Abstract
Chaperone-enriched domains are formed in the nuclei of cells lytically infected with herpes simplex virus type 1 (HSV-1). These domains, called VICE, for virus induced chaperone enriched, contain Hsc70, Hsp70, Hsp40, Hsp90, polyubiquitinated proteins, and components of the proteasome machinery. Accumulating evidence indicates that these sites may be utilized during infection to sequester misfolded, modified, or otherwise unwanted proteins away from viral replication compartments, sites of robust transcription, DNA synthesis, and capsid maturation. To further explore the role of cellular chaperones and VICE domains during HSV-1 infection, we have analyzed the cytoprotective chaperone Hsp27. Here we present evidence that Hsp27, which is known to possess several antioxidant functions, is rapidly reorganized and modified at early stages in response to HSV-1 infection and signaling from the mitogen-activated protein kinase p38. Immunofluorescence analysis and fractionation experiments reveal disparate subcellular localizations of nonphosphorylated and phosphorylated forms of Hsp27 during wild-type HSV-1 infection. Unmodified forms of Hsp27 are localized in nuclear foci that are outside of replication compartments, adjacent to VICE domains, and in the cytoplasm. Conversely, we find that phosphorylated forms of Hsp27 are localized exclusively in the cytoplasm. Last, in cells depleted of all forms of Hsp27, virus replication is significantly reduced.
Often during viral infection, stress pathways are activated in response to foreign nucleic acids, changes in the amount of unfolded proteins, or the oxidation state of the cell (28). Viruses have evolved so as to not only counter this response but to also exploit it in the creation of a favorable environment for production of progeny. We have shown that in herpes simplex virus type 1 (HSV-1)-infected cells, components of several chaperone systems are redistributed within the cell (6, 7). Notably, both specialized (Hsp90) and classical (Hsc70/Hsp70) chaperones, as well as cochaperone proteins (Hsp40), are enriched within foci that are adjacent to viral replication compartments, which are the sites of viral DNA replication, virus assembly, and genome encapsidation (6, 7). These foci, termed virus-induced chaperone-enriched or VICE domains, are also rich in polyubiquitinated proteins, ubiquitinated viral proteins, and components of the 20S proteasome. Interestingly, the specialized chaperone Hsp90 has also been found to have a strongly localized distribution within viral replication compartments (6). We determined that the activity of Hsp90 is required for nuclear localization of the viral polymerase (6); however, possible additional roles for Hsp90 within replication compartments remain to be determined. We speculate that VICE domains are sites of protein remodeling and/or degradation and that the active sequestration of the chaperones and other proteolytic proteins evolved either as a means to delay the apoptosis cascade that would be triggered by massive amounts of misfolded proteins or as a mechanism to remove a specific population of proteins during infection.
The multifunctional mammalian heat shock protein 27 (Hsp27) is a member of the small heat shock protein family, which includes α- and β-crystallins (1, 2). Its ability to adopt many conformations through oligomerization allows this small protein to perform numerous important and complex functions in cells. Through its α-crystallin domain, Hsp27 assembles into higher-order oligomers ranging from 60 to 800 MDa in size (17). These multimeric forms of Hsp27 have been shown to bind to misfolded or oxidized proteins and to remodel them into intermediates suitable for refolding by other chaperone systems (Hsp70 or Hsp90). This ability to transfer proteins for remodeling or refolding by other chaperones is unique to Hsp27 and has caused Hsp27 to be termed a “holdase” rather than a classic folding chaperone. Hsp27 can promote damaged protein turnover through ubiquitin-dependent (23, 24) and -independent (3) mechanisms, both of which utilize the proteasome for degradation. Interestingly, several studies indicate that oxidized proteins are degraded in a 20S proteasome-dependent, ubiquitin-independent fashion (11).
The balance between large oligomers and smaller forms of Hsp27 is regulated by the cellular stress response kinase system (1, 2). During times of stress, phosphorylation by the mitogen-activated protein kinase p38 can occur on three amino acid residues (Ser15, Ser78, and Ser82) of the Hsp27 molecule (19). This modification stimulates the dissociation of the oligomeric Hsp27 complex into dimeric and monomeric subunits. Dimers and monomers of Hsp27 possess exclusive functions that are dependent on the protein's phosphorylation state. It has been shown that p38 is activated in HSV-1-infected cells through a mechanism that is dependent upon viral immediate-early gene products, in particular the immediate-early protein ICP27 (Infected Cell Protein 27) (14, 16). Treatment with chemical inhibitors that block p38 activation resulted in reduction in HSV-1 titers in tissue culture, although the levels of viral transcription were not interrupted (16). It is unknown whether HSV-1 replication is influenced by some of the functions of Hsp27, a known substrate of p38, or whether the reduction in virus production when p38 is inhibited is a consequence of compromised Hsp27 function. In this report, we describe experiments designed to characterize the modification state, subcellular localization, and viral requirement for this complex cellular chaperone.
MATERIALS AND METHODS
Cells, viruses, standard techniques, and reagents.
African green monkey kidney cells (Vero; American Type Culture Collection, Rockville, MD) were propagated and maintained by the Wadsworth Center Tissue Culture Core facility. HeLa cells used for small interfering RNA (siRNA) applications were propagated according to ATCC instructions by the Wadsworth Center Tissue Culture Core facility. The KOS strain of herpes simplex virus type 1 was used as the wild-type virus in these studies. Wild-type virus handling was performed as described previously (31). Western blot analysis was performed as described previously (7). Virus infection was performed as described previously (31). All the infections were performed at a multiplicity of infection (MOI) of 5 PFU/ml except where indicated otherwise. Viral plaque assays were performed using methyl cellulose overlay and crystal violet staining. After viral infection, methyl cellulose (2% solution made in 2× Dulbecco's modified Eagle medium; Sigma Chemical, St. Louis, MO) was added to the infected cell monolayers. After plaque formation, the monolayer was fixed with 3.7% formaldehyde (in phosphate-buffered saline [PBS]) for 3 h. The monolayers were then washed and stained with 0.5% crystal violet solution (in 1:3 ethanol:H20) for 3 h to overnight. The crystal violet was rinsed, and viral plaques were subsequently counted. Antibodies used in these studies include the following: anti-γ-tubulin (rabbit polyclonal; Sigma Chemical, St. Louis, MO), anti-Hsp27 (rabbit polyclonal and mouse monoclonal; Stressgen, Ann Arbor, MI), anti-phosphorylated Hsp27 Ser15, Ser78, and Ser82 (rabbit polyclonal antibodies; Stressgen, Ann Arbor, MI), anti-Hsc70 (rat monoclonal; Stressgen, Ann Arbor, MI), anti-ICP8 (mouse monoclonal; Abcam, Cambridge, MA), and anti-L26 (rabbit polyclonal; Sigma, St. Louis, MO). Protease inhibitor tablets were purchased from Roche Diagnostics (Indianapolis, IN). Protease inhibitor cocktails, dimethylsulfoxide, and the p38 inhibitor SB203580 (SB) were purchased from Sigma Chemical (St. Louis, MO) and were prepared as per the manufacturer's instructions. SB was used at a concentration of 10 μM.
RNA interference (RNAi) analysis.
HeLa cells seeded into six-well (35 mm/well) plates at 30% confluence were transfected with siRNA according to the manufacturer's specifications (Dharmacon Technologies) at 100 pmol/well. Controls for siRNA experiments were a siRNA against cyclophilin B (siGLO cyclophilin B control siRNA D-001610-01-05) and a nonspecific siRNA (ON- TARGET Plus nontargeting siRNA #1 D-001810-01-05). siRNA pools specific for human Hspb1 (siGENOME ON-TARGETplus SMARTpool L-005269-0005) were used to knock down the expression of Hsp27. Levels of the Hsp27 protein after siRNA transfection were analyzed by Western blotting and immunofluorescence. HeLa cells were incubated with siRNA for a minimum of 96 h before infection with HSV-1 at an MOI of 0.1 PFU/ml for various times of infection. Assessment of protein levels and viral growth kinetics was performed. Data were compiled and plotted using the Graphpad Prism 5.0a software program.
Nuclear/cytoplasmic fractionation.
Flasks (75 cm2) of confluent Vero cells were either infected with wild-type HSV (KOS strain) for 6 h or were left uninfected. The flasks were washed twice with cold PBS. A 1.5-ml volume of freshly prepared cold buffer A (10 mM HEPES [pH 7.9], 10 mM KCl, 0.1 mM EDTA, protease inhibitor cocktail [Sigma], 1 mM dithiothreitol [DTT], 0.01% NP-40 substitute) was added directly to each flask, with incubation at room temperature for 5 min. Following this, the cells were scraped with a sterile scraper, pipetted several times to disrupt the clumps, transferred to a sterile tube, and placed on ice. Lysates were centrifuged at 4°C at 9,300 × g for 5 min, and the tubes were returned to the ice. The supernatant (cytosolic fraction) was transferred to a fresh tube and stored at −20°C. A 300-μl volume of cold SDS buffer (200 mM Tris [pH 8.8], 2% sodium dodecyl sulfate [SDS], 10% glycerol, and 100 mM DTT) was added to each tube, and the pellet was resuspended by pipetting. Following this, the samples were subjected to cup sonication at 50% duty. The samples were then centrifuged at 4°C at 9,300 × g for 5 min. The supernatant (nuclear fraction) was transferred to a fresh tube and stored at −20°C until further use.
Immunofluorescence studies.
African green monkey kidney cells (Vero; American Type Culture Collection, Rockville, MD) were grown on sterile glass coverslips to 60% confluence. Cells were infected at an MOI of 5 for 6 h. After infection, the medium was removed by aspiration and the cells were washed three times with PBS at room temperature. The cells were then fixed with 4% paraformaldehyde for 10 min, after which the coverslips were again washed three times with PBS. Cells analyzed for Hsp27 levels after siRNA treatment were fixed with 200-proof ice-cold methanol. Cells were permeabilized with 1% NP-40 (in PBS) for 10 min, and after three washes with PBS, the coverslips were treated with blocking solution (3% bovine serum albumin in PBS) for at least 1 h at room temperature. The coverslips were inverted and placed on 100 μl of diluted primary antibody for 1 h. Hsp27, ppHsp27 (Ser15, Ser78, and Ser82), and ICP8 antibodies were diluted 1:600 in blocking solution. The coverslips were then washed with PBS seven times before inversion on 100 μl of diluted secondary antibody for 1 h. All of the secondary antibodies were highly cross-absorbed Alexa Fluor antibodies (Molecular Probes) at a 1:600 dilution. Coverslips were washed seven times with PBS, rinsed with distilled water, and mounted on slides using ProLong Gold with 4′,6′-diamidino-2-phenylindole (DAPI) (Molecular Probes). After the mounted slides had set, clear nail polish was used to seal the coverslips. Images were obtained using the Openlab 4.0.4 software program at magnification ×63 or ×100 with a Zeiss Axiovert 200 M inverted microscope and a Q Imaging or Hamamatsu Orca ER camera. Images were deconvolved from 0.3-μm-thick optical sections using Openlab software. The Adobe Photoshop CS software program was also used to prepare the images for figures. For evaluation of cross-reactivity, controls included mock-infected cells and infected cells that had been treated with different combinations of primary and secondary antibodies.
RESULTS
Hsp27 localizes to foci adjacent to VICE domains early in HSV-1 infection.
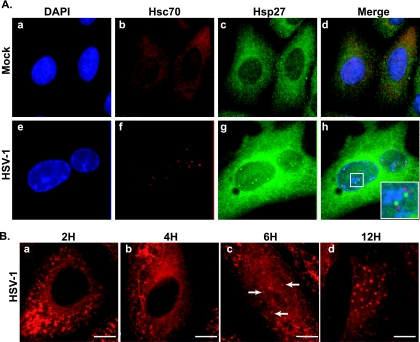
Upon infection, HSV-1 establishes replication compartments within the nucleus (25). It has previously been shown that the host cell chaperone proteins Hsp90, Hsp/Hsc70, and Hsp40 are sequestered by the virus in VICE domains, which are located at sites adjacent to replication compartments (6, 7). Since Hsp27 has many roles that are potentially beneficial to the virus and can coordinate the transfer of misfolded proteins to other chaperone systems, we wanted to determine the protein's subcellular distribution in HSV-1-infected cells. In uninfected cells, both Hsc70 and Hsp27 are detected in a diffuse staining pattern throughout the cell (Fig. 1A, frames b and c). In infected cells, Hsc70 is detected at nuclear foci, which are adjacent to viral replication compartments as described previously (7) (Fig. 1A, frame f). Using a polyclonal antibody that recognizes all forms of Hsp27, we observed a strong signal for Hsp27 in the cytoplasm, at the nuclear periphery, and at foci within the nucleus adjacent to replication compartments (Fig. 1A, frames g and h). The outlines of the replication compartments can be observed in the DAPI channel (Fig. 1A, frame e). Interestingly, Hsp27-enriched foci did not coincide with the Hsc70 distribution but rather were distinct and often peripheral to VICE domains in infected cells (Fig. 1A, frame h, inset). The formation of VICE domains was shown previously to occur at very early time points postinfection, in response to immediate-early gene expression (7, 20). To address the timing of Hsp27 focus formation, we conducted a time course immunofluorescence microscopic assay. At an MOI of 5 PFU/ml, Hsp27 can be detected in nuclear foci at 6 h postadsorption (Fig. 1B, frame c) but not at 4 h (Fig. 1B, frame b), indicating that its sequestration in the nucleus occurs after VICE domain formation. Hsp27 foci (like VICE domains) are long-lived, persisting in infected cells for up to 12 h postinfection (Fig. 1B, frame d).
FIG. 1.
Hsp27 localization in HSV-1-infected cells. (A) Immunofluorescence analysis of cellular chaperones at 6 h in uninfected cells (a to d) or HSV-1-infected cells (e to h). Shown are staining profiles for DNA using DAPI (blue in frames a and e and the merged images), the host chaperone Hsc70—a marker of VICE domains (red in frames b and f and the merged images), and Hsp27 (green in frames c and g and the merged images). The Hsp27 antibody used in this experiment recognizes all forms of Hsp27 (unmodified and phosphorylated). Composite images showing the three signals merged are shown in frames d and h. The inset in frame h shows a magnified view of VICE domains and Hsp27 foci. (B) Time course immunofluorescence analysis of Hsp27. Staining profiles of Hsp27 at 2, 4, 6, and 12 h (a to d, respectively) after HSV-1 infection are shown. White arrows show Hsp27 foci in the nucleus during viral infection.
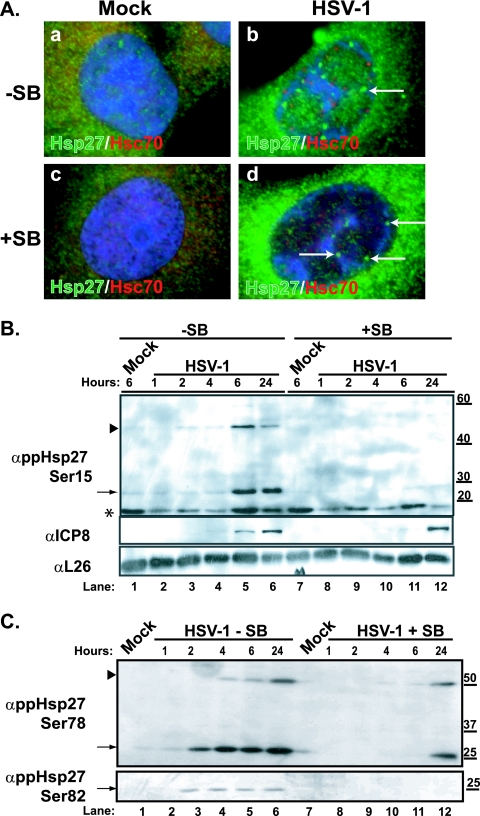
We next sought to define whether the phosphorylation state of Hsp27 affects the formation of nuclear foci. We treated cells with a chemical inhibitor of p38, the kinase that phosphorylates Hsp27 during periods of stress, and analyzed the subcellular distribution of Hsp27. Hsp27 staining was similar between uninfected cells treated with the p38 inhibitor and untreated uninfected cells (Fig. 2A, green in frames a and c). Treatment with the p38 inhibitor SB203580 did not alter the ability of HSV-1 to induce the formation of VICE domains (Fig. 2A, red in frames b and d). In independent experiments, we confirmed the inhibitory activity of SB on phosphorylation of ATF-2, a well-characterized substrate of the p38 kinase (data not shown). Likewise, sequestration of Hsp27 in the nucleus was unaffected (Fig. 2A, frames b and d), which indicates that the phosphorylation state of Hsp27 proteins does not influence the nuclear distribution in infected cells. Moreover, this observation suggests that Hsp27 proteins at foci adjacent to VICE domains are not phosphorylated, a fact that may be important in understanding the function of Hsp27 in the nuclei of HSV-1-infected cells.
FIG. 2.
Hsp27 focus formation and modification and p38 activity during HSV-1 infection. (A) Merged images of staining profile for the cellular chaperone and marker for VICE domains, Hsc70 (red) and Hsp27 species (green), in uninfected (a and c) or HSV-1-infected cells (b and d) fixed at 6 h are shown. Cells shown in frames c and d have been treated with the p38 inhibitor SB during the time of infection (6 h). White arrows show Hsp27 foci. (B) Western blotting analysis of phosphorylated Hsp27 (Ser15) (upper panel) in HSV-1-infected or mock-infected Vero cell lysates. Cells were either left untreated (−SB) or treated with SB. Samples for 1-, 2-, 4-, 6-, and 24-h time points were collected. The slower-migrating phospho-reactive form is indicated by a black arrowhead. The viral ICP8 protein (middle panel) is used as a marker for infection, and the cellular L26 protein is used as a loading control (bottom panel). (C) Western blotting of phosphorylated Hsp27 (Ser78 and Ser82) of HSV-1-infected Vero cell lysates. Cells were either left untreated (−SB) or treated with 10 μM SB (+SB). Samples for 1-, 2-, 4-, 6-, and 24-h time points were collected. The slower-migrating phospho-reactive form is indicated by a black arrowhead. A faster-migrating cross-reacting species detected in ppHsp27Ser15 Western blots is indicated with an asterisk. Molecular weight markers are indicated on the right. “α” indicates antibody.
Hsp27 is rapidly phosphorylated during HSV-1 infection.
The many and varied functions of human Hsp27 are performed by different oligomeric species, and the balance among these species is governed by the mitogen-activated stress kinases within the cell. During stress, the stress-activated kinase p38 phosphorylates Hsp27 on any of three serines (Ser15, Ser78, and/or Ser82) and this phosphorylation triggers the dissociation from the higher-order oligomer (26). The phosphorylated forms perform auxiliary roles within the stressed cell. We analyzed the phosphorylation state of Hsp27 during wild-type HSV-1 infection, using monoclonal antibodies specific for each of the phosphorylated forms. Western blot analysis indicated that the overall levels of Hsp27 did not dramatically change during the first few hours after infection (data not shown). Use of phospho-specific Hsp27 antibodies revealed that Hsp27 was phosphorylated on Ser15 (Fig. 2B, lane 5) and at Ser78 and Ser82 (Fig. 2C, lane 3) between 2 and 6 h postinfection. The phosphorylation of Hsp27 was blocked by the p38 inhibitor SB (Fig. 2B, lanes 8 to 12, and C, lanes 8 to 12) but not by the chemical carrier dimethylsulfoxide (data not shown). The half-life of SB has been reported to be 12 h (30), likely explaining why phospho-reactive species were detected in some samples at 24 h postinfection in cells treated with SB (Fig. 2C, lane 12), indicating that p38 activity is required to phosphorylate Hsp27 in HSV-1-infected cells.
During infection, slower-migrating phospho-reactive species were observed in Ser15 and Ser78 Western blots (Fig. 2B and C). These forms were not observed in lysates derived from SB-treated cells (Fig. 2B and C, lanes 8 to 11) or in lysates from uninfected or heat-stressed cells (data not shown), indicating that they may be cross-linked phosphorylated Hsp27 or a cross-reacting viral protein. Hsp27 monomers have been reported to form cross-links to one another in times of severe oxidative stress and/or to “client” proteins in certain protein folding disorders (e.g., Huntington's disease and Parkinson's disease); the latter linking occurs via the activity of tissue transglutaminases (4, 22). We were unable to prevent the accumulation of these forms of Hsp27 by chemical treatment with cystamine, an inhibitor of tissue transglutaminase function in vitro. Moreover, these forms were resistant to the effects of reducing agents such as β-mercaptoethanol or DTT (data not shown). Although more studies are needed to determine the exact nature and function of these forms of Hsp27, our results indicate that these species may be Hsp27 monomers cross-linking to one another or to client proteins during infection. Notwithstanding, the accumulation of Hsp27 cross-links may be indicative of a severe oxidative imbalance in infected cells at this time.
Phosphorylated Hsp27 is distributed in the cytoplasm of HSV-1-infected cells.
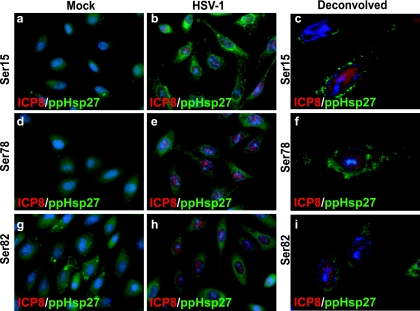
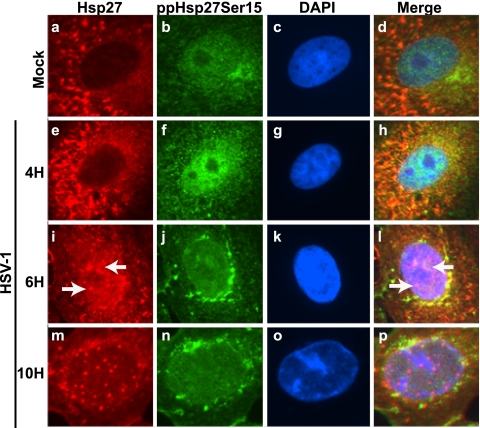
We next analyzed the subcellular localization of phosphorylated forms of Hsp27 in infected cells. In uninfected subconfluent cells, faint cytoplasmic staining for ppHsp27Ser15 (Fig. 3, frame a) and ppHsp27Ser78 forms (Fig. 3, frame d) was detected. The antibody specific for the form of Hsp27 that is phosphorylated on Ser82 revealed a dense, uneven staining pattern in the cytoplasm of uninfected cells (Fig. 3, frame g). In HSV-1-infected cells, strong staining for each of the phosphorylated forms was observed in the cytoplasm of infected cells at 6 h postinfection (Fig. 3, frames b, e, and h). This finding is consistent with the results of Western blot analysis of samples collected at that time, which indicated an increase in the quantity of phosphorylated protein species at this time. Although hundreds of cells were imaged, we were unable to detect signal above the background level for the phosphorylated forms in the nucleus at this time point. Deconvolution was applied to several optical sections of cells to refine the signal acquired on our light microscope (Fig. 3, frames c, f, and i). No nuclear signal was observed with any of the Hsp27 phospho-specific antibodies after this processing, a finding that was confirmed by confocal microscopy experiments performed subsequently (data not shown). When Hsp27 and ppHsp27 signals were collected simultaneously from cells at discrete time points over the course of an infection, a similar disparity in staining patterns was observed (Fig. 4). Hsp27 foci were present as early as 6 h postinfection (Fig. 4, frames i and I) and remained visible throughout the course of infection. Phosphorylated Hsp27 signal, on the other hand, was detectable by immunofluorescence microscopy in the nucleus at 4 h postinfection and in the cytoplasm subsequently (Fig. 4, frames f, j, and n).
FIG. 3.
Immunofluorescence analysis of phospho-reactive species of Hsp27 in HSV-1-infected cells. Merged images of staining profiles for the viral single-strand DNA binding protein (ICP8; red) and phospho-reactive Hsp27 species (ppHsp27; green) in uninfected (a, d, and g) or HSV-1-infected (b, e, and h) cells collected at 6 h are shown. Deconvolved images of each ppHsp27 species are shown in panels c, f, and i.
FIG. 4.
Immunofluorescence time course analysis of Hsp27 and phosphorylated Hsp27 in virus-infected cells. Immunofluorescence analysis of cellular chaperones at various time points in uninfected cells (top row, Mock) or HSV-1-infected cells (bottom three rows) is shown. Infected cells were collected at 4, 6, and 10 h after infection with HSV-1. Uninfected cells were fixed at 10 h after the start of the infection. Staining profiles for Hsp27 (red), for phospho-reactive Hsp27 species (ppHsp27Ser15; green), and for DNA using DAPI (blue) are shown. Merged images are shown in the last column. Hsp27 localization at nuclear foci is observed as early as 6 h after infection (white arrows in frames i and l).
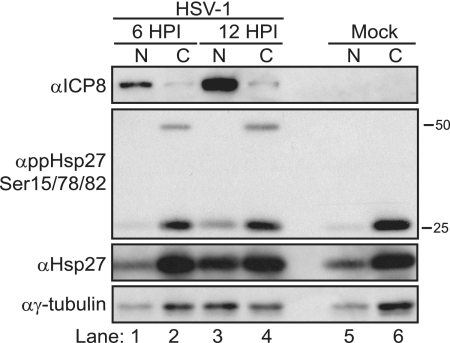
To investigate this further, we fractionated nuclear and cytoplasmic material from HSV-1-infected or uninfected cells at two time points to compare Hsp27 protein profiles in the two compartments by Western blot analysis. Viral ICP8 (Fig. 5, top panel) served as a marker for infection and an indicator of the quality of our fractionation procedure because it is a strictly nuclear protein (Fig. 5, lanes 1 and 3, first panel from the top). This protein increased over the course of infection. We used the housekeeping protein γ-tubulin as a loading control to ensure that similar amounts of total protein were assayed among samples (Fig. 5, bottom panel). The increase in γ-tubulin signal detected in nuclear fractions is most likely due to the fragility of the infected cell nuclear membrane at later times (Fig. 5, bottom panel, lane 3). Western blot analysis of the phosphorylated forms of Hsp27 confirmed that these forms are found primarily in the cytoplasm during infection at this time (Fig. 5, second panel from the top, lanes 2 and 4). In uninfected cells, some phospho-specific Hsp27 was detected 12 h after the experiment began (Fig. 5, lane 6, second panel from the top). The presence of phospho-specific Hsp27 is unlike what we observe when subconfluent uninfected cells are analyzed by Western blotting (Fig. 2A). We suspect that the discrepancy is due to some stress signaling that occurs when cells approach confluence, signaling that does not necessarily occur to the same extent in the subconfluent cells used in our infection studies. Modest amounts of the 25-kDa phospho-reactive species and of γ-tubulin were found in the nuclear fraction at 12 h postinfection (Fig. 5, second panel from the top, lanes 1 and 3). As in our previous experiments (Fig. 2), a slower-migrating 50-kDa dimeric form of phospho-Hsp27 was detected but only in the cytoplasmic fractions and only in HSV-1-infected cells (Fig. 5, second panel from the top, lanes 2 and 4). Hsp27 was found in both nuclear and cytoplasmic compartments in uninfected and HSV-1-infected cells (Fig. 5, third panel from the top, lanes 1 to 6). The amount of Hsp27 in the nuclear fraction increased at later times (Fig. 5, third panel from the top, lane 3). This pattern is consistent with what was observed by immunofluorescence microscopy (Fig. 4). Collectively, these data indicate that phosphorylated Hsp27 accumulates over the course of HSV-1 infection and is not enriched in the nucleus for at least the first 12 h of infection.
FIG. 5.
Localization of phosphorylated Hsp27 during HSV-1 infection. Nuclear (N; lanes 1, 3, and 5) and cytosolic (C; lanes 2, 4, and 6) fractions from HSV-1-infected (lanes 1 to 4) or uninfected (lanes 5 and 6) cells were collected and subjected to SDS-polyacrylamide gel electrophoresis and Western blotting. Western blots of viral (ICP8) or cellular (ppHsp27Ser15/78/82, Hsp27, and γ-tubulin) proteins are shown. The viral ICP8 protein is used as a marker for infection and an indicator of the quality of fractionation because it is a nuclear protein. “α” indicates antibody.
RNAi-mediated depletion of Hsp27 reduces HSV-1 viral yields.
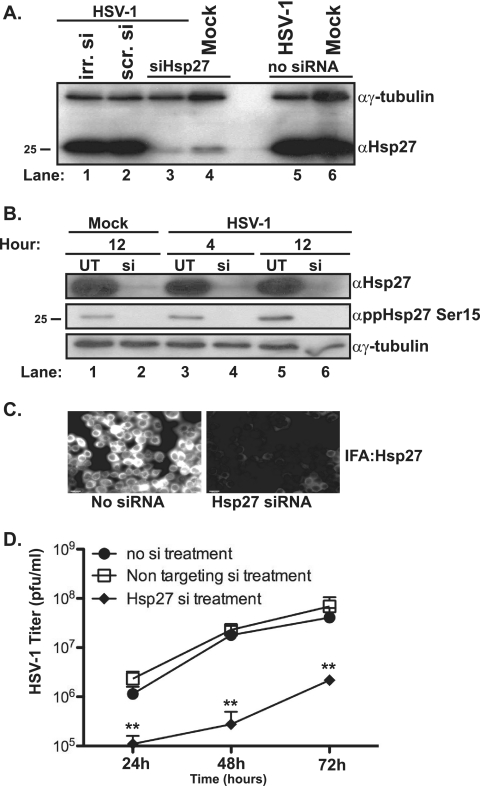
To address the role(s) of Hsp27 during HSV-1 infection, we employed an RNAi approach to deplete the levels of Hsp27 and then determined the consequences for viral yield. siRNAs (ONtarget Plus; Dharmacon), either sequence specific for Hsp27 or sequence nonspecific, were transfected into HeLa cells, and the levels of Hsp27 and γ-tubulin were monitored by Western blotting (Fig. 6A, lanes 1 to 6). Similar amounts of Hsp27 and γ-tubulin were detected in untransfected cells (Fig. 6B, lanes 1, 3, and 5), in cells treated with transfection reagent alone, and in cells transfected with a nonspecific siRNA after 96 h (Fig. 6A, lanes 1 and 2). In contrast, a significant reduction in the levels of Hsp27 was observed in cells treated with the Hsp27-specific siRNA pool (Fig. 6A, lanes 3 and 4, and B, upper immunoblot, lanes 2, 4, and 6). Levels of γ-tubulin in these samples were similar, suggesting that the Hsp27 reduction that we observed was due neither to cell loss nor to unequal loading. Roughly the same amounts of Hsp27 depletion were observed in HSV-1-infected cells and in uninfected cells (Fig. 6A, compare lane 3 to lane 4), indicating that viral infection did not affect the ability of the siRNA pool to stimulate a reduction in protein levels. To ensure that the siRNA treatment targeted all forms of Hsp27 (unmodified and modified), we analyzed the levels of phosphorylated species of Hsp27 in siRNA-treated cells at two time points in uninfected cells and HSV-1-infected cells by Western blotting (Fig. 6B). The analysis revealed that the siRNA pool specific to Hsp27 is also effective in reducing levels of the phosphorylated forms of the protein (Fig. 6B, middle immunoblot, lanes 2, 4, and 6). Immunofluorescence microscopy of Hsp27 signal in siRNA-treated cells also showed a dramatic reduction in the intensity of Hsp27-specific staining (Fig. 6C). Multistep growth experiments with HSV-1 were conducted in Hsp27-depleted cells. Significantly fewer virions were recovered from cells depleted for all forms of Hsp27 (Fig. 6D) compared to results for untreated cells (Fig. 6D) or cells treated with a nontargeting control siRNA (Fig. 6D).
FIG. 6.
Hsp27 knockdown and HSV-1 virus yield in Hsp27-depleted cells. (A) Western blot analysis of Hsp27 and γ-tubulin proteins in cells treated with either Hsp27 siRNA (lanes 3 and 4), irrelevant siRNA against cyclophilin D (irr. si, lane 1), or a nonspecific siRNA (ns si, lane 2). Untreated infected (lane 5) or uninfected (lane 6) cells are shown. (B) Western blot analysis of Hsp27, ppHsp27Ser15, and γ-tubulin proteins in untreated cells (UT, lanes 1, 3, and 5) or in cells treated with Hsp27 siRNA (si, lanes 2, 4, and 6). Western blot analysis was performed on infected-cell lysates collected at 4 h (lanes 3 and 4) or 12 h (lanes 5 and 6). (C) Immunofluorescence analysis (IFA) of Hsp27 levels in untreated (left) or Hsp27 siRNA-treated (right) cells. (D) Multistep growth analysis of HSV-1 (MOI, 0.1 PFU/ml) in no siRNA-treated, nontargeting siRNA-treated, or Hsp27 siRNA-treated cells at 24, 48, or 72 h postinfection. The asterisks represent statistically significant differences in growth of HSV-1 in the Hsp27-depleted cells compared to that with the other treatments (P values of <0.05) from two independent experiments. “α” indicates antibody.
DISCUSSION
Previously we demonstrated that HSV-1 infection stimulates the accumulation of cellular chaperones at nuclear foci adjacent to replication compartments termed VICE domains (6, 7). The function of cellular chaperone proteins found at these domains is under active investigation. So far, the activities of Hsp90 (6) and Hsc70 (20) have been shown to be essential for HSV-1 replication. We sought to expand our understanding of the roles of cellular chaperones during virus infection by analyzing the antioxidant Hsp27 chaperone system. We report here that Hsp27 is rapidly phosphorylated during HSV-1 infection in a p38-dependent fashion. Viral infection stimulates the accumulation of a phospho-reactive species of Hsp27 that has a mobility in SDS-polyacrylamide gel electrophoresis gels that is slower than the mobility of the unphosphorylated forms. This species and other phosphorylated forms of Hsp27 are excluded from the nuclear compartment, whereas unphosphorylated Hsp27 is dispersed throughout the cell and is also present in nuclear foci adjacent to VICE domains. Last, we found that HSV-1 virus production is significantly compromised in cells depleted of all forms of Hsp27, indicating that this cellular chaperone is required for robust virus production in tissue culture.
Cellular chaperones are complex, multifunctional proteins that have roles extending beyond protein folding in both normal and stressed cells. Unlike classic chaperones, such as Hsp/Hsc70, that assist in protein folding (32), Hsp90 and Hsp27 have auxiliary functions that are regulated by cochaperones or the oligomeric state, respectively. The Hsp27 chaperone can perform many diverse functions depending on its oligomeric and phosphorylation states (1, 2). Oligomeric, unphosphorylated Hsp27 primarily binds aggregation-prone proteins that have been damaged by oxidative stress or are misfolded and transfers them to other chaperone molecules or to the 20S proteasome for degradation. After phosphorylation and dissociation from the oligomer, lower-order Hsp27 species (dimeric and monomeric) have other roles in the cell, such as binding to and capping actin stress fibers, preventing activity of the apoptotic modulator proteins like DAXX (death-domain- associated protein), and delivering oxidized proteins to the 20S proteasome for degradation (2, 3). Functions of both the phosphorylated and unphosphorylated forms may be useful to the virus, as indicated by this study.
Hsp27 modification during HSV-1 infection. (i) Phosphorylated Hsp27.
Studies by Bachenheimer and colleagues showed that the viral immediate-early protein ICP27 is necessary and sufficient for p38 activation; further, in cells treated with a specific p38 inhibitor, virus yields are reduced, indicating the importance of this signaling pathway (14, 16). Although Hsp27 is just one among many protein substrates in the p38 pathway, we speculated that its modification by activated p38 would influence virus infection. We find that the activity of p38 is required for Hsp27 phosphorylation during HSV-1 infection, and as early as 2 h postinfection, we detect phosphorylation at the previously documented serine residues (Ser15, Ser78, and Ser82). Although Hsp27 phosphorylation may simply be a host response to virus infection, it is possible that these forms could be potentially beneficial to the virus during infection and may contribute to the significant reduction in virus yield in Hsp27-depleted cells. Additionally, virus infection also stimulates the accumulation of an SDS-resistant, slower-migrating species of phosphorylated Hsp27. Hsp27 monomers have been reported to cross-link to one another via formation of a single disulfide bond or through transglutamination (4, 12, 22); the cross-linking processes may well be enhanced under oxidative conditions. Our studies indicate that the phosphorylated form of Hsp27 is insensitive to transglutaminase inhibitors and DTT (data not shown), suggesting it to be an unusual form of cross-linked Hsp27 or Hsp27 attached to a client protein, a cross-reacting viral protein, or even an Hsp27-viral protein cross-linked species. More work is needed to identify the composition of the slower-migrating species and determine if it has a function in infected cells.
Our studies indicate that phosphorylated Hsp27 species are excluded from the nuclei of HSV-1-infected cells (Fig. 3, 4, and 5). During infection, phosphorylated forms accumulate in the perinuclear region in a pattern reminiscent of endoplasmic reticular staining and partially colocalize with unphosphorylated Hsp27 at later time points during the course of infection. The activities of phosphorylated forms of Hsp27 in capping actin and binding DAXX to prevent FasL interaction (8, 9, 18) may be useful to the virus. It is also possible that Hsp27 modification during HSV-1 infection is related to a newly described role for the protein in ARE (AU-rich RNA element)-mediated mRNA decay. AREs are instability motifs found within the 3′ untranslated region of some transcripts and serve as markers for rapid degradation. Recently published research has shown that knockdown of Hsp27 (all forms) resulted in decreased mRNA decay (29). During HSV-1 infection, although most cellular mRNAs are degraded through the activity of the viral vhs (virion host shutoff) protein (27), some, like that of the cytokine regulator IEX-1, are stabilized (15). Further studies have revealed that the viral ICP27 protein and its activation by p38 are essential for such stabilization (10). It is important to determine whether Hsp27 is needed for ARE-containing mRNA stability in HSV-1 infection.
(ii) Unphosphorylated Hsp27.
The majority of unphosphorylated Hsp27 is found in the cytoplasm of uninfected cells. Early after HSV-1 infection (1 to 6 h), unphosphorylated Hsp27 staining becomes less reticular and more clustered (Fig. 1B and 4). At later times (6 to 12 h), unphosphorylated Hsp27 is found in discrete foci adjacent to VICE domains at 6 h postinfection. These foci, like the VICE domains themselves, are long-lived within infected cells and are formed in the presence of the p38 inhibitor, suggesting that they are primarily composed of unphosphorylated Hsp27. Hsp70, Hsc70, Hsp40, and Hsp90 all localize to VICE domains during HSV-1 infection, and these domains are also enriched in components of the 20S proteasome (7). We do not yet understand the importance of the difference in Hsp27 localization noted above and its significance in Hsp27 activity during viral infection. Based on the proximity of Hsp27 foci to VICE domains, Hsp27's known holdase activity, and the ability of Hsp27 to direct oxidized protein turnover via the 20S proteasome, we speculate that cross talk may be occurring between subnuclear foci.
Based on previous reports and on our unpublished observations, we surmise that the unphosphorylated form is composed of higher-order oligomers. Unphosphorylated, oligomeric Hsp27 has been shown to modulate glutathione levels, to prevent cytochrome c release from the mitochondria, and to bind to caspase 9 and thereby prevent the latter's downstream activity (1, 2, 5, 13, 21). We speculate that cytoplasmic unphosphorylated Hsp27 during HSV-1 infection would be able to perform these functions. Oligomeric Hsp27 has also been shown to bind to aggregation-prone proteins or proteins that have been damaged by oxidative stress in vitro and in vivo. These clients are passed on to the proteasome for degradation in a ubiquitin-dependent or -independent fashion. Hsp27 has been shown to colocalize with the 26S proteasome and to promote ubiquitin-dependent protein turnover (23). It is possible that nuclear unphosphorylated Hsp27 at foci adjacent to VICE domains is performing such a role during HSV-1 infection. Other work from our laboratory indicates that oxidized proteins accumulate in the nucleus at early times during HSV-1 infection and are then rapidly degraded by the proteasome at the onset of robust DNA synthesis in a process that involves Hsp27 (S. S. Mathew and A. D. Burch, submitted for publication). Studies are under way in our laboratory to confirm the role(s) of this complex chaperone in this process and during HSV-1 replication.
In conclusion, new information on how host chaperones can positively or negatively influence virus infection not only could provide useful details about the auxiliary roles of these essential cellular proteins but also could reveal novel antiviral targets and provide information on antioxidant modalities that treat viral disease.
Acknowledgments
We thank Todd Gray, David Anders, Paul Masters, and all the members of the Burch Laboratory for helpful suggestions throughout the study and for critically reading the manuscript. We are grateful for the support of the Wadsworth Center Light Microscopy, Tissue Culture, Media & Glassware, and Genomics Core Facilities.
These studies were supported by funds from the Wadsworth Center New Investigator Fund and by a Career Development Award (K22AI062991) from NIH to A.D.B. S.S.M. is supported by New Investigator Funds provided to A.D.B. by the Wadsworth Center.
Footnotes
Published ahead of print on 8 July 2009.
REFERENCES
- 1.Arrigo, A. P. 2001. Hsp27: novel regulator of intracellular redox state. IUBMB Life 52:303-307. [DOI] [PubMed] [Google Scholar]
- 2.Arrigo, A. P. 2007. The cellular “networking” of mammalian Hsp27 and its functions in the control of protein folding, redox state and apoptosis. Adv. Exp. Med. Biol. 594:14-26. [DOI] [PubMed] [Google Scholar]
- 3.Arrigo, A. P., S. Virot, S. Chaufour, W. Firdaus, C. Kretz-Remy, and C. Diaz-Latoud. 2005. Hsp27 consolidates intracellular redox homeostasis by upholding glutathione in its reduced form and by decreasing iron intracellular levels. Antioxid. Redox Signal. 7:414-422. [DOI] [PubMed] [Google Scholar]
- 4.Boros, S., B. Kamps, L. Wunderink, W. de Bruijn, W. W. de Jong, and W. C. Boelens. 2004. Transglutaminase catalyzes differential crosslinking of small heat shock proteins and amyloid-beta. FEBS Lett. 576:57-62. [DOI] [PubMed] [Google Scholar]
- 5.Bruey, J. M., C. Ducasse, P. Bonniaud, L. Ravagnan, S. A. Susin, C. Diaz-Latoud, S. Gurbuxani, A. P. Arrigo, G. Kroemer, E. Solary, and C. Garrido. 2000. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat. Cell Biol. 2:645-652. [DOI] [PubMed] [Google Scholar]
- 6.Burch, A. D., and S. K. Weller. 2005. Herpes simplex virus type 1 DNA polymerase requires the mammalian chaperone hsp90 for proper localization to the nucleus. J. Virol. 79:10740-10749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burch, A. D., and S. K. Weller. 2004. Nuclear sequestration of cellular chaperone and proteasomal machinery during herpes simplex virus type 1 infection. J. Virol. 78:7175-7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charette, S. J., and J. Landry. 2000. The interaction of HSP27 with Daxx identifies a potential regulatory role of HSP27 in Fas-induced apoptosis. Ann. N. Y. Acad. Sci. 926:126-131. [DOI] [PubMed] [Google Scholar]
- 9.Charette, S. J., J. N. Lavoie, H. Lambert, and J. Landry. 2000. Inhibition of Daxx-mediated apoptosis by heat shock protein 27. Mol. Cell. Biol. 20:7602-7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corcoran, J. A., W. L. Hsu, and J. R. Smiley. 2006. Herpes simplex virus ICP27 is required for virus-induced stabilization of the ARE-containing IEX-1 mRNA encoded by the human IER3 gene. J. Virol. 80:9720-9729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies, K. J. 2001. Degradation of oxidized proteins by the 20S proteasome. Biochimie 83:301-310. [DOI] [PubMed] [Google Scholar]
- 12.Diaz-Latoud, C., E. Buache, E. Javouhey, and A. P. Arrigo. 2005. Substitution of the unique cysteine residue of murine Hsp25 interferes with the protective activity of this stress protein through inhibition of dimer formation. Antioxid. Redox Signal. 7:436-445. [DOI] [PubMed] [Google Scholar]
- 13.Garrido, C., J. M. Bruey, A. Fromentin, A. Hammann, A. P. Arrigo, and E. Solary. 1999. HSP27 inhibits cytochrome c-dependent activation of procaspase-9. FASEB J. 13:2061-2070. [DOI] [PubMed] [Google Scholar]
- 14.Hargett, D., T. McLean, and S. L. Bachenheimer. 2005. Herpes simplex virus ICP27 activation of stress kinases JNK and p38. J. Virol. 79:8348-8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu, W. L., H. A. Saffran, and J. R. Smiley. 2005. Herpes simplex virus infection stabilizes cellular IEX-1 mRNA. J. Virol. 79:4090-4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karaca, G., D. Hargett, T. I. McLean, J. S. Aguilar, P. Ghazal, E. K. Wagner, and S. L. Bachenheimer. 2004. Inhibition of the stress-activated kinase, p38, does not affect the virus transcriptional program of herpes simplex virus type 1. Virology 329:142-156. [DOI] [PubMed] [Google Scholar]
- 17.Lambert, H., S. J. Charette, A. F. Bernier, A. Guimond, and J. Landry. 1999. HSP27 multimerization mediated by phosphorylation-sensitive intermolecular interactions at the amino terminus. J. Biol. Chem. 274:9378-9385. [DOI] [PubMed] [Google Scholar]
- 18.Landry, J., and J. Huot. 1995. Modulation of actin dynamics during stress and physiological stimulation by a signaling pathway involving p38 MAP kinase and heat-shock protein 27. Biochem. Cell Biol. 73:703-707. [DOI] [PubMed] [Google Scholar]
- 19.Landry, J., H. Lambert, M. Zhou, J. N. Lavoie, E. Hickey, L. A. Weber, and C. W. Anderson. 1992. Human HSP27 is phosphorylated at serines 78 and 82 by heat shock and mitogen-activated kinases that recognize the same amino acid motif as S6 kinase II. J. Biol. Chem. 267:794-803. [PubMed] [Google Scholar]
- 20.Li, L., L. A. Johnson, J. Q. Dai-Ju, and R. M. Sandri-Goldin. 2008. Hsc70 focus formation at the periphery of HSV-1 transcription sites requires ICP27. PLoS ONE 3:e1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehlen, P., E. Hickey, L. A. Weber, and A. P. Arrigo. 1997. Large unphosphorylated aggregates as the active form of hsp27 which controls intracellular reactive oxygen species and glutathione levels and generates a protection against TNFα in NIH-3T3-ras cells. Biochem. Biophys. Res. Commun. 241:187-192. [DOI] [PubMed] [Google Scholar]
- 22.Nemes, Z., B. Devreese, P. M. Steinert, J. Van Beeumen, and L. Fesus. 2004. Cross-linking of ubiquitin, HSP27, parkin, and alpha-synuclein by gamma-glutamyl-epsilon-lysine bonds in Alzheimer's neurofibrillary tangles. FASEB J. 18:1135-1137. [DOI] [PubMed] [Google Scholar]
- 23.Parcellier, A., M. Brunet, E. Schmitt, E. Col, C. Didelot, A. Hammann, K. Nakayama, K. I. Nakayama, S. Khochbin, E. Solary, and C. Garrido. 2006. HSP27 favors ubiquitination and proteasomal degradation of p27Kip1 and helps S-phase re-entry in stressed cells. FASEB J. 20:1179-1181. [DOI] [PubMed] [Google Scholar]
- 24.Parcellier, A., E. Schmitt, S. Gurbuxani, D. Seigneurin-Berny, A. Pance, A. Chantome, S. Plenchette, S. Khochbin, E. Solary, and C. Garrido. 2003. HSP27 is a ubiquitin-binding protein involved in I-κBα proteasomal degradation. Mol. Cell. Biol. 23:5790-5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quinlan, M. P., L. B. Chen, and D. M. Knipe. 1984. The intranuclear location of a herpes simplex virus DNA-binding protein is determined by the status of viral DNA replication. Cell 36:857-868. [DOI] [PubMed] [Google Scholar]
- 26.Rogalla, T., M. Ehrnsperger, X. Preville, A. Kotlyarov, G. Lutsch, C. Ducasse, C. Paul, M. Wieske, A. P. Arrigo, J. Buchner, and M. Gaestel. 1999. Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor alpha by phosphorylation. J. Biol. Chem. 274:18947-18956. [DOI] [PubMed] [Google Scholar]
- 27.Schek, N., and S. L. Bachenheimer. 1985. Degradation of cellular mRNAs induced by a virion-associated factor during herpes simplex virus infection of Vero cells. J. Virol. 55:601-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sen, G. C., and G. A. Peters. 2007. Viral stress-inducible genes. Adv. Virus Res. 70:233-263. [DOI] [PubMed] [Google Scholar]
- 29.Sinsimer, K. S., F. M. Gratacos, A. M. Knapinska, J. Lu, C. D. Krause, A. V. Wierzbowski, L. R. Maher, S. Scrudato, Y. M. Rivera, S. Gupta, D. K. Turrin, M. P. De La Cruz, S. Pestka, and G. Brewer. 2008. Chaperone Hsp27, a novel subunit of AUF1 protein complexes, functions in AU-rich element-mediated mRNA decay. Mol. Cell. Biol. 28:5223-5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ward, K. W., J. W. Prokscht, L. M. Azzaranot, J. A. Mumawa, T. J. Roethke, G. J. Stelman, M. J. Walsh, K. S. Zeigler, J. E. McSurdy-Freed, J. R. Kehlert, J. Chokshi, M. A. Levy, and B. R. Smith. 2001. Preclinical pharmacokinetics of SB-203580, a potent inhibitor of p38 mitogen-activated protein kinase. Xenobiotica 31:783-797. [DOI] [PubMed] [Google Scholar]
- 31.Weller, S. K., D. P. Aschman, W. R. Sacks, D. M. Coen, and P. A. Schaffer. 1983. Genetic analysis of temperature-sensitive mutants of HSV-1: the combined use of complementation and physical mapping for cistron assignment. Virology 130:290-305. [DOI] [PubMed] [Google Scholar]
- 32.Young, J. C., V. R. Agashe, K. Siegers, and F. U. Hartl. 2004. Pathways of chaperone-mediated protein folding in the cytosol. Nat. Rev. Mol. Cell Biol. 5:781-791. [DOI] [PubMed] [Google Scholar]