Abstract
Heterologous prime-boost is a common vaccination strategy to elicit CD8+ T cells (TCD8+), and vaccinia virus (VACV) has been widely used as a boosting vector. Studies with other viruses have suggested that priming may reduce responses to native epitopes in boosting vectors as well as improve responses to primed epitopes. We explored this possibility with a VACV model in mice and find that irrespective of an epitope's dominance, prior priming was able to double TCD8+ responses. More surprisingly, and in contrast to findings for other viruses, responses to remaining epitopes were undisturbed, leaving the overall dominance hierarchy unchanged.
CD8+ T cells (TCD8+) are important effectors in antiviral immunity (4, 16), recognizing virus peptides presented on infected cells by major histocompatibility complex class I (20). Not all antigenic virus peptides are immunogenic, and for those that are, the response sizes range widely. This phenomenon, known as immunodominance, is well recognized, but the underlying mechanisms are complex (12, 17, 18). One potential mechanism is immunodomination, where TCD8+ that recognize a dominant epitope reduce responses to epitopes lower in the hierarchy.
Vaccination strategies that induce robust TCD8+ immunity are being pursued for many pathogens and cancers (19), and a variety of vectors, including vaccinia virus (VACV), have been examined. However, where explored, antivector immunity to these vaccines is vastly superior to the immunity induced to the recombinant antigen (1, 3, 14). Immunodomination may contribute to this problem if native epitopes in the vector are more highly ranked in the dominance hierarchy than are epitopes in the recombinant antigen. This might be especially so for large vectors, such as poxviruses, that have many native epitopes (8, 9).
Heterologous prime-boost systems have been used in animal models and humans to increase TCD8+ responses (6, 7, 13). It has also been suggested that memory TCD8+ induced by prime-boost immunization are of higher avidity than those elicited by other strategies (2). Another potential benefit is that priming with an antigen of choice might reduce responses to vector antigens via immunodomination. Once primed, TCD8+ recognizing the recombinant epitope will be numerically superior and able to respond more rapidly upon boosting than naïve TCD8+ encountering vector epitopes for the first time. Indeed, precedents for this have been established with lymphocytic choriomeningitis virus (LCMV) and influenza A virus (1, 10). In both cases, at least some epitopes that are subdominant after virus infection could be shifted to the top of the hierarchy by prior priming. This rearrangement of the hierarchy has been shown to result from increases in responses to the subdominant epitope and a concomitant decrease in responses to the usually dominant epitope (1, 10).
Here, we report a series of experiments that answer two questions for prime-boost strategies that use VACV as the boosting vector. First, can any epitope benefit from priming irrespective of its position in the overall immunodominance hierarchy? Second, does prior priming reduce responses to nonprimed epitopes in VACV, as was shown for LCMV and influenza virus?
The main model system used was priming C57BL/6 mice with peptides and α-galactosylceramide (α-GalCer) (13) and boosting with VACV. Native VACV epitopes and VACV strain WR were used rather than recombinant antigens in an engineered VACV (as is usual in prime-boost experiments) to take advantage of an already-established immunodominance hierarchy (15). The peptides examined were (from most to least dominant): B8R20-27 (B8R, TSYKFESV, H-2 Kb restricted), K3L6-15 (K3L, YSLDNAGDVI, H-2 Db restricted) and A19L47-55 (A19L, VSLDYINTM, H-2 Kb restricted) (15). All mice were at least 8 weeks old, and experiments done in accordance with ethical requirements.
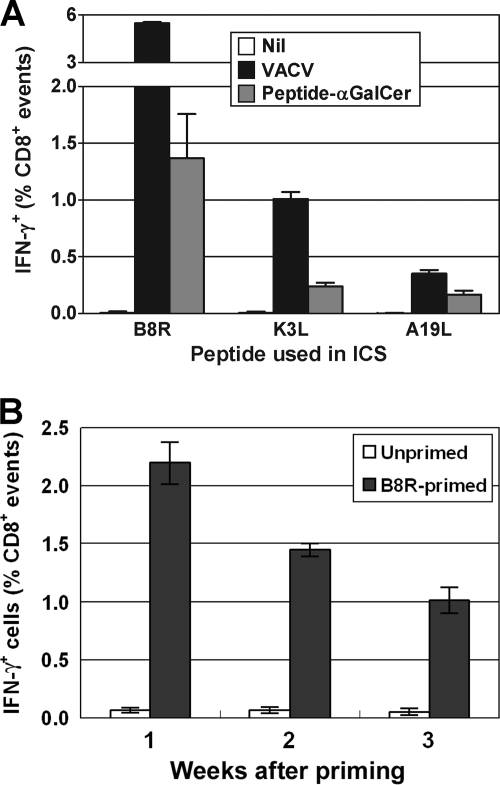
First, we determined the number of TCD8+ induced by peptide/α-GalCer immunization compared with the number induced by VACV infection. Mice were injected intraperitoneally (i.p.) with 100 μg of B8R, K3L, or A19L peptide (Genscript, Piscataway, NJ) mixed with 1 μg αGalCer (Alexis Biochemicals, Farmingdale, NY) in phosphate-buffered saline (13) or with 1 × 106 PFU of VACV. Peptide-specific TCD8+ in spleens were measured after 7 days using intracellular cytokine staining (ICS) for gamma interferon (IFN-γ) after a 4-h ex vivo culture with peptide in the presence of brefeldin A (15). All peptides primed a measurable TCD8+ response when used in immunizations, and the hierarchy mirrored that seen after VACV infection, but responses were lower (approximately one-fifth for B8R and K3L and one-half for A19L) (Fig. 1A). No peptide-specific TCD8+ were found in naïve mice. Next, we wanted to see how rapidly TCD8+ declined after immunization with peptide/α-GalCer to choose the best time for boosting with VACV. This was done using the same method as that described above, but only B8R was examined. The magnitude of the anti-B8R response was highest after 7 days, at over 2% of TCD8+ in the spleen, and waned over the following 2 weeks (Fig. 1B), and so we chose to boost at 7 days for the next set of experiments.
FIG. 1.
TCD8+ responses after a single injection of peptide/αGalCer. Groups of three C57BL/6 mice were injected i.p. with 100 μg peptide (in panel A, same as used in ICS; in panel B, B8R) with 1 μg αGalCer in 100 μl of phosphate-buffered saline/0.5% Tween 20 buffer, or with 1 × 106 PFU VACV (A, black bar) or 100 μl buffer (B, white bar). A group of naïve controls were included in panel A (Nil). Peptide-specific TCD8+ in spleens were measured using ICS for IFN-γ at 7 days (A) and the indicated time points (B). (A) Data are percentages of TCD8+ that produce IFN-γ in ex vivo stimulations with relevant peptides after subtraction of background (determined using mock stimulation in ICS). (B) Data are raw values of percent TCD8+ that produce IFN-γ in ex vivo stimulations with B8R; means and standard errors of the means (SEM) are plotted.
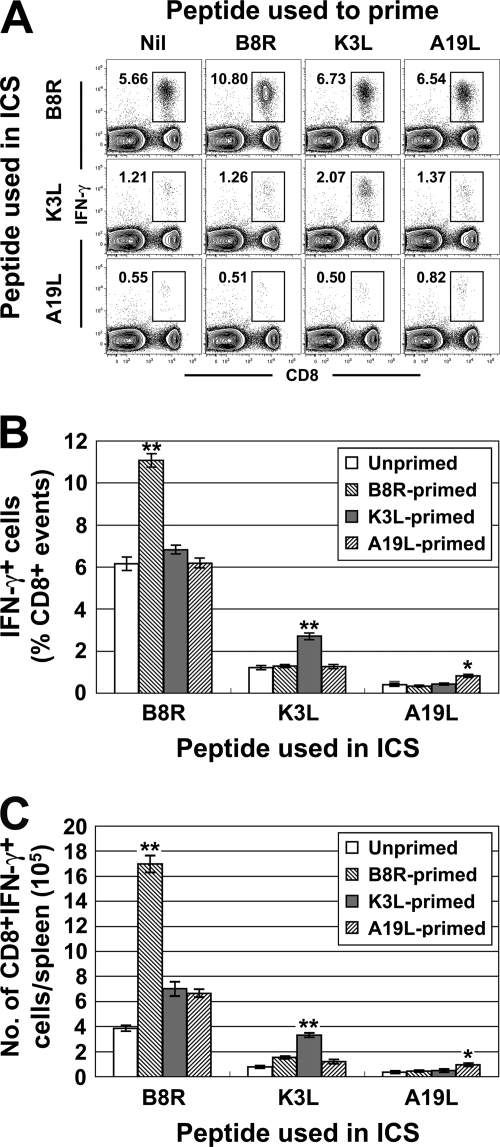
To examine our main question, groups of three mice were primed with VACV peptides in αGalCer and boosted after 7 days by i.p. injection of 1 × 106 PFU of VACV. After 7 days, splenic TCD8+ responses to the three peptides were determined by ICS (Fig. 2). This experiment was repeated twice, and because data across these experiments were highly consistent, they were pooled for analysis. In the absence of peptide priming, TCD8+ responses were similar to those in published data and in Fig. 1A (15). In all mice primed with a VACV peptide, the TCD8+ response to that peptide was increased nearly twofold. Strikingly, however, preimmunization with a single peptide had no effect on responses to any other peptides; even a near doubling of the B8R-specific response had no impact on responses to the much weaker K3L and A19L peptides. This result held, irrespective of whether responses were analyzed as percent TCD8+ (Fig. 2B) or total number of peptide-specific cells (Fig. 2C) in the spleen.
FIG. 2.
TCD8+ responses after peptide priming and a VACV boost. Groups of C57BL/6 mice were primed with 100 μg of peptide (shown across the top of panel A and in the legends of panels B and C) in 1 μg αGalCer and boosted with 1 × 106 PFU VACV-WR (both i.p.) after 7 days. Peptide-specific TCD8+ in spleens were measured by ICS 7 days after the boost. (A) Representative flow cytometry analysis showing lymphocyte gated events on a plot of IFN-γ+ cells versus CD8+ events. The bold number in each plot is the number of IFN-γ+, CD8+ events as a percent of CD8+ events. (B) Percent of TCD8+ that produce IFN-γ in ex vivo stimulations with the peptides shown after subtraction of background (determined using mock stimulation in ICS). Data are from three independent experiments, each with groups of three mice (n = 9), and means and SEM are plotted. (C) Data are from the same mice as in panel B, but total numbers of peptide-specific TCD8+ in the spleen are shown. *, P < 0.05; **, P < 0.01 compared to any other group.
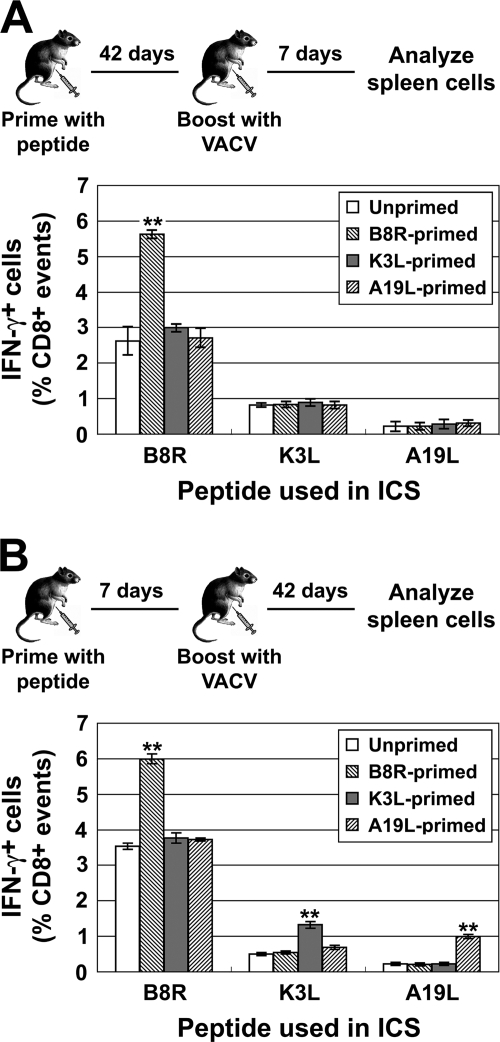
To explore this result further, we asked whether the time between prime and boost was important in increasing TCD8+ responses to selected epitopes by boosting with VACV 42 days after peptide priming (Fig. 3A). At this time, there should be no residual effect of α-GalCer, and primed TCD8+ should have a memory phenotype. In these experiments, only responses to B8R, the most dominant peptide, were consistently improved by peptide priming (Fig. 3A). However, even when B8R responses were significantly increased, there was no impact on responses to K3L and A19L. This general picture remained when TCD8+ responses were analyzed as total numbers of peptide-specific cells per spleen, although there was some evidence of a boost for K3L after priming (not shown). We speculated that the number of K3L- and A19L-specific TCD8+ may have fallen below a functional threshold by 6 weeks after priming. In support of this idea, further experiments showed that only B8R responses were measurable 4 and 6 weeks after peptide priming (means of 0.3% and 0.17% of TCD8+, respectively). K3L-specific responses were just above 0.1% of TCD8+ at 4 weeks and at background levels after 6 weeks (∼0.05% TCD8+), as were A19L-specific TCD8+ at both times. It remains possible that functional characteristics of K3L- and A19L-specific TCD8+ were also compromised, but this was not pursued.
FIG. 3.
TCD8+ responses when boosting is delayed after priming or responses are measured 6 weeks after boosting. Mice were primed with peptides/αGalCer as in previous experiments and boosted with 1 × 106 PFU VACV 42 (A) or 7 (B) days later. Peptide-specific TCD8+ in spleens were measured by ICS 7 (A) or 42 (B) days after boosting and are shown as a percentage of CD8+ cells in the spleen (after subtraction of background). Data plotted are means and SEM from six mice, derived from two independent experiments, each using groups of three mice. **, P < 0.01 compared to any other group.
For vaccination protocols, it is important to establish that immunity is durable. To examine this, peptide priming and boosting were done within 7 days, but the final readout of TCD8+ responses was done 42 days after boosting (Fig. 3B). As was seen at 7 days after infection, peptide priming increased the number of TCD8+ responding to the relevant epitope without altering responses to other specificities (Fig. 3B). In this experiment, it appears that less-dominant epitopes are boosted to a greater extent than more-dominant epitopes, with A19L responses being increased 3-fold while B8R responses were improved 1.5-fold. This leads to more A19L-specific TCD8+ than K3L-specific TCD8+ in mice primed with A19L peptide, but no specificity ever comes close to that of B8R in terms of dominance. These data demonstrate that the advantage conferred by prior priming is retained in long-term memory responses.
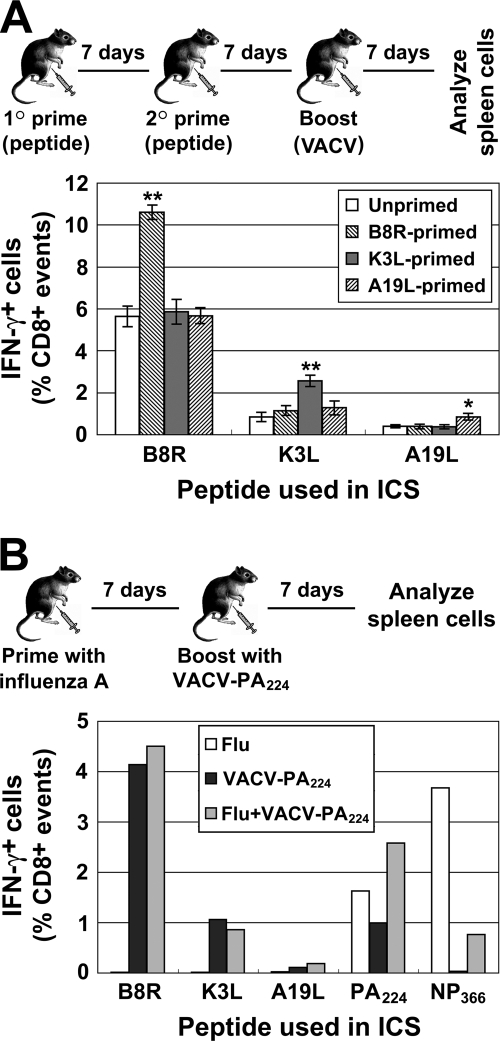
Finally, given the disparity between our results and those published previously for LCMV and influenza A virus (1, 10), we wanted to increase the chance of shifting VACV immunodominance hierarchies by stronger priming. Two experiments were done. First, mice were primed twice with peptide before boosting with VACV. Second, mice were primed by i.p. injection with influenza A virus and boosted with a recombinant VACV expressing the PA224-233 epitope (VACV-PA224) (5). In each case, the size of peptide-specific responses was determined a week after VACV boosting (Fig. 4). The first experiment, in which mice were primed twice with peptide, gave a result that was almost identical to that obtained by a single peptide prime (Fig. 4a). In the second experiment, influenza A virus injection primed a substantial PA224-specific response, which exceeded that induced by VACV-PA224 (Fig. 4B). Priming with influenza virus before immunizing with VACV-PA224 increased PA224-specific responses approximately 2.5 times. This boost is similar to that seen for K3L (which ranks with PA224) after peptide/α-GalCer priming (Fig. 2 and 4B). This experiment also confirmed that priming for a recombinant epitope does not alter TCD8+ responses to native VACV peptides. We speculate that even stronger priming may boost responses to an epitope more than twofold. However, it seems unlikely that this will disturb responses to unprimed epitopes, as the experiments described in the present study already represent an advantage in the order of 1,000-fold for primed TCD8+ over the naïve precursor frequency of those not primed. It is not clear why VACV should behave so differently in these kinds of experiments compared with influenza virus and LCMV. We propose the most likely reason is the >10-fold-larger genome size and, thus, the increase in epitope diversity.
FIG. 4.
TCD8+ responses after repeated peptide or influenza A priming followed by a VACV boost. (A) Mice were primed with peptides/αGalCer i.p. twice and boosted with 1 × 106 PFU VACV i.p with 7 days between each injection. (B) Mice were infected with 500 hemagglutination units of influenza virus strain A/PR/8/34 (Flu), 1 × 106 PFU VACV-PA224 (which encodes the influenza PA224 peptide as an ER-targeted minigene; VACV-PA224), or A/PR/8/34 followed by a VACV-PA224 boost 7 days later (Flu+VACV-PA224). Peptide-specific TCD8+ in spleens were measured by ICS 7 days after the last immunization and are shown as a percentge of CD8+ cells in the spleen (after subtraction of background). (A) Data are means and SEM from six mice derived from two independent experiments, each using groups of three mice. *, P < 0.05; **, P < 0.01 compared to any other group. (B) Data are the mean from groups of three mice.
In conclusion, data presented here demonstrate three points of relevance to vaccination with recombinant VACV. (i) Irrespective of an epitope's position in the VACV dominance hierarchy, peptide-specific TCD8+ can always be increased by prior priming. (ii) This increase approaches twofold but is in the context of a dominance hierarchy that spans at least 100-fold. Simply priming before boosting with VACV is then unlikely to produce the very large numbers of TCD8+ that may be required for protection against some pathogens (11). (iii) The immunodominance hierarchy of VACV is exceptionally stable, and even large numbers of extra TCD8+ with a dominant specificity do not reduce responses to other, less-dominant peptides. Therefore, immunodomination is unlikely to be a major problem for VACV vaccines despite the apparently overwhelming size of anti-VACV TCD8+ responses.
Acknowledgments
We thank Lisa Alleva for titered stocks of influenza A virus and Jon Yewdell and Jack Bennink for VACV-PA224.
This work was funded by grants from the National Health and Medical Research Council (Australia) to D.C.T. (Biomedical CDA 418108 and project 389819) and Y.W. (training fellowship 316978) and the National Institute of Allergy and Infectious Diseases, NIH, to D.C.T. (R01 AI067401).
Footnotes
Published ahead of print on 17 June 2009.
REFERENCES
- 1.Chen, W., L. C. Anton, J. R. Bennink, and J. W. Yewdell. 2000. Dissecting the multifactorial causes of immunodominance in class I-restricted T cell responses to viruses. Immunity 1283-93. [DOI] [PubMed] [Google Scholar]
- 2.Estcourt, M. J., A. J. Ramsay, A. Brooks, S. A. Thomson, C. J. Medveckzy, and I. A. Ramshaw. 2002. Prime-boost immunization generates a high frequency, high-avidity CD8+ cytotoxic T lymphocyte population. Int. Immunol. 1431-37. [DOI] [PubMed] [Google Scholar]
- 3.Harrington, L. E., R. van der Most, J. L. Whitton, and R. Ahmed. 2002. Recombinant vaccinia virus-induced T-cell immunity: quantitation of the response to the virus vector and the foreign epitope. J. Virol. 763329-3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaech, S. M., E. J. Wherry, and R. Ahmed. 2002. Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2251-262. [DOI] [PubMed] [Google Scholar]
- 5.Lev, A., K. Takeda, D. Zanker, J. C. Maynard, P. Dimberu, E. Waffarn, J. Gibbs, N. Netzer, M. F. Princiotta, L. Neckers, D. Picard, C. V. Nicchitta, W. Chen, Y. Reiter, J. R. Bennink, and J. W. Yewdell. 2008. The exception that reinforces the rule: crosspriming by cytosolic peptides that escape degradation. Immunity 28787-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McConkey, S. J., W. H. Reece, V. S. Moorthy, D. Webster, S. Dunachie, G. Butcher, J. M. Vuola, T. J. Blanchard, P. Gothard, K. Watkins, C. M. Hannan, S. Everaere, K. Brown, K. E. Kester, J. Cummings, J. Williams, D. G. Heppner, A. Pathan, K. Flanagan, N. Arulanantham, M. T. Roberts, M. Roy, G. L. Smith, J. Schneider, T. Peto, R. E. Sinden, S. C. Gilbert, and A. V. Hill. 2003. Enhanced T-cell immunogenicity of plasmid DNA vaccines boosted by recombinant modified vaccinia virus Ankara in humans. Nat. Med. 9729-735. [DOI] [PubMed] [Google Scholar]
- 7.Moore, A. C., and A. V. Hill. 2004. Progress in DNA-based heterologous prime-boost immunization strategies for malaria. Immunol. Rev. 199126-143. [DOI] [PubMed] [Google Scholar]
- 8.Moutaftsi, M., B. Peters, V. Pasquetto, D. C. Tscharke, J. Sidney, H. H. Bui, H. Grey, and A. Sette. 2006. A consensus epitope prediction approach identifies the breadth of murine TCD8+-cell responses to vaccinia virus. Nat. Biotechnol. 24817-819. [DOI] [PubMed] [Google Scholar]
- 9.Oseroff, C., B. Peters, V. Pasquetto, M. Moutaftsi, J. Sidney, V. Panchanathan, D. C. Tscharke, B. Maillere, H. Grey, and A. Sette. 2008. Dissociation between epitope hierarchy and immunoprevalence in CD8 responses to vaccinia virus Western Reserve. J. Immunol. 1807193-7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez, F., S. Harkins, M. K. Slifka, and J. L. Whitton. 2002. Immunodominance in virus-induced CD8+ T-cell responses is dramatically modified by DNA immunization and is regulated by gamma interferon. J. Virol. 764251-4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt, N. W., R. L. Podyminogin, N. S. Butler, V. P. Badovinac, B. J. Tucker, K. S. Bahjat, P. Lauer, A. Reyes-Sandoval, C. L. Hutchings, A. C. Moore, S. C. Gilbert, A. V. Hill, L. C. Bartholomay, and J. T. Harty. 2008. Memory CD8 T cell responses exceeding a large but definable threshold provide long-term immunity to malaria. Proc. Natl. Acad. Sci. USA 10514017-14022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sercarz, E. E., P. V. Lehmann, A. Ametani, G. Benichou, A. Miller, and K. Moudgil. 1993. Dominance and crypticity of T cell antigenic determinants. Annu. Rev. Immunol. 11729-766. [DOI] [PubMed] [Google Scholar]
- 13.Silk, J. D., I. F. Hermans, U. Gileadi, T. W. Chong, D. Shepherd, M. Salio, B. Mathew, R. R. Schmidt, S. J. Lunt, K. J. Williams, I. J. Stratford, A. L. Harris, and V. Cerundolo. 2004. Utilizing the adjuvant properties of CD1d-dependent NK T cells in T cell-mediated immunotherapy. J. Clin. Investig. 1141800-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith, C. L., F. Mirza, V. Pasquetto, D. C. Tscharke, M. J. Palmowski, P. R. Dunbar, A. Sette, A. L. Harris, and V. Cerundolo. 2005. Immunodominance of poxviral-specific CTL in a human trial of recombinant-modified vaccinia Ankara. J. Immunol. 1758431-8437. [DOI] [PubMed] [Google Scholar]
- 15.Tscharke, D. C., G. Karupiah, J. Zhou, T. Palmore, K. R. Irvine, S. M. Haeryfar, S. Williams, J. Sidney, A. Sette, J. R. Bennink, and J. W. Yewdell. 2005. Identification of poxvirus CD8+ T cell determinants to enable rational design and characterization of smallpox vaccines. J. Exp. Med. 20195-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yap, K. L., G. L. Ada, and I. F. McKenzie. 1978. Transfer of specific cytotoxic T lymphocytes protects mice inoculated with influenza virus. Nature 273238-239. [DOI] [PubMed] [Google Scholar]
- 17.Yewdell, J. W., and J. R. Bennink. 1999. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu. Rev. Immunol. 1751-88. [DOI] [PubMed] [Google Scholar]
- 18.Yewdell, J. W., and M. Del Val. 2004. Immunodominance in TCD8+ responses to viruses: cell biology, cellular immunology, and mathematical models. Immunity 21149-153. [DOI] [PubMed] [Google Scholar]
- 19.Yewdell, J. W., and S. M. Haeryfar. 2005. Understanding presentation of viral antigens to CD8+ T cells in vivo: the key to rational vaccine design. Annu. Rev. Immunol. 23651-682. [DOI] [PubMed] [Google Scholar]
- 20.Zinkernagel, R. M., and P. C. Doherty. 1979. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv. Immunol. 2751-177. [DOI] [PubMed] [Google Scholar]