Abstract
Despite many efforts to develop AIDS vaccines eliciting virus-specific T-cell responses, whether induction of these memory T cells by vaccination before human immunodeficiency virus (HIV) exposure can actually contribute to effective T-cell responses postinfection remains unclear. In particular, induction of HIV-specific memory CD4+ T cells may increase the target cell pool for HIV infection because the virus preferentially infects HIV-specific CD4+ T cells. However, virus-specific CD4+ helper T-cell responses are thought to be important for functional CD8+ cytotoxic-T-lymphocyte (CTL) induction in HIV infection, and it has remained unknown whether HIV-specific memory CD8+ T cells induced by vaccination without HIV-specific CD4+ T-cell help can exert effective responses after virus exposure. Here we show the impact of CD8+ T-cell memory induction without virus-specific CD4+ T-cell help on the control of a simian immunodeficiency virus (SIV) challenge in rhesus macaques. We developed a prophylactic vaccine by using a Sendai virus (SeV) vector expressing a single SIV Gag241-249 CTL epitope fused with enhanced green fluorescent protein (EGFP). Vaccination resulted in induction of SeV-EGFP-specific CD4+ T-cell and Gag241-249-specific CD8+ T-cell responses. After a SIV challenge, the vaccinees showed dominant Gag241-249-specific CD8+ T-cell responses with higher effector memory frequencies in the acute phase and exhibited significantly reduced viral loads. These results demonstrate that virus-specific memory CD8+ T cells induced by vaccination without virus-specific CD4+ T-cell help could indeed facilitate SIV control after virus exposure, indicating the benefit of prophylactic vaccination eliciting virus-specific CTL memory with non-virus-specific CD4+ T-cell responses for HIV control.
Virus-specific T-cell responses are crucial for controlling human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) replication (3, 4, 12, 20, 28, 36, 37). Therefore, a great deal of effort has been exerted to develop AIDS vaccines eliciting virus-specific T-cell responses (23, 27, 30, 47), but whether this approach actually results in HIV control remains unclear (1, 6). It is important to determine which T-cell responses need to be induced by prophylactic vaccination for HIV control after virus exposure.
Because HIV preferentially infects HIV-specific CD4+ T cells (5), induction of HIV-specific memory CD4+ T cells by vaccination may increase the target cell pool for HIV infection and could enhance viral replication (42). However, CD4+ helper T-cell responses are important for functional CD8+ cytotoxic-T-lymphocyte (CTL) induction (11, 40, 43, 46), and it has remained unknown whether HIV-specific memory CD8+ T cells induced by vaccination with non-virus-specific CD4+ T-cell help (but without HIV-specific CD4+ T-cell help) can exert effective responses after virus exposure. Indeed, the real impact of prophylactic induction of CTL memory itself on HIV replication has not been well documented thus far.
We previously developed a prophylactic AIDS vaccine consisting of DNA priming followed by boosting with a recombinant Sendai virus (SeV) vector expressing SIVmac239 Gag (26). Evaluation of this vaccine's efficacy against a SIVmac239 challenge in Burmese rhesus macaques showed that some vaccinees contained SIV replication whereas unvaccinated animals developed AIDS (15, 27). In particular, vaccination consistently resulted in control of SIV replication in those animals possessing the major histocompatibility complex class I (MHC-I) haplotype 90-120-Ia. Gag206-216 (IINEEAADWDL) and Gag241-249 (SSVDEQIQW) epitope-specific CD8+ T-cell responses were shown to be involved in SIV control in these vaccinated macaques (14, 16).
In the present study, focusing on CD8+ T-cell responses directed against one of these epitopes, we have evaluated the efficacy of a vaccine expressing the Gag241-249 epitope fused with enhanced green fluorescent protein (EGFP) against a SIVmac239 challenge in 90-120-Ia-positive rhesus macaques. The animals exhibited this single-epitope-specific CD8+ T-cell response and SeV-EGFP-specific CD4+ T-cell responses after vaccination and showed rapid, dominant induction of potent secondary Gag241-249-specific CD8+ T-cell responses after a SIV challenge. Plasma viral loads in these vaccinees were significantly reduced compared to those of naive controls. These results indicate that induction of CD8+ T-cell memory without virus-specific CD4+ T-cell help by prophylactic vaccination can result in effective CD8+ T-cell responses after virus exposure.
MATERIALS AND METHODS
Animal experiments.
Burmese rhesus macaques (Macaca mulatta) possessing the MHC-I haplotype 90-120-Ia were divided into three groups: unvaccinated group I (n = 6), control-vaccinated group II (n = 6), and Gag236-250-vaccinated group III (n = 6). The MHC-I haplotype was determined by reference strand-mediated conformation analysis as described previously (2, 27, 44). Macaque R06-019, administered nonspecific immunoglobulin G 1 week after a SIV challenge, and previously reported macaque R02-007 (15) were included in group I. pGag236-250-EGFP-N1 DNA expressing a Gag236-250-EGFP fusion protein was constructed from pEGFP-N1 DNA (BD, Tokyo, Japan). The fusion protein was designed to have 31 amino acids including SIVmac239 Gag236-250-sequences (IAGTTSSVDEQIQWM) added to the amino-terminal portion of EGFP (Fig. 1A). The group III macaques received 5 mg of pGag236-250-EGFP-N1 DNA intramuscularly and 6 weeks later received a single intranasal boost with 6 × 109 cell infectious units of F deletion-containing, replication-defective SeV (24) expressing the Gag236-250-EGFP fusion protein (F[−]SeV-Gag236-250-EGFP). The group II macaques were primed with pEGFP-N1 DNA and boosted with F(−)SeV-EGFP instead. Approximately 3 months after the boost, these animals and the unvaccinated group I animals were challenged intravenously with 1,000 50% tissue culture infective doses of SIVmac239 (17). All animals were maintained in accordance with the guidelines for animal experiments performed at the National Institute of Infectious Diseases (32).
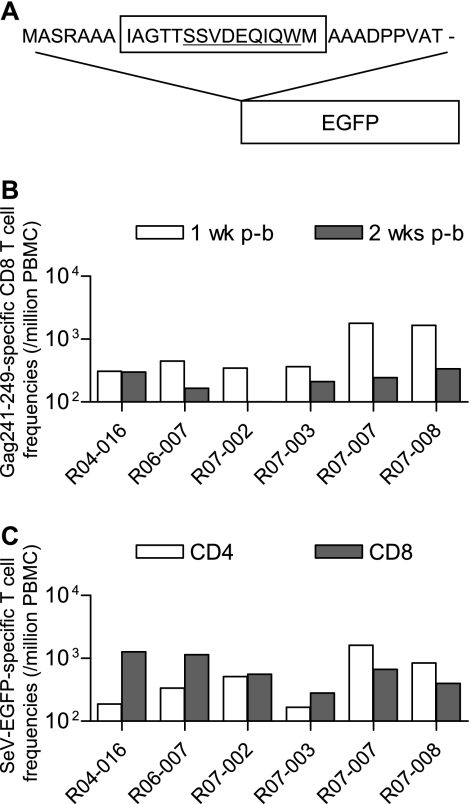
FIG. 1.
Gag241-249-specific CD8+ T-cell induction by prophylactic vaccination. (A) Schema of the cDNA construct encoding the Gag236-250-EGFP fusion protein. A DNA fragment that encodes a 31-mer peptide including the Gag236-250 sequence was introduced into the 5′ end of the EGFP cDNA. (B) Gag241-249-specific CD8+ T-cell frequencies 1 (open boxes) and 2 weeks (closed boxes) after F(−)SeV-Gag236-250-EGFP boosting in group III macaques. (C) SeV-EGFP-specific CD4+ (open boxes) or CD8+ (closed boxes) T-cell frequencies 2 weeks after F(−)SeV-Gag236-250-EGFP boosting in group III macaques. p-b, postboost.
Analysis of virus-specific CD8+ T-cell responses.
We measured virus-specific CD8+ T-cell levels by flow cytometric analysis of gamma interferon (IFN-γ) induction after specific stimulation as described previously (15, 27). In brief, peripheral blood mononuclear cells (PBMCs) were cocultured with autologous herpesvirus papio-immortalized B-lymphoblastoid cell lines pulsed with 1 μM SIVmac239 Gag241-249 or Gag206-216 peptides for Gag241-249-specific or Gag206-216-specific stimulation. Alternatively, PBMCs were cocultured with B-lymphoblastoid cell lines infected with vesicular stomatitis virus G protein-pseudotyped SIVGP1 for SIV-specific stimulation. The pseudotyped virus was obtained by cotransfection of COS-1 cells with a vesicular stomatitis virus G protein expression plasmid and env and nef deletion-containing simian-human immunodeficiency virus molecular clone (SIVGP1) DNA (26, 41). Intracellular IFN-γ staining was performed with a CytofixCytoperm kit (BD) and fluorescein isothiocyanate-conjugated anti-human CD4, peridinin chlorophyll protein-conjugated anti-human CD8, allophycocyanin (APC)-conjugated anti-human CD3, and phycoerythrin (PE)-conjugated anti-human IFN-γ monoclonal antibodies (BD). Specific T-cell levels were calculated by subtracting nonspecific IFN-γ+ T-cell frequencies from those after peptide-specific or SIV-specific stimulation. Specific T-cell levels lower than 100 per million PBMCs were considered negative.
Analysis of Gag241-249-specific cytolytic CD8+ T-cell responses.
We analyzed Gag241-249-specific induction of IFN-γ and CD107a in CD8+ T cells. PBMCs were stained with custom-made, PE-conjugated Gag241-249 epitope-Mamu-A*90120-5 tetrameric complexes, Gag241-249-A*90120-5 tetramers (Medical and Biological Laboratories Co. Ltd., Nagoya, Japan) (45), for 15 min at 37°C and subsequently incubated with anti-human CD107a antibody (BD) for 6 h in the absence or presence of 1 μM Gag241-249 peptide for unstimulated controls or Gag241-249-specific stimulation. In both cultures, anti-human CD28 and anti-human CD49d antibodies (5 μg/ml) (BD) were added for costimulation and monensin (BD) and brefeldin A (Sigma-Aldrich, Tokyo, Japan) were used for inhibition of cytokine secretion. Immunostaining was performed with a CytofixCytoperm kit and the following monoclonal antibodies: fluorescein isothiocyanate-conjugated anti-human perforin (MABTECH), peridinin chlorophyll protein-conjugated anti-human CD4 (BD), APC-conjugated anti-human granzyme B (Invitrogen, Tokyo, Japan), PE-cyanine 7 (PE-Cy7)-conjugated anti-human IFN-γ (BD), APC-Cy7-conjugated anti-human CD3 (BD), energy-coupled-dye-conjugated anti-human CD69 (Beckman Coulter, Tokyo, Japan), Alexa700-conjugated anti-human CD8 (BD), and anti-human CD107a conjugated with Pacific Blue with a Zeon mouse immunoglobulin G1 labeling kit (Invitrogen). Flow cytometric analysis was performed with the FACSaria system (BD). The data were analyzed with FlowJo (version 8.2; TreeStar Inc., Ashland, OR), FACSDiva (BD), PESTLE (version 1.5.4), and SPICE (version 4.1.6) software.
Statistical analysis.
Statistical analysis of plasma viral loads in the acute phase (at the peak and week 5) was performed with R version 2.7.1 (R Development Core Team; http://www.R-project.org/). Data were log transformed, and a two-tailed one-way analysis of variance, followed by the Shaffer sequentially rejective method of multiple-comparison analysis (39), was performed to estimate differences among groups I, II, and III with overall significance levels set to α = 0.05 (two tailed). Statistical analysis of set point plasma viral loads was performed by the nonparametric Kruskal-Wallis test with the sequentially rejective pairwise Mann-Whitney exact test, because we did not assume residual normality and homoscedasticity in set point viral loads, which were mostly below the lower limit of detection in group III animals. Antigen-specific T-cell frequencies were log transformed and compared by unpaired two-tailed t test with significance levels set at P < 0.05, and correlation was analyzed by using Prism software version 4.03 (GraphPad Software, Inc., San Diego, CA).
RESULTS
Gag241-249-specific CD8+ T-cell induction following prophylactic vaccination.
Eighteen Burmese rhesus macaques possessing MHC-I haplotype 90-120-Ia were divided into three groups of six animals each (Table 1). Group I received no vaccination, group II received a control vaccine, and group III received a vaccine eliciting Gag241-249-specific CD8+ T-cell responses. We refer to groups I and II as naive controls in the present study. We constructed a plasmid DNA (pGag236-250-EGFP-N1) and an F deletion-containing SeV (F[−]SeV-Gag236-250-EGFP) vector both expressing an SIVmac239 Gag236-250 (IAGTTSSVDEQIQWM)-EGFP fusion protein to be used for group III vaccination (Fig. 1A). SeV proteins and EGFP have no amino acid sequence identity with SIVmac239. These group III animals received a single intramuscular pGag236-250-EGFP DNA injection, followed by a single intranasal boost with the F(−)SeV-Gag236-250-EGFP vector. Group II animals were administered pEGFP-N1 DNA and the F(−)SeV-EGFP vector, both expressing EGFP instead of Gag236-250-EGFP, as a control vaccine.
TABLE 1.
Macaques used in this study
| Group | Animal identification codes | Vaccinationa |
|---|---|---|
| I | R02-007, R06-037, R07-001, R07-004, R07-009, R06-019 | None |
| II | R02-008, R05-026, R06-004, R06-014, R06-040, R07-006 | Control vaccination [pEGFP-N1 DNA prime, F(−)SeV-EGFP boost] |
| III | R04-016, R06-007, R07-002, R07-003, R07-007, R07-008 | Gag241-249-specific vaccination [pGag236-250-EGFP-N1 DNA prime, F[(−)SeV-Gag236-250-EGFP boost] |
All animals were challenged with SIVmac239.
We measured the antigen-specific CD8+ T-cell responses in these macaques 1 or 2 weeks after the SeV boost by detection of specific IFN-γ induction. All group III macaques showed efficient Gag241-249-specific CD8+ T-cell induction after the F(−)SeV-Gag236-250-EGFP boost (Fig. 1B). In these animals, we also confirmed SeV-EGFP-specific CD8+ and CD4+ T-cell responses (Fig. 1C) but did not detect Gag206-216-specific CD8+ T-cell responses, which are dominantly induced in 90-120-Ia-positive macaques by Gag-expressing SeV vaccination (14). We have never found Gag236-250-specific CD4+ T-cell responses in any previously examined animals, and as expected, analyses with the Gag236-250 peptide did not detect Gag236-250-specific CD4+ T-cell responses in any of the group III animals in the present study. In group II animals, we detected SeV-EGFP-specific T-cell responses but not Gag236-250-specific T-cell responses after the F(−)SeV-EGFP boost (data not shown).
Control of an SIV challenge in vaccinees.
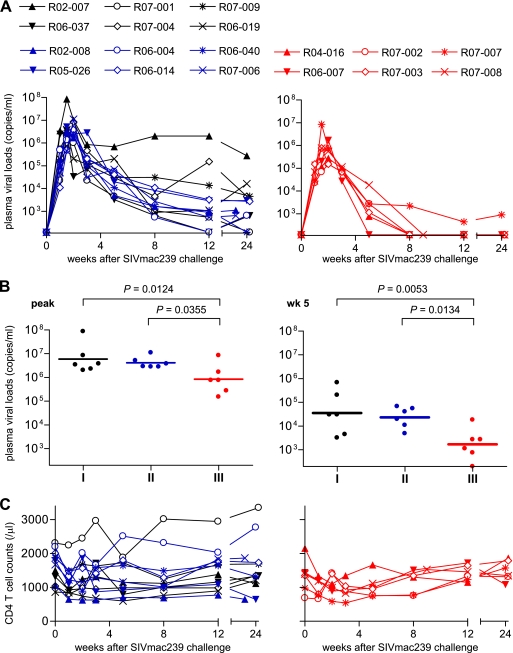
Group I (unvaccinated), II (control-vaccinated), and III (Gag236-250-vaccinated) macaques were challenged intravenously with SIVmac239. Plasma viral loads in these animals were examined after the challenge (Fig. 2A). Most of the group I and II animals failed to contain SIV replication, although plasma viremia became undetectable at week 12 in one animal in group I (R06-037) and one in group II (R06-004). No significant differences were observed between groups I and II in plasma viral loads at the peak, at week 5, at week 12, or around week 24 after the challenge. In contrast, most group III animals contained SIV replication; plasma viral loads became undetectable after week 5 in five of the six animals (Fig. 2A). Plasma viral loads in these animals were significantly lower than those in unvaccinated group I and those in control-vaccinated group II at the peak, at week 5, and at the set point (Fig. 2B). Thus, the prophylactic vaccination inducing Gag241-249 single-epitope-specific CD8+ T-cell responses resulted in a significant reduction of peak and subsequent viral loads after the SIV challenge. No significant difference in peripheral CD4+ T-cell counts was observed among these three groups (Fig. 2C).
FIG. 2.
Plasma viral loads and peripheral CD4+ T-cell counts after a SIV challenge. (A) Changes in plasma viral loads (SIV gag RNA copies/ml plasma) in unvaccinated group I animals (black lines in the left panel), control-vaccinated group II animals (blue lines in the left panel), and Gag236-250-vaccinated group III animals (red lines in the right panel) after a SIVmac239 challenge. Plasma viral loads were determined as described previously (27). The lower limit of detection is approximately 4 × 102 copies/ml. (B) Comparisons of plasma viral loads in groups I (n = 6), II (n = 6), and III (n = 6) at the peak (left panel) and at week 5 (right panel). The bar indicates the geometric mean of each group. Viral loads at the peak and at week 5 in group III were significantly lower than in group I (P = 0.0124 at the peak and P = 0.0053 at week 5) and group II (P = 0.0355 at the peak and P = 0.0134 at week 5). There were no significant differences between groups I and II either at the peak or at week 5 (P = 0.6047 at the peak and P = 0.6536 at week 5). Set point viral loads in group III were significantly lower than those in group I and group II at week 12 by nonparametric analysis (P = 0.3939 between I and II, P = 0.0152 between I and III, and P = 0.0152 between II and III; P = 0.1797 between I and II, P = 0.0260 between I and III, and P = 0.0411 between II and III around week 24). (C) Changes in peripheral CD4+ T-cell counts (per μl) in groups I (black lines) and II (blue lines) in the left panel and in group III (red lines) in the right panel after a SIVmac239 challenge.
Dominant Gag241-249-specific CD8+ T-cell responses in vaccinees after a SIV challenge.
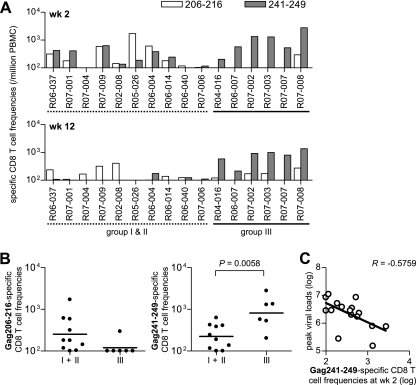
We assessed virus-specific CD8+ T-cell responses at weeks 2 and 12 after a SIV challenge by measuring antigen-specific IFN-γ induction. Gag241-249-specific CD8+ T-cell responses were undetectable or marginal in some naive controls (group I and II) but were efficiently induced in all of the group III animals (Fig. 3A). In most of the naive controls, Gag206-216-specific CD8+ T-cell responses were induced equivalently or more efficiently than Gag241-249-specific CD8+ responses, whereas all of the group III animals showed dominant induction of Gag241-249-specific CD8+ T-cell responses. In these group III animals, Gag206-216-specific CD8+ T-cell responses were inefficient but frequencies of CD8+ T cells exhibiting Gag241-249-specific IFN-γ induction were significantly higher than in naive controls at week 2 (Fig. 3B) and week 12. Gag241-249-specific CD8+ T-cell frequencies at week 2 inversely correlated with peak viral loads (Fig. 3C).
FIG. 3.
Gag epitope-specific CD8+ T-cell frequencies after a SIV challenge. (A) Frequencies of CD8+ T cells (per million PBMCs) showing Gag206-216-specific (open boxes) or Gag241-249-specific (closed boxes) IFN-γ induction in naive controls and group III macaques at week 2 (upper panel) and week 12 (lower panel). (B) Comparison of the Gag206-216-specific (left panel) or Gag241-249-specific (right panel) CD8+ T-cell frequencies in naive controls (n = 10) and group III animals (n = 6) at week 2. The bar indicates the geometric mean of each group. Frequencies of Gag241-249-specific (P = 0.0058) but not Gag206-216-specific (P = 0.0922) CD8+ T cells in group III were significantly higher than in naive controls. The Gag241-249-specific frequencies at week 12 in group III were significantly higher than those in naive controls (P < 0.0001). (C) Analysis of the correlation between Gag241-249-specific CD8+ T-cell frequencies (log) at week 2 and peak plasma viral loads (log). An inverse correlation is shown (P = 0.0196, R = −0.5759). Samples from macaques R02-007 and R06-019 in group I were unavailable for this analysis.
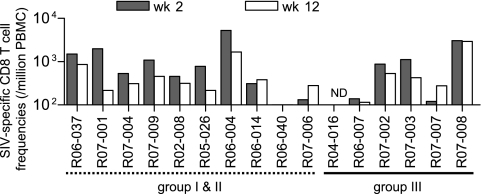
We also tested SIV-specific CD8+ T-cell responses in these animals (Fig. 4). We used env and nef deletion-containing simian-human immunodeficiency virus molecular clone DNA SIVGP1 containing the genes encoding SIVmac239 Gag, Pol, Vif, Vpx, and a part of Vpr and measured the frequencies of CD8+ T cells responding to SIVGP1-transduced cells (referred to as SIV-specific CD8+ T cells) as described previously (15). Naive controls (groups I and II) and vaccinees (group III) were found to possess similar levels of SIV-specific CD8+ T cells at week 2 and week 12.
FIG. 4.
SIV-specific CD8+ T-cell frequencies after a SIV challenge. SIV-specific CD8+ T-cell frequencies (per million PBMCs) in naive controls and group III macaques at week 2 (closed boxes) and week 12 (open boxes) are shown. ND, not determined.
In our previous study (27), all of the 90-120-Ia-positive macaques vaccinated with Gag-expressing SeV contained SIV replication with rapid selection of a gag mutation (GagL216S), resulting in escape from Gag206-216-specific CD8+ T-cell recognition at week 5, implicating Gag206-216-specific CD8+ T-cell responses (rather than Gag241-249-specific CD8+ T-cell responses) in viral control. In the present study, however, five of six Gag236-250-vaccinated animals controlled SIV replication and had undetectable set point viremia without selection of gag mutation over 5 weeks (data not shown). No gag mutation was selected at week 5 in naive controls, either. These results indicate that in the group III macaques, dominantly induced Gag241-249-specific CD8+ T-cell responses in the acute phase play an important role in this vaccine-based SIV control.
Higher Gag241-249-specific effector memory CD8+ T-cell frequencies in vaccinees.
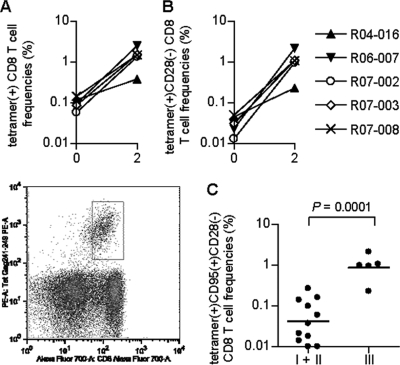
We then examined Gag241-249-specific CD8+ T-cell frequencies in these macaques by using PE-conjugated Gag241-249-A*90120-5 tetramers. In group III animals, Gag241-249-specific tetramer+ CD8+ T cells were still detectable just before the SIV challenge, and their frequencies increased greatly after the challenge; most of the vaccinees exhibited a >10-fold increase at week 2 compared to prechallenge levels (Fig. 5A). Increases in tetramer+ CD28− CD8+ T-cell frequencies after a challenge were especially marked (>30-fold) (Fig. 5B). Indeed, within the tetramer+ cells, the ratio of CD28− cells increased after a challenge and these cells became predominant at week 2. Analysis of an effector memory subset delineated by the CD95+ CD28− phenotype (29, 34) revealed significantly higher frequencies of Gag241-249-specific tetramer+ CD95+ CD28− CD8+ T cells in group III than in naive controls (Fig. 5C). These results suggest efficient responses of Gag241-249-specific CD8+ T cells with effector function in the acute phase in group III animals.
FIG. 5.
Frequencies of Gag241-249-specific CD8+ T cells detected by Gag241-249-Mamu-A*90120-5 tetramers after a SIV challenge. (A) Frequencies of Gag241-249-Mamu-A*90120-5 tetramer+ cells within CD8+ T cells in group III animals before a challenge (week 0) or at week 2 after a challenge. A representative dot plot gated on CD3+ lymphocytes for determining tetramer+ CD8+ T cells (x axis, CD8; y axis, tetramer) in macaque R07-008 is shown in the lower panel. (B) Tetramer+ CD28− cell frequencies in CD8+ T cells in group III animals at weeks 0 and 2. Data on tetramer+ CD95+ CD28− CD8+ T-cell frequencies at week 0 are unavailable. (C) Tetramer+ CD95+ CD28− CD8+ T-cell frequencies in naive controls (groups I and II) and group III animals at week 2. The bar indicates the geometric mean of each group. The frequencies in group III were significantly higher than those in naive controls (P = 0.0001 by unpaired t test). Samples from macaques R06-019 in group I and R07-007 in group III were unavailable for this analysis.
Gag241-249-specific cytolytic CD8+ T-cell responses in vaccinees.
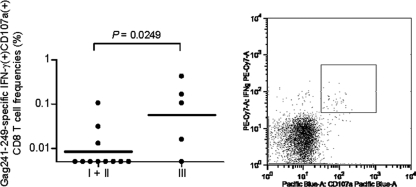
To further investigate the cytolytic quality of Gag241-249-specific CD8+ T-cell responses after a challenge, we examined Gag241-249-specific induction of CD107a (a degranulation marker), which is related to cytolytic activity (21, 38), in CD8+ T cells at week 2. Frequencies of CD8+ T cells exhibiting Gag241-249-specific induction of CD107a, as well as IFN-γ, within the CD8+ T-cell pool were significantly higher in group III than in naive controls (P = 0.0249 by unpaired t test) (Fig. 6). One animal, R04-016, in group III did not show Gag241-249-specific CD107a+ IFN-γ+ CD8+ T-cell responses, but further analysis revealed that this animal had Gag241-249-specific granzyme B+ IFN-γ+ CD8+ T cells. Indeed, group III animals had significantly higher frequencies of Gag241-249-specific IFN-γ+ CD8+ T cells producing CD107a, granzyme B, or perforin (P = 0.0076; data not shown). These results indicate efficient induction of Gag241-249-specific CD8+ T cells with higher cytolytic activity in the acute phase in group III animals.
FIG. 6.
Gag241-249-specific cytolytic CD8+ T-cell frequencies at week 2 after a challenge. PBMCs were cultured in the absence or the presence of the Gag241-249 peptide for unstimulated controls or Gag241-249-specific stimulation, and the frequencies of CD8+ T cells exhibiting Gag241-249-specific induction of both IFN-γ and CD107a in the total CD8+ T cells were examined. The bar indicates the geometric mean of each group. The frequencies in group III were significantly higher than those in naive controls (P = 0.0249 by unpaired t test). The right panel is a representative dot plot showing the CD107a (x axis) and IFN-γ (y axis) responses in CD8+ T cells in macaque R07-008 after Gag241-249-specific stimulation. Samples from macaques R06-019 in group I and R07-007 in group III were unavailable for this analysis.
DISCUSSION
In the present study, induction of CD8+ T cells specific for a single Gag241-249 epitope by prophylactic vaccination resulted in a significant reduction of plasma viral loads after a SIV challenge. Even if vaccines are designed to express multiple antigens, of the vaccine-induced CD8+ T cells generated, at most one or only a few epitope-specific cells may recognize the incoming HIV because of viral diversity and host MHC polymorphisms (10). Our finding, however, implies that even a CD8+ T-cell memory response to a single epitope which can recognize the incoming HIV could facilitate HIV control.
Group III macaques showed more effective CD8+ T-cell responses than did naive controls after a SIV challenge. Our previous trial of a vaccine inducing Gag-specific T-cell responses resulted in SIV control in 90-120-Ia-positive macaques with rapid selection of the GagL216S mutation escaping from Gag206-216-specific CD8+ T-cell recognition at week 5 (27). In contrast, the Gag236-250 vaccination resulted in SIV control without gag mutation selection over 5 weeks in the present study, reflecting the fact that, rather than Gag206-216-specific CD8+ T-cell responses, dominantly induced Gag241-249-specific CD8+ T-cell responses played a central role in the reduction of viral loads in the acute phase. These results suggest that this vaccination approach altered the dominance pattern of CD8+ T-cell responses and resulted in dominant induction of effective Gag241-249-specific CD8+ T-cell responses in the acute phase after a SIV challenge, facilitating a reduction in peak viral loads. Selection of vaccine epitopes for induction of CD8+ T-cell responses might be important for viral control because the antiviral efficacy of CD8+ T cells could be affected by MHC-I-restricted target epitopes (10, 19, 25, 35).
Gag241-249-specific CD8+ T-cell induction by prophylactic vaccination resulted in higher frequencies of these T-cell responses during the acute phase after the SIV challenge. The induction of Gag241-249-specific effector memory CD8+ T cells was especially marked. We did not examine polyfunctionality, but analyses of a cytolytic marker, CD107a, indicated higher frequencies of Gag241-249-specific cytolytic CD8+ T-cell responses, implying that these T cells originating from vaccine-induced memory may have higher cytolytic activity in the acute phase. These results suggest that group III animals with Gag241-249-specific memory CD8+ T cells showed induction of a high magnitude of Gag241-249-specific CD8+ T cells with effector function after a SIV challenge, resulting in reduction of viral loads in the acute phase.
In this study, some 90-120-Ia-positive unvaccinated macaques showed lower viral loads. However, in our previous studies with Burmese rhesus macaques (reference 15 and unpublished data), all unvaccinated 90-120-Ia-negative animals failed to contain a SIVmac239 challenge and animals, including vaccinees, that failed to control SIVmac239 replication developed AIDS in 1 to 4 years; even R-90-120 descendants possessing the MHC-I haplotype 90-120-Ib but not 90-120-Ia (both 90-120-Ia and 90-120-Ib are derived from breeder R-90-120) showed high viral loads. Additionally, 90-120-Ia-positive animals failed to control the replication of SIVmac239 carrying CTL escape mutations (16). Thus, a SIVmac239 challenge of Burmese rhesus macaques mostly results in persistent viremia and progression to AIDS but some 90-120-Ia-positive animals may show lower viral loads due to 90-120-Ia-associated SIV-specific CTL responses. However, a previously reported 90-120-Ia-positive unvaccinated macaque, R02-007, developed AIDS around 3 years after a SIVmac239 challenge. Furthermore, two of the 90-120-Ia-positive vaccinees that controlled a SIVmac239 challenge but showed reappearance of viremia around 1 year later developed AIDS (15). Thus, it is inferred that the majority of 90-120-Ia-positive unvaccinated macaques develop AIDS after a SIVmac239 challenge. Several MHC-I alleles are known to be associated with lower viral loads in HIV and SIV infections, and potent CTLs directed against these MHC-I-restricted epitopes have been implicated in the suppression of viral replication (7, 8, 9, 10, 13, 18, 22, 31, 33, 48). The Gag241-249-specific CTL may also be naturally potent (10, 16), but the impact of memory induction of even these potent CTLs on viral control has not yet been determined. Thus, this is the first study documenting the benefit of single-epitope-specific memory CD8+ T-cell induction by prophylactic vaccination for HIV/SIV control. Further analysis with a vaccine expressing a single helper epitope, as well as a CTL epitope, would contribute to evaluation of the impact of HIV/SIV-specific CD4+ T-cell memory induction on HIV/SIV replication.
Because CCR5+ memory CD4+ T cells, especially HIV-specific CD4+ T cells, are themselves targets of this virus, whether virus-specific CD4+ T-cell induction by prophylactic vaccination can result in effective virus-specific CD4+ T-cell responses postinfection and contribute to HIV control remains unclear. On the other hand, it has been unknown whether HIV-specific memory CD8+ T cells induced by vaccination without HIV-specific CD4+ T-cell help can elicit effective responses after virus exposure. In the present study, the pGag236-250-EGFP/F(−)SeV-Gag236-250-EGFP vaccination elicited Gag241-249-specific CD8+ T-cell responses without SIV-specific CD4+ T-cell help but possibly with EGFP-specific or SeV-specific CD4+ T-cell help; i.e., SeV-EGFP-specific CD4+ T cells would confer cognate help for Gag241-249-specific CD8+ T-cell induction. The Gag241-249-specific memory CD8+ T cells induced by prophylactic vaccination without SIV-specific CD4+ T-cell help but with non-SIV-specific CD4+ T-cell responses responded efficiently to a SIV challenge, showing dominant Gag241-249-specific CD8+ T-cell responses resulting in SIV control; infection-induced SIV-specific CD4+ T-cell responses may be involved in Gag241-249-specific CD8+ T-cell induction postinfection. Therefore, this study documents that prophylactic vaccination eliciting virus-specific CD8+ T-cell memory even without virus-specific CD4+ T-cell responses (but with cognate non-virus-specific CD4+ T-cell responses) can facilitate SIV control after a challenge.
Taken together, the present study demonstrates that induction of single-epitope-specific CD8+ T-cell memory without virus-specific CD4+ T-cell help by prophylactic vaccination can result in dominant potent CD8+ T-cell responses and control of SIV replication after a challenge. These results imply possible HIV control by prophylactic vaccination eliciting virus-specific CD8+ T-cell memory with non-virus-specific CD4+ T-cell help and provide valuable insights into AIDS vaccine development.
Acknowledgments
This work was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology and grants from the Ministry of Health, Labor, and Welfare in Japan. The animal experiments were conducted through the Cooperative Research Program in the Tsukuba Primate Research Center, National Institute of Biomedical Innovation, with the help of the Corporation for Production and Research of Laboratory Primates.
We thank H. Akari, Y. Yasutomi, F. Ono, A. Hiyaoka, K. Komatsuzaki, K. Oto, K. Ishikawa, and T. Nakasone for their assistance in animal experiments; DNAVEC Corp. for providing SeV vectors; M. Roederer for providing the PESTLE and SPICE software; and Y. Tsunetsugu-Yokota, H. Igarashi, A. Kimura, T. Naruse, M. Miyazawa, K. Mori, N. Yamamoto, A. Nomoto, and Y. Nagai for their help.
Footnotes
Published ahead of print on 8 July 2009.
REFERENCES
- 1.Anonymous. 2007. Cold shower for AIDS vaccines. Nat. Med. 13:1389-1390. [DOI] [PubMed] [Google Scholar]
- 2.Argüello, J. R., A. M. Little, A. L. Pay, D. Gallardo, I. Rojas, S. G. Marsh, J. M. Goldman, and J. A. Madrigal. 1998. Mutation detection and typing of polymorphic loci through double-strand conformation analysis. Nat. Genet. 18:192-194. [DOI] [PubMed] [Google Scholar]
- 3.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brander, C., and B. D. Walker. 1999. T lymphocyte responses in HIV-1 infection: implication for vaccine development. Curr. Opin. Immunol. 11:451-459. [DOI] [PubMed] [Google Scholar]
- 5.Douek, D. C., J. M. Brenchley, M. R. Betts, D. R. Ambrozak, B. J. Hill, Y. Okamoto, J. P. Casazza, J. Kuruppu, K. Kunstman, S. Wolinsky, Z. Grossman, M. Dybul, A. Oxenius, D. A. Price, M. Connors, and R. A. Koup. 2002. HIV preferentially infects HIV-specific CD4+ T cells. Nature 417:95-98. [DOI] [PubMed] [Google Scholar]
- 6.Feinberg, M. B., and J. P. Moore. 2002. AIDS vaccine models: challenging challenge viruses. Nat. Med. 8:207-210. [DOI] [PubMed] [Google Scholar]
- 7.Friedrich, T. C., E. J. Dodds, L. J. Yant, L. Vojnov, R. Rudersdorf, C. Cullen, D. T. Evans, R. C. Desrosiers, B. R. Mothe, J. Sidney, A. Sette, K. Kunstman, S. Wolinsky, M. Piatak, J. Lifson, A. L. Hughes, N. Wilson, D. H. O'Connor, and D. I. Watkins. 2004. Reversion of CTL escape-variant immunodeficiency viruses in vivo. Nat. Med. 10:275-281. [DOI] [PubMed] [Google Scholar]
- 8.Goulder, P. J., M. Bunce, P. Krausa, K. McIntyre, S. Crowley, B. Morgan, A. Edwards, P. Giangrande, R. E. Phillips, and A. J. McMichael. 1996. Novel, cross-restricted, conserved, and immunodominant cytotoxic T lymphocyte epitopes in slow progressors in HIV type 1 infection. AIDS Res. Hum. Retrovir. 12:1691-1698. [DOI] [PubMed] [Google Scholar]
- 9.Goulder, P. J., and D. I. Watkins. 2004. HIV and SIV CTL escape: implications for vaccine design. Nat. Rev. Immunol. 4:630-640. [DOI] [PubMed] [Google Scholar]
- 10.Goulder, P. J. R., and D. I. Watkins. 2008. Impact of MHC class I diversity on immune control of immunodeficiency virus replication. Nat. Rev. Immunol. 8:619-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janssen, E. M., E. E. Lemmens, T. Wolfe, U. Christen, M. G. von Herrath, and S. P. Schoenberger. 2003. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421:852-856. [DOI] [PubMed] [Google Scholar]
- 12.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189:991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaslow, R. A., M. Carrington, R. Apple, L. Park, A. Muñoz, A. J. Saah, J. J. Goedert, C. Winkler, S. J. O'Brien, C. Rinaldo, R. Detels, W. Blattner, J. Phair, H. Erlich, and D. L. Mann. 1996. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat. Med. 2:405-411. [DOI] [PubMed] [Google Scholar]
- 14.Kawada, M., H. Igarashi, A. Takeda, T. Tsukamoto, H. Yamamoto, S. Dohki, M. Takiguchi, and T. Matano. 2006. Involvement of multiple epitope-specific cytotoxic T-lymphocyte responses in vaccine-based control of simian immunodeficiency virus replication in rhesus macaques. J. Virol. 80:1949-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawada, M., T. Tsukamoto, H. Yamamoto, A. Takeda, H. Igarashi, D. I. Watkins, and T. Matano. 2007. Long-term control of simian immunodeficiency virus replication with central memory CD4+ T-cell preservation after nonsterile protection by a cytotoxic T-lymphocyte-based vaccine. J. Virol. 81:5202-5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawada, M., T. Tsukamoto, H. Yamamoto, N. Iwamoto, K. Kurihara, A. Takeda, C. Moriya, H. Takeuchi, H. Akari, and T. Matano. 2008. Gag-specific cytotoxic T lymphocyte-based control of primary simian immunodeficiency virus replication in a vaccine trial. J. Virol. 82:10199-10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kestler, H. W., III, D. J. Ringler, K. Mori, D. L. Panicali, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65:651-662. [DOI] [PubMed] [Google Scholar]
- 18.Kiepiela, P., A. J. Leslie, I. Honeyborne, D. Ramduth, C. Thobakgale, S. Chetty, P. Rathnavalu, C. Moore, K. J. Pfafferott, L. Hilton, P. Zimbwa, S. Moore, T. Allen, C. Brander, M. M. Addo, M. Altfeld, I. James, S. Mallal, M. Bunce, L. D. Barber, J. Szinger, C. Day, P. Klenerman, J. Mullins, B. Korber, H. M. Coovadia, B. D. Walker, and P. J. Goulder. 2004. Dominant influence of HLA-B in mediating the potential co-evolution of HIV and HLA. Nature 432:769-775. [DOI] [PubMed] [Google Scholar]
- 19.Kiepiela, P., K. Ngumbela, C. Thobakgale, D. Ramduth, I. Honeyborne, E. Moodley, S. Reddy, C. de Pierres, Z. Mncube, N. Mkhwanazi, K. Bishop, M. van der Stok, K. Nair, N. Khan, H. Crawford, R. Payne, A. Leslie, J. Prado, A. Prendergast, J. Frater, N. McCarthy, C. Brander, G. H. Learn, D. Nickle, C. Rousseau, H. Coovadia, J. I. Mullins, D. Heckerman, B. D. Walker, and P. Goulder. 2007. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat. Med. 13:46-53. [DOI] [PubMed] [Google Scholar]
- 20.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamoreaux, L., M. Roederer, and R. Koup. 2006. Intracellular cytokine optimization and standard operating procedure. Nat. Protoc. 1:1507-1516. [DOI] [PubMed] [Google Scholar]
- 22.Leslie, A. J., K. J. Pfafferott, P. Chetty, R. Draenert, M. M. Addo, M. Feeney, Y. Tang, E. C. Holmes, T. Allen, J. G. Prado, M. Altfeld, C. Brander, C. Dixon, D. Ramduth, P. Jeena, S. A. Thomas, A. St. John, T. A. Roach, B. Kupfer, G. Luzzi, A. Edwards, G. Taylor, H. Lyall, G. Tudor-Williams, V. Novelli, J. Martinez-Picado, P. Kiepiela, B. D. Walker, and P. J. Goulder. 2004. HIV evolution: CTL escape mutation and reversion after transmission. Nat. Med. 10:282-289. [DOI] [PubMed] [Google Scholar]
- 23.Letvin, N. L., J. R. Mascola, Y. Sun, D. A. Gorgone, A. P. Buzby, L. Xu, Z. Y. Yang, B. Chakrabarti, S. S. Rao, J. E. Schmitz, D. C. Montefiori, B. R. Barker, F. L. Bookstein, and G. J. Nabel. 2006. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science 312:1530-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li, H. O., Y. F. Zhu, M. Asakawa, H. Kuma, T. Hirata, Y. Ueda, Y. S. Lee, M. Fukumura, A. Iida, A. Kato, Y. Nagai, and M. Hasegawa. 2000. A cytoplasmic RNA vector derived from nontransmissible Sendai virus with efficient gene transfer and expression. J. Virol. 74:6564-6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loffredo, J. T., A. T. Bean, D. R. Beal, E. J. León, G. E. May, S. M. Piaskowski, J. R. Furlott, J. Reed, S. K. Musani, E. G. Rakasz, T. C. Friedrich, N. A. Wilson, D. B. Allison, and D. I. Watkins. 2008. Patterns of CD8+ immunodominance may influence the ability of Mamu-B*08-positive macaques to naturally control simian immunodeficiency virus SIVmac239 replication. J. Virol. 82:1723-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matano, T., M. Kano, H. Nakamura, A. Takeda, and Y. Nagai. 2001. Rapid appearance of secondary immune responses and protection from acute CD4 depletion after a highly pathogenic immunodeficiency virus challenge in macaques vaccinated with a DNA-prime/Sendai viral vector-boost regimen. J. Virol. 75:11891-11896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matano, T., M. Kobayashi, H. Igarashi, A. Takeda, H. Nakamura, M. Kano, C. Sugimoto, K. Mori, A. Iida, T. Hirata, M. Hasegawa, T. Yuasa, M. Miyazawa, Y. Takahashi, M. Yasunami, A. Kimura, D. H. O'Connor, D. I. Watkins, and Y. Nagai. 2004. Cytotoxic T lymphocyte-based control of simian immunodeficiency virus replication in a preclinical AIDS vaccine trial. J. Exp. Med. 199:1709-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matano, T., R. Shibata, C. Siemon, M. Connors, H. C. Lane, and M. A. Martin. 1998. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J. Virol. 72:164-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mattapallil, J. J., N. L. Letvin, and M. Roederer. 2004. T-cell dynamics during acute SIV infection. AIDS 18:13-23. [DOI] [PubMed] [Google Scholar]
- 30.McMichael, A. J., and T. Hanke. 2003. HIV vaccines 1983-2003. Nat. Med. 9:874-880. [DOI] [PubMed] [Google Scholar]
- 31.Migueles, S. A., M. S. Sabbaghian, W. L. Shupert, M. P. Bettinotti, F. M. Marincola, L. Martino, C. W. Hallahan, S. M. Selig, D. Schwartz, J. Sullivan, and M. Connors. 2000. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc. Natl. Acad. Sci. USA 97:2709-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Institute of Infectious Diseases. 2007. Guides for animal experiments performed at National Institute of Infectious Diseases. National Institute of Infectious Diseases, Tokyo, Japan. (In Japanese.)
- 33.O'Connor, D. H., B. R. Mothe, J. T. Weinfurter, S. Fuenger, W. M. Rehrauer, P. Jing, R. R. Rudersdorf, M. E. Liebl, K. Krebs, J. Vasquez, E. Dodds, J. Loffredo, S. Martin, A. B. McDermott, T. M. Allen, C. Wang, G. G. Doxiadis, D. C. Montefiori, A. Hughes, D. R. Burton, D. B. Allison, S. M. Wolinsky, R. Bontrop, L. J. Picker, and D. I. Watkins. 2003. Major histocompatibility complex class I alleles associated with slow simian immunodeficiency virus disease progression bind epitopes recognized by dominant acute-phase cytotoxic-T-lymphocyte responses. J. Virol. 77:9029-9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pitcher, C. J., S. I. Hagen, J. M. Walker, R. Lum, B. L. Mitchell, V. C. Maino, M. K. Axthelm, and L. J. Picker. 2002. Development and homeostasis of T cell memory in rhesus macaques. J. Immunol. 168:29-43. [DOI] [PubMed] [Google Scholar]
- 35.Reynolds, M. R., A. M. Weiler, K. L. Weisgrau, S. M. Piaskowski, J. R. Furlott, J. T. Weinfurter, M. Kaizu, T. Soma, E. J. León, C. MacNair, D. P. Leaman, M. B. Zwick, E. Gostick, S. K. Musani, D. A. Price, T. C. Friedrich, E. G. Rakasz, N. A. Wilson, A. B. McDermott, R. Boyle, D. B. Allison, D. R. Burton, W. C. Koff, and D. I. Watkins. 2008. Macaques vaccinated with live-attenuated SIV control replication of heterologous virus. J. Exp. Med. 205:2537-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenberg, E. S., J. M. Billingsley, A. M. Caliendo, S. L. Boswell, P. E. Sax, S. A. Kalams, and B. D. Walker. 1997. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 278:1447-1450. [DOI] [PubMed] [Google Scholar]
- 37.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 38.Seder, R. A., P. A. Darrah, and M. Roederer. 2008. T-cell quality in memory and protection: implications for vaccine design. Nat. Rev. Immunol. 8:247-258. [DOI] [PubMed] [Google Scholar]
- 39.Shaffer, J. P. 1986. Modified sequentially rejective multiple test procedures. J. Am. Stat. Assoc. 81:826-831. [Google Scholar]
- 40.Shedlock, D. J., and H. Shen. 2003. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 300:337-339. [DOI] [PubMed] [Google Scholar]
- 41.Shibata, R., F. Maldarelli, C. Siemon, T. Matano, M. Parta, G. Miller, T. Fredrickson, and M. A. Martin. 1997. Infection and pathogenicity of chimeric simian-human immunodeficiency viruses in macaques: determinants of high virus loads and CD4 cell killing. J. Infect. Dis. 176:362-373. [DOI] [PubMed] [Google Scholar]
- 42.Staprans, S. I., A. P. Barry, G. Silvestri, J. T. Safrit, N. Kozyr, B. Sumpter, H. Nguyen, H. McClure, D. Montefiori, J. I. Cohen, and M. B. Feinberg. 2004. Enhanced SIV replication and accelerated progression to AIDS in macaques primed to mount a CD4 T cell response to the SIV envelope protein. Proc. Natl. Acad. Sci. USA 101:13026-13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun, J. C., and M. J. Bevan. 2003. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 300:339-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanaka-Takahashi, Y., M. Yasunami, T. Naruse, K. Hinohara, T. Matano, K. Mori, M. Miysazawa, M. Honda, Y. Yasutomi, Y. Nagai, and A. Kimura. 2007. Reference strand-mediated conformation analysis (RSCA)-based typing of multiple alleles in the rhesus macaque MHC class I Mamu-A and Mamu-B loci. Electrophoresis 28:918-924. [DOI] [PubMed] [Google Scholar]
- 45.Tsukamoto, T., S. Dohki, T. Ueno, M. Kawada, A. Takeda, M. Yasunami, T. Naruse, A. Kimura, M. Takiguchi, and T. Matano. 2008. Determination of a major histocompatibility complex class I restricting simian immunodeficiency virus Gag241-249 epitope. AIDS 22:993-994. [DOI] [PubMed] [Google Scholar]
- 46.Vaccari, M., J. Mattapallil, K. Song, W. P. Tsai, A. Hryniewicz, D. Venzon, M. Zanetti, K. A. Reimann, M. Roederer, and G. Franchini. 2008. Reduced protection from simian immunodeficiency virus SIVmac251 infection afforded by memory CD8+ T cells induced by vaccination during CD4+ T-cell deficiency. J. Virol. 82:9629-9638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson, N. A., J. Reed, G. S. Napoe, S. Piaskowski, A. Szymanski, J. Furlott, E. J. Gonzalez, L. J. Yant, N. J. Maness, G. E. May, T. Soma, M. R. Reynolds, E. Rakasz, R. Rudersdorf, A. B. McDermott, D. H. O'Connor, T. C. Friedrich, D. B. Allison, A. Patki, L. J. Picker, D. R. Burton, J. Lin, L. Huang, D. Patel, G. Heindecker, J. Fan, M. Citron, M. Horton, F. Wang, X. Liang, J. W. Shiver, D. R. Casimiro, and D. I. Watkins. 2006. Vaccine-induced cellular immune responses reduce plasma viral concentrations after repeated low-dose challenge with pathogenic simian immunodeficiency virus SIVmac239. J. Virol. 80:5875-5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yant, L. J., T. C. Friedrich, R. C. Johnson, G. E. May, N. J. Maness, A. M. Enz, J. D. Lifson, D. H. O'Connor, M. Carrington, and D. I. Watkins. 2006. The high-frequency major histocompatibility complex class I allele Mamu-B*17 is associated with control of simian immunodeficiency virus SIVmac239 replication. J. Virol. 80:5074-5077. [DOI] [PMC free article] [PubMed] [Google Scholar]