Abstract
Here, we report that the S-acyl-2-mercaptobenzamide thioester (SAMT) class of human immunodeficiency virus type 1 (HIV-1) nucleocapsid protein (NCp7) inhibitors was able to prevent transmission of HIV-1 from infected cells, including primary cells. Furthermore, when SAMTs were introduced during an HIV-1 challenge of cervical explant tissue, inhibition of dissemination of infectious virus by cells emigrating from the tissue explants was observed. Preliminary studies using a rhesus macaque vaginal challenge model with mixed R5 and X4 simian-human immunodeficiency virus infection found that five of six monkeys were completely protected, with the remaining animal being partially protected, infected only by the R5 virus. These data suggest that SAMTs may be promising new drug candidates for further development in anti-HIV-1 topical microbicide applications.
As the human immunodeficiency virus type 1 (HIV-1)/AIDS pandemic continues into its third decade, new prevention strategies are urgently required to prevent sexual transmission of HIV-1. This need is perhaps most acute for women in the developing world, where there is a growing discrepancy between infection rates of men and women and where gender inequalities mean that condom use is often controlled by the male partner (16). Development of an effective vaccine or microbicide offers the greatest hope for protection for this vulnerable group. Recent setbacks in both fields, however, suggest that mucosal protection may be harder to achieve than previously anticipated (8, 14, 20, 33).
The nucleocapsid protein (NCp7) of HIV-1 has been suggested as a target for therapeutic and microbicide intervention (29). NCp7 is a small highly basic protein of 55 amino acids, generated by protease processing of the Gag polyprotein during virion maturation. It contains two zinc-binding domains which form tight rigid loops (22, 25, 34). Each loop contains three cysteines and one histidine organized in a Cys-X2-Cys-X4-His-X4-Cys motif (X is any amino acid) that coordinates a single zinc ion.
A combination of factors encourages the investigation of NCp7 inhibitors as potential microbicides against HIV-1 infection. NCp7 has many essential functions throughout the viral life cycle which render the protein highly intolerant to mutation (7). It is important for reverse transcription and integration in the early stage of the life cycle, and NCp7 is required for dimerization and packaging of the viral genome late in the life cycle (1, 2, 5, 9, 18, 26, 28). Additionally, the protein is highly conserved among all retroviruses except spumavirus (35). The high genetic barrier to mutation combined with its role in multiple stages of the infectious cycle makes NCp7 an ideal candidate for targeting the transmission of HIV.
A number of compounds have been developed to date that interact with the retroviral zinc-binding domains with various degrees of efficacy and toxicity (29, 30, 35, 38, 39). In this study, we have examined three analogs of the latest class of inhibitor—the S-acyl-2-mercaptobenzamide thioesters (SAMTs) (Fig. 1). These compounds function through ejection of the zinc ion, an irreversible process due to covalent modification of the zinc-coordinating cysteine residues and lysine residues required for proper tetrahedral geometry (21). The SAMTs inhibit a range of wild-type retroviruses, as well as a multidrug-resistant isolate of HIV-1 (35). Furthermore, the compounds show virucidal activity, the ability to inhibit virus expression from latently infected cells, and inhibition of cell-to-cell transmission (35). Here, we have evaluated these compounds for their efficacy in inhibiting HIV-1 transmission in cell-based antiviral assays, ex vivo cervical tissue explant culture, and the rhesus macaque challenge model, with a view to further develop these candidates as topical microbicides for the prevention of the sexual transmission of HIV.
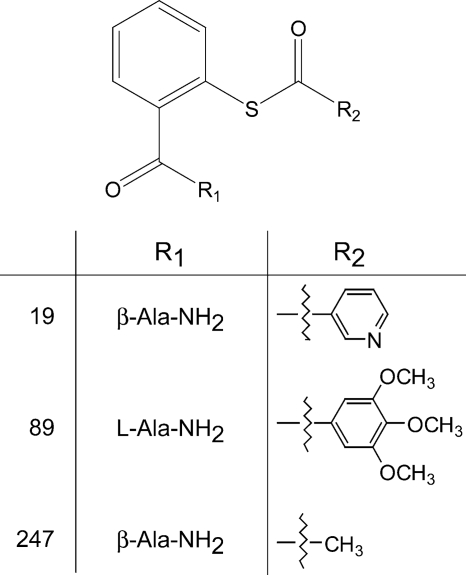
FIG. 1.
Chemical structure of SAMT compounds (SAMT-19, SAMT-89, SAMT-247) analyzed. The synthesis of these compounds has been previously described in detail (35).
MATERIALS AND METHODS
SAMT synthesis and structure.
The syntheses and chemical properties of the three SAMTs used, N-[2-(nicotinoylthio)benzoyl]-β-alaninamide (SAMT-19), N-[2-(3,4,5-trimethoxybenzoylthio)benzoyl]-l-alaninamide (SAMT-89), and N-[2-(acetylthio)benzoyl]-β-alaninamide (SAMT-247), have been reported previously (35). Compound synthesis for these experiments was carried out as described previously (35).
Cell lines.
The following nonadherent cell lines were used in this study: PM-1, CCR5+ Jurkat tat (generous donation from Q. Sattentau, Dunn School, University of Oxford, United Kingdom), and Raji B cell lines (DC-SIGN+/−) (by generous donation from V. N. KewalRamani, HIV Drug Resistance Program, NCI, Frederick, MD). All cells were cultured at 37°C, 5% CO2, and 80% humidity in RPMI 1640 medium, 10% fetal calf serum supplemented with penicillin, streptomycin, and l-glutamine (complete RPMI) and were passaged twice weekly. The CCR5 transfection of Jurkat tat cells and DC-SIGN transfection of the Raji cells were sustained with Geneticin at 500 μg/ml, while the tat transfection of Jurkat tat cells was sustained with hygromycin B at 250 μg/ml.
Viruses.
HIV-1RF and HIV-1BaL were grown in CCR5+ Jurkat tat T cells or peripheral blood mononuclear cells. The 50% tissue culture infectious dose (TCID50)/ml was determined in peripheral blood mononuclear cells. X4 simian-human immunodeficiency virus SHIVSF33A and R5 SHIVSF162P3 were propagated and titrated in a CEMX174.CCR5 cell line; the in vitro titer for both viruses was 5.9 × 103 TCID50/ml. Cell-free virus was filtered through a 22-μm filter and stored at −180°C.
Antiviral activity of SAMTs in the presence of mucin and seminal plasma.
To evaluate the effect of mucin on the activity of SAMT compounds, 0.5% pig mucin was solubilized in colorless complete RPMI as previously described (6). SAMTs were diluted 1:4 in the mucin-RPMI mixture and incubated for 2 h at 37°C. The SAMT-mucin mixture was titrated in complete RPMI and added to immobilized (centrifugation at 2,000 × g for 90 min) HIV-1RF (105 TCID50) in 96-well plates and then incubated for 1 h at 37°C. After the addition of PM-1 cells (2 × 104), plates were cultured for 7 days in the presence of mucin/compound. Viral replication was determined by the presence of reverse transcriptase (reverse transcriptase assay) in culture supernatants (27). The effect of seminal plasma (final concentration, 33.3% [vol/vol]), purchased from Vital Products, Inc. (Boynton Beach, FL), and obtained with written consent according to the local research ethics committee, on the inhibitory and virucidal activities of SAMT-89 and SAMT-247 was determined as described previously (24).
Inhibition of DC-SIGN binding and transfer. (i) Viral binding by DC-SIGN.
Raji/DC-SIGN+ cells (11) (2 × 105/well) were resuspended in complete RPMI containing mannan (200 μg/ml; Sigma-Aldrich Ltd., United Kingdom) or SAMT compounds (2× final concentration) and incubated for 1 h at 37°C. An equal volume of virus (105 TCID50) was then added to each well and incubated for a further 2 h at 37°C. Wild-type Raji/DC-SIGN− cells pulsed with virus alone were run as controls for each SAMT compound to ascertain background levels of HIV binding. After washing (four times with phosphate-buffered saline [PBS]) to remove unbound virus/compound, cell pellets were lysed with 1% Triton X-100 in PBS (100 μl) for 20 min at 25°C. DC-SIGN-bound virus was quantified by p24 enzyme-linked immunosorbent assay (ELISA; HIV-1 p24 ELISA; SAIC, Frederick, MD) as per the manufacturer's instructions.
(ii) DC-SIGN transfer assay.
Raji/DC-SIGN+ or Raji/DC-SIGN− cells (1.5 × 105) (11) were resuspended in complete medium containing SAMT (2× final concentration) and incubated for 1 h at 37°C. Cell suspensions were then exposed to an equal volume of virus (105 TCID50) in the presence of compound and incubated for a further 2 h at 37°C. Cells were washed to remove compound/unbound virus and cell pellets resuspended in complete RPMI. This cell suspension was then divided into three wells before being cocultured with PM-1 T cells (0.8 × 105/well) for 10 days, after which viral replication was determined by the presence of reverse transcriptase in culture supernatants (27). There was no significant toxicity associated with the SAMT compounds up to 1 mM on Raji or PM-1 cells as assessed by MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide] assays (50% toxic dose [TD50] > 1 mM).
Supply and culture of human genital tract tissue explants.
Cervical tissue samples were obtained from women undergoing planned therapeutic hysterectomy at St George's, St Helier's, and Kingston Hospitals (London, United Kingdom) (written consent was obtained according to the local research ethics committee). Cervical tissue samples were cut into 3-mm explants, comprising both mucosal epithelium and underlying stromal tissue as previously described (13, 15). Explants were preexposed to SAMTs for 20 min, prior to the addition of HIV-1BaL (2× tissue half-maximal infective dose for cervical tissue, equivalent to 105 TCID50 when determined in PM-1 cells). Explants were then incubated for 2 h at 37°C in the presence of compound. Explants were washed four times with PBS and transferred to fresh culture plates. Following overnight culture in the absence of compound, explants were again transferred to fresh culture plates and cultured for 10 days, with 50% medium feeds every 2 to 3 days. Cells that had spontaneously migrated out of tissue explants (11, 15) during overnight culture were washed (twice with PBS), transferred to fresh plates, and cocultured with 4 × 104 PM-1 cells/well to assess the blockade of virus transfer by migrating cells. Cultures were maintained for 10 to 14 days, with 50% medium feeds every 2 to 3 days. At the end of the assay (day 10), HIV-1 infection was determined by the measurement of p24 in culture supernatants by ELISA (HIV-1 p24 ELISA; SAIC, Frederick, MD, or Beckman Coulter). Each condition was tested in triplicate within each independent donor. For the evaluation of virion infectivity, cervical tissue was treated with SAMTs as described above. Following overnight culture to separate explants and migratory cells, explants were subsequently cocultured with PM-1 T cells (4 × 104/well). Cultures were maintained for 7 to 14 days with 50% medium feeds every 2 to 3 days. Viral replication was determined by the presence of p24 in culture supernatants on day 7.
Determination of compound toxicity.
Viability of explant tissues was then determined by the principle of MTT dye reduction. Following overnight exposure to compound, explants were exposed to MTT (0.5 mg/ml) for 3 h at 37°C. The formazan product was then released by overnight incubation in methanol (1 ml). Methanol-formazan absorbance was then determined at 570 nm (Bio-Tek Synergy HT plate reader with KC4 software). All data are expressed as the percentage of viability (normalized to tissue weight) of compound-treated wells compared to that of untreated control wells, and the 50% cytotoxic concentration (CC50) is defined as the concentration of drug at which the tissue viability was reduced to 50% of the drug-free control value.
Macaque challenge studies.
All infections were carried out in adult female rhesus macaques (Macaca mulatta) individually housed at the Tulane National Primate Research Center (TNPRC) in compliance with the Guide for the Care and Use of Laboratory Animals, and the studies were reviewed and approved by the Institutional Animal Care and Use Committee at TNPRC. Animals were confirmed to be serologically negative for simian type D retrovirus, simian immunodeficiency virus (SIV), and simian T-cell lymphotropic virus and treated with a single intramuscular dose of 30 mg medroxyprogesterone acetate (Depo-Provera; Pharmacia and Upjohn, Kalamazoo, MI) 5 weeks prior to use. For protection studies, 2 ml of 1% SAMT-247 formulated in the universal placebo gel hydroxy ethyl cellulose (HEC) (36) was applied to the vaginal cavities of the animals 20 min prior to challenge with a mixed inoculum containing 150 TCID50 each of X4 SHIVSF33A and R5 SHIVSF162P3. The animals were kept with their pelvises elevated for 20 min after virus exposure. Whole-blood samples were collected at designated time intervals postchallenge. Plasma virus was quantified by branch DNA analysis (Siemens Medical Solutions Diagnostic Clinical Lab, Emeryville, CA), and T-cell subsets (CD3, CD4, and CD8 lymphocytes) were determined by Trucount flow cytometry analysis (Becton Dickinson, San Jose, CA). For all procedures, macaques were sedated with ketamine-HCl (10 mg/kg). At the end of the study, the animals were euthanized by intravenous administration of ketamine-HCl followed by an overdose of sodium pentobarbital.
Data manipulations and statistical analyses.
Unless otherwise stated, data were analyzed to produce means with the standard deviation (SD; for individual experiment variation) or the standard error of the mean (SEM; used when producing the mean of the results for more than three individual donor/experimental means) (23), using Microsoft Excel or GraphPad PRISM. The results of drug susceptibility assays were expressed as IC50, defined as the concentration of drug at which there was 50% infection compared to a drug-free control. IC50 and CC50 were calculated by nonlinear regression analysis using GraphPad PRISM. Significance tested was completed using paired Student's t tests with unequal variance.
RESULTS
Efficacy of SAMTs against DC-SIGN binding and cell-mediated HIV-1 transmission.
Prior studies of the antiviral activity of SAMT compounds found that SAMT-89 and SAMT-19 (Fig. 1) were able to inhibit both cell-free and cell-associated viruses (35). Indeed, we observed that SAMT-19 and SAMT-89 inhibit virus replication due to cell-cell transmission at 0.08 and 0.1 μM, respectively (35). Additionally, we have determined that the more water soluble SAMT-247 shows similar antiviral activity against HIV-1RF in CEM-SS cells, with a 50% effective concentration of 3.2 μM by reverse transcriptase assay, as do SAMT-19 and SAMT-89 (50% effective concentrations of 2.9 and 2.1 μM, respectively). Thus, we continued to explore the antiviral activities of these three SAMT compounds. To investigate the ability of the SAMTs to inhibit the binding of HIV-1 to DC-SIGN, DC-SIGN-transfected Raji cells were incubated with compounds for 1 h and then with HIV-1RF or HIV-1BaL for 2 h in the continued presence of compound. Mannan, a known inhibitor of virus-DC-SIGN interaction, was able to reduce viral attachment to DC-SIGN+ Raji cells (Fig. 2A and B). In contrast, all three compounds failed to alter virus-DC-SIGN interaction (Fig. 2A and B). Thus, the SAMT compounds do not inhibit viral binding to DC-SIGN.
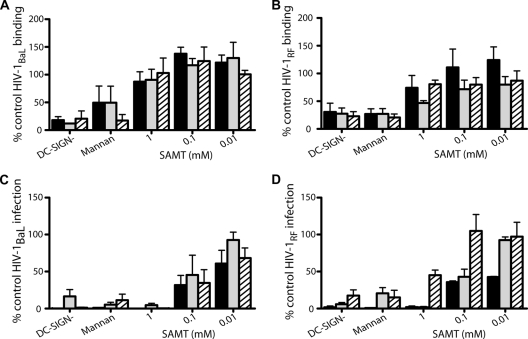
FIG. 2.
SAMTs block DC-SIGN cell-mediated HIV-1 infection. Raji/DC-SIGN+ cells were preincubated with complete media (no compound control), SAMT compounds, or mannan (200 μg/ml) for 1 h before addition of HIV-1BaL (A and C) or HIV-1RF (B and D) for 2 h in the presence of compound. Control Raji/DC-SIGN− cells were treated with virus alone to ascertain background levels of viral binding. Cells were then washed and either lysed to determine viral binding by the presence of p24 (A and B) or cocultured with permissive T cells (PM-1) (C and D) to evaluate DC-SIGN-mediated infection, determined by the presence of reverse transcriptase activity in culture supernatants following 7 days in culture. Black bars, SAMT-19; gray bars, SAMT-89; and hatched bars, SAMT-247. Data represent the mean ± SEM of the results for three independent experiments, where each condition was tested in triplicate. For the mannan controls, one set of experiments was performed for each SAMT compound.
When DC-SIGN+ cells, pulsed with virus with or without compound, were incubated with PM-1 T cells, the SAMTs inhibited trans infection by both HIV-1RF and HIV-1BaL in a dose-dependent manner (Fig. 2C and D). Raji cells cultured alone (both DC-SIGN− and DC-SIGN+) were not infected by either virus (data not shown). trans infection by HIV-1BaL was reduced by approximately 50% at 0.1 mM (P values of 0.035, 0.18, and 0.069 for SAMT-19, SAMT-89, and SAMT-247, respectively) (Fig. 2C). The IC50 values for inhibition of HIV-1BaL trans infection were 0.02, 0.08, and 0.03 mM for SAMT-19, SAMT-89, and SAMT-247, respectively. SAMT-247 inhibited HIV-1RF trans infection by approximately 50% at 1 mM (P = 0.004) but did not inhibit HIV-1RF trans infection at 0.1 mM, although SAMT-19 and SAMT-89 both did significantly inhibit trans infection at this concentration (P = 0.0008 and 0.03, respectively) (Fig. 2D). The IC50s for HIV-1RF trans infection were 0.01, 0.08, and 0.99 mM for SAMT-19, SAMT-89, and SAMT-247, respectively. It is unclear as to why SAMT-247 was less active against HIV-1RF trans infection than it was against HIV-1BaL, as the NCp7 sequence is common to both viruses.
Efficacy of SAMTs in cervical mucus and in the presence of seminal fluid.
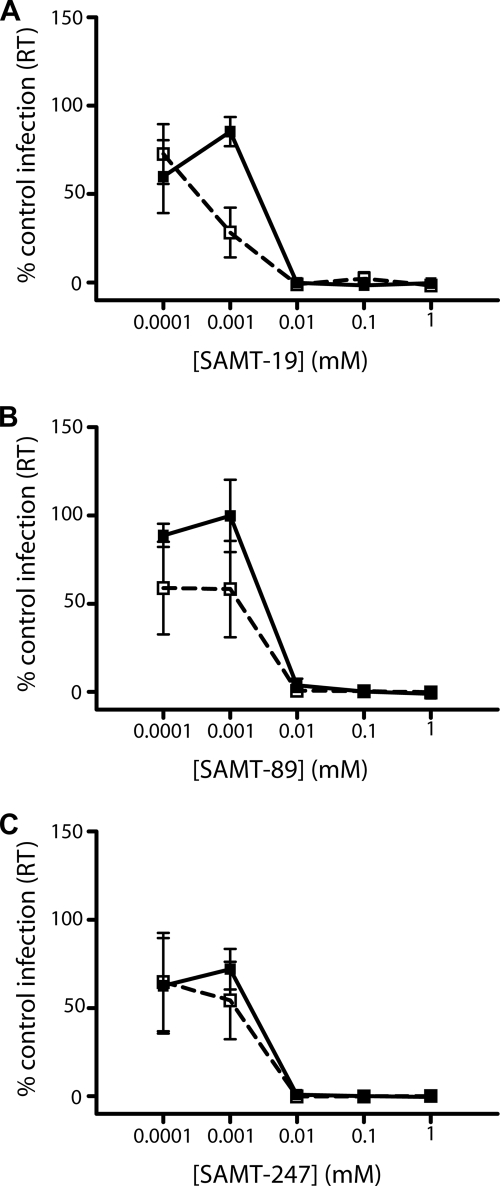
As the SAMTs have been proposed to be used as microbicides, it was important to determine whether their activity is affected by the presence of cervical mucus or seminal fluid. Therefore, the efficacy of the three compounds was first tested in the presence of synthetic cervical mucus containing 0.5% pig mucin, equivalent to the concentration of mucin in cervical mucus. SAMT-19 was the only inhibitor affected by the presence of mucin, resulting in a significant increase in efficacy at 0.001 mM with a P value of 0.03 (Fig. 3A). Controls in which mucin without compound was added to determine if the mucin alone affects HIV replication were run concurrently with this study. As there was no significant effect of the mucin alone, the increased inhibitory action of SAMT-19 is likely to be real. Both SAMT-89 and SAMT-247 showed no change in efficacy in the presence of mucin (Fig. 3B and C). SAMT-89 and SAMT-247 maintain their inhibitory activities in the presence of human seminal fluid (SAMT-19 was not tested). At 0.008 mM SAMT-89, 34% of the decrease in infectivity was observed without seminal fluid, compared with a 61% decrease in the presence of seminal fluid; at 0.032 mM SAMT-89, 10% and 16% decreases in infectivity were observed without and in the presence of seminal fluid, respectively. Similarly, for SAMT-247, 34% and 32% reductions were observed without and with seminal fluid, respectively, at 0.008 mM, whereas at 0.512 mM, 14% and 15% decreases were observed without and with seminal fluid, respectively.
FIG. 3.
Efficacy of SAMTs in the presence of synthetic cervical mucus. SAMTs were incubated in synthetic mucus for 2 h at 37°C before titrations were made. The mixtures were then added to solid-phase immobilized HIV-1RF and incubated for 1 hour prior to addition of 2 × 104 PM-1 T cells. Cultures were maintained for 7 days, when viral replication was determined by the presence of reverse transcriptase (RT) in culture supernatants. (A) SAMT-19; (B) SAMT-89; (C) SAMT-247. Solid lines, compound alone; dashed lines, compound in the presence of synthetic cervical mucus. Data represent the mean ± SEM of the results for three independent experiments, where each condition was tested in triplicate.
Biocompatibility of SAMTs.
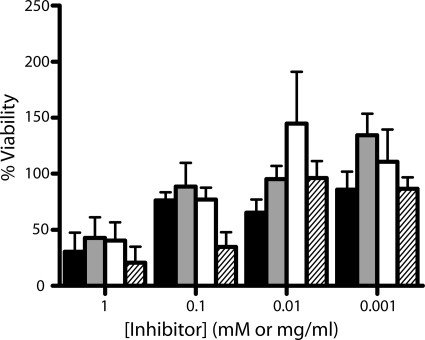
The three SAMT candidates were next assessed for their effect on cervical tissue viability. Ectocervical explants were incubated overnight in the presence of SAMT-19, SAMT-89, SAMT-247, or nonoxynol-9, after which tissue viability was assessed by the MTT assay. Nonoxynol-9 exposure resulted in a significant loss of ex vivo tissue viability (Fig. 4) with a CC50 of 0.07 mg/ml. In contrast, the biocompatibility of the SAMT compounds was higher, with CC50 values of 0.41, 0.66, and 0.40 mM for SAMT-19, SAMT-89, and SAMT-247, respectively.
FIG. 4.
Biocompatibility of SAMTs with ectocervical tissue. Cervical explants were incubated in SAMT-19 (mM), SAMT-89 (mM), SAMT-247 (mM), or nonoxynol-9 (mg/ml) overnight at 37°C. Explant viability was then assessed by the MTT dye reduction assay, as described in Materials and Methods. Each explant was weighed to allow the final absorbance reading to be normalized to account for differences in explant weight, and viability is expressed as the percentage without compound control. Black bars, SAMT-19 (n = 4); white bars, SAMT-89 (n = 6); gray bars, SAMT-247 (n = 4) (all SAMT concentrations are in mM); and hatched bars, nonoxynol-9 (mg/ml) (n = 7). Data represent the mean ± SD (SAMT-19 and SAMT-247) or mean ± SEM (SAMT-89 and nonoxynol-9) of the results. Each experimental determination was made in triplicate.
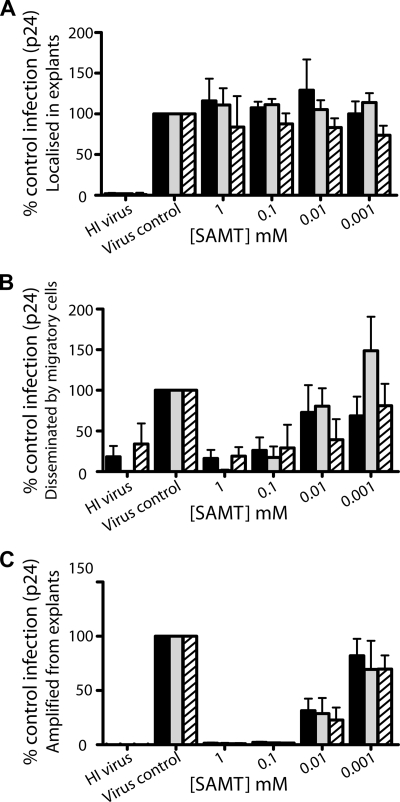
Efficacy of SAMTs against HIV-1 infection of human cervical tissue and dissemination by migratory cells.
The previous experiments demonstrated that the SAMTs were capable of inhibiting both direct and HIV-1 trans infection in cellular models. We next determined whether such efficacy would also be seen in a previously described ex vivo model of human cervical HIV-1 transmission (13). The efficacy of SAMTs against HIV-1BaL infection of ectocervical tissue and the transfer of infection via migratory cells is shown in Fig. 5A and B. Heat-inactivated virus was used as a negative control; thus any p24 recorded above this baseline was due to viral replication in either mucosal tissue explants (Fig. 5A) or PM-1 cells after transfer from migratory cells (Fig. 5B). In controls with no compound added, significant p24 production was observed (Fig. 5A and B). Interestingly, none of the compounds had any inhibitory effect on p24 production from the ectocervical explants (Fig. 5A) (P > 0.5) even though they all inhibited cell-free HIV-1 infection in cellular models (this study and reference 35). However, all three inhibitors showed excellent efficacy in blocking migratory cell-disseminated HIV-1 infection to PM-1 T cells (Fig. 5B). Significant inhibition was observed for all three inhibitors at either 0.1 mM (SAMT-19, P < 0.001; SAMT-247, P = 0.007) or 1 mM (SAMT-89, P < 0.001). All three compounds demonstrated similar levels of efficacy, though SAMT-247 was more active, with an IC50 of 0.005 mM, than SAMT-89 (IC50 = 0.02 mM) and SAMT-19 (IC50 = 0.03 mM). Thus, while the SAMTs were unable to inhibit p24 production by ectocervical explants, they were able to effectively inhibit production of infectious virus.
FIG. 5.
Effect of SAMTs on HIV-1BaL infection in human mucosal explants. Ectocervical explants were preexposed to SAMTs for 20 min, prior to exposure to HIV-1BaL for 2 h in the presence of a compound. Heat-inactivated (HI) virus was used as a negative control. The compound and virus were then removed by washing, and explants were cultured overnight in the absence of compound. Following overnight culture, explants were separated from any cells that had migrated out of the explants and cultured separately (localized infection) in the absence of compound (A), and migratory cells were washed and then cocultured with PM-1 cells (4 × 104/well) (migratory cell coculture) in the absence of compound (B). Explants were separated from any cells that had migrated out of the explants and cocultured with PM-1 cells (4 × 104/well) in the absence of compound (C). Explants/cocultures were cultured for up to 10 days and fed every 2 to 3 days with 50% medium exchange. Infection was quantified by ELISA measurement of p24 antigen released into the supernatant on day 10 of culture. Black bars, SAMT-19 (n = 3 experiments); gray bars, SAMT-89 (n = 6 in panels A and B; n = 3 in panel C); hatched bars, SAMT-247 (n = 2 in panel A; n = 4 in panel B; n = 3 in panel C). Data shown represent the mean ± SEM percentage of untreated control infection (>600 pg for panel A and >8 ng for panels B and C), where each condition was tested in triplicate or more within each independent donor.
Infectivity of virus produced by explants exposed to HIV-1.
As infectivity can be lost without reducing total virus output as measured by reverse transcriptase or p24 levels and we and others have observed that SAMTs can reduce HIV infectivity (35), we next examined the infectivity of the virus released from the ectocervical explants. Ectocervical explants were treated with SAMTs and virus and then incubated for 2 h. Tissues were washed to remove the compound and virus, incubated overnight and washed again to remove any residual unbound virus or compound. PM-1 cells were added immediately after and cultured for a further 7 to 14 days. Viral replication was assessed by the presence of p24 on day 7. Untreated cultures produced significant amounts of virus, whereas coculture of PM-1 cells with SAMT-treated explants and virus failed to demonstrate infection (Fig. 5C). Release of infectious virus was significantly inhibited (>90%) at 0.1 mM, with IC50s of 0.0045, 0.0035, and 0.0027 mM for SAMT-19, SAMT-89, and SAMT-247, respectively. Thus, the SAMTs were able to inhibit the transmission of virus from ectocervical explants to PM-1 cells over 7 days without the addition of more compound.
Efficacy of SAMT 247 in rhesus macaque models of HIV infection.
Having displayed good activity against mucosal transmission of HIV-1 in the cellular and cervical explant models, SAMT-247, as a representative of these inhibitors, was taken into a small, proof-of-concept study to investigate its efficacy in a gel formulation against a vaginal SHIV challenge in rhesus macaques (4). SAMT-247 was used for this study because it was the most water soluble and thus was easily dissolved in the gel formulation. A mixed R5 and X4 SHIV challenge was performed to allow for assessment of differential effects of the SAMT compound on phenotypically diverse viruses under the same experimental conditions. This was critical, as some microbicide candidates have been shown to be more effective against X4 than against R5 viruses (e.g., Carraguard [10]).
Having demonstrated that the SAMTs did not lose efficacy in the presence of synthetic cervical mucus, six adult female rhesus macaques were treated with Depo-Provera (30 mg, intramuscular) 5 weeks before viral challenge. Treatment with Depo-Provera served to thin the vaginal epithelium, ensuring that close to 100% of the macaques would be consistently infected even with low doses of virus (19, 37). Two milliliters of 1% SAMT-247 in HEC vehicle (corresponding to 37.6 mM) was applied to the cervicovaginal region 20 min before intravaginal exposure to a mixture of the R5 SHIVSF162P3 and the X4 SHIVSF33A. This concentration of SAMT-247 had previously been shown to be efficacious and safe in transgenic mouse studies (31).
Control experiments (23 macaques) were run both before and after this study, using the same viral stocks for challenging. In those studies, 6/23 macaques were treated with HEC, the universal placebo gel, before challenge; 12/23 were challenged in the absence of any placebo gel, and 5/23 were challenged in the presence of hydroxypropyl methylcellulose. Of these control macaques, 21 out of 23 macaques became infected with both R5 and X4 viruses, while the remaining two animals became infected solely with R5 virus, using the same protocol and SHIV challenge viruses, with all six of the HEC-treated macaques becoming infected with both viruses.
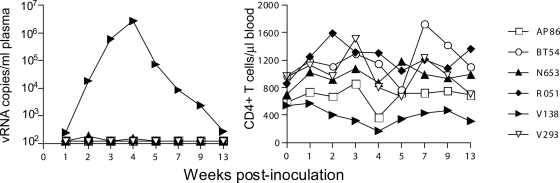
Protection from infection is defined as the absence of detectable plasma viremia (assay threshold of 125 RNA copies per ml) at two consecutive time points examined. Results showed that SAMT-247 protected five out of six macaques from X4 and R5 SHIV transmission (Fig. 6). Transient peripheral CD4+ T-cell depletion accompanied acute virus replication in the sole infected macaque, V138, and genotyping analysis of replicating virus demonstrated the presence of only the R5 virus (data not shown). In macaque N653, 188 and 151 RNA copies/ml were detected in the plasma 2 and 4 weeks after infection, respectively. However, as viral RNA was not detected at two consecutive time points, this animal was not considered to be infected.
FIG. 6.
Protective efficacy of SAMT-247 against HIV-1 infection of rhesus macaques. Virologic and immunologic outcome in macaques challenged intravaginally with 300 TCID50 of mixed X4 and R5 SHIV inoculum 20 min after SAMT-247 formulation application. Codes shown on the right side are the macaque designations.
As part of the proof-of-concept study, it was critical to determine if vaginal treatment with the SAMT compounds resulted in any irritation to the vaginal epithelium. To determine the safety of these compounds, rabbit vaginal irritation studies were performed with SAMT-19 and SAMT-247. The rabbits were dosed intravaginally with 1 ml of 1% SAMT-19 or 0.5% SAMT-247 in HEC gel formulation each day for 10 days. On day 11, the animals were sacrificed for evaluation. The severity and scope of changes associated with the intravaginal administration of SAMT-19 and -247 formulations were associated with prominent epithelial cell vacuolation, with no epithelial erosion or distinguishing leukocyte infiltrations seen (see Fig. S1 in the supplemental material). The vacuolation was considered a response to minor irritation, and similar results were observed in the presence of the HEC formulation vehicle alone. In contrast, animals treated with the positive control Conceptrol showed significantly higher vaginal irritation, with prominent erosion, epithelial thinning, and leukocyte infiltration. Thus, the results of the irritation study demonstrate that both SAMT-19 and SAMT-247 HEC gel formulations did not cause vaginal irritation and should be safe for use as microbicides.
DISCUSSION
The SAMT compounds have previously been shown to have antiviral activity against HIV-1, HIV-2, SIV, and a multidrug-resistant isolate of HIV-1 (35). Here, we have demonstrated that SAMT compounds were able to inhibit trans infection of T cells by DC-SIGN-expressing cells (Fig. 2). Inhibition occurred in the absence of toxicity for human cervical tissue (Fig. 4 and 5), and the efficacy of the inhibitors was preserved in the presence of human seminal fluid or synthetic cervical mucus (Fig. 3). The antiviral efficacy of the SAMTs was further examined in an ex vivo model for human cervical HIV-1 transmission. None of the SAMTs appeared to have inhibitory effects upon direct infection of ectocervical explants and human cervical tissues as measured by p24 production (Fig. 5A). However, the SAMTs were effective in blocking virus dissemination by migratory cells (Fig. 5B). We have previously demonstrated that this pathway is dependent upon both CD4-mediated (cis) and DC-SIGN (trans) infection of cocultured T cells (15), indicating that SAMTs are likely to be active against both pathways. Furthermore, infected explants treated with SAMTs were shown to release noninfectious virions (Fig. 5C), suggesting that localized infection could not be disseminated following SAMT application. As the compounds displayed good activity against transmission of HIV-1 in both the in vitro model systems and the ex vivo cervical explant model, a representative SAMT was used in a preliminary investigation of efficacy in gel formulation at 1% (wt/vol) against vaginal SHIV challenge of rhesus macaques. SAMT-247 protected six of six macaques from X4 virus transmission and five of six from R5 virus transmission. Thus, the SAMTs are able to inhibit HIV-1 transmission not only in in vitro model systems and ex vivo cervical explant models, but also in vivo in rhesus macaques.
The SAMT-247 concentration in the gel formulation was approximately 3 orders of magnitude higher than that required for rapid HIV-1 inactivation as measured in vitro. By comparison, candidate microbicide compounds typically require concentrations 100- to 10 million-fold higher for protection against vaginal transmission in macaques than for blocking infection in vitro (17). Therefore, the protection of macaques by a 1% SAMT-247 gel is comparable to that by the best microbicide candidates studied thus far.
Continued production of p24 at a decreased infectious titer has previously been seen with NCp7 inhibitors in cellular models. Treatment of latently infected U1 cells with disulfide benzamide compounds resulted in continuous virion release, but the viruses were replication incompetent (39). A transgenic mouse model that held an integrated HIV-1 provirus induced by Mycobacterium avium infection used to study NCp7 inhibitors (32) demonstrated that spleen cells from these animals still produced high levels of p24 but the virus was not able to establish an infection when cocultured with MAGI cells. The most likely reason for the production of noninfectious virus is the disruption of the zinc finger motifs by the inhibitors, such that NCp7 could no longer bind the genomic RNA for incorporation into new virions (3). Thus, virions that had the same gross architecture as wild-type virions, but without any viral RNA, may have been released, preventing further replication. This is also consistent with early studies conducted on NCp7 inhibitors in which virion release was observed following mutation of the zinc-coordinating residues, but viral replication was inhibited due to deficiency of genomic RNA (1, 12).
The most likely mechanism for the observed inhibition of trans infection of T cells by virus from migratory cells is that the SAMTs were inhibiting direct infection of dendritic cells within the cervical explant so that any virus produced from these dendritic cells would have been noninfectious. Indeed, the observed lack of infection of PM-1 cells upon coculture with infected explants suggests that any virus produced by the infected cells is itself noninfectious (Fig. 5C). There are two primary levels at which the SAMTs could inhibit HIV-1 infection of dendritic cells in the cervical explant model. First, they could have exerted some level of inhibition during the incubation of explant, virus, and inhibitor at the start of the assay. Inhibition in this case would be against cell-free virus, assuming it had not yet infected any susceptible cells. Second, inhibition could have occurred against virus that had infected target cells within the explant, in which case the SAMTs were working against cell-associated virus. The pattern of inhibition observed with the migratory cell model was more in keeping with inhibition of cell-associated virus, as the efficacy of inhibition was higher for cell-associated virus than for cell-free virus. Whichever route of viral transfer is responsible, and probably both can be implicated, this study demonstrates that the SAMT compounds were able to prevent virus from disseminating from infected cells in biologically relevant tissue.
In summary, our data show that the SAMTs are capable of potently inhibiting HIV-1 dissemination. SAMT-247, formulated as a topical microbicide, conferred protection against both X4 and R5 SHIV infection of rhesus macaques in a preliminary proof-of-concept study, demonstrating its potential for the prevention of sexual HIV-1 transmission. The activity of the SAMTs in the presence of synthetic cervical mucus and human seminal plasma suggests that they will retain their efficacy within the physical environment of the human vagina, and the rabbit vaginal irritation studies demonstrate that their use does not induce vaginal irritation. These compounds do not work against cellular receptors but are able to inhibit cell-free and cell-associated virus. Furthermore, cell-free virus was rapidly inactivated when in contact with SAMT-247 at concentrations similar to those needed for inhibition of infectious HIV-1 production in cell cultures. Therefore, if incorporated into a topical microbicide, they could be applied very shortly before intercourse, since no incubation period is required to inactivate cellular receptors or to metabolize the drug into an active form. This renders the formulation more versatile, with no preplanning of application necessary. In light of these results and with the knowledge that NCp7 functions in many stages of the replication, these inhibitors show promise for further development as topical microbicides.
Supplementary Material
Acknowledgments
This study was supported in part by the NIH Intramural AIDS Targeted Antiretroviral Program (IATAP) (L.M.M.J., R.H., and E.A.) and NIH grant PO1 HD41761 (A.R.N.).
The technical assistance of Y.-Y. Li and Naomi Armanasco is greatly appreciated.
Footnotes
Published ahead of print on 8 July 2009.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Aldovini, A., and R. A. Young. 1990. Mutations of RNA and protein sequences involved in human immunodeficiency virus type 1 packaging result in production of noninfectious virus. J. Virol. 64:1920-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allain, B., M. Lapadat-Tapolsky, C. Berlioz, and J. L. Darlix. 1994. Transactivation of the minus-strand DNA transfer by nucleocapsid protein during reverse transcription of the retroviral genome. EMBO J. 13:973-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berthoux, L., C. Pechoux, and J. L. Darlix. 1999. Multiple effects of an anti-human immunodeficiency virus nucleocapsid inhibitor on virus morphology and replication. J. Virol. 73:10000-10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boadi, T., E. Schneider, S. Chung, L. Tsai, A. Gettie, M. Ratterree, J. Blanchard, A. R. Neurath, and C. Cheng-Mayer. 2005. Cellulose acetate 1,2-benzenedicarboxylate protects against challenge with pathogenic X4 and R5 simian/human immunodeficiency virus. AIDS 19:1587-1594. [DOI] [PubMed] [Google Scholar]
- 5.Buckman, J. S., W. J. Bosche, and R. J. Gorelick. 2003. Human immunodeficiency virus type 1 nucleocapsid Zn2+ fingers are required for efficient reverse transcription, initial integration processes, and protection of newly synthesized viral DNA. J. Virol. 77:1469-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burruano, B. T., R. L. Schnaare, and D. Malamud. 2002. Synthetic cervical mucus formulation. Contraception 66:137-140. [DOI] [PubMed] [Google Scholar]
- 7.Cruceanu, M., M. A. Urbaneja, C. V. Hixson, D. G. Johnson, S. A. Datta, M. J. Fivash, A. G. Stephen, R. J. Fisher, R. J. Gorelick, J. R. Casas-Finet, A. Rein, I. Rouzina, and M. C. Williams. 2006. Nucleic acid binding and chaperone properties of HIV-1 Gag and nucleocapsid proteins. Nucleic Acids Res. 34:593-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cutler, B., and J. Justman. 2008. Vaginal microbicides and the prevention of HIV transmission. Lancet Infect. Dis. 8:685-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darlix, J. L., C. Gabus, M. T. Nugeyre, F. Clavel, and F. Barre-Sinoussi. 1990. Cis elements and trans-acting factors involved in the RNA dimerization of the human immunodeficiency virus HIV-1. J. Mol. Biol. 216:689-699. [DOI] [PubMed] [Google Scholar]
- 10.Dezzutti, C. S., V. N. James, A. Ramos, S. T. Sullivan, A. Siddig, T. J. Bush, L. A. Grohskopf, L. Paxton, S. Subbarao, and C. E. Hart. 2004. In vitro comparison of topical microbicides for prevention of human immunodeficiency virus type 1 transmission. Antimicrob. Agents Chemother. 48:3834-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fletcher, P. S., G. S. Wallace, P. M. Mesquita, and R. J. Shattock. 2006. Candidate polyanion microbicides inhibit HIV-1 infection and dissemination pathways in human cervical explants. Retrovirology 3:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorelick, R. J., S. M. Nigida, Jr., J. W. Bess, Jr., L. O. Arthur, L. E. Henderson, and A. Rein. 1990. Noninfectious human immunodeficiency virus type 1 mutants deficient in genomic RNA. J. Virol. 64:3207-3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenhead, P., P. Hayes, P. S. Watts, K. G. Laing, G. E. Griffin, and R. J. Shattock. 2000. Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. J. Virol. 74:5577-5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hendrix, C. W., Y. J. Cao, and E. J. Fuchs. 2009. Topical microbicides to prevent HIV: clinical drug development challenges. Annu. Rev. Pharmacol. Toxicol. 49:349-375. [DOI] [PubMed] [Google Scholar]
- 15.Hu, Q., I. Frank, V. Williams, J. J. Santos, P. Watts, G. E. Griffin, J. P. Moore, M. Pope, and R. J. Shattock. 2004. Blockade of attachment and fusion receptors inhibits HIV-1 infection of human cervical tissue. J. Exp. Med. 199:1065-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klasse, P. J., R. Shattock, and J. P. Moore. 2008. Antiretroviral drug-based microbicides to prevent HIV-1 sexual transmission. Annu. Rev. Med. 59:455-471. [DOI] [PubMed] [Google Scholar]
- 17.Klasse, P. J., R. J. Shattock, and J. P. Moore. 2006. Which topical microbicides for blocking HIV-1 transmission will work in the real world? PLoS Med. 3:e351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li, X., Y. Quan, E. J. Arts, Z. Li, B. D. Preston, H. de Rocquigny, B. P. Roques, J. L. Darlix, L. Kleiman, M. A. Parniak, and M. A. Wainberg. 1996. Human immunodeficiency virus type 1 nucleocapsid protein (NCp7) directs specific initiation of minus-strand DNA synthesis primed by human tRNA3Lys in vitro: studies of viral RNA molecules mutated in regions that flank the primer binding site. J. Virol. 70:4996-5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marx, P. A., A. I. Spira, A. Gettie, P. J. Dailey, R. S. Veazey, A. A. Lackner, C. J. Mahoney, C. J. Miller, L. E. Claypool, D. D. Ho, and N. J. Alexander. 1996. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat. Med. 2:1084-1089. [DOI] [PubMed] [Google Scholar]
- 20.Miedema, F. 2008. A brief history of HIV vaccine research: stepping back to the drawing board? AIDS 22:1699-1703. [DOI] [PubMed] [Google Scholar]
- 21.Miller Jenkins, L. M., T. Hara, S. R. Durell, R. Hayashi, J. K. Inman, J. P. Piquemal, N. Gresh, and E. Appella. 2007. Specificity of acyl transfer from 2-mercaptobenzamide thioesters to the HIV-1 nucleocapsid protein. J. Am. Chem. Soc. 129:11067-11078. [DOI] [PubMed] [Google Scholar]
- 22.Morellet, N., N. Jullian, H. De Rocquigny, B. Maigret, J. Darlix, and B. P. Roques. 1992. Determination of the structure of the nucleocapsid protein from the human immunodeficiency virus type 1 by 1H NMR. EMBO J. 11:3059-3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagele, P. 2003. Misuse of standard error of the mean (SEM) when reporting variability of a sample. A critical evaluation of four anaesthesia journals. Br. J. Anaesth. 90:514-516. [DOI] [PubMed] [Google Scholar]
- 24.Neurath, A. R., N. Strick, and Y. Y. Li. 2006. Role of seminal plasma in the anti-HIV-1 activity of candidate microbicides. BMC Infect. Dis. 6:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Omichinski, J. G., G. M. Clore, K. Sakaguchi, E. Appella, and A. M. Gronenborn. 1991. Structural characterization of a 39-residue synthetic peptide containing the two zinc binding domains from the HIV-1 p7 nucleocapsid protein by CD and NMR spectroscopy. FEBS Lett. 292:25-30. [DOI] [PubMed] [Google Scholar]
- 26.Poljak, L., S. M. Batson, D. Ficheux, B. P. Roques, J. L. Darlix, and E. Kas. 2003. Analysis of NCp7-dependent activation of HIV-1 cDNA integration and its conservation among retroviral nucleocapsid proteins. J. Mol. Biol. 329:411-421. [DOI] [PubMed] [Google Scholar]
- 27.Potts, B. 1990. Mini reverse transcriptase (RT) assay. In A. Aldovini and B. D. Walker (ed.), Techniques in HIV research. Stockton Press/MacMillan Publishers, New York, NY.
- 28.Rein, A., L. E. Henderson, and J. G. Levin. 1998. Nucleic-acid-chaperone activity of retroviral nucleocapsid proteins: significance for viral replication. Trends Biochem. Sci. 23:297-301. [DOI] [PubMed] [Google Scholar]
- 29.Rice, W. G., C. A. Schaeffer, B. Harten, F. Villinger, T. L. South, M. F. Summers, L. E. Henderson, J. W. Bess, Jr., L. O. Arthur, J. S. McDougal, S. L. Orloff, J. Mendeleyev, and E. Kun. 1993. Inhibition of HIV-1 infectivity by zinc-ejecting aromatic C-nitroso compounds. Nature 361:473-475. [DOI] [PubMed] [Google Scholar]
- 30.Rice, W. G., J. G. Supko, L. Malspeis, R. W. Buckheit, Jr., D. Clanton, M. Bu, L. Graham, C. A. Schaeffer, J. A. Turpin, J. Domagala, R. Gogliotti, J. P. Bader, S. M. Halliday, L. Coren, R. C. Sowder II, L. O. Arthur, and L. E. Henderson. 1995. Inhibitors of HIV nucleocapsid protein zinc fingers as candidates for the treatment of AIDS. Science 270:1194-1197. [DOI] [PubMed] [Google Scholar]
- 31.Schito, M. L., A. Goel, Y. Song, J. K. Inman, R. J. Fattah, W. G. Rice, J. A. Turpin, A. Sher, and E. Appella. 2003. In vivo antiviral activity of novel human immunodeficiency virus type 1 nucleocapsid p7 zinc finger inhibitors in a transgenic murine model. AIDS Res. Hum. Retroviruses 19:91-101. [DOI] [PubMed] [Google Scholar]
- 32.Schito, M. L., P. E. Kennedy, R. P. Kowal, E. A. Berger, and A. Sher. 2001. A human immunodeficiency virus-transgenic mouse model for assessing interventions that block microbial-induced proviral expression. J. Infect. Dis. 183:1592-1600. [DOI] [PubMed] [Google Scholar]
- 33.Sekaly, R. P. 2008. The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? J. Exp. Med. 205:7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.South, T. L., P. R. Blake, D. R. Hare, and M. F. Summers. 1991. C-terminal retroviral-type zinc finger domain from the HIV-1 nucleocapsid protein is structurally similar to the N-terminal zinc finger domain. Biochemistry 30:6342-6349. [DOI] [PubMed] [Google Scholar]
- 35.Srivastava, P., M. Schito, R. J. Fattah, T. Hara, T. Hartman, R. W. Buckheit, Jr., J. A. Turpin, J. K. Inman, and E. Appella. 2004. Optimization of unique, uncharged thioesters as inhibitors of HIV replication. Bioorg. Med. Chem. 12:6437-6450. [DOI] [PubMed] [Google Scholar]
- 36.Tien, D., R. L. Schnaare, F. Kang, G. Cohl, T. J. McCormick, T. R. Moench, G. Doncel, K. Watson, R. W. Buckheit, M. G. Lewis, J. Schwartz, K. Douville, and J. W. Romano. 2005. In vitro and in vivo characterization of a potential universal placebo designed for use in vaginal microbicide clinical trials. AIDS Res. Hum. Retroviruses 21:845-853. [DOI] [PubMed] [Google Scholar]
- 37.Trunova, N., L. Tsai, S. Tung, E. Schneider, J. Harouse, A. Gettie, V. Simon, J. Blanchard, and C. Cheng-Mayer. 2006. Progestin-based contraceptive suppresses cellular immune responses in SHIV-infected rhesus macaques. Virology 352:169-177. [DOI] [PubMed] [Google Scholar]
- 38.Turpin, J. A., Y. Song, J. K. Inman, M. Huang, A. Wallqvist, A. Maynard, D. G. Covell, W. G. Rice, and E. Appella. 1999. Synthesis and biological properties of novel pyridinioalkanoyl thiolesters (PATE) as anti-HIV-1 agents that target the viral nucleocapsid protein zinc fingers. J. Med. Chem. 42:67-86. [DOI] [PubMed] [Google Scholar]
- 39.Turpin, J. A., S. J. Terpening, C. A. Schaeffer, G. Yu, C. J. Glover, R. L. Felsted, E. A. Sausville, and W. G. Rice. 1996. Inhibitors of human immunodeficiency virus type 1 zinc fingers prevent normal processing of Gag precursors and result in the release of noninfectious virus particles. J. Virol. 70:6180-6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.