Abstract
The herpes simplex virus 1 protein ICP27 is methylated on arginine residues within an RGG box, and arginine methylation regulates ICP27 export to the cytoplasm. Arginine methylation can regulate protein-protein interactions; therefore, we examined the effect of hypomethylation on ICP27's interactions with cellular proteins SRPK1 and Aly/REF, which bind to ICP27 through the RGG box region. During infections with viral mutants containing lysine substitutions or the methylation inhibitor adenosine dialdehyde, the interaction of ICP27 with SRPK1 and Aly/REF was decreased, as determined by coimmunoprecipitation and colocalization studies, indicating that ICP27 RGG box methylation regulates interaction with these proteins.
The herpes simplex virus 1 (HSV-1) protein ICP27 is a multifunctional regulatory protein that acts at the transcriptional and posttranscriptional levels. Posttranscriptionally, ICP27 inhibits cellular pre-mRNA splicing early in infection (3, 13) by mediating the aberrant phosphorylation of essential splicing proteins, termed SR proteins (13). ICP27 accomplishes this by interacting with SRPK1, a predominantly cytoplasmic SR protein-specific kinase, and recruits SRPK1 to the nucleus (13). ICP27 interacts with SRPK1 through its RGG box RNA binding motif (13). Beginning at about 5 h after infection, ICP27 disassociates from spliceosomal sites and begins shuttling between the nucleus and the cytoplasm in its role as a viral RNA export protein (5, 7, 12, 15). ICP27 interacts with the TREX complex RNA export protein Aly/REF and recruits it to viral replication compartments (4, 5, 7). The region of ICP27 that is required for interaction with Aly/REF includes the RGG box (5).
ICP27 is modified posttranslationally by methylation on arginine residues (10, 16). The major site of arginine methylation, as determined by mass spectrometric analysis, is the RGG box from residues 138 to 152 (16). Specifically, arginine residues 138, 148, and 150 were found to be methylated during infection (16). RGG box motifs, which consist of closely spaced arginine and glycine repeats, are often found in RNA binding proteins, and they function in both RNA binding and protein-protein interactions (1). Arginine methylation plays a functional role in regulating the import and export of proteins and in regulating protein-protein interactions (1, 2, 8). We reported that arginine methylation regulates ICP27's export from the nucleus, so that under conditions of hypomethylation, ICP27 was exported from the nucleus earlier and more rapidly (16). Viral mutants with substitutions of lysine for arginine at positions 138, 148, and 150 singly and in combination were constructed. These mutants were defective in viral replication and gene expression compared to wild-type HSV-1, and similar effects could be achieved with the methylation inhibitor adenosine dialdehyde (AdOx), indicating that hypomethylation was responsible for the observed phenotypes (16).
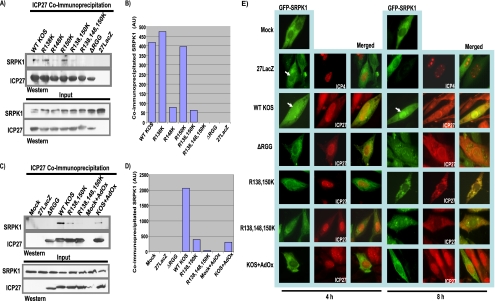
Arginine methylation also regulates protein-protein interactions (2). Because the region of interaction of SRPK1 and Aly/REF with ICP27 includes the RGG box, we asked what effect hypomethylation would have on these interactions. Hypomethylation was achieved by using the viral mutants ΔRGG, in which the RGG box is deleted (10, 16), and R138K, R148K, R150K, R138,150K, and R138,148,150K, which have substitutions of lysine residues for arginine residues at the indicated positions (16), and by adding AdOx to HSV-1 KOS-infected cells. We first looked at the interaction of ICP27 with SRPK1. HeLa cells were infected with HSV-1 KOS, 27-LacZ (14), or mutant ΔRGG, R138K, R148K, R150K, R138,150K, or R138,148,150K or were infected with HSV-1 KOS in the presence of AdOx (20 μM), which was added 2 h after infection (Fig. 1). Immunoprecipitation with the ICP27 monoclonal antibody P1119 (Virusys) was performed on cell lysates harvested 8 h after infection, and Western blot analysis was performed on fractionated samples with anti-SRPK1 monoclonal antibody (BD Transduction Laboratories) and anti-ICP27 antibody, as described previously (13, 16). No reduction in the amount of SRPK1 that coimmunoprecipitated with ICP27 was seen in the R138K and R150K samples (Fig. 1A and B) compared to that of the wild type; however, there was an 80% reduction in the amount of SRPK1 that coprecipitated with R148K (Fig. 1A and B) and a 90% reduction in the amount of SRPK1 that coimmunoprecipitated with R138,150K. SRPK1 was not detected in the R138,148,150K-immunoprecipitated samples (Fig. 1A to D), and HSV-1 KOS infection in the presence of AdOx resulted in about a 90% reduction in the amount of SRPK1 coimmunoprecipitating with ICP27 (Fig. 1C and D), suggesting that hypomethylation of the RGG box is the cause of the reduced interaction between ICP27 and SRPK1.
FIG. 1.
Coimmunoprecipitation of ICP27 and SRPK1. (A) HeLa cells were mock infected or infected with HSV-1 KOS or the ICP27 viral mutants as indicated, and immunoprecipitation was performed with anti-ICP27 antibody. Western blots were probed with anti-SRPK1 and anti-ICP27 antibodies. Samples of each lysate were analyzed in parallel with the immunoprecipitated samples, and the Western blot is labeled input. WT, wild-type. (B) Western blots in panel A were scanned and quantified by densitometry. AU, arbitrary units. (C) HeLa cells were infected as indicated in the presence or absence of AdOx. Immunoprecipitation was performed, as shown in panel A. (D) Western blots shown in panel C were quantified by densitometry. (E) RSF cells were transfected with pEGFP-SRPK1 and were infected 24 h later with the indicated viruses. Immunofluorescent staining was performed with anti-ICP27 antibody, and GFP fluorescence was visualized directly.
We reported that ICP27 recruits SRPK1 from the cytoplasm to the nucleus, and the ICP27 RGG box was required (13). We looked at the functional interaction between ICP27 and SRPK1 by using immunofluorescence microscopy. Rabbit skin fibroblast (RSF) cells were transfected, as described previously (13), with plasmid pEGFP-SRPK1 (13). Twenty-four hours later, cells were mock infected or were infected at a multiplicity of infection of 10 with KOS in the presence and absence of AdOx, 27-LacZ, ΔRGG, R138,150K, or R138,148,150K for 4 and 8 h. Cells were stained with anti-ICP27 antibody. 27-LacZ-infected cells were stained with anti-ICP4 antibody P1101 (Virusys) to mark the nuclei. Green fluorescent protein (GFP) was visualized directly at 100× magnification with a Zeiss Axiovert S100 microscope. The images shown are representative of the results visualized, with greater than 90% of the GFP-SRPK1-expressing cells displaying the phenotypes shown. GFP-SRPK1 was predominantly cytoplasmic in mock-infected cells and in cells infected with 27-LacZ (Fig. 1E). GFP-SRPK1 localization in the nucleus was seen in KOS-infected cells at 4 h after infection, and strong nuclear fluorescence of GFP-SRPK1 was visualized in KOS-infected cells at 8 h (Fig. 1E). In contrast, GFP-SRPK1 remained predominantly cytoplasmic in ΔRGG-, R138,150K-, R138,148,150K-, and KOS-plus-AdOx-infected cells (Fig. 1E). Thus, hypomethylation of the ICP27 RGG box prevents the nuclear recruitment of SRPK1 by ICP27.
ICP27 interacts with the RNA export protein Aly/REF (5, 7) and recruits Aly/REF away from splicing speckles, where it is localized in uninfected cells (18), to HSV-1 replication compartments (4). We mapped the region of ICP27 that is required for interaction with Aly/REF to amino acids 104 to 153, which includes the RGG box (5). To determine if methylation of the RGG box can regulate the interaction between ICP27 and Aly/REF, coimmunoprecipitation experiments were performed. HeLa cells were mock infected or infected with KOS with or without AdOx or with 27-LacZ, ΔRGG, R138K, R148K, R150K, R138,150K, or R138,148,150K for 8 h. Immunoprecipitation was performed using anti-ICP27 antibody, as described previously (4). RNase was added to ensure that RNA was not bridging the interaction between the two RNA binding proteins. Western blots were probed with anti-ICP27 and anti-Aly/REF antibodies (Sigma). Aly/REF was coimmunoprecipitated with wild-type ICP27 and with the mutants R138K, R148K, and R150K (Fig. 2A and B). However, Aly/REF was barely detectable with the ΔRGG protein and was reduced by about 60 to 70% for R138,150K and R138,148,150K (Fig. 2), with about a 55% reduction in the amount of Aly/REF that coprecipitated with ICP27 in the KOS-plus-AdOx sample (Fig. 2C and D). AdOx has been shown to reduce arginine methylation of proteins by about 50% (11), so ICP27 would still be partially methylated. These results again point to hypomethylation as the cause of the decrease in the interaction of Aly/REF and ICP27.
FIG. 2.
Coimmunoprecipitation of ICP27 and Aly/REF. (A) HeLa cells were mock infected or were infected with KOS or ICP27 viral mutants, as indicated. Immunoprecipitation was performed with anti-ICP27 antibody, and Western blots were probed with anti-ICP27 and anti-Aly/REF antibodies. Samples of each lysate were analyzed in parallel with the immunoprecipitated samples, and the blot is labeled “Input.” (B) Western blots shown in panel A were quantified by densitometry. WT, wild-type; AU, arbitrary units. (C) HeLa cells were infected as indicated in the presence or absence of AdOx. Immunoprecipitation was performed, as shown in panel A. (D) Western blots were quantified by densitometry.
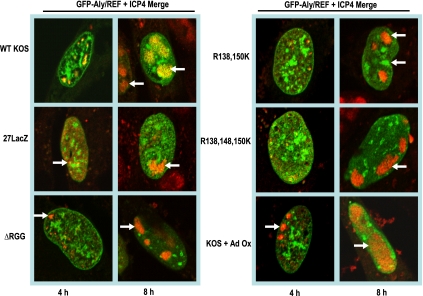
ICP27 colocalizes with Aly/REF at early times of infection and recruits Aly/REF to HSV-1 replication compartments (4). We investigated the localization of Aly/REF under conditions of ICP27 hypomethylation. RSF cells were transfected with pEGFP-Aly/REF, and 24 h later, cells were infected with KOS in the presence or absence of AdOx, ΔRGG, R138,150K, or R138,148,150K for 4 and 8 h. Immunofluorescent staining was performed using anti-ICP27 antibody, and GFP fluorescence was visualized directly. Representative images are shown, and greater than 85% of the GFP-expressing cells displayed this phenotype. Areas of colocalization were seen in KOS-infected cells at 4 h after infection, and some colocalization was also seen at 8 h (Fig. 3). Localization of Aly/REF and the ΔRGG, R138,150K, and R138,148,150K proteins appeared to be distinct rather than colocalized at 4 h (data not shown) or 8 h after infection (Fig. 3). Similarly, under conditions of hypomethylation induced by AdOx, ICP27 and Aly/REF did not appear to be colocalized (Fig. 3).
FIG. 3.
Aly/REF localization in HSV-1-infected cells. RSF cells were transfected with pEGFP-Aly/REF and were infected as indicated 24 h later for the times shown. ICP27 staining is shown in red, and GFP staining is shown in green. Yellow arrows point to areas of colocalization, and white arrows point to distinct Aly/REF staining that is not colocalized with ICP27. WT, wild-type.
To determine if Aly/REF can be recruited to viral transcription/replication compartments, immunofluorescence experiments were performed using anti-ICP4 antibody to mark viral transcription sites. RSF cells were transfected with pEGFP- Aly/REF and, 24 h later, were infected with KOS in the presence or absence of AdOx or with 27-Lacz, ΔRGG, R138,150K, or R138,148,150K for 4 and 8 h. Cells were stained with anti-ICP4 antibody, and GFP fluorescence was viewed directly with a Zeiss LSM 510 confocal microscope. In KOS-infected cells at 4 h postinfection, Aly/REF was localized in splicing speckles, as we reported previously (4), but at 8 h, Aly/REF was relocalized into ICP4-containing replication compartments (Fig. 4). In contrast, Aly/REF remained in splicing speckles and was not recruited to replication compartments in cells infected with ICP27 mutant viruses or in KOS-infected cells treated with AdOx (Fig. 4). Therefore, under conditions of hypomethylation, the interaction of ICP27 with Aly/REF was decreased, and Aly/REF was not recruited to viral replication compartments.
FIG. 4.
Aly/REF recruitment to HSV-1 replication compartments. RSF cells were transfected with pEGFP-Aly/REF and were infected as indicated 24 h later for the times shown. Immunofluorescent staining of ICP4 was performed with anti-ICP4 antibody, and GFP was visualized directly. Merged confocal images are shown in which GFP staining is green and ICP4 staining is red. Arrows point to ICP4-containing transcription/replication sites. WT, wild-type.
These studies have shown that methylation of the ICP27 RGG box plays an important role in regulating the interaction of ICP27 with two cellular proteins. ICP27 recruits SRPK1 from the cytoplasm to the nucleus, which results in the aberrant phosphorylation of SR splicing proteins. This results in stalled spliceosomal complexes and an inhibition of pre-mRNA splicing (13). Although we have not investigated the effect of hypomethylation of ICP27 on SR protein phosphorylation and splicing, this will be an important direction to pursue in future experiments.
Aly/REF is a component of the TREX complex that is recruited to the 5′ end of mRNA (9), where it interacts with CBP80 to direct mRNA to the export receptor TAP/NXF1, with which Aly/REF directly binds (17). Although Aly/REF is recruited to viral replication compartments by ICP27 (4), we showed that Aly/REF may be dispensable for viral RNA export (6). Knockdown of Aly/REF with small interfering RNA had little effect on the export of RNA in HSV-1-infected cells (6). We also found that ICP27 interacts with another TREX component, UAP56, and Aly/REF stabilized the interaction (L. A. Johnson and R. M. Sandri-Goldin, unpublished results). Thus, Aly/REF may not be essential for viral RNA export, but its recruitment to replication compartments may contribute to RNA export efficiency by assembling TREX components on viral RNAs.
Acknowledgments
This work was supported by National Institute of Allergy and Infectious Diseases (NIAID) grants AI61397 and AI21215.
Footnotes
Published ahead of print on 24 June 2009.
REFERENCES
- 1.Bedford, M. T., and S. G. Clarke. 2009. Protein arginine methylation in mammals: who, what and why. Mol. Cell 331-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bedford, M. T., and S. Richard. 2005. Arginine methylation: an emerging regulator of protein function. Mol. Cell 18263-272. [DOI] [PubMed] [Google Scholar]
- 3.Bryant, H. E., S. Wadd, A. I. Lamond, S. J. Silverstein, and J. B. Clements. 2001. Herpes simplex virus IE63 (ICP27) protein interacts with spliceosome-associated protein 145 and inhibits splicing prior to the first catalytic step. J. Virol. 754376-4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, I. B., L. Li, L. Silva, and R. M. Sandri-Goldin. 2005. ICP27 recruits Aly/REF but not TAP/NXF1 to herpes simplex virus type 1 transcription sites although TAP/NXF1 is required for ICP27 export. J. Virol. 793949-3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, I. B., K. S. Sciabica, and R. M. Sandri-Goldin. 2002. ICP27 interacts with the export factor Aly/REF to direct herpes simplex virus 1 intronless RNAs to the TAP export pathway. J. Virol. 7612877-12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson, L. A., L. Li, and R. M. Sandri-Goldin. 2009. The cellular RNA export receptor TAP/NXF1 is required for ICP27-mediated export of herpes simplex virus 1 RNA, but the TREX complex adaptor protein Aly/REF appears to be dispensable. J. Virol. 836335-6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koffa, M. D., J. B. Clements, E. Izaurralde, S. Wadd, S. A. Wilson, I. W. Mattaj, and S. Kuersten. 2001. Herpes simplex virus ICP27 protein provides viral mRNAs with access to the cellular mRNA export pathway. EMBO J. 205769-5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lukong, K. E., and S. Richard. 2004. Arginine methylation signals mRNA export. Nat. Struct. Mol. Biol. 11914-915. [DOI] [PubMed] [Google Scholar]
- 9.Masuda, S., R. Das, H. Cheng, E. Hurt, N. Dorman, and R. Reed. 2005. Recruitment of the human TREX complex to mRNA during splicing. Genes Dev. 191512-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mears, W. E., and S. A. Rice. 1996. The RGG box motif of the herpes simplex virus ICP27 protein mediates an RNA-binding activity and determines in vivo methylation. J. Virol. 707445-7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pahlich, S., R. Zakaryan, and H. Gehring. 2006. Protein arginine methylation: cellular functions and methods of analysis. Biochim. Biophys. Acta 17641890-1903. [DOI] [PubMed] [Google Scholar]
- 12.Sandri-Goldin, R. M. 1998. ICP27 mediates herpes simplex virus RNA export by shuttling through a leucine-rich nuclear export signal and binding viral intronless RNAs through an RGG motif. Genes Dev. 12868-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sciabica, K. S., Q. J. Dai, and R. M. Sandri-Goldin. 2003. ICP27 interacts with SRPK1 to mediate HSV-1 inhibition of pre-mRNA splicing by altering SR protein phosphorylation. EMBO J. 221608-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith, I. L., M. A. Hardwicke, and R. M. Sandri-Goldin. 1992. Evidence that the herpes simplex virus immediate early protein ICP27 acts post-transcriptionally during infection to regulate gene expression. Virology 18674-86. [DOI] [PubMed] [Google Scholar]
- 15.Soliman, T. M., R. M. Sandri-Goldin, and S. J. Silverstein. 1997. Shuttling of the herpes simplex virus type 1 regulatory protein ICP27 between the nucleus and cytoplasm mediates the expression of late proteins. J. Virol. 719188-9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Souki, S. K., P. D. Gerson, and R. M. Sandri-Goldin. 2009. Arginine methylation of the ICP27 RGG box regulates ICP27 export and is required for efficient herpes simplex virus 1 replication. J. Virol. 835309-5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stutz, F., A. Bachi, T. Doerks, I. C. Braun, B. Seraphin, M. Wilm, P. Bork, and E. Izaurralde. 2000. REF, an evolutionary conserved family of hnRNP-like proteins, interacts with TAP/Mex67p and participates in mRNA nuclear export. RNA 6638-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou, Z., M. J. Luo, K. Straesser, J. Katahira, E. Hurt, and R. Reed. 2000. The protein Aly links pre-messenger-RNA splicing to nuclear export in metazoans. Nature 407401-405. [DOI] [PubMed] [Google Scholar]