Abstract
The Epstein-Barr virus (EBV), a human B-lymphotropic gamma herpesvirus, contains multiple repetitive sequences within its genome. A group of repetitive sequences, known as the family of repeats (FR), contains multiple binding sites for the viral trans-acting protein EBNA-1. The FR sequences are important for viral genome maintenance and for the regulation of the promoter involved in viral latent gene expression. It has been reported that a palindromic sequence with a putative secondary structure exists at the 3′ end of the FR in the genome of the EBV B95-8 strain and that this palindromic sequence has been deleted from the FR of the commonly used EBV miniplasmids. For the first time, we cloned an EBV B95-8 DNA fragment containing the full-length FR, which enabled us to examine the functional difference between full-length and deleted FRs. The full-length FR, like the deleted FR, functioned as a transcriptional enhancer of the viral latent gene promoter, but that transactivation was significantly attenuated in the case of the full-length FR. No significant enhancement of replication was observed when the deleted FR was replaced with the full-length FR in an EBV miniplasmid. By contrast, when the same set of FR sequences were tested in the context of the complete EBV genome, the full-length FR resulted in more-efficient B-cell transformation than the deleted FR. We propose that the presence of the full-length FR contributes to the precise regulation of the viral latent promoter and increases the efficiency of B-cell transformation.
The Epstein-Barr virus (EBV), the etiologic agent of infectious mononucleosis, is known to be associated with various lymphoid and epithelial malignancies, such as Burkitt's lymphoma (BL), nasopharyngeal carcinoma, Hodgkin's lymphoma, gastric carcinoma, and lymphomas in immunosuppressed patients (36). The EBV infects B-lymphocytes in vitro, establishes a latent infection, and expresses various virally encoded gene products. As a result, infected B-lymphocytes become transformed and can grow indefinitely as lymphoblastoid cell lines (LCLs) (36).
The EBV genome is a linear, double-stranded, 175-kb DNA containing multiple repetitive sequences, which are a common feature of the herpesvirus family. The major repetitive sequences include 6 to 12 tandem repeats of 3 kb, known as internal direct repeats (IR1), and 0.5-kb direct repeats at both termini, known as terminal repeats (TRs) (36). The copy number of the EBV tandem repeats varies during viral DNA replication. For example, when EBV infects a cell, its linear genome becomes a circular episome with a characteristic number of TRs. Thus, the homogeneity or heterogeneity of the number of TRs is useful in determining whether, or not, a group of infected cells arose from a single cell (32).
EBV genomes are maintained as episomes in latently infected cells. Episomal maintenance is mediated by the following two viral elements: a cis-acting sequence, oriP, and a gene encoding a trans-acting protein, EBNA-1 (48). The oriP sequence consists of two functional elements, the dyad symmetry element (DS) and the FR (35). The DS contains four copies of the EBNA-1 binding site (33) and functions as an origin of bidirectional replication in oriP-containing miniplasmids (40, 47). The FR consists of multiple copies of a 30-bp repeat unit, each unit consisting of a 16-bp palindromic sequence that constitutes an EBNA-1 binding site (1).
A recent study reported that the number of 30-bp repeat units within the EBV FR varies between strains; for example, the FR of the EBV B95-8 strain, a prototype EBV, consists of 29 copies of the 30-bp repeat unit (5). Unlike other repetitive sequences that alter their copy numbers during viral DNA replication, the size of the FR appears to be strictly conserved within each EBV strain. Each viral strain maintains its characteristic number of FRs during long-term passaging of the cells (5). Thus, the length of the FR can be used to categorize different EBV strains. These observations support the idea that maintaining the respective size of the FR is somehow critical for the life cycle of each EBV strain.
Curiously, there is a discrepancy between the number of 30-bp units within the FR of the so-called oriP miniplasmid (such as pCEP4 [Invitrogen]) and that of the original EBV B95-8 strain from which it is derived (5). The FR of the commonly used oriP plasmid consists of only 20 copies of the 30-bp unit (21). A 252-bp DNA sequence, which corresponds to almost nine copies of the 30-bp repeats, is missing from the oriP miniplasmid, as well as from the GenBank sequence of the EBV B95-8 strain (V01555) (2). It is most likely that the 252-bp deletion in the FR occurred while subcloning EBV genomic DNA fragments into plasmid vectors (5). By contrast, the DS sequence is stably maintained before, and after, subcloning, demonstrating that the FR is subject to instability.
These observations raise the interesting possibility that the full-length FR, consisting of 29 copies of the 30-bp repeats, may have important biological functions, which have been lost from the deleted version of the repeats which contain only 20 copies.
Several functional roles have been assigned to the FR. First, the FR sequence can operate as a transcriptional enhancer. When the FR sequence is positioned in either orientation, upstream or downstream, of a heterologous promoter, the FR enhances the expression of the reporter gene (34). Deletion analysis has revealed that a minimum of seven to nine copies of the 30-bp repeats are enough to fully transactivate reporter gene expression (46). The binding of the EBNA-1 protein to the FR sequence can enhance the activity of the BamHI C promoter (Cp) (30), which drives the expression of viral transforming proteins, such as EBV nuclear antigens (EBNAs), in LCLs.
Second, the FR contributes to the nuclear retention of oriP-containing plasmids (19, 25, 46). The nuclear retention ability of the FR is partly mediated by tethering the oriP-containing plasmids to cellular chromosomes via EBNA-1 (12, 14, 22, 41). Thus, although the FR itself is not essential for the replication of the oriP plasmid (40, 47), it can contribute indirectly to the efficiency of oriP plasmid replication by minimizing plasmid loss from nuclei in dividing cells.
For the first time, we have succeeded in subcloning an EBV B95-8 strain DNA fragment containing the full-length FR (29 copies of the 30-bp repeat unit) into a plasmid vector. We then used the plasmid to generate reporter constructs and to investigate whether the full-length FR plays a role in specific biological functions that cannot be mediated by the deleted FR. The study was further extended to a B-cell transformation assay to investigate the significance of the full-length FR in the context of the EBV genome.
MATERIALS AND METHODS
Cells.
The Akata cell line is an EBV-positive BL cell line derived from a Japanese patient (42). Raji is an EBV-genome-positive cell line obtained from a BL biopsy (31). BJAB is an EBV-genome-negative B lymphoblast derived from an EBV-negative BL biopsy (17). B95-8 is a nonadherent lymphoblastoid cell line derived from a marmoset cell immortalized by EBV (26). B95a is an adherent derivative of B95-8 (18). P3HR-1 is an EBV-genome-positive BL cell line (9). All cells were maintained in RPMI 1640 medium (Sigma-Aldrich Fine Chemicals, St. Louis, MO) supplemented with 10% fetal bovine serum.
Cloning of the EBV B95-8 fragment containing the full-length FR.
Genomic DNAs of three derivatives of Akata cells and two derivatives of B95-8 cells (B95-8 cells and B95a cells) were digested with EcoRI and MluI, and the sizes of the FRs were examined by Southern blotting by using the PstI fragment of the EBV BamHI C fragment as a probe.
Cosmid clone p5 (7), containing an intact BamHI C fragment of the B95-8 strain EBV genome, was digested with BamHI, and the BamHI C fragment (9,473 bp) was subcloned into the pMBL19 vector (27), a pACYC-derived cloning vector which is capable of maintaining highly repetitive sequences. The resultant plasmid, B95-8(FR29)-BamC, was digested with EcoRI-MluI, and the size of the FR was verified by Southern blotting as described above.
Plasmid constructions.
pFR20-BamC was made by replacing the MluI-XcmI fragment of B95-8(FR29)-BamC with the 906-bp MluI-XcmI fragment of pCEP4 (Invitrogen). Plasmids pFR29-SfiI and pFR20-SfiI were constructed by inserting annealed oligonucleotides (5-CTAGCAGATCTGGCCATGTAGGCCA-3 and 5-AGCTTGGCCTACATGGCCAGATCTG-3), constituting an SfiI site, into the NheI-HindIII-digested B95-8(FR29)-BamC and pFR20-BamC plasmids, respectively. The BamHI-SspI fragment of PGV-B2 (Toyo Ink), consisting of a luciferase gene and upstream and downstream poly(A) signals, was subcloned into the BamHI-SspI-digested pMBL19 vector to generate pMBL19-Luc. pLuc was made by inserting annealed oligonucleotides (5-GATCTGGCCTACATGGCCAGGCCTA-3 and 5-CGCGTAGGCCTGGCCATGTAGGCCA-3), constituting an SfiI site, into BglII-MluI-digested pMBL19-Luc. The luciferase reporter constructs (pFR29-Luc or pFR20-Luc), containing the oriP region with the FR (either with 29 [FR29] or 20 [FR20] copies of 30-bp repeats) and DS, located upstream of the EBV Cp, were constructed by inserting the SfiI fragments of the pFR29-SfiI or pFR20-SfiI plasmid into the SfiI site of the pLuc plasmid, respectively.
The FR9 reporter constructs containing either the first nine copies of the 30-bp repeats (FR9) from the deleted FR or the nine copies of the 30-bp repeats with a 128-bp palindromic sequence [FR9(hairpin+)] from the 3′ end of the full-length FR were constructed as follows. pFR20-Luc was cut with NsiI and BstXI to remove the FR sequence, and then annealed oligonucleotides (5-TCCAGATATTTGGTGGC-3 and 5-CCAAATATCTGGATGCA-3), constituting a BstXI site, were inserted to NsiI-BstXI-digested pFR20-Luc to generate the pΔFR20 vector. The reporter construct pFR9-Luc was constructed by inserting an NsiI-BstXI fragment of pFR20-Luc vector into the NsiI-BstXI-digested pΔFR20 plasmid. The pFR29-Luc plasmid was digested with NcoI and self-ligated to generate the pFR29-ΔN plasmid, which was then cut with BstXI and self-ligated to generate pFR29-ΔNB. Annealed oligonucleotides (5-CATGGCCAGATATTTGGAGATCT-3 and 5-GTACAGATCTCCAAATATCTGGC-3), constituting a BstXI site, were then inserted into NcoI-BsrGI-digested pFR29-ΔNB to generate pFR29-BstXI. The BstXI fragment of the pFR29-BstXI plasmid contains the 252-bp region that is missing in the EBV B95-8 strain sequence (5). The reporter construct pFR9(hairpin+)-Luc was constructed by cloning the BstXI fragment of pFR29-BstXI into the BstXI site of pΔFR20.
The test plasmids used for the replication assay were constructed as follows. The pΔLuc plasmid, a control plasmid, was constructed by deleting the luciferase gene from pLuc by BamHI-BglII digestion, followed by self-ligation. Test plasmid pFR29-ΔLuc was made by digesting pFR29-Luc with DraIII and SalI, followed by Klenow enzyme (Takara) treatment and self-ligation. Another test plasmid, pFR20-ΔLuc, was cloned by inserting the SpeI-XcmI fragment of pFR20-BamC into SpeI-XcmI-digested pFR29-ΔLuc.
Reporter assay.
Cells (5 × 106 for the Akata, P3HR-1, and BJAB cell lines) were transfected with 1 to 5 μg DNA of each reporter plasmid [pFR29-Luc, pFR20-Luc, pFR9-Luc, and pFR9(hairpin+)-Luc or the control plasmid (pLuc)] together with 2.5 to 50 ng of Renilla internal control plasmid (pRL-TK) via electroporation (Bio-Rad Gene Pulser II, 190 V, 950 μF). In the case of the B95-8 cells, the cells (1 × 106) were plated onto a six-well dish and transfected with DNAs via Lipofectamine Plus reagent (Invitrogen). At either 48 h or 72 h posttransfection, the cell lysates were prepared and luciferase activities were measured by using a dual-luciferase reporter system according to the manufacturer's protocol (Promega). The obtained absolute values of firefly luciferase activities of various reporter constructs were normalized using the values of Renilla luciferase activities.
Transient replication assay and Southern blotting.
A transient replication assay was performed as described previously (46). Briefly, exponentially growing Raji cells (1 × 107) were transfected with 5 μg each of test plasmid DNA via electroporation (250 V, 950 μF), and the transfected cells were plated onto a 6-cm dish. At 24 h posttransfection, the cells were washed once with phosphate-buffered saline (PBS) to remove the untransfected DNAs, replated in 10-cm dishes, and cultured for 3 days. At 4 days posttransfection, low-molecular-weight DNA (LMW DNA) was extracted by Hirt's method (10). Briefly, cells (7 × 106) were harvested, washed once with PBS, and resuspended in 300 μl cold PBS. The cells were then lysed by adding 600 μl Hirt's solution (10 mM Tris-HCl [pH 8.0], 10 mM EDTA [pH 8.0], and 1% sodium dodecyl sulfate). Following incubation for 5 min at room temperature, the cell lysates were neutralized by adding 225 μl of 5 M NaCl and chilled at 4°C overnight. The cell lysates were centrifuged at 14,000 rpm at 4°C for 30 min, and the supernatants containing LMW DNA were extracted twice with Tris-saturated phenol. Samples were ethanol-precipitated at −80°C for 10 min, followed by centrifugation at 13,000 rpm at 4°C for 15 min. The DNA pellet was rinsed with 70% ethanol and redissolved to 50 μl of Tris-EDTA buffer (10 mM Tris-HCl, 1 mM EDTA [pH 8.0]) containing 20 μg/ml RNaseA (Sigma).
LMW DNAs prepared by Hirt's extraction were linearized by SspI digestion (14 units; New England Biolabs) in the presence or absence of DpnI (4 units; New England Biolabs) in a total volume of 40 μl. Digested samples were separated by 0.8% agarose gel electrophoresis and transferred to a Hybond N+ nylon membrane (Amersham Pharmacia Biotech). The DNA fragment of the ampicillin gene (AhdI-SspI fragment of pFR29-SfiI) was used as a probe. Probe labeling was carried out using the AlkPhos direct labeling kit (Amersham), and signals were detected with CDP-Star detection reagent (Amersham).
Bacterial artificial chromosome (BAC) engineering.
The test BACmids harboring either the FR20 or FR29 were generated by modifying the Akata-derived BAC clone with a green fluorescent protein (GFP) transgene (AK-BAC-GFP) via GET recombination (29) as described previously (15). A unique BsrGI site of B95-8(FR29)-BamC was converted to a unique NotI site by using a synthetic NotI linker to get B95-8(FR29)-BamC-NotI. A NotI fragment of the zeocin marker gene (flanked by mutated loxP sites) was then subcloned into B95-8(FR29)-BamC-NotI to get B95-8(FR29)-BamC-zeo. A linear BamHI fragment (10,248 bp) of B95-8(FR29)-BamC-zeo was used to modify the BamHI C region of AK-BAC-GFP via GET recombination. The successfully recombined BACmid DNA was treated with Cre recombinase (Novagen, Madison, WI) in vitro to remove the zeocin marker. The resultant BACmid, designated FR29-BAC, is free of the zeocin marker gene in its BamHI C region. A similar strategy was employed to generate another test BACmid, FR20-BAC.
Recombinant EBV production.
P3HR-1 cells (5 × 106) were transfected with the test BACmids (5 μg each of FR29-BAC and FR20-BAC) via electroporation (190 V, 950 μF). At 2 days posttransfection, the cells were resuspended in G418-containing medium (1,000 μg per milliliter) at a density of 3 × 105 cells per milliliter and plated in 10-cm dishes. Culture medium was replaced with fresh G418-containing medium every 5 days. The pools of G418-resistant cells, which were more than 95% GFP positive, were obtained at 2 weeks posttransfection.
Lytic EBV infection was induced in BAC-transduced P3HR-1 cells by BZLF1 transfection as described previously (23). Cell-free virus solutions were prepared by filtration (0.8-μm-pore-size filter) of the culture supernatants. EBV-negative Daudi cells (28) were infected with serially diluted mixture viruses. The infected cells were harvested at 2 days postinfection, fixed with 0.5% paraformaldehyde in PBS, and the frequency of cells expressing GFP was determined by fluorescence-activated cell sorting analyses using a FACSCalibur flow cytometer (Becton Dickinson Co., San Jose, CA). The obtained values, designated GFP-inducing titers, were used to ensure that comparable amounts of BAC-derived viruses were used for the B-cell transformation assay.
B-cell transformation assay.
Peripheral blood mononuclear cells (derived from healthy donors) were subjected to the depletion of T-lymphocytes by using CD3 Dynabeads (Invitrogen) according to the manufacturer's protocol. The T-cell-depleted mononuclear cells were infected with serially diluted (10−1 to 10−5) virus solutions at the volume ratio of 1:1, and the infected cells were plated at the density of 3 × 105 cells per well (0.2 ml per well) in 96-well plates (48 wells for each dilution). Thus, 0.1 ml of virus solution per well was used for the inoculation of each well in the assay. Half of the culture medium was replaced with fresh medium every 5 days. The number of wells with growing cells was counted at 6 weeks postinfection. The 50% transforming doses (TD50/ml) were calculated by the Reed-Muench method.
PCR analysis for discriminating type 1 and type 2 EBV.
PCR was used to discriminate the strain-specific sequence variations within the EBNA-3C gene of type 1 EBV (an Akata strain, from which the test BACmids are derived) and type 2 EBV (P3HR-1 strain) as described previously (37). The PCR parameters were as follows: 94°C for 1 min, 40 cycles at 94°C for 5 s, 63°C for 30 s, and 72°C for 30 s, followed by 72°C for 3 min. The PCR products were analyzed by 2% agarose gel electrophoresis.
RESULTS
Cloning of the full-length FR sequence into a plasmid vector.
We first examined whether the FR sequences of the EBV episomes were stably maintained in latently infected cells. Genomic DNA from several derivative cell lines of the BL cell line Akata, the B95-8 cell line, and the B95a cell line (a B95-8 derivative) (18) were prepared, and the sizes of the FRs were examined by Southern blotting using the FR sequence as a probe. We found that the FR sequences were stably maintained in the Akata-derived cell lines as well as in the B95-8-derived cell lines (Fig. 1A). The results agree with a previous report which stated that, although the copy number of the FR repeats varies substantially in different EBV strains, the length of the FR within each EBV strain is maintained stably during long-term passage (5).
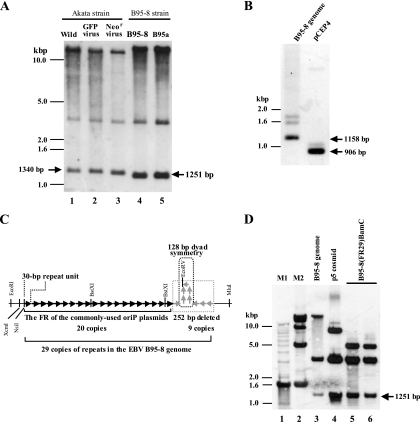
FIG. 1.
(A) Southern blot analysis demonstrating the stability of the sizes of the FRs of the different EBV strains in latently infected cells. The results of the Akata cells harboring the wild-type Akata EBV (Wild, lane 1), two Akata derivatives harboring either a recombinant EBV expressing GFP (24) (GFP virus, lane 2) or a recombinant EBV expressing the neomycin resistance gene (39) (Neor virus, lane 3), B95-8 cells (lane 4), and B95a cells (lane 5) are shown. The calculated sizes of the FR fragments (EcoRI-MluI fragments) are also indicated. (B) The sizes of the FRs in the EBV B95-8 strain genome and in pCEP4 were examined by Southern blot analysis (XcmI-MluI digestion) by using the XcmI-MluI fragment of pCEP4 as a probe. The calculated sizes of the XcmI-MluI fragments (containing the FR) are indicated. (C) Schematic representation of the full-length FR and the deleted FR. Each arrow represents a 30-bp repeat unit. The region of the 252-bp sequence (nine copies of repeats with a 128-bp palindromic sequence) that is missing from the 3′ end of the FR of the commonly used oriP plasmids is indicated. The restriction endonuclease sites of EcoRI, XcmI, NsiI, BstXI, EcoRV, and MluI are indicated. Note that 30-bp repeat units around the palindromic sequence are inversely oriented (5). (D) Successful cloning of the full-length FR of EBV B95-8 strain into a cloning vector. The Southern blot results of the B95-8 genomic DNA (lane 3), the p5 cosmid (lane 4), and B95-8(FR29)-BamC (lanes 5 and 6) are shown. Signals hybridizing to DNA size markers (M1 and M2) (lanes 1 and 2) and the calculated size of the full-length FR fragments are indicated.
The sizes of the EcoRI-MluI fragments, containing the FR region, in the EBV Akata strain were slightly bigger than those in the EBV B95-8 strain, and both of them were approximately 1,300 bp. The result allowed us to estimate the copy numbers of the FR sequences in the Akata strain and the B95-8 strain as 32 copies and 29 copies, respectively. We then directly compared the size of the FR in the EBV B95-8 strain with that of the commonly used oriP miniplasmid (pCEP4) by Southern blotting and found that the former was substantially bigger than the latter (Fig. 1B). The result confirmed the discrepancy between the size of the FR in the EBV B95-8 strain genome and its size in pCEP4. This agrees with the previous study which reported that almost nine copies (252 bp) of the repeats are missing in the oriP miniplasmids (5). The missing DNA fragment contains a 128-bp region of perfect dyad symmetry. Such a DNA sequence is likely to form a stable stem-loop structure, which tends to be deleted in Escherichia coli (Fig. 1C).
We then set out to subclone the full-length FR derived from the EBV B95-8 strain. We used the cosmid clone p5 (7), which spans the BamHI C fragment of the B95-8 genome, as the source of the full-length FR, since this cosmid clone has been shown to retain the intact FR (5). We found that the BamHI C fragment of the p5 cosmid, containing the FR region, could be subcloned into pMBL19, a low-copy-number plasmid (27), without affecting the size of the FR. Southern blotting revealed that the length of the FR in the subcloned BamHI C fragment was identical to that in the original EBV genome maintained in B95-8 cells (Fig. 1D). To our knowledge, this is the first successful cloning of the fully intact FR into a plasmid-based vector.
Attenuated transactivation of the reporter construct containing the full-length FR in EBV latently infected cells.
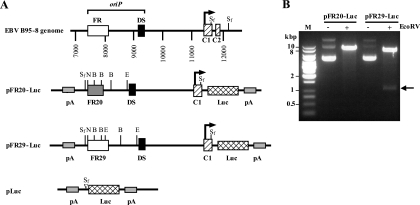
The successful cloning of the full-length FR (29 copies) and the availability of the deleted FR (20 copies), obtained from the commonly used oriP plasmids, enabled us to examine possible functional differences between the full-length and deleted FRs. Based on the long-term stability of the FR within the EBV genome in latently infected cells, we hypothesized that retaining the full-length FR should somehow be advantageous for the EBV life cycle. One possibility is that the full-length FR has stronger enhancer activity than the deleted FR on the EBV latent gene promoter Cp (3). We therefore made reporter constructs in which EBV genomic DNA fragments, spanning the region from oriP (which includes the FR and DS) to the transcription start site of Cp, were cloned upstream of a luciferase reporter gene. Two reporter constructs were made, pFR29-Luc with the full-length FR (FR29) within its oriP sequence and pFR20-Luc with the deleted FR (FR20) (Fig. 2A). The restriction enzyme EcoRV was used to distinguish between these constructs, as there is an extra EcoRV site within the FR region of pFR29-Luc which is missing in pFR20-Luc. The presence of the EcoRV fragment (1,122 bp) confirmed the existence of the full-length FR in pFR29-Luc (Fig. 2B).
FIG. 2.
(A) Schematic diagrams of the reporter constructs containing either the deleted or the full-length FR. The locations of oriP and the Cp promoter region of EBV B95-8 strain DNA are indicated on top. Two functional elements of the oriP (the FR and DS), the transcription start site of Cp (arrows), the luciferase reporter gene (Luc), and polyadenylation signals (pA) are indicated. C1 and C2 (top) are exons giving rise to the leader sequence of EBNA-encoding messages. pFR20-Luc harbors the FR with 20 copies of 30-bp repeats, while pFR29-Luc harbors the FR with 29 copies of 30-bp repeats (FR29, open box). pLuc serves as a control plasmid having only the SfiI synthetic linker (white arrowhead) instead of oriP and Cp. Restriction endonuclease sites are shown for BstXI (B), EcoRV (E), NsiI (N), and SfiI (Sf). (B) Plasmid DNAs of pFR20-Luc or pFR29-Luc, either undigested (−) or digested with EcoRV (+), were electrophoresed along with a DNA size marker (M) in 0.8% agarose gel. The EcoRV fragment (1,122 bp) being excised from pFR29-Luc is indicated by an arrow.
We examined the EBNA-1-mediated transactivation of the reporter constructs (pFR29-Luc, pFR20-Luc, and control plasmid pLuc) by introducing them into several EBV-positive cell lines. First, we transfected them into Akata cells, in which EBV expresses type I latency genes (38). We found that luciferase gene expression from both pFR20-Luc and pFR29-Luc was strongly transactivated but that stronger transactivation was observed with pFR20-Luc. The pFR20-Luc reporter exhibited approximately 40-fold transactivation compared to that of the control plasmid, whereas the pFR29-Luc reporter exhibited only 25-fold transactivation (Fig. 3A).
FIG. 3.
The enhancer activities of either the deleted FR (FR20) or the full-length FR (FR29) were determined by luciferase reporter assay. EBV-positive Akata cells (A), B95-8 cells (B), P3HR-1 cells (C), and BJAB cells (D) were used as recipient cells for transfection. Indicated amounts of an EBNA-1 expression plasmid were cotransfected together with the reporter constructs in the case of panel D. The results are expressed as the mean values ± the standard errors of the means (n = 5 in panels A and B and 3 in panels C and D).
We then performed similar experiments using B95-8 and P3HR-1 cells as the recipient cells. EBV expresses type III latency genes in these cells, with the exception of EBNA-2 expression that is missing in P3HR-1 cells. The results revealed that the absolute levels of luciferase activities of the reporter constructs were much higher than those of the Akata cells, presumably due to the high Cp activity as well as to the good transfection efficiencies of these cells (Fig. 3B and C). In B95-8 cells, pFR20-Luc and pFR29-Luc exhibited 4.2- and 2.5-fold transactivation compared to that of pLuc, while in the P3HR-1 cells, the transactivations of pFR20-Luc and pFR29-Luc were 109- and 85-fold, respectively. The induction was much lower in B95-8 cells than in Akata and P3HR-1 cells, because pLuc backbone vector itself exhibited strong transactivation in B95-8 cells (Fig. 3). In any case, the pFR29-Luc plasmid exhibited an attenuated transactivation, compared to that of pFR20-Luc, in all of the tested EBV-positive cells.
Each of the reporter constructs, together with an EBNA-1 expression vector, was then cotransfected into the EBV-negative BJAB cells in order to exclude the possibility that the attenuated transactivation observed with the reporter construct containing FR29 is peculiar to EBV-positive cells. BJAB cells were transfected with various amounts of EBNA-1 expression vector (0, 1, or 5 μg) together with constant amounts (1 μg) of either pFR20-Luc or pFR29-Luc. Both reporter constructs exhibited strong transactivation in an EBNA-1 dose-dependent manner (Fig. 3D). Absolute levels of EBNA-1-mediated transactivation of pFR29-Luc were significantly less than those of pFR20-Luc (Fig. 3D).
Taken together, the results indicate that the reporter construct containing the full-length FR does not exhibit stronger Cp transactivation than the reporter with the deleted FR. Rather, having the full-length FR actually results in attenuated transactivation, at least under these experimental conditions.
A DNA sequence containing a putative secondary structure within the FR region affects its enhancer activity.
The attenuated enhancer activity of the full-length FR could be due to the 252-bp sequence that is missing from the 3′ end of the deleted FR (Fig. 1C) (5). It was previously reported that a mutant FR containing only the first nine copies of the 30-bp repeats (designated FR9) exhibited maximal enhancer activity (46). This prompted us to test whether the last nine copies of the 30-bp repeats in the full-length FR (FR29), spanning the 252-bp sequence, exhibit similarly strong enhancer activity. These nine copies of the 30-bp repeats contain a 128-bp palindromic sequence that would be expected to form a hairpin-like secondary structure and are referred to as FR9(hairpin+).
We made two reporter constructs, pFR9-Luc and pFR9(hairpin+)-Luc, in which either FR9 or FR9(hairpin+) was placed upstream of the DS, the Cp transcription start site, and the luciferase gene (Fig. 4A). Importantly, the sizes of FR9 and FR9(hairpin+) are quite similar (264-bp and 284-bp, respectively), thus minimizing a possible effect due to a difference in size. Since FR9(hairpin+) is relatively unstable in E. coli, we verified the integrity of the FR fragments in the reporter constructs by restriction enzyme digestion. NsiI-SpeI double digestion excised similarly sized bands, representing either FR9 or FR9(hairpin+), from these two reporter constructs (Fig. 4B). By contrast, EcoRV digestion excised a 789-bp band only from pFR9(hairpin+)-Luc but not from pFR9-Luc (Fig. 4C). The result indicates that pFR9(hairpin+)-Luc retains the additional EcoRV site (as does pFR29-Luc [Fig. 2B]), which exists only in the FR9(hairpin+) region but not in the FR9 region.
FIG. 4.
(A) Schematic diagrams of the reporter constructs having nine copies of 30-bp repeats with and without a putative secondary structure. Plasmids pFR20-Luc and pFR29-Luc are identical to those depicted in Fig. 2A, and the DS of oriP, the transcription start site of Cp, the first exon (C1), the luciferase gene (Luc), and polyadenylation signals (pA) are also as depicted in Fig. 2A. pFR9-Luc harbors the first nine copies of FR that are not expected to form any secondary structure, while pFR9(hairpin+)-Luc contains the last nine copies of the full-length FR that possibly forms a stable stem-loop structure (5). The 273-bp sequences that have been artificially deleted during the plasmid construction are indicated. Restriction endonuclease sites are shown for BstXI (B), EcoRV (E), NsiI (N), and SpeI (Sp). (B, C) Plasmid DNAs of pFR9-Luc and pFR9(hairpin+)-Luc, either undigested (−) or enzyme-digested (+) (NsiI-SpeI [B] and EcoRV [C]), were electrophoresed along with a DNA size marker (M) in 0.8% agarose gel. The NsiI-SpeI fragments being excised from both of the plasmids are indicated by an arrowhead in panel B, while the EcoRV fragment being excised only from pFR9(hairpin+)-Luc is indicated by an arrow in panel C.
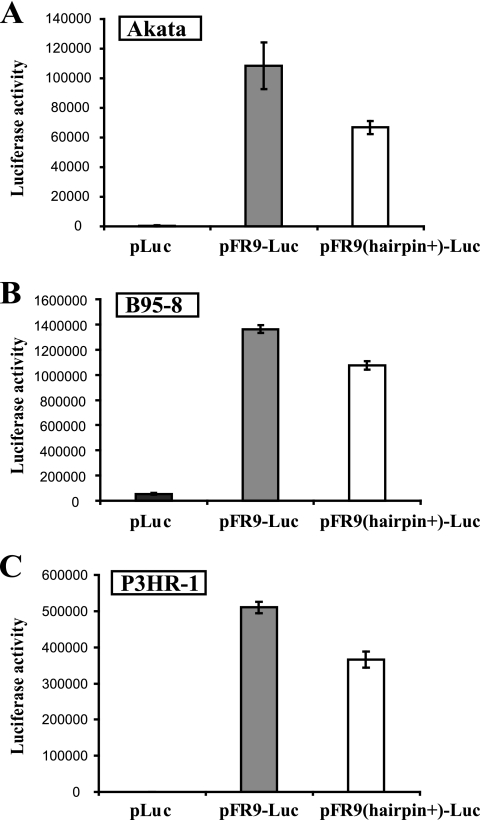
We then compared the enhancer activities of FR9 and FR9(hairpin+) by introducing the reporter constructs into various EBV-positive cells (Akata, B95-8, and P3HR-1). As expected, pFR9-Luc exhibited very strong transactivation in these cell lines. The inductions of luciferase expression of pFR9-Luc compared to those of pLuc were approximately 270-fold in Akata cells, 25-fold in B95-8 cells, and 650-fold in P3HR-1 cells. On the other hand, those of pFR9(hairpin+)-Luc were approximately 160-fold, 20-fold, and 460-fold, respectively. The induction was much lower in B95-8 cells than in Akata and P3HR-1 cells, because the pLuc backbone vector itself exhibited strong transactivation in B95-8 cells. Thus, in all of the tested EBV-positive cells, pFR9-Luc exhibited the strongest transactivation effect, whereas the transactivation observed with pFR9(hairpin+)-Luc was attenuated in all three cell lines (Fig. 5A to C).
FIG. 5.
The reporter construct having nine copies of the 30-bp repeat, which possibly forms a secondary structure, exhibits attenuated transactivation in EBV latently infected B cells. EBV-positive Akata cells (A), B95-8 cells (B), and P3HR-1 cells (C) were used as recipient cells for the luciferase reporter assay. The results are expressed as the mean values ± standard errors of the means (n = 4 for panel A and 5 for panels B and C).
The results indicate that the 252-bp region containing the 128-bp palindromic sequence can function as an enhancer but does so less efficiently than the FR9 construct lacking the palindrome. These results support the idea that the sequences represented by FR9 and FR9(hairpin+) have different biological functions and that the attenuated enhancer activity observed with the reporter construct containing the full-length FR (Fig. 3) could be due to the presence of the hairpin-like secondary structure.
Similar replication efficiencies for full-length and deleted FR test plasmids.
The FR is also well known for its ability to ensure the nuclear retention of oriP-containing plasmids. The different numbers of EBNA-1 binding sites within the FR sequences may affect EBNA-1-mediated nuclear retention abilities (25), which in turn can indirectly affect the replication efficiency of oriP-containing plasmids by minimizing the catastrophic loss of replicated plasmids from nuclei of dividing cells. Thus, we examined the replication efficiencies of test plasmids containing either the full-length FR (FR29) or the deleted FR (FR20).
The test plasmids, pFR29-ΔLuc and pFR20-ΔLuc, used for the replication assay, are identical to the reporter constructs described above, except that these plasmids had both the luciferase gene and the Cp promoter deleted. The plasmid pΔLuc, lacking the oriP sequence, was used as a negative control for the assay.
The test plasmids were transfected into Raji cells, and LMW DNA was harvested from the cells 4 days after transfection. The replication efficiencies of the test plasmids were assessed using a DpnI assay. We found that pFR29-ΔLuc and pFR20-ΔLuc replicated in Raji cells, while pΔLuc did not replicate at all (Fig. 6). The amount of DpnI-resistant DNA, representing DNA molecules that had replicated in Raji cells, was similar for both pFR29-ΔLuc and pFR20-ΔLuc. Therefore, it is unlikely that replacing the deleted FR, found in conventional oriP plasmids, with the full-length FR would confer enhanced replication efficiency on the oriP miniplasmid.
FIG. 6.
Replication activities of the test plasmids after being transiently introduced into Raji cells. Hirt extracts of transduced Raji cells were digested with restriction enzymes as indicated, and the digested DNA samples were analyzed by Southern blotting. The DNA fragment of an ampicillin resistance gene was used as a probe. The results obtained by SspI-DpnI digestion (top blot) or SspI digestion alone (bottom blot) are shown. The test plasmids, either with the deleted FR (pFR20-ΔLuc) or the full-length FR (pFR29-ΔLuc), and a control plasmid (pΔLuc) are indicated at the top. Two samples per test plasmid were analyzed, as the experiment was performed in a duplicated manner. In the top blot, only the top bands representing the DpnI-resistant replicated molecules are shown.
These results show clearly that plasmids containing either the full-length FR or the deleted FR have similar replication efficiencies. It was difficult to determine, therefore, whether the extra sequence in the full-length FR performed a specific function, missing from the deleted FR, at least under these experimental conditions.
Enhanced transformation ability of a recombinant EBV derived from an EBV-BAC clone harboring the full-length FR.
Our results thus far raised the possibility that the full-length FR might be functionally significant specifically in the context of the EBV genome. We therefore proceeded to investigate the importance of the full-length FR by using a recombinant EBV technology.
We utilized the Akata-BAC system (15) and generated EBV genomes with different FR sizes. The FR20-BAC had 20 copies of the 30-bp FR repeats and the FR29-BAC contained the full-length FR. The FR sizes of the obtained BAC clones were verified by Southern hybridization. The results indicated that the FR fragment of the FR20-BAC was identical to that of pCEP4, whereas the FR fragment of the FR29-BAC was identical to that of the B95-8 EBV genome (Fig. 7A). The size difference between the FR20-BAC and FR29-BAC BamHI C fragments (containing the FR region) could also be identified by BamHI digestion of the BAC clone DNAs, followed by agarose gel electrophoresis (Fig. 7B). Importantly, except for the bands corresponding to the FR fragments, FR20-BAC and FR29-BAC exhibited identical DNA digestion patterns (Fig. 7B). For example, in the case of the NcoI-digested samples, the sizes of the slowest-migrating bands, representing IR1, were identical between FR20-BAC and FR29-BAC (Fig. 7B). Thus, FR20-BAC and FR29-BAC are appropriate as test BAC clones.
FIG. 7.
(A) Verification of the FR sizes of the test BACmids. DNA samples were simultaneously digested by NsiI and MluI (indicated in Fig. 1C) and analyzed by Southern hybridization by using the FR20 sequence as a probe. The calculated sizes of the NsiI-MluI fragments are indicated. (B) DNA restriction enzyme mapping of the test BACmids. DNAs of AK-BAC-GFP, FR20-BAC (two independent clones), and FR29-BAC (two independent clones) were digested by either BamHI or NcoI, and the digested samples were analyzed by 0.8% agarose gel electrophoresis. The bands containing the FR region are indicated by white dots. The bands representing the IR1 are also indicated. (C) Comparable GFP-inducing titers of virus mixtures containing either FR20-BAC virus or FR29-BAC virus. EBV-negative Daudi cells were infected with serially diluted (10-fold or 100-fold) mixture viruses containing either FR20-BAC or FR29-BAC. Infected cells were analyzed by fluorescence-activated cell sorting using FL1 and FL2 channels, and GFP-expressing cells were identified by the shift of fluorescence intensity in the FL1 channel. Numbers represent the percentages of GFP-positive cells (surrounded by dotted line) after infection. The result of uninfected cells is also shown (top left). (D) PCR amplification of DNA from the EBNA-3C coding region of the latently infected EBV genomes in the established LCLs. The PCR products of the Akata EBV genome (type 1 EBV) and those of the P3HR-1 EBV genome (type 2 EBV) served as controls. (E) Five independent LCLs harboring only type 1 EBV were selected for FR20-BAC and FR29-BAC, respectively, and BAC clones were rescued from such LCLs. Three independent BAC clones from each LCL were digested by BamHI and analyzed. The BamHI C fragments containing the FR region are indicated by white dots, while the bands representing terminal repeats are indicated by black dots. Note the size difference between the BamHI C fragments of FR20-BAC (indicated by a black arrow) and those of FR29-BAC (comigrating with BamHI B fragment, indicated by a white arrowhead).
We then introduced the BAC clone DNAs into P3HR-1 cells, and transfected cells were selected using G418. We obtained four independent pools of G418-resistant cells, two pools transduced with FR20-BAC and the other two pools transduced with FR29-BAC.
The pools of transduced P3HR-1 cells were induced by BZLF1 transfection to produce mixtures of viruses consisting of endogenous P3HR-1 viruses, BAC-derived viruses, and possibly their recombinants. Both FR20-BAC and FR29-BAC contained sequences for GFP expression. The viral mixtures were initially examined for their abilities to induce GFP expression in recipient cells (EBV-negative Daudi cells) following infection. GFP-inducing titers should correlate with the amounts of BAC-derived viruses in the viral mixtures. The GFP-inducing titers were similar for the viral mixtures derived from the FR20-BAC-transduced P3HR-1 cells and those derived from the FR29-BAC-transduced P3HR-1 cells (representative data are shown in Fig. 7C), indicating that they contained comparable amounts of BAC-derived recombinant viruses.
Peripheral blood mononuclear cells were subjected to the depletion of T-lymphocytes and then infected with serially diluted viral mixtures containing either FR20-BAC or FR29-BAC, which exhibited comparable GFP-inducing titers. The parental P3HR-1 virus itself could not transform B-lymphocytes due to a deletion of its EBNA-2 gene (16). By contrast, the FR20-BAC and FR29-BAC viral mixtures were both transformation competent. Two independent experiments were performed, and in each experiment, 48 replicates were used for each dilution of viruses to accurately determine the transformation titers (Table 1). Importantly, both experiments revealed that the TD50 values of the FR29-BAC viral mixture were significantly higher than those of the FR20-BAC viral mixture.
TABLE 1.
Primary data of transformation assay and TD50 of virus mixtures containing either FR20-BAC virus or FR29-BAC virus
| Expt and clone | No. of wells with proliferating cells for indicated virus dilution factorsa |
TD50/mlb | ||||
|---|---|---|---|---|---|---|
| 10−1 | 10−2 | 10−3 | 10−4 | 10−5 | ||
| Expt 1 | ||||||
| FR20-BAC | ||||||
| Clone 1 | 48 | 48 | 27 | 1 | 0 | 104.1 |
| Clone 2 | 48 | 48 | 28 | 4 | 0 | 104.2 |
| FR29-BAC | ||||||
| Clone 1 | 48 | 48 | 44 | 15 | 3 | 104.7 |
| Clone 2 | 48 | 48 | 46 | 17 | 1 | 104.8 |
| Expt 2 | ||||||
| FR20-BAC | ||||||
| Clone 1 | 48 | 48 | 48 | 15 | 5 | 104.8 |
| Clone 2 | 48 | 48 | 48 | 15c | 0 | 104.7 |
| FR29-BAC | ||||||
| Clone 1 | 48 | 48 | 48 | 31 | 2 | 105.3 |
| Clone 2 | 48 | 48 | 48 | 34c | 2 | 105.2 |
Numbers given are out of a total of 48 wells.
Per well, 0.1 ml of diluted virus solution was used for inoculation, and the TD50 values were calculated per milliliter of virus solution.
Soundly proliferating LCLs obtained from these wells were analyzed and the results depicted in Fig. 7D.
The outgrowing LCLs were then examined to find out whether they were actually transformed by the test BAC clones. We used PCR analysis amplifying the EBNA-3C region to distinguish type 1 EBV (Akata strain EBV from which the test BAC clones are derived) from type 2 EBV (P3HR-1 EBV) (37). When we examined a panel of LCLs established by the virus solutions of dilution factors 10−1 to 10−3, all of the established LCLs were dually infected with type 1 and type 2 EBVs (data not shown). We then focused on the LCLs that were established by the virus mixture of dilution factor 10−4. Eight soundly proliferating LCLs were obtained out of 15 wells (indicated in Table 1, experiment 2) in the case of FR20-BAC (clone 2), while 14 soundly proliferating LCLs were obtained out of 34 wells (indicated in Table 1, experiment 2) in the case of FR29-BAC (clone 2). The PCR analysis revealed that, in the case of the FR20-BAC-derived LCLs, 6 out of 8 LCLs were solely infected with type 1 virus, and in the case of the FR29-BAC-derived LCLs, 13 out of 14 were solely infected with type 1 virus (Fig. 7D). Thus, most of the LCLs that were obtained by the infection of highly diluted virus mixtures were solely infected with the BAC-derived viruses, and they lacked P3HR-1 coinfection.
Furthermore, we found that such LCLs did contain the intact test BACmids. BAC clones were rescued from the LCLs that were solely infected with type 1 virus and subjected to restriction enzyme mapping. The results revealed that the rescued BAC clones were identical to the BAC clones that were used for transfection, with the only exception of the varied lengths of the terminal repeats (Fig. 7E). Importantly, when we focused on the sizes of the BamHI C fragments (containing the FR region), the difference between FR20 and FR29 was clearly identified in the rescued BAC clones (Fig. 7E). Namely, the result indicates that the established LCLs are actually transformed by EBV genomes with the expected FR copy numbers.
Thus, the overall results demonstrate that P3HR-1 coinfection and possible recombination between the test BACmids and the P3HR-1 genome should have little effect, if any, on calculating the TD50 values of the BAC-derived recombinant EBVs. Therefore, although we used the virus mixtures of BACmids and P3HR-1 for the B-cell transformation assay, it is most likely that the obtained results faithfully reflect the transformation abilities of the test BACmids FR20-BAC and FR29-BAC.
Taken together, these results strongly suggest that the FR29-BAC recombinant virus can transform B-lymphocytes more efficiently than the FR20-BAC recombinant virus and support the idea that the full-length FR is of biological significance in the context of the EBV genome.
DISCUSSION
It has been widely accepted that the FR of the B95-8 strain EBV genome consists of 20 copies of the 30-bp repeats (2). Similarly, all the available EBV-based miniplasmids also contain an FR with 20 copies of the repeats (21). These plasmids can replicate efficiently in human cells in an EBNA-1-dependent manner (48), confirming that 20 copies of the 30-bp repeats (FR20) are sufficient for normal function.
However, a recent report clearly demonstrated that FR20 has, in fact, undergone a deletion and that the full-length FR actually consists of 29 copies of the 30-bp repeats (5). In this study, we examined the size of the FR in the EBV B95-8 strain and confirmed the previous report that the FR with 29 copies of the repeats (FR29) is stably maintained in B95-8 cells. While we failed to clone the full-length FR from the EBV Akata cell line (which contains the equivalent of 32 copies of the repeats) due to its instability in E. coli (data not shown), we were able to subclone FR29 from the EBV B95-8 strain into a low-copy-number plasmid. Thus, we used two versions of the FR, the full-length (FR29) and the deleted (FR20) versions, both derived from the EBV B95-8 strain, to clarify their possible functional differences regarding transcriptional regulation and plasmid replication.
Quite unexpectedly, the inclusion of the full-length FR (FR29) in a reporter construct did not result in enhanced transcriptional regulation, or in enhanced replication, compared to the deleted FR (FR20), at least under the experimental conditions used in this study. Instead, we observed an attenuated enhancer activity with FR29 compared to that for FR20. Furthermore, no significant difference was observed between the replication efficiencies of the test plasmids with either FR29 or FR20.
A previous study demonstrated that the maximal enhancer activity of the FR did not require even 20 copies of the 30-bp repeats (20 copies were assumed to be full-length) (46). Instead, only nine copies of the 30-bp repeats were required for maximal enhancer activity. Taken together with our results, it is apparent that increased copy number does not necessarily result in stronger enhancer activity. There are at least two possible explanations for the attenuated enhancer activity of the full-length FR. The first possibility is simply that too many copies of the 30-bp repeats somehow result in attenuated enhancer activity. The second possibility is that the 3′ end of FR29, which is missing from FR20, plays some regulatory role in the attenuation of enhancer activity. Our reporter assay data for FR9-Luc and FR9(hairpin+)-Luc demonstrated that FR9-Luc exhibited stronger transactivation than FR9(hairpin+)-Luc which contained a palindromic sequence, even though the sizes of the FRs of these constructs were similar. Thus, it appears that the size of the FR is not the sole determinant of enhancer activity. Rather, the data support the idea that a putative hairpin-like secondary structure in FR9(hairpin+) plays a role in regulating enhancer activity in the EBV genome.
The primary nucleotide sequence at the 3′ end of the FR in the EBV B95-8 strain contains a 128-bp perfect palindrome. This palindromic sequence separates the FR region into two clusters of repeats (the first cluster having 23 copies and the second 6 copies), which are inverted with respect to each other (Fig. 1C). Thus, the FR should form a stable loop structure with inverse repeats surrounding the loop. Interestingly, an investigation into the primary sequences of EBV genomes derived from other lymphoblastoid cell lines has revealed that they all share a similar characteristic secondary structure (5). Therefore, although the primary sequences and the sizes of the FR are heterogeneous among various EBV strains, it appears that they may share a common secondary structure.
By contrast to the observed attenuated Cp transactivation of the full-length FR in plasmid-based systems, slightly enhanced B-cell transformation was observed when the biological activity of the full-length FR was examined in the context of the EBV genome (Table 1). Although the mixture viruses of P3HR-1 and the test BAC clones were used for the assay, we argue that the result faithfully reflects the difference of transformation potentials of the two test BAC clones, as most of the LCLs that had been established by highly diluted mixture viruses were solely infected BAC-derived viruses.
Then why does the recombinant virus of FR29-BAC transform B-lymphocytes more efficiently than that of FR20-BAC? One possible explanation is that the full-length FR is specifically required for episomal maintenance of the full EBV genome. It was demonstrated that EBNA-1 molecules constitutively bind to the oriP region of the EBV genomes (11) and that EBNA-1 serves as a bridging molecule which ensures that oriP plasmids, as well as EBV episomes, remain associated with their host's mitotic chromosomes (12, 14, 22). EBNA-1 also appears to be responsible for the faithful partitioning of EBV episomes during mitosis (13), further highlighting the importance of EBNA-1-mediated interactions between EBV episomes and host chromosomes. The full-length FR (FR29) is expected to accommodate approximately 40% more EBNA-1 molecules than the deleted FR (FR20). It is thus possible that the whole EBV genome may require more EBNA-1 molecules for a stable association with the host's mitotic chromosomes, compared to EBV-based miniplasmids, due to its larger size.
Another possibility is that the attenuation of Cp-driven transcription by the FR9(hairpin+) sequence is critical for fine-tuning the expression of viral transforming genes. In relation to this point, the FR sequence is also known to be critical for the upregulation of EBV latent membrane protein-1 (LMP-1) promoter (6). The truncated FR (FR20) may result in the overstimulation of the LMP-1 promoter and the overproduction of the LMP-1 protein, which may be disadvantageous for B-cell transformation due to its cytostatic or cytotoxic effects (4, 8, 20). Presumably, the preservation of the full-length FR (FR29) is important for the appropriate expression levels of viral transforming genes in addition to its contribution to the episomal persistence of the complete EBV genome.
It is equally likely that the observed attenuation of Cp-driven transcription by FR29 may not reflect the physiological situation of EBV infection and transformation. It was reported that the transcriptional regulation of expression of latent genes is complex. Two promoters (Cp and Wp) are involved in driving the expression of the 6 EBNA genes (44), and promoter switching occurs during the initial stages of infection (43, 45). How the FR deletion affects viral promoter usages and their transactivations remains unknown. Unfortunately, the use of viral mixtures (consisting of the nontransforming P3HR-1 virus, the transforming BAC-derived virus, and possibly their recombinants) in our current experimental system hindered the detailed analyses of viral promoter usages during the initial stages of infection. We recently obtained a BAC clone of the B95-8 EBV genome retaining the full-length FR, and succeeded in producing recombinant EBV with high transforming titer (T. Kanda, unpublished data). The system should be applicable for further analyzing the effect of the FR deletion on the expression profile of viral transforming proteins during the course of the B-cell transformation.
This study has clearly demonstrated that the integrity of repetitive sequences, known to be scattered throughout viral genomes, is sometimes necessary for viruses to function efficiently, although the mechanisms involved, at least with respect to the EBV FR, are unclear. Much of interest still remains to be clarified, including the evolutionary origin of the heterogeneity of the FRs between the various EBV strains and how such heterogeneity affects the biological processes in the different strains.
Acknowledgments
We thank B. E. Griffin for providing us with the p5 cosmid and K. Takeuchi for the B95a cells. We also thank B. Sugden, T. Tsurumi, S. Maruo, and H. Yoshiyama for helpful discussions and M. Sato for technical assistance.
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology Japan (K.T. and T.K.) and by a grant from the Akiyama Memorial Foundation (T.K.).
Footnotes
Published ahead of print on 1 July 2009.
REFERENCES
- 1.Ambinder, R. F., W. A. Shah, D. R. Rawlins, G. S. Hayward, and S. D. Hayward. 1990. Definition of the sequence requirements for binding of the EBNA-1 protein to its palindromic target sites in Epstein-Barr virus DNA. J. Virol. 64:2369-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baer, R., A. T. Bankier, M. D. Biggin, P. L. Deininger, P. J. Farrell, T. J. Gibson, G. Hatfull, G. S. Hudson, S. C. Satchwell, C. Seguin, et al. 1984. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature 310:207-211. [DOI] [PubMed] [Google Scholar]
- 3.Bodescot, M., M. Perricaudet, and P. J. Farrell. 1987. A promoter for the highly spliced EBNA family of RNAs of Epstein-Barr virus. J. Virol. 61:3424-3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Floettmann, J. E., K. Ward, A. B. Rickinson, and M. Rowe. 1996. Cytostatic effect of Epstein-Barr virus latent membrane protein-1 analyzed using tetracycline-regulated expression in B cell lines. Virology 223:29-40. [DOI] [PubMed] [Google Scholar]
- 5.Fruscalzo, A., G. Marsili, V. Busiello, L. Bertolini, and D. Frezza. 2001. DNA sequence heterogeneity within the Epstein-Barr virus family of repeats in the latent origin of replication. Gene 265:165-173. [DOI] [PubMed] [Google Scholar]
- 6.Gahn, T. A., and B. Sugden. 1995. An EBNA-1-dependent enhancer acts from a distance of 10 kilobase pairs to increase expression of the Epstein-Barr virus LMP gene. J. Virol. 69:2633-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffin, B. E., and L. Karran. 1984. Immortalization of monkey epithelial cells by specific fragments of Epstein-Barr virus DNA. Nature 309:78-82. [DOI] [PubMed] [Google Scholar]
- 8.Hammerschmidt, W., B. Sugden, and V. R. Baichwal. 1989. The transforming domain alone of the latent membrane protein of Epstein-Barr virus is toxic to cells when expressed at high levels. J. Virol. 63:2469-2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hinuma, Y., M. Konn, J. Yamaguchi, D. J. Wudarski, J. R. Blakeslee, Jr., and J. T. Grace, Jr. 1967. Immunofluorescence and herpes-type virus particles in the P3HR-1 Burkitt lymphoma cell line. J. Virol. 1:1045-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirt, B. 1967. Selective extraction of polyoma DNA from infected mouse cell cultures. J. Mol. Biol. 26:365-369. [DOI] [PubMed] [Google Scholar]
- 11.Hsieh, D. J., S. M. Camiolo, and J. L. Yates. 1993. Constitutive binding of EBNA1 protein to the Epstein-Barr virus replication origin, oriP, with distortion of DNA structure during latent infection. EMBO J. 12:4933-4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hung, S. C., M. S. Kang, and E. Kieff. 2001. Maintenance of Epstein-Barr virus (EBV) oriP-based episomes requires EBV-encoded nuclear antigen-1 chromosome-binding domains, which can be replaced by high-mobility group-I or histone H1. Proc. Natl. Acad. Sci. USA 98:1865-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanda, T., M. Kamiya, S. Maruo, D. Iwakiri, and K. Takada. 2007. Symmetrical localization of extrachromosomally replicating viral genomes on sister chromatids. J. Cell Sci. 120:1529-1539. [DOI] [PubMed] [Google Scholar]
- 14.Kanda, T., M. Otter, and G. M. Wahl. 2001. Coupling of mitotic chromosome tethering and replication competence in Epstein-Barr virus-based plasmids. Mol. Cell. Biol. 21:3576-3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanda, T., M. Yajima, N. Ahsan, M. Tanaka, and K. Takada. 2004. Production of high-titer Epstein-Barr virus recombinants derived from Akata cells by using a bacterial artificial chromosome system. J. Virol. 78:7004-7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King, W., T. Dambaugh, M. Heller, J. Dowling, and E. Kieff. 1982. Epstein-Barr virus DNA XII. A variable region of the Epstein-Barr virus genome is included in the P3HR-1 deletion. J. Virol. 43:979-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein, G., T. Lindahl, M. Jondal, W. Leibold, J. Menezes, K. Nilsson, and C. Sundstrom. 1974. Continuous lymphoid cell lines with characteristics of B cells (bone-marrow-derived), lacking the Epstein-Barr virus genome and derived from three human lymphomas. Proc. Natl. Acad. Sci. USA 71:3283-3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobune, F., H. Sakata, and A. Sugiura. 1990. Marmoset lymphoblastoid cells as a sensitive host for isolation of measles virus. J. Virol. 64:700-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krysan, P. J., S. B. Haase, and M. P. Calos. 1989. Isolation of human sequences that replicate autonomously in human cells. Mol. Cell. Biol. 9:1026-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Clorennec, C., T. S. Ouk, I. Youlyouz-Marfak, S. Panteix, C. C. Martin, J. Rastelli, E. Adriaenssens, U. Zimber-Strobl, J. Coll, J. Feuillard, and C. Jayat-Vignoles. 2008. Molecular basis of cytotoxicity of Epstein-Barr virus (EBV) latent membrane protein 1 (LMP1) in EBV latency III B cells: LMP1 induces type II ligand-independent autoactivation of CD95/Fas with caspase 8-mediated apoptosis. J. Virol. 82:6721-6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mackey, D., and B. Sugden. 1999. Applications of oriP plasmids and their mode of replication. Methods Enzymol. 306:308-328. [DOI] [PubMed] [Google Scholar]
- 22.Marechal, V., A. Dehee, R. Chikhi-Brachet, T. Piolot, M. Coppey-Moisan, and J. C. Nicolas. 1999. Mapping EBNA-1 domains involved in binding to metaphase chromosomes. J. Virol. 73:4385-4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maruo, S., Y. Wu, S. Ishikawa, T. Kanda, D. Iwakiri, and K. Takada. 2006. Epstein-Barr virus nuclear protein EBNA3C is required for cell cycle progression and growth maintenance of lymphoblastoid cells. Proc. Natl. Acad. Sci. USA 103:19500-19505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maruo, S., L. Yang, and K. Takada. 2001. Roles of Epstein-Barr virus glycoproteins gp350 and gp25 in the infection of human epithelial cells. J. Gen. Virol. 82:2373-2383. [DOI] [PubMed] [Google Scholar]
- 25.Middleton, T., and B. Sugden. 1994. Retention of plasmid DNA in mammalian cells is enhanced by binding of the Epstein-Barr virus replication protein EBNA1. J. Virol. 68:4067-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller, G., T. Shope, H. Lisco, D. Stitt, and M. Lipman. 1972. Epstein-Barr virus: transformation, cytopathic changes, and viral antigens in squirrel monkey and marmoset leukocytes. Proc. Natl. Acad. Sci. USA 69:383-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakano, Y., Y. Yoshida, Y. Yamashita, and T. Koga. 1995. Construction of a series of pACYC-derived plasmid vectors. Gene 162:157-158. [DOI] [PubMed] [Google Scholar]
- 28.Nanbo, A., K. Inoue, K. Adachi-Takasawa, and K. Takada. 2002. Epstein-Barr virus RNA confers resistance to interferon-alpha-induced apoptosis in Burkitt's lymphoma. EMBO J. 21:954-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narayanan, K., R. Williamson, Y. Zhang, A. F. Stewart, and P. A. Ioannou. 1999. Efficient and precise engineering of a 200 kb beta-globin human/bacterial artificial chromosome in E. coli DH10B using an inducible homologous recombination system. Gene Ther. 6:442-447. [DOI] [PubMed] [Google Scholar]
- 30.Puglielli, M. T., M. Woisetschlaeger, and S. H. Speck. 1996. oriP is essential for EBNA gene promoter activity in Epstein-Barr virus-immortalized lymphoblastoid cell lines. J. Virol. 70:5758-5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pulvertaft, J. V. 1965. A study of malignant tumours in Nigeria by short-term tissue culture. J. Clin. Pathol. 18:261-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raab-Traub, N., and K. Flynn. 1986. The structure of the termini of the Epstein-Barr virus as a marker of clonal cellular proliferation. Cell 47:883-889. [DOI] [PubMed] [Google Scholar]
- 33.Rawlins, D. R., G. Milman, S. D. Hayward, and G. S. Hayward. 1985. Sequence-specific DNA binding of the Epstein-Barr virus nuclear antigen (EBNA-1) to clustered sites in the plasmid maintenance region. Cell 42:859-868. [DOI] [PubMed] [Google Scholar]
- 34.Reisman, D., and B. Sugden. 1986. trans activation of an Epstein-Barr viral transcriptional enhancer by the Epstein-Barr viral nuclear antigen 1. Mol. Cell. Biol. 6:3838-3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reisman, D., J. Yates, and B. Sugden. 1985. A putative origin of replication of plasmids derived from Epstein-Barr virus is composed of two cis-acting components. Mol. Cell. Biol. 5:1822-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rickinson, A. B., and E. Kieff. 2001. Epstein-Barr virus, p. 2575-2627. In M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 37.Sample, J., L. Young, B. Martin, T. Chatman, E. Kieff, A. Rickinson, and E. Kieff. 1990. Epstein-Barr virus types 1 and 2 differ in their EBNA-3A, EBNA-3B, and EBNA-3C genes. J. Virol. 64:4084-4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimizu, N., A. Tanabe-Tochikura, Y. Kuroiwa, and K. Takada. 1994. Isolation of Epstein-Barr virus (EBV)-negative cell clones from the EBV-positive Burkitt's lymphoma (BL) line Akata: malignant phenotypes of BL cells are dependent on EBV. J. Virol. 68:6069-6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimizu, N., H. Yoshiyama, and K. Takada. 1996. Clonal propagation of Epstein-Barr virus (EBV) recombinants in EBV-negative Akata cells. J. Virol. 70:7260-7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shirakata, M., and K. Hirai. 1998. Identification of minimal oriP of Epstein-Barr virus required for DNA replication. J. Biochem. 123:175-181. [DOI] [PubMed] [Google Scholar]
- 41.Simpson, K., A. McGuigan, and C. Huxley. 1996. Stable episomal maintenance of yeast artificial chromosomes in human cells. Mol. Cell. Biol. 16:5117-5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takada, K., K. Horinouchi, Y. Ono, T. Aya, T. Osato, M. Takahashi, and S. Hayasaka. 1991. An Epstein-Barr virus-producer line Akata: establishment of the cell line and analysis of viral DNA. Virus Genes 5:147-156. [DOI] [PubMed] [Google Scholar]
- 43.Woisetschlaeger, M., X. W. Jin, C. N. Yandava, L. A. Furmanski, J. L. Strominger, and S. H. Speck. 1991. Role for the Epstein-Barr virus nuclear antigen 2 in viral promoter switching during initial stages of infection. Proc. Natl. Acad. Sci. USA 88:3942-3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woisetschlaeger, M., J. L. Strominger, and S. H. Speck. 1989. Mutually exclusive use of viral promoters in Epstein-Barr virus latently infected lymphocytes. Proc. Natl. Acad. Sci. USA 86:6498-6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woisetschlaeger, M., C. N. Yandava, L. A. Furmanski, J. L. Strominger, and S. H. Speck. 1990. Promoter switching in Epstein-Barr virus during the initial stages of infection of B lymphocytes. Proc. Natl. Acad. Sci. USA 87:1725-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wysokenski, D. A., and J. L. Yates. 1989. Multiple EBNA1-binding sites are required to form an EBNA1-dependent enhancer and to activate a minimal replicative origin within oriP of Epstein-Barr virus. J. Virol. 63:2657-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yates, J. L., S. M. Camiolo, and J. M. Bashaw. 2000. The minimal replicator of Epstein-Barr virus oriP. J. Virol. 74:4512-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yates, J. L., N. Warren, and B. Sugden. 1985. Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. Nature 313:812-815. [DOI] [PubMed] [Google Scholar]