Abstract
The widely used hepatitis B virus (HBV) vaccine is based on three doses of hepatitis B surface antigen (HBsAg) protein. We previously showed that vectored measles viruses (MV) expressing HBsAg retain measles vaccine function in monkeys but do not induce a protective anti-HBs response in all animals. We show here that a single dose of HBsAg protein following a three-dose vaccination regimen with an optimized HBsAg-expressing MV elicits protective anti-HBs responses in all four vaccinated Rhesus monkeys. Vaccination strategies coupling the effective, long-term immunity elicited by the high-coverage MV vaccine to prophylactic HBV immunity are discussed.
Despite an effective hepatitis B virus (HBV) vaccine, chronic HBV infection remains an important cause of liver cirrhosis and hepatocellular carcinoma, with an estimated 350 million chronic carriers and 620,000 annual deaths (9). Thus, universal immunization against HBV remains an important goal which can be achieved through the inclusion of recombinant hepatitis B surface antigen (HBsAg) protein in multivalent formulations used for routine pediatric immunization (16). Nonetheless, due to vaccine and distribution network costs, this alternative is not an option worldwide (10). An attractive strategy to include HBV vaccination in the extended program of immunization is to use the globally distributed, safe (21) and efficacious measles vaccine as a viral vector to deliver HBsAg.
Indeed, vaccine safety and efficiency, induction of long-lasting immunity, and established production methods give measles virus (MV) great appeal as a vector to deliver foreign antigens (3). Toward this, we previously generated vaccine-identical MVs expressing HBsAg at different levels as a function of the location of an additional transcription unit (ATU) in the MV genome. Cells infected with these viruses secreted HBsAg with a density of 1.12 to 1.15 g/ml, corresponding to that of subviral HBV particles, and no indication of incorporation of HBsAg in MV particles was obtained (6). Importantly, both recombinant and parental MVs tested in monkeys maintained vaccine function against a wild-type MV challenge (6). However, after a single dose, even the vectored MV expressing HBsAg at the highest level, MVvac2(HBsAg)P, induced protective levels of anti-HBs antibodies in two of four experimentally infected monkeys. We have explored here three strategies to improve the efficacy of vaccination: HBsAg expression at higher levels, repeated vaccination, and an HBsAg protein boost. The third strategy was successful.
Generation and characterization of a MV expressing HBsAg at the highest tolerated levels.
We previously showed that different levels of HBsAg expression from MV-based vectors elicit vastly different anti-HBs antibody levels in mice (6). In particular, HBsAg expression from the L-trailer, H-L, or P-M intergenic regions elicited progressively higher levels of anti-HBs. However, HBsAg expression at the theoretically highest possible level interfered with efficient viral replication; a vectored MV expressing HBsAg from upstream of N was generated but grew to low titers, incompatible with vaccine use (6).
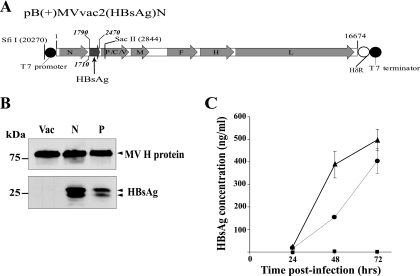
We thus attempted to express HBsAg from the N-P intergenic region (position 1710) (Fig. 1A), the second highest theoretically possible expression level. Using standard techniques (22), we generated MVvac2(HBsAg)N (Fig. 1A). This vector reached a maximum titer of 107 50% tissue culture infective dose (TCID50) at 48 h postinfection in the cell-associated fraction, and 24 h later in the medium, a growth kinetics equivalent to that of the parental strain MVvac2 or the other vectored strain, MVvac2(HBsAg)P (data not shown).
FIG. 1.
Generation of MVvac2(HBsAg)N and characterization of HBsAg expression and secretion. (A) Map of pB(+)MVvac2(HBsAg)N, which was generated by exchanging the SfiII-SacII restriction fragment from pUCHindIII(HBsAg)N, an intermediate plasmid containing an ATU with the HBsAg open reading frame, with the corresponding fragment of pB(+)MVvac2 (7). The coding regions of the MV genes are represented by gray arrows and those of the HBsAg gene by the dark-gray arrows. The T7 promoter and terminator, the hepatitis delta ribozyme (HδR), and unique restriction sites used for plasmid construction are indicated. The insertion site of the ATU and the first and last nucleotide of the HBsAg coding region are indicated. (B) Proteins produced by Vero/hSLAM cells infected with MVvac2 (Vac), MVvac2(HBsAg)N (N), and MVvac2(HBsAg)P (P) were analyzed by immunoblotting using anti-MV H (top)- and anti-HBsAg (bottom)-specific antibodies. The positions of molecular-mass standards are indicated on the left in kilodaltons. (C) HBsAg secretion by different viruses. Vero/hSLAM cells were infected with MVvac2 (squares), MVvac2(HBsAg)N (triangles), or MVvac2(HBsAg)P (circles); media were collected at the time points indicated and clarified. HBsAg was assayed by ELISA and quantified by comparison with a standard curve (6). Averages and standard deviations of the results for a triplicate experiment are shown.
To characterize the HBsAg expressed from the new vector, proteins were extracted from infected cells at 24 h postinoculation and assayed by immunoblotting, using the MV H protein and anti-HBs antibodies (Fig. 1B). Higher HBsAg expression was observed in cells infected with MVvac2(HBsAg)N (Fig. 1B, lane N) than in cells infected with MVvac2(HBsAg)P (Fig. 1B, lane P). Expression levels of the MV H protein were equivalent in cells infected with the two vectors and the parental strain MVvac2 (Fig. 1B, lane Vac). This expression profile was corroborated when infected cells were analyzed by flow cytometry (data not shown). Moreover, secretion of HBsAg was measured with a quantitative enzyme-linked immunosorbent assay (ELISA) in media from infected cells collected at different times. At 72 h postinfection, cells infected with MVvac2(HBsAg)N secreted approximately 500 ng/ml antigen, whereas cells infected with MVvac2(HBsAg)P secreted about 400 ng/ml (Fig. 1C).
Higher levels of HBsAg expression do not enhance the anti-HBs response of MV-susceptible mice.
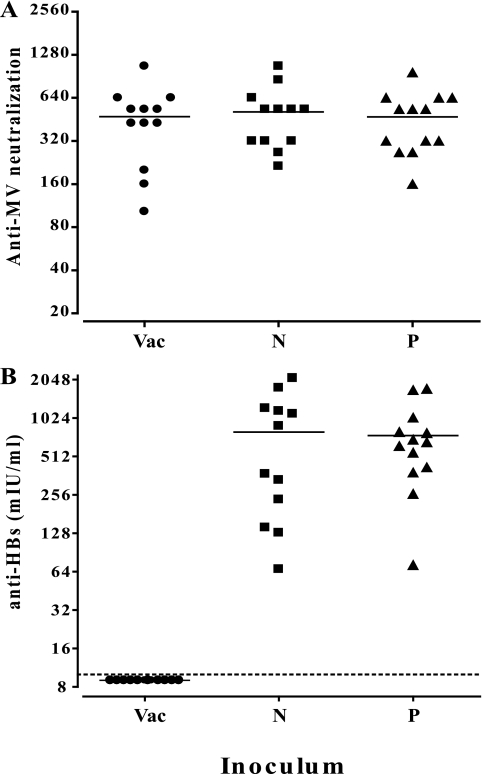
To assess the immunogenicity of HBsAg expressed from the new vector, MV-susceptible mice were infected. These Ifnarko-CD46Ge mice express the MV vaccine strain receptor human CD46 with human-like tissue specificity in a type I interferon receptor knockout background (20). Mice were inoculated with the new HBsAg-expressing vector or with the reference MVvac2(HBsAg)P vector. Additionally, 12 mice were inoculated with the parental strain, MVvac2, as a negative control. The anti-MV neutralizing titer and the anti-HBsAg response were assessed at 28 days postimmunization. As shown in Fig. 2A, all animal groups showed average MV neutralization titers in the 1:320-to-1:640 range.
FIG. 2.
Humoral immune reaction against MV and HBsAg in MV-susceptible mice. Groups of 12 or 13 animals were inoculated by the intraperitoneal route with 5 × 104 TCID50 of MVvac2 (Vac), MVvac2(HBsAg)N (N), and MVvac2(HBsAg)P (P). Serum samples obtained at 28 days postimmunization were assayed for MV neutralization (A) (reciprocals of neutralizing titer) and anti-HBs levels (B) (quantitative ELISA, Bioelisa anti-HBs; Biokit, Barcelona, Spain). Each dot represents one animal; the mean is indicated by a horizontal bar. All experimental procedures were performed as sanctioned by the Mayo Clinic Institutional Animal Care and Use Committee.
The anti-HBs responses were equivalent in the two experimental groups immunized with either HBsAg-expressing recombinant virus (Fig. 2B). As expected, none of the MVvac2-immunized mice had a positive anti-HBsAg response. Anti-HBs titers in animals immunized with MVvac2(HBsAg)N ranged from 67 to 2,100 mIU/ml and from 72 to 1731 mIU/ml in the other group. Averages were 793 versus 744 mIU/ml; the annotated P value was 0.839, corroborating the lack of statistical significance. Thus, it was not possible to further augment the anti-HBs response of mice by enhancing the HBsAg expression levels of the vectored MV.
Revaccination with vectored MV enhances the anti-HBs response in only one of four monkeys.
To characterize the replication of MVvac2(HBsAg)N and the HBsAg immune response in a primate model, a group of four monkeys was immunized subcutaneously using a measles vaccine-equivalent dose (104 TCID50) of MVvac2(HBsAg)N; two additional doses were administered 10 and 16 weeks thereafter. Additionally, 26 weeks after the initial MV inoculation, a single pediatric dose of the commercially available HBsAg vaccine (Merck and Co.)-based vaccine was administered by the intramuscular route. To compare MV-specific host responses, neutralization titers and the number of gamma interferon (IFN-γ) spot-forming cells were measured. To assess the immune response against HBsAg, antibodies and T-cell proliferation were measured.
At 7 days postimmunization, levels of viremia ranged from undetectable to 10 infectious units per 106 peripheral blood mononuclear cells (PBMC), which is the expected range for MV vaccine in rhesus monkeys (6). As expected, none of the animals developed MV-related rashes or any sign of immunosuppression. To assess the genetic stability of the vectored MV after replication in a monkey, we propagated virus reisolated from PBMC and analyzed the sequence of about 1.4 kb of genome including the 0.7-kb HBsAg gene. None of the viral sequences recovered from the three viremic animals presented mutations or deletions.
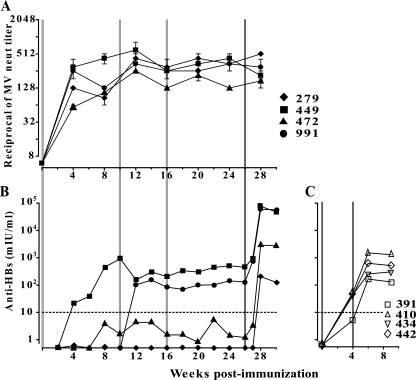
As illustrated in Fig. 3A, levels of anti-MV neutralizing antibodies steadily rose up to a 1:80-to-1:320 range at 8 weeks after the initial dose of the vectored vaccine. This response increased up to four times 2 weeks after the first boost at 10 weeks, to subsequently reach a plateau at around 1:256. MV-neutralizing immunity remained unchanged by the second revaccination. The cellular immune response against MV was assayed by IFN-γ enzyme-linked immunospot assay (Table 1). Three of four animals presented and maintained a cell-mediated immune response of 30 IFN-γ spot-forming cells/106 PBMC on average for the duration of the study. The reasons for the negative response in the fourth monkey are not known.
FIG. 3.
Humoral immune response against MV (A) or HBsAg (B and C) in rhesus monkeys immunized with MVvac2(HBsAg)N (A and B) or recombinant HBsAg protein vaccine (C). MV seronegative rhesus monkeys (A and B; ID in top panel) were housed at the California National Primate Research Center in accordance with the regulations of the Association for the Assessment and Accreditation of Laboratory Animal Care and were vaccinated subcutaneously with an MV vaccine-equivalent dose (104 TCID50) of MVvac2(HBsAg)N at day 1 and at 10 and 16 weeks afterwards (light-gray vertical lines) and with one dose of pediatric recombinant HBsAg protein vaccine (Engerix-B) at 26 weeks (dark-gray vertical line). Animals were monitored daily for MV-related symptoms. They were bled, under ketamine sedation, on days 0, 4, 7, and 14 and every 2 weeks thereafter. Measles viremia was quantified as previously described (18). (C) Rhesus monkeys were immunized with two doses of pediatric recombinant HBsAg protein vaccine at day 1 and 4 weeks afterwards (gray vertical lines). In panel A, averages and standard deviations of the results for at least four independent determinations are shown. In panels B and C, protective levels of anti-HBs (10 mIU/ml) are indicated by an interrupted line. Anti-HBs titers were determined by a quantitative automated anti-HBs assay (Vitros ECiQ Immunodiagnostic System, Ortho Clinical Diagnostics, Inc., Raritan, NJ). neut, neutralizing.
TABLE 1.
Viremia, rash, sequence of replicating MV, and cellular immunity in infected rhesus monkeys
| Monkey ID | Viremiaa | Rash | Sequencec | CMI at indicated no. of wk p.i.d
|
|
|---|---|---|---|---|---|
| 4 | 12 | ||||
| 279 | 0 | No | Not available | 33 | 26 |
| 449 | 5 | No | Unchanged | 23 | 36 |
| 472 | 1b | No | Unchanged | 25 | 30 |
| 991 | 10 | No | Unchanged | 0 | 0 |
Viremia levels are expressed as TCID50/106 PBMC, assayed at 7 days after the first MV inoculation.
MV cytopathic effect is documented, but the titer is too low to be measured.
Sequences covered nucleotides 1244 to 2664 of the vectored MV genome, including the complete HBsAg coding region (nucleotides 1790 to 2470).
Cell-mediated immunity (CMI) was assayed at 4 or 12 weeks postinfection (wk p.i.) by IFN-γ enzyme-linked immunospot assay; numbers of spot-forming cells are after subtraction of the paired medium control and adjusted to 106 PBMC.
The humoral immune responses against HBsAg differed between monkeys (Fig. 3B). After the first inoculation, the anti-HBs response steadily rose up to 1,000 mIU/ml in one monkey. In a second monkey, anti-HBs levels rose to nonprotective levels (protective levels, >10 mIU/ml) at week 8 and stayed constant, even after two revaccinations. After the first revaccination, one monkey seroconverted, showing protective levels of anti-HBs in the range of 100 to 500 mIU/ml. The fourth monkey remained negative in spite of receiving three doses of the vectored vaccine. The titers of MV antibodies rose slightly after the first revaccination and minimally, if at all, after the second revaccination. Thus, the efficacy of revaccination in boosting anti-HBs titers was minimal.
Single boost with HBsAg strongly enhances anti-HBs levels in all animals.
In an alternative strategy, the monkeys received a pediatric dose (10 μg) of yeast-expressed HBsAg (Fig. 3B, week 26). This resulted in a very robust response only 2 weeks after the protein boost. In each monkey, anti-HBs titers increased 100 to 1,000 times, reaching more than 50,000 mIU/ml for two animals, 2,770 mIU/ml for the animal with only subprotective levels before protein boost, and 204 mIU/ml, or about 20 times the protective level, in the animal which did not seroconvert after three immunizations with the vectored MV. To assess cellular immunity against HBsAg, we used a pool of 54 peptides in a lymphocyte proliferation assay. Lymphocytes of three out of four monkeys were positive in this assay (stimulation indices ranging from 2 to 4.2) 24 weeks after the first immunization (data not shown). Thus, a single boost with HBsAg strongly enhances anti-HBV immunity.
We have explored three strategies to improve the efficacy of vaccination with vectored MV expressing HBsAg. Two strategies failed. First, expressing HBsAg at the highest tolerated levels did not enhance anti-HBs titers in mice, or in monkeys. Second, repeated vaccination of monkeys improved anti-HBs titers in only one of four animals, and a second revaccination had no effect. We suspect that high titers of neutralizing antibodies contributed to this lack of efficacy. The third strategy worked; a single pediatric dose of HBsAg protein vaccine elicited a robust response and significantly boosted anti-HBs titers in every monkey inoculated with MVvac2(HBsAg)N. The kinetics of this response suggest that immune memory was established in all monkeys examined here, and that memory B cells sustained the rapid 100- to 1,000-times boost in anti-HBs levels.
Anti-HBs response and protection.
Anti-HBs levels elicited by the alternative HBV immunization strategy based on priming the host with a vectored MV and a single dose of protein compare favorably with those obtained with another protocol based on monkey immunization with two 10-μg doses of HBsAg vaccine at a 1-month interval. In this protocol, anti-HBs levels of 5, 45, 42, and 54 and 135, 321, 554, and 1,414 mIU/ml were documented 30 days after the first and second doses, respectively (Fig. 3C); these levels are in the same range as those observed in the weakly responding monkey in the above-described protocol, but about 100 times below those observed in the two strongly responding animals. In children and adolescents, anti-HBs titers in the 690- to 10,316-mIU/ml range are observed 30 days after standard three-dose vaccination (8, 13; reviewed in reference 12).
Even an immunized individual who does not present protective anti-HBs titers may be protected from accidental HBV challenge, acute hepatitis, and HBV chronicity. This notion has been corroborated by challenge experiments performed with chimpanzees immunized with DNA-coding HBsAg (5) or vectored adeno- or vaccinia viruses (14, 19). We also note that after vaccination with the recommended three-dose recombinant HBsAg schedule, specific antibodies decline within the first year and more slowly thereafter (12). Among children (11) and adults (23) as well, low or undetectable anti-HBs titers are common within 15 years after vaccination. An anamnestic response can be readily evoked even if sero-protective levels are no longer detectable (2, 17, 24), protecting nearly all vaccinated persons against HBV infection. Thus, even without a protein boost, vaccination with an HBsAg-vectored MV may effectively protect children from HBV contagion.
Alternative vaccination strategies.
Current HBsAg-based vaccination strategies foresee three or four doses, the first one given at birth to reduce the incidence of perinatal virus transmission (1). An HBsAg-vectored MV divalent vaccine cannot be administered before about 4 to 6 months of age due to maternal antibodies to MV, and would not prevent HBV perinatal transmission. Nevertheless, even if administered at about the 1-year mark, MVvac2(HBsAg)N may extend HBV vaccination to a majority of the 73% of children worldwide who do not receive a dose of HBsAg at birth (4). This regimen can be tested experimentally in monkeys. High coverage would contribute to preventing HBV transmission to the next generation.
It can also be envisaged to use the HBsAg-vectored MV vaccine, which can be given as early as 4.5 months of age (15), to bolster the anti-HBs response conferred by an HBsAg dose at birth, reducing the number of doses of the HBsAg protein vaccine. Both of these vaccination alternatives would link the strong immunity elicited by the attenuated MV vaccine with prophylactic immunity to HBV at minimal additional cost.
Acknowledgments
We thank D. Granger and J. Yao, Department of Laboratory Medicine and Pathology, Mayo Clinic, for determining anti-HBs serum titers in monkey samples with the automated system; P. Devaux and V. Leonard for helpful discussions; J. Yao for comments on the manuscript; and Marie Frenzke for excellent technical support.
This work was supported by the National Institutes of Health (AI57761, RR11069) and the Mayo Foundation for Medical Education and Research.
Footnotes
Published ahead of print on 17 June 2009.
REFERENCES
- 1.Anonymous. 2004. Hepatitis B vaccines. Wkly. Epidemiol. Rec. 79255-263. [PubMed] [Google Scholar]
- 2.Bauer, T., and W. Jilg. 2006. Hepatitis B surface antigen-specific T and B cell memory in individuals who had lost protective antibodies after hepatitis B vaccination. Vaccine 24572-577. [DOI] [PubMed] [Google Scholar]
- 3.Billeter, M. A., H. Y. Naim, and S. A. Udem. 2009. Reverse genetics of measles virus and resulting multivalent recombinant vaccines: applications of recombinant measles viruses. Curr. Top. Microbiol. Immunol. 329129-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2008. Implementation of newborn hepatitis B vaccination—worldwide, 2006. MMWR Morb. Mortal. Wkly. Rep. 571249-1252. [PubMed] [Google Scholar]
- 5.Davis, H. L., M. J. McCluskie, J. L. Gerin, and R. H. Purcell. 1996. DNA vaccine for hepatitis B: evidence for immunogenicity in chimpanzees and comparison with other vaccines. Proc. Natl. Acad. Sci. USA 937213-7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.del Valle, J. R., P. Devaux, G. Hodge, N. J. Wegner, M. B. McChesney, and R. Cattaneo. 2007. A vectored measles virus induces hepatitis B surface antigen antibodies while protecting macaques against measles virus challenge. J. Virol. 8110597-10605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devaux, P., V. von Messling, W. Songsungthong, C. Springfeld, and R. Cattaneo. 2007. Tyrosine 110 in the measles virus phosphoprotein is required to block STAT 1 phosphorylation. Virology 36072-83. [DOI] [PubMed] [Google Scholar]
- 8.Dobson, S., D. Scheifele, and A. Bell. 1995. Assessment of a universal, school-based hepatitis B vaccination program. JAMA 2741209-1213. [PubMed] [Google Scholar]
- 9.Goldstein, S. T., F. Zhou, S. C. Hadler, B. P. Bell, E. E. Mast, and H. S. Margolis. 2005. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int. J. Epidemiol. 341329-1339. [DOI] [PubMed] [Google Scholar]
- 10.Griffiths, U. K., V. S. Korczak, D. Ayalew, and A. Yigzaw. 2009. Incremental system costs of introducing combined DTwP-hepatitis B-Hib vaccine into national immunization services in Ethiopia. Vaccine 271426-1432. [DOI] [PubMed] [Google Scholar]
- 11.Huang, L. M., B. L. Chiang, C. Y. Lee, P. I. Lee, W. K. Chi, and M. H. Chang. 1999. Long-term response to hepatitis B vaccination and response to booster in children born to mothers with hepatitis B e antigen. Hepatology 29954-959. [DOI] [PubMed] [Google Scholar]
- 12.Keating, G. M., and S. Noble. 2003. Recombinant hepatitis B vaccine (Engerix-B): a review of its immunogenicity and protective efficacy against hepatitis B. Drugs 631021-1051. [DOI] [PubMed] [Google Scholar]
- 13.Leroux-Roels, G., B. Abraham, M. Fourneau, N. De Clercq, and A. Safary. 2000. A comparison of two commercial recombinant vaccines for hepatitis B in adolescents. Vaccine 19937-942. [DOI] [PubMed] [Google Scholar]
- 14.Lubeck, M. D., A. R. Davis, M. Chengalvala, R. J. Natuk, J. E. Morin, K. Molnar-Kimber, B. B. Mason, B. M. Bhat, S. Mizutani, P. P. Hung, et al. 1989. Immunogenicity and efficacy testing in chimpanzees of an oral hepatitis B vaccine based on live recombinant adenovirus. Proc. Natl. Acad. Sci. USA 866763-6767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martins, C. L., M. L. Garly, C. Bale, A. Rodrigues, H. Ravn, H. C. Whittle, I. M. Lisse, and P. Aaby. 2008. Protective efficacy of standard Edmonston-Zagreb measles vaccination in infants aged 4.5 months: interim analysis of a randomised clinical trial. BMJ 337a661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mast, E. E., H. S. Margolis, A. E. Fiore, E. W. Brink, S. T. Goldstein, S. A. Wang, L. A. Moyer, B. P. Bell, and M. J. Alter. 2005. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recommend. Rep. 541-31. [PubMed] [Google Scholar]
- 17.Mast, E. E., and J. W.Ward. 2008. Hepatitis B vaccines, p. 205-241. In S. Plotkin, W. Orenstein, and P. Offit (ed.), Vaccines, 5th ed. Saunders Elsevier, Philadelphia, PA.
- 18.McChesney, M. B., C. J. Miller, P. A. Rota, Y. D. Zhu, L. Antipa, N. W. Lerche, R. Ahmed, and W. J. Bellini. 1997. Experimental measles. I. Pathogenesis in the normal and the immunized host. Virology 23374-84. [DOI] [PubMed] [Google Scholar]
- 19.Moss, B., G. L. Smith, J. L. Gerin, and R. H. Purcell. 1984. Live recombinant vaccinia virus protects chimpanzees against hepatitis B. Nature 31167-69. [DOI] [PubMed] [Google Scholar]
- 20.Mrkic, B., J. Pavlovic, T. Rulicke, P. Volpe, C. J. Buchholz, D. Hourcade, J. P. Atkinson, A. Aguzzi, and R. Cattaneo. 1998. Measles virus spread and pathogenesis in genetically modified mice. J. Virol. 727420-7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pless, R. P., A. D. Bentsi-Enchill, and P. Duclos. 2003. Monitoring vaccine safety during measles mass immunization campaigns: clinical and programmatic issues. J. Infect. Dis. 187(Suppl. 1)S291-S298. [DOI] [PubMed] [Google Scholar]
- 22.Radecke, F., P. Spielhofer, H. Schneider, K. Kaelin, M. Huber, C. Dotsch, G. Christiansen, and M. A. Billeter. 1995. Rescue of measles viruses from cloned DNA. EMBO J. 145773-5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wainwright, R. B., L. R. Bulkow, A. J. Parkinson, C. Zanis, and B. J. McMahon. 1997. Protection provided by hepatitis B vaccine in a Yupik Eskimo population—results of a 10-year study. J. Infect. Dis. 175674-677. [DOI] [PubMed] [Google Scholar]
- 24.Watson, B., D. J. West, A. Chilkatowsky, S. Piercy, and V. A. Ioli. 2001. Persistence of immunologic memory for 13 years in recipients of a recombinant hepatitis B vaccine. Vaccine 193164-3168. [DOI] [PubMed] [Google Scholar]