Abstract
The process by which nonenveloped viruses cross cell membranes during host cell entry remains poorly defined; however, common themes are emerging. Here, we use correlated in vivo and in vitro studies to understand the mechanism of Flock House virus (FHV) entry and membrane penetration. We demonstrate that low endocytic pH is required for FHV infection, that exposure to acidic pH promotes FHV-mediated disruption of model membranes (liposomes), and particles exposed to low pH in vitro exhibit increased hydrophobicity. In addition, FHV particles perturbed by heating displayed a marked increase in liposome disruption, indicating that membrane-active regions of the capsid are exposed or released under these conditions. We also provide evidence that autoproteolytic cleavage, to generate the lipophilic γ peptide (4.4 kDa), is required for membrane penetration. Mutant, cleavage-defective particles failed to mediate liposome lysis, regardless of pH or heat treatment, suggesting that these particles are not able to expose or release the requisite membrane-active regions of the capsid, namely, the γ peptides. Based on these results, we propose an updated model for FHV entry in which (i) the virus enters the host cell by endocytosis, (ii) low pH within the endocytic pathway triggers the irreversible exposure or release of γ peptides from the virus particle, and (iii) the exposed/released γ peptides disrupt the endosomal membrane, facilitating translocation of viral RNA into the cytoplasm.
Flock House virus (FHV), a nonenveloped, positive-sense RNA virus, has been employed as a model system in several important studies to address a wide range of biological questions (reviewed in reference 55). FHV has been instrumental in understanding virus structure and assembly (17, 19, 45), RNA replication (2, 3, 37), and specific packaging of the genome (33, 44, 53, 54). Studies of FHV infection in Drosophila melanogaster flies have provided valuable information about the antiviral innate immune response in invertebrate hosts (29, 57). FHV is also used in nanotechnology applications as an epitope-presenting platform to develop novel vaccines and medical therapies (31, 48). In this report, we use FHV as a model system to further elucidate the means by which nonenveloped viruses enter host cells and traverse cellular membranes.
During cell entry enveloped and nonenveloped viral capsid proteins undergo structural rearrangements that enable the virus to breach the membrane bilayer, ultimately releasing the viral genome or nucleocapsid into the cytoplasm. These entry-related conformational changes have been well characterized for enveloped viruses, which use membrane fusion to cross membrane bilayers (reviewed in reference 59). However, the mechanisms nonenveloped viruses employ to breach cellular membranes are poorly defined. Recently, significant parallels in the mechanisms of cell entry have emerged for a diverse group of nonenveloped viruses. Specifically, programmed capsid disassembly and release of small membrane-interacting peptides appear to be a common theme (reviewed in references 4 and 50).
The site of membrane penetration depends upon the route of virus entry into the cell. Viruses can enter host cells via several distinct pathways, including clathrin-mediated endocytosis, caveolae-mediated endocytosis, lipid raft-mediated endocytosis, and macropinocytosis (reviewed in reference 40). The two primary routes of virus entry are clathrin-mediated endocytosis, where viruses encounter an acidic environment, and caveolae-mediated endocytosis, which is pH neutral. Many nonenveloped viruses, including adenovirus (24, 52), parvovirus (6), and reovirus (34, 49), require acidic pH during entry. However, numerous nonenveloped viruses have acid-independent entry mechanisms, including rotavirus (28), polyomavirus (43), simian virus 40 (41, 51), and several members of the picornavirus family (7, 14, 32, 42).
Upon reaching the appropriate site of membrane penetration, nonenveloped virus capsid proteins are triggered by cellular factors, such as receptor binding and/or exposure to low pH within endosomes, to undergo conformational changes necessary for membrane interactions. These tightly regulated structural rearrangements may include capsid disassembly, exposure of hydrophobic regions, and/or release of membrane-lytic factors. For example, low pH within endosomes triggers adenovirus capsid disassembly, leading to the release of the membrane lytic protein VI (24, 60). In contrast, poliovirus is activated for membrane penetration by a pH-independent mechanism. Receptor binding triggers the poliovirus capsid to undergo a conformational change, resulting in the exposure of the N terminus of VP1 and the release of VP4 (18, 23), both of which facilitate membrane interactions (20). Notably, even though some viruses, such as reovirus, enter cells via an acidic endocytic pathway, membrane penetration is not acid activated (16), indicating that exposure to low pH and membrane penetration are not always mutual events.
The overall simplicity of the FHV capsid, composed of a single gene product, along with the wealth of available high-resolution structural information (reviewed in reference 45) make FHV an ideal candidate for understanding nonenveloped virus entry and infection. FHV, a member of the family Nodaviridae, is a nonenveloped insect virus with a bipartite RNA genome surrounded by an icosahedral protein capsid. The quasi-equivalent T=3 virion (∼300-Å diameter) is initially composed of 180 copies of a single coat precursor protein α (44 kDa). Following capsid assembly the α protein undergoes autocatalytic cleavage to generate two particle-associated cleavage products, a large N-terminal fragment, β (39 kDa), and a small C-terminal fragment, γ (4.4 kDa) (22), creating the infectious virion (46). Mutant FHV particles that do not undergo autocatalytic cleavage, and therefore cannot release the γ peptide, are not infectious (46). It has been hypothesized that these particles are noninfectious because they cannot mediate membrane penetration, but this has never been shown directly.
The FHV X-ray structure revealed that the γ peptides were located inside the capsid shell with residues 364 to 385 forming amphipathic helices (19). Subsequent studies showed that the FHV capsid is dynamic, with γ transiently exposed to the exterior of the capsid (11). These findings led to a structure-based model of FHV membrane disruption in which the dynamic γ peptides are reversibly exposed to the surface of the capsid (11), “sampling” the environment until they encounter the appropriate cellular trigger. The virus is then activated to undergo an irreversible conformational change in which the γ helical bundles located at each fivefold axis are externalized and released from the virus particle (17, 19). Upon release, the γ pentameric helical bundles are predicted to insert into and create a local disruption of the membrane bilayer to allow the RNA to enter the cytoplasm (10).
While biochemical and structural studies have provided the foundation for a model of FHV cell entry, more rigorous in vivo and in vitro studies are necessary to confirm the ideas put forth in this model. Here, we clarify the route of FHV entry and characterize the tightly regulated events required for FHV membrane penetration. We demonstrate for the first time that low endocytic pH is required for FHV infection, that acidic pH promotes FHV membrane penetration, and that particles exposed to low pH exhibit increased hydrophobicity. In addition, we provide evidence that mutant, cleavage-defective particles are blocked specifically at the membrane penetration step during cell entry. Taken together, these findings offer an experimentally supported model of FHV entry into host cells. In addition, these results add to the accumulating evidence that nonenveloped viruses employ common mechanisms to traverse cellular membranes.
MATERIALS AND METHODS
Cells and viruses.
Schneider's line 1 and line 2 Drosophila melanogaster cells (DL-1 and S2, respectively) were grown in Schneider's insect medium (Sigma) supplemented with 15% heat-inactivated fetal bovine serum (Gibco) and penicillin (100 U/ml)-streptomycin (100 μg/ml) (Gibco) as described previously (21). FHV particles were purified from infected or transfected DL-1 cells using established protocols (44). Mutant virus particles were prepared from existing clones as previously described (46).
Electron microscopy.
Samples were prepared for electron microscopy as previously described (56). FHV particles were stained with 2% Nano-W (methylamine tungstate; Nanoprobes). The samples were viewed using a Phillips CM 100 transmission electron microscope at 100 keV.
Infectivity experiments.
FHV growth in DL-1 cells was monitored in the presence or absence of NH4Cl or bafilomycin A1 (Sigma). DL-1 cells (2 × 106 cells/ml) were preincubated in growth medium with or without inhibitor at 4°C for 1 h. Following pretreatment, the cells were pelleted (500 × g) and resuspended in growth medium (with or without inhibitor) at a concentration of 4 × 107 cells/ml. FHV was added at a multiplicity of infection (MOI) of 1 PFU per cell and the virus was allowed to attach to cells at 4°C for 1 h. Following attachment, cells were diluted to a concentration of 2 × 106 cells/ml in growth medium (with or without inhibitor) and 2 × 106 cells/well were added to a 12-well plate. The plates were transferred to 27°C, and samples were collected and frozen at either 0 or 24 h postinfection. Cell lysates were generated by freeze-thawing the samples, and FHV infectious titers of the lysates were determined by plaque assay as described previously (46). The amount of FHV progeny produced during the 24-h growth period was determined by the following equation: (log10 titer at 24 h) − (log10 titer at 0 h).
Determination of cell viability.
Trypan blue staining was used to determine cell viability. Cells were incubated (2 × 106 cells/well in a 12-well plate) at 27°C for 24 h in growth medium containing the indicated concentration of NH4Cl or bafilomycin A1. Cells were resuspended, mixed 1:1 with 0.4% trypan blue solution (Sigma), and scored as dead/dying (stained) or living (not stained). At least 500 cells were scored for each sample.
Liposome dye release assay.
Liposomes composed of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC; Avanti Polar Lipids, Inc.) containing the entrapped fluorescent dye sulforhodamine B (SulfoB; Invitrogen/Molecular Probes) were prepared as described previously, with slight modifications (8). Briefly, DOPC lipids dissolved in chloroform were dried under nitrogen gas and rehydrated in 50 mM HEPES (pH 7.0) containing 100 mM SulfoB to a final lipid concentration of 1.0 mg/ml (1.2 × 10−3 M). The lipid suspension was disrupted by three freeze-thaw cycles in liquid nitrogen and passed through a lipid extruder (Avanti Polar Lipids, Inc.) equipped with a 0.1-μm polycarbonate membrane filter. The liposomes containing entrapped SulfoB were separated from free fluorescent dye using a PD-10 size exclusion column (GE Healthcare) equilibrated with 50 mM HEPES (pH 7.0).
To measure FHV-mediated dye release from liposomes, a 100-μl reaction mixture was prepared containing 1.5 μl FHV (1.1 × 10−9 to 5.3 × 10−8 M final concentration), 1.0 μl SulfoB liposomes (1.2 × 10−5 M final concentration), and 97.5 μl buffer of the indicated pH. The molar concentration of gamma peptides was determined by multiplying the FHV molar concentration by 180 (the number of copies of γ peptides per virus particle). The amount of liposomes used in these experiments was determined empirically to optimize the level of signal to noise, and a 1-μl volume was used for all experiments. All reactions were carried out at 20°C. The extent of FHV-mediated liposome rupture was determined by measuring the increase in fluorescence due to dequenching of SulfoB. Fluorescence intensity was measured with a Cary Eclipse fluorescence spectrophotometer (Varian) using an excitation wavelength of 535 nm and an emission wavelength of 585 nm. Triton X-100 was added to liposomes to a final concentration of 0.1% (wt/vol) to determine 100% SulfoB release. The percent SulfoB released was calculated using the following equation: percent SulfoB released = 100 × [(Fsample − Fblank)/(FTX100 − Fblank)], where Fsample is the fluorescence intensity measured for each sample, FTX100 is the fluorescence intensity in the presence of 0.1% TX-100, and Fblank is the fluorescence intensity in the absence of virus.
To test the reversibility of FHV pH-dependent membrane disruption, FHV particles were incubated in 20 mM bis-Tris, pH 6.0, for 1 h at 22°C, diluted 10-fold in 100 mM HEPES, pH 7.0, and incubated for 1 to 20 h at 22°C. The pH of the neutralized solution was confirmed by performing the same dilution on a larger scale and measuring the resulting pH with a pH meter (Accumet Research). The neutralized particles were added to SulfoB-loaded liposomes at a concentration of 0.1 mg/ml and incubated for 20 min at 20°C, and SulfoB fluorescence was measured as described above.
bis-ANS fluorescence of FHV.
bis-4,4-Dianilino-1,1-binaphthyl-5,5-disulfonic acid, dipotassium salt (bis-ANS) was obtained from Invitrogen/Molecular Probes. To measure bis-ANS binding to FHV, a 100-μl reaction mixture was prepared containing 10 μg FHV, 50 μg bis-ANS, and buffer of the indicated pH. The amount of bis-ANS fluorescence was measured for each pH condition following a 60-min incubation. Fluorescence intensity was measured with a Cary Eclipse fluorescence spectrophotometer (Varian) using an excitation wavelength of 405 nm and an emission wavelength of 485 nm. bis-ANS fluorescence intensity (I) was calculated using the following equation: Icorrected = Isample − Iblank, where Isample is the fluorescence intensity measured for each sample and Iblank is the fluorescence intensity in the absence of virus.
Preparation of heat-treated FHV for the liposome dye release assay.
In order to minimize nonspecific protein binding to the surface of the tube, all reactions were carried out in siliconized low-retention microtubes (Fisher Scientific). For each sample, 0.1 mg/ml FHV in 50 mM HEPES, pH 7.0, was incubated as indicated at 22°C, 45°C, 65°C, or boiled for 10 min. Following incubation at the appropriate temperature, the samples were cooled on ice for 10 min. The liposome dye release assay was performed as described above; each reaction mixture contained 10 μl of heat-treated virus particles (0.01 mg/ml; 1.1 × 10−9 M final concentration), 1 μl SulfoB-loaded liposomes, and 89 μl 50 mM HEPES, pH 7.0.
RESULTS
FHV infection requires acidic endocytic pH.
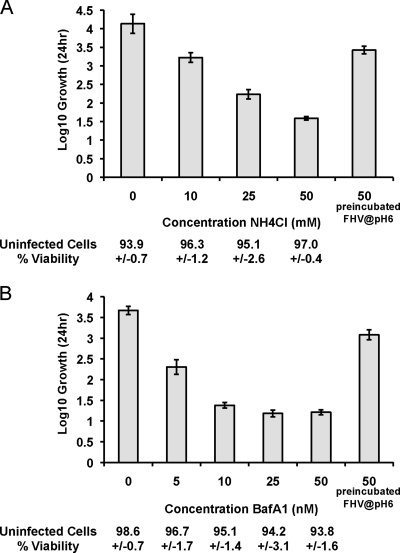
To determine whether FHV entry is pH dependent, the lysosomotropic agent NH4Cl or the vacuolar H+ ATPase inhibitor bafilomycin A1 was used to raise the pH of the endocytic compartment during FHV infection. DL-1 cells were infected with FHV at an MOI of 1 PFU/cell in growth medium containing increasing concentrations of NH4Cl or bafilomycin A1. At 24 h postinfection, viral growth was measured by plaque assay. As the concentration of NH4Cl or bafilomycin A1 in the growth medium was increased, the infectivity decreased (Fig. 1), indicating low endocytic pH is required for FHV infection. In addition, trypan blue exclusion was used to show that the concentrations of NH4Cl and bafilomycin A1 used in these experiments were not toxic to uninfected DL-1 cells (Fig. 1).
FIG. 1.
Infectivity of FHV in DL-1 cells in the presence and absence of NH4Cl or bafilomycin A1. DL-1 cells were infected with FHV at an MOI of 1 PFU/cell in the presence or absence of NH4Cl (A) or bafilomycin A1 (BafA1) (B) in the growth medium. Where indicated, FHV was incubated at pH 6.0 for 1 h prior to infection. Samples were harvested at 24 h postinfection, and infectious titers were determined by plaque assay. The amount of FHV progeny produced (log10 growth) was determined by the log10 titer at 24 h minus the log10 titer at 0 h. Each bar represents the average ± standard deviation of three independent infections. Cell viability was determined by incubating uninfected cells in growth medium containing the indicated concentration of NH4Cl or bafilomycin A1. After 24 h of incubation, the proportion of viable cells (percent viability) was determined by trypan blue staining.
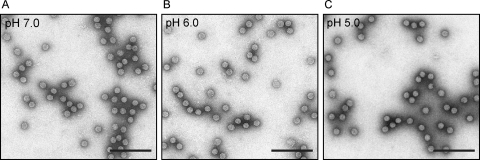
We then investigated whether FHV particles exposed to low pH in vitro prior to infection could circumvent the need for low endocytic pH during infection. FHV particles were incubated at pH 6.0 at 22°C for 1 h prior to infection. The virus was then diluted in growth medium (pH 7.6) containing 50 mM NH4Cl or 50 nM bafilomycin A1 and added to DL-1 cells at an MOI of 1 PFU/cell. At 24 h postinfection, viral growth was measured by plaque assay. In contrast to untreated FHV, virus particles that were preincubated at pH 6.0 were highly infectious in the presence of 50 mM NH4Cl or 50 nM bafilomycin A1 (Fig. 1). This indicates that low endocytic pH is not required during FHV entry if virus particles have already been exposed to low pH in vitro. In addition, this result provides further evidence that NH4Cl and bafilomycin A1 specifically block infection by inhibiting endosomal acidification. To address the possibility that the FHV capsid is denatured or unstable at low pH, particles were analyzed by negative stain electron microscopy. FHV particles that had been incubated at pH 5.0, 6.0, or 7.0 for 1 h prior to staining with methylamine tungstate (pH 6.8) revealed no apparent morphological differences (Fig. 2A to C). In addition, these low pH-treated FHV particles displayed identical sucrose gradient sedimentation profiles, suggesting no large-scale conformational changes had taken place (data not shown).
FIG. 2.
Electron micrographs of FHV particles. FHV particles were incubated for 1 h at pH 7.0 (A), 6.0 (B), or 5.0 (C). The virus particles were stained with methylamine tungstate and visualized by transmission electron microscopy at a magnification of ×52,000. Bar, 200 nm.
FHV mediates fluorescent dye release from preloaded liposomes in a concentration- and pH-dependent manner.
Considering the finding that low pH is required for FHV entry into cells, we hypothesized that low pH may be required to trigger virus-membrane interactions. To test this, liposomes composed of phospatidylcholine were used as an artificial membrane system, and the capacity of FHV to disrupt the integrity of the liposomal membranes was monitored by the release of an entrapped self-quenching fluorescent dye. For these experiments, the fluorescent dye SulfoB was used, since its fluorescence emission is insensitive to pH.
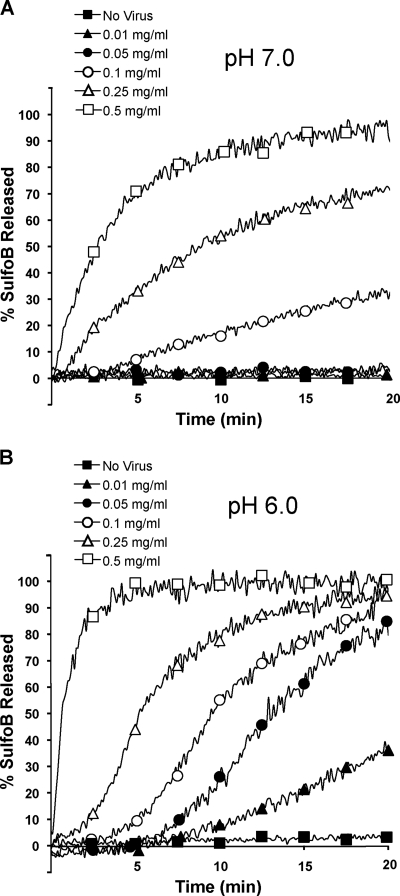
To test whether FHV can permeabilize membranes using this in vitro assay, SulfoB-loaded liposomes were incubated with increasing concentrations of FHV at pH 7.0, and fluorescent dye release was measured as a function of time. As the concentration of FHV increased from 0.01 mg/ml to 0.5 mg/ml, the extent of dye release increased (Fig. 3A). To test the effect of pH on FHV-mediated membrane penetration, the same experiment was repeated at pH 6.0. At pH 6.0, a similar pattern of concentration dependence was observed; the extent of dye release from liposomes increased as the concentration of FHV increased from 0.01 mg/ml to 0.5 mg/ml (Fig. 3B). However, for each FHV concentration tested, the kinetics of fluorescent dye release from liposomes was higher at pH 6.0 than at pH 7.0 (Fig. 3A and B). In addition, liposomes incubated at pH 6.0 in the absence of FHV did not release dye, indicating the integrity of the liposomal membrane was not affected by pH alone (Fig. 3B). These results agree with our hypothesis that when FHV particles are exposed to low pH within endosomes, the virus becomes activated to permeabilize cellular membranes.
FIG. 3.
Concentration and pH dependence of FHV-mediated dye release from SulfoB-loaded liposomes. FHV particles (0.01 to 0.5 mg/ml) were incubated with liposomes containing the entrapped fluorescent dye SulfoB at pH 7.0 (A) or pH 6.0 (B). The release of SulfoB from liposomes was measured as a function of time over the course of a 20-min incubation.
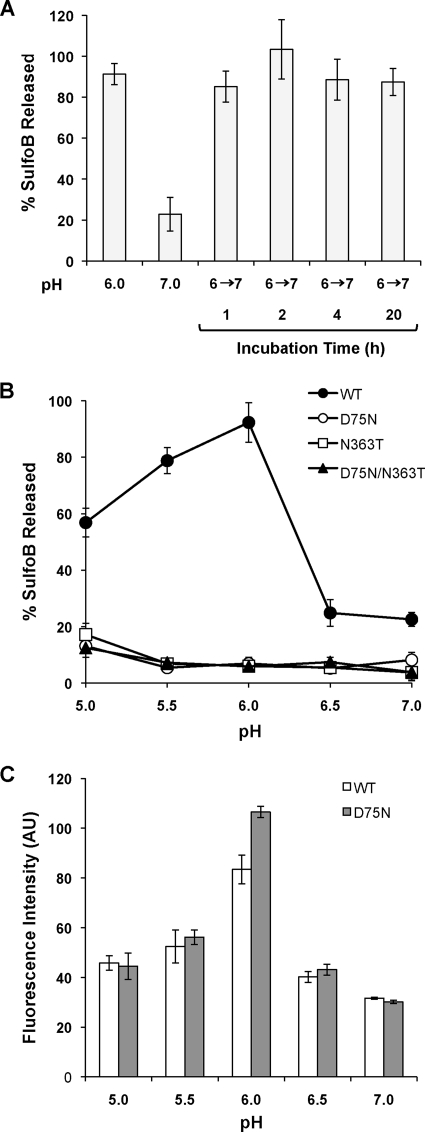
We next sought to examine whether the observed increase in membrane activity at pH 6.0 was reversible. To test this, FHV particles were incubated at pH 6.0 for 1 h, after which the particles were neutralized in pH 7.0 buffer and incubated for an additional 1 to 20 h. These particles were added to SulfoB-loaded liposomes and the fluorescence was measured following a 20-min incubation. As controls, FHV particles that had been preincubated at pH 6.0 or pH 7.0 for 1 h mediated ∼91% and ∼23% dye release from liposomes, respectively (Fig. 4A). Particles that had been incubated at pH 6.0 prior to being neutralized maintained high levels of membrane-lytic activity, regardless of the neutralization time, and mediated similar levels of liposome dye release as pH 6.0-treated particles that had not been neutralized (Fig. 4A). These findings indicate that the observed low-pH-induced membrane-lytic activity is not reversible.
FIG. 4.
Liposome lysis and bis-ANS fluorescence of wild-type and cleavage-defective FHV particles at various pHs. (A) Reversibility of FHV pH-dependent membrane disruption. FHV particles were incubated at pH 6.0 for 1 h, diluted into pH 7.0 buffer (indicated as 6→7), and incubated for 1 to 20 h. The neutralized particles were added to SulfoB-loaded liposomes at a concentration of 0.1 mg/ml, and the percent SulfoB released was determined following a 20-min incubation. As controls, FHV particles preincubated at pH 6.0 or 7.0 for 1 h were added to SulfoB-loaded liposomes at a concentration of 0.1 mg/ml, and the percent SulfoB released was determined after a 20-min incubation. (B) SulfoB-loaded liposomes were incubated with 0.1 mg/ml of either wt-FHV, FHVD75N, FHVN363T, or FHVD75N/N363T at different pHs (pH 5.0 to 7.0). The release of SulfoB from liposomes was measured after 20 min. (C) FHVwt or FHVD75N particles (1 μg) were incubated in buffer ranging from pH 5.0 to 7.0 in the presence of bis-ANS (50 μM). The amount of bis-ANS fluorescence was measured for each pH condition following a 60-min incubation. Fluorescence intensity is given in arbitrary units (AU). Each error bar represents the average ± standard deviation of three independent experiments.
To further investigate the effect of pH on FHV-mediated dye release, FHV was incubated with dye-loaded liposomes in buffer ranging from pH 5.0 to 7.0, and SulfoB release was measured following a 20-min incubation. The highest level of FHV-mediated fluorescent dye released from liposomes occurred at pH 6.0 (Fig. 4B), with ∼90% of the dye released. At pH 5.0 and 5.5, ∼60% and ∼80% dye release from liposomes was observed, respectively. However, little fluorescent dye release, ∼20 to 25%, was observed at pH 6.5 or pH 7.0. In addition, liposomes incubated in buffer pH 5.0 to 7.0 in the absence of FHV did not release dye, indicating that dye release was not affected by pH alone (data not shown). These results support a model in which FHV has little membrane-lytic activity while in the extracellular space (pH ∼6.5 to 7.0) and is triggered for membrane penetration within early endosomes (pH ∼5.9 to 6.0), late endosomes (pH ∼5.6 to 6.0), or lysosomes (pH ∼5.0 to 5.5) (36, 61).
Cleavage-defective FHV particles do not mediate liposome disruption.
The model for FHV entry posits that the small hydrophobic γ peptide inserts into and ruptures membranes. To address the role of γ in membrane penetration, three different mutant particles that do not undergo maturation cleavage of α into β and γ, FHVD75N, FHVN363T, and FHVD75N/N363T, were tested in the liposome dye release assay. These cleavage-defective particles assemble and package the FHV genome normally; however, these particles are noninfectious (46), presumably because the γ peptide is not available to mediate membrane penetration. To investigate the role of γ in FHV-mediated liposome dye release, cleavage-defective particles were incubated with liposomes in buffer ranging from pH 5 to 7 and SulfoB release was measured after 20 min. Under all of the pH conditions tested, cleavage-defective particles mediated only a low level, ∼10 to 20%, of dye release from liposomes (Fig. 4B). This result indicates that maturation cleavage, to generate the γ peptide, is required for FHV membrane penetration and supports the current model of FHV entry.
Hydrophobicity of FHV particles increases at acidic pH.
Based on the finding that low pH promotes FHV membrane penetration, we predicted acidic pH may induce the virus particles to undergo a conformational change in which previously buried hydrophobic sequences become exposed. To examine changes in hydrophobicity we used the small molecule bis-ANS, which fluoresces upon binding to surface-accessible hydrophobic protein sequences (35). bis-ANS binding is driven primarily by interactions between the aromatic rings of the bis-ANS molecule and hydrophobic regions of proteins (13). Either wild-type FHV (FHVwt) or FHVD75N particles were incubated with bis-ANS in buffer ranging from pH 5 to 7, and fluorescence was measured following a 1-h incubation. Both FHVwt and FHVD75N particles displayed a similar pattern of bis-ANS fluorescence, with the largest increase in bis-ANS binding at pH 6.0 (Fig. 4C). The finding that capsid hydrophobicity peaks at pH 6.0 correlates with the pH dependence of FHV membrane-lytic activity, suggesting this pH optimally induces FHV particles to expose hydrophobic membrane-active regions of the capsid. The finding that FHVD75N particles undergo this pH-dependent increase in hydrophobicity suggests that these particles can undergo some, but not all, of the transitions required for membrane penetration.
Disrupted FHV particles mediate membrane penetration.
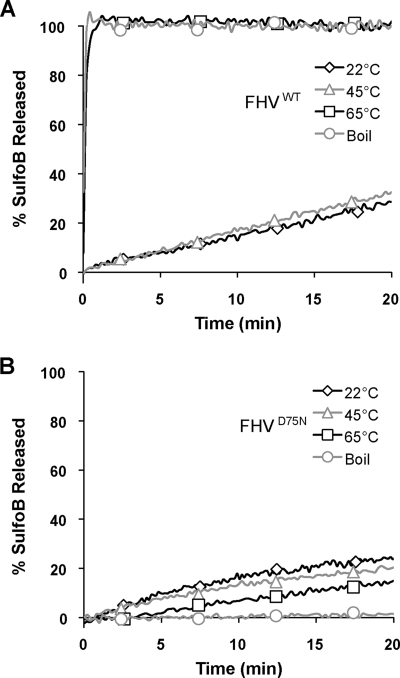
One role of acidic pH within the endosomal compartment may be to destabilize the FHV capsid, allowing membrane-active regions to be exposed or released. To test whether destabilization of the FHV capsid by heating promotes membrane disruption in vitro, FHV particles in pH 7.0 buffer were incubated at 22°C, 45°C, or 65°C or boiled. Following this incubation, large protein aggregates were removed by low-speed centrifugation, and the resulting supernatant was added to SulfoB-loaded liposomes to test for membrane lytic activity. FHV particles that had been incubated at 22°C or 45°C mediated only a low level of fluorescent dye release from liposomes, 19% and 20% SulfoB release after 20 min, respectively (Fig. 5A). In contrast, FHV particles that had been heated at 65°C or boiled readily mediated 100% SulfoB release from liposomes (Fig. 5A). These results suggest that the portion of the capsid that mediates membrane penetration is made accessible or released when the FHV capsid is perturbed by heating. Analysis of these heat-treated particles by negative stain microscopy confirmed that the capsid integrity of these particles was severely compromised (data not shown).
FIG. 5.
Disrupted FHV particles and fluorescent dye release from liposomes. FHVwt or FHVD75N particles were incubated at 22°C, 45°C, 65°C, or boiled in pH 7.0 buffer as indicated. Following this incubation, large protein aggregates were removed by low-speed centrifugation, and the resulting supernatant was analyzed by the liposome dye release assay. FHVwt (A) or FHVD75N (B) heat-treated virus particles were added to SulfoB-loaded liposomes, and fluorescent dye release was measured as a function of time during a 20-min incubation.
To test whether cleavage-defective particles can facilitate membrane penetration once disrupted by heating, the experiment described above was repeated with FHVD75N particles. Consistent with previous results, FHVD75N particles that had been incubated at 22°C in pH 7.0 buffer mediated approximately 20% SulfoB release after 20 min (Fig. 5B). In contrast to FHVwt particles, FHVD75N particles incubated at higher temperatures did not show an increased capacity to mediate dye release from liposomes (Fig. 5B). The levels of FHVD75N-mediated liposome lysis incrementally decreased as the particles were incubated at higher temperature, likely due to particle aggregation. These findings confirm that autocatalytic cleavage, to generate the γ peptide, is required for membrane penetration. Furthermore, these results suggest that heat-treated FHVD75N particles cannot mediate membrane disruption because these particles are not able to expose or release the requisite membrane-active regions of the capsid, the γ peptides.
DISCUSSION
Models of FHV entry have been previously proposed based on structural and limited biochemical information; however, little concrete data were provided to substantiate these models. Here, we provide the first evidence that FHV enters cells via an acidic endocytic pathway and that low pH is required for FHV infection. In addition, we used dye-loaded liposomes to demonstrate that virus particles can disrupt model membranes in the absence of any other cellular components and that FHV-mediated dye release from liposomes occurs optimally at acidic pH (pH ≤ 6.0). Particles incubated at low pH exhibited increased hydrophobicity, which correlated with the optimal pH of FHV membrane-lytic activity. We also show that noninfectious cleavage-defective particles fail to permeabilize liposomes, providing the first biochemical evidence that these mutant particles are blocked at the membrane penetration step during cell entry. These results offer new and novel insights into the mechanism of FHV entry and membrane penetration and differ significantly from prior studies of FHV membrane disruption, as physiologically relevant virus particles were used in the membrane penetration assays rather than a synthetic version of the γ peptide.
The findings described here support a model in which FHV enters cells by acid-dependent endocytosis and that low pH within the endocytic pathway triggers the virus to undergo the transitions necessary to penetrate the endosomal membrane (Fig. 6). We predict that at neutral pH the γ peptides are largely sequestered inside the capsid shell. Exposure to low endocytic pH during cell entry induces the capsid to undergo an irreversible conformational change in which the γ peptides are externalized or released from the virus particle, and ultimately, these liberated or particle-associated γ peptides facilitate disruption of the endosomal membrane. This is a new and updated model of FHV membrane disruption, as previous models did not include an acidic pH component. In addition, this model now specifies where in the infectivity pathway cleavage-defective particles are blocked. This model fits a common paradigm of nonenveloped virus entry in which virus penetration proteins undergo primed and triggered conformational changes, leading to capsid disassembly and release of small membrane-interacting peptides (4, 50).
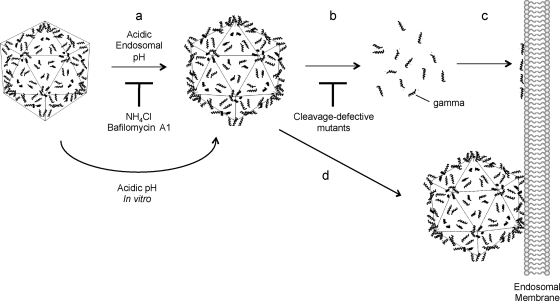
FIG. 6.
Updated model of FHV membrane penetration. Prior to infection, the γ peptides (shown as helices) are noncovalently associated with the interior of the FHV capsid. During uptake into the host cell, FHV is exposed to acidic pH within the endocytic compartment. Acidic endosomal pH irreversibly triggers the virus to undergo partial disassembly and the γ peptides are externalized (step a) or released from the virus particle (step b). Released or particle-associated γ peptides insert into and create a local disruption of the endosomal membrane to facilitate translocation of the RNA or nucleocapsid into the cytoplasm (steps c and d, respectively). Inhibitors that raise endocytic pH, such as NH4Cl and bafilomycin A1, block the low-pH-induced uncoating step (blocked at step a). However, capsid disassembly can be initiated by exposing FHV particles to acidic pH in vitro prior to infection, and these particles no longer require low endosomal pH (step a is bypassed). FHV cleavage-defective mutants also undergo a conformational change upon exposure to acidic pH inside endosomes but cannot fully permeabilize membranes because these particles cannot release the γ peptide (blocked at step b).
We found that FHV particles that had been preincubated at pH 6.0 in vitro (equivalent to the pH found inside early endosomes) no longer required low endocytic pH during infection. Based upon these findings, we hypothesize that during a normal FHV infection, low pH inside endosomes triggers the virus particle to adopt a membrane-active conformation. However, if virus particles are pretreated at pH 6.0, the virus can undergo this low pH conformational change in vitro prior to infection (Fig. 6). While the nature of this conformational change remains to be fully characterized, experiments using bis-ANS revealed that FHV particles expose hydrophobic regions when incubated at pH 6.0. Furthermore, this increase in capsid hydrophobicity at pH 6.0 correlated with the optimal pH of FHV membrane lytic activity, consistent with a model in which endocytic pH induces FHV particles to expose hydrophobic, membrane-active regions of the capsid.
The finding that cleavage-defective particles have compromised membrane penetration activity has significantly advanced our understanding of the role of autocatalytic cleavage during FHV infection. Previously, cleavage-defective mutants were shown to be noninfectious (46); however, it was not determined which specific step in the infectivity pathway was blocked. It has been hypothesized that cleavage-defective particles are noninfectious because these particles cannot release the putative membrane-lytic γ peptide, and as a result, cannot facilitate membrane penetration during entry. In this report we provide the first biochemical evidence to support this model by demonstrating that cleavage-defective mutants have a decreased capacity to disrupt model membranes. Furthermore, perturbation of these particles by low pH or heating does not enhance membrane-lytic activity, suggesting partial capsid disassembly is not sufficient to restore membrane activity and the γ peptides must dissociate from the particle.
It remains possible that either particle-associated γ peptides or released γ peptides mediate membrane disruption (Fig. 6). The finding that cleavage-defective particles, which do not contain a dissociable form of the γ peptide, are competent to mediate low levels of liposome disruption (albeit, much less than wild-type FHV) suggests γ peptide release is not required. The residual membrane penetration activity of cleavage-defective particles can be explained by the finding that the γ peptide region of cleavage-defective particles is transiently exposed to the capsid surface, similar to what is observed in wild-type virus particles (12); therefore, it is likely that γ peptides can mediate a low level of membrane-lytic activity when surface exposed (a similar line of reasoning can be used to explain why FHVwt particles mediate low levels of liposome disruption at neutral pH). Nonetheless, cleavage-defective particles did not approach wild-type levels of membrane-lytic activity, even when the capsid was severely disrupted by a denaturing temperature, suggesting release of the γ peptides may be a critical component of FHV membrane disruption.
The finding that heat-treated FHVwt particles readily mediate liposome disruption suggests membrane-interacting regions of the capsid that were previously buried or inaccessible become exposed or released once the capsid is partially disassembled by heat. The finding that particles incubated at ≥65°C, but not at ≤45°C, exhibited increased membrane-lytic activity is consistent with a previous report which demonstrated that the FHV capsid is highly stable at temperatures lower than 65°C (47). It is interesting that particles incubated at boiling temperature for 10 min maintained a high level of membrane-lytic activity, indicating that the membrane-active moieties are not denatured and remain functional following this harsh treatment. Perhaps the most significant result of these heat treatment studies is the finding that heat-treated cleavage-defective particles do not mediate dye release from liposomes, suggesting partial capsid disassembly is not sufficient to supply membrane-lytic activity and the γ peptides must dissociate from the particle.
An intriguing observation is the concentration dependence of FHV-mediated liposome rupture. At both pH 6.0 and 7.0, the amount of dye released from liposomes increased as the concentration of FHV increased. A similar pattern of concentration-dependent dye release from liposomes was observed using a synthetic version of the γ peptide corresponding to the 21-residue N-terminal amphipathic helical region of γ (denoted γ1) (10, 30); notably, a much lower concentration (25- to 100-fold-lower molar equivalent) was required to disrupt liposomes when full-length γ was supplied from FHV particles versus the synthetic γ1 peptide. Based on this observed concentration dependence, it is tempting to speculate that the released γ peptides may act in concert to form a pore or cause disruption of the membrane bilayer. It is also possible that endocytosis may function to compartmentalize and concentrate released γ peptides at the site of membrane penetration. If the γ peptides do act in concert, one might expect the released peptides to form higher-order oligomers. Indeed, the structure-based model of FHV membrane penetration predicts that pentameric helical bundles of γ peptides are released from each fivefold axis (19).
The model of FHV entry put forth in this report is supported by a recent study that suggests acidic pH and γ peptide release may play a role during the FHV entry process (56). A putative entry intermediate, termed the eluted particle, is a particle type that dissociates from cells after initial binding. Eluted particles were shown to have lost approximately 25% of their γ peptides (56), consistent with γ being released from virus particles during entry. In addition, eluted particles, but not naïve FHV particles, were shown to aggregate (as judged by sucrose gradient sedimentation) when exposed to a pH below 6.2 (56). This sensitivity to low pH may be indicative of conformational changes that occur as a result of receptor binding, which render the virus capsid susceptible to disassembly once exposed to low pH within the endocytic pathway. The findings presented in the current study agree with the previous report, as we also showed that low pH-treated naïve particles do not undergo any large-scale conformational changes (as judged by negative stain electron microscopy and sucrose gradient sedimentation). However, we have now extended the analysis to demonstrate that low-pH-treated naïve particles undergo subtle conformational changes not detected in the previous study, even in the absence of receptor binding. Eluted particles provide evidence that binding of FHV to its cellular receptor initiates entry-related conformational changes, and viral uncoating progresses within acidic endosomes. Since the FHV receptor has yet to be identified, the role of receptor binding was not addressed in this study. However, it is possible that a combination of cellular factors contribute to FHV uncoating and membrane penetration.
Whether FHV forms a small membrane-spanning pore or causes a large-scale disruption of the membrane remains to be determined. Biophysical studies of the γ1 peptide bound to membrane bilayers in vitro demonstrate that the γ1 peptide is positioned parallel to the lipid bilayer, with the hydrophobic face of the helix packed against the membrane surface (9, 10, 27). These findings suggest that the γ1 peptide inserts only into the outer leaflet of the lipid bilayer, favoring a model in which the peptide causes localized disruption of the membrane rather than forming a membrane-spanning pore. One caveat of these studies is that the synthetic γ1 peptides may not behave the same as full-length γ peptides in the context of the virus particle. Therefore, now that we have developed a method to study membrane penetration in the context of the virus particle, we plan to further address γ peptide membrane interactions.
While this study has provided new details and advanced our understanding of the mechanism of FHV membrane penetration, numerous questions remain. One unresolved issue is whether the RNA genome segments or the entire nucleocapsid is translocated across the membrane into the cytoplasm. Since the FHV genome segments are functional mRNAs, it has been postulated that only the viral RNA enters the cell cytoplasm, but this has never been shown conclusively. Another outstanding question is whether, in addition to its role as a membrane-lytic factor, γ serves additional functions during membrane penetration. An additional role of the γ peptide may be to deliver the viral RNA to the site of membrane penetration or into to the cytoplasm. The C terminus of γ is known to bind RNA (44). Therefore, it is possible that the viral RNA remains associated with the γ peptides as they are released from the capsid interior, and the RNA travels along with the γ peptides to the site of membrane penetration or into the cytoplasm. Studies are currently under way to address the role of the RNA binding region of γ in membrane penetration (5).
The model of FHV membrane penetration presented herein bears striking resemblance to that of other nonenveloped viruses. Several nonenveloped viruses are primed by proteolytic cleavage (in many cases autoproteolytic cleavage) to generate a small hydrophobic peptide. During infection, these mature virions undergo conformational changes in response to cellular stimuli, whereby this small lipophilic peptide is released from the particle and ultimately mediates membrane penetration. For example, poliovirus is primed by autocatalytic cleavage to generate the N-myristolyated VP4 peptide (26). Poliovirus binding to its cellular receptor triggers the release of the VP4, which (along with the N terminus of VP1) facilitates membrane penetration (18, 23, 20). Similarly, reovirus is primed by autocatalytic cleavage to generate the N-myristolyated μ1N peptide (38). While the cellular trigger is not known, the μ1N peptide is released from virus particles during membrane penetration (15, 39), and liberated μ1N directly mediates pore formation in membranes (1, 25). Adenovirus is primed by proteolytic cleavage (by a virally encoded cysteine protease) to generate the lipophilic protein VI peptide (58). During infection, endosomal pH triggers adenovirus to undergo a conformational change in which protein VI dissociates from the capsid and mediates membrane disruption (24, 60).
In this report, we have complemented in-cell infectivity experiments with in vitro assays to show that FHV enters cells by endocytosis and is activated by acidic pH to adopt a membrane active conformation. We propose a model in which acidic endosomal pH prompts virus disassembly, leading to the irreversible exposure and/or release of the membrane-lytic γ peptides. These studies offer new insights into the early steps of FHV infection and support a model of virus entry that is widely applicable to a diverse group of nonenveloped viruses.
Acknowledgments
This work was supported by NIH grant GM034220 (to J.E.J.). A.L.O. was supported by NIH training grant T32 AI007354.
Footnotes
Published ahead of print on 24 June 2009.
REFERENCES
- 1.Agosto, M. A., T. Ivanovic, and M. L. Nibert. 2006. Mammalian reovirus, a nonfusogenic nonenveloped virus, forms size-selective pores in a model membrane. Proc. Natl. Acad. Sci. USA 10316496-16501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ball, L. A. 1994. Replication of the genomic RNA of a positive-strand RNA animal virus from negative-sense transcripts. Proc. Natl. Acad. Sci. USA 9112443-12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ball, L. A., and K. L. Johnson. 1998. Nodaviruses of insects, p. 225-267. In L. K. Miller and L. A. Ball (ed.), The insect viruses. Plenum, New York, NY.
- 4.Banerjee, M., and J. E. Johnson. 2008. Activation, exposure and penetration of virally encoded, membrane-active polypeptides during non-enveloped virus entry. Curr. Protein Pept. Sci. 916-27. [DOI] [PubMed] [Google Scholar]
- 5.Banerjee, M., R. Khayat, H. E. Walukiewicz, A. L. Odegard, A. Schneemann, and J. E. Johnson. 2009. Dissecting the functional domains of a non-enveloped virus membrane penetration peptide. J. Virol. 836929-6933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basak, S., and H. Turner. 1992. Infectious entry pathway for canine parvovirus. Virology 186368-376. [DOI] [PubMed] [Google Scholar]
- 7.Bayer, N., E. Prchla, M. Schwab, D. Blaas, and R. Fuchs. 1999. Human rhinovirus HRV14 uncoats from early endosomes in the presence of bafilomycin. FEBS Lett. 463175-178. [DOI] [PubMed] [Google Scholar]
- 8.Blumenthal, R., P. Seth, M. C. Willingham, and I. Pastan. 1986. pH-dependent lysis of liposomes by adenovirus. Biochemistry 252231-2237. [DOI] [PubMed] [Google Scholar]
- 9.Bong, D. T., A. Janshoff, C. Steinem, and M. R. Ghadiri. 2000. Membrane partitioning of the cleavage peptide in flock house virus. Biophys. J. 78839-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bong, D. T., C. Steinem, A. Janshoff, J. E. Johnson, and M. Reza Ghadiri. 1999. A highly membrane-active peptide in Flock House virus: implications for the mechanism of nodavirus infection. Chem. Biol. 6473-481. [DOI] [PubMed] [Google Scholar]
- 11.Bothner, B., X. F. Dong, L. Bibbs, J. E. Johnson, and G. Siuzdak. 1998. Evidence of viral capsid dynamics using limited proteolysis and mass spectrometry. J. Biol. Chem. 273673-676. [DOI] [PubMed] [Google Scholar]
- 12.Bothner, B., A. Schneemann, D. Marshall, V. Reddy, J. E. Johnson, and G. Siuzdak. 1999. Crystallographically identical virus capsids display different properties in solution. Nat. Struct. Biol. 6114-116. [DOI] [PubMed] [Google Scholar]
- 13.Bothra, A., A. Bhattacharyya, C. Mukhopadhyay, K. Bhattacharyya, and S. Roy. 1998. A fluorescence spectroscopic and molecular dynamics study of bis-ANS/protein interaction. J. Biomol. Struct. Dyn. 15959-966. [DOI] [PubMed] [Google Scholar]
- 14.Brandenburg, B., L. Y. Lee, M. Lakadamyali, M. J. Rust, X. Zhuang, and J. M. Hogle. 2007. Imaging poliovirus entry in live cells. PLoS Biol. 5e183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandran, K., D. L. Farsetta, and M. L. Nibert. 2002. Strategy for nonenveloped virus entry: a hydrophobic conformer of the reovirus membrane penetration protein μ1 mediates membrane disruption. J. Virol. 769920-9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandran, K., and M. L. Nibert. 2003. Animal cell invasion by a large nonenveloped virus: reovirus delivers the goods. Trends Microbiol. 11374-382. [DOI] [PubMed] [Google Scholar]
- 17.Cheng, R. H., V. S. Reddy, N. H. Olson, A. J. Fisher, T. S. Baker, and J. E. Johnson. 1994. Functional implications of quasi-equivalence in a T = 3 icosahedral animal virus established by cryo-electron microscopy and X-ray crystallography. Structure 2271-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Sena, J., and B. Mandel. 1977. Studies on the in vitro uncoating of poliovirus. II. Characteristics of the membrane-modified particle. Virology 78554-566. [DOI] [PubMed] [Google Scholar]
- 19.Fisher, A. J., and J. E. Johnson. 1993. Ordered duplex RNA controls capsid architecture in an icosahedral animal virus. Nature 361176-179. [DOI] [PubMed] [Google Scholar]
- 20.Fricks, C. E., and J. M. Hogle. 1990. Cell-induced conformational change in poliovirus: externalization of the amino terminus of VP1 is responsible for liposome binding. J. Virol. 641934-1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friesen, P. D., and R. R. Rueckert. 1981. Synthesis of Black beetle virus proteins in cultured Drosophila cells: differential expression of RNAs 1 and 2. J. Virol. 37876-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallagher, T. M., and R. R. Rueckert. 1988. Assembly-dependent maturation cleavage in provirions of a small icosahedral insect ribovirus. J. Virol. 623399-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomez Yafal, A., G. Kaplan, V. R. Racaniello, and J. M. Hogle. 1993. Characterization of poliovirus conformational alteration mediated by soluble cell receptors. Virology 197501-505. [DOI] [PubMed] [Google Scholar]
- 24.Greber, U. F., M. Willetts, P. Webster, and A. Helenius. 1993. Stepwise dismantling of adenovirus 2 during entry into cells. Cell 75477-486. [DOI] [PubMed] [Google Scholar]
- 25.Ivanovic, T., M. A. Agosto, L. Zhang, K. Chandran, S. C. Harrison, and M. L. Nibert. 2008. Peptides released from reovirus outer capsid form membrane pores that recruit virus particles. EMBO J. 271289-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobson, M. F., and D. Baltimore. 1968. Polypeptide cleavages in the formation of poliovirus proteins. Proc. Natl. Acad. Sci. USA 6177-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janshoff, A., D. T. Bong, C. Steinem, J. E. Johnson, and M. R. Ghadiri. 1999. An animal virus-derived peptide switches membrane morphology: possible relevance to nodaviral transfection processes. Biochemistry 385328-5336. [DOI] [PubMed] [Google Scholar]
- 28.Keljo, D. J., M. Kuhn, and A. Smith. 1988. Acidification of endosomes is not important for the entry of rotavirus into the cell. J. Pediatr. Gastroenterol. Nutr. 7257-263. [DOI] [PubMed] [Google Scholar]
- 29.Li, H., W. X. Li, and S. W. Ding. 2002. Induction and suppression of RNA silencing by an animal virus. Science 2961319-1321. [DOI] [PubMed] [Google Scholar]
- 30.Maia, L. F., M. R. Soares, A. P. Valente, F. C. Almeida, A. C. Oliveira, A. M. Gomes, M. S. Freitas, A. Schneemann, J. E. Johnson, and J. L. Silva. 2006. Structure of a membrane-binding domain from a non-enveloped animal virus: insights into the mechanism of membrane permeability and cellular entry. J. Biol. Chem. 28129278-29286. [DOI] [PubMed] [Google Scholar]
- 31.Manayani, D. J., D. Thomas, K. A. Dryden, V. Reddy, M. E. Siladi, J. M. Marlett, G. J. Rainey, M. E. Pique, H. M. Scobie, M. Yeager, J. A. Young, M. Manchester, and A. Schneemann. 2007. A viral nanoparticle with dual function as an anthrax antitoxin and vaccine. PLoS Pathog. 31422-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marjomaki, V., V. Pietiainen, H. Matilainen, P. Upla, J. Ivaska, L. Nissinen, H. Reunanen, P. Huttunen, T. Hyypia, and J. Heino. 2002. Internalization of echovirus 1 in caveolae. J. Virol. 761856-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marshall, D., and A. Schneemann. 2001. Specific packaging of nodaviral RNA2 requires the N-terminus of the capsid protein. Virology 285165-175. [DOI] [PubMed] [Google Scholar]
- 34.Martinez, C. G., R. Guinea, J. Benavente, and L. Carrasco. 1996. The entry of reovirus into L cells is dependent on vacuolar proton-ATPase activity. J. Virol. 70576-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McClure, W. O., and G. M. Edelman. 1966. Fluorescent probes for conformational states of proteins. I. Mechanism of fluorescence of 2-p-toluidinylnaphthalene-6-sulfonate, a hydrophobic probe. Biochemistry 51908-1919. [DOI] [PubMed] [Google Scholar]
- 36.Mellman, I., R. Fuchs, and A. Helenius. 1986. Acidification of the endocytic and exocytic pathways. Annu. Rev. Biochem. 55663-700. [DOI] [PubMed] [Google Scholar]
- 37.Miller, D. J., M. D. Schwartz, and P. Ahlquist. 2001. Flock house virus RNA replicates on outer mitochondrial membranes in Drosophila cells. J. Virol. 7511664-11676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nibert, M. L., L. A. Schiff, and B. N. Fields. 1991. Mammalian reoviruses contain a myristoylated structural protein. J. Virol. 651960-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Odegard, A. L., K. Chandran, X. Zhang, J. S. Parker, T. S. Baker, and M. L. Nibert. 2004. Putative autocleavage of outer capsid protein μ1, allowing release of myristoylated peptide μ1N during particle uncoating, is critical for cell entry by reovirus. J. Virol. 788732-8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pelkmans, L., and A. Helenius. 2003. Insider information: what viruses tell us about endocytosis. Curr. Opin. Cell Biol. 15414-422. [DOI] [PubMed] [Google Scholar]
- 41.Pelkmans, L., J. Kartenbeck, and A. Helenius. 2001. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 3473-483. [DOI] [PubMed] [Google Scholar]
- 42.Perez, L., and L. Carrasco. 1993. Entry of poliovirus into cells does not require a low-pH step. J. Virol. 674543-4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richterova, Z., D. Liebl, M. Horak, Z. Palkova, J. Stokrova, P. Hozak, J. Korb, and J. Forstova. 2001. Caveolae are involved in the trafficking of mouse polyomavirus virions and artificial VP1 pseudocapsids toward cell nuclei. J. Virol. 7510880-10891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schneemann, A., and D. Marshall. 1998. Specific encapsidation of nodavirus RNAs is mediated through the C terminus of capsid precursor protein alpha. J. Virol. 728738-8746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schneemann, A., V. Reddy, and J. E. Johnson. 1998. The structure and function of nodavirus particles: a paradigm for understanding chemical biology. Adv. Virus Res. 50381-446. [DOI] [PubMed] [Google Scholar]
- 46.Schneemann, A., W. Zhong, T. M. Gallagher, and R. R. Rueckert. 1992. Maturation cleavage required for infectivity of a nodavirus. J. Virol. 666728-6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwarcz, W. D., S. P. Barroso, A. M. Gomes, J. E. Johnson, A. Schneemann, A. C. Oliveira, and J. L. Silva. 2004. Virus stability and protein-nucleic acid interaction as studied by high-pressure effects on nodaviruses. Cell Mol. Biol. (Noisy-le-grand) 50419-427. [PubMed] [Google Scholar]
- 48.Scodeller, E. A., S. G. Tisminetzky, F. Porro, M. Schiappacassi, A. De Rossi, L. Chiecco-Bianchi, and F. E. Baralle. 1995. A new epitope presenting system displays a HIV-1 V3 loop sequence and induces neutralizing antibodies. Vaccine 131233-1239. [DOI] [PubMed] [Google Scholar]
- 49.Sturzenbecker, L. J., M. Nibert, D. Furlong, and B. N. Fields. 1987. Intracellular digestion of reovirus particles requires a low pH and is an essential step in the viral infectious cycle. J. Virol. 612351-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsai, B. 2007. Penetration of nonenveloped viruses into the cytoplasm. Annu. Rev. Cell. Dev. Biol. 2323-43. [DOI] [PubMed] [Google Scholar]
- 51.Upcroft, P. 1987. Simian virus 40 infection is not mediated by lysosomal activation. J. Gen. Virol. 682477-2480. [DOI] [PubMed] [Google Scholar]
- 52.Varga, M. J., C. Weibull, and E. Everitt. 1991. Infectious entry pathway of adenovirus type 2. J. Virol. 656061-6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Venter, P. A., N. K. Krishna, and A. Schneemann. 2005. Capsid protein synthesis from replicating RNA directs specific packaging of the genome of a multipartite, positive-strand RNA virus. J. Virol. 796239-6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Venter, P. A., and A. Schneemann. 2007. Assembly of two independent populations of Flock House virus particles with distinct RNA packaging characteristics in the same cell. J. Virol. 81613-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Venter, P. A., and A. Schneemann. 2008. Recent insights into the biology and biomedical applications of Flock House virus. Cell. Mol. Life Sci. 652675-2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walukiewicz, H. E., J. E. Johnson, and A. Schneemann. 2006. Morphological changes in the T=3 capsid of Flock House virus during cell entry. J. Virol. 80615-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang, X. H., R. Aliyari, W. X. Li, H. W. Li, K. Kim, R. Carthew, P. Atkinson, and S. W. Ding. 2006. RNA interference directs innate immunity against viruses in adult Drosophila. Science 312452-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weber, J. M. 1995. Adenovirus endopeptidase and its role in virus infection. Curr. Top. Microbiol. Immunol. 199227-235. [DOI] [PubMed] [Google Scholar]
- 59.Weissenhorn, W., A. Hinz, and Y. Gaudin. 2007. Virus membrane fusion. FEBS Lett. 5812150-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wiethoff, C. M., H. Wodrich, L. Gerace, and G. R. Nemerow. 2005. Adenovirus protein VI mediates membrane disruption following capsid disassembly. J. Virol. 791992-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamashiro, D. J., and F. R. Maxfield. 1984. Acidification of endocytic compartments and the intracellular pathways of ligands and receptors. J. Cell. Biochem. 26231-246. [DOI] [PubMed] [Google Scholar]