Abstract
The treatment of human immunodeficiency virus type 1 (HIV-1) infection with highly active antiretroviral therapy (HAART), a combination of three or more antiretroviral drugs, suppresses viremia below the clinical limit of detection (50 HIV-1 RNA copies/ml), but latently infected resting CD4+ T cells serve as lifelong reservoirs, and low-level viremia can be detected with special assays. Recent studies have provided evidence for additional reservoirs that contribute to residual viremia but are not present in circulating cells. Identification of all the sources of residual viremia in humans may be difficult. These discoveries highlight the need for a tractable model system to identify additional viral reservoirs that could represent barriers to eradication. In this study, simian immunodeficiency virus (SIV)-infected pig-tailed macaques (Macaca nemestrina) were treated with four antiretroviral drugs to develop an animal model for viral suppression during effective HAART. Treatment led to a biphasic decay in viremia and a significant rise in levels of circulating CD4+ T cells. At terminal infection time points, the frequency of circulating resting CD4+ T cells harboring replication-competent virus was reduced to a low steady-state level similar to that observed for HIV-infected patients on HAART. The frequencies of resting CD4+ T cells harboring replication-competent virus in the pooled head lymph nodes, gut lymph nodes, spleen, and peripheral blood were reduced relative to those for untreated SIV-infected animals. These observations closely parallel findings for HIV-infected humans on suppressive HAART and demonstrate the value of this animal model to identify and characterize viral reservoirs persisting in the setting of suppressive antiretroviral drugs.
Early studies of human immunodeficiency virus type 1 (HIV-1) dynamics in patients on antiretroviral therapy demonstrated that potent antiretroviral drugs caused an initial rapid exponential decay in viremia, reflecting the short half-life of the infected cells that produce most of the virus in plasma (36, 55, 72). This finding led to the belief that eradication of HIV-1 infection could be achieved with the prolonged use of combinations of antiretroviral drugs (54). Subsequent studies, however, indicated that HIV-1 infection cannot be cured by long-term treatment with highly active antiretroviral therapy (HAART) because HIV-1 can persist in a latent form in resting memory CD4+ T cells, which function as a stable reservoir for the virus (13, 15, 18, 23, 73) for the lifetimes of most infected individuals (22, 62). In addition, new techniques for the ultrasensitive detection of HIV-1 RNA have since demonstrated that free virus particles are present in the plasma of patients on suppressive HAART at levels below 50 copies of HIV-1 RNA/ml (20, 52). In principle, this residual viremia could reflect ongoing cycles of replication that continue despite HAART (16, 20, 25, 26, 32, 37, 49, 66, 74), the release of virus from latently infected cells that become activated (35, 40, 50, 66, 74), or the release of virus from other stable cellular reservoirs (14, 75).
Data from recent studies of patients on suppressive HAART support the existence of other stable viral reservoirs in addition to latently infected resting memory CD4+ T cells (4, 11, 53, 64). Bailey and colleagues previously showed that viral sequences isolated from the plasma of patients on HAART were not entirely derived from activated or resting memory CD4+ T cells in the circulation. Phylogenetic analyses of free virus in the plasma and proviruses in resting CD4+ T cells revealed the presence of viral clones in some patients that persisted in the plasma but were not found in resting CD4+ T cells or other cells in the circulation, suggesting that long-lived noncirculating cells may be the source (4). In a 7-year longitudinal study, Palmer and colleagues further characterized the decay kinetics of viremia under suppressive HAART and revealed third and fourth phases of decay in addition to the well-described first two phases (53). As with the first two phases, the third and fourth phases of decay represent different virus-producing compartments. However, the half-lives are very long: 39 weeks for the third phase and essentially infinite (no decay) for the fourth phase (53). The compartments responsible for these decay phases have not been identified and are thought to represent long-lived stable reservoirs. These recent findings demonstrate the complexity of HIV-1 persistence and how stable reservoirs are significant contributors to residual viremia. Furthermore, these findings highlight the need for an animal model of HAART suppression to ascertain the identity of all the stable reservoirs that contribute to HIV-1 persistence, including those not present in the circulation. With such a model, a full-body analysis could be performed to identify and enumerate viral reservoirs in various tissues to include various candidate cell types. This knowledge is essential if eradication is to be achieved.
Simian immunodeficiency virus (SIV)-infected nonhuman primates have served as important models for understanding HIV pathogenesis (1, 5, 8, 21, 27, 34, 42, 51, 60, 63, 69-71). Here, we report on such a HAART model in which SIV-infected pig-tailed macaques were treated with four-drug combination antiretroviral therapy. The animals were infected simultaneously with SIV/17E-Fr and SIV/Delta B670, a protocol that has been shown to yield high levels of plasma viremia and reproducible progression to AIDS in 3 months (2, 3, 24, 44, 76, 77). SIV-infected animals were treated with a potent four-drug regimen of antiretrovirals consisting of a nucleoside reverse transcriptase inhibitor, an integrase inhibitor, and two protease inhibitors beginning during primary infection. The suppression of plasma viremia and the persistence of virus in resting CD4+ T cells in peripheral blood and lymphoid tissues establish this as a robust model for studying viral persistence in patients on HAART.
MATERIALS AND METHODS
Animal studies.
Eleven juvenile pig-tailed macaques (Macaca nemestrina) were studied. Of the 11 animals, 9 were inoculated intravenously with 10,000 50% animal infectious doses of neurovirulent SIV/17E-Fr and 50 50% animal infectious doses of immunosuppressive SIV/Delta B670. Previous studies have shown that this combination of viral inoculants, which includes both macrophage-tropic and lymphocyte-tropic as well as neurovirulent and nonneurovirulent strains, induces reproducible disease in a relatively short period of time, 3 months postinoculation (p.i.) (2, 3, 24, 44, 76, 77). Of the nine infected animals, five were treated with a four-drug combination, referred to hereafter as HAART, beginning on day 12 p.i. and continuing until necropsy (range, days 161 to 175). These animals were administered 205 mg/kg body weight saquinavir (SQV); 10 mg/kg of L-870812, an integrase inhibitor; and 270 mg/kg atazanavir (ATV) orally twice/day. In addition, 30 mg/kg of 9-R-(2-phosphonomethoxypropyl)adenine (PMPA) was administered once daily intramuscularly (i.m.). The doses for SQV and ATV for macaques were established after performing pharmacokinetic studies in which macaques were administered increasing doses of the drugs orally, and plasma samples were taken every 2 h from 2 to 12 h after drug administration. Plasma drug concentrations measured by high-performance liquid chromatography were plotted as a function of time, and the area under the curve was calculated. Doses of 205 mg/kg of SQV and 270 mg/kg of ATV provided areas under the curve most similar to those of humans administered these drugs. A dose of 30 mg/kg of PMPA was used, as this is the established dose for the reduction of SIV replication in macaques (67). The dose of 10 mg/kg of L-870812 was used effectively in studies of macaques (33). Plasma levels of the drugs were measured by testing plasma samples weekly.
The PMPA dosage of three of the animals receiving HAART, animals PTa2, PYd2, and POy1, was reduced to 10 mg/kg at day 71 until necropsy, and animal POy1 was taken off PMPA entirely at day 107 because creatinine levels in this animal were elevated, an indication of nephropathy. Nephropathy has been shown to occur in animals (68) and humans taking tenofovir disoproxil fumarate (TDF) (6, 56, 57), a prodrug of PMPA. Discontinuation of TDF was shown to reverse renal toxicity in humans (56). Four of the nine infected animals were not treated with HAART and served as positive controls for infection. Two animals were not infected and served as negative controls. Throughout the study, blood was collected for measurements of viremia and CD4+ T-cell counts and to monitor the frequency of circulating resting CD4+ T cells harboring replication-competent virus. Data for all the animals studied are summarized in Table 1.
TABLE 1.
Animal groups and characteristics
| Group and animal | Result for animala |
Viremia on day 10 p.i. (SIV RNA copy equivalents/ml) | Necropsy (days p.i.) | |
|---|---|---|---|---|
| Inoculated with SIV | Treated with HAART | |||
| SIV infected, HAART treated | ||||
| PEz1 | + | + | 3.5 × 108 | 165 |
| PFy1 | + | + | 1.4 × 108 | 172 |
| PTa2 | + | +* | 9.8 × 107 | 161 |
| PYd2 | + | +* | 1.5 × 108 | 168 |
| POy1 | + | +** | 1.4 × 108 | 175 |
| SIV infected, untreated | ||||
| PDi2 | + | − | 1.1 × 108 | 83 |
| PSk2 | + | − | 9.9 × 107 | 87 |
| PUi2 | + | − | 8.8 × 108 | 88 |
| PEi2 | + | − | 2.0 × 108 | 84 |
| Uninfected animals | ||||
| PBk2 | − | − | − | 89 |
| PNk2 | − | − | − | 90 |
*, doses of PMPA were reduced to 10 mg/kg at day 71 p.i. until necropsy; **, doses of PMPA were reduced to 10 mg/kg at day 71 p.i. PMPA was removed from the HAART regimen at day 107.
The animals were euthanized in accordance with recommendations of the Panel on Euthanasia of the American Veterinary Medical Association. Untreated SIV-infected animals were euthanized between day 83 and day 88 p.i., as all animals had AIDS and most had SIV-associated central nervous system disease at this time (2, 3, 24, 44, 76, 77). Uninfected animals were euthanized day 89 and day 90 p.i. relative to the untreated SIV-infected animals. HAART-treated animals were euthanized between day 161 and day 175 p.i. Prior to necropsy, animals were placed under deep anesthesia with pentobarbital and were perfused with phosphate-buffered saline to remove virus-containing blood from tissues.
Plasma SIV RNA levels.
Levels of plasma SIV RNA were measured by reverse transcriptase-mediated quantitative PCR, as previously described (60, 65). No-template controls and no-reverse-transcriptase negative controls were used to ensure that each measurement was derived from RNA. SIV RNA copy numbers were calculated from a regression curve derived from RNA transcript standards. This value was divided by the volume of the extracted plasma specimen to obtain values in units of SIV RNA copy equivalents/ml of plasma.
Mathematical modeling of decay in viremia with HAART.
The decay of viremia following the initiation of HAART was evaluated using a mathematical model similar to that developed previously by Perelson et al. to study the decay of HIV-1 viremia in patients starting HAART (54). Briefly, two distinct cellular compartments were assumed to contribute to the viremia: CD4+ T cells, T, which are infected with a rate constant, k, die with a rate constant, δ, and have a burst size of N. The second compartment consists of cells, M, which become infected with a rate constant, kM, die with a rate, μM, and produce p virions per cell. Free virus is degraded with a rate constant, c. Due to limitations in measuring levels of viremia, we have not considered the contribution of latently infected resting CD4+ T cells. We have assumed 100% efficacy for the drugs used to treat the animals.
Using the parameters described above, the overall viremia levels can be described by the following equation:
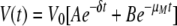 |
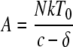 |
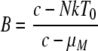 |
A and B represent the proportion of contributions made by T and M, respectively, before therapy. The Curve Fitting Toolbox (version 1.2) of Matlab (R2007b, 7.5) was used to estimate δ, μM, A, B, and V0 using the technique of nonlinear least-squares regression.
Quantification of CD4+ T cells in blood and tissues.
Circulating CD4+ T cells from whole blood were quantified. Tissue-associated CD4+ T cells from single-cell suspensions obtained by mechanical agitation of pooled head lymph nodes (cervical, retropharyngeal, and submandibular), pooled gut lymph nodes (mesenteric and colonic), pooled peripheral lymph nodes (axillary and inguinal), and spleen were quantified. One million cells from blood and tissues were labeled with phycoerythrin (PE)-conjugated anti-CD4 monoclonal antibody (Becton Dickinson). Analysis of the proportion of lymphocytes expressing CD4 was performed using flow cytometry with a FACSCalibur instrument (Becton Dickinson). CD4+ T cells were defined as cells in the lymphocyte population (forward- and side-scatter profiles) that express CD4. Circulating CD4+ T-cell measurements were expressed in units of cells per μl of blood. Tissue-associated CD4+ T-cell measurements were expressed as a percentage of total lymphocyte cells.
Isolation of resting memory CD4+ T cells.
Pooled head lymph nodes, pooled gut lymph nodes, and spleen were subjected to mechanical agitation to obtain single-cell suspensions. Peripheral blood mononuclear cells (PBMC) were isolated from whole blood by discontinuous density gradient centrifugation using 25% Percoll (GE Healthcare). Cell populations were then enriched for resting (HLA-DR−) CD4+ T cells using magnetic bead separation (Miltenyi Biotec). CD4+ cells were first isolated from lymph node and spleen suspensions and from PBMC by using antibody-conjugated microbeads to deplete cells expressing CD8, CD11b, CD16, CD20, CD56, or CD66abce, thereby leaving an untouched population of CD4+ T cells. HLA-DR-expressing cells were depleted from this enriched cell population with microbeads conjugated to an antibody against HLA-DR to yield a population of CD4+ HLA-DR− cells. The enriched cell population was incubated overnight at 37°C to allow contaminating monocytes to adhere to the tissue culture vessel. Nonadherent cells were labeled with PE-conjugated anti-CD4 and fluorescein isothiocyanate-conjugated anti-HLA-DR monoclonal antibodies (Becton Dickinson) and sorted for small CD4+ HLA-DR− cells using a MoFlo cell sorter (Cytomation). Populations of small CD4+ HLA-DR− cells were isolated with 98 to 99% purity.
Measurement of the frequency of resting CD4+ T cells harboring replication-competent virus.
The frequency of resting CD4+ T cells harboring replication-competent virus was measured using a coculture assay in which resting CD4+ T cells were cultured with a cell line, CEMx174, as previously described (60). Resting CD4+ T cells were cultured in a fivefold dilution series in duplicate ranging from 1 × 106 cells to 3.2 × 102 cells per well. In the event that the isolation of resting CD4+ T cells did not yield enough cells for culturing 1 × 106 cells, 2 × 105 cells were cultured, which raised the limit of quantification from 0.32 infectious units per million resting CD4+ T cells (IUPM) to 1.61 IUPM. The CEMx174 cells serve both to activate resting macaque T cells through CD2-CD58 interactions and to expand viruses released from latently infected cells that become activated (59). The presence of replication-competent SIV was determined by p27 enzyme-linked immunosorbent assay of culture supernatants after 3 weeks of culture. The cultures were set up in limiting-dilution format, and the frequencies of cells harboring replication-competent virus were determined as previously described (48). In brief, reported values are a reflection of the results obtained from all the dilutions performed in duplicate. The confidence intervals for each data point are ±0.7 logs.
Statistical analysis.
Paired Student t tests were performed to analyze changes in circulating CD4+ T-cell counts and changes in the frequencies of circulating CD4+ T cells harboring replication-competent virus over time within an animal. Unpaired Student t tests were performed to analyze differences in the proportions of CD4+ T cells in tissues between animal groups. An analysis of variance was performed to analyze differences in the frequencies of latently infected CD4+ T cells between different tissues in the HAART-treated animals. Frequencies of resting CD4+ T cells harboring replication-competent virus below the limit of detection were included with a value of the limit of detection for statistical analysis.
RESULTS
Suppression of viremia with HAART.
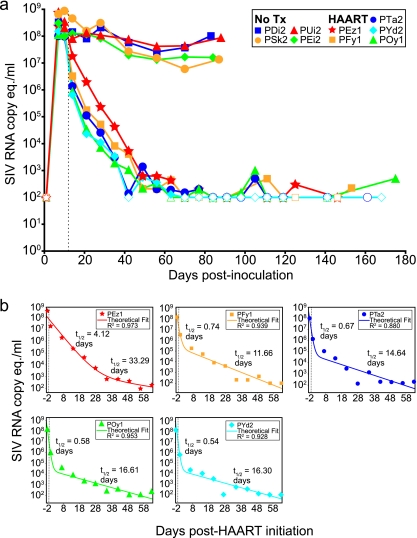
Eleven pig-tailed macaques were used in this study. Of the 11 animals, 9 were inoculated intravenously with both SIV/17E-Fr and SIV/Delta B670. These animals demonstrated high levels of viremia by day 10 p.i. (median, 1.40 × 108 copies of SIV RNA/ml; range, 0.98 × 108 to 8.75 × 108 copies/ml). Of the nine animals infected, five were treated with HAART at day 12 p.i., and all five animals displayed a rapid decay of viremia to below the limit of detection (100 RNA copies/ml) by day 70 p.i. (Fig. 1a). From day 70 postinfection to the day of necropsy, there were 47 viral load measurements (mean, 9 measurements per animal), and of those, 36 (77%) were ≤100 copies/ml (mean, 7 per animal). Of the 36 measurements that were ≤100 copies/ml, 14 (39%) (mean, 3 measurements per animal) were completely undetectable, with no signal detected in the three replicate quantitative PCR reactions.
FIG. 1.
Suppression of SIV viremia with HAART. (a) Levels of viremia were measured from day 0 to the day of necropsy for untreated animals (No Tx) (n = 4) and HAART-treated animals (n = 5). In the HAART-treated animals, viremia was reduced at least by ∼5 log10 units relative to the untreated animals, to levels below the limit of detection. Uninfected animals (n = 2) were consistently negative for SIV RNA. (b) Mathematical modeling of the decay of viremia for each animal revealed a biphasic decay that occurred upon initiation of HAART. Half-lives (t1/2) for each phase of decay are shown for each animal. These values are similar to half-lives reported for humans initiating HAART. Open symbols represent measurements at or below the limit of detection (100 SIV RNA copy equivalents [eq.]/ml). The vertical dotted line represents the day when HAART was initiated (day 12) for the treated animals.
Mathematical modeling of the decay of viremia was carried out using a model previously described by Perelson and colleagues. This analysis revealed a biphasic decay consistent with observations made for humans (Fig. 1b). In most animals, half-lives were similar to those reported previously for humans (54), with first-phase half-lives ranging from 0.54 to 4.12 days and second-phase half-lives ranging from 11.66 to 33.30 days. Animal PEz1 displayed decay kinetics that were slower during both phases than those of the other animals (Fig. 1b, top left). In contrast, the four SIV-infected, untreated animals experienced a slight decay in viremia from the peak at day 10 p.i. to a set point viremia that was maintained above 1 × 107 copies/ml until the day of necropsy (Fig. 1). Therefore, the treated animals experienced at least a 5-log10 decrease in viremia relative to the untreated animals, consistent with what was reported previously for humans (29, 30, 54). The uninfected animals consistently yielded negative results for plasma SIV RNA. Taken together, these results suggest that the virological response to HAART in HIV-1-infected humans is similar to that in SIV-infected pig-tailed macaques.
Rebound of CD4+ T cells with HAART.
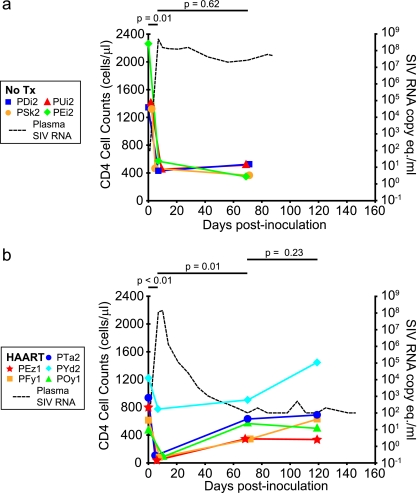
Circulating CD4+ T cells were measured during the course of the study (Fig. 2). In the four untreated SIV-infected animals, counts of circulating CD4+ T cells were measured on day 0 prior to infection; on day 7 p.i., during the establishment of peak viremia; and on day 70 p.i., before necropsy. There was a significant reduction in levels of circulating CD4+ T cells from day 0 (mean, 1,587 cells/μl) to day 7 (mean, 483 cells/μl) (P = 0.01). From day 7 to day 70, there was not a significant reduction in levels of circulating CD4+ T cells (from a mean of 483 cells/μl to a mean of 442 cells/μl; P = 0.62) (Fig. 2a). In the five HAART-treated animals, counts of circulating CD4+ T cells were also significantly reduced from day 0 (mean, 812 cells/μl) to day 7 p.i. (mean, 213 cells/μl) (P < 0.01). However, in contrast to the untreated animals, the HAART-treated animals experienced a significant rebound in circulating CD4+ T-cells count from day 7 (mean, 213 cells/μl) to day 70 (mean, 564 cells/μl) (P = 0.01). From day 70 (mean, 583 cells/μl) to day 119 (mean, 724 cells/μl), CD4 T-cell counts did not significantly change (P = 0.23) (Fig. 2b). In the two uninfected animals, mean circulating CD4+ T-cell counts were 2,207, 1,916, and 1,549 cells/μl at days 0, 7, and 70, respectively.
FIG. 2.
Effect of HAART on CD4+ T-cell counts in peripheral blood. Counts of circulating CD4+ T cells were measured at days 0, 7, and 70 p.i. for both untreated (No Tx) (n = 4) (a) and HAART-treated (n = 5) (b) animals and at day 119 for HAART-treated animals. For both untreated and HAART-treated animals, cell counts were significantly reduced between day 0 and day 7, the time interval during which viremia peaks. CD4+ T-cell counts were significantly increased by day 70 in the HAART-treated animals but not in untreated animals. Changes in cell counts between day 70 and day 119 were not significant.
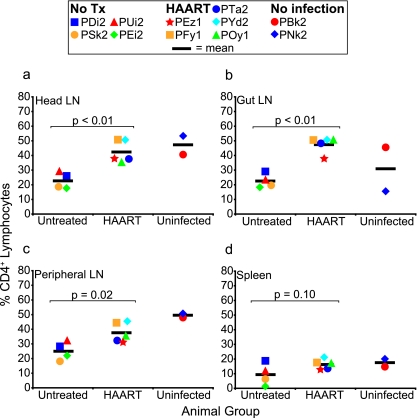
At necropsy, single-cell suspensions from pooled head lymph nodes (cervical, retropharyngeal, and submandibular), pooled gut lymph nodes (mesenteric and colonic), pooled peripheral lymph nodes (axillary and inguinal), and spleen were examined for proportions of CD4+ T cells (Fig. 3). In head, gut, and peripheral lymph nodes, the proportion of CD4+ T cells was significantly greater in HAART-treated animals than in untreated SIV-infected animals (P < 0.02 for all tissues). In the spleen, the proportion of CD4+ T cells was not significantly different between the two groups (P = 0.10); however, the proportion of CD4+ T cells in the spleen was low in both groups relative to that in the other tissues examined.
FIG. 3.
Fraction of CD4+ T cells in tissues. The percentage of lymphocytes that were CD4+ was measured in head lymph nodes (LN) (cervical, retropharyngeal, and submandibular) (a), gut lymph nodes (mesenteric and colonic) (b), peripheral lymph nodes (axillary and inguinal) (c), and spleen (d) in untreated (n = 4), HAART-treated (n = 5), and uninfected (n = 2) animals. Differences between untreated (No Tx) and HAART-treated animals were significant for the lymph node tissue but not spleen. Mean measurements are indicated by horizontal bars.
Taken together, these results indicate that circulating CD4+ T-cell counts were significantly increased in HAART-treated animals, and this increase occurred during the first 58 days of treatment, as counts between day 70 and day 119 were not significantly different. Furthermore, the proportion of CD4+ T cells in lymph nodes was significantly greater in the HAART-treated animals than in the untreated animals. Interestingly, this was not the case for the spleen. Overall, these data suggest that HAART induces a rebound in the number of CD4+ T cells in treated SIV-infected macaques, consistent with what was shown previously for treated HIV-infected humans (29, 30).
Detection of resting CD4+ T cells harboring replication-competent SIV in animals with undetectable levels of viremia.
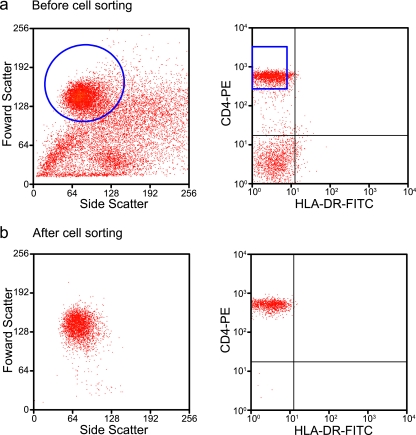
For patients on HAART, HIV-1 can persist in a latent form in resting CD4+ T cells. To determine whether SIV could persist in animals on HAART, we used an SIV coculture assay to measure frequencies of resting CD4+ T cells harboring replication-competent virus from multiple time points starting shortly after viremia became undetectable (<100 SIV copies/ml). Resting CD4+ T cells were isolated by a combination of magnetic bead depletion and cell sorting to obtain a highly pure population of cells (mean purity, 98.8%) (Fig. 4). To activate resting CD4+ T cells and amplify the released virus, purified resting CD4+ T cells were cocultured with CEMx174, a human hybrid T-B-lymphocyte cell line (see Materials and Methods). After 3 weeks of culture, supernatants were assayed for the presence of SIV p27 to identify the frequency of resting CD4+ T cells harboring replication-competent virus. In all SIV-infected animals, blood samples yielded detectable frequencies of resting CD4+ T cells harboring replication-competent virus during the course of low-level viremia, an observation consistent with previously reported data for humans on HAART (22, 62).
FIG. 4.
Isolation of resting CD4+ T cells. Resting CD4+ T cells were isolated from blood and various lymphoid tissues by magnetic bead depletion followed by sorting for small morphologically homogeneous populations of CD4+ HLA-DR− cells. (a) After magnetic bead enrichment, enriched cells contained a morphologically heterogeneous population of cells (left) and some CD4− cells (right). (b) Upon cell sorting using CD4-PE and HLA-DR-fluorescein isothiocyanate (FITC), isolated cells were morphologically (left) and phenotypically (CD4+ HLA-DR−) (right) homogeneous, with 98 to 99% purity.
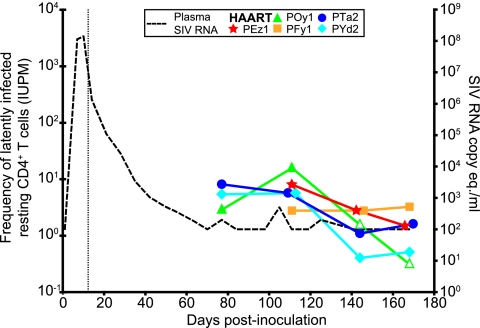
For the animals treated with HAART, frequencies of circulating resting CD4+ T cells harboring replication-competent virus were measured at multiple time points (Fig. 5). The average frequencies were 5.5 IUPM CD4+ T cells at day 77, 7.7 IUPM at day 111, 1.7 IUPM at day 141, and 1.4 IUPM at the day of necropsy (mean, day 168). Animal POy1 had frequencies below the limit of quantification for the last two measurements. The decay in the frequencies of resting CD4+ T cells harboring replication-competent virus likely reflects the fact that resting CD4+ T cells can harbor a labile unintegrated form of SIV as well as a more stable integrated form. By analogy with humans infected with HIV-1, it takes several months for the unintegrated form to decay completely (7). At the time of necropsy, frequencies of resting CD4+ T cells harboring replication-competent virus showed no evidence of decay, as shown by the lack of a significant difference between the last two measurements (P = 0.51). Furthermore, measurements at necropsy ranged between <0.32 and 3.25 IUPM, within the range of values observed for humans on long-term HAART (62). These results illustrate that this model will be useful for evaluating strategies for eradicating the latent reservoir. However, the animals should be treated with HAART for at least 150 days to allow labile forms of SIV to decay.
FIG. 5.
Frequency of circulating resting CD4+ T cells harboring replication-competent virus. At various times after undetectable viremia (dotted line, right y axis) was achieved with HAART, circulating resting CD4+ T cells (small CD4+ HLA-DR− cells) were isolated and assayed for the presence of inducible, replication-competent SIV. For HAART-treated animals, the frequencies of cells harboring replication-competent virus in circulating resting CD4+ T cells isolated at days 77, 111, and 141 and at necropsy (days 161 to 175) were measured. The vertical dotted line represents the day when HAART was initiated (day 12). Open symbols represent measurements less than the indicated values. The confidence intervals at each data point are ±0.7 logs.
Frequency of resting CD4+ T cells harboring replication-competent virus in tissues and blood at necropsy.
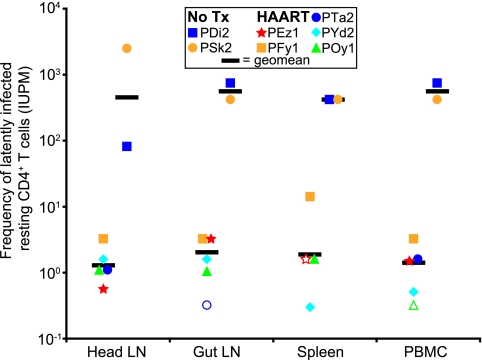
The frequency of resting CD4+ T cells harboring replication-competent virus was measured in pooled head (cervical, retropharyngeal, and submandibular) lymph nodes, pooled gut (mesenteric and colonic) lymph nodes, spleen, and PBMC that were isolated at necropsy (Fig. 6). Tissues were homogenized to single-cell suspensions by mechanical agitation, and resting CD4+ T cells were isolated with magnetic bead separation and subsequent cell sorting for small CD4+ HLA-DR− cells. Cell purity from tissues ranged from 98 to 99% (mean, 98.9%). In two of the four untreated SIV-infected animals (animals PDi2 and PSk2), we measured frequencies of resting CD4+ T cells harboring replication-competent virus and found geometric means of 452 IUPM for head lymph nodes, 561 IUPM for gut lymph nodes, 421 IUPM for spleen, and 564 IUPM for PBMC. For all five HAART-treated animals, frequencies were substantially lower than those for untreated animals. Geometric mean frequencies for HAART-treated animals were 1.3 IUPM for head lymph nodes, 1.4 IUPM for gut lymph nodes, 1.8 IUPM for spleen, and 1.1 IUPM for PBMC. Animals PTa2, PEz1, and POy1 yielded frequencies below the limit of detection in gut lymph nodes, spleen, and PBMC, respectively. Within the HAART-treated animal group, frequencies of latently infected resting CD4+ T cells were not significantly different (P = 0.47) between the tissues, a finding that is consistent with previously reported observations for untreated HIV-1-infected humans (13). For the one uninfected animal (animal PNk2) evaluated, coculture assays were negative for latently infected cells for all tissues. Taken together, these results show that the model of HAART closely mimics the virological state achieved by HIV-1-infected humans on HAART with respect to the degree of suppression of viremia, the rebound in circulating and lymphoid tissue-associated CD4+ T-cell counts, and the persistence of replication-competent virus from resting CD4+ T cells.
FIG. 6.
Frequency of resting CD4+ T cells harboring replication-competent virus in tissues and blood at necropsy. Resting CD4+ T cells (small CD4+ HLA-DR− cells) were isolated from head lymph nodes (LN), gut lymph nodes, spleen, and PBMC at necropsy. Purified cells were assayed for the presence of cells harboring replication-competent virus. Geometric mean frequencies for HAART-treated animals were ≥2 log10 values lower than those for untreated (No Tx) animals and were not statistically different between tissues (P = 0.47). Open symbols represent measurements less than the indicated values. The confidence intervals at each data point are ±0.7 logs.
DISCUSSION
Current treatment guidelines for HIV-1 infection recommend the use of specified combinations of three or more antiretroviral drugs, an approach that is commonly referred to as HAART. HIV-1-infected individuals who are adherent to therapy maintain levels of viremia below the clinical limit of detection, 50 HIV-1 RNA copies/ml of plasma (29, 30, 54). However, residual viremia continues at levels below 50 copies/ml despite years of therapy (20, 52). The median level of residual viremia is 2 copies/ml (43, 53). Another hallmark of treatment with HAART is a rise in CD4+ T-cell counts relative to pretreatment levels (29, 30).
Despite the success of HAART, HIV-1-infected individuals cannot be cured of the infection, in part because latently infected resting memory CD4+ T cells serve as lifelong viral reservoirs (18, 22, 23, 62, 73). To complicate matters, extensive sequence analysis of residual viremia in a cohort of HIV-1-infected individuals on suppressive HAART regimens for several years has shown that there is another long-lived reservoir contributing to residual viremia that appears to be distinct from the latent reservoir in resting memory CD4+ T cells (4). A recent study analyzing the sequence diversity of plasma virus that rebounded after an interruption of HAART showed that rebound viremia was derived from a source that was ancestral to viruses present at the time that HAART was started (38). In other words, rebound viremia was more closely related to the most recent common ancestor than virus isolated just before the initiation of HAART, suggesting that its source was established earlier in infection and persisted throughout the course of treatment. Altogether, the evidence suggests that the sources of residual viremia are stable reservoirs established before HAART that are able to persist during therapy independent of ongoing replication. Identification of all cell and tissue sources of residual viremia will be essential for developing strategies for eradicating the infection, but this is difficult in humans since for some patients, much of the residual viremia appears to be derived from cells not in circulation (4, 10, 58). Identifying all sources of residual virus and testing strategies for eradication require an animal model of HAART that directly parallels HIV-infected patients on HAART.
Here, we describe the development of an animal model that reproduces many key features of HIV-1 infection in the setting of suppressive HAART. We infected pig-tailed macaques with SIV/17E-Fr and SIV/Delta B670, an infection model that has been shown to yield high levels of plasma viremia and reproducible disease in 3 months (2, 3, 24, 44, 76, 77). Infection with this combination of viruses consistently yields high levels of viremia, and no animals spontaneously controlled infection. Consistent infection is important when establishing a model to identify new reservoirs and approaches to eradicate them. During acute infection, when levels of plasma viremia were high, the animals were treated with a four-drug antiretroviral combination consisting of a nucleotide reverse transcriptase inhibitor, two different protease inhibitors, and an integrase inhibitor, thereby targeting three different steps in the viral life cycle. Using this HAART regimen, we observed a dramatic biphasic decline in viremia. Each phase exhibited a decay constant similar to that reported previously for HIV-1-infected humans on HAART (54). In addition, infected animals showed an increase in the counts of CD4+ T cells following the initiation of HAART similar to that observed previously for treatment of HIV-1 infection (29, 30). At necropsy, we quantified the proportion of CD4+ T cells in total lymphocytes isolated from gut, head, peripheral lymph nodes, and spleen and showed that there was a significantly greater proportion of CD4+ T cells in HAART-treated animals than in the untreated group. For the untreated animals, levels of viremia reached a high set point, and CD4+ T-cell counts did not increase during the study interval. Levels of plasma viremia and CD4+ T-cell counts have proven to be important indicators of the response to antiretroviral therapy (29, 30), and our model displayed responses consistent with those for humans receiving HAART.
By using a coculture assay described elsewhere (60), we isolated resting small CD4+ HLA-DR− T cells from blood and tissues and measured the frequency of latently infected cells. In this population, only ∼6% of cells express CD25, and a similarly small fraction expresses CD69. None were dual positive for these early activation markers. Thus, the overwhelming majority of cells isolated by this procedure are truly resting, as assessed by their small size and the lack of early and late activation markers. It is unlikely that the small fraction of recently activated cells contributed significantly to the frequency of latently infected cells which we quantified since levels of infection in activated and resting CD4+ T cells are similar (17). Furthermore, CD69 and CD25 are expressed early after T-cell activation. Therefore, virus released from CD25+ or CD69+ cells during suppressive HAART would most likely represent a reactivation of latently infected cells. Given the results of recent pharmacodynamic (61) and HAART intensification (19) studies, it is unlikely that de novo infection of these recently activated cells occurs during suppressive HAART. Given the low frequency of CD25- and CD69-expressing cells and the low probability of new infection during suppressive HAART, our cell sorting method and coculture assay likely measure the frequency of latently infected cells. In the HAART-treated animals, the frequency of circulating resting CD4+ T cells harboring replication-competent virus showed an initial decay similar to that observed for HIV-1-infected humans on HAART. Presumably, the initial decay reflects the turnover of the labile preintegration complexes that cannot integrate into the host genome due to the resting state of the infected cell. Before this decay is complete, it is thought that the release of virus from resting CD4+ T cells that have become activated can arise from two different infection states: one state in which preintegration complexes integrate into the host genome upon the activation of the cell and produce virus and another state in which the resting cell carries a stably integrated but latent viral genome and becomes activated to produce virus. Our results suggest that the frequencies measured at necropsy are representative of cells in the stable postintegration state of latency, although a contribution from cells in the labile preintegration state cannot be excluded. To date, this has not been shown for any animal model of HIV-1 infection and HAART. Thus, with regard to virus in resting CD4+ T cells, this system provides an excellent model of HIV-1 infection and HAART as defined by previous work with HIV-1-infected humans (7).
For HIV-1-infected humans on HAART, latently infected CD4+ T cells harboring replication-competent virus can be detected in the circulation after many years of treatment. However, it is not clear how frequent these cells are in the lymphoid tissue during suppressive HAART. In the animal model described here, we show that HAART-treated animals had latently infected CD4+ T cells in the lymphoid tissue but at lower frequencies than those of untreated SIV-infected animals. This is not surprising given that the frequencies measured for the untreated animals reflect the two states of latent infection described above. Consistent with studies of humans with untreated HIV-1 infection, the frequencies were not significantly different between tissues and blood (13). Furthermore, the frequencies in the lymphoid tissues were within a range observed for blood from humans on HAART. These results confirm the persistence of latently infected resting CD4+ T cells in the lymphoid tissue and raise the possibility that there is an even distribution of this reservoir in the lymphoid tissues throughout the body of an infected individual on HAART.
This study also contributed to our understanding of the pharmacology of antiretroviral drugs in macaques. For three of the five HAART-treated animals, dosages of PMPA were reduced, and for one animal, PMPA was removed from the regimen. In these animals, creatinine levels were elevated, which is indicative of nephrotoxicity. In humans, TDF, the prodrug of PMPA, can cause nephrotoxicity, and the discontinuation of TDF has been shown to mitigate nephrotoxicity. In the animals studied here, a reduction of the PMPA dosage was shown to reduce levels of creatinine. In future studies, the choice of an appropriate dose of an antiretroviral drug could be guided by toxicity considerations and by a more accurate evaluation of the activity of the drugs against SIV. We have recently shown that the dose-response curve slope is a critical missing dimension in the analysis of antiviral activity (61). The use of measures that incorporate this parameter may facilitate the choice of drug doses that control SIV replication without substantial toxicity.
Other studies previously described treatment of SIV-infected rhesus macaques (8, 27, 28, 42, 51, 69-71) and pig-tailed macaques (1, 60) with antiretroviral drugs. The pig-tailed macaque model described previously by Ambrose and colleagues used an HIV reverse transcriptase-encoding SIV and three-drug HAART (1). In this model, there was also a dramatic reduction in viremia and a rise in the number of CD4+ T cells in blood. However, latently infected cells were not measured. In contrast, latently infected cells were studied in the pig-tailed macaque model described previously by Shen and colleagues; however, only two antiretroviral drugs were used, both of the same drug class (60). Thus, that model is not fully comparable to current HAART regimens. In addition, the SIV strain used did not consistently give high levels of viremia, and the disease course was variable in untreated animals. In comparison to other pig-tailed macaque models, we believe that the model described here represents the best available model for viral persistence in the setting of HAART.
In addition to demonstrating a dramatic biphasic decay in SIV viremia and an increase in circulating CD4+ T-cell counts following the initiation of HAART, this study also tracks the decay in circulating resting CD4+ T cells harboring replication-competent virus to frequencies observed for humans on long-term HAART. After 150 days of treatment, the frequency of resting CD4+ T cells harboring replication-competent virus appeared to have plateaued. It will be important in future studies to treat animals for longer periods of time to determine whether the frequencies of latently infected cells remain stable as they do in humans. In addition, we demonstrate for the first time the presence of latently infected resting CD4+ T cells in lymphoid tissue in the setting of suppressive HAART and quantify their frequencies. Our approach could be extended to a total-body analysis of various tissues to quantify the frequency of latently infected resting CD4+ T cells residing in other tissues. Our data suggest that there is an equal distribution of latently infected cells among lymphoid cells in the tissues sampled.
The gut-associated lymphoid tissue (GALT) has been shown to be massively depleted of CD4+ T cells in HIV-1 and SIV infections (9, 12, 28, 39, 42, 45-47). It has also been shown that antiretroviral therapy leads to a reconstitution of CD4+ T cells in the GALT but not to the same degree as that observed for peripheral blood (39, 46). These qualities make this tissue compartment very unique in HIV-1/SIV infection. The animal model described here may be a useful tool for exploring viral persistence in this compartment and other effector sites such as the lung, as dual infection of pig-tailed macaques with SIV/17E-Fr and SIV/Delta B670 was previously shown to lead to a massive depletion of CD4+ T cells in the GALT (12). Furthermore, evaluation of memory CD4+ T-cell subsets and the depletion of CD4+ T cells at effector sites would provide a more sensitive indication of disease progression (9, 12, 28, 31, 39, 41, 42, 45-47). However, previously reported observations of CD4+ T-cell depletion in GALT (12) and clinical manifestations of disease in a period of 3 months in untreated animals (2, 3, 24, 44, 76, 77) suggest that this system does model the ability of HAART to prevent or reverse clinical immunodeficiency disease seen for humans with HIV-1 infection.
A quantitative understanding of the tissues that harbor latently infected cells will be essential for an eradication effort, first to target drugs that activate latent virus to tissues that harbor latently infected cells and then to determine whether the therapy was effective in eradicating the latently infected cells. In addition to the quantification of the well-characterized resting CD4+ T-cell reservoir, this model could be used to identify uncharacterized stable reservoirs persisting in anatomical sites that are difficult to screen for humans. Examples of potential reservoirs include hematopoietic stem and progenitor cells that reside in the bone marrow. In observing predominant plasma clones persisting for years in the context of suppressive HAART, Bailey and colleagues proposed a self-renewing CD34+ stem or progenitor cell of the hematopoietic lineage as a potential source of residual viremia (4). However, these cells reside in the bone marrow and are difficult to study for HIV-infected patients on HAART because of the invasive procedures required to obtain bone marrow. Finally, this animal model would serve as a model to validate the proposed cell types that have demonstrated stable viral reservoir qualities ex vivo (11, 64).
Acknowledgments
This work was supported by NIH grants PO1 MH 070306 and RO143222 and by the Howard Hughes Medical Institute.
We thank Charles Flexner and Teresa Parsons for performing drug-level monitoring; Ming Li, Brandon Bullock, Suzanne Queen, Erin Shirk, Christopher Bartizal, John Varrone, and Jamie Karper for excellent technical assistance; and Joseph Margolick and Hao Zhang for cell sorting. We thank Merck, Bristol-Myers Squibb, Gilead Sciences, and Roche for providing anti-HIV-1 drugs.
Footnotes
Published ahead of print on 1 July 2009.
REFERENCES
- 1.Ambrose, Z., S. Palmer, V. F. Boltz, M. Kearney, K. Larsen, P. Polacino, L. Flanary, K. Oswald, M. Piatak, Jr., J. Smedley, W. Shao, N. Bischofberger, F. Maldarelli, J. T. Kimata, J. W. Mellors, S. L. Hu, J. M. Coffin, J. D. Lifson, and V. N. KewalRamani. 2007. Suppression of viremia and evolution of human immunodeficiency virus type 1 drug resistance in a macaque model for antiretroviral therapy. J. Virol. 81:12145-12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babas, T., J. B. Dewitt, J. L. Mankowski, P. M. Tarwater, J. E. Clements, and M. C. Zink. 2006. Progressive selection for neurovirulent genotypes in the brain of SIV-infected macaques. AIDS 20:197-205. [DOI] [PubMed] [Google Scholar]
- 3.Babas, T., E. Vieler, D. A. Hauer, R. J. Adams, P. M. Tarwater, K. Fox, J. E. Clements, and M. C. Zink. 2001. Pathogenesis of SIV pneumonia: selective replication of viral genotypes in the lung. Virology 287:371-381. [DOI] [PubMed] [Google Scholar]
- 4.Bailey, J. R., A. R. Sedaghat, T. Kieffer, T. Brennan, P. K. Lee, M. Wind-Rotolo, C. M. Haggerty, A. R. Kamireddi, Y. Liu, J. Lee, D. Persaud, J. E. Gallant, J. Cofrancesco, Jr., T. C. Quinn, C. O. Wilke, S. C. Ray, J. D. Siliciano, R. E. Nettles, and R. F. Siliciano. 2006. Residual human immunodeficiency virus type 1 viremia in some patients on antiretroviral therapy is dominated by a small number of invariant clones rarely found in circulating CD4+ T cells. J. Virol. 80:6441-6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barouch, D. H., J. Kunstman, M. J. Kuroda, J. E. Schmitz, S. Santra, F. W. Peyerl, G. R. Krivulka, K. Beaudry, M. A. Lifton, D. A. Gorgone, D. C. Montefiori, M. G. Lewis, S. M. Wolinsky, and N. L. Letvin. 2002. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature 415:335-339. [DOI] [PubMed] [Google Scholar]
- 6.Barrios, A., A. L. Rendon, O. Gallego, L. Martin-Carbonero, L. Valer, P. Rios, I. Maida, T. Garcia-Benayas, I. Jimenez-Nacher, J. Gonzalez-Lahoz, and V. Soriano. 2004. Predictors of virological response to atazanavir in protease inhibitor-experienced patients. HIV Clin. Trials 5:201-205. [DOI] [PubMed] [Google Scholar]
- 7.Blankson, J. N., D. Finzi, T. C. Pierson, B. P. Sabundayo, K. Chadwick, J. B. Margolick, T. C. Quinn, and R. F. Siliciano. 2000. Biphasic decay of latently infected CD4+ T cells in acute human immunodeficiency virus type 1 infection. J. Infect. Dis. 182:1636-1642. [DOI] [PubMed] [Google Scholar]
- 8.Boyer, J. D., S. Kumar, T. Robinson, R. Parkinson, L. Wu, M. Lewis, and D. B. Weiner. 2006. Initiation of antiretroviral therapy during chronic SIV infection leads to rapid reduction in viral loads and the level of T-cell immune response. J. Med. Primatol. 35:202-209. [DOI] [PubMed] [Google Scholar]
- 9.Brenchley, J. M., T. W. Schacker, L. E. Ruff, D. A. Price, J. H. Taylor, G. J. Beilman, P. L. Nguyen, A. Khoruts, M. Larson, A. T. Haase, and D. C. Douek. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200:749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brennan, T. P., J. O. Woods, A. R. Sedaghat, J. D. Siliciano, R. F. Siliciano, and C. O. Wilke. 2009. Analysis of human immunodeficiency virus type 1 viremia and provirus in resting CD4+ T cells reveals a novel source of residual viremia in patients on antiretroviral therapy. J. Virol. 83:8470-8481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown, A., H. Zhang, P. Lopez, C. A. Pardo, and S. Gartner. 2006. In vitro modeling of the HIV-macrophage reservoir. J. Leukoc. Biol. 80:1127-1135. [DOI] [PubMed] [Google Scholar]
- 12.Chase, A. J., A. R. Sedaghat, J. R. German, L. Gama, M. C. Zink, J. E. Clements, and R. F. Siliciano. 2007. Severe depletion of CD4+ CD25+ regulatory T cells from the intestinal lamina propria but not peripheral blood or lymph nodes during acute simian immunodeficiency virus infection. J. Virol. 81:12748-12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chun, T. W., L. Carruth, D. Finzi, X. Shen, J. A. DiGiuseppe, H. Taylor, M. Hermankova, K. Chadwick, J. Margolick, T. C. Quinn, Y. H. Kuo, R. Brookmeyer, M. A. Zeiger, P. Barditch-Crovo, and R. F. Siliciano. 1997. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 387:183-188. [DOI] [PubMed] [Google Scholar]
- 14.Chun, T. W., R. T. Davey, Jr., M. Ostrowski, J. S. Justement, D. Engel, J. I. Mullins, and A. S. Fauci. 2000. Relationship between pre-existing viral reservoirs and the re-emergence of plasma viremia after discontinuation of highly active anti-retroviral therapy. Nat. Med. 6:757-761. [DOI] [PubMed] [Google Scholar]
- 15.Chun, T. W., D. Finzi, J. Margolick, K. Chadwick, D. Schwartz, and R. F. Siliciano. 1995. In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nat. Med. 1:1284-1290. [DOI] [PubMed] [Google Scholar]
- 16.Chun, T. W., D. C. Nickle, J. S. Justement, D. Large, A. Semerjian, M. E. Curlin, M. A. O'Shea, C. W. Hallahan, M. Daucher, D. J. Ward, S. Moir, J. I. Mullins, C. Kovacs, and A. S. Fauci. 2005. HIV-infected individuals receiving effective antiviral therapy for extended periods of time continually replenish their viral reservoir. J. Clin. Investig. 115:3250-3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chun, T. W., D. C. Nickle, J. S. Justement, J. H. Meyers, G. Roby, C. W. Hallahan, S. Kottilil, S. Moir, J. M. Mican, J. I. Mullins, D. J. Ward, J. A. Kovacs, P. J. Mannon, and A. S. Fauci. 2008. Persistence of HIV in gut-associated lymphoid tissue despite long-term antiretroviral therapy. J. Infect. Dis. 197:714-720. [DOI] [PubMed] [Google Scholar]
- 18.Chun, T. W., L. Stuyver, S. B. Mizell, L. A. Ehler, J. A. Mican, M. Baseler, A. L. Lloyd, M. A. Nowak, and A. S. Fauci. 1997. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA 94:13193-13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dinoso, J. B., S. Y. Kim, A. M. Wiegand, S. E. Palmer, S. J. Gange, L. Cranmer, A. O'Shea, M. Callender, A. Spivak, T. Brennan, M. F. Kearney, M. A. Proschan, J. M. Mican, C. A. Rehm, J. M. Coffin, J. W. Mellors, R. F. Siliciano, and F. Maldarelli. 2009. Treatment intensification does not reduce residual HIV-1 viremia in patients on highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA 106:9403-9408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dornadula, G., H. Zhang, B. VanUitert, J. Stern, L. Livornese, Jr., M. J. Ingerman, J. Witek, R. J. Kedanis, J. Natkin, J. DeSimone, and R. J. Pomerantz. 1999. Residual HIV-1 RNA in blood plasma of patients taking suppressive highly active antiretroviral therapy. JAMA 282:1627-1632. [DOI] [PubMed] [Google Scholar]
- 21.Evans, D. T., J. E. Bricker, H. B. Sanford, S. Lang, A. Carville, B. A. Richardson, M. Piatak, Jr., J. D. Lifson, K. G. Mansfield, and R. C. Desrosiers. 2005. Immunization of macaques with single-cycle simian immunodeficiency virus (SIV) stimulates diverse virus-specific immune responses and reduces viral loads after challenge with SIVmac239. J. Virol. 79:7707-7720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finzi, D., J. Blankson, J. D. Siliciano, J. B. Margolick, K. Chadwick, T. Pierson, K. Smith, J. Lisziewicz, F. Lori, C. Flexner, T. C. Quinn, R. E. Chaisson, E. Rosenberg, B. Walker, S. Gange, J. Gallant, and R. F. Siliciano. 1999. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat. Med. 5:512-517. [DOI] [PubMed] [Google Scholar]
- 23.Finzi, D., M. Hermankova, T. Pierson, L. M. Carruth, C. Buck, R. E. Chaisson, T. C. Quinn, K. Chadwick, J. Margolick, R. Brookmeyer, J. Gallant, M. Markowitz, D. D. Ho, D. D. Richman, and R. F. Siliciano. 1997. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278:1295-1300. [DOI] [PubMed] [Google Scholar]
- 24.Flaherty, M. T., D. A. Hauer, J. L. Mankowski, M. C. Zink, and J. E. Clements. 1997. Molecular and biological characterization of a neurovirulent molecular clone of simian immunodeficiency virus. J. Virol. 71:5790-5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frenkel, L. M., Y. Wang, G. H. Learn, J. L. McKernan, G. M. Ellis, K. M. Mohan, S. E. Holte, S. M. De Vange, D. M. Pawluk, A. J. Melvin, P. F. Lewis, L. M. Heath, I. A. Beck, M. Mahalanabis, W. E. Naugler, N. H. Tobin, and J. I. Mullins. 2003. Multiple viral genetic analyses detect low-level human immunodeficiency virus type 1 replication during effective highly active antiretroviral therapy. J. Virol. 77:5721-5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furtado, M. R., D. S. Callaway, J. P. Phair, K. J. Kunstman, J. L. Stanton, C. A. Macken, A. S. Perelson, and S. M. Wolinsky. 1999. Persistence of HIV-1 transcription in peripheral-blood mononuclear cells in patients receiving potent antiretroviral therapy. N. Engl. J. Med. 340:1614-1622. [DOI] [PubMed] [Google Scholar]
- 27.George, M. D., E. Reay, S. Sankaran, and S. Dandekar. 2005. Early antiretroviral therapy for simian immunodeficiency virus infection leads to mucosal CD4+ T-cell restoration and enhanced gene expression regulating mucosal repair and regeneration. J. Virol. 79:2709-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guadalupe, M., S. Sankaran, M. D. George, E. Reay, D. Verhoeven, B. L. Shacklett, J. Flamm, J. Wegelin, T. Prindiville, and S. Dandekar. 2006. Viral suppression and immune restoration in the gastrointestinal mucosa of human immunodeficiency virus type 1-infected patients initiating therapy during primary or chronic infection. J. Virol. 80:8236-8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gulick, R. M., J. W. Mellors, D. Havlir, J. J. Eron, C. Gonzalez, D. McMahon, D. D. Richman, F. T. Valentine, L. Jonas, A. Meibohm, E. A. Emini, and J. A. Chodakewitz. 1997. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N. Engl. J. Med. 337:734-739. [DOI] [PubMed] [Google Scholar]
- 30.Hammer, S. M., K. E. Squires, M. D. Hughes, J. M. Grimes, L. M. Demeter, J. S. Currier, J. J. Eron, Jr., J. E. Feinberg, H. H. Balfour, Jr., L. R. Deyton, J. A. Chodakewitz, M. A. Fischl, et al. 1997. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. N. Engl. J. Med. 337:725-733. [DOI] [PubMed] [Google Scholar]
- 31.Hansen, S. G., C. Vieville, N. Whizin, L. Coyne-Johnson, D. C. Siess, D. D. Drummond, A. W. Legasse, M. K. Axthelm, K. Oswald, C. M. Trubey, M. Piatak, Jr., J. D. Lifson, J. A. Nelson, M. A. Jarvis, and L. J. Picker. 2009. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat. Med. 15:293-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Havlir, D. V., M. C. Strain, M. Clerici, C. Ignacio, D. Trabattoni, P. Ferrante, and J. K. Wong. 2003. Productive infection maintains a dynamic steady state of residual viremia in human immunodeficiency virus type 1-infected persons treated with suppressive antiretroviral therapy for five years. J. Virol. 77:11212-11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hazuda, D. J., S. D. Young, J. P. Guare, N. J. Anthony, R. P. Gomez, J. S. Wai, J. P. Vacca, L. Handt, S. L. Motzel, H. J. Klein, G. Dornadula, R. M. Danovich, M. V. Witmer, K. A. Wilson, L. Tussey, W. A. Schleif, L. S. Gabryelski, L. Jin, M. D. Miller, D. R. Casimiro, E. A. Emini, and J. W. Shiver. 2004. Integrase inhibitors and cellular immunity suppress retroviral replication in rhesus macaques. Science 305:528-532. [DOI] [PubMed] [Google Scholar]
- 34.Hel, Z., D. Venzon, M. Poudyal, W. P. Tsai, L. Giuliani, R. Woodward, C. Chougnet, G. Shearer, J. D. Altman, D. Watkins, N. Bischofberger, A. Abimiku, P. Markham, J. Tartaglia, and G. Franchini. 2000. Viremia control following antiretroviral treatment and therapeutic immunization during primary SIV251 infection of macaques. Nat. Med. 6:1140-1146. [DOI] [PubMed] [Google Scholar]
- 35.Hermankova, M., S. C. Ray, C. Ruff, M. Powell-Davis, R. Ingersoll, R. T. D'Aquila, T. C. Quinn, J. D. Siliciano, R. F. Siliciano, and D. Persaud. 2001. HIV-1 drug resistance profiles in children and adults with viral load of <50 copies/ml receiving combination therapy. JAMA 286:196-207. [DOI] [PubMed] [Google Scholar]
- 36.Ho, D. D., A. U. Neumann, A. S. Perelson, W. Chen, J. M. Leonard, and M. Markowitz. 1995. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 373:123-126. [DOI] [PubMed] [Google Scholar]
- 37.Imamichi, H., K. A. Crandall, V. Natarajan, M. K. Jiang, R. L. Dewar, S. Berg, A. Gaddam, M. Bosche, J. A. Metcalf, R. T. Davey, Jr., and H. C. Lane. 2001. Human immunodeficiency virus type 1 quasi species that rebound after discontinuation of highly active antiretroviral therapy are similar to the viral quasi species present before initiation of therapy. J. Infect. Dis. 183:36-50. [DOI] [PubMed] [Google Scholar]
- 38.Joos, B., M. Fischer, H. Kuster, S. K. Pillai, J. K. Wong, J. Boni, B. Hirschel, R. Weber, A. Trkola, H. F. Gunthard, and the Swiss HIV Cohort Study. 2008. HIV rebounds from latently infected cells, rather than from continuing low-level replication. Proc. Natl. Acad. Sci. USA 105:16725-16730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kader, M., W. M. Hassan, M. Eberly, M. Piatak, J. D. Lifson, M. Roederer, and J. J. Mattapallil. 2008. Antiretroviral therapy prior to acute viral replication preserves CD4 T cells in the periphery but not in rectal mucosa during acute simian immunodeficiency virus infection. J. Virol. 82:11467-11471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kieffer, T. L., M. M. Finucane, R. E. Nettles, T. C. Quinn, K. W. Broman, S. C. Ray, D. Persaud, and R. F. Siliciano. 2004. Genotypic analysis of HIV-1 drug resistance at the limit of detection: virus production without evolution in treated adults with undetectable HIV loads. J. Infect. Dis. 189:1452-1465. [DOI] [PubMed] [Google Scholar]
- 41.Lay, M. D. H., J. Petravic, S. N. Gordon, J. Engram, G. Silvestri, and M. P. Davenport. 2009. Is the gut the major source of virus in early simian immunodeficiency virus infection? J. Virol. 83:7517-7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lifson, J. D., M. Piatak, Jr., A. N. Cline, J. L. Rossio, J. Purcell, I. Pandrea, N. Bischofberger, J. Blanchard, and R. S. Veazey. 2003. Transient early post-inoculation anti-retroviral treatment facilitates controlled infection with sparing of CD4+ T cells in gut-associated lymphoid tissues in SIVmac239-infected rhesus macaques, but not resistance to rechallenge. J. Med. Primatol. 32:201-210. [DOI] [PubMed] [Google Scholar]
- 43.Maldarelli, F., S. Palmer, M. S. King, A. Wiegand, M. A. Polis, J. Mican, J. A. Kovacs, R. T. Davey, D. Rock-Kress, R. Dewar, S. Liu, J. A. Metcalf, C. Rehm, S. C. Brun, G. J. Hanna, D. J. Kempf, J. M. Coffin, and J. W. Mellors. 2007. ART suppresses plasma HIV-1 RNA to a stable set point predicted by pretherapy viremia. PLoS Pathog. 3:e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mankowski, J. L., M. T. Flaherty, J. P. Spelman, D. A. Hauer, P. J. Didier, A. M. Amedee, M. Murphey-Corb, L. M. Kirstein, A. Munoz, J. E. Clements, and M. C. Zink. 1997. Pathogenesis of simian immunodeficiency virus encephalitis: viral determinants of neurovirulence. J. Virol. 71:6055-6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mehandru, S., M. A. Poles, K. Tenner-Racz, A. Horowitz, A. Hurley, C. Hogan, D. Boden, P. Racz, and M. Markowitz. 2004. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 200:761-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mehandru, S., M. A. Poles, K. Tenner-Racz, P. Jean-Pierre, V. Manuelli, P. Lopez, A. Shet, A. Low, H. Mohri, D. Boden, P. Racz, and M. Markowitz. 2006. Lack of mucosal immune reconstitution during prolonged treatment of acute and early HIV-1 infection. PLoS Med. 3:e484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mehandru, S., M. A. Poles, K. Tenner-Racz, V. Manuelli, P. Jean-Pierre, P. Lopez, A. Shet, A. Low, H. Mohri, D. Boden, P. Racz, and M. Markowitz. 2007. Mechanisms of gastrointestinal CD4+ T-cell depletion during acute and early human immunodeficiency virus type 1 infection. J. Virol. 81:599-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Myers, L. E., L. J. McQuay, and F. B. Hollinger. 1994. Dilution assay statistics. J. Clin. Microbiol. 32:732-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Natarajan, V., M. Bosche, J. A. Metcalf, D. J. Ward, H. C. Lane, and J. A. Kovacs. 1999. HIV-1 replication in patients with undetectable plasma virus receiving HAART. Highly active antiretroviral therapy. Lancet 353:119-120. [DOI] [PubMed] [Google Scholar]
- 50.Nettles, R. E., T. L. Kieffer, P. Kwon, D. Monie, Y. Han, T. Parsons, J. Cofrancesco, Jr., J. E. Gallant, T. C. Quinn, B. Jackson, C. Flexner, K. Carson, S. Ray, D. Persaud, and R. F. Siliciano. 2005. Intermittent HIV-1 viremia (Blips) and drug resistance in patients receiving HAART. JAMA 293:817-829. [DOI] [PubMed] [Google Scholar]
- 51.North, T. W., K. K. Van Rompay, J. Higgins, T. B. Matthews, D. A. Wadford, N. C. Pedersen, and R. F. Schinazi. 2005. Suppression of virus load by highly active antiretroviral therapy in rhesus macaques infected with a recombinant simian immunodeficiency virus containing reverse transcriptase from human immunodeficiency virus type 1. J. Virol. 79:7349-7354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palmer, S., A. P. Wiegand, F. Maldarelli, H. Bazmi, J. M. Mican, M. Polis, R. L. Dewar, A. Planta, S. Liu, J. A. Metcalf, J. W. Mellors, and J. M. Coffin. 2003. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J. Clin. Microbiol. 41:4531-4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palmer, S., F. Maldarelli, A. Wiegand, B. Bernstein, G. J. Hanna, S. C. Brun, D. J. Kempf, J. W. Mellors, J. M. Coffin, and M. S. King. 2008. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc. Natl. Acad. Sci. USA 105:3879-3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perelson, A. S., P. Essunger, Y. Cao, M. Vesanen, A. Hurley, K. Saksela, M. Markowitz, and D. D. Ho. 1997. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature 387:188-191. [DOI] [PubMed] [Google Scholar]
- 55.Perelson, A. S., A. U. Neumann, M. Markowitz, J. M. Leonard, and D. D. Ho. 1996. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science 271:1582-1586. [DOI] [PubMed] [Google Scholar]
- 56.Peyriere, H., J. Reynes, I. Rouanet, N. Daniel, C. M. de Boever, J. M. Mauboussin, H. Leray, L. Moachon, D. Vincent, and D. Salmon-Ceron. 2004. Renal tubular dysfunction associated with tenofovir therapy: report of 7 cases. J. Acquir. Immune Defic. Syndr. 35:269-273. [DOI] [PubMed] [Google Scholar]
- 57.Rollot, F., E. M. Nazal, L. Chauvelot-Moachon, C. Kelaidi, N. Daniel, M. Saba, S. Abad, and P. Blanche. 2003. Tenofovir-related Fanconi syndrome with nephrogenic diabetes insipidus in a patient with acquired immunodeficiency syndrome: the role of lopinavir-ritonavir-didanosine. Clin. Infect. Dis. 37:e174-e176. [DOI] [PubMed] [Google Scholar]
- 58.Sahu, G. K., D. Paar, S. D. Frost, M. M. Smith, S. Weaver, and M. W. Cloyd. 2009. Low-level plasma HIVs in patients on prolonged suppressive highly active antiretroviral therapy are produced mostly by cells other than CD4 T-cells. J. Med. Virol. 81:9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shen, A., H. C. Yang, Y. Zhou, A. J. Chase, J. D. Boyer, H. Zhang, J. B. Margolick, M. C. Zink, J. E. Clements, and R. F. Siliciano. 2007. Novel pathway for induction of latent virus from resting CD4+ T cells in the simian immunodeficiency virus/macaque model of human immunodeficiency virus type 1 latency. J. Virol. 81:1660-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shen, A., M. C. Zink, J. L. Mankowski, K. Chadwick, J. B. Margolick, L. M. Carruth, M. Li, J. E. Clements, and R. F. Siliciano. 2003. Resting CD4+ T lymphocytes but not thymocytes provide a latent viral reservoir in a simian immunodeficiency virus-Macaca nemestrina model of human immunodeficiency virus type 1-infected patients on highly active antiretroviral therapy. J. Virol. 77:4938-4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shen, L., S. Peterson, A. R. Sedaghat, M. A. McMahon, M. Callender, H. Zhang, Y. Zhou, E. Pitt, K. S. Anderson, E. P. Acosta, and R. F. Siliciano. 2008. Dose-response curve slope sets class-specific limits on inhibitory potential of anti-HIV drugs. Nat. Med. 14:762-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Siliciano, J. D., J. Kajdas, D. Finzi, T. C. Quinn, K. Chadwick, J. B. Margolick, C. Kovacs, S. J. Gange, and R. F. Siliciano. 2003. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat. Med. 9:727-728. [DOI] [PubMed] [Google Scholar]
- 63.Silvestri, G., D. L. Sodora, R. A. Koup, M. Paiardini, S. P. O'Neil, H. M. McClure, S. I. Staprans, and M. B. Feinberg. 2003. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity 18:441-452. [DOI] [PubMed] [Google Scholar]
- 64.Sundstrom, J. B., J. E. Ellis, G. A. Hair, A. S. Kirshenbaum, D. D. Metcalfe, H. Yi, A. C. Cardona, M. K. Lindsay, and A. A. Ansari. 2007. Human tissue mast cells are an inducible reservoir of persistent HIV infection. Blood 109:5293-5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suryanarayana, K., T. A. Wiltrout, G. M. Vasquez, V. M. Hirsch, and J. D. Lifson. 1998. Plasma SIV RNA viral load determination by real-time quantification of product generation in reverse transcriptase-polymerase chain reaction. AIDS Res. Hum. Retrovir. 14:183-189. [DOI] [PubMed] [Google Scholar]
- 66.Tobin, N. H., G. H. Learn, S. E. Holte, Y. Wang, A. J. Melvin, J. L. McKernan, D. M. Pawluk, K. M. Mohan, P. F. Lewis, J. I. Mullins, and L. M. Frenkel. 2005. Evidence that low-level viremias during effective highly active antiretroviral therapy result from two processes: expression of archival virus and replication of virus. J. Virol. 79:9625-9634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tsai, C. C., K. E. Follis, T. W. Beck, A. Sabo, N. Bischofberger, and P. J. Dailey. 1997. Effects of (R)-9-(2-phosphonylmethoxypropyl)adenine monotherapy on chronic SIV infection in macaques. AIDS Res. Hum. Retrovir. 13:707-712. [DOI] [PubMed] [Google Scholar]
- 68.Van Rompay, K. K., L. L. Brignolo, D. J. Meyer, C. Jerome, R. Tarara, A. Spinner, M. Hamilton, L. L. Hirst, D. R. Bennett, D. R. Canfield, T. G. Dearman, W. Von Morgenland, P. C. Allen, C. Valverde, A. B. Castillo, R. B. Martin, V. F. Samii, R. Bendele, J. Desjardins, M. L. Marthas, N. C. Pedersen, and N. Bischofberger. 2004. Biological effects of short-term or prolonged administration of 9-[2-(phosphonomethoxy)propyl]adenine (tenofovir) to newborn and infant rhesus macaques. Antimicrob. Agents Chemother. 48:1469-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Van Rompay, K. K., L. Durand-Gasselin, L. L. Brignolo, A. S. Ray, K. Abel, T. Cihlar, A. Spinner, C. Jerome, J. Moore, B. P. Kearney, M. L. Marthas, H. Reiser, and N. Bischofberger. 2008. Chronic administration of tenofovir to rhesus macaques from infancy through adulthood and pregnancy: summary of pharmacokinetics and biological and virological effects. Antimicrob. Agents Chemother. 52:3144-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Van Rompay, K. K., R. P. Singh, W. Heneine, J. A. Johnson, D. C. Montefiori, N. Bischofberger, and M. L. Marthas. 2006. Structured treatment interruptions with tenofovir monotherapy for simian immunodeficiency virus-infected newborn macaques. J. Virol. 80:6399-6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Veazey, R. S., P. J. Klasse, T. J. Ketas, J. D. Reeves, M. Piatak, Jr., K. Kunstman, S. E. Kuhmann, P. A. Marx, J. D. Lifson, J. Dufour, M. Mefford, I. Pandrea, S. M. Wolinsky, R. W. Doms, J. A. DeMartino, S. J. Siciliano, K. Lyons, M. S. Springer, and J. P. Moore. 2003. Use of a small molecule CCR5 inhibitor in macaques to treat simian immunodeficiency virus infection or prevent simian-human immunodeficiency virus infection. J. Exp. Med. 198:1551-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wei, X., S. K. Ghosh, M. E. Taylor, V. A. Johnson, E. A. Emini, P. Deutsch, J. D. Lifson, S. Bonhoeffer, M. A. Nowak, and B. H. Hahn. 1995. Viral dynamics in human immunodeficiency virus type 1 infection. Nature 373:117-122. [DOI] [PubMed] [Google Scholar]
- 73.Wong, J. K., M. Hezareh, H. F. Gunthard, D. V. Havlir, C. C. Ignacio, C. A. Spina, and D. D. Richman. 1997. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 278:1291-1295. [DOI] [PubMed] [Google Scholar]
- 74.Zhang, L., B. Ramratnam, K. Tenner-Racz, Y. He, M. Vesanen, S. Lewin, A. Talal, P. Racz, A. S. Perelson, B. T. Korber, M. Markowitz, and D. D. Ho. 1999. Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. N. Engl. J. Med. 340:1605-1613. [DOI] [PubMed] [Google Scholar]
- 75.Zhu, T., D. Muthui, S. Holte, D. Nickle, F. Feng, S. Brodie, Y. Hwangbo, J. I. Mullins, and L. Corey. 2002. Evidence for human immunodeficiency virus type 1 replication in vivo in CD14+ monocytes and its potential role as a source of virus in patients on highly active antiretroviral therapy. J. Virol. 76:707-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zink, M. C., A. M. Amedee, J. L. Mankowski, L. Craig, P. Didier, D. L. Carter, A. Munoz, M. Murphey-Corb, and J. E. Clements. 1997. Pathogenesis of SIV encephalitis. Selection and replication of neurovirulent SIV. Am. J. Pathol. 151:793-803. [PMC free article] [PubMed] [Google Scholar]
- 77.Zink, M. C., K. Suryanarayana, J. L. Mankowski, A. Shen, M. Piatak, Jr., J. P. Spelman, D. L. Carter, R. J. Adams, J. D. Lifson, and J. E. Clements. 1999. High viral load in the cerebrospinal fluid and brain correlates with severity of simian immunodeficiency virus encephalitis. J. Virol. 73:10480-10488. [DOI] [PMC free article] [PubMed] [Google Scholar]