however, HtrA is thought to be anchored to the cell membrane via a single, hydrophobic transmembrane domain located near the N terminus (463). HtrA is a serine protease, and homologs in other bacteria serve to degrade abnormal exported proteins in response to environmental stresses (297). A similar role is proposed for streptococci, as the deletion of htrA in S. mutans altered its ability to withstand environmental stresses (temperature, pH, and oxidative stress) (131), while in GAS, the biogenesis of secreted virulence factors was affected (371).
Aside from enzymatic capabilities, other members of the anchorless adhesin family share an ability to bind ECM proteins and so promote adhesion to host tissues via an ECM protein bridge. One of the first such adhesins to be reported was Fn/fibrinogen-binding protein Fbp54 in GAS (114). Homologs in a number of other streptococci have since been discovered, exhibiting high percent identities: FbpA in S. gordonii (81%), PavA in S. pneumoniae (70%), and SmFnB in S. mutans (70%). All have been shown to adhere directly to immobilized Fn (Table 1), although FbpA also exerts its effects through the modulation of CshA expression, another Fn-binding adhesin of S. gordonii (102). The role of these adhesins in streptococcal colonization is implied by the fact that an N-terminal fragment of Fbp54 can impair GAS adhesion to buccal epithelial cells (112), while PavA was shown to be essential for virulence in a mouse model of pneumococcal sepsis (241). A further Fn-binding anchorless adhesin identified in GAS is Shr (165). Unlike Fbp54, this 145-kDa protein bears an N-terminal signal peptide and is predicted to be anchored to the cell membrane via a transmembrane domain at its C terminus. Shr binds myoglobin and hemoglobin and is thought to mediate heme acquisition through the ATP-binding cassette (ABC)-type SiaABC transporter (32). Shr also binds laminin and Fn but does not contain Fn-binding repeat blocks or the Fn-binding consensus motif (see above).
Two further anchorless adhesins of GAS that adhere to Igs have been described, Sib35 and Lzp. Both possess an N-terminal signal sequence, but whereas Lzp has five C-terminal hydrophobic regions that are predicted to hold it within the cell membrane, no such domains exist in Sib35. Sib35 exhibits adhesion to human IgG, IgA, and IgM (289) and has been shown to act as a mitogen, triggering proliferation and differentiation of mouse B cells (435).
In summary, the anchorless adhesins complement the functions of cell wall-anchored adhesins because they provide positional as well as functional flexibility. They have the potential to act as scouts in the inevitable battle between bacteria and host defenses. They could be released from the cell surface to sense the environment and may be bound back to the cell surface, perhaps in complex with their ligands. The possibility exists, therefore, that they may represent a mechanism for feeling the environmental waters, interacting with host molecules at a distance and with a surface dispensability that cannot be achieved through activities of cell wall-anchored adhesins.
Host Cell Modulation
Receptor recognition by bacterial surface adhesins is an essential step in mediating attachment to host tissues. It is increasingly evident, however, that these events can also trigger host cell signaling cascades and so modulate host cell behavior. In many cases, the precise sequence of signaling events triggered by streptococci remains to be defined. Nonetheless, the outcomes of streptococcal host cell modulation can be grouped into three general categories: (i) internalization of streptococci by host cells, (ii) induction of cytokine release, and (iii) induction of apoptosis.
Internalization.
A common property shared by host cells and a number of streptococcal adhesins is the ability to bind ECM proteins. For mammalian cells, this is often facilitated by integrin receptors. Integrins are a large family of αβ heterodimeric glycoproteins that regulate diverse cellular processes including cell migration, proliferation, and adhesion. To date, 18 α and 8 β subunits that combine to form 24 distinct heterodimers, each with various affinities for ECM proteins, have been identified (249). Using common ECM protein ligands as bridging molecules, several streptococci have been found to interact with integrins and exploit their signaling pathways to promote their own adhesion, colonization, and invasion. One of the best-characterized examples of this is signaling via Fn-binding integrin α5β1 by GAS, a process that leads to GAS internalization by nonprofessional phagocytic cells. Two Fn-binding adhesins of GAS are principally associated with this process, SfbI and M protein, and trigger distinct uptake mechanisms (refer to reference 426 for a comprehensive review of this process). SfbI interacts with the N terminus of Fn, which in turn binds integrin α5β1 via its RGD motif. Interactions with multiple Fn molecules leads to integrin clustering and the formation of focal complexes and focal adhesions, which contain numerous signaling and structural components linked to the host cytoskeleton. These are the sites of activation for two independent signaling pathways. One requires tyrosine phosphorylation of focal adhesion kinase (FAK) and paxillin and involves the Src kinases Src, Yes, and Fyn. The second requires the recruitment and activation of the GTPases Rac and Cdc42. These pathways then ultimately converge to trigger the formation and fusion of caveolae beneath the attached bacteria, resulting in large invaginations via which GAS species are internalized (441) (Fig. 4B).
M protein also interacts with integrin α5β1 via a Fn bridge, but the subsequent downstream signaling events differ from those triggered by SfbI. The reason for this variation is not fully understood, but as SfbI and M protein interact with different domains of Fn, it has been postulated that this may affect the way in which Fn binds to integrin, which in turn may influence subsequent signaling events. For M protein, major cytoskeletal rearrangements are triggered, and this is dependent upon the activation of phosphatidylinositol 3-kinase (472). This activates a classic endocytotic pathway, the result of which is the recruitment and fusion of microvilli around the streptococcal cells and their uptake via a zipper-like mechanism (137). Rather than caveolae, bacteria ingested by this pathway ultimately enter phagolysosomes, as characterized by the lysosomal marker LAMP-1 (137). Similar entry mechanisms are also utilized by GBS (79, 620) and S. dysgalactiae (82).
It should be noted, however, that adhesins that bind ECM proteins are not the only mediators of streptococcal host cell invasion. GAS strain A8, which lacks Fn-binding capabilities, has been shown to trigger its uptake in a Fn- and α5β1 integrin-independent manner (409), although details of the surface proteins and signaling networks in this process remain to be defined. Furthermore, both SDH (GAPDH) (Table 1) and hyaluronic acid capsular polysaccharide (CPS) have been shown to promote GAS internalization. SDH-mediated invasion triggers a series of signaling events following adhesion to two pharyngeal membrane proteins, one outcome of which is the phosphorylation of nuclear core histone H3 (449). In contrast, CPS signals via keratinocyte receptor CD44 to induce membrane ruffling, a process that requires the activation of Rac1 and the cytoskeleton linker protein ezrin (123). Rather than leading to direct host cell invasion, the latter mechanism disrupts intercellular junctions, allowing the passage of GAS to underlying tissues via a paracellular route. This alternative method of promoting streptococcal dissemination has also been described for GBS and is associated with pilus expression (459, 564).
Invasion may enable bacteria to translocate or disseminate to other parts of the host, but there is growing evidence that an ability to enter nonphagocytic cells may also provide bacteria with an increased opportunity for survival. Once internalized, bacteria are protected against host innate immune defenses and antibiotic therapies, and this is thought to explain, at least in part, the recurrence of some GAS infections (439). The notion of host cells as a reservoir of bacteria has implications not only for pathogens but also for commensal microorganisms. Rudney et al. (514) demonstrated that an extensive polymicrobial flora can be found within buccal epithelial cells, a large proportion of which corresponds to commensal streptococci. This implies that commensals are also able to become internalized, and the commensal S. gordonii was shown to trigger its internalization by HEp-2 epithelial cells via an interaction of its SspA and SspB (AgI/II family polypeptides) with integrin α5β1 (427).
Cytokine release.
Proinflammatory responses resulting from de novo or increased cytokine synthesis are a frequent outcome of streptococcal interactions with a range of host cells. These can significantly influence the progression of disease by affecting a number of processes including immune cell infiltration, tissue inflammation, and tissue damage. In some cases, the streptococcal triggers are known and include both cell-bound adhesins and secreted factors. One powerful inducer of inflammation is the GAS M protein. This has been shown to trigger the release of the cytokines IL-6, IL-1β, and tumor necrosis factor alpha (TNF-α) following recognition by Toll-like receptor 2 on the surface of monocytes (444). In S. mutans, rhamnose glucose polymers, GTFs, and AgI/II polypeptides have been shown to induce IL-6 and IL-8 release from epithelial, endothelial, and T cells (12, 97, 625). For the AgI/II proteins, this process is associated with binding to integrin α5β1 and the activation of FAK, paxillin, and phospholipase Cγ, fundamental components of the mitogen-activated protein kinase signaling pathways (12). In contrast, S. gordonii was found to inhibit the release of IL-6 and IL-8 from epithelial cells (222), a phenomenon that may contribute to the ability of this organism to persist in the oral cavity as a commensal. Interestingly, S. gordonii and other oral streptococci were also shown to suppress the IL-8 release normally induced by the opportunistic periodontopathogen Fusobacterium nucleatum (664), providing insight into how the presence of such commensals might promote a “healthy” oral environment (273). Aside from proinflammatory effects, cytokine release may also influence mechanisms such as streptococcal invasion, as described above. Interactions of GAS with epithelial cells and tonsillar fibroblasts have been shown to trigger the production of transforming growth factor β1 (635). This is a multifunctional cytokine and a positive regulator of integrin signaling, upregulating several proteins including FAK, paxillin, and phosphatidylinositol 3-kinase. GAS-mediated transforming growth factor β1 release was found to upregulate integrin α5 expression (635). Thus, GAS is able to manipulate host cytokine production to increase levels of expression of target receptors and so promote its own adherence and internalization.
Apoptosis.
Apoptosis is often considered a host strategy for clearance of infection by eliminating infected cells and removing potential targets for adhesion and internalization. However, the ability to induce apoptosis may be beneficial to bacteria, enabling escape from phagocytic activity or damaging host tissues and so promoting deeper penetration and dissemination. There are two main pathways via which mammalian cells commit to apoptosis. The first results from the deregulation of mitochondrial function. Cytochrome c is released from mitochondria into the cytoplasm, where it binds APAf-1 and cleaves procaspase-9 to form the apoptosome, which in turn activates caspase-3 (intrinsic apoptosis). Alternatively, the ligation of death receptors such as CD95 and TNF receptors on the cell surface leads to the cleavage of procaspase-8. This then cleaves procaspase-3, leading to DNA fragmentation and cell death (extrinsic apoptosis). Bacterial components with proapoptotic effects can be broadly differentiated into four groups: (i) superantigens, (ii) pore-forming toxins, (iii) nonenzymatic structural components, and (iv) metabolic end products (comprehensively reviewed in reference 614). Knowledge of apoptosis regulation by streptococci is limited but has been best studied for pathogenic species, including GAS and GBS.
GAS induces apoptosis in a variety of cell types, including neutrophils (301), macrophages (323), and epithelial cells (416, 417, 612), and can activate both pathways. Extrinsic apoptosis can be attributed in part to the activities of SpeB, which has been shown to activate matrix metalloproteinases on the surface of mammalian cells and trigger TNF-α and Fas ligand release (594). Alternatively, interactions of GAS with epithelial cells induce caspase-9 activation, cytochrome c release, and Bax translocation to the mitochondria (416, 417). These events are associated with intrinsic apoptosis and are dependent upon SfbI-mediated internalization (416), as described previously. The GAS-secreted toxin NADase has also been shown to promote the apoptosis of keratinocytes in conjunction with the hemolysin streptolysin O (SLO). SLO forms pores in the cell membrane, and NADase enters the cell through these pores and exerts its effects (66). Similarly, the hemolysin CylE of GBS triggers the apoptosis of macrophages, but the mechanism is not yet understood (163). It should be noted, however, that not all modulatory effects lead to enhanced host cell apoptosis. For example, GBS infection of HeLa cells has been shown to trigger prosurvival signaling events and protection against caspase-3 cleavage (79). It seems, therefore, that a delicate balance exists between the pro- and antiapoptotic effects of streptococci upon host cells and that this can significantly influence disease progression. By promoting apoptosis, bacteria can escape phagocytic killing and enhance their dissemination, while apoptosis inhibition can allow bacteria to create an intracellular refuge and so persist in the host.
Platelet Interactions
The adherence of streptococci to platelets is thought to be crucial for the colonization of damaged heart valves, which may result in the life-threatening condition infective endocarditis (IE). Streptococci that can induce platelet activation, and the resulting thrombus formation, are believed to play a role in disseminated intravascular coagulation by causing increased platelet and clotting factor consumption (Fig. 4C). Interactions of streptococci with platelets usually occurs by direct binding to platelets via streptococcal surface proteins (293). It may also occur indirectly via streptococci binding a plasma-bridging molecule, such as fibrinogen (171), engaged with a platelet surface receptor. Bacteria may activate platelets or simply adhere to them, suggesting that platelet adherence and activation are separate events that are perhaps mediated by different bacterial and platelet components.
Viridans group streptococci including S. sanguinis, S. oralis, S. mutans, S. mitis, S. parasanguinis, and S. gordonii (31) have long been recognized as major etiological agents of IE. They gain entry to the vascular system via the oral cavity, and their adherence to platelets is thought to be pivotal to pathogenic potential (234). Components of streptococci involved in colonization and virulence in IE include lipoproteins, polysaccharides, and LPxTz-anchored proteins. The lipoproteins shown to be virulence factors in IE are FimA from S. parasanguinis and SloC from S. mutans. These are divalent metal ion-binding proteins of ABC transport systems for the uptake of Mn2+ or Fe2+/Fe3+ (430, 446). S. mutans mutants that do not produce extracellular glucan show decreased virulence in rat models of endocarditis (414). Moreover, the serotype-specific cell wall polysaccharides from S. mutans comprised of rhamnose-glucose polymers induce platelet aggregation in the presence of plasma (98).
The Srr proteins (see above) SrpA (460) and GspB/Hsa (39) have been identified as recognizing sialyl-T antigen found on platelet integrin GPIbα. GspB/Hsa are strain specific to S. gordonii and have been shown to induce platelet adherence by interacting with sialic acid residues on the sialomucin core and the N terminus of GPIbα, respectively (39, 588). They act in concert with the AgI/II polypeptides SspA (172 kDa) and SspB (164 kDa) (427) to aggregate platelets (261). The M1 protein of S. pyogenes triggers thrombus formation by forming complexes with fibrinogen, which, in the presence of IgG against M protein, engage the Fc receptor on platelets and activate them (545). This leads to platelet aggregation and the formation of platelet-rich thrombi. Streptococci thus utilize a range of different components and mechanisms for adherence to, and activation of, platelets. It is important to decipher further the molecular mechanisms involved in Streptococcus-induced thrombus formation so that potential new targets for the prevention of IE or disseminated intravascular coagulation may be identified.
STREPTOCOCCUS SOCIETY DEVELOPMENT
After the first encounter of Streptococcus with a surface, and adherence to that surface, the bacterial cells may begin to multiply if the environmental conditions are favorable. A small society of streptococci can then form, sometimes referred to as a linking film, and provides a new surface onto which other bacteria may deposit (Fig. 2). Alternatively, some species may attach but become internalized before a linking film can develop. Within the society, cell-cell signaling ensures coordinated growth and development, while the production of extracellular hydrolytic enzymes provides new nutrients, and polysaccharide formation promotes the retention and accumulation of streptococci (Fig. 2). All these activities occurring within the society are central to the overall colonization processes, which may then lead to carriage, to the formation of mixed-species communities, or on to infection.
Biofilm Formation
Dental plaque, comprising many hundreds of bacterial species, is the best example of a natural biofilm in the human body. Complex biofilms are also formed on the intestinal linings and on the mucosal surfaces of the vagina, but these biofilms are conceptually different for this article because they do not generally incorporate commensal streptococci. Single-species biofilms (biofilm societies) may occur in some instances in nature, such as in the colonization of prosthetic implants by Staphylococcus epidermidis or of the middle ear by S. pneumoniae. However, many studies of single-species biofilm formation, although providing information on the growth of the organisms as sessile societies, do not truly represent the biofilm communities encountered in vivo, where there is intense competition as well as metabolic synergism among many microbial species. S. mutans, S. gordonii, and other oral streptococci readily form single-species biofilms in the laboratory on a variety of surfaces, and biofilm formation is dependent upon activities of multiple genetic loci (Fig. 6), as discussed below. Thus, mutations in genes encoding components of quorum-sensing systems, e.g., comCDE (354); TCSS, e.g., vicRK (543); GBPs (370); and metal ion permeases (503) can impair S. mutans biofilm formation. Optimal biofilm formation by S. gordonii and S. sanguinis also requires multiple gene products including sortase (428) and proteins involved with β-glucoside metabolism and carbon catabolite control (296, 364), nucleotide biosynthesis (181), oxidative stress (322, 363), and environmental sensing and signaling (193). The growth of non-mitis-group streptococci as biofilms is only just beginning to be studied, but the complexities of M serotypes, capsular serotypes, and the expression of pilus types and antigenically variable surface proteins will probably make interpretations of the molecular pathways involved extremely difficult. Already, it is clear that strains of S. pyogenes encompassing clinically important serotypes behave very differently and quite unpredictably in biofilm formation (341). Pili (382), M protein, and streptococcal protective antigen (Spa) (115) are some factors that have been implicated, but the complements of surface components required for biofilm development may turn out to be strain and serotype specific. Interestingly, the inactivation of the regulator Srv in S. pyogenes results in the upregulation of the cysteine protease SpeB and a significant decrease in biofilm formation (136). Thus, it is possible that the production of SpeB may be associated with a transition from a colonizing to an invasive phenotype.
FIG. 6.
Integration of adhesins, receptors, signals, adaptation, and nutrition in Streptococcus biofilm formation. The colonization process depends upon the expression of genes encoding adhesins (gbpA and srpA, etc.), transporters (scaABC, etc.), transcriptional regulators (codY, etc.), posttranslational processing (htrA, etc.), TCSS (vikRK, etc.), and cell-cell communication (pheromones and AI-2, etc.), with only a few selected examples shown.
Polysaccharide Production
The production of extracellular polysaccharides by oral streptococci plays a key role in the development of biofilms and dental plaque (Fig. 6). The accumulation of S. mutans is enhanced through the binding of extracellular glucans by cell surface-associated GBPs (Table 1). GTFs and fructosyltransferases are enzymes secreted by viridans group streptococci (Table 2) that are responsible for the synthesis of glucans and fructans. There are three genes encoding GTFs in S. mutans, designated gtfB, gtfC, and gtfD. The GtfB enzyme (162 kDa) synthesizes a water-insoluble glucan comprised of α1-3-linked glucose (Glc) residues, GtfC (149 kDa) produces a low-molecular-mass partly water-soluble glucan, and GtfD (155 kDa) synthesizes a water-soluble glucan. Sucrose induces the expression of gtfB, while Glc and fructose repress it (246, 646) at acidic pHs, possibly through the activity of the RegM transcriptional regulator (similar to catabolite control protein A) (74). The GBPs help determine the architecture of the biofilm, with GbpC being the principal glucan receptor (370). The transcriptional regulator CovR represses gbpC (53) as well as gtfB and gtfC (55). The water-insoluble glucans contribute to adherence and society formation, while the water-soluble glucans are akin to trapped nutrient supplies, which are readily degraded into metabolizable sugars by extracellular or cell-bound dextranases (Fig. 6).
A large amount of work carried out over the last few decades has confirmed that colonization and dental caries production by S. mutans in rodents fed high-sucrose diets depend upon the synthesis of water-insoluble glucans. However, it is clear that the polysaccharide-synthesizing enzyme products of all four genes gtfBC, gtfD, and ftf are involved in eliciting dental caries (415). The GTFs secreted by oral streptococci contain a common four-domain structure: a signal (leader) peptide of 36 to 38 aa residues, followed by a nonconserved region (200 aa residues), a highly conserved catalytic domain (800 aa residues), and a C-terminal region of approximately 500 aa residues that binds glucans. The latter regions are composed of a series of YG repeats, each of which comprises a 21-aa-residue sequence with one or more aromatic residues (190). The YG repeats are present in regular arrays within GtfB, GtfC, GtfD, and GtfI (S. downei), etc., and have arisen from multiple duplication events, with evidence for a functional selection of conserved residues for carbohydrate binding.
The virulence of S. pyogenes correlates closely with the expression of hyaluronic acid CPS. Heavily capsulated strains are spread more readily, while capsule production is repressed during carriage. The upregulation of capsule during systemic infection protects S. pyogenes from opsonophagocytosis and masks other immunogenic determinants on the cell surface, constituting a mechanism for the evasion of antigen-specific antibodies (133). The biosynthesis of the capsule is directed by the hasABC gene cluster (20), and there is evidence that the antimicrobial protein human cathelicidin LL-37, perhaps with other signals from the host, may trigger increased CPS production through the CsrRS TCSS (202).
A major factor contributing to the virulence of S. agalactiae is the polysaccharide capsule. It is composed of repeating units of Glc, Gal, GlcNAc, and NeuNAc (sialic acid), and variations in saccharide linkages and composition result in the designation of nine capsular serotypes to date. All GBS capsule types share a terminal NeuNAc that is similar to the sialic acid on human cells. It has been envisaged that this may play a role in resistance to complement-mediated killing by binding factor H, as well as binding CD33rSiglecs on neutrophils and macrophages to exert suppressive effects on the innate immune system (84). The oral streptococci S. sanguinis, S. oralis, and S. gordonii also express antigenically diverse cell wall polysaccharides on their cell surfaces that contain the host-like recognition motifs GalNAcβ1→3Gal or Galβ1→3GalNAc (103). These are receptor polysaccharides for the fimbrial lectins of Actinomyces naeslundii in the development of oral microbial communities, but the host glycoconjugate-like features of the receptor polysaccharides suggest that they may also be involved in the evasion of the secretory immune response by oral streptococci.
Two-Component Signal Transduction Systems
To successfully colonize and persist within the host, bacteria must adapt their gene expression profiles in response to environmental changes. Streptococci utilize two main types of transcriptional regulators: TCSS and stand-alone regulators (see below). TCSS (more formally known as histidine-aspartate phosphorelay systems) comprise two signaling proteins: a sensor kinase, which is anchored to the cell membrane, and a cognate response regulator found within the cytoplasm. Sensor kinases typically possess a highly variable N-terminal sensor region, containing one to several transmembrane domains, and a more conserved C terminus, containing the kinase and transmitter domains. Comparative analyses across the sensor kinase family have identified five amino acid motifs (or boxes) within the C-terminal region, named after the most conserved residue (H, N, D, F, and G). Response regulator proteins can be divided into an N-terminal region, which carries a highly conserved “receiver” domain, and the C-terminal “effector” domain, which often exhibits DNA-binding capabilities. Upon receipt of an appropriate stimulus, the sensor kinase is activated and autophosphorylates at the conserved histidine residue of the H box. The kinase then donates the high-energy phosphate group to an invariant aspartate residue within the receiver domain of the response regulator. This phosphorylation leads to structural changes in the regulator, enabling the modulation of gene expression or protein function.
Analyses of complete genomes have revealed 13 independent TCSS in GAS (161), S. pneumoniae (334, 605) and S. mutans (6), while 20 TCSS were found in GBS (194). TCSS are not, however, restricted to these species of streptococci and have been associated with the regulation of an array of cellular responses including pathogenesis, nutrient utilization, competence, and stress resistance (Table 3). Nonetheless, it should be noted that for many TCSS, the precise regulatory networks and functional roles are still not understood.
TABLE 3.
TCSS
| TCSS | Species | Homolog(s) | Function(s) and/or substrate(s)b | Reference(s) |
|---|---|---|---|---|
| CsrRS (CovRS) | S. pyogenes, S. agalactiae, S. dysgalactiae | GcrR/TarC (S. mutans); orphan | Virulence, biofilm formation, negative global regulator | 330, 349, 575 |
| VicRK | S. pyogenes, S. pneumoniae, S. mutans | Virulence, stress resistance; ↑ GtfBCD, GbpB, Ftf; biofilm formation, competence, nutrient uptake | 360, 543, 630 | |
| CiaRH | S. pneumoniae, S. pyogenes, S. mutans, S. mitis, S. agalactiae, S. gordonii, S. sanguinis | Virulence, competence, biofilm formation | 5, 186, 203, 495 | |
| BfrAB | S. gordonii, S. sanguinis | Biofilm formation | 668, 669 | |
| ComED | S. mutans, S. pneumoniae, S. gordonii, S. oralis, S. anginosus, S. cristatus, S. sanguinis, S. mitis | Competence (CSP), mutacin production, biofilm formation | 122, 225, 352, 453 | |
| HdrRM | S. mutans | High cell density, bacteriocin production, biofilm formation, competence | 404 | |
| HK11/RR11 | S. mutans | Acid resistance, biofilm formation | 353 | |
| TcbRK | S. mutans | SpiHR (S. pneumoniae) | Biofilm formation | 50 |
| TcdRK (TCS3) | S. mutans | ScnRK (S. pyogenes) | Mutacin production | 50, 348 |
| SpiHR | S. pneumoniae | TcbRK (S. mutans) | Bacteriocin production | 491 |
| BlpRH | S. pneumoniae | Bacteriocin production | 130 | |
| ScnRK | S. pyogenes | TcdRK (TCS3) (S. mutans) | Lantibiotic production | 393 |
| SalKR | S. salivarius | Lantibiotic (SalA) production | 617 | |
| LytRS | S. pyogenes, S. agalactiae, S. pneumoniae | Virulence | 197, 280, 607 | |
| Ihk-Irr | S. pyogenes | Polymorphonuclear neutrophil signal, virulence | 629 | |
| FasBCA | S. pyogenes | FasCAX (S. dysgalactiae), RgfAC (S. agalactiae) | Local tissue destruction | 309 |
| TrxSR | S. pyogenes | Mga virulence regulon | 337 | |
| SptRS | S. pyogenes | Carbohydrate uptake and utilization | 549 | |
| SilAB | S. pyogenes | Virulence | 151 | |
| PnpRS (TCS04) | S. pneumoniae | Phosphate regulation; ↑ PsaA; oxidative stress | 334, 390, 429 | |
| VncRS | S. pneumoniae | Vancomycin resistance | 429 | |
| MicAB | S. pneumoniae | Oxidative stress | 144 | |
| CbpRS | S. pneumoniae | ↓ pili | 508 | |
| CpsXY | S. pneumoniae | Capsule expression | 307 | |
| HK/RR03 | S. pneumoniae | ↓ pili | 508 | |
| HK06/RR06 | S. pneumoniae | Virulence (CbpA, PspA) | 573, 574 | |
| TCS08 | S. pneumoniae | Virulence, cellobiose metabolism | 334, 392 | |
| RelRS | S. mutans | (p)ppGpp production | 344 | |
| LiaSR | S. mutans | Virulence; ↓ Gbp; mutacin production | 54, 101 | |
| LevRS | S. mutans | ↑ FruA | 663 | |
| LytTS | S. mutans | Autolysin regulation, cell division | 91 | |
| TCS9 | S. mutans | Competence | 348 | |
| GcrR/TarC | S. mutans | CovR (S. pyogenes) | Acid tolerance response; ↓ GtfS, GbpC | 141, 253 |
| RgfAC | S. agalactiae | FasBCA (S. pyogenes) | Surface adhesins (↓ ScpB) | 566 |
| DltRS | S. agalactiae | LTA biosynthesis | 464 | |
| FasCAX | S. dysgalactiae | FasBCA (S. pyogenes) | Streptokinase and SLS production | 575 |
| StkP/PhpP/RitRa | S. pneumoniae | Global; stress resistance, iron homeostasis | 142, 615, 616 | |
| Stk1/Stp1/Cova | S. agalactiae | Beta-hemolysin/cytolysin production, growth, virulence | 477, 478 |
Serine/threonine kinase-phosphatase signaling via regulator.
↑, gene/protein expression is upregulated; ↓, gene/protein expression is downregulated.
CsrRS.
One of the best-characterized TCSS is CsrRS (capsule synthesis regulator), otherwise known as CovRS (control of virulence genes). As its names suggest, CsrRS was first shown to repress the expression of the capsule synthesis has operon in GAS (349) but has since been found to affect the expression of a wide range of genes associated with GAS colonization and virulence (159, 197). These include genes encoding surface adhesins (e.g., lmb and scl2), extracellular enzymes (e.g., speB, ska, and sagA), and stress response proteins (e.g., dnaJK and grpE). Furthermore, CsrRS regulates other TCSS (e.g., ciaRH, ihk-irr, and trxSR) and global regulators (e.g., rgg and RofA-like protein [RALP] family genes), thereby mediating indirect effects on the expression of a secondary set of genes (197, 337). Taken together, CsrRS influences the transcription of 15% of GAS chromosomal genes (197), and as the majority of these are downregulated, CsrRS is often referred to as a negative global regulator. Accordingly, mutants attenuated in csrRS exhibit increased virulence in mouse models of infection (150). Gryllos et al. (201) found that CsrRS responds to the Mg2+ concentration, being repressed at low concentrations and becoming fully active only once concentrations of ≥10 mM are reached. As physiologic Mg2+ levels in body fluids such as mucosal secretions and blood are approximately 1 mM, this means that the expression of Csr-regulated virulence genes is maximal under conditions encountered during human infection.
Similar to GAS, a CsrRS homolog in S. dysgalactiae was shown to repress the expression of streptokinase and streptolysin S (SLS) (575). In contrast, CsrRS of GBS was found to upregulate almost as many genes (63 genes) as were repressed (76 genes), and a ΔcsrRS mutant is attenuated for virulence in a neonate rat sepsis model (330). Such differences may reflect the adaptation of these pathogens to the specific environments encountered during the course of infection. S. mutans expresses an orphan regulator with homology to CsrR, known as GcrR/TarC (253, 526). This acts as a transcriptional repressor of GTF GtfD and GBP GbpC expression, thereby affecting S. mutans attachment to tooth surfaces and subsequent biofilm formation (253). GcrR has also been shown to upregulate the expression of genes required for the acid tolerance response, which enables S. mutans to survive at pHs as low as 3.0. Rather than responding directly to acidic pHs, however, the transcription of gcrR is itself regulated by the transcriptional repressor SloR, whose repression is relieved under manganese-deprived and acidic conditions (141).
VicRK.
Another TCSS of S. mutans that affects GTFs and GBPs is VicRK (Table 3). This TCSS positively regulates the expression of GtfBCD, Ftf, and GbpB (543), thereby modulating glucan and fructan synthesis and sucrose-mediated bacterial aggregation. No environmental stimulus has been defined for VicRK, but a vicK mutant was found to form aberrant biofilms that were readily disrupted compared to the wild type, implying an important role for VicRK in S. mutans colonization of tooth surfaces (Fig. 6). VicRK was also implicated in competence development by S. mutans, indicating that it may act in conjunction with the TCSS ComED to promote genetic variation and the uptake of heterologous genes, including those associated with antibiotic resistance (543). S. pneumoniae and GAS possess VicRK homologs, and mutants in this TCSS are attenuated for virulence in mouse models of infection (360, 630). This is associated with the fact that for both pathogens, VicRK positively regulates the expression of PcsB, a murein hydrolase that is homologous to GbpB and that is required to maintain cell wall integrity (360, 423). VicRK in GAS has also been shown to modulate nutrient uptake, by the regulation of a carbohydrate transport system, and osmotic protection, by targeting the osmoprotectant transporter OpuA (360). Interestingly, VicK contains a PAS domain, a motif associated with proteins that detect oxygen and cytoplasmic redox potentials (630). It is possible, therefore, that the signal recognized by VicRK may originate from the cytoplasm.
CiaRH.
CiaRH was the first TCSS to be identified in S. pneumoniae (203) and has since been reported for a number of streptococcal species (Table 3). For S. pneumoniae, mutations in ciaRH are associated with increased antibiotic resistance (186, 203, 386), a phenotype that is likely linked to the regulation of genes related to cell wall polysaccharide biochemistry (186, 386). In addition, CiaRH affects competence development by repressing the comCDE operon (143, 186, 203), and a ΔciaRH mutant failed to effectively colonize the mouse nasopharynx, a finding that is associated with a reduced level of expression of the anchorless protease HtrA (252, 537). Conversely, the inactivation of ciaH in S. mutans diminishes competence development and increases the level of expression of HtrA, although the mutation does disrupt biofilm formation (5, 476). It is clear, therefore, that there has been a significant divergence in the function of this TCSS across streptococci, meaning that it influences their colonization capabilities in a variety of ways. This is further highlighted by the role of CiaRH in GAS. As GAS is not naturally transformable, the mutation of ciaH has little effect on competence development. Rather, the mutant exhibits decreased levels of expression of sugar transport systems (495), implying a role in maintaining sugar homeostasis. CiaRH of GAS was also found to regulate the expression of some virulence factors, including the adhesins PrtF2 and SfbX, a function that influences GAS adhesion and the invasion of HEp-2 epithelial cells (495). As for the functions of CiaRH homologs, the environmental stimuli to which they respond also vary. For GAS, ciaRH transcription was shown to be altered in the presence of various concentrations of Co2+, Cu2+, and Zn2+ (495), while CiaRH of S. pneumoniae and S. mutans senses Ca2+ (186, 226). For S. mutans, a third gene of the operon has been found, CiaX, which encodes a double-glycine-containing peptide. This acts as a calcium signaling peptide, which, when bound by Ca2+, undergoes a conformational change that prevents its activation of CiaRH (226).
BfrAB.
In S. gordonii, the TCSS BfrAB is involved in biofilm development (666, 668). BfrAB regulates the expression of the bfrCD and bfrEFG operons, which encode two ABC transporters and a CAAX amino-terminal protease family protein. Interestingly, bfrC (ccmA) and bfrG are also required for the maturation of dual-species communities with P. gingivalis (322). As an organism that is predominantly in the biofilm mode of existence, S. gordonii may benefit from homotypic and heterotypic community-related genes being in relatively close proximity.
Other TCSS.
Several other TCSS that are implicated in biofilm formation have been found in streptococci, including HdrRM, TcbRK, and HK11/RR11 of S. mutans (50, 353, 404). However, aside from ComED, a TCSS found in many streptococci that is associated with competence development and biofilm formation (see “Peptide-Mediated Signaling”), the regulatory networks of these TCSS remain to be defined.
A further function that can influence the ability of streptococci to colonize an environmental niche, and which TCSS often regulate, is the production of bacteriocins. Bacteriocins are proteinaceous toxins that inhibit the growth of closely related bacterial strains. Thus, by their production, streptococci can effectively compete with neighboring bacteria for a given site. Two types of bacteriocins are produced by streptococci, classified according to their posttranslational modifications: lantibiotics (class I) and nonlantibiotics (class II). Lantibiotics correspond to ribosomally synthesized and posttranslationally modified peptides that contain lanthionine or β-methyllanthionine. These can be further divided into class A lantibiotics, which have a linear structure, and class B lantibiotics, which have a more globular conformation. One such example is SA-FF22, a lantibiotic expressed by GAS. SA-FF22 production is regulated by the TCSS ScnRK (393), a homolog of which is found in S. mutans, designated TcdRK (50). Similarly, a second TCSS of S. mutans, TcbRK, is homologous to SpiHR of S. pneumoniae (50), which regulates the putative bacteriocin cluster comprised of PncA to PncM (491). No environmental signal has yet been determined for these TCSS. However, Guerra and Pastrana (204) showed that bacteriocin production was stimulated by acidic pHs in Lactococcus lactis, a model that may also be relevant for streptococci, particularly S. mutans. Another environmental stimulus associated with bacteriocin expression is cell density. Thus, by expressing bacteriocins when cell numbers are high, bacteria are able to kill competing species when nutrients become limited. Such a strategy has been seen for S. mutans expression of the lantibiotic mutacin I, which acts against most gram-positive bacteria, and the nonlantibiotic mutacin IV, which targets predominantly mitis group streptococci and is associated with the LuxS/HdrRM and ComED regulatory systems, respectively (318, 402, 404). Interestingly, the coordination of mutacin IV and competence development has been proposed to be a mechanism for S. mutans to acquire DNA from bacteriocin-sensitive streptococci, thereby promoting genetic diversity (317). The production of the bacteriocin Blp in S. pneumoniae is also cell density dependent but, unlike S. mutans, is controlled independently of ComED by the TCSS BlpRH (130).
One final mechanism of bacteriocin production that has important implications for colonization and community development is interspecies signaling. This has been demonstrated for lantibiotic production by S. salivarius and GAS (617). S. salivarius expresses salivaricin A (SalA), regulated by the TCSS SalKR, which is active against GAS (513). GAS possesses a homologous sal locus that encodes the lantibiotic SalA1 and is effective against S. salivarius (555). In both cases, lantibiotic expression is autoregulated and provides increased immunity to the inhibitory effects of SalA and SalA1 compared to SalA/SalA1-negative strains. Of particular interest, however, is that although not identical, the TCSS of S. salivarius and GAS do not discriminate between SalA and SalA1 and are capable of sensing and responding to either one (617). As such, lantibiotic production by one species could be detected by another, inducing its own lantibiotic production. In this way, the outgrowth of a single streptococcal population could be restricted and, instead, a state of cocolonization could be promoted. By modulating the coexistence of streptococcal populations, such mechanisms are likely to have a significant influence on the development of polymicrobial community structures.
Transporters
When streptococci deposit upon a surface, one of the first requirements is that they acquire nutrients in order to form a society. Streptococcal species differ considerably in their growth requirements, with oral species generally being far less fastidious than pyogenic group organisms (Fig. 1). The sugar transport systems in S. mutans, especially the phosphoenolpyruvate-dependent phosphotransferase systems, are critical for growth and for extracellular polysaccharide production. There have been 10 or more phosphotransferase systems identified within the S. mutans and other streptococcal genomes, with more than 50 putative carbohydrate transporters in S. sanguinis (657). The streptococci also have transporter systems for complex carbohydrates such as maltodextrins, e.g., Msm in S. mutans. Maltodextrin utilization is important for S. pyogenes growth in human saliva and for colonization of the oropharynx in an animal model (548).
Specific ABC transporters have been implicated in streptococcal colonization processes (Table 2). The oligopeptide permeases HppABCDF in S. gordonii and OppABCDF in S. pyogenes are involved in adherence and virulence, respectively. The Hpp transporter is necessary for the response of S. gordonii to an extracellular factor that regulates the expression of the fibril adhesin CshA (397). Mutations in the genes encoding the oligopeptide permease in S. pyogenes lead to a reduced level of production of the cysteine protease SpeB (461) and cause less mortality and tissue damage in an air pouch model of virulence (637). Other permeases that have been specifically shown to influence adherence and biofilm formation are the ABC-type metal ion transporters (Fig. 6). The ScaA lipoprotein component of the ATP binding protein-dependent permease ScaABCD was first identified as being a putative adhesin mediating the binding of S. gordonii to A. naeslundii (304) in mixed-species community development. The orthologous FimA protein in S. parasanguinis was shown to adhere to salivary components (436). More recently, the PsaA protein ortholog from S. pneumoniae has been shown to bind E-cadherin (16). In addition to their putative roles in adherence, these proteins are substrate-binding components of transporters for Mn2+ ions (262) and possibly Fe3+ ions (430). Metal ion homeostasis in streptococci is crucial for many aspects of metabolism, particularly responses to oxidative stress (260). Therefore, the disruption of genes encoding these transporters has deleterious effects on the growth and survival of bacteria in vivo. In animal models of endocarditis, S. parasanguinis FimA− mutants (78) or S. mutans Slo− (Mn2+/Fe3+) permease mutants (446) are severely attenuated. The Adc permease, encoded by adcRCBA, is involved in Zn2+ and Mn2+ uptake in S. gordonii, and the inactivation of adcR results in a competence- and biofilm-defective phenotype (365). Downstream of the adc operon is a copper transport operon (copYAZ), which is involved in biofilm detachment (408). Taken collectively, these observations suggest that metal ion homeostasis in streptococci is central to their abilities to express adhesins for interacting with host surfaces, grow in the face of environments that may be limiting in essential metal ions such as Mn2+ or Fe2+/Fe3+, resist oxidative stress and host defenses, and form biofilm communities.
Iron is essential for most bacterial pathogens. The substrate-binding components of ATP-type transporters are able to bind Fe3+, the ferric siderophore complex, or heme, and these are taken across the plasma membrane concomitant with ATP hydrolysis. S. pyogenes expresses three transporters, FtsABCD, HtsABC (SiaABC), and MtsABC (Table 2), that acquire ferric ferrichrome, heme, and Fe3+/Mn2+, respectively. S. pyogenes can utilize heme derived from human hemoproteins as a source of iron, but the affinity by which heme is bound to host proteins is extremely high. The acquisition machinery for heme must therefore be specialized and comprises the cell surface proteins Shr and Shp and HtsABC. Shp and HtsA are both able to bind heme, but Shp cannot acquire heme from methemoglobin. Instead, it was suggested that Shr acquires heme from host proteins and transfers this directly to Shp, which then relays the heme to the lipoprotein HtsA of the heme transporter (671). In this way, heme at infection sites is effectively sequestered from host proteins bound at the streptococcal cell surface and delivered to the uptake system sited at the plasma membrane.
Regulators
Streptococci are able to coordinate changes in their environment with the modulation of a given set of genes (regulon), thereby ensuring expression of the factors necessary for survival. One way in which this is achieved is through the activities of TCSS (see above). Alternatively, streptococci possess transcriptional regulators, both activators and repressors, for which the sensory element is unknown. These are the so-called “stand-alone” regulators. Multiple examples exist across the streptococci, regulating a vast range of genes (Table 4). However, the regulatory networks and functional roles have been defined in detail for relatively few of them, examples of which will be described here.
TABLE 4.
Stand-alone regulators
| Protein family | Protein(s) | Species | Function(s) and/or substrate(s)a | Reference(s) |
|---|---|---|---|---|
| Mga | Mga/VirR/Mry | S. pyogenes | Virulence (↑ emm, sof, scpA, mrp, arp, scl1, sic, fbaA, nra) | 83, 242, 312 |
| DmgA/MgrC | S. dysgalactiae | Unknown | 183, 623 | |
| MgrA | S. pneumoniae | Virulence (↓ PI-1) | 230 | |
| RALP | Nra | S. pyogenes | Virulence (↓ pfbp, cpa, speB, speA, sagA, mga) | 169, 462 |
| RofA | S. pyogenes | Virulence (↑ sfbI; ↓ speB, sagA, mga) | 35, 198 | |
| RALP3 | S. pyogenes | Virulence (↓ sda1, speB, hasA, lsa, mga) | 325 | |
| RALP4 | S. pyogenes | Unknown | 198 | |
| RALP5/RlrA | S. pneumoniae | Colonization, virulence (↑ PI-1) | 198, 224 | |
| RogB | S. agalactiae | Virulence (↑ fbsA, PI-2a; ↓ cpsA) | 139, 206 | |
| Rgg | Rgg/RopB | S. pyogenes | Virulence (↑ csrRS, fasBCAX, ihk-irr; ↓ mga, sagA) | 93, 312 |
| RovS | S. agalactiae | Virulence (↑ cylE, sodA, rogB; ↓ fbsa) | 521 | |
| MutR | S. mutans | ↑ mutA | 474 | |
| Rgg | S. gordonii, S. sanguinis, S. oralis | ↑ gtfG | 578, 579, 627 | |
| LacI/GalR | CcpA | S. pyogenes | Carbon catabolite repression, virulence (↑ mga) | 11 |
| CcpA/RegM | S. pneumoniae | Carbon catabolite repression, virulence (↑ cps) | 185 | |
| CcpA/RegG | S. gordonii | Carbon catabolite repression (↓ abpA) | 502 | |
| CcpA/RegM | S. mutans | Carbon catabolite repression | 554 | |
| CcpA | S. salivarius, S. sanguinis | Carbon catabolite repression | 324 | |
| RegR | S. pneumoniae | Competence, virulence (↑ comDE, ciaRH; ↓ hyl) | 90 | |
| MalR | S. pneumoniae | Maltose utilization (↓ malXCD, malMP) | 473 | |
| ScrR | S. mutans | Sucrose utilization (↓ scrAB) | 238, 634 | |
| ScrR, SusR | S. pneumoniae | Sucrose utilization, colonization (↓ scrTKH, susT1T2X) | 255, 256 | |
| DeoR | FruR | S. mutans, S. gordonii, S. pneumoniae, S. pyogenes, S. agalactiae, S. mitis | Fructose utilization (↓ fruRKI) | 38, 364 |
| FxpA | S. mutans, S. pneumoniae, S. pyogenes | Fructose and xylitol utilization (↓ fxpABC) | 38 | |
| LacR | S. mutans | Lactose utilization (↓ lacABCDFE) | 511 | |
| DtxR | MtsR | S. pyogenes | Iron homeostasis, virulence (↓ siaABC, shr) | 33 |
| PsaR | S. pneumoniae | Mn homeostasis, virulence (↓ psaBCA, pcpA, rlrA) | 278 | |
| SloR/Dlg | S. mutans | Mn homeostasis, virulence (↓ sloABC, fimA; ↑ ropA, spaP, comDE, sod, gbpB, gtfB, gcrR) | 141, 503 | |
| ScaR | S. gordonii | Mn homeostasis (↓ scaCBA) | 262 | |
| AdcR | S. pneumoniae | Mn homeostasis, competence (↓ adcCBA, phtA, phtB, phtD, phtE) | 134, 432 | |
| AdcR | S. gordonii | Mn homeostasis, biofilm formation (↓ adcCBA) | 365 | |
| AdcR | S. pyogenes | Mn homeostasis, competence, biofilm formation (↓ adcCBA, phtD, phtY, rpsN2, lsp) | 65 | |
| Fur | PerR | S. pyogenes | Iron and sugar homeostasis, peroxide and phagocytic killing resistance, virulence (↑ sodA, mtsA, csp, czcD; ↓ mrgA, pmtA) | 64, 65, 200, 496 |
| LysR | MtaR | S. agalactiae | Methionine utilization, virulence (↑ metQINP, pdsM, artPQ, artGH, manB, cspA; ↓ fbsB) | 75, 550 |
| MetR | S. mutans | Methionine utilization (↑ metEF, atmBDE) | 568 | |
| Crp/Fnr | Srv | S. pyogenes | Virulence, biofilm formation (↑ sic, mga, dpp, ropA, htrA, luxS; ↓ speB) | 136, 492, 493 |
| CodY | CodY | S. pneumoniae | Amino acid metabolism, colonization (↑ pcpA; ↓ ilvBNC, ilvA, ilvE, livJ, amiA, aliB, gdhA, fatD, acuB, asd, gapN) | 231 |
| CodY | S. pyogenes | Amino acid metabolism, virulence (↑ pel [sagA], mga, dppA, prtS; ↓ covRS, ropB, pyrR, graB, pncA) | 380 | |
| CodY | S. mutans | Amino acid metabolism, acid tolerance, biofilm formation (↑ feoA; ↓ ilvC, livK, gdhA, hisE) | 345 | |
| AraC | MsmR | S. pyogenes | Colonization, cytolysin-mediated translocation (↑ nra, cpa, prtF2, sclA, fbaA, scpA, sof, sfbX, hasA, nga, slo) | 419 |
| MsmR | S. mutans | Sugar metabolism (↑ aga, dexB) | 518 | |
| PblR | S. mitis | Unknown | 40 | |
| Other regulators | Frp | S. mutans | Exopolysaccharide synthesis, biofilm formation, competence (↑ ftf, gtfB, gtfC, comC) | 551, 633 |
| BrpA | S. mutans | Biofilm formation, virulence (↑ fruC, atpD, recA; ↓ sod, nox, dpr) | 418, 642, 643 | |
| RelA, RelP, RelQ | S. mutans, S. pyogenes, S. agalactiae, S. dysgalactiae | Stringent response [(p)ppGpp synthesis] | 183, 343, 344, 400, 421 | |
| Alternative regulatory mechanisms | LacD.1 | S. pyogenes | Class I tagatose-1,6-bisphosphate aldolase; virulence (↓ speB) | 367 |
| LuxS | S. mutans, S. pneumoniae, S. pyogenes, S. anginosus, S. agalactiae, S. gordonii, S. oralis | Acid and oxidative stress tolerance, carbohydrate metabolism, biofilm formation, virulence (AI-2 production) | 220, 372, 395, 403, 440, 454, 497, 582 |
↑, gene/protein expression is upregulated; ↓, gene/protein expression is downregulated.
Mga.
One of the first regulators to be studied in detail was the multiple-gene regulator of GAS, Mga, previously known as VirR (virulence regulator) (565) or Mry (M protein RNA yield) (83). Mga is ubiquitous in GAS and, due to its regulation of a vast array of genes implicated in GAS pathogenesis, is often referred to as a global regulator of virulence (see references 242 and 312 for comprehensive reviews). Such genes include those encoding adhesins associated with colonization (e.g., emm, scl1, sof, and fba) and immune evasion factors (e.g., sic, scpA, and mrp), along with mga itself. Located within a region of DNA closely linked to mga, these genes form part of the core regulon, and Mga binds directly to their promoter regions, although a consensus binding site has yet to be defined. Mga can also regulate genes outside of this region, which include sugar utilization and metabolic operons, although its control is thought to be indirect. The expression of the Mga regulon is maximal during exponential growth, while during stationary-phase growth, the regulators RofA/Nra and Rgg/RopB repress mga transcription. The activation of Mga also occurs in response to elevated CO2 levels, normal body temperature, and iron-limiting conditions. Homologs of Mga have been identified for a number of streptococci, including DmgA/MgrC of S. dysgalactiae and MgrA of S. pneumoniae. Similar to Mga, DmgA was shown to regulate transcription of the emm homolog demA and is responsive to CO2 (183, 623). MgrA represses the expression of PI-1 in S. pneumoniae (230) and was found to be essential for nasopharyngeal colonization of mice (224).
RofA.
Another group of regulators, first identified in GAS, is the RALP family (Table 4). Four members have been found in GAS, of which RofA and Nra are the best characterized. These regulators modulate the expression of a variety of MSCRAMMS (e.g., SfbI and Cpa) and extracellular enzymes (e.g., SLS and SpeB), along with the regulator Mga, and are associated with the abilities of GAS strains to attach to and invade host cells and to persist intracellularly (312). The expression of Nra is maximal at early stationary phase (462), while the transcription of rofA has been shown to respond to changes in temperature and O2 levels (168, 562). RALP family members have also been identified in GBS and S. pneumoniae and have been shown to regulate virulence mechanisms including pilus expression (139, 206, 224).
Rgg.
Rgg family proteins are a group of regulators that control the expression of extracellular products. Rgg was first identified in S. gordonii and was shown to positively regulate the expression of the GTF GtfG (578, 579). Homologs of Rgg and GtfG were then found in strains of S. sanguinis and S. oralis (627), implying an important role for Rgg in regulating the expression of these colonization determinants in oral streptococci. An Rgg regulator has also been described for S. mutans, designated MutR, but rather than controlling Gtf expression, MutR regulates lantibiotic mutacin II (MutA). While these examples describe Rgg regulation of a single gene, Rgg (or RopB) and RovS of GAS and S. pneumoniae, respectively, have been shown to control the expression of multiple genes. In GAS, mutation of Rgg affects the transcription of a number of virulence factor genes, including speB, mac, sagA, and slo (312). However, rather than direct regulation, these effects are associated predominantly with Rgg modulation of other regulatory systems such as Mga, CsrRS, FasBCA, and Ihk-Irr (93). Conversely, RovS of S. pneumoniae was found to regulate the expression of FbsA, SodA, and CylE by binding directly to the promoter regions of their genes (521). Despite the implied importance of Rgg regulators to streptococcal colonization and pathogenesis, little is yet known about the stimuli to which they respond.
LacI/GalR.
Efficient nutrient utilization is critical for bacterial survival. Consequently, the genes involved in these processes are tightly controlled, and many representative regulators can be found in Table 4. Of these, one of the largest protein families is the LacI/GalR family, which includes regulators of carbohydrate utilization. Streptococci, particularly those that colonize the oral cavity, must often survive under conditions of carbohydrate starvation that alternate with periods of sugar excess. One mechanism that streptococci have developed to cope with this situation is carbohydrate catabolite repression. This enables bacteria to repress the metabolism of a complex sugar source in favor of one that is readily metabolizable and is controlled by the global regulatory protein CcpA (see reference 638 for a detailed review of this mechanism). Upon activation by glycolytic intermediates, CcpA binds catabolite response elements in the promoters of a specific set of genes associated with carbohydrate utilization, blocking their transcription. Interestingly, CcpA regulation has also been shown to contribute to streptococcal virulence (547). The mutation of CcpA (RegM) in S. pneumoniae resulted in reduced virulence in mouse models of bacteremia and nasopharyngeal colonization. This was associated with changes in the pneumococcal cell surface, including reduced levels of expression of polysaccharide capsule and enolase (185, 255). Similarly, in the absence of CcpA, GAS was significantly impaired in its ability to colonize the mouse oropharynx, and CcpA was found to modulate the expression of a number of virulence factors both via the regulator Mga and by direct promoter binding (11, 547). Given the widespread distribution of CcpA homologs across streptococci, this may represent an important regulatory mechanism of streptococcal colonization.
Virulence Factors
Pyogenic streptococci produce an array of virulence factors (recently reviewed in references 437 and 599) that include extracellular enzymes, toxins, and surface proteins, all of which have the ability to modulate antibody recognition and immune cell function (119). The activities of some of these, e.g., M protein and C5a peptidase, have already been considered above. Those not already discussed and of notable relevance to adherence and the initiation of disease are SpeB, SLO, streptokinase, and superantigens. SpeB cysteine protease is a crucial virulence factor, which is able to modulate functions of S. pyogenes cell surface proteins in colonization and significantly contribute to tissue destruction in necrotizing fasciitis. SpeB can cleave host ECM proteins, e.g., Fn, as well as immune system components, e.g., the antimicrobial peptide cathelicidin LL-37, and activates matrix metalloproteinases to promote further tissue damage and the release of proapoptotic factors (594). SLO is a human-specific cytolysin (Table 2) with a range of properties, including the ability to form pores through which effector proteins, e.g., NAD+ glycohydrolase (SN), may be injected into the host cell cytoplasm (184). An important property of SLO in colonization is that it prevents the internalization of GAS by lysosomes, thus enhancing the intracellular survival of GAS within epithelial cells (208). This is believed to be a feature involved in the long-term carriage of not only GAS but also commensal oral streptococci (514), which may potentially survive for long periods within epithelial cells, partially protected from immune defenses and from antibiotics.
A common defense mechanism to prevent the systemic spread of infecting bacteria involves the encapsulation of bacteria within fibrin networks. The acquisition of plasmin at the bacterial cell surface circumvents this defense process. Streptokinase is a plasminogen-activating protein produced by GAS that combines with plasminogen to make functional plasmin. This can hydrolyze fibrin and promote the spread of GAS through tissues. Streptokinase is an important virulence factor that acts in concert with plasmin(ogen)-binding proteins (Table 1), and the reader is referred to a recent article for further information (388).
A significant feature of GAS and group C and group G Streptococcus is the production of a range of superantigens (Sags), with 11 found to date in GAS. Sags are released as toxins that can activate a large proportion of the T-cell population, eliciting an inflammatory response. The excessive uncoordinated release of cytokines such as IL-1, IL-2, IL-6, TNF-α, IFN-γ, and macrophage inflammatory protein 1α, etc., overloads the body, resulting in rash, fever, organ failure, coma, and death. These effects are associated more with overt infection than with initial colonization and therefore will not be further advanced in this article, but the reader is referred to a recent detailed review (172).
COMMUNITY DEVELOPMENT
Left undisturbed, assemblages of adherent organisms develop into complex communities. Increases in biomass can occur through growth and division or through the recruitment and retention of additional organisms from the fluid phase. Bacteria within these communities encounter higher cell densities than their planktonic counterparts. In consequence, community living involves adaptation to higher (and unevenly distributed) levels of metabolic by-products, secondary metabolites, and other secreted molecules, along with lower levels of nutrients and oxygen. Bacterial inhabitants of biofilms are known to both collaborate (e.g., through nutritional cross-feeding) and compete (e.g., through the production of bacteriocins) as they strive to optimize their adaptation to these environmental constraints. More recently, it has become apparent that bacteria can communicate with one another through a variety of sensing and response systems based on either cell-to-cell contact or the detection of soluble mediators. The signaling molecules are processed through transcriptional and posttranscriptional networks, and they allow bacterial inhabitants of biofilms to coordinate activities at a group or community level.
Peptide-Mediated Signaling
Many gram-positive bacteria utilize competence-stimulating peptide (CSP), such as the pneumococcal 21-aa-peptide pheromone, to trigger readiness for natural transformation (reviewed in references 542 and 581). CSP is also a quorum-sensing signal that is secreted into the milieu and initiates competence development throughout the bacterial culture after exceeding a threshold level. In the archetypal pneumococcal system, extracellular CSP binds to a sensor histidine kinase, ComD, which, with the response regulator ComE, forms a TCSS (Table 3) that initiates temporally distinct waves of transcriptional activity. First, there is an upregulation of the CSP precursor (comC), which, along with comDE, forms an operon. ComE also controls comAB and comX. ComA is an ABC transporter that, together with its accessory protein ComB, exports CSP following the removal of the leader peptide distal to a Gly-Gly motif (double-glycine leader) of the pre-CSP (453). ComX induces the second phase of gene regulation that includes components of the DNA uptake and integration systems (the late-competence genes) through the recognition of a com box (or cin box) consensus sequence in the upstream regulatory region of target genes. This is followed by an induction of the delayed class of stress-related genes and then by the repression of genes involved in protein synthesis. Many of the mitis group and anginosus group streptococci have a genetic arrangement similar to that of the pneumococci; however, in S. mutans, comC has a distinct promoter (581). The repertoire of genes controlled by the CSP system extends beyond competence and includes bacteriocin production, adaptation to low pH, virulence-associated properties, and biofilm formation.
In S. mutans, the loss of CSP results in biofilms with altered architectures, whereas mutants defective in comD, comE, or comX are deficient in adherence and form biofilms with reduced masses (354). The loss of ComA and ComB also attenuates biofilm formation (662). Collectively, these results indicate that multiple biofilm control pathways in S. mutans are influenced by CSP (Fig. 6). In addition, the TCSS HK/RR11 (Table 3) controls biofilm architecture, and HK11 may act as a second receptor for CSP (355). CSP-mediated communication has also been shown to be important for biofilm formation in S. gordonii (362) and S. intermedius (457). Structure-function studies have revealed that S. mutans CSP possesses at least two functional domains. The C-terminal structural motif comprises polar hydrophobic charged residues and is required for the activation of the signal transduction pathway, while the core α-helical structure extending from residue 5 to the end of the peptide is necessary for receptor binding (585). Some strains of GAS possess a putative CSP-like communication system known as Sil (341). The sil locus encodes an ABC transporter comprised of SilD and SilE, a TCSS (SilAB), and two small converging overlapping open reading frames (SilC and SilCR). The CSP-like peptide is encoded by SilCR, whereas SilC encodes a signaling peptide. Together, SilC and SilCR form a novel regulatory circuit that controls the transcription of the sil locus (151). The deletion of silC drastically reduces biofilm accumulation by GAS (341). The aggregation of GAS into microcolonies is also dependent on AHP, a conserved 19-aa-residue peptide present in M protein and in protein H (174).
The evolutionary driving force that ties CSP to biofilm formation may be related to the ability of streptococcal cells in a biofilm to incorporate foreign DNA more efficiently than equivalent cells in suspension (352). Transcriptional upregulation of comCDE and comX has been demonstrated in actively growing biofilms of S. mutans and S. gordonii (21, 193, 354). Moreover, the production of bacteriocins and bacteriocin immunity proteins is coordinated by CSP through ComE binding to direct repeats in the promoter regions (315, 387). Along with the regulation of autolysis by the CSP system (315, 475, 666), these mechanisms will ensure the availability of DNA in biofilms. DNA may be used to increase genetic variability or as a nutrient source (570). In addition, however, DNA can comprise a major component of the ECM in S. mutans biofilms (458) and contribute to biofilm formation in a structural capacity.
Diffusible Signals and Environmental Factors
The close proximity of bacteria in biofilms provides an environment conducive to signaling through short-range diffusible mediators such as metabolites and autoinducers (Fig. 6). Oral streptococci, GAS, and GBS all produce autoinducer-2 (AI-2). While CSP signaling is species or strain specific, the AI-2 quorum-sensing system, originally identified in Vibrio harveyi, is considered to be not species specific and may function as a universal bacterial language (654). AI-2 is produced through the action of the LuxS enzyme, which cleaves S-ribosylhomocysteine to produce homocysteine and the AI-2 precursor 4,5-dihydroxy-2,3-pentanedione. This reaction is also a major component of the activated methyl cycle that recycles homocysteine from S-adenosyl methionine (220). AI-2 is more accurately a collective term for a group of structurally related molecules generated from the spontaneous cyclization of 4,5-dihydroxy-2,3-pentanedione (405, 569). In V. harveyi, AI-2 above a threshold concentration is detected by LuxPQ, which channel information to the phosphotransferase protein LuxU. LuxU, in turn, transfers the signal to LuxO, a σ54-dependent transcriptional activator (37). Other bacterial species may process AI-2 by different mechanisms (546, 586), and indeed, AI-2 signaling is not always density dependent (264). In some cases, AI-2 may have a physiological role related to the activated methyl cycle rather than a signaling role (220).
Many gram-positive bacteria, including streptococci, possess luxS and respond to AI-2, and this signaling has been associated with biofilm formation. However, despite intense study, a clearly defined role for AI-2 has not yet emerged, and the impact of AI-2 on streptococcal biofilm formation appears to be assay and growth condition dependent. For example, an early study with a luxS mutant of S. mutans reported no difference in the abilities of parent and mutant strains to form biofilms in microtiter plates (643). However, a subsequent microscopic visualization found architectural differences between biofilms of parent and luxS mutant S. mutans strains (403). The role of AI-2 in S. mutans biofilm structure has since been observed consistently (644, 661) and is dependent on the presence of sucrose in the growth medium (661). Sucrose dependence is related to the regulation of the GTF genes gtfB and gtfC by LuxS (661). In S. mutans, LuxS also controls the production of the lantibiotic bacteriocin mutacin I (which is not controlled by the CSP system) at the transcriptional level (402). In the absence of AI-2, the level of expression of the transcriptional regulator IrvA is increased, which leads to the repression of mutA (encoding mutacin I) and mutR (encoding a specific transcriptional activator of mutA) and reduced levels of mutacin I production. AI-2 also controls biofilm development by S. gordonii, and the inability to produce AI-2 is associated with an altered microcolony architecture (58). LuxS controls GTF activity, and indeed, AI-2 affects several aspects of carbohydrate metabolism in S. gordonii. The presence of AI-2 favors the utilization of sucrose by S. gordonii, whereas in its absence, lactose and other galactose-containing sugars are metabolized preferentially (395).
Many streptococcal biofilms in nature, particularly in the oral cavity, are polymicrobial, and AI-2 can play a role in the formation of these heterotypic communities. Mutualistic biofilm growth of S. oralis and A. naeslundii in flowing saliva is dependent upon the production of AI-2 by S. oralis (498). Furthermore, this effect is dependent on the AI-2 concentration, and heterotypic biofilm formation is suppressed above and below threshold concentrations. AI-2 is also required for the accumulation of mixed biofilms of S. gordonii with the periodontal pathogen P. gingivalis (395). Interestingly, the bacterial source of AI-2 is unimportant, as luxS mutants of either S. gordonii or P. gingivalis form biofilms in the presence of the wild-type partner organisms. Heterotypic communities do not accumulate, however, when AI-2 production is disrupted in both organisms.
Metabolic communication that extends beyond the provision of nutrients is a feature of high-density heterotypic biofilms. For example, oral streptococci coaggregate with Veillonella atypica, and these organisms are also metabolically compatible, as S. gordonii ferments carbohydrates to form lactic acid, which is a preferred fermentation substrate for V. atypica (Fig. 4D). In addition, however, a short-range diffusible signal is produced by V. atypica that induces expression of the α-amylase-encoding gene amyB in S. gordonii (146).
Related species of oral streptococci, in contrast, compete for space and resources. For example, under aerobic conditions, S. sanguinis and S. gordonii produce hydrogen peroxide more efficiently than S. mutans and, consequently, S. mutans is inhibited in mixed communities of these three species (319). Furthermore, in the presence of oxygen, S. sanguinis and S. gordonii release more DNA, which can stabilize biofilm structure, but may also be acquired by S. mutans cells that are more competent within biofilms (discussed above). In retaliation, S. mutans within biofilms increases the production of bacteriocins that target S. sanguinis and S. gordonii (316). The “arms race” escalates as S. gordonii in biofilms can interfere with bacteriocin production by S. mutans (636). The ongoing blows and counterblows struck by these organisms result in “competitive exclusion,” and hence, the establishment of S. gordonii/S. sanguinis or of S. mutans in a niche precludes colonization by the antagonistic species (316).
Cell-to-Cell Communication
Signaling mediated by surface contact is emerging as an important means of information transfer in bacteria. The streptococci dominate the densely packed biofilms that develop on supragingival tooth surfaces and will thereby be in direct contact with numerous other oral biofilm constituents. Signaling can ensue from adhesin-receptor binding or from the activation of surface proteins not associated with adherence. One of the best-studied examples is coadhesion and communication between S. gordonii and P. gingivalis. On the streptococcal surface, the AgI/II proteins SspA and SspB interact with short fimbriae (containing the Mfa major subunit protein) of P. gingivalis (273). The engagement of SspA or SspB with the short fimbriae initiates a signaling event within P. gingivalis cells that facilitates biofilm accumulation (68, 327). Structure-function analyses of the mechanism of the Mfa-SspB interaction identified a functional domain of SspB, designated BAR (SspB adherence region), spanning aa 1167 to 1193, that is fully conserved between SspA and SspB (68, 124). Within BAR, the N1182 and V1185 of an NITVK motif are essential for the Mfa recognition of SspB, and these residues, along with T1184, are not conserved in SpaP, the S. mutans homolog of Ssp that does not bind to Mfa (124, 128). The substitution of basic amino acids or serine for N1182 and the substitution of the hydrophobic residue Ile, Trp, or Phe for V1185 enhance the level of P. gingivalis binding to BAR, suggesting that both electrostatic and hydrophobic interactions contribute to BAR-Mfa binding. Furthermore, the substitution of the α-helix-breaking residue Pro or Gly is detrimental to P. gingivalis adherence, consistent with secondary structure playing a role in P. gingivalis adherence.
BAR also possesses a region resembling a known protein-protein-interacting motif, the eukaryotic nuclear receptor (NR) box (528). Interactions of NRs with their coactivating proteins are driven by the association of a hydrophobic α-helix of consensus sequence LXXLL, the NR box, with a hydrophobic pocket in the NR protein. This initial interaction is stabilized by electrostatic interactions that form with charged amino acids that flank LXXLL (528). The specificity of the coactivator interaction with a given NR is thus determined by the residues that reside on either side of LXXLL. The BAR peptide contains a predicted hydrophobic α-helix of the sequence VXXLL that is flanked on each side by positively charged lysine residues (125). This region resides immediately upstream from the NITVK motif. The introduction of amino acids with the potential to disrupt the secondary structure of VXXLL reduced the specific binding activity of BAR, suggesting that the putative α-helical character of VXXLL is important for the interaction of BAR with Mfa (125). Furthermore, replacing the lysines that flank VQDLL with acidic amino acids also reduced the binding activity, indicating that the association of VQDLL with Mfa may be stabilized by a charge clamp. The outcome of SspB-Mfa binding is a shift in the metabolic activity of P. gingivalis, including an increase in levels of extracellular polysaccharide synthesis, which enhances structure and leads to the recruitment of additional P. gingivalis cells (375, 552).
Conversely, contact with S. cristatus propagates a signal in P. gingivalis that causes the downregulation of fimA expression, with a resultant reduction in the amount of long fimbriae (containing the FimA major subunit protein) on the surface of P. gingivalis and the failure of these organisms to develop a heterotypic biofilm (655). Signaling is mediated by arginine deiminase (ArcA) on the surface of S. cristatus (656). While ArcA is an enzyme involved in the arginine metabolism pathway that converts arginine to ornithine, ammonia, and CO2, the signaling function of ArcA does not depend on enzymatic activity (356). Although S. gordonii also expresses ArcA, the ability of S. cristatus to repress FimA production is related to the elevated level of expression of arcA due to differences in the cis catabolite response elements of arcA and in the expression of trans-acting regulatory proteins (356).
Cell-to-cell communication in densely packed communities can also contribute to optimal metabolic activity in the participating organisms. Contact between S. gordonii and A. naeslundii induces the differential expression of genes involved in streptococcal arginine biosynthesis and transport (258). As a result, S. gordonii in a dual-species community is capable of aerobic growth when exogenous arginine is limited. Moreover, A. naeslundii produces catalase that can remove H2O2 from dual-species cultures and consequently decrease the level of oxidation of arginine residues in S. gordonii proteins (259). Interspecies interactions thus drive these organisms toward an ecologically balanced community.
CONCLUSION
Streptococcal adherence and colonization are complex multilevel processes that define the success or failure of the organism in human ecosystems. As such, streptococci devote considerable resources to ensuring the availability of an appropriate repertoire of effector molecules (Fig. 3) with sufficient redundancy to be robust in situations where one particular activity is unavailable or impeded by host or other bacterial factors. Moreover, these are not passive events; rather, streptococci are continuously monitoring the local environment and fine-tuning the expression of adhesins, communication systems, and metabolic pathways to optimize fitness under the prevailing conditions. The universal presence of streptococci in humans (and in many other animal hosts) reflects the success of these strategies. Paradoxically, the strengths of the streptococcal colonization mechanisms may also turn out to be weaknesses that can be exploited to develop new ways to control colonization and infection.
Vaccination against pathogenic species is very much a preferred strategy because it reduces the usage of antibiotics. These may be harmful, as in the case of application to pregnant mothers to act against potential GBS infection of neonates, or simply add to the ongoing problem of antibiotic resistance development. However, the vaccine route is fraught with difficulties, a major one being that the best protective (opsonic) antibodies are directed against cell surface components that are highly antigenically variable. A strategy being adopted for GAS is to string together multiple A regions of M proteins representing the most invasive serotypes and then use this as a polyvalent vaccine. This avoids the conformational regions of M protein that may generate cross-reactive antibodies to heart, brain, kidney, or joint cartilage and includes the opsonic epitopes. A 26-valent vaccine of this type has been shown to be well tolerated in human adult volunteers (126). However, the experience with pneumococcus conjugate vaccine comprised of seven or nine CPS serotypes is that the elimination of vaccine serotypes leads to serotype replacement and the emergence of new virulent strains. The careful construction of vaccine epitopes may go some way to reducing this, but nevertheless, the antigens to which protective antibodies have to be made are subject to immune selection. The many facets of GAS pathogenesis have been recently summarized in an excellent review (437), and the reader is referred to that article for detailed information about virulence factors.
The sialic acid-containing CPS has been a focus of most vaccine strategies for GBS to date, and clinical trials with conjugate vaccines covering five CPS serotypes have shown them to be safe and immunogenic (24). There are nine distinct CPS serotypes, but even a 9-valent vaccine would not protect against the increasing number of nontypeable isolates (24). A protein-based vaccine could, on the other hand, provide antigenic targets that are conserved across all GBS serotypes. Major surface proteins include the Alp family of proteins, e.g., α, Rib, R28, and Alp2 (357, 571, 572), that elicit protective immunity; ScpB C5a peptidase (96); β protein that binds IgA and factor H (373, 374); Lmb (228, 567); FbsA fibrinogen-binding protein (535); Sip (67, 385); LrrG (538); and CspA protease (221). Some of these proteins are promising vaccine candidates, but their potential for cross-protection is relatively unknown (279). More recently, the components of GAS and GBS pili have been a focus of vaccine design. Backbone and ancillary subunits from each GBS PI have been shown to elicit protective immunity against GBS in a neonatal mouse model of immunization (376, 383, 512). A vaccine comprising three selected pilus protein subunits could potentially confer protection against 94% GBS strains currently found in the United States and Italy (383).
Anti-dental-caries vaccines have been under study since it was first shown that antigens from S. mutans cells, such as Gtf, Gbp, and AgI/II, were protective against S. mutans colonization and caries in animal models (557). Clinical trails have tested the delivery of S. mutans antigens via the nasal route to utilize the inductive characteristics of nasally associated lymphoid tissue for secretory IgA and IgG antibodies. Immunization with Gtf or GbpB consistently produces high levels of salivary antibodies and reductions in experimental dental caries. The oral health impact on children would be huge, especially for those who are at high risk, if an effective dental caries vaccine were to be developed that was stable and could be administered mucosally (560). There are many interesting strategies under development that could affect longer-term colonization patterns of bacteria on mucosal surfaces or hard surfaces, e.g., teeth, to promote health. It is thought that the early retention of some organisms, e.g., S. salivarius and S. sanguinis, within the oral cavity of infants may be beneficial to their future oral health (88). It is possible, then, that by supplementing the oropharynx or nasopharynx with selected streptococcal species shortly after birth and during infancy, less desirable bacterial species may be excluded. There is evidence that colonization of the tongue and throat by bacteriocin-producing S. salivarius may assist in reducing the incidence of GAS pharyngitis in children (132). Replacement therapy has been considered to have potential for controlling S. mutans levels. By introducing an engineered nonpathogenic strain of S. mutans with a selective advantage, it might be possible to replace pathogenic strains, but the efficacy when applied to human subjects remains to be established.
Biofilm formation occurs as a result of initial adherence, the growth of societies involving quorum sensing, and then community development associated with a range of intermicrobial communication reactions. In terms of preventing specific organisms from depositing onto surfaces or being incorporated into communities, promising results have been obtained by blocking the adherence of S. mutans to salivary pellicles with peptides that mimic the streptococcal AgI/II surface protein adhesin (292). Variable-Fc-chain antibodies to AgI/II expressed by lactobacilli have been shown to prevent S. mutans colonization and caries development in rodent models of infection (321). These observations suggest that there may be alternatives to vaccination and antibiotics for modulating Streptococcus colonization. However, in removing a component of the natural microflora, there is a potential for exposing a niche that could be colonized by another less desirable organism.
Although not yet tried with complex biofilm communities, inhibitors of quorum sensing have been shown to effectively impair normal biofilm formation. Bromofuranone interferes with AI-2 signaling pathways and inhibits the formation of single-species biofilms of S. mutans and S. intermedius (361). However, halogenated furanones show toxic side effects on human cells and possible carcinogenic properties that make them unsuitable for use as pharmaceuticals for humans (56). It is possible that nontoxic microbial signaling inhibitors (486) or biomimetics (391) that disrupt the interactions that occur between streptococci in establishing biofilms might be found.
It is clear that new approaches are required, informed by microbiology, immunology, and molecular biology, to control components of microbial communities that form on or in the human body. The increased use of DNA sequencing for the characterization of pathogens, commensals, and complex ecosystems such as the oral microbiome has led to new approaches in the study of host-bacterium interactions. The in silico prediction of streptococcal surface-exposed proteins (27) is of interest for the rational development of vaccines or new inhibitors. The continuing use of broad-spectrum antibiotics to resolve problems associated with streptococcal infections might not be sustainable in the future, with the increasing incidence of antibiotic resistance. Since streptococci need to colonize mucosal surfaces, mainly in the upper respiratory tract, to be carried long-term or prior to causing infection, it seems crucial that nonantibiotic measures be developed in order to control colonization. The oropharynx and microflora therein provide an ideal system for experimental studies. The results of such studies might lead to an extension of methodologies to control the colonization of streptococci at other body sites.
Acknowledgments
We thank members of our laboratories for their excellent work on many aspects of Streptococcus molecular genetics and for their helpful discussions. We are very grateful to Mogens Kilian, Manfred Rohde, Rob Palmer, and Steve Kerrigan for providing figures. We are also grateful to many colleagues for their invaluable suggestions and insightful comments over the years. Owing to the complexity of this area of research, there are undoubtedly references that we have inadvertently not cited, or not been able to cite, for which we sincerely apologize.
Research in the laboratory of H.F.J. is currently supported by the NIH/NIDCR (grant R01 DE0166690) and the Wellcome Trust (grant 081855), and research in the laboratory of R.J.L. is currently supported by the NIH/NIDCR (grant R01 DE12505).
Biography
Angela H. Nobbs received her B.Sc. (Hons.) in Applied Microbiology at the University of Manchester in 1999. In 2003, she was awarded a Ph.D. in Molecular Microbiology at the University of Bristol, where her studies focused on streptococcal interactions with human epithelial cells and mechanisms of streptococcal colonization. This theme was continued with her first postdoctoral appointment at the University of Minnesota, where she studied the role of streptococcal surface adhesins in mediating interbacterial competition and worked on the characterization of housekeeping transpeptidase sortase A. She then expanded this area to investigate the role of sortase A in pilus assembly by group B Streptococcus with a Marie Curie Fellowship at Novartis Vaccines, Siena, Italy. Most recently, she has returned to the University of Bristol, where current projects focus on the interactions of streptococci with the fungus Candida albicans and the impact on candidal colonization and pathogenesis.
Richard J. Lamont was educated in Scotland at the University of Edinburgh and at the University of Aberdeen, where he received a Ph.D. in Microbiology in 1985. His postdoctoral training was in Bob Rosan's laboratory at the University of Pennsylvania, working on oral streptococcal adherence mechanisms. He spent 14 years on the faculty at the University of Washington, where his research interests expanded into the area of gene regulation in streptococcal biofilms and the assembly of complex multispecies bacterial communities. He also began to examine the molecular dialog between opportunistic pathogens and epithelial cells that allow host cell responses to be tailored to the threat level of colonizing organisms. In 2002, he moved his research group to the University of Florida, where the group continues with the study of the regulatory networks that control biofilm community development and facilitate cohabitation between oral bacteria and host epithelial cells.
Howard F. Jenkinson obtained a B.Sc. (Hons.) in Microbiology and Virology at the University of Warwick and a Ph.D. in Applied Biochemistry at the University of Nottingham, United Kingdom, in 1978. He then undertook nearly 5 years of postdoctoral research with Joel Mandelstam in the Microbiology Unit, University of Oxford, United Kingdom, on the biochemistry and genetics of Bacillus subtilis sporulation. He then spent 13 years on the faculty at the University of Otago, Dunedin, New Zealand, where he established research interests in oral streptococcus genetics and the molecular basis of microbial adhesion. At Otago, he also worked collaboratively with the Candida albicans research group and developed genetic and adhesion studies. In 1997, he moved to the University of Bristol, United Kingdom, where his group continues work on adhesion, colonization, and virulence properties of human oral microorganisms. Currently, the research program focuses on Streptococcus, Candida, and Treponema mechanisms of biofilm formation, microbial community development, and invasion of oral tissues.
REFERENCES
- 1.Aas, J. A., B. J. Paster, L. N. Stokes, I. Olsen, and F. E. Dewhirst. 2005. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 435721-5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbot, E. L., W. D. Smith, G. P. Siou, C. Chiriboga, R. J. Smith, J. A. Wilson, B. H. Hirst, and M. A. Kehoe. 2007. Pili mediate specific adhesion of Streptococcus pyogenes to human tonsil and skin. Cell. Microbiol. 91822-1833. [DOI] [PubMed] [Google Scholar]
- 3.Adamou, J. E., J. H. Heinrichs, A. L. Erwin, W. Walsh, T. Gayle, M. Dormitzer, R. Dagan, Y. A. Brewah, P. Barren, R. Lathigra, S. Langermann, S. Koenig, and S. Johnson. 2001. Identification and characterization of a novel family of pneumococcal proteins that are protective against sepsis. Infect. Immun. 69949-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adderson, E. E., S. Takahashi, Y. Wang, J. Armstrong, D. V. Miller, and J. F. Bohnsack. 2003. Subtractive hybridization identifies a novel predicted protein mediating epithelial cell invasion by virulent serotype III group B Streptococcus agalactiae. Infect. Immun. 716857-6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahn, S. J., Z. T. Wen, and R. A. Burne. 2006. Multilevel control of competence development and stress tolerance in Streptococcus mutans UA159. Infect. Immun. 741631-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ajdic, D., W. M. McShan, R. E. McLaughlin, G. Savic, J. Chang, M. B. Carson, C. Primeaux, R. Tian, S. Kenton, H. Jia, S. Lin, Y. Qian, S. Li, H. Zhu, F. Najar, H. Lai, J. White, B. A. Roe, and J. J. Ferretti. 2002. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl. Acad. Sci. USA 9914434-14439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akerstrom, B., E. Nielsen, and L. Bjorck. 1987. Definition of IgG- and albumin-binding regions of streptococcal protein G. J. Biol. Chem. 26213388-13391. [PubMed] [Google Scholar]
- 8.Akesson, P., J. Cooney, F. Kishimoto, and L. Bjorck. 1990. Protein H—a novel IgG binding bacterial protein. Mol. Immunol. 27523-531. [DOI] [PubMed] [Google Scholar]
- 9.Akesson, P., A. G. Sjoholm, and L. Bjorck. 1996. Protein SIC, a novel extracellular protein of Streptococcus pyogenes interfering with complement function. J. Biol. Chem. 2711081-1088. [DOI] [PubMed] [Google Scholar]
- 10.Allen, B. L., and M. Hook. 2002. Isolation of a putative laminin binding protein from Streptococcus anginosus. Microb. Pathog. 3323-31. [DOI] [PubMed] [Google Scholar]
- 11.Almengor, A. C., T. L. Kinkel, S. J. Day, and K. S. McIver. 2007. The catabolite control protein CcpA binds to Pmga and influences expression of the virulence regulator Mga in the group A Streptococcus. J. Bacteriol. 1898405-8416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Okla, S., C. Chatenay-Rivauday, J. P. Klein, and D. Wachsmann. 1999. Involvement of α5β1 integrins in interleukin 8 production induced by oral viridans streptococcal protein I/IIf in cultured endothelial cells. Cell. Microbiol. 1157-168. [DOI] [PubMed] [Google Scholar]
- 13.Alouf, J. E. 1980. Streptococcal toxins (streptolysin O, streptolysin S, erythrogenic toxin). Pharmacol. Ther. 11661-717. [DOI] [PubMed] [Google Scholar]
- 14.Alouf, J. E., and H. Muller-Alouf. 2003. Staphylococcal and streptococcal superantigens: molecular, biological and clinical aspects. Int. J. Med. Microbiol. 292429-440. [DOI] [PubMed] [Google Scholar]
- 15.Andersen, R. N., N. Ganeshkumar, and P. E. Kolenbrander. 1993. Cloning of the Streptococcus gordonii PK488 gene, encoding an adhesin which mediates coaggregation with Actinomyces naeslundii PK606. Infect. Immun. 61981-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderton, J. M., G. Rajam, S. Romero-Steiner, S. Summer, A. P. Kowalczyk, G. M. Carlone, J. S. Sampson, and E. W. Ades. 2007. E-cadherin is a receptor for the common protein pneumococcal surface adhesin A (PsaA) of Streptococcus pneumoniae. Microb. Pathog. 42225-236. [DOI] [PubMed] [Google Scholar]
- 17.Andre, I., J. Persson, A. M. Blom, H. Nilsson, T. Drakenberg, G. Lindahl, and S. Linse. 2006. Streptococcal M protein: structural studies of the hypervariable region, free and bound to human C4BP. Biochemistry 454559-4568. [DOI] [PubMed] [Google Scholar]
- 18.Archambaud, C., M. A. Nahori, J. Pizarro-Cerda, P. Cossart, and O. Dussurget. 2006. Control of Listeria superoxide dismutase by phosphorylation. J. Biol. Chem. 28131812-31822. [DOI] [PubMed] [Google Scholar]
- 19.Areschoug, T., M. Stalhammar-Carlemalm, I. Karlsson, and G. Lindahl. 2002. Streptococcal β protein has separate binding sites for human factor H and IgA-Fc. J. Biol. Chem. 27712642-12648. [DOI] [PubMed] [Google Scholar]
- 20.Ashbaugh, C. D., S. Albertí, and M. R. Wessels. 1998. Molecular analysis of the capsule gene region of group A Streptococcus: the hasAB genes are sufficient for capsule expression. J. Bacteriol. 1804955-4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aspiras, M. B., R. P. Ellen, and D. G. Cvitkovitch. 2004. ComX activity of Streptococcus mutans growing in biofilms. FEMS Microbiol. Lett. 238167-174. [DOI] [PubMed] [Google Scholar]
- 22.Attali, C., C. Frolet, C. Durmort, J. Offant, T. Vernet, and A. M. Di Guilmi. 2008. Streptococcus pneumoniae choline-binding protein E interaction with plasminogen/plasmin stimulates migration across the extracellular matrix. Infect. Immun. 76466-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bagnoli, F., M. Moschioni, C. Donati, V. Dimitrovska, I. Ferlenghi, C. Facciotti, A. Muzzi, F. Giusti, C. Emolo, A. Sinisi, M. Hilleringmann, W. Pansegrau, S. Censini, R. Rappuoli, A. Covacci, V. Masignani, and M. A. Barocchi. 2008. A second pilus type in Streptococcus pneumoniae is prevalent in emerging serotypes and mediates adhesion to host cells. J. Bacteriol. 1905480-5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker, C. J., and M. S. Edwards. 2003. Group B streptococcal conjugate vaccines. Arch. Dis. Child. 88375-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banas, J. A., R. R. Russell, and J. J. Ferretti. 1990. Sequence analysis of the gene for the glucan-binding protein of Streptococcus mutans Ingbritt. Infect. Immun. 58667-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banas, J. A., and M. M. Vickerman. 2003. Glucan-binding proteins of the oral streptococci. Crit. Rev. Oral Biol. Med. 1489-99. [DOI] [PubMed] [Google Scholar]
- 27.Barinov, A., V. Loux, A. Hammani, P. Nicolas, P. Langella, D. Ehrlich, E. Maguin, and M. van de Guchte. 2009. Prediction of surface exposed proteins in Streptococcus pyogenes, with a potential application to other gram-positive bacteria. Proteomics 961-73. [DOI] [PubMed] [Google Scholar]
- 28.Barlow, G. H., E. Devine, and R. Finley. 1975. Immunological and biochemical comparison of streptokinase and the streptokinase plasminogen complex. Res. Commun. Chem. Pathol. Pharmacol. 10465-471. [PubMed] [Google Scholar]
- 29.Barocchi, M. A., J. Ries, X. Zogaj, C. Hemsley, B. Albiger, A. Kanth, S. Dahlberg, J. Fernebro, M. Moschioni, V. Masignani, K. Hultenby, A. R. Taddei, K. Beiter, F. Wartha, A. von Euler, A. Covacci, D. W. Holden, S. Normark, R. Rappuoli, and B. Henriques-Normark. 2006. A pneumococcal pilus influences virulence and host inflammatory responses. Proc. Natl. Acad. Sci. USA 1032857-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baron, M. J., G. R. Bolduc, M. B. Goldberg, T. C. Auperin, and L. C. Madoff. 2004. Alpha C protein of group B Streptococcus binds host cell surface glycosaminoglycan and enters cells by an actin-dependent mechanism. J. Biol. Chem. 27924714-24723. [DOI] [PubMed] [Google Scholar]
- 31.Barrau, K., A. Boulamery, G. Imbert, J. P. Casalta, G. Habib, T. Messana, J. L. Bonnet, E. Rubinstein, and D. Raoult. 2004. Causative organisms of infective endocarditis according to host status. Clin. Microbiol. Infect. 10302-308. [DOI] [PubMed] [Google Scholar]
- 32.Bates, C. S., G. E. Montanez, C. R. Woods, R. M. Vincent, and Z. Eichenbaum. 2003. Identification and characterization of a Streptococcus pyogenes operon involved in binding of hemoproteins and acquisition of iron. Infect. Immun. 711042-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bates, C. S., C. Toukoki, M. N. Neely, and Z. Eichenbaum. 2005. Characterization of MtsR, a new metal regulator in group A Streptococcus, involved in iron acquisition and virulence. Infect. Immun. 735743-5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beachey, E. H., and H. S. Courtney. 1987. Bacterial adherence: the attachment of group A streptococci to mucosal surfaces. Rev. Infect. Dis. 9475-481. [DOI] [PubMed] [Google Scholar]
- 35.Beckert, S., B. Kreikemeyer, and A. Podbielski. 2001. Group A streptococcal rofA gene is involved in the control of several virulence genes and eukaryotic cell attachment and internalization. Infect. Immun. 69534-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beg, A. M., M. N. Jones, T. Miller-Torbert, and R. G. Holt. 2002. Binding of Streptococcus mutans to extracellular matrix molecules and fibrinogen. Biochem. Biophys. Res. Commun. 29875-79. [DOI] [PubMed] [Google Scholar]
- 37.Bejerano-Sagie, M., and K. B. Xavier. 2007. The role of small RNAs in quorum sensing. Curr. Opin. Microbiol. 10189-198. [DOI] [PubMed] [Google Scholar]
- 38.Benchabane, H., L. A. Lortie, N. D. Buckley, L. Trahan, and M. Frenette. 2002. Inactivation of the Streptococcus mutans fxpC gene confers resistance to xylitol, a caries-preventive natural carbohydrate sweetener. J. Dent. Res. 81380-386. [DOI] [PubMed] [Google Scholar]
- 39.Bensing, B. A., J. A. Lopez, and P. M. Sullam. 2004. The Streptococcus gordonii surface proteins GspB and Hsa mediate binding to sialylated carbohydrate epitopes on the platelet membrane glycoprotein Ibα. Infect. Immun. 726528-6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bensing, B. A., C. E. Rubens, and P. M. Sullam. 2001. Genetic loci of Streptococcus mitis that mediate binding to human platelets. Infect. Immun. 691373-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bensing, B. A., and P. M. Sullam. 2002. An accessory sec locus of Streptococcus gordonii is required for export of the surface protein GspB and for normal levels of binding to human platelets. Mol. Microbiol. 441081-1094. [DOI] [PubMed] [Google Scholar]
- 42.Berge, A., and U. Sjobring. 1993. PAM, a novel plasminogen-binding protein from Streptococcus pyogenes. J. Biol. Chem. 26825417-25424. [PubMed] [Google Scholar]
- 43.Bergmann, S., and S. Hammerschmidt. 2006. Versatility of pneumococcal surface proteins. Microbiology 152295-303. [DOI] [PubMed] [Google Scholar]
- 44.Bergmann, S., M. Rohde, G. S. Chhatwal, and S. Hammerschmidt. 2001. α-Enolase of Streptococcus pneumoniae is a plasmin(ogen)-binding protein displayed on the bacterial cell surface. Mol. Microbiol. 401273-1287. [DOI] [PubMed] [Google Scholar]
- 45.Bergmann, S., M. Rohde, and S. Hammerschmidt. 2004. Glyceraldehyde-3-phosphate dehydrogenase of Streptococcus pneumoniae is a surface-displayed plasminogen-binding protein. Infect. Immun. 722416-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berry, A. M., R. A. Lock, S. M. Thomas, D. P. Rajan, D. Hansman, and J. C. Paton. 1994. Cloning and nucleotide sequence of the Streptococcus pneumoniae hyaluronidase gene and purification of the enzyme from recombinant Escherichia coli. Infect. Immun. 621101-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bessen, D. E. 1994. Localization of immunoglobulin A-binding sites within M or M-like proteins of group A streptococci. Infect. Immun. 621968-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bessen, D. E., and V. A. Fischetti. 1992. Nucleotide sequences of two adjacent M or M-like protein genes of group A streptococci: different RNA transcript levels and identification of a unique immunoglobulin A-binding protein. Infect. Immun. 60124-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bevanger, L., and J. Iversen. 1981. The Ibc protein fraction of group B streptococci: characterization of protein antigens extracted by HCL. Acta Pathol. Microbiol. Scand. B 89205-209. [DOI] [PubMed] [Google Scholar]
- 50.Bhagwat, S. P., J. Nary, and R. A. Burne. 2001. Effects of mutating putative two-component systems on biofilm formation by Streptococcus mutans UA159. FEMS Microbiol. Lett. 205225-230. [DOI] [PubMed] [Google Scholar]
- 51.Bhakdi, S., J. Tranum-Jensen, and A. Sziegoleit. 1985. Mechanism of membrane damage by streptolysin-O. Infect. Immun. 4752-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bikker, F. J., A. J. Ligtenberg, K. Nazmi, E. C. Veerman, W. van't Hof, J. G. Bolscher, A. Poustka, A. V. N. Amerongen, and J. Mollenhauer. 2002. Identification of the bacteria-binding peptide domain on salivary agglutinin (gp-340/DMBT1), a member of the scavenger receptor cysteine-rich superfamily. J. Biol. Chem. 27732109-32115. [DOI] [PubMed] [Google Scholar]
- 53.Biswas, I., L. Drake, and S. Biswas. 2007. Regulation of gbpC expression in Streptococcus mutans. J. Bacteriol. 1896521-6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Biswas, I., L. Drake, D. Erkina, and S. Biswas. 2008. Involvement of sensor kinases in the stress tolerance response of Streptococcus mutans. J. Bacteriol. 19068-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Biswas, S., and I. Biswas. 2006. Regulation of the glucosyltransferase (gtfBC) operon by CovR in Streptococcus mutans. J. Bacteriol. 188988-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bjarnsholt, T., and M. Givskov. 2007. Quorum-sensing blockade as a strategy for enhancing host defences against bacterial pathogens. Philos. Trans. R. Soc. Lond. B Biol. Sci. 3621213-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bjorck, L., and G. Kronvall. 1984. Purification and some properties of streptococcal protein G, a novel IgG-binding reagent. J. Immunol. 133969-974. [PubMed] [Google Scholar]
- 58.Blehert, D. S., R. J. Palmer, Jr., J. B. Xavier, J. S. Almeida, and P. E. Kolenbrander. 2003. Autoinducer 2 production by Streptococcus gordonii DL1 and the biofilm phenotype of a luxS mutant are influenced by nutritional conditions. J. Bacteriol. 1854851-4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boackle, R. J., M. H. Connor, and J. Vesely. 1993. High molecular weight non-immunoglobulin salivary agglutinins (NIA) bind C1Q globular heads and have the potential to activate the first complement component. Mol. Immunol. 30309-319. [DOI] [PubMed] [Google Scholar]
- 60.Bolduc, G. R., M. J. Baron, C. Gravekamp, C. S. Lachenauer, and L. C. Madoff. 2002. The alpha C protein mediates internalization of group B Streptococcus within human cervical epithelial cells. Cell. Microbiol. 4751-758. [DOI] [PubMed] [Google Scholar]
- 61.Boyle, M. D., J. Hawlitzky, R. Raeder, and A. Podbielski. 1994. Analysis of genes encoding two unique type IIa immunoglobulin G-binding proteins expressed by a single group A streptococcal isolate. Infect. Immun. 621336-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brady, L. J., D. A. Piacentini, P. J. Crowley, P. C. Oyston, and A. S. Bleiweis. 1992. Differentiation of salivary agglutinin-mediated adherence and aggregation of mutans streptococci by use of monoclonal antibodies against the major surface adhesin P1. Infect. Immun. 601008-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bray, B. A., I. C. Sutcliffe, and D. J. Harrington. 2009. Expression of the MtsA lipoprotein of Streptococcus agalactiae A909 is regulated by manganese and iron. Antonie van Leeuwenhoek 95101-109. [DOI] [PubMed] [Google Scholar]
- 64.Brenot, A., K. Y. King, and M. G. Caparon. 2005. The PerR regulon in peroxide resistance and virulence of Streptococcus pyogenes. Mol. Microbiol. 55221-234. [DOI] [PubMed] [Google Scholar]
- 65.Brenot, A., B. F. Weston, and M. G. Caparon. 2007. A PerR-regulated metal transporter (PmtA) is an interface between oxidative stress and metal homeostasis in Streptococcus pyogenes. Mol. Microbiol. 631185-1196. [DOI] [PubMed] [Google Scholar]
- 66.Bricker, A. L., C. Cywes, C. D. Ashbaugh, and M. R. Wessels. 2002. NAD+-glycohydrolase acts as an intracellular toxin to enhance the extracellular survival of group A streptococci. Mol. Microbiol. 44257-269. [DOI] [PubMed] [Google Scholar]
- 67.Brodeur, B. R., M. Boyer, I. Charlebois, J. Hamel, F. Couture, C. R. Rioux, and D. Martin. 2000. Identification of group B streptococcal Sip protein, which elicits cross-protective immunity. Infect. Immun. 685610-5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brooks, W., D. R. Demuth, S. Gil, and R. J. Lamont. 1997. Identification of a Streptococcus gordonii SspB domain that mediates adhesion to Porphyromonas gingivalis. Infect. Immun. 653753-3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brown, A. E., J. D. Rogers, E. M. Haase, P. M. Zelasko, and F. A. Scannapieco. 1999. Prevalence of the amylase-binding protein A gene (abpA) in oral streptococci. J. Clin. Microbiol. 374081-4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brown, C. K., Z. Y. Gu, Y. V. Matsuka, S. S. Purushothaman, L. A. Winter, P. P. Cleary, S. B. Olmsted, D. H. Ohlendorf, and C. A. Earhart. 2005. Structure of the streptococcal cell wall C5a peptidase. Proc. Natl. Acad. Sci. USA 10218391-18396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brown, J. S., S. M. Gilliland, and D. W. Holden. 2001. A Streptococcus pneumoniae pathogenicity island encoding an ABC transporter involved in iron uptake and virulence. Mol. Microbiol. 40572-585. [DOI] [PubMed] [Google Scholar]
- 72.Brown, J. S., S. M. Gilliland, J. Ruiz-Albert, and D. W. Holden. 2002. Characterization of pit, a Streptococcus pneumoniae iron uptake ABC transporter. Infect. Immun. 704389-4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brown, J. S., A. D. Ogunniyi, M. C. Woodrow, D. W. Holden, and J. C. Paton. 2001. Immunization with components of two iron uptake ABC transporters protects mice against systemic Streptococcus pneumoniae infection. Infect. Immun. 696702-6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Browngardt, C. M., Z. T. Wen, and R. A. Burne. 2004. RegM is required for optimal fructosyltransferase and glucosyltransferase gene expression in Streptococcus mutans. FEMS Microbiol. Lett. 24075-79. [DOI] [PubMed] [Google Scholar]
- 75.Bryan, J. D., R. Liles, U. Cvek, M. Trutschl, and D. Shelver. 2008. Global transcriptional profiling reveals Streptococcus agalactiae genes controlled by the MtaR transcription factor. BMC Genomics 9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bunick, F. J., and S. Kashket. 1981. Enolases from fluoride-sensitive and fluoride-resistant streptococci. Infect. Immun. 34856-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Burne, R. A., and J. E. Penders. 1992. Characterization of the Streptococcus mutans GS-5 fruA gene encoding exo-β-d-fructosidase. Infect. Immun. 604621-4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Burnette-Curley, D., V. Wells, H. Viscount, C. L. Munro, J. C. Fenno, P. Fives-Taylor, and F. L. Macrina. 1995. FimA, a major virulence factor associated with Streptococcus parasanguis endocarditis. Infect. Immun. 634669-4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Burnham, C. A., S. E. Shokoples, and G. J. Tyrrell. 2007. Invasion of HeLa cells by group B Streptococcus requires the phosphoinositide-3-kinase signalling pathway and modulates phosphorylation of host-cell Akt and glycogen synthase kinase-3. Microbiology 1534240-4252. [DOI] [PubMed] [Google Scholar]
- 80.Busscher, H. J., R. Bos, and H. C. van der Mei. 1995. Initial microbial adhesion is a determinant for the strength of biofilm adhesion. FEMS Microbiol. Lett. 128229-234. [DOI] [PubMed] [Google Scholar]
- 81.Busscher, H. J., B. van de Belt-Gritter, R. J. Dijkstra, W. Norde, F. C. Petersen, A. A. Scheie, and H. C. van der Mei. 2007. Intermolecular forces and enthalpies in the adhesion of Streptococcus mutans and an antigen I/II-deficient mutant to laminin films. J. Bacteriol. 1892988-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Calvinho, L. F., and S. P. Oliver. 1998. Characterization of mechanisms involved in uptake of Streptococcus dysgalactiae by bovine mammary epithelial cells. Vet. Microbiol. 63261-274. [DOI] [PubMed] [Google Scholar]
- 83.Caparon, M. G., and J. R. Scott. 1987. Identification of a gene that regulates expression of M protein, the major virulence determinant of group A streptococci. Proc. Natl. Acad. Sci. USA 848677-8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carlin, A. F., A. L. Lewis, A. Varki, and V. Nizet. 2007. Group B streptococcal capsular sialic acids interact with Siglecs (immunoglobulin-like lectins) on human leukocytes. J. Bacteriol. 1891231-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carlsson, F., M. Stalhammar-Carlemalm, K. Flardh, C. Sandin, E. Carlemalm, and G. Lindahl. 2006. Signal sequence directs localized secretion of bacterial surface proteins. Nature 442943-946. [DOI] [PubMed] [Google Scholar]
- 86.Caswell, C. C., M. Barczyk, D. R. Keene, E. Lukomska, D. E. Gullberg, and S. Lukomski. 2008. Identification of the first prokaryotic collagen sequence motif that mediates binding to human collagen receptors, integrins α2β1 and α11β1. J. Biol. Chem. 28336168-36175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Caswell, C. C., R. Han, K. M. Hovis, P. Ciborowski, D. R. Keene, R. T. Marconi, and S. Lukomski. 2008. The Scl1 protein of M6-type group A Streptococcus binds the human complement regulatory protein, factor H, and inhibits the alternative pathway of complement. Mol. Microbiol. 67584-596. [DOI] [PubMed] [Google Scholar]
- 88.Caufield, P. W., A. P. Dasanayake, Y. Li, Y. Pan, J. Hsu, and J. M. Hardin. 2000. Natural history of Streptococcus sanguinis in the oral cavity of infants: evidence for a discrete window of infectivity. Infect. Immun. 684018-4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chalmers, N. I., R. J. Palmer, Jr., J. O. Cisar, and P. E. Kolenbrander. 2008. Characterization of a Streptococcus sp.-Veillonella sp. community micromanipulated from dental plaque. J. Bacteriol. 1908145-8154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chapuy-Regaud, S., A. D. Ogunniyi, N. Diallo, Y. Huet, J. F. Desnottes, J. C. Paton, S. Escaich, and M. C. Trombe. 2003. RegR, a global LacI/GalR family regulator, modulates virulence and competence in Streptococcus pneumoniae. Infect. Immun. 712615-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chatfield, C. H., H. Koo, and R. G. Quivey, Jr. 2005. The putative autolysin regulator LytR in Streptococcus mutans plays a role in cell division and is growth-phase regulated. Microbiology 151625-631. [DOI] [PubMed] [Google Scholar]
- 92.Chaudhuri, B., S. Paju, E. M. Haase, M. M. Vickerman, J. M. Tanzer, and F. A. Scannapieco. 2008. Amylase-binding protein B of Streptococcus gordonii is an extracellular dipeptidyl-peptidase. Infect. Immun. 764530-4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chaussee, M. S., G. L. Sylva, D. E. Sturdevant, L. M. Smoot, M. R. Graham, R. O. Watson, and J. M. Musser. 2002. Rgg influences the expression of multiple regulatory loci to coregulate virulence factor expression in Streptococcus pyogenes. Infect. Immun. 70762-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen, C. C., and P. P. Cleary. 1990. Complete nucleotide sequence of the streptococcal C5a peptidase gene of Streptococcus pyogenes. J. Biol. Chem. 2653161-3167. [PubMed] [Google Scholar]
- 95.Cheng, Q., D. Finkel, and M. K. Hostetter. 2000. Novel purification scheme and functions for a C3-binding protein from Streptococcus pneumoniae. Biochemistry 395450-5457. [DOI] [PubMed] [Google Scholar]
- 96.Cheng, Q., D. Stafslien, S. S. Purushothaman, and P. Cleary. 2002. The group B streptococcal C5a peptidase is both a specific protease and an invasin. Infect. Immun. 702408-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chia, J. S., H. T. Lien, P. R. Hsueh, P. M. Chen, A. Sun, and J. Y. Chen. 2002. Induction of cytokines by glucosyltransferases of Streptococcus mutans. Clin. Diagn. Lab. Immunol. 9892-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chia, J. S., Y. L. Lin, H. T. Lien, and J. Y. Chen. 2004. Platelet aggregation induced by serotype polysaccharides from Streptococcus mutans. Infect. Immun. 722605-2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chia, J. S., C. Y. Yeh, and J. Y. Chen. 2000. Identification of a fibronectin binding protein from Streptococcus mutans. Infect. Immun. 681864-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chmouryguina, I., A. Suvorov, P. Ferrieri, and P. P. Cleary. 1996. Conservation of the C5a peptidase genes in group A and B streptococci. Infect. Immun. 642387-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chong, P., L. Drake, and I. Biswas. 2008. LiaS regulates virulence factor expression in Streptococcus mutans. Infect. Immun. 763093-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Christie, J., R. McNab, and H. F. Jenkinson. 2002. Expression of fibronectin-binding protein FbpA modulates adhesion in Streptococcus gordonii. Microbiology 1481615-1625. [DOI] [PubMed] [Google Scholar]
- 103.Cisar, J. O., A. L. Sandberg, C. Abeygunawardana, G. P. Reddy, and C. A. Bush. 1995. Lectin recognition of host-like saccharide motifs in streptococcal cell wall polysaccharides. Glycobiology 5655-662. [DOI] [PubMed] [Google Scholar]
- 104.Clarke, V. A., N. Platt, and T. D. Butters. 1995. Cloning and expression of the β-N-acetylglucosaminidase gene from Streptococcus pneumoniae. Generation of truncated enzymes with modified aglycon specificity. J. Biol. Chem. 2708805-8814. [DOI] [PubMed] [Google Scholar]
- 105.Cleary, P., and D. Retnoningrum. 1994. Group A streptococcal immunoglobulin-binding proteins: adhesins, molecular mimicry or sensory proteins? Trends Microbiol. 2131-136. [DOI] [PubMed] [Google Scholar]
- 106.Cleary, P. P., U. Prahbu, J. B. Dale, D. E. Wexler, and J. Handley. 1992. Streptococcal C5a peptidase is a highly specific endopeptidase. Infect. Immun. 605219-5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cockeran, R., R. Anderson, and C. Feldman. 2002. The role of pneumolysin in the pathogenesis of Streptococcus pneumoniae infection. Curr. Opin. Infect. Dis. 15235-239. [DOI] [PubMed] [Google Scholar]
- 108.Collin, M., and A. Olsen. 2001. EndoS, a novel secreted protein from Streptococcus pyogenes with endoglycosidase activity on human IgG. EMBO J. 203046-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Comfort, D., and R. T. Clubb. 2004. A comparative genome analysis identifies distinct sorting pathways in gram-positive bacteria. Infect. Immun. 722710-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cook, G. S., J. W. Costerton, and R. J. Lamont. 1998. Biofilm formation by Porphyromonas gingivalis and Streptococcus gordonii. J. Periodont. Res. 33323-327. [DOI] [PubMed] [Google Scholar]
- 111.Correia, F. F., J. M. DiRienzo, T. L. McKay, and B. Rosan. 1996. scbA from Streptococcus crista CC5A: an atypical member of the lraI gene family. Infect. Immun. 642114-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Courtney, H. S., J. B. Dale, and D. I. Hasty. 1996. Differential effects of the streptococcal fibronectin-binding protein, FBP54, on adhesion of group A streptococci to human buccal cells and HEp-2 tissue culture cells. Infect. Immun. 642415-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Courtney, H. S., J. B. Dale, and D. L. Hasty. 2002. Mapping the fibrinogen-binding domain of serum opacity factor of group A streptococci. Curr. Microbiol. 44236-240. [DOI] [PubMed] [Google Scholar]
- 114.Courtney, H. S., Y. Li, J. B. Dale, and D. L. Hasty. 1994. Cloning, sequencing, and expression of a fibronectin/fibrinogen-binding protein from group A streptococci. Infect. Immun. 623937-3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Courtney, H. S., I. Ofek, T. Penfound, V. Nizet, M. A. Pence, B. Kreikemeyer, A. Podbielski, D. L. Hasty, and J. B. Dale. 2009. Relationship between expression of the family of M proteins and lipoteichoic acid to hydrophobicity and biofilm formation in Streptococcus pyogenes. PLoS ONE 4e4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Courtney, H. S., C. von Hunolstein, J. B. Dale, M. S. Bronze, E. H. Beachey, and D. L. Hasty. 1992. Lipoteichoic acid and M protein: dual adhesins of group A streptococci. Microb. Pathog. 12199-208. [DOI] [PubMed] [Google Scholar]
- 117.Cowan, M. M., K. G. Taylor, and R. J. Doyle. 1987. Energetics of the initial phase of adhesion of Streptococcus sanguis to hydroxylapatite. J. Bacteriol. 1692995-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cue, D., P. E. Dombek, H. Lam, and P. P. Cleary. 1998. Streptococcus pyogenes serotype M1 encodes multiple pathways for entry into human epithelial cells. Infect. Immun. 664593-4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cunningham, M. W. 2008. Pathogenesis of group A streptococcal infections and their sequelae. Adv. Exp. Med. Biol. 60929-42. [DOI] [PubMed] [Google Scholar]
- 120.Cunningham, M. W. 2000. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 13470-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cunningham, M. W., and E. H. Beachey. 1974. Peptic digestion of streptococcal M protein. I. Effect of digestion at suboptimal pH upon the biological and immunochemical properties of purified M protein extracts. Infect. Immun. 9244-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cvitkovitch, D. G. 2001. Genetic competence and transformation in oral streptococci. Crit. Rev. Oral Biol. Med. 12217-243. [DOI] [PubMed] [Google Scholar]
- 123.Cywes, C., and M. R. Wessels. 2001. Group A Streptococcus tissue invasion by CD44-mediated cell signalling. Nature 414648-652. [DOI] [PubMed] [Google Scholar]
- 124.Daep, C. A., D. M. James, R. J. Lamont, and D. R. Demuth. 2006. Structural characterization of peptide-mediated inhibition of Porphyromonas gingivalis biofilm formation. Infect. Immun. 745756-5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Daep, C. A., R. J. Lamont, and D. R. Demuth. 2008. Interaction of Porphyromonas gingivalis with oral streptococci requires a motif that resembles the eukaryotic nuclear receptor box protein-protein interaction domain. Infect. Immun. 763273-3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dale, J. B. 2008. Current status of group A streptococcal vaccine development. Adv. Exp. Med. Biol. 60953-63. [DOI] [PubMed] [Google Scholar]
- 127.Daniely, D., M. Portnoi, M. Shagan, A. Porgador, N. Givon-Lavi, E. Ling, R. Dagan, and N. Y. Mizrachi. 2006. Pneumococcal 6-phosphogluconate-dehydrogenase, a putative adhesin, induces protective immune response in mice. Clin. Exp. Immunol. 144254-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Demuth, D. R., D. C. Irvine, J. W. Costerton, G. S. Cook, and R. J. Lamont. 2001. Discrete protein determinant directs the species-specific adherence of Porphyromonas gingivalis to oral streptococci. Infect. Immun. 695736-5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Derbise, A., Y. P. Song, S. Parikh, V. A. Fischetti, and V. Pancholi. 2004. Role of the C-terminal lysine residues of streptococcal surface enolase in Glu- and Lys-plasminogen-binding activities of group A streptococci. Infect. Immun. 7294-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.de Saizieu, A., C. Gardes, N. Flint, C. Wagner, M. Kamber, T. J. Mitchell, W. Keck, K. E. Amrein, and R. Lange. 2000. Microarray-based identification of a novel Streptococcus pneumoniae regulon controlled by an autoinduced peptide. J. Bacteriol. 1824696-4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Diaz-Torres, M. L., and R. R. Russell. 2001. HtrA protease and processing of extracellular proteins of Streptococcus mutans. FEMS Microbiol. Lett. 20423-28. [DOI] [PubMed] [Google Scholar]
- 132.Dierksen, K. P., C. J. Moore, M. Inglis, P. A. Wescombe, and J. R. Tagg. 2007. The effect of ingestion of milk supplemented with salivaricin A-producing Streptococcus salivarius on the bacteriocin-like inhibitory activity of streptococcal populations on the tongue. FEMS Microbiol. Ecol. 59584-591. [DOI] [PubMed] [Google Scholar]
- 133.Dinkla, K., I. Sastalla, A. W. Godehardt, N. Janze, G. S. Chhatwal, M. Rohde, and E. Medina. 2007. Upregulation of capsule enables Streptococcus pyogenes to evade immune recognition by antigen-specific antibodies directed to the G-related α2-macroglobulin-binding protein GRAB located on the bacterial surface. Microbes Infect. 9922-931. [DOI] [PubMed] [Google Scholar]
- 134.Dintilhac, A., and J. P. Claverys. 1997. The adc locus, which affects competence for genetic transformation in Streptococcus pneumoniae, encodes an ABC transporter with a putative lipoprotein homologous to a family of streptococcal adhesins. Res. Microbiol. 148119-131. [DOI] [PubMed] [Google Scholar]
- 135.Di Virgilio, F. 1995. The P2Z purinoceptor: an intriguing role in immunity, inflammation and cell death. Immunol. Today 16524-528. [DOI] [PubMed] [Google Scholar]
- 136.Doern, C. D., A. L. Roberts, W. Hong, J. Nelson, S. Lukomski, W. E. Swords, and S. D. Reid. 2009. Biofilm formation by group A Streptococcus: a role for the streptococcal regulator of virulence (Srv) and streptococcal cysteine protease (SpeB). Microbiology 15546-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dombek, P. E., D. Cue, J. Sedgewick, H. Lam, S. Ruschkowski, B. B. Finlay, and P. P. Cleary. 1999. High-frequency intracellular invasion of epithelial cells by serotype M1 group A streptococci: M1 protein-mediated invasion and cytoskeletal rearrangements. Mol. Microbiol. 31859-870. [DOI] [PubMed] [Google Scholar]
- 138.Douglas, C. W. 1990. Characterization of the α-amylase receptor of Streptococcus gordonii NCTC 7868. J. Dent. Res. 691746-1752. [DOI] [PubMed] [Google Scholar]
- 139.Dramsi, S., E. Caliot, I. Bonne, S. Guadagnini, M. C. Prevost, M. Kojadinovic, L. Lalioui, C. Poyart, and P. Trieu-Cuot. 2006. Assembly and role of pili in group B streptococci. Mol. Microbiol. 601401-1413. [DOI] [PubMed] [Google Scholar]
- 140.Dramsi, S., P. Trieu-Cuot, and H. Bierne. 2005. Sorting sortases: a nomenclature proposal for the various sortases of gram-positive bacteria. Res. Microbiol. 156289-297. [DOI] [PubMed] [Google Scholar]
- 141.Dunning, D. W., L. W. McCall, W. F. Powell, Jr., W. T. Arscott, E. M. McConocha, C. J. McClurg, S. D. Goodman, and G. A. Spatafora. 2008. SloR modulation of the Streptococcus mutans acid tolerance response involves the GcrR response regulator as an essential intermediary. Microbiology 1541132-1143. [DOI] [PubMed] [Google Scholar]
- 142.Echenique, J., A. Kadioglu, S. Romao, P. W. Andrew, and M. C. Trombe. 2004. Protein serine/threonine kinase StkP positively controls virulence and competence in Streptococcus pneumoniae. Infect. Immun. 722434-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Echenique, J. R., S. Chapuy-Regaud, and M. C. Trombe. 2000. Competence regulation by oxygen in Streptococcus pneumoniae: involvement of ciaRH and comCDE. Mol. Microbiol. 36688-696. [DOI] [PubMed] [Google Scholar]
- 144.Echenique, J. R., and M. C. Trombe. 2001. Competence repression under oxygen limitation through the two-component MicAB signal-transducing system in Streptococcus pneumoniae and involvement of the PAS domain of MicB. J. Bacteriol. 1834599-4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Edwards, A. M., A. G. Manetti, F. Falugi, C. Zingaretti, S. Capo, S. Buccato, G. Bensi, J. L. Telford, I. Margarit, and G. Grandi. 2008. Scavenger receptor gp340 aggregates group A streptococci by binding pili. Mol. Microbiol. 681378-1394. [DOI] [PubMed] [Google Scholar]
- 146.Egland, P. G., R. J. Palmer, Jr., and P. E. Kolenbrander. 2004. Interspecies communication in Streptococcus gordonii-Veillonella atypica biofilms: signaling in flow conditions requires juxtaposition. Proc. Natl. Acad. Sci. USA 10116917-16922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ellen, R. P., and R. J. Gibbons. 1972. M protein-associated adherence of Streptococcus pyogenes to epithelial surfaces: prerequisite for virulence. Infect. Immun. 5826-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Elliott, D., E. Harrison, P. S. Handley, S. K. Ford, E. Jaffray, N. Mordan, and R. McNab. 2003. Prevalence of Csh-like fibrillar surface proteins among mitis group oral streptococci. Oral Microbiol. Immunol. 18114-120. [DOI] [PubMed] [Google Scholar]
- 149.Elsner, A., B. Kreikemeyer, A. Braun-Kiewnick, B. Spellerberg, B. A. Buttaro, and A. Podbielski. 2002. Involvement of Lsp, a member of the LraI-lipoprotein family in Streptococcus pyogenes, in eukaryotic cell adhesion and internalization. Infect. Immun. 704859-4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Engleberg, N. C., A. Heath, A. Miller, C. Rivera, and V. J. DiRita. 2001. Spontaneous mutations in the CsrRS two-component regulatory system of Streptococcus pyogenes result in enhanced virulence in a murine model of skin and soft tissue infection. J. Infect. Dis. 1831043-1054. [DOI] [PubMed] [Google Scholar]
- 151.Eran, Y., Y. Getter, M. Baruch, I. Belotserkovsky, G. Padalon, I. Mishalian, A. Podbielski, B. Kreikemeyer, and E. Hanski. 2007. Transcriptional regulation of the sil locus by the SilCR signalling peptide and its implications on group A Streptococcus virulence. Mol. Microbiol. 631209-1222. [DOI] [PubMed] [Google Scholar]
- 152.Erdogan, S., P. K. Fagan, S. R. Talay, M. Rohde, P. Ferrieri, A. E. Flores, C. A. Guzman, M. J. Walker, and G. S. Chhatwal. 2002. Molecular analysis of group B protective surface protein, a new cell surface protective antigen of group B streptococci. Infect. Immun. 70803-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fachon-Kalweit, S., B. L. Elder, and P. Fives-Taylor. 1985. Antibodies that bind to fimbriae block adhesion of Streptococcus sanguis to saliva-coated hydroxyapatite. Infect. Immun. 48617-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fagan, P. K., D. Reinscheid, B. Gottschalk, and G. S. Chhatwal. 2001. Identification and characterization of a novel secreted immunoglobulin binding protein from group A Streptococcus. Infect. Immun. 694851-4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fahnestock, S. R., P. Alexander, J. Nagle, and D. Filpula. 1986. Gene for an immunoglobulin-binding protein from a group G Streptococcus. J. Bacteriol. 167870-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Falker, S., A. L. Nelson, E. Morfeldt, K. Jonas, K. Hultenby, J. Ries, O. Melefors, S. Normark, and B. Henriques-Normark. 2008. Sortase-mediated assembly and surface topology of adhesive pneumococcal pili. Mol. Microbiol. 70595-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Falugi, F., C. Zingaretti, V. Pinto, M. Mariani, L. Amodeo, A. G. Manetti, S. Capo, J. M. Musser, G. Orefici, I. Margarit, J. L. Telford, G. Grandi, and M. Mora. 2008. Sequence variation in group A Streptococcus pili and association of pilus backbone types with Lancefield T serotypes. J. Infect. Dis. 1981834-1841. [DOI] [PubMed] [Google Scholar]
- 158.Faulmann, E. L., J. L. Duvall, and M. D. Boyle. 1991. Protein B: a versatile bacterial Fc-binding protein selective for human IgA. BioTechniques 10748-755. [PubMed] [Google Scholar]
- 159.Federle, M. J., K. S. McIver, and J. R. Scott. 1999. A response regulator that represses transcription of several virulence operons in the group A Streptococcus. J. Bacteriol. 1813649-3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fenno, J. C., A. Shaikh, G. Spatafora, and P. Fives-Taylor. 1995. The fimA locus of Streptococcus parasanguis encodes an ATP-binding membrane transport system. Mol. Microbiol. 15849-863. [DOI] [PubMed] [Google Scholar]
- 161.Ferretti, J. J., W. M. McShan, D. Ajdic, D. J. Savic, G. Savic, K. Lyon, C. Primeaux, S. Sezate, A. N. Suvorov, S. Kenton, H. S. Lai, S. P. Lin, Y. Qian, H. G. Jia, F. Z. Najar, Q. Ren, H. Zhu, L. Song, J. White, X. Yuan, S. W. Clifton, B. A. Roe, and R. McLaughlin. 2001. Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc. Natl. Acad. Sci. USA 984658-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ferretti, J. J., R. R. Russell, and M. L. Dao. 1989. Sequence analysis of the wall-associated protein precursor of Streptococcus mutans antigen A. Mol. Microbiol. 3469-478. [DOI] [PubMed] [Google Scholar]
- 163.Fettucciari, K., E. Rosati, L. Scaringi, P. Cornacchione, G. Migliorati, R. Sabatini, I. Fetriconi, R. Rossi, and P. Marconi. 2000. Group B Streptococcus induces apoptosis in macrophages. J. Immunol. 1653923-3933. [DOI] [PubMed] [Google Scholar]
- 164.Fischetti, V. A. 2006. Surface proteins on gram-positive bacteria, p. 12-25. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. ASM Press, Washington, DC.
- 165.Fisher, M., Y.-S. Huang, X. Li, K. S. McIver, C. Toukoki, and Z. Eichenbaum. 2008. Shr is a broad-spectrum surface receptor that contributes to adherence and virulence in group A Streptococcus. Infect. Immun. 765006-5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fives-Taylor, P. M., and D. W. Thompson. 1985. Surface properties of Streptococcus sanguis FW213 mutants nonadherent to saliva-coated hydroxyapatite. Infect. Immun. 47752-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Flores, A. E., and P. Ferrieri. 1989. Molecular species of R-protein antigens produced by clinical isolates of group B streptococci. J. Clin. Microbiol. 271050-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fogg, G. C., and M. G. Caparon. 1997. Constitutive expression of fibronectin binding in Streptococcus pyogenes as a result of anaerobic activation of rofA. J. Bacteriol. 1796172-6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fogg, G. C., C. M. Gibson, and M. G. Caparon. 1994. The identification of rofA, a positive-acting regulatory component of prtF expression: use of an mγδ-based shuttle mutagenesis strategy in Streptococcus pyogenes. Mol. Microbiol. 11671-684. [DOI] [PubMed] [Google Scholar]
- 170.Fontana, M., L. E. Gfell, and R. L. Gregory. 1995. Characterization of preparations enriched for Streptococcus mutans fimbriae: salivary immunoglobulin A antibodies in caries-free and caries-active subjects. Clin. Diagn. Lab. Immunol. 2719-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Foster, T. J., and M. Hook. 1998. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 6484-488. [DOI] [PubMed] [Google Scholar]
- 172.Fraser, J. D., and T. Proft. 2008. The bacterial superantigen and superantigen-like proteins. Immunol. Rev. 225226-243. [DOI] [PubMed] [Google Scholar]
- 173.Frick, I. M., K. L. Crossin, G. M. Edelman, and L. Bjorck. 1995. Protein H—a bacterial surface protein with affinity for both immunoglobulin and fibronectin type III domains. EMBO J. 141674-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Frick, I. M., M. Morgelin, and L. Bjorck. 2000. Virulent aggregates of Streptococcus pyogenes are generated by homophilic protein-protein interactions. Mol. Microbiol. 371232-1247. [DOI] [PubMed] [Google Scholar]
- 175.Fujiwara, T., T. Hoshino, T. Ooshima, S. Sobue, and S. Hamada. 2000. Purification, characterization, and molecular analysis of the gene encoding glucosyltransferase from Streptococcus oralis. Infect. Immun. 682475-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ganeshkumar, N., N. Arora, and P. E. Kolenbrander. 1993. Saliva-binding protein (SsaB) from Streptococcus sanguis 12 is a lipoprotein. J. Bacteriol. 175572-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ganeshkumar, N., P. M. Hannam, P. E. Kolenbrander, and B. C. McBride. 1991. Nucleotide sequence of a gene coding for a saliva-binding protein (SsaB) from Streptococcus sanguis 12 and possible role of the protein in coaggregation with actinomyces. Infect. Immun. 591093-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ganeshkumar, N., M. Song, and B. C. McBride. 1988. Cloning of a Streptococcus sanguis adhesin which mediates binding to saliva-coated hydroxyapatite. Infect. Immun. 561150-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gase, K., A. Gase, H. Schirmer, and H. Malke. 1996. Cloning, sequencing and functional overexpression of the Streptococcus equisimilis H46A gapC gene encoding a glyceraldehyde-3-phosphate dehydrogenase that also functions as a plasmin(ogen)-binding protein. Purification and biochemical characterization of the protein. Eur. J. Biochem. 23942-51. [DOI] [PubMed] [Google Scholar]
- 180.Ge, J., D. M. Catt, and R. L. Gregory. 2004. Streptococcus mutans surface α-enolase binds salivary mucin MG2 and human plasminogen. Infect. Immun. 726748-6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ge, X., T. Kitten, Z. Chen, S. P. Lee, C. L. Munro, and P. Xu. 2008. Identification of Streptococcus sanguinis genes required for biofilm formation and examination of their role in endocarditis virulence. Infect. Immun. 762551-2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gerlach, D., B. Fleischer, M. Wagner, K. Schmidt, S. Vettermann, and W. Reichardt. 2000. Purification and biochemical characterization of a basic superantigen (SPEX/SMEZ3) from Streptococcus pyogenes. FEMS Microbiol. Lett. 188153-163. [DOI] [PubMed] [Google Scholar]
- 183.Geyer, A., and K. H. Schmidt. 2000. Genetic organisation of the M protein region in human isolates of group C and G streptococci: two types of multigene regulator-like (mgrC) regions. Mol. Gen. Genet. 262965-976. [DOI] [PubMed] [Google Scholar]
- 184.Ghosh, J., and M. G. Caparon. 2006. Specificity of Streptococcus pyogenes NAD(+) glycohydrolase in cytolysin-mediated translocation. Mol. Microbiol. 621203-1214. [DOI] [PubMed] [Google Scholar]
- 185.Giammarinaro, P., and J. C. Paton. 2002. Role of RegM, a homologue of the catabolite repressor protein CcpA, in the virulence of Streptococcus pneumoniae. Infect. Immun. 705454-5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Giammarinaro, P., M. Sicard, and A. M. Gasc. 1999. Genetic and physiological studies of the CiaH-CiaR two-component signal-transducing system involved in cefotaxime resistance and competence of Streptococcus pneumoniae. Microbiology 1451859-1869. [DOI] [PubMed] [Google Scholar]
- 187.Gianfaldoni, C., S. Censini, M. Hilleringmann, M. Moschioni, C. Facciotti, W. Pansegrau, V. Masignani, A. Covacci, R. Rappuoli, M. A. Barocchi, and P. Ruggiero. 2007. Streptococcus pneumoniae pilus subunits protect mice against lethal challenge. Infect. Immun. 751059-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Giannakis, E., T. S. Jokiranta, R. J. Ormsby, T. G. Duthy, D. A. Male, D. Christiansen, V. A. Fischetti, C. Bagley, B. E. Loveland, and D. L. Gordon. 2002. Identification of the streptococcal M protein binding site on membrane cofactor protein (CD46). J. Immunol. 1684585-4592. [DOI] [PubMed] [Google Scholar]
- 189.Giffard, P. M., D. M. Allen, C. P. Milward, C. L. Simpson, and N. A. Jacques. 1993. Sequence of the gtfK gene of Streptococcus salivarius ATCC 25975 and evolution of the gtf genes of oral streptococci. J. Gen. Microbiol. 1391511-1522. [DOI] [PubMed] [Google Scholar]
- 190.Giffard, P. M., and N. A. Jacques. 1994. Definition of a fundamental repeating unit in streptococcal glucosyltransferase glucan-binding regions and related sequences. J. Dent. Res. 731133-1141. [DOI] [PubMed] [Google Scholar]
- 191.Giffard, P. M., C. L. Simpson, C. P. Milward, and N. A. Jacques. 1991. Molecular characterization of a cluster of at least two glucosyltransferase genes in Streptococcus salivarius ATCC 25975. J. Gen. Microbiol. 1372577-2593. [DOI] [PubMed] [Google Scholar]
- 192.Gilmore, K. S., R. R. Russell, and J. J. Ferretti. 1990. Analysis of the Streptococcus downei gtfS gene, which specifies a glucosyltransferase that synthesizes soluble glucans. Infect. Immun. 582452-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gilmore, K. S., P. Srinivas, D. R. Akins, K. L. Hatter, and M. S. Gilmore. 2003. Growth, development, and gene expression in a persistent Streptococcus gordonii biofilm. Infect. Immun. 714759-4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Glaser, P., C. Rusniok, C. Buchrieser, F. Chevalier, L. Frangeul, T. Msadek, M. Zouine, E. Couve, L. Lalioui, C. Poyart, P. Trieu-Cuot, and F. Kunst. 2002. Genome sequence of Streptococcus agalactiae, a pathogen causing invasive neonatal disease. Mol. Microbiol. 451499-1513. [DOI] [PubMed] [Google Scholar]
- 195.Goldstein, J. M., A. Banbula, T. Kordula, J. A. Mayo, and J. Travis. 2001. Novel extracellular x-prolyl dipeptidyl-peptidase (DPP) from Streptococcus gordonii FSS2: an emerging subfamily of viridans streptococcal x-prolyl DPPs. Infect. Immun. 695494-5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gomi, H., T. Hozumi, S. Hattori, C. Tagawa, F. Kishimoto, and L. Bjorck. 1990. The gene sequence and some properties of protein H. A novel IgG-binding protein. J. Immunol. 1444046-4052. [PubMed] [Google Scholar]
- 197.Graham, M. R., L. M. Smoot, C. A. Migliaccio, K. Virtaneva, D. E. Sturdevant, S. F. Porcella, M. J. Federle, G. J. Adams, J. R. Scott, and J. M. Musser. 2002. Virulence control in group A Streptococcus by a two-component gene regulatory system: global expression profiling and in vivo infection modeling. Proc. Natl. Acad. Sci. USA 9913855-13860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Granok, A. B., D. Parsonage, R. P. Ross, and M. G. Caparon. 2000. The RofA binding site in Streptococcus pyogenes is utilized in multiple transcriptional pathways. J. Bacteriol. 1821529-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gravekamp, C., D. S. Horensky, J. L. Michel, and L. C. Madoff. 1996. Variation in repeat number within the alpha C protein of group B streptococci alters antigenicity and protective epitopes. Infect. Immun. 643576-3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Gryllos, I., R. Grifantini, A. Colaprico, M. E. Cary, A. Hakansson, D. W. Carey, M. Suarez-Chavez, L. A. Kalish, P. D. Mitchell, G. L. White, and M. R. Wessels. 2008. PerR confers phagocytic killing resistance and allows pharyngeal colonization by group A Streptococcus. PLoS Pathog. 4e1000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Gryllos, I., J. C. Levin, and M. R. Wessels. 2003. The CsrR/CsrS two-component system of group A Streptococcus responds to environmental Mg2+. Proc. Natl. Acad. Sci. USA 1004227-4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Gryllos, I., H. J. Tran-Winkler, M. F. Cheng, H. Chung, R. Bolcome III, W. Lu, R. I. Lehrer, and M. R. Wessels. 2008. Induction of group A Streptococcus virulence by a human antimicrobial peptide. Proc. Natl. Acad. Sci. USA 10516755-16760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Guenzi, E., A. M. Gasc, M. A. Sicard, and R. Hakenbeck. 1994. A two-component signal-transducing system is involved in competence and penicillin susceptibility in laboratory mutants of Streptococcus pneumoniae. Mol. Microbiol. 12505-515. [DOI] [PubMed] [Google Scholar]
- 204.Guerra, N. P., and L. Pastrana. 2003. Influence of pH drop on both nisin and pediocin production by Lactococcus lactis and Pediococcus acidilactici. Lett. Appl. Microbiol. 3751-55. [DOI] [PubMed] [Google Scholar]
- 205.Guss, B., M. Eliasson, A. Olsson, M. Uhlen, A. K. Frej, H. Jornvall, J. I. Flock, and M. Lindberg. 1986. Structure of the IgG-binding regions of streptococcal protein G. EMBO J. 51567-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Gutekunst, H., B. J. Eikmanns, and D. J. Reinscheid. 2003. Analysis of RogB-controlled virulence mechanisms and gene repression in Streptococcus agalactiae. Infect. Immun. 715056-5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Gutekunst, H., B. J. Eikmanns, and D. J. Reinscheid. 2004. The novel fibrinogen-binding protein FbsB promotes Streptococcus agalactiae invasion into epithelial cells. Infect. Immun. 723495-3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hakansson, A., C. C. Bentley, E. A. Shakhnovic, and M. R. Wessels. 2005. Cytolysin-dependent evasion of lysosomal killing. Proc. Natl. Acad. Sci. USA 1025192-5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hammerschmidt, S., S. R. Talay, P. Brandtzaeg, and G. S. Chhatwal. 1997. SpsA, a novel pneumococcal surface protein with specific binding to secretory immunoglobulin A and secretory component. Mol. Microbiol. 251113-1124. [DOI] [PubMed] [Google Scholar]
- 210.Han, T. K., C. Zhang, and M. L. Dao. 2006. Identification and characterization of collagen-binding activity in Streptococcus mutans wall-associated protein: a possible implication in dental root caries and endocarditis. Biochem. Biophys. Res. Commun. 343787-792. [DOI] [PubMed] [Google Scholar]
- 211.Hanada, N., K. Fukushima, Y. Nomura, H. Senpuku, M. Hayakawa, H. Mukasa, T. Shiroza, and Y. Abiko. 2002. Cloning and nucleotide sequence analysis of the Streptococcus sobrinus gtfU gene that produces a highly branched water-soluble glucan. Biochim. Biophys. Acta 157075-79. [DOI] [PubMed] [Google Scholar]
- 212.Hanada, N., Y. Isobe, Y. Aizawa, T. Katayama, S. Sato, and M. Inoue. 1993. Nucleotide sequence analysis of the gtfT gene from Streptococcus sobrinus OMZ176. Infect. Immun. 612096-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Handley, P. S. 1990. Structure, composition and functions of surface structures on oral bacteria. Biofouling 2239-264. [Google Scholar]
- 214.Handley, P. S., P. L. Carter, and J. Fielding. 1984. Streptococcus salivarius strains carry either fibrils or fimbriae on the cell surface. J. Bacteriol. 15764-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Handley, P. S., P. L. Carter, J. E. Wyatt, and L. M. Hesketh. 1985. Surface structures (peritrichous fibrils and tufts of fibrils) found on Streptococcus sanguis strains may be related to their ability to coaggregate with other oral genera. Infect. Immun. 47217-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Handley, P. S., F. F. Correia, K. Russell, B. Rosan, and J. M. DiRienzo. 2005. Association of a novel high molecular weight, serine-rich protein (SrpA) with fibril-mediated adhesion of the oral biofilm bacterium Streptococcus cristatus. Oral Microbiol. Immunol. 20131-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Handley, P. S., D. W. Harty, J. E. Wyatt, C. R. Brown, J. P. Doran, and A. C. Gibbs. 1987. A comparison of the adhesion, coaggregation and cell-surface hydrophobicity properties of fibrillar and fimbriate strains of Streptococcus salivarius. J. Gen. Microbiol. 1333207-3217. [DOI] [PubMed] [Google Scholar]
- 218.Hanks, T. S., M. Liu, M. J. McClure, and B. Lei. 2005. ABC transporter FtsABCD of Streptococcus pyogenes mediates uptake of ferric ferrichrome. BMC Microbiol. 562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Hanski, E., and M. Caparon. 1992. Protein F, a fibronectin-binding protein, is an adhesin of the group A streptococcus Streptococcus pyogenes. Proc. Natl. Acad. Sci. USA 896172-6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hardie, K. R., and K. Heurlier. 2008. Establishing bacterial communities by ‘word of mouth’: LuxS and autoinducer 2 in biofilm development. Nat. Rev. Microbiol. 6635-643. [DOI] [PubMed] [Google Scholar]
- 221.Harris, T. O., D. W. Shelver, J. F. Bohnsack, and C. E. Rubens. 2003. A novel streptococcal surface protease promotes virulence, resistance to opsonophagocytosis, and cleavage of human fibrinogen. J. Clin. Investig. 11161-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Hasegawa, Y., J. J. Mans, S. Mao, M. C. Lopez, H. V. Baker, M. Handfield, and R. J. Lamont. 2007. Gingival epithelial cell transcriptional responses to commensal and opportunistic oral microbial species. Infect. Immun. 752540-2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Hasty, D. L., I. Ofek, H. S. Courtney, and R. J. Doyle. 1992. Multiple adhesins of streptococci. Infect. Immun. 602147-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hava, D. L., and A. Camilli. 2002. Large-scale identification of serotype 4 Streptococcus pneumoniae virulence factors. Mol. Microbiol. 451389-1406. [PMC free article] [PubMed] [Google Scholar]
- 225.Havarstein, L. S., R. Hakenbeck, and P. Gaustad. 1997. Natural competence in the genus Streptococcus: evidence that streptococci can change pherotype by interspecies recombinational exchanges. J. Bacteriol. 1796589-6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.He, X., C. Wu, D. Yarbrough, L. Sim, G. Niu, J. Merritt, W. Shi, and F. Qi. 2008. The cia operon of Streptococcus mutans encodes a unique component required for calcium-mediated autoregulation. Mol. Microbiol. 70112-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Heath, D. G., and P. P. Cleary. 1989. Fc-receptor and M-protein genes of group A streptococci are products of gene duplication. Proc. Natl. Acad. Sci. USA 864741-4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Heath, P. T., and R. G. Feldman. 2005. Vaccination against group B Streptococcus. Expert Rev. Vaccines 4207-218. [DOI] [PubMed] [Google Scholar]
- 229.Heden, L. O., E. Frithz, and G. Lindahl. 1991. Molecular characterization of an IgA receptor from group B streptococci: sequence of the gene, identification of a proline-rich region with unique structure and isolation of N-terminal fragments with IgA-binding capacity. Eur. J. Immunol. 211481-1490. [DOI] [PubMed] [Google Scholar]
- 230.Hemsley, C., E. Joyce, D. L. Hava, A. Kawale, and A. Camilli. 2003. MgrA, an orthologue of Mga, acts as a transcriptional repressor of the genes within the rlrA pathogenicity islet in Streptococcus pneumoniae. J. Bacteriol. 1856640-6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Hendriksen, W. T., H. J. Bootsma, S. Estevao, T. Hoogenboezem, A. de Jong, R. de Groot, O. P. Kuipers, and P. W. Hermans. 2008. CodY of Streptococcus pneumoniae: link between nutritional gene regulation and colonization. J. Bacteriol. 190590-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Henneke, P., S. Morath, S. Uematsu, S. Weichert, M. Pfitzenmaier, O. Takeuchi, A. Muller, C. Poyart, S. Akira, R. Berner, G. Teti, A. Geyer, T. Hartung, P. Trieu-Cuot, D. L. Kasper, and D. T. Golenbock. 2005. Role of lipoteichoic acid in the phagocyte response to group B Streptococcus. J. Immunol. 1746449-6455. [DOI] [PubMed] [Google Scholar]
- 233.Herzberg, M. C. 1996. Platelet-streptococcal interactions in endocarditis. Crit. Rev. Oral Biol. Med. 7222-236. [DOI] [PubMed] [Google Scholar]
- 234.Herzberg, M. C., G. D. MacFarlane, K. Gong, N. N. Armstrong, A. R. Witt, P. R. Erickson, and M. W. Meyer. 1992. The platelet interactivity phenotype of Streptococcus sanguis influences the course of experimental endocarditis. Infect. Immun. 604809-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Hidalgo-Grass, C., M. Ravins, M. Dan-Goor, J. Jaffe, A. E. Moses, and E. Hanski. 2002. A locus of group A Streptococcus involved in invasive disease and DNA transfer. Mol. Microbiol. 4687-99. [DOI] [PubMed] [Google Scholar]
- 236.Hill, J. 1976. Purification and properties of streptococcal hyaluronate lyase. Infect. Immun. 14726-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Hilleringmann, M., F. Giusti, B. C. Baudner, V. Masignani, A. Covacci, R. Rappuoli, M. A. Barocchi, and I. Ferlenghi. 2008. Pneumococcal pili are composed of protofilaments exposing adhesive clusters of RrgA. PLoS Pathog. 4e1000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Hiratsuka, K., B. Wang, Y. Sato, and H. Kuramitsu. 1998. Regulation of sucrose-6-phosphate hydrolase activity in Streptococcus mutans: characterization of the scrR gene. Infect. Immun. 663736-3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Hogg, S. D., and J. E. Manning. 1988. Inhibition of adhesion of viridans streptococci to fibronectin-coated hydroxyapatite beads by lipoteichoic acid. J. Appl. Bacteriol. 65483-489. [DOI] [PubMed] [Google Scholar]
- 240.Holmes, A. R., R. McNab, and H. F. Jenkinson. 1996. Candida albicans binding to the oral bacterium Streptococcus gordonii involves multiple adhesin-receptor interactions. Infect. Immun. 644680-4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Holmes, A. R., R. McNab, K. W. Millsap, M. Rohde, S. Hammerschmidt, J. L. Mawdsley, and H. F. Jenkinson. 2001. The pavA gene of Streptococcus pneumoniae encodes a fibronectin-binding protein that is essential for virulence. Mol. Microbiol. 411395-1408. [DOI] [PubMed] [Google Scholar]
- 242.Hondorp, E. R., and K. S. McIver. 2007. The Mga virulence regulon: infection where the grass is greener. Mol. Microbiol. 661056-1065. [DOI] [PubMed] [Google Scholar]
- 243.Horstmann, R. D., H. J. Sievertsen, J. Knobloch, and V. A. Fischetti. 1988. Antiphagocytic activity of streptococcal M protein: selective binding of complement control protein factor H. Proc. Natl. Acad. Sci. USA 851657-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Hoshino, T., M. Kawaguchi, N. Shimizu, N. Hoshino, T. Ooshima, and T. Fujiwara. 2004. PCR detection and identification of oral streptococci in saliva samples using gtf genes. Diagn. Microbiol. Infect. Dis. 48195-199. [DOI] [PubMed] [Google Scholar]
- 245.Hryniewicz, W., B. Lipinski, and J. Jeljaszewicz. 1972. Nature of the interaction between M protein of Streptococcus pyogenes and fibrinogen. J. Infect. Dis. 125626-630. [DOI] [PubMed] [Google Scholar]
- 246.Hudson, M. C., and R. Curtiss III. 1990. Regulation of expression of Streptococcus mutans genes important to virulence. Infect. Immun. 58464-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Hughes, R. C., and R. W. Jeanloz. 1964. The extracellular glycosidases of Diplococcus pneumoniae. I. Purification and properties of a neuraminidase and a β-galactosidase. Action on the α-1-acid glycoprotein of human plasma. Biochemistry 31535-1543. [DOI] [PubMed] [Google Scholar]
- 248.Humtsoe, J. O., J. K. Kim, Y. Xu, D. R. Keene, M. Hook, S. Lukomski, and K. K. Wary. 2005. A streptococcal collagen-like protein interacts with the α2β1 integrin and induces intracellular signaling. J. Biol. Chem. 28013848-13857. [DOI] [PubMed] [Google Scholar]
- 249.Hynes, R. O. 2002. Integrins: bidirectional, allosteric signaling machines. Cell 110673-687. [DOI] [PubMed] [Google Scholar]
- 250.Hytonen, J., S. Haataja, and J. Finne. 2003. Streptococcus pyogenes glycoprotein-binding strepadhesin activity is mediated by a surface-associated carbohydrate-degrading enzyme, pullulanase. Infect. Immun. 71784-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Hytonen, J., S. Haataja, D. Gerlach, A. Podbielski, and J. Finne. 2001. The SpeB virulence factor of Streptococcus pyogenes, a multifunctional secreted and cell surface molecule with strepadhesin, laminin-binding and cysteine protease activity. Mol. Microbiol. 39512-519. [DOI] [PubMed] [Google Scholar]
- 252.Ibrahim, Y. M., A. R. Kerr, J. McCluskey, and T. J. Mitchell. 2004. Control of virulence by the two-component system CiaR/H is mediated via HtrA, a major virulence factor of Streptococcus pneumoniae. J. Bacteriol. 1865258-5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Idone, V., S. Brendtro, R. Gillespie, S. Kocaj, E. Peterson, M. Rendi, W. Warren, S. Michalek, K. Krastel, D. Cvitkovitch, and G. Spatafora. 2003. Effect of an orphan response regulator on Streptococcus mutans sucrose-dependent adherence and cariogenesis. Infect. Immun. 714351-4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Igarashi, T., A. Yamamoto, and N. Goto. 1995. Sequence analysis of the Streptococcus mutans Ingbritt dexA gene encoding extracellular dextranase. Microbiol. Immunol. 39853-860. [DOI] [PubMed] [Google Scholar]
- 255.Iyer, R., N. S. Baliga, and A. Camilli. 2005. Catabolite control protein A (CcpA) contributes to virulence and regulation of sugar metabolism in Streptococcus pneumoniae. J. Bacteriol. 1878340-8349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Iyer, R., and A. Camilli. 2007. Sucrose metabolism contributes to in vivo fitness of Streptococcus pneumoniae. Mol. Microbiol. 661-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Jaffe, J., S. Natanson-Yaron, M. G. Caparon, and E. Hanski. 1996. Protein F2, a novel fibronectin-binding protein from Streptococcus pyogenes, possesses two binding domains. Mol. Microbiol. 21373-384. [DOI] [PubMed] [Google Scholar]
- 258.Jakubovics, N. S., S. R. Gill, S. E. Iobst, M. M. Vickerman, and P. E. Kolenbrander. 2008. Regulation of gene expression in a mixed-genus community: stabilized arginine biosynthesis in Streptococcus gordonii by coaggregation with Actinomyces naeslundii. J. Bacteriol. 1903646-3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Jakubovics, N. S., S. R. Gill, M. M. Vickerman, and P. E. Kolenbrander. 2008. Role of hydrogen peroxide in competition and cooperation between Streptococcus gordonii and Actinomyces naeslundii. FEMS Microbiol. Ecol. 66637-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Jakubovics, N. S., and H. F. Jenkinson. 2001. Out of the iron age: new insights into the critical role of manganese homeostasis in bacteria. Microbiology 1471709-1718. [DOI] [PubMed] [Google Scholar]
- 261.Jakubovics, N. S., S. W. Kerrigan, A. H. Nobbs, N. Stromberg, C. J. van Dolleweerd, D. M. Cox, C. G. Kelly, and H. F. Jenkinson. 2005. Functions of cell surface-anchored antigen I/II family and Hsa polypeptides in interactions of Streptococcus gordonii with host receptors. Infect. Immun. 736629-6638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Jakubovics, N. S., A. W. Smith, and H. F. Jenkinson. 2000. Expression of the virulence-related Sca (Mn2+) permease in Streptococcus gordonii is regulated by a diphtheria toxin metallorepressor-like protein ScaR. Mol. Microbiol. 38140-153. [DOI] [PubMed] [Google Scholar]
- 263.Jakubovics, N. S., N. Stromberg, C. J. van Dolleweerd, C. G. Kelly, and H. F. Jenkinson. 2005. Differential binding specificities of oral streptococcal antigen I/II family adhesins for human or bacterial ligands. Mol. Microbiol. 551591-1605. [DOI] [PubMed] [Google Scholar]
- 264.James, C. E., Y. Hasegawa, Y. Park, V. Yeung, G. D. Tribble, M. Kuboniwa, D. R. Demuth, and R. J. Lamont. 2006. LuxS involvement in the regulation of genes coding for hemin and iron acquisition systems in Porphyromonas gingivalis. Infect. Immun. 743834-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Jameson, M. W., H. F. Jenkinson, K. Parnell, and P. S. Handley. 1995. Polypeptides associated with tufts of cell-surface fibrils in an oral Streptococcus. Microbiology 1412729-2738. [DOI] [PubMed] [Google Scholar]
- 266.Janulczyk, R., F. Iannelli, A. G. Sjoholm, G. Pozzi, and L. Bjorck. 2000. Hic, a novel surface protein of Streptococcus pneumoniae that interferes with complement function. J. Biol. Chem. 27537257-37263. [DOI] [PubMed] [Google Scholar]
- 267.Janulczyk, R., J. Pallon, and L. Bjorck. 1999. Identification and characterization of a Streptococcus pyogenes ABC transporter with multiple specificity for metal cations. Mol. Microbiol. 34596-606. [DOI] [PubMed] [Google Scholar]
- 268.Janulczyk, R., S. Ricci, and L. Bjorck. 2003. MtsABC is important for manganese and iron transport, oxidative stress resistance, and virulence of Streptococcus pyogenes. Infect. Immun. 712656-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Jeng, A., V. Sakota, Z. Li, V. Datta, B. Beall, and V. Nizet. 2003. Molecular genetic analysis of a group A Streptococcus operon encoding serum opacity factor and a novel fibronectin-binding protein, SfbX. J. Bacteriol. 1851208-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Jenkinson, H. F. 1994. Cell surface protein receptors in oral streptococci. FEMS Microbiol. Lett. 121133-140. [DOI] [PubMed] [Google Scholar]
- 271.Jenkinson, H. F., and D. R. Demuth. 1997. Structure, function and immunogenicity of streptococcal antigen I/II polypeptides. Mol. Microbiol. 23183-190. [DOI] [PubMed] [Google Scholar]
- 272.Jenkinson, H. F., and R. J. Lamont. 1997. Streptococcal adhesion and colonization. Crit. Rev. Oral Biol. Med. 8175-200. [DOI] [PubMed] [Google Scholar]
- 273.Jenkinson, H. F., and R. J. Lamont. 2005. Oral microbial communities in sickness and in health. Trends Microbiol. 13589-595. [DOI] [PubMed] [Google Scholar]
- 274.Jenkinson, H. F., S. D. Terry, R. McNab, and G. W. Tannock. 1993. Inactivation of the gene encoding surface protein SspA in Streptococcus gordonii DL1 affects cell interactions with human salivary agglutinin and oral actinomyces. Infect. Immun. 613199-3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Jerlstrom, P. G., G. S. Chhatwal, and K. N. Timmis. 1991. The IgA-binding β antigen of the C protein complex of group B streptococci: sequence determination of its gene and detection of two binding regions. Mol. Microbiol. 5843-849. [DOI] [PubMed] [Google Scholar]
- 276.Jerlstrom, P. G., S. R. Talay, P. Valentin-Weigand, K. N. Timmis, and G. S. Chhatwal. 1996. Identification of an immunoglobulin A binding motif located in the β-antigen of the C protein complex of group B streptococci. Infect. Immun. 642787-2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Jin, H., Y. P. Song, G. Boel, J. Kochar, and V. Pancholi. 2005. Group A streptococcal surface GAPDH, SDH, recognizes uPAR/CD87 as its receptor on the human pharyngeal cell and mediates bacterial adherence to host cells. J. Mol. Biol. 35027-41. [DOI] [PubMed] [Google Scholar]
- 278.Johnston, J. W., D. E. Briles, L. E. Myers, and S. K. Hollingshead. 2006. Mn2+-dependent regulation of multiple genes in Streptococcus pneumoniae through PsaR and the resultant impact on virulence. Infect. Immun. 741171-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Johri, A. K., L. C. Paoletti, P. Glaser, M. Dua, P. K. Sharma, G. Grandi, and R. Rappuoli. 2006. Group B Streptococcus: global incidence and vaccine development. Nat. Rev. Microbiol. 4932-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Jones, A. L., K. M. Knoll, and C. E. Rubens. 2000. Identification of Streptococcus agalactiae virulence genes in the neonatal rat sepsis model using signature-tagged mutagenesis. Mol. Microbiol. 371444-1455. [DOI] [PubMed] [Google Scholar]
- 281.Jonsson, H., C. Burtsoff-Asp, and B. Guss. 1995. Streptococcal protein MAG—a protein with broad albumin binding specificity. Biochim. Biophys. Acta 124965-71. [DOI] [PubMed] [Google Scholar]
- 282.Jonsson, H., L. Frykberg, L. Rantamaki, and B. Guss. 1994. MAG, a novel plasma protein receptor from Streptococcus dysgalactiae. Gene 14385-89. [DOI] [PubMed] [Google Scholar]
- 283.Jonsson, H., and H. P. Muller. 1994. The type-III Fc receptor from Streptococcus dysgalactiae is also an α2-macroglobulin receptor. Eur. J. Biochem. 220819-826. [DOI] [PubMed] [Google Scholar]
- 284.Kadioglu, A., J. N. Weiser, J. C. Paton, and P. W. Andrew. 2008. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat. Rev. Microbiol. 6288-301. [DOI] [PubMed] [Google Scholar]
- 285.Kahn, F., M. Morgelin, O. Shannon, A. Norrby-Teglund, H. Herwald, A. I. Olin, and L. Bjorck. 2008. Antibodies against a surface protein of Streptococcus pyogenes promote a pathological inflammatory response. PLoS Pathog. 4e1000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Kamezawa, Y., T. Nakahara, S. Nakano, Y. Abe, J. Nozaki-Renard, and T. Isono. 1997. Streptococcal mitogenic exotoxin Z, a novel acidic superantigenic toxin produced by a T1 strain of Streptococcus pyogenes. Infect. Immun. 653828-3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Kang, H. J., F. Coulibaly, F. Clow, T. Proft, and E. N. Baker. 2007. Stabilizing isopeptide bonds revealed in gram-positive bacterial pilus structure. Science 3181625-1628. [DOI] [PubMed] [Google Scholar]
- 288.Kang, W., and K. B. Reid. 2003. DMBT1, a regulator of mucosal homeostasis through the linking of mucosal defense and regeneration? FEBS Lett. 54021-25. [DOI] [PubMed] [Google Scholar]
- 289.Kawabata, S., Y. Tamura, J. Murakami, Y. Terao, I. Nakagawa, and S. Hamada. 2002. A novel, anchorless streptococcal surface protein that binds to human immunoglobulins. Biochem. Biophys. Res. Commun. 2961329-1333. [DOI] [PubMed] [Google Scholar]
- 290.Kehoe, M. A. 1994. Cell-wall-associated proteins in gram-positive bacteria, p. 217-261. In J. M. Ghuysen and R. Hakenbeck (ed.), Bacterial cell wall. Elsevier Science, Amsterdam, The Netherlands.
- 291.Kelly, C., P. Evans, L. Bergmeier, S. F. Lee, A. Progulske-Fox, A. C. Harris, A. Aitken, A. S. Bleiweis, and T. Lehner. 1989. Sequence analysis of the cloned streptococcal cell surface antigen I/II. FEBS Lett. 258127-132. [DOI] [PubMed] [Google Scholar]
- 292.Kelly, C. G., J. S. Younson, B. Y. Hikmat, S. M. Todryk, M. Czisch, P. I. Haris, I. R. Flindall, C. Newby, A. I. Mallet, J. K. Ma, and T. Lehner. 1999. A synthetic peptide adhesion epitope as a novel antimicrobial agent. Nat. Biotechnol. 1742-47. [DOI] [PubMed] [Google Scholar]
- 293.Kerrigan, S. W., I. Douglas, A. Wray, J. Heath, M. F. Byrne, D. Fitzgerald, and D. Cox. 2002. A role for glycoprotein Ib in Streptococcus sanguis-induced platelet aggregation. Blood 100509-516. [DOI] [PubMed] [Google Scholar]
- 294.Kihlberg, B. M., M. Collin, A. Olsen, and L. Bjorck. 1999. Protein H, an antiphagocytic surface protein in Streptococcus pyogenes. Infect. Immun. 671708-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Kilian, M. 2005. Streptococcus and Lactobacillus, p. 833-881. In P. Borriello, P. R. Murray, and G. Funke (ed.), Topley and Wilson's microbiology and microbial infections. Hodder Arnold, London, United Kingdom.
- 296.Kilic, A. O., L. Tao, Y. Zhang, Y. Lei, A. Khammanivong, and M. C. Herzberg. 2004. Involvement of Streptococcus gordonii β-glucoside metabolism systems in adhesion, biofilm formation, and in vivo gene expression. J. Bacteriol. 1864246-4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Kim, D. Y., and K. K. Kim. 2005. Structure and function of HtrA family proteins, the key players in protein quality control. J. Biochem. Mol. Biol. 38266-274. [DOI] [PubMed] [Google Scholar]
- 298.Kinnby, B., N. A. Booth, and G. Svensater. 2008. Plasminogen binding by oral streptococci from dental plaque and inflammatory lesions. Microbiology 154924-931. [DOI] [PubMed] [Google Scholar]
- 299.Kitten, T., C. L. Munro, S. M. Michalek, and F. L. Macrina. 2000. Genetic characterization of a Streptococcus mutans LraI family operon and role in virulence. Infect. Immun. 684441-4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Kline, J. B., S. Xu, A. L. Bisno, and C. M. Collins. 1996. Identification of a fibronectin-binding protein (GfbA) in pathogenic group G streptococci. Infect. Immun. 642122-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Kobayashi, S. D., K. R. Braughton, A. R. Whitney, J. M. Voyich, T. G. Schwan, J. M. Musser, and F. R. DeLeo. 2003. Bacterial pathogens modulate an apoptosis differentiation program in human neutrophils. Proc. Natl. Acad. Sci. USA 10010948-10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Kolberg, J., A. Aase, S. Bergmann, T. K. Herstad, G. Rodal, R. Frank, M. Rohde, and S. Hammerschmidt. 2006. Streptococcus pneumoniae enolase is important for plasminogen binding despite low abundance of enolase protein on the bacterial cell surface. Microbiology 1521307-1317. [DOI] [PubMed] [Google Scholar]
- 303.Kolenbrander, P. E., and R. N. Andersen. 1990. Characterization of Streptococcus gordonii (S. sanguis) PK488 adhesin-mediated coaggregation with Actinomyces naeslundii PK606. Infect. Immun. 583064-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Kolenbrander, P. E., R. N. Andersen, R. A. Baker, and H. F. Jenkinson. 1998. The adhesion-associated sca operon in Streptococcus gordonii encodes an inducible high-affinity ABC transporter for Mn2+ uptake. J. Bacteriol. 180290-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Kolenbrander, P. E., R. N. Andersen, and N. Ganeshkumar. 1994. Nucleotide sequence of the Streptococcus gordonii PK488 coaggregation adhesin gene, scaA, and ATP-binding cassette. Infect. Immun. 624469-4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Kolenbrander, P. E., N. Ganeshkumar, F. J. Cassels, and C. V. Hughes. 1993. Coaggregation: specific adherence among human oral plaque bacteria. FASEB J. 7406-413. [DOI] [PubMed] [Google Scholar]
- 307.Koskiniemi, S., M. Sellin, and M. Norgren. 1998. Identification of two genes, cpsX and cpsY, with putative regulatory function on capsule expression in group B streptococci. FEMS Immunol. Med. Microbiol. 21159-168. [DOI] [PubMed] [Google Scholar]
- 308.Kotarsky, H., J. Hellwage, E. Johnsson, C. Skerka, H. G. Svensson, G. Lindahl, U. Sjobring, and P. F. Zipfel. 1998. Identification of a domain in human factor H and factor H-like protein-1 required for the interaction with streptococcal M proteins. J. Immunol. 1603349-3354. [PubMed] [Google Scholar]
- 309.Kreikemeyer, B., M. D. Boyle, B. A. Buttaro, M. Heinemann, and A. Podbielski. 2001. Group A streptococcal growth phase-associated virulence factor regulation by a novel operon (Fas) with homologies to two-component-type regulators requires a small RNA molecule. Mol. Microbiol. 39392-406. [DOI] [PubMed] [Google Scholar]
- 310.Kreikemeyer, B., M. Klenk, and A. Podbielski. 2004. The intracellular status of Streptococcus pyogenes: role of extracellular matrix-binding proteins and their regulation. Int. J. Med. Microbiol. 294177-188. [DOI] [PubMed] [Google Scholar]
- 311.Kreikemeyer, B., D. R. Martin, and G. S. Chhatwal. 1999. SfbII protein, a fibronectin binding surface protein of group A streptococci, is a serum opacity factor with high serotype-specific apolipoproteinase activity. FEMS Microbiol. Lett. 178305-311. [DOI] [PubMed] [Google Scholar]
- 312.Kreikemeyer, B., K. S. McIver, and A. Podbielski. 2003. Virulence factor regulation and regulatory networks in Streptococcus pyogenes and their impact on pathogen-host interactions. Trends Microbiol. 11224-232. [DOI] [PubMed] [Google Scholar]
- 313.Kreikemeyer, B., M. Nakata, S. Oehmcke, C. Gschwendtner, J. Normann, and A. Podbielski. 2005. Streptococcus pyogenes collagen type I-binding Cpa surface protein. Expression profile, binding characteristics, biological functions, and potential clinical impact. J. Biol. Chem. 28033228-33239. [DOI] [PubMed] [Google Scholar]
- 314.Kreikemeyer, B., S. R. Talay, and G. S. Chhatwal. 1995. Characterization of a novel fibronectin-binding surface protein in group A streptococci. Mol. Microbiol. 17137-145. [DOI] [PubMed] [Google Scholar]
- 315.Kreth, J., D. C. Hung, J. Merritt, J. Perry, L. Zhu, S. D. Goodman, D. G. Cvitkovitch, W. Shi, and F. Qi. 2007. The response regulator ComE in Streptococcus mutans functions both as a transcription activator of mutacin production and repressor of CSP biosynthesis. Microbiology 1531799-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Kreth, J., J. Merritt, W. Shi, and F. Qi. 2005. Competition and coexistence between Streptococcus mutans and Streptococcus sanguinis in the dental biofilm. J. Bacteriol. 1877193-7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Kreth, J., J. Merritt, W. Shi, and F. Qi. 2005. Co-ordinated bacteriocin production and competence development: a possible mechanism for taking up DNA from neighbouring species. Mol. Microbiol. 57392-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Kreth, J., J. Merritt, L. Zhu, W. Shi, and F. Qi. 2006. Cell density- and ComE-dependent expression of a group of mutacin and mutacin-like genes in Streptococcus mutans. FEMS Microbiol. Lett. 26511-17. [DOI] [PubMed] [Google Scholar]
- 319.Kreth, J., Y. Zhang, and M. C. Herzberg. 2008. Streptococcal antagonism in oral biofilms: Streptococcus sanguinis and Streptococcus gordonii interference with Streptococcus mutans. J. Bacteriol. 1904632-4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Krishnan, V., A. H. Gaspar, N. Ye, A. Mandlik, H. Ton-That, and S. V. Narayana. 2007. An IgG-like domain in the minor pilin GBS52 of Streptococcus agalactiae mediates lung epithelial cell adhesion. Structure 15893-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Kruger, C., A. Hultberg, C. van Dollenweerd, H. Marcotte, and L. Hammarstrom. 2005. Passive immunization by lactobacilli expressing single-chain antibodies against Streptococcus mutans. Mol. Biotechnol. 31221-231. [DOI] [PubMed] [Google Scholar]
- 322.Kuboniwa, M., G. D. Tribble, C. E. James, A. O. Kilic, L. Tao, M. C. Herzberg, S. Shizukuishi, and R. J. Lamont. 2006. Streptococcus gordonii utilizes several distinct gene functions to recruit Porphyromonas gingivalis into a mixed community. Mol. Microbiol. 60121-139. [DOI] [PubMed] [Google Scholar]
- 323.Kuo, C.-F., J.-J. Wu, P.-J. Tsai, F.-J. Kao, H.-Y. Lei, M. T. Lin, and Y.-S. Lin. 1999. Streptococcal pyrogenic exotoxin B induces apoptosis and reduces phagocytic activity in U937 cells. Infect. Immun. 67126-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Kuster, E., E. J. Luesink, W. M. de Vos, and W. Hillen. 1996. Immunological crossreactivity to the catabolite control protein CcpA Bacillus megaterium is found in many gram-positive bacteria. FEMS Microbiol. Lett. 139109-115. [DOI] [PubMed] [Google Scholar]
- 325.Kwinn, L. A., A. Khosravi, R. K. Aziz, A. M. Timmer, K. S. Doran, M. Kotb, and V. Nizet. 2007. Genetic characterization and virulence role of the RALP3/LSA locus upstream of the streptolysin S operon in invasive M1T1 group A Streptococcus. J. Bacteriol. 1891322-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Lachenauer, C. S., and L. C. Madoff. 1996. A protective surface protein from type V group B streptococci shares N-terminal sequence homology with the alpha C protein. Infect. Immun. 644255-4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Lamont, R. J., A. El-Sabaeny, Y. Park, G. S. Cook, J. W. Costerton, and D. R. Demuth. 2002. Role of the Streptococcus gordonii SspB protein in the development of Porphyromonas gingivalis biofilms on streptococcal substrates. Microbiology 1481627-1636. [DOI] [PubMed] [Google Scholar]
- 328.Lamont, R. J., S. Gil, D. R. Demuth, D. Malamud, and B. Rosan. 1994. Molecules of Streptococcus gordonii that bind to Porphyromonas gingivalis. Microbiology 140867-872. [DOI] [PubMed] [Google Scholar]
- 329.Lamont, R. J., and B. Rosan. 1990. Adherence of mutans streptococci to other oral bacteria. Infect. Immun. 581738-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Lamy, M. C., M. Zouine, J. Fert, M. Vergassola, E. Couve, E. Pellegrini, P. Glaser, F. Kunst, T. Msadek, P. Trieu-Cuot, and C. Poyart. 2004. CovS/CovR of group B Streptococcus: a two-component global regulatory system involved in virulence. Mol. Microbiol. 541250-1268. [DOI] [PubMed] [Google Scholar]
- 331.Lancefield, R. C. 1933. A serological differentiation of human and other groups of hemolytic streptococci. J. Exp. Med. 57571-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Lancefield, R. C., and G. E. Perlmann. 1952. Preparation and properties of a protein (R antigen) occurring in streptococci of group A, type 28 and in certain streptococci of other serological groups. J. Exp. Med. 9683-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Lancy, P., Jr., J. M. DiRienzo, B. Appelbaum, B. Rosan, and S. C. Holt. 1983. Corncob formation between Fusobacterium nucleatum and Streptococcus sanguis. Infect. Immun. 40303-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Lange, R., C. Wagner, A. de Saizieu, N. Flint, J. Molnos, M. Stieger, P. Caspers, M. Kamber, W. Keck, and K. E. Amrein. 1999. Domain organization and molecular characterization of 13 two-component systems identified by genome sequencing of Streptococcus pneumoniae. Gene 237223-234. [DOI] [PubMed] [Google Scholar]
- 335.LaPolla, R. J., J. A. Haron, C. G. Kelly, W. R. Taylor, C. Bohart, M. Hendricks, J. P. Pyati, R. T. Graff, J. K. Ma, and T. Lehner. 1991. Sequence and structural analysis of surface protein antigen I/II (SpaA) of Streptococcus sobrinus. Infect. Immun. 592677-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Lauer, P., C. D. Rinaudo, M. Soriani, I. Margarit, D. Maione, R. Rosini, A. R. Taddei, M. Mora, R. Rappuoli, G. Grandi, and J. L. Telford. 2005. Genome analysis reveals pili in group B Streptococcus. Science 309105. [DOI] [PubMed] [Google Scholar]
- 337.Leday, T. V., K. M. Gold, T. L. Kinkel, S. A. Roberts, J. R. Scott, and K. S. McIver. 2008. TrxR, a new CovR-repressed response regulator that activates the Mga virulence regulon in group A Streptococcus. Infect. Immun. 764659-4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Lee, S. Y., K. K. Kim, and S. J. Choe. 2001. Binding of oral streptococci to human fibrinogen. Oral Microbiol. Immunol. 1688-93. [DOI] [PubMed] [Google Scholar]
- 339.Lei, B., F. R. DeLeo, N. P. Hoe, M. R. Graham, S. M. Mackie, R. L. Cole, M. Liu, H. R. Hill, D. E. Low, M. J. Federle, J. R. Scott, and J. M. Musser. 2001. Evasion of human innate and acquired immunity by a bacterial homolog of CD11b that inhibits opsonophagocytosis. Nat. Med. 71298-1305. [DOI] [PubMed] [Google Scholar]
- 340.Lei, B., M. Liu, J. M. Voyich, C. I. Prater, S. V. Kala, F. R. DeLeo, and J. M. Musser. 2003. Identification and characterization of HtsA, a second heme-binding protein made by Streptococcus pyogenes. Infect. Immun. 715962-5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Lembke, C., A. Podbielski, C. Hidalgo-Grass, L. Jonas, E. Hanski, and B. Kreikemeyer. 2006. Characterization of biofilm formation by clinically relevant serotypes of group A streptococci. Appl. Environ. Microbiol. 722864-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.LeMieux, J., D. L. Hava, A. Basset, and A. Camilli. 2006. RrgA and RrgB are components of a multisubunit pilus encoded by the Streptococcus pneumoniae rlrA pathogenicity islet. Infect. Immun. 742453-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Lemos, J. A., T. A. Brown, Jr., and R. A. Burne. 2004. Effects of RelA on key virulence properties of planktonic and biofilm populations of Streptococcus mutans. Infect. Immun. 721431-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Lemos, J. A., V. K. Lin, M. M. Nascimento, J. Abranches, and R. A. Burne. 2007. Three gene products govern (p)ppGpp production by Streptococcus mutans. Mol. Microbiol. 651568-1581. [DOI] [PubMed] [Google Scholar]
- 345.Lemos, J. A., M. M. Nascimento, V. K. Lin, J. Abranches, and R. A. Burne. 2008. Global regulation by (p)ppGpp and CodY in Streptococcus mutans. J. Bacteriol. 1905291-5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Levesque, C., C. Vadeboncoeur, F. Chandad, and M. Frenette. 2001. Streptococcus salivarius fimbriae are composed of a glycoprotein containing a repeated motif assembled into a filamentous nondissociable structure. J. Bacteriol. 1832724-2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Levesque, C., C. Vadeboncoeur, and M. Frenette. 2004. The csp operon of Streptococcus salivarius encodes two predicted cell-surface proteins, one of which, CspB, is associated with the fimbriae. Microbiology 150189-198. [DOI] [PubMed] [Google Scholar]
- 348.Levesque, C. M., R. W. Mair, J. A. Perry, P. C. Lau, Y. H. Li, and D. G. Cvitkovitch. 2007. Systemic inactivation and phenotypic characterization of two-component systems in expression of Streptococcus mutans virulence properties. Lett. Appl. Microbiol. 45398-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Levin, J. C., and M. R. Wessels. 1998. Identification of csrR/csrS, a genetic locus that regulates hyaluronic acid capsule synthesis in group A Streptococcus. Mol. Microbiol. 30209-219. [DOI] [PubMed] [Google Scholar]
- 350.Lewis, M. J., M. Meehan, P. Owen, and J. M. Woof. 2008. A common theme in interaction of bacterial immunoglobulin-binding proteins with immunoglobulins illustrated in the equine system. J. Biol. Chem. 28317615-17623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Li, L., J. M. Tanzer, and F. A. Scannapieco. 2002. Identification and analysis of the amylase-binding protein B (AbpB) and gene (abpB) from Streptococcus gordonii. FEMS Microbiol. Lett. 212151-157. [DOI] [PubMed] [Google Scholar]
- 352.Li, Y.-H., P. C. Y. Lau, J. H. Lee, R. P. Ellen, and D. G. Cvitkovitch. 2001. Natural genetic transformation of Streptococcus mutans growing in biofilms. J. Bacteriol. 183897-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Li, Y.-H., P. C. Y. Lau, N. Tang, G. Svensäter, R. P. Ellen, and D. G. Cvitkovitch. 2002. Novel two-component regulatory system involved in biofilm formation and acid resistance in Streptococcus mutans. J. Bacteriol. 1846333-6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Li, Y.-H., N. Tang, M. B. Aspiras, P. C. Y. Lau, J. H. Lee, R. P. Ellen, and D. G. Cvitkovitch. 2002. A quorum-sensing signaling system essential for genetic competence in Streptococcus mutans is involved in biofilm formation. J. Bacteriol. 1842699-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Li, Y. H., X. L. Tian, G. Layton, C. Norgaard, and G. Sisson. 2008. Additive attenuation of virulence and cariogenic potential of Streptococcus mutans by simultaneous inactivation of the ComCDE quorum-sensing system and HK/RR11 two-component regulatory system. Microbiology 1543256-3265. [DOI] [PubMed] [Google Scholar]
- 356.Lin, X., R. J. Lamont, J. Wu, and H. Xie. 2008. Role of differential expression of streptococcal arginine deiminase in inhibition of fimA expression in Porphyromonas gingivalis. J. Bacteriol. 1904367-4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Lindahl, G., M. Stalhammar-Carlemalm, and T. Areschoug. 2005. Surface proteins of Streptococcus agalactiae and related proteins in other bacterial pathogens. Clin. Microbiol. Rev. 18102-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Lindgren, P. E., M. J. McGavin, C. Signas, B. Guss, S. Gurusiddappa, M. Hook, and M. Lindberg. 1993. Two different genes coding for fibronectin-binding proteins from Streptococcus dysgalactiae. The complete nucleotide sequences and characterization of the binding domains. Eur. J. Biochem. 214819-827. [DOI] [PubMed] [Google Scholar]
- 359.Lindgren, P. E., P. Speziale, M. McGavin, H. J. Monstein, M. Hook, L. Visai, T. Kostiainen, S. Bozzini, and M. Lindberg. 1992. Cloning and expression of two different genes from Streptococcus dysgalactiae encoding fibronectin receptors. J. Biol. Chem. 2671924-1931. [PubMed] [Google Scholar]
- 360.Liu, M., T. S. Hanks, J. Zhang, M. J. McClure, D. W. Siemsen, J. L. Elser, M. T. Quinn, and B. Lei. 2006. Defects in ex vivo and in vivo growth and sensitivity to osmotic stress of group A Streptococcus caused by interruption of response regulator gene vicR. Microbiology 152967-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Lonn-Stensrud, J., F. C. Petersen, T. Benneche, and A. A. Scheie. 2007. Synthetic bromated furanone inhibits autoinducer-2-mediated communication and biofilm formation in oral streptococci. Oral Microbiol. Immunol. 22340-346. [DOI] [PubMed] [Google Scholar]
- 362.Loo, C. Y., D. A. Corliss, and N. Ganeshkumar. 2000. Streptococcus gordonii biofilm formation: identification of genes that code for biofilm phenotypes. J. Bacteriol. 1821374-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Loo, C. Y., K. Mitrakul, S. Jaafar, C. Gyurko, C. V. Hughes, and N. Ganeshkumar. 2004. Role of a nosX homolog in Streptococcus gordonii in aerobic growth and biofilm formation. J. Bacteriol. 1868193-8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Loo, C. Y., K. Mitrakul, I. B. Voss, C. V. Hughes, and N. Ganeshkumar. 2003. Involvement of an inducible fructose phosphotransferase operon in Streptococcus gordonii biofilm formation. J. Bacteriol. 1856241-6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Loo, C. Y., K. Mitrakul, I. B. Voss, C. V. Hughes, and N. Ganeshkumar. 2003. Involvement of the adc operon and manganese homeostasis in Streptococcus gordonii biofilm formation. J. Bacteriol. 1852887-2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Lottenberg, R., C. C. Broder, M. D. Boyle, S. J. Kain, B. L. Schroeder, and R. Curtiss III. 1992. Cloning, sequence analysis, and expression in Escherichia coli of a streptococcal plasmin receptor. J. Bacteriol. 1745204-5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Loughman, J. A., and M. G. Caparon. 2006. A novel adaptation of aldolase regulates virulence in Streptococcus pyogenes. EMBO J. 255414-5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Love, R. M., M. D. McMillan, and H. F. Jenkinson. 1997. Invasion of dentinal tubules by oral streptococci is associated with collagen recognition mediated by the antigen I/II family of polypeptides. Infect. Immun. 655157-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Lukomski, S., K. Nakashima, I. Abdi, V. J. Cipriano, R. M. Ireland, S. D. Reid, G. G. Adams, and J. M. Musser. 2000. Identification and characterization of the scl gene encoding a group A Streptococcus extracellular protein virulence factor with similarity to human collagen. Infect. Immun. 686542-6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Lynch, D. J., T. L. Fountain, J. E. Mazurkiewicz, and J. A. Banas. 2007. Glucan-binding proteins are essential for shaping Streptococcus mutans biofilm architecture. FEMS Microbiol. Lett. 268158-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Lyon, W. R., and M. G. Caparon. 2004. Role for serine protease HtrA (DegP) of Streptococcus pyogenes in the biogenesis of virulence factors SpeB and the hemolysin streptolysin S. Infect. Immun. 721618-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Lyon, W. R., J. C. Madden, J. C. Levin, J. L. Stein, and M. G. Caparon. 2001. Mutation of luxS affects growth and virulence factor expression in Streptococcus pyogenes. Mol. Microbiol. 42145-157. [DOI] [PubMed] [Google Scholar]
- 373.Madoff, L. C., J. L. Michel, E. W. Gong, A. K. Rodewald, and D. L. Kasper. 1992. Protection of neonatal mice from group B streptococcal infection by maternal immunization with beta C protein. Infect. Immun. 604989-4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Madoff, L. C., L. C. Paoletti, J. Y. Tai, and D. L. Kasper. 1994. Maternal immunization of mice with group B streptococcal type III polysaccharide-beta C protein conjugate elicits protective antibody to multiple serotypes. J. Clin. Investig. 94286-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Maeda, K., G. D. Tribble, C. M. Tucker, C. Anaya, S. Shizukuishi, J. P. Lewis, D. R. Demuth, and R. J. Lamont. 2008. A Porphyromonas gingivalis tyrosine phosphatase is a multifunctional regulator of virulence attributes. Mol. Microbiol. 691153-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Maione, D., I. Margarit, C. D. Rinaudo, V. Masignani, M. Mora, M. Scarselli, H. Tettelin, C. Brettoni, E. T. Iacobini, R. Rosini, N. D'Agostino, L. Miorin, S. Buccato, M. Mariani, G. Galli, R. Nogarotto, V. Nardi Dei, F. Vegni, C. Fraser, G. Mancuso, G. Teti, L. C. Madoff, L. C. Paoletti, R. Rappuoli, D. L. Kasper, J. L. Telford, and G. Grandi. 2005. Identification of a universal group B Streptococcus vaccine by multiple genome screen. Science 309148-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Maisey, H. C., M. Hensler, V. Nizet, and K. S. Doran. 2007. Group B streptococcal pilus proteins contribute to adherence to and invasion of brain microvascular endothelial cells. J. Bacteriol. 1891464-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Maisey, H. C., D. Quach, M. E. Hensler, G. Y. Liu, R. L. Gallo, V. Nizet, and K. S. Doran. 2008. A group B streptococcal pilus protein promotes phagocyte resistance and systemic virulence. FASEB J. 221715-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Malke, H., B. Roe, and J. J. Ferretti. 1985. Nucleotide sequence of the streptokinase gene from Streptococcus equisimilis H46A. Gene 34357-362. [DOI] [PubMed] [Google Scholar]
- 380.Malke, H., K. Steiner, W. M. McShan, and J. J. Ferretti. 2006. Linking the nutritional status of Streptococcus pyogenes to alteration of transcriptional gene expression: the action of CodY and RelA. Int. J. Med. Microbiol. 296259-275. [DOI] [PubMed] [Google Scholar]
- 381.Manco, S., F. Hernon, H. Yesilkaya, J. C. Paton, P. W. Andrew, and A. Kadioglu. 2006. Pneumococcal neuraminidases A and B both have essential roles during infection of the respiratory tract and sepsis. Infect. Immun. 744014-4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Manetti, A. G., C. Zingaretti, F. Falugi, S. Capo, M. Bombaci, F. Bagnoli, G. Gambellini, G. Bensi, M. Mora, A. M. Edwards, J. M. Musser, E. A. Graviss, J. L. Telford, G. Grandi, and I. Margarit. 2007. Streptococcus pyogenes pili promote pharyngeal cell adhesion and biofilm formation. Mol. Microbiol. 64968-983. [DOI] [PubMed] [Google Scholar]
- 383.Margarit, I., C. D. Rinaudo, C. L. Galeotti, D. Maione, C. Ghezzo, E. Buttazzoni, R. Rosini, Y. Runci, M. Mora, S. Buccato, M. Pagani, E. Tresoldi, A. Berardi, R. Creti, C. J. Baker, J. L. Telford, and G. Grandi. 2009. Preventing bacterial infections with pilus-based vaccines: the group B Streptococcus paradigm. J. Infect. Dis. 199108-115. [DOI] [PubMed] [Google Scholar]
- 384.Marraffini, L. A., A. C. Dedent, and O. Schneewind. 2006. Sortases and the art of anchoring proteins to the envelopes of gram-positive bacteria. Microbiol. Mol. Biol. Rev. 70192-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Martin, D., S. Rioux, E. Gagnon, M. Boyer, J. Hamel, N. Charland, and B. R. Brodeur. 2002. Protection from group B streptococcal infection in neonatal mice by maternal immunization with recombinant Sip protein. Infect. Immun. 704897-4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Mascher, T., D. Zahner, M. Merai, N. Balmelle, A. B. de Saizieu, and R. Hakenbeck. 2003. The Streptococcus pneumoniae cia regulon: CiaR target sites and transcription profile analysis. J. Bacteriol. 18560-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Matsumoto-Nakano, M., and H. K. Kuramitsu. 2006. Role of bacteriocin immunity proteins in the antimicrobial sensitivity of Streptococcus mutans. J. Bacteriol. 1888095-8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.McArthur, J. D., F. C. McKay, V. Ramachandran, P. Shyam, A. J. Cork, M. L. Sanderson-Smith, J. N. Cole, U. Ringdahl, U. Sjobring, M. Ranson, and M. J. Walker. 2008. Allelic variants of streptokinase from Streptococcus pyogenes display functional differences in plasminogen activation. FASEB J. 223146-3153. [DOI] [PubMed] [Google Scholar]
- 389.McArthur, J. D., and M. J. Walker. 2006. Domains of group A streptococcal M protein that confer resistance to phagocytosis, opsonization and protection: implications for vaccine development. Mol. Microbiol. 591-4. [DOI] [PubMed] [Google Scholar]
- 390.McCluskey, J., J. Hinds, S. Husain, A. Witney, and T. J. Mitchell. 2004. A two-component system that controls the expression of pneumococcal surface antigen A (PsaA) and regulates virulence and resistance to oxidative stress in Streptococcus pneumoniae. Mol. Microbiol. 511661-1675. [DOI] [PubMed] [Google Scholar]
- 391.McDougald, D., S. A. Rice, and S. Kjelleberg. 2007. Bacterial quorum sensing and interference by naturally occurring biomimics. Anal. Bioanal. Chem. 387445-453. [DOI] [PubMed] [Google Scholar]
- 392.McKessar, S. J., and R. Hakenbeck. 2007. The two-component regulatory system TCS08 is involved in cellobiose metabolism of Streptococcus pneumoniae R6. J. Bacteriol. 1891342-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.McLaughlin, R. E., J. J. Ferretti, and W. L. Hynes. 1999. Nucleotide sequence of the streptococcin A-FF22 lantibiotic regulon: model for production of the lantibiotic SA-FF22 by strains of Streptococcus pyogenes. FEMS Microbiol. Lett. 175171-177. [DOI] [PubMed] [Google Scholar]
- 394.McNab, R., H. Forbes, P. S. Handley, D. M. Loach, G. W. Tannock, and H. F. Jenkinson. 1999. Cell wall-anchored CshA polypeptide (259 kilodaltons) in Streptococcus gordonii forms surface fibrils that confer hydrophobic and adhesive properties. J. Bacteriol. 1813087-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.McNab, R., S. K. Ford, A. El-Sabaeny, B. Barbieri, G. S. Cook, and R. J. Lamont. 2003. LuxS-based signaling in Streptococcus gordonii: autoinducer 2 controls carbohydrate metabolism and biofilm formation with Porphyromonas gingivalis. J. Bacteriol. 185274-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.McNab, R., A. R. Holmes, J. M. Clarke, G. W. Tannock, and H. F. Jenkinson. 1996. Cell surface polypeptide CshA mediates binding of Streptococcus gordonii to other oral bacteria and to immobilized fibronectin. Infect. Immun. 644204-4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.McNab, R., and H. F. Jenkinson. 1998. Altered adherence properties of a Streptococcus gordonii hppA (oligopeptide permease) mutant result from transcriptional effects on cshA adhesin gene expression. Microbiology 144127-136. [DOI] [PubMed] [Google Scholar]
- 398.McNab, R., H. F. Jenkinson, D. M. Loach, and G. W. Tannock. 1994. Cell-surface-associated polypeptides CshA and CshB of high molecular mass are colonization determinants in the oral bacterium Streptococcus gordonii. Mol. Microbiol. 14743-754. [DOI] [PubMed] [Google Scholar]
- 399.McNamara, C., A. S. Zinkernagel, P. Macheboeuf, M. W. Cunningham, V. Nizet, and P. Ghosh. 2008. Coiled-coil irregularities and instabilities in group A Streptococcus M1 are required for virulence. Science 3191405-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Mechold, U., M. Cashel, K. Steiner, D. Gentry, and H. Malke. 1996. Functional analysis of a relA/spoT gene homolog from Streptococcus equisimilis. J. Bacteriol. 1781401-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Medina, E., G. Molinari, M. Rohde, B. Haase, G. S. Chhatwal, and C. A. Guzman. 1999. Fc-mediated nonspecific binding between fibronectin-binding protein I of Streptococcus pyogenes and human immunoglobulins. J. Immunol. 1633396-3402. [PubMed] [Google Scholar]
- 402.Merritt, J., J. Kreth, W. Shi, and F. Qi. 2005. LuxS controls bacteriocin production in Streptococcus mutans through a novel regulatory component. Mol. Microbiol. 57960-969. [DOI] [PubMed] [Google Scholar]
- 403.Merritt, J., F. Qi, S. D. Goodman, M. H. Anderson, and W. Shi. 2003. Mutation of luxS affects biofilm formation in Streptococcus mutans. Infect. Immun. 711972-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Merritt, J., L. Zheng, W. Shi, and F. Qi. 2007. Genetic characterization of the hdrRM operon: a novel high-cell-density-responsive regulator in Streptococcus mutans. Microbiology 1532765-2773. [DOI] [PubMed] [Google Scholar]
- 405.Miller, S. T., K. B. Xavier, S. R. Campagna, M. E. Taga, M. F. Semmelhack, B. L. Bassler, and F. M. Hughson. 2004. Salmonella typhimurium recognizes a chemically distinct form of the bacterial quorum-sensing signal AI-2. Mol. Cell 15677-687. [DOI] [PubMed] [Google Scholar]
- 406.Miller-Torbert, T. A., S. Sharma, and R. G. Holt. 2008. Inactivation of a gene for a fibronectin-binding protein of the oral bacterium Streptococcus mutans partially impairs its adherence to fibronectin. Microb. Pathog. 4553-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Mitchell, T. J. 2003. The pathogenesis of streptococcal infections: from tooth decay to meningitis. Nat. Rev. Microbiol. 1219-230. [DOI] [PubMed] [Google Scholar]
- 408.Mitrakul, K., C. Y. Loo, C. V. Hughes, and N. Ganeshkumar. 2004. Role of a Streptococcus gordonii copper-transport operon, copYAZ, in biofilm detachment. Oral Microbiol. Immunol. 19395-402. [DOI] [PubMed] [Google Scholar]
- 409.Molinari, G., M. Rohde, C. A. Guzman, and G. S. Chhatwal. 2000. Two distinct pathways for the invasion of Streptococcus pyogenes in non-phagocytic cells. Cell. Microbiol. 2145-154. [DOI] [PubMed] [Google Scholar]
- 410.Mollick, J. A., G. G. Miller, J. M. Musser, R. G. Cook, and R. R. Rich. 1992. Isolation and characterization of a novel streptococcal superantigen. Trans. Assoc. Am. Physicians 105110-122. [PubMed] [Google Scholar]
- 411.Montanez, G. E., M. N. Neely, and Z. Eichenbaum. 2005. The streptococcal iron uptake (Siu) transporter is required for iron uptake and virulence in a zebrafish infection model. Microbiology 1513749-3757. [DOI] [PubMed] [Google Scholar]
- 412.Mora, M., G. Bensi, S. Capo, F. Falugi, C. Zingaretti, A. G. Manetti, T. Maggi, A. R. Taddei, G. Grandi, and J. L. Telford. 2005. Group A Streptococcus produce pilus-like structures containing protective antigens and Lancefield T antigens. Proc. Natl. Acad. Sci. USA 10215641-15646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Müller, H.-P., and L. K. Rantamäki. 1995. Binding of native α2-macroglobulin to human group G streptococci. Infect. Immun. 632833-2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Munro, C. L., and F. L. Macrina. 1993. Sucrose-derived exopolysaccharides of Streptococcus mutans V403 contribute to infectivity in endocarditis. Mol. Microbiol. 8133-142. [DOI] [PubMed] [Google Scholar]
- 415.Munro, C. L., S. M. Michalek, and F. L. Macrina. 1995. Sucrose-derived exopolymers have site-dependent roles in Streptococcus mutans-promoted dental decay. FEMS Microbiol. Lett. 128327-332. [DOI] [PubMed] [Google Scholar]
- 416.Nakagawa, I., M. Nakata, S. Kawabata, and S. Hamada. 2001. Cytochrome c-mediated caspase-9 activation triggers apoptosis in Streptococcus pyogenes-infected epithelial cells. Cell. Microbiol. 3395-405. [DOI] [PubMed] [Google Scholar]
- 417.Nakagawa, I., M. Nakata, S. Kawabata, and S. Hamada. 2004. Transcriptome analysis and gene expression profiles of early apoptosis-related genes in Streptococcus pyogenes-infected epithelial cells. Cell. Microbiol. 6939-952. [DOI] [PubMed] [Google Scholar]
- 418.Nakano, K., K. Fujita, K. Nishimura, R. Nomura, and T. Ooshima. 2005. Contribution of biofilm regulatory protein A of Streptococcus mutans to systemic virulence. Microbes Infect. 71246-1255. [DOI] [PubMed] [Google Scholar]
- 419.Nakata, M., A. Podbielski, and B. Kreikemeyer. 2005. MsmR, a specific positive regulator of the Streptococcus pyogenes FCT pathogenicity region and cytolysin-mediated translocation system genes. Mol. Microbiol. 57786-803. [DOI] [PubMed] [Google Scholar]
- 420.Nanbu, A., M. Hayakawa, K. Takada, N. Shinozaki, Y. Abiko, and K. Fukushima. 2000. Production, characterization, and application of monoclonal antibodies which distinguish four glucosyltransferases from Streptococcus sobrinus. FEMS Immunol. Med. Microbiol. 279-15. [DOI] [PubMed] [Google Scholar]
- 421.Nascimento, M. M., J. A. Lemos, J. Abranches, V. K. Lin, and R. A. Burne. 2008. Role of RelA of Streptococcus mutans in global control of gene expression. J. Bacteriol. 19028-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Nelson, A. L., J. Ries, F. Bagnoli, S. Dahlberg, S. Falker, S. Rounioja, J. Tschop, E. Morfeldt, I. Ferlenghi, M. Hilleringmann, D. W. Holden, R. Rappuoli, S. Normark, M. A. Barocchi, and B. Henriques-Normark. 2007. RrgA is a pilus-associated adhesin in Streptococcus pneumoniae. Mol. Microbiol. 66329-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Ng, W. L., K. M. Kazmierczak, and M. E. Winkler. 2004. Defective cell wall synthesis in Streptococcus pneumoniae R6 depleted for the essential PcsB putative murein hydrolase or the VicR (YycF) response regulator. Mol. Microbiol. 531161-1175. [DOI] [PubMed] [Google Scholar]
- 424.Nilson, B. H., I. M. Frick, P. Akesson, S. Forsen, L. Bjorck, B. Akerstrom, and M. Wikstrom. 1995. Structure and stability of protein H and the M1 protein from Streptococcus pyogenes. Implications for other surface proteins of gram-positive bacteria. Biochemistry 3413688-13698. [DOI] [PubMed] [Google Scholar]
- 425.Nitsche-Schmitz, D. P., H. M. Johansson, I. Sastalla, S. Reissmann, I. M. Frick, and G. S. Chhatwal. 2007. Group G streptococcal IgG binding molecules FOG and protein G have different impacts on opsonization by C1q. J. Biol. Chem. 28217530-17536. [DOI] [PubMed] [Google Scholar]
- 426.Nitsche-Schmitz, D. P., M. Rohde, and G. S. Chhatwal. 2007. Invasion mechanisms of gram-positive pathogenic cocci. Thromb. Haemost. 98488-496. [PubMed] [Google Scholar]
- 427.Nobbs, A. H., B. H. Shearer, M. Drobni, M. A. Jepson, and H. F. Jenkinson. 2007. Adherence and internalization of Streptococcus gordonii by epithelial cells involves β1 integrin recognition by SspA and SspB (antigen I/II family) polypeptides. Cell. Microbiol. 965-83. [DOI] [PubMed] [Google Scholar]
- 428.Nobbs, A. H., R. M. Vajna, J. R. Johnson, Y. Zhang, S. L. Erlandsen, M. W. Oli, J. Kreth, L. J. Brady, and M. C. Herzberg. 2007. Consequences of a sortase A mutation in Streptococcus gordonii. Microbiology 1534088-4097. [DOI] [PubMed] [Google Scholar]
- 429.Novak, R., A. Cauwels, E. Charpentier, and E. Tuomanen. 1999. Identification of a Streptococcus pneumoniae gene locus encoding proteins of an ABC phosphate transporter and a two-component regulatory system. J. Bacteriol. 1811126-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Oetjen, J., P. Fives-Taylor, and E. H. Froeliger. 2002. The divergently transcribed Streptococcus parasanguis virulence-associated fimA operon encoding an Mn2+-responsive metal transporter and pepO encoding a zinc metallopeptidase are not coordinately regulated. Infect. Immun. 705706-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Ofek, I., and R. J. Doyle. 1994. Bacterial adhesion to cells and tissues. Chapman & Hall, New York, NY.
- 432.Ogunniyi, A. D., M. Grabowicz, L. K. Mahdi, J. Cook, D. L. Gordon, T. A. Sadlon, and J. C. Paton. 2009. Pneumococcal histidine triad proteins are regulated by the Zn2+-dependent repressor AdcR and inhibit complement deposition through the recruitment of complement factor H. FASEB J. 23731-738. [DOI] [PubMed] [Google Scholar]
- 433.Okada, N., M. K. Liszewski, J. P. Atkinson, and M. Caparon. 1995. Membrane cofactor protein (CD46) is a keratinocyte receptor for the M protein of the group A Streptococcus. Proc. Natl. Acad. Sci. USA 922489-2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Okamoto, S., Y. Terao, K. Hasuike, S. Hamada, and S. Kawabata. 2008. A novel streptococcal leucine zipper protein (Lzp) binds to human immunoglobulins. Biochem. Biophys. Res. Commun. 3771128-1134. [DOI] [PubMed] [Google Scholar]
- 435.Okamoto, S., Y. Terao, Y. Tamura, S. Hamada, and S. Kawabata. 2008. Streptococcal immunoglobulin-binding protein Sib35 exerts stimulatory and mitogenic effects toward mouse B lymphocytes. FEMS Microbiol. Lett. 28173-80. [DOI] [PubMed] [Google Scholar]
- 436.Oligino, L., and P. Fives-Taylor. 1993. Overexpression and purification of a fimbria-associated adhesin of Streptococcus parasanguis. Infect. Immun. 611016-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Olsen, R. J., S. A. Shelburne, and J. M. Musser. 2009. Molecular mechanisms underlying group A streptococcal pathogenesis. Cell. Microbiol. 111-12. [DOI] [PubMed] [Google Scholar]
- 438.Olsson, A., M. Eliasson, B. Guss, B. Nilsson, U. Hellman, M. Lindberg, and M. Uhlen. 1987. Structure and evolution of the repetitive gene encoding streptococcal protein G. Eur. J. Biochem. 168319-324. [DOI] [PubMed] [Google Scholar]
- 439.Osterlund, A., R. Popa, T. Nikkila, A. Scheynius, and L. Engstrand. 1997. Intracellular reservoir of Streptococcus pyogenes in vivo: a possible explanation for recurrent pharyngotonsillitis. Laryngoscope 107640-647. [DOI] [PubMed] [Google Scholar]
- 440.Ouyang, Q., W. L. Ma, C. H. Liu, R. Shi, and W. L. Zheng. 2006. Phenotypic analysis of luxS gene deletion mutants and its application in virulence regulation research in group B Streptococcus. J. South. Med. Uni. 26117-121. [PubMed] [Google Scholar]
- 441.Ozeri, V., I. Rosenshine, A. Ben-Ze'Ev, G. M. Bokoch, T. S. Jou, and E. Hanski. 2001. De novo formation of focal complex-like structures in host cells by invading streptococci. Mol. Microbiol. 41561-573. [DOI] [PubMed] [Google Scholar]
- 442.Ozeri, V., I. Rosenshine, D. F. Mosher, R. Fassler, and E. Hanski. 1998. Roles of integrins and fibronectin in the entry of Streptococcus pyogenes into cells via protein F1. Mol. Microbiol. 30625-637. [DOI] [PubMed] [Google Scholar]
- 443.Ozeri, V., A. Tovi, I. Burstein, S. Natanson-Yaron, M. G. Caparon, K. M. Yamada, S. K. Akiyama, I. Vlodavsky, and E. Hanski. 1996. A two-domain mechanism for group A streptococcal adherence through protein F to the extracellular matrix. EMBO J. 15989-998. [PMC free article] [PubMed] [Google Scholar]
- 444.Pahlman, L. I., M. Morgelin, J. Eckert, L. Johansson, W. Russell, K. Riesbeck, O. Soehnlein, L. Lindbom, A. Norrby-Teglund, R. R. Schumann, L. Bjorck, and H. Herwald. 2006. Streptococcal M protein: a multipotent and powerful inducer of inflammation. J. Immunol. 1771221-1228. [DOI] [PubMed] [Google Scholar]
- 445.Pahlman, L. I., A. I. Olin, J. Darenberg, M. Morgelin, M. Kotb, H. Herwald, and A. Norrby-Teglund. 2008. Soluble M1 protein of Streptococcus pyogenes triggers potent T cell activation. Cell. Microbiol. 10404-414. [DOI] [PubMed] [Google Scholar]
- 446.Paik, S., A. Brown, C. L. Munro, C. N. Cornelissen, and T. Kitten. 2003. The sloABCR operon of Streptococcus mutans encodes an Mn and Fe transport system required for endocarditis virulence and its Mn-dependent repressor. J. Bacteriol. 1855967-5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Pancholi, V., and V. A. Fischetti. 1992. A major surface protein on group A streptococci is a glyceraldehyde-3-phosphate-dehydrogenase with multiple binding activity. J. Exp. Med. 176415-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Pancholi, V., and V. A. Fischetti. 1997. A novel plasminogen/plasmin binding protein on the surface of group A streptococci. Adv. Exp. Med. Biol. 418597-599. [DOI] [PubMed] [Google Scholar]
- 449.Pancholi, V., and V. A. Fischetti. 1997. Regulation of the phosphorylation of human pharyngeal cell proteins by group A streptococcal surface dehydrogenase: signal transduction between streptococci and pharyngeal cells. J. Exp. Med. 1861633-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Pancholi, V., and V. A. Fischetti. 1998. α-Enolase, a novel strong plasmin(ogen) binding protein on the surface of pathogenic streptococci. J. Biol. Chem. 27314503-14515. [DOI] [PubMed] [Google Scholar]
- 451.Pandiripally, V., E. Gregory, and D. Cue. 2002. Acquisition of regulators of complement activation by Streptococcus pyogenes serotype M1. Infect. Immun. 706206-6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Paterson, G. K., L. Nieminen, J. M. Jefferies, and T. J. Mitchell. 2008. PclA, a pneumococcal collagen-like protein with selected strain distribution, contributes to adherence and invasion of host cells. FEMS Microbiol. Lett. 285170-176. [DOI] [PubMed] [Google Scholar]
- 453.Pestova, E. V., L. S. Havarstein, and D. A. Morrison. 1996. Regulation of competence for genetic transformation in Streptococcus pneumoniae by an auto-induced peptide pheromone and a two-component regulatory system. Mol. Microbiol. 21853-862. [DOI] [PubMed] [Google Scholar]
- 454.Petersen, F. C., N. A. Ahmed, A. Naemi, and A. A. Scheie. 2006. LuxS-mediated signalling in Streptococcus anginosus and its role in biofilm formation. Antonie van Leeuwenhoek 90109-121. [DOI] [PubMed] [Google Scholar]
- 455.Petersen, F. C., S. Assev, H. C. van der Mei, H. J. Busscher, and A. A. Scheie. 2002. Functional variation of the antigen I/II surface protein in Streptococcus mutans and Streptococcus intermedius. Infect. Immun. 70249-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Petersen, F. C., S. Pasco, J. Ogier, J. P. Klein, S. Assev, and A. A. Scheie. 2001. Expression and functional properties of the Streptococcus intermedius surface protein antigen I/II. Infect. Immun. 694647-4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Petersen, F. C., D. Pecharki, and A. A. Scheie. 2004. Biofilm mode of growth of Streptococcus intermedius favored by a competence-stimulating signaling peptide. J. Bacteriol. 1866327-6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Petersen, F. C., L. Tao, and A. A. Scheie. 2005. DNA binding-uptake system: a link between cell-to-cell communication and biofilm formation. J. Bacteriol. 1874392-4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Pezzicoli, A., I. Santi, P. Lauer, R. Rosini, D. Rinaudo, G. Grandi, J. L. Telford, and M. Soriani. 2008. Pilus backbone contributes to group B Streptococcus paracellular translocation through epithelial cells. J. Infect. Dis. 198890-898. [DOI] [PubMed] [Google Scholar]
- 460.Plummer, C., H. Wu, S. W. Kerrigan, G. Meade, D. Cox, and C. W. I. Douglas. 2005. A serine-rich glycoprotein of Streptococcus sanguis mediates adhesion to platelets via GPIb. Br. J. Haematol. 129101-109. [DOI] [PubMed] [Google Scholar]
- 461.Podbielski, A., B. Pohl, M. Woischnik, C. Korner, K. H. Schmidt, E. Rozdzinski, and B. A. Leonard. 1996. Molecular characterization of group A streptococcal (GAS) oligopeptide permease (opp) and its effect on cysteine protease production. Mol. Microbiol. 211087-1099. [DOI] [PubMed] [Google Scholar]
- 462.Podbielski, A., M. Woischnik, B. A. Leonard, and K. H. Schmidt. 1999. Characterization of nra, a global negative regulator gene in group A streptococci. Mol. Microbiol. 311051-1064. [DOI] [PubMed] [Google Scholar]
- 463.Poquet, I., V. Saint, E. Seznec, N. Simoes, A. Bolotin, and A. Gruss. 2000. HtrA is the unique surface housekeeping protease in Lactococcus lactis and is required for natural protein processing. Mol. Microbiol. 351042-1051. [DOI] [PubMed] [Google Scholar]
- 464.Poyart, C., M. C. Lamy, C. Boumaila, F. Fiedler, and P. Trieu-Cuot. 2001. Regulation of d-alanyl-lipoteichoic acid biosynthesis in Streptococcus agalactiae involves a novel two-component regulatory system. J. Bacteriol. 1836324-6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Pracht, D., C. Elm, J. Gerber, S. Bergmann, M. Rohde, M. Seiler, K. S. Kim, H. F. Jenkinson, R. Nau, and S. Hammerschmidt. 2005. PavA of Streptococcus pneumoniae modulates adherence, invasion, and meningeal inflammation. Infect. Immun. 732680-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Prakobphol, A., F. Xu, V. M. Hoang, T. Larsson, J. Bergstrom, I. Johansson, L. Frangsmyr, U. Holmskov, H. Leffler, C. Nilsson, T. Boren, J. R. Wright, N. Stromberg, and S. J. Fisher. 2000. Salivary agglutinin, which binds Streptococcus mutans and Helicobacter pylori, is the lung scavenger receptor cysteine-rich protein gp-340. J. Biol. Chem. 27539860-39866. [DOI] [PubMed] [Google Scholar]
- 467.Pramanik, A., and V. Braun. 2006. Albomycin uptake via a ferric hydroxamate transport system of Streptococcus pneumoniae R6. J. Bacteriol. 1883878-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Pritzlaff, C. A., J. C. Chang, S. P. Kuo, G. S. Tamura, C. E. Rubens, and V. Nizet. 2001. Genetic basis for the beta-haemolytic/cytolytic activity of group B Streptococcus. Mol. Microbiol. 39236-247. [DOI] [PubMed] [Google Scholar]
- 469.Proft, T., and E. N. Baker. 2009. Pili in gram-negative and gram-positive bacteria—structure, assembly and their role in disease. Cell. Mol. Life Sci. 66613-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Proft, T., S. L. Moffatt, C. J. Berkahn, and J. D. Fraser. 1999. Identification and characterization of novel superantigens from Streptococcus pyogenes. J. Exp. Med. 18989-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Purushothaman, S. S., H. S. Park, and P. P. Cleary. 2004. Promotion of fibronectin independent invasion by C5a peptidase into epithelial cells in group A Streptococcus. Indian J. Med. Res. 11944-47. [PubMed] [Google Scholar]
- 472.Purushothaman, S. S., B. Wang, and P. P. Cleary. 2003. M1 protein triggers a phosphoinositide cascade for group A Streptococcus invasion of epithelial cells. Infect. Immun. 715823-5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Puyet, A., A. M. Ibanez, and M. Espinosa. 1993. Characterization of the Streptococcus pneumoniae maltosaccharide regulator MalR, a member of the LacI-GalR family of repressors displaying distinctive genetic features. J. Biol. Chem. 26825402-25408. [PubMed] [Google Scholar]
- 474.Qi, F., P. Chen, and P. W. Caufield. 1999. Functional analyses of the promoters in the lantibiotic mutacin II biosynthetic locus in Streptococcus mutans. Appl. Environ. Microbiol. 65652-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Qi, F., J. Kreth, C. M. Levesque, O. Kay, R. W. Mair, W. Shi, D. G. Cvitkovitch, and S. D. Goodman. 2005. Peptide pheromone induced cell death of Streptococcus mutans. FEMS Microbiol. Lett. 251321-326. [DOI] [PubMed] [Google Scholar]
- 476.Qi, F., J. Merritt, R. Lux, and W. Shi. 2004. Inactivation of the ciaH gene in Streptococcus mutans diminishes mutacin production and competence development, alters sucrose-dependent biofilm formation, and reduces stress tolerance. Infect. Immun. 724895-4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Rajagopal, L., A. Clancy, and C. E. Rubens. 2003. A eukaryotic-type serine/threonine kinase and phosphatase in Streptococcus agalactiae reversibly phosphorylate an inorganic pyrophosphatase and affect growth, cell segregation, and virulence. J. Biol. Chem. 27814429-14441. [DOI] [PubMed] [Google Scholar]
- 478.Rajagopal, L., A. Vo, A. Silvestroni, and C. E. Rubens. 2006. Regulation of cytotoxin expression by converging eukaryotic-type and two-component signalling mechanisms in Streptococcus agalactiae. Mol. Microbiol. 62941-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Rakonjac, J. V., J. C. Robbins, and V. A. Fischetti. 1995. DNA sequence of the serum opacity factor of group A streptococci: identification of a fibronectin-binding repeat domain. Infect. Immun. 63622-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Ramachandran, V., J. D. McArthur, C. E. Behm, C. Gutzeit, M. Dowton, P. K. Fagan, R. Towers, B. Currie, K. S. Sriprakash, and M. J. Walker. 2004. Two distinct genotypes of prtF2, encoding a fibronectin binding protein, and evolution of the gene family in Streptococcus pyogenes. J. Bacteriol. 1867601-7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Ramsland, P. A., N. Willoughby, H. M. Trist, W. Farrugia, P. M. Hogarth, J. D. Fraser, and B. D. Wines. 2007. Structural basis for evasion of IgA immunity by Staphylococcus aureus revealed in the complex of SSL7 with Fc of human IgA1. Proc. Natl. Acad. Sci. USA 10415051-15056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Rapport, M. M., A. Linker, and K. Meyer. 1951. The hydrolysis of hyaluronic acid by pneumococcal hyaluronidase. J. Biol. Chem. 192283-291. [PubMed] [Google Scholar]
- 483.Rasmussen, M., and L. Bjorck. 2001. Unique regulation of SclB—a novel collagen-like surface protein of Streptococcus pyogenes. Mol. Microbiol. 401427-1438. [DOI] [PubMed] [Google Scholar]
- 484.Rasmussen, M., A. Eden, and L. Bjorck. 2000. SclA, a novel collagen-like surface protein of Streptococcus pyogenes. Infect. Immun. 686370-6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Rasmussen, M., H. P. Muller, and L. Bjorck. 1999. Protein GRAB of Streptococcus pyogenes regulates proteolysis at the bacterial surface by binding α2-macroglobulin. J. Biol. Chem. 27415336-15344. [DOI] [PubMed] [Google Scholar]
- 486.Rasmussen, T. B., and M. Givskov. 2006. Quorum sensing inhibitors: a bargain of effects. Microbiology 152895-904. [DOI] [PubMed] [Google Scholar]
- 487.Rathsam, C., P. M. Giffard, and N. A. Jacques. 1993. The cell-bound fructosyltransferase of Streptococcus salivarius: the carboxyl terminus specifies attachment in a Streptococcus gordonii model system. J. Bacteriol. 1754520-4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Ray, C., J. R. Brown, and B. B. Akhremitchev. 2006. Single-molecule force spectroscopy measurements of “hydrophobic bond” between tethered hexadecane molecules. J. Phys. Chem. B 11017578-17583. [DOI] [PubMed] [Google Scholar]
- 489.Ray, C. A., L. E. Gfell, T. L. Buller, and R. L. Gregory. 1999. Interactions of Streptococcus mutans fimbria-associated surface proteins with salivary components. Clin. Diagn. Lab. Immunol. 6400-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Raz, A., and V. A. Fischetti. 2008. Sortase A localizes to distinct foci on the Streptococcus pyogenes membrane. Proc. Natl. Acad. Sci. USA 10518549-18554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Reichmann, P., and R. Hakenbeck. 2000. Allelic variation in a peptide-inducible two-component system of Streptococcus pneumoniae. FEMS Microbiol. Lett. 190231-236. [DOI] [PubMed] [Google Scholar]
- 492.Reid, S. D., M. S. Chaussee, C. D. Doern, M. A. Chaussee, A. G. Montgomery, D. E. Sturdevant, and J. M. Musser. 2006. Inactivation of the group A Streptococcus regulator srv results in chromosome wide reduction of transcript levels, and changes in extracellular levels of Sic and SpeB. FEMS Immunol. Med. Microbiol. 48283-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Reid, S. D., A. G. Montgomery, and J. M. Musser. 2004. Identification of srv, a PrfA-like regulator of group A Streptococcus that influences virulence. Infect. Immun. 721799-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Reyes, A., A. Leiva, V. Cambiazo, M. A. Mendez, and M. Gonzalez. 2006. Cop-like operon: structure and organization in species of the Lactobacillale order. Biol. Res. 3987-93. [DOI] [PubMed] [Google Scholar]
- 495.Riani, C., K. Standar, S. Srimuang, C. Lembke, B. Kreikemeyer, and A. Podbielski. 2007. Transcriptome analyses extend understanding of Streptococcus pyogenes regulatory mechanisms and behavior toward immunomodulatory substances. Int. J. Med. Microbiol. 297513-523. [DOI] [PubMed] [Google Scholar]
- 496.Ricci, S., R. Janulczyk, and L. Bjorck. 2002. The regulator PerR is involved in oxidative stress response and iron homeostasis and is necessary for full virulence of Streptococcus pyogenes. Infect. Immun. 704968-4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Rickard, A. H., S. R. Campagna, and P. E. Kolenbrander. 2008. Autoinducer-2 is produced in saliva-fed flow conditions relevant to natural oral biofilms. J. Appl. Microbiol. 1052096-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Rickard, A. H., R. J. Palmer, Jr., D. S. Blehert, S. R. Campagna, M. F. Semmelhack, P. G. Egland, B. L. Bassler, and P. E. Kolenbrander. 2006. Autoinducer 2: a concentration-dependent signal for mutualistic bacterial biofilm growth. Mol. Microbiol. 601446-1456. [DOI] [PubMed] [Google Scholar]
- 499.Rigel, N. W., and M. Braunstein. 2008. A new twist on an old pathway—accessory Sec systems. Mol. Microbiol. 69291-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Ringdahl, U., and U. Sjobring. 2000. Analysis of plasminogen-binding M proteins of Streptococcus pyogenes. Methods 21143-150. [DOI] [PubMed] [Google Scholar]
- 501.Rogers, J. D., E. M. Haase, A. E. Brown, C. W. Douglas, J. P. Gwynn, and F. A. Scannapieco. 1998. Identification and analysis of a gene (abpA) encoding a major amylase-binding protein in Streptococcus gordonii. Microbiology 1441223-1233. [DOI] [PubMed] [Google Scholar]
- 502.Rogers, J. D., and F. A. Scannapieco. 2001. RegG, a CcpA homolog, participates in regulation of amylase-binding protein A gene (abpA) expression in Streptococcus gordonii. J. Bacteriol. 1833521-3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Rolerson, E., A. Swick, L. Newlon, C. Palmer, Y. Pan, B. Keeshan, and G. Spatafora. 2006. The SloR/Dlg metalloregulator modulates Streptococcus mutans virulence gene expression. J. Bacteriol. 1885033-5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Romero-Steiner, S., T. Pilishvili, J. S. Sampson, S. E. Johnson, A. Stinson, G. M. Carlone, and E. W. Ades. 2003. Inhibition of pneumococcal adherence to human nasopharyngeal epithelial cells by anti-PsaA antibodies. Clin. Diagn. Lab. Immunol. 10246-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Rosan, B. 1973. Antigens of Streptococcus sanguis. Infect. Immun. 7205-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Rosch, J., and M. Caparon. 2004. A microdomain for protein secretion in gram-positive bacteria. Science 3041513-1515. [DOI] [PubMed] [Google Scholar]
- 507.Rosch, J. W., F. F. Hsu, and M. G. Caparon. 2007. Anionic lipids enriched at the ExPortal of Streptococcus pyogenes. J. Bacteriol. 189801-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Rosch, J. W., B. Mann, J. Thornton, J. Sublett, and E. Tuomanen. 2008. Convergence of regulatory networks on the pilus locus of Streptococcus pneumoniae. Infect. Immun. 763187-3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Rose, L., P. Shivshankar, E. Hinojosa, A. Rodriguez, C. J. Sanchez, and C. J. Orihuela. 2008. Antibodies against PsrP, a novel Streptococcus pneumoniae adhesin, block adhesion and protect mice against pneumococcal challenge. J. Infect. Dis. 198375-383. [DOI] [PubMed] [Google Scholar]
- 510.Rosenow, C., P. Ryan, J. N. Weiser, S. Johnson, P. Fontan, A. Ortqvist, and H. R. Masure. 1997. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol. Microbiol. 25819-829. [DOI] [PubMed] [Google Scholar]
- 511.Rosey, E. L., and G. C. Stewart. 1992. Nucleotide and deduced amino acid sequences of the lacR, lacABCD, and lacFE genes encoding the repressor, tagatose 6-phosphate gene cluster, and sugar-specific phosphotransferase system components of the lactose operon of Streptococcus mutans. J. Bacteriol. 1746159-6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Rosini, R., C. D. Rinaudo, M. Soriani, P. Lauer, M. Mora, D. Maione, A. Taddei, I. Santi, C. Ghezzo, C. Brettoni, S. Buccato, I. Margarit, G. Grandi, and J. L. Telford. 2006. Identification of novel genomic islands coding for antigenic pilus-like structures in Streptococcus agalactiae. Mol. Microbiol. 61126-141. [DOI] [PubMed] [Google Scholar]
- 513.Ross, K. F., C. W. Ronson, and J. R. Tagg. 1993. Isolation and characterization of the lantibiotic salivaricin A and its structural gene salA from Streptococcus salivarius 20P3. Appl. Environ. Microbiol. 592014-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Rudney, J. D., R. Chen, and G. Zhang. 2005. Streptococci dominate the diverse flora within buccal cells. J. Dent. Res. 841165-1171. [DOI] [PubMed] [Google Scholar]
- 515.Russell, M. W., L. A. Bergmeier, E. D. Zanders, and T. Lehner. 1980. Protein antigens of Streptococcus mutans: purification and properties of a double antigen and its protease-resistant component. Infect. Immun. 28486-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Russell, M. W., and B. Mansson-Rahemtulla. 1989. Interaction between surface protein antigens of Streptococcus mutans and human salivary components. Oral Microbiol. Immunol. 4106-111. [DOI] [PubMed] [Google Scholar]
- 517.Russell, R. R. 1979. Glucan-binding proteins of Streptococcus mutans serotype c. J. Gen. Microbiol. 112197-201. [DOI] [PubMed] [Google Scholar]
- 518.Russell, R. R., J. Aduse-Opoku, I. C. Sutcliffe, L. Tao, and J. J. Ferretti. 1992. A binding protein-dependent transport system in Streptococcus mutans responsible for multiple sugar metabolism. J. Biol. Chem. 2674631-4637. [PubMed] [Google Scholar]
- 519.Russell, R. R., M. L. Gilpin, H. Mukasa, and G. Dougan. 1987. Characterization of glucosyltransferase expressed from a Streptococcus sobrinus gene cloned in Escherichia coli. J. Gen. Microbiol. 133935-944. [DOI] [PubMed] [Google Scholar]
- 520.Samen, U., B. J. Eikmanns, D. J. Reinscheid, and F. Borges. 2007. The surface protein Srr-1 of Streptococcus agalactiae binds human keratin 4 and promotes adherence to epithelial HEp-2 cells. Infect. Immun. 755405-5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Samen, U. M., B. J. Eikmanns, and D. J. Reinscheid. 2006. The transcriptional regulator RovS controls the attachment of Streptococcus agalactiae to human epithelial cells and the expression of virulence genes. Infect. Immun. 745625-5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Sampson, J. S., S. P. O'Connor, A. R. Stinson, J. A. Tharpe, and H. Russell. 1994. Cloning and nucleotide sequence analysis of psaA, the Streptococcus pneumoniae gene encoding a 37-kilodalton protein homologous to previously reported Streptococcus sp. adhesins. Infect. Immun. 62319-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Santi, I., A. Pezzicoli, M. Bosello, F. Berti, M. Mariani, J. L. Telford, G. Grandi, and M. Soriani. 2008. Functional characterization of a newly identified group B Streptococcus pullulanase eliciting antibodies able to prevent α-glucans degradation. PLoS ONE 3e3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Santi, I., M. Scarselli, M. Mariani, A. Pezzicoli, V. Masignani, A. Taddei, G. Grandi, J. L. Telford, and M. Soriani. 2007. BibA: a novel immunogenic bacterial adhesin contributing to group B Streptococcus survival in human blood. Mol. Microbiol. 63754-767. [DOI] [PubMed] [Google Scholar]
- 525.Sato, Y., K. Okamoto, A. Kagami, Y. Yamamoto, T. Igarashi, and H. Kizaki. 2004. Streptococcus mutans strains harboring collagen-binding adhesin. J. Dent. Res. 83534-539. [DOI] [PubMed] [Google Scholar]
- 526.Sato, Y., Y. Yamamoto, and H. Kizaki. 2000. Construction of region-specific partial duplication mutants (merodiploid mutants) to identify the regulatory gene for the glucan-binding protein C gene in vivo in Streptococcus mutans. FEMS Microbiol. Lett. 186187-191. [DOI] [PubMed] [Google Scholar]
- 527.Sato, Y., Y. Yamamoto, and H. Kizaki. 1997. Cloning and sequence analysis of the gbpC gene encoding a novel glucan-binding protein of Streptococcus mutans. Infect. Immun. 65668-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Savkur, R. S., and T. P. Burris. 2004. The coactivator LXXLL nuclear receptor recognition motif. J. Pept. Res. 63207-212. [DOI] [PubMed] [Google Scholar]
- 529.Scannapieco, F. A. 1994. Saliva-bacterium interactions in oral microbial ecology. Crit. Rev. Oral Biol. Med. 5203-248. [DOI] [PubMed] [Google Scholar]
- 530.Schachtele, C. F., A. Nobbs, Y. Zhang, M. Costalonga, and M. C. Herzberg. 2007. Oral streptococci: commensals and opportunistic pathogens, p. 411-462. In R. M. Hakenbeck and S. Chhatwal (ed.), Molecular biology of streptococci. Horizon Bioscience, Norwich, United Kingdom.
- 531.Schenkels, L. C., A. J. Ligtenberg, E. C. Veerman, and A. Van Nieuw Amerongen. 1993. Interaction of the salivary glycoprotein EP-GP with the bacterium Streptococcus salivarius HB. J. Dent. Res. 721559-1565. [DOI] [PubMed] [Google Scholar]
- 532.Schroder, N. W., S. Morath, C. Alexander, L. Hamann, T. Hartung, U. Zahringer, U. B. Gobel, J. R. Weber, and R. R. Schumann. 2003. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J. Biol. Chem. 27815587-15594. [DOI] [PubMed] [Google Scholar]
- 533.Schroeder, V. A., S. M. Michalek, and F. L. Macrina. 1989. Biochemical characterization and evaluation of virulence of a fructosyltransferase-deficient mutant of Streptococcus mutans V403. Infect. Immun. 573560-3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Schubert, A., K. Zakikhany, G. Pietrocola, A. Meinke, P. Speziale, B. J. Eikmanns, and D. J. Reinscheid. 2004. The fibrinogen receptor FbsA promotes adherence of Streptococcus agalactiae to human epithelial cells. Infect. Immun. 726197-6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Schubert, A., K. Zakikhany, M. Schreiner, R. Frank, B. Spellerberg, B. J. Eikmanns, and D. J. Reinscheid. 2002. A fibrinogen receptor from group B Streptococcus interacts with fibrinogen by repetitive units with novel ligand binding sites. Mol. Microbiol. 46557-569. [DOI] [PubMed] [Google Scholar]
- 536.Schwandner, R., R. Dziarski, H. Wesche, M. Rothe, and C. J. Kirschning. 1999. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by Toll-like receptor 2. J. Biol. Chem. 27417406-17409. [DOI] [PubMed] [Google Scholar]
- 537.Sebert, M. E., L. M. Palmer, M. Rosenberg, and J. N. Weiser. 2002. Microarray-based identification of htrA, a Streptococcus pneumoniae gene that is regulated by the CiaRH two-component system and contributes to nasopharyngeal colonization. Infect. Immun. 704059-4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Seepersaud, R., S. B. Hanniffy, P. Mayne, P. Sizer, R. Le Page, and J. M. Wells. 2005. Characterization of a novel leucine-rich repeat protein antigen from group B streptococci that elicits protective immunity. Infect. Immun. 731671-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Seifert, K. N., E. E. Adderson, A. A. Whiting, J. F. Bohnsack, P. J. Crowley, and L. J. Brady. 2006. A unique serine-rich repeat protein (Srr-2) and novel surface antigen (epsilon) associated with a virulent lineage of serotype III Streptococcus agalactiae. Microbiology 1521029-1040. [DOI] [PubMed] [Google Scholar]
- 540.Seifert, K. N., W. P. McArthur, A. S. Bleiweis, and L. J. Brady. 2003. Characterization of group B streptococcal glyceraldehyde-3-phosphate dehydrogenase: surface localization, enzymatic activity, and protein-protein interactions. Can. J. Microbiol. 49350-356. [DOI] [PubMed] [Google Scholar]
- 541.Seifert, T. B., A. S. Bleiweis, and L. J. Brady. 2004. Contribution of the alanine-rich region of Streptococcus mutans P1 to antigenicity, surface expression, and interaction with the proline-rich repeat domain. Infect. Immun. 724699-4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Senadheera, D., and D. G. Cvitkovitch. 2008. Quorum sensing and biofilm formation by Streptococcus mutans. Adv. Exp. Med. Biol. 631178-188. [DOI] [PubMed] [Google Scholar]
- 543.Senadheera, M. D., B. Guggenheim, G. A. Spatafora, Y.-C. C. Huang, J. Choi, D. C. I. Hung, J. S. Treglown, S. D. Goodman, R. P. Ellen, and D. G. Cvitkovitch. 2005. A VicRK signal transduction system in Streptococcus mutans affects gtfBCD, gbpB, and ftf expression, biofilm formation, and genetic competence development. J. Bacteriol. 1874064-4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Shah, D. S., and R. R. Russell. 2004. A novel glucan-binding protein with lipase activity from the oral pathogen Streptococcus mutans. Microbiology 1501947-1956. [DOI] [PubMed] [Google Scholar]
- 545.Shannon, O., E. Hertzen, A. Norrby-Teglund, M. Morgelin, U. Sjobring, and L. Bjorck. 2007. Severe streptococcal infection is associated with M protein-induced platelet activation and thrombus formation. Mol. Microbiol. 651147-1157. [DOI] [PubMed] [Google Scholar]
- 546.Shao, H., D. James, R. J. Lamont, and D. R. Demuth. 2007. Differential interaction of Aggregatibacter (Actinobacillus) actinomycetemcomitans LsrB and RbsB proteins with autoinducer 2. J. Bacteriol. 1895559-5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Shelburne, S. A., III, D. Keith, N. Horstmann, P. Sumby, M. T. Davenport, E. A. Graviss, R. G. Brennan, and J. M. Musser. 2008. A direct link between carbohydrate utilization and virulence in the major human pathogen group A Streptococcus. Proc. Natl. Acad. Sci. USA 1051698-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Shelburne, S. A., III, D. B. Keith, M. T. Davenport, N. Horstmann, R. G. Brennan, and J. M. Musser. 2008. Molecular characterization of group A Streptococcus maltodextrin catabolism and its role in pharyngitis. Mol. Microbiol. 69436-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Shelburne, S. A., III, P. Sumby, I. Sitkiewicz, C. Granville, F. R. DeLeo, and J. M. Musser. 2005. Central role of a bacterial two-component gene regulatory system of previously unknown function in pathogen persistence in human saliva. Proc. Natl. Acad. Sci. USA 10216037-16042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Shelver, D., L. Rajagopal, T. O. Harris, and C. E. Rubens. 2003. MtaR, a regulator of methionine transport, is critical for survival of group B Streptococcus in vivo. J. Bacteriol. 1856592-6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Shibata, Y., and H. K. Kuramitsu. 1996. Identification of the Streptococcus mutans frp gene as a potential regulator of fructosyltransferase expression. FEMS Microbiol. Lett. 14049-54. [DOI] [PubMed] [Google Scholar]
- 552.Simionato, M. R., C. M. Tucker, M. Kuboniwa, G. Lamont, D. R. Demuth, G. D. Tribble, and R. J. Lamont. 2006. Porphyromonas gingivalis genes involved in community development with Streptococcus gordonii. Infect. Immun. 746419-6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Simpson, C. L., P. M. Giffard, and N. A. Jacques. 1995. Streptococcus salivarius ATCC 25975 possesses at least two genes coding for primer-independent glucosyltransferases. Infect. Immun. 63609-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Simpson, C. L., and R. R. Russell. 1998. Identification of a homolog of CcpA catabolite repressor protein in Streptococcus mutans. Infect. Immun. 662085-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Simpson, W. J., N. L. Ragland, C. W. Ronson, and J. R. Tagg. 1995. A lantibiotic gene family widely distributed in Streptococcus salivarius and Streptococcus pyogenes. Dev. Biol. Stand. 85639-643. [PubMed] [Google Scholar]
- 556.Smith, B. L., A. Flores, J. Dechaine, J. Krepela, A. Bergdall, and P. Ferrieri. 2004. Gene encoding the group B streptococcal protein R4, its presence in clinical reference laboratory isolates & R4 protein pepsin sensitivity. Indian J. Med. Res. 119213-220. [PubMed] [Google Scholar]
- 557.Smith, D. J. 2002. Dental caries vaccines: prospects and concerns. Crit. Rev. Oral Biol. Med. 13335-349. [DOI] [PubMed] [Google Scholar]
- 558.Smith, D. J., H. Akita, W. F. King, and M. A. Taubman. 1994. Purification and antigenicity of a novel glucan-binding protein of Streptococcus mutans. Infect. Immun. 622545-2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Smith, D. J., W. F. King, C. D. Wu, B. I. Shen, and M. A. Taubman. 1998. Structural and antigenic characteristics of Streptococcus sobrinus glucan binding proteins. Infect. Immun. 665565-5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Smith, D. J., and R. O. Mattos-Graner. 2008. Secretory immunity following mutans streptococcal infection or immunization. Curr. Top. Microbiol. Immunol. 319131-156. [DOI] [PubMed] [Google Scholar]
- 561.Smith, M. W., J. E. Schmidt, J. E. Rehg, C. J. Orihuela, and J. A. McCullers. 2007. Induction of pro- and anti-inflammatory molecules in a mouse model of pneumococcal pneumonia after influenza. Comp. Med. 5782-89. [PMC free article] [PubMed] [Google Scholar]
- 562.Smoot, L. M., J. C. Smoot, M. R. Graham, G. A. Somerville, D. E. Sturdevant, C. A. Migliaccio, G. L. Sylva, and J. M. Musser. 2001. Global differential gene expression in response to growth temperature alteration in group A Streptococcus. Proc. Natl. Acad. Sci. USA 9810416-10421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Sommer, P., C. Gleyzal, S. Guerret, J. Etienne, and J. A. Grimaud. 1992. Induction of a putative laminin-binding protein of Streptococcus gordonii in human infective endocarditis. Infect. Immun. 60360-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Soriani, M., I. Santi, A. Taddei, R. Rappuoli, G. Grandi, and J. L. Telford. 2006. Group B Streptococcus crosses human epithelial cells by a paracellular route. J. Infect. Dis. 193241-250. [DOI] [PubMed] [Google Scholar]
- 565.Spanier, J. G., S. J. Jones, and P. Cleary. 1984. Small DNA deletions creating avirulence in Streptococcus pyogenes. Science 225935-938. [DOI] [PubMed] [Google Scholar]
- 566.Spellerberg, B., E. Rozdzinski, S. Martin, J. Weber-Heynemann, and R. Lutticken. 2002. rgf encodes a novel two-component signal transduction system of Streptococcus agalactiae. Infect. Immun. 702434-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567.Spellerberg, B., E. Rozdzinski, S. Martin, J. Weber-Heynemann, N. Schnitzler, R. Lutticken, and A. Podbielski. 1999. Lmb, a protein with similarities to the LraI adhesin family, mediates attachment of Streptococcus agalactiae to human laminin. Infect. Immun. 67871-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.Sperandio, B., C. Gautier, S. McGovern, D. S. Ehrlich, P. Renault, I. Martin-Verstraete, and E. Guedon. 2007. Control of methionine synthesis and uptake by MetR and homocysteine in Streptococcus mutans. J. Bacteriol. 1897032-7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Sperandio, V., A. G. Torres, B. Jarvis, J. P. Nataro, and J. B. Kaper. 2003. Bacteria-host communication: the language of hormones. Proc. Natl. Acad. Sci. USA 1008951-8956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Spoering, A. L., and M. S. Gilmore. 2006. Quorum sensing and DNA release in bacterial biofilms. Curr. Opin. Microbiol. 9133-137. [DOI] [PubMed] [Google Scholar]
- 571.Stalhammar-Carlemalm, M., T. Areschoug, C. Larsson, and G. Lindahl. 1999. The R28 protein of Streptococcus pyogenes is related to several group B streptococcal surface proteins, confers protective immunity and promotes binding to human epithelial cells. Mol. Microbiol. 33208-219. [DOI] [PubMed] [Google Scholar]
- 572.Stalhammar-Carlemalm, M., L. Stenberg, and G. Lindahl. 1993. Protein Rib: a novel group B streptococcal cell surface protein that confers protective immunity and is expressed by most strains causing invasive infections. J. Exp. Med. 1771593-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Standish, A. J., U. H. Stroeher, and J. C. Paton. 2005. The two-component signal transduction system RR06/HK06 regulates expression of cbpA in Streptococcus pneumoniae. Proc. Natl. Acad. Sci. USA 1027701-7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Standish, A. J., U. H. Stroeher, and J. C. Paton. 2007. The pneumococcal two-component signal transduction system RR/HK06 regulates CbpA and PspA by two distinct mechanisms. J. Bacteriol. 1895591-5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Steiner, K., and H. Malke. 2002. Dual control of streptokinase and streptolysin S production by the covRS and fasCAX two-component regulators in Streptococcus dysgalactiae subsp. equisimilis. Infect. Immun. 703627-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Stenberg, L., P. O'Toole, and G. Lindahl. 1992. Many group A streptococcal strains express two different immunoglobulin-binding proteins, encoded by closely linked genes: characterization of the proteins expressed by four strains of different M-type. Mol. Microbiol. 61185-1194. [DOI] [PubMed] [Google Scholar]
- 577.Stenberg, L., P. W. O'Toole, J. Mestecky, and G. Lindahl. 1994. Molecular characterization of protein Sir, a streptococcal cell surface protein that binds both immunoglobulin A and immunoglobulin G. J. Biol. Chem. 26913458-13464. [PubMed] [Google Scholar]
- 578.Sulavik, M. C., and D. B. Clewell. 1996. Rgg is a positive transcriptional regulator of the Streptococcus gordonii gtfG gene. J. Bacteriol. 1785826-5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Sulavik, M. C., G. Tardif, and D. B. Clewell. 1992. Identification of a gene, rgg, which regulates expression of glucosyltransferase and influences the Spp phenotype of Streptococcus gordonii Challis. J. Bacteriol. 1743577-3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Sun, J. W., S. Y. Wanda, A. Camilli, and R. Curtiss III. 1994. Cloning and DNA sequencing of the dextranase inhibitor gene (dei) from Streptococcus sobrinus. J. Bacteriol. 1767213-7222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581.Suntharalingam, P., and D. G. Cvitkovitch. 2005. Quorum sensing in streptococcal biofilm formation. Trends Microbiol. 133-6. [DOI] [PubMed] [Google Scholar]
- 582.Surette, M. G., M. B. Miller, and B. L. Bassler. 1999. Quorum sensing in Escherichia coli, Salmonella typhimurium, and Vibrio harveyi: a new family of genes responsible for autoinducer production. Proc. Natl. Acad. Sci. USA 961639-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Suvorov, A. N., A. E. Flores, and P. Ferrieri. 1997. Cloning of the glutamine synthetase gene from group B streptococci. Infect. Immun. 65191-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Switalski, L. M., H. Murchison, R. Timpl, R. Curtiss III, and M. Hook. 1987. Binding of laminin to oral and endocarditis strains of viridans streptococci. J. Bacteriol. 1691095-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Syvitski, R. T., X. L. Tian, K. Sampara, A. Salman, S. F. Lee, D. L. Jakeman, and Y. H. Li. 2007. Structure-activity analysis of quorum-sensing signaling peptides from Streptococcus mutans. J. Bacteriol. 1891441-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Taga, M. E., S. T. Miller, and B. L. Bassler. 2003. Lsr-mediated transport and processing of AI-2 in Salmonella typhimurium. Mol. Microbiol. 501411-1427. [DOI] [PubMed] [Google Scholar]
- 587.Takahashi, Y., K. Konishi, J. O. Cisar, and M. Yoshikawa. 2002. Identification and characterization of hsa, the gene encoding the sialic acid-binding adhesin of Streptococcus gordonii DL1. Infect. Immun. 701209-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588.Takahashi, Y., A. L. Sandberg, S. Ruhl, J. Muller, and J. O. Cisar. 1997. A specific cell surface antigen of Streptococcus gordonii is associated with bacterial hemagglutination and adhesion to α2-3-linked sialic acid-containing receptors. Infect. Immun. 655042-5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.Takahashi, Y., A. Yajima, J. O. Cisar, and K. Konishi. 2004. Functional analysis of the Streptococcus gordonii DL1 sialic acid-binding adhesin and its essential role in bacterial binding to platelets. Infect. Immun. 723876-3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Takamatsu, D., B. A. Bensing, H. Cheng, G. A. Jarvis, I. R. Siboo, J. A. Lopez, J. M. Griffiss, and P. M. Sullam. 2005. Binding of the Streptococcus gordonii surface glycoproteins GspB and Hsa to specific carbohydrate structures on platelet membrane glycoprotein Ibα. Mol. Microbiol. 58380-392. [DOI] [PubMed] [Google Scholar]
- 591.Talay, S. R., P. Valentin-Weigand, P. G. Jerlstrom, K. N. Timmis, and G. S. Chhatwal. 1992. Fibronectin-binding protein of Streptococcus pyogenes: sequence of the binding domain involved in adherence of streptococci to epithelial cells. Infect. Immun. 603837-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Talay, S. R., P. Valentin-Weigand, K. N. Timmis, and G. S. Chhatwal. 1994. Domain structure and conserved epitopes of Sfb protein, the fibronectin-binding adhesin of Streptococcus pyogenes. Mol. Microbiol. 13531-539. [DOI] [PubMed] [Google Scholar]
- 593.Talay, S. R., A. Zock, M. Rohde, G. Molinari, M. Oggioni, G. Pozzi, C. A. Guzman, and G. S. Chhatwal. 2000. Co-operative binding of human fibronectin to Sfbl protein triggers streptococcal invasion into respiratory epithelial cells. Cell. Microbiol. 2521-535. [DOI] [PubMed] [Google Scholar]
- 594.Tamura, F., R. Nakagawa, T. Akuta, S. Okamoto, S. Hamada, H. Maeda, S. Kawabata, and T. Akaike. 2004. Proapoptotic effect of proteolytic activation of matrix metalloproteinases by Streptococcus pyogenes thiol proteinase (Streptococcus pyrogenic exotoxin B). Infect. Immun. 724836-4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Tamura, G. S., J. R. Hull, M. D. Oberg, and D. G. Castner. 2006. High-affinity interaction between fibronectin and the group B streptococcal C5a peptidase is unaffected by a naturally occurring four-amino-acid deletion that eliminates peptidase activity. Infect. Immun. 745739-5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Tamura, H., T. Kikuchi, R. Shirato, and H. Kato. 2001. Cloning and DNA sequencing of the surface protein antigen I/II (PAa) of Streptococcus cricetus. FEMS Microbiol. Lett. 196251-256. [DOI] [PubMed] [Google Scholar]
- 597.Tamura, H., A. Yamada, and H. Kato. 2008. Identification and characterization of an antigen I/II homologous gene, pah, from Streptococcus downei. Curr. Microbiol. 56518-523. [DOI] [PubMed] [Google Scholar]
- 598.Tamura, H., A. Yamada, H. Saito, S. Murai, and H. Kato. 2004. Identification of another surface protein antigen I/II gene, paaB, and a putative transcriptional regulator gene, par, from Streptococcus cricetus. Genes Genet. Syst. 79129-137. [DOI] [PubMed] [Google Scholar]
- 599.Tart, A. H., M. J. Walker, and J. M. Musser. 2007. New understanding of the group A Streptococcus pathogenesis cycle. Trends Microbiol. 15318-325. [DOI] [PubMed] [Google Scholar]
- 600.Tenenbaum, T., B. Spellerberg, R. Adam, M. Vogel, K. S. Kim, and H. Schroten. 2007. Streptococcus agalactiae invasion of human brain microvascular endothelial cells is promoted by the laminin-binding protein Lmb. Microbes Infect. 9714-720. [DOI] [PubMed] [Google Scholar]
- 601.Terao, Y., S. Kawabata, E. Kunitomo, J. Murakami, I. Nakagawa, and S. Hamada. 2001. Fba, a novel fibronectin-binding protein from Streptococcus pyogenes, promotes bacterial entry into epithelial cells, and the fba gene is positively transcribed under the Mga regulator. Mol. Microbiol. 4275-86. [DOI] [PubMed] [Google Scholar]
- 602.Terao, Y., S. Kawabata, E. Kunitomo, I. Nakagawa, and S. Hamada. 2002. Novel laminin-binding protein of Streptococcus pyogenes, Lbp, is involved in adhesion to epithelial cells. Infect. Immun. 70993-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603.Terao, Y., S. Kawabata, M. Nakata, I. Nakagawa, and S. Hamada. 2002. Molecular characterization of a novel fibronectin-binding protein of Streptococcus pyogenes strains isolated from toxic shock-like syndrome patients. J. Biol. Chem. 27747428-47435. [DOI] [PubMed] [Google Scholar]
- 604.Tettelin, H., V. Masignani, M. J. Cieslewicz, J. A. Eisen, S. Peterson, M. R. Wessels, I. T. Paulsen, K. E. Nelson, I. Margarit, T. D. Read, L. C. Madoff, A. M. Wolf, M. J. Beanan, L. M. Brinkac, S. C. Daugherty, R. T. DeBoy, A. S. Durkin, J. F. Kolonay, R. Madupu, M. R. Lewis, D. Radune, N. B. Fedorova, D. Scanlan, H. Khouri, S. Mulligan, H. A. Carty, R. T. Cline, S. E. Van Aken, J. Gill, M. Scarselli, M. Mora, E. T. Iacobini, C. Brettoni, G. Galli, M. Mariani, F. Vegni, D. Maione, D. Rinaudo, R. Rappuoli, J. L. Telford, D. L. Kasper, G. Grandi, and C. M. Fraser. 2002. Complete genome sequence and comparative genomic analysis of an emerging human pathogen, serotype V Streptococcus agalactiae. Proc. Natl. Acad. Sci. USA 9912391-12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605.Tettelin, H., K. E. Nelson, I. T. Paulsen, J. A. Eisen, T. D. Read, S. Peterson, J. Heidelberg, R. T. DeBoy, D. H. Haft, R. J. Dodson, A. S. Durkin, M. Gwinn, J. F. Kolonay, W. C. Nelson, J. D. Peterson, L. A. Umayam, O. White, S. L. Salzberg, M. R. Lewis, D. Radune, E. Holtzapple, H. Khouri, A. M. Wolf, T. R. Utterback, C. L. Hansen, L. A. McDonald, T. V. Feldblyum, S. Angiuoli, T. Dickinson, E. K. Hickey, I. E. Holt, B. J. Loftus, F. Yang, H. O. Smith, J. C. Venter, B. A. Dougherty, D. A. Morrison, S. K. Hollingshead, and C. M. Fraser. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293498-506. [DOI] [PubMed] [Google Scholar]
- 606.Thern, A., L. Stenberg, B. Dahlback, and G. Lindahl. 1995. Ig-binding surface proteins of Streptococcus pyogenes also bind human C4b-binding protein (C4BP), a regulatory component of the complement system. J. Immunol. 154375-386. [PubMed] [Google Scholar]
- 607.Throup, J. P., K. K. Koretke, A. P. Bryant, K. A. Ingraham, A. F. Chalker, Y. Ge, A. Marra, N. G. Wallis, J. R. Brown, D. J. Holmes, M. Rosenberg, and M. K. Burnham. 2000. A genomic analysis of two-component signal transduction in Streptococcus pneumoniae. Mol. Microbiol. 35566-576. [DOI] [PubMed] [Google Scholar]
- 608.Touhami, A., B. Hoffmann, A. Vasella, F. A. Denis, and Y. F. Dufrene. 2003. Aggregation of yeast cells: direct measurement of discrete lectin-carbohydrate interactions. Microbiology 1492873-2878. [DOI] [PubMed] [Google Scholar]
- 609.Touhami, A., M. H. Jericho, J. M. Boyd, and T. J. Beveridge. 2006. Nanoscale characterization and determination of adhesion forces of Pseudomonas aeruginosa pili by using atomic force microscopy. J. Bacteriol. 188370-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 610.Towers, R. J., P. K. Fagan, S. R. Talay, B. J. Currie, K. S. Sriprakash, M. J. Walker, and G. S. Chhatwal. 2003. Evolution of sfbI encoding streptococcal fibronectin-binding protein I: horizontal genetic transfer and gene mosaic structure. J. Clin. Microbiol. 415398-5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611.Troffer-Charlier, N., J. Ogier, D. Moras, and J. Cavarelli. 2002. Crystal structure of the V-region of Streptococcus mutans antigen I/II at 2.4 Å resolution suggests a sugar preformed binding site. J. Mol. Biol. 318179-188. [DOI] [PubMed] [Google Scholar]
- 612.Tsai, P.-J., Y.-S. Lin, C.-F. Kuo, H.-Y. Lei, and J.-J. Wu. 1999. Group A Streptococcus induces apoptosis in human epithelial cells. Infect. Immun. 674334-4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613.Tu, A. H., R. L. Fulgham, M. A. McCrory, D. E. Briles, and A. J. Szalai. 1999. Pneumococcal surface protein A inhibits complement activation by Streptococcus pneumoniae. Infect. Immun. 674720-4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614.Ulett, G. C., and E. E. Adderson. 2006. Regulation of apoptosis by gram-positive bacteria: mechanistic diversity and consequences for immunity. Curr. Immunol. Rev. 2119-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615.Ulijasz, A. T., D. R. Andes, J. D. Glasner, and B. Weisblum. 2004. Regulation of iron transport in Streptococcus pneumoniae by RitR, an orphan response regulator. J. Bacteriol. 1868123-8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616.Ulijasz, A. T., S. P. Falk, and B. Weisblum. 2009. Phosphorylation of the RitR DNA-binding domain by a Ser-Thr phosphokinase: implications for global gene regulation in the streptococci. Mol. Microbiol. 71382-390. [DOI] [PubMed] [Google Scholar]
- 617.Upton, M., J. R. Tagg, P. Wescombe, and H. F. Jenkinson. 2001. Intra- and interspecies signaling between Streptococcus salivarius and Streptococcus pyogenes mediated by SalA and SalA1 lantibiotic peptides. J. Bacteriol. 1833931-3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618.Vacca-Smith, A. M., C. A. Jones, M. J. Levine, and M. W. Stinson. 1994. Glucosyltransferase mediates adhesion of Streptococcus gordonii to human endothelial cells in vitro. Infect. Immun. 622187-2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.Vadillo-Rodriguez, V., T. J. Beveridge, and J. R. Dutcher. 2008. Surface viscoelasticity of individual gram-negative bacterial cells measured using atomic force microscopy. J. Bacteriol. 1904225-4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620.Valentin-Weigand, P., H. Jungnitz, A. Zock, M. Rohde, and G. S. Chhatwal. 1997. Characterization of group B streptococcal invasion in HEp-2 epithelial cells. FEMS Microbiol. Lett. 14769-74. [DOI] [PubMed] [Google Scholar]
- 621.van Bueren, A. L., M. Higgins, D. Wang, R. D. Burke, and A. B. Boraston. 2007. Identification and structural basis of binding to host lung glycogen by streptococcal virulence factors. Nat. Struct. Mol. Biol. 1476-84. [DOI] [PubMed] [Google Scholar]
- 622.van Dolleweerd, C. J., C. G. Kelly, D. Chargelegue, and J. K. Ma. 2004. Peptide mapping of a novel discontinuous epitope of the major surface adhesin from Streptococcus mutans. J. Biol. Chem. 27922198-22203. [DOI] [PubMed] [Google Scholar]
- 623.Vasi, J., L. Frykberg, L. E. Carlsson, M. Lindberg, and B. Guss. 2000. M-like proteins of Streptococcus dysgalactiae. Infect. Immun. 68294-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 624.Vats, N., and S. F. Lee. 2001. Characterization of a copper-transport operon, copYAZ, from Streptococcus mutans. Microbiology 147653-662. [DOI] [PubMed] [Google Scholar]
- 625.Vernier, A., M. Diab, M. Soell, G. Haan-Archipoff, A. Beretz, D. Wachsmann, and J. P. Klein. 1996. Cytokine production by human epithelial and endothelial cells following exposure to oral viridans streptococci involves lectin interactions between bacteria and cell surface receptors. Infect. Immun. 643016-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Vickerman, M. M., and D. B. Clewell. 1997. Deletions in the carboxyl-terminal region of Streptococcus gordonii glucosyltransferase affect cell-associated enzyme activity and sucrose-associated accumulation of growing cells. Appl. Environ. Microbiol. 631667-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627.Vickerman, M. M., M. C. Sulavik, and D. B. Clewell. 1995. Oral streptococci with genetic determinants similar to the glucosyltransferase regulatory gene, rgg. Infect. Immun. 634524-4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628.Vickerman, M. M., M. C. Sulavik, J. D. Nowak, N. M. Gardner, G. W. Jones, and D. B. Clewell. 1997. Nucleotide sequence analysis of the Streptococcus gordonii glucosyltransferase gene, gtfG. DNA Seq. 783-95. [DOI] [PubMed] [Google Scholar]
- 629.Voyich, J. M., D. E. Sturdevant, K. R. Braughton, S. D. Kobayashi, B. Lei, K. Virtaneva, D. W. Dorward, J. M. Musser, and F. R. DeLeo. 2003. Genome-wide protective response used by group A Streptococcus to evade destruction by human polymorphonuclear leukocytes. Proc. Natl. Acad. Sci. USA 1001996-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 630.Wagner, C., A. A. Saizieu, H. J. Schonfeld, M. Kamber, R. Lange, C. J. Thompson, and M. G. Page. 2002. Genetic analysis and functional characterization of the Streptococcus pneumoniae vic operon. Infect. Immun. 706121-6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 631.Wahid, R. M., M. Yoshinaga, J. Nishi, N. Maeno, J. Sarantuya, T. Ohkawa, A. M. Jalil, K. Kobayashi, and K. Miyata. 2005. Immune response to a laminin-binding protein (Lmb) in group A streptococcal infection. Pediatr. Int. 47196-202. [DOI] [PubMed] [Google Scholar]
- 632.Wanda, S. Y., and R. Curtiss III. 1994. Purification and characterization of Streptococcus sobrinus dextranase produced in recombinant Escherichia coli and sequence analysis of the dextranase gene. J. Bacteriol. 1763839-3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 633.Wang, B., and H. K. Kuramitsu. 2006. A pleiotropic regulator, Frp, affects exopolysaccharide synthesis, biofilm formation, and competence development in Streptococcus mutans. Infect. Immun. 744581-4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634.Wang, B., and H. K. Kuramitsu. 2003. Control of enzyme IIscr and sucrose-6-phosphate hydrolase activities in Streptococcus mutans by transcriptional repressor ScrR binding to the cis-active determinants of the scr regulon. J. Bacteriol. 1855791-5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 635.Wang, B., S. Li, P. J. Southern, and P. P. Cleary. 2006. Streptococcal modulation of cellular invasion via TGF-β1 signaling. Proc. Natl. Acad. Sci. USA 1032380-2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 636.Wang, B. Y., and H. K. Kuramitsu. 2005. Interactions between oral bacteria: inhibition of Streptococcus mutans bacteriocin production by Streptococcus gordonii. Appl. Environ. Microbiol. 71354-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.Wang, C.-H., C.-Y. Lin, Y.-H. Luo, P.-J. Tsai, Y.-S. Lin, M. T. Lin, W.-J. Chuang, C.-C. Liu, and J.-J. Wu. 2005. Effects of oligopeptide permease in group A streptococcal infection. Infect. Immun. 732881-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638.Warner, J. B., and J. S. Lolkema. 2003. CcpA-dependent carbon catabolite repression in bacteria. Microbiol. Mol. Biol. Rev. 67475-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639.Weerkamp, A. H., P. S. Handley, A. Baars, and J. W. Slot. 1986. Negative staining and immunoelectron microscopy of adhesion-deficient mutants of Streptococcus salivarius reveal that the adhesive protein antigens are separate classes of cell surface fibril. J. Bacteriol. 165746-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Weerkamp, A. H., H. C. van der Mei, and R. S. B. Liem. 1986. Structural properties of fibrillar proteins isolated from the cell surface and cytoplasm of Streptococcus salivarius (K+) cells and nonadhesive mutants. J. Bacteriol. 165756-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 641.Weerkamp, A. H., H. C. van der Mei, and J. W. Slot. 1987. Relationship of cell surface morphology and composition of Streptococcus salivarius K+ to adherence and hydrophobicity. Infect. Immun. 55438-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 642.Wen, Z. T., H. V. Baker, and R. A. Burne. 2006. Influence of BrpA on critical virulence attributes of Streptococcus mutans. J. Bacteriol. 1882983-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 643.Wen, Z. T., and R. A. Burne. 2002. Functional genomics approach to identifying genes required for biofilm development by Streptococcus mutans. Appl. Environ. Microbiol. 681196-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 644.Wen, Z. T., and R. A. Burne. 2004. LuxS-mediated signaling in Streptococcus mutans is involved in regulation of acid and oxidative stress tolerance and biofilm formation. J. Bacteriol. 1862682-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 645.Wexler, D. E., and P. P. Cleary. 1985. Purification and characteristics of the streptococcal chemotactic factor inactivator. Infect. Immun. 50757-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 646.Wexler, D. L., M. C. Hudson, and R. A. Burne. 1993. Streptococcus mutans fructosyltransferase (ftf) and glucosyltransferase (gtfBC) operon fusion strains in continuous culture. Infect. Immun. 611259-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 647.Whittaker, C. J., D. L. Clemans, and P. E. Kolenbrander. 1996. Insertional inactivation of an intrageneric coaggregation-relevant adhesin locus from Streptococcus gordonii DL1 (Challis). Infect. Immun. 644137-4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 648.Wilkins, J. C., D. Beighton, and K. A. Homer. 2003. Effect of acidic pH on expression of surface-associated proteins of Streptococcus oralis. Appl. Environ. Microbiol. 695290-5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 649.Wilkinson, H. W. 1972. Comparison of streptococcal R antigens. Appl. Microbiol. 24669-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 650.Willcox, M. D., and D. B. Drucker. 1989. Surface structures, co-aggregation and adherence phenomena of Streptococcus oralis and related species. Microbios 5919-29. [PubMed] [Google Scholar]
- 651.Wistedt, A. C., U. Ringdahl, W. Muller-Esterl, and U. Sjobring. 1995. Identification of a plasminogen-binding motif in PAM, a bacterial surface protein. Mol. Microbiol. 18569-578. [DOI] [PubMed] [Google Scholar]
- 652.Wu, H., and P. M. Fives-Taylor. 1999. Identification of dipeptide repeats and a cell wall sorting signal in the fimbriae-associated adhesin, Fap1, of Streptococcus parasanguis. Mol. Microbiol. 341070-1081. [DOI] [PubMed] [Google Scholar]
- 653.Wu, H., K. P. Mintz, M. Ladha, and P. M. Fives-Taylor. 1998. Isolation and characterization of Fap1, a fimbriae-associated adhesin of Streptococcus parasanguis FW213. Mol. Microbiol. 28487-500. [DOI] [PubMed] [Google Scholar]
- 654.Xavier, K. B., and B. L. Bassler. 2003. LuxS quorum sensing: more than just a numbers game. Curr. Opin. Microbiol. 6191-197. [DOI] [PubMed] [Google Scholar]
- 655.Xie, H., G. S. Cook, J. W. Costerton, G. Bruce, T. M. Rose, and R. J. Lamont. 2000. Intergeneric communication in dental plaque biofilms. J. Bacteriol. 1827067-7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 656.Xie, H., X. Lin, B. Y. Wang, J. Wu, and R. J. Lamont. 2007. Identification of a signalling molecule involved in bacterial intergeneric communication. Microbiology 1533228-3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 657.Xu, P., J. M. Alves, T. Kitten, A. Brown, Z. Chen, L. S. Ozaki, P. Manque, X. Ge, M. G. Serrano, D. Puiu, S. Hendricks, Y. Wang, M. D. Chaplin, D. Akan, S. Paik, D. L. Peterson, F. L. Macrina, and G. A. Buck. 2007. Genome of the opportunistic pathogen Streptococcus sanguinis. J. Bacteriol. 1893166-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 658.Yamaguchi, T., and N. Matsunoshita. 2004. Isolation and some properties of fimbriae of oral Streptococcus intermedius. Curr. Microbiol. 4959-65. [DOI] [PubMed] [Google Scholar]
- 659.Yamashita, Y., W. H. Bowen, R. A. Burne, and H. K. Kuramitsu. 1993. Role of the Streptococcus mutans gtf genes in caries induction in the specific-pathogen-free rat model. Infect. Immun. 613811-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 660.Yilmaz, O., L. Yao, K. Maeda, T. M. Rose, E. L. Lewis, M. Duman, R. J. Lamont, and D. M. Ojcius. 2008. ATP scavenging by the intracellular pathogen Porphyromonas gingivalis inhibits P2X7-mediated host-cell apoptosis. Cell. Microbiol. 10863-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 661.Yoshida, A., T. Ansai, T. Takehara, and H. K. Kuramitsu. 2005. LuxS-based signaling affects Streptococcus mutans biofilm formation. Appl. Environ. Microbiol. 712372-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 662.Yoshida, A., and H. K. Kuramitsu. 2002. Multiple Streptococcus mutans genes are involved in biofilm formation. Appl. Environ. Microbiol. 686283-6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 663.Zeng, L., Z. T. Wen, and R. A. Burne. 2006. A novel signal transduction system and feedback loop regulate fructan hydrolase gene expression in Streptococcus mutans. Mol. Microbiol. 62187-200. [DOI] [PubMed] [Google Scholar]
- 664.Zhang, G., R. Chen, and J. D. Rudney. 2008. Streptococcus cristatus attenuates Fusobacterium nucleatum-induced interleukin-8 expression in oral epithelial cells. J. Periodont. Res. 43408-416. [DOI] [PubMed] [Google Scholar]
- 665.Zhang, J. R., K. E. Mostov, M. E. Lamm, M. Nanno, S. Shimida, M. Ohwaki, and E. Tuomanen. 2000. The polymeric immunoglobulin receptor translocates pneumococci across human nasopharyngeal epithelial cells. Cell 102827-837. [DOI] [PubMed] [Google Scholar]
- 666.Zhang, K., M. Ou, W. Wang, and J. Ling. 2009. Effects of quorum sensing on cell viability in Streptococcus mutans biofilm formation. Biochem. Biophys. Res. Commun. 379933-938. [DOI] [PubMed] [Google Scholar]
- 667.Zhang, S., N. M. Green, I. Sitkiewicz, R. B. Lefebvre, and J. M. Musser. 2006. Identification and characterization of an antigen I/II family protein produced by group A Streptococcus. Infect. Immun. 744200-4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 668.Zhang, Y., Y. Lei, A. Khammanivong, and M. C. Herzberg. 2004. Identification of a novel two-component system in Streptococcus gordonii V288 involved in biofilm formation. Infect. Immun. 723489-3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 669.Zhang, Y., M. Whiteley, J. Kreth, Y. Lei, A. Khammanivong, J. N. Evavold, J. Fan, and M. C. Herzberg. 2009. The two-component system BfrAB regulates expression of ABC transporters in Streptococcus gordonii and Streptococcus sanguinis. Microbiology 155165-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 670.Zhou, M., and H. Wu. 2009. Glycosylation and biogenesis of a family of serine-rich bacterial adhesins. Microbiology 155317-327. [DOI] [PubMed] [Google Scholar]
- 671.Zhu, H., M. Liu, and B. Lei. 2008. The surface protein Shr of Streptococcus pyogenes binds heme and transfers it to the streptococcal heme-binding protein Shp. BMC Microbiol. 815. [DOI] [PMC free article] [PubMed] [Google Scholar]