Abstract
CXCR3 is a G-protein-coupled receptor preferentially expressed by activated T cells, NK cells, and dendritic cells. Signaling through gamma interferon-regulated chemokines CXCL9, CXCL10, CXCL11, and CXCR3 plays a critical role in the immune response of many viral pathogens. However, the relevance of CXCR3 for optimal T-cell activation and the induction of regulatory transcription factors (i.e., T-bet and eomesodermin) relative to host immune defense against genital herpes simplex virus type 2 (HSV-2) infection have been poorly defined. In this study, we evaluated the requirement of CXCR3 expression during genital HSV-2 infection using mice deficient in CXCR3 (CXCR3−/−) along with wild-type (WT) controls, assessing the resistance of mice to viral infection and focusing on the cytokine/chemokine response, phenotypic analysis of recruited leukocytes, and functional analysis of CD8+ T cells. CXCR3−/− mice showed a heightened sensitivity to infection compared to WT animals in terms of the viral burden in infected tissues as well as elevated mortality. The poor response of CXCR3−/− mice to viral infection was associated with reduced cytotoxic T-lymphocyte activity through the impairment of T-bet, perforin, and granzyme B expression by CD8+ T cells. Corresponding with the defective cytolytic activity, a reduction in recruitment of plasmacytoid dendritic cells and CD80 expression in CD11c+ dendritic cells in the draining lymph nodes of CXCR3−/− mice were detected. Collectively, the results provide a new perspective to CXCR3 signaling for the appropriate activation of CD8+ T cells required for host defense against genital HSV-2 infection.
Herpes simplex virus type 2 (HSV-2), the most common cause of genital herpes, is a highly successful neurotropic virus which can lead to lifelong infection with episodic reactivation (11, 12, 56). Evidence suggests that the development and activation of CD8+ T cells are critical to the control of genital HSV-2 infection in both the human population and mice (9, 23, 33). However, asymptomatic shedding of HSV-2, even in the presence of CD8+ cytotoxic T lymphocytes (CTLs), and the production of a viral glycoprotein that indirectly inactivates NK cells are significant attributes for the successful maintenance of the pathogen in the population (4, 43). The worldwide prevalence of genital herpes and incidence of HSV-2 among human immunodeficiency virus/AIDS patients are indicative of a major public health impact (36, 37).
The development of a mouse model of genital HSV-2 infection has allowed investigators to identify and characterize cells and pathways critical to the control of virus replication and spread (30, 40). A number of cellular constituents of the innate and adaptive immune response, including neutrophils, macrophages, NK cells, NK T cells, dendritic cells (DCs), T cells, and B cells, have been shown to contribute to resistance to genital HSV-2 infection (2, 9, 10, 13, 17, 32). These cells operate through direct physical contact with virally infected cells or via the secretion of soluble factors that inactivate virus or block viral replication. Leukocyte migration to sites of infection (e.g., vaginal tract) is associated with the upregulation of adhesion molecules and expression of chemokines, including CCL2 and CCL5 (14, 22, 41). Mice deficient in one of the receptors for CCL5, CCR5, show an increase in susceptibility to genital HSV-2 infection associated with a reduction in the expansion of NK cells in the spleen and a deficiency in the recruitment of NK cells to the nervous system (51). A more recent study reported a number of chemokines expressed in vaginal tissue following HSV-2 infection in addition to CCL2 and CCL5, including CCL3, CXCL1, CXCL9, and CXCL10 (49). Like CCR5-deficient mice, CXCL9-deficient (CXCL9−/−) and CXCL10-deficient (CXCL10−/−) mice are more susceptible to genital HSV-2 infection than fully competent, wild-type (WT) animals, as shown by a decrease in cumulative survival as well as an increase in viral titers recovered in vaginal, spinal cord, and brain stem tissue (52). In the case of CXCL9−/− and CXCL10−/− mice, the susceptibility correlates with a transient delay or decrease in the recruitment of NK cells and HSV-specific CD8+ T cells to vaginal tissue and the nervous system, even though comparable levels of effector cells are recovered in the spleen and draining lymph nodes of infected mice.
The present study was undertaken to determine if the fate of mice deficient in the lone receptor for CXCL9 and CXCL10, CXCR3, paralleled that of CXCL9−/− and CXCL10−/− mice in response to genital HSV-2 infection. Precedence for such a study stems from previous findings that show that mice deficient in CXCR3 (CXCR3−/−) do not respond in a manner consistent with the response of CXCL10−/− mice following ocular HSV-1 infection (57, 59). The present findings clearly show that CXCR3−/− mice are susceptible to genital HSV-2 infection, similar to CXCL9−/− and CXCL10−/− mice. However, the sensitivity to the pathogen is not attributable to a recruitment deficiency of effector T cells and/or NK cells but, rather, to a defect in the cytolytic activity of CD8+ effector T cells.
MATERIALS AND METHODS
Virus.
A clinical isolate of HSV-2 obtained from Charity Hospital (New Orleans) was maintained at −80°C and propagated in Vero cells (African green monkey kidney fibroblasts, ATCC CCL-81; American Type Culture Collection). The virus stock (3 × 107 PFU/ml) was diluted in RPMI 1640 medium containing 10% fetal bovine serum, gentamicin, and anti-mycotic-antibiotic solution (complete medium; Invitrogen Life Technologies, Gaithersburg, MD) at 37°C, 5% CO2, and 95% humidity immediately before infection.
Mice and infection.
Six- to eight-week-old female C57BL/6 mice (The Jackson Laboratory, Bar Harbor, ME) and CXCR3−/− female mice backcrossed to the C57BL/6 genetic background for eight and nine generations were used (54). The transgenic gBT.I-1 mice were originally generated by Francis R. Carbone (University of Melbourne) (34). Mice were rendered susceptible to genital HSV-2 using medroxyprogesterone acetate (Depo-Provera; Pharmacia & Upjohn Diagnostics, New York, NY), infected with HSV-2 (2,000 PFU/vagina) intravaginally, as described previously (40), and exsanguinated at various times postinfection (p.i.) for analysis. Bone marrow (BM) chimeras were generated by irradiating C57BL/6 female mice (CD45.1+) using 2 sublethal doses of 650 rad at 4-h intervals. Mice were reconstituted with BM cells (5 × 106 cells) from either congenic WT (CD45.2+) or CXCR3−/− mice retro-orbitally (intravenously [i.v.]). Mouse chimeras were kept under sterile conditions for 14 days and maintained for 12 weeks before implementation of the experiments. All procedures and use of animals were approved by the University of Oklahoma Health Sciences Center and Dean A. McGee Eye Institute animal care and use committees.
Virus plaque assay.
Tissue (vagina, spinal cord, and brain stem) was removed from infected mice at various times p.i., placed into complete medium (500 μl), and homogenized using a tissue homogenizer (Fisher Scientific). Supernatants were clarified (10,000 × g for 1 min) and assessed for viral titers by plaque assay, as described previously (15).
Histological analysis.
Spinal cords and brain stems were removed from infected mice on day 7 p.i., placed into 10% neutral buffered formalin at 4°C for 24 h, and frozen, and paraffin sections were generated. Sections (10 μm) were processed for immunohistological analysis using hematoxylin and eosin staining and Nikon epifluorescence microscopy.
Suspension array and ELISA.
Detection of CXCL1, CCL2, CCL3, CCL5, and gamma interferon (IFN-γ) was performed using a suspension array system (Bio-Rad, Richmond, CA) or an enzyme-linked immunosorbent assay (ELISA) (CXCL9 and CXCL10; R&D Systems, Minneapolis, MN). Samples were analyzed in duplicate, along with a standard provided. The weight of each tissue was determined in order to normalize the amount of each cytokine/chemokine per mg of tissue weight.
Flow cytometry.
Cells residing in organized lymphoid, vaginal, spinal cord, and brain stem tissue were phenotyped by flow cytometry, as described previously (52). In some experiments, 500-μl peripheral blood mononuclear cell samples were taken from the ventricle of anesthetized mice prior to perfusion with saline. The following antibodies were used for the identification of cell populations or to determine expression of chemokine receptors; anti-CD3 (17A2), anti-CD4 (RM4-5), anti-CD8α (53-6.7) or anti-NK1.1 (PK136), anti-CD45 (30-F11), anti-F4/80 (MCA497FA), anti-GR1 (RB6-8C5), anti-CD11c (HL3), anti-B220 (RA3-6B2), anti-CCR5 (C34-3448), and anti-CXCR3 (220803). The antibodies were obtained from BD Pharmingen, except for anti-F4/80 (Serotec) and anti-CXCR3 (R&D Systems). After being labeled, cells were washed, fixed, and resuspended in 1× phosphate-buffered saline (PBS) containing 1% bovine serum albumin. A known number of fluorescent beads (Invitrogen Life Technologies) was immediately added to the sample and then analyzed on a Coulter Epics XL flow cytometer (Beckman Coulter). The absolute number of leukocytes (CD45hi) in the tissue was determined by multiplying the ratio of the total number of beads added per sample divided by the number of beads collected by the number of CD45hi events by the sample dilution factor. For tetramer staining, cells were labeled with HSV peptide gB498-505 (SSIEFARL)-specific major histocompatibility complex tetramer (MHC Tetramer Lab, Houston, TX) for 60 min. Cells were washed and labeled with anti-CD8 and anti-CD45, fixed in 1% paraformaldehyde, resuspended in 1× PBS, and analyzed by flow cytometry.
CTL assay.
The CTL assay was performed, as described previously (52). In brief, MC57G (CRL-2295, American Type Culture Collection) were infected with HSV-2 at a multiplicity of infection of 3 for 8 h at 37°C, 5% CO2, and 95% humidity. The cells were labeled with 0.5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE, Invitrogen) and mixed with the desired number of isolated leukocytes from the processed spinal cords and inguinal/iliac lymph nodes (ILN) of mice at an effector-to-target cell ratio of 10:1. After 4 h of incubation, cells were stained with propidium iodide (PI) (0.5 μg), washed, resuspended in 1× PBS, and analyzed by flow cytometry. The percent cytotoxicity was calculated by dividing the number of PI-labeled CFSE-expressing cells by the total number of CFSE-expressing cells multiplied by 100. The background level was determined by using target cells without effector cells and target cells incubated with spleen cells from uninfected mice.
In vivo proliferation assay.
CD8+ T cells were purified from transgenic gBT-I.1 mice by using MACS columns (Miltenyi Biotec, Auburn, CA). The enriched CD8+ T cells were labeled with 5 μM CFSE and transferred (1 × 106) to WT or CXCR3−/− mice infected 24 h earlier with HSV-2 (2,000 PFU/vagina) via a retro-orbital route. A total of 24 or 72 h after cell transfer, mice were exsanguinated, and ILN and spleens were removed and processed for analysis by flow cytometry. The number of CFSE-positive CD8+ T cells residing in ILN and the spleen were determined, as were the number of cell divisions.
Adoptive transfer experiment.
Equivalent numbers (1 × 106 cells) of CD8+ T cells purified from the spleens of HSV-2-infected WT, gBT.I-1, and CXCR3−/− mice using MACS columns were transferred retro-orbitally to CXCR3−/− recipient mice separately and immediately infected with HSV-2 (2,000 PFU/vagina). On day 7 p.i., mice were exsanguinated, and tissues were processed for viral titers by plaque assay.
Intracellular staining.
Cells (1 × 106) isolated from infected ILN or spinal cords were restimulated with 20 μg of HSV gB498-505 peptide (SSIEFARL) in the presence of monensin (Sigma Aldrich, St. Louis, MO) in a 24-well culture plate at 37°C for 4 h. Cultured cells were washed, incubated with anti-mouse CD16/32, and stained for surface antigens with fluorescein isothiocyanate-CD8 (53-6.7) and phycoerythrin (PE)-Cy5-anti-CD45 (30-F11). Cells were washed, fixed, and permeabilized with 100 μl of BD Cytofix/Cytoperm buffer (BD Pharmingen, San Diego, CA). Next, cells were washed and stained with PE-conjugated granzyme B (16G6; eBioscience, San Diego, CA), PE-conjugated perforin (eBioOMAK-D; eBioscience), or PE-conjugated T-bet (4B10; Santa Cruz Biotechnology, Santa Cruz, CA). Then, cells were washed and resuspended in 1 ml of 1× PBS with 1% bovine serum albumin and analyzed by flow cytometry.
Statistics.
All statistical analyses were carried out using the GB-STAT program (Dynamic Microsystems, Silver Spring, MD). One-way analysis of variance and Tukey's post hoc t test were used to determine significant (P < 0.05) differences between WT and CXCR3−/− mice.
RESULTS
CXCR3−/− mice are highly susceptible to genital HSV-2 infection.
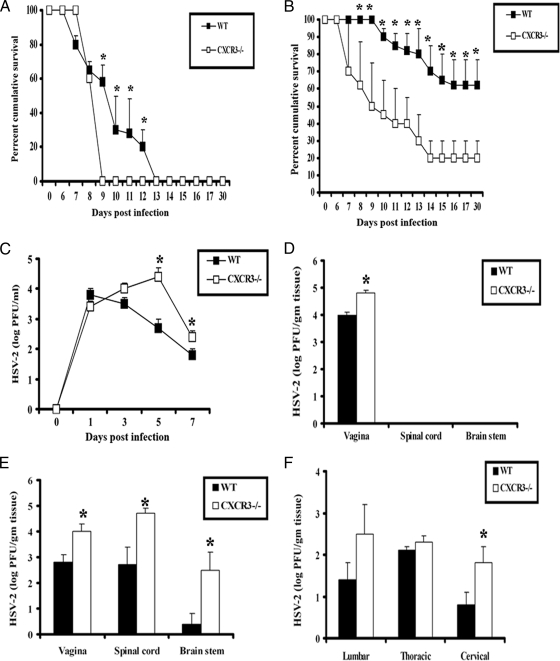
CXCR3 signaling plays a critical role in the immune response to many viral pathogens, including lymphocytic choriomeningitis virus (LCMV), murine gammaherpesvirus 68, dengue virus, and respiratory syncytial virus (16, 24, 25, 28). To understand the contribution of CXCR3 in the host defense against genital HSV-2 infection, sensitivity of WT and CXCR3−/− mice was initially evaluated by measuring the survival and viral burden of HSV-2-infected mice. The lack of CXCR3 confers mice that are highly susceptible to virus infection, with elevated mortality and an increase in perigenital lesions at two different inocula of HSV-2 (20,000 PFU/vagina and 2,000 PFU/vagina) (Fig. 1A and B). In contrast, WT mice were found to show greater resistance to infection, as determined by a delay in mortality and the number of survivors at each infectious dose (Fig. 1A and B). Consistent with this finding, CXCR3−/− mice were found to shed more infectious virus in the vaginal lumen from day 5 to 7 p.i. (Fig. 1C) and more virus in the vaginal tissue on day 3 p.i. compared to the shedding of WT mice (Fig. 1D). Likewise, CXCR3−/− mice harbored significantly higher viral loads in the vagina, spinal cord, and brain stem on day 7 p.i. (Fig. 1E). Interestingly, significantly higher viral titers were recovered only from the cervical section of the spinal cord but not from the thoracic or lumber sections in CXCR3−/− mice (Fig. 1F). Taken together, CXCR3−/− mice show enhanced sensitivity to genital HSV-2 infection based on an increase in mortality and viral titers in vaginal tissue and the central nervous system (CNS). Consistent with this observation, a significant increase in neuropathology, evident by an increase in infiltrating cells, loss of neurons, and degeneration of tissue, was found in the spinal cord and brain stem of CXCR3−/− mice (Fig. 2).
FIG. 1.
CXCR3−/− mice are highly susceptible to genital HSV-2 infection. (A and B) C57BL/6 (WT) and CXCR3−/− mice (14 mice/group) were rendered susceptible to infection using medroxyprogesterone acetate, infected with 20,000 PFU HSV-2/vagina (A) or 2,000 PFU HSV-2/vagina (B), and monitored for survival over 30 days p.i. The results are displayed as survival means ± standard errors of the means for each time point, summarized from three independent experiments. *, P was <0.05 when comparing WT and CXCR3−/− mice. (C) Virus titers obtained from vaginal lavage fluid from HSV-2 (2,000 PFU/vagina)-infected WT and CXCR3−/− mice (six mice/group) at the indicated times. *, P was <0.05 when comparing WT and CXCR3−/− mice. (D and E) Vaginal, spinal cord, and brain stem tissue were processed and assayed for viral titers on day 3 (D) and day 7 (E) p.i. *, P was <0.05 when comparing WT and CXCR3−/− mice. (F) Spinal cords were processed and dissected into three sections (cervical, thoracic, and lumbar) and assayed for viral titers. The viral titer is expressed as the mean log PFU ± standard error of the mean. The bars represent means ± standard errors of the means from three independent experiments. *, P was <0.05 when comparing WT and CXCR3−/− mice.
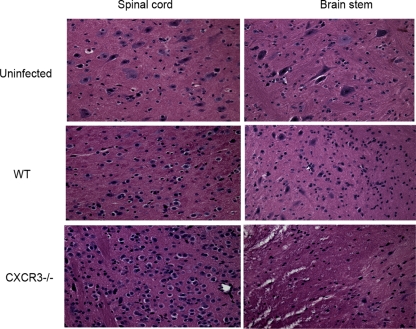
FIG. 2.
An increase in neuropathology is evident in HSV-2-infected CXCR3−/− mice. Following medroxyprogesterone acetate treatment, WT and CXCR3−/− mice (six mice/group) were infected with HSV-2 (2,000 PFU/vagina). On day 7 p.i., mice were exsanguinated, and the brain stem and spinal cord were removed from each mouse and processed for histological analysis using hematoxylin and eosin staining. Tissues from uninfected WT mice were used as a control. The magnification used is ×200. The data are representative of three independent experiments.
CXCR3 expression on hematopoietic cells is important in order to contain viral infection.
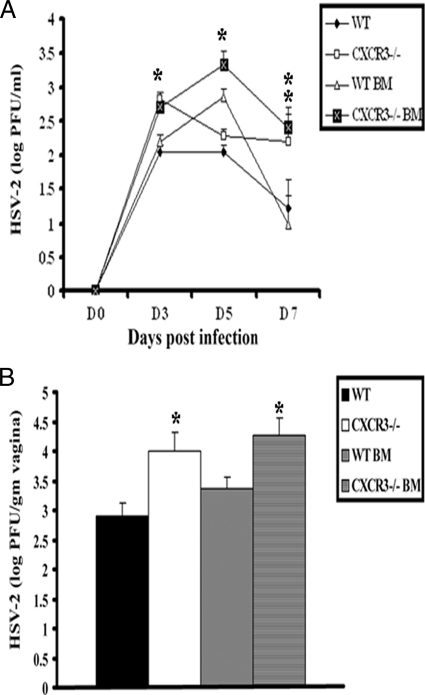
To examine the consequences of CXCR3 expression on hematopoietic-derived cells versus resident cells for protection against genital HSV-2 infection, WT and CXCR3−/− BM chimeras were generated and evaluated for sensitivity to HSV-2. As controls, WT and CXCR3−/− mice were also included in the experiment, and sensitivity was assessed, measuring viral shedding and viral titers at the primary site of infection (i.e., vaginal tissue). CXCR3−/− BM chimeras were found to shed more infectious virus in the vaginal lumen from day 3 to day 7 p.i. than WT or WT BM chimeras but were consistent with levels shed by CXCR3−/− mice (Fig. 3A). In addition, WT and WT BM chimeric mice harbored less virus in vaginal tissue on day 7 p.i. than CXCR3−/− or CXCR3−/− BM chimeric mice (Fig. 3B). Taken together, CXCR3 expression on leukocytes but not nonhematopoietic-derived resident cells contributes to resistance to HSV-2 infection in vaginal tissue. Whether the contribution is at the level of recruitment of leukocytes to the infected tissue or because of other events associated with induction of the adaptive immune response is addressed below.
FIG. 3.
Viral titers of HSV-2-infected WT and CXCR3−/− BM chimera mice. (A) WT, CXCR3−/−, WT BM chimera, and CXCR3−/− BM chimera mice (nine mice/group) were infected with HSV-2 (2,000 PFU/vagina), and at the indicated time points, vaginal lavage fluid was collected and assayed for viral titers. *, P was <0.05 when comparing WT and CXCR3−/− mice. (B) Vaginal tissue collected from HSV-2-infected mice (nine mice/group) on day 7 p.i. was assayed for determination of the viral load. The viral titers are expressed as log PFU ± standard errors of the means. The bars represent means ± standard errors of the means from three independent experiments. *, P was <0.05 when comparing WT and CXCR3−/− mice.
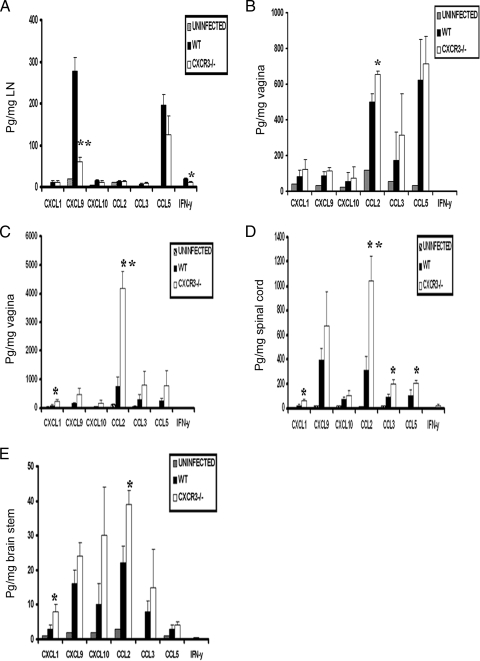
CXCR3−/− mice express elevated chemokine/cytokine levels in infected tissue.
The local production of cytokines/chemokines in response to genital HSV-2 infection influences leukocyte recruitment (49). To determine if cytokine/chemokine levels were modified in the absence of CXCR3 expression following HSV-2 infection, candidate cytokine/chemokines were surveyed at various times p.i. Of all the cytokine/chemokines evaluated, only CXCL9 and IFN-γ were significantly reduced in the draining inguinal/iliac lymph nodes (ILN) of CXCR3−/− mice compared to those of WT mice on day 3 p.i. (Fig. 4A) but not on day 7 p.i. (data not shown). Whereas CCL2 was the only chemokine significantly increased in the vaginal tissue of CXCR3−/− mice on day 3 p.i. (Fig. 4B), elevated levels of CXCL1 and CCL2 were found on day 7 p.i. (Fig. 4C). In comparison, there was an increase in CXCL1, CCL2, CCL3, and CCL5 levels in the spinal cord of the receptor knockout mice on day 7 p.i. (Fig. 4D). Within the brain stem, CXCL1 and CCL2 were elevated in CXCR3−/− mice on day 7 p.i. (Fig. 4E). Since no infectious virus was recovered in the spinal cord or brain stem of mice at day 3 p.i., no analysis of the levels of cytokines/chemokines were conducted at this time point. Collectively, the results suggest that an increase in select chemokines (i.e., CXCL1, CCL2, CCL3, and CCL5) in HSV-2-infected CXCR3−/− mice corresponds with elevated infectious virus recovered in the tissue.
FIG. 4.
Chemokine/cytokine levels in the tissue of HSV-2-infected WT and CXCR3−/− mice. WT and CXCR3−/− mice (six mice/group) were infected with HSV-2 (2,000 PFU/vagina). The mice were exsanguinated at the indicated times p.i., and ILN (day 3) (A), vaginal tissue (day 3) (B), vaginal tissue (day 7) (C), spinal cords (day 7) (D), and brain stems (day 7) (E) were removed, processed, and assessed for the indicated analyte using a suspension array system or an ELISA (for CXCL9 and CXCL10). Samples were analyzed in duplicate, along with a standard provided to generate standard curves for each analyte. The weight of the tissue was used to normalize the amount of cytokine/chemokine per milligram of tissue weight, and bars represent the means ± standard errors of the means from two independent experiments. P was <0.01 (**) and <0.05 (*) when comparing the WT and CXCR3−/− mice.
Recruitment of Gr1+ F4/80+ macrophages into infected tissue is elevated in HSV-2-infected CXCR3−/− mice.
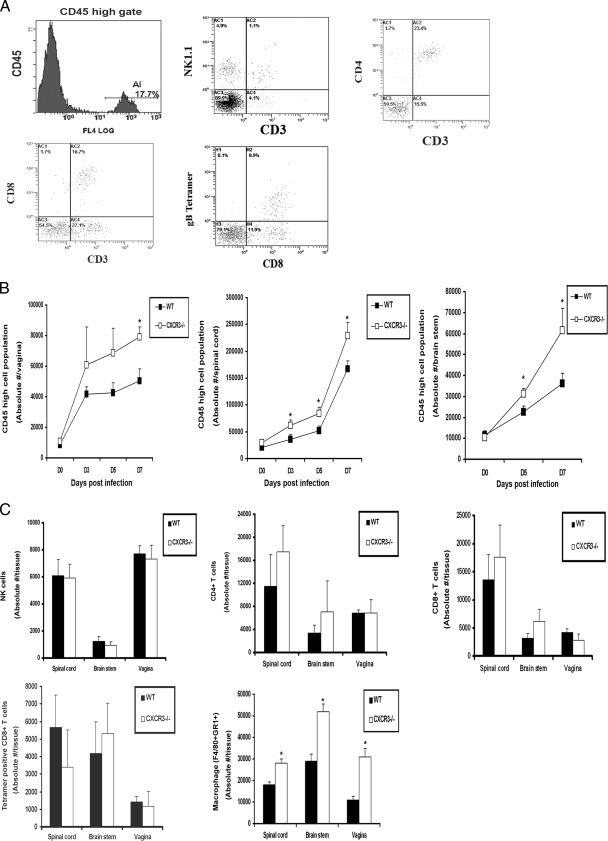
CXCR3 signaling has been reported to be critical for the chemoattraction of activated Th1 cells, CTLs, NK cells, macrophages, and DCs to inflammatory sites (1, 26, 44). To identify possible changes in leukocyte recruitment to infected tissue as well as cells residing in the ILN and the spleen in CXCR3−/− mice following HSV-2 infection, tissues were analyzed for leukocyte populations gating on CD45hi-expressing cells that discriminate infiltrating leukocytes from the resident microglia population (exhibiting CD45lo-to-CD45med phenotype) in the CNS (5) (Fig. 5A). The total number of leukocytes recruited to the vaginal, spinal cord, and brain stem tissue of CXCR3−/− mice was elevated throughout the acute infection, which became clearly significant in all tissues surveyed by day 7 p.i. (Fig. 5B). Ironically, no significant difference in the absolute number of neutrophils (data not shown), NK, CD4+ T cells, CD8+ T cells, or HSV-specific CD8+ T cells was observed infiltrating the spinal cord, brain stem or vagina on day 7 p.i. (Fig. 5C) or at any earlier time points (data not shown). In addition to a lack of change in the recruitment of T cells, neutrophils, or NK cells to infected tissue, there was no numerical difference in cell types residing in the ILN or the spleen (data not shown). However, the total number of activated macrophages (F4/80+ Gr1+) infiltrating the CNS and vagina of CXCR3−/− mice was significantly elevated by day 7 p.i. (Fig. 5C), consistent with the elevation in CCL2 expression at these sites at this time point (Fig. 4).
FIG. 5.
Leukocyte infiltration into HSV-2-infected tissue. (A) WT and CXCR3−/− mice (six mice/group) were infected with HSV-2 (2,000 PFU/vagina) and exsanguinated at the indicated time p.i., and tissues were removed, processed, and analyzed for CD45hi cell population and surface characteristics, as shown by the representative flow plots in the spinal cord indicating CD45hi gate cells, NK cells (NK1.1+ CD3−), CD4+ T cells (CD3+ CD4+), CD8+ T cells (CD3+ CD8+), and HSV gB-specific CD8+ T cells (CD8+ Tetramer+). (B) The absolute number of CD45hi cell population in vaginal, spinal cord, and brain stem tissue samples were determined by flow cytometry. The day 0 time point represents uninfected controls. *, P was <0.05 when comparing WT and CXCR3−/− mice. (C) The absolute number of NK cells, CD4+ T cells, CD8+ T cells, HSV gB-specific CD8+ T cells, and macrophages in infected tissues on day 7 p.i. were determined by flow cytometry. The bars represent standard errors of the means from three independent experiments. *, P was <0.05 when comparing WT and CXCR3−/− mice.
Change in dynamics of CXCR3 expression in circulation and tissue following genital HSV-2 infection.
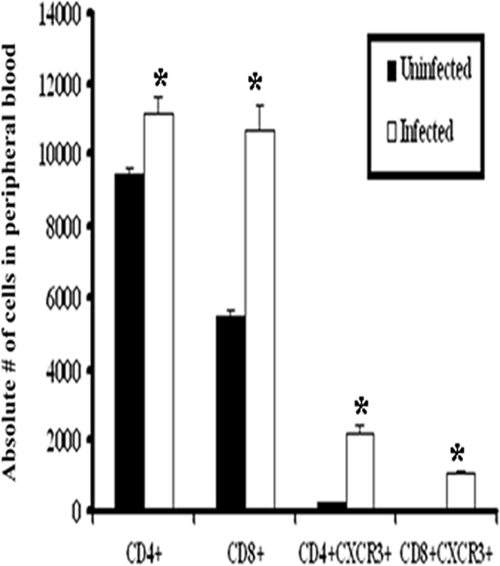
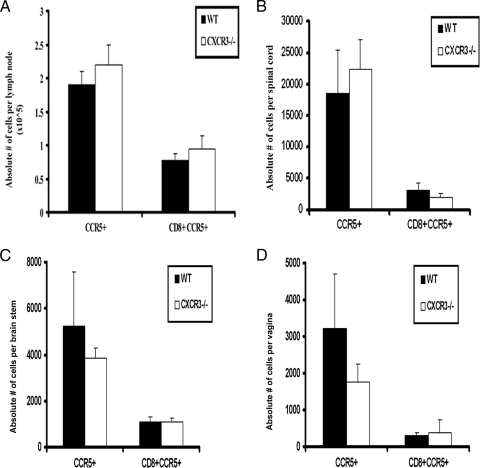
Since the data listed above indicated that the absence of CXCR3 had no significant effect on the recruitment of T cells to sites of infection following exposure to HSV-2, we compared the expression levels of chemokine receptors on these cells. Following HSV-2 exposure, there was a significant increase in the number of circulating CD4+ and CD8+ T cells in WT mice (Fig. 6). Moreover, there was a significant increase in the number of circulating CD4+ and CD8+ T cells expressing CXCR3 following infection in WT mice (Fig. 6). In contrast to peripheral blood T lymphocytes, only intracellular but not surface expression of CXCR3 was detected in T-cell subsets residing in vaginal, spinal cord, and brain stem tissue following infection (data not shown). In contrast, CCR5 expression was unaltered on CD4+ T cells (data not shown) and CD8+ T cells localized in the infected tissue or ILN in response to HSV-2 infection (Fig. 7). Since T cells and NK cells express both CXCR3 and CCR5, it is likely that the lack of a significant change in the recruitment of these cells to HSV-2-infected tissue, when comparing WT and CXCR3−/− mice, may be due, in part, to the CCR5 expression in the effector cells of CXCR3−/− mice that can respond to CCR5 ligands (e.g., CCL3 and CCL5) expressed at equivalent or elevated levels in the tissue of infected CXCR3−/− mice compared to the CCR5 expression in WT mice (Fig. 4).
FIG. 6.
CXCR3 expression in peripheral blood T cells. Blood was drawn from uninfected WT or HSV-2-infected WT mice (six mice/group) on day 7 p.i. and processed to isolate cells. Purified cells were stained with anti-CXCR3, anti-CD4, or anti-CD8 and were analyzed using a flow cytometer, as described in Materials and Method. Bars represent means ± standard errors of the means from three independent experiments. *, P was <0.05 when comparing infected and uninfected mice.
FIG. 7.
CCR5 expression in HSV-2-infected T cells. Following medroxyprogesterone acetate treatment, WT and CXCR3−/− mice (six mice/group) were infected with HSV-2 (2,000 PFU/vagina). On day 7 p.i., mice were exsanguinated, and the ILN, vaginal tissue, brain stem, and spinal cord were removed from each mouse and processed for CCR5 expression by flow cytometry. Cells were stained with anti-CCR5, anti-CD4 or anti-CD8, and anti-CD45 and analyzed by flow cytometry. Only CD8+ CCR5+ cell data are shown. Bars represent the means ± standard errors of the means from three independent experiments.
The cytolytic activity of CD8+ T cells is reduced in HSV-2-infected CXCR3−/− mice.
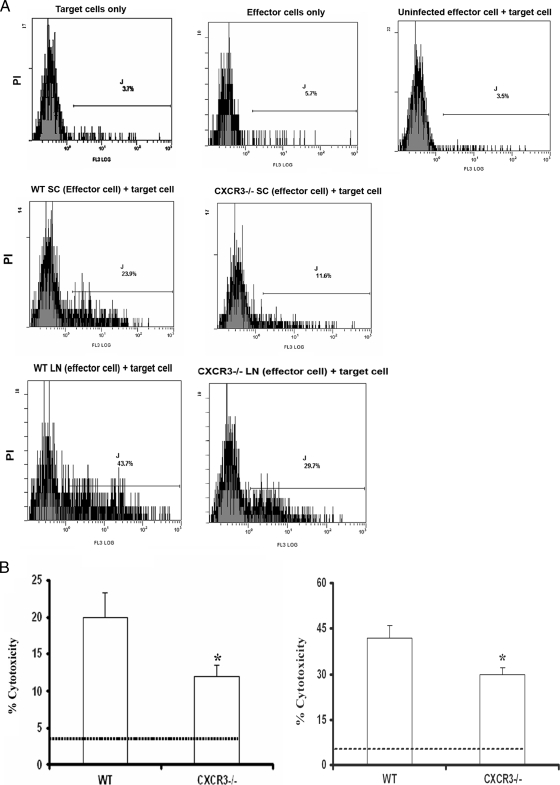
Although the absence of CXCR3 was found to increase sensitivity to genital HSV-2 infection, there was no demonstrable difference in effector cell (NK cell, total T-cell, or HSV gB-specific CD8+ T-cell) numbers in the infected tissue of CXCR3−/− mice (Fig. 5C). To address the conundrum, Percoll gradient-enriched leukocytes from the spinal cord or ILN of WT and CXCR3−/− mice were evaluated for CTL activity (Fig. 8A). Cells extracted from spinal cord preparations of CXCR3−/− mice were less efficient in the lysis of HSV-2-infected target cells than WT leukocytes (Fig. 8B, left). Likewise, ILN cells obtained from CXCR3−/− HSV-2-infected mice also showed a reduction in cytolytic activity compared to ILN cells obtained from WT animals (Fig. 8B, right). Since the cytolytic activity levels of effector cells from CXCR3−/− ILN and spinal cord samples were reduced in comparison to that of matching WT samples, we interpreted the results to suggest that the environment driving effector cell development in CXCR3−/− mice may not be optimal in comparison to that of WT mice.
FIG. 8.
Cytolytic activity of CD8+ T cells is reduced in HSV-2-infected CXCR3−/− mice. WT and CXCR3−/− female mice (six mice/group) were infected with HSV-2 (2,000 PFU/vagina. On day 7 p.i., mice were exsanguinated, and spinal cords and ILN were processed and assessed for CTL activity. (A) Representative flow histograms showing background PI incorporation into CFSE-labeled HSV-2-infected target cells incubated without effector cells, effector cells incubated without HSV-2-infected target cells, target cells incubated with uninfected WT splenocytes (SC), and cytolytic activity of effector cells from spinal cords and lymph nodes (LN) of WT mice and CXCR3−/− mice. (B) Preparations from spinal cords (left) and ILN (right) were assayed for CTL activity at an effector-to-target cell ratio of 10:1. The dotted line indicates the background PI incorporation in CSFE-labeled targets cells. The bars represent the means ± standard errors of the means from two independent experiments. *, P was <0.05 when comparing WT and CXCR3−/− mice.
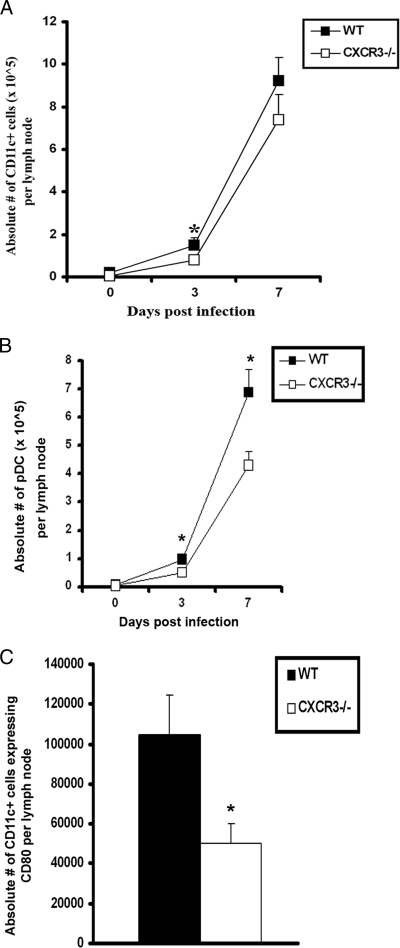
Mobilization and activation of DCs are reduced in draining lymph nodes of CXCR3−/− mice.
As antigen presentation is a requisite for the generation of CTLs, the mobilization and activation of DC populations in the ILN of HSV-2-infected CXCR3−/− and WT mice were analyzed. A significant reduction in the number of conventional DCs (B220− CD11c+) in the lymph nodes of CXCR3−/− mice was observed on day 3 p.i. (Fig. 9A). Similarly, the number of plasmacytoid DC (B220+ CD11c+) in the lymph nodes of CXCR3−/− mice was also reduced compared to that of WT animals on day 3 and 7 p.i. (Fig. 9B). In addition, both the cell number and percentage of CD80-expressing DCs from CXCR3−/− mice were reduced in comparison to the those of the WT DCs on day 3 p.i. (Fig. 9C).
FIG. 9.
DC infiltration into the ILN of HSV-2-infected mice is reduced in the absence of CXCR3. WT and CXCR3−/− mice (six mice/group) were infected with HSV-2 (2,000 PFU/vagina) and subsequently exsanguinated at the indicated time points p.i. ILN samples were processed and analyzed for B220− CD11c+ DCs (A), plasmacytoid DCs (CD11c+ B220+) (B), and total CD11c+ DCs expressing CD80 (C) by flow cytometry on day 3 p.i. The data are displayed as the means ± standard errors of the means from three independent experiments. *, P was <0.05 when comparing WT and CXCR3−/− mice.
In addition to investigating costimulatory molecule expression, other factors may also play a role, such as IFN-α, which has been associated with CD8+ T-cell activation (38). However, there was no difference in the level of IFN-α in the ILN of WT and that of CXCR3−/− mice at day 3 or day 7 p.i. (data not shown). Interleukin-18 (IL-18), which plays a significant role in stimulating DC migration and activation for antigen presentation in draining lymph nodes (7, 45), was also investigated. Like IFN-α, IL-18 levels within ILN were not different when comparing the two genotypes (data not shown). To test the possibility that negative signaling molecules, including programmed death ligand 1 (PDL-1) and PDL-2 (39), might also be involved, the expression of these molecules was analyzed on ILN DCs. However, no significant difference in PDL-1 or PDL-2 levels on draining lymph node DCs from HSV-2-infected WT and CXCR3−/− mice was observed (data not shown). Taken together, IFN-α, IL-18, PDL-1, and PDL-2 do not appear to play a role. Instead, CXCR3 expression influences mobilization as well as CD80 expression in DCs in the lymph nodes, which is consistent with aberrant effector cell maturation, which in this case is CTL activity.
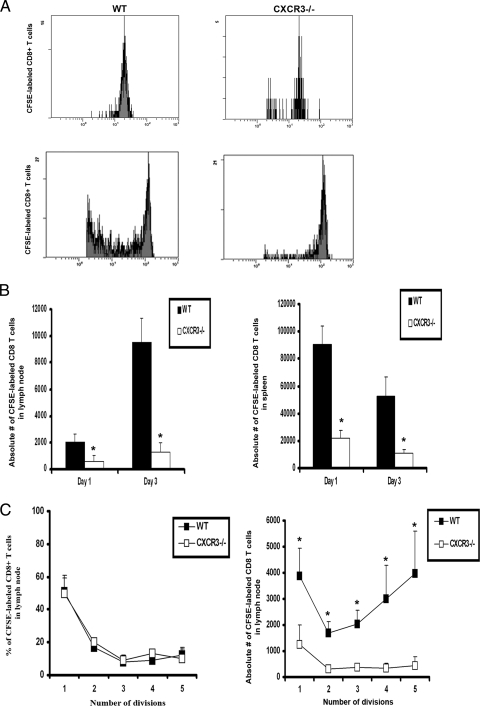
Migration but not proliferation of transferred HSV-specific CD8+ T cells is affected in organized lymphoid tissue of CXCR3−/− mice.
Since CD80 expression was diminished on DCs, and CXCL9 levels were reduced in the ILN of CXCR3−/− mice, it stands to reason that such changes may alter migration and/or proliferation of cells in situ. To address this hypothesis, carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled CD8+ effector T cells from gBT-I.1 transgenic mice, in which the T-cell receptor (TCR) is specific for HSV gB (34), were adoptively transferred into WT and CXCR3−/− mice infected 24 h earlier. The labeled transgenic T cells were monitored for migration to the organized lymphoid tissue as well as for the capacity to divide. Twenty-four hours posttransfer, the number of transgenic T cells migrating to the ILN and the spleen of WT mice was greater than the number of those migrating to the ILN and the spleen of CXCR3−/− mice (Fig. 10A and B). However, the proliferation of the cells residing in the lymph nodes was equivalent between these mouse genotypes (Fig. 10A and C). Within 72 h posttransfer, there was a significant reduction in the proliferation of CFSE-labeled gBT-I.1 T cells in the ILN and the spleen of HSV-2-infected CXCR3−/− mice in comparison to that of infected WT controls (Fig. 10A to C). However, the deficiency in the number of CFSE-labeled gBT-I.1 transgenic T cells to undergo multiple divisions is likely due to the number of labeled cells entering the lymphoid tissue, since the percentage of cells undergoing each round of division did not change when comparing WT to CXCR3−/− mice (Fig. 10C).
FIG. 10.
In vivo proliferation of virus-specific CD8+ T cells. CD8+ T cells from gBT.I-1 mice were enriched from spleen preparations by using MACS columns and labeled with CFSE (5 μM) for 15 min. Cells (1 × 106) were transferred retro-orbitally (i.v.) to WT and CXCR3−/− mice (six mice/group) infected 24 h earlier with HSV-2 (2,000 PFU/vagina). At the indicated times, mice were exsanguinated, and ILN and spleens were processed to analyze the number of CFSE-labeled CD8+ T cells. (A) Representative histograms showing the divisions of CFSE-labeled CD8+ T cells from lymph nodes of WT and CXCR3−/− mice at 24 h (top) and 72 h (bottom) p.i. (B) Absolute number of CFSE-labeled CD8+ T cells in lymph nodes (left) and spleens (right) on day 1 and day 3 p.i., with results shown as the means ± standard errors of the means. (C) Percentage of dividing CFSE-labeled CD8+ T cells (left) and absolute number of those (right) in lymph nodes. Each point represents the mean ± standard error of the mean from two independent experiments. *, P was <0.05 when comparing WT and CXCR3−/− mice.
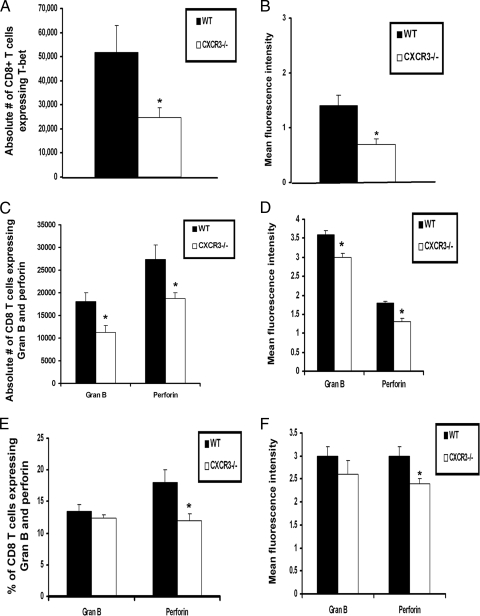
T-bet, perforin, and granzyme B expression by CD8+ T cells are reduced in CXCR3−/− mice.
Previous studies suggest that CD8+ T cells control viral infection through two main pathways, the perforin- and granzyme-mediated cytolytic pathway and/or Fas-Fas ligand (FasL)-mediated apoptosis (3, 9, 21, 61). Since T-bet has been found to regulate perforin, granzyme B, and FasL expression by CD8+ T cells (35, 46, 48), T-bet expression in CD8+ T cells was assessed. The results show that the number of CD8+ T cells expressing T-bet was reduced 50% in CXCR3−/− mice compared to that in WT mice following viral infection (Fig. 11A). Furthermore, the mean fluorescence intensity (MFI) of T-bet expression by CD8+ T cells from CXCR3−/− mice was also reduced, suggesting that on a per-cell basis of cells expressing T-bet, there was less T-bet protein present (Fig. 11B).
FIG. 11.
T-bet, perforin, and granzyme B expression by CD8+ T cells is reduced in HSV-2-infected CXCR3−/− mice. WT and CXCR3−/− mice (six mice/group) were infected with HSV-2 (2,000 PFU/vagina) and subsequently exsanguinated on day 7 p.i. The spleens were processed, and CD8+ T cells were purified using MACS columns. Enriched CD8+ T cells were analyzed for T-bet expression (A) and MFI of T-bet (log scale) by intracellular staining (B). ILN (C and D) and spinal cords (E and F) from WT and CXCR3−/− mice were processed and analyzed for the percentage of CD8+ T cells expressing granzyme B (Gran B) and perforin and for the MFI of granzyme B and perforin by intracellular staining, respectively. For spinal cord samples, lymphocytes were purified by Percoll gradient, and 100,000 cells were pooled from three animals and assayed in duplicate per experiment. Error bars represent the means ± standard errors of the means. *, P was <0.05 when comparing WT and CXCR3−/− mice.
To address the cytolytic machinery involved in CD8+ T cell effector activity, effector molecule expression was determined. FasL expression could not be detected in CD8+ T cells residing in the spinal cord and ILN of HSV-2-infected mice (data not shown). Therefore, this pathway was eliminated as a likely contributor to CD8+ T cell-directed cytolysis. In comparison, perforin and granzyme B were readily detected in CD8+ T cells in the draining lymph nodes (Fig. 11C and D) and spinal cord (Fig. 11E and F). There was a reduction in the absolute number of CD8+ T cells expressing perforin and granzyme B in the ILN of CXCR3−/− mice compared to that of WT mice (Fig. 11C). MFIs of both perforin and granzyme were also reduced by CD8+ T cells from lymph nodes of CXCR3−/− mice in comparison to those of WT mice (Fig. 11D). In addition, the percentage and number (data not shown) of CD8+ T cells expressing perforin and perforin MFI were also reduced in the spinal cord of CXCR3−/− mice (Fig. 11E and F). However, there was no significant difference in the number of CD8+ T cells expressing granzyme B residing in the spinal cord of CXCR3−/− mice compared to that of WT mice.
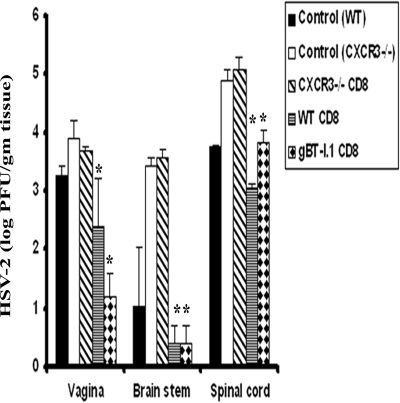
Adoptive transfer of WT or transgenic HSV gB-specific CD8+ T cells into CXCR3−/− recipients restores resistance to genital HSV-2 infection.
The outcome of this study has found an association between greater sensitivity to genital HSV-2 infection in CXCR3−/− mice and CD8+ T-cell activation, as measured by cytolytic activity and effector molecule expression. If indeed a deficiency in CD8+ T cell activation and function is the principal reason for the phenotype displayed by CXCR3−/− mice in response to HSV-2, then the establishment of a intact complementary CD8+ T-cell population should restore resistance to the infected CXCR3−/− host. To address this hypothesis, CD8+ T cells from HSV-2-infected mice were adoptively transferred into CXCR3−/− recipients, and viral replication was monitored in the infected tissue of recipient animals. CXCR3−/− mice that did or did not (control) receive CD8+ T cells from immunized CXCR3−/− mice possessed significantly more virus in the vagina, brain stem, and spinal cord than WT mice (Fig. 12). In contrast, CXCR3−/− recipients of CD8+ T cells from immunized WT or HSV gB-specific TCR transgenic mice possessed virus titers at or below WT controls (Fig. 12). This result underscores the importance of developing an optimal CTL response against genital HSV-2 infection that is compromised in CXCR3−/− mice as a result of improper CD8+ T-cell activation.
FIG. 12.
Adoptive transfer of transgenic CD8+ T cells protects CXCR3−/− mice. WT, gBT.I-1, and CXCR3−/− mice (six mice/group) were infected with HSV-2 (2,000 PFU/vagina), and spleens were removed on day 7 p.i and processed to isolate CD8+ T cells using MACS columns. The enriched CD8+ T cells (1 × 106) from WT mice (WT CD8), transgenic mice gBT.I-1 (gBT-I.1 CD8), or CXCR3−/− mice (CXCR3−/− CD8) were separately transferred i.v. to CXCR3−/− mice (six mice/group) and subsequently infected with HSV-2 (2,000 PFU/vagina). WT and CXCR3−/− mice that received no cells at the time of infection were used as control groups. Mice were exsanguinated on day 7 p.i., and vaginas, spinal cords, and brain stems were removed and processed for viral content. The error bars represent standard errors of the means from two independent experiments. *, P was <0.05 when comparing groups of mice receiving cells and CXCR3−/− mice.
DISCUSSION
CXCR3 signaling induces multiple functions of CD8+ T cells, including the chemotaxis, activation, and antiviral immune response elicited by IFN-γ-regulated ligands CXCL9, CXCL10, and CXCL11 (1, 6, 8, 26, 44). In the present study, we demonstrate that the effector function of CD8+ T cells relies on CXCR3 signaling, which is critical for protection against genital HSV-2 infection. Although a similar clinical outcome was reported for CXCR3−/− mice infected with dengue virus (16), other viral pathogens, including LCMV (28), gammaherpesvirus 68 (24), and HSV-1 (57), were found to have no apparent effect or modestly improved clinical outcome to infection. Consequently, the role of CXCR3 in host resistance to virus infection is likely dependent on the specific pathogen, the site of infection, and the effector cells required to control virus replication. In the case of genital HSV-2 infection, the results demonstrate the necessity for an optimal CTL response that is greatly influenced by CXCR3 signaling.
TCR stimulation induces expression of the T-box transcription factor T-bet, the master regulator of T-cell function capable of controlling the expression of effector molecules, including IFN-γ, perforin, granzymes, and CXCR3 (35, 46, 48). T-bet has been reported to play an important role for induction of a vaccine-induced, anti-HSV-2 immune response associated with IFN-γ production by CD4+ T cells (47). However, no function has been attributed for CD8+ T cells. In response to other pathogens, T-bet-deficient mice are unable to clear LCMV and vaccinia virus in part through a defect in IFN-γ production by virus-specific CD8+ T cells (19, 29). However, T-bet is not required for protection against murine cytomegalovirus or the bacterial pathogen Listeria monocytogenes (53, 55). The conflicting results could be due to a number of factors that contribute to the host-adaptive immune response. One candidate molecule, eomesodermin (Eomes), a paralogue of T-bet, is believed to complement the function of T-bet in the differentiation of CD8+ T cells (42). The redundant role of T-bet and Eomes has been demonstrated using compound mutant Eomes+/− Tbx21−/− mice, which display reduced CD122, perforin, and granzyme B expression by NK cells and CD8+ T cells (18). Though Eomes protein expression was not measured in this study, mRNA levels were evaluated by real-time PCR. The results showed that Eomes mRNA expression by CD8+ T cells from CXCR3−/− mice was not significantly different compared to that from WT controls (data not shown). T-bet has been reported to regulate CXCR3 expression in Th1 cells, coinciding with cell trafficking to inflammatory sites (27). In response to infection, CXCR3−/− mice showed no defect in the recruitment of T cells to sites of infection but displayed diminished cytolytic activity associated with reduced expression of T-bet, perforin, and granzyme B by CD8+ T cells. As a result, viral clearance from the infected tissues was grossly affected, leading to the elevated mortality of mice. These findings are consistent with previous studies that reported that impairment in T-bet expression leads to reduced perforin and granzyme B production by CD8+ T cells (20, 46). Since perforin, granzyme B, and T-bet expression were evaluated following stimulation of CD8+ T cells to the HSV gB498-505 peptide, it is assumed that the cells responding to the peptide are HSV gB-specific CD8+ T cells.
Like a previous study that reported the internalization of surface CXCR3 after ligand binding (31), our results also demonstrate that activated T cells lose surface expression of CXCR3 once they are recruited to the tissue, suggesting strict regulation of expression in T cells. In addition, the absence of CXCR3 does not alter CCR5 expression in T cells, suggesting functional redundancy between CXCR3 and CCR5 in the recruitment of T cells following viral infection. Previously, we reported that the lack of CCR5 expression had no significant impact on T cell recruitment, but rather, was associated with NK cell mobilization to the CNS following genital HSV-2 infection (51). Taken together, the results suggest that CXCR3- and CCR5-expressing effector T and NK cells act in concert to maximize resistance to genital HSV-2, in terms of reacting to local replication in the vaginal tract and spread into the CNS.
The increase in viral titers recovered from HSV-2-infected CXCR3−/− mice correlated with the levels of activated macrophages presumably recruited by CCL2 expression to the spinal cord and brain stem. Consequently, CCL2 may be a product of macrophages that have migrated in excessive numbers in response to an elevation in viral burden within the tissue. Alternatively, resident cells, including microglia cells, may also be a source of this chemokine. An additional product of activated macrophages, tumor necrosis factor alpha, has been implicated in neuropathology associated with genital HSV-2 infection (50). Consequently, we cannot rule out the detrimental contribution that activated macrophages may have within the CNS of CXCR3−/− mice relative to mortality.
CXCR3 expression by plasmacytoid DCs has been found to facilitate migration and appropriate priming of HSV-specific CTLs likely through the production of IFN-α, which can act on conventional DCs in antigen presentation (62). Consistent with these findings, in the present study, DC trafficking to the draining ILN of CXCR3−/− in response to genital HSV-2 infection was impaired. Of the chemokines assessed in the ILN, only CXCL9 was significantly reduced in CXCR3−/− mice at an early time point (day 3 p.i.), which matches the migration impairment of DCs. While not formally proven, it is likely that CXCL9 is a major chemoattractant for pDC and other DCs to the ILN following genital HSV-2 infection through interaction with CXCR3. In addition to a reduction in the number of DCs recruited to the ILN of CXCR3−/−-infected mice, CD80 expression on CXCR3−/− mouse DCs was reduced by fifty percent. Other costimulatory molecules are known to provide a second signal in the generation of CTLs in the absence of CD80 (58). However, as CD80 levels were present but reduced in DCs of HSV-2-infected CXCR3−/− mice, it is likely that the deficit in cytolytic activity and the reduction in T-bet, perforin, and granzyme B expression by the effector CD8+ T cells from HSV-2-infected, CXCR3−/− mice is a direct result of reduced CD80 expression.
Like CXCL9-deficient (CXCL9−/−) and CXCL10-deficient (CXCL10−/−) mice, CXCR3−/− mice were more sensitive to genital HSV-2 infection than WT animals. However, unlike CXCL9−/− and CXCL10−/− mice, which showed a reduction in the recruitment of NK cells and HSV gB-specific CD8+ T cells to infected sites (52), CXCR3−/− mice did not display any deficiency in recruitment of these cells. This discrepancy may be due in part to the redundancy between CXCR3 and CCR5, which are also expressed in NK cells and T cells. In fact, in the absence of CCR5, NK cell mobilization to the brain stem in response to HSV-2 infection is significantly diminished, whereas T-cell recruitment is not altered (51). Consequently, the absence of a specific chemokine receptor as a result of genetic manipulation in mice may facilitate a greater role for redundant receptors on effector T and NK cells within these animals that are responsive to distinct chemokines expressed at levels equivalent to or greater than those found in WT mice. CXCR3−/− mice were also found to possess a greater total cellular infiltrate in the nervous system than WT mice (Fig. 2 and 5). Although the only leukocyte population found to be significantly increased in such tissue of CXCR3−/− mice were “activated” macrophages (defined as Gr1+ F4/80+), other cell populations were increased in the CXCR3−/− samples, including CD4+ and CD8+ T cells, neutrophils, and DCs, which collectively are likely to contribute to the elevation in the total leukocyte infiltration into the nervous system.
Collectively, the present study highlights the chemokine receptor CXCR3 as an important signaling molecule required to maximize the host immune (CTL) response to genital HSV-2 infection. Even though CCR5 expression in T cells is normal in HSV-2-infected CXCR3−/− mice, which may explain a lack of a deficiency in the recruitment of T cells, the data suggest that optimal CD8+ T-cell effector function is dependent on CXCR3 signaling in response to genital HSV-2 infection. Therefore, CXCR3 is a pivotal molecular signaling receptor that operates in the recruitment of antigen-presenting cells to organized lymphoid tissue and facilitates the activation of CD8+ effector T cells by direct or indirect means. With the seroprevalence of HSV-2 in individuals ages 12 and older hovering between 40 and 50 million Americans (12, 60), the identification of chemokines and chemokine receptors that contribute to the immune response to infection is important in the development of successful intervention strategies.
Acknowledgments
This work was supported by Public Health Service grant AI067309. Additional support includes grant P20 RR017703 and NEI core grant EY12190.
We thank John Ash, Todd Wuest, Gabriel Nyugen, and the histology core unit of the Dean A. McGee Eye Institute and OUHSC for their help.
Footnotes
Published ahead of print on 8 July 2009.
REFERENCES
- 1.Agostini, C., F. Calabrese, F. Rea, M. Facco, A. Tosoni, M. Loy, G. Binotto, M. Valente, L. Trentin, and G. Semenzato. 2001. CXCR3 and its ligand CXCL10 are expressed by inflammatory cells infiltrating lung allografts and mediate chemotaxis of cells at sites of rejection. Am. J. Pathol. 158:1703-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashkar, A. A., and K. L. Rosenthal. 2003. Interleukin-15 and natural killer and NKT cells play a critical role in innate protection against genital herpes simplex virus type 2 infection. J. Virol. 77:10168-10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barry, M., and R. C. Bleackley. 2002. Cytotoxic T lymphocytes: all roads to death. Nat. Rev. Immunol. 2:401-409. [DOI] [PubMed] [Google Scholar]
- 4.Bellner, L., F. Thoren, E. Nygren, J. A. Liljeqvist, A. Karlsson, and K. Eriksson. 2005. A proinflammatory peptide from herpes simplex virus type 2 glycoprotein G affects neutrophil, monocyte, and NK cell functions. J. Immunol. 174:2235-2241. [DOI] [PubMed] [Google Scholar]
- 5.Carson, M. J., C. R. Reilly, J. G. Sutcliffe, and D. Lo. 1998. Mature macroglia resemble immature antigen-presenting cell. Glia 22:72-85. [DOI] [PubMed] [Google Scholar]
- 6.Christensen, J. E., C. D. Lemos, T. Moos, J. P. Christensen, and A. R. Thomsen. 2006. CXCL10 is the key ligand for CXCR3 on CD8+ effector T cells involved in immune surveillance of the lymphocytic choriomeningitis virus-infected central nervous system. J. Immunol. 176:4235-4243. [DOI] [PubMed] [Google Scholar]
- 7.Cumberbatch, M., R. J. Dearman, C. Antonopoulos, R. W. Groves, and I. Kimber. 2001. Interleukin (IL)-18 induces Langerhans cell migration by a tumor necrosis factor-α and IL-1β-dependent mechanism. Immunology 102:323-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dar, W. A., and S. A. Knechtle. 2007. CXCR3-mediated T-cell chemotaxis involves ZAP-70 and is regulated by signaling through the T-cell receptor. Immunology 120:467-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dobbs, M. E., J. E. Strasser, C.-F. Chu, C. Chalk, and G. N. Milligan. 2005. Clearance of herpes simplex virus type 2 by CD8+ T cells requires gamma interferon and either perforin- or Fas-mediated cytolytic mechanisms. J. Virol. 79:14546-14554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudley, K. L., N. Bourne, and G. N. Milligan. 2000. Immune protection against HSV-2 in B-cell-deficient mice. Virology 270:454-463. [DOI] [PubMed] [Google Scholar]
- 11.Duerst, R. J., and L. A. Morrison. 2003. Innate immunity to herpes simplex virus type 2. Viral Immunol. 16:475-490. [DOI] [PubMed] [Google Scholar]
- 12.Fleming, D. T., G. M. McQuillan, R. E. Johnson, A. J. Nahmias, S. O. Aral, F. K. Lee, and M. E. St. Louis. 1997. Herpes simplex virus type 1 in the United States, 1976 to 1994. N. Engl. J. Med. 337:1105-1111. [DOI] [PubMed] [Google Scholar]
- 13.Harandi, A. M., B. Svennerholm, J. Holmgren, and K. Eriksson. 2001. Differential roles of B cells and IFN-γ-secreting CD4+ T cells in innate and adaptive immune control of genital herpes simplex virus type 2 infection in mice. J. Gen. Virol. 82:845-853. [DOI] [PubMed] [Google Scholar]
- 14.Harandi, A. M., B. Svennerholm, J. Holmgren, and K. Eriksson. 2001. Protective vaccination against genital herpes simplex virus type 2 (HSV-2) infection in mice is associated with a rapid induction of local IFN-gamma-dependent RANTES production following vaginal viral challenge. Am. J. Reprod. Immunol. 46:420-424. [DOI] [PubMed] [Google Scholar]
- 15.Härle, P., V. F. Cull, M. P. Agbaga, R. F. Silverman, B. R. Williams, C. James, and D. J. J. Carr. 2002. Differential effect of murine alpha/beta interferon transgenes on antagonization of herpes simplex virus type 1 replication. J. Virol. 76:6558-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsieh, M., S. Lai, J. Chen, J. Sung, Y. Lin, B. A. Wu-Hsieh, C. Gerard, A. Luster, and F. Liao. 2006. Both CXCR3 and CXCL10/IFN-inducible protein 10 are required for resistance to primary infection by dengue virus. J. Immunol. 177:1855-1863. [DOI] [PubMed] [Google Scholar]
- 17.Iijima, N., J. M. Thompson, and A. Iwasaki. 2008. Dendritic cells and macrophages in the genitourinary tract. Mucosal Immunol. 1:451-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Intlekofer, A. M., N. Takemoto, E. J. Wherry, S. A. Longworth, J. T. Northrup, V. R. Palanivel, A. C. Mullen, C. R. Gasink, S. M. Kaech, J. D. Miller, L. Gapin, K. Ryan, A. P. Russ, T. Lindsten, J. S. Orange, A. W. Goldrath, R. Ahmed, and S. L. Reiner. 2005. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat. Immunol. 6:1236-1244. [DOI] [PubMed] [Google Scholar]
- 19.Juedes, A. E., E. Rodrigo, L. Togher, L. H. Glimcher, and M. G. Herrath. 2004. T-bet controls autoaggressive CD8 lymphocytes in type 1 diabetes. J. Exp. Med. 199:1153-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaech, S. M., S. Hemby, E. Kersh, and R. Ahmed. 2002. Molecular and functioning profiling of memory CD8 T cell differentiation. Cell 111:837-851. [DOI] [PubMed] [Google Scholar]
- 21.Keckler, M. S. 2007. Dodging the CTL response: viral evasion of Fas and granzyme induced apoptosis. Front. Biosci. 12:725-732. [DOI] [PubMed] [Google Scholar]
- 22.King, N. J. C., E. L. Parr, and M. B. Parr. 1998. Migration of lymphoid cells from vaginal epithelium to iliac lymph nodes in relation to vaginal infection by herpes simplex virus type 2. J. Immunol. 160:1173-1180. [PubMed] [Google Scholar]
- 23.Koelle, D. M., C. M. Posavad, G. R. Barnum, M. L. Johnson, J. M. Frank, and L. Corey. 1998. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J. Clin. Investig. 101:1500-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee, B. J., F. Giannoni, A. Lyon, S. Yada, B. Lu, C. Gerard, and S. R. Sarawar. 2005. Role of CXCR3 in the immune response to murine gammaherpesvirus 68. J. Virol. 79:9351-9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindell, D. M., T. E. Lane, and N. W. Lukacs. 2008. CXCL10/CXCR3-mediated responses promote immunity to respiratory syncytial virus infection by augmenting dendritic cell and CD8(+) T cell efficacy. Eur. J. Immunol. 38:2168-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loetscher, M., B. Gerber, P. Loetscher, S. A. Jones, L. Piali, I. Clark-Lewis, M. Baggiolini, and B. Moser. 1996. Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytes. J. Exp. Med. 184:963-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lord, G. M., R. M. Rao, H. Choe, B. M. Sullivan, A. H. Lichtman, F. W. Luscinskas, and L. H. Glimcher. 2005. T-bet is required for optimal proinflammatory CD4+ T-cell trafficking. Blood 106:3432-3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahalingam, S., J. M. Farber, and G. Karupiah. 1999. The interferon-inducible chemokines MuMig and Crg-2 exhibit antiviral activity in vivo. J. Virol. 73:1479-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsui, M., O. Moriya, T. Yoshimoto, and T. Akatsuka. 2005. T-bet is required for protection against vaccinia virus infection. J. Virol. 79:12798-12806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDermott, M. R., C. H. Goldsmith, K. L. Rosenthal, and L. J. Brais. 1989. T lymphocytes in genital lymph nodes protect mice from intravaginal infection with herpes simplex virus type 2. J. Infect. Dis. 159:460-466. [DOI] [PubMed] [Google Scholar]
- 31.Meiser, A., A. Mueller, E. L. Wise, E. M. McDonagh, S. J. Petit, N. Saran, P. C. Clark, T. J. Williams, and J. E. Pease. 2008. The chemokine receptor CXCR3 is degraded following internalization and is replenished at the cell surface by de novo synthesis of receptor. J. Immunol. 180:6713-6724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milligan, G. N. 1999. Neutrophils aid in protection of the vaginal mucosae of immune mice against challenge with herpes simplex virus type 2. J. Virol. 73:6380-6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milligan, G. N., D. I. Bernstein, and N. Bourne. 1998. T lymphocytes are required for protection of the vaginal mucosae and sensory ganglia of immune mice against reinfection with herpes simplex virus type 2. J. Immunol. 160:6093-6100. [PubMed] [Google Scholar]
- 34.Mueller, S. N., W. Heath, J. D. McLain, F. R. Carbone, and C. M. Jones. 2002. Characterization of two TCR transgenic mouse lines specific for herpes simplex virus. Immunol. Cell Biol. 80:156-163. [DOI] [PubMed] [Google Scholar]
- 35.Mullen, A. C., F. A. High, A. S. Hutchins, H. W. Lee, A. V. Villarino, D. M. Livingston, A. L. Kung, N. Cereb, T. P. Yao, S. Y. Yang, and S. L. Reiner. 2001. Role of T-bet in commitment of Th1 cells before IL-12-dependent selection. Science 292:1907-1910. [DOI] [PubMed] [Google Scholar]
- 36.Nagot, N., A. Ouedraogo, M. C. Defer, R. Vallo, P. Mayaud, and P. Van de Perre. 2007. Association between bacterial vaginosis and herpes simplex virus type 2 infection: implications for HIV acquisition studies. Sex. Transm. Infect. 83:365-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Farrell, N., P. Moodley, and A. W. Sturm. 2007. Genital herpes in Africa: a time to rethink treatment. Lancet 370:2164-2166. [DOI] [PubMed] [Google Scholar]
- 38.Ogasawara, K., S. Hida, Y. Weng, A. Saiura, K. Sato, H. Takayanagi, S. Sakaguchi, T. Yokochi, T. Kodama, M. Naitoh, J. A. De Martino, and T. Taniguchi. 2002. Requirement of the IFN-α/β-induced CXCR3 chemokine signaling for CD8+ T cell activation. Genes Cells 7:309-320. [DOI] [PubMed] [Google Scholar]
- 39.Okazaki, T., and T. Honzo. 2007. PD-1 and PD-1 ligands: from discovery to clinical application. Int. Immunol. 19:813-824. [DOI] [PubMed] [Google Scholar]
- 40.Parr, M. B., L. Kepple, M. R. McDermott, M. D. Drew, J. J. Bozzola, and E. L. Parr. 1994. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab. Investig. 70:369-380. [PubMed] [Google Scholar]
- 41.Parr, M. B., and E. L. Parr. 2000. Interferon-γ up-regulates intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 and recruits lymphocytes into the vagina of immune mice challenged with herpes simplex virus-2. Immunology 99:540-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pearce, E. L., A. C. Mullen, G. A. Martins, C. M. Krawczyk, A. S. Hutchins, V. P. Zediak, M. Banica, C. B. DiCioccio, D. A. Gross, C. A. Mao, H. Shen, N. Cereb, S. Y. Yang, T. Lindsten, J. Rossant, C. A. Hunter, and S. L. Reiner. 2003. Control of effector CD8+ T cell function by the transcription factor eomesodermin. Science 302:1041-1043. [DOI] [PubMed] [Google Scholar]
- 43.Posavad, C. M., M. L. Huang, S. Barcy, D. M. Koelle, and L. Corey. 2000. Long term persistence of herpes simplex virus-specific CD8+ CTL in persons with frequently recurring genital herpes. J. Immunol. 165:1146-1152. [DOI] [PubMed] [Google Scholar]
- 44.Qin, S., J. B. Rottman, P. Myers, N. Kassam, M. Weinblatt, M. Loetscher, A. E. Koch, B. Moser, and C. R. Mackay. 1998. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J. Clin. Investig. 101:746-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stoll, S., H. Jonuleit, S. Schmitt, G. Muller, H. Yamauchi, M. Kurimoto, J. Knop, and A. H. Enk. 1998. Production of functional IL-18 by different subtypes of murine and human dendritic cells (DC): DC-derived IL-18 enhances IL-12-dependent Th1 development. Eur. J. Immunol. 28:3231-3239. [DOI] [PubMed] [Google Scholar]
- 46.Sullivan, B. M., A. Juedes, S. J. Szabo, M. V. Herrath, and L. H. Glimcher. 2003. Antigen-driven effector CD8 T cell function regulated by T-bet. Proc. Natl. Acad. Sci. USA 100:15818-15823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Svensson, A., I. Nordstrom, J. Sun, and K. Eriksson. 2005. Protective immunity to genital herpes simplex virus infection is mediated by T-bet. J. Immunol. 174:6266-6273. [DOI] [PubMed] [Google Scholar]
- 48.Szabo, S. J., S. T. Kim, G. L. Costa, X. Zhang, C. G. Fathman, and L. H. Glimcher. 2000. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100:655-669. [DOI] [PubMed] [Google Scholar]
- 49.Thapa, M., and D. J. J. Carr. 2008. Chemokines and chemokine receptors critical to host resistance following genital herpes simplex virus type 2 (HSV-2) infection. Open Immunol. J. 1:33-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thapa, M., and D. J. J. Carr. 2008. Herpes simplex virus type 2 (HSV-2)-induced mortality following genital infection is blocked by anti-TNF-alpha antibody in CXCL10-deficient (CXCL10−/−) mice. J. Virol. 82:10295-10301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thapa, M., W. A. Kuziel, and D. J. J. Carr. 2007. Susceptibility of CCR5-deficient mice to genital herpes simplex virus type 2 is linked to NK cell mobilization. J. Virol. 81:3704-3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thapa, M., R. S. Welner, R. Pelayo, and D. J. J. Carr. 2008. CXCL9 and CXCL10 expression are critical for control of genital herpes simplex virus type 2 infection through mobilization of HSV-specific CTL and NK cells to the nervous system. J. Immunol. 180:1098-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Townsend, M. J., A. S. Weinmann, J. L. Matsuda, R. Salomon, P. J. Farnham, C. A. Biron, L. Gapin, and L. H. Glimcher. 2004. T-bet regulates the terminal maturation and homeostasis of NK and Vα14i NKT cells. Immunity 20:477-494. [DOI] [PubMed] [Google Scholar]
- 54.Wareing, M. D., A. B. Lyon, B. Lu, C. Gerard, and S. R. Sarawar. 2004. Chemokine expression during development and resolution of a pulmonary leukocyte response to influenza A virus infection in mice. J. Leukoc. Biol. 76:886-895. [DOI] [PubMed] [Google Scholar]
- 55.Way, S. S., and C. B. Wilson. 2004. Immunity and IFN-γ production during Listeria monocytogenes infection in the absence of T-bet. J. Immunol. 173:5918-5922. [DOI] [PubMed] [Google Scholar]
- 56.Whitley, R. J., and R. L. Miller. 2001. Immunologic approach to herpes simplex virus. Viral Immunol. 14:111-118. [DOI] [PubMed] [Google Scholar]
- 57.Wickham, S., B. Lu, J. Ash, and D. J. J. Carr. 2005. Chemokine receptor deficiency is associated with increased chemokine expression in the peripheral and central nervous systems and increased resistance to herpetic encephalitis. J. Neuroimmunol. 162:51-59. [DOI] [PubMed] [Google Scholar]
- 58.Williams, M. A., and M. J. Bevan. 2007. Effector and memory CTL differentiation. Annu. Rev. Immunol. 25:171-192. [DOI] [PubMed] [Google Scholar]
- 59.Wuest, T. R., and D. J. J. Carr. 2008. Dysregulation of CXCR3 signaling due to CXCL10 deficiency impairs the antiviral response to herpes simplex virus 1 infection. J. Immunol. 181:7985-7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu, F., M. R. Sternberg, B. J. Kottiri, G. M. McQuillan, F. K. Lee, A. J. Nahmias, S. M. Berman, and L. E. Markowitz. 2006. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 296:864-973. [DOI] [PubMed] [Google Scholar]
- 61.Yasukawa, M., H. Ohminami, A. Junko, Y. Kasahara, Y. Ishida, and S. Fujita. 2000. Granule exocytosis, and not the Fas/Fas ligand system, is the main pathway mediated by alloantigen-specific CD4+ as well as CD8+ cytotoxic T lymphocytes in humans. Blood 95:2352-2355. [PubMed] [Google Scholar]
- 62.Yoneyama, H., K. Matsuno, E. Toda, T. Nishiwaki, N. Matsuo, A. Nakano, S. Narumi, B. Lu, C. Gerard, S. Ishikawa, and K. Matsushima. 2005. Plasmacytoid DCs help lymph node DCs to induce anti-HSV CTLs. J. Exp. Med. 202:425-435. [DOI] [PMC free article] [PubMed] [Google Scholar]