Abstract
Simian virus 40 large T antigen (TAg) contributes to cell transformation, in part, by targeting two well-characterized tumor suppressors, pRb and p53. TAg expression affects the transcriptional circuits controlled by Rb and by p53. We have performed a microarray analysis to examine the global change in gene expression induced by wild-type TAg (TAgwt) and TAg mutants, in an effort to link changes in gene expression to specific transforming functions. For this analysis we have used enterocytes from the mouse small intestine expressing TAg. Expression of TAgwt in the mouse intestine results in hyperplasia and dysplasia. Our analysis indicates that practically all gene expression regulated by TAg in enterocytes is dependent upon its binding and inactivation of the Rb family proteins. To further dissect the role of the Rb family in the induction of intestinal hyperplasia, we have screened several lines of transgenic mice expressing a truncated TAg (TAgN136), which is able to interfere with the Rb pathway but lacks the functions associated with the carboxy terminus of the protein. This analysis confirmed the pivotal association between the Rb pathway and the induction of intestinal hyperplasia and revealed that upregulation of p53 target genes is not associated with the tumorigenic phenotype. Furthermore, we found that TAgN136 was sufficient to induce intestinal hyperplasia, although the appearance of dysplasia was significantly delayed.
The large tumor antigen (TAg) encoded by simian virus 40 (SV40) induces transformation of multiple cell lines as well as tumors in experimental animals, and therefore it serves as an important tool to understand the mechanisms of cellular transformation (1). TAg induces transformation, in part, by binding to and disabling the functions of tumor suppressors such as pRb and p53 (6, 7, 14, 22, 23). The Rb protein family (pRb, p107, and p130) regulates cell cycle entry and progression by repressing the E2F family of transcription factors. This, in turn, blocks the expression of a large collection of cellular genes that are E2F dependent. In the absence of active Rb proteins, E2F-dependent genes are expressed, resulting in S-phase entry and progression through the cell cycle. TAg is thought to stimulate cell proliferation by blocking the ability of pRb, p107, and p130 to repress E2F-dependent transcription. The loss of Rb-mediated growth suppression often results in the stabilization of p53 and consequently in a large increase in p53 steady-state levels. Under normal circumstances, this would lead to the expression of p53-dependent genes that arrest cell cycle progression and induce apoptosis. However, TAg binds to p53 and blocks its ability to stimulate gene expression. Thus, TAg-expressing cells proliferate and survive under conditions that would result in growth arrest and/or apoptosis of nontransformed cells.
While the roles of TAg in blocking pRb and p53 functions in SV40-mediated transformation are well established, it is also clear that action on additional cellular factors is sometimes required. For example, TAg interactions with Bub1, Cul7, and CBP/p300 have been implicated in SV40-mediated transformation (2, 5, 24). Furthermore, the SV40-encoded small TAg, which targets the cellular protein phosphatase pp2A, is required for transformation under some conditions (27).
Mouse intestinal epithelial cells offer a useful model for understanding how TAg induces neoplastic transformation. The mouse intestinal epithelium is organized into numerous finger-like projections, the villi, and the structures responsible for their renewal, the crypts of Lieberkühn. Intestinal villi and crypts are localized in different regions of the tissue and therefore can be readily isolated from the underlying submucosa and muscularis. This allows us to prepare proteins or nucleic acids from cell populations greatly enriched for nonproliferating, terminally differentiated cells located in the villi or from their proliferating, multipotent progenitors located in the crypts (20, 33).
We and others have previously described the generation of a series of transgenic mice expressing wild-type TAg (TAgwt) or TAg mutants (TAg1137, TAg3213, and TAgD44N) in terminally differentiated enterocytes, using the intestinal fatty acid binding protein promoter (Fig. 1) (12, 15, 16, 26). Under these conditions, expression of TAg extends from the base of the villi to the apical extrusion zone of the villi (12). These transgenic lines do not express detectable levels of small TAg (4). Expression of TAgwt results in intestinal hyperplasia that progresses to dysplasia by 4 to 6 months of age, while TAg3213, an LXCXE motif mutant unable to bind to the Rb family of proteins, and TAgD44N, a J domain mutant unable to inactivate Rb family members, do not result in any observable phenotype (15, 26). Thus, TAg-mediated transformation of enterocytes requires pRb binding and a functional J domain. Furthermore, the ability of TAgwt to induce ectopic enterocyte proliferation depends on the presence of E2F2 (28). Surprisingly, p53 is not stabilized by TAg in enterocytes, nor do TAg-p53 interactions play any role in enterocyte transformation (20).
FIG. 1.
Domain maps of SV40 TAgwt and mutants, showing the J domain (J), Rb protein (pRb, p130, and p107) binding motif (LXCXE), nuclear localization signal (NLS), origin binding domain (OBD), Zn domain (Zn), ATPase domain (ATPase), and host range domain (HR). Gray circles represent cellular proteins interacting with TAg or mutants. The phenotypic effects of expression of TAg or the mutants in intestinal enterocytes are indicated.
We have also characterized a single line of transgenic mice expressing TAg1137, a truncated TAg that is capable of inactivating Rb proteins and stimulating cell proliferation. Like TAgwt, TAg1137 induces enterocyte proliferation and intestinal hyperplasia (15). However, these mice did not progress to dysplasia, suggesting a role for the carboxy-terminal portion of TAg in tumorigenic progression. However, only one line of TAg1137 mice was analyzed, and it is therefore possible that the failure to progress to dysplasia was specific to this line. In this study we report the characterization of independent lines of mice expressing an amino-terminally truncated TAg mutant, and we compare the gene expression patterns from transgenic mice expressing TAgwt or different mutants of TAg.
MATERIALS AND METHODS
Production and maintenance of transgenic mice.
Transgenic mice expressing SV40 TAgwt under the control of the rat intestinal fatty acid binding protein promoter (pedigree 103) were obtained from Jeff Gordon (16). The construction of transgenic mice expressing SV40 TAgN136, TAg3213, and TAgD44N under the control of the rat intestinal fatty acid binding protein promoter is described elsewhere (26, 28). For simplification, we refer to the mice as TAgwt (Fabpi-SV40 TAgwt), TAgN136 (Fabpi-SV40 TAgN136), TAg3213 (Fabpi-SV40 TAg3213), and TAgD44N (Fabpi-SV40 TAgD44N). All lines were maintained by crosses to nontransgenic FVB mice. The genotype of each mouse was determined by PCR amplification as described previously (28). All mice were between the ages of 2.4 and 5 months.
Preparation of intestinal villi samples by LCM.
The intestine was removed in its entirety from the abdominal cavities of mice, from the exit of the stomach to the entry into the large intestine. The intestines were then opened along their cephalocaudal axis, washed with phosphate-buffered saline, and rolled from the duodenum to the ileum. Rolls from TAgwt and TAg mutant transgenic mice were then processed to isolate villus cell types by laser capture microscopy (LCM). Briefly, the rolls were quickly frozen and stored at −80°C. Frozen sections were cut and immediately dehydrated in graded alcohol solutions. The dried sections were examined microscopically, and the top two-thirds of 50 villous segments from each section were collected using a laser capture microscope (Pix Cell II; Arcturus, Mountain View, CA). The captured tissue was extracted in Pico Pure extraction buffer (Arcturus) according to the manufacturer's directions and stored at −80°C.
RNA extraction.
For the microarray experiments, total RNA was isolated from LCM-prepared villi samples with the PicoPure kit (Arcturus) and amplified twice with the RiboAmp kit (Arcturus). Independent RNA preparations were made from three nontransgenic mice and from three TAgD44N, three TAg3213, four TAgwt, and five TAgN136 transgenic mice.
Microarrays.
Amplified total RNA was sent to the Genomics and Proteomics Core Laboratories (University of Pittsburgh) for hybridization to the Mouse 430 2.0 whole genome array (Affymetrix), which contains 45,101 probe sets representing 21,635 unique genes. CEL files for each array were converted into RMA expression values using BRB-Array Tools (Rich Simon, National Cancer Institute; http://linus.nci.nih.gov/BRB-ArrayTools.html). An average fold change ratio (experimental/control) and a one-sample t test were calculated for each probe set.
Microarray analysis.
The Affymetrix mouse whole-genome chip consisting of 21,635 unique genes was used for microarray analysis. Genes which are threefold up- or downregulated in an individual experimental class (TAgwt/TAgN136/TAg3213/TAgD44N) were selected for further analysis. We applied additional criteria of present and absent calls provided by Affymetrix data files. To be included for consideration, an upregulated gene needed to be present in all the replicates of an experimental class and a downregulated gene needed to be present in all the replicates of the control class (nontransgenic mice).
To analyze the effect of individual domains/regions of TAg on gene expression, we did independent comparisons between TAgwt and each mutant. Genes with a TAgwt/control ratio of greater than or equal to 3 and a TAg mutant/control ratio of less than 1.2 were considered upregulated by TAg but not by the mutant. Genes upregulated by the mutant were identified similarly. Furthermore, genes with a TAgwt/control ratio of less than or equal to 0.33 and a mutant/control ratio of greater than 0.8 were considered downregulated by TAgwt but not by the mutant. Genes downregulated by the mutant were identified similarly. This strict selection criterion was used in order to identify genes which are up- or downregulated by one class but not by the other. In addition, we have considered present and absent calls as described above.
Reverse transcription-PCR (RT-PCR) analysis.
cDNA synthesis from 1 μg of total RNA was performed using Superscript II reverse transcriptase (Invitrogen). PCR was performed with equal amounts of cDNA using GoTaq polymerase (Promega) for 25 cycles with specific primers for the different transcripts. Amplification with primers for the alcohol dehydrogenase 5 (Adh5) transcript was used as a normalizing control. PCR products were resolved through a 2% agarose gel in 1× Tris-acetate-EDTA and stained with GelStar (Cambrex Bio Science). The cDNAs were amplified with PCR using primers specific for Adh5 (5′-TGCACCACCAACTGCTTAG and 5′-GATGCAGGGATGATGTTC), Fas (5′-GCCGAATGTCGCAGAACCTTAG and 5′-CAGGAGTTGCCAATGTCAATACAG), Lrdd (5′-GAGAGCAGGTTGCCCATACTTG and 5′-GAGCCATCAGGTAGGTAGAGGAAAC), Noxa (5′-TTGTTTGGAGACAAGGGTCCC and 5′-GACCATAAATACCCATTGGGCAAG), and Pten (5′-ATTTGGTCACCCTTTGAGTCCTC and 5′-AGTTTGTCTTGTTGTTAGCCCACC).
To ensure that these reactions were within the linear range of the assay, we optimized the number of cycles required to obtain nonsaturated signals. Exponential amplifications of PCR products were obtained as follows: 2 min at 94°C; a series of 25 cycles of 94°C for 30 s, variable annealing temperatures for 30 s, and 72°C for 30 s; and a final extension step of 5 min at 72°C. The annealing temperatures and product sizes were as follows: 58°C and 152 bp for Adh5, 54.4°C and 204 bp for Fas, 55.7°C and 151 bp for Lrdd, 52.8°C and 189 bp for Noxa, and 52.9°C and 231 bp for Pten. The products were resolved on 2% agarose gels and stained with GelStar (BioWhittaker Molecular Applications).
Immunoblot analysis.
Material enriched in intestinal villi and crypts was prepared and analyzed by Western blotting as described previously (20). An appropriate dilution of TAg-mouse monoclonal PAb416 (11) was used as a primary antibody. Goat anti-mouse antibody linked to peroxidase (Sigma A2554) was used as a secondary antibody and developed with ECL (GE Healthcare) according to the manufacturer's instructions.
Immunohistochemistry.
Small intestines were processed for immunohistochemistry as described elsewhere (20). Assessment of the proliferative status was obtained after mice were given an intraperitoneal injection of 5-bromo-2′-deoxyuridine (BrdU) (Sigma) (120 mg/kg of body weight) 2 hours prior to their sacrifice. Sections were stained with monoclonal rat BU1/75 (dilution, 1:2,000) (Accurate Scientific, Westbury, NY). Antigen-antibody complexes were detected with an ABC Elite Kit Rat (Vector Labs, Burlingame, CA), and development of the peroxidase reaction was performed with diaminobenzidine substrate (DAKO). Stained sections of murine intestines were photographed under a Nikon FXA microscope (original magnification, ×200).
Detection of TAg proteins was performed in 5-μm-thick histological sections using appropriate dilutions of the anti-TAg antibody PAb419 (for TAgwt) (11) and PAb416 (for TAgN136) as primary antibodies. Biotinylated anti-mouse-Fab was used as the secondary antibody, followed by use of the streptavidin-peroxidase ARK kit plus development of the peroxidase reaction with diaminobenzidine substrate (DAKO), according to the manufacturer's instructions.
Microarray data accession number.
The microarray data have been submitted to GEO under accession number GSE16389.
RESULTS
Global patterns of cellular gene expression in villus enterocytes of transgenic mice expressing TAgwt or mutant TAgs.
The intestinal villus enterocytes are composed mainly of nonproliferating, terminally differentiated cells. Expression of full-length TAg in murine enterocytes forces a nonproliferating cell population to restart the cell cycle and results in intestinal hyperplasia that progresses to dysplasia as the animal ages. We have analyzed the RNA profile of villus enterocytes of transgenic mice expressing TAgwt or mutants using microarray technology. The Affymetrix GeneChip Mouse Genome 430 2.0 Array platform consisting of 21,635 unique genes was used for the microarray analysis. Genes whose expression levels changed at least threefold up or down relative to control nontransgenic enterocytes were considered to be regulated by TAg. Enterocytes isolated from TAgwt mice showed upregulation of 291 genes and downregulation of 140 genes in comparison to those from their nontransgenic littermates, while those isolated from TAgN136 mice showed upregulation of 274 genes and downregulation of 197 genes. In contrast, TAg3213 mice showed upregulation of only 19 genes and downregulation of 28 genes, while TAgD44N mice showed upregulation of 20 genes and downregulation of 61 genes. Thus, mutation of either the LXCXE motif or the J domain results in a large reduction of the number of genes upregulated and downregulated by TAg. These data indicate that nearly all TAg-mediated gene regulation in enterocytes requires a functional J domain and LXCXE motif.
TAgwt and TAgN136 regulate similar sets of cellular genes.
The J domain and LXCXE motif of TAg act in cis to disrupt Rb-E2F complexes and to activate E2F-dependent transcription. Amino-terminal fragments of TAg possessing both of these elements are sufficient for this action both in vitro and in vivo (28, 31). We compared the global patterns of cellular gene expression in enterocytes expressing TAgwt and TAgN136 with those in enterocytes expressing TAgD44N and TAg3213 by hierarchical clustering. The average log ratios for the gene probe sets (n = 796) that were threefold up- or downregulated in at least one class of mice (TAgwt, TAgN136, TAg3213, and TAgD44N) were clustered using the Euclidean distance metric and complete linkage (Fig. 2A).
FIG. 2.
Hierarchical clustering of regulated genes reveals two distinct patterns of gene expression. (A) The average log ratios for the probe sets (n = 796) that were threefold upregulated or downregulated by at least one class were clustered using the Euclidean distance metric and complete linkage. The classes are listed at the top of the cluster diagram (left). Probe sets colored red are upregulated (green, downregulated) relative to normal villi. (B) Three groups of probe sets (347 total) showed a pattern of being upregulated in mice expressing TAgwt and TAgN136 but not TAgD44N and TAg3213 (red bar). (C) Conversely, there are two groups of probe sets (44 total) that are downregulated by TAgwt and TAgN136 but are not changed in TAgD44N and TAg3213 (green bar). The line plots for these groups are shown on the right, where the average log ratio is plotted on the y axis.
Three groups of probe sets (347 total) show a pattern of upregulation in mice expressing TAgwt and TAgN136 but not in those expressing TAgD44N and TAg3213 (Fig. 2B). The majority of the genes in these upregulated clusters are involved in replication, mitosis/cytokinesis, transcriptional control, and DNA repair. A list of some of the genes in the major categories are shown in Table 1. Two groups of probe sets (44 total) were downregulated by TAgwt and TAgN136 but were not changed by TAgD44N and TAg3213 (Fig. 2C). The downregulated cluster includes genes belonging to the transmembrane, transcriptional control, immune response, and signaling classes.
TABLE 1.
Genes regulated by TAgwt and TAgN136 grouped by ontologya
| Category and gene description | AFFY symbol |
|---|---|
| Genes upregulated by Tagwt and TAgN136 | |
| Replication | |
| Minichromosome maintenance deficient 5, cell division cycle | Mcm5 |
| 46 (Saccharomyces cerevisiae) | |
| Minichromosome maintenance deficient 7 (S. cerevisiae) | Mcm7 |
| Minichromosome maintenance deficient 2 mitotin | Mcm2 |
| (S. cerevisiae) | |
| Flap structure specific endonuclease 1 | Fen1 |
| Ribonucleotide reductase M1 | Rrm1 |
| Ribonucleotide reductase M2 | Rrm2 |
| Minichromosome maintenance deficient 4 homolog | Mcm4 |
| (S. cerevisiae) | |
| Minichromosome maintenance deficient 6 (MIS5 homolog, | Mcm6 |
| Schizosaccharomyces pombe) (S. cerevisiae) | |
| Thymidine kinase 1 | Tk1 |
| Replication protein A2 | Rpa2 |
| Ligase I, DNA, ATP dependent | Lig1 |
| Proliferating cell nuclear antigen | Pcna |
| Polymerase (DNA directed), alpha 1 | Pola1 |
| Minichromosome maintenance deficient 3 (S. cerevisiae) | Mcm3 |
| Replication factor C (activator 1) 4 | Rfc4 |
| Topoisomerase (DNA) II alpha | Top2a |
| DNA primase, p49 subunit | Prim1 |
| Cell division cycle 45 homolog (S. cerevisiae)-like | Cdc45l |
| Geminin | Gmnn |
| GINS complex subunit 4 (Sld5 homolog) | Gins4 |
| DNA primase, p58 subunit | Prim2 |
| Deoxyuridine triphosphatase | Dut |
| Replication protein A1 | Rpa1 |
| Polymerase (DNA directed), epsilon 4 (p12 subunit) | Pole4 |
| Polymerase (DNA directed), epsilon 2 (p59 subunit) | Pole2 |
| Lin-9 homolog (Caenorhabditis elegans) | Lin9 |
| GINS complex subunit 2 (Psf2 homolog) | Gins2 |
| Minichromosome maintenance deficient 8 (S. cerevisiae) | Mcm8 |
| Minichromosome maintenance deficient 10 (S. cerevisiae) | Mcm10 |
| Deoxycytidine kinase | Dck |
| Replication protein A3 | Rpa3 |
| Bloom syndrome homolog (human) | Blm |
| Deoxycytidine kinase | Dck |
| DNA2 DNA replication helicase 2-like (yeast) | Dna2l |
| GINS complex subunit 1 (Psf1 homolog) | Gins1 |
| Deoxythymidylate kinase | Dtymk |
| Replication factor C (activator 1) 5 | Rfc5 |
| Replication factor C (activator 1) 2 | Rfc2 |
| Origin recognition complex, subunit 6-like (S. cerevisiae) | Orc6l |
| Topoisomerase (DNA) II beta binding protein | Topbp1 |
| Topoisomerase (DNA) II alpha | Top2a |
| Mitosis/cytokinesis | |
| Stathmin 1 | Stmn1 |
| Cell division cycle 20 homolog (S. cerevisiae) | Cdc20 |
| Cyclin-dependent kinase inhibitor 2C (p18, inhibits CDK4) | Cdkn2c |
| Budding uninhibited by benzimidazoles 1 homolog | Bub1 |
| (S. cerevisiae) | |
| Cyclin A2 | Ccna2 |
| Cyclin B1 | Ccnb1 |
| Antigen identified by monoclonal antibody Ki 67 | Mki67 |
| Kinesin family member 2C | Kif2c |
| Cyclin B2 | Ccnb2 |
| Cell division cycle 6 homolog (S. cerevisiae) | Cdc6 |
| Cyclin E1 | Ccne1 |
| Cell division cycle 2 homolog A (S. pombe) | Cdc2a |
| Kinesin family member 22 | Kif22 |
| Cytoskeleton associated protein 2 | Ckap2 |
| Thymopoietin | Tmpo |
| CDC28 protein kinase regulatory subunit 2 | Cks2 |
| Kinesin family member 23 | Kif23 |
| Inner centromere protein | Incenp |
| Kinesin family member 11 | Kif11 |
| Polo-like kinase 4 (Drosophila) | Plk4 |
| Centromere autoantigen A | Cenpa |
| Cell division cycle associated 2 | Cdca2 |
| Cell division cycle associated 3 | Cdca3 |
| Cell division cycle associated 5 | Cdca5 |
| Cell division cycle associated 8 | Cdca8 |
| Protein regulator of cytokinesis 1 | Prc1 |
| Anillin, actin binding protein (scraps homolog, Drosophila) | Anln |
| Nucleolar and spindle-associated protein 1 | Nusap1 |
| NIMA (never in mitosis gene a)-related expressed kinase 2 | Nek2 |
| MAD2 (mitotic arrest deficient, homolog)-like 1 (yeast) | Mad2l1 |
| Thymopoietin | Tmpo |
| Transcriptional control/chromatin | |
| H2A histone family, member X | H2afx |
| Retinoblastoma-like 1 (p107) | Rbl1 |
| SMC2 structural maintenance of chromosomes 2-like 1 | Smc2l1 |
| (yeast) | |
| E2F transcription factor 2 | E2f2 |
| Enhancer of zeste homolog 2 (Drosophila) | Ezh2 |
| High-mobility-group box 2 | Hmgb2 |
| DNA methyltransferase (cytosine-5) 1 | Dnmt1 |
| Transcription factor 19 | Tcf19 |
| ASF1 anti-silencing function 1 homolog B (S. cerevisiae) | Asf1b |
| Histone aminotransferase 1 | Hat1 |
| Chromobox homolog 5 (Drosophila HP1a) | Cbx5 |
| Thyroid hormone receptor interactor 13 | Trip13 |
| SMC4 structural maintenance of chromosomes 4-like 1 | Smc4l1 |
| (yeast) | |
| DEK oncogene (DNA binding) | Dek |
| Lamin B receptor | Lbr |
| Retinoblastoma-like 1 (p107) | Rbl1 |
| DNA repair | |
| Meiotic recombination 11 homolog A (S. cerevisiae) | Mre11a |
| MutS homolog 6 (Escherichia coli) | Msh6 |
| Exonuclease 1 | Exo1 |
| Breast cancer 2 | Brca2 |
| RAD54-like (S. cerevisiae) | Rad54l |
| Timeless homolog (Drosophila) | Timeless |
| RAD51-associated protein 1 | Rad51ap1 |
| Denticleless homolog (Drosophila) | Dtl |
| Checkpoint kinase 1 homolog (S. pombe) | Chek1 |
| Topoisomerase (DNA) II beta binding protein | Topbp1 |
| Claspin homolog (Xenopus laevis) | Clspn |
| Ubiquitin-like, containing PHD and RING finger domains, 1 | Uhrf1 |
| RAD51 homolog (S. cerevisiae) | Rad51 |
| Breast cancer 1 | Brca1 |
| Timeless-interacting protein | Tipin |
| Apoptosis | |
| Caspase 8-associated protein 2 | Casp8ap2 |
| Genes downregulated by Tagwt and TAgN136 | |
| Transmembrane | |
| Secreted and transmembrane 1 | Sectm1 |
| Aquaporin 1 | Aqp1 |
| Protein tyrosine phosphatase, receptor type, E | Ptpre |
| Solute carrier family 5 (neutral amino acid transporters, | Slc5a4b |
| system A), member 4b | |
| Inositol 1,4,5-triphosphate receptor 2 | Itpr2 |
| Adenosine A2b receptor | Adora2b |
| Grina | |
| Solute carrier family 5 (sodium/glucose cotransporter), | Slc5a12 |
| member 12 | |
| Integrin, alpha E, epithelium associated | Itgae |
| F-box only protein 32 | Fbxo32 |
| Transcriptional control | |
| Laminin, alpha 3 | Lama3 |
| Avian musculoaponeurotic fibrosarcoma (v-maf) AS42 | Maf |
| oncogene homolog | |
| High-mobility group box transcription factor 1 | Hbp1 |
| Signaling | |
| 5′-Nucleotidase, ecto | Nt5e |
| Tensin 4 | Tns4 |
| Insulin receptor substrate 2 | Irs2 |
| Oxidoreductase | |
| Aldo-keto reductase family 1, member B7 | Akr1b7 |
| Leukotriene B4 12-hydroxydehydrogenase | Ltb4dh |
| Immune response | |
| Mannose binding lectin (C) | Mbl2 |
| Tumor necrosis factor (ligand) superfamily, member 13b | Tnfsf13b |
| CD36 antigen | Cd36 |
Genes in the upregulated cluster from Fig. 2 include genes involved in the control of cell growth and division, such as DNA replication, mitosis, chromatin remodeling, and DNA repair. Genes in the downregulated cluster from Fig. 2 include genes involved in transmembrane signaling, transcriptional control, and immune response.
To further determine the effect of individual domains/regions of TAg on gene expression, we did independent comparisons between each mutant and TAgwt (Fig. 3). Genes with a TAgwt/control ratio of greater than or equal to 3 and a TAg mutant/control ratio of less than 1.2 were considered upregulated by TAg but not by the mutant. Genes upregulated by the mutant were identified similarly. Furthermore, genes with a TAgwt/control ratio of less than or equal to 0.33 and a mutant/control ratio of greater than 0.8 were considered downregulated by TAgwt but not by the mutant. Genes downregulated by the mutant were identified similarly. We used the DAVID 2.0 bioinformatics resource (http://david.abcc.ncifcrf.gov/home.jsp) to group the identified genes into different biological classes.
FIG. 3.
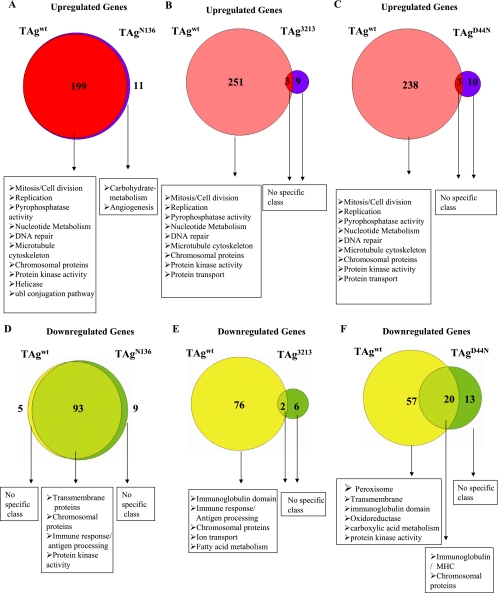
The majority of gene regulation by TAgwt requires the LXCXE motif and J domain. In each Venn diagram, the intersection represents the number of genes that are commonly upregulated (A, B, and C) or downregulated (D, E, and F) threefold or more by TAgwt, TAgN136, TAg3213, or TAgD44N. The left circle represents genes which are uniquely upregulated or downregulated by TAgwt, while the right circle represents genes which are uniquely upregulated or downregulated by TAgN136, TAg3213, or TAgD44N (see Materials and Methods for details). Genes were assigned to different biological classes using the DAVID 2.0 bioinformatics resource. Biological classes are sorted to show genes with low P values (high enrichment score) in the first position of the list.
Comparison of the gene expression patterns between TAgwt and TAgN136 revealed that all the genes upregulated by TAgwt (199) are also upregulated by TAgN136 (Fig. 3A). These genes are associated with E2F-dependent transcription, DNA replication and repair, cell cycle regulation, chromatin assembly/modification, and the microtubule cytoskeleton (Table 1). A small number of genes (11) were upregulated by TAgN136 but not by TAgwt. These belonged to biological classes such as carbohydrate metabolism and angiogenesis. Similarly, there was a large overlap (93 genes) between genes downregulated by TAgwt and TAgN136 (Fig. 3D). These genes belong to different biological classes, such as transmembrane proteins, chromosomal proteins, immune response/antigen processing, and protein kinases (Table 1).
Comparison of the gene expression patterns between TAgwt and TAg3213 revealed that TAg3213 expression does not affect the genes (251) upregulated by TAgwt (Fig. 3B), and therefore there is not a significant overlap of genes regulated by both TAgwt and TAg3213. In fact, no specific class of genes was uniquely upregulated by TAg3213, indicating that gene upregulation by TAgwt in enterocytes requires an intact LXCXE motif. A total of 76 genes are uniquely downregulated by TAgwt (Fig. 3E). These genes belong to the class of immunoglobulins, immune response/antigen processing, chromatin/histones, ion transport, and fatty acid metabolism. Only a few genes (6) were uniquely downregulated by TAg3213, but they do not belong to any specific class. As observed with the upregulated genes, there was no significant overlap between the genes downregulated by TAgwt and TAg3213.
The comparison between gene upregulation in TAgwt and TAgD44N shows a pattern similar to that observed in the comparison between TAgwt and TAg3213. TAgD44N mice do not show regulation of the genes (238) upregulated by TAgwt (Fig. 3C), nor did we find any specific class of genes uniquely upregulated by TAgD44N. Therefore, we did not find any significant overlap of genes between TAgwt and TAgD44N, suggesting that all gene upregulation by TAgwt in enterocytes requires an intact J domain. We found a group of genes commonly downregulated by both TAgwt and TAgD44N belonging to the class of major histocompatibility complex and chromatin/histones, suggesting J domain-independent regulation of these genes. A total of 57 genes are uniquely downregulated by TAgwt (Fig. 3F). These genes belong to the class of peroxisomal proteins, transmembrane proteins, immunoglobulins, oxidoreductase, carboxylic acid metabolism, and protein kinases. Additionally, 13 genes are uniquely downregulated by TAgD44N, but they do not belong to any specific class. If we relax the cutoff for selecting genes from threefold to twofold, we find additional potential genes downregulated by TAgD44N. These genes correspond to nucleic acid metabolism, mitosis, cell cycle, and cell division.
Regulation of E2F-dependent gene expression by TAg in villus enterocytes requires inactivation of the Rb family.
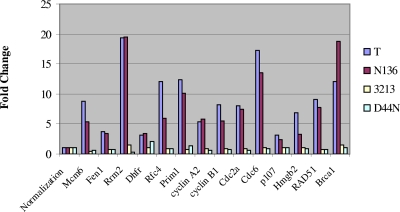
The LXCXE motif and J domain are required for the complete inactivation of Rb family members and thus for the induction of E2F target genes (29, 30, 34). Therefore we hypothesized that TAgN136 should be able to regulate the same set of E2F target genes as TAgwt, while TAg3213 and TAgD44N should be defective for this regulation. Consistent with this hypothesis, our microarray data showed upregulation of E2F target genes that was equal to or greater than threefold in mice expressing either TAgwt or TAgN136 but not in those expressing TAg3213 and TAgD44N (Fig. 4). The array results were confirmed by RT-PCR analysis of a series of selected E2F target genes using RNA isolated from enterocytes expressing TAgwt or mutant TAg proteins (data not shown).
FIG. 4.
Regulation of E2F target genes by TAg and mutants. Mice expressing TAgwt and TAgN136 show significant upregulation of E2F target genes, while mice expressing TAg3213 and TAgD44N do not. The average fold change of the genes was determined by microarray analysis of each category (n = 3): TAgwt, TAgN136, TAg3213, and TAgD44N. “Normalization” represents a fold change of 1 (not regulated).
Induction of intestinal hyperplasia by TAg does not involve regulation of p53-dependent genes.
In many cell culture and transgenic systems, TAgwt binds the tumor suppressor p53 and blocks p53-dependent transcription (3, 13, 17, 18). In contrast, the expression of amino-terminal truncations of TAg (such as TAgN136) usually results in p53 stabilization and the activation of p53-dependent transcription and growth suppression (21). However, we have previously shown that enterocytes do not express detectable levels of p53, even in the presence of TAg, and that the interaction between TAg and p53 does not play a role in TAg-mediated enterocyte transformation (20). Consistent with these observations, the array experiments did not show any evidence to indicate that p53-target genes are regulated by TAgwt or any of the mutants (data not shown). We confirmed the expression levels of selected p53 target genes (Fas, Lrdd, Noxa, and Pten) by semiquantitative RT-PCR analysis (Fig. 5). Nontransformed mouse embryo fibroblasts (MEFs) and MEFs expressing TAgwt or TAgN136 were used as controls. As expected, all the p53 target genes were significantly upregulated in MEFs expressing TAgN136 (lane 3) in comparison to control MEFs (lane 1) and MEFs expressing TAgwt (lane 2). In contrast, none of the p53 target genes tested was regulated by TAgwt (lane 5) or mutant TAg (lanes 6 to 8) proteins in enterocytes.
FIG. 5.
p53 target genes are not affected in villus enterocytes expressing TAg and mutants. Transcript levels of p53 target genes in nontransgenic (NT), TAgwt, TAgN136, TAg3213, and TAgD44N villus samples were analyzed. Normal MEFs (control) and MEFs expressing TAgwt and TAgN136 were used to show the presence of active p53 in MEFs. cDNAs were reverse transcribed from equal amounts of total RNA extracts and subjected to PCR using specific primers. The transcript level of Adh5 was used as a loading control.
Transgenic mice expressing an amino-terminal truncated TAg develop hyperplasia and show signs of dysplasia.
Comparison of the corresponding gene expression patterns suggests that in villus enterocytes, TAgN136 regulates almost the same set of genes as TAgwt. Based on this observation, we predicted that mice expressing TAgN136 will show the same intestinal phenotype as TAgwt. To test this, we screened TAgwt and TAgN136 mice for their capability to induce neoplasia.
Mice expressing TAgwt in the intestinal enterocytes develop hyperplasia by 6 weeks after birth, a phenotype that becomes dysplastic by 4 to 6 months (12, 15, 20). Additionally, these mice show a significant increase in the length of their intestines and in the size of intestinal crypts (20). We have previously reported that a line of transgenic mice expressing one amino-terminal truncation of TAg in enterocytes, TAg1137, also develops hyperplasia. However, these mice did not show progression to intestinal dysplasia or increased intestinal length or crypt size (15). We considered two possible explanations for the different phenotypes in TAgwt and TAg1137 mice. First, one or more TAg functions mapping to the carboxy-terminal end of the molecule could be required for progression to dysplasia and/or increased intestinal length and crypt size. The second possibility is that the failure to display dysplasia and increased intestinal length was specific to the single line of TAg1137 mice used in the study.
To distinguish these possibilities we generated additional lines of transgenic mice expressing a truncated TAg, TAgN136 (Fig. 1). TAgN136 is similar to TAg1137, but it contains a nuclear localization signal. Like TAg1137, TAgN136 is capable of disrupting Rb-E2F complexes and of activating E2F-dependent transcription (28). We confirmed the expression of the N136 protein in transgenic mice by immunostaining and Western blot analysis. Paraffin sections stained with anti-TAg antibody revealed that TAgN136 expression is confined to the top three-fourths of the villi and is not observed in the villus/crypt junction or the top of the crypts (Fig. 6B). TAgwt mice also exhibited TAg expression in the villi, but in this case the staining extended to the villus/crypt junction and the top of the crypts (Fig. 6A and C). These results were confirmed by Western blot analysis of fractionated cell extracts (Fig. 6D). The TAgN136 protein levels remained constant as the animals aged (Fig. 6E).
FIG. 6.
Expression patterns of TAgwt and TAgN136 in villus enterocytes. Intestinal tissues were embedded in paraffin and 5-μm sections were stained with anti-TAg antibodies. A brown color reflects the presence of TAg. Mice from 3 to 6 months of age were analyzed and a representative case is shown. The TAgwt mice present a clear dysplastic phenotype, while TAgN136 mice are hyperplastic. (A) Nuclear expression of TAg in TAgwt mice extends from the bottom three-fourths of villus enterocytes into the top of the crypts. (B) TAgN136 mice express N136 in the nucleus and cytoplasm of villus enterocytes (top three-fourths). (C) Higher magnification showing expression of TAg on the top of the crypts in TAgwt mice. (D) Expression levels of TAg in TAgwt and TAgN136 villus (V) and crypt (C) samples. Protein extracts from villi and crypts of nontransgenic (NT) and TAgwt and TAgN136 transgenic mice were subjected to immunoblotting for TAg. Protein levels for β-tubulin were used as loading controls. (E) Expression levels of N136 protein through development. Protein extracts from the middle intestines of nontransgenic (NT) and TAgwt and TAgN136 transgenic mice were subjected to immunoblotting for TAg. TAgwt mice (4 and 7 months) and TAgN136 mice (4, 9, 12, and 18 months) were used. β-Tubulin was used as a loading control.
To assess the proliferative status of transgenic intestines, mice were injected with BrdU and histological sections were stained with anti-BrdU (Fig. 7). We found numerous BrdU-positive cells in enterocytes of both TAgwt and TAgN136 mice, irrespective of the age tested (Fig. 7B and C). Thus, like TAgwt, TAgN136 induces enterocytes to reenter the cell cycle. TAgN136 mice also develop hyperplasia and progress to dysplasia, although these histological changes progress at a slower pace than in TAgwt mice. We also assessed the intestinal lengths of mice in different age groups (Table 2). As expected, the average intestinal length of TAgwt mice was much greater than that of control mice (51 cm versus 40 cm). Similarly, TAgN136 mice show a significant increase in intestinal length (48 cm) in comparison to nontransgenic littermates (40 cm). Unlike TAgwt mice, TAgN136 mice do not exhibit enlarged crypts. We conclude that like TAgwt, TAgN136 is capable of driving enterocyte proliferation, of inducing hyperplasia, and of increasing intestinal length.
FIG. 7.
TAgN136 induces ectopic proliferation in villus enterocytes. TAgwt and TAgN136 mice (B and C) show ectopic proliferation of enterocytes, while proliferation is restricted to the crypts in nontransgenic mice (A). Intestinal sections were stained with anti-BrdU and show numerous BrdU-positive cells (indicated by arrows) in villus enterocytes of mice expressing TAgwt and TAgN136. Mice from 3 to 6 months of age were analyzed, and a representative case is shown. The TAgwt mice present a clear dysplastic phenotype, while TAgN136 mice are hyperplastic.
TABLE 2.
Intestinal length of mice according to genetic background and age
| Genetic background | Avg total length of small intestine (cm) ± SE (total no. of mice analyzed) for mice at age (mo): |
|||
|---|---|---|---|---|
| 0-3 | 3-6 | 6-9 | ≥9 | |
| Nontransgenic | 39.6 ± 0.45 (70) | 40.3 ± 0.41 (75) | 40.3 ± 0.52 (46) | 40.7 ± 0.61 (35) |
| TAgwt | 49.4 ± 1.15 (61) | 51.5 ± 0.97 (90) | 52.6 ± 1.45 (42) | 52.1 ± 1.75 (29) |
| TAgN136 | 43.6 ± 0.57 (11) | 47.3 ± 1.04 (14) | 50.5 ± 1.21 (15) | 51.4 ± 0.76 (28) |
DISCUSSION
The action of TAg on the Rb family proteins is central to our current understanding of SV40-mediated transformation. Our studies with transgenic mice expressing TAgwt and mutant TAg in enterocytes is consistent with this view. While TAgwt induces enterocyte proliferation, TAg mutants that are defective either for Rb binding (TAg3213) or for chaperone action on Rb-E2F complexes (TAgD44N) fail to do so. Like TAgwt, truncation mutants such as TAg1137and TAgN136, which contain a functional J domain and Rb binding motif, induce enterocyte proliferation. The expression of TAgwt or TAgN136 results in the degradation of p130 and the loss of p130-E2F4 complexes, the upregulation of E2F2 and E2F3a, and increased expression of E2F-dependent genes (28). Finally, the ectopic enterocyte proliferation induced by TAg is dependent on the presence of E2F2 and perhaps E2F3a (28). Small TAg is not expressed in this system (4), and thus all of the observed effects are due to large TAg.
In this study we have used gene microarray experiments to assess the effects of TAgwt and mutant TAg on enterocyte gene expression. We have previously reported that TAgwt alters the levels of a large collection of cellular genes in enterocytes, including an upregulation of E2F-dependent genes (4). In the current study we have used whole-mouse genome arrays to compare mRNA levels in enterocytes expressing TAgwt, TAgN136, TAgD44N, or TAg3213. We found that TAgwt regulates over 400 genes and that essentially all TAg-mediated gene regulation in enterocytes requires a functional J domain and LXCXE motif. In the present study we have used a strict criterion of a threefold cutoff, which likely results in an underestimation of regulated genes. Nevertheless, TAgN136 upregulated the same genes as TAgwt, including E2F-dependent genes. Similarly, almost all of the cellular genes downregulated by TAgwt were also downregulated by TAgN136. Taken together, these results indicate that TAgN136 is fully competent to antagonize Rb proteins and to activate E2F-dependent transcription in intestinal enterocytes.
The fact that all of the DNA tumor viruses alter some aspect of the Rb-E2F axis indicates the central importance of this pathway to cell cycle regulation and tumorigenesis. Thus, each of these viruses would be expected to alter cellular gene expression in a manner similar to that of SV40. However, two factors complicate this prediction. First, each virus exhibits its transforming properties in different host species and/or different cell lineages. For example, we have previously reported that TAgwt regulates unique sets of cellular genes in transformed MEFs compared to mouse enterocytes (4). Second, the transforming proteins encoded by each of the DNA tumor viruses act by unique mechanisms, and each can have specific effects on cellular gene expression. Nevertheless, in an attempt to discern common effects among these different systems, we have compared our results with three other microarray data sets: (i) the so-called TAg signature, obtained by expression of TAg in three different mouse tissues (8); (ii) human papillomavirus (HPV)-positive tumors (25); and (iii) MEFs conditionally depleted of pRb (19). This comparison is illustrated in Table 3.
TABLE 3.
Comparison of genes regulated in three tumorigenic systems and TAgwt-expressing enterocytes
| System | Upregulated genes |
Downregulated genes |
||
|---|---|---|---|---|
| No. of genesa | % Overlap with TAgwt enterocytesb | No. of genesa | % Overlap with TAgwt enterocytesb | |
| TAg signature | 83 | 78 | 33 | 9 |
| HPV+ | 79 | 35 | 10 | 0 |
| Rb−/− | 126 | 67 | 46 | 7 |
Genes up- or downregulated in the TAg signature (Table 1) (8), HPV-positive tumors (25), and MEFs conditionally depleted of pRb (Fig. 7) (19) were matched to genes on the TAgwt enterocyte microarray. A majority of the genes were found.
The percentage represents the overlapping genes between TAgwt-expressing enterocytes and the other three systems at a twofold cutoff.
We found that a relatively large number of genes upregulated by transformation in the three systems were also upregulated by TAgwt in enterocytes. For example, of 83 genes identified as being upregulated in the TAg signature, 78% were also upregulated by TAgwt in enterocytes. This indicates that TAg utilizes some of the same molecular pathways to induce transformation in different systems. Similarly, 35% of the genes upregulated in HPV tumors and 67% of the genes upregulated in MEFs following pRb ablation were also upregulated in TAgwt-expressing enterocytes. Nearly all of these genes are related to cell cycle regulation and E2F-dependent gene transcription. Thus, transformation by TAgwt in different tissues and cell types, transformation by HPV, and the loss of pRb activity all appear to lead to similar changes in gene expression. Nevertheless, each system also harbored a collection of genes that were uniquely upregulated. In contrast, there was little or no overlap of cellular genes downregulated in any of the systems. Therefore, the mechanism of gene repression and, consequently, the specific genes downregulated by transformation appear to depend both on the transforming system and on the cell type.
While TAg action on the Rb-E2F axis is necessary for enterocyte transformation, it is unclear if this action is sufficient. If this were the case, then transformed properties of TAgN136-expressing mice should phenocopy those of mice expressing TAgwt. To test this, we generated several new lines of TAgN136-expressing mice and assessed phenotypic characteristics, including enterocyte proliferation, progression to hyperplasia and/or dysplasia, intestinal length, and crypt size. In addition to hyperproliferation, dysplasia is characterized by the release of the cells from the basement membrane, resulting in multiple cell layers of epithelium as well as branching of the villi, increased crypt size, and overall general disorganization of the tissue. The induction of enterocyte proliferation and E2F-dependent gene expression was identical in TAgwt and TAgN136 mice. Like TAgwt mice, TAgN136 mice exhibited dysplasia and increased intestinal length. However, TAgN136 induced these phenotypes at a much slower pace. Unlike TAgwt mice, TAgN136 mice did not exhibit an increase in crypt size. We have not observed a significant difference in the expression levels of TAgwt or TAgN136 with the age of the animal (Fig. 6E), and thus we do not believe that the delayed phenotype observed in TAgN136 mice is related to a change in protein expression.
Instead, we suggest two possible explanations for this observation. First, we found some significant differences in the patterns of TAg expression in villus enterocytes between TAgwt and TAgN136 mice. TAgN136 mice showed staining only in the top three-fourths of villi, and the staining never went down to the villus/crypt differentiation zone (Fig. 6B). On the other hand, TAgwt mice showed a dense nuclear staining throughout the villi and villus/crypt differentiation zone which occasionally extended to the top of the crypts (Fig. 6A and C). The expression of TAg in cells that have not differentiated completely could result in a higher degree of dysplastic phenotype and/or the increase in crypt size observed in TAgwt mice. Experiments directing the expression of TAgwt and TAgN136 to the intestinal crypts would test this possibility.
Alternatively, the carboxy terminus of TAg could contribute to the differences observed between TAgwt and TAgN136 mice. One obvious candidate that could interact with TAgwt but not with TAgN136 is p53, but TAg action on p53 does not appear to play a role in the transformation of enterocytes. TAg expression in enterocytes does not result in p53 stabilization, nor can TAg-p53 complexes be detected in these cells (20). Furthermore, the genetic ablation of p53 has no effect on enterocyte transformation induced by either TAgwt or truncated TAgs (20). In this study we show that neither TAgwt nor truncated TAgs alter the transcriptional levels of p53-dependent genes in enterocytes. In this regard, enterocytes differ from some other epithelial cell types, such as the choroid plexus, which exhibit robust p53-dependent apoptosis in response to truncated TAg (32). It is possible that as-yet-unidentified cellular targets are affected by TAg through its carboxy terminus and that their action plays a role in determining dysplasia and/or crypt size.
This is the first study which compares the global changes in the gene expression by TAgwt and its mutants side by side. Our results confirm and complete previous studies indicating the central and perhaps unique role played by Rb proteins (pRb, p130, and p107) in the control of intestinal tumorigenesis (9, 10). Not only are the first 136 amino acids of TAg sufficient to induce hyperplasia, but the inactivation of Rb proteins appears to be the primary requirement for this induction. The requirement of the LXCXE motif and the J domain for TAg-mediated gene regulation is evident by the fact that TAgwt and TAgN136 regulate the same set of genes and the regulation is lost by TAg3213 and TAgD44N. Furthermore, p53 does not play any role in TAg-mediated gene regulation in the intestinal epithelium, as reflected by the inability of TAgN136 to induce p53 target genes.
Acknowledgments
We thank M. Judith Tevethia and Satvir S. Tevethia (Pennsylvania State University College of Medicine, Hershey, PA) for providing the protocol for TAg staining. We thank Anthony L. Frazier for his assistance in preparing tissue for laser capture microdissection.
This work was supported by National Institutes of Health grant CA098956 to J.M.P.
Footnotes
Published ahead of print on 1 July 2009.
REFERENCES
- 1.Ahuja, D., M. T. Saenz-Robles, and J. M. Pipas. 2005. SV40 large T antigen targets multiple cellular pathways to elicit cellular transformation. Oncogene 24:7729-7745. [DOI] [PubMed] [Google Scholar]
- 2.Ali, S. H., J. S. Kasper, T. Arai, and J. A. DeCaprio. 2004. Cul7/p185/p193 binding to simian virus 40 large T antigen has a role in cellular transformation. J. Virol. 78:2749-2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bargonetti, J., J. J. Manfredi, X. Chen, D. R. Marshak, and C. Prives. 1993. A proteolytic fragment from the central region of p53 has marked sequence-specific DNA-binding activity when generated from wild-type but not from oncogenic mutant p53 protein. Genes Dev. 7:2565-2574. [DOI] [PubMed] [Google Scholar]
- 4.Cantalupo, P. G., M. T. Saenz-Robles, A. V. Rathi, R. W. Beerman, W. H. Patterson, R. H. Whitehead, and J. M. Pipas. 2009. Cell-type specific regulation of gene expression by simian virus 40 T antigens. Virology 386:183-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cotsiki, M., R. L. Lock, Y. Cheng, G. L. Williams, J. Zhao, D. Perera, R. Freire, A. Entwistle, E. A. Golemis, T. M. Roberts, P. S. Jat, and O. V. Gjoerup. 2004. Simian virus 40 large T antigen targets the spindle assembly checkpoint protein Bub1. Proc. Natl. Acad. Sci. USA 101:947-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeCaprio, J. A. 2009. How the Rb tumor suppressor structure and function was revealed by the study of adenovirus and SV40. Virology 384:274-284. [DOI] [PubMed] [Google Scholar]
- 7.DeCaprio, J. A., J. W. Ludlow, J. Figge, J. Y. Shew, C. M. Huang, W. H. Lee, E. Marsilio, E. Paucha, and D. M. Livingston. 1988. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell 54:275-283. [DOI] [PubMed] [Google Scholar]
- 8.Deeb, K. K., A. M. Michalowska, C. Y. Yoon, S. M. Krummey, M. J. Hoenerhoff, C. Kavanaugh, M. C. Li, F. J. Demayo, I. Linnoila, C. X. Deng, E. Y. Lee, D. Medina, J. H. Shih, and J. E. Green. 2007. Identification of an integrated SV40 T/t-antigen cancer signature in aggressive human breast, prostate, and lung carcinomas with poor prognosis. Cancer Res. 67:8065-8080. [DOI] [PubMed] [Google Scholar]
- 9.Guo, J., S. Longshore, R. Nair, and B. W. Warner. 2009. Retinoblastoma protein (pRb), but not p107 or p130, is required for maintenance of enterocyte quiescence and differentiation in small intestine. J. Biol. Chem. 284:134-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haigis, K., J. Sage, J. Glickman, S. Shafer, and T. Jacks. 2006. The related retinoblastoma (pRb) and p130 proteins cooperate to regulate homeostasis in the intestinal epithelium. J. Biol. Chem. 281:638-647. [DOI] [PubMed] [Google Scholar]
- 11.Harlow, E., L. V. Crawford, D. C. Pim, and N. M. Williamson. 1981. Monoclonal antibodies specific for simian virus 40 tumor antigens. J. Virol. 39:861-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hauft, S. M., S. H. Kim, G. H. Schmidt, S. Pease, S. Rees, S. Harris, K. A. Roth, J. R. Hansbrough, S. M. Cohn, D. J. Ahnen, et al. 1992. Expression of SV-40 T antigen in the small intestinal epithelium of transgenic mice results in proliferative changes in the crypt and reentry of villus-associated enterocytes into the cell cycle but has no apparent effect on cellular differentiation programs and does not cause neoplastic transformation. J. Cell Biol. 117:825-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang, D., A. Srinivasan, G. Lozano, and P. D. Robbins. 1993. SV40 T antigen abrogates p53-mediated transcriptional activity. Oncogene 8:2805-2812. [PubMed] [Google Scholar]
- 14.Kierstead, T. D., and M. J. Tevethia. 1993. Association of p53 binding and immortalization of primary C57BL/6 mouse embryo fibroblasts by using simian virus 40 T-antigen mutants bearing internal overlapping deletion mutations. J. Virol. 67:1817-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim, S. H., K. A. Roth, C. M. Coopersmith, J. M. Pipas, and J. I. Gordon. 1994. Expression of wild-type and mutant simian virus 40 large tumor antigens in villus-associated enterocytes of transgenic mice. Proc. Natl. Acad. Sci. USA 91:6914-6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim, S. H., K. A. Roth, A. R. Moser, and J. I. Gordon. 1993. Transgenic mouse models that explore the multistep hypothesis of intestinal neoplasia. J. Cell Biol. 123:877-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lane, D. P., and L. V. Crawford. 1979. T antigen is bound to a host protein in SV40-transformed cells. Nature 278:261-263. [DOI] [PubMed] [Google Scholar]
- 18.Linzer, D. I., and A. J. Levine. 1979. Characterization of a 54K dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell 17:43-52. [DOI] [PubMed] [Google Scholar]
- 19.Markey, M. P., J. Bergseid, E. E. Bosco, K. Stengel, H. Xu, C. N. Mayhew, S. J. Schwemberger, W. A. Braden, Y. Jiang, G. F. Babcock, A. G. Jegga, B. J. Aronow, M. F. Reed, J. Y. Wang, and E. S. Knudsen. 2007. Loss of the retinoblastoma tumor suppressor: differential action on transcriptional programs related to cell cycle control and immune function. Oncogene 26:6307-6318. [DOI] [PubMed] [Google Scholar]
- 20.Markovics, J. A., P. A. Carroll, M. T. Robles, H. Pope, C. M. Coopersmith, and J. M. Pipas. 2005. Intestinal dysplasia induced by simian virus 40 T antigen is independent of p53. J. Virol. 79:7492-7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Shea, C. C., and M. Fried. 2005. Modulation of the ARF-p53 pathway by the small DNA tumor viruses. Cell Cycle 4:449-452. [DOI] [PubMed] [Google Scholar]
- 22.Peden, K. W., A. Srinivasan, J. M. Farber, and J. M. Pipas. 1989. Mutants with changes within or near a hydrophobic region of simian virus 40 large tumor antigen are defective for binding cellular protein p53. Virology 168:13-21. [DOI] [PubMed] [Google Scholar]
- 23.Pipas, J. M., and A. J. Levine. 2001. Role of T antigen interactions with p53 in tumorigenesis. Semin. Cancer Biol. 11:23-30. [DOI] [PubMed] [Google Scholar]
- 24.Poulin, D. L., A. L. Kung, and J. A. DeCaprio. 2004. p53 targets simian virus 40 large T antigen for acetylation by CBP. J. Virol. 78:8245-8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pyeon, D., M. A. Newton, P. F. Lambert, J. A. den Boon, S. Sengupta, C. J. Marsit, C. D. Woodworth, J. P. Connor, T. H. Haugen, E. M. Smith, K. T. Kelsey, L. P. Turek, and P. Ahlquist. 2007. Fundamental differences in cell cycle deregulation in human papillomavirus-positive and human papillomavirus-negative head/neck and cervical cancers. Cancer Res. 67:4605-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rathi, A. V., M. T. Saenz Robles, and J. M. Pipas. 2007. Enterocyte proliferation and intestinal hyperplasia induced by simian virus 40 T antigen require a functional J. domain. J. Virol. 81:9481-9489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sablina, A. A., and W. C. Hahn. 2008. SV40 small T antigen and PP2A phosphatase in cell transformation. Cancer Metastasis Rev. 27:137-146. [DOI] [PubMed] [Google Scholar]
- 28.Saenz-Robles, M. T., J. A. Markovics, J. L. Chong, R. Opavsky, R. H. Whitehead, G. Leone, and J. M. Pipas. 2007. Intestinal hyperplasia induced by simian virus 40 large tumor antigen requires E2F2. J. Virol. 81:13191-13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srinivasan, A., A. J. McClellan, J. Vartikar, I. Marks, P. Cantalupo, Y. Li, P. Whyte, K. Rundell, J. L. Brodsky, and J. M. Pipas. 1997. The amino-terminal transforming region of simian virus 40 large T and small t antigens functions as a J domain. Mol. Cell. Biol. 17:4761-4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stubdal, H., J. Zalvide, K. S. Campbell, C. Schweitzer, T. M. Roberts, and J. A. DeCaprio. 1997. Inactivation of pRB-related proteins p130 and p107 mediated by the J domain of simian virus 40 large T antigen. Mol. Cell. Biol. 17:4979-4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sullivan, C. S., P. Cantalupo, and J. M. Pipas. 2000. The molecular chaperone activity of simian virus 40 large T antigen is required to disrupt Rb-E2F family complexes by an ATP-dependent mechanism. Mol. Cell. Biol. 20:6233-6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Symonds, H., L. Krall, L. Remington, M. Saenz-Robles, S. Lowe, T. Jacks, and T. Van Dyke. 1994. p53-dependent apoptosis suppresses tumor growth and progression in vivo. Cell 78:703-711. [DOI] [PubMed] [Google Scholar]
- 33.Whitehead, R. H., A. Brown, and P. S. Bhathal. 1987. A method for the isolation and culture of human colonic crypts in collagen gels. In Vitro Cell Dev. Biol. 23:436-442. [DOI] [PubMed] [Google Scholar]
- 34.Zalvide, J., H. Stubdal, and J. A. DeCaprio. 1998. The J domain of simian virus 40 large T antigen is required to functionally inactivate RB family proteins. Mol. Cell. Biol. 18:1408-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]