Abstract
For the final stages in the eradication of poliovirus type 1 (P1), the World Health Organization advocates the selective use of monovalent type 1 oral poliovirus vaccine (mOPV1). To compare the immunogenicity of mOPV1 with that of trivalent OPV (tOPV) in infants, a study was performed in Egypt in 2005. Newborns were vaccinated with mOPV1 or tOPV immediately after birth and were challenged with mOPV1 after 1 month. Vaccination with mOPV1 at birth resulted in significantly higher seroconversion against P1 viruses and lower excretion of P1 viruses than vaccination with tOPV. Intratypic differentiation of the viruses shed by the newborns revealed the presence of remarkably high numbers of antigenically divergent (AD) P1 isolates, especially in the mOPV1 study group. The majority of these AD P1 isolates (71%) were mOPV1 challenge derived and were shed by newborns who did not seroconvert to P1 after the birth dose. Genetic characterization of the viruses revealed that amino acid 60 of the VP3 region was mutated in all AD P1 isolates. Isolates with substitution of residue 99 of the VP1 region had significantly higher numbers of nonsynonymous mutations in the VP1 region than isolates without this substitution and were preferentially shed in the mOPV1 study group. The widespread use of mOPV1 has proven to be a powerful tool for fighting poliovirus circulation in the remaining areas of endemicity. This study provides another justification for the need to achieve high vaccination coverage in order to prevent the circulation of AD strains.
Polioviruses are the causative agents of human poliomyelitis and belong to the genus Enterovirus in the family Picornaviridae. The virus is transmitted primarily by the fecal-oral route and replicates in the human intestinal tract. The virus may also be transmitted through respiratory droplets and may replicate for a short period in the upper respiratory tract and tonsillar tissue. From either site of primary replication, the virus may invade the central nervous system and cause paralysis following infection and destruction of motor neurons. Three serologically different types of poliovirus can be distinguished (poliovirus type 1 [P1], P2, and P3), and only limited cross-protection exists between serotypes (35).
In 1988, the World Health Assembly passed a resolution to eradicate wild poliovirus globally. A worldwide vaccination campaign with the trivalent oral poliovirus vaccine (tOPV) was launched by the World Health Organization (WHO). This vaccine contains the three attenuated poliovirus vaccine strains developed by Albert Sabin in the proportion of 10:1:6 for P1, P2, and P3, respectively. These OPV strains have been selected to replicate successfully in the human intestinal tract but not in the cells of the central nervous system. In addition to a strong humoral response, these strains generate strong intestinal immunity (12). Sabin type 1 is considered to be the most stable of the three attenuated poliovirus serotypes (19). This strain has 54 mutations compared to the parental Mahoney strain, of which 6 are primarily responsible for attenuation. Sabin type 2 has two major determinants of attenuation, and Sabin type 3 has three determinants of attenuation (11, 32). Upon replication in the human intestinal tract, the sites of attenuation can mutate, which results in reversion of the Sabin strains toward a parental neurovirulent phenotype. Also as a consequence of replication in the host, antibodies are produced that recognize the antigenic sites of the Sabin strains (42). This immunogenic pressure could favor the selection of antigenically divergent (AD) viruses with substituted residues in parts of these antigenic sites. AD Sabin viruses might circulate among a population for a long period and evolve into vaccine-derived polioviruses (VDPVs; with differences of >1% from the prototype Sabin viruses in the VP1 region) capable of causing outbreaks. These viruses might escape current diagnostic screening methods, and the risk for generation of these viruses should be reduced as much as possible (1, 9, 16).
The tOPV vaccination campaigns have been very successful, since the number of countries with endemic wild poliovirus circulation decreased from >125 in 1988 to 4 in 2006, and wild type 2 poliovirus has likely been eradicated since 1999 (5). The tOPV vaccine, however, is known to be less immunogenic against type 1 and 3 polioviruses. After tOPV administration, the superior replicative capacity of the P2 vaccine strain interferes with effective replication of the other two serotype viruses in the human intestine (30). To eradicate wild P1 as well, vaccination with monovalent type 1 oral poliovirus vaccine (mOPV1) was introduced in the remaining countries where poliovirus is endemic, since this vaccine is more immunogenic for type 1 than the tOPV (4, 20).
In 2005/2006, a clinical study was conducted in Egypt to compare the immunogenicity of mOPV1 with that of the tOPV in newborns (15). Newborns were vaccinated with mOPV1 or tOPV as soon as possible after birth and were challenged with mOPV1 4 weeks later. Vaccination with mOPV1 at birth resulted in a higher humoral and mucosal protection against P1 at day 28 than vaccination with tOPV at birth.
In line with the recommendations of the WHO Polio Laboratory Network, we determined the antigenic characters of all the viruses shed by the newborns of the Egyptian study by using an intratypic differentiation (ITD) enzyme-linked immunosorbent assay (ELISA). The outcome of this analysis, an unexpectedly high percentage of AD isolates, prompted further investigation. To determine the possible presence of VDPVs and to gain insight into the genetic and antigenic evolution of the mOPV1 and tOPV isolates shed by the newborns in this study, we determined the sequences of the capsid regions of these isolates. We looked for correlates with antigenic change and rates of mutagenesis in the viruses and compared the evolution rates of the viruses shed by vaccinees of both study groups. We also linked the serological data collected during the study to the excretion of Sabin 1 isolates.
MATERIALS AND METHODS
Study design.
The design of the study of the comparative immunogenicity of mOPV1 versus tOPV has been described previously (15). Briefly, 530 newborns from three sites in Egypt located in Greater Cairo and Alexandria were randomly distributed into two groups (A and B). Group A received mOPV1 with a titer of 106.8 50% cell culture infective doses per dose as soon as possible after birth and before receiving breast milk (median interval, 60 min). Group B received tOPV (titers of 106.9 for type 1, 105.5 for type 2, and 106.5 for type 3) as soon as possible after birth and before receiving breast milk. The newborns of both group A and group B were challenged with mOPV1 (titer, 106.8) 28 days after the birth dose. Three blood samples were collected per child: one at birth (cord blood), one at day 28 just before the challenge with mOPV1, and one at day 56. Five stool samples per child were collected: at day 28 just before the challenge dose was administered and at days 35, 42, 49, and 56. The blood samples were shipped to the Enterovirus Diagnostic Laboratory at the Centers for Disease Control and Prevention (CDC) in Atlanta, GA, where the antibody titers were determined by using a modified neutralization assay for antibodies to poliovirus types 1, 2, and 3 (15). Serological results were reported as reciprocal titers of serum dilutions that exhibited 50% neutralization. Seroconversion was defined as a ≥4-fold increase over the expected decline in the maternal antibody titer. The half-life of maternal antibody decay was assumed to be 28 days, consistent with direct measurements from previous studies. Change from a seronegative immune status to a reciprocal titer of ≥8 was also considered seroconversion. The stool samples were shipped to the National Institute for Public Health and the Environment (RIVM, The Netherlands) for virological analysis.
Randomization and inoculation of stool samples.
A total of 2,459 fecal samples were received at the RIVM in four shipments and were labeled with blinded code numbers that could not be used to deduce the identities of individual vaccinees or vaccination groups by the testing laboratory. For each fecal sample, a 20% (wt/vol) suspension was prepared in phosphate-buffered saline containing 20% chloroform. For each sample, two tubes with monolayers of L20B cells, a transgenic murine cell line expressing the human poliovirus receptor, were inoculated with 0.1 ml stool suspension and observed for cytopathogenic effect (CPE) for 10 days. Cytopathogenic effect-positive cultures were passed once more in L20B cells (41).
Sabin-specific PCR assay and ITD.
The presence and identities of poliovirus serotypes in the viral cultures were determined by a Sabin-specific PCR assay (43). Viral RNA was isolated from the viral culture, eluted in a final volume of 50 μl using the MagnaPure LC method (Roche Diagnostics, Mannheim, Germany) according to the manufacturer's instructions, and amplified in a two-step reverse transcriptase PCR (RT-PCR) assay according to standard procedures recommended by the WHO (41). Virus harvests containing a mixture of serotypes were passed in the presence of polyclonal antisera against two of the three serotypes in order to separate the virus mixture (40).
The antigenic characters of the isolates were determined by the ITD-ELISA described in the WHO Polio Laboratory Manual (40). With this assay the isolates were characterized as Sabin-like (SL), non-Sabin-like (NSL), double-reactive (DR), or nonreactive (NR), based on their reactivities with Sabin-like- and wild-type-specific cross-absorbed polyclonal antibodies (39).
Capsid sequencing.
The VP1 regions of all isolates were amplified in a two-step RT-PCR assay using the Y7 forward (5′-GGT TTT GTG TCA GCG TGT AAT GA-3′) and Q8 reverse (5′-AAG AGG TCT CTA TTC CAC AT-3′) primers (33). Viral RNA was converted to cDNA by incubation of 3.0 μl of the isolated RNA at 42°C for 60 min with 4.0 μl 50 μM Q8 primer (Isogen), 3.0 μl 10× PCR buffer (670 mM Tris-HCl [pH 8.8], 20 mM MgCl2, 170 mM ammonium sulfate, 0.06 mM EDTA [pH 8], 0.12 mM β-mercaptoethanol), 16 μl 2.5 mM deoxynucleoside triphosphates (Roche), 1.25 μl 10-U/μl avian myeloblastosis virus RT (Promega), 0.16 μl 125-U/μl RNase inhibitor (Amersham, Life Science), and 2.59 μl water. The reverse transcription reaction was terminated by incubation of the samples at 94°C for 3 min and subsequent chilling on ice. In total, 15 μl of the cDNA was used for the PCR amplification, together with 3.5 μl 10× PCR buffer, 2 μl 50 μM Y7 primer, 0.5 μl 5-U/μl Taq polymerase (Roche), and 29 μl water. PCR was carried out for 25 cycles of 30 s at 94°C, 45 s at 42°C, and 1 min at 60°C, with a final cycle of 7 min at 60°C. The PCR products were purified according to the manufacturer's protocol for the QIAquick PCR purification kit (Qiagen, 2002) and were sequenced using a fluorescence-labeled dideoxynucleotide technology from Applied Biosystems (Foster City, CA) with forward primer Y7 and reverse primer Q8. The VP2 and VP3 regions of selected P1 isolates were amplified by RT-PCR and sequenced by the procedures described above using primers 3F2 (5′-GAG CCC ATC AAG GAT GTC C-3′) and n5R (5′-CCT AGG ATC TGA AGC TGG-3′) for VP2 and primers 5F (5′-ATA TCT YAC TGC AGA CAA-3′), n6R (5′-TGACCTAACCCCTGTGCT-3′), 6F (5′-CTCATGTACTATGGTAGT-3′), and 7R (5′-CACTTGATTTAAGGCATG-3′) for VP3 (3).
Sequence data analysis.
The nucleotide sequences were assembled using Seqman software (version 3.61; DNAStar). Sequence data were obtained by using both the sense and antisense primers. The P1, P2, and P3 sequences were aligned with the Sabin reference strains obtained from mOPV1 and tOPV by using Clustal W (38) from BioEdit software, version 7.0.0 (21). Synonymous and nonsynonymous mutations were determined with DNA Sequence Polymorphism, version 4.10 (37). Nucleotide mutation rates were estimated by linear regression. At ambiguous sites, only the base found in the higher molar proportion was scored.
Statistical methods.
Data were analyzed with the statistical software package R (36). Synonymous and nonsynonymous mutation rates were estimated by linear regression. The serological data of the Egyptian study were plotted in boxplots using Excel.
Nucleotide sequence accession numbers.
The nucleotide sequence data reported in this paper will appear in the DDBJ/EMBL/GenBank nucleotide sequence databases with accession numbers AB467375 to AB467543 and AB467720 to AB467724 for the P1 isolates of the mOPV1 study group, accession numbers AB467544 to AB467719 for the P1 isolates of the tOPV study group, accession numbers AB467725 to AB467814 for the P2 isolates, and accession numbers AB467815 to AB467867 for the P3 isolates.
RESULTS
Mucosal immunity following the birth dose of mOPV1 or tOPV.
Of the 530 children initially enrolled, 421 newborns participated throughout the study: 231 in group A (mOPV1 at birth) and 190 in group B (tOPV at birth) (Table 1). These children represented the group used for the final analyses, and the sample sizes were sufficient for analyzing differences in the responses to mOPV1 and tOPV and for some subset comparisons of the groups and subgroups. For the primary outcome measure, seroconversion to P1, mOPV1 demonstrated approximately twofold greater seroconversion to the birth dose than tOPV1, as reported previously (15). Mucosal immunity as measured by virus excretion following challenge was the secondary outcome measure of the study. Significantly more newborns who received mOPV1 at birth shed P1 at 28 days before administration of the challenge dose than newborns who received tOPV at birth (P = 0.02; odds ratio [OR], 2.34; 95% confidence interval [CI95], 1.09 to 5.33) (Table 1), consistent with the previously reported higher seroconversion in group A (15). After the mOPV1 challenge at day 28, however, a higher proportion of newborns in group B than in group A shed P1 virus at all four sampling times, consistent with higher mucosal immunity in newborns of group A. Newborns never shedding P1 virus on any of the sampling days were significantly more common in the mOPV1 group (136/231) than in the tOPV group (79/190) (P = 0.000; OR, 2.01; CI95, 1.34 to 3.03).
TABLE 1.
Shedding of Sabin 1 virus before and after challengea
| P1 virus shedding | % of newborns (no./total tested) in groupb:
|
Pc | |
|---|---|---|---|
| A (mOPV1) | B (tOPV) | ||
| None | 58.9 (136/231) | 41.6 (79/190) | 0.000 |
| Before challenge | 12.6 (29/231) | 5.8 (11/190) | 0.020 |
| 7 days after challenge | 25.9 (59/228) | 41.5 (78/188) | 0.001 |
| 14 days after challenge | 22.3 (50/224) | 29.3 (54/184) | 0.111 |
| 21 days after challenge | 12.6 (28/222) | 15.3 (28/183) | 0.471 |
| 28 days after challenge | 8.2 (18/220) | 13.2 (24/182) | 0.140 |
Data are from reference 15.
In group A, 294 newborns were enrolled for the study and 231 completed the study requirements. In group B, 236 newborns were enrolled for the study and 190 completed the study requirements.
Calculated by Fisher's two-tailed test.
Virus isolation.
The Sabin-specific PCR detected the presence of 186 P1 isolates, 35 P2 isolates, and 19 P3 isolates in group A (231 newborns). In group B (190 newborns), 202 P1, 67 P2, and 44 P3 isolates were detected. All L20B-positive cultures contained only polioviruses that reacted as Sabin-like viruses in the Sabin-specific PCR.
Antigenic characterization.
In addition to screening for wild poliovirus by PCR, all these isolates were antigenically characterized by the ITD-ELISA (40) using cross-absorbed polyclonal Sabin- and wild-type-specific antibodies (Table 2). In total, 44.6% of the P1 isolates in the mOPV1 study group (83/186 isolates from 53/95 children) and 21.8% of the P1 isolates in the tOPV study group (44/202 isolates from 34/111 children) had an AD character (NR, NSL, or DR). These percentages were much higher than the percentages of AD P2 and P3 isolates in both study groups and also much higher than the percentages of isolates or children observed in routine screening of Sabin-related isolates in the global surveillance network (<5% for P1) (27). The elevated percentage of AD P3 isolates in the mOPV1 study group is not significant and is most likely due to the low number of P3 isolates shed by this group (n = 3). The difference in the percentage of AD P1 isolates shed by newborns between group A and group B was significant (P = 0.001; OR, 2.89; CI95, 1.82 to 4.62).
TABLE 2.
Antigenic characterization by ITD-ELISA
| Antigenic characterization | % of isolates
|
|||||
|---|---|---|---|---|---|---|
| Group A (mOPV1)
|
Group B (tOPV)
|
|||||
| P1 (n = 186) | P2 (n = 35) | P3 (n = 19) | P1 (n = 202) | P2 (n = 67) | P3 (n = 44) | |
| SL | 55.4 | 94.3 | 84.2 | 78.2 | 91 | 97.7 |
| NR | 22.6 | 5.7 | 15.8 | 12.4 | 4.5 | 2.3 |
| NSL | 16.1 | 0 | 0 | 6.4 | 0 | 0 |
| DR | 5.9 | 0 | 0 | 3.0 | 4.5 | 0 |
VP1 capsid sequence analysis.
Because the ITD-ELISA revealed remarkably high numbers of AD P1 isolates, it was necessary to characterize these isolates further in consistency with WHO-recommended procedures (40). All known P1 VDPVs and most, but not all, of the known P2 and P3 VDPVs react as AD in the ITD-ELISA (9). The VP1 capsid region was sequenced to determine whether any isolate could be defined as a VDPV and to understand the antigenic character and evolution of these isolates. The VP1 regions of 345 P1 isolates (involving 81 newborns of group A and 94 newborns of group B), 90 P2 isolates, and 53 P3 isolates were sequenced successfully. Sequences could not be obtained from the remaining 43 P1 isolates, 12 P2 isolates, and 10 P3 isolates because of poor sequencing quality, possibly caused by the presence of multiple strains of the same serotype. These isolates without definitive sequences were distributed randomly among newborns of both groups A and B; all of these isolates had a Sabin-like character by ITD-ELISA and therefore most likely contained only a small number of nucleotide changes and had no amino acid changes associated with AD isolates.
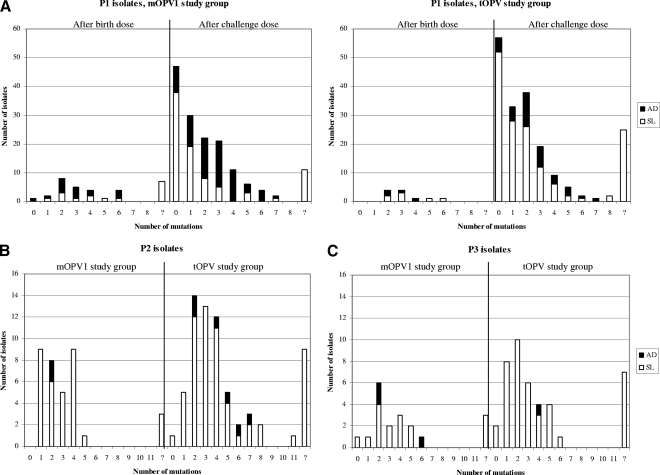
No VDPVs (defined as 10 or more changes in the VP1 region) of type 1 or type 3 were found in the study groups (Fig. 1A and 1C). The number of nucleotide mutations in the VP1 regions of P1 isolates ranged from none to eight. The maximum number of nucleotide mutations found in the VP1 regions of the P3 isolates was six. One newborn of group B shed a type 2 VDPV with a VP1 divergence of 1.22% (11 mutations in VP1) at 56 days after tOPV vaccination (Fig. 1B). Two earlier stool specimens collected from the same vaccinee at days 42 and 49 contained viruses with 5 and 7 mutations, respectively, all of which were also present in the isolate collected at day 56, demonstrating the accumulation of 6 mutations in a 2-week period in this child and implying the accumulation of 11 mutations within 8 weeks of receipt of the birth dose. The child had already seroconverted to P2 by day 28, also consistent with the response to the tOPV birth dose this child received. In addition, one newborn who had received an extra dose of mOPV1 during the routine vaccination activities, and was therefore excluded from the study, shed a type 1 VDPV with a VP1 divergence of 1.3% at 49 days after the birth dose. No evidence of immunodeficiency or disease was found during clinical follow-up of these two newborns with VDPVs.
FIG. 1.
Numbers of nucleotide mutations in the VP1 regions of isolates. (A) Birth dose- and challenge dose-derived P1 isolates; (B) P2 isolates; (C) P3 isolates.
VP2 and VP3 capsid sequencing.
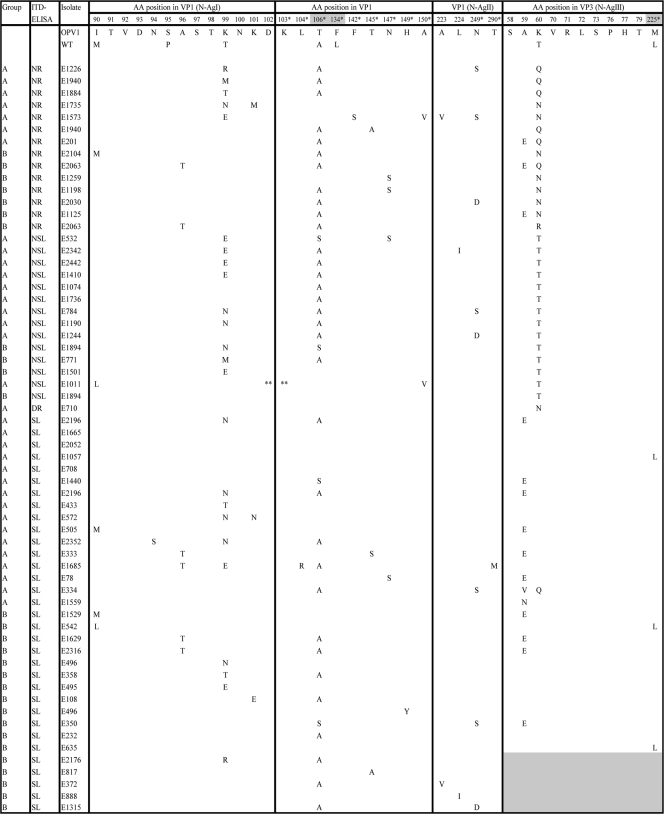
In the VP1 regions of the P1 isolates, no substitutions were found that distinguished the Sabin-like from the AD isolates (Table 3). The VP2 and VP3 capsid regions of 133 P1 isolates (91 Sabin-like isolates and 42 isolates with an AD character) were sequenced to identify the determinants that correlated with the observation of antigenic divergence. In the VP2 region, no amino acid substitutions were found that distinguished the Sabin-like P1 isolates from the AD P1 isolates. In the VP3 region, Ala59 and Lys60 were preferred sites of nonsynonymous mutation (Table 3). Both of these amino acids are associated with neutralization antigenic site III (N-AgIII). Isolates with a mutation of Ala59 to a glutamic acid kept the Sabin-like antigenic character. However, replacement of Lys60 with a threonine, asparagine, or glutamine resulted in an AD character by the ITD-ELISA (Table 3). It therefore appears that the mutation of Lys60 is the major determinant of the AD characteristics observed among the P1 isolates in this study. One isolate (E334) had mutations of Ala59 to valine and Lys60 to glutamine but was characterized as Sabin like by the ITD-ELISA, indicating the possibility of a more complex antigenic structure in this region.
TABLE 3.
Amino acid mutations in the VP1 and VP3 regions of P1 isolatesa
The VP3 regions of E2176, E817, E372, E888, and E1315 were not sequenced. The isolates in this table are presented to show that in the VP1 region, no substitutions that distinguish SL isolates from AD (NSL, NR, or DR) isolates were present. Major determinants of attenuation are boldfaced. *, the amino acid (AA) is not located in an antigenic site; **, the amino acid was deleted.
Linkage of P1 shedding and serological data.
Using the serological data and VP1 sequence data, it was possible to determine whether observed P1 isolates were most likely derived from the birth dose or the challenge dose. Seroconversion to P1 following the birth dose and/or shedding of P1 virus before administration of the challenge dose at day 28 (observed for 37 newborns) (Table 4) was interpreted as evidence of replication of the birth dose vaccine viruses. Isolates shed after the challenge dose (170 newborns) were assumed to be derived from the challenge dose unless the isolates included mutations in the VP1 region identical to those of the isolate detected at day 28 (6 newborns). The absence of seroconversion to P1 after the birth dose and the absence of shedding of P1 virus at day 28, in combination with seroconversion to P1 after administration of the challenge dose and/or shedding of P1 virus any time after the challenge dose, were interpreted as evidence of replication of the challenge virus (Table 4). It could not be determined whether 11 children (8 in group A and 3 in group B) had undergone seroconversion. Two of these indeterminate children (both in group A) never shed P1 virus; two (both group A) shed birth dose virus; and seven (four in group A and three in group B) shed virus following the challenge dose.
TABLE 4.
Overview of shedding of P1 isolates and seroconversion against P1
| Shedding of P1after birth dose or challenge dose | Groupa | No. of newborns:
|
Seroconversion to P1b
|
Avg (range) log 2 titer of antibody against P1
|
No. of newborns with seroconversion to:
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Shedding at least 1 AD P1 isolate | Before dose | After dose | Day 0 | Day 28 | Day 56 | P2 | P3 | ||
| No shedding | A | 26 | 0 | − | − | 8.65 (5.17-10.5) | 7.07 (3.5-10.5) | 5.99 (2.83-10.17) | 5 | 4 |
| B | 19 | 0 | 8.96 (6.17-10.5) | 7.39 (4.5-9.83) | 5.94 (3.5-8.5) | 10 | 7 | |||
| A | 11 | 0 | − | + | 8.38 (7.5-9.5) | 7.65 (4.83-9.83) | 10.17 (9.5-10.5) | 1 | 4 | |
| B | 12 | 0 | 7.44 (5.17-9.17) | 5.64 (3.83-7.83) | 10.06 (9.17-10.5) | 9 | 5 | |||
| A | 97 | 0 | + | 6.14 (2.5-10.17) | 10.14 (7.17-10.5) | 10.00 (6.5-10.5) | 13 | 5 | ||
| B | 48 | 0 | 5.60 (2.5-9.83) | 9.74 (6.83-10.5) | 9.25 (4.5-10.5) | 34 | 14 | |||
| A | 2 | 0 | ? | ? | 10.5 | 10.5 | 10.5 | 0 | 0 | |
| B | 0 | 0 | 0 | 0 | ||||||
| Subtotal | A | 136 | 0 | 19 | 13 | |||||
| B | 79 | 0 | 53 | 26 | ||||||
| Shedding after birth dose | A | 2 | 0 | − | − | 9.5 (9.17-9.83) | 9.5 (8.83-10.17) | 8.84 (8.5-9.17) | 1 | 2 |
| B | 1 | 0 | 7.83 | 5.17 | 4.83 | 0 | 0 | |||
| A | 1 | 0 | − | + | 7.5 | 4.83 | 10.5 | 0 | 1 | |
| B | 3 | 0 | 9.61 (8.83-10.17) | 9.61 (9.17-10.17) | 10.5 | 3 | 1 | |||
| A | 21 | 12 | + | 7.47 (5.17-9.5) | 10.13 (7.5-10.5) | 10.39 (9.83-10.5) | 2 | 0 | ||
| B | 7 | 4 | 6.17 (3.5-8.17) | 10.02 (9.5-10.5) | 10.07 (8.5-10.5) | 7 | 2 | |||
| A | 2 | 2 | ? | ? | 10.5 | 10.5 | 10.5 | 0 | 0 | |
| B | 0 | 0 | 0 | 0 | ||||||
| Subtotal | A | 26 | 14 | 3 | 3 | |||||
| B | 11 | 4 | 10 | 3 | ||||||
| Shedding after challenge dose | A | 6 | 0 | − | − | 10.06 (8.83-10.5) | 9.17 (6.5-10.17) | 8.17 (4.83-10.17) | 1 | 1 |
| B | 9 | 0 | 9.43 (6.5-10.5) | 8.54 (5.5-9.83) | 8.02 (6.5-9.83) | 5 | 3 | |||
| A | 49 | 30 | − | + | 7.5 (2.83-10.5) | 5.95 (2.5-10.17) | 10.3 (7.17-10.5) | 6 | 5 | |
| B | 78 | 28 | 7.35 (3.17-10.5) | 5.6 (2.5-10.17) | 9.98 (5.17-10.5) | 49 | 19 | |||
| A | 10 | 6 | + | 5.43 (2.5-10.17) | 9.9 (7.83-10.5) | 10.5 | 1 | 0 | ||
| B | 11 | 2 | 6.83 (3.5-10.17) | 9.23 (5.5-10.5) | 9.65 (5.5-10.5) | 11 | 2 | |||
| A | 4 | 3 | ? | ? | 10.5 | 10.5 | 10.5 | 0 | 0 | |
| B | 3 | 0 | 10.5 | 10.5 | 10.5 | 2 | 0 | |||
| Subtotal | A | 69 | 39 | 8 | 6 | |||||
| B | 101 | 30 | 67 | 24 | ||||||
| Total | A | 231 | 53 | 30 | 22 | |||||
| B | 191c | 34 | 130 | 53 | ||||||
Group A, mOPV1 study group; group B, tOPV study group.
−, no seroconversion; +, seroconversion; ?, indeterminable.
One newborn who was already shedding the birth dose virus started shedding the challenge dose virus as well.
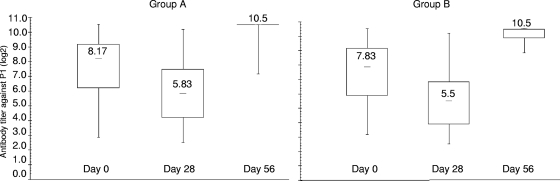
In total, P1 virus shedding was detected at any time in 95 newborns of group A and 111 newborns of group B. Birth dose P1 virus was shed from 26 newborns (27%) of the mOPV1 study group (group A) and 11 newborns (10%) of the tOPV study group (group B), of whom the majority (21/26 in group A and 7/11 in group B) also demonstrated seroconversion to P1 following the birth dose (Table 4). The majority of the newborns who ever shed P1 virus shed challenge-derived P1 viruses (73% [69/95] in group A and 91% [101/111] in group B). In total, 71% of these newborns in group A (49/69) and 77% of these newborns in group B (78/101) seroconverted only after administration of the challenge dose virus. AD P1 isolates were shed mainly by the newborns belonging to this category, especially in the mOPV1 study group, and were thus mOPV1 (challenge dose) derived. The difference between groups A and B in the number of newborns shedding challenge dose P1 virus with an AD character who seroconverted against P1 only after the challenge dose could not be ascribed to a difference in the neutralizing antibody titers against P1 at day 28 (mean titers, 5.95 log 2 for group A and 5.6 log 2 for group B; P = 0.34 by a one-way analysis of variance [ANOVA] test) (Fig. 2). In addition, these titers did not differ significantly between newborns shedding only Sabin-like isolates (mean titer for group A, 5.39 log 2 [range, 2.83 to 8.5 log 2]; mean titer for group B, 5.5 log 2 [range, 2.5 to 10.17 log 2]) and newborns shedding at least one AD isolate (mean titer for group A, 5.93 log 2 [range, 2.5 to 10.17 log 2]; mean titer for group B, 5.46 log 2 [range, 2.83 to 9.17 log 2]) (P by one-way ANOVA, 0.396 for group A and 0.927 for group B).
FIG. 2.
Serological data for newborns with seroconversion against P1 after the challenge dose and shedding of challenge dose P1 virus (49 newborns in group A and 78 in group B). Values given in the boxplots are median titers.
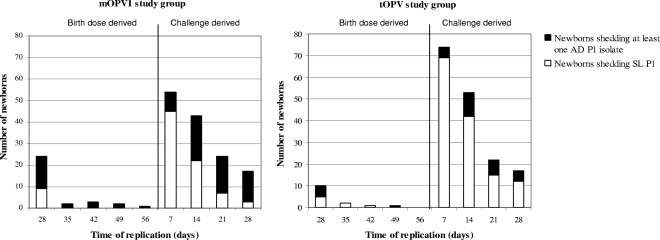
In total, 56% of the newborns shedding P1 virus in group A (53/95) shed at least one AD P1 isolate. In group B, this percentage was 31.6% (34/111). In group A, 26% of the newborns who shed AD P1 virus (14/53) were shedding virus from the birth dose (Table 4). In group B, this percentage was 12% (4/34). The majority of the newborns shedding AD P1 virus (74% [39/53] in group A and 88% [30/34] in group B), however, shed challenge dose virus. These shedding newborns mainly seroconverted to P1 after administration of the challenge dose. In this category, 30 of the 49 newborns in group A (61%) shed AD P1 virus. In group B, this number was significantly lower: 28 of the 78 newborns (36%). The proportion of AD P1 isolates (birth dose and challenge derived) increased with time of shedding, particularly in the mOPV1 study group (Fig. 3). There were 21 infants who shed virus after the challenge dose but seroconverted after the birth dose, and it is therefore uncertain which dose was the source of the virus observed after challenge. If these children are excluded from the analysis, then the percentage of newborns shedding birth dose-derived AD P1 isolates was 56% in the mOPV1 group (20/36) versus 27% in the tOPV group (6/22). The percentage of newborns shedding challenge dose-derived AD P1 isolates was 56% in the mOPV1 group (33/59) versus 31% in the tOPV group (28/90). These results are not significantly different from those with the 21 infants included.
FIG. 3.
Shedding of antigenically divergent P1 isolates over time.
A significant proportion of children who did not shed virus following vaccination nevertheless seroconverted during the study. Among newborns in group A, 42.0% (97/231) seroconverted to the mOPV1 birth dose although shedding of virus was never detected in any sample, whereas 80% (49/61) of children in group A who seroconverted to the challenge dose shed virus at some point after challenge. Because of the study design, the first time point where shedding of birth dose-derived virus is possible is 28 days following vaccination. In contrast, shedding as a result of the challenge dose can be measured at 7, 14, 21, and 28 days following vaccination. This indicates the likelihood that the measurement of birth dose shedding is underestimated; therefore, interpretation of the comparison of shedding rates from the birth dose and the challenge dose is not possible.
Sites of mutations.
The average number of nucleotide mutations in isolates of both study groups increased with time of shedding following vaccination (Table 5). Nonsynonymous/synonymous mutation ratios were calculated from the total numbers of nonsynonymous and synonymous mutations for all isolates at the same sampling instance and were similar among isolates of both study groups. For the challenge dose isolates, the first phase of evolution (14 to 28 days of replication in group A and 7 to 28 days of replication in group B) was characterized primarily by the accumulation of nonsynonymous mutations (nonsynonymous/synonymous mutation ratio, >1) (Table 5). Reversion of Thr106, one of the major determinants of attenuation, occurred relatively quickly after vaccination. At 14 days, 49% of the P1 isolates of the mOPV1 study group and 60% of the P1 isolates of the tOPV study group had replaced this residue. After 28 days, however, the ratio of nonsynonymous to synonymous mutations decreased in group B. This resulted from the accumulation and fixation of silent mutations and the decline in the rate of nonsynonymous mutations.
TABLE 5.
Average percentage of VP1 difference and nonsynonymous/synonymous mutation ratio
| Group, virus, and day | VP1 difference (%) | No. of mutations
|
Nonsynonymous/synonymous mutation ratio | No. of isolates | |
|---|---|---|---|---|---|
| Synonymous | Nonsynonymous | ||||
| Group A (mOPV1) | |||||
| Birth dose-derived virus | |||||
| Day 28 | 0.35 (±0.20) | 24 | 39 | 1.63 | 23 |
| Day 35 | 0.39 (±0.04) | 2 | |||
| Day 42 | 0.41 (±0.10) | 3 | |||
| Day 49 | 0.55 (±0.16) | 2 | |||
| Day 56 | 0.66 | 1 | |||
| Challenge dose-derived virus | |||||
| Day 7 | 0.08 (±0.15) | 19 | 17 | 0.89 | 50 |
| Day 14 | 0.18 (±0.11) | 23 | 48 | 2.09 | 41 |
| Day 21 | 0.36 (±0.18) | 23 | 46 | 2.00 | 22 |
| Day 28 | 0.32 (±0.15) | 16 | 33 | 2.00 | 16 |
| Group B (tOPV) | |||||
| Birth dose-derived virus | |||||
| Day 28 | 0.34 (±0.15) | 14 | 16 | 1.14 | 10 |
| Day 35 | 0.41 (±0.12) | 2 | |||
| Day 42 | 0.33 | 1 | |||
| Day 49 | 0.39 | 1 | |||
| Day 56 | 0 | ||||
| Challenge dose-derived virus | |||||
| Day 7 | 0.08 (±0.13) | 18 | 24 | 1.33 | 64 |
| Day 14 | 0.24 (±0.19) | 32 | 66 | 2.06 | 45 |
| Day 21 | 0.26 (±0.17) | 11 | 34 | 3.1 | 19 |
| Day 28 | 0.33 (±0.17) | 19 | 30 | 1.5 | 15 |
In addition to Thr106, Lys99 was a preferred site for mutation (Table 3). Significantly more newborns in group A (28.4% [23/81]) shed at least one isolate with a mutation of Lys99 than newborns in group B (11.7% [11/94]) (P = 0.007; OR, 2.99; CI95, 1.28 to 7.32). Up to 4 weeks of replication, isolates with a mutation of residue 99 had a significantly higher number of nonsynonymous nucleotide mutations in the VP1 region than isolates without this amino acid mutation (P = 0.000 by a mixed-effect Poisson regression model) (see the supplemental material). In group A, 89.7% (26/29) of the isolates with a Lys99 mutation were AD, compared to 41.7% (5/12) of the isolates with a Lys99 mutation in group B.
Sequence comparison of the P1 isolates obtained from different newborns showed that each newborn whose isolates had more than three mutations had a unique mutation pattern. The synonymous- and nonsynonymous-mutation rates in the VP1 region were estimated from the slope of the regression lines using the numbers of mutations of all isolates shed by an individual (Table 6). In both groups A and B, the average nonsynonymous-mutation rate was higher than the average synonymous-mutation rate (Table 6, “Total”). For group A, however, this difference was not significant (P = 0.107). When these rates were observed separately for newborns shedding P1 isolates without (−Δ99) and with (+Δ99) replacement of lysine 99, the isolates from both groups A and B with replacement of residue 99 had a ∼3-fold higher nonsynonymous-mutation rate than isolates without this replacement (P = 0.000) (Table 6, −Δ99 and +Δ99; see also supplemental material). The synonymous-mutation rates, on the other hand, did not differ significantly between isolates with and without replacement of residue 99 in the mOPV1 study group. In the tOPV study group this difference was significant (P = 0.005), but the number of isolates with replacement of this residue was small in this study group.
TABLE 6.
Substitution rates for VP1 capsid sequences of isolates without and with replacement of residue 99a
| Group and type of mutation | Rate (10−2 mutation/site/yr) (variation)b
|
Pc | ||
|---|---|---|---|---|
| Total | −Δ99 | +Δ99 | ||
| A | ||||
| Synonymous | 2.4 (0.20) | 2.4 (0.16) | 2.5 (0.28) | 0.949 |
| Nonsynonymous | 3.9 (0.27) | 2.2 (0.04) | 7.6 (0.64) | 0.000 |
| B | ||||
| Synonymous | 1.9 (0.09) | 1.6 (0.07) | 4.7 (0.22) | 0.005 |
| Nonsynonymous | 3.3 (0.14) | 2.6 (0.09) | 8.8 (0.16) | 0.000 |
−Δ99 and +Δ99, without and with replacement of residue 99, respectively.
For group A, the P values for the significance of differences between rates of synonymous and nonsynonymous mutations were 0.107 for all isolates, 0.078 for −Δ99 isolates, and 0.028 for +Δ99 isolates. For group B, the corresponding P values were 0.013, 0.033, and 0.085, respectively.
Calculated for −Δ99 and +Δ99 isolates using the one-way ANOVA test (alpha = 0.05).
The titers of neutralizing antibody to P1 at day 28 did not differ significantly between newborns shedding Sabin-like and AD P1 isolates without replacement of lysine 99 and newborns shedding P1 isolates with replacement of residue 99 after administration of the challenge dose (mean titer, 6.76 log 2 [range, 2.5 to 10.17 log 2]) (P by a one-way ANOVA test, 0.081 for group A and 0.114 for group B).
Shedding of P2 and P3 isolates.
In the tOPV study group, 130 newborns seroconverted to P2 and 53 seroconverted to P3 (Table 4); of these, 47 newborns shed P2 virus (36%) and 26 shed P3 virus (49%) at any time, while 39 shed P2 virus (30%) and 24 shed P3 virus (45%) at the time of the challenge dose. This is consistent with the preferential replication of P2 following the first dose of tOPV. In the VP1 regions of the P2 isolates, four preferred sites of mutation were observed: Ile143 (a major determinant of attenuation), Lys169 (antigenic site II), Asn171, and Arg103 (Table 7). VP1 sequencing of the P3 isolates revealed the presence of two preferred sites of mutation: Ala54 and Thr6 (an important determinant of attenuation) (Table 8).
TABLE 7.
Amino acid mutations in the VP1 regions of P2 isolates
| Group | Antigenic characterization by ITD-ELISA | Isolate | Amino acid mutation at the following position in VP1:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 101 | 102 | 103 | 104 | 105 | 142 | 143a | 144 | 168 | 169 | 170 | 171 | |||
| Sabin 2 | A | S | R | L | F | Y | I | D | G | K | W | N | ||
| WT | K | R | ||||||||||||
| A | DR/NR | E382 | T | |||||||||||
| B | NR | E2135 | T | E | ||||||||||
| B | NR | E1815 | K | T | ||||||||||
| B | DR | E2214 | N | |||||||||||
| B | NR | E2400 | D | R | ||||||||||
| B | DR | E808 | ||||||||||||
| B | NR | E1630 | T | |||||||||||
| B | DR | E514 | K | T | ||||||||||
| A | SL | E216 | ||||||||||||
| A | SL | E1560 | D | |||||||||||
| A | SL | E561 | N | |||||||||||
| A | SL | E137 | S | |||||||||||
| B | SL | E1104 | N | D | ||||||||||
| B | SL | E2363 | T | D | ||||||||||
| B | SL | E958 | T | |||||||||||
| B | SL | E983 | T | E | ||||||||||
| B | SL | E700 | E | |||||||||||
| B | SL | E196 | D | |||||||||||
| B | SL | E74 | E | |||||||||||
| B | SL | E13 | K | T | ||||||||||
| B | SL | E972 | V | |||||||||||
| B | SL | E483 | N | E | ||||||||||
| B | SL | E2148 | T | D | ||||||||||
| B | SL | E694 | S | D | ||||||||||
A determinant of attenuation.
TABLE 8.
Amino acid mutations in the VP1 regions of P3 isolates
| Group | Antigenic characterization by ITD-ELISA | Isolate | Amino acid mutation at the following position in VP1:
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| 4 | 5 | 6a | 7 | 53 | 54 | 55 | |||
| Sabin 3 | D | L | T | S | L | A | P | ||
| WT | I | ||||||||
| A | NR | E1305 | G | ||||||
| B | NR | E229 | I | V | |||||
| A | SL | E2352 | I | V | |||||
| A | SL | E127 | G | ||||||
| A | SL | E428 | T | ||||||
| A | SL | E28 | G | I | |||||
| A | SL | E21 | |||||||
| B | SL | E1771 | I | ||||||
| B | SL | E643 | I | T | |||||
| B | SL | E299 | |||||||
A determinant of attenuation.
Although newborns of group A were vaccinated solely with mOPV1, 30 newborns seroconverted against P2 (13%) and 22 against P3 (9.5%) (Table 4). In addition, 21 of these newborns also shed a P2 isolate (2 of which were AD) and 6 newborns shed a P3 isolate (3 of which were AD). Seroconversion of these newborns to P2 and/or P3 is consistent with the isolation of P2 and P3 viruses and makes contamination of these fecal samples highly unlikely. This finding strongly implies that the study population was exposed secondarily to vaccine-related viruses from the families or communities outside the vaccine doses administered as part of the study protocol. The effect is to overestimate the rate of seroconversion and shedding that can be attributed to the vaccines administered.
DISCUSSION
This study describes the antigenic and genetic variety of the vaccine viruses shed by newborns participating in a clinical trial in Egypt in 2005 and 2006 to determine the relative efficacy of mOPV1 versus tOPV in newborns (15). The participants were enrolled in the study immediately after birth and did not participate in routine vaccination activities. Therefore, their initial immunity was provided solely by maternal antibody that could be directly measured, which made possible the comparative evaluation of the immunogenicity of mOPV1 versus tOPV. The average titers of maternal antibodies against P1, P2, and P3 were similar among the newborns of the mOPV1 and tOPV study groups (15). The superiority of mOPV1 in humoral response was also observed in mucosal response. Priming of newborns with mOPV1 resulted in reduced shedding of vaccine virus compared to priming with tOPV.
Unexpectedly, another finding was that the proportion of AD vaccine viruses shed by the newborns was much higher than that previously observed, especially in the mOPV1 study group. Although it is likely that no strictly comparable study, with a birth dose of mOPV1 in an Egyptian study population and using these methods of virus characterization, has been done, from which an expectation could be derived, it is clear that these rates have not been observed elsewhere in unselected healthy individuals or through the global AFP (acute flaccid paralysis) surveillance activities (27). The majority of these AD isolates appeared to be derived from the mOPV1 challenge dose provided at the age of 28 days. Replacement of lysine 60 in the VP3 capsid region, as described by previous studies, explains the AD character observed in this study (3, 42). This residue is located in the loop of antigenic site III and is also involved in the interaction between the virus and the cellular receptor CD155 (2, 22). This effect was compensated for in one strain by an additional mutation of Ala59 to valine as shown previously (42).
Excretion of more AD P1 isolates, coupled with an overall lower rate of shedding, suggests high immunogenic pressure at some of the antigenic sites in the mOPV1 study group. This, however, could not be specifically correlated with antibody titers, and the lack of correlation implies the presence of other parameters that may be more predictive for the shedding of AD P1 isolates.
In both study groups, the majority of the AD P1 isolates were shed by newborns who did not seroconvert to P1 after the birth dose (mOPV1 or tOPV). The majority of these newborns in the tOPV study group (88%), however, did seroconvert to P2 and/or P3 after the birth dose, indicating replication of P2 and P3 in the gut prior to the mOPV1 challenge dose (Table 4). Interference between these replicating P2 and P3 strains and the mOPV1 challenge strain might result in a more limited and less widespread replication of P1 within the tOPV study group and possibly in a lower likelihood of AD P1 isolates than within the mOPV1 study group (3). The amounts of P1 virus in the stool samples could be quantified in order to study the replication of P1 viruses in both study groups.
VDPVs are presently defined as viruses with more than 1% sequence difference in the VP1 gene, compared to the corresponding OPV strain. This degree of sequence variation has been equated to approximately 1 year of replication after infection and, by inference, also after the administration of an OPV dose (28). This period is considerably longer than the 4- to 6-week excretion period of the immunocompetent vaccinees of this study (8). In the present study, the mean overall VP1 synonymous- and nonsynonymous-mutation rates are severalfold higher than those reported previously from circulating viruses or those in immunodeficient patients (18, 25, 26, 28, 31, 42, 43). It has been reported previously that rapid reversion of attenuating amino acids in the capsid region can occur in primary vaccinees (14); however, the overall rate of mutations in a large study using molecular characterization by genomic sequencing of a large number of isolates has only rarely been determined.
Isolates with mutations at residue 99 had nonsynonymous-mutation rates ∼3 times higher than those of isolates without this mutation and were inclined to have an AD character, whereas P1 isolates with Thr106 did not have significantly more nonsynonymous changes than isolates without this mutation. Previous studies have shown that residue 99, which forms a trypsin cleavage site in antigenic site I of the Sabin virus, is a hot spot for change in the VP1 region of VDPVs (10, 13, 17, 19, 23, 24, 28, 29, 34, 42, 43). It is not clear what role mutation of residue 99 could play in the evolution of VDPVs. The observation of a much faster accumulation of nonsynonymous mutations shortly after vaccination in this study should be considered in estimating the duration of replication or circulation of Sabin viruses and VDPVs. The difference in the age and dose of origin for P1 virus shedding complicates the comparison of mutation rates between the different groups. The limited number of children shedding at each time point also limits the determination of difference between intervals and rates of mutation. These study design differences and observed rates may influence differences in the nonsynonymous/synonymous mutation ratios at the different time points and should be kept in mind when these ratios are compared. Regardless, these observations imply that VDPVs analyzed in the past might not be as old as expected.
It should be noted that the P2 VDPV observed in this study was not detected by the current WHO-recommended screening algorithm for VDPVs and was detected only by sequencing of the complete VP1 gene. This finding reiterates the need for new assays for the rapid screening of P2 (and P3) OPV isolates for VDPV detection. The present WHO-recommended VDPV screening methods still detect all known VDPVs of serotype 1.
There are some limitations in this study. The degree to which the study participants would be exposed to poliovirus through family or community contacts during the 8-week study period was unknown at the start of the study. This would have the effect of reducing the specificity of the measured outcomes that could be attributed to the study vaccines. Somewhat surprisingly, 52 newborns vaccinated with mOPV1 at birth seroconverted to P2 and/or P3 during their first month, indicating significant exposure of the study participants to P2 and P3 vaccine strains through their environments. Alternatively, some of these children may represent false-positive seroconversions because of the method of determining seroconversion relative to a projected decay of maternal antibody (15). As noted in Table 4, there were also 11 children whose dose-specific seroconversion could not be determined. On the basis of previous studies, the proportion of misclassification due to these factors is expected to be small but could not be verified within this study design. Because of these observations, we cannot entirely rule out the possibility that some of the AD viruses isolated from the mOPV1 group were secondary infections, but the rates of AD isolates were much higher than the numbers of P2/P3 isolates observed. The significant difference in the proportion of AD P1 isolates shed between the mOPV1 and tOPV study groups, however, cannot be easily ascribed to the circulation of P1 in the environment, because newborns of both groups should have been equally likely to come into contact with environmentally derived P1 isolates, and the proportion of AD P1 viruses would be expected to be no higher than the ∼5% observed in AFP surveillance.
Another limitation in the study design is the limited number of stool specimens following the birth dose compared to the more frequent sampling following the challenge dose. As noted previously, this almost ensures that the rate of shedding from the birth dose is underestimated compared to that the challenge dose. In addition, it makes the two groups of children inherently noncomparable for a variety of factors. Even though many of these can be addressed directly, by subdividing groups as presented, absolute specificity cannot be ensured. These limitations, however, do not seem to negate the primary conclusions of the study, even if the explanation of the causation is ambiguous.
In summary, this study shows the reduced shedding of mOPV1 challenge virus associated with seroconversion to a birth dose of mOPV1 compared to a comparable dose of tOPV. Unexpectedly, however, vaccination with mOPV1 at birth results in the excretion of a significantly higher proportion of AD P1 viruses, possibly due to antigenic pressure and a longer and more widespread replication of P1 viruses than occurs in the presence of heterologous strains in the case of vaccination with tOPV. In the mOPV1 study group, more isolates with mutations of VP1 amino acid residue 99 occurred, which resulted in a significant increase in additional nonsynonymous mutations. It is uncertain what role this could play in the evolution of VDPVs, but it does suggest the possibility of additional studies of this population. Results from this study, however, also showed that newborns vaccinated with mOPV1 at birth were better protected against P1 and had a clearly lower excretion rate of Sabin viruses after the challenge dose than newborns vaccinated with tOPV at birth. In turn, a high mucosal protection against P1 reduces shedding of vaccine-derived P1 and therefore reduces the risk for transmission and evolution of VDPVs. In areas with good vaccination coverage and thus a high level of mucosal protection, transmission of vaccine virus is expected to be minimal and vaccination with mOPV1 could be very effective in eradicating the last polioviruses of type 1. In areas with low vaccination coverage and a high number of susceptible individuals, vaccination with mOPV1 could potentially lead to transmission of AD P1 isolates like those observed in this study and could possibly increase the risk for the development of VDPVs. However, until now, regular surveillance activities (based on AFP and environmental surveillance) have not provided evidence for a contribution of vaccination with mOPV1 to the evolution and circulation of VDPVs in Egypt after this study or in countries where mOPV1 has been used for several years already (Nigeria, India) (6, 7). This study describes accelerated genetic and antigenic changes observed following vaccination of newborns and may provide some insight into the earliest steps in the process of the generation of VDPVs.
Supplementary Material
Acknowledgments
This work was supported by the World Health Organization.
We acknowledge the contribution of the laboratory staff at the National Institute for Public Health and the Environment (Ron Altena, Edin Jusic, and Gökhan Uslu) and the staff at the Enterovirus Diagnostic Laboratory at the CDC (Debbie Moore, Naomi Dybdahl-Sissoko, and Barbara Anderson). We also thank Hein Boot for critical reading of the manuscript.
Footnotes
Published ahead of print on 10 June 2009.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Agol, V. I. 2006. Vaccine-derived polioviruses. Biologicals 34103-108. [DOI] [PubMed] [Google Scholar]
- 2.Belnap, D. M., B. M. McDermott, Jr., D. J. Filman, N. Cheng, B. L. Trus, H. J. Zuccola, V. R. Racaniello, J. M. Hogle, and A. C. Steven. 2000. Three-dimensional structure of poliovirus receptor bound to poliovirus. Proc. Natl. Acad. Sci. USA 9773-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boot, H. J., J. Sonsma, F. van Nunen, F. Abbink, T. G. Kimman, and A. M. Buisman. 2007. Determinants of monovalent oral polio vaccine mutagenesis in vaccinated elderly people. Vaccine 254706-4714. [DOI] [PubMed] [Google Scholar]
- 4.Cáceres, V. M., and R. W. Sutter. 2001. Sabin monovalent oral polio vaccines: review of past experiences and their potential use after polio eradication. Clin. Infect. Dis. 33531-541. [DOI] [PubMed] [Google Scholar]
- 5.CDC. 2007. Progress toward interruption of wild poliovirus transmission—worldwide, January 2006-May 2007. MMWR Morb. Mortal. Wkly. Rep. 56682-685. [PubMed] [Google Scholar]
- 6.CDC. 2006. Progress toward poliomyelitis eradication—India, January 2005-June 2006. MMWR Morb. Mortal. Wkly. Rep. 57772-776. [PubMed] [Google Scholar]
- 7.CDC. 2008. Progress toward poliomyelitis eradication—Nigeria, January 2007-August 12, 2008. MMWR Morb. Mortal. Wkly. Rep. 57942-946. [PubMed] [Google Scholar]
- 8.CDC. 2006. Update on vaccine-derived polioviruses. MMWR Morb. Mortal. Wkly. Rep. 551093-1097. [PubMed] [Google Scholar]
- 9.CDC. 2007. Update on vaccine-derived polioviruses—worldwide, January 2006-August 2007. MMWR Morb. Mortal. Wkly. Rep. 56996-1001. [PubMed] [Google Scholar]
- 10.Cherkasova, E. A., E. A. Korotkova, M. L. Yakovenko, O. E. Ivanova, T. P. Eremeeva, K. M. Chumakov, and V. I. Agol. 2002. Long-term circulation of vaccine-derived poliovirus that causes paralytic disease. J. Virol. 766791-6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chumakov, K. M., L. P. Norwood, M. L. Parker, E. M. Dragunsky, Y. X. Ran, and I. S. Levenbook. 1992. RNA sequence variants in live poliovirus vaccine and their relation to neurovirulence. J. Virol. 66966-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crainic, R., and O. Kew. 1993. Evolution and polymorphism of poliovirus genomes. Biologicals 21379-384. [DOI] [PubMed] [Google Scholar]
- 13.Dedepsidis, E., I. Karakasiliotis, et al. 2006. Detection of unusual mutation within the VP1 region of different re-isolates of poliovirus Sabin vaccine. Virus Genes 33183-191. [DOI] [PubMed] [Google Scholar]
- 14.Dunn, G., N. T. Begg, N. Cammack, and P. D. Minor. 1990. Virus excretion and mutation by infants following primary vaccination with live oral polio vaccine from two sources. J. Med. Virol. 3292-95. [DOI] [PubMed] [Google Scholar]
- 15.el-Sayed, N., Y. el-Gamal, A. A. Abbassy, I. Seoud, M. Salama, A. Kandeel, E. Hossny, A. Shawky, H. A. Hussein, M. A. Pallansch, H. G. van der Avoort, A. H. Burton, M. Sreevatsava, P. Malankar, M. H. Wahdan, and R. W. Sutter. 2008. Monovalent type 1 oral poliovirus vaccine in newborns. N. Engl. J. Med. 3591655-1665. [DOI] [PubMed] [Google Scholar]
- 16.Fine, P. E., R. W. Sutter, and W. A. Orenstein. 2001. Stopping a polio outbreak in the post-eradication era. Dev. Biol. (Basel) 105129-147. [PubMed] [Google Scholar]
- 17.Fricks, C. E., J. P. Icenogle, and J. M. Hogle. 1985. Trypsin sensitivity of the Sabin strain of type 1 poliovirus: cleavage sites in virions and related particles. J. Virol. 54856-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gavrilin, G. V., E. A. Cherkasova, G. Y. Lipskaya, O. M. Kew, and V. I. Agol. 2000. Evolution of circulating wild poliovirus and of vaccine-derived poliovirus in an immunodeficient patient: a unifying model. J. Virol. 747381-7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Georgescu, M. M., J. Balanant, A. Macadam, D. Otelea, M. Combiescu, A. A. Combiescu, R. Crainic, and F. Delpeyroux. 1997. Evolution of the Sabin type 1 poliovirus in humans: characterization of strains isolated from patients with vaccine-associated paralytic poliomyelitis. J. Virol. 717758-7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grassly, N. C., J. Wenger, S. Durrani, S. Bahl, J. M. Deshpande, R. W. Sutter, D. L. Heymann, and R. B. Aylward. 2007. Protective efficacy of a monovalent oral type 1 poliovirus vaccine: a case-control study. Lancet 3691356-1362. [DOI] [PubMed] [Google Scholar]
- 21.Hall, T. A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 4195-98. [Google Scholar]
- 22.He, Y., V. D. Bowman, et al. 2000. Interaction of the poliovirus receptor with poliovirus. Proc. Natl. Acad. Sci. USA 9779-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He, Y., S. Mueller, P. R. Chipman, C. M. Bator, X. Peng, V. D. Bowman, S. Mukhopadhyay, E. Wimmer, R. J. Kuhn, and M. G. Rossmann. 2003. Complexes of poliovirus serotypes with their common cellular receptor, CD155. J. Virol. 774827-4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Icenogle, J. P., P. D. Minor, M. Ferguson, and J. M. Hogle. 1986. Modulation of humoral response to a 12-amino-acid site on the poliovirus virion. J. Virol. 60297-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jorba, J., R. Campagnoli, L. De, and O. Kew. 2008. Calibration of multiple poliovirus molecular clocks covering an extended evolutionary range. J. Virol. 824429-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kew, O., V. Morris-Glasgow, M. Landaverde, C. Burns, J. Shaw, Z. Garib, J. Andre, E. Blackman, C. J. Freeman, J. Jorba, R. Sutter, G. Tambini, L. Venczel, C. Pedreira, F. Laender, H. Shimizu, T. Yoneyama, T. Miyamura, H. van der Avoort, M. S. Oberste, D. Kilpatrick, S. Cochi, M. Pallansch, and C. de Quadros. 2002. Outbreak of poliomyelitis in Hispaniola associated with circulating type 1 vaccine-derived poliovirus. Science 296356-359. [DOI] [PubMed] [Google Scholar]
- 27.Kew, O. M., R. W. Sutter, E. M. de Gourville, W. R. Dowdle, and M. A. Pallansch. 2005. Vaccine-derived polioviruses and the endgame strategy for global polio eradication. Annu. Rev. Microbiol. 59587-635. [DOI] [PubMed] [Google Scholar]
- 28.Kew, O. M., R. W. Sutter, B. K. Nottay, M. J. McDonough, D. R. Prevots, L. Quick, and M. A. Pallansch. 1998. Prolonged replication of a type 1 vaccine-derived poliovirus in an immunodeficient patient. J. Clin. Microbiol. 362893-2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laassri, M., E. Dragunsky, J. Enterline, T. Eremeeva, O. Ivanova, K. Lottenbach, R. Belshe, and K. Chumakov. 2005. Genomic analysis of vaccine-derived poliovirus strains in stool specimens by combination of full-length PCR and oligonucleotide microarray hybridization. J. Clin. Microbiol. 432886-2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lentz, K. N., A. D. Smith, S. C. Geisler, S. Cox, P. Buontempo, A. Skelton, J. DeMartino, E. Rozhon, J. Schwartz, V. Girijavallabhan, J. O'Connell, and E. Arnold. 1997. Structure of poliovirus type 2 Lansing complexed with antiviral agent SCH48973: comparison of the structural and biological properties of three poliovirus serotypes. Structure 5961-978. [DOI] [PubMed] [Google Scholar]
- 31.Liu, H. M., D. P. Zheng, L. B. Zhang, M. S. Oberste, M. A. Pallansch, and O. M. Kew. 2000. Molecular evolution of a type 1 wild-vaccine poliovirus recombinant during widespread circulation in China. J. Virol. 7411153-11161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Macadam, A. J., S. R. Pollard, G. Ferguson, R. Skuce, D. Wood, J. W. Almond, and P. D. Minor. 1993. Genetic basis of attenuation of the Sabin type 2 vaccine strain of poliovirus in primates. Virology 19218-26. [DOI] [PubMed] [Google Scholar]
- 33.Manor, Y., R. Handsher, T. Halmut, M. Neuman, A. Bobrov, H. Rudich, A. Vonsover, L. Shulman, O. Kew, and E. Mendelson. 1999. Detection of poliovirus circulation by environmental surveillance in the absence of clinical cases in Israel and the Palestinian Authority. J. Clin. Microbiol. 371670-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martín, J., K. Odoom, G. Tuite, G. Dunn, N. Hopewell, G. Cooper, C. Fitzharris, K. Butler, W. W. Hall, and P. D. Minor. 2004. Long-term excretion of vaccine-derived poliovirus by a healthy child. J. Virol. 7813839-13847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Modlin, J. F. 1995. Poliomyelitis and poliovirus immunization, p. 195-220. In H. A. Rotbart (ed.), Human enterovirus infections. American Society for Microbiology, Washington, DC.
- 36.R Development Core Team. 2008. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
- 37.Rozas, J., and R. Rozas. 1999. DnaSP, version 3: an integrated program for molecular population genetics and molecular evolution analysis. Bioinformatics 15174-175. [DOI] [PubMed] [Google Scholar]
- 38.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. ClustalW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 224673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Avoort, H. G., B. P. Hull, T. Hovi, M. A. Pallansch, O. M. Kew, R. Crainic, D. J. Wood, M. N. Mulders, and A. M. van Loon. 1995. Comparative study of five methods for intratypic differentiation of polioviruses. J. Clin. Microbiol. 332562-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.WHO. 2004. Polio laboratory manual, 4th ed., p. 101-108. WHO, Geneva, Switzerland.
- 41.WHO. 2004. Polio laboratory manual, 4th ed., p. 88-91. WHO, Geneva, Switzerland.
- 42.Yakovenko, M. L., E. A. Cherkasova, G. V. Rezapkin, O. E. Ivanova, A. P. Ivanov, T. P. Eremeeva, O. Y. Baykova, K. M. Chumakov, and V. I. Agol. 2006. Antigenic evolution of vaccine-derived polioviruses: changes in individual epitopes and relative stability of the overall immunological properties. J. Virol. 802641-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang, C. F., L. De, B. P. Holloway, M. A. Pallansch, and O. M. Kew. 1991. Detection and identification of vaccine-related polioviruses by the polymerase chain reaction. Virus Res. 20159-179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.