Abstract
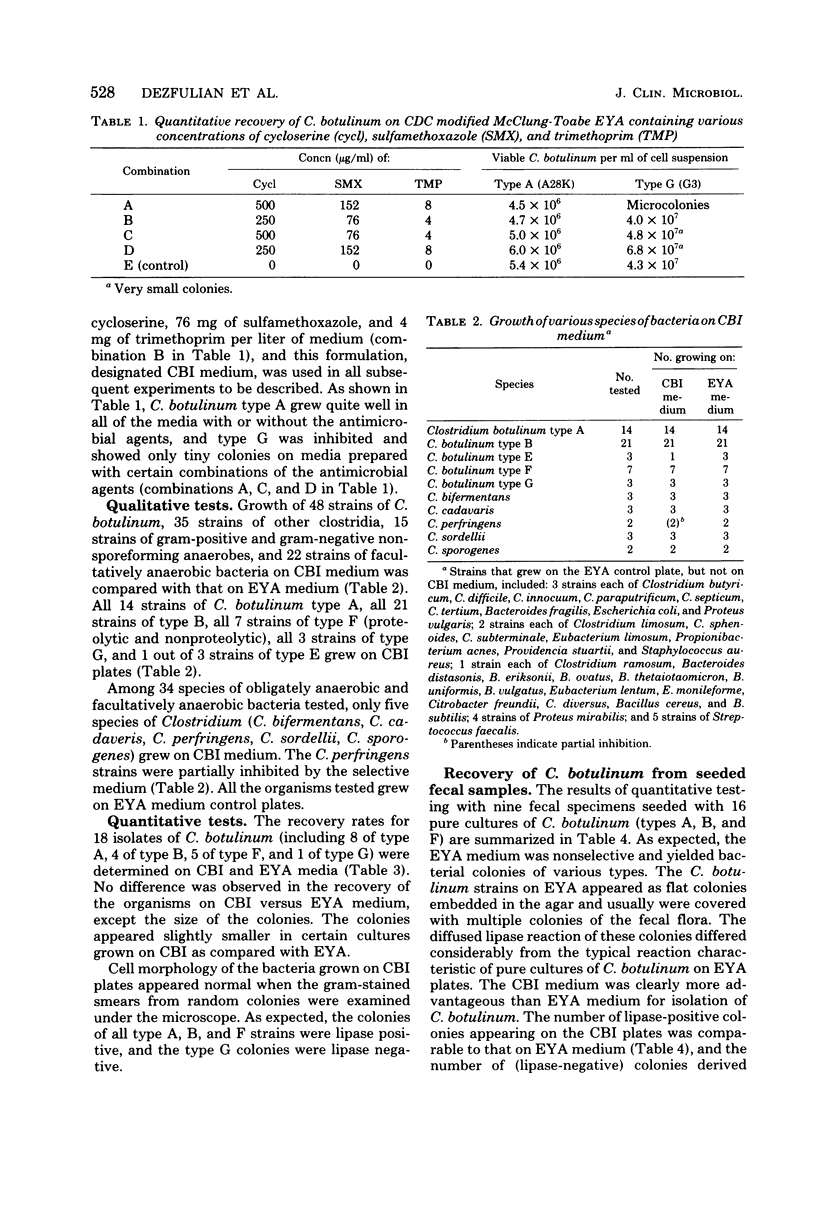
A selective medium, Clostridium botulinum isolation (CBI) agar, was developed for the isolation of C. botulinum from human feces. This medium contains cycloserine (250 microgram/ml), sulfamethoxazole (76 microgram/ml), and trimethoprim (4 microgram/ml) as selective inhibitory agents. Qualitative tests indicated complete recovery of C. botulinum types A, B, F, and G on CBI medium. It was more difficult to recognize type G colonies on the medium because of their lack of lipase activity. Except for a few species of Clostridium, the growth of other obligate anaerobes and of the facultative anaerobes tested on CBI medium was suppressed. Quantitative studies of C. botulinum on the selective medium yielded counts comparable to those obtained on egg yolk agar control plates. Isolation of C. botulinum types A, B, and F from seeded fecal specimens was easily achieved with CBI medium. The use of CBI agar should aid the rapid isolation of C. botulinum from fecal specimens associated with foodborne and infant botulism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ciccarelli A. S., Whaley D. N., McCroskey L. M., Gimenez D. F., Dowell V. R., Jr, Hatheway C. L. Cultural and physiological characteristics of Clostridium botulinum type G and the susceptibility of certain animals to its toxin. Appl Environ Microbiol. 1977 Dec;34(6):843–848. doi: 10.1128/aem.34.6.843-848.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezfulian M., Dowell V. R., Jr Cultural and physiological characteristics and antimicrobial susceptibility of Clostridium botulinum isolates from foodborne and infant botulism cases. J Clin Microbiol. 1980 Jun;11(6):604–609. doi: 10.1128/jcm.11.6.604-609.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell V. R., Jr, McCroskey L. M., Hatheway C. L., Lombard G. L., Hughes J. M., Merson M. H. Coproexamination for botulinal toxin and clostridium botulinum. A new procedure for laboratory diagnosis of botulism. JAMA. 1977 Oct 24;238(17):1829–1832. [PubMed] [Google Scholar]
- George W. L., Sutter V. L., Citron D., Finegold S. M. Selective and differential medium for isolation of Clostridium difficile. J Clin Microbiol. 1979 Feb;9(2):214–219. doi: 10.1128/jcm.9.2.214-219.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giménez D. F., Ciccarelli A. S. Another type of Clostridium botulinum. Zentralbl Bakteriol Orig. 1970;215(2):221–224. [PubMed] [Google Scholar]
- Gunn B. A., Ohashi D. K., Gaydos C. A., Holt E. S. Selective and enhanced recovery of group A and B streptococci from throat cultures with sheep blood agar containing sulfamethoxazole and trimethoprim. J Clin Microbiol. 1977 Jun;5(6):650–655. doi: 10.1128/jcm.5.6.650-655.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatheway C. L. Laboratory procedures for cases of suspected infant botulism. Rev Infect Dis. 1979 Jul-Aug;1(4):647–651. doi: 10.1093/clinids/1.4.647. [DOI] [PubMed] [Google Scholar]
- Koch A. E., Burchall J. J. Reversal of the antimicrobial activity of trimethoprim by thymidine in commercially prepared media. Appl Microbiol. 1971 Nov;22(5):812–817. doi: 10.1128/am.22.5.812-817.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midura T. F., Arnon S. S. Infant botulism. Identification of Clostridium botulinum and its toxins in faeces. Lancet. 1976 Oct 30;2(7992):934–936. doi: 10.1016/s0140-6736(76)90894-1. [DOI] [PubMed] [Google Scholar]
- Moss C. W., Hatheway C. L., Lambert M. A., McCroskey L. M. Production of phenylacetic and hydroxyphenylacetic acids by clostridium botulinum type G. J Clin Microbiol. 1980 Jun;11(6):743–745. doi: 10.1128/jcm.11.6.743-745.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skirrow M. B. Campylobacter enteritis: a "new" disease. Br Med J. 1977 Jul 2;2(6078):9–11. doi: 10.1136/bmj.2.6078.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson J. M., Thornsberry C., McCroskey L. M., Hatheway C. L., Dowell V. R., Jr Susceptibility of Clostridium botulinum to thirteen antimicrobial agents. Antimicrob Agents Chemother. 1980 Jul;18(1):13–19. doi: 10.1128/aac.18.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcke B. W., Jr, Midura T. F., Arnon S. S. Quantitative evidence of intestinal colonization by Clostridium botulinum in four cases of infant botulism. J Infect Dis. 1980 Apr;141(4):419–423. doi: 10.1093/infdis/141.4.419. [DOI] [PubMed] [Google Scholar]
- Willey S. H., Bartlett J. G. Cultures for Clostridium difficile in stools containing a cytotoxin neutralized by Clostridium sordellii antitoxin. J Clin Microbiol. 1979 Dec;10(6):880–884. doi: 10.1128/jcm.10.6.880-884.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


