Abstract
Parapoxvirus ovis (PPVO) is a member of the Poxviridae family and belongs to the genus Parapoxvirus. It displays only limited homology with orthopoxviruses and has some molecular features such as an unusual high GC content distinct from orthopoxviruses. Inactivated PPVO (iPPVO) displays strong immunostimulatory capacities mediating antiviral activity in vivo. The role of dendritic cells (DC) and the pattern recognition receptors and signaling requirements responsible for immunostimulation by iPPVO are unknown. We demonstrate here that bone marrow-derived plasmacytoid DC (BM-pDC) and bone marrow-derived conventional DC (BM-cDC) secrete alpha/beta interferon (IFN-α/β) in response to iPPVO. Furthermore, iPPVO induces tumor necrosis factor alpha (TNF-α) and interleukin-12/23p40 (IL-12/23p40) release and major histocompatibility complex class II (MHC-II), MHC-I, and CD86 upregulation by bone marrow-derived DC (BMDC). After engulfment, iPPVO is located in endosomal compartments and in the cytosol of BMDC. iPPVO elicits IFN-α/β by Toll-like receptor (TLR)-independent pathways in BM-cDC, since IFN-α/β release does not require myeloid differentiation primary response gene 88 (MyD88) or TIR-domain containing adaptor protein inducing interferon (TRIF). In contrast, iPPVO-induced TNF-α release and enhanced expression of MHC-I and CD86 but not of MHC-II by BMDC chiefly requires MyD88 but not TLR2 or TLR4. Induction of IFN-α by iPPVO in BM-cDC occurred in the absence of IFN regulatory factor 3 (IRF3) but required the presence of IRF7, whereas iPPVO-triggered IFN-β production required the presence of either IRF7 or IRF3. These results provide the first evidence that iPPVO mediates its immunostimulatory properties by TLR-independent and TLR-dependent pathways and demonstrate an important role of cDC for IFN-α/β production.
Parapoxvirus (PPV) ovis (PPVO), a member of the Parapoxvirus genus of the Chordopoxvirinae subfamily within the Poxviridae family, is an ovoid-shaped epitheliotrophic double-stranded DNA (dsDNA) virus with a genome of approximately 140 kb and a particle size of about 200 nm. It infects damaged or scarified skin causing ecthyma contagiosum (contagious pustular dermatitis known as orf) in sheep and goats worldwide (22) and occasionally infects humans, leading to a localized pustule or blister (milker's nodule) (49). PPVO has been shown to stimulate the immune system for release of inflammatory cytokines (9). Thus, PPVO infection might be able to modulate the outcome of concomitant infections by other pathogens. In fact, chemically inactivated PPVO (iPPVO) was demonstrated to efficiently reduce susceptibility to hepatitis B virus and herpes simplex virus infection (57). Protective nonspecific effects were also reported for modified vaccinia virus Ankara (MVA) (46), another member of the Poxviridae family. However, MVA and PPVO belong to different genera (Orthopoxvirus and Parapoxvirus), showing not only a different virion morphology but also exhibiting different virus-host interactions. The genomes of PPV in general have a base content of ca. 63% G+C. In contrast to the majority of the other poxviruses, which are A+T-rich, PPV DNA thus has by far the highest G+C content of all poxvirus DNAs (59). In electron microscopic pictures, PPVO displays a characteristic basket weave pattern, that distinguishes PPVO from other poxviruses (22).
Currently available data suggest that treatment with iPPVO leads to the activation of protective innate immune mechanisms. Among the front-line innate sentinel cells that initiate a response to immunomodulators such as iPPVO are dendritic cells (DC). DC are heterogeneous comprising several subsets expressing different morphological shapes and functions. Conventional DC (cDC) have a dendritic shape and exhibit typical DC functions such as antigen uptake, processing, and presentation. By flow cytometry, murine cDC are characterized as CD11c+ CD11b+ B220− (58). In addition to cDC, plasmacytoid DC (pDC), which are non-dendritic-shaped round cells defined by flow cytometry as CD11clow CD11b− B220+ (6) with the ability to produce large amounts of the type I interferons (IFNs) IFN-α and IFN-β in response to viral pathogens, recently came into focus for protective immune responses to viral infections (3, 19). However, the role of pDC and cDC in the enhancement of innate resistance by iPPVO and the responsible pattern recognition receptor(s) (PRR) and/or signaling requirements have not been investigated up to date.
Until today two groups of innate PRR have been linked to virus detection and the induction of immune responses. The first group, the membrane-associated Toll-like receptors (TLR), is primarily expressed by DC and MΦ and comprises at least 12 TLR (54). Ligands for TLR3, TLR7, and TLR9, which are expressed at the membranes of endosomal compartments are double-stranded RNA, single-stranded RNA, and dsDNA with unmethylated CpG motifs, respectively (31). Among the TLR expressed on the cell surface, TLR1, TLR2, and TLR6 are involved in the recognition of hepatitis C virus core protein (10). Moreover, TLR2 binds herpes simplex virus (2, 19), human cytomegalovirus (8), and hemagglutinin protein of measles virus (5), whereas TLR4 recognizes the fusion protein of respiratory syncytial virus (19, 36) and mouse mammary tumor virus (8). Among poxviruses, TLR2 (60) and TLR4 (29) were reported to be involved in immunity to vaccinia virus (VACV), whereas a role for TLR9 was proposed for recognition of MVA and ectromelia virus (ECTV) (46). Ligand binding to TLR triggers complex signaling cascades which depend on the intracellular adaptor molecules myeloid differentiation primary response gene 88 (MyD88) and/or TIR-domain containing adaptor protein inducing IFN (TRIF). Activation of MyD88 and/or TRIF triggers further downstream signaling cascades leading to the transcription of proinflammatory cytokines such as IFN-α/β, tumor necrosis factor alpha (TNF-α) and interleukin-12/23p40 (IL-12/23p40). All known TLR use either MyD88 or TRIF, only TLR4 is known to signal via both adaptor molecules (31). Importantly, proinflammatory cytokines and IFN are critically regulated by either MyD88 or TRIF alone but not by pathways using both adaptor molecules (23).
The second group of innate PRR for viruses belongs to the family of RNA helicases such as RIG-1 and MDA-5 involved in the detection of cytosolic double-stranded RNA. MDA-5 recognizes poly(I:C) and measles virus (4, 20), while RIG-I senses influenza virus and Epstein-Barr virus (37, 42, 45) leading to the expression of type I IFN. Lately, the existence of a novel cytosolic DNA sensor activating innate immune responses was reported (52) and provides evidence that additional antimicrobial recognition mechanisms exist.
PRR signaling leads to activation of transcription factors such as NF-κB or IFN regulatory factors (IRFs). IRFs induce genes that encode type I IFNs. The mammalian IRF family comprises nine family members, of which IRF3 and IRF7 came into focus as key regulators of type I IFN induction. IRF3 is constitutively expressed (26) and is required for lipopolysaccharide (LPS)/TLR4-mediated IFN-β induction (32, 44). Moreover, IRF3 is activated by signaling cascades initiated by cytosolic RNA sensors, including RIG-I or MDA-5 in response to RNA viruses (26). The cytosolic RNA pathway was also reported to be important for maximal type I IFN induction by poxviruses such as MVA and myxoma virus (35, 56). In addition to its role in cytosolic RNA pathways, IRF3 is involved in signaling pathways triggered by cytosolic DNA (14, 41). IRF7 is expressed in small amounts in most cells (26); however, in lymphoid tissues, the majority of IRF7 is expressed in pDC (43). IRF7 has been shown to play an essential role for the induction of type I IFN by RNA viruses, including encephalomyocarditis virus, and DNA viruses such as herpes simplex virus type 1 and adenovirus (14, 27).
The purpose of the present study was (i) to investigate the interaction of iPPVO with novel cellular targets such as DC and (ii) to define viral sensors and/or signaling requirements for iPPVO. In the present study, we used PPVO in its inactivated form to exclusively examine mechanisms in the absence of viral replication and escape mechanisms established by live PPVO. Flt3L-generated bone marrow-derived cDC (BM-cDC) and pDC (BM-pDC) were analyzed for the production of IFN-α/β, TNF-α, and IL-12/23p40, as well as for the expression of major histocompatibility complex class II (MHC-II), MHC-I, and CD86 in response to iPPVO and compared to BM-cDC generated in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF). To define viral sensors/signaling requirements used by iPPVO, we primarily analyzed the effects of iPPVO on cDCs and the downstream adaptor molecules MyD88 and TRIF in BM-cDC.
MATERIALS AND METHODS
Mice.
C57BL/6J wild-type mice were bred at the Max Planck Institute of evolutionary Anthropology (Leipzig, Germany). C57BL/6J TLR2−/−, TLR4−/−, and MyD88−/− mice were bred at the Technische Universität München (Munich, Germany). C57BL/6J TRIF−/−, IRF3−/−, IRF7−/−, and IRF3/7−/− mice were bred at the Max Planck Institute for Immunobiology (Freiburg, Germany). Mice were kept under specific-pathogen-free conditions. Bone marrow from mice 8 to 16 weeks old was used for all experiments.
Cell culture.
For generation of a mixed culture of bone marrow-derived pDC and cDC (BMDC), murine bone marrow cells from C57BL/6J wild-type, TLR2−/−, TLR4−/−, MyD88−/−, TRIF−/−, IRF3−/−, IRF7−/−, and IRF3/7−/− mice were cultivated for 7 days at 37°C in a humidified atmosphere containing 5% CO2 in RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum (FCS), 2 mM l-glutamine, 100 U of penicillin/ml, 100 μg of streptomycin/ml, 50 μM β-mercaptoethanol (Sigma, Taufkirchen, Germany), 0.2% vitamins, essential and nonessential amino acids, and 1% murine Flt3L-containing supernatant generated in our laboratory. A culture of pure BM-cDC was generated in the presence of GM-CSF-containing supernatant as described elsewhere (38). For stimulation, cells were seeded into 24-well plates at a final concentration of 3 × 105 to 5 × 105 cells/ml in culture medium and stimulated for the indicated time periods.
For differentiation of bone marrow-derived macrophages as defined by adherence properties, morphological features after Pappenheim staining, esterase staining, and phagocytic activity (18), murine bone marrow cells (105 cells/ml) were cultivated in Pluznik's medium containing M-CSF-containing supernatant as described elsewhere (48). After a 10-day differentiation period, bone marrow-derived macrophages were washed twice with a serum-free, high-glucose formulation of Dulbecco modified Eagle medium and stimulated at a final concentration of 3 × 105 to 5 × 105 cells/ml in 24-well plates for 48 h.
For electron microscopy, cells were washed several times after stimulation by vigorous pipetting and centrifugation to ensure that no iPPVO particles adhered to the cell surface. Subsequently, the cells were centrifuged, and the cell pellets were fixed in 2.5% Sörensen-buffered glutaraldehyde (pH 7.35). After postfixation in cacodylate-buffered 1% osmium tetroxide, the tissues were rinsed in buffer, dehydrated in graded alcohols, and processed into polymerized blocks of Epon resin. Sections for initial light microscopy to determine tissue quality and architecture suitable for ultrastructure were cut at 1 μm and stained with toluidene blue. For electron microscopy, thin sections were cut with a diamond knife, mounted on copper grids, stained with 0.2% lead citrate and 5% uranyl acetate, and examined by using a Philips EM 208 electron microscope (Philips, Eindhoven, The Netherlands) operating at 80 kV. Photography was performed with a Morada digital camera (Olympus SIS, Muenster, Germany).
Cell purification.
Where indicated, cells were purified prior to stimulation by high-gradient cell separation (MACS; Miltenyi Biotec, Bergisch-Gladbach, Germany). Either CD11b+ BM-cDC or B220+ BM-pDC were removed with fluorescein isothiocyanate (FITC)/phycoerythrin (PE)-labeled anti-mouse-CD11b (clone M1/70.15; Caltag Laboratories, Burlingame, CA) or anti-mouse-B220 (clone RA3-6B2; Caltag Laboratories), respectively, followed by incubation with an anti-FITC/PE-antibody coupled to magnetic beads (Miltenyi Biotec) according to the manufacturer's instructions using LD MACS columns and the VarioMACS system (Miltenyi Biotec). The success of depletion was confirmed by fluorescence-activated cell sorting (FACS) analysis prior to stimulation. Purified BM-pDC contained at most less than 4% BM-cDC. Purified BM-cDC contained at most less than 0.6% BM-pDC.
Stimuli.
Chemically inactivated and purified PPVO strain D1701 was kindly provided by Pfizer, Ltd. (Sandwich, United Kingdom). Highly purified iPPVO obtained by sucrose gradient purification was produced as described previously (39). Inactivation was performed using binary ethyleneimine or β-propiolactone, two agents commonly used for virus inactivation. The inactivation efficacy was tested by three blind passages of undiluted iPPVO in highly permissive primary bovine esophagus cells. iPPVO was used, if not indicated otherwise, at a multiplicity of infection (MOI) of 5. Similar results were obtained using differently purified and inactivated iPPVO batches (data not shown). For the control, cells were stimulated with the synthetic ligands PAM3CSK4 (2 μg/ml; EMC Microcollections, Tübingen, Germany), polyinosinic-poly(C) potassium salt [poly(I:C), 100 μg/ml; Amersham, Piscataway, NJ], LPS from Salmonella Abortus Equi S-Form (TLRgrade, 5 μg/ml; Alexis, Grünberg, Germany), or CpG-ODN 2216 (1 μM, TIB MOLBIOL; Syntheselabor GmbH, Berlin, Germany) with the sequence 5′-GsGsGGGACGATCGTCsGsGsGsGsGsG-3′ (s = phosphothioate) for TLR2, TLR3, TLR4, or TLR9, respectively. All stimuli were diluted in the appropriate cell culture medium prior to cell stimulation.
Cytokine analysis.
The cytokine concentrations of stimulated BMDC and bone marrow-derived macrophage supernatants were measured by sandwich enzyme-linked immunosorbent assay (ELISA). Murine IFN-β was quantified by using a VeriKine Mouse Interferon Beta ELISA kit from PBL Biomedical Laboratories (Piscataway, NJ). Murine TNF-α was analyzed according to the manufacturer's description with capture and detection antibody pairs supplied by R&D Systems GmbH (Wiesbaden-Nordenstadt, Germany). Murine IFN-α and IL-12/23p40 were analyzed using the monoclonal antibodies RMMA-1 (2 μg/ml; R&D Systems GmbH) and 5C3 (25 μg/ml), respectively, as capture antibodies. For detection, polyclonal rabbit anti-mouse IFN-α-purified immunoglobulin G (IgG; 1:4,000; R&D Systems GmbH) and biotinylated goat anti-mouse IL- 12/23p40-purified IgG (1:1,000; provided by M. Gately, F. Hoffmann-La Roche, Ltd., Nutley, NJ) were used, respectively. Murine IFN-α as an ELISA standard was purchased from Cytimmune (Lee Biomolecular Research, Inc., San Diego, CA) and was measured in international reference units (IRU)/ml as provided by the National Institutes of Health (7, 15). IL-12/23p40 as an ELISA standard was kindly provided by Schering-Plough Biopharma (Palo Alto, CA). The polyclonal rabbit anti-mouse IFN-α-purified IgG was detected by using donkey anti-rabbit IgG F(ab′)2-horseradish peroxidase (1:2,500; Dianova, Hamburg, Germany), whereas the biotinylated goat anti-mouse IL-12/23p40-purified IgG was detected by using streptavidin-horseradish peroxidase (1:3,000; Southern Biotechnology Associates, Birmingham, AL). For the final colorimetric reaction, the TMB Microwell peroxidase system (KPL, Gaithersburg, MD) was used as a substrate. The ELISA plates were measured at 650 and 480 nm with a Spectra-max 340 ELISA reader (Molecular Devices, Munich, Germany).
Flow cytometry.
If not noted otherwise, antibodies were acquired from BD (Heidelberg, Germany). Cells were incubated with anti-CD16/CD32 FcR block (clone 2.4G2) and stained with rat IgG isotype control-FITC/PE, mouse IgG isotype control-PE, hamster IgG isotype control-allophycocyanin, anti-mouse F4/80-FITC (clone CI:A3-1; Serotec GmbH, Düsseldorf, Germany), anti-mouse F4/80-PE (clone CI:A3-1; Caltag Laboratories), anti-mouse-CD45R-FITC/PE (B220; clone RA3-6B2; Caltag Laboratories), anti-mouse CD86-PE (clone RMMP-2; Caltag Laboratories), anti-mouse I-Ab-FITC/PE (clone AF6-120.1), anti-mouse H-2Db-PE (clone KH95), or anti-mouse CD11c-allophycocyanin (clone HL3). After 15 min of incubation at 4 to 8°C, the cells were washed twice in FACS buffer (3% FCS and 0.1% NaN3 in phosphate-buffered saline [PBS]) and fixed with 1.0% (vol/vol) formaldehyde in PBS. Analyses of the cells were performed with a FACSCalibur flow cytometer (BD, Heidelberg, Germany) by using the software CellQuest Pro (BD).
For intracellular IFN-α and IL-12/23p40 staining, BMDC were stimulated with iPPVO at an MOI of 5 as described above in the presence of GolgiPlug (BD) for the final 2 to 3 h. At least 106 cells were incubated in the presence of 25 μg of anti-CD16/CD32/ml for 10 min at 4°C, washed once in FACS buffer (3% FCS and 0.1% NaN3 in PBS), and fixed in 4% formaldehyde in PBS. Subsequently, BMDC were incubated in permeabilization buffer (0.55% saponin, 5% FCS, and 2 mM HEPES in PBS) for 20 min and stained for intracellular IFN-α with the unlabeled monoclonal antibody RMMA-1 and polyclonal rabbit anti-mouse-IFN-α IgG (R&D Systems GmbH) or PE-labeled IL-12/23p40 (clone C15.6) as primary antibodies. The unlabeled anti-IFN-α antibodies were detected by using PE-labeled donkey anti-rat IgG F(ab′)2 donkey anti-rabbit IgG F(ab′)2 (2.5 μg/ml; Dianova). This was followed by surface staining with anti-CD11c (allophycocyanin labeled) and anti-B220 (FITC labeled) as described above. Cells were analyzed within 24 h of staining.
Statistics.
Statistical analysis was performed by using analysis of variance and Bonferroni's post test. Statistical significance was defined to be based on a P value smaller than 0.05.
RESULTS
BMDC release type I IFN and the proinflammatory cytokines in response to iPPVO.
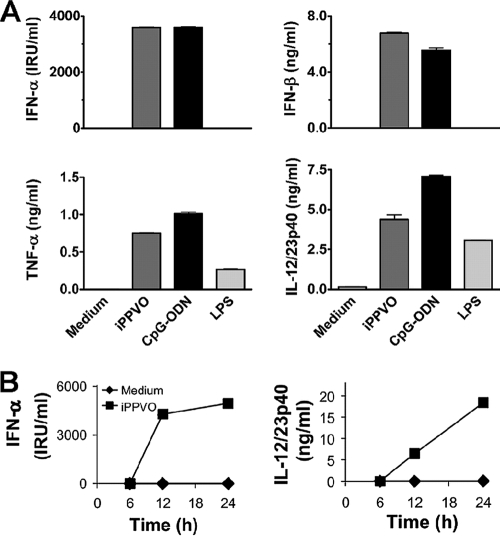
Type I IFN including IFN-α and IFN-β induce important antiviral effector mechanisms such as the enhanced expression of antiviral proteins, sensitization of bystander cells to apoptosis upon subsequent viral infection, and stimulation of antiviral effector cells such as NK cells and cytotoxic T lymphocytes (50). Important cellular sources of type I IFN are pDC (12). Nevertheless, besides pDC also other cells, including cDC, are able to produce type I IFN, albeit usually at lower levels than pDC (17). The activation of DC by iPPVO has not been investigated yet. To explore whether immunostimulatory effects of iPPVO were associated with the induction of IFN-α and IFN-β in DC, Flt3L-generated BMDC comprising BM-cDC and BM-pDC (6) were compared in terms of IFN-α/β production upon iPPVO stimulation. In addition, the release of the proinflammatory cytokines TNF-α and IL-12/23p40 was investigated. CpG-ODN, characterized as a potent IFN-α inducer (33), and LPS, known as a strong stimulator of BMDC (48), were used for control. Upon iPPVO stimulation, BMDC were potent producers of IFN-α, IFN-β, TNF-α, and IL-12/23p40 (Fig. 1A). Similar to iPPVO, CpG-ODN induced the release of IFN-α, IL-12/23p40, and TNF-α in BMDC, whereas upon LPS stimulation TNF-α and IL- 12/23p40 but not IFN-α/β were produced. LPS-induced levels of IFN-β were below detection in most experiments; however, we observed detectable production of IFN-β by BMDC in response to LPS in single experiments (about 30 pg/ml; data not shown). The induction of cytokine responses of BMDC by iPPVO was dose dependent between MOI 5 and 0.05 (data not shown). Interestingly, the production of IFN-α and IL- 12/23p40 occurred with different kinetics (Fig. 1B), indicating that differential signaling modes of production of these cytokines are operative. As expected from a previous study using human PBMC (56), murine bone marrow-derived macrophages also responded with substantial IFN-α (270-2100 IRU/ml) and IFN-β (0.6-1.0 ng/ml) production upon iPPVO stimulation. However, bone marrow-derived macrophages did not produce IL-12/23p40 and only released marginal levels of TNF-α in response to iPPVO (data not shown). Taken together, these data demonstrate that BMDC are potent producers of IFN-α, IFN-β, TNF-α, and IL-12/23p40 after iPPVO stimulation, pointing to DC as important cells sensing iPPVO and mediating immunostimulatory effects upon iPPVO encounter.
FIG. 1.
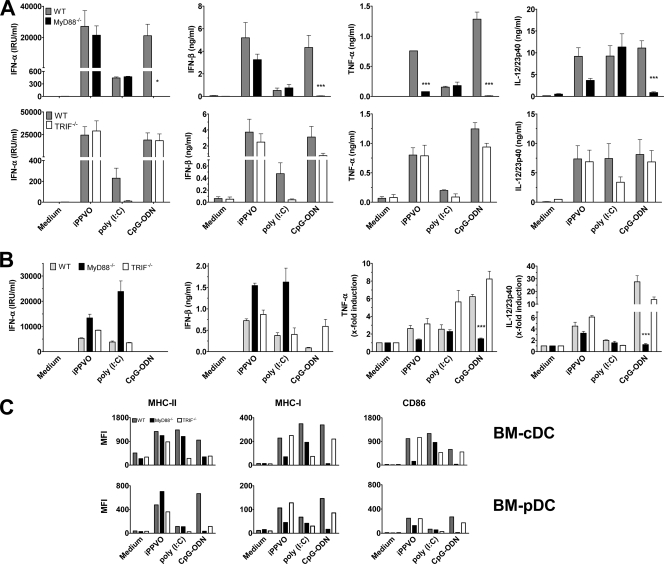
Production of IFN-α, IFN-β, TNF-α, and IL-12/23p40 by BMDC in response to iPPVO. (A) Flt3L-generated BMDC were stimulated with iPPVO (MOI = 5), CpG-ODN (1 μM), and LPS (5 μg/ml). After 48 h of incubation, the concentrations of IFN-α, IFN-β, TNF-α, and IL-12/23p40 in the culture supernatants were measured by sandwich ELISA. Each column represents the mean ± the standard error of the mean (SEM) of at least duplicate cultures. The results of one representative experiment of at least three independent experiments are shown. The levels of LPS-induced IFN-β were below detection in this experiment. However, small amounts of LPS-induced IFN-β could be observed in single experiments (data not shown). (B) Supernatants of iPPVO-stimulated Flt3L-generated BMDC were harvested at the indicated time points, and the amounts of IFN-α and IL-12/23p40 were determined. The results of one of two independent experiments are shown (duplicate cultures).
BM-pDC and BM-cDC contribute to iPPVO-induced IFN-α/β production.
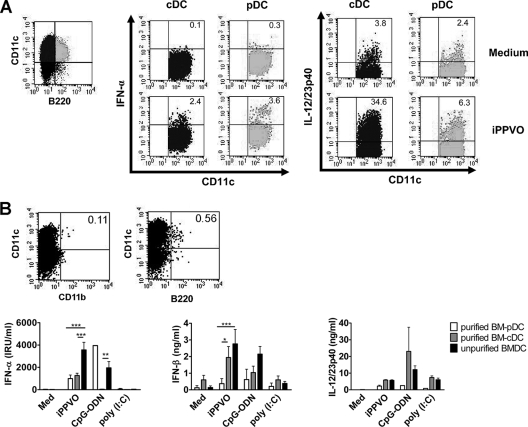
Flt3L-generated BMDC represent a mixed culture of BM-pDC and BM-cDC (6). To address the question, which BMDC subset(s) contributed to IFN-α production in response to iPPVO, the presence of intracellular IFN-α and, for comparison, of intracellular IL-12/23p40 was monitored by flow cytometry in iPPVO-stimulated BM-cDC and BM-pDC. BM-cDC and BM-pDC were distinguished by the expression of the pDC-specific surface molecule B220 (3, 6). CpG-ODN served as control stimulus. Upon stimulation with iPPVO, surprisingly, both BM-pDC and BM-cDC produced IFN-α in similar amounts, whereas IL-12/23p40 production was, as expected, biased to BM-cDC (Fig. 2A). To investigate whether interactions between these two BMDC subsets influenced cytokine secretion, we analyzed purified BM-cDC and BM-pDC for the release of IFN-α, IL-12/23p40, and IFN-β in response to iPPVO. Consistent with the finding of the flow cytometric analysis, after iPPVO stimulation purified BM-cDC and purified BM-pDC both contributed to IFN-α production, whereas IL-12/23p40 production was dominated by BM-cDC (Fig. 2B). Furthermore, purified BM-cDC significantly contributed to IFN-β production compared to BM-pDC upon iPPVO encounter (Fig. 2B). IFN-α but not IFN-β levels were enhanced about ∼3-fold if both BMDC subsets, BM-cDC and BM-pDC, were present during stimulation, indicating only limited interactions between DC subsets for IFN production. These results indicate that both cDC and pDC are critically involved in mediating immunostimulatory effects in response to iPPVO and suggest an important role of cDC in addition to pDC for IFN-α and IFN-β production.
FIG. 2.
iPPVO-induced IFN-α and IL-12/23p40 production by BM-pDC and BM-cDC. (A) Flt3L-generated BM-cDC (cDC; upper left quadrant, black) and BM-pDC (pDC; upper right quadrant, gray) were stimulated for 12 h with medium or iPPVO (MOI = 5) and analyzed for intracellular IFN-α and IL-12/23p40 synthesis by flow cytometry. The numbers in the upper right corners indicate the proportion of IFN-α+ (left) and IL-12/23p40+ (right) cells. Quadrants were set according to the isotype control staining; all background staining signals were below 0.2% (data not shown). (B) Prior to stimulation with iPPVO (MOI = 5), Flt3L-generated BM-cDC and BM-pDC were purified by depleting contaminating cells with magnetic beads. The numbers in the upper right corners indicate the percentages of contaminating CD11b+ BM-cDC in purified BM-pDC (left) and of contaminating B220+ BM-pDC in purified BM-cDC (right) after the depletion procedure. The results of one of two independent experiments are shown (top panels). The cell numbers were adjusted to 5 × 105 cells/ml. After stimulation for 24 h, cell culture supernatants were analyzed for IFN-α, IL-12/23p40, and IFN-β by sandwich ELISA. Each column represents the mean ± the SEM of at least duplicate cultures. Pooled data of two independent experiments are shown (bottom panels). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
iPPVO induces upregulation of MHC-II, MHC-I, and CD86 chiefly on BM-cDC.
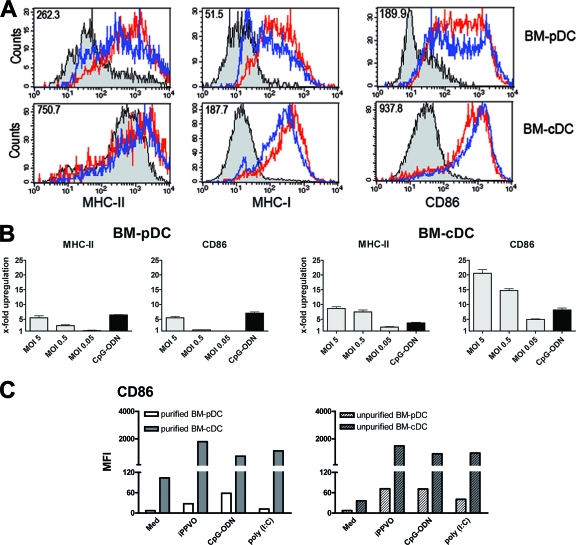
The expression of MHC and costimulatory molecules on antigen-presenting cells (APC) directly correlates with their ability to present antigen to T cells and to activate naive T cells. Therefore, it was of interest to characterize and compare the expression of MHC-II, MHC-I, and the costimulatory molecule CD86 on BM-pDC versus BM-cDC by flow cytometry. Again, BM-cDC and BM-pDC were distinguished by the expression of B220. On BM-pDC, MHC-II, MHC-I, and CD86 were found to be upregulated to some extent upon stimulation with iPPVO (Fig. 3A, top panels, blue line). However, upregulation of MHC-II, MHC-I, and CD86 was much stronger on BM-cDC in response to iPPVO (Fig. 3A, bottom panels, blue line). A more pronounced upregulation on BM-cDC than on BM-pDC was also observed for CD40 and CD80 (data not shown). Upon CpG-ODN stimulation, BM-pDC and BM-cDC expressed similar levels of MHC-II, MHC-I, and CD86 as upon iPPVO stimulation (Fig. 3A, red line). The iPPVO-induced upregulation of MHC-II and CD86 on BM-cDC and BM-pDC was dose dependent (Fig. 3B). The x-fold upregulation of CD86 upon CpG-ODN stimulation was identical in BM-cDC and BM-pDC, showing that BM-pDC had no intrinsic defect in surface molecule expression (Fig. 3B). Bone marrow-derived macrophages did not markedly enhance expression of MHC-II or CD86 upon iPPVO stimulation (data not shown). These data point to cDC as potent APC in response to iPPVO. Upregulation of CD86 was similar on purified BM-cDC and BM-pDC compared to unpurified BM-cDC and BM-pDC (Fig. 3C), indicating that cell-cell contact or soluble factors did not lead to enhanced BMDC maturation.
FIG. 3.
iPPVO-induces upregulation of MHC-II, MHC-I, and CD86 on BM-cDC rather than BM-pDC. (A) After cultivation for 48 h in the presence of iPPVO (MOI = 5; blue line), medium (gray curve), and CpG-ODN (1 μM; red line) for control, Flt3L-generated BM-pDC and BM-cDC were stained for MHC-II, MHC-I, and CD86 and analyzed separately by FACS. The numbers in the top left corners indicate the difference in the median fluorescence intensities (MFI) of the indicated molecule of iPPVO- versus medium-cultured cells. (B) The x-fold upregulation of MHC-II and CD86 on Flt3L-generated BM-pDC and BM-cDC after stimulation as in panel A was calculated by division of the MFI of the indicated surface molecule after cultivation in the presence of the indicated MOIs of iPPVO by those of medium cultured cells. The upregulation of the indicated surface molecules of medium-cultured cells was normalized to 1. (C) Purified Flt3L-generated BM-cDC and BM-pDC were analyzed for the expression of CD86 after 24 h of culture in the presence of the indicated stimuli by FACS measurement (left panel). For comparison, BM-pDC and BM-cDC within unpurified Flt3L-generated BMDC were electronically gated and investigated for CD86 expression by flow cytometry (right panel). The results of one of two independent experiments are shown.
iPPVO-induced BMDC activation partially depends on MyD88 but does not require TRIF.
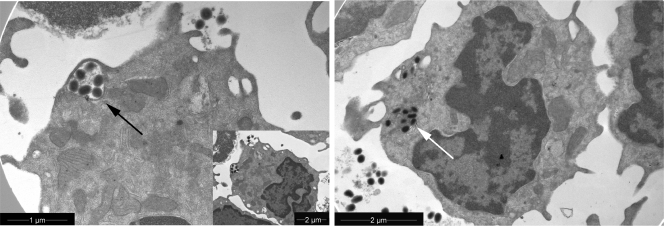
It was further investigated whether iPPVO was taken up by BMDC. By electron microscopy we found that iPPVO was indeed taken up by BMDC. Engulfed iPPVO particles located in groups in endosomal compartments (Fig. 4, black arrow). Single iPPVO particles were also observed in the cytosol (Fig. 4, white arrow). The endosomal localization of iPPVO indicates phagocytosis as one mode of iPPVO uptake and provides new insights in the localization and processing mechanisms of iPPVO.
FIG. 4.
Electron micrograph of Flt3L-generated BMDC cultured in the presence of iPPVO. Flt3L-generated BMDC cultured for 6 h in the presence of iPPVO (MOI = 5). iPPVO particles were located in the endosomal compartments of the cell (black arrow) and in the cytosol (white arrow).
We wanted to investigate the potential involvement of TLR in iPPVO-induced BMDC activation. Activation of TLR requires either the intracellular adaptor molecule MyD88 or TRIF (54). Therefore, analysis of the role of MyD88 and TRIF in iPPVO-induced BMDC activation allows conclusions on the TLR involved in iPPVO recognition. Thus, we monitored IFN-α/β production by BMDC originating from wild-type, MyD88−/−, and TRIF−/− mice upon iPPVO stimulation. The number and phenotype of BMDC derived from MyD88−/− and TRIF−/− mice were similar to those of wild-type BMDC (data not shown). Poly(I:C) or CpG-ODN, ligands known to signal via TRIF or MyD88, respectively, were used as control stimuli. Induction of IFN-α and IFN-β by CpG-ODN were triggered only in the presence of MyD88. In contrast, wild-type and MyD88−/− BMDC released IFN-α and IFN-β upon iPPVO stimulation (Fig. 5A, top panels). However, IFN-β levels tended to be slightly reduced in MyD88−/− BMDC. Upon analyzing the requirement of TRIF for induction of IFN-α/β by iPPVO, we found that wild-type and TRIF−/− BMDC were able to produce IFN-α/β to a similar degree after iPPVO stimulation. In contrast, IFN-α/β induction by poly(I:C) required the presence TRIF (Fig. 5A, bottom panels). These data provide evidence that induction of IFN-α/β in BMDC by iPPVO does not depend on MyD88- and TRIF-dependent pathways.
FIG. 5.
Role of intracellular adaptors MyD88 and TRIF in iPPVO-induced BMDC activation. (A) Flt3L-generated BMDC originating from wild-type (WT), MyD88−/− (upper panels), or TRIF−/− (lower panels) bone marrow were cultured in the presence of iPPVO (MOI = 5). Poly(I:C) (100 μg/ml; TRIF dependent) and CpG-ODN (1 μM; MyD88 dependent) were used as controls. Cell culture supernatants of at least duplicate cultures were harvested and analyzed by sandwich ELISA. Pooled data of two independent experiments are shown. Each column represents mean ± the SEM. (B) BM-cDC originating from wild-type (WT), MyD88−/−, or TRIF−/− bone marrow were generated in the presence of GM-CSF containing supernatant and analyzed as in panel A. Pooled data of two independent experiments (triplicate cultures) are shown. Each column represents mean ± the SEM. *, P < 0.05; ***, P < 0.001 (compared to the wild type). (C) Flt3L-generated BMDC originating from wild-type, MyD88−/−, or TRIF−/− bone marrow were cultured as in panel A. BM-pDC and BM-cDC were electronically gated and analyzed for the expression of the indicated molecules measuring the median fluorescence intensities (MFIs). The results of one of two independent experiments are shown.
For comparison, we analyzed iPPVO-induced TNF-α and IL-12/23p40 release in wild-type, MyD88−/−, and TRIF−/− BMDC. Again, poly(I:C) and CpG-ODN served as controls. As expected, CpG-ODN did not require TRIF but MyD88 to trigger TNF-α and IL-12/23p40 release by BMDC. We found that after iPPVO stimulation, the release of TNF-α by BMDC was markedly reduced in MyD88−/− BMDC compared to wild-type BMDC (Fig. 5A, top panels). Moreover, the levels of IL-12/23p40 in supernatants of iPPVO-stimulated BMDC were diminished; however, the differences were not statistically significant. Nevertheless, marginal levels of TNF-α were also induced by iPPVO in MyD88−/− BMDC, indicating that MyD88-dependent receptors are chiefly but not exclusively involved in the induction of TNF-α by iPPVO. The amounts of TNF-α and IL-12/23p40 were similar, however, in supernatants of wild-type and TRIF−/− BMDC. This suggests that MyD88-dependent rather TRIF-independent receptors are involved in TNF-α and IL-12/23p40 production in response to iPPVO. As expected, poly(I:C) triggered TNF-α and IL-12/23p40 production independently of MyD88. The observed induction of IL-12/23p40 by poly(I:C) in TRIF−/− BMDC is likely due to the ability of poly(I:C) to trigger TRIF-independent MDA5-dependent signaling (20).
We had observed that in a mixed culture of BM-pDC and BM-cDC the majority of IFN-β was produced by BM-cDC (Fig. 2B). Therefore, we wanted to investigate the activation of pure BM-cDC by iPPVO more closely. Analysis of MyD88−/− and TRIF−/− BM-cDC generated in the presence of GM-CSF showed that induction of IFN-α/β by iPPVO occurred in the absence of MyD88 or TRIF (Fig. 5B). This indicates that iPPVO induces IFN-α/β in BM-cDC by TLR-independent pathways. In contrast to BM-cDC, iPPVO-induced type I IFN production by BM-pDC proved to depend on MyD88, pointing to a TLR-dependent pathway of type I IFN production for pDC. In a representative experiment, purified wild-type BM-pDC derived from Flt3L-generated BMDC showed significant IFN-α production (i.e., 1,033 IRU/ml) compared to purified MyD88-deficient BM-pDC (i.e., <62.5 IRU/ml) in response to iPPVO stimulation. CpG-ODN did not induce IFN-α in BM-cDC, supporting the observation that IFN-α production in BM-cDC is not triggered via TLR9-dependent signaling (24). Low levels of IFN-β, however, were induced as expected upon CpG-ODN activation of wild-type and TRIF−/− BM-cDC but not MyD88−/− BM-cDC. Poly(I:C) induced IFN-α/β in MyD88−/− and TRIF−/− BM-cDC. This is consistent with the observation that BM-cDC generated in the presence of GM-CSF preferentially signal via MDA-5 upon poly(I:C) stimulation (20). In contrast to the observed TLR-independent IFN-α/β induction in BM-cDC, the induction of TNF-α and IL- 12/23p40 by iPPVO was impaired in MyD88−/− BM-cDC, although the differences did not become statistically significant. TNF-α and IL-12/23p40 production did not require TRIF (Fig. 5B, right panels). These results demonstrate that TNF-α and IL-12/23p40 are induced by MyD88-dependent rather than TRIF-dependent receptors in BM-cDC in response to iPPVO.
We wanted to clarify whether the iPPVO-induced upregulation of MHC-II, MHC-I, and CD86 required MyD88 and/or TRIF. Therefore, the expression of MHC-II, MHC-I, and CD86 on wild-type, MyD88−/−, and TRIF−/− BM-cDC and BM-pDC after stimulation with iPPVO or poly(I:C) and CpG-ODN as controls was compared to the expression on medium-cultured wild-type, MyD88−/−, and TRIF−/− BM-cDC and BM-pDC. Interestingly, the expression of MHC-II, MHC-I, and CD86 was regulated in different ways. Whereas MHC-II was upregulated to the same extent on wild-type, MyD88−/−, and TRIF−/− BM-cDC and BM-pDC, the lack of MyD88 but not of TRIF diminished expression of MHC-I and CD86 on BM-cDC and BM-pDC after iPPVO stimulation (Fig. 5C). As expected, poly(I:C) required TRIF to enhance expression of MHC-II, MHC-I, and CD86, whereas CpG-ODN depended on MyD88. Similar observations were made for BM-cDC generated in the presence of GM-CSF-containing supernatant (data not shown). Taken together, these data point to MyD88-dependent and MyD88-independent mechanisms involved in iPPVO-induced BMDC activation.
iPPVO-induced MyD88-dependent cytokine induction is not mediated by TLR2 or TLR4.
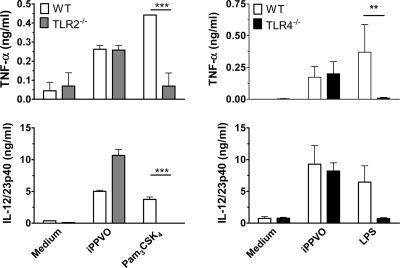
Recent data indicated a role for TLR2 and TLR4 for the recognition of VACV, which is another member of the family Poxviridae (29, 60). To analyze a potential role of TLR2 for the activation of BMDC, we assessed TNF-α and IL-12/23p40 release and CD86 upregulation by iPPVO-stimulated wild-type and TLR2−/− BMDC. Moreover, to elucidate a potential role of TLR4 for iPPVO sensing and to additionally make sure that the observed MyD88-dependent effects were not caused by endotoxin contamination, we determined TNF-α and IL-12/23p40 release and CD86 upregulation using TLR4−/− BMDC. Pam3CSK4 and LPS served as positive controls for TLR2 and TLR4, respectively. In contrast to VACV, we found no reduction of TNF-α or IL-12/23p40 in supernatants of TLR2−/− or TLR4−/− BMDC upon iPPVO stimulation, whereas Pam3CSK4 and LPS failed to induce cytokine secretion in TLR2−/− and TLR4−/− BMDC, respectively (Fig. 6). Consistent with these data, CD86 upregulation was not diminished in TLR2−/− or TLR4−/− BMDC upon iPPVO stimulation (data not shown). These data exclude TLR2 and TLR4 for BMDC activation by iPPVO and dismiss endotoxin contamination of the virus preparation.
FIG. 6.
TLR2 and TLR4 are not involved in iPPVO-triggered TNF-α and IL-12/23p40 release. Wild-type (WT), TLR2−/−, and TLR4−/− BMDC generated in the presence of Flt3L were stimulated with iPPVO at an MOI of 5 and with the indicated control stimuli for 48 h. Subsequently, supernatants were harvested and levels of TNF-α and IL-12/23p40 determined by sandwich ELISA. Pooled data of two independent experiments are shown. ***, P < 0.001; **, P < 0.01.
iPPVO-induced IFN-α induction is mediated by IRF7 independently of IRF3.
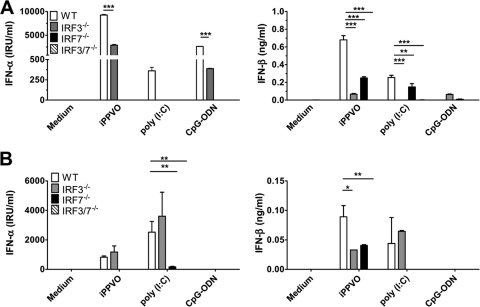
The transcription factors IRF3 and IRF7 are important for the induction of type I IFN responses to viruses (26). We wanted to investigate the involvement of IRF3 and IRF7 in iPPVO-induced type I IFN induction. Upon analyzing the IFN-α/β responses of BMDC generated in the presence of Flt3L originating from IRF7−/−, IRF3−/−, and IRF3/7−/− mice, we found that IFN-α induction by iPPVO depends on IRF7 consistent with IRF7-dependent type I IFN production by both cDC and pDC (26). In addition, we observed iPPVO-induced IFN-α production in the absence of IRF3, although the levels of IFN-α were significantly reduced (Fig. 7A, left panel). Strict IRF7-dependent and IRF3-independent IFN-α production was also observed for CpG-ODN, whereas no IFN-α release was triggered in IRF3−/−, IRF7−/−, or IRF3/7−/− BMDC by poly(I:C). Moreover, after encountering iPPVO, BMDC released IFN-β in the absence of IRF3 or IRF7, but not if both IRF3 and IRF7 were absent (Fig. 7A, right panel). Poly(I:C) required IRF3 for IFN-β induction, whereas CpG-ODN triggered only low-level production of IFN-β in this experiment. Interestingly, we also observed reduced TNF-α production by IRF3−/− BMDC in response to iPPVO (data not shown). Involvement of IRF3 for TNF-α induction has also been shown in response to adenovirus DNA and myxoma virus (41, 56). However, BMDC of all genotypes produced levels of IL-12/23p40 upon iPPVO stimulation similar to those for wild-type BMDC, indicating that IRF7−/−, IRF3−/−, and IRF3/7−/− BMDC had no intrinsic defect in cytokine production (data not shown).
FIG. 7.
IRF7 is involved in iPPVO-induced IFN-α release. (A) Wild-type (WT), IRF3−/−, IRF7−/−, and IRF3/7−/− BMDC generated in the presence of Flt3L were cultured in the presence of iPPVO (MOI = 5) and the indicated control stimuli. After 48 h of incubation, the concentrations of IFN-α and IFN-β were determined by sandwich ELISA. One representative of two independent experiments (triplicate samples) is shown. (B) Wild-type (WT), IRF3−/−, IRF7−/−, and IRF3/7−/− BM-cDC generated in the presence of GM-CSF-containing supernatant were cultured in the presence of the indicated stimuli as in panel A. The levels of IFN-α and IFN-β were determined by sandwich ELISA. Shown are pooled data of two independent experiments. Each column represents means ± the SEM. ***, P < 0.001; **, P < 0.01; *, P < 0.05.
To investigate the role of IRF3 and IRF7 for the induction of IFN-α/β in cDC, we analyzed the production of IFN-α and IFN-β by wild-type, IRF3−/−, IRF7−/−, and IRF3/7−/− BM-cDC generated in the presence of GM-CSF containing supernatant after stimulation with iPPVO, CpG-ODN, or poly(I:C). These BM-cDC were free of contaminating BM-pDC (data not shown). The numbers and phenotypes of wild-type, IRF3−/−, IRF7−/−, and IRF3/7−/− BM-cDC were similar (data not shown). Surprisingly, we observed that iPPVO-induced release of IFN-α occurred in the absence of IRF3 but required IRF7 (Fig. 7B, left panel). This finding points to a currently unknown signaling pathway with a critical role of IRF7 for the induction of IFN-α by iPPVO in cDC. The production of IFN-β depended on the presence of either IRF3 or IRF7; however, maximum production of IFN-β was observed if both IRF3 and IRF7 were present (Fig. 7B, right panel). As expected, BM-cDC did not release IFN-α/β upon stimulation with CpG-ODN. Poly(I:C) induced strong production of IFN-α/β in the absence of IRF3 but not in the absence of IRF7.
DISCUSSION
The activation of DC by orthopoxviruses such as VACV, MVA, and ECTV has been well characterized (46, 55). However, PPVO is a member of the genus Parapoxvirus which displays only limited homology with orthopoxviruses, e.g., in virion morphology, protein composition, and genome organization. Thus, it is of great importance to characterize PPVO interactions with BMDC to elucidate mechanisms of APC activation by the prototypic member of the genus Parapoxvirus. We used PPVO in its inactivated form to exclude involvement of viral replication and escape mechanisms established by live PPVO. The present data provide the first evidence that iPPVO is able to potently activate DC. Moreover, several receptors and/or modes of activation appear to contribute to DC activation. Whereas iPPVO is able to induce IFN-α/β release and MHC-II upregulation by DC independently of MyD88 or TRIF, MyD88-dependent pathways are involved in TNF-α production and in MHC-I and CD86 upregulation. However, TLR2 and TLR4 do not contribute to DC activation by iPPVO, demonstrating that iPPVO activates immune cells in a different way than well-studied orthopoxviruses such as MVA, ECTV, and VACV.
iPPVO was reported to mediate immunostimulatory effects (21), enhancing antiviral activity against hepatitis B virus and herpes simplex virus type 1 (57). The presented study demonstrates that iPPVO triggers IFN-α/β, TNF-α, and IL-12/23p40 secretion by BMDC (Fig. 1). iPPVO-induced activation of APC correlated with enhanced expression of MHC-II, MHC-I, and CD86 mainly on BM-cDC (Fig. 3A). Thus, these data indicate that DC are involved in mediating immunostimulatory effects with a major role for cDC in antigen presentation.
These data also provide evidence that cDC, in addition to pDC, substantially contribute to IFN-α/β production (Fig. 2A and B). The role of pDC for the production of type I IFN in response to viral encounter is well documented (16). However, little is known about the role of cDC for type I IFN production during virus infection. Although recent studies demonstrate a major role for pDC for type I IFN induction by orthopoxviruses such as MVA or ECTV (46, 55), we show here that BM-cDC and BM-pDC both contribute to a similar degree to IFN-α production in response to iPPVO (Fig. 2A and B). Moreover, our data point to cDC as an important source of IFN-β in response to iPPVO (Fig. 2B).
The pathway of cell entry for iPPVO is yet unknown. As demonstrated in the present study, other than single iPPVO particles in the cytosol (Fig. 4, right), iPPVO is localized in groups in endosomes after uptake by BMDC (Fig. 4, left), suggesting endocytosis as one mechanism of uptake. Recently, endocytosis was demonstrated to mediate the uptake of VACV mature intracellular virions (28). An important advantage for cellular uptake of poxviruses is their large size and complexity that will favor phagocytosis, endocytosis, or macropinocytosis. Phosphatidylserine is enriched in the membrane of VACV strain MVA and plays an important role in MVA uptake by apoptotic mimicry which, along with macropinocytosis, might allow VACV to enter many different cell types (40). Further studies with host cell-restricted poxviruses such as PPVO or fowlpox virus should clarify if a similar process for cellular entry exists in all poxviruses.
It was demonstrated in the present study that production of IFN-α/β and MHC-II upregulation by BM-cDC in response to iPPVO occurred independently of MyD88 and TRIF (Fig. 5B and C), indicating that other receptors than currently known TLR are involved in cDC activation by iPPVO. Activation of TLR-dependent and -independent pathways was also demonstrated for VACV. In the case of VACV, proinflammatory cytokines were induced by TLR2, whereas IFN-β production was triggered independently of MyD88 and TRIF (55). Moreover, a protective role for TLR4 in pulmonary VACV infection was reported (29). In the same study, however, TLR4 had no effect on IFN-β and proinflammatory cytokine production in the lung, supporting the idea of involvement of several PRR in virus recognition. A TLR-independent DNA sensing mechanism involving IRF-3 was reported in response to adenovirus (41). These data provide evidence for the existence of as-yet-unknown receptors sensing viral dsDNA. Ishii et al. demonstrated that intracellular administration of double-stranded B-form DNA leads to TLR-independent type I IFN production (30). Very recently, the existence of a novel cytoplasmic DNA receptor termed DAI (for DNA-dependent activator of IFN-regulatory factors) has been reported which could be involved in the recognition of viral DNA (52). Thus, the involvement of cytosolic DNA receptors inducing IFN-α/β production in response to iPPVO seems conceivable.
Intriguingly, iPPVO-induced BMDC activation by additional MyD88-dependent pathways resulting in production of TNF-α and upregulation of MHC-I and CD86 suggests the involvement of more than one receptor or signaling pathway activated by iPPVO. However, TLR2 and TLR4 were not required for iPPVO-triggered MyD88-dependent TNF-α and IL-12/23p40 production or MHC-I and CD86 upregulation. MyD88 is also involved in IL-1R family signaling (53) and could be indirectly involved in iPPVO-triggered TNF-α and IL-12/23p40 release. These potential effects would be further downstream in the induction of an IL-1R-dependent cascade after initial iPPVO recognition. Intriguingly, Sun et al. reported that MyD88 was required to stabilize the half-life of TNF mRNA induced by IFN-γ (51), demonstrating that MyD88 can be involved in other mechanisms, such as posttranscriptional regulation, in addition to TLR/IL-1R signaling. A recent Mycobacterium tuberculosis infection study showed that MyD88−/− mice, in contrast to TLR2/4/9−/− mice, were highly susceptible to M. tuberculosis infection due to impaired restriction of M. tuberculosis growth in MyD88−/− mice but not in TLR2/4/9−/− mice (25). These data also indicate novel roles of MyD88 beyond its TLR adapter function. Interestingly, MyD88 was required for enhanced expression of MHC-I but not of MHC-II (Fig. 5C). MHC-I is known to present endogenous antigens. One way to deliver antigens to the MHC class I pathway is cross-presentation via the uptake of apoptotic material (1). iPPVO was reported to induce apoptosis in murine APC (34), arguing for the ability of iPPVO cross-presentation. However, no signs of apoptosis could be detected in BMDC cultured in the presence of iPPVO as evaluated by electron microscopy (data not shown). Chen et al. reported that MyD88 is required for efficient cross-presentation of viral antigens (11). Therefore, the observed MyD88-dependent effects might also reflect cross-presentation of iPPVO by BMDC.
For the induction of IFN-α/β genes in response to viruses, the transcription factors IRF3 and IRF7 are essential (47). IRF7 is a potent activator of both IFNA and IFNB genes, whereas IRF3 efficiently activates IFNB but not IFNA genes except IFNA4 (26). IRF3 is activated by signaling cascades initiated by cytosolic RNA and DNA sensors (26, 41). The cytosolic RNA pathway was reported to be important for type I IFN induction by poxviruses such as MVA and myxoma virus (35, 56). Purified adenovirus DNA was shown to signal via IRF3 (41). We show that IFN-α/β induction by iPPVO in BM-cDC surprisingly does not require IRF3 but strictly depends on IRF7 (Fig. 7B). This suggests that the known pathways of type I IFN induction by viral DNA or RNA are not activated by iPPVO in cDC. Thus, iPPVO may stimulate a novel signaling pathway for the induction of type I IFN in cDC. IRF3-independent IFN-α/β production by cDC was recently reported for the first time in vivo with adenovirus (14). Experiments conducted with HEK cells transfected with TLR3 and TLR7 suggested no involvement of TLR3 and TLR7 in iPPVO sensing (data not shown).
Our data demonstrate that cDC sense iPPVO by TLR-independent pathways. In contrast, our data analyzing pDC stimulated with iPPVO indicate TLR-dependent type I IFN production for pDC. The genetic material of iPPVO is dsDNA (13) with a notably high base content of ca. 63% cytosine and guanosine (21). Therefore, TLR9 sensing double-stranded CpG DNA motifs, which are common in bacterial and viral DNA, is a likely candidate for type I IFN induction in pDC by iPPVO. Future experiments with purified pDC have to be done to carefully analyze a potential contribution of TLR9 to iPPVO sensing.
In conclusion, the data presented here demonstrate for the first time that iPPVO activates MyD88-dependent and -independent signaling pathways in BM-cDC leading to the release of proinflammatory cytokines, type I IFN, and the enhanced expression of surface molecules required for T-cell activation. Moreover, our data provide evidence for an as-yet-unknown signaling pathway for the induction of type I IFN in cDC that does not require IRF3. Therefore, the present study sheds new light on the interaction of PPVO with innate immune cells pointing to distinct immunostimulatory activities for members of the genus Parapoxvirus compared to orthopoxviruses.
Acknowledgments
We thank C. Westermann for help with electron microscopy and U. Müller for carefully reading the manuscript.
This study was financially supported by Pfizer Animal Health, United Kingdom.
Footnotes
Published ahead of print on 1 July 2009.
REFERENCES
- 1.Albert, M. L., B. Sauter, and N. Bhardwaj. 1998. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392:86-89. [DOI] [PubMed] [Google Scholar]
- 2.Aravalli, R. N., S. Hu, T. N. Rowen, J. M. Palmquist, and J. R. Lokensgard. 2005. Cutting edge: TLR2-mediated proinflammatory cytokine and chemokine production by microglial cells in response to herpes simplex virus. J. Immunol. 175:4189-4193. [DOI] [PubMed] [Google Scholar]
- 3.Barchet, W., M. Cella, and M. Colonna. 2005. Plasmacytoid dendritic cells: virus experts of innate immunity. Semin. Immunol. 17:253-261. [DOI] [PubMed] [Google Scholar]
- 4.Berghall, H., J. Siren, D. Sarkar, I. Julkunen, P. B. Fisher, R. Vainionpaa, and S. Matikainen. 2006. The interferon-inducible RNA helicase, mda-5, is involved in measles virus-induced expression of antiviral cytokines. Microbes. Infect. 8:2138-2144. [DOI] [PubMed] [Google Scholar]
- 5.Bieback, K., E. Lien, I. M. Klagge, E. Avota, J. Schneider-Schaulies, W. P. Duprex, H. Wagner, C. J. Kirschning, V. Ter Meulen, and S. Schneider-Schaulies. 2002. Hemagglutinin protein of wild-type measles virus activates Toll-like receptor 2 signaling. J. Virol. 76:8729-8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brawand, P., D. R. Fitzpatrick, B. W. Greenfield, K. Brasel, C. R. Maliszewski, and T. De Smedt. 2002. Murine plasmacytoid pre-dendritic cells generated from Flt3 ligand-supplemented bone marrow cultures are immature APCs. J. Immunol. 169:6711-6719. [DOI] [PubMed] [Google Scholar]
- 7.Brennan, G. L., and L. H. Kronenberg. 1983. BioTechniques. 1:78. [Google Scholar]
- 8.Burzyn, D., J. C. Rassa, D. Kim, I. Nepomnaschy, S. R. Ross, and I. Piazzon. 2004. Toll-like receptor 4-dependent activation of dendritic cells by a retrovirus. J. Virol. 78:576-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buttner, M., C. P. Czerny, K. H. Lehner, and K. Wertz. 1995. Interferon induction in peripheral blood mononuclear leukocytes of man and farm animals by poxvirus vector candidates and some poxvirus constructs. Vet. Immunol. Immunopathol. 46:237-250. [DOI] [PubMed] [Google Scholar]
- 10.Chang, S., A. Dolganiuc, and G. Szabo. 2007. Toll-like receptors 1 and 6 are involved in TLR2-mediated macrophage activation by hepatitis C virus core and NS3 proteins. J. Leukoc. Biol. [DOI] [PubMed]
- 11.Chen, M., C. Barnfield, T. I. Naslund, M. N. Fleeton, and P. Liljestrom. 2005. MyD88 expression is required for efficient cross-presentation of viral antigens from infected cells. J. Virol. 79:2964-2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colonna, M., G. Trinchieri, and Y. J. Liu. 2004. Plasmacytoid dendritic cells in immunity. Nat. Immunol. 5:1219-1226. [DOI] [PubMed] [Google Scholar]
- 13.Cottone, R., M. Buttner, B. Bauer, M. Henkel, E. Hettich, and H. J. Rziha. 1998. Analysis of genomic rearrangement and subsequent gene deletion of the attenuated Orf virus strain D1701. Virus Res. 56:53-67. [DOI] [PubMed] [Google Scholar]
- 14.Fejer, G., L. Drechsel, J. Liese, U. Schleicher, Z. Ruzsics, N. Imelli, U. F. Greber, S. Keck, B. Hildenbrand, A. Krug, C. Bogdan, and M. A. Freudenberg. 2008. Key role of splenic myeloid DCs in the IFN-α/β response to adenoviruses in vivo. PLoS Pathog. 4:e1000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finter, N. B. 1969. Dye uptake methods for assessing viral cytopathogenicity and their application to interferon assays. J. Gen. Virol. 5:419. [Google Scholar]
- 16.Fitzgerald-Bocarsly, P., J. Dai, and S. Singh. 2008. Plasmacytoid dendritic cells and type I IFN: 50 years of convergent history. Cytokine Growth Factor Rev. 19:3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fitzgerald-Bocarsly, P., and D. Feng. 2007. The role of type I interferon production by dendritic cells in host defense. Biochimie 89:843-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freudenberg, M. A., D. Keppler, and C. Galanos. 1986. Requirement for lipopolysaccharide-responsive macrophages in galactosamine-induced sensitization to endotoxin. Infect. Immun. 51:891-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friebe, A., A. Siegling, S. Friederichs, H. D. Volk, and O. Weber. 2004. Immunomodulatory effects of inactivated parapoxvirus ovis (ORF virus) on human peripheral immune cells: induction of cytokine secretion in monocytes and Th1-like cells. J. Virol. 78:9400-9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gitlin, L., W. Barchet, S. Gilfillan, M. Cella, B. Beutler, R. A. Flavell, M. S. Diamond, and M. Colonna. 2006. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc. Natl. Acad. Sci. USA 103:8459-8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haig, D. M. 2006. Orf virus infection and host immunity. Curr. Opin. Infect. Dis. 19:127-131. [DOI] [PubMed] [Google Scholar]
- 22.Haig, D. M., and C. J. McInnes. 2002. Immunity and counter-immunity during infection with the parapoxvirus orf virus. Virus Res. 88:3-16. [DOI] [PubMed] [Google Scholar]
- 23.Hirotani, T., M. Yamamoto, Y. Kumagai, S. Uematsu, I. Kawase, O. Takeuchi, and S. Akira. 2005. Regulation of lipopolysaccharide-inducible genes by MyD88 and Toll/IL-1 domain containing adaptor inducing IFN-β. Biochem. Biophys. Res. Commun. 328:383-392. [DOI] [PubMed] [Google Scholar]
- 24.Hochrein, H., B. Schlatter, M. O'Keeffe, C. Wagner, F. Schmitz, M. Schiemann, S. Bauer, M. Suter, and H. Wagner. 2004. Herpes simplex virus type-1 induces IFN-α production via Toll-like receptor 9-dependent and -independent pathways. Proc. Natl. Acad. Sci. USA 101:11416-11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holscher, C., N. Reiling, U. E. Schaible, A. Holscher, C. Bathmann, D. Korbel, I. Lenz, T. Sonntag, S. Kroger, S. Akira, H. Mossmann, C. J. Kirschning, H. Wagner, M. Freudenberg, and S. Ehlers. 2008. Containment of aerogenic Mycobacterium tuberculosis infection in mice does not require MyD88 adaptor function for TLR2, -4, and -9. Eur. J. Immunol. 38:680-694. [DOI] [PubMed] [Google Scholar]
- 26.Honda, K., and T. Taniguchi. 2006. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat. Rev. Immunol. 6:644-658. [DOI] [PubMed] [Google Scholar]
- 27.Honda, K., H. Yanai, H. Negishi, M. Asagiri, M. Sato, T. Mizutani, N. Shimada, Y. Ohba, A. Takaoka, N. Yoshida, and T. Taniguchi. 2005. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434:772-777. [DOI] [PubMed] [Google Scholar]
- 28.Huang, C. Y., T. Y. Lu, C. H. Bair, Y. S. Chang, J. K. Jwo, and W. Chang. 2008. A novel cellular protein, VPEF, facilitates vaccinia virus penetration into HeLa cells through fluid phase endocytosis. J. Virol. 82:7988-7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hutchens, M. A., K. E. Luker, J. Sonstein, G. Nunez, J. L. Curtis, and G. D. Luker. 2008. Protective effect of Toll-like receptor 4 in pulmonary vaccinia infection. PLoS Pathog. 4:e1000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishii, K. J., C. Coban, H. Kato, K. Takahashi, Y. Torii, F. Takeshita, H. Ludwig, G. Sutter, K. Suzuki, H. Hemmi, S. Sato, M. Yamamoto, S. Uematsu, T. Kawai, O. Takeuchi, and S. Akira. 2006. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat. Immunol. 7:40-48. [DOI] [PubMed] [Google Scholar]
- 31.Kawai, T., and S. Akira. 2006. Innate immune recognition of viral infection. Nat. Immunol. 7:131-137. [DOI] [PubMed] [Google Scholar]
- 32.Kawai, T., O. Takeuchi, T. Fujita, J. Inoue, P. F. Muhlradt, S. Sato, K. Hoshino, and S. Akira. 2001. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J. Immunol. 167:5887-5894. [DOI] [PubMed] [Google Scholar]
- 33.Krug, A., S. Rothenfusser, V. Hornung, B. Jahrsdorfer, S. Blackwell, Z. K. Ballas, S. Endres, A. M. Krieg, and G. Hartmann. 2001. Identification of CpG oligonucleotide sequences with high induction of IFN-α/β in plasmacytoid dendritic cells. Eur. J. Immunol. 31:2154-2163. [DOI] [PubMed] [Google Scholar]
- 34.Kruse, N., and O. Weber. 2001. Selective induction of apoptosis in antigen-presenting cells in mice by parapoxvirus ovis. J. Virol. 75:4699-4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar, H., T. Kawai, H. Kato, S. Sato, K. Takahashi, C. Coban, M. Yamamoto, S. Uematsu, K. J. Ishii, O. Takeuchi, and S. Akira. 2006. Essential role of IPS-1 in innate immune responses against RNA viruses. J. Exp. Med. 203:1795-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurt-Jones, E. A., L. Popova, L. Kwinn, L. M. Haynes, L. P. Jones, R. A. Tripp, E. E. Walsh, M. W. Freeman, D. T. Golenbock, L. J. Anderson, and R. W. Finberg. 2000. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 1:398-401. [DOI] [PubMed] [Google Scholar]
- 37.Le Goffic, R., J. Pothlichet, D. Vitour, T. Fujita, E. Meurs, M. Chignard, and M. Si-Tahar. 2007. Cutting edge: influenza A virus activates TLR3-dependent inflammatory and RIG-I-dependent antiviral responses in human lung epithelial cells. J. Immunol. 178:3368-3372. [DOI] [PubMed] [Google Scholar]
- 38.Lutz, M. B., N. Kukutsch, A. L. Ogilvie, S. Rossner, F. Koch, N. Romani, and G. Schuler. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223:77-92. [DOI] [PubMed] [Google Scholar]
- 39.Mayr, A., M. Buttner, G. Wolf, H. Meyer, and C. Czerny. 1989. Experimental detection of the paraspecific effects of purified and inactivated poxviruses. Zentralbl. Veterinarmed. B 36:81-99. (In German.) [PubMed] [Google Scholar]
- 40.Mercer, J., and A. Helenius. 2008. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science 320:531-535. [DOI] [PubMed] [Google Scholar]
- 41.Nociari, M., O. Ocheretina, J. W. Schoggins, and E. Falck-Pedersen. 2007. Sensing infection by adenovirus: Toll-like receptor-independent viral DNA recognition signals activation of the interferon regulatory factor 3 master regulator. J. Virol. 81:4145-4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Opitz, B., A. Rejaibi, B. Dauber, J. Eckhard, M. Vinzing, B. Schmeck, S. Hippenstiel, N. Suttorp, and T. Wolff. 2007. IFNbeta induction by influenza A virus is mediated by RIG-I which is regulated by the viral NS1 protein. Cell Microbiol. 9:930-938. [DOI] [PubMed] [Google Scholar]
- 43.Prakash, A., and D. E. Levy. 2006. Regulation of IRF7 through cell type-specific protein stability. Biochem. Biophys. Res. Commun. 342:50-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sakaguchi, S., H. Negishi, M. Asagiri, C. Nakajima, T. Mizutani, A. Takaoka, K. Honda, and T. Taniguchi. 2003. Essential role of IRF-3 in lipopolysaccharide-induced interferon-beta gene expression and endotoxin shock. Biochem. Biophys. Res. Commun. 306:860-866. [DOI] [PubMed] [Google Scholar]
- 45.Samanta, M., D. Iwakiri, T. Kanda, T. Imaizumi, and K. Takada. 2006. EB virus-encoded RNAs are recognized by RIG-I and activate signaling to induce type I IFN. EMBO J. 25:4207-4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samuelsson, C., J. Hausmann, H. Lauterbach, M. Schmidt, S. Akira, H. Wagner, P. Chaplin, M. Suter, M. O'Keeffe, and H. Hochrein. 2008. Survival of lethal poxvirus infection in mice depends on TLR9, and therapeutic vaccination provides protection. J. Clin. Investig. 118:1776-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sato, M., H. Suemori, N. Hata, M. Asagiri, K. Ogasawara, K. Nakao, T. Nakaya, M. Katsuki, S. Noguchi, N. Tanaka, and T. Taniguchi. 2000. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity 13:539-548. [DOI] [PubMed] [Google Scholar]
- 48.Siegemund, S., N. Schutze, M. A. Freudenberg, M. B. Lutz, R. K. Straubinger, and G. Alber. 2008. Production of IL-12, IL-23 and IL-27p28 by bone marrow-derived conventional dendritic cells rather than macrophages after LPS/TLR4-dependent induction by Salmonella enteritidis. Immunobiology 212:739-750. [DOI] [PubMed] [Google Scholar]
- 49.Smith, K. J., H. G. Skelton III, W. D. James, and G. P. Lupton. 1991. Parapoxvirus infections acquired after exposure to wildlife. Arch. Dermatol. 127:79-82. [PubMed] [Google Scholar]
- 50.Stetson, D. B., and R. Medzhitov. 2006. Type I interferons in host defense. Immunity 25:373-381. [DOI] [PubMed] [Google Scholar]
- 51.Sun, D., and A. Ding. 2006. MyD88-mediated stabilization of interferon-gamma-induced cytokine and chemokine mRNA. Nat. Immunol. 7:375-381. [DOI] [PubMed] [Google Scholar]
- 52.Takaoka, A., Z. Wang, M. K. Choi, H. Yanai, H. Negishi, T. Ban, Y. Lu, M. Miyagishi, T. Kodama, K. Honda, Y. Ohba, and T. Taniguchi. 2007. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448:501-505. [DOI] [PubMed] [Google Scholar]
- 53.Takeuchi, O., and S. Akira. 2002. MyD88 as a bottle neck in Toll/IL-1 signaling. Curr. Top. Microbiol. Immunol. 270:155-167. [DOI] [PubMed] [Google Scholar]
- 54.Trinchieri, G., and A. Sher. 2007. Cooperation of Toll-like receptor signals in innate immune defence. Nat. Rev. Immunol. 7:179-190. [DOI] [PubMed] [Google Scholar]
- 55.Waibler, Z., M. Anzaghe, H. Ludwig, S. Akira, S. Weiss, G. Sutter, and U. Kalinke. 2007. Modified vaccinia virus Ankara induces Toll-like receptor-independent type I interferon responses. J. Virol. 81:12102-12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang, F., X. Gao, J. W. Barrett, Q. Shao, E. Bartee, M. R. Mohamed, M. Rahman, S. Werden, T. Irvine, J. Cao, G. A. Dekaban, and G. McFadden. 2008. RIG-I mediates the co-induction of tumor necrosis factor and type I interferon elicited by myxoma virus in primary human macrophages. PLoS Pathog. 4:e1000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weber, O., A. Siegling, A. Friebe, A. Limmer, T. Schlapp, P. Knolle, A. Mercer, H. Schaller, and H. D. Volk. 2003. Inactivated parapoxvirus ovis (Orf virus) has antiviral activity against hepatitis B virus and herpes simplex virus. J. Gen. Virol. 84:1843-1852. [DOI] [PubMed] [Google Scholar]
- 58.Wick, M. J. 2007. Monocyte and dendritic cell recruitment and activation during oral Salmonella infection. Immunol. Lett. 112:68-74. [DOI] [PubMed] [Google Scholar]
- 59.Wittek, R., C. C. Kuenzle, and R. Wyler. 1979. High C+G content in parapoxvirus DNA. J. Gen. Virol. 43:231-234. [DOI] [PubMed] [Google Scholar]
- 60.Zhu, J., J. Martinez, X. Huang, and Y. Yang. 2007. Innate immunity against vaccinia virus is mediated by TLR2 and requires TLR-independent production of IFN-β. Blood 109:619-625. [DOI] [PMC free article] [PubMed] [Google Scholar]