Abstract
Persistent viral infections are a major health concern worldwide. During persistent infection, overwhelming viral replication and the rapid loss of antiviral T-cell function can prevent immune-mediated clearance of the infection, and therapies to reanimate the immune response and purge persistent viruses have been largely unsuccessful. Adoptive immunotherapy using memory T cells is a highly successful therapeutic approach to eradicate a persistent viral infection. Understanding precisely how therapeutically administered memory T cells achieve clearance should improve our ability to terminate states of viral persistence in humans. Mice persistently infected from birth with lymphocytic choriomeningitis virus are tolerant to the pathogen at the T-cell level and thus provide an excellent model to evaluate immunotherapeutic regimens. Previously, we demonstrated that adoptively transferred memory T cells require recipient dendritic cells to effectively purge an established persistent viral infection. However, the mechanisms that reactivate and sustain memory T-cell responses during clearance of such an infection remain unclear. Here we establish that therapeutic memory T cells require CD80 and CD86 costimulatory signals to efficiently clear an established persistent viral infection in vivo. Early blockade of costimulatory pathways with CTLA-4-Fc decreased the secondary expansion of virus-specific CD8+ and CD4+ memory T cells as well as their ability to produce antiviral cytokines and purge the persistent infection. Late costimulation blockade also reduced virus-specific T-cell numbers, illustrating that sustained interactions with costimulatory molecules is required for efficient T-cell expansion. These findings indicate that antiviral memory T cells require costimulation to efficiently clear a persistent viral infection and that costimulatory pathways can be targeted to modulate the magnitude of an adoptive immunotherapeutic regimen.
Persistent viruses, such as human immunodeficiency virus, hepatitis B virus, and hepatitis C virus, cause major health problems worldwide and are extraordinarily difficult to clear following the establishment of persistence. Given the challenges associated with clearing persistent infections, it is important to develop and mechanistically understand therapeutic strategies that successfully achieve viral eradication without inducing permanent damage in the host. Studies using the lymphocytic choriomeningitis virus (LCMV) model system have convincingly demonstrated that a systemic persistent viral infection can be completely purged from a murine host by using a therapeutic approach referred to as adoptive immunotherapy (1, 15, 22, 29, 30). Remarkably, total body control of multiple persistent viral infections in both the mouse (1, 15, 22, 29, 30) and humans (8, 14, 24, 26, 31) can be achieved using adoptive immunotherapy. When mice are persistently infected at birth or in utero with LCMV (referred to as carrier mice), the virus establishes systemic persistence (6). Adult LCMV carrier mice are tolerant to the virus at the T-cell level and thus are unable to eradicate the pathogen (23), which provides an excellent model to study immunotherapeutic regimens. Immunocytotherapy relies on the adoptive transfer of virus-specific memory CD8 and CD4 T cells from LCMV-immune donor mice into recipient carrier mice (1, 15, 22, 29, 30). Following the therapeutic administration of memory cells, LCMV is purged from most peripheral tissues of carrier mice in 14 days, whereas more than 100 days are required to clear virus from the central nervous system (CNS) and kidneys (1, 15, 22). Furthermore, successful viral clearance requires antiviral “memory” but not “effector” T cells (11). Thus, in addition to its proven therapeutic relevance, this model also provides a paradigm to understand factors that regulate memory T cells following secondary exposure to pathogens in vivo.
The mechanisms leading to activation of naïve T cells have been well described and involve recognition of major histocompatibility complex (MHC) peptide through the T-cell receptor (TCR) as well as costimulation (e.g., CD80 and CD86 interactions) (4, 25, 27). On the other hand, the factors that govern the activation and secondary expansion of memory CD8+ and CD4+ T cells are less clearly defined, particularly in an in vivo therapeutic setting. When memory T cells reencounter cognate antigen, they respond rapidly by producing cytokines and dividing. Previous studies indicated that there was no role for dendritic cells or costimulation (4, 27) in the reactivation of memory T cells; however, three recent studies have shown that dendritic cells (DCs) stimulate memory T-cell activity upon antigen rechallenge (2, 33) and during adoptive immunotherapy (15). Because MHC class I antigen (MHC-I) is expressed on nearly all cell types but costimulatory molecules are not, these three studies strongly suggested that DCs were influencing memory T cells with costimulatory pathways thought only to be required during priming. Indeed, when the issue was reexamined, it was revealed that memory CD8+ and CD4+ T cells require CD28-CD80/CD86 costimulation to be fully reactivated upon secondary exposure to antigen (3, 7, 21).
Because therapeutically administered memory T cells require effective interactions with the host hematopoietic system (10), in particular dendritic cells (15), to achieve successful viral clearance, we set out to address several unanswered questions. First, is costimulation required for the immunotherapeutic clearance of an established persistent viral infection? This is a particularly important question because the requirements imposed on therapeutically administered memory T cells, which encounter immediate and overwhelmingly high levels of virus, heightened antigenic stimulation, and a unique inflammatory milieu, are likely to be different than those faced by endogenous memory T cells following pathogen rechallenge in an otherwise-quiescent environment. The second question we set out to address in this study was whether costimulation blockade could modulate the activities of an immunotherapeutic regimen consisting of memory T cells. This question is of great importance in a clinical setting where pathogen-specific memory T cells can induce severe tissue pathology through the release of effector molecules (12). Thus, it is critical to have a strategy to limit the magnitude of an undesirable response without impeding viral clearance.
MATERIALS AND METHODS
Mice.
C57BL/6 (B6) and B6 Thy1.1+DbGP33-41 TCR-transgenic (TCR-tg) mice (Thy1.1+ P14 mice) were bred and maintained in a closed breeding facility. The handling of all mice conformed to the requirements of the National Institutes of Health.
Virus.
The parental Armstrong b53 strain of LCMV (5) was used for all experiments. Viral titers were determined by plaque assay on Vero cells.
LCMV carrier colony.
A persistent LCMV infection was originally established by infecting 1-day-old C57BL/6 mice with 103 PFU of LCMV Armstrong intracerebrally (22). Because LCMV is known to spread through vertical transmission (i.e., mother to offspring), the resultant carrier mice were interbred to establish and maintain a colony of persistently infected mice for experimentation. Carrier mice obtained from this colony were used as recipients for adoptive immunotherapy experiments.
Mononuclear cell isolations and tissue processing.
To obtain cell suspensions for flow cytometric analysis and in vitro stimulation, spleens, mesenteric lymph nodes, lungs, livers, and kidneys were harvested after an intracardiac perfusion with a 0.9% saline solution to remove the contaminating blood leukocytes. Tissues were infused with collagenase solution (1 mg/ml) and incubated for 30 min at 37°C. Single-cell suspensions were then prepared by mechanically disrupting the organs through a 100-μm filter. Splenocytes were then treated with red cell lysis buffer (ammonium chloride; Tris-HCl, 0.02 M, and NH4Cl, 0.14 M), washed, and finally resuspended in cell culture medium or phosphate-buffered saline (PBS). To isolate organ-infiltrating leukocytes from livers, lungs, and kidneys, homogenates were resuspended in 35% Percoll (Amersham Biosciences). The mononuclear cells were then extracted, washed, and resuspended in cell culture medium or PBS. The number of mononuclear cells was determined for each tissue preparation and used to calculate the absolute number of the denoted cell populations. Peripheral blood mononuclear cells (PBMCs) were obtained by mixing 200 μl of blood with 10 ml of red blood cell lysis buffer on ice for 1 h. Afterward, PBMCs were centrifuged, washed, and resuspended in cell culture medium or PBS.
Adoptive immunotherapy.
To generate LCMV memory donors for immunocytotherapy experiments, C57BL/6 mice were seeded intravenously with 104 naive Thy1.1+ P14 CD8+ T cells (18, 19). All naive cells were purified from Thy1.1+ P14 mice by negative selection (StemCell Technologies Inc.). The enrichment procedure resulted in a purity of >98%. One day after adoptive transfer, mice were infected intraperitoneally with 105 PFU of LCMV Armstrong. Splenocytes were harvested from all mice at ∼45 days after infection, when LCMV-specific memory T lymphocytes are known to be present in the spleen (11, 20). For all immunocytotherapy experiments, 1 × 107 to 2 × 107 memory splenocytes seeded with traceable DbGP33-41-specific CD8+ T cells were injected intraperitoneally into LCMV carrier mice.
Costimulation blockade.
To simultaneously block CD80 and CD86, immunotherapy-treated mice were injected intraperitoneally with noncytolytic mouse CTLA-4-Fc chimeric protein (Sigma-Aldrich) (16). For early treatment, CTLA-4-Fc was injected intraperitoneally on days −1, 0, 1, 3, and 5 postimmunotherapy (200 μg per injection in 200 μl PBS). For late blockade experiments, CTLA-4-Fc was injected on days 4, 6, 8, and 10 postimmunotherapy.
Surface staining and antibodies.
Mononuclear cell suspensions were incubated for 15 min with a rat anti-CD16/32 to block Fc receptors and then with primary antibodies for 30 min on ice. Cells were stained with the following antibodies: CD45.2-fluorescein isothiocyanate (FITC) (clone 104; BD Biosciences), CD86-phycoerythrin (PE) (GL1; BD Biosciences), Thy1.1-peridinin chlorophyll protein (PerCP)-Cy5.5 (OX7; BD Biosciences), Thy1.2-PerCP-Cy5.5 (30H12; Biolegend), NK1.1-PerCP-Cy5.5 (PK136; BD Biosciences), CD19-PerCP-Cy5.5 (1D3; BD Biosciences), CD11b-PE-Cy7 (M1/70; eBioscience), CD11c-allophycocyanin (APC) (N418; BD Biosciences), CD80 biotinylated (16-10A1; Biolegend), streptavidin-APC-Cy7 (Biolegend), CD8-Pacific Blue (53.6.7; Caltag), CD4-Pacific Blue (RMA4.5; eBioscience), and B220-Pacific Blue (RA3-6B2; BD Biosciences). For MHC-I tetramer stains, mononuclear cells were stained for 1 h on ice with DbGP33-41-PE. For MHC-II tetramer stains, mononuclear cells were stained for 1 h 30 min at room temperature with I-AbGP67-77-PE. Biotinylated DbGP33-41 and I-AbGP67-77 monomers were obtained from the NIH Tetramer Core Facility. All cells were acquired using a digital flow cytometer (Digital LSR II; Becton Dickinson), and data were analyzed with FlowJo software (Tree Star).
Intracellular cytokine analyses.
Splenocytes were stimulated for 5 h at 37°C with 2 μg/ml of MHC class I-restricted LCMV peptides (GP33-41 or NP396-404) or 5 μg/ml of an MHC class II-restricted peptide (GP61-80) in the presence of 50 U/ml recombinant murine interleukin-2 (IL-2; National Institutes of Health) and 1 mg/ml brefeldin A (Sigma). All peptides were greater than 95% pure. Following the 5-h in vitro stimulation, cells were surface stained with anti-CD8-Pacific Blue (clone 53.6.7; Caltag) or anti-CD4-PerCP-Cy5.5 (clone RMA4-5; BD Biosciences). Afterward, cells were fixed, permeabilized, and stained with the following cytokine antibodies: tumor necrosis factor alpha (TNF-α)-FITC (clone MP6-XT22; eBioscience), gamma interferon (IFN-γ)-PE (clone XMG1.2; BD Biosciences), and IL-2-APC (clone JES6-5H4; BD Biosciences).
In vivo T-cell proliferation assay.
Thy1.2+ memory splenocytes from B6 mice seeded with Thy1.1+ P14 T cells were labeled with 5 μM of carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes) and injected intraperitoneally into B6 LCMV carrier mice (Thy1.2+). Three days later the proliferation of splenic Thy1.1+ P14 T cells was determined by quantifying the degree of CFSE dilution. This was done by staining splenocytes from carrier mice with Thy1.1-PerCP-Cy5.5 (clone OX-7; BD Pharmingen) and CD8-Pacific Blue (clone 53.6.7; Caltag). The cells were analyzed by digital flow cytometry.
In vitro T-cell proliferation assay.
CD8+ T cells from the spleens of B6 memory mice seeded with Thy1.1+ P14 T cells were isolated by positive selection (Miltenyi Biotec) and labeled with 0.5 μM of CFSE. Following positive selection, the CD8+ T cells were >98% pure. One million CFSE-labeled CD8+ T cells were then stimulated for 3.5 days in a 37°C, 5% CO2 incubator with 50,000 CD11c+ cells positively selected (Stem Cell Technologies) from spleens of B6 carrier or uninfected control mice. During the incubation period, cells were treated with 10 μg/ml CTLA-4-Fc, anti-CD80 antibody, or anti-CD86 antibody (13). Three and a half days later, the proliferation of Thy1.1+ P14 T cells was determined based on CFSE dilution. Cultured cells were stained with Thy1.1-PerCP-Cy5.5 (clone OX-7; BD Biosciences) and CD8-Pacific Blue (clone 53.6.7; Caltag) and analyzed by digital flow cytometry.
In vitro T-cell cytokine assay.
Splenocytes from B6 memory mice seeded with Thy1.1+ P14 T cells were stained with Thy1.1-PerCP-Cy5.5 and sorted by flow cytometry (FACSAria cell sorter; Becton Dickinson). A total of 50,000 Thy1.1+ P14 cells were stimulated for 24 h in a 37°C, 5% CO2 incubator with 50,000 CD11c+ cells positively selected (Stem Cell Technologies) from the spleens of B6 carrier or uninfected control mice. During the incubation period, cells were treated with 10 μg/ml CTLA-4-Fc, anti-CD80 antibody, or anti-CD86 antibody (13). Brefeldin A (1 mg/ml; Sigma-Aldrich) was added for the last 6 h of stimulation. Cells were surface stained with Thy1.1-PerCP-Cy5.5 (clone OX-7; BD Biosciences) and with anti-CD8-Pacific Blue (clone 53.6.7; Caltag). Afterwards, cells were fixed, permeabilized, and stained with the following cytokine antibodies: TNF-α-FITC (clone MP6-XT22; eBioscience), IFN-γ-PE (clone XMG1.2; BD Biosciences), and IL-2-APC (clone JES6-5H4; BD Biosciences).
Statistical analysis.
Data handling, analysis, and graphical representations were performed using Microsoft Excel and SigmaPlot 10.0. Statistical differences (P < 0.05) were determined with Student's t test using SigmaStat 3.5.
RESULTS
Adoptive immunotherapy induces systemic antigen-presenting cell influx into lymphoid and nonlymphoid tissues.
Antigen-presenting cells play an important role in the success of adoptive immunotherapy (15); therefore, we first set out to evaluate the magnitude and kinetics of systemic antigen-presenting cell migration induced by the therapeutic administration of memory splenocytes into mice persistently infected from birth with LCMV (referred to as LCMV carrier mice). To simultaneously follow LCMV-specific cytotoxic lymphocytes (CTL), memory T cells were generated by intravenously seeding Thy1.2+ C57BL/6 (B6) mice with 1 × 104 naïve Thy1.1+ TCR-tg CD8+ T cells specific to amino acids 33 to 41 of the LCMV glycoprotein (referred to as P14 cells) as we have performed previously (15). One day following transfer, mice were infected with 1 × 105 PFU of LCMV Armstrong, which is cleared in 8 to 10 days and generates a memory T-cell pool by day 45 postinfection (11). At day 45, memory splenocytes were harvested and transferred into LCMV carrier mice persistently infected from birth.
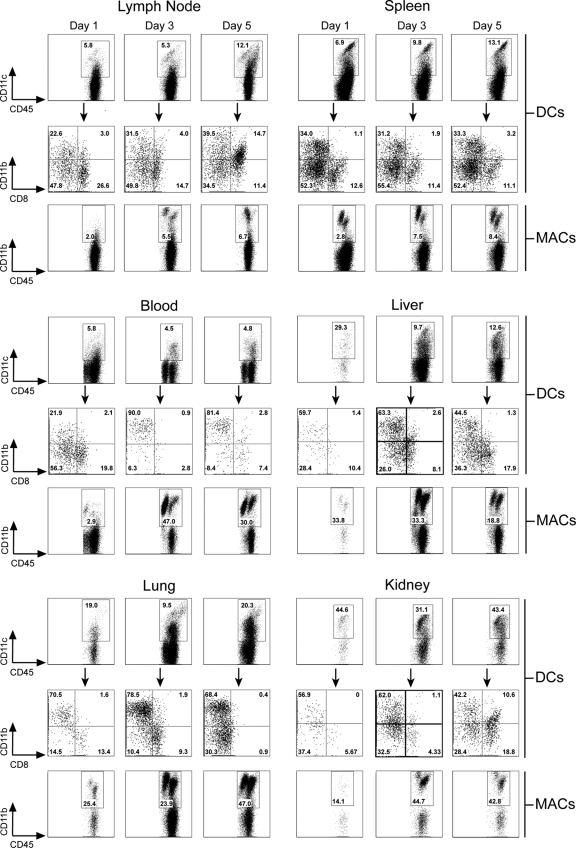
Dendritic cells (CD45+ CD3− NK1.1− CD11c+) and monocytes/macrophages (CD45+ CD3− NK1.1− CD11c− CD11b+) in representative lymphoid (spleen and lymph nodes) and nonlymphoid (liver, lung, and kidney) tissues as well as the blood were quantified on days 1, 3, and 5 postimmunotherapy (Fig. 1). We also subdefined DCs phenotypically based on the expression of two conventional DC markers: CD11b and CD8α. Between days 1 and 3 postimmunotherapy, we noted a sizeable increase in the frequency/number of DCs and macrophages found in nonlymphoid tissues such as the liver, lung, and kidney. Note that the quantity of cells on representative flow cytometric plots increased considerably between these two time points and remained elevated on day 5. The majority of DCs found in nonlymphoid tissues at days 3 and 5 expressed CD11b, suggesting that these cells were derived from blood monocytes. Consistent with this supposition, monocytes increased markedly in the blood between days 1 and 3 postimmunotherapy, and the majority (90%) of the DCs found in the blood at this time expressed CD11b. Within lymphoid tissues such as the spleen and lymph nodes, DCs and macrophages also increased over this time frame, but the percentages of CD11b+ and CD8α+ DCs remained remarkably stable, indicating that both populations increased equally in number over time. When absolute numbers of DCs were analyzed temporally in a representative lymphoid (spleen) and nonlymphoid (liver) tissue over the entire course of immunotherapy, a marked elevation in DC numbers was observed that coincided perfectly with the secondary expansion of traceable Thy1.1+ P14 CTL (data not shown). Taken together, these data demonstrate that DCs and monocytes/macrophages increase systemically early after the administration of immunotherapy.
FIG. 1.
Kinetics of antigen-presenting cell influx following adoptive immunotherapy. At the denoted time points postimmunotherapy, mononuclear cells were isolated from mesenteric lymph nodes, spleen, blood, liver, lungs, and kidneys, and the frequencies of DCs (CD45+ CD3− NK1.1− CD11c+) and monocytes/macrophages (MACs; CD45+ CD3− NK1.1− CD11c− CD11b+) were determined cytometrically. DCs in each compartment were further analyzed based on expression of two conventional phenotypic markers (black arrows), CD11b (“myeloid” DCs) and CD8α (“lymphoid” DCs). Representative flow cytometric plots gated on the aforementioned markers are shown. Boxes and quadrants denote the frequencies of the denoted antigen-presenting cell subsets. Note the marked systemic elevation in the frequency of DCs and macrophages between days 1 and 3 postimmunotherapy. Data are representative of three mice per group and two independent experiments.
Immunotherapy induces upregulation of costimulatory molecules on DCs.
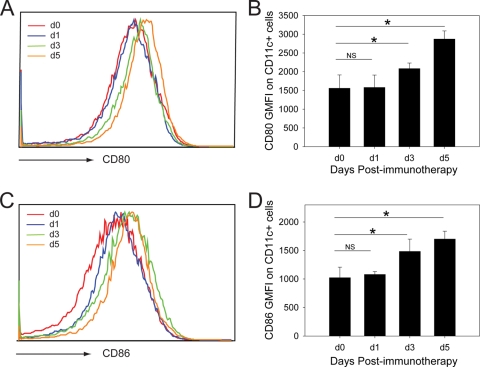
Because host DCs had been shown to be important for viral clearance following adoptive immunotherapy (15), we evaluated the kinetics of costimulatory molecule upregulation on DCs residing in a representative secondary lymphoid tissue (spleen) at days 1, 3, and 5 postimmunotherapy (Fig. 2), which coincided with their systemic distribution (Fig. 1). On day 1 postimmunotherapy, no detectable increase in CD80 and CD86 expression was observed on CD11c+ cells. However, increased expression of both costimulatory molecules was observed on CD11c+ cells at day 3 and 5 postimmunotherapy. These data indicate that immunocytotherapy induces an upregulation of costimulatory molecules on DCs in recipient carrier mice.
FIG. 2.
Upregulation of costimulatory molecules on DCs following adoptive immunotherapy. Carrier mice were injected with 2 × 107 memory splenocytes. At days 0, 1, 3, and 5 following adoptive immunotherapy, CD80 (A and B) and CD86 (C and D) expression was examined cytometrically on splenic CD11chi DCs (CD45+ CD19− Th1.2− NK1.1− CD11chi). Representative histograms gated on CD45+ CD19− Th1.2− NK1.1− CD11chi cells are shown in panels A and C. The geometric mean fluorescent intensities (GMFI) of CD80 and CD86 are graphed (mean ± standard deviation) in panels B and D. Data are representative of four mice per group and two independent experiments. Asterisks denote statistical significance (P < 0.05).
Early costimulation blockade decreases the secondary expansion of virus-specific memory T cells and delays viral clearance.
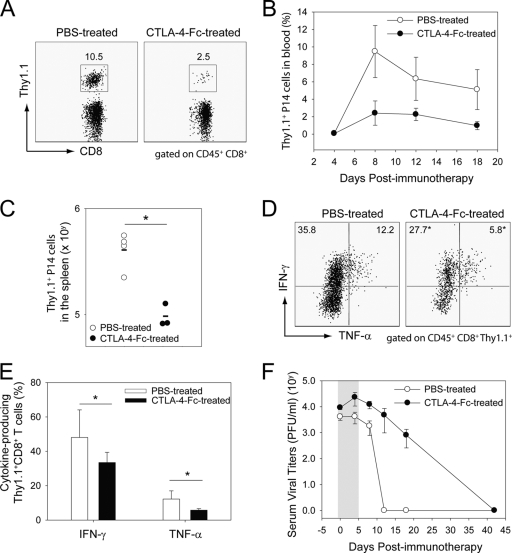
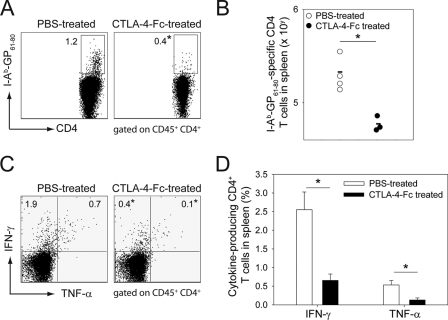
To evaluate the importance of the rapid upregulation of costimulatory molecules following adoptive therapy, we simultaneously blocked CD80 and CD86 by injection of CTLA-4-Fc (16) on days −1, 0, 1, 3, and 5 after adoptive immunotherapy and monitored virus-specific memory CD8+ and CD4+ T-cell responses (Fig. 3 and 4). CD8+ T-cell responses were monitored using traceable Thy1.1+ P14 (Fig. 3), and CD4+ T cells were evaluated using I-AbGP67-77 tetramers (Fig. 4). Costimulation blockade reduced the expansion of LCMV-specific CD8+ (Fig. 3A and B) and CD4+ (data not shown) T cells in the blood. To further evaluate the quantity of LCMV-specific CD8+ and CD4+ memory T cells in immunotherapy recipients that received costimulation blockade, we performed ex vivo functional analyses on cells extracted from a representative lymphoid tissue (spleen) at the peak of the response (day 9). Confirming our analyses of the blood, the absolute numbers of Thy1.1+ CD8+ (Fig. 3C) and I-AbGP67-77 CD4+ (Fig. 4A and B) T cells were significantly reduced in mice treated with CTLA-4-Fc.
FIG. 3.
Effect of early costimulation blockade on therapeutic memory CD8+ T cells and viral clearance. A total of 2 × 107 memory splenocytes seeded with Thy1.1+ P14 cells were adoptively transferred in LCMV carrier mice treated with PBS or CTLA-4-Fc. (A to C) The frequency and absolute number of Thy1.1+P14 CTL was quantified over time in the blood (A and B) and at day 9 postimmunotherapy in the spleen (C) of B6 carrier mice. Representative dot plots gated on CD45+ CD8+ cells at day 8 postimmunotherapy are shown for the blood in panel A. Boxes depict the frequencies of CD8+ Thy1.1+ P14 cells in each group. The frequencies of Thy1.1+ P14 CTL in the blood are represented graphically in panel B as means ± standard deviations. Absolute numbers of Thy1.1+ P14 cells in the spleen for individual mice are shown in panel C. Note that the frequency and absolute number of Thy1.1+ P14 CTL were reduced in immunotherapy recipients that received CTLA-4-Fc. (D and E) On day 8 postimmunotherapy, the function of splenic Thy1.1+ P14 cells from PBS-treated and CTLA-4-Fc-treated carrier mice was assessed by ex vivo peptide stimulation with GP33-41 peptide. Representative dot plots in panel D gated on CD45+ CD8+ Thy1.1+ cells show the frequencies of P14 cells that produce IFN-γ and TNF-α. Data are represented graphically in panel E as means ± standard deviations. Note the statistically significant reduction in the frequency of IFN-γ- and TNF-α-producing P14 cells in CTLA-4-Fc-treated mice. (F) Serum viral titers were quantified over time by plaque assay. A delay in the viral clearance was also noted in immunotherapy recipients treated with CTLA-4-Fc. Data in panel F are expressed as PFU per ml of serum (mean ± standard deviation). All graphs include four mice per group and represent at least two independent experiments. Asterisks denote statistical significance (P < 0.05). The grey area in panel F corresponds to the CTLA-4-Fc administration period.
FIG. 4.
Effect of early costimulation blockade on therapeutic memory CD4+ T cells. On day 8 postimmunotherapy, the number of splenic I-AbGP61-80-specific CD4+ T cells from PBS-treated and CTLA-4-Fc-treated carrier mice was assessed by MHC-II tetramers staining (A and B). Effector function was evaluated by ex vivo peptide stimulation with GP61-80 peptide (C and D). Representative dot plots gated on CD45+ CD4+ T cells show the frequency tetramer-positive (A) and cytokine-producing (IFN-γ and TNF-α) (C) cells. Data are represented graphically in panels B and D. Data in panel D are means ± standard deviations. Note the statistically significant reduction in the frequency of tetramer-positive and cytokine-producing CD4+ T cells in CTLA-4-Fc-treated mice. All graphs include four mice per group and represent at least two independent experiments. Asterisks denote statistical significance (P < 0.05).
To examine effector functions following costimulation blockade, day 9 splenocytes were stimulated with an immunodominant CD8 (GP33-41) or CD4 (GP61-80) peptide, and T cells were subsequently analyzed for the production of two antiviral cytokines (IFN-γ and TNF-α) (Fig. 3 and 4) as well as IL-2. CTLA-4-Fc treatment reduced the percentages (Fig. 3D and E and 4C and D) and absolute numbers (data not shown) of IFN-γ- and TNF-α-producing CD8+ and CD4+ T cells following peptide stimulation. No IL-2 production was detected following ex vivo peptide stimulation of CD8+ T cells at day 9, and CTLA-4-Fc treatment had no impact on the ability of CD4+ T cells to produce this cytokine (data not shown). The observed reduction in the number of IFN-γ- and TNF-α-producing antiviral T cells could be explained by a failure of the transferred memory T cells to efficiently expand and/or an inability to engage effector functions. The use of transgenic Thy1.1+ P14 cells allowed us to address this issue for antiviral CD8+ T cells. When GP33-41 splenocytes were gated on Thy1.1+ CD8+ T cells, a statistically significant reduction in the frequency of IFN-γ- and TNF-α-producing Thy1.1+ P14 cells was observed in CTLA-4-Fc-treated mice (Fig. 3E). This suggests that costimulation blockade interfered with the ability of memory CD8+ T cells to reacquire certain effector functions. However, cytokine-producing P14 cells found in PBS and CTLA-4-Fc-treated mice did express similar quantities of cytokine on a per cell basis (analyzed as the geometric mean fluorescent intensity) (data not shown), indicating that when memory CD8+ T cells did engage effector functions in CTLA-4-Fc-treated mice, they produced amounts similar to those observed in control mice. To evaluate whether costimulation blockade also interfered with the activation status of memory T cells following secondary expansion, we quantified the geometric fluorescent intensities of activation markers on Thy1.1 P14+ cells at day 9 postimmunotherapy. No statistically significant difference in CD44 (28,451 ± 3,619 versus 25,040 ± 4,222), CD69 (339 ± 136 versus 369 ± 67), CD62L (280 ± 187 versus 278 ± 75), CD25 (219 ± 32 versus 189 ± 56), LFA-1 (18,361 ± 1,329 versus 20,079 ± 1,381), 41BB ligand (33.0 ± 15.2 versus 38.3 ± 3.8), or PD-1 (1,628 ± 574 versus 1,923 ± 482) expression was detected on P14 cells extracted from the spleens of PBS-treated versus CTLA-4-Fc-treated mice (means ± standard deviations; n = 5 mice per group). These data indicate that costimulation blockade interferes with memory T-cell proliferation and function but not activation.
Given that costimulation blockade significantly interfered with memory T-cell expansion and function following transfer into carrier mice, we next addressed the impact of this treatment on therapeutic viral clearance. When serum viral titers were monitored over time in mice treated with PBS and CTLA-4-Fc, a significant delay in viral clearance was observed. Peripheral viral clearance was extended over a 40-day time window instead of the 12 days observed in PBS-treated controls (Fig. 3F). However, it is important to note that viral clearance was attained in CTLA-4-Fc-treated mice despite its negative impacts on secondary expansion and effector functions. To ensure that viral clearance was attained even in a compartment with delayed clearance kinetics such as the CNS (1, 15, 22), we quantified CNS viral titers by plaque assay in PBS-treated and CTLA-4-Fc-treated mice at 126 days postimmunotherapy. Virus was reduced to an undetectable level in both groups (data not shown). In concert, these data indicate that costimulation is required for maximal secondary T-cell expansion and antiviral activity during adoptive immunotherapy as well as timely clearance of an established persistent viral infection.
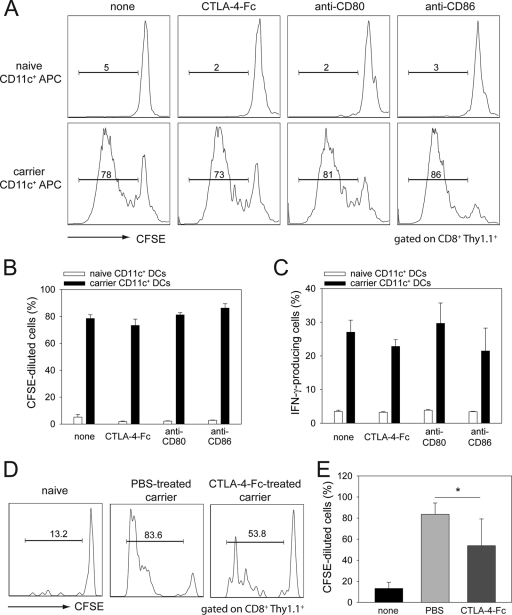
Memory T cells proliferate less when costimulatory molecules are blocked in vivo, but not in vitro.
Upon reexposure to cognate antigen, memory T cells rapidly acquire effector functions and, after a lag period, proliferate (32). To test whether LCMV-specific memory CD8 T cells specifically require costimulation to proliferate and/or produce antiviral cytokines, we cultured memory CD8+ P14 T cells with persistently infected dendritic cells (antigen-presenting cells) in vitro while blocking CD80, CD86, or both. Memory CD8+ T cells seeded with Thy1.1+ P14 cells were purified from the spleens of B6 mice infected 45 days earlier with LCMV Armstrong. These cells were labeled with CFSE and cultured with CD11c+ DCs purified from LCMV carrier mice or uninfected mice as a control. DCs from LCMV carrier mice are known to contain an abundance of LCMV antigen (9). Memory P14 T cells did not proliferate or produce antiviral cytokines when exposed to naïve DCs, and as expected costimulation blockade did not impact this profile (Fig. 5A to C). In contrast, memory P14 cells proliferated substantially following stimulation by persistently infected DCs (Fig. 5A and B) and produced IFN-γ (Fig. 5C), TNF-α (data not shown), and IL-2 (data not shown); however, blockade of costimulatory pathways in this in vitro assay did not interfere with proliferation or cytokine production (Fig. 5A to C).
FIG. 5.
Impact of costimulation blockade on memory T-cell proliferation. (A) Memory CD8 T cells seeded with Thy1.1+ P14 cells were isolated from the spleens of B6 memory mice at day 45 postinfection, labeled with CFSE, and incubated with CD11c+ DCs obtained from LCMV carrier or uninfected control mice. Cultures were incubated for 3.5 days in the presence of CTLA-4-Fc, anti-CD80, or anti-CD86, and then Thy1.1+P14 cells were analyzed for the degree of CFSE dilution. Representative histograms are gated on CD45+ CD8+ Thy1.1+ P14 T cells. (B) The frequencies of CFSElow Thy1.1+ CD8+ T cells are plotted as means ± standard deviations. (C) Flow cytometrically sorted memory Thy1.1+ P14 T cells were stimulated with either naïve or LCMV carrier CD11c+ cells for 24 h in the presence of CTLA-4-Fc, anti-CD80, or anti-CD86. Brefeldin A was added during the last 6 h of culture. The frequencies of IFN-γ-producing Thy1.1+ CD8+ P14 T cells are presented as means ± standard deviations. All cultures were performed in triplicate. (D) A total of 2 × 107 CFSE-labeled Thy1.1+ P14-seeded memory splenocytes were injected into uninfected mice, PBS-treated carrier mice, and CTLA-4-Fc-treated carrier mice. Representative histograms gated on splenic CD45+ CD8+ Thy1.1+ T cells at day 3 postinjection show reduced CFSE dilution in CTLA-4-Fc-treated mice compared to PBS controls. Minimal CFSE dilution was observed in uninfected mice. (E) The frequency of CFSE-diluted Thy1.1+ CD8+ T cells, plotted as means ± standard deviations, revealed a statistically significant reduction in CTLA-4-Fc-treated mice. All graphs show results for five mice per group and represent two independent experiments. Asterisks denote statistical significance (P < 0.05).
To assess whether costimulation blockade had an effect on memory T cells in vivo, we transferred CFSE-labeled memory splenocytes seeded with Thy1.1+ P14 cells into persistently infected LCMV carrier mice and treated them with CTLA-4-Fc or PBS on days −1, 0, and 1 postimmunotherapy. As an additional control for this study, we transferred CFSE-labeled memory cells into naïve mice. CFSE dilution in Thy1.1+ P14 T cells was evaluated for all groups on day 3 posttransfer. A statistically significant reduction in the frequency of CFSE-diluted Thy1.1+ P14 cells was observed in carrier mice treated with CTLA-4-Fc when compared to PBS controls (Fig. 5D and E). These data indicate that costimulation is required for optimal proliferation of virus-specific memory T cells when used to therapeutically purge a persistent viral infection.
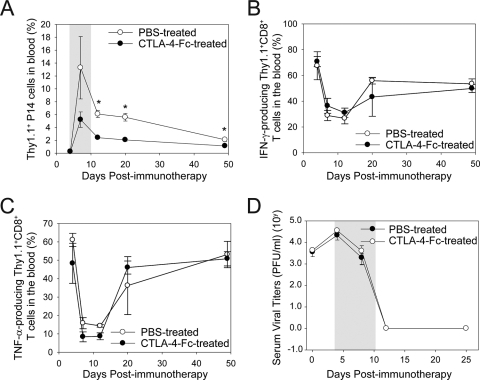
Late costimulation blockade reduces LCMV-specific T-cell expansion but has a minimal impact on viral clearance.
We demonstrated that CTLA-4-Fc impedes memory T-cell expansion and viral clearance when administered 1 day before adoptive immunotherapy. To evaluate the role of costimulation at a point that followed the first exposure to cognate antigen, we injected CTLA-4-Fc on days 4, 6, 8, and 10 postimmunotherapy. We observed a statistically significant reduction in Thy1.1+ P14 cells in the blood of CTLA-4-Fc-treated mice compared to controls (Fig. 6A), although the reduction was not as substantial as that observed when CTLA-4-Fc was administered 1 day before immunotherapy (Fig. 3A and B) (2.5-fold reduction compared to 4.0-fold reduction at day 8). Interestingly, despite the reduction in secondary CTL expansion, CTLA-4-Fc treatment had no impact on cytokine production (Fig. 6B and C) or the kinetics of viral clearance (Fig. 6D) following immunotherapy. In addition, no infectious virus was detected by plaque assay in the brains of CTLA-4-Fc-treated immunotherapy recipients at 100 days postimmunotherapy (data not shown), indicating that even this “hard-to-clear” reservoir (1, 22) was purged with normal kinetics despite costimulation blockade. Collectively, these data demonstrate that antiviral T-cell number can be modulated during immunotherapy without necessarily impacting viral clearance or T-cell function.
FIG. 6.
Effect of late costimulation blockade on therapeutic memory T-cell expansion, function, and viral clearance. (A) The frequency of Thy1.1+ P14 CTL was quantified over time in the blood of B6 carrier mice treated with PBS or CTLA-4-Fc. Note that the frequency of P14 CTL (mean ± standard deviation) was significantly reduced in immunotherapy recipients that received CTLA-4-Fc. (B and C) PBMC were isolated from PBS-treated and CTLA-4-Fc-treated groups at the denoted time points, stimulated in vitro with GP33-41 peptide, and analyzed for the production of IFN-γ (B) and TNF-α (C) production. (D) Serum viral titers are represented as PFU per ml of serum sampled from PBS-treated and CTLA-4-Fc-treated immunotherapy recipients (means ± standard deviations). All graphs include four mice per group and represent two independent experiments. Asterisks denote statistical significance (P < 0.05). The grey areas in panels A and D correspond to the CTLA-4-Fc administration period.
DISCUSSION
In this study we examined the role of costimulation in modulating the activities of therapeutically administered memory T cells during the clearance of a persistent viral infection. Our results revealed that administration of adoptive immunotherapy induced marked systemic changes in host APC distribution and number. Within 3 days, DCs as well as monocytes/macrophages increased systemically in both lymphoid and nonlymphoid tissues. It was shown previously using CD11c-DTR carrier mice that successful adoptive immunotherapy requires host DCs (15). We, therefore, examined the expression of costimulatory molecules on this potent APC population and observed that DCs in carrier mice upregulated CD80 and CD86 with kinetics that coincided with their numerical increase systemically. Blockade of these costimulatory molecules in vivo using CTLA-4-Fc resulted in a significantly reduced expansion of memory CD8+ and CD4+ T cells, decreased antiviral effector functions (i.e., cytokine-producing ability), and a delayed ability to clear an established persistent viral infection. Interestingly, our finding that costimulation blockade did not impede memory T-cell responses in vitro indicates that the in vivo environment imposes an additional set of constraints on antiviral memory T cells that cannot be adequately assessed through in vitro experimentation alone. It is important to note that the virus was cleared despite the impact on secondary expansion and function. These data indicate that an adoptive immunotherapeutic regimen consisting of antiviral T cells can be muted without necessarily impacting pathogen clearance. This fact was confirmed in late costimulation blockade experiments, which revealed that secondary memory T-cell expansion could be halved without impacting antiviral function or viral clearance kinetics. In concert, these data demonstrate that sustained costimulation is important in optimizing therapeutically administered memory T cells and that costimulation blockade can be used as a means to modulate or “apply the brakes” to an overly robust response without disrupting the ability to ultimately purge a persistent virus.
The landmark studies of Volkert and colleagues first revealed that memory immune cells could be used to eradicate an established persistent viral infection (29, 30), and it was demonstrated subsequently that the kinetics of this memory T-cell-dependent process differed between tissues (1, 22). LCMV carrier mice provide an excellent in vivo paradigm to uncover factors that modulate memory T-cell responses throughout the body. In this study we focused on the importance of costimulation using an inhibitor that is approved for clinical use, CTLA-4-Fc (16). One of the original studies to suggest LCMV-specific memory T cells do not require costimulation was conducted in CD28-deficient mice (27). CD28-deficient mice are able to clear LCMV Armstrong, and CD28-deficient mice that clear LCMV Armstrong are not susceptible to LCMV-induced meningitis or infection with immunosuppressive LCMV clone 13, suggesting normal memory T-cell formation. However, because the immune response to LCMV is so robust (17), these assessments conducted in CD28-deficient mice would not have been expected to reveal a role for costimulation in antiviral memory T-cell function. In fact, it was subsequently revealed in different models of viral rechallenge that costimulation does in fact facilitate the activities of memory T cells (3, 7, 21).
Here we set out to examine whether memory T cells used therapeutically do indeed require costimulatory molecules to purge an already persistent viral infection. We thought that the taxing and systemic antigenic environment found in LCMV carrier mice would provide a very stringent system to explore the absolute requirements of memory T cells because the experimental end point is total body viral eradication. One of the most interesting findings in our study is that costimulation blockade can be used as a means to fine tune an immunotherapeutic regimen consisting of memory T cells without preventing viral clearance. Both early and late costimulation blockade reduced the secondary expansion of memory T cells, but neither treatment completely prevented the therapeutic clearance of the persistent viral infection. Early (but not late) costimulation blockade also interfered with the ability of therapeutic memory T cells to produce antiviral cytokines but did not interfere with activation status or ability to produce IL-2. This knowledge is important for the treatment of persistent viral infections in humans, as it suggests that an overly robust and potentially injurious immunotherapeutic regimen can be dampened using a short treatment with a costimulation blocker. This is particularly important in light of a recent study demonstrating that costimulation blockade can reduce the immunopathology caused by memory CD4+ T cells in the lungs of mice rechallenged with influenza virus (28). The upside of using CTLA-4-Fc treatment is that memory T-cell proliferation and function can be reduced without preventing the desired outcome of viral clearance. The fact that viral clearance is still attained suggests that the quantity of memory T cells that expand under normal conditions is well above the number required for clearance of a persistence viral infection. A fourfold reduction in the absolute number of antiviral CTL (as we observed when CTLA-4-Fc was administered 1 day before immunotherapy) is enough to delay but not prevent clearance, and a twofold reduction in CTL number (observed when CTLA-4-Fc was given on day 4) did not alter viral kinetics at all. These data, considered in the context of the aforementioned influenza study (28), suggest that immunotherapeutic regimens can be finely tuned using CTLA-4-Fc to minimize the pathology induced by antiviral T cells while still preserving their ability to purge virus.
In our studies we also observed that costimulation blockade impeded memory T-cell proliferation in vivo but not in vitro. This difference is not surprising given the abundance and diversity of infected targets in LCMV carrier mice, an environment that simply cannot be replicated in a cell culture system. Previous in vitro studies by Croft et al. led to the conclusion that memory T cells are less dependent on costimulation than their naïve counterparts and respond to a broader range of APC types (4). However, the consensus of several recent in vivo studies is that DCs represent an important bottleneck in primary and secondary responses (2, 15, 33) and that costimulation does play an important role in the reactivation antiviral memory T cells (3, 7, 21). Imposing this bottleneck makes sense evolutionarily, as memory CD8+ T cells receiving equivalent reactivation instructions from every MHC class I-bearing cell in the body could easily give rise to fatal immunopathology.
In conclusion, our findings not only demonstrate an important role for costimulation in optimizing the therapeutic clearance of a persistent viral infection, but they also offer new possibilities to those performing adoptive immunotherapy in a clinical setting. Costimulation blockade can be used before or following the therapeutic administration of antiviral T cells to modulate the robustness of the response. This intervention could be used to dull an overly aggressive therapeutic response and potentially limit undesirable immunopathology without preventing the eventual clearance of the persistent viral infection.
Acknowledgments
This work was supported by National Institutes of Health grants AI070967-01 (to D. B. McGavern) and AI77012-03 (to D. G. Brooks) and a grant from the Ray Thomas Edwards Foundation (to D. B. McGavern). L. Garidou was supported by a fellowship from the Association pour la recherche sur la sclerose en plaques.
Footnotes
Published ahead of print on 24 June 2009.
REFERENCES
- 1.Ahmed, R., B. D. Jamieson, and D. D. Porter. 1987. Immune therapy of a persistent and disseminated viral infection. J. Virol. 613920-3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belz, G. T., N. S. Wilson, C. M. Smith, A. M. Mount, F. R. Carbone, and W. R. Heath. 2006. Bone marrow-derived cells expand memory CD8+ T cells in response to viral infections of the lung and skin. Eur. J. Immunol. 36327-335. [DOI] [PubMed] [Google Scholar]
- 3.Borowski, A. B., A. C. Boesteanu, Y. M. Mueller, C. Carafides, D. J. Topham, J. D. Altman, S. R. Jennings, and P. D. Katsikis. 2007. Memory CD8+ T cells require CD28 costimulation. J. Immunol. 1796494-6503. [DOI] [PubMed] [Google Scholar]
- 4.Croft, M., L. M. Bradley, and S. L. Swain. 1994. Naive versus memory CD4 T cell response to antigen. Memory cells are less dependent on accessory cell costimulation and can respond to many antigen-presenting cell types including resting B cells. J. Immunol. 1522675-2685. [PubMed] [Google Scholar]
- 5.Dutko, F. J., and M. B. Oldstone. 1983. Genomic and biological variation among commonly used lymphocytic choriomeningitis virus strains. J. Gen. Virol. 641689-1698. [DOI] [PubMed] [Google Scholar]
- 6.Fazakerley, J. K., P. Southern, F. Bloom, and M. J. Buchmeier. 1991. High resolution in situ hybridization to determine the cellular distribution of lymphocytic choriomeningitis virus RNA in the tissues of persistently infected mice: relevance to arenavirus disease and mechanisms of viral persistence. J. Gen. Virol. 721611-1625. [DOI] [PubMed] [Google Scholar]
- 7.Fuse, S., W. Zhang, and E. J. Usherwood. 2008. Control of memory CD8+ T cell differentiation by CD80/CD86-CD28 costimulation and restoration by IL-2 during the recall response. J. Immunol. 1801148-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heslop, H. E., C. Y. Ng, C. Li, C. A. Smith, S. K. Loftin, R. A. Krance, M. K. Brenner, and C. M. Rooney. 1996. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat. Med. 2551-555. [DOI] [PubMed] [Google Scholar]
- 9.Homann, D., D. B. McGavern, and M. B. Oldstone. 2004. Visualizing the viral burden: phenotypic and functional alterations of T cells and APCs during persistent infection. J. Immunol. 1726239-6250. [DOI] [PubMed] [Google Scholar]
- 10.Jamieson, B. D., L. D. Butler, and R. Ahmed. 1987. Effective clearance of a persistent viral infection requires cooperation between virus-specific Lyt2+ T cells and nonspecific bone marrow-derived cells. J. Virol. 613930-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaech, S. M., S. Hemby, E. Kersh, and R. Ahmed. 2002. Molecular and functional profiling of memory CD8 T cell differentiation. Cell 111837-851. [DOI] [PubMed] [Google Scholar]
- 12.Kagi, D., B. Ledermann, K. Burki, R. M. Zinkernagel, and H. Hengartner. 1996. Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in immunological protection and pathogenesis in vivo. Annu. Rev. Immunol. 14207-232. [DOI] [PubMed] [Google Scholar]
- 13.Krummel, M. F., and J. P. Allison. 1995. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 182459-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lau, G. K., D. Suri, R. Liang, E. I. Rigopoulou, M. G. Thomas, I. Mullerova, A. Nanji, S. T. Yuen, R. Williams, and N. V. Naoumov. 2002. Resolution of chronic hepatitis B and anti-HBs seroconversion in humans by adoptive transfer of immunity to hepatitis B core antigen. Gastroenterology 122614-624. [DOI] [PubMed] [Google Scholar]
- 15.Lauterbach, H., E. I. Zuniga, P. Truong, M. B. Oldstone, and D. B. McGavern. 2006. Adoptive immunotherapy induces CNS dendritic cell recruitment and antigen presentation during clearance of a persistent viral infection. J. Exp. Med. 2031963-1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linsley, P. S., P. M. Wallace, J. Johnson, M. G. Gibson, J. L. Greene, J. A. Ledbetter, C. Singh, and M. A. Tepper. 1992. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science 257792-795. [DOI] [PubMed] [Google Scholar]
- 17.Masopust, D., K. Murali-Krishna, and R. Ahmed. 2007. Quantitating the magnitude of the lymphocytic choriomeningitis virus-specific CD8 T-cell response: it is even bigger than we thought. J. Virol. 812002-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGavern, D. B., U. Christen, and M. B. Oldstone. 2002. Molecular anatomy of antigen-specific CD8+ T cell engagement and synapse formation in vivo. Nat. Immunol. 3918-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGavern, D. B., and P. Truong. 2004. Rebuilding an immune-mediated central nervous system disease: weighing the pathogenicity of antigen-specific versus bystander T cells. J. Immunol. 1734779-4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murali-Krishna, K., J. D. Altman, M. Suresh, D. J. Sourdive, A. J. Zajac, J. D. Miller, J. Slansky, and R. Ahmed. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8177-187. [DOI] [PubMed] [Google Scholar]
- 21.Ndejembi, M. P., J. R. Teijaro, D. S. Patke, A. W. Bingaman, M. R. Chandok, A. Azimzadeh, S. G. Nadler, and D. L. Farber. 2006. Control of memory CD4 T cell recall by the CD28/B7 costimulatory pathway. J. Immunol. 1777698-7706. [DOI] [PubMed] [Google Scholar]
- 22.Oldstone, M. B., P. Blount, P. J. Southern, and P. W. Lampert. 1986. Cytoimmunotherapy for persistent virus infection reveals a unique clearance pattern from the central nervous system. Nature 321239-243. [DOI] [PubMed] [Google Scholar]
- 23.Pircher, H., K. Burki, R. Lang, H. Hengartner, and R. M. Zinkernagel. 1989. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature 342559-561. [DOI] [PubMed] [Google Scholar]
- 24.Riddell, S. R., K. S. Watanabe, J. M. Goodrich, C. R. Li, M. E. Agha, and P. D. Greenberg. 1992. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science 257238-241. [DOI] [PubMed] [Google Scholar]
- 25.Salomon, B., and J. A. Bluestone. 2001. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu. Rev. Immunol. 19225-252. [DOI] [PubMed] [Google Scholar]
- 26.Smith, C. A., C. Y. Ng, S. K. Loftin, C. Li, H. E. Heslop, M. K. Brenner, and C. M. Rooney. 1996. Adoptive immunotherapy for Epstein-Barr virus-related lymphoma. Leuk. Lymphoma 23213-220. [DOI] [PubMed] [Google Scholar]
- 27.Suresh, M., J. K. Whitmire, L. E. Harrington, C. P. Larsen, T. C. Pearson, J. D. Altman, and R. Ahmed. 2001. Role of CD28-B7 interactions in generation and maintenance of CD8 T cell memory. J. Immunol. 1675565-5573. [DOI] [PubMed] [Google Scholar]
- 28.Teijaro, J. R., M. N. Njau, D. Verhoeven, S. Chandran, S. G. Nadler, J. Hasday, and D. L. Farber. 2009. Costimulation modulation uncouples protection from immunopathology in memory T cell responses to influenza virus. J. Immunol. 1826834-6843. [DOI] [PubMed] [Google Scholar]
- 29.Volkert, M. 1962. Studies on immunological tolerance to LCM virus. A preliminary report on adoptive immunization of virus carrier mice. Acta Pathol. Microbiol. Scand. 56305-301. [PubMed] [Google Scholar]
- 30.Volkert, M. 1963. Studies on immunological tolerance to LCM virus. Treatment of virus carrier mice by adoptive immunization. Acta Pathol. Microbiol. Scand. 57465-487. [PubMed] [Google Scholar]
- 31.Walter, E. A., P. D. Greenberg, M. J. Gilbert, R. J. Finch, K. S. Watanabe, E. D. Thomas, and S. R. Riddell. 1995. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N. Engl. J. Med. 3331038-1044. [DOI] [PubMed] [Google Scholar]
- 32.Whitmire, J. K., B. Eam, and J. L. Whitton. 2008. Tentative T cells: memory cells are quick to respond, but slow to divide. PLoS Pathog. 4e1000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zammit, D. J., L. S. Cauley, Q. M. Pham, and L. Lefrancois. 2005. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity 22561-570. [DOI] [PMC free article] [PubMed] [Google Scholar]