Abstract
As tumors grow larger, they often experience an insufficient supply of oxygen and nutrients. Hence, cancer cells must develop mechanisms to overcome these stresses. Using an in vitro transformation model where the presence of the simian virus 40 (SV40) small T (ST) antigen has been shown to be critical for tumorigenic transformation, we investigated whether the ST antigen has a role to play in regulating the energy homeostasis of cancer cells. We find that cells expressing the SV40 ST antigen (+ST cells) are more resistant to glucose deprivation-induced cell death than cells lacking the SV40 ST antigen (−ST cells). Mechanistically, we find that the ST antigen mediates this effect by activating a nutrient-sensing kinase, AMP-activated protein kinase (AMPK). The basal level of active, phosphorylated AMPK was higher in +ST cells than in −ST cells, and these levels increased further in response to glucose deprivation. Additionally, inhibition of AMPK in +ST cells increased the rate of cell death, while activation of AMPK in −ST cells decreased the rate of cell death, under conditions of glucose deprivation. We further show that AMPK mediates its effects, at least in part, by inhibiting mTOR (mammalian target of rapamycin), thereby shutting down protein translation. Finally, we show that +ST cells exhibit a higher percentage of autophagy than −ST cells upon glucose deprivation. Thus, we demonstrate a novel role for the SV40 ST antigen in cancers, where it functions to maintain energy homeostasis during glucose deprivation by activating AMPK, inhibiting mTOR, and inducing autophagy as an alternate energy source.
The localization of most mammalian cells within a 100- to 150-μm distance from blood vessels ensures a continuous supply of oxygen and nutrients, a prerequisite for cell survival. However, tumors often grow beyond this limit, thereby experiencing oxygen and nutrient deprivation (28). Tumors overcome this barrier by initiating neoangiogenesis, a process that supplies new blood vessels (44). However, before neoangiogenesis can set in, incipient tumors must survive the stresses of nutrient deprivation. Therefore, an understanding of the molecular mechanisms that regulate cancer cell survival under conditions of nutrient deprivation is fundamental in cancer biology. Additionally, targeting the ability of cancer cells to survive under nutrient-deprived conditions can be exploited for designing novel cancer therapeutics.
Glucose is the major source of energy for mammalian cells. Several types of cancer cells exhibit marked resistance to cell death upon glucose deprivation (22). In this study we have attempted to delineate the mechanisms that allow cancer cells to survive under conditions of glucose deprivation by using human foreskin fibroblasts that have been transformed by the serial introduction of the simian virus 40 (SV40) early region (coding for the large T [LT] and small T [ST] antigens), the catalytic subunit of human telomerase (hTERT), and an oncogenic allele of H-Ras (H-Ras V12) (referred to below as +ST cells) (32). In this model, human cells lacking the ST antigen but expressing the rest of these genetic elements (referred to below as −ST cells) are nontumorigenic (16, 32), highlighting the importance of the ST antigen in human cell transformation. However, little is known about the specific cellular functions moderated by the ST antigen that aid in transformation (3).
Since glucose is the major source of energy for mammalian cells, and cancer cells experience glucose deprivation when they are beyond the diffusion limit, we investigated whether the ST antigen has any role to play under conditions of glucose deprivation. We report here a novel link between the ST antigen and AMP-activated protein kinase (AMPK) activation that enables cancer cell survival under glucose deprivation via inhibition of protein synthesis and activation of autophagy as an alternate energy source.
MATERIALS AND METHODS
Cell culture and pharmacological chemical inhibitors.
The following cell lines were used in this study: human foreskin fibroblasts carrying hTERT, LT antigen, ST antigen, and Ras (+ST cells); human foreskin fibroblasts carrying only hTERT, LT antigen, and Ras (−ST cells); human embryonic kidney (HEK) cells carrying hTERT, LT antigen, ST antigen, and Ras (referred to below as HEK+ST cells); HEK cells carrying only hTERT, LT antigen, and Ras (referred to below as HEK−ST cells); and HEK cells carrying hTERT, LT antigen, Ras, and mutant ST antigen cDNA encoding N-terminal amino acids 1 to 100 (referred to below as HEKΔST 1-100 cells). Cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum at 37°C under 5% CO2 (32). Pharmacological inhibitors used include rapamycin (1 μM; Calbiochem), 5-aminoimidazole-4-carboxyamide ribonucleoside (AICAR) (1 mM; Sigma), okadaic acid (OA) (0.1 nM; Calbiochem), and 6-[4-(2-piperidin-1-yl-ethoxy-phenyl)]-3-pyridin-4-yl-pyrrazolo[1,5-a]-pyrimidine (compound C) (5 μM; Calbiochem). Absolute ethanol and dimethyl sulfoxide were used as solvents for rapamycin and OA, respectively.
Glucose deprivation.
A total of 2 × 105 cells were seeded in a 60-mm-diameter tissue culture dish (Tarsons). After 24 h of seeding, the cells were washed twice with DMEM (without glucose) (Sigma) and then incubated in DMEM (without glucose) supplemented with 10% dialyzed fetal bovine serum for 16 to 18 h. The cells were monitored for 16 to 18 h for detection of phenotypic changes. For all protein analyses, the cells were lysed after 2 h of glucose deprivation.
Cell viability and apoptosis detection.
Cell viability was determined by a trypan blue dye exclusion assay.
Antibodies and immunoblotting.
Primary antibodies against phosphorylated AMPK (pAMPK) (α172), phosphorylated acetyl coenzyme A carboxylase (pACC) (Ser79), phosphorylated eukaryotic initiation factor 4E binding protein 1 (phospho-p4EBP1) (Ser65), cleaved poly(ADP-ribose) polymerase (PARP) (Asp214), and phosphorylated mammalian target of rapamycin (phosphorylated mTOR) (Ser2448) were obtained from Cell Signaling Technologies, and α-tubulin was obtained from Calbiochem. Horseradish peroxidase-conjugated anti-mouse and anti-rabbit antibodies were obtained from BD Pharmingen and Jackson ImmunoResearch Laboratories. For Western blotting, cells were lysed in lysis buffer (1% NP-40 detergent, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 50 mM sodium fluoride, 1 tablet of protease inhibitor [Roche], 1 mM sodium orthovanadate, and 10 mM sodium pyrophosphate) for 30 min on ice. Two hundred micrograms of protein per lane was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membranes, and probed with appropriate antibodies.
Transfection.
Lipofectamine (Invitrogen) was used to transfect eGFP-LC3 (enhanced green fluorescent protein fused to light chain 3) DNA into +ST and −ST cells, and Fugene (Roche) was used to transfect CSCG ΔST 1-100 into HEK−ST cells according to the manufacturer's guidelines.
Generation of a mutant ST cell line.
From a vector expressing the full-length ST antigen (pMIG-ST) (16), bp 1 to 300, corresponding to N-terminal amino acids 1 to 100, was PCR amplified using forward primer 5′-CGCAGGATCCATGGATAAAGTTTTAAACAG-3′ and reverse primer 5′-CCGAGAATTCACACTCAGGCCATTGTTT-3′. The PCR-amplified mutant ST antigen cDNA (ΔST 1-100) was cloned between the BamHI and EcoRI restriction sites of the CSCG lentiviral vector. The resultant CSCG-ΔST 1-100 construct was transfected into HEK−ST cells to obtain stable clones.
RESULTS
The ST antigen protects transformed human cells under glucose-deprived conditions.
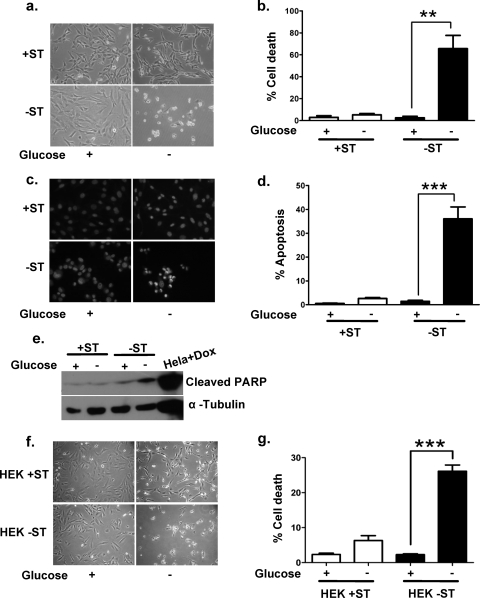
One of the major nutrients that acts as fuel for mammalian cells is glucose. Cancer cells have a heavy demand for glucose in order to maintain their high proliferative rate. How cancers overcome this demand under nutrient stress conditions remains poorly understood. In order to investigate a possible role for the SV40 ST antigen under glucose-deprived conditions, we cultured +ST and −ST cells in glucose-free medium. After 18 h of glucose deprivation, −ST cells were completely circularized and detached from the culture dish, whereas +ST cells showed only a slight change in morphology (Fig. 1a). Trypan blue staining revealed 65% cell death among −ST cells, in contrast to only 5% among +ST cells, indicating that the ST antigen confers a survival advantage on cancer cells under glucose deprivation (Fig. 1b).
FIG. 1.
Effect of glucose deprivation on the survival of +ST and −ST cells. (a) Phase-contrast microscopic images of +ST and −ST cells in the presence and absence of glucose. (b) Graph representing quantification of trypan blue-positive (dead) cells upon glucose deprivation. (c) Photomicrographs showing fluorescent images of glucose-deprived cells stained with Hoechst 33342 to detect apoptosis. (d) Graph representing quantification of Hoechst 33342-positive (apoptotic) cells upon glucose deprivation. (e) Immunoblot analysis of whole-cell lysates of glucose-deprived +ST and −ST cells for cleaved PARP using an antibody that specifically recognizes the large fragment of cleaved PARP protein. Doxorubicin (Dox)-treated HeLa cells served as a positive control. (f) Phase-contrast microscopic images of HEK+ST and HEK−ST cells in the presence and absence of glucose. (g) Graph representing quantification of trypan blue-positive (dead) cells upon glucose deprivation (n = 6). ***, P < 0.001; **, P value between 0.001 and 0.01. In all experiments, statistical significance was determined using one-way analysis of variance. Error bars, standard errors of the means (n = 3).
Glucose deprivation has previously been shown to trigger apoptosis in mammalian cells (41). In order to determine if −ST cells also underwent apoptosis when subjected to glucose deprivation, we assayed for chromatin condensation and PARP cleavage, two hallmarks of apoptosis (2). Staining with Hoechst 33342 revealed condensed nuclei in 36% of −ST cells, in contrast to only 3% in +ST cells (Fig. 1c and d). Immunoblot analysis revealed that compared to control cells, −ST cells growing under glucose-deprived conditions showed an increase in the density of the 89-kDa band corresponding to cleaved PARP (Fig. 1e), showing that indeed −ST cells undergo apoptosis when deprived of glucose.
In order to address the question of whether this protective effect of the ST antigen under glucose deprivation is restricted to fibroblasts or is a general phenomenon, we tested yet another cell type that had been transformed by the introduction of the same genetic elements. Like fibroblasts, HEK cells carrying the SV40 LT antigen, hTERT, and oncogenic Ras and also expressing the ST antigen (HEK+ST cells) show a transformed phenotype, whereas those lacking the ST antigen (HEK−ST cells) fail to be transformed (15). When +ST and −ST cells derived from HEK cells were deprived of glucose, phenotypic differences similar to those for fibroblasts became evident by 36 h, and at 48 h a clear difference could be seen in viability, with HEK−ST cells showing 26% cell death compared to 6% for HEK+ST cells (Fig. 1f and g). Taken together, these results demonstrate that ST confers a survival advantage on cancer cells under glucose-deprived conditions.
Inhibition of PP2A mimics ST function.
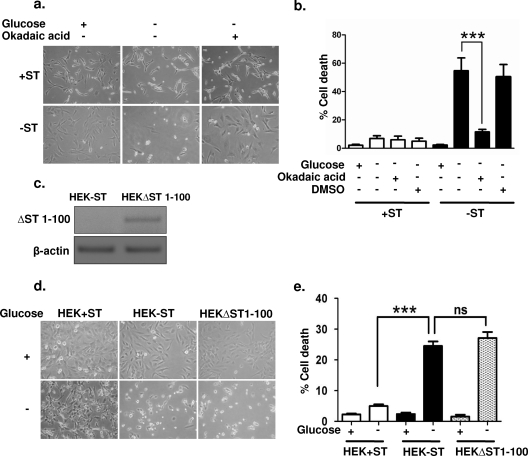
The SV40 ST antigen binds to and inhibits protein phosphatase 2A (PP2A), a principal serine/threonine phosphatase found in mammalian cells (16). PP2A regulates diverse activities, including metabolism, DNA replication, transcription, RNA splicing, translation, cell cycle progression, morphogenesis, development, and transformation (12). ST antigen-mediated inhibition of PP2A has been shown to be essential for human cell transformation. We investigated whether the protective effects of the ST antigen on glucose-deprived cells are also mediated via PP2A. In order to address this question, we used the selective and cell-permeant pharmacological inhibitor OA (26) to determine whether inhibition of PP2A in −ST cells would confer protection against cell death. In parallel with glucose deprivation, +ST and −ST cells were treated with 0.1 nM OA (26). The addition of OA caused a marked reduction in the rate of cell death for −ST cells upon glucose deprivation, from 54.3% to 11.4%, while no change was detected for +ST cells (Fig. 2a and b). Thus, inhibition of PP2A by OA mimicked the effects of the ST antigen, suggesting that an ST antigen-PP2A interaction is critical for the survival effects mediated by the SV40 ST antigen under glucose deprivation.
FIG. 2.
OA mimics the effects of the ST antigen. (a) Phase-contrast microscopic images of +ST and −ST cells in the presence and absence of OA. (b) Quantification of trypan blue-positive cells in the presence and absence of OA. Dimethyl sulfoxide (DMSO) was used as a vehicle control (n = 3). (c) Reverse transcription-PCR analysis for ΔST 1-100 in HEK−ST and HEKΔST 1-100 cells. (d) Phase-contrast microscopic images of HEK+ST, HEK−ST, and HEKΔST 1-100 cells in the presence and absence of glucose. (e) Quantification of trypan blue-positive cells in the presence and absence of glucose. ***, P < 0.001; ns, nonsignificant.
To further confirm that the ST antigen-PP2A interaction is critical for the protective role of the ST antigen in glucose deprivation, we employed a C-terminally truncated construct of the ST antigen that is known to prevent its interaction with PP2A (16, 43). To this end, we PCR amplified bp 1 to 300 of ST antigen cDNA (corresponding to N-terminal amino acids 1 to 100) using specific primers (see Materials and Methods) and ligated the product into the CSCG lentiviral expression plasmid. The HEK−ST cells transfected with the ST (1-100) construct (HEKΔST 1-100 cells) showed expression of the truncated ST antigen as detected by reverse transcription-PCR (Fig. 2c). When deprived of glucose, compared to HEK+ST cells (which showed marginal cell death), both HEK−ST cells and HEKΔST 1-100 cells showed ∼28% cell death (Fig. 2d and e), clearly highlighting the requirement of ST antigen-PP2A interactions for the survival of cancer cells under glucose-deprived conditions.
The SV40 ST antigen activates AMPK.
We next investigated the mechanism downstream of the ST antigen-PP2A interaction that may bring about the observed effects. Since PP2A has several substrates (3), we reasoned that an ideal downstream candidate to mediate the cellular effects of glucose deprivation would be a molecule whose function is regulated by the cellular energy status. One likely candidate is AMPK, which consists of a catalytic subunit (α) and two regulatory subunits (β and γ). In response to nutrient deprivation, AMPK is allosterically activated by an increase in the intracellular AMP/ATP ratio, followed by phosphorylation of threonine 172 within its α subunit by the upstream kinases LKB1 and Ca2+/calmodulin-dependent protein kinase kinase (40, 45). Upon activation, AMPK turns off energy-consuming anabolic processes while triggering energy-producing catabolic processes, thereby regulating cellular energy homeostasis (4).
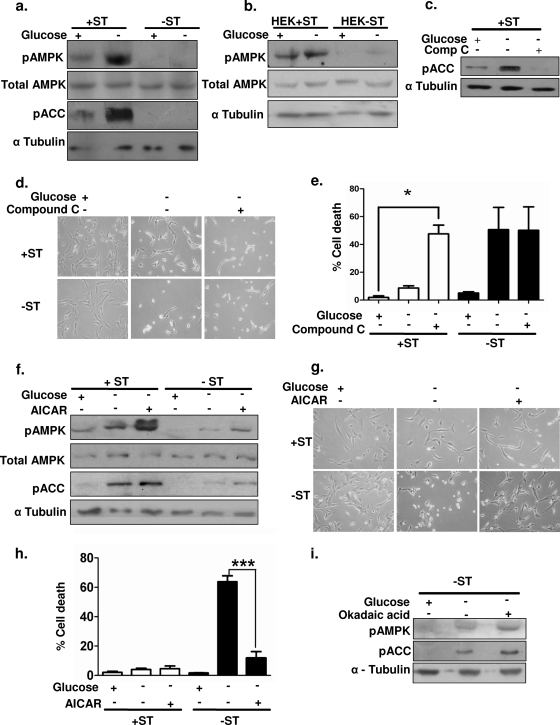
In order to address the involvement of AMPK in ST antigen-mediated protection, we first measured the levels of active pAMPK by immunoblotting using phospho-Thr172-specific antibodies. We found that the basal levels of pAMPK Thr172 were higher in +ST cells than in −ST cells (Fig. 3a). In response to glucose deprivation, a further increase in pAMPK Thr172 levels was detected in +ST cells, while −ST cells showed marginal changes (Fig. 3a). No change was detected in the levels of total AMPK (Fig. 3a). Since AMPK activation leads to phosphorylation of its downstream effector ACC, we further measured the levels of pACC as a readout of AMPK activity. In parallel with higher levels of pAMPK, we detected elevated levels of pACC in +ST cells upon glucose deprivation (Fig. 3a). A similar trend in pAMPK Thr172 levels was observed in HEK+ST and HEK−ST cells upon glucose deprivation (Fig. 3b). Thus, these results revealed that ST-mediated inhibition of PP2A enables the accumulation of higher levels of pAMPK within the cells, which may in turn regulate energy homeostasis under glucose-deprived conditions.
FIG. 3.
Involvement of AMPK in ST antigen-mediated protection under glucose-deprived conditions. (a) Whole-cell extracts from +ST and −ST cells in the presence and absence of glucose were subjected to immunoblot analysis for pAMPK (Thr172), total AMPK, and pACC (Ser79). α-Tubulin was used as a loading control for all immunoblots. (b) Whole-cell extracts of HEK+ST and HEK−ST cells in the presence and absence of glucose were subjected to immunoblot analysis for pAMPK (Thr172) and total AMPK. (c) Whole-cell extracts from +ST cells deprived of glucose and treated with compound C (Comp C) were subjected to immunoblot analysis for pACC (Ser79). (d) Phase-contrast microscopic images of +ST and −ST cells in the presence and absence of compound C. (e) Quantification of trypan blue-positive cells in the presence and absence of compound C (n = 3). (f) Whole-cell extracts of +ST and −ST cells deprived of glucose in the presence and absence of AICAR were subjected to immunoblot analysis for pAMPK (Thr172), total AMPK, and pACC (Ser79). (g) Phase-contrast microscopic images of +ST and −ST cells in the presence and absence of AICAR. (h) Quantification of trypan blue-positive cells in the presence and absence of AICAR (n = 3). (i) Whole-cell extracts of −ST cells deprived of glucose and treated with OA were subjected to immunoblot analysis for pAMPK (Thr172) and pACC (Ser79). *, P value between 0.01 and 0.05; ***, P < 0.001.
In order to further assess the involvement of AMPK under glucose-deprived conditions, we inhibited AMPK activity in +ST cells using 5 mM compound C, a selective inhibitor of AMPK (10). Immunoblot analysis revealed a clear decrease in the levels of pACC (Fig. 3c), demonstrating the inhibition of AMPK by the addition of compound C. Like −ST cells, some of the +ST cells treated with compound C started rounding up upon glucose deprivation (Fig. 3d). Furthermore, trypan blue analysis revealed a higher frequency of cell death in compound C-treated +ST cells (47%) than in untreated control cells (2%) (Fig. 3e). Treatment with compound C had no significant additional effects on −ST cells. Thus, inhibition of AMPK rendered +ST cells susceptible to cell death upon glucose deprivation.
We next gauged if activation of AMPK is sufficient to mimic the effects of the ST antigen. In order to do so, we subjected −ST cells to an artificial activator of AMPK, AICAR (33). AICAR is a cell-permeant adenosine analog that is taken up by the cells and phosphorylated to form its monophosphate derivative, ZMP. ZMP mimics AMP and acts as an activator of AMPK. Treatment of −ST cells with 1 mM AICAR resulted in an increase in pAMPK levels, as determined by immunoblotting (Fig. 3f). Upon glucose deprivation, AICAR-treated −ST cells exhibited 11% cell death, whereas untreated cells exhibited 63% cell death (Fig. 3g and h). No significant difference was noticed in the survival of +ST cells upon glucose deprivation in the presence or absence of AICAR. Thus, activation of AMPK is sufficient to protect −ST cells from glucose deprivation.
Since we demonstrated above that addition of OA (PP2A inhibitor) rescued −ST cells from glucose deprivation-induced cell death (Fig. 2a and b), we investigated whether PP2A inhibition also involved AMPK activation. We observed increases in the levels of pAMPK and pACC in OA-treated −ST cells deprived of glucose (Fig. 3i), suggesting that the protection conferred by the inhibition of PP2A in −ST cells also involves AMPK activation. Taken together, these results clearly demonstrate that ST mediates its protective effects on glucose deprivation via activation of AMPK. Thus, the ability to activate AMPK and maintain it in a phosphorylated form may be critical for the prolonged survival of cancer cells under glucose-deprived conditions.
AMPK inhibits mTOR.
mTOR has been shown to coordinate nutrient response and cell growth by regulating translation in response to cellular energy status (19). Under conditions of energy stress, AMPK has been shown to negatively regulate the TOR pathway in HEK 293 cells cultured in glucose-free medium (20). Since protein translation utilizes a major proportion of cellular energy, we reasoned that cancer cells might survive better by limiting their energy demand under glucose-deprived conditions by inhibiting mTOR.
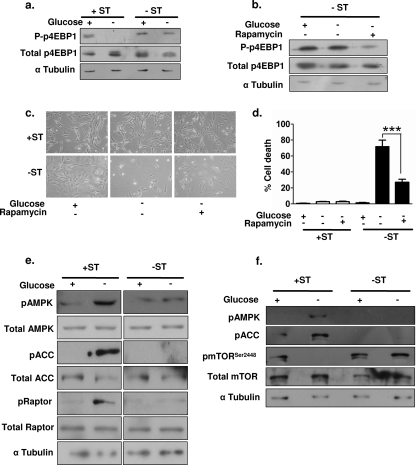
In order to address the question of whether ST antigen-mediated activation of AMPK further led to the inhibition of mTOR, we undertook immunoblot analysis to assess the functional status of the mTOR pathway. To do so, we measured the phosphorylation status of p4EBP1 (Ser65), a downstream substrate of mTOR (35). We observed reductions in phospho-p4EBP1 levels upon glucose deprivation only in +ST cells (Fig. 4a), indicating that the mTOR pathway may be inhibited in these cells. Furthermore, inhibition of mTOR in −ST cells by use of 20 nM rapamycin both reduced the levels of mTOR activity, as detected by lowered levels of phospho-p4EBP1 (Fig. 4b), and decreased the rate of cell death from 71% to 26% (Fig. 4c and d), indicating that mTOR inhibition is sufficient to rescue −ST cells from glucose deprivation. Thus, ST-mediated AMPK activation may promote survival, at least in part, by inhibiting the mTOR pathway.
FIG. 4.
Involvement of mTOR in ST antigen-AMPK-mediated rescue. (a) Immunoblot analysis of whole-cell extracts of glucose-deprived +ST and −ST cells for phospho-p4EBP1 (P-p4EBP1) (Ser65). (b) Immunoblot analysis of whole-cell extracts of rapamycin-treated −ST cells for P-p4EBP1 (Ser65). (c) Photomicrographs represent phase-contrast images of +ST and −ST cells treated with rapamycin. (d) Quantification of trypan blue-positive +ST and −ST cells upon glucose deprivation and rapamycin treatment (n = 3). ***, P < 0.001. (e) Whole-cell extracts of glucose-deprived +ST and −ST cells were subjected to immunoblot analysis for pAMPK (Thr172), total AMPK, pACC (Ser79), total ACC, phospho-raptor (pRaptor) (Ser792), and total raptor. (f) Whole-cell extracts of glucose-deprived +ST and −ST cells were subjected to immunoblot analysis for pAMPK (Thr172), pACC (Ser79), phosphorylated mTOR (pmTOR) (Ser2448), and total mTOR.
We next investigated how AMPK may negatively regulate mTOR signaling under glucose deprivation. Recently, AMPK-mediated phosphorylation of the mTOR binding protein, raptor, at Ser792 has been shown to inactivate mTOR signaling in HEK 293 cells (13). In order to investigate if AMPK-mediated inhibition of mTOR in transformed human fibroblasts also involved raptor phosphorylation, we probed for raptor Ser792 phosphorylation status in +ST and −ST cells. Our results showed that upon glucose deprivation, the levels of phospho-raptor Ser792 increased in +ST cells concomitantly with increases in pAMPK and pACC levels, whereas no such changes were detected in −ST cells (Fig. 4e). In addition to the phosphorylation of raptor, direct phosphorylation of mTOR has also been shown to regulate its function. In HEK 293 cells, S6K phosphorylates mTOR at Ser2448 in response to insulin signaling, while AMPK phosphorylates mTOR at Thr2446 in response to serum deprivation. Phosphorylation of these two sites on mTOR has been shown to be mutually exclusive and to act as a switch to control positive and negative signals regulating protein translation (6, 7). We found that mTOR Ser2448 phosphorylation decreased to undetectable levels in +ST cells upon glucose deprivation (Fig. 4f), while no change was detected in the levels of total mTOR. In contrast, the levels of phospho-Ser2448 remained unperturbed in −ST cells (Fig. 4f). The decrease in phospho-Ser2448 mTOR levels in +ST cells coincides with the increase in pAMPK levels, suggesting that activated AMPK-mediated phosphorylation of mTOR under glucose-deprived conditions in +ST cells may prevent its phosphorylation at Ser2448. Taken together, these results reveal that activation of AMPK by the ST antigen leads to mTOR inactivation in multiple ways, thereby inhibiting protein translation, reducing energy consumption, and promoting survival under glucose-deprived conditions.
The ST antigen triggers autophagy upon glucose deprivation.
Autophagy probably factors into both the promotion and the prevention of cancer (25), and its role may be altered during tumor progression (42). During the early phase of cancer progression, autophagy is shown to facilitate tumor progression in colorectal cancer cells under amino acid deprivation (38). We reasoned that while prolonged autophagy may result in cell death, short-term autophagy can provide an alternate energy supply to incipient tumors facing energy deprivation. Interestingly, under nutrient starvation, activation of AMPK and inhibition of mTOR signaling have also been shown to activate autophagy in colon cancer cells (25, 38). Since we find that mTOR is inhibited in +ST cells under glucose deprivation, we investigated whether +ST cells survived better under glucose deprivation by upregulating autophagy.
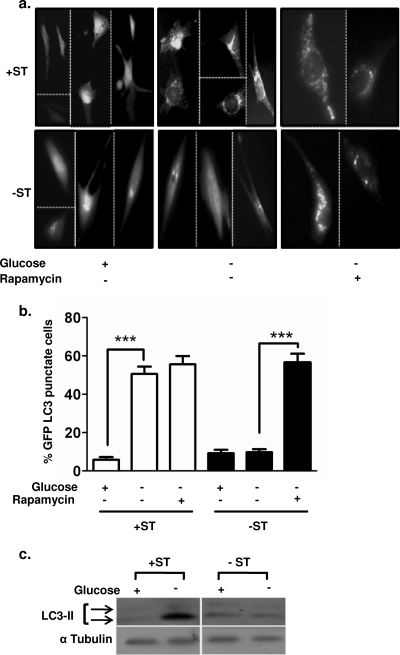
We observed a distinct, punctate appearance of GFP-LC3, a marker of autophagy (25), in 51% of +ST cells, compared to only 10% of −ST cells, under glucose-deprived conditions (Fig. 5a and b). Additionally, immunoblot analysis revealed higher levels of cleaved LC3 protein (yet another marker of autophagy) in +ST cells than in −ST cells, revealing that the ST antigen indeed triggers autophagy during energy stress (Fig. 5c). We demonstrated above that rapamycin treatment rescued −ST cells from cell death upon glucose deprivation (Fig. 4d). To ask if rapamycin treatment did so by activating autophagy in −ST cells, we once again measured autophagy using the GFP-LC3 construct in rapamycin-treated −ST cells. We found that −ST cells now showed increased autophagy concomitant with cell survival (Fig. 5a and b). Thus, induction of autophagy by the ST antigen may account for the increased survival of +ST cells under glucose-deprived conditions.
FIG. 5.
Autophagy in +ST and −ST cells upon glucose deprivation. (a) eGFP-LC3 localization in +ST and −ST cells upon glucose deprivation and rapamycin treatment. (b) Quantification of eGFP-LC3 punctate cells among +ST and −ST cells upon glucose deprivation and rapamycin treatment (n = 8). ***, P < 0.001. (c) Immunoblot analysis of whole-cell extracts for the cleaved LC3 fragment upon glucose deprivation.
DISCUSSION
The study of transforming viruses, and the oncoproteins they encode, has shaped our current understanding of the mechanisms involved in cellular transformation (17). For example, the E6 and E7 oncoproteins encoded by human papillomaviruses, adenoviral E1A and E1B proteins, and the SV40 LT antigen have provided key insights into the functions of two major cellular-growth-regulatory proteins, p53 and pRb (1, 5, 30). Both these proteins are also found to be mutated in several cancers (18). In addition to the LT antigen, the SV40 early region encodes the ST antigen, a 174-amino-acid protein. The LT and ST antigens share 82 amino acids at their N termini. Earlier studies indicated that the ST antigen replaces growth factor requirements in Chinese hamster ovary cells during transformation by SV40 (27). Further studies showed that even though the ST antigen by itself was not sufficient for transformation, it facilitated transformation by the LT antigen in rodent cells (34). More recently, in a human cell transformation model, it was shown that normal human cells can be transformed into their tumorigenic counterparts by the serial introduction of the SV40 early region, hTERT, and oncogenic Ras (15). However, when similar studies were carried out using human papillomavirus E6 and E7 proteins (which, like the LT antigen, inactivate p53 and pRB), normal human fibroblasts failed to be transformed (15, 29, 46). Subsequent studies revealed that the ST antigen, encoded by the early region of SV40, was additionally required for the experimental transformation of human cells (16). However, the mechanisms of action of the ST antigen in cellular transformation remain poorly understood. In this study we report that in the absence of glucose, cancer cells that express the ST antigen survive longer than those that do not (Fig. 1a, b, f, and g), thereby revealing a hitherto unidentified function for the ST antigen in the survival of cancer cells under glucose-deprived conditions.
Studies using SV40 ST antigen mutants have revealed that the major transforming functions of the ST antigen are mediated via its interaction with and inhibition of PP2A complexes (31), suggesting that alteration of the phosphorylation status of signaling molecules is the primary mode of action of the ST antigen in transformation. ST antigen-mediated PP2A inhibition has been shown to regulate mitogen-activated protein kinase and Wnt pathways at multiple steps (3) and to activate Akt and telomerase in human fetal keratinocyes (46). Inhibition of PP2A activity by the ST antigen stabilizes c-Myc (47). More recently, the PP2Aβ subunit was shown to form a complex with, and decrease the transforming functions of, RalA (37). Thus, PP2A complexes are involved in the regulation of several cellular proteins, and many of these interactions could play important roles in transformation. In this report we identify AMPK as a novel target downstream of the ST antigen-PP2A interaction in transformation. Our results reveal that indeed Thr172 phosphorylation of AMPK is maintained at a higher level in +ST cells than in −ST cells (Fig. 3a), suggesting that PP2A may directly regulate AMPK function by dephosphorylating the key Thr172 residue. Alternatively, PP2A may dephosphorylate a phosphorylated residue that negatively influences AMPK activation, thereby permitting the phosphorylation of AMPK at the critical Thr172 residue by its upstream kinases, as has been shown for ST antigen-PP2A-mediated regulation of c-Myc activity (24). Taken together, our results reveal that in the presence of functional PP2A, −ST cells are unable to maintain a high level of pAMPK, suggesting that strong activation of AMPK requires the phosphatase (PP2A) to be inactivated.
Once activated, AMPK regulates the cellular energy demand by inhibiting the energy-consuming metabolic pathways while activating the energy-producing pathways. For example, in muscle, AMPK activation decreases the activity of glycogen synthase, thereby diverting the glucose for energy production. AMPK activation is known to phosphorylate and inactivate the enzymes essential in lipid and cholesterol synthesis, namely, ACC2 and 3-hydroxy-3-methylglutaryl coenzyme A reductase, in HeLa cells and rat liver cells, respectively (8, 14). Protein synthesis is one of the highest-energy-consuming reactions within the cell, and AMPK has been shown to regulate protein synthesis in mouse embryonic fibroblasts in response to AICAR treatment (39). Our results reveal that ST antigen-mediated activation of AMPK leads to the downmodulation of mTOR activity, as revealed by a reduction in phospho-p4EBP1 levels (Fig. 4a). Reduction of energy-consuming reactions would favor longer cell survival under conditions of nutrient deprivation. Thus, it appears that when cancer cells are faced with starvation, a shutdown of protein synthesis, a major energy-consuming reaction within the cells, is better suited for survival (and cancer progression) (see the model in Fig. 6) than an attempt to divide, thereby facing death. Indeed, we note that under glucose deprivation, +ST cells show an arrest in the G0/G1 phase of the cell cycle (data not shown).
FIG. 6.
Schematic of tumor development. As incipient tumors (or micrometastases) grow beyond the homeostatic limit (∼150 μM), they often experience insufficient supplies of oxygen and nutrients. Tumor cells must overcome this stress before replenishment arrives by way of neoangiogenesis. Our data show that ST antigen-mediated induction of autophagy can be one such mechanism that provides an alternate source of energy for tumor growth and the establishment of metastases. Thus, pathways activating autophagy can be targeted for cancer therapy.
Autophagy is known to play a critical role in normal development (23), while its role in cancer is just beginning to be revealed. Limited self-eating has been shown to be required both for the initial phase of development and for regular organelle turnover within cells. However, progressive autophagy can be detrimental, leading to cell death (25). The relationship of autophagy to tumors is complex, with evidence supporting both a tumor-suppressive and a tumor-promoting role. Loss of autophagy has been correlated with tumorigenesis (9). Furthermore, several inducers of autophagy are tumor suppressors, while overexpression of genes involved in autophagy decreases tumorigenicity (11). Conversely, under conditions of metabolic stress, induction of the autophagic pathway has been hypothesized to help cancer progression. In keeping with this, autophagy has been detected in regions of metabolic stress in tumors but not in vascularized areas, suggesting that autophagy may be involved in promoting tumor cell survival under conditions of energy deprivation (9). Indeed, our experiments provide direct evidence that ST antigen-expressing cells initiate autophagy, which enables them to survive longer under glucose-deprived conditions, while in the absence of the ST antigen, cells die earlier. Thus, autophagy may provide an alternate source of energy to cancer cells under nutrient-deprived conditions. Autophagy, unlike apoptosis, is reversible. While apoptosis, once triggered, will lead to cell death, the use of autophagy might allow cells to survive stress conditions until the arrival of new resources. Indeed, replenishing the supply of glucose to +ST cells that had been starved for 24 h resulted in the downregulation of autophagy, followed by the restoration of cell proliferation, while prolonged autophagy resulted in major cell death (data not shown).
Our results additionally show that ST antigen-mediated inactivation of PP2A is required for cancer cell survival under glucose-deprived conditions, lending further support to the growing notion that PP2A is a tumor suppressor. Indeed, recent studies have reported multiple ways in which PP2A functions are compromised in naturally arising tumors. Several mutations have already been mapped to the PP2A protein that led to the compromise of its functions in lung, colon, breast, and cervical carcinomas and primary glioblastoma (3, 36). In addition, overexpression of SET (a phosphoprotein) or CIP2A (cancerous inhibitor of PP2A) (36) in chronic myelogenous leukemia or colon cancers, respectively, has been reported to inhibit PP2A function (21). Thus, exploration of the physiological mechanisms that lead to the disruption of the PP2A gene or its function in cancers and identification of specific downstream targets of PP2A are likely to provide new mechanistic insights into cancer progression.
Taken together, our results demonstrate a novel function for the SV40 ST antigen in protecting cancer cells under glucose deprivation by activating AMPK, inhibiting mTOR, and triggering autophagy (Fig. 6). Our results provide direct proof of the use of autophagy as a crutch for tumor cell survival under energy stress conditions. Therefore, the prevention of cancer cell survival under nutrient-deprived conditions, either by targeting the AMPK/mTOR pathway or by restricting the ability of cancer cells to initiate the autophagy response, is likely to offer new therapeutic strategies to curb tumor growth and progression.
Acknowledgments
We thank Robert A. Weinberg for +ST and −ST cells and Maria Colombo for the eGFP-LC3 construct.
This work was supported in part by a grant from TWAS.
Footnotes
Published ahead of print on 10 June 2009.
REFERENCES
- 1.Ali, S. H., and J. A. DeCaprio. 2001. Cellular transformation by SV40 large T antigen: interaction with host proteins. Semin. Cancer Biol. 1115-23. [DOI] [PubMed] [Google Scholar]
- 2.Allen, S., J. Sotos, M. J. Sylte, and C. J. Czuprynski. 2001. Use of Hoechst 33342 staining to detect apoptotic changes in bovine mononuclear phagocytes infected with Mycobacterium avium subsp. paratuberculosis. Clin. Diagn. Lab. Immunol. 8460-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arroyo, J. D., and W. C. Hahn. 2005. Involvement of PP2A in viral and cellular transformation. Oncogene 247746-7755. [DOI] [PubMed] [Google Scholar]
- 4.Carling, D. 2005. AMP-activated protein kinase: balancing the scales. Biochimie 8787-91. [DOI] [PubMed] [Google Scholar]
- 5.Chellappan, S., V. B. Kraus, B. Kroger, K. Munger, P. M. Howley, W. C. Phelps, and J. R. Nevins. 1992. Adenovirus E1A, simian virus 40 tumor antigen, and human papillomavirus E7 protein share the capacity to disrupt the interaction between transcription factor E2F and the retinoblastoma gene product. Proc. Natl. Acad. Sci. USA 894549-4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng, S. W., L. G. Fryer, D. Carling, and P. R. Shepherd. 2004. Thr2446 is a novel mammalian target of rapamycin (mTOR) phosphorylation site regulated by nutrient status. J. Biol. Chem. 27915719-15722. [DOI] [PubMed] [Google Scholar]
- 7.Chiang, G. G., and R. T. Abraham. 2005. Phosphorylation of mammalian target of rapamycin (mTOR) at Ser-2448 is mediated by p70S6 kinase. J. Biol. Chem. 28025485-25490. [DOI] [PubMed] [Google Scholar]
- 8.Clarke, P. R., and D. G. Hardie. 1990. Regulation of HMG-CoA reductase: identification of the site phosphorylated by the AMP-activated protein kinase in vitro and in intact rat liver. EMBO J. 92439-2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Degenhardt, K., R. Mathew, B. Beaudoin, K. Bray, D. Anderson, G. Chen, C. Mukherjee, Y. Shi, C. Gelinas, Y. Fan, D. A. Nelson, S. Jin, and E. White. 2006. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell 1051-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans, A. M., K. J. Mustard, C. N. Wyatt, C. Peers, M. Dipp, P. Kumar, N. P. Kinnear, and D. G. Hardie. 2005. Does AMP-activated protein kinase couple inhibition of mitochondrial oxidative phosphorylation by hypoxia to calcium signaling in O2-sensing cells? J. Biol. Chem. 28041504-41511. [DOI] [PubMed] [Google Scholar]
- 11.Gozuacik, D., and A. Kimchi. 2004. Autophagy as a cell death and tumor suppressor mechanism. Oncogene 232891-2906. [DOI] [PubMed] [Google Scholar]
- 12.Groves, M. R., N. Hanlon, P. Turowski, B. A. Hemmings, and D. Barford. 1999. The structure of the protein phosphatase 2A PR65/A subunit reveals the conformation of its 15 tandemly repeated HEAT motifs. Cell 9699-110. [DOI] [PubMed] [Google Scholar]
- 13.Gwinn, D. M., D. B. Shackelford, D. F. Egan, M. M. Mihaylova, A. Mery, D. S. Vasquez, B. E. Turk, and R. J. Shaw. 2008. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 30214-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ha, J., S. Daniel, S. S. Broyles, and K. H. Kim. 1994. Critical phosphorylation sites for acetyl-CoA carboxylase activity. J. Biol. Chem. 26922162-22168. [PubMed] [Google Scholar]
- 15.Hahn, W. C., C. M. Counter, A. S. Lundberg, R. L. Beijersbergen, M. W. Brooks, and R. A. Weinberg. 1999. Creation of human tumour cells with defined genetic elements. Nature 400464-468. [DOI] [PubMed] [Google Scholar]
- 16.Hahn, W. C., S. K. Dessain, M. W. Brooks, J. E. King, B. Elenbaas, D. M. Sabatini, J. A. DeCaprio, and R. A. Weinberg. 2002. Enumeration of the simian virus 40 early region elements necessary for human cell transformation. Mol. Cell. Biol. 222111-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hahn, W. C., and R. A. Weinberg. 2002. Modelling the molecular circuitry of cancer. Nat. Rev. Cancer 2331-341. [DOI] [PubMed] [Google Scholar]
- 18.Hahn, W. C., and R. A. Weinberg. 2002. Rules for making human tumor cells. N. Engl. J. Med. 3471593-1603. [DOI] [PubMed] [Google Scholar]
- 19.Inoki, K., H. Ouyang, T. Zhu, C. Lindvall, Y. Wang, X. Zhang, Q. Yang, C. Bennett, Y. Harada, K. Stankunas, C. Y. Wang, X. He, O. A. MacDougald, M. You, B. O. Williams, and K. L. Guan. 2006. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 126955-968. [DOI] [PubMed] [Google Scholar]
- 20.Inoki, K., T. Zhu, and K.-L. Guan. 2003. TSC2 mediates cellular energy response to control cell growth and survival. Cell 115577-590. [DOI] [PubMed] [Google Scholar]
- 21.Junttila, M. R., P. Puustinen, M. Niemela, R. Ahola, H. Arnold, T. Bottzauw, R. Ala-aho, C. Nielsen, J. Ivaska, Y. Taya, S. L. Lu, S. Lin, E. K. Chan, X. J. Wang, R. Grenman, J. Kast, T. Kallunki, R. Sears, V. M. Kahari, and J. Westermarck. 2007. CIP2A inhibits PP2A in human malignancies. Cell 13051-62. [DOI] [PubMed] [Google Scholar]
- 22.Kato, K., T. Ogura, A. Kishimoto, Y. Minegishi, N. Nakajima, M. Miyazaki, and H. Esumi. 2002. Critical roles of AMP-activated protein kinase in constitutive tolerance of cancer cells to nutrient deprivation and tumor formation. Oncogene 216082-6090. [DOI] [PubMed] [Google Scholar]
- 23.Klionsky, D. J., and S. D. Emr. 2000. Autophagy as a regulated pathway of cellular degradation. Science 2901717-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klucky, B., and E. Wintersberger. 2007. Polyomavirus small T antigen transactivates genes by its ability to provoke the synthesis and the stabilization of MYC. Oncogene 266356-6360. [DOI] [PubMed] [Google Scholar]
- 25.Kondo, Y., T. Kanzawa, R. Sawaya, and S. Kondo. 2005. The role of autophagy in cancer development and response to therapy. Nat. Rev. Cancer 5726-734. [DOI] [PubMed] [Google Scholar]
- 26.Laderoute, K. R., K. Amin, J. M. Calaoagan, M. Knapp, T. Le, J. Orduna, M. Foretz, and B. Viollet. 2006. 5′-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Mol. Cell. Biol. 265336-5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin, R. G., V. P. Setlow, C. A. Edwards, and D. Vembu. 1979. The roles of the simian virus 40 tumor antigens in transformation of Chinese hamster lung cells. Cell 17635-643. [DOI] [PubMed] [Google Scholar]
- 28.Minchinton, A. I., and I. F. Tannock. 2006. Drug penetration in solid tumours. Nat. Rev. Cancer 6583-592. [DOI] [PubMed] [Google Scholar]
- 29.Morales, C. P., S. E. Holt, M. Ouellette, K. J. Kaur, Y. Yan, K. S. Wilson, M. A. White, W. E. Wright, and J. W. Shay. 1999. Absence of cancer-associated changes in human fibroblasts immortalized with telomerase. Nat. Genet. 21115-118. [DOI] [PubMed] [Google Scholar]
- 30.Moran, E. 1993. Interaction of adenoviral proteins with pRB and p53. FASEB J. 7880-885. [DOI] [PubMed] [Google Scholar]
- 31.Pallas, D. C., L. K. Shahrik, B. L. Martin, S. Jaspers, T. B. Miller, D. L. Brautigan, and T. M. Roberts. 1990. Polyoma small and middle T antigens and SV40 small t antigen form stable complexes with protein phosphatase 2A. Cell 60167-176. [DOI] [PubMed] [Google Scholar]
- 32.Rangarajan, A., S. J. Hong, A. Gifford, and R. A. Weinberg. 2004. Species- and cell type-specific requirements for cellular transformation. Cancer Cell 6171-183. [DOI] [PubMed] [Google Scholar]
- 33.Ruderman, N. B., A. K. Saha, D. Vavvas, and L. A. Witters. 1999. Malonyl-CoA, fuel sensing, and insulin resistance. Am. J. Physiol. 276E1-E18. [DOI] [PubMed] [Google Scholar]
- 34.Rundell, K., and R. Parakati. 2001. The role of the SV40 ST antigen in cell growth promotion and transformation. Semin. Cancer Biol. 115-13. [DOI] [PubMed] [Google Scholar]
- 35.Sabatini, D. M. 2006. mTOR and cancer: insights into a complex relationship. Nat. Rev. Cancer 6729-734. [DOI] [PubMed] [Google Scholar]
- 36.Sablina, A., and W. Hahn. 2008. SV40 small T antigen and PP2A phosphatase in cell transformation. Cancer Metastasis Rev. 27137-146. [DOI] [PubMed] [Google Scholar]
- 37.Sablina, A. A., W. Chen, J. D. Arroyo, L. Corral, M. Hector, S. E. Bulmer, J. A. DeCaprio, and W. C. Hahn. 2007. The tumor suppressor PP2A Aβ regulates the RalA GTPase. Cell 129969-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sato, K., K. Tsuchihara, S. Fujii, M. Sugiyama, T. Goya, Y. Atomi, T. Ueno, A. Ochiai, and H. Esumi. 2007. Autophagy is activated in colorectal cancer cells and contributes to the tolerance to nutrient deprivation. Cancer Res. 679677-9684. [DOI] [PubMed] [Google Scholar]
- 39.Shaw, R. J., N. Bardeesy, B. D. Manning, L. Lopez, M. Kosmatka, R. A. DePinho, and L. C. Cantley. 2004. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell 691-99. [DOI] [PubMed] [Google Scholar]
- 40.Shaw, R. J., M. Kosmatka, N. Bardeesy, R. L. Hurley, L. A. Witters, R. A. DePinho, and L. C. Cantley. 2004. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. USA 1013329-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shim, H., Y. S. Chun, B. C. Lewis, and C. V. Dang. 1998. A unique glucose-dependent apoptotic pathway induced by c-Myc. Proc. Natl. Acad. Sci. USA 951511-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shintani, T., and D. J. Klionsky. 2004. Autophagy in health and disease: a double-edged sword. Science 306990-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sontag, E., S. Fedorov, C. Kamibayashi, D. Robbins, M. Cobb, and M. Mumby. 1993. The interaction of SV40 small tumor antigen with protein phosphatase 2A stimulates the MAP kinase pathway and induces cell proliferation. Cell 75887-897. [DOI] [PubMed] [Google Scholar]
- 44.Tozer, G. M., C. Kanthou, and B. C. Baguley. 2005. Disrupting tumour blood vessels. Nat. Rev. Cancer 5423-435. [DOI] [PubMed] [Google Scholar]
- 45.Woods, A., K. Dickerson, R. Heath, S. P. Hong, M. Momcilovic, S. R. Johnstone, M. Carlson, and D. Carling. 2005. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 221-33. [DOI] [PubMed] [Google Scholar]
- 46.Yuan, H., T. Veldman, K. Rundell, and R. Schlegel. 2002. Simian virus 40 small tumor antigen activates AKT and telomerase and induces anchorage-independent growth of human epithelial cells. J. Virol. 7610685-10691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao, J. J., O. V. Gjoerup, R. R. Subramanian, Y. Cheng, W. Chen, T. M. Roberts, and W. C. Hahn. 2003. Human mammary epithelial cell transformation through the activation of phosphatidylinositol 3-kinase. Cancer Cell 3483-495. [DOI] [PubMed] [Google Scholar]