Abstract
Ebolavirus (EBOV) is the etiological agent of a severe hemorrhagic fever with a high mortality rate. The spike glycoprotein (GP) is believed to be one of the major determinants of virus pathogenicity. In this study, we demonstrated the molecular mechanism responsible for the downregulation of surface markers caused by EBOV GP expression. We showed that expression of mature GP on the plasma membrane results in the masking of cellular surface proteins, including major histocompatibility complex class I. Overexpression of GP also results in the masking of certain antigenic epitopes on GP itself, causing an illusory effect of disappearance from the plasma membrane.
Ebolavirus (EBOV) and Marburgvirus form the family Filoviridae, a group of enveloped, negative-strand RNA viruses responsible for severe hemorrhagic fevers in humans. The EBOV genome is about 19 kb long and codes for seven structural proteins and at least two nonstructural proteins (17). A single spike glycoprotein (GP) of EBOV is responsible for cell targeting and virus entry (8, 15). GP bears a signal peptide at the N terminus, which targets the protein to the endoplasmic reticulum (GPer). Glycosylation in the endoplasmic reticulum (ER) and later in the Golgi apparatus contributes to approximately half of the mass of GP, with O-linked glycans conferring a mucin-like property to the C terminus of the GP1 subunit (5, 17). Mature GP represents a complex of the disulfide-linked subunits GP1 and GP2 (19).
Transient expression of EBOV GP causes cytotoxicity and modifications in the surface expression of cellular proteins (2, 6, 13, 14, 22). The cytotoxicity caused by GP has been proposed to play a major role in the high-level pathogenicity of EBOV (13). An increased endosomal uptake induced by the interaction of GP with cellular dynamin was suggested as a mechanism for the downregulation of surface proteins. Interestingly, the same mechanism was proposed to play a role in the simultaneous disappearance of GP, namely GP self-downregulation (14). The importance of a mucin-like domain for these cytotoxic properties has been emphasized in several publications (13, 14). Since moderate levels of GP expression, as occur in cells stably expressing GP or during natural EBOV infection, do not cause early cell rounding, the role of GP cytotoxicity in EBOV pathogenesis remains unclear (1).
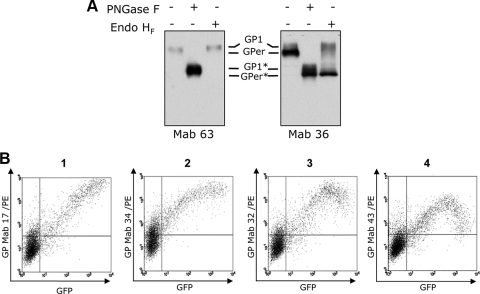
In this study, we first investigated GP downregulation by using a bank of 87 anti-GP monoclonal antibodies. The recognition pattern of antibodies was assessed by Western blot analysis of cells expressing GP (Fig. 1A). The antibody panel was divided into two major groups based on the patterns of GP recognition. Figure 1A shows one example of each group. Antibodies from group A recognized exclusively the GP1 subunit (approximately 140 kDa), and antibodies from group B recognized GP1 and GPer (approximately 110 kDa) (19).
FIG. 1.
Analysis of EBOV GP expression by using a panel of monoclonal antibodies (Mab). (A) Western blot analysis. HEK293T cells were transfected with pIRES2-EBOVGP/GFP, collected 20 h posttransfection, and lysed, and samples were treated with endoglycosidases endo-β-N-acetylglucosaminidase H (endo HF) or peptide N-glycosidase F (PNGase F), followed by Western blot analysis using monoclonal antibodies from group A (Mab 63) and group B (Mab 36). GP1 and GPer are indicated. GP1 and GPer digested with endoglycosidases are indicated with asterisks. (B) Flow cytometry. The cells were incubated unfixed with different anti-GP antibodies at +4°C and then with secondary polyclonal goat anti-mouse PE immunoglobulins. The data presented represent at least three independent experiments.
To investigate the nature of GP self-downregulation, HEK293T cells were transfected with pIRES2-EBOVGP/GFP, a bicistronic vector that allows the expression of both GP and green fluorescent protein (GFP) from the same mRNA by using an internal ribosome entry site sequence. The expression of GP was estimated at 20 h posttransfection by using anti-GP antibodies and GFP fluorescence by flow cytometry (Fig. 1B). Staining of the cells was performed at +4°C without any pretreatment. A decrease in the level of surface GP (in cells with high levels of GFP synthesis), a benchmark for GP self-downregulation, was seen with a number of monoclonal antibodies, confirming previous observations (Fig. 1B, panels 3 and 4) (14, 23). However, the antibodies appeared to differ in the levels of GP downregulation even though the same batch of GP-expressing cells was used. Strikingly, several anti-GP antibodies did not reveal any evidence of a self-downregulation pattern. Indeed, the highest level of surface GP expression correlates here with the highest level of GFP expression (Fig. 1B, panels 1 and 2). These results clearly indicate that GP self-downregulation cannot be explained by a decrease in its presence on the cell surface. We thus speculate that some epitopes on the GP are masked when a certain concentration of the protein is reached at the plasma membrane.
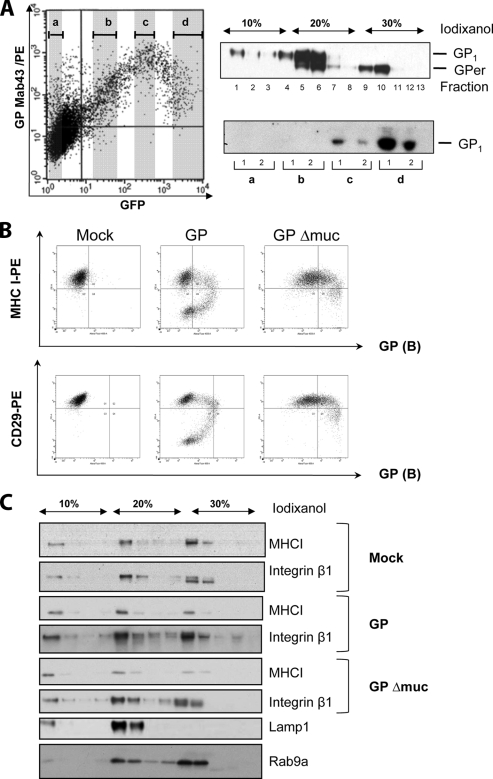
To further investigate the phenomenon of GP self-downregulation, we sorted the cells expressing GP by fluorescence-activated cell sorter using GFP expression. Four groups of cells, each corresponding to a different level of GFP, were selected (Fig. 2A). Equal amounts of cells (5 × 106) from each group were lysed in hypotonic lysis buffer, and after Potter homogenization, the cell lysates were subjected to isopycnic flotation in iodixanol gradients (10 to 20 to 30%) (7, 9). Centrifugation was performed in a SW60 Ti rotor in a Beckman LX100 centrifuge for 3 h at 250,000 × g. The fractions were analyzed by Western blotting using a mixture of monoclonal anti-GP antibodies from groups A and B (Fig. 2A). Three major GP-containing fractions were distinguished. Mature plasma membrane GP was found on the top of the 10% iodixanol gradient. The interface between 10% and 20% iodixanol contained mature GP present in Golgi vesicles. These fractions also contained GPer precursor present in the ER. The interface between 20% and 30% iodixanol contained predominantly GPer. When samples containing plasma membranes were compared, the highest amount of mature GP was found in the cells with the highest level of GFP expression (Fig. 2A, lower-right panel), thus confirming our observations. “Disappearance” of GP from the plasma membrane observed with some antibodies is likely to be explained by the loss of the ability to recognize corresponding epitopes shielded by neighboring GP molecules. Because of the high degree of GP glycosylation, including N- and O-linked sugars and the fact that surface GP is a trimer, the masking of surface epitopes on GP seems to be a very likely phenomenon. We thus speculate that the antibodies with a downregulation pattern recognize epitopes adjacent to the plasma membrane which are hidden by overexpressed GP. Antibodies that showed no GP downregulation, on the contrary, likely recognize epitopes located at the top surface of the GP. This hypothesis is reinforced by the recent findings that the epitope for KZ52, a human anti-GP neutralizing monoclonal antibody (11) which belongs to group B (1), maps to the base of the GP spike (10). Moreover, we also demonstrate that antibodies from group A do not recognize GP with the mucin domain deleted (GPΔmuc) (data not shown). This domain maps to the top of the GP spike (10). GPΔmuc, which was generated as previously described (13), showed no GP self-downregulation and only a minor downregulation of major histocompatibility complex class I (MHC-I) and integrin β molecules in comparison to full-length GP (Fig. 2B).
FIG. 2.
Isopycnic fractionation in iodixanol gradients. (A) HEK293T cells were transfected by pIRES2-EBOVGP/GFP and then sorted by fluorescence-activated cell sorter (BD FACS Aria) into four groups (a, b, c, and d) according to the level of GFP expression (left graph). (Upper-right panel) Each group of cells was subjected to subcellular fractionation, and fractions were analyzed for GP expression by Western blotting (equal numbers of cells per each group were analyzed). The data presented represent three independent experiments. (Lower-right panel) Western blot analysis of fractions containing plasma membranes for each group of sorted cells (a, b, c, and d). GP1 and GPer are indicated. Mab, monoclonal antibody. (B) Flow cytometry. HEK293T cells were transfected with phCMV empty vector, phCMVGP, or phCMVGPΔmuc and collected 24 h posttransfection. The cells were incubated unfixed at 4°C with anti-GP antibodies (group B), then with secondary polyclonal donkey anti-mouse Alexa-488, and finally with anti-MHC-I-PE or anti-CD29-PE (integrin β1). (C) Western blot analysis of fractions collected upon fractionation of cell lysates on iodixanol isopycnic gradients. Mouse anti-MHC-I (Abcam), goat anti-integrin β1 (R&D Systems), mouse anti-Rab9a (Santa Cruz Biotechnology), and mouse anti-lamp1 (Becton Dickinson) antibodies were used for Western blot analysis together with species-specific secondary antibodies labeled with horseradish peroxidase (Dako).
The ability of GP to shield its own epitopes raises the question of whether the same phenomenon can explain the downregulation of cellular proteins. The implication of EBOV GP in the downregulation of cell surface molecules, including integrins and MHC-I, has been postulated in numerous publications (13, 14, 16, 22, 23). In this study, we have focused on the analysis of the surface expression of MHC-I and integrin β1 molecules in cells transfected with phCMVGP. We used two assays, flow cytometry and isopycnic flotation in iodixanol gradients, followed by Western blot analysis. HEK293T cells were transfected with phCMVGP or phCMVGPΔmuc, and 24 h posttransfection, the cells were collected and incubated with an anti-GP antibody (group A) and an anti-MHC-I-phycoerythrin (PE) or an anti-CD29-PE antibody (Pharmingen). In control experiments, the cells were transfected with empty phCMV plasmid. As shown in Fig. 2B, cytometry analysis revealed MHC-I, CD29, and GP downregulation patterns in GP-expressing cells similar to what has been described in previous publications (6, 13, 14). Expression of GPΔmuc resulted only in a minor reduction of surface markers expression, while the downregulation of GP itself was not observed at all. On the contrary, analysis of MHC-I and integrin β1 surface expression by using a fractionation assay showed no sign of protein downregulation at the plasma membrane, as phCMVGP-, phCMVGPΔmuc-, and mock-transfected cells showed the same amounts of MHC-I and integrin β1 in the plasma membrane fractions (Fig. 2C).
Finally, in order to confirm that GP has a masking effect on cellular surface proteins, we used the nerve growth factor receptor TrkA-GFP, a membrane protein coupled to GFP at the intracellular domain. Using antibodies against the extracellular part of the protein and GFP fluorescence, we were able to efficiently monitor its surface expression through visualization of both extracellular and intracellular parts of the fusion protein (Fig. 3A and B). TrkA is not naturally expressed in HEK293T cells, and thus, this avoids possible problems related to background staining. The cells were transfected with pCML-TrkA-GFP and cotransfected with phCMVGP, phCMVGPΔmuc, or the empty vector. Cells were collected 16 h posttransfection and stained with anti-GP antibodies (group A) and anti-TrkA antibodies at +4°C. Goat anti-mouse PE (Dako) and donkey anti-rabbit Alexa-647 (Invitrogen) antibodies were used for detection by flow cytometry. After being stained, the cells were fixed in 3% paraformaldehyde. Aliquots of the cells were also analyzed by confocal microscopy after they were spread on glass slides using cytospin. In cells transfected with pCML-TrkA-GFP alone, flow cytometry analysis (Fig. 3A) demonstrated that the presence of TrkA at the cell surface correlated well with GFP expression (upper-right graph). Approximately half of the cells which expressed GP and were positive for GFP (TrkA-GFP) showed an absence of surface staining or low-level surface staining of TrkA (Fig. 3A, lower-right graph). Therefore, the use of TrkA-GFP as an example of proteins with a downregulation pattern is justified. As expected, GPΔmuc did not show such effect (Fig. 3A, middle-right graph). In an attempt to visualize the ability of GP to mask cellular proteins, we used confocal microscopy (Fig. 3B). The cells with a high level of GP expression showed a very low level of staining for surface TrkA, if any. The same cells, however, showed no evidence of TrkA-GFP disappearance from the plasma membrane. Cells expressing no GP and, even more important, those expressing GP at a moderate level did not show any disappearance of extracellular TrkA. As expected, GPΔmuc-expressing cells showed no sign of TrkA masking. These experiments strongly support our predictions that masking of cellular surface proteins by overexpressed GP prevents antibody recognition. Overexpression of highly glycosylated protein plays a key role in the downregulation of surface markers observed in cells transfected with EBOV GP. However, in virus-infected cells, the overexpression of GP is less likely, especially at early stages of infection. Indeed, several mechanisms are involved in the control of GP expression. Transcriptional RNA editing that is required for GP appearance reduces the level of GP mRNA synthesis (18, 20). Shedding of surface GP by TACE removes a portion of GP from the cell surface (4). Moreover, budding of virus particles obviously reduces the amounts of GP present on the cell surface during infection.
FIG. 3.
EBOV GP masks cellular surface proteins. (A) Flow cytometry analysis. HEK293T cells were transfected with pCML-TrkA-GFP and phCMVGP or phCMVGPΔmuc and were collected 16 h posttransfection and stained unfixed for TrkA and GP at +4°C. Cells were fixed in 3% paraformaldehyde prior to analysis. Flow cytometry analysis was performed on a Becton-Dickinson Canto machine. The data presented represent at least three independent experiments. (B) Confocal immunofluorescence analysis. Aliquots of the cells used in flow cytometry were analyzed by confocal microscopy using a Leica SP5 confocal microscope. Light blue staining corresponds to surface TrkA, green staining corresponds to TrkA-GFP, and red straining corresponds to GP. A red arrow shows the cell with a disappearance of surface TrkA staining in the presence of EBOV GP. The white arrow shows the surface staining of TrkA in GP-negative cells. The data presented represent four independent experiments. (C) Schematic representation of a model of EBOV GP masking both its own epitopes and the presence of cellular proteins on the plasma membrane. Type A antibodies do not show the GP self-downregulation pattern, whereas this effect is observed with type B antibodies. Mab, monoclonal antibody.
Importantly, masking, as a mechanism of downregulation, correlates with data from other publications demonstrating that the GP mucin-like domain plays a key role in the cytotoxicity of this protein (14, 24). Cellular proteins containing mucin-like domains have also been shown to be involved in cell detachment through modification of integrin function (21). Indeed, transmembrane proteins with mucin-like domains have a common property: masking other cell surface molecules through steric hindrance. For instance, it has been proposed that masking of MHC molecules by epiglycanin results in a reduced immune response against cells expressing this protein (3, 12).
In conclusion, we have demonstrated (Fig. 3C), at least in part, that overexpression of mature GP on the plasma membrane results in the masking of antigenic epitopes on GP itself and the shielding of MHC-I and integrin β. This suggests that shielding may impact other surface proteins. This function of GP may play an important role in the evasion of antiviral immunity. We speculate that masking of surface proteins may result in the inhibition of receptor-ligand interactions and signaling and thus contribute to a functional destabilization of virus-infected cells.
Acknowledgments
We are grateful to Robin Buckland and St. Patrick Reid for their helpful comments on the manuscript. pCML-TrkA-GFP and antibody against TrkA were kindly provided by B. Rudkin. We thank Chantal Bella and Sebastien Dussurgey of the cytometry platform and Christophe Chamot of the confocal microscopy platform (PLATIM) of the IFR128 for providing excellent technical support.
This work was supported by INSERM, the Biotox program, the French Ministère de la Recherché (grant 04G537), the Agence National de la Recherché, the National Institute of Health (grant AI059536), and the Deutsche Forschungsgemeinschaft (grant SFB 593 to V.E.V.). M.B. was supported by the Socrates-Erasmus program. S.D. was supported by a grant from the Délégation française Générale pour l'Armement and Fondation pour la Recherche Médicale (FDT20080913612). M.M. was supported by the Région Rhône-Alpes Cluster Infectiologie.
Footnotes
Published ahead of print on 8 July 2009.
REFERENCES
- 1.Alazard-Dany, N., V. Volchkova, O. Reynard, C. Carbonnelle, O. Dolnik, M. Ottmann, A. Khromykh, and V. E. Volchkov. 2006. Ebola virus glycoprotein GP is not cytotoxic when expressed constitutively at a moderate level. J. Gen. Virol. 87:1247-1257. [DOI] [PubMed] [Google Scholar]
- 2.Chan, S. Y., M. C. Ma, and M. A. Goldsmith. 2000. Differential induction of cellular detachment by envelope glycoproteins of Marburg and Ebola (Zaire) viruses. J. Gen. Virol. 81:2155-2159. [DOI] [PubMed] [Google Scholar]
- 3.Codington, J. F., G. Klein, A. G. Cooper, N. Lee, M. C. Brown, and R. W. Jeanloz. 1978. Further studies on the relationship between large glycoprotein molecules and allotransplantability in the TA3 tumor of the mouse: studies on segregating TA3-HA hybrids. J. Natl. Cancer Inst. 60:811-818. [DOI] [PubMed] [Google Scholar]
- 4.Dolnik, O., V. Volchkova, W. Garten, C. Carbonnelle, S. Becker, J. Kahnt, U. Stroher, H. D. Klenk, and V. Volchkov. 2004. Ectodomain shedding of the glycoprotein GP of Ebola virus. EMBO J. 23:2175-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feldmann, H., V. E. Volchkov, V. A. Volchkova, U. Stroher, and H. D. Klenk. 2001. Biosynthesis and role of filoviral glycoproteins. J. Gen. Virol. 82:2839-2848. [DOI] [PubMed] [Google Scholar]
- 6.Francica, J. R., M. K. Matukonis, and P. Bates. 2009. Requirements for cell rounding and surface protein down-regulation by Ebola virus glycoprotein. Virology 383:237-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grigorov, B., F. Arcanger, P. Roingeard, J. L. Darlix, and D. Muriaux. 2006. Assembly of infectious HIV-1 in human epithelial and T-lymphoblastic cell lines. J. Mol. Biol. 359:848-862. [DOI] [PubMed] [Google Scholar]
- 8.Ito, H., S. Watanabe, A. Takada, and Y. Kawaoka. 2001. Ebola virus glycoprotein: proteolytic processing, acylation, cell tropism, and detection of neutralizing antibodies. J. Virol. 75:1576-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolesnikova, L., S. Bamberg, B. Berghofer, and S. Becker. 2004. The matrix protein of Marburg virus is transported to the plasma membrane along cellular membranes: exploiting the retrograde late endosomal pathway. J. Virol. 78:2382-2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee, J. E., M. L. Fusco, A. J. Hessell, W. B. Oswald, D. R. Burton, and E. O. Saphire. 2008. Structure of the Ebola virus glycoprotein bound to an antibody from a human survivor. Nature 454:177-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maruyama, T., P. W. Parren, A. Sanchez, I. Rensink, L. L. Rodriguez, A. S. Khan, C. J. Peters, and D. R. Burton. 1999. Recombinant human monoclonal antibodies to Ebola virus. J. Infect. Dis. 179(Suppl. 1):S235-S239. [DOI] [PubMed] [Google Scholar]
- 12.Miller, S. C., J. F. Codington, and G. Klein. 1982. Further studies on the relationship between allotransplantability and the presence of the cell surface glycoprotein epiglycanin in the TA3-MM mouse mammary carcinoma ascites cell. J. Natl. Cancer Inst. 68:981-988. [PubMed] [Google Scholar]
- 13.Simmons, G., R. J. Wool-Lewis, F. Baribaud, R. C. Netter, and P. Bates. 2002. Ebola virus glycoproteins induce global surface protein down-modulation and loss of cell adherence. J. Virol. 76:2518-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sullivan, N. J., M. Peterson, Z. Y. Yang, W. P. Kong, H. Duckers, E. Nabel, and G. J. Nabel. 2005. Ebola virus glycoprotein toxicity is mediated by a dynamin-dependent protein-trafficking pathway. J. Virol. 79:547-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takada, A., C. Robison, H. Goto, A. Sanchez, K. G. Murti, M. A. Whitt, and Y. Kawaoka. 1997. A system for functional analysis of Ebola virus glycoprotein. Proc. Natl. Acad. Sci. USA 94:14764-14769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takada, A., S. Watanabe, H. Ito, K. Okazaki, H. Kida, and Y. Kawaoka. 2000. Downregulation of beta1 integrins by Ebola virus glycoprotein: implication for virus entry. Virology 278:20-26. [DOI] [PubMed] [Google Scholar]
- 17.Volchkov, V. E., V. A. Volchkova, O. Dolnik, H. Feldmann, and H. D. Klenk. 2005. Polymorphism of filovirus glycoproteins. Adv. Virus Res. 64:359-381. [DOI] [PubMed] [Google Scholar]
- 18.Volchkov, V. E., S. Becker, V. A. Volchkova, V. A. Ternovoj, A. N. Kotov, S. V. Netesov, and H. D. Klenk. 1995. GP mRNA of Ebola virus is edited by the Ebola virus polymerase and by T7 and vaccinia virus polymerases. Virology 214:421-430. [DOI] [PubMed] [Google Scholar]
- 19.Volchkov, V. E., H. Feldmann, V. A. Volchkova, and H. D. Klenk. 1998. Processing of the Ebola virus glycoprotein by the proprotein convertase furin. Proc. Natl. Acad. Sci. USA 95:5762-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Volchkov, V. E., V. A. Volchkova, E. Muhlberger, L. V. Kolesnikova, M. Weik, O. Dolnik, and H. D. Klenk. 2001. Recovery of infectious Ebola virus from complementary DNA: RNA editing of the GP gene and viral cytotoxicity. Science 291:1965-1969. [DOI] [PubMed] [Google Scholar]
- 21.Wesseling, J., S. W. van der Valk, H. L. Vos, A. Sonnenberg, and J. Hilkens. 1995. Episialin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. J. Cell Biol. 129:255-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang, Z. Y., H. J. Duckers, N. J. Sullivan, A. Sanchez, E. G. Nabel, and G. J. Nabel. 2000. Identification of the Ebola virus glycoprotein as the main viral determinant of vascular cell cytotoxicity and injury. Nat. Med. 6:886-889. [DOI] [PubMed] [Google Scholar]
- 23.Zampieri, C. A., J. F. Fortin, G. P. Nolan, and G. J. Nabel. 2007. The ERK mitogen-activated protein kinase pathway contributes to Ebola virus glycoprotein-induced cytotoxicity. J. Virol. 81:1230-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zampieri, C. A., N. J. Sullivan, and G. J. Nabel. 2007. Immunopathology of highly virulent pathogens: insights from Ebola virus. Nat. Immunol. 8:1159-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]