Abstract
In immunocompetent individuals, the stability of the herpesvirus-host balance limits opportunities to study the disappearance of a virus-specific CD8+ T-cell response. However, we noticed that in HLA-A*0201-positive infectious mononucleosis (IM) patients undergoing primary Epstein-Barr virus (EBV) infection, the initial CD8 response targets three EBV lytic antigen-derived epitopes, YVLDHLIVV (YVL), GLCTLVAML (GLC), and TLDYKPLSV (TLD), but only the YVL and GLC reactivities persist long-term; the TLD response disappears within 10 to 27 months. While present, TLD-specific cells remained largely indistinguishable from YVL and GLC reactivities in many phenotypic and functional respects but showed unique temporal changes in two markers of T-cell fate, interleukin 7 receptor alpha (IL-7Rα; CD127) and programmed death 1 (PD-1). Thus, following the antigen-driven downregulation of IL-7Rα seen on all populations in acute IM, in every case, the TLD-specific population recovered expression unusually quickly post-IM. As well, in four of six patients studied, TLD-specific cells showed very strong PD-1 upregulation in the last blood sample obtained before the cells’ disappearance. Our data suggest that the disappearance of this individual epitope reactivity from an otherwise stable EBV-specific response (i) reflects a selective loss of cognate antigen restimulation (rather than of IL-7-dependent signals) and (ii) is immediately preceded, and perhaps mediated, by PD-1 upregulation to unprecedented levels.
Virus-specific CD8+ T cells play a major role in controlling primary virus infections, but what determines the long-term fate of these cells is poorly understood (23, 31, 42). Studies of lymphocytic choriomeningitis virus (LCMV) infection in mice show that, where the virus is completely cleared in vivo, entry into and maintenance within the CD8 memory pool depends upon the homeostatic cytokine interleukin 7 (IL-7) and is restricted to LCMV-specific T cells that reacquire the high-affinity IL-7 receptor alpha (IL-7Rα; CD127) (22). However, in situations in which LCMV is not cleared, the virus-specific-T-cell pool never becomes fully IL-7Rα positive, and its maintenance depends upon chronic antigen stimulation rather than IL-7 (24, 36, 43). Furthermore, with ongoing virus replication, the LCMV-specific CD8+ T cells remain detectable but become functionally exhausted, responding poorly to antigen in ex vivo assays (16, 42, 44, 47). This is marked by the cells’ upregulation of programmed death 1 (PD-1), one member of the CD28 family of proteins that modulate T-cell responses through specific receptor-ligand interactions with B7 family members (5). PD-1 normally acts as an inhibitor of T-cell function (18), and indeed, in mice with chronic LCMV infection, monoclonal antibody blockade of the PD-1-PD-1 ligand interaction reversed functional exhaustion and reduced viral load (5). Likewise, chronic uncontrolled infection with human immunodeficiency virus (HIV) or hepatitis B (HBV) or C (HCV) viruses in humans can lead to functional impairment of the virus-specific CD8+ T cells, again marked by their increased PD-1 expression (8, 13, 27, 28, 30, 38, 39).
Human herpesviruses, such as Epstein-Barr virus (EBV) and cytomegalovirus (CMV), also elicit strong CD8+ T-cell responses to virus replicative (lytic) cycle proteins (21, 37), especially during primary infection when they are manifest as infectious mononucleosis (IM) (20, 45). These viruses are never cleared but persist by establishing niches of latent infection, thus evading CD8+ T-cell surveillance rather than compromising its function. Occasional low-level reactivations from latency into the lytic cycle provide recurrent antigen challenge and, as a result, the full range of virus-specific CD8+ T-cell responses tend to be maintained in the immunocompetent host throughout long-term virus carriage. Interestingly, recent prospective studies of EBV-positive IM patients provided a rare exception to the general stability of herpesvirus-specific CD8+ T-cell memory in virus carriers (19). Thus, all HLA-A*0201-positive patients make a primary response to three A*0201-restricted EBV epitopes, YVLDHLIVV, GLCTLVAML, and TLDYKPLSV (designated YVL, GLC, and TLD, respectively). These are derived from three viral proteins, BRLF1, BMLF1, and BMRF1, respectively, that are expressed in the immediate early (BRLF1) and early (BMLF1 and BMRF1) phases of the virus replicative cycle. While YVL- and GLC-specific cells persist in the longer term, as do responses to many other EBV lytic and latent cycle epitopes studied (11, 21, 46), the TLD response almost always disappears (19). Here, we followed the TLD-specific cell population over time post-IM and found that its disappearance is preceded by distinctive changes in the expression of two key markers associated with T-cell fate, IL-7Rα and PD-1.
MATERIALS AND METHODS
Donors.
IM patients were bled during the acute phase and at intervals up to 27 months later; in all cases, acute symptoms resolved within 1 month. Peripheral blood mononuclear cells (PBMCs) were cryopreserved on each occasion, and all samples from an individual patient were tested together. Healthy donors of known HLA type and known EBV/CMV serologic status, as controls, were also bled. Blood was taken with informed consent, and the study was approved by the South Birmingham Health Authority Local Research Ethics Committee.
Phenotypic analysis. (i) Tetramer staining.
Phycoerythrin (PE)- or TricolorFluorochrome-conjugated HLA class I-peptide tetramers were prepared and used as previously described (3, 19), representing EBV lytic cycle epitopes A2/YVLDHLIVV, A2/GLCTLVAML, A2/TLDYKPLSV, B8/RAKFKQLL, and B35/EPLPQGQLTAY; EBV latent cycle epitopes A2/CLGGLLTMV, B8/FLRGRAYGL, B8/QAKWRLQTL, B35/YPLHEQHGM, and B35/HPVGEADYFEY; and CMV epitopes A2/NLVPMVATV, A2/VLEETSVML, B8/ELRRKMMYM, B8/ELKRKMIYM, B8/QIKVRVDMV, and B35/IPSINVHHY.
(ii) IL-7Rα staining.
Goat anti-human IL-7Rα antibody (R&D Systems, Minneapolis, MN) was detected by fluorescein isothiocyanate (FITC)-conjugated swine anti-goat immunoglobulin G antibody (Caltag Laboratories, Burlingame, CA) as previously described (33, 34); cells were dually stained with Tricolor-labeled anti-human CD8 monoclonal antibody (MAb) (Caltag). In selected cases, IL-7Rα staining was also carried out using directly conjugated MAbs, namely, mouse anti-human CD127-PE (clone R34.34; Beckman-Coulter, Fullerton, CA) and mouse anti-human CD127-allophycocyanin (APC) (clone 40131; R&D Systems). In one case where cell numbers allowed, responsiveness to IL-7 was studied by exposing PBMCs to 5 μΜ epitope peptide and then culturing for 7 days in medium supplemented with 5 ng/ml IL-7; tetramer-positive cell numbers were determined at the start and end of the culture period by tetramer staining of counted cell populations.
(iii) PD-1 staining.
Cells first stained with Tricolor-labeled tetramer were exposed to the PE-labeled anti-human PD-1 specific antibody (at 0.5 μg/ml) as described previously (14) and to FITC-labeled CD8 MAb (BD Biosciences, San Jose, CA). In some experiments, staining was also confirmed using a different PD-1-PE antibody (PD-1; eBioscience, San Diego, CA) (used at 0.5 μg/ml).
(iv) Other markers.
PBMCs were stained with pretitrated concentrations of tetramer (conjugated to PE or Tricolor) and then stained with directly conjugated or unconjugated antibodies plus the appropriate second step as described previously (4). The antibodies were anti-CD38 (APC; BD Biosciences), -Bcl2 (FITC), -Ki67 (FITC), -CCR7 (PE-Cy7; all Beckman Coulter), -CD45RA (PE-Texas Red), -CD8 (Alexa405; Caltag), and -CD3 (Cascade Yellow; Dako).
Functional assays (intracellular multifunction staining).
For stimulation, at least 106 PBMCs, prestained with tetramer, were incubated in the presence of specific peptide (5 μM) and anti-PE-Cy5-conjugated CD107a for 1 h at 37°C in a 5% CO2 incubator, followed by an additional 5 h in the presence of the secretion inhibitors monensin (2.5 μg/ml; Sigma-Aldrich) and brefeldin A (5 μg/ml; Sigma-Aldrich). After incubation and washes, BD Cytofix/Cytoperm was used for permeabilization of the cells prior to MAb staining using Alexa405-labeled anti-CD8 and one of the intracellular markers, i.e., APC-conjugated anti-IL-2 (BD Biosciences), Alexa700-labeled anti-gamma interferon (IFN-γ) (BD Biosciences), PE-Cy7-conjugated anti-tumor necrosis factor alpha (TNF-α) (BD Biosciences), or FITC-labeled anti-MIP-1β (R&D Systems). PBMCs processed in parallel but without peptide stimulation served as controls.
All stainings were analyzed either on an Epics flow cytometer (Beckman Coulter, Fullerton, CA) or on an LSR2 flow cytometer (Becton Dickinson) with appropriate isotype controls and color compensation. Data from cell stainings for CD107a or cytokines were expressed as a percentage of the tetramer-positive, CD8+ population.
Statistical analysis.
Statistical analysis was performed using GraphPad Prism software. Data were compared using a Mann-Whitney test, and significant differences were verified with 95% confidence intervals.
RESULTS
Characterization of the disappearing TLD response.
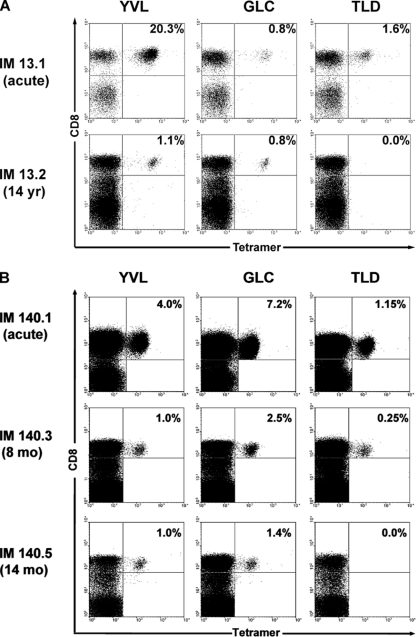
Figure 1A illustrates a result that we consistently observed in an earlier study of HLA-A*0201-positive IM patients (19). Tetramer staining, in this case for samples from patient IM13, detected CD8+ T-cell responses to three EBV-encoded lytic cycle epitopes, YVL, GLC, and TLD, in the blood during the acute phase of the disease. However, in a follow-up blood sample from the same individual, in this case, obtained 14 years later, the YVL- and GLC-specific populations were still detectable but the TLD response had disappeared. Here, we focused on six HLA-A*0201 patients from the earlier study, from whom blood samples had been taken over the first 2 years post-IM (19), and reexamined additional aliquots of cryopreserved PBMCs by tetramer/CD8 dual staining. Figure 1B presents the results from one such patient, IM140, showing that in this case, the TLD response became undetectable within 14 months of the acute disease. For the results for all six prospective study patients, see Table S1 in the supplemental material. In each case, the TLD-specific population disappeared within 10 to 27 months post-IM, even in patients for whom this was the largest of the three epitope-specific responses in the acute phase (IM146 and IM179); by contrast, the YVL and GLC responses always persisted. Also available upon request are data from 12 HLA-A*0201-positive long-term virus carriers with no history of IM. Such individuals are consistently positive for YVL- and GLC-specific cells but lack detectable TLD reactivity; this is also true for tonsillar cell preparations from such virus carriers (20), indicating that these findings reflect a genuine absence, and not a relocation, of the TLD response.
FIG. 1.
Tetramer staining of PBMCs from HLA-A*0201-positive IM patients. (A) IM13, sampled during acute primary infection (IM13.1) and about 14 years later (IM13.2). (B) IM140, sampled during acute primary infection (IM140.1) and at intervals up to 14 months later (IM140.5) as indicated. FACS profiles from cells dually stained with a Tricolor-conjugated anti-CD8 MAb and a PE-labeled tetramer specific for the YVL, GLC, or TLD epitope are shown. Numbers in the upper-right quadrant refer to the percentages of CD8+ T cells that stained with the tetramer.
We then compared the YVL-, GLC-, and TLD-specific populations in post-IM blood samples to look for distinguishing features of the TLD response that might explain its inability to persist. We hypothesized that TLD-specific cells might show one of three characteristics. (i) The first is prolonged retention of the activated (CD38+ Ki67+) and apoptosis-prone (Bcl2lo) phenotype shown by primary effectors in acute IM and associated with the marked culling of virus-specific CD8+ T-cell numbers immediately post-acute phase (9, 29). (ii) The second is unusual distribution along the central memory (CCR7+ CD45RA−), effector memory (CCR7− CD45RA−), and effector terminal (CCR7− CD45RA+) differentiation pathway post-IM (19), in particular, a greater movement into the effector terminal subset which some have associated with proliferative exhaustion (31). (iii) The third is overreliance upon T-cell clones of unusually low functional avidity, such that the response might be outcompeted by higher-avidity responses to other epitopes. In fact, the TLD response was not unusual in any of the respects listed above. Thus, within any individual patient, the YVL, GLC, and TLD responses showed similar rates of transition from their initial CD38+ Ki67+ Bcl2lo status toward the CD38− Ki67− Bcl2+ phenotype typical of longer-term memory (data not shown) and assumed similar distributions across the central memory, effector memory, and effector terminal subsets (data shown). Likewise, CD8+ T-cell clones specific for the three epitopes could be established with equal facility from 3- to 4-month-post-IM bleedings, and their analysis in peptide titration assays showed that, in the three post-IM patients studied, TLD-specific clones showed higher functional avidity than GLC-specific clones and were either equal to YVL-specific clones in this respect or intermediate between the more-avid YVL and less-avid GLC values (data not shown). Since these experiments did not reveal any defining feature of the TLD response, we went on to examine two other potentially relevant markers that, in mouse model systems (5, 22), can influence virus-specific CD8+ T-cell fate, IL-7Rα and PD-1.
Accelerated recovery of IL-7Rα expression on TLD-specific cells post-IM.
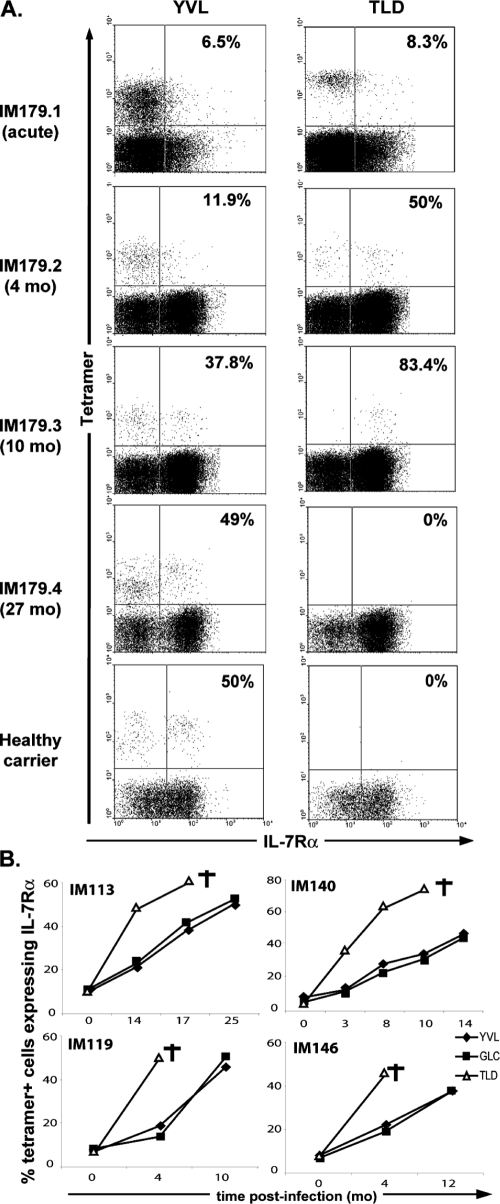
Earlier work has shown that IL-7Rα expression is lost on the activated CD8+ T cells in acute IM (33); thereafter, while expression quickly recovers on the bulk CD8+ T-cell population, EBV lytic cycle epitope-specific populations typically take 2 years to acquire the 50 to 70% IL-7Rα positivity shown by these populations in long-term EBV carriers (34, 40). This incomplete recovery of IL-7Rα expression on EBV-specific CD8+ T cells is typical of responses to persistent, as opposed to cleared, infections (24, 43) and is likely to reflect the effects of ongoing chronic antigen restimulation (7). Interestingly, the TLD response proved different in this respect. Figure 2A shows tetramer/IL-7Rα dual stains, analyzed on CD8+ T cells, in successively obtained blood samples from patient IM179. Recovery of IL-7Rα expression was characteristically slow on the YVL-specific (and GLC-specific [data not shown]) populations, reaching the 50 to 60% levels seen in an HLA-A*0201-positive healthy carrier control only after 27 months. By contrast, the TLD response had become 50% IL-7Rα positive by 4 months post-IM, and at 10 months (i.e., the last blood draw before the response disappeared), >80% cells were positive. As summarized in Fig. 2B, this same pattern was observed for four other IM patients analyzed in detail (IM113, IM119, IM140, and IM146). In each case, the disappearance of the TLD response was preceded by markedly accelerated recovery of IL-7Rα expression on TLD-specific T-cell populations relative to YVL- and GLC-specific T-cell populations. Note that another patient, IM123, was examined only at the last blood draw before the TLD response disappeared (IM123.2). In this case, the TLD-specific population was 48% IL-7Rα positive, whereas the corresponding value for the GLC-specific population was 18% (data not shown). We further checked these findings by using two directly conjugated mouse anti-IL-7Rα MAbs. Similar IL-7Rα staining profiles were obtained for TLD-specific cells in successively obtained blood samples from IM140 by using the original protocol and using the two directly conjugated reagents (data not shown). IL-7Rα expression was absent on the great majority of TLD-specific cells in acute primary infection (IM140.1) but had recovered on 38 to 48% of the population in the 3-month blood samples (IM140.2) and on 71 to 89% of the population in the 10-month blood samples (IM140.4). To look for evidence that the enhanced recovery of IL-7Rα on TLD-specific cells as opposed to GLC-specific cells was also reflected in IL-7 responsiveness in vitro, we exposed small remaining aliquots of IM140.2 and IM140.4 PBMCs to the TLD and GLC peptides and then cultured for 7 days in 5 ng/ml IL-7. Over this period, tetramer staining showed 1.8-fold (IM140.2) and 2.2-fold (IM140.4) expansions of TLD-specific cell numbers, whereas the corresponding values for GLC-specific cell numbers were 0.4-fold and 0.8-fold, respectively.
FIG. 2.
IL-7Rα statuses of YVL-, GLC-, and TLD-specific responses over time post-IM. (A) FACS profiles of IL-7Rα and either YVL- or TLD-tetramer staining from the blood samples of an HLA-A*0201-positive IM patient (IM179) taken during acute infection (IM179.1), and after a further 4 (IM179.2), 10 (IM179.3), and 27 (IM179.4) months. Data from a healthy EBV carrier are shown for comparison. Profiles are gated on CD8+ T cells. Numbers in the upper-right quadrants refer to the percentages of tetramer-positive, CD8+ cells that express IL-7Rα. (B) Summary of data from another four prospectively analyzed HLA-A*0201-positive IM patients (IM113, IM119, IM140, and IM146), showing the percentages of YVL-, GLC-, and TLD-specific tetramer-positive cells that expressed IL-7Rα, plotted against time from the acute disease. In each case, the last blood sample taken before disappearance of the TLD-specific population is marked with a dagger. Comparing values from the last available blood sample in which all three epitope-specific populations were detectable for the five patients analyzed plus IM123 (see the text), the difference between the percentages of TLD-specific cells versus YVL/GLC-specific cells that were IL-7Rα positive is significant (P = 0.001).
Marked upregulation of PD-1 on TLD-specific cells preceding their disappearance.
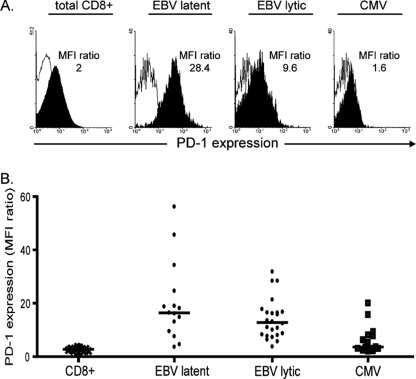
For a prelude to PD-1 phenotyping of the TLD response, we determined PD-1 levels on EBV lytic cycle, EBV latent cycle, and CMV epitope-specific populations in the blood of 11 healthy carriers. Figure 3 shows individual profiles from one representative donor and composite results from all assays. Similar results were obtained with a second independent anti-PD-1 MAb. The results were expressed as ratios of the mean fluorescence intensity (MFI) of anti-PD-1 antibody staining to that of an irrelevant antibody control, and PD-1 levels were very low on bulk CD8+ populations (mean ratio, 2.7) but progressively higher on CMV, EBV lytic cycle, and EBV latent cycle populations (mean ratios, 3.6, 12.8, and 16.4, respectively).
FIG. 3.
PD-1 staining of PBMCs from healthy donors. (A) PD-1 expression on PBMCs from an HLA-A*0201, B*0801-positive EBV/CMV carrier. The FACS profiles show distribution of PD-1 staining (filled area) versus staining with an irrelevant isotype control (open area) for total CD8+ T cells and for CD8+ tetramer-stained T cells specific for the HLA-B*0801-restricted EBV latent cycle epitope FLR, for the HLA-A*0201-restricted EBV lytic cycle epitope YVL, and for the HLA-A*0201-restricted CMV epitope NLV. For each cell population, the level of PD-1 staining is expressed as a ratio of the MFI of PD-1 antibody staining relative to that of isotype control antibody staining; isotype control MFI values were between 11 and 29, with a mean of 19.2. (B). Scatter plot showing composite PD-1 data from blood samples from 11 healthy EBV and/or CMV carriers stained as described above. Results, expressed as MFI ratios as described above, are shown for total CD8+ T cells in PBMCs and for CD8+ tetramer-stained cells specific for panels of EBV latent cycle, EBV lytic cycle, and CMV epitopes. Individual symbols refer to individual results from each donor, and horizontal lines show the median levels. Statistically significant differences in levels of PD-1 expression were observed between EBV latent cycle and CMV epitopes (P = 0.002) and EBV lytic cycle and CMV epitopes (P = 0.001) but not between EBV latent cycle and EBV lytic cycle epitopes (P = 0.09).
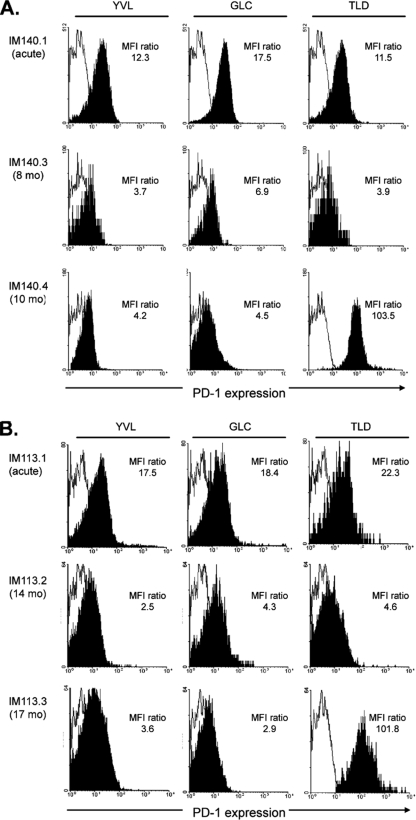
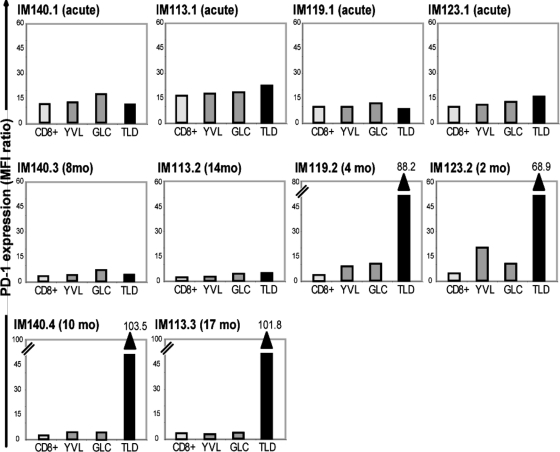
We then used the same protocols to follow the PD-1 status of YVL-, GLC-, and TLD-specific responses in IM patients. Figure 4 shows data from two patients, IM140 and IM113. In both cases, all three epitope-specific populations showed moderate PD-1 staining in the acute phase, but all had fallen to a low level in samples IM140.3 and IM113.2, taken after 8 and 14 months, respectively. This contrasts with the situation just 2 to 3 months later for the last blood samples, IM140.4 and IM113.3, in which the TLD response was still detectable. In both cases, while PD-1 staining of the YVL- and GLC-specific cells was still at a low level, the TLD-specific population had upregulated PD-1 expression to an unprecedented level, with MFI ratios of >100, at least 5-fold higher than the peak levels seen on epitope-specific cells in the acute phase of the disease and at least 20-fold higher than seen on YVL- and GLC-specific cells in the same late blood samples.
FIG. 4.
PD-1 staining of YVL-, GLC-, and TLD-specific T cells in the blood of HLA-A*0201-positive IM patients studied over time. (A) IM140 blood samples, taken during acute primary infection (IM140.1) and after a further 8 (IM140.3) and 10 (IM140.4) months. (B) IM113 blood samples, taken during acute primary infection (IM113.1) and after a further 14 (IM113.2) and 17 (IM113.3) months. At each time point, the FACS profiles show distribution of PD-1 staining (filled area) versus staining with an irrelevant isotype control (open area) on the tetramer-positive, CD8+ population. For each cell population, the level of PD-1 staining is expressed as a ratio of the MFI of PD-1 antibody staining relative to that of isotype control antibody staining; isotype control MFI values were between 8.9 and 26.5, with a mean of 14.9.
The results of PD-1 staining assays are summarized in Fig. 5 as histograms of MFI ratios. In addition to IM140 and IM113, another two patients, IM119 and IM123, also showed marked upregulation of PD-1 on TLD-specific cells in the last blood samples taken before that response disappeared. In these two cases, the key samples (IM119.2 and IM123.2) were taken just 2 to 4 months post-IM, at which time both the YVL and GLC responses still retained the moderately high PD-1 levels typical of the acute disease. However, the disappearing TLD response was again quite distinct, showing levels of PD-1 upregulation that were by comparison between 3- and 12-fold higher than these coresident reactivities. Note that for the two remaining patients (IM146 and IM179; data not shown), PD-1 expression was again moderate on epitope-specific CD8+ T cells in acute IM and then fell to a low level; in these patients, for whom there was a 8- to 17-month gap between blood draws, encompassing the disappearance of the TLD response, we did not detect any change in PD-1 levels in the last blood sample in which TLD-specific cells were present.
FIG. 5.
Histograms showing levels of PD-1 expression on total CD8+ T cells and on the YVL, GLC, and TLD epitope-specific populations in PBMCs from patients IM140, IM113, IM119, and IM123 during acute IM and at later times. Results are expressed as MFI ratios as described in the legend to Fig. 4. Note that the IM140.4, IM113.3, IM119.2, and IM123.2 samples are the last samples obtained in which a TLD-specific response was detectable; where PD-1 staining on TLD-specific cells exceeds the vertical scale, the actual MFI ratios for these TLD-specific populations are shown above the relevant columns. In a comparison of values from the last available blood sample in which all three epitope-specific populations were detectable for the four patients analyzed, the difference between PD-1 expression on TLD-specific and YVL/GLC-specific cells was significant (P = 0.028).
Functional competence of TLD-specific responses in ex vivo assays.
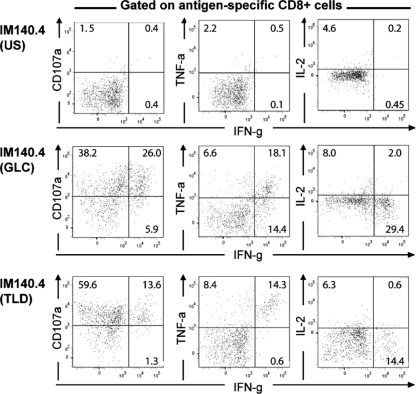
In models of chronic uncontrolled viral infection, PD-1 upregulation on virus-specific CD8+ T cells has been linked to their progressive functional impairment, as detected in cytotoxicity and cytokine release assays when the cells are stimulated ex vivo with cognate peptide (28). We therefore compared the functional competences of TLD-specific cells and their GLC-specific counterparts. Acute and post-IM PBMC samples from one patient (IM140.1, IM140.3, and IM140.4) and the key post-IM samples from two other patients for whom TLD-specific cells were PD-1hi (IM123.2 and IM119.2) were stained for CD8 and tetramers and stimulated with epitope peptide, and the responses were monitored by surface staining for CD107a as a marker of cytolytic function and by intracytoplasmic staining for the cytokines IFN-γ, TNF-α, IL-2, and MIP-1β. Non-peptide-stimulated cells served as controls. Figure 6 shows data from the IM140.4 blood sample (gated on epitope-specific populations) and fluorescence-activated cell sorter (FACS) profiles of the IFN-γ response relative to a second marker. The majority of both GLC-specific (64%) and TLD-specific (73%) cells mobilized CD107a in response to epitope stimulation. In terms of cytokine production, both epitope-specific populations gave uniformly low IL-2 (and MIP-1β [Fig. 6 and data not shown]) responses, whereas some difference was observed in the IFN-γ and TNF-α assays, with high proportions of GLC-specific cells responding compared to TLD-specific cells. The full set of functional results from GLC- and TLD-specific cells in the successive IM140 samples, alongside the phenotypic data obtained from these populations in the CD38/Ki67/Bcl2, CCR7/CD45RA, IL-7Rα, and PD-1 staining assays, are summarized in Table 1. Table 1 also shows the corresponding functional results and phenotype data for GLC- and TLD-specific cells in the IM123.2 and IM119.2 samples. Here again, the PD-1hi TLD-specific cells still made IFN-γ and TNF-α responses to peptide in ex vivo assays, though less strongly then their GLC-specific counterparts, while their responsiveness in CD107a mobilization assays remained entirely intact.
FIG. 6.
Analysis of the functional capacity of GLC- and TLD-specific populations within the IM140.4 blood sample, taken 10 months post-IM, at a time when the TLD-specific cells had upregulated PD-1 expression prior to the disappearance of that response. Cells were stained with tetramer and then exposed to epitope peptide in the presence of appropriately conjugated anti-CD107a MAb and then (after fixation and permeabilization) to appropriately conjugated MAbs to CD8 and to the intracellular cytokines IFN-γ (IFN-g), TNF-α (TNF-a), and IL-2. Data are shown as FACS profiles (gating on tetramer-labeled CD8+ T cells) that plot the IFN-γ response against each of the other response markers. Responses induced by epitope peptide stimulation are compared with those seen for the unstimulated (US) cells (the unstimulated control values illustrated are for the TLD-specific population; the GLC-specific population gave similar control data). Numbers refer to the percentages of tetramer-positive cells that, in dual staining for IFN-γ and a second marker (CD107a, TNF-α, or IL-2), respond by IFN-γ alone (bottom-right quadrant), by the second marker alone (top-left quadrant), or by both IFN-γ and the second marker (top-right quadrant).
TABLE 1.
Summary of phenotypic and functional analysis of GLC- versus TLD-specific CD8+ T cellsa
| Sample (no. of mo) and T-cell epitope | % Tetramer-positive cells with indicated phenotype/marker |
MFI ratio for PD-1b | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CCR7+ CD45RA− | CCR7− CD45RA− | CCR7− CD44RA+ | CD38 | Ki67 | Bcl2 | IL-7Rα | CD107α | IFN-γ | TNF-α | MIP-1β | IL-2 | ||
| IM140.1 | |||||||||||||
| GLC | 0.4 | 88.4 | 10.9 | 98.8 | 74.1 | 4.5 | 5.8 | NT | NT | NT | NT | NT | 17.5 |
| TLD | 1.5 | 73.2 | 24.6 | 100 | 50.0 | 9.5 | 5.8 | 87.9 | 51.1 | 51.2 | 7.8 | 11.5 | 11.5 |
| IM140.3 (8) | |||||||||||||
| GLC | 1.3 | 37.2 | 59.7 | 12.5 | 5.8 | 52.4 | 12.0 | 57.8 | 31.5 | 27.9 | 8.2 | 9.5 | 6.9 |
| TLD | 1.9 | 19.5 | 76.5 | 19.5 | 6.1 | 52.8 | 36.0 | 71.7 | 14.8 | 26.4 | 7.0 | 6.0 | 3.9 |
| IM140.4 (10) | |||||||||||||
| GLC | 1.7 | 26.2 | 70.9 | 40.5 | 7.5 | 54.3 | 24.0 | 64.2 | 31.9 | 24.7 | 8.8 | 10.0 | 4.5 |
| TLD | 3.2 | 14.3 | 80.8 | 57.9 | 9.0 | 50.8 | 64.0 | 73.2 | 14.9 | 22.7 | 7.7 | 6.9 | 103.5 |
| IM119.2 (4) | |||||||||||||
| GLC | 4.6 | 73.7 | 20.1 | 71.4 | 11.1 | 46.7 | 11.6 | 50.0 | 52.1 | 61.7 | 17.0 | 4.9 | 7.4 |
| TLD | 1.4 | 75.9 | 21.3 | 58.8 | 10.5 | 51.5 | 41.6 | 59.4 | 11.8 | 28.3 | 8.6 | 12.3 | 88.2 |
| IM123.2 (2) | |||||||||||||
| GLC | 1.5 | 80.5 | 6.5 | 92.5 | 8.6 | 31.2 | 18.0 | 48.7 | 25.2 | 26.4 | 10.7 | 12.5 | 20.3 |
| TLD | 4.8 | 74.7 | 20.5 | 84.6 | 10.7 | 36.1 | 48.0 | 52.8 | 14.8 | 17.4 | 5.5 | 7.7 | 68.9 |
Numbers refer to the percentages of tetramer-positive cells with the relevant phenotype or (following peptide stimulation) functional markers. NT, not tested. Underlined values identify key differences between GLC- and TLD-specific cell phenotypes.
Data for PD-1 staining of tetramer-positive cells are expressed as MFI ratios.
DISCUSSION
Herpesvirus carriage, as opposed to chronic progressive infection with viruses such as HIV, HBV, and HCV, is characterized by a stable virus-host balance and long-term maintenance of the virus-specific CD8+ T-cell response. The consistent disappearance of reactivity to the EBV-encoded TLD epitope in HLA-A*0201-positive IM patients (Fig. 1) provided a rare opportunity to examine virus-specific CD8+ T-cell fate in a situation where the virus infection as a whole remains under strong host control. We asked whether nonpersistence of the TLD response might be associated with an overreliance on low-avidity clones or with prolonged retention of the highly activated phenotype of primary effectors post-IM or with extreme differentiation into the effector terminal memory compartment. In fact, none of these scenarios proved to be the case. Distinguishing features of the TLD response were identified only when we turned to two other markers that, in a mouse model system, had been implicated as determinants of CD8+ T-cell fate, IL-7Rα and PD-1 (5, 22).
The percentage of IL-7Rα-positive cells in TLD-specific populations rose much faster post-IM than that in YVL- or GLC-specific populations (Fig. 2). Since all three responses contract at this time, it might be argued that any rise in percent IL-7Rα positivity simply reflects the preferential survival of IL-7Rα-positive cells at the expense of IL-7Rα-negative counterparts in the same population (22). If this were true, such a rise would be seen most quickly in the epitope-specific response that is most heavily culled post-IM. This cannot explain the present findings, however, since the rapid switch to IL-7Rα positivity in the TLD response was seen for all six HLA-A*0201-positive IM patients studied, even for cases such as IM140 and IM146, from whom YVL-, GLC-, and TLD-specific populations were culled equivalently in the months following the acute phase (see Table S1 in the supplemental material). The reacquisition of IL-7Rα by T cells that are destined to disappear might seem paradoxical, given the work on LCMV infection in mice in which IL-7Rα reexpression was identified as a positive marker of CD8+ T-cell selection into memory and IL-7 was identified as a key homeostatic cytokine mediating memory cell survival (22). However, those findings were made in the context of an acute LCMV infection that was rapidly cleared, generating memory cells that subsequently persisted in the absence of antigen. In complete contrast, CD8+ T-cell responses raised in a setting of ongoing chronic LCMV infection appear to remain dependent upon continual antigen stimulation for their survival. Even though some of those virus-specific cells become IL-7Rα positive, they could not be sustained by IL-7-mediated homeostatic signals (24, 36, 43). A similar antigen dependence is also suggested from observations of CD8+ T-cell responses to persistent HIV infection in humans, in which a virus mutation leading to epitope loss leads to the decline and/or disappearance of T cells specific for that particular epitope while other epitope reactivities persist (2, 10, 15, 17).
From such studies and from the results from the mouse herpesvirus 68 model (26), we would expect EBV-specific CD8+ T-cell survival post-IM to be similarly dependent upon recurrent antigen exposure and not upon signals from IL-7. Indeed, note that just as with LCMV-specific CD8+ T cells in chronically infected animals, EBV-specific CD8+ populations (including those specific to YVL and GLC) persist for life in healthy carriers without ever becoming fully IL-7Rα positive (33, 40). More importantly, when CD8+ T-cell memory populations against a range of EBV and CMV epitopes were compared, the percentage of IL-7Rα-positive cells within an epitope response was found to be inversely proportional to the size of that response (34). We infer from this that the degree of IL-7Rα downregulation within an epitope-specific population might actually reflect the frequency with which these cells reencounter cognate antigen in vivo. As such, we take the rapid recovery of IL-7Rα expression on TLD-specific cells post-IM to reflect a loss of sustained epitope stimulation, with the cells eventually disappearing as a result. To date, our findings are restricted to the TLD epitope, and there may be features of TLD epitope stimulation that render it unique in this regard. However, it becomes important to look for other examples of EBV lytic cycle epitopes/antigens that induce but cannot sustain CD8+ T-cell responses. One possibility is that responses to some delayed early as well as late antigens of the lytic cycle are preferentially affected in this way because their presentation is most sensitive to EBV's blockade of the HLA class I pathway in lytically infected cells (12, 48, 49). In such circumstances, as the viral load falls post-IM, the relevant T cells may no longer receive antigen stimulation.
A second distinguishing feature of the TLD-specific response relates to its PD-1 status over time. (18) Initially, PD-1 was upregulated on all EBV-specific CD8+ T-cell effectors in acute IM, consistent with other reports that its expression is transiently increased on T cells reactive to a primary virus infection (39, 41). After resolution of the acute infection, PD-1 levels on YVL-, GLC-, and TLD-specific populations fell over time to levels typical of lytic cycle epitope-specific cells in the blood of healthy carriers (i.e., above the low baseline shown by the bulk CD8+ population yet, interestingly, slightly below that of latent cycle epitope-specific cells). However, in four of six patients tested (IM140, IM113, IM119, and IM123), PD-1 was markedly upregulated on TLD-specific cells in the last blood sample in which such cells were detectable. These patients’ TLD responses must have disappeared at most within 4 to 9 months of that sample being taken or perhaps much earlier, soon after those blood samples were taken (see Table S1 in the supplemental material). For the other two patients (IM146 and IM179), therefore, it seems possible that PD-1 upregulation on TLD-specific cells could have occurred, but could have been missed, in the 7 to 17 months between the penultimate and final blood draws.
Two aspects of these findings in the EBV system contrast with those seen in chronic uncontrolled viral infections. The first is the relationship between PD-1 phenotype and function. Studies of chronic LCMV infection in mice identified PD-1 upregulation as being instrumental in the functional exhaustion of virus-specific CD8+ T cells, with rapid loss of cytotoxic responses and progressive loss of cytokine secretion responses to antigenic challenge (5). Likewise, in patients with progressive HIV, HBV, or HCV infection, while CD8+ T cells reactive to multiple viral epitopes remain detectable in the blood by tetramer staining, these cells have upregulated PD-1 expression and show impaired cytokine responses to cognate peptide stimulation in either overnight or 6-day culture assays (8, 13, 27, 28, 30, 38, 39), though in some cases, they retain activity in CD107a mobilization assays (1, 32). However, in our study, the disappearing TLD-specific cells remained largely functional in ex vivo peptide stimulation assays despite very high PD-1 expression. Thus, they still gave detectable cytokine (IFN-γ and TNF-α) responses, albeit somewhat reduced compared to those of GLC-specific control cells, and their cytotoxic response, as measured in CD107a mobilization assays, was completely unimpaired. It may be that, in the special case of late TLD-specific cells, other factors have overridden the effects of PD-1-mediated functional impairment. As recent work has shown, a T cell's functional state is not determined by PD-1 levels alone but by the overall balance of multiple stimulatory and inhibitory receptors on the T-cell surface (6, 18).
A second unusual aspect of the phenotype of TLD-specific cells immediately before their disappearance is the combination of high PD-1 expression and IL-7Rα positivity. While coexpression of these markers has been noted in circulating CD8+ T cells specific for certain HBV and HCV epitopes (8, 30), most studies of CD8+ T-cell responses to chronic viral infections in humans report progressive PD-1 upregulation and IL-7Rα downregulation (30, 38). This PD-1-positive, IL-7Rα-negative phenotype is likely a direct consequence of chronic stimulation in a situation of high antigen load (25, 36), since it is known that antigen stimulation upregulates PD-1 with the kinetics of an activation antigen (32) and downregulates IL-7Rα (35). In contrast, the asymptomatic carrier state established following primary EBV infection is characterized by low virus loads which, we infer, provide a sufficiently strong antigenic stimulus to maintain most reactivities within the EBV-specific CD8 response. Those well-maintained epitope-specific populations therefore express PD-1 at low to intermediate levels and IL-7Rα at intermediate levels. In the case of the TLD-specific cells, we suggest that they suffer inadequate antigenic stimulation; one consequence of this is that they move to an IL-7Rα-positive state, and another is that they are destined to disappear. In at least some of the cases studied here, this was associated with, perhaps executed through (28), marked upregulation of PD-1. Our study therefore suggests that PD-1 upregulation on virus-specific CD8+ T cells may have different implications in different contexts. In a chronically replicating infection, moderate levels of PD-1 upregulation are seen on multiple components of the virus-specific response, indicating its progressive impairment. In a persistent but controlled infection, much higher expression of PD-1 can identify an individual epitope response that is apparently still largely functional but, unlike coresident responses, is destined to be lost.
Supplementary Material
Acknowledgments
This study was supported by the United Kingdom Medical Research Council and NIH grant AI56299 (Gordon Freeman). D.S. benefited from a Lavoisier grant from the Ministère des Affaires Etrangères, France.
We declare no financial conflict of interest.
Footnotes
Published ahead of print on 15 July 2009.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Almeida, J. R., D. A. Price, L. Papagno, Z. A. Arkoub, D. Sauce, E. Bornstein, T. E. Asher, A. Samri, A. Schnuriger, I. Theodorou, D. Costagliola, C. Rouzioux, H. Agut, A.-G. Marcelin, D. Douek, B. Autran, and V. Appay. 2007. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J. Exp. Med. 204:2473-2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altfeld, M., T. M. Allen, X. G. Yu, M. N. Johnston, D. Agrawal, B. T. Korber, D. C. Montefiori, D. H. O'Connor, B. T. Davis, P. K. Lee, E. L. Maier, J. Harlow, P. J. R. Goulder, C. Brander, E. S. Rosenberg, and B. D. Walker. 2002. HIV-1 superinfection despite broad CD8+ T-cell responses containing replication of the primary virus. Nature 420:434-439. [DOI] [PubMed] [Google Scholar]
- 3.Annels, N. E., M. F. Callan, L. Tan, and A. B. Rickinson. 2000. Changing patterns of dominant TCR usage with maturation of an EBV-specific cytotoxic T cell response. J. Immunol. 165:4831-4841. [DOI] [PubMed] [Google Scholar]
- 4.Appay, V., and S. L. Rowland-Jones. 2002. The assessment of antigen-specific CD8+ T cells through the combination of MHC class I tetramer and intracellular staining. J. Immunol. Methods 268:9-19. [DOI] [PubMed] [Google Scholar]
- 5.Barber, D. L., E. J. Wherry, D. Masopust, B. Zhu, J. P. Allison, A. H. Sharpe, G. J. Freeman, and R. Ahmed. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439:682-687. [DOI] [PubMed] [Google Scholar]
- 6.Blackburn, S. D., H. Shin, W. N. Haining, T. Zou, C. J. Workman, A. Polley, M. R. Betts, G. J. Freeman, D. A. A. Vignali, and E. J. Wherry. 2009. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 10:29-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blattman, J. N., E. J. Wherry, S.-J. Ha, R. G. van der Most, and R. Ahmed. 2009. Impact of epitope escape on PD-1 expression and CD8 T-cell exhaustion during chronic infection. J. Virol. 83:4386-4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boni, C., P. Fisicaro, C. Valdatta, B. Amadei, P. Di Vincenzo, T. Giuberti, D. Laccabue, A. Zerbini, A. Cavalli, G. Missale, A. Bertoletti, and C. Ferrari. 2007. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J. Virol. 81:4215-4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Callan, M. F., C. Fazou, H. Yang, T. Rostron, K. Poon, C. Hatton, and A. J. McMichael. 2000. CD8(+) T-cell selection, function, and death in the primary immune response in vivo. J. Clin. Investig. 106:1251-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao, J., J. McNevin, U. Malhotra, and M. J. McElrath. 2003. Evolution of CD8+ T cell immunity and viral escape following acute HIV-1 infection. J. Immunol. 171:3837-3846. [DOI] [PubMed] [Google Scholar]
- 11.Catalina, M. D., J. L. Sullivan, K. R. Bak, and K. Luzuriaga. 2001. Differential evolution and stability of epitope-specific CD8(+) T cell responses in EBV infection. J. Immunol. 167:4450-4457. [DOI] [PubMed] [Google Scholar]
- 12.Croft, N. P., C. Shannon-Lowe, A. I. Bell, D. Horst, E. Kremmer, M. E. Ressing, E. J. H. J. Wiertz, J. M. Middeldorp, M. Rowe, A. B. Rickinson, and A. D. Hislop. 2009. Stage-specific inhibition of MHC class I presentation by the Epstein-Barr virus BNLF2a protein during virus lytic cycle. PLoS Pathog. 5:e1000490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Day, C. L., D. E. Kaufmann, P. Kiepiela, J. A. Brown, E. S. Moodley, S. Reddy, E. W. Mackey, J. D. Miller, A. J. Leslie, C. Depierres, Z. Mncube, J. Duraiswamy, B. Zhu, Q. Eichbaum, M. Altfeld, E. J. Wherry, H. M. Coovadia, P. J. Goulder, P. Klenerman, R. Ahmed, G. J. Freeman, and B. D. Walker. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443:350-354. [DOI] [PubMed] [Google Scholar]
- 14.Dorfman, D. M., J. A. Brown, A. Shahsafaei, and G. J. Freeman. 2006. Programmed death-1 (PD-1) is a marker of germinal center-associated T cells and angioimmunoblastic T-cell lymphoma. Am. J. Surg. Pathol. 30:802-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Draenert, R., T. M. Allen, Y. Liu, T. Wrin, C. Chappey, C. L. Verrill, G. Sirera, R. L. Eldridge, M. P. Lahaie, L. Ruiz, B. Clotet, C. J. Petropoulos, B. D. Walker, and J. Martinez-Picado. 2006. Constraints on HIV-1 evolution and immunodominance revealed in monozygotic adult twins infected with the same virus. J. Exp. Med. 203:529-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallimore, A., T. Dumrese, H. Hengartner, R. M. Zinkernagel, and H. G. Rammensee. 1998. Protective immunity does not correlate with the hierarchy of virus-specific cytotoxic T cell responses to naturally processed peptides. J. Exp. Med. 187:1647-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goulder, P. J. R., A. K. Sewell, D. G. Lalloo, D. A. Price, J. A. Whelan, J. Evans, G. P. Taylor, G. Luzzi, P. Giangrande, R. E. Phillips, and A. J. McMichael. 1997. Patterns of immunodominance in HIV-1-specific cytotoxic T lymphocyte responses in two human histocompatibility leukocyte antigens (HLA)-identical siblings with HLA-A*0201 are influenced by epitope mutation. J. Exp. Med. 185:1423-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenwald, R. J., G. J. Freeman, and A. H. Sharpe. 2005. The B7 family revisited. Annu. Rev. Immunol. 23:515-548. [DOI] [PubMed] [Google Scholar]
- 19.Hislop, A. D., N. E. Annels, N. H. Gudgeon, A. M. Leese, and A. B. Rickinson. 2002. Epitope-specific evolution of human CD8(+) T cell responses from primary to persistent phases of Epstein-Barr virus infection. J. Exp. Med. 195:893-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hislop, A. D., M. Kuo, A. B. Drake-Lee, A. N. Akbar, W. Bergler, N. Hammerschmitt, N. Khan, U. Palendira, A. M. Leese, J. M. Timms, A. I. Bell, C. D. Buckley, and A. B. Rickinson. 2005. Tonsillar homing of Epstein-Barr virus-specific CD8+ T cells and the virus-host balance. J. Clin. Investig. 115:2546-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hislop, A. D., G. S. Taylor, D. Sauce, and A. B. Rickinson. 2007. Cellular responses to viral infection in humans: lessons from Epstein-Barr virus. Annu. Rev. Immunol. 25:587-617. [DOI] [PubMed] [Google Scholar]
- 22.Kaech, S. M., J. T. Tan, E. J. Wherry, B. T. Konieczny, C. D. Surh, and R. Ahmed. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 4:1191-1198. [DOI] [PubMed] [Google Scholar]
- 23.Kaech, S. M., E. J. Wherry, and R. Ahmed. 2002. Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2:251-262. [DOI] [PubMed] [Google Scholar]
- 24.Lang, K. S., M. Recher, A. A. Navarini, N. L. Harris, M. Lohning, T. Junt, H. C. Probst, H. Hengartner, and R. M. Zinkernagel. 2005. Inverse correlation between IL-7 receptor expression and CD8 T cell exhaustion during persistent antigen stimulation. Eur. J. Immunol. 35:738-745. [DOI] [PubMed] [Google Scholar]
- 25.Mueller, S. N., and R. Ahmed. 2009. High antigen levels are the cause of T cell exhaustion during chronic viral infection. Proc. Natl. Acad. Sci. USA 106:8623-8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obar, J. J., S. G. Crist, E. K. Leung, and E. J. Usherwood. 2004. IL-15-independent proliferative renewal of memory CD8+ T cells in latent gammaherpesvirus infection. J. Immunol. 173:2705-2714. [DOI] [PubMed] [Google Scholar]
- 27.Penna, A., M. Pilli, A. Zerbini, A. Orlandini, S. Mezzadri, L. Sacchelli, G. Missale, and C. Ferrari. 2007. Dysfunction and functional restoration of HCV-specific CD8 responses in chronic hepatitis C virus infection. Hepatology 45:588-601. [DOI] [PubMed] [Google Scholar]
- 28.Petrovas, C., J. P. Casazza, J. M. Brenchley, D. A. Price, E. Gostick, W. C. Adams, M. L. Precopio, T. Schacker, M. Roederer, D. C. Douek, and R. A. Koup. 2006. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 203:2281-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plunkett, F. J., M. V. Soares, M. Salmon, and A. N. Akbar. 2000. Regulation of apoptosis and replicative senescence in CD8+ T cell following acute viral infection. Apoptosis 5:431-434. [DOI] [PubMed] [Google Scholar]
- 30.Radziewicz, H., C. C. Ibegbu, M. L. Fernandez, K. A. Workowski, K. Obideen, M. Wehbi, H. L. Hanson, J. P. Steinberg, D. Masopust, E. J. Wherry, J. D. Altman, B. T. Rouse, G. J. Freeman, R. Ahmed, and A. Grakoui. 2007. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J. Virol. 81:2545-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sallusto, F., J. Geginat, and A. Lanzavecchia. 2004. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 22:745-763. [DOI] [PubMed] [Google Scholar]
- 32.Sauce, D., J. R. Almeida, M. Larsen, L. Haro, B. Autran, G. J. Freeman, and V. Appay. 2007. PD-1 expression on human CD8+ T-cells depends on both state of differentiation and activation status. AIDS 21:2005-2013. [DOI] [PubMed] [Google Scholar]
- 33.Sauce, D., M. Larsen, S. J. Curnow, A. M. Leese, P. A. Moss, A. D. Hislop, M. Salmon, and A. B. Rickinson. 2006. EBV-associated mononucleosis leads to long-term global deficit in T cell responsiveness to IL-15. Blood 108:11-18. [DOI] [PubMed] [Google Scholar]
- 34.Sauce, D., M. Larsen, A. M. Leese, D. Millar, N. Khan, A. D. Hislop, and A. B. Rickinson. 2007. IL-7R alpha versus CCR7 and CD45 as markers of virus-specific CD8+ T cell differentiation: contrasting pictures in blood and tonsillar lymphoid tissue. J. Infect. Dis. 195:268-278. [DOI] [PubMed] [Google Scholar]
- 35.Schluns, K. S., W. C. Kieper, S. C. Jameson, and L. Lefrancois. 2000. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 1:426-432. [DOI] [PubMed] [Google Scholar]
- 36.Shin, H., S. D. Blackburn, J. N. Blattman, and E. J. Wherry. 2007. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J. Exp. Med. 204:941-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sylwester, A. W., B. L. Mitchell, J. B. Edgar, C. Taormina, C. Pelte, F. Ruchti, P. R. Sleath, K. H. Grabstein, N. A. Hosken, F. Kern, J. A. Nelson, and L. J. Picker. 2005. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J. Exp. Med. 202:673-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trautmann, L., L. Janbazian, N. Chomont, E. A. Said, G. Wang, S. Gimmig, B. Bessette, M. R. Boulassel, E. Delwart, H. Sepulveda, R. S. Balderas, J. P. Routy, E. K. Haddad, and R. P. Sekaly. 2006. Upregulation of PD-1 expression on HIV-specific CD8 + T cells leads to reversible immune dysfunction. Nat. Med. 12:1198-1202. [DOI] [PubMed] [Google Scholar]
- 39.Urbani, S., B. Amadei, D. Tola, M. Massari, S. Schivazappa, G. Missale, and C. Ferrari. 2006. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J. Virol. 80:11398-11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Leeuwen, E. M., G. J. de Bree, E. B. Remmerswaal, S. L. Yong, K. Tesselaar, I. J. Ten Berge, and R. A. van Lier. 2005. IL-7 receptor {alpha}-chain expression discriminates functional subsets of virus-specific human CD8+ T cells. Blood 106:2091-2098. [DOI] [PubMed] [Google Scholar]
- 41.Velu, V., S. Kannanganat, C. Ibegbu, L. Chennareddi, F. Villinger, G. J. Freeman, R. Ahmed, and R. R. Amara. 2007. Elevated expression levels of inhibitory receptor programmed death 1 on simian immunodeficiency virus-specific CD8 T cells during chronic infection but not after vaccination. J. Virol. 81:5819-5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wherry, E. J., and R. Ahmed. 2004. Memory CD8 T-cell differentiation during viral infection. J. Virol. 78:5535-5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wherry, E. J., D. L. Barber, S. M. Kaech, J. N. Blattman, and R. Ahmed. 2004. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc. Natl. Acad. Sci. USA 101:16004-16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wherry, E. J., J. N. Blattman, K. Murali-Krishna, R. van der Most, and R. Ahmed. 2003. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77:4911-4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wills, M. R., A. J. Carmichael, M. P. Weekes, K. Mynard, G. Okecha, R. Hicks, and J. G. Sissons. 1999. Human virus-specific CD8+ CTL clones revert from CD45ROhigh to CD45RAhigh in vivo: CD45RAhighCD8+ T cells comprise both naive and memory cells. J. Immunol. 162:7080-7087. [PubMed] [Google Scholar]
- 46.Woodberry, T., T. J. Suscovich, L. M. Henry, J. K. Davis, N. Frahm, B. D. Walker, D. T. Scadden, F. Wang, and C. Brander. 2005. Differential targeting and shifts in the immunodominance of Epstein-Barr virus-specific CD8 and CD4 T cell responses during acute and persistent infection. J. Infect. Dis. 192:1513-1524. [DOI] [PubMed] [Google Scholar]
- 47.Zajac, A. J., J. N. Blattman, K. Murali-Krishna, D. J. Sourdive, M. Suresh, J. D. Altman, and R. Ahmed. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zuo, J., A. Currin, B. D. Griffin, C. Shannon-Lowe, W. A. Thomas, M. E. Ressing, E. J. H. J. Wiertz, and M. Rowe. 2009. The Epstein-Barr virus G-protein-coupled receptor contributes to immune evasion by targeting MHC class I molecules for degradation. PLoS Pathog. 5:e1000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zuo, J., W. Thomas, D. van Leeuwen, J. M. Middeldorp, E. J. H. J. Wiertz, M. E. Ressing, and M. Rowe. 2008. The DNase of gammaherpesviruses impairs recognition by virus-specific CD8+ T cells through an additional host shutoff function. J. Virol. 82:2385-2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.