Abstract
Bluetongue virus (BTV) is the etiological agent of bluetongue, a hemorrhagic disease of ruminants (particularly sheep), which causes important economic losses around the world. BTV is transmitted primarily via the bites of infected midges, which inject the virus into the ruminant's skin during blood feeding. The virus initially replicates in the draining lymph node and then disseminates to secondary organs where it induces edema, hemorrhages, and necrosis. In this study, we show that ovine conventional dendritic cells (cDCs) are the primary targets of BTV that contribute to the primary dissemination of BTV from the skin to draining lymph nodes. Lymph cDCs support BTV RNA and protein synthesis, as well as the production of infectious virus belonging to several different BTV serotypes, regardless of their level of attenuation. Afferent lymph cell subsets, other than cDCs, showed only marginal levels of BTV protein expression. BTV infection provoked a massive recruitment of cDCs to the sheep skin and afferent lymph, providing cellular targets for infection. Although BTV productively infects cDCs, no negative impact on their physiology was detected. Indeed, BTV infection and protein expression in cDCs enhanced their survival rate. Several serotypes of BTV stimulated the surface expression of the CD80 and CD86 costimulatory molecules on cDCs as well as the mRNA synthesis of cytokines involved in inflammation and immunity, i.e., interleukin-12 (IL-12), IL-1β, and IL-6. BTV-infected cDCs stimulated antigen-specific CD4 and CD8 proliferation as well as gamma interferon production. BTV initially targets cDCs while preserving their functional properties, reflecting the optimal adaptation of the virus to its host cells for its first spread.
Bluetongue virus (BTV) is the prototype species of the genus Orbivirus within the family Reoviridae (16). There are 25 distinct serotypes of BTV (24, 28) which are transmitted between their ruminant hosts, primarily by adult females of certain species of biting midge. The BTV particle is icosahedral and nonenveloped and has a three-layered protein capsid, with a genome composed of 10 distinct segments of linear double-stranded RNA (16). BTV causes the hemorrhagic disease bluetongue in sheep and some other species of ruminants (41). Depending on the virus strain and host factors (including breed and species), BTV infection induces clinical signs that can vary from being asymptomatic to mild disease to severe or even fatal disease, with many organs showing vascular degeneration, hemorrhage, edema, disseminated intravascular coagulation, vascular thrombosis, and tissue infarction (9, 32). Some sheep breeds such as Merinos or Dorset Poll are more sensitive than others (12, 30, 45). After a BTV-infected vector insect bites a susceptible ruminant host, the virus initially replicates in the draining lymph node (3, 31) and then disseminates further, via efferent lymph and blood to secondary sites of infection. These include the spleen, lungs, and other organs, where it replicates in a large number of endothelium cells and mononuclear phagocytes (3). Viremia in some infected animals persists for long periods, despite concurrent, specific humoral (31) and cellular immunity (25). The variations observed in individual animals in both the pathogenesis and duration of viremia caused by BTV have not been fully explained, although it has been suggested that variations or deficiencies in immunity to BTV could be involved (9).
Infectious BTV has been found to be associated mainly with red blood cells and monocytes in the blood of cattle (46) and sheep (9), indicating that these cells contribute to the secondary dissemination of BTV via the blood of these animals. Although BTV infection of erythrocytes does not progress beyond adsorption, the virus replicates productively in monocytes, with formation of viral inclusion bodies and virus-specific tubules (8). In addition to the well-established infection of monocytes, contradictory reports suggest that other leukocytes may also become infected, including activated lymphocytes (4, 9) and γ/δ T cells (26, 43). Viral RNA transcripts have also been detected within the cytoplasm of monocytes/macrophages, as well as within endothelial cells of the lymph nodes and spleens of infected sheep (9).
The viral hemorrhagic fevers include a group of human diseases that are caused by enveloped, single-stranded RNA viruses, such as the Lassa, Rift Valley fever, Ebola, dengue, and yellow fever viruses (7). These diseases are all characterized by fever and malaise, increased vascular permeability, and altered coagulation, resulting in hemorrhages and, in some cases, intravascular-disseminated coagulation. It is notable that the early replication of all of these viruses occurs in monocytes/macrophages, as well as in conventional dendritic cells (cDCs), which may contribute to viral dissemination within the infected host (6, 7, 19, 22). cDCs are the main type of DCs involved in antigen presentation and trafficking from tissues to lymph nodes, and plasmacytoid DCs (pDCs) are the main type I interferon producers (42). In addition, the infection of these mononuclear phagocytes triggers undesirable cytokine and chemokine responses, and even mediators associated with vascular dysfunctions and blood coagulation, that likely contribute to viral pathogenesis (hemorrhages and inflammation) (7, 17, 27). Furthermore, the infection of cDCs by highly virulent Ebola or Lassa viruses resulted in the impaired activation and production of cytokines that are associated with a reduced and delayed adaptive response (2, 33).
In view of the similar pathology caused by the human hemorrhagic viruses and BTV, and the known tropism of BTV for monocytes, interactions between BTV and cDCs need to be investigated further to establish the role of these cells in BTV dissemination, pathogenesis, and immunity within the infected host.
A permanent catheter can be placed in a pseudoafferent duct allowing the collection of sheep lymph cells (including migrating cDCs) after the intracutaneous administration of pathogens. Sheep therefore provide an exceptional opportunity to examine the early stages of pathogen dissemination via the afferent lymph compartment of the skin. This experimental system was therefore used to intercept cells migrating from the skin to the draining lymph node, providing a direct evaluation of the early phase of its dissemination in a natural host. The results obtained indicate that BTV uses cDCs as a vehicle for dissemination while maintaining their function, indicating a high level of adaptation to these cells as a primary target for infection.
MATERIALS AND METHODS
Animals, surgery, and statement of ethics.
Prealpe BTV-seronegative female sheep (2 to 4 years old), originating from the Unité Commune d' Expérimentation Animale in Jouy-en-Josas, France, were cannulated. Pseudoafferent prescapular (21) and cervical efferent (1) lymph duct cannulations were conducted at the Centre de Recherche en Imagerie Interventionnelle in Jouy-en-Josas. A total of 18 sheep were used for the cannulation, among which 9 were successfully cannulated. Low-molecular heparin (enoxaparin [Lovenox]; Sanofi-Aventis) was injected intradermally in the shoulder skin every 12 h (2,000 IU anti-Xa per injection). The animal experiments were carried out under license (accreditation numbers A78-93, A78-15, and A78-730), issued by the Direction of the Veterinary Services of Versailles with agreement from the Regional Paris South Ethics Committee (number 08-002) in 2008.
Lymph collections, lymph fluid, and cell subset isolations.
Lymph was collected twice a day in flasks containing 500 IU heparin, 10,000 IU penicillin, and 10 mg streptomycin. The volume and the number of cells per ml were recorded. Total lymph (TL) cells were spun down to 700 g. The lymph supernatant was filtered through polyethersulfone Minisart syringe filters (0.2-μm pores; Sartorius). Low-density lymph (LDL) cells were obtained after centrifugation on a 1.065 density iodixanol gradient (Optiprep; Nycomed Pharma, Denmark) as previously described (40). In many instances, TL and LDL cells were frozen in liquid nitrogen as previously described (40). Lymph cell subsets were enriched by either positive immunomagnetic cell sorting (Myltenyi Biotech) or negative immunomagnetic cell sorting (Dynal). For the positive selection of CD4+ T cells or cDCs, the anti-CD4 (ST4) or anti-CD11c (mix of OM1 and BAQ153) monoclonal antibodies (MAbs) were used as previously described (37). In some experiments involving “untouched” cDCs, the cells were enriched by negative sorting using a mixture of anti-CD4 (ST4), -CD8 (CACT80C), -γ/δ T cells (86D), -B cells (DU2-104), and -CD14 (CAM36), followed by a mix of magnetic Dynabeads (Dynal) coated with human MAb anti-mouse immunoglobulin G (IgG) and rat MAb anti-mouse IgM, according to the manufacturer's recommendations. The efficiency of positive and negative selections was examined by fluorescence-activated cell sorting (FACS) after incubating the sorted cells with fluorescein isothiocyanate (FITC)-labeled goat Ig anti-mouse IgG (H+L). The quality of the DC selection obtained after negative sorting was also estimated by the microscopic examination of cDC morphology.
Production of viruses and infections.
Wild-type field strains of BTV serotypes 2 and 8 (BTV2wt and BTV8wt, respectively) were isolated in Corsica, France, in 2001 and in the north of France in 2006, respectively. These viruses are still virulent in sheep. Modified live vaccine strains (BTV1at, BTV2at, BTV4at, BTV9at, and BTV16at [where at indicates that it is attenuated]) were produced by Onderstepoort Biological Products in the Republic of South Africa.
Virus particles of the South African reference strain of BTV1 were purified as previously described by Mertens et al. (34). This strain of BTV is held in the Institute for Animal Health (IAH; United Kingdom) reference collection (www.reoviridae.org/dsRNA_virus_proteins/ReoID/btv-1.htm) and is identified by strain number RSArrrr/01.
Infections with BTV strains and production of viral stocks were performed in the secure BL3 facilities at Maisons-Alfort, France, or at IAH Pirbright. Viral cell culture supernatants and purified BTV1wt were titrated on baby hamster kidney 21 (BHK21) cells. Briefly, BHK21 cells (2,500 per well) were plated on 96-well plates in Dulbecco's modified Eagle's medium containing 5% fetal calf serum (FCS), 100 IU/ml penicillin, and 100 μg/ml streptomycin. Dilutions of virus suspensions (1:3) were added to a row of six wells. After 5 days, the medium was removed. The cells were fixed with formalin and stained with methylene blue. Each titer was expressed as the inverse of the last dilution that showed a cytopathic effect in 50% of the wells (50% tissue culture infectious dose [TCID50]) according to the method of Spearman-Karber.
Lymph cells (TL, LDL, and selected cDCs) were incubated at 37°C for 2 h with 0.004 TCID50 BTV per cell in 400 μl X-Vivo 15 medium (Biowhittaker). The cells were then washed three times in X-Vivo 15 medium containing 1% FCS. They were plated in X-Vivo 15 medium containing 20% autologous lymph cell supernatants, 100 IU/ml penicillin, and 100 μg/ml streptomycin at 37°C.
Two cannulated sheep (nos. 17 and 04) and two noncannulated sheep (nos. 45 and 69) were inoculated intradermally with 105 TCID50 BTV2at at five different skin spots (200 μl in total). Skin fragments, lymph nodes, or lymph were harvested at different time points as indicated, and the sheep were killed at the end of the experiment.
RNA extractions and reverse transcriptase PCR (RT-PCR).
Total RNA (5 × 105 cells) was extracted using the Arcturus pico-pure kit (Arcturus).
BTV RNA was detected in each sample by subjecting 100 ng of the extracted RNA to a one-step quantitative RT-PCR (qRT-PCR) using the SuperScript III Platinum one-step kit, with primers targeting the NS3 gene (BTV genome segment 10 [Seg-10]) of BTV2at (forward, ACCATTTCTCAACCACCAAGGT; reverse, GCAACCGTTGGCATAGATGA). Initial testing demonstrated that the denaturation of the double-stranded RNA using dimethyl sulfoxide and heating did not improve the sensitivity of detection. Consequently, the RT-PCR does not distinguish between genomic and newly synthesized viral mRNA. The data were normalized by performing a one-step qRT-PCR on the same RNA sample (20 ng) using primers targeting the sheep GAPDH (glyceraldehyde-3-phosphate dehydrogenase) housekeeping gene (forward, CACCATCTTCCAGGAGCGAG, and reverse, CCAGCATCACCCCACTTGAT). Each experiment included a calibrator RNA derived from BTV2at-infected BHK21 cells, allowing threshold-cycle calculations using SDS 2.1 software (Applied Biosystems). The viral RNA in the calibrator BTV2at-infected BHK21 cell was quantified using a standard curve of in vitro-transcribed NS3 RNA. The limit of detection was 100 copies of the Seg-10 gene. For each normalized cell RNA sample, the copy number of the viral NS3 RNA was calculated.
For the detection of viral-induced cytokine mRNAs in DC preparations, RNA (400 ng) was reverse transcribed using random primers and Multiscribe RT (Applied Biosystems). Quantitative real-time PCR was carried out using 100 ng cDNA with 300 nM of each primer in a final reaction volume of 25 μl of 1× SYBR green PCR master mix (Applied Biosystems). The primers used to amplify ovine interleukin-12 (IL-12) (forward, GAATTCTCGGCAGGTGGAAG, and reverse, GTGCTCCACGTGTCAGGGTA) and IL-6 (forward, GCTGCTCCTGGTGATGACTTC, and reverse, GGTGGTGTCATTTTTGAAATCTTCT) were designed using Primer Express software (version 2.0). The primers used to amplify IL-1β were as previously described (10). PCR cycling conditions were 95°C for 10 min, linked to 40 cycles of 95°C for 15 s and 60°C for 1 min. Real-time PCR data were collected by the ABI 7900HT sequence detection system (Applied Biosystems), and threshold-cycle calculations for the relative expression of the different genes (arbitrary units) were performed with SDS 2.1 software (Applied Biosystems) using GAPDH for normalization and cDNA from mock-cultured DC preparations.
Immunolabeling of cells and detection of NS2 by flow cytometry.
Labeling of lymph cells was conducted with mouse MAb antiruminant determinants that have been described previously (15, 37) and included anti-CD11c (OM1 [IgG1] or BAQ153A [IgM]), -CD4 (ST4 [IgG1] or GC50A1 [IgM]), -γ/δ T cells (86D [IgG1]), -CD8 (CACT80C [IgG1] or 7C2 [IgG2a]), -B cells (DU2-104 [IgM]), -CD172 (ILA24 [IgG1]), -CD45RB (CC76 [IgG1]), -CD26 (CC69 [IgG1]), -major histocompatibility complex class II (MHC-II) (Th14B [CAT82A]), -CD80 (ILA159), and -CD86 (ILA190). Irrelevant IgG1, IgG2a, and IgM were used as negative controls. Lymph cells (5 × 106) were treated with primary antibodies (2 μg/ml) in FACS medium (RPMI 1640 containing 4% horse serum) for 30 min at 4°C, washed twice, and further incubated with FITC, phycoerythrin, cyanine 5, or tricolor-conjugated purified goat IgG directed to specific murine isotypes (Caltag Laboratories) at a 1:200 final dilution. In some instances, double labeling involving two primary IgG1 were conducted as previously described (37). For the intracellular detection of NS2, labeled cells were treated with the Fix and Perm kit (Caltag Laboratories), following the manufacturer's instructions, and then intracellularly labeled with rabbit serum directed against NS2 (1:500; raised in our lab by immunizing rabbits with purified recombinant NS2). Labeling was revealed with Alexa Fluor 488-conjugated goat anti-rabbit IgG (1:200; Molecular Probes). The cells were analyzed by flow cytometry (FASCalibur; Becton Dickinson), collecting 200,000 events, with Cellquest software.
DC recruitment in skin.
Two sheep (nos. 45 and 69) were inoculated intradermally in the shoulder with 2 × 104 TCID50 BTV2at in a final volume of 200 μl RPMI, and the area of inoculation was identified with permanent marker. Five days later, the injected skin area was harvested at necropsy, along with an equivalent skin area injected with control RPMI medium from the other side of the animal. Skin cryosections (4 μm) were prepared on charged slides and fixed in 1:1 cold methanol/acetone (15 min at −20°C). DC recruitment in skin was performed on three independent sections from the same skin biopsies. The labeling of dermal DCs was done with an anti-MHC-II MAb (1 μg/ml; TH14B) followed by Alexa Fluor 555-conjugated goat anti-mouse IgG (1:100; Molecular Probes), both antibodies being diluted in RPMI plus 10% FCS. The cell nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole) for 5 min, and the slides were mounted in Vectashield. The slides were observed using an ApoTome microscope, equipped with a Plan-Neofluar 20× oil objective with a numerical aperture of 0.8 (Zeiss), and four independent fields of the dermis were captured per tissue section. All the MHC-II-positive (MHC-IIpos) cells and DAPI-positive (DAPIpos) nuclei (>150) per field were counted, and the ratio of MHC-IIpos to DAPIpos cells was established for each independent tissue section.
Immunodetections in prescapular lymph nodes.
The prescapular lymph node was harvested at necropsy (sheep 45 and 69) 5 days after intradermal inoculation of 105 TCID50 BTV2at and processed as above. The tissue sections were treated with rabbit anti-NS2 (1:500) in RPMI plus 10% FCS, in combination with murine MAb (1 μg/ml) and anti-CD1 (clone 20.27 [IgG1]; Serotec), -CD11c (OM1 [IgG1]), -CD4 (ST4 [IgG1]), -γ/δ T cells (86D [IgG1]), -CD172 (ILA24 [IgG1]), or -CD11b (MM12A [IgG1] was from Veterinary Medical Research Development, Pullman, WA; ILA130 [IgG2a] was provided by Jan Naessens [Nairobi, Kenya]). NS2 was revealed with Alexa Fluor 488-conjugated goat anti-rabbit IgG (1:100), and the cellular marker was revealed with Alexa Fluor 555-conjugated goat anti-mouse IgG (1:100). In most cases, the cell nuclei were stained with DAPI for 5 min, and the slides were mounted in Vectashield. The slides were observed using a Leica DMR microscope (X63).
Measurement of cDC survival.
After incubation/infection with BTV (BTV2wt, BTV2at, BTV1at, and BTV8wt), cDCs were incubated with MitoTracker Deep Red 633 (Molecular Probes) for 30 min at 37°C (following the manufacturer's instructions) with 2 μg/ml 7-amino-actinomycin D (7-AAD; Sigma) and were directly processed for FACS analysis. The surviving cDCs were identified as cells positive for MitoTracker Deep Red 633 (MitoTrackerpos) and negative for 7-AAD (7-AADneg). Enriched cDCs that had been cultured with BTV8wt for 72 h were subsequently incubated with MitoTracker Deep Red 633 with 2 μg/ml 7-AAD for 30 min at 37°C and then further processed for the intracellular detection of NS2 (see above) in the presence of 4 μg/ml actinomycin D to prevent any false staining by residual 7-AAD due to cell fixation.
Activation of autologous immune T cells by BTV-infected DCs.
After the termination of afferent lymph collection by removal of the catheter, the same sheep was vaccinated twice with inactivated BTV2wt (provided by Merial S.A.) in the skin area drained by the cervical nodes. Thirteen days after the second vaccine injection, the cervical efferent lymph duct was cannulated. Efferent lymph cells collected at 15 and 30 days after the second vaccine injection were depleted of B and putative CD11b+ cells and then incubated with 500 nM carboxyfluorescein succinimidyl ester (CFSE). In parallel, autologous LDL cells were recovered from liquid nitrogen storage, and cDCs were positively selected and then incubated with BTV2at (0.004 TCID50/cell) for 1 h at 37°C. These BTV-treated/BTV-infected cDCs were cocultured with CFSE-labeled autologous T cells at a cDC-to-T-cell ratio of 1:20. Uninfected cDCs were used as controls for basal autologous cDC-induced proliferation. After 3 days in culture, the cell supernatants were collected for the detection of gamma interferon (IFN-γ) and IL-10, using a ruminant-specific enzyme-linked immunosorbent assay (37). After the 5-day cultures were complete, the cells were labeled for CD4 (GC50A1) and CD8 (7C2) detection as described above, and the CD4+ and CD8+ cells that had divided were analyzed by FACS.
RESULTS
BTV RNA association with cDCs in skin lymph.
Skin-afferent lymph was collected before and sequentially after the intradermal injection of BTV2at into the shoulders of two cannulated sheep (sheep 17 and 04). TL and LDL cells were prepared for RNA extraction, and BTV NS3 RNA (genomic or mRNA) was detected by qRT-PCR (Fig. 1A). LDL cells are enriched in cDCs (38% ± 10% DCs) (40; unpublished data) compared to TL cells (3.1% ± 0.7% DCs) (15). In a few instances, uncontrollable events in the animal management (flow blockade in the catheter or catheter removal from the collecting bottle) prevented the preparation of RNA samples.
FIG. 1.
BTV2at migrates in afferent lymph associated with lymph cDCs. (A) BTV2at (105 TCID50) was injected intradermally in two cannulated sheep (17 and 04), and lymph was sequentially collected. RNA was extracted from TL and LDL cells, and 100 ng (corresponding to 2 × 105 cells) was subjected to qRT-PCR for Seg-10 (NS3 gene) RNA quantitation, which was normalized between samples using GAPDH. ND, not done. (B) Left panel, results for CD4+, CD11c+, and CD11c− cells (>95% purity) which were selected using immunomagnetic beads from a mix of lymph cells collected on days 1 to 3 (D 1-3) after BTV injection. Right panel, results for CD4+ and CD11c+ cells (>95% purity) which were sorted using immunomagnetic beads from lymph cells collected on day 4, 5, or 6 (D4, D5, and D6, respectively) post-BTV2at injection (sheep 17) or from a pool of lymph cells collected on days 4 to 6 (D 4-5-6) post-BTV2at injection (sheep 04).
BTV Seg-10 (NS3 gene) was detected within the first 4 h of lymph cell collection (Fig. 1A), possibly due to the passive translocation of virus particles in the inoculum and adsorption/capture by migrating cells, as previously observed with latex particles (39). Low levels of Seg-10 RNA were detected that were associated with lymph cells during the first 3 days postinoculation (p.i.) (<1.5 × 103 copies per 2 × 105 cells). Seg-10 RNA levels peaked in lymph cells at day 4 p.i. in sheep 17 (9.5 × 103 and 1.9 × 104 NS3 copies per 2 × 105 TL and LDL cells, respectively) and at day 5 p.i. in sheep 04 (7.5 × 103 and 1.5 × 104 NS3 copies per 2 × 105 TL and LDL cells, respectively). Seg-10 RNA detection corresponds to genomic RNA or to newly synthesized mRNA. At the peak of Seg-10 RNA detection and days after, Seg-10 RNA is detected in higher amounts in LDL cells than in TL cells, suggesting that cDCs may contribute to BTV transportation.
Sheep cDCs can be labeled with anti-CD1 (36, 38), -CD1b, -MHC-II, or -CD11c (11, 23). CD11c, which is not expressed by pDCs (37), was chosen to sort the lymph cDCs and proceed to BTV RNA detection. As controls, CD4+ T cells, which represent the major subset in afferent lymph (60% of TL cells [39]) were sorted from the same samples (Fig. 1B). The amount of Seg-10 detected in cDCs was 10-fold higher than in CD4+ T cells at early time points (days 1 to 3) and at the peak of BTV migration (days 4 to 6) (Fig. 1B). The amount of BTV RNA that was detected to be associated with cDCs increased by ∼60-fold between days 1 to 3 p.i. (1.8 × 103 copies of Seg-10 per 2 × 105 cells) and days 4 to 6 p.i. (1.1 × 105 to 1.2 × 105 NS3 copies per 2 × 105 cells) (Fig. 1B). Altogether, the results obtained in vivo indicate that BTV utilizes cDC for its transportation to draining lymph nodes.
BTV RNA and NS2 are expressed by lymph cDCs.
In order to determine whether BTV can replicate in cDCs, cDCs and CD4 T cells were purified from afferent lymph collected at steady state from two uninfected sheep, 02 and 55. These cells were infected in vitro with BTV2at at a multiplicity of infection (MOI) of 0.004 TCID50/cell. This represents an MOI comparable to that previously used for the infection of monocytes in vitro (4, 46). cDCs showed a 30-fold increase of NS3 RNA content within 16 h p.i. at 37°C, compared to that at 4°C, while CD4 T cells show no NS3 RNA accumulation under the same conditions (Fig. 2A).
FIG. 2.
BTV2at RNA and nonstructural protein NS2 expression in cDCs in in vitro-infected lymph cells. (A) CD4+ and CD11c+ lymph cells (>95% purity) from two sheep (sheep 02 and 55) were incubated at 4°C or 37°C for 1 h with BTV2at, washed twice, and further incubated at the same temperatures for 16 h. The RNA was processed by qRT-PCR for Seg-10 (NS3 gene) and GAPDH detection. The increase in NS3 mRNA copies was calculated in the cells incubated at 37°C relative to 4°C. (B) LDL cells were incubated with BTV2at for several time periods, placed at 4°C, labeled with anti-CD11c MAb, and fixed for further intracellular detection of NS2. The percentage of infected DCs is indicated. Uninfected control cultures (control) were processed in parallel to check for the specificity of the NS2 labeling, and an isotype IgM (IC) was used to check for the specificity of CD11c labeling. (C) BTV2at LDL cells at 48 h postinfection were cytocentrifuged and stained with anti-MHC-II (red) and anti-NS2 (green), and the nuclei were stained with DAPI.
For the detection of viral protein synthesis, LDL cells (5 × 106) were incubated with BTV2at at an MOI of 0.004 TCID50/cell for various periods. Under these conditions, the expression of the BTV nonstructural protein NS2 was first detected in cDCs after 24 h at 37°C (4.8% cDCs; Fig. 2B). The proportion of cDCs, showing strong cytosolic staining for NS2, had increased to 20% by 48 h p.i. (Fig. 2B and C). In contrast, NS2 expression was only observed in a small number of non-cDCs by 48 h (1.5%) (Fig. 2B).
A previous study of BTV3 and BTV11 infection in sheep detected a small number of cells that expressed BTV RNA in the lymph nodes where infection started to be detectable, in that case, at day 4 postinfection (9). The expression of BTV proteins by cDCs in vivo was thus evaluated after the injection of BTV2at and the collection of the draining lymph node at 5 days p.i. that corresponds to the peak of BTV transportation in lymph. Very strong NS2 staining was detected in a few dispersed cells within the lymph node sections (Fig. 3). Some of these cells were positive for CD1 (Fig. 3A) or CD11c (Fig. 3B), although no NS2+ cells were detected that were also positive for CD4 (Fig. 3C), γ/δ T cells (Fig. 3D), B cells, or CD11b (data not shown). In some cases, the identity of the NS2-expressing cells could not be assigned to known lymph node subsets (including cDCs, T and B cells, or macrophages), possibly due to a shutoff of the surface protein synthesis induced by the virus.
FIG. 3.
BTV2at NS2 expression in cDCs in lymph nodes. Five days after the intradermal injection of BTV2at, lymph node sections were processed for detection of NS2 (green), cDC (CD1 and CD11c) and T-cell (CD4 or γ/δ) markers (red), and DAPI staining (blue). Cells colabeled for NS2 and the DC markers appear in yellow. Two sheep were used (45 and 69) and gave similar results.
BTV strain and virulence variations were evaluated for any significant effect on the susceptibilities of different lymph cell subsets. TL cells from naive sheep (nos. 31, 55, and 64) were incubated/infected with BTV2at, BTV-2wt, and BTV-8wt in vitro and then stained both for intracellular NS2 and for surface markers (CD4, CD8, γ/δ T cells, B cells, and CD11c). cDCs represented the afferent lymph cell subset most frequently infected with BTV2at (9.5% of cDCs expressed NS2 [Fig. 4A]), BTV2wt (14.2% [Fig. 4B]), and BTV8wt (10.7% [Fig. 4C]). Although other cell types were generally much more abundant in lymph than cDCs, they were far less receptive (<2.5%) to BTV infection.
FIG. 4.
BTV NS2 is preferentially expressed by cDCs in in vitro lymph cells infected with BTV2at, BTV2wt, and BTV8wt. (A to C) TL cells were infected with BTV2at, BTV2wt, and BTV8wt (0.004 TCID50/cell) for 48 h. Cells were then labeled with anti-CD4, -CD8, -B cells, -CD11c, and -γ/δ T cells; fixed; and stained for the intracellular detection of NS2. The specificity of NS2 labeling was controlled using uninfected TL cultures, and the specificity of the TL subset labeling was determined using isotype controls. The experiment has been reproduced three times with different sheep, and a representative experiment is shown (sheep 64). (D) LDL cells from sheep 64 were incubated with BTV2at (0.004 TCID50/cell) for 48 h. For cDC subset analysis, cells were stained with anti-CD11c and -CD172 and fixed for NS2 detection; the percentages of NS2+ in the CD11c+ CD172+ and CD11c+ CD172− cells were established. For pDC analysis, cells were stained with anti-CD11c, -B cells, and -CD45RB and fixed for NS2 detection. The percentage of NS2+ in the CD11c− B− CD45RB+ cells was evaluated.
Overall, cDCs represented more than 65% of the TL cells that were identified as expressing NS2 (66% for BTV2at, 69% for BTV2wt, and 74% for BTV8wt [Table 1]). Each of the three BTV strains tested showed a significant tropism for cDCs in lymph that did not appear to be related to virulence or to serotype. However, the percentages of total DCs that were infected (expressing NS2) showed large variations between individual sheep, from 1.2 to 19% (Table 2).
TABLE 1.
Representation of leukocyte subsets in BTV-infected (NS2+) TL cells from sheep 64 at 48 h
| BTV type | % Cell subset in NS2+ cells
|
|||||
|---|---|---|---|---|---|---|
| CD11c | CD4 | CD8 | γ/δ T cells | B cells | Unknowna | |
| BTV2at | 66 | 13 | 8 | 5.8 | 4.8 | 2.4 |
| BTV2wt | 69 | 7 | 2 | 0.8 | 16 | 5.2 |
| BTV8wt | 74 | 4.5 | 4.4 | 1.1 | 4.2 | 11.8 |
Some lymph cells are not defined.
TABLE 2.
Intersheep variations of the cDC receptivity to BTV in TL cells
| BTV type | cDC receptivity (% NS2+ at 48 h) for indicated sheep
|
||
|---|---|---|---|
| 64 | 55 | 31 | |
| BTV2at | 9.5 | 1.25 | 19 |
| BTV2wt | 14.2 | 2 | 6 |
| BTV8wt | 10.7 | NDa | 7 |
ND, not done.
The susceptibilities of different lymph DC subsets to BTV were also evaluated after the in vitro infection of LDL cells with BTV2at (Fig. 4D). Lymph pDC (CD11c− B− CD45RB+ cells) (37) showed no detectable NS2 expression. In contrast, the CD11c+ CD172− cDC subset, which shares common functional features with cross-priming CD8α mouse DCs (15, 44), showed a similar level of susceptibility to BTV infection (18 to 20%) compared to the other lymph cDCs (i.e., CD11c+ CD172+ cells). These data demonstrate that the two lymph cDC subsets (CD11c+ CD172+ and CD11c+ CD172−) are primarily responsible for BTV expression by lymph leukocytes.
BTV infection in lymph cDCs is productive.
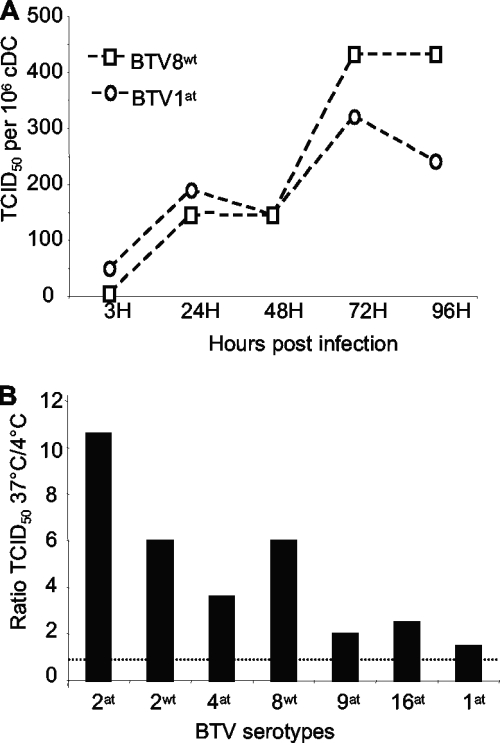
cDCs were enriched by negative sorting in order to obtain untouched cDCs and optimize their in vitro viability. These cDCs were then infected with BTV8wt and BTV1at at an MOI of 0.004 (as described above), and the amount of virus in the supernatant was measured by titration on BHK21 cells. cDC supernatants collected at 3 h p.i. contained only residual amounts of infectivity (<50 or 5 TCID50/106 DCs for BTV1at and BTV8wt, respectively). However, after 24 h, virus titers had risen to 190 TCID50/106 DCs for BTV1at and 140 TCID50/106 DCs for BTV8wt, and by 72 h p.i., they had reached 320 and 430 TCID50/106 DCs, respectively (Fig. 5A).
FIG. 5.
Productive BTV infection of cDCs. (A) Negatively selected cDCs were purified from sheep 66 (>87% purity based on cDC morphology and FACS) and infected at 37°C for 1 h with 0.004 TCID50/cell BTV1at, BTV8wt, or cultured alone (mock) and extensively washed. The culture supernatants were harvested after 3 h, 24 h, 48 h, 72 h, and 96 h and tested for cytopathic effect on BHK21 cells. The viral titers per 106 DCs were evaluated using the Spearman-Karber method. (B) Negatively selected cDCs were prepared (>85% purity based on cDC morphology and FACS) from sheep 33 and were incubated for 1 h with 0.004 TCID50/cell at 37°C or at 4°C (control), washed three times and further incubated at 37°C and 4°C for 24 h, respectively. The supernatants were harvested and the ratios of the TCID50 obtained at 37°C and 4°C were calculated. The dashed line represents a ratio of 1. The experiment has been reproduced twice.
In order to establish if different BTV strains/serotypes could productively replicate in cDCs, these cells were also cultured for 24 h with BTV2at, BTV2wt, BTV4at, BTV8wt, BTV9at, BTV16at, and BTV1at. The TCID50 ratio between the cDC supernatants obtained at 4°C (residual viral inoculum) and 37°C was always higher than 1 for all BTV serotypes/strains tested (in three independent experiments), indicating active viral production (Fig. 5B). Although the extent of viral replication was variable between individual sheep and different BTV strains, none of the viral strains consistently replicated more efficiently in cDCs (data not shown).
BTV-induced recruitment of cDCs in the dermis and in skin lymph.
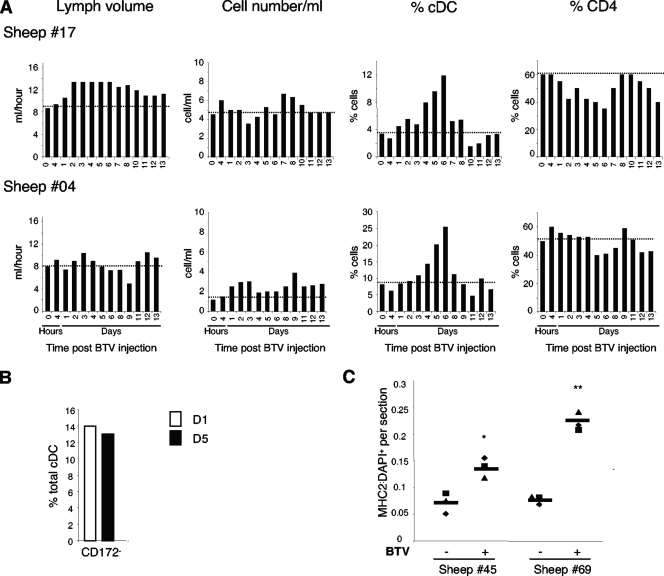
Data already obtained here indicate that lymph cDCs cannot only transport BTV but can also become infected and produce infectious progeny virus. Thus, cDCs could potentially play a very significant role in the dissemination of infectious viruses within the infected ruminant host. Since inflammation has been shown to trigger cDC recruitment to lymph (5, 44), we investigated whether the skin inoculation of BTV2at would also favor DC influx into the lymph, thus further increasing virus trafficking within the host by recruiting the BTV cellular target. The administration of BTV2at did not significantly alter the lymph volume or the total cell output from the skin (Fig. 6A). However, the percentage of cDCs in lymph cells was increased significantly, starting at day 4 p.i., peaking at day 6 (Fig. 6A), which parallels the rise in BTV transportation (Fig. 1A). In sheep 17, cDCs increased from 4% of TL cells (basal level) to 12% (day 6) and from 10% to 26% in sheep 04. BTV did not alter the proportions of cDC subsets in the lymph, with the CD11c+ CD172− subset representing 14% of the cDCs at day 1 and 13% at day 5 (Fig. 6B). Conversely, the percentages of CD4+ T cells were slightly reduced by BTV inoculation (Fig. 6A), and the percentages of the other subsets analyzed did not change (pDCs and B cells), except for CD8+ T cells that were very significantly augmented by days 13 to 15 (data not shown).
FIG. 6.
BTV2at increases cDC output in skin lymph and cDC accumulation in dermis. (A) Afferent lymph cells were sequentially collected from two sheep (17 and 04) before and after the intradermal inoculation of BTV2at. The total number of TL cells per ml, the lymph volume per h, and the percentages of CD11c and CD4 cells are reported. (B) The percentage of the CD172− subset within the cDCs was analyzed in TL cells 1 and 5 days post-BTV injection (sheep 04). (C) MHC-II+/DAPI+ ratios in three dermis sections of biopsies from BTV-injected (+) and contralateral (−) sides (see Materials and Methods) were established from the same two sheep as those for which results are depicted in Fig. 3 (45 and 69). The horizontal bar represents the mean MHC-IIpos/DAPIpos ratio per biopsy. Significant differences between BTV-inoculated and control skin were evaluated with a Student's t test (*, P < 0.05; **, P < 0.005).
The increased output of cDCs in afferent skin lymph could be associated with the emigration of cDCs from the skin, possibly resulting in a depletion of skin cDCs. Alternatively, the augmented output of cDCs could be the result of an increased recruitment of cDCs in the skin induced by BTV. We thus analyzed the skin sites of BTV inoculation of sheep 45 and 69 at 5 days p.i. The skin biopsies were labeled with DAPI in order to estimate total cell numbers per section and with anti-MHC-II MAb to count the number of cDCs. Anti-MHC-II staining was chosen, as it only colabels CD1b+ DCs in sheep dermis (data not shown) and generates a strong signal that facilitates cell counting. A two- to fourfold increase in the ratio of the MHC-IIpos/DAPIpos cells was observed after the inoculation of BTV2at (Fig. 6C), indicating that BTV induces a chemoattraction for their cDC targets in the dermis.
BTV infection does not alter lymph cDC survival.
Mortola et al. (35) reported that the BTV infection of susceptible mammalian cells can cause apoptosis. The possibility that the BTV infection of cDCs could have a similar effect was therefore investigated. In the first experiment, naive cDCs were selected by negative sorting and then infected with BTV in vitro. Cell viability was monitored each day by the activation of MitoTracker, which indicates the efficiency of mitochondrial metabolism, associated with the exclusion of 7-AAD, a late apoptosis/necrosis indicator. Culture with BTV8wt for 72 h both increased the percentage of MitoTrackerpos cDCs from 35% to 80% in mock cultures and decreased the percentage of 7-AADpos cDCs from 55% to 9% in mock cultures (Fig. 7A). Similarly, cultures with BTV2at, BTV2wt, or BTV8wt enhanced the percentage of MitoTrackerpos 7-AADneg cDCs at any of the tested times between 24 h and 96 h (Fig. 7B). In the second experiment, we costained the cDCs with MitoTracker and anti-NS2 in order to directly evaluate cDC survival in each BTV-infected cell. As shown for BTV8wt (Fig. 7C), most (>95%) of the cDCs that express NS2 were alive, whereas the non-NS2-expressing cDCs showed a lower survival rate (64%). The cultured cDCs that were mock infected also showed a 60% survival rate (Fig. 7C). We can conclude that the expression of BTV proteins in cDCs does not induce apoptosis in short-term cultures but actually enhances cDC survival.
FIG. 7.
BTV expression is associated with increased cDC survival. (A and B) Negatively selected cDCs were purified (>88% purity) and cultured with BTV2wt, BTV2at, and BTV8wt for 24 h, 48 h, 72 h, and 96 h. The percentage of surviving cDCs was evaluated by the activation of MitoTracker Deep Red 633 and the exclusion of 7-AAD that labels late apoptosis cells. Panel A shows the MitoTracker and 7-AAD staining of cDCs cultured for 72 h in complete medium (mock) and of cDCs cultured with BTV8wt. The percentages of MitoTrackerpos 7-AADneg (surviving) cDCs are indicated in the top left rectangles, and the percentages of MitoTrackerneg 7-AADpos (necrotic) cDCs are in the bottom right rectangles. Panel B reports the percentages of surviving MitoTrackerpos cDCs cultured with different BTV strains for the indicated lengths of time. The experiment was repeated three times with similar results using different sheep (results with sheep 81 are shown). (C) Negatively selected cDCs were obtained (>85% purity) and infected with BTV8wt. After 72 h, cells were incubated in MitoTracker Deep Red 633 and 7-AAD and stained for NS2 detection. The percentages of MitoTrackerpos cells were evaluated in the NS2+ and NS2− gated cDCs that had been cultured with BTV8wt and without BTV (mock cDC cultures, sheep 33).
Induction of DC activation and specific-antigen presentation by interaction with BTV.
Some hemorrhagic fever viruses, such as the Ebola and Marburg viruses, replicate in human monocyte-derived DCs and inhibit the expression of costimulatory molecules and cytokines (33). The impairment of cytokine and costimulatory molecule expression by the Ebola and Marburg viruses in DCs may contribute to immune evasion.
The possibility that BTV alters the expression of MHC-II, costimulatory CD80 and CD86 molecules, immune cytokines (IL-12), or inflammatory cytokines (IL-1β and IL-6), was investigated in negatively selected cDCs from two sheep (nos. 33 and 66). BTV can be difficult to purify (34), and all of the previous experiments were carried out using virus in supernatants from infected BHK21 cells, although they may secrete cytokines that activate cDCs. Thus, BTV2at and BTV2wt were used in parallel with sucrose gradient-purified BTV1. The infection of lymph cDCs with BTV2wt, BTV2at, or purified BTV1wt for 24 h clearly upregulated CD80 and CD86 expression but did not augment MHC-II expression (Fig. 8). IL-12 mRNA expression was slightly enhanced (1.2- to 3.5-fold) in cells from both sheep at 24 h p.i. by all of the BTV strains tested and was still further increased at 48 h by BTV2at in sheep 33 (Table 3). IL-1β mRNA was also increased in cells from both sheep at 24 h p.i. (1.5- to 16-fold) by each of the BTV strains used (Table 3). IL-1β mRNA was also increased by all BTV at 48 h (3.2- to 16-fold) except for BTV2wt in sheep 66 (Table 3). IL-6 mRNA expression was enhanced at 24 h (4- to 14.5-fold) by all of the BTV strains tested, in cells from both sheep, and remained high at 48 h in sheep 33 (5- to 45-fold) and less so in sheep 66 (Table 3). Although the increase of cytokine mRNA by BTV is modest, it is observed in most instances (in 34 out of 36 viral cultures [Table 3]). Thus, the tested BTV strains (purified BTV1wt, BTV2wt, and BTV2at) activated cDCs with increased costimulatory molecule expression in vitro and increased production of immune and inflammatory cytokine mRNAs, indicating that BTV positively activates lymph cDCs.
FIG. 8.
BTV activates the expression of CD80 and CD86 by lymph cDCs. Negatively selected cDCs (>85% purity based on morphology and FACS analysis) were obtained from sheep 33 and were either cultured alone (mock cultures), with 0.004 TCID50/cell BTV2at, BTV2wt, or sucrose gradient-purified BTV1wt. After 24 h, the cells were labeled with anti-CD80 (ILA159), -CD86 (ILA190), and -MHC-II (CAT82A) and IgG1 isotype control, followed by FITC-conjugated goat anti-mouse IgG1.
TABLE 3.
BTV activates the expression of sheep mRNA cytokines (IL-12, IL-1β, and IL-6) in cDCs
| BTV type | Ratioa of:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-12
|
IL-1β
|
IL-6
|
||||||||||
| Sheep 33
|
Sheep 66
|
Sheep 33
|
Sheep 66
|
Sheep 33
|
Sheep 66
|
|||||||
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | |
| BTV1wt | 3 | 3 | 1.2 | <1 | 1.5 | 1.8 | 13 | 3.2 | 4 | 5 | 5 | 1.5 |
| BTV2at | 3.4 | 12 | 2 | 2.2 | 8.5 | 7.2 | 16 | 4.5 | 14.5 | 45 | 7 | 6 |
| BTV2wt | 2.5 | 2 | 1.6 | 1.2 | 8.5 | 5.5 | 5 | <1 | 7 | 7 | 5.2 | 1.8 |
Negatively enriched cDCs were obtained from two different sheep (33 and 66) and were cultured alone (mock) or with 0.004 TCID50/cell BTV2at, BTV2wt, or BTV1wt. After 24- and 48-h cultures, cells were lysed for RNA extraction for real-time RT-PCR. The ratio of the normalized cytokine mRNA signals in BTV-stimulated versus nonstimulated cultures was calculated.
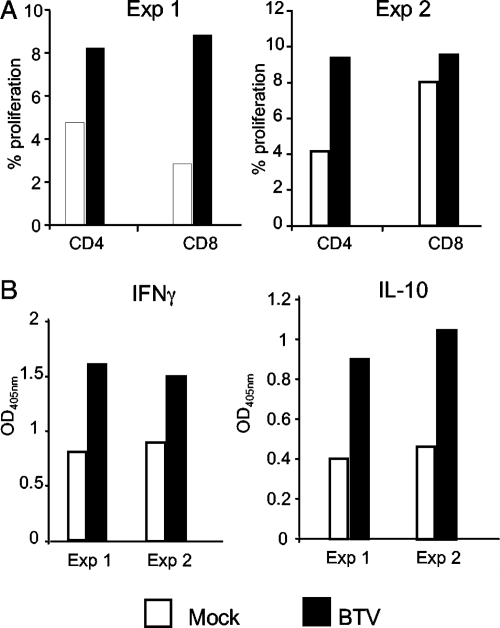
The ability of BTV-infected DCs to activate immune T cells was also tested. Preliminary experiments indicated that efferent lymph from immunized animals was the best source of BTV immune T cells. These cells were therefore cocultured with BTV-infected autologous cDCs, which were purified from LDL cells that had been frozen prior to immunization. Both CD4+ and CD8+ T cells increased their proliferation as a result of coculturing with BTV-infected cDCs in two independent experiments (Fig. 9). BTV-infected cDCs also enhanced IFN-γ and IL-10 secretion from BTV-immune T cells (Fig. 9).
FIG. 9.
BTV infection of DCs triggers activation of autologous immune CD4 and CD8 lymphocytes. cDCs were positively selected, infected with BTV2at for 1 h, and CFSE-labeled autologous efferent lymph T cells depleted of B and CD11b+ cells were added at a ratio of 1:20. (A) The percentages of CD4 and CD8 T cells that have divided over 5 days in the absence or presence of BTV2at are shown. (B) The production of IL-10 and IFN-γ at day 3 was measured by ELISA. The results of two independent experiments are shown (Exp 1 at day 15 postvaccination and Exp 2 at day 30 postvaccination with inactivated BTV).
Collectively, these results demonstrate that BTV infects and replicates in ovine cDCs, increasing their survival and their usual response to pathogens, including the expression of costimulatory molecules, cytokine production, and T-cell activation.
DISCUSSION
A dynamic in vivo model was used to show that BTV is initially transported via migrating cDCs from the skin to draining lymph nodes, where it undergoes a primary round of replication. The dissemination of the virus is coupled with cDC recruitment in the skin and a large increase in cDC output in skin lymph. Skin lymph cDCs can be infected by BTV, supporting productive virus replication. BTV infection also appears to preserve cDC functions, promoting their survival, expression of costimulatory molecules/cytokines, and capacity to stimulate specific T cells and allowing them to spread infection to other target cells within the body, including other cDCs, monocytes, and endothelial cells.
The preferential tropism of the virus for cDCs in afferent lymph was confirmed for viral expression in vitro with several BTVat and BTVwt strains. There was no clear difference in the initial receptivity (percentage of cDCs expressing NS2) for infection by the BTV strains tested, using replicate cDCs from the same sheep. However, there were large variations in cDC receptivity for BTV among three individual sheep (Table 2). Altogether, these findings suggest that cDCs can themselves become infected at an early stage, contributing to the initial dissemination of the virus in infected sheep. The interaction of BTV with cDCs may influence the subsequent viral burden and the severity of the disease, controlling the acute infection and/or viral persistence, which have been reported to vary significantly between sheep hosts (9, 30).
The onset of clinical signs of bluetongue usually occurs between 6 to 10 days p.i. with virulent strains of BTV (18). Previous reports indicate that BTV viremia (the peak of detection in blood) is biphasic in sheep at 6 and 10 days p.i. (±2 days) (18). The results presented here showed the peak transportation of BTV by cDCs in afferent lymph at 4 to 5 days after inoculation, which is therefore immediately before the onset of viremia and clinical disease. The timing of these events provides further support for a link between viral dissemination by lymph cDCs and the disease itself.
Free BTV is unlikely to play a role in viral dissemination as BTV has been reported to be strongly cell associated (34), and BTV was not detected in blood plasma or in the efferent lymph plasma of experimentally infected calves, despite significant viral titers associated with erythrocytes and mononuclear cells (3). In BTV-infected sheep and cattle, viral RNA and infectious BTV were found associated with blood monocytes (1 in 105 sheep monocytes) and mainly with erythrocytes in the circulation (9, 46). Several in vitro studies with bovine leukocytes have demonstrated that monocytes represent a subset that is preferentially targeted by BTV, with 10% to 20% of cells producing viral proteins at 24 h p.i. (4, 46). These results are essentially similar to those described here for ovine lymph cDCs. Bovine blood monocytes in short-term culture medium also produced low titers of infectious BTV within the first 24 to 48 h (4, 46), indicating that both blood monocytes and lymph cDCs can support productive although low-level BTV infections. Coculture with other mesenchymal cells, such as endothelial cells might promote viral synthesis by monocytes and cDCs, as previously observed with γ/δ T cells and skin fibroblasts (9). Bovine blood lymphocytes were comparatively either less susceptible (4) or nonsusceptible to BTV infection (8), except when activated (4). A significant but low proportion of lymph CD4 T cells expressed NS2 after infection, possibly due to a low activation state of the recirculating memory CD4 T cells in afferent lymph in culture (29). Conversely, lymph γ/δ T cells were only very marginally infected, indicating that γ/δ T cells probably do not play a significant role in the dissemination of the virus.
The effect of BTV infection on cell survival appears to depend on cell type and cell culture conditions. Indeed, BTV induces potent apoptosis in many mammalian cell lines (35) and microvascular cell cultures (13). The two viral outer proteins, VP2 and VP5, are sufficient to trigger apoptosis independently of viral replication, involving NF-κB activation in mammalian cell lines (35). On the other hand, insect cells were resistant to BTV-induced apoptosis, despite productive viral replication (35). An infected γ/δ T-cell line persistently produced BTV without inducing cell death, except when induced to G1 cell cycle arrest by coculturing with skin fibroblasts or treated with the WC1 MAb (43). Plastic adherent bovine mononuclear cells producing BTV also failed to show strong cytopathic effects (4). It has been suggested that the capacity for viral production without cytopathic effect involves viral budding, instead of cell membrane permeabilization, by association of the viroporin NS3 to the sorting Tsg101 molecule (47). Probably, the signal transduction cascades induced by VP2 and VP5 vary between different cell types. Whatever the mechanism(s) involved, the short-term increased viability of BTV-infected cDCs may benefit BTV dissemination in its mammalian host.
Purified BTV1wt and unpurified BTV2wt or BTV2at positively activated the expression of costimulatory CD80 and CD86 molecules and the expression of cytokine mRNAs in enriched lymph cDCs prepared in vitro. The enrichment of cDC preparations by negative sorting helped ensure their in vitro viability. However, these cDCs were still contaminated by other cell types (<15% of the preparation) that may influence the activation of the cDCs. Even with this possibility, the level of IL-1β mRNA was around 100 times less in the enriched cDCs than reported in microvascular cells (14). This suggests that cDCs make a much lower contribution to the inflammatory syndrome induced by BTV than endothelial cells.
The infection of cDCs by BTV did not trigger tissue factor, a primary initiator of the coagulation cascade, or metalloproteinase 9 mRNA synthesis (data not shown). In contrast, tissue factor is induced by Ebola virus in infected monocytes (20), and metalloproteinase 9 mRNA is induced by dengue virus in exposed monocyte-derived DCs (27), implicating monocytes and/or DCs in viral-induced thrombohemorrhagic syndromes (20). However, a more complete picture of BTV interaction with lymph DCs will be generated using microarray analyses that are ongoing in our laboratory to determine whether lymph DC productions play a role in the hemorrhagic syndrome induced by BTV.
The studies described here demonstrated that BTV disseminates via cDCs in lymph. BTV preserved cDC functions and appeared to activate cDCs in an appropriate way to elicit an efficient immune response, as observed in animals that survive the acute phase of infection. Further studies are required to test whether the differences observed in cDC receptivity and cDC responses to BTV between individual hosts could explain the differences also observed in the expression of clinical signs and differences in the kinetics of viral clearance in individual sheep or between different sheep breeds.
Acknowledgments
We are extremely grateful to Sanofi-Aventis for the gracious gift of Lovenox. We are especially grateful for the enlightening advice of Bernard Charley and for his valuable comments on the manuscript. We thank the Unité Commune d' Expérimentation Animale in Jouy-en-Josas, France, for providing sheep and for their care of the cannulated sheep. We thank the technicians of the Centre d'Imagerie Interventionnelle for their help in managing the surgery. We thank the BSL3 staff (Maison-Alfort). We thank the MIMA-2 platform (Jouy en Josas) for their support in confocal microscopy. We are grateful to the PICT platform and Claudia Bevilacqua for their support in qRT-PCR. We thank Merial SA for providing us with inactivated BTV2 vaccine.
We are grateful to the Islamic Azad University—Karaj Branch for their support to B.H. We also thank the EU Commission, BBSRC, Defra (United Kingdom), and the Agence Nationale for Research (Genanimal program, France) for financial support.
Footnotes
Published ahead of print on 24 June 2009.
REFERENCES
- 1.Au, B., M. R. Boulton, P. P. Narini, C. A. McCulloch, and J. B. Hay. 1996. Lymph and interstitial fluid dynamics in labial gingival tissues of sheep. J. Periodontal Res. 31570-578. [DOI] [PubMed] [Google Scholar]
- 2.Baize, S., J. Kaplon, C. Faure, D. Pannetier, M. C. Georges-Courbot, and V. Deubel. 2004. Lassa virus infection of human dendritic cells and macrophages is productive but fails to activate cells. J. Immunol. 1722861-2869. [DOI] [PubMed] [Google Scholar]
- 3.Barratt-Boyes, S. M., and N. J. MacLachlan. 1994. Dynamics of viral spread in bluetongue virus infected calves. Vet. Microbiol. 40361-371. [DOI] [PubMed] [Google Scholar]
- 4.Barratt-Boyes, S. M., P. V. Rossitto, J. L. Stott, and N. J. MacLachlan. 1992. Flow cytometric analysis of in vitro bluetongue virus infection of bovine blood mononuclear cells. J. Gen. Virol. 731953-1960. [DOI] [PubMed] [Google Scholar]
- 5.Bonneau, M., M. Epardaud, F. Payot, V. Niborski, M. I. Thoulouze, F. Bernex, B. Charley, S. Riffault, L. A. Guilloteau, and I. Schwartz-Cornil. 2006. Migratory monocytes and granulocytes are major lymphatic carriers of Salmonella from tissue to draining lymph node. J. Leukoc. Biol. 79268-276. [DOI] [PubMed] [Google Scholar]
- 6.Bosio, C. M., M. J. Aman, C. Grogan, R. Hogan, G. Ruthel, D. Negley, M. Mohamadzadeh, S. Bavari, and A. Schmaljohn. 2003. Ebola and Marburg viruses replicate in monocyte-derived dendritic cells without inducing the production of cytokines and full maturation. J. Infect. Dis. 1881630-1638. [DOI] [PubMed] [Google Scholar]
- 7.Bray, M. 2005. Pathogenesis of viral hemorrhagic fever. Curr. Opin. Immunol. 17399-403. [DOI] [PubMed] [Google Scholar]
- 8.Brewer, A. W., and N. J. MacLachlan. 1994. The pathogenesis of bluetongue virus infection of bovine blood cells in vitro: ultrastructural characterization. Arch. Virol. 136287-298. [DOI] [PubMed] [Google Scholar]
- 9.Brodie, S. J., W. C. Wilson, P. M. O'Hearn, D. Muthui, K. Diem, and L. D. Pearson. 1998. The effects of pharmacological and lentivirus-induced immune suppression on orbivirus pathogenesis: assessment of virus burden in blood monocytes and tissues by reverse transcription-in situ PCR. J. Virol. 725599-5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Budhia, S., L. F. Haring, I. McConnell, and B. A. Blacklaws. 2006. Quantitation of ovine cytokine mRNA by real-time RT-PCR. J. Immunol. Methods 309160-172. [DOI] [PubMed] [Google Scholar]
- 11.Bujdoso, R., J. Hopkins, B. M. Dutia, P. Young, and I. McConnell. 1989. Characterization of sheep afferent lymph dendritic cells and their role in antigen carriage. J. Exp. Med. 1701285-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darpel, K. E., C. A. Batten, E. Veronesi, A. E. Shaw, S. Anthony, K. Bachanek-Bankowska, L. Kgosana, A. bin-Tarif, S. Carpenter, U. U. Muller-Doblies, H. H. Takamatsu, P. S. Mellor, P. P. Mertens, and C. A. Oura. 2007. Clinical signs and pathology shown by British sheep and cattle infected with bluetongue virus serotype 8 derived from the 2006 outbreak in northern Europe. Vet. Rec. 161253-261. [DOI] [PubMed] [Google Scholar]
- 13.DeMaula, C. D., M. A. Jutila, D. W. Wilson, and N. J. MacLachlan. 2001. Infection kinetics, prostacyclin release and cytokine-mediated modulation of the mechanism of cell death during bluetongue virus infection of cultured ovine and bovine pulmonary artery and lung microvascular endothelial cells. J. Gen. Virol. 82787-794. [DOI] [PubMed] [Google Scholar]
- 14.DeMaula, C. D., C. M. Leutenegger, K. R. Bonneau, and N. J. MacLachlan. 2002. The role of endothelial cell-derived inflammatory and vasoactive mediators in the pathogenesis of bluetongue. Virology 296330-337. [DOI] [PubMed] [Google Scholar]
- 15.Epardaud, M., M. Bonneau, F. Payot, C. Cordier, J. Megret, C. Howard, and I. Schwartz-Cornil. 2004. Enrichment for a CD26hi SIRP− subset in lymph dendritic cells from the upper aero-digestive tract. J. Leukoc. Biol. 76553-561. [DOI] [PubMed] [Google Scholar]
- 16.Fauquet, C. M., M. A. Mayo, J. Maniloff, U. Desselberger, and L. A. Ball (ed.). 2005. Virus taxonomy: eighth report of the International Committee on Taxonomy of Viruses. Academic Press, San Diego, CA.
- 17.Feldmann, H., H. Bugany, F. Mahner, H. D. Klenk, D. Drenckhahn, and H. J. Schnittler. 1996. Filovirus-induced endothelial leakage triggered by infected monocytes/macrophages. J. Virol. 702208-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foster, N. M., A. J. Luedke, I. M. Parsonson, and T. E. Walton. 1991. Temporal relationships of viremia, interferon activity, and antibody responses of sheep infected with several bluetongue virus strains. Am. J. Vet. Res. 52192-196. [PubMed] [Google Scholar]
- 19.Geisbert, T. W., L. E. Hensley, T. Larsen, H. A. Young, D. S. Reed, J. B. Geisbert, D. P. Scott, E. Kagan, P. B. Jahrling, and K. J. Davis. 2003. Pathogenesis of Ebola hemorrhagic fever in cynomolgus macaques: evidence that dendritic cells are early and sustained targets of infection. Am. J. Pathol. 1632347-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geisbert, T. W., H. A. Young, P. B. Jahrling, K. J. Davis, E. Kagan, and L. E. Hensley. 2003. Mechanisms underlying coagulation abnormalities in Ebola hemorrhagic fever: overexpression of tissue factor in primate monocytes/macrophages is a key event. J. Infect. Dis. 1881618-1629. [DOI] [PubMed] [Google Scholar]
- 21.Gohin, I. 1997. The lymphatic system and its functioning in sheep. Vet. Res. 28417-438. [PubMed] [Google Scholar]
- 22.Gowen, B. B., and M. R. Holbrook. 2008. Animal models of highly pathogenic RNA viral infections: hemorrhagic fever viruses. Antivir. Res. 7879-90. [DOI] [PubMed] [Google Scholar]
- 23.Hein, W. R., T. Barber, S. A. Cole, L. Morrison, and A. Pernthaner. 2004. Long-term collection and characterization of afferent lymph from the ovine small intestine. J. Immunol. Methods 293153-168. [DOI] [PubMed] [Google Scholar]
- 24.Hofmann, M. A., S. Renzullo, M. Mader, V. Chaignat, G. Worwa, and B. Thuer. 2008. Genetic characterization of Toggenburg orbivirus, a new bluetongue virus, from goats, Switzerland. Emerg. Infect. Dis. 141855-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeggo, M. H., and R. C. Wardley. 1982. Generation of cross-reactive cytotoxic T lymphocytes following immunization of mice with various bluetongue virus types. Immunology 45629-635. [PMC free article] [PubMed] [Google Scholar]
- 26.Lunt, R. A., L. Melville, N. Hunt, S. Davis, C. L. Rootes, K. M. Newberry, L. I. Pritchard, D. Middleton, J. Bingham, P. W. Daniels, and B. T. Eaton. 2006. Cultured skin fibroblast cells derived from bluetongue virus-inoculated sheep and field-infected cattle are not a source of late and protracted recoverable virus. J. Gen. Virol. 873661-3666. [DOI] [PubMed] [Google Scholar]
- 27.Luplertlop, N., D. Misse, D. Bray, V. Deleuze, J. P. Gonzalez, V. Leardkamolkarn, H. Yssel, and F. Veas. 2006. Dengue-virus-infected dendritic cells trigger vascular leakage through metalloproteinase overproduction. EMBO Rep. 71176-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maan, S., N. S. Maan, A. R. Samuel, S. Rao, H. Attoui, and P. P. Mertens. 2007. Analysis and phylogenetic comparisons of full-length VP2 genes of the 24 bluetongue virus serotypes. J. Gen. Virol. 88621-630. [DOI] [PubMed] [Google Scholar]
- 29.Mackay, C. R., W. L. Marston, and L. Dudler. 1990. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J. Exp. Med. 171801-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacLachlan, N. J. 1994. The pathogenesis and immunology of bluetongue virus infection of ruminants. Comp. Immunol. Microbiol. Infect. Dis. 17197-206. [DOI] [PubMed] [Google Scholar]
- 31.MacLachlan, N. J., G. Jagels, P. V. Rossitto, P. F. Moore, and H. W. Heidner. 1990. The pathogenesis of experimental bluetongue virus infection of calves. Vet. Pathol. 27223-229. [DOI] [PubMed] [Google Scholar]
- 32.MacLachlan, N. J., R. A. Nunamaker, J. B. Katz, M. M. Sawyer, G. Y. Akita, B. I. Osburn, and W. J. Tabachnick. 1994. Detection of bluetongue virus in the blood of inoculated calves: comparison of virus isolation, PCR assay, and in vitro feeding of Culicoides variipennis. Arch. Virol. 1361-8. [DOI] [PubMed] [Google Scholar]
- 33.Mahanty, S., K. Hutchinson, S. Agarwal, M. McRae, P. E. Rollin, and B. Pulendran. 2003. Cutting edge: impairment of dendritic cells and adaptive immunity by Ebola and Lassa viruses. J. Immunol. 1702797-2801. [DOI] [PubMed] [Google Scholar]
- 34.Mertens, P. P., J. N. Burroughs, and J. Anderson. 1987. Purification and properties of virus particles, infectious subviral particles, and cores of bluetongue virus serotypes 1 and 4. Virology 157375-386. [DOI] [PubMed] [Google Scholar]
- 35.Mortola, E., R. Noad, and P. Roy. 2004. Bluetongue virus outer capsid proteins are sufficient to trigger apoptosis in mammalian cells. J. Virol. 782875-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parsons, K. R., C. J. Howard, and P. Sopp. 1991. Immunohistology of workshop monoclonal antibodies to the bovine homologue of CD1. Vet. Immunol. Immunopathol. 27201-206. [DOI] [PubMed] [Google Scholar]
- 37.Pascale, F., V. Contreras, M. Bonneau, A. Courbet, S. Chilmonczyk, C. Bevilacqua, M. Eparaud, V. Niborski, S. Riffault, A. M. Balazuc, E. Foulon, L. Guzylack-Piriou, B. Riteau, J. Hope, N. Bertho, B. Charley, and I. Schwartz-Cornil. 2008. Plasmacytoid dendritic cells migrate in afferent skin lymph. J. Immunol. 1805963-5972. [DOI] [PubMed] [Google Scholar]
- 38.Rhind, S. M., B. M. Dutia, C. J. Howard, and J. Hopkins. 1996. Discrimination of two subsets of CD1 molecules in the sheep. Vet. Immunol. Immunopathol. 52265-270. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz-Cornil, I., M. Epardaud, J. P. Albert, C. Bourgeois, F. Gerard, I. Raoult, and M. Bonneau. 2005. Probing leukocyte traffic in lymph from oro-nasal mucosae by cervical catheterization in a sheep model. J. Immunol. Methods 305152-161. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz-Cornil, I., M. Epardaud, and M. Bonneau. 2006. Cervical duct cannulation in sheep for collection of afferent lymph dendritic cells from head tissues. Nat. Protoc. 1874. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz-Cornil, I., P. P. Mertens, V. Contreras, B. Hemati, F. Pascale, E. Breard, P. S. Mellor, N. J. Maclachlan, and S. Zientara. 2008. Bluetongue virus: virology, pathogenesis and immunity. Vet. Res. 3946. [DOI] [PubMed] [Google Scholar]
- 42.Segura, E., and J. A. Villadangos. 2009. Antigen presentation by dendritic cells in vivo. Curr. Opin. Immunol. 21105-110. [DOI] [PubMed] [Google Scholar]
- 43.Takamatsu, H., P. S. Mellor, P. P. Mertens, P. A. Kirkham, J. N. Burroughs, and R. M. Parkhouse. 2003. A possible overwintering mechanism for bluetongue virus in the absence of the insect vector. J. Gen. Virol. 84227-235. [DOI] [PubMed] [Google Scholar]
- 44.Turnbull, E. L., U. Yrlid, C. D. Jenkins, and G. G. Macpherson. 2005. Intestinal dendritic cell subsets: differential effects of systemic TLR4 stimulation on migratory fate and activation in vivo. J. Immunol. 1741374-1384. [DOI] [PubMed] [Google Scholar]
- 45.Veronesi, E., C. Hamblin, and P. S. Mellor. 2005. Live attenuated bluetongue vaccine viruses in Dorset Poll sheep, before and after passage in vector midges (Diptera: Ceratopogonidae). Vaccine 235509-5516. [DOI] [PubMed] [Google Scholar]
- 46.Whetter, L. E., N. J. Maclachlan, D. H. Gebhard, H. W. Heidner, and P. F. Moore. 1989. Bluetongue virus infection of bovine monocytes. J. Gen. Virol. 701663-1676. [DOI] [PubMed] [Google Scholar]
- 47.Wirblich, C., B. Bhattacharya, and P. Roy. 2006. Nonstructural protein 3 of bluetongue virus assists virus release by recruiting ESCRT-I protein Tsg101. J. Virol. 80460-473. [DOI] [PMC free article] [PubMed] [Google Scholar]