Abstract
Bovine spongiform encephalopathy (BSE) is a fatal, transmissible, neurodegenerative disease of cattle. BSE can be transmitted experimentally between cattle through the oral route, and in this study, brain tissue samples from animals at different time points postinoculation were analyzed for changes in gene expression. The aims of this study were to identify differentially regulated genes during the progression of BSE using microarray-based gene expression profiling and to understand the effect of prion pathogenesis on gene expression. A total of 114 genes were found to be differentially regulated over the time course of the infection, and many of these 114 genes encode proteins involved in immune response, apoptosis, cell adhesion, stress response, and transcription. This study also revealed a broad correlation between gene expression profiles and the progression of BSE in cattle. At 21 months postinoculation, the largest number of differentially regulated genes was detected, suggesting that there are many pathogenic processes in the animal brain even prior to the detection of infectivity in the central nervous systems of these orally infected cattle. Moreover, evidence is presented to suggest that it is possible to predict the infectious status of animals using the expression profiles from this study.
Bovine spongiform encephalopathy (BSE) in cattle belongs to the group of prion diseases or transmissible spongiform encephalopathies (TSEs), which includes Creutzfeldt-Jakob disease (CJD) in humans and scrapie in sheep and goats. Prion diseases are transmissible, neurodegenerative disorders that are invariably fatal. The neuropathological hallmarks of these diseases are vacuolation of the neuropil, gliosis, neuronal loss, and the accumulation of an abnormally folded isoform (PrPSc) of the cellular prion protein (PrPC). PrPSc differs from PrPC by its relatively high beta-sheet content and its resistance to protease treatment. It is thought that PrPSc is an essential, if not the sole, part of the prion, the infectious agent in prion diseases (1a, 2, 7, 24).
BSE was first reported in the United Kingdom in 1986, and its most likely cause was contaminated meat and bone meal, a dietary supplement for cattle (33, 34). The resulting BSE epidemic reached its maximum in 1992, and to date, more than 185,000 cattle succumbed to BSE in the United Kingdom. BSE is also considered to be the origin of the human disease variant CJD (15, 31). Worldwide, there have been almost 200 cases of variant CJD, and most have been found in the United Kingdom.
In recent years, few cases of unusual forms of BSE (bovine amyloidotic spongiform encephalopathy, lower-molecular-weight BSE, and higher-molecular-weight BSE [known as BASE, L-type BSE, and H-type BSE, respectively]) were identified mainly in Europe (4, 6, 12). The molecular signature of PrPSc of these cases differs from that of classical BSE, and the distribution of PrPSc in the brains of the affected animals also differ where data are available. These unusual (or atypical) forms of BSE mainly affect older cattle, and the etiology is currently unclear.
Many aspects of the pathogenesis of classical BSE have been studied extensively following oral infection of cattle (32). Calves orally exposed to BSE by ingestion of 100-g portions of homogenized brain stem samples from cattle with BSE developed BSE with a mean incubation time of 45 months (between 33 and 55 months). The earliest detection of infectivity in the central nervous system was 32 months postinoculation (34). The correlation between BSE pathogenesis and the transcriptional activities of genes is however not explored in any detail. Gene expression profiling studies of mice with scrapie have shown that these studies can provide valuable insights in the possible pathomechanisms of the disease (5, 25, 27, 29, 36, 37).
To determine the correlation between BSE pathogenesis and gene expression, we analyzed brain stem samples from cattle experimentally infected with the BSE agent over the time course of the infection by using microarray-based gene expression profiling.
MATERIALS AND METHODS
Tissue samples.
Experimental cattle were from United Kingdom farms that had no history of BSE, and the breed of the cattle was either Friesian or Holstein-Friesian (3). Four- to 6-month-old calves were infected orally with a dose of 100 g brain stem homogenate of cattle infected with the BSE agent (3, 32). Samples were taken from the euthanized infected animals at 6 months postinoculation (mpi) (three samples), 21 mpi (two samples), 27 mpi (three samples), 36 mpi (two samples), 39 mpi (three samples), and 45 mpi (two samples) when there were no clinical signs consistent with detection of BSE. No PrPSc, judged by PrP immunohistochemistry, was found in the brain stems of these animals. Three animals that were positive for PrPSc by immunochemical methods and that were terminally diseased at 41, 42, and 44 mpi were used as positive controls. The negative controls were age matched with the positive controls, and brain tissue samples were taken from the animals that were not infected with BSE. From all 21 animals, tissue samples of the brain stem, which is the standard tissue for reliable BSE diagnosis, were taken. All samples were supplied by the TSE archive at the Veterinary Laboratories Agency, United Kingdom.
Microarray analysis.
The preparation of samples and reagents used were as specified by the Affymetrix GeneChip Expression Analysis manual (1). Briefly, total RNA was extracted from 0.2-g brain stem tissue samples by the Trizol method (Invitrogen), cleaned by RNeasy columns (Qiagen), and checked for integrity by gel electrophoresis. Total RNA (5 μg) was used as a template for T7-oligo(dT)-primed reverse transcription to cDNA according to the Affymetrix manual (1). The double-stranded cDNA was cleaned using the GeneChip sample cleanup module (Affymetrix). Synthesis of biotin-labeled cRNA from the cDNA was performed by Affymetrix one-cycle target labeling assays. The cRNA was cleaned with the GeneChip sample cleanup module and quantified according to the Affymetrix manual (1). The biotin-labeled cRNA (20 μg) was fragmented in fragmentation buffer at 94°C for 35 min (Affymetrix). The quality of the fragmented cRNA was examined by electrophoresis using 1% agarose gel (Sigma-Aldrich). The fragmented cRNA (15 μg) was used to prepare the hybridization cocktail (Affymetrix 49 format). To ensure that the RNA samples were of high quality, Test 3 probe arrays (Affymetrix) were used to evaluate seven RNA samples, and all of them showed satisfactory quality (3′/5′ ratios of the probe sets for glyceraldehyde-3-phosphate dehydrogenase [GAPDH] < 3). The cRNA cocktails were hybridized to Affymetrix bovine genome GeneChips, which contain 24,128 probe sets, at 60 rpm for 16 h at 45°C in Affymetrix hybridization oven 640. The microarrays were washed and stained in Affymetrix fluidics station 450DX, and the data from the microarrays were collected by an Affymetrix scanner.
The raw data were first imported to Affymetrix GeneChip operating software version 1.4. After initial analysis of the data and inspection of the images, the pivot formatted data were analyzed further with GeneSpring version 7 software (Silicon Genetics). The data were normalized by three steps: (i) transform data transformation set measurements less than 0.01 to 0.01, (ii) normalize each chip to the 50th percentile, and (iii) normalize each gene to the median. These steps are the default settings for the GeneSpring package.
One-way analysis of variance (ANOVA) statistical analysis was used as a filter tool that compares mean expression levels between two or more groups of samples. The comparison is performed for each gene, and the genes with the most significant differential expression (smallest P value) are returned. The parameters for the analysis were as follows. (i) The P value cutoff was 0.05. (ii) The parametric test did not assume that variances were equal. (iii) Multiple testing correction was used. (iv) The post hoc tests were Student-Newman-Keuls. The post hoc tests were carried out in conjunction with ANOVA to determine which specific group pair(s) was statistically different.
Quantitative reverse transcription (RT)-PCR.
The RNA samples were treated with DNA-free DNase treatment and removal reagents (Ambion) for 1 h at 37°C to remove any trace DNA. The treated RNA was then used as a template for cDNA synthesis with a TaqMan reverse transcription kit (Applied Biosystems). The real-time PCR was carried out by denaturing at 95°C for 15 s, annealing at 50°C for 2 min, and extension at 60°C for 1 min for 40 cycles using ABI Prism 7700 sequencing detector. The GAPDH gene was used as an internal control to normalize the expression levels of target mRNA.
The primer sets were chosen by using Primer Express 1.5 for TaqMan software. The sequences of the primer sets and probes follow. For Arg2 (arginase, type II), the primers were 5′-GGC AGT GGA CGT CAT TGC T and 5′-GGT CGT AGA CAA TAT GCC CTC C, and the probe was 5′-FAM-CGA GTT TCG GGC AGA CGA GGG A (FAM is 6-carboxyfluorescein). For ITGB5 (integrin, beta 5), the primers were 5′-TGC TCG TCA CCA TCC ACG and 5′-GGCCCT GGA TCG CTC ACT, and the probe was 5′-FAM-CCG GAG AGA GTT CGC CAA GTT CCA. For ITGA4 (integrin, alpha 4), the primers were 5′-TGC TTT CCT TCA TTT CTT ATG TTA TGT G and 5′-TCC AAC TGT CTC TTC TGT TTT CTT TTT, and the probe was 5′-FAM-AAG GCT GGC TTC TTC AAA AGA CAG TAC CAA TC. For BOLA-DMA (major histocompatibility complex, class II, DM alpha-chain), the primers were 5′-TGT GCG GCG TGG CC and 5′-AGA GGA CCA AGC CAA CAA TGA, and the probe was 5′-FAM-TTG GCC TGG GTG TGC TGG GC. For COL9A1 (collagen, type IX, alpha 1), the primers were 5′-GCC ACT GGG AAT CGA ACA AG and 5′-GGG CTG GAT GGA AGT CTC C, and the probe was 5′-FAM-AGG GCA AAT TCG AAA AGG CTG CAG TT. For GSTA2 (glutathione S-transferase, alpha 2), the primers were 5′-GAC CCT AGC CTT TTG GCC A and 5′-CCG GGA GAC TGC TGA CTC TG, and the probe was 5′-FAM-TTC CCT CTG CTG AAG GGC CTG AAA G. For GAPDH, the primers were 5′-TCA GCA ATG CCT CCT GCA C and 5′-CAG TCT TCT GGG TGG CAG TGA, and the probe was 5′-VIC-CCC CTG GCC AAG GTC ATC CAT.
RESULTS
Gene expression studies of cattle infected with the BSE agent at various time points postinfection were performed in order to investigate transcriptional changes during progression of the disease. RNA from brain stem tissue samples of animals 6, 21, 27, 36, 39 mpi, BSE-positive controls (41, 42 and 44 mpi), and BSE-negative controls were analyzed using Affymetrix bovine genomic GeneChips.
Clustering analysis of the time course samples.
To identify any relationship between the changes in gene expression of the various samples, clustering analysis was performed using the GeneSpring package to group individual samples according to the similarities in expression patterns using the condition tree with the Spearman correlation.
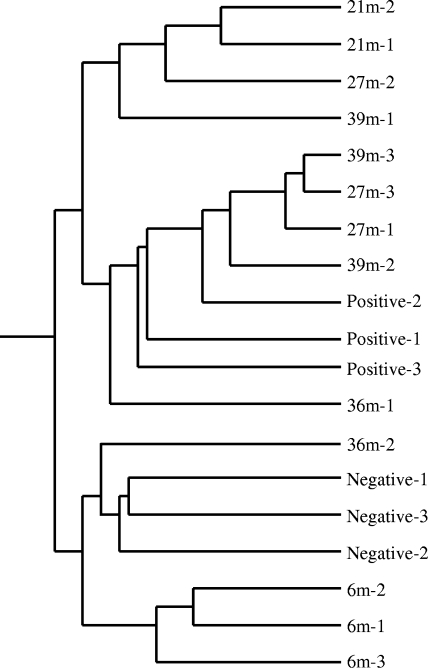
The results showed that the samples were divided into two major groups (Fig. 1). One group combined all the negative samples and all 6 mpi samples plus one 36 mpi sample. The other group was divided into two subgroups: one subgroup combined all 21 mpi samples with one 27 mpi sample and one 39 mpi sample; the other subgroup combined all positive samples together with two 27 mpi samples, two 39 mpi samples, and one 36 mpi sample. This clustering analysis indicated that there was a broad correlation between disease progression and expression patterns. It is also noteworthy that the highest degree of difference was between 21 mpi samples and the negative controls, a finding that was confirmed in further analyses (see below).
FIG. 1.
Condition tree of clustering analysis for BSE time course samples. The analysis was performed by GeneSpring using all 24,128 genes (probe sets) on Affymetrix bovine microarray GeneChips. Similarity was measured using the Spearman correlation with a value of 1 for the separation ratio and a value if 0.001 for the minimum distance to merge similar branches. Three positive-control animals (Positive-1, -2, and -3) and three negative-control animals (Negative-1, -2, and -3) were used in the study. 21m-2, sample from 21 mpi from animal 2.
Identification of differentially regulated genes during development of BSE.
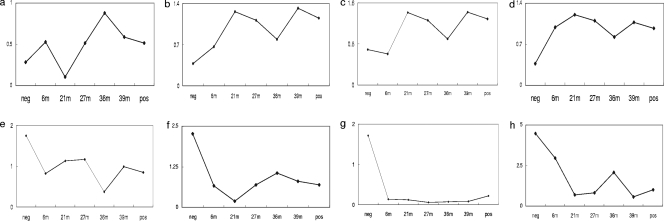
To search for differentially regulated genes during the time course of BSE, one-way ANOVA analysis was chosen and applied to the microarray results. A total of 205 probe sets (a technical term that describes a transcript on the microarray) were identified to be associated with statistically significant differences among the time course groups. As some of these probe sets represent the same genes, the 205 probe sets in the Affymetrix GeneChips describe 187 genes. Among these 187 genes, there were 114 genes whose functions are known or are similar to genes with known function (Table 1), 73 encoded functionally unknown genes. Of the 114 genes with known function, 53 genes were upregulated (46%) and 40 genes were downregulated (35%) during the time course of BSE, and the remaining genes showed complex expression patterns (19%). The functional group with most of the genes was the metabolism group (14 genes), followed by the transcription and translation group (13 genes), the immune response group (8 genes), and the transport group (8 genes) (Table 1). The expression profiles of eight exemplary genes known to play a role in various neurodegenerative diseases are shown in detail in Fig. 2. T-cell receptor gamma variable 3-1, proteasome 26S subunit ATPase, 4, a gene similar to 14-3-3 protein theta and a gene similar to metalloprotease 1 were overall upregulated (Fig. 2a to d), while nuclear receptor (NR1H3), T-cell receptor delta chain variable region, acetylcholine receptor (nicotinic, beta 4), and a gene similar to glycine dehydrogenase were overall downregulated (Fig. 2e to h) during progression of the disease. The maximal increase was 3.54-fold for proteasome 26S subunit ATPase 4 at 39 mpi (Fig. 2b), and the maximal decrease was 30.8-fold for nicotinic acetylcholine receptor (nicotinic, beta 4) at 36 mpi (Fig. 2g) compared with the negative controls. The maximal value for up- or downregulation was between 21 and 39 mpi compared to the negative controls. The degree of up- or downregulation and the pattern of expression changes of the genes shown in Fig. 2 were representative for most of the genes in Table 1.
TABLE 1.
Relative levels of differentially expressed genes during the progression of BSE
| Functional group | Gene identification | Gene name or descriptiona | P valueb | Relative level of gene in samples from animals |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Negative controls | 6 mpi | 21 mpi | 27 mpi | 36 mpi | 39 mpi | Positive controls | ||||
| Cell adhesion | Bt.13062.1.S1 | Collagen, type IX, alpha 1 | 0.0164 | 0.243 | 0.189 | 0.185 | 0.0909 | 0.591 | 0.143 | 0.23 |
| Bt.13201.1.S1 | Desmocollin 2 | 0.0482 | 0.633 | 1.079 | 0.721 | 1.392 | 1.847 | 0.892 | 0.264 | |
| Bt.4055.1.S1 | Bone gamma-carboxyglutamate (gla) protein (osteocalcin) | 0.0447 | 0.431 | 1.507 | 1.14 | 1.582 | 0.053 | 0.785 | 0.653 | |
| Bt.5536.1.S1 | Integrin, beta 5 | 0.0448 | 0.417 | 0.835 | 1.398 | 1.063 | 1.287 | 1.231 | 1.025 | |
| Bt.8130.1.S1 | Integrin, alpha 4 (antigen CD49D, alpha 4 subunit of VLA-4 receptor) | 0.0456 | 0.903 | 0.677 | 0.636 | 0.609 | 0.0351 | 0.266 | 0.239 | |
| Bt.20596.2.S1 | Epithelial V-like antigen 1 (EVA1) | 0.0238 | 1.706 | 0.321 | 0.149 | 0.88 | 1.455 | 0.941 | 0.214 | |
| Immune responses | Bt.13003.1.S1 | T-cell receptor, alpha | 0.0443 | 0.282 | 0.431 | 0.0777 | 0.0345 | 0.134 | 0.0105 | 0.0937 |
| Bt.1673.2.S1 | Similar to interleukin 17 receptor C isoform 2 precursor | 0.0487 | 0.627 | 0.69 | 0.333 | 0.926 | 1.458 | 0.99 | 0.724 | |
| Bt.29692.1.A1 | T-cell receptor delta chain variable region | 0.0295 | 2.261 | 0.658 | 0.188 | 0.693 | 1.054 | 0.797 | 0.695 | |
| Bt.29694.1.S1 | T-cell receptor gamma variable 3-1 | 0.0456 | 0.286 | 0.529 | 0.109 | 0.519 | 0.883 | 0.587 | 0.514 | |
| Bt.12809.1.S1 | Immunoglobulin delta heavy-chain membrane-bound form (IgD) | 0.0158 | 0.567 | 0.53 | 0.121 | 0.488 | 0.257 | 0.336 | 0.149 | |
| Bt.344.1.S1 | Major histocompatibility complex, class II, DM alpha-chain | 0.0303 | 0.428 | 0.613 | 0.896 | 1.212 | 1.069 | 1.437 | 1.115 | |
| Bt.4660.1.S1 | Colony-stimulating factor 3 (granulocyte) | 0.0456 | 1.098 | 0.56 | 0.152 | 0.396 | 1.263 | 1.152 | 0.614 | |
| Bt.7031.1.A1 | Similar to cytokine receptor-like factor 3 | 0.0284 | 0.825 | 0.851 | 1.016 | 1.052 | 0.824 | 1.022 | 1.095 | |
| Transcription or translation | Bt.10777.1.S1 | Similar to Forkhead box protein P1 | 0.0415 | 0.817 | 0.936 | 1.026 | 0.999 | 0.837 | 1.145 | 1.351 |
| Bt.11345.1.A1 | Transcriptional adaptor 3-like isoform b | 0.0368 | 1.536 | 1.12 | 0.77 | 0.866 | 1.396 | 0.881 | 0.976 | |
| Bt.12412.1.S1 | HMG-box transcription factor 1 | 0.0387 | 0.879 | 0.762 | 0.965 | 0.859 | 1.048 | 1.09 | 1.054 | |
| Bt.14175.1.A1 | Ribosomal protein L3 | 0.0467 | 0.503 | 1.363 | 0.907 | 0.979 | 1.155 | 0.545 | 0.742 | |
| Bt.15436.2.S1 | Ribosomal protein L10 | 0.0223 | 0.348 | 0.598 | 0.985 | 0.964 | 0.968 | 1.295 | 1.189 | |
| Bt.17265.2.S1 | Nuclear receptor subfamily 1, group H, member 3 | 0.0325 | 1.748 | 0.818 | 1.128 | 1.162 | 0.372 | 0.983 | 0.846 | |
| Bt.21763.2.S1 | Similar to nuclear factor 1 B-type (nuclear factor 1/B) | 0.0422 | 1.812 | 1.375 | 0.969 | 0.795 | 1.105 | 0.837 | 0.833 | |
| Bt.2466.1.S1 | Similar to DNA-directed RNA polymerase II 14.5-kDa polypeptide (RPB9) (RPB14.5) | 0.0253 | 0.68 | 0.878 | 1.168 | 1.037 | 0.848 | 1.117 | 1.014 | |
| Bt.22449.2.S1 | Similar to CCR4-NOT transcription complex, subunit 6-like isoform 1 | 0.0137 | 1.343 | 0.751 | 0.526 | 0.906 | 0.873 | 0.953 | 0.995 | |
| Bt.23590.1.S1 | Polymerase (RNA) I, polypeptide D, 16 kDa | 0.0325 | 0.537 | 0.815 | 1.07 | 1.131 | 0.966 | 1.08 | 1.152 | |
| Bt.5344.1.S1 | Tu translation elongation factor, mitochondrial | 0.0015 | 0.327 | 1.051 | 1.139 | 0.966 | 0.752 | 1.131 | 0.847 | |
| Bt.6390.1.S1 | Mitochondrial ribosomal protein S17 | 0.0147 | 0.932 | 0.771 | 1.266 | 1.085 | 0.989 | 1.049 | 1.13 | |
| Bt.27316.2.A1 | Similar to retinoblastoma-binding protein 8 (RBBP-8) (CtBP-interacting protein) | 0.0447 | 1.182 | 0.91 | 0.934 | 0.969 | 0.913 | 0.648 | 0.764 | |
| Transport | Bt.19028.1.A1 | Transient receptor potential channel 6 | 0.048 | 0.272 | 0.635 | 0.0744 | 0.149 | 0.122 | 0.444 | 0.52 |
| Bt.21173.1.S1 | Similar to HECT, UBA, and WWE domain containing 1 | 0.0381 | 0.327 | 0.75 | 1.166 | 1.076 | 0.752 | 1.177 | 1.243 | |
| Bt.23698.1.A1 | Albumin | 0.0325 | 0.364 | 0.0855 | 0.0569 | 0.131 | 0.868 | 0.11 | 0.0458 | |
| Bt.26069.1.A1 | FXYD domain containing ion transport regulator 7 | 0.0456 | 0.381 | 0.768 | 0.964 | 1.036 | 1.013 | 1.344 | 1.102 | |
| Bt.28684.1.S1 | Karyopherin, alpha 6 | 0.0147 | 0.788 | 0.859 | 1.278 | 0.984 | 0.893 | 1.084 | 1.088 | |
| Bt.3551.1.S1 | Similar to putative transporter C20orf59 | 0.0179 | 0.665 | 0.6 | 1.242 | 1.052 | 1.089 | 0.665 | 0.945 | |
| Bt.9978.1.S1 | Cholinergic receptor, nicotinic, beta polypeptide 4 | 0.0344 | 1.714 | 0.141 | 0.123 | 0.0557 | 0.0703 | 0.0844 | 0.22 | |
| Bt.9360.1.S1 | Similar to calgranulin A (S100 calcium-binding protein A8) | 0.0408 | 0.793 | 1.052 | 1.982 | 0.823 | 2.029 | 1.281 | 1.008 | |
| Signal transduction | Bt.18259.1.A1 | Similar to serine/threonine-protein kinase p21-activated kinase 6 (PAK-6) (PAK-5) | 0.0247 | 1.841 | 0.919 | 0.983 | 1.089 | 1.32 | 0.562 | 0.627 |
| Bt.409.1.S1 | Guanine nucleotide-binding protein (G protein), gamma 5 | 0.0295 | 0.108 | 0.0843 | 0.0744 | 0.0987 | 0.0393 | 0.141 | 0.637 | |
| Bt.28596.1.S1 | Similar to RAS-like GTP-binding protein REM | 0.0344 | 0.5 | 0.427 | 0.766 | 1.004 | 1.486 | 0.748 | 0.516 | |
| Bt.4990.1.S1 | Activin A receptor, type IIB | 0.0253 | 0.719 | 1.763 | 0.745 | 0.935 | 1.098 | 0.781 | 0.984 | |
| Cell growth | Bt.11042.1.A1 | Similar to thyroid hormone receptor-associated protein complex 240-kDa component (Trap240) | 0.0289 | 2.659 | 0.942 | 0.857 | 1.16 | 1.099 | 0.628 | 0.974 |
| Bt.2.1.S1 | Cell division cycle 2, G1 to S and G2 to M (CDC2) | 0.0456 | 1.446 | 0.857 | 0.835 | 0.827 | 0.486 | 1.146 | 0.957 | |
| Bt.29681.1.S1 | Prolactin-related protein VI | 0.0462 | 0.104 | 0.0422 | 0.0172 | 0.0281 | 0.0249 | 0.0127 | 0.0453 | |
| Bt.13326.1.S1 | Similar to growth arrest-specific protein 7 (GAS-7) | 0.029 | 0.571 | 1.507 | 1.031 | 0.956 | 1.094 | 0.95 | 0.793 | |
| Bt.27010.1.A1 | Similar to craniofacial development protein 1 | 0.0341 | 1.016 | 0.942 | 1.049 | 0.83 | 1.066 | 1.048 | 0.955 | |
| Phosphatase or kinase | Bt.21050.1.S1 | Similar to WNK lysine-deficient protein kinase 2 | 0.0078 | 0.972 | 1.05 | 1.08 | 0.988 | 1.174 | 0.898 | 1.068 |
| Bt.7619.3.S1 | Similar to calcium/calmodulin-dependent protein kinase IIA isoform 1 | 0.0454 | 0.835 | 0.237 | 0.471 | 1.272 | 0.787 | 1.165 | 1.052 | |
| Bt.6626.1.S1 | Similar to phosphatidic acid phosphatase type 2A isoform 1 | 0.0387 | 0.48 | 0.534 | 1.285 | 0.96 | 0.767 | 1.315 | 1.058 | |
| Bt.7870.1.S1 | Pyrophosphatase (inorganic) | 0.0035 | 0.816 | 0.643 | 1.062 | 0.988 | 0.748 | 1.007 | 1.213 | |
| Bt.9542.2.S1 | Similar to tyrosine-protein phosphatase, non-receptor type 1 (protein-tyrosine phosphatase 1B [PTP-1B]) | 0.0462 | 2.089 | 1.313 | 0.598 | 0.401 | 1.919 | 0.2 | 0.65 | |
| Metabolism | Bt.13095.1.S1 | Hyaluronan synthase 3 | 0.0325 | 0.0964 | 0.117 | 0.35 | 0.0837 | 0.0759 | 0.133 | 0.0941 |
| Bt.1582.1.S1 | Nonmetastatic cells 1, protein (NM23A) | 0.0472 | 0.412 | 0.632 | 1.435 | 1.037 | 0.971 | 1.147 | 1.154 | |
| Bt.15900.1.A1 | Similar to putative adenosylhomocysteinase 3 (S-adenosyl-l-homocysteine hydrolase) | 0.0478 | 1.681 | 1.102 | 0.942 | 0.918 | 1.156 | 0.772 | 1.02 | |
| Bt.16561.1.A1 | Aldo-keto reductase family 1, member C1 (dihydrodiol dehydrogenase 1; 20-alpha (3-alpha)-hydroxysteroid dehydrogenase) | 0.0004 | 0.335 | 0.313 | 0.0294 | 0.6 | 0.118 | 0.143 | 0.217 | |
| Bt.1663.1.A1 | Biphenyl hydrolase-like (serine hydrolase; breast epithelial mucin-associated antigen) | 0.0467 | 0.589 | 0.859 | 1.164 | 0.952 | 0.929 | 1.265 | 1.022 | |
| Bt.16685.1.A1 | Similar to GTPase, IMAP family member 5 | 0.0357 | 1.253 | 0.321 | 0.789 | 0.188 | 0.624 | 0.0365 | 0.0846 | |
| Bt.16774.1.A1 | Similar to glycine dehydrogenase (decarboxylating) | 0.0493 | 4.465 | 2.95 | 0.7 | 0.819 | 2.072 | 0.574 | 0.991 | |
| Bt.20584.1.S1 | Similar to NAD(P)-dependent steroid dehydrogenase-like | 0.0454 | 0.664 | 1.347 | 1.283 | 1.022 | 0.881 | 0.953 | 1.015 | |
| Bt.2169.1.S1 | Similar to tissue alpha-l-fucosidase precursor (alpha-l-fucosidase I) | 0.0046 | 0.673 | 0.803 | 1.3 | 1.13 | 1.168 | 1.261 | 1.03 | |
| Bt.21820.2.S1 | Similar to GTPase, IMAP family member 5 | 0.0443 | 1.804 | 1.722 | 0.635 | 0.496 | 0.369 | 0.381 | 0.799 | |
| Bt.4093.1.S1 | Biliverdin reductase B (flavin reductase [NADPH]) | 0.0244 | 0.0317 | 0.773 | 1.233 | 1.015 | 0.687 | 1.38 | 1.05 | |
| Bt.832.1.S1_ | Dehydrogenase/3-ketoacyl-coenzyme A thiolase/enoyl-coenzyme A hydratase (trifunctional protein), beta subunit | 0.0121 | 0.671 | 0.717 | 1.06 | 1.184 | 0.777 | 1.241 | 1.021 | |
| Bt.8624.2.S1 | Arginase, type II | 0.0341 | 0.336 | 0.27 | 0.0623 | 0.094 | 0.146 | 0.0856 | 0.0841 | |
| Bt.9591.2.S1 | Similar to glutaminase kidney isoform, mitochondrial precursor (GLS) (l-glutamine amidohydrolase) (K-glutaminase) | 0.0456 | 2.253 | 1.616 | 0.931 | 0.96 | 1.287 | 0.853 | 0.898 | |
| Stress response | Bt.1731.1.S1 | DnaJ (Hsp40) homolog, subfamily B, member 6 | 0.0088 | 0.917 | 0.865 | 1.092 | 1.065 | 0.839 | 1.072 | 1.051 |
| Bt.227.2.A1 | Glutathione S-transferase A2 | 0.0104 | 0.29 | 0.1 | 0.256 | 0.61 | 0.157 | 0.046 | 0.0524 | |
| Bt.11662.1.A1 | Similar to glutathione peroxidase 7 | 0.010 | 0.454 | 0.78 | 1.036 | 0.986 | 1.362 | 1.236 | 0.815 | |
| Extracellular matrix | Bt.13124.1.A1 | Tissue inhibitor of metalloproteinase TIMP4 | 0.0226 | 1.672 | 0.87 | 0.0844 | 0.0788 | 0.298 | 0.279 | 0.697 |
| Bt.27262.1.S1 | Similar to metalloprotease 1 | 0.0405 | 0.374 | 0.99 | 1.208 | 1.104 | 0.824 | 1.071 | 0.971 | |
| DNA or RNA binding | Bt.13291.1.S1 | Similar to pogo transposable element with ZNF domain isoform 1 | 0.0004 | 1.215 | 1.245 | 0.734 | 0.945 | 1.122 | 0.898 | 0.965 |
| Bt.13501.3.S1 | Similar to XPA-binding protein 1 | 0.0104 | 0.695 | 0.963 | 1.13 | 1.239 | 0.907 | 1.617 | 0.974 | |
| Bt.13515.2.A1 | RNA pseudouridylate synthase domain containing 3 | 0.0244 | 0.905 | 1.537 | 0.988 | 1.042 | 1.213 | 0.887 | 1.162 | |
| Bt.18116.1.S1 | Similar to zinc finger CCCH-type domain containing 1 | 0.0128 | 0.564 | 0.0794 | 0.0415 | 0.111 | 0.203 | 0.0675 | 0.62 | |
| Bt.27054.2.S1 | Similar to histone-lysine N-methyltransferase, H3 lysine-9 specific 4 (histone H3-K9 methyltransferase 4) | 0.0193 | 0.69 | 0.857 | 1.182 | 1.186 | 0.971 | 1.221 | 1.131 | |
| Bt.27564.1.A1 | Likely ortholog of mouse D11lgp2 | 0.0456 | 0.869 | 1.182 | 0.815 | 1.071 | 1.417 | 1.238 | 0.9 | |
| Bt.2944.2.S1 | Similar to protein C14orf21 homolog | 0.0383 | 0.549 | 0.34 | 0.142 | 1.057 | 0.654 | 0.786 | 0.338 | |
| Bt.5079.1.S1 | Proteasome (prosome, macropain) 26S subunit, ATPase, 4 | 0.0325 | 0.373 | 0.662 | 1.257 | 1.112 | 0.791 | 1.322 | 1.147 | |
| Metal ion binding | Bt.11329.1.S1 | Similar to vacuolar protein sorting 11 (yeast homolog) | 0.0244 | 0.524 | 0.667 | 1.26 | 1.068 | 0.877 | 1.188 | 1.034 |
| Bt.16117.1.A1 | Tripartite motif-containing 38 | 0.0244 | 1.349 | 1.331 | 0.877 | 0.664 | 1.251 | 0.766 | 0.875 | |
| Bt.17228.1.A1 | Similar to RING-box protein 2 (Rbx2) | 0.0447 | 2.829 | 1.716 | 0.814 | 0.753 | 1.03 | 0.777 | 0.853 | |
| Bt.26131.1.S1 | Cytidine and dCMP deaminase domain containing 1 (CDADC1) | 0.0454 | 0.596 | 0.864 | 0.71 | 0.838 | 0.683 | 1.401 | 0.535 | |
| Protein binding | Bt.16187.1.A1 | Similar to kelch repeat and BTB (POZ) domain-containing 6 | 0.0291 | 3.096 | 1.229 | 0.694 | 0.746 | 0.703 | 0.811 | 0.844 |
| Bt.18506.1.S1 | Discs, large homolog 5 (Drosophila) | 0.0078 | 0.185 | 0.285 | 0.802 | 0.102 | 0.0746 | 0.0567 | 0.193 | |
| Bt.23254.1.S1 | Similar to 14-3-3 protein theta (14-3-3 protein tau) | 0.0169 | 0.687 | 0.608 | 1.414 | 1.262 | 0.9 | 1.418 | 1.286 | |
| Other | Bt.10890.1.S1 | Similar to positive cofactor 2, glutamine/Q-rich-associated protein isoform b | 0.011 | 0.943 | 1.301 | 1.059 | 1.022 | 1.27 | 0.96 | 1.019 |
| Bt.11228.1.S1 | Similar to CLN3 protein (battenin) | 0.0121 | 0.612 | 1.122 | 0.864 | 0.967 | 0.995 | 1.149 | 1.223 | |
| Bt.11522.1.A1 | Troponin T2, cardiac | 0.0447 | 0.152 | 0.254 | 0.0673 | 0.123 | 0.536 | 0.0926 | 0.388 | |
| Bt.11934.1.S1 | Similar to CG7071-PA, isoform A | 0.0046 | 0.81 | 0.484 | 1.195 | 0.985 | 0.837 | 1.105 | 1.255 | |
| Bt.13291.1.S1 | Similar to pogo transposable element with ZNF domain isoform 1 | 0.0004 | 1.215 | 1.245 | 0.734 | 0.945 | 1.122 | 0.898 | 0.965 | |
| Bt.1536.2.S1 | Similar to nuclear factor of kappa light polypeptide gene enhancer in B-cell inhibitor, epsilon | 0.019 | 0.153 | 0.0696 | 0.57 | 0.146 | 0.0994 | 0.138 | 0.166 | |
| Bt.16647.1.S1 | Similar to amyotrophic lateral sclerosis 2 (juvenile) | 0.0276 | 3.682 | 2.059 | 0.682 | 0.965 | 1.204 | 0.864 | 1.005 | |
| Bt.17716.1.A1 | Similar to charged multivesicular body protein 4b (chromatin-modifying protein 4b [CHMP4b]) | 0.0431 | 0.161 | 0.138 | 0.0379 | 0.247 | 0.265 | 0.0587 | 0.0976 | |
| Bt.1992.1.S1 | Similar to protein HSPC020 | 0.0217 | 0.476 | 1.052 | 1.18 | 1.077 | 0.842 | 1.083 | 1.006 | |
| Bt.21925.1.A1 | Similar to AT-rich interactive domain 1B (SWI1-like) isoform 3 | 0.0478 | 1.205 | 1.518 | 0.976 | 0.819 | 1.276 | 0.826 | 0.968 | |
| Bt.23596.1.S1 | Similar to Leydig cell tumor 10-kDa protein homolog | 0.0084 | 0.563 | 0.635 | 1.036 | 1.077 | 0.873 | 1.292 | 1.05 | |
| Bt.24423.1.A1 | Low-density-lipoprotein receptor-related protein 5 | 0.0343 | 0.395 | 0.114 | 0.0294 | 0.484 | 0.0393 | 0.399 | 0.329 | |
| Bt.25773.1.A1 | Similar to IQ motif containing AAA domain | 0.0447 | 1.115 | 1.259 | 0.651 | 0.81 | 1.3 | 1.073 | 0.82 | |
| Bt.26569.1.S1 | Similar to SHQ1 homolog | 0.03 | 0.741 | 0.729 | 1.072 | 0.916 | 1.034 | 1.116 | 1.193 | |
| Bt.26615.1.S1 | Similar to Y73E7A.1 | 0.0492 | 0.547 | 0.804 | 1.098 | 1.221 | 1.006 | 1.071 | 1.212 | |
| Bt.26708.1.A1 | Similar to leucine-rich repeat containing 16 | 0.0467 | 0.563 | 0.839 | 1.274 | 1.022 | 0.856 | 1.065 | 1.126 | |
| Bt.26845.1.S1 | Similar to flotillin-1 | 0.0276 | 0.492 | 1.22 | 0.648 | 0.837 | 0.097 | 0.876 | 1.504 | |
| Bt.27011.1.A1 | Translokin | 0.0117 | 2.116 | 1.173 | 0.856 | 0.877 | 1.146 | 0.973 | 0.988 | |
| Bt.29253.1.S1 | Similar to protein disulfide-isomerase A5 precursor (protein disulfide isomerase-related protein) | 0.0482 | 0.351 | 0.211 | 0.13 | 0.145 | 0.155 | 0.168 | 0.177 | |
| Bt.29835.1.S1 | Leucine-rich glycoprotein homolog | 0.0325 | 0.381 | 0.211 | 0.995 | 1.154 | 0.667 | 1.492 | 1.362 | |
| Bt.29854.1.S1 | Myosin, heavy polypeptide 1, skeletal muscle, adult | 0.0225 | 0.0984 | 0.124 | 0.0536 | 0.118 | 0.0556 | 0.018 | 0.0307 | |
| Bt.3605.1.S1 | Inner membrane protein, mitochondrial (mitofilin) | 0.0107 | 0.649 | 0.839 | 1.208 | 1.12 | 0.755 | 1.083 | 1.069 | |
| Bt.4091.3.S1 | Similar to epsin 1 | 0.0295 | 0.108 | 0.0843 | 0.0744 | 0.0987 | 0.0393 | 0.141 | 0.637 | |
| Bt.4825.1.A1 | Similar to 1810010N17Rik protein | 0.0419 | 0.713 | 1.615 | 1.136 | 1.029 | 1.166 | 0.882 | 0.824 | |
| Bt.5177.1.S1 | Similar to BMP and activin membrane-bound inhibitor homolog precursor (putative transmembrane protein NMA[nonmetastatic gene A protein]) | 0.0492 | 0.736 | 0.712 | 1.032 | 0.965 | 1.247 | 1.933 | 0.992 | |
| Bt.6434.1.S1 | Similar to ring finger protein 149 | 0.0325 | 0.846 | 0.664 | 1.369 | 1.024 | 0.875 | 1.31 | 1.013 | |
| Bt.6771.1.S1 | Similar to EH domain-binding protein 1 | 0.0465 | 0.933 | 0.661 | 1.192 | 1.025 | 0.997 | 1.023 | 1.077 | |
| Bt.6898.2.S1 | Similar to SPRY domain-containing SOCS box protein SSB-2 | 0.0249 | 1.449 | 0.838 | 1.759 | 0.874 | 0.861 | 0.979 | 0.551 | |
| Bt.7311.1.S1 | Similar to necdin-like 2 | 0.0001 | 0.69 | 1.023 | 1.227 | 1.07 | 0.876 | 1.148 | 1.048 | |
| Bt.7562.1.S1 | Similar to CG6962-PA | 0.0211 | 0.846 | 1.7 | 1.077 | 1.041 | 1.074 | 0.775 | 0.973 | |
| Bt.8828.2.S1 | Similar to CG30394-PB, isoform B | 0.0244 | 0.181 | 0.606 | 0.665 | 0.626 | 0.304 | 1.751 | 0.423 | |
Prion disease-associated genes are shown in boldface type.
P values were determined by ANOVA.
FIG. 2.
Gene expression profiles of T-cell receptor gamma variable 3-1 (a), proteasome 26S subunit (b), a gene similar to 14-3-3 protein theta (c), a gene similar to metalloprotease 1 (d), nuclear receptor (NR1H3) (e), T-cell receptor delta chain variable region (f), acetylcholine receptor (nicotinic, beta 4) (g), and a gene similar to glycine dehydrogenase during the progression of BSE (h). neg, negative control; pos, positive control; 6m, 6 mpi.
Validation of gene expression profiling by quantitative RT-PCR.
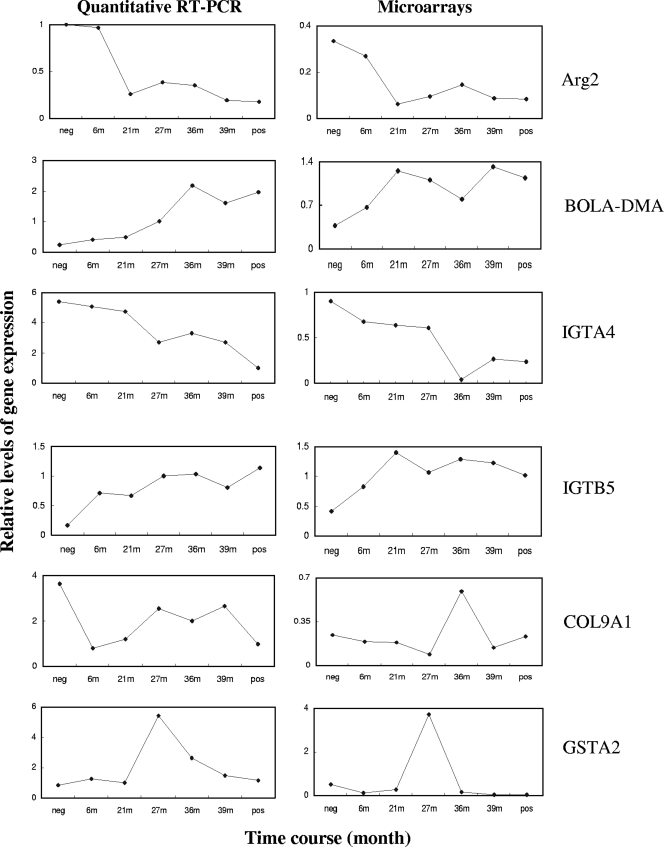
To confirm the results of the microarray and ANOVA analyses, we carried out quantitative RT-PCR using TaqMan assays. We chose two upregulated genes, two downregulated genes, and two complex regulated genes for the validation, and two of these genes (arginase and MHC class II [MHC-II]) have been reported to be associated with prion diseases (26, 30). The following genes were tested: Arg2 (arginase), ITGB5 (integrin), ITGA4 (integrin), COL9A1 (collagen), GSTA2 (glutathione S-transferase) and BOLA-DMA (major histocompatibility complex, class II, DM alpha-chain) (Fig. 3). Five quantitative RT-PCR analyses out of six showed very similar results compared to the data from microarrays (Arg2, ITGB5, ITGA4, GSTA2, and BOLA-DMA). One analysis (COL9A1) showed only partial correspondence. On the whole, the quantitative RT-PCR was, therefore, able to confirm the microarray data.
FIG. 3.
Relative levels of expression of Arg2, BOLA-DMA, IGTA4, IGTB5, COL9A1, and GSTA2 during the progression of BSE. The graphs of quantitative RT-PCR and microarrays are shown side by side for comparison. The time points are 6 mpi (6m), 21 mpi (21m), 27 mpi (27m), 36 mpi (36m), and 39 mpi (39m). neg, negative control; pos, positive control.
Regulation of genes associated with prion diseases.
The genes differentially regulated during development of BSE were then compared with genes that have been reported to be associated with prion diseases in other studies. Seven of these genes were found in this study: MHC-II (30), nicotinic beta 4 acetylcholine receptor (22), S100 calcium-binding protein (36), arginase (26), proteasome 26S subunit (17), 14-3-3 protein (18), and flotillin-1 (23) (Table 1). Many other genes have been found to be differentially regulated by other microarray studies using mice with scrapie. Several of these genes could be identified on the bovine arrays, but they were not differentially regulated using the stringent criteria in this study. However, the trends in their changes of expression (e.g., upregulation of the GFAP [glial fibrillary acidic protein] gene) were in agreement with the results of previously published studies (Table 2). The expression of the prion protein gene itself (Prnp) was not changed during infection, and again this has been observed in many studies before.
TABLE 2.
Relative levels of expression for genes previously associated with prion diseases
| Genea | Relative level of expression of gene from samples from animalsb |
Expression in other studies (reference) | ||||||
|---|---|---|---|---|---|---|---|---|
| Neg | 6 mpi | 21 mpi | 27 mpi | 36 mpi | 39 mpi | Pos | ||
| PRNP | 1.2 | 0.8 | 1.15 | 0.97 | 1.04 | 1.01 | 1.07 | No change (16) |
| GFAP | 0.325 | 0.84 | 1.2 | 1.11 | 0.824 | 1.15 | 1.06 | Up (36) |
| Cathepsin D | 0.46 | 1.2 | 1.27 | 0.96 | 1.22 | 1.06 | 0.85 | Up (25) |
| Cathepsin S | 0.78 | 0.64 | 1.0 | 1.07 | 1.04 | 1.41 | 1.25 | Up (25) |
| Cathepsin Z | 0.53 | 0.8 | 1.1 | 0.89 | 1.39 | 1.13 | 1.0 | Up (25) |
| Integrin B2 | 0.4 | 0.85 | 1.1 | 1.18 | 1.27 | 1.31 | 1.27 | Up (25) |
| CD9 | 0.43 | 0.68 | 1.25 | 1.09 | 0.83 | 1.19 | 1.17 | Up (25) |
| CD53 | 0.53 | 0.35 | 0.9 | 1.1 | 1.0 | 1.52 | 1.4 | Up (25) |
| CD63 | 0.5 | 0.83 | 1.14 | 1.03 | 1.15 | 1.24 | 1.0 | Up (25) |
| B2M | 0.57 | 0.58 | 1.26 | 1.1 | 0.82 | 1.52 | 1.17 | Up (25) |
| SOX9 | 0.44 | 0.23 | 0.25 | 0.19 | 0.24 | 0.21 | 0.22 | Down (29)c |
| Vimentin | 0.93 | 1.03 | 1.41 | 1.03 | 1.71 | 1.38 | 0.58 | Down (29) |
| Clusterin | 0.31 | 0.39 | 1.08 | 0.98 | 0.97 | 1.27 | 1.07 | Up (29) |
PRNP, prion protein; GFAP, glial fibrillary acidic protein.
Levels of expression of genes from samples from positive controls (Pos) and negative controls (Neg) and from animals at the time points postinoculation (6 mpi to 39 mpi).
The SOX9 gene is downregulated in ME7 mice and upregulated in 22L mice and in Chandler mice infected with RML prion.
The largest amount of differentially regulated genes was found at 21 mpi.
To examine the degree of differential expression between the negative controls and the various time points postincubation including the positive controls, we added three different change (fold) filters to analyze the 205 probe sets from ANOVA analysis. The analysis showed that at 21 mpi, there were more genes differentially regulated than at any other time points for all three different changes (1.5-, 2-, and 4-fold; Table 3).
TABLE 3.
Number of genes that pass ANOVA analysis and change (fold) tests at each time pointa
| Time point | No. of genes that pass the following change test: |
||
|---|---|---|---|
| 1.5-fold | 2-fold | 4-fold | |
| 6 mpi | 103 | 58 | 13 |
| 21 mpi | 154 | 105 | 35 |
| 27 mpi | 130 | 80 | 15 |
| 36 mpi | 120 | 76 | 23 |
| 39 mpi | 140 | 90 | 27 |
| Positive controls | 135 | 83 | 17 |
Number of genes that pass ANOVA analysis and change tests at each time point compared to the values for the negative controls.
We next applied a twofold change filter to the genes in Table 1 to examine which gene changed expression twofold or more between the negative controls and 21 mpi samples. Out of 114 genes, 51 passed the twofold change filter, and among them were 6 out of 7 genes previously described as associated with prion disease. This analysis indicates that even at 21 mpi, most of prion disease-associated genes found in this study have been differentially regulated.
Differentially regulated genes correlated well with the time course of BSE.
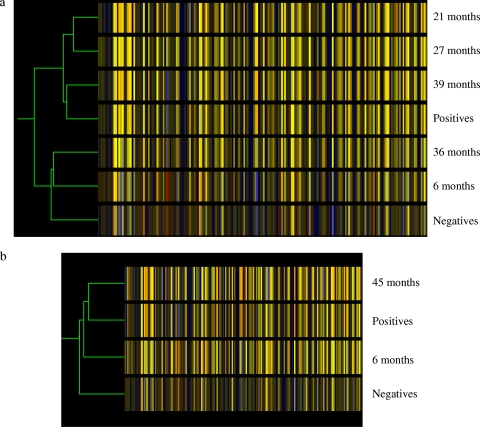
We next carried out clustering analysis to investigate whether those 205 probe sets from the ANOVA analysis reflect the progression of BSE. For this purpose, individual samples of each time point postincubation were grouped together. Figure 4a showed that according to gene expression profiles of the 205 probe sets, there was a good reflection of the BSE time course except for the samples at 36 mpi.
FIG. 4.
Condition trees of clustering analysis. The analysis was performed by GeneSpring using 205 genes found by ANOVA analysis. The individual samples were grouped by time point. Similarity was measured using the standard correlation with a value of 1 for the separation ratio and a value of 0.001 for the minimum distance to merge similar branches. Each colored bar represents a gene, and the color represents the level of expression. The relative levels of expression are displayed in different colors: red, 5; orange, 2; yellow, 1; dark yellow, 0.7; dark blue, 0.4; and blue, 0.1. (a) Samples taken from negative controls, samples taken from animals at 6 mpi, 21 mpi, 27 mpi, 36 mpi, and 39 mpi, and samples taken from positive controls. (b) Samples taken from negative controls and positive controls and samples taken from animals at 6 mpi and 45 mpi.
Furthermore, in addition to the time course samples that were used in the analysis so far, there were two additional samples for animals at 45 mpi, which did not show any clinical signs of BSE when they were culled. These samples were not used in the ANOVA analysis because the animals were older than the positive-control animals (41 to 44 mpi). Clustering analysis was carried out to predict whether they were closer to the negative- or positive-control animals. The results showed that the gene expression profiles of the 205 genes were very similar to the profiles of positive-control animals and very dissimilar to the negative-control animals or animals at 6 mpi (Fig. 4b). These results suggest that it is possible to predict BSE in animals during their incubation time while they are still clinically healthy.
DISCUSSION
Gene expression profiling in the BSE time course study revealed a broad correlation between the expression of genes and the progression of BSE. The incubation state most probably varied from animal to animal even at the same time point, as the efficacy of the oral exposure can be quite varied (32). This is exemplified by the clinical onset of BSE from 33 to 55 mpi in cattle following oral exposure with 100 g BSE material (34). The finding that one of the 36 mpi samples exhibited an expression profile similar to profiles from negative-controls and samples taken from animals at 6 mpi might indicate that the oral infection in this animal might have been much delayed or even failed.
ANOVA analysis defined 205 significantly changed probe sets, of which 114 genes with a known function could be identified. The association of seven of those genes with prion diseases had been described previously. The expression of three T-cell receptors was found to have significantly changed. This may be due to T cells infiltrating the brain of an animal infected with the BSE agent in the same way as occurs in murine and human transmissible spongiform encephalopathies (19).
Also, in the immune response group, the expression of the MHC-II gene was upregulated with an expression maximum at 39 mpi. This may indicate an immune response in the brain either through microglia activation or T-cell infiltration (14, 19). In the brains of CJD patients, the increased expression of MHC-II correlates well with neuronal apoptosis (14).
The microarray data also showed that there was a 12-fold reduction in expression of acetylcholine receptor (nicotinic, beta 4) at 6 mpi and that expression remained low throughout the time course of BSE. The regulation of acetylcholine receptor is linked to many neurodegenerative diseases. In Parkinson's disease and Alzheimer's disease, one of the pathogenic features is the loss of several subunits of the nicotinic acetylcholine receptor (13).
Furthermore, the expression of NR1H3 was reduced from the 6 mpi level. At 36 mpi, the expression of NR1H3 reached its lowest level (−4.7-fold of the negative controls). This might be linked to the downregulation of cholesterol synthesis similar to the findings in mice with scrapie (36). The nuclear oxysterol receptors liver X receptor-alpha [LXRalpha (NR1H3)] and LXRbeta (NR1H2) regulate genes involved in cholesterol homeostasis in a coordinate manner. Perturbations of cholesterol metabolism are associated with the development of other neurodegenerative diseases (21).
There were not many genes used both in this study and in the study by Sawiris and colleagues using VM mice infected with the BSE agent (27). This may be due to three fundamental differences between these two studies. First, Sawiris and colleagues used mice inoculated intraperitoneally with the mouse-adapted BSE strain 301V, while in this study cattle were orally infected with BSE. Second, in the mouse system, the whole brain had to be used for the expression analysis, while in this study the samples were from the brain stem. The third major difference is that in the mouse model only the negative and positive samples at the time point of terminal disease were analyzed, while in this study the progression of BSE was analyzed by using time course samples.
In this study we found that at 21 mpi, more genes passed both the ANOVA and change (fold) filters than at any other time point. Many of them are known to be associated with prion diseases. This may explain why at 24 to 25 mpi some cattle have shown some early clinical signs of BSE even though there is no detectable PrPSc in the brain at this stage of the disease, and the earliest detection of infectivity of BSE in the central nervous system starts at 32 mpi (3, 32, 34). Our results indicate that the global changes of gene expression activities are prior to the PrPSc accumulation and the appearance of clinical signs. Some of the genes identified at 21 mpi may therefore be responsible for the subsequent pathological and clinical events. While there is no detectable pathology present at this time point, microglia activation might already occur as it coincides with the earliest changes in neuronal morphology (11, 35). Furthermore, in a recent systems biology study based on gene expression changes in mice with scrapie, mRNA levels of C1qa/b and C3ar1 reflecting activation of microglia and astrocytes are among the first detectable expression changes (10 weeks postinfection) and precede, e.g., clinical signs by many weeks (16). Not only are our results consistent with early changes of gene expression in mice infected with the TSE agent (16, 36), they are also consistent with the behavioral changes in the early stages of prion infection in mice (9, 10, 20). Prion-infected mice have shown significant inability to discriminate a novel object from 7 week postinoculation compared with healthy mice (20). In this study mice succumb to RML prion at around 13 weeks postinoculation. Early cognitive deficits are therefore preceding the clinically recognizable prion disease in this model. The authors reason that an unidentified transient neurotoxic species is generated within neurons when PrPC is converted to PrPSc, which rapidly impairs neuronal function and synaptic responses. Recently, Collinge and Clarke (8) have proposed that it is PrPL that is responsible for the neurotoxicity instead of PrPSc. PrPL is a templated intermediate or side product during the accumulation of PrPSc. It is possible that the early changes in gene expression in this study are the responses to neurotoxicity produced by PrPL. Other studies have shown that soluble, low-molecular-weight oligomers of the full-length prion protein (PrP) are neurotoxic both in vitro and in vivo (28). The pronounced changes in gene expression at 21 mpi might therefore be a consequence of the emergence of low-molecular-weight oligomers. These oligomers are probably very difficult to detect with current assay technology, but sensitive methods, such as protein misfolding cyclic amplification might be able to detect the presence of these oligomers in the future.
The differentially regulated genes identified by the ANOVA analysis correlated well with the development of BSE. Therefore, the profiles of those genes might be able to tell the infectivity status of any given sample as shown for the samples at 45 mpi that were still clinically and pathologically negative. Their expression profiles were very similar to those of the positive controls. It will be interesting to know whether the results from this study can be applied to field cases of BSE, which are far more complex than the samples from the time course experiments regarding genotype, breed, feed, age, and environment.
In summary, there are two major findings of this study. First, at 21 mpi, there were more changes in gene expression compared to the negative controls than at any other period during the time course of BSE. Second, the 205 probe sets found by ANOVA might be used to predict the incubation period using clustering analysis.
Acknowledgments
We thank T. Haferlach and Leukemia Diagnostics, Ludwig- Maximilians University, Munich, Germany, for the use of the Affymetrix workstation. We thank Gerald Wells and Danny Matthews for the design of and advice on the BSE pathogenesis study, and we thank Linda Terry for useful discussions.
This work was supported by a development grant from the Veterinary Laboratories Agency and a grant from the European Network of Excellence Neuroprion (FOOD-CT-2004-506579).
Footnotes
Published ahead of print on 8 July 2009.
REFERENCES
- 1.Affymetrix UK Ltd. 2001. GeneChip expression analysis technical manual. Affymetrix, Ltd., Santa Clara, CA. http://www.affymetrix.com/support/downloads/manuals/expression_analysis_technical_manual.pdf.
- 1a.Aguzzi, A. 2006. Prion diseases of humans and farm animals: epidemiology, genetics, and pathogenesis. J. Neurochem. 97:1726-1739. [DOI] [PubMed] [Google Scholar]
- 2.Aguzzi, A., C. Sigurdson, and M. Heikenwalder. 2008. Molecular mechanisms of prion pathogenesis. Annu. Rev. Pathol. 3:11-40. [DOI] [PubMed] [Google Scholar]
- 3.Arnold, M. E., J. B. Ryan, T. Konold, M. M. Simmons, Y. I. Spencer, A. Wear, M. Chaplin, M. Stack, S. Czub, R. Mueller, P. R. Webb, A. Davis, J. Spiropoulos, J. Holdaway, S. A. Hawkins, A. R. Austin, and G. A. Wells. 2007. Estimating the temporal relationship between PrPSc detection and incubation period in experimental bovine spongiform encephalopathy of cattle. J. Gen. Virol. 88:3198-3208. [DOI] [PubMed] [Google Scholar]
- 4.Biacabe, A. G., J. L. Laplanche, S. Ryder, and T. Baron. 2004. Distinct molecular phenotypes in bovine prion diseases. EMBO Rep. 5:110-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Booth, S., C. Bowman, R. Baumgartner, G. Sorensen, C. Robertson, M. Coulthart, C. Phillipson, and R. L. Somorjai. 2004. Identification of central nervous system genes involved in the host response to the scrapie agent during preclinical and clinical infection. J. Gen. Virol. 85:3459-3471. [DOI] [PubMed] [Google Scholar]
- 6.Caramelli, M., P. Acutis, E. Bozzetta, C. Casalone, C. Gagna, and G. Ru. 2003. Bovine spongiform encephalopathy in Italian herds. Vet. Rec. 153:711-712. [PubMed] [Google Scholar]
- 7.Collinge, J. 2005. Molecular neurology of prion disease. J. Neurol. Neurosurg. Psychiatry 76:906-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collinge, J., and A. R. Clarke. 2007. A general model of prion strains and their pathogenicity. Science 318:930-936. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham, C., R. M. Deacon, K. Chan, D. Boche, J. N. Rawlins, and V. H. Perry. 2005. Neuropathologically distinct prion strains give rise to similar temporal profiles of behavioral deficits. Neurobiol. Dis. 18:258-269. [DOI] [PubMed] [Google Scholar]
- 10.Dell'Omo, G., E. Vannoni, A. L. Vyssotski, M. A. Di Bari, R. Nonno, U. Agrimi, and H. P. Lipp. 2002. Early behavioural changes in mice infected with BSE and scrapie: automated home cage monitoring reveals prion strain differences. Eur. J. Neurosci. 16:735-742. [DOI] [PubMed] [Google Scholar]
- 11.Eikelenboom, P., C. Bate, W. A. Van Gool, J. J. Hoozemans, J. M. Rozemuller, R. Veerhuis, and A. Williams. 2002. Neuroinflammation in Alzheimer's disease and prion disease. Glia 40:232-239. [DOI] [PubMed] [Google Scholar]
- 12.Gavier-Widen, D., M. J. Stack, T. Baron, A. Balachandran, and M. Simmons. 2005. Diagnosis of transmissible spongiform encephalopathies in animals: a review. J. Vet. Diagn. Investig. 17:509-527. [DOI] [PubMed] [Google Scholar]
- 13.Gotti, C., M. Moretti, I. Bohr, I. Ziabreva, S. Vailati, R. Longhi, L. Riganti, A. Gaimarri, I. G. McKeith, R. H. Perry, D. Aarsland, J. P. Larsen, E. Sher, R. Beattie, F. Clementi, and J. A. Court. 2006. Selective nicotinic acetylcholine receptor subunit deficits identified in Alzheimer's disease, Parkinson's disease and dementia with Lewy bodies by immunoprecipitation. Neurobiol. Dis. 23:481-489. [DOI] [PubMed] [Google Scholar]
- 14.Gray, F., F. Chretien, H. Adle-Biassette, A. Dorandeu, T. Ereau, M. B. Delisle, N. Kopp, J. W. Ironside, and C. Vital. 1999. Neuronal apoptosis in Creutzfeldt-Jakob disease. J. Neuropathol. Exp. Neurol. 58:321-328. [DOI] [PubMed] [Google Scholar]
- 15.Hilton, D. A. 2006. Pathogenesis and prevalence of variant Creutzfeldt-Jakob disease. J. Pathol. 208:134-141. [DOI] [PubMed] [Google Scholar]
- 16.Hwang, D., I. Y. Lee, H. Yoo, N. Gehlenborg, J. H. Cho, B. Petritis, D. Baxter, R. Pitstick, R. Young, D. Spicer, N. D. Price, J. G. Hohmann, S. J. Dearmond, G. A. Carlson, and L. E. Hood. 2009. A systems approach to prion disease. Mol. Syst. Biol. 5:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kristiansen, M., P. Deriziotis, D. E. Dimcheff, G. S. Jackson, H. Ovaa, H. Naumann, A. R. Clarke, F. W. van Leeuwen, V. Menendez-Benito, N. P. Dantuma, J. L. Portis, J. Collinge, and S. J. Tabrizi. 2007. Disease-associated prion protein oligomers inhibit the 26S proteasome. Mol. Cell 26:175-188. [DOI] [PubMed] [Google Scholar]
- 18.Lee, K. H., and M. G. Harrington. 1997. The assay development of a molecular marker for transmissible spongiform encephalopathies. Electrophoresis 18:502-506. [DOI] [PubMed] [Google Scholar]
- 19.Lewicki, H., A. Tishon, D. Homann, H. Mazarguil, F. Laval, V. C. Asensio, I. L. Campbell, S. DeArmond, B. Coon, C. Teng, J. E. Gairin, and M. B. Oldstone. 2003. T cells infiltrate the brain in murine and human transmissible spongiform encephalopathies. J. Virol. 77:3799-3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mallucci, G. R., M. D. White, M. Farmer, A. Dickinson, H. Khatun, A. D. Powell, S. Brandner, J. G. Jefferys, and J. Collinge. 2007. Targeting cellular prion protein reverses early cognitive deficits and neurophysiological dysfunction in prion-infected mice. Neuron 53:325-335. [DOI] [PubMed] [Google Scholar]
- 21.Patel, N. V., and B. M. Forman. 2004. Linking lipids, Alzheimer's and LXRs? Nucl. Recept. Signal. 2:e001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrakis, S., T. Irinopoulou, C. H. Panagiotidis, R. Engelstein, J. Lindstrom, A. Orr-Urtreger, R. Gabizon, N. Grigoriadis, and T. Sklaviadis. 2008. Cellular prion protein co-localizes with nAChR beta4 subunit in brain and gastrointestinal tract. Eur. J. Neurosci. 27:2212. [DOI] [PubMed] [Google Scholar]
- 23.Pimpinelli, F., S. Lehmann, and I. Maridonneau-Parini. 2005. The scrapie prion protein is present in flotillin-1-positive vesicles in central- but not peripheral-derived neuronal cell lines. Eur. J. Neurosci. 21:2063-2072. [DOI] [PubMed] [Google Scholar]
- 24.Prusiner, S. B. 1998. Prions. Proc. Natl. Acad. Sci. USA 95:13363-13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riemer, C., S. Neidhold, M. Burwinkel, A. Schwarz, J. Schultz, J. Kratzschmar, U. Monning, and M. Baier. 2004. Gene expression profiling of scrapie-infected brain tissue. Biochem. Biophys. Res. Commun. 323:556-564. [DOI] [PubMed] [Google Scholar]
- 26.Roikhel, V. M., G. I. Fokina, A. I. Khokhlov, S. G. Sobolev, I. A. Zavalishin, M. B. Korolev, and V. V. Pogodina. 1990. Alterations of arginase activity in scrapie-infected mice and in amyotrophic lateral sclerosis. Acta Virol. 34:545-553. [PubMed] [Google Scholar]
- 27.Sawiris, G. P., K. G. Becker, E. J. Elliott, R. Moulden, and R. G. Rohwer. 2007. Molecular analysis of bovine spongiform encephalopathy infection by cDNA arrays. J. Gen. Virol. 88:1356-1362. [DOI] [PubMed] [Google Scholar]
- 28.Simoneau, S., H. Rezaei, N. Sales, G. Kaiser-Schulz, M. Lefebvre-Roque, C. Vidal, J. G. Fournier, J. Comte, F. Wopfner, J. Grosclaude, H. Schatzl, and C. I. Lasmezas. 2007. In vitro and in vivo neurotoxicity of prion protein oligomers. PLoS Pathog. 3:e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skinner, P. J., H. Abbassi, B. Chesebro, R. E. Race, C. Reilly, and A. T. Haase. 2006. Gene expression alterations in brains of mice infected with three strains of scrapie. BMC Genomics 7:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.von Eitzen, U., R. Egensperger, S. Kosel, E. M. Grasbon-Frodl, Y. Imai, K. Bise, S. Kohsaka, P. Mehraein, and M. B. Graeber. 1998. Microglia and the development of spongiform change in Creutzfeldt-Jakob disease. J. Neuropathol. Exp. Neurol. 57:246-256. [DOI] [PubMed] [Google Scholar]
- 31.Wadsworth, J. D., and J. Collinge. 2007. Update on human prion disease. Biochim. Biophys. Acta 1772:598-609. [DOI] [PubMed] [Google Scholar]
- 32.Wells, G. A., T. Konold, M. E. Arnold, A. R. Austin, S. A. Hawkins, M. Stack, M. M. Simmons, Y. H. Lee, D. Gavier-Widen, M. Dawson, and J. W. Wilesmith. 2007. Bovine spongiform encephalopathy: the effect of oral exposure dose on attack rate and incubation period in cattle. J. Gen. Virol. 88:1363-1373. [DOI] [PubMed] [Google Scholar]
- 33.Wells, G. A., A. C. Scott, C. T. Johnson, R. F. Gunning, R. D. Hancock, M. Jeffrey, M. Dawson, and R. Bradley. 1987. A novel progressive spongiform encephalopathy in cattle. Vet. Rec. 121:419-420. [DOI] [PubMed] [Google Scholar]
- 34.Wells, G. A. H., and J. W. Wilesmith. 2004. Bovine spongiform encephalopathy and related diseases, p. 595-628. In S. B. Prusiner (ed.), Prion biology and diseases. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 35.Williams, A., P. J. Lucassen, D. Ritchie, and M. Bruce. 1997. PrP deposition, microglial activation, and neuronal apoptosis in murine scrapie. Exp. Neurol. 144:433-438. [DOI] [PubMed] [Google Scholar]
- 36.Xiang, W., M. Hummel, G. Mitteregger, C. Pace, O. Windl, U. Mansmann, and H. A. Kretzschmar. 2007. Transcriptome analysis reveals altered cholesterol metabolism during the neurodegeneration in mouse scrapie model. J. Neurochem. 102:834-847. [DOI] [PubMed] [Google Scholar]
- 37.Xiang, W., O. Windl, G. Wunsch, M. Dugas, A. Kohlmann, N. Dierkes, I. M. Westner, and H. A. Kretzschmar. 2004. Identification of differentially expressed genes in scrapie-infected mouse brains by using global gene expression technology. J. Virol. 78:11051-11060. [DOI] [PMC free article] [PubMed] [Google Scholar]