Abstract
The current concept is that Tsc-deficient cells are sensitized to apoptosis due to the inhibition of Akt activity by the negative feedback mechanism induced by the hyperactive mTORC1. Unexpectedly, however, we found that Tsc1/2-deficient cells exhibit increased resistance to serum deprivation-induced apoptosis. mTORC1 hyperactivity contributes to the apoptotic resistance of serum-deprived Tsc1/2-deficient cells in part by increasing the growth factor-independent expression of hexokinase II (HKII) and GLUT1. mTORC1-mediated increase in hypoxia-inducible factor 1α (HIF1α) abundance, which occurs in the absence of serum in normoxic Tsc2-deficient cells, contributes to these changes. Increased HIF1α abundance in these cells is attributed to both an increased level and the sustained translation of HIF1α mRNA. Sustained glycogen synthase kinase 3β inhibition and Mcl-1 expression also contribute to the apoptotic resistance of Tsc2-deficient cells to serum deprivation. The inhibition of mTORC1 activity by either rapamycin or Raptor knockdown cannot resensitize these cells to serum deprivation-induced apoptosis because of elevated Akt activity that is an indirect consequence of mTORC1 inhibition. However, the increased HIF1α abundance and the maintenance of Mcl-1 protein expression in serum-deprived Tsc2−/− cells are dependent largely on the hyperactive eIF4E in these cells. Consistently, the reduction of eIF4E levels abrogates the resistance of Tsc2−/− cells to serum deprivation-induced apoptosis.
Growth factors are obligatory for the survival of mammalian cells. The evolutionarily conserved kinase Akt has emerged as the predominant and indispensable mediator of the ability of growth factors to promote cell survival in mammalian cells (reviewed in reference 9). Akt promotes cell survival by multiple mechanisms, including key roles in regulating cellular energy metabolism. Akt maintains mitochondrial integrity and inhibits apoptosis at least in part through effects on mitochondrial hexokinases and their functionally coupled facilitated glucose transporters (reviewed in reference 18). One of the most crucial functions of Akt involves the activation of the mammalian target of rapamycin complex 1 (mTORC1), which integrates growth factor signaling with nutritional cues and synchronizes these upstream signals with the downstream stimulation of cell growth and proliferation (reviewed in reference 1). Akt activates mTORC1 in part by inhibiting the heterodimeric tuberous sclerosis complex (Tsc1/Tsc2). Tsc2 (or tuberin) functions as a GTPase-activating protein (GAP) to specifically inhibit the small GTPase Rheb, which activates mTORC1. The formation of a functional heterodimeric complex between Tsc2 and Tsc1 (or hamartin) is required for mTORC1 inhibition. As such, the disruption of the expression or function of either Tsc1 or Tsc2 is sufficient to activate mTORC1. Mammalian cells have evolved a negative feedback mechanism between mTORC1 and Akt to maintain an optimal balance between their activities. When Akt activates mTORC1, it initiates a negative feedback loop that serves to attenuate Akt activity. As such, mTORC1 serves as both an upstream and a downstream effector of Akt signaling. The loss of a functional Tsc1/Tsc2 complex disrupts this delicate balance, resulting in mTORC1 hyperactivity, which greatly reduces Akt activation (reviewed in reference 1). This is relevant to the heritable development of tuberous sclerosis in humans, which is caused by the mutational inactivation of either the TSC1 or TSC2 gene, leading to benign hamartoma formation and growth in a variety of organs (11).
It is widely appreciated that low basal Akt activity renders Tsc1/2-deficient cells more sensitive to proapoptotic stimuli (4, 19). Unexpectedly, however, we found that both Tsc1 and Tsc2 null cells exhibit increased apoptotic resistance to growth factor withdrawal despite greatly reduced Akt activity relative to that of their wild-type counterparts. This implies that Tsc1/2 deficiency promotes or unmasks potent antiapoptotic mechanisms that reduce mammalian cell dependence upon growth factors and Akt for survival. Further investigation has uncovered a critical role for mTORC1 in promoting cell survival in the absence of growth factors.
Trophic growth factors found in serum play a pivotal role in the cellular uptake and utilization of glucose, and serum withdrawal results in attenuated glucose metabolism. The maintenance of glucose utilization by the overexpression of the rate-limiting glycolytic enzyme hexokinase and its functionally coupled facilitative glucose transporters maintains cell survival in the absence of growth factors (reviewed in reference 18). We found that serum deprivation markedly increased both hexokinase II (HKII) and GLUT1 abundance in Tsc2-deficient cells, and the knockdown of HKII and GLUT1 increased the apoptotic susceptibility of these cells to serum deprivation. The elevated expression of HKII and GLUT1 is mediated by hypoxia-inducible factor 1α (HIF1α) protein, which is markedly induced by mTORC1 in serum-deprived Tsc2−/− cells.
In addition to increased HKII and GLUT1 expression, Tsc2−/− cells display the sustained inhibition of glycogen synthase kinase 3 (GSK3) activity and stable Mcl-1 abundance following serum withdrawal, which also contribute to their apoptotic resistance under these conditions. Mcl-1 abundance, which normally declines following serum deprivation, is sustained in Tsc2−/− cells by the constitutive inhibition of GSK3 and the activation of eIF4E.
MATERIALS AND METHODS
Cells and retroviruses.
All cells were cultured in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% fetal bovine serum (FBS; Gemini Bio-Products), 100 IU/ml penicillin, and 100 μg/ml streptomycin. The Tsc2+/− murine embryonic fibroblasts (MEFs) and Tsc2−/− MEFs (gifts from D. J. Kwiatkowski) were derived from p53−/− mouse embryos. The Tsc1+/+ MEFs and Tsc1−/− MEFs were obtained as primary cells containing wild-type p53 (gifts from D. J. Kwiatkowski) and were immortalized by infecting them with a retrovirus (pBabePuro-GSE56) expressing dominant-negative p53 (20).
All of the retroviruses used were generated by the transient transfection of the retroviral vectors into Phoenix ecotropic packaging cell lines, and then the retrovirus-containing culture medium was harvested. The retroviruses were used to infect cells in the presence of 8 μg/ml polybrene for 24 h. The pBabe(GFP)-BAD and pBabe(GFP)-BAD S112,136A plasmids were described before (10). The retroviral vector pLPCX-Tsc2 was constructed by inserting rat Tsc2 into the NotI site of pLPCX. Retroviruses encoding either pLPCX or pLPCX-Tsc2 were made and used to infect Tsc2−/− p53−/− MEFs, which then were selected using puromycin (3.5 μg/ml), resulting in the generation of Tsc2−/−/pLPCX MEFs and Tsc2−/−/pLPCX-Tsc2 MEFs.
RNA interference-mediated gene knockdown.
The short interfering RNAs (siRNAs) were transfected into the Tsc2−/− MEFs using Lipofectamine 2000 transfection reagent. GLUT1 knockdown was achieved by transfecting cells with GLUT1 Stealth siRNA from Invitrogen (GGCGGAACUCCAUGCUGAUGAUGAA); the corresponding negative control used was Stealth negative control medium GC siRNA (Invitrogen). Mcl-1 knockdown was achieved by transfecting cells with Mcl-1 SMARTpool siRNA from Dharmacon. HIF1α knockdown was achieved by transfecting cells with HIF1A ON-TARGETplus SMARTpool siRNA from Dharmacon. For short hairpin RNA (shRNA)-mediated gene knockdown, the shRNAs were cloned into the lentiviral vector pLenti6/BLOCK-iT-DEST (Invitrogen), and lentiviruses encoding these vectors were generated. The lentiviruses were used to infect Tsc2−/− MEFs in the presence of polybrene (8 μg/ml) for about 8 h. After about 48 h of infection, the cells were selected using Blasticidin (20 μg/ml for 3 days). The Raptor and LacZ shRNA sequences were described before (20). The sequences of the HKII shRNA are 5′CACCGCATATGATCGCCTGCTTATTCGAAAATAAGCAGGCGATCATATGC 3′ (top strand) and 5′AAAAGCATATGATCGCCTGCTTATTTTCGAATAAGCAGGCGATCATATGC 3′ (bottom strand). The sequences of the Akt1 shRNA are 5′CACCGCTACTTCCTCCTCAAGAATGTTCAAGAGACATTCTTGAGGAGGAAGTAGC 3′ (top strand) and 5′AAAAGCTACTTCCTCCTCAAGAATGTCTCTTGAACATTCTTGAGGAGGAAGTAGC 3′ (bottom strand). The LacZ-targeting shRNA was used as a negative control for the Raptor-, HKII-, and Akt1-targeting shRNAs.
Antisense knockdown of eIF4E.
To reduce eIF4E expression, eIF4E antisense oligonucleotides (ASO) with a phosphorothioate backbone and flanking bases modified with a methoxyethyl group at the 2′ position of the sugar were obtained from Eli Lilly and were used as previously described (6). Cells were washed twice with phosphate-buffered saline (PBS) 24 and 48 h posttransfection, and cells were subjected to serum withdrawal for 72 h. Cells then were lysed for protein isolation and immunoblotting or fixed with formaldehyde and subjected to 4′,6′-diamidino-2-phenylindole (DAPI) staining for apoptosis determination.
Apoptosis assay.
Cells were plated at low density (∼50,000 cells/well of a 6-well plate) and allowed to grow overnight before being subjected to treatments that induce apoptosis. At the end of the apoptosis induction period, the cells were fixed by the direct addition of formaldehyde (final concentration, 12%) to the culture medium. After overnight fixation, the cells were washed with PBS, stained with DAPI (1 μg/ml for 6 min), and then left in PBS. The cells were visualized using fluorescence microscopy, and cells with the condensed and/or fragmented chromatin that is characteristic of apoptosis were counted as apoptotic.
Immunoblot analysis.
For immunoblot analysis, the cells were plated at low density (∼125,000 cells/6-cm plate) and allowed to grow for 24 h before the application of the various experimental conditions. Afterwards, the cells were harvested, and cell extracts were made using ice-cold lysis buffer (20 mM HEPES, 1% Triton X-100, 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 10 mM sodium pyrophosphate, 100 mM NaF, 5 mM iodoacetic acid, 20 nM okadaic acid, 0.2 mM phenylmethylsulfonyl fluoride, and Complete protease inhibitor cocktail [Roche Diagnostics]). The extracts were run on sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to a polyvinyl difluoride membrane, and probed with the following antibodies: anti-Raptor (a gift from N. Sonenberg), anti-phospho-p70 S6 kinase T389 (Cell Signaling), anti-p70 S6 kinase (Cell Signaling), anti-phospho-Akt S473 (Cell Signaling), anti-Akt (Cell Signaling), anti-Akt1 pH domain (Upstate), antiactin (Sigma), anti-BAD (Cell Signaling), anti-phospho-BAD S112 (Cell Signaling), anti-phospho-BAD S136 (Cell Signaling), anti-GLUT1 (Alpha Diagnostic), anti-Mcl-1 (Santa Cruz), anti-HKII (a gift from R. B. Robey), anti-GSK3β (QCB), anti-phospho-GSK3β Ser9 (Cell Signaling), HIF1α (Novus), anti-eIF4E (obtained from N. Sonenebrg), and anti-tubulin (Sigma). The quantification of Western blots was done using ImageJ software.
Real-time reverse transcription-PCR (RT-PCR).
Total RNA was isolated from cells and reverse transcribed into cDNA, which then was subjected to a real-time PCR using the iQ SYBR green supermix reagent (Bio-Rad). The GLUT1 PCR primers used were 5′CAGTTCGGCTATAACACTGGTG3′ and 5′GCCCCCGACAGAGAAGATG3′. The HKII PCR primers used were 5′TGATCGCCTGCTTATTCACGG3′ and 5′AACCGCCTAGAAATCTCCAGA3′. The HIF1α PCR primers used were 5′ACCTTCATCGGAAACTCCAAAG3′ and 5′ACTGTTAGGCTCAGGTGAACT3′.
BrdU labeling.
To determine actively replicating cells after serum withdrawal or upon serum stimulation, cells were incubated in the absence of serum for 48 h and then either fixed or rinsed twice with PBS and stimulated with 10% FBS for 24 h before fixation. In both conditions, 3 h prior to fixation, cells were incubated with 3 μg/ml bromodeoxyuridine (BrdU) and stained for incorporated BrdU. Briefly, cells were washed with PBS, fixed for 10 min in 70% ice-cold ethanol at −20°C, and washed. The DNA then was denatured to single strands for the antibody to bind incorporated BrdU. This was done by incubating the cells in 2 M HCl-0.5% Triton X-100 for 1 h (at room temperature). Denaturation was stopped by washing the cells with 0.1 M sodium borate and then with PBS. The cells subsequently were incubated overnight at 4°C with a monoclonal antibody against BrdU (Dako) and fluorescein isothiocyanate-conjugated secondary antibody for 2 h (room temperature). Data are expressed as the percentages of cells positive for BrdU staining compared to the total cell number determined by DAPI staining.
RESULTS
Tsc1/2-deficient MEFs are highly resistant to apoptosis induced by growth factor withdrawal but are hypersensitive to other proapoptotic stimuli.
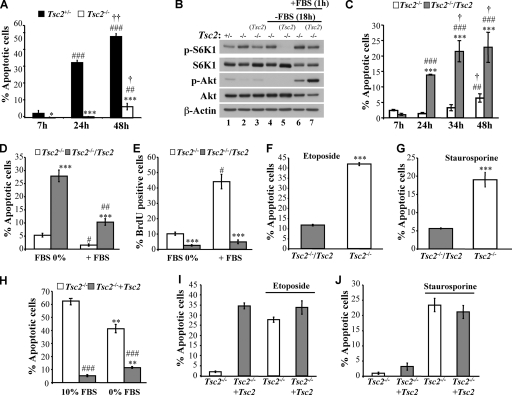
Because Tsc1/2-deficient cells display diminished Akt activity, it is expected that they are hypersensitive to apoptosis induced by growth factor withdrawal. However, we found that despite reduced Akt activity, Tsc2−/− MEFs are markedly more resistant to serum deprivation-induced apoptosis than their Tsc2+/− counterparts (Fig. 1A). To address the specificity of this observation, Tsc2−/− MEFs were stably transduced with a Tsc2-expressing retrovirus to generate a polyclonal Tsc2-reconstituted cell line (designated Tsc2−/−/Tsc2 MEFs) as previously described (20). A control polyclonal cell line transduced by the corresponding empty vector also was generated in parallel. As expected, the Tsc2 expression in Tsc2−/− cells restored Akt activity (Fig. 1B). As shown in Fig. 1C, Tsc2−/−/Tsc2 MEFs were dramatically more sensitive to apoptosis induced by serum withdrawal than control Tsc2−/− MEFs. Furthermore, after 48 h of serum deprivation, the Tsc2−/− cells completely recovered when they were reexposed to serum and continued to proliferate as measured by BrdU incorporation (Fig. 1D and E).
FIG. 1.
Tsc2−/− MEFs are resistant to serum deprivation-induced apoptosis but hypersensitive to other apoptotic stimuli in the presence of serum. (A) Apoptosis induced by the serum deprivation of Tsc2+/− or Tsc2−/− MEFs. The apoptosis was measured after 7, 24, or 48 h of serum deprivation. Data represent the means ± standard errors of the means from three independent experiments. * and ***, P < 0.05 and P < 0.001, respectively, compared to results for Tsc2+/− MEFs. ## and ###, P < 0.01 and P < 0.001, respectively, compared to results at 7 h. † and ††, P < 0.05 and P < 0.01, respectively, compared to results at 24 h. (B) Immunoblot showing mTORC1 activity and Akt activity. Tsc2+/− MEFs (lane 1), Tsc2−/− MEFs (lanes 2, 4, and 6), and Tsc2−/− MEFs reconstituted with Tsc2 (lanes 3, 5, and 7) were cultured in the presence (lanes 1 to 3) or absence of 10% serum (lanes 4 to 7) for 18 h and then were left untreated (lanes 4 and 5) or restimulated with 10% serum for 1 h (lanes 6 and 7). Lysates of these cells were analyzed by immunoblotting using antibodies specific for Akt phosphorylation (p-Akt) on Ser473 (an indicator of Akt activity), total Akt, p70S6 kinase phosphorylation on Thr389 (p-S6K1; an indicator of mTORC1 activity), total p70S6 kinase, and actin. (C) Apoptosis induced by the serum deprivation of Tsc2−/− MEFs or reconstituted Tsc2−/− (Tsc2−/−/Tsc2) MEFs. The apoptosis was measured after 7, 24, 34, or 48 h of serum deprivation. Data represent the means ± standard errors of the means of three independent experiments. ***, P < 0.001 compared to results for Tsc2−/− cells. ## and ###, P < 0.01 and P < 0.001, respectively, compared to results at 7 h. †, P < 0.05 compared to results at 24 h. (D) Apoptosis induced by the serum deprivation of Tsc2−/− MEFs or reconstituted Tsc2−/− (Tsc2−/−/Tsc2) MEFs. The cells were incubated in the absence of FBS for 48 h and then were fixed for apoptosis determination or rinsed twice with PBS and incubated with 10% FBS for 24 h. The apoptosis was measured after 48 h of serum deprivation or 48 h of serum deprivation, followed by 24 h of serum stimulation. Data represent the means ± standard errors of the means from three independent experiments. ***, P < 0.001 compared to results for Tsc2−/− MEFs. # and ##, P < 0.05 and P < 0.01, respectively, compared to results at 48 h with 0% FBS. (E) Proliferation rate of Tsc2−/− MEFs or reconstituted Tsc2−/− (Tsc2−/−/Tsc2) MEFs. The cells were incubated as described for panel D, and the proliferation rate was measured by labeling cells for 3 h with BrdU prior to fixation and staining. BrdU incorporation was carried out as described in Materials and Methods and measured by counting at least 150 cells from at least five fields in triplicate plates. Data represent the means ± standard errors of the means from three independent experiments. ***, P < 0.001 compared to results for Tsc2−/− MEFs. #, P < 0.05 compared to results at 48 h with 0% FBS. (F) Apoptosis induced by etoposide treatment of Tsc2−/− and Tsc2−/−/Tsc2 MEFs. Data represent the means ± standard errors of the means from three independent experiments. ***, P < 0.001 compared to results for Tsc2−/−/Tsc2. The cells were exposed to 75 μM etoposide in the presence of 10% serum for 24 h. (G) Apoptosis induced by the staurosporine treatment of Tsc2−/− MEFs or Tsc2−/−/Tsc2 MEFs. The cells were exposed to 100 nM staurosporine in the presence of 10% serum for 7 h. Data represent the means ± standard errors of the means from three independent experiments. ***, P < 0.001 compared to results for Tsc2−/−/Tsc2 MEFs. Apoptosis induced by the glucose deprivation of Tsc2−/− MEFs or Tsc2−/−/Tsc2 MEFs. (H) Cells were subjected to glucose deprivation in the presence or in the absence of serum for 48 h, and then apoptosis was assessed by DAPI staining. Data represent the means ± standard errors of the means from three independent experiments. **, P < 0.01 compared to results for 10% FBS; ###, P < 0.001 compared to results for Tsc2−/− MEFs. (I) Apoptosis induced by etoposide treatment of Tsc2−/− and reconstituted Tsc2−/−/Tsc2 MEFs. The cells were treated with etoposide (75 μM) for 24 h in the absence of FBS. Data represent the means ± standard errors of the means from three independent experiments. (J) Apoptosis induced by the staurosporine treatment of Tsc2−/− MEFs or Tsc2−/−/Tsc2 MEFs. The cells were exposed to 100 nM staurosporine in the absence of serum for 7 h. Data represent the means ± standard errors of the means from three independent experiments.
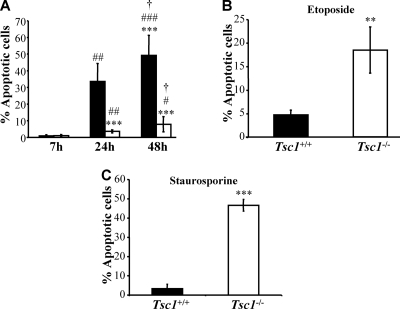
These findings validate the notion that Tsc2−/− MEFs are highly resistant to apoptosis induced by growth factor withdrawal. Because it had been shown previously that Tsc2−/− cells are hypersensitive to apoptosis induced by etoposide and other proapoptotic stimuli in the presence of serum (4, 19), we also examined the sensitivity of Tsc2−/− cells to apoptotic stimuli other than growth factor deprivation. We confirmed that in the presence of serum, Tsc2−/− MEFs are more sensitive to apoptosis induced by etoposide, staurosporine, and glucose withdrawal than Tsc2−/−/Tsc2 MEFs (Fig. 1F, G, and H). Notably, in the absence of serum, Tsc2−/− MEFs were not more sensitive to etoposide and staurosporine than Tsc2−/−/Tsc2 MEFs (Fig. 1I and J). The results also were confirmed when apoptosis was quantified by annexin V/propidium iodide staining (data not shown). The apoptotic sensitivities of Tsc1+/+ and Tsc1−/− MEFs in response to different proapoptotic stimuli also were compared, and Tsc1 deficiency was found to result in changes indistinguishable from those observed in Tsc2−/− MEFs (Fig. 2). Collectively, these results show that despite diminished Akt activity, which is a major downstream effector of growth factor-mediated cell survival, Tsc1/2-deficient cells display a survival pathway that promotes apoptotic resistance to growth factor withdrawal. We therefore focused our attention upon the identification of this unknown prosurvival pathway that is activated in Tsc1/2-deficient cells.
FIG. 2.
Tsc1−/− MEFs are resistant to serum deprivation-induced apoptosis but hypersensitive to other apoptotic stimuli in the presence of serum. (A) Apoptosis induced by the serum deprivation of Tsc1+/+ (▪) or Tsc1−/− (□) MEFs. The apoptosis was measured after 7, 24, or 48 h of serum deprivation. Data represent the means ± standard errors of the means from three independent experiments. ***, P < 0.001 compared to results for Tsc1+/+ MEFs. #, ##, and ###, P < 0.05, P < 0.01, and P < 0.001, respectively, compared to results at 7 h. †, P < 0.05 compared to results at 24 h. (B) Apoptosis induced by etoposide treatment of Tsc1+/+ or Tsc1−/− MEFs. The cells were exposed to 75 μM etoposide in the presence of 10% serum for 24 h. **, P < 0.01 compared to results for Tsc1+/+ MEFs. (C) Apoptosis induced by the staurosporine treatment of Tsc1+/+ or Tsc1−/− MEFs. The cells were exposed to 100 nM staurosporine in the presence of 10% serum for 6 h. ***, P < 0.001 compared to results for Tsc1+/+ MEFs.
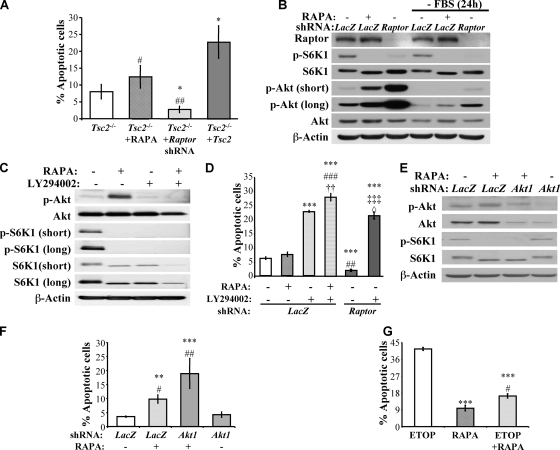
Inhibition of mTORC1 failed to overcome the resistance of Tsc2−/− MEFs to serum withdrawal as a consequence of indirect Akt activation.
To determine whether increased mTORC1 signaling in Tsc2−/− MEFs is responsible for the apoptotic resistance to growth factor withdrawal, these cells were subjected to serum withdrawal for 48 h in both the absence and presence of rapamycin. No significant effect of rapamycin was observed after 48 h of serum deprivation (Fig. 3A). Moreover, Raptor knockdown, which markedly decreased mTORC1 activity (Fig. 3B), did not increase, but rather decreased, the apoptotic susceptibility of Tsc2−/− MEFs to serum deprivation (Fig. 3A). These results seem to suggest that mTORC1 activity does not directly contribute to the mechanism by which Tsc2−/− MEFs resist apoptosis due to serum withdrawal. However, the inhibition of mTORC1 leads to an increase in Akt activity (Fig. 3B). The increase in Akt activity by rapamycin or by Raptor knockdown correlates with their relative effect on the sensitivity of Tsc2−/− cells to growth factor withdrawal-induced apoptosis and could explain the increased resistance induced by Raptor knockdown. Therefore, these results raised the possibility that the hyperactivated mTORC1 in Tsc2−/− cells mediate the resistance of these cells to growth factor withdrawal-induced apoptosis, but mTORC1 inhibition is unable to reverse this apoptotic resistance because of the associated increase in Akt activity that results from disrupted feedback inhibition.
FIG. 3.
Inhibition of mTORC1 activity by rapamycin (RAPA) or the knockdown of Raptor in Tsc2−/− MEFs could not abrogate resistance to serum deprivation-induced apoptosis because of the elevated Akt activity. (A) Effect of mTORC1 inhibition on apoptosis triggered by serum deprivation. Tsc2−/− MEFs in the absence or presence of 20 nM rapamycin, Tsc2−/− MEFs expressing LacZ shRNA (control) or Raptor shRNA, and Tsc2−/−/Tsc2 MEFs were subjected to serum deprivation. Apoptosis was quantified after 48 h of serum deprivation. Data represent the means ± standard errors of the means from three independent experiments. *, P < 0.05 compared to results for Tsc2−/− MEFs; # and ##, P < 0.05 and P < 0.01, respectively, compared to results for Tsc2−/−/Tsc2 MEFs. (B) Immunoblot showing mTORC1 activity and Akt activity upon rapamycin treatment or Raptor knockdown. Tsc2−/− MEFs in the absence or presence of rapamycin (20 nM) and Tsc2−/− MEFs expressing LacZ shRNA (control) or Raptor shRNA were cultured in the presence or absence of 10% serum for 24 h. Lysates of these cells were analyzed by immunoblotting using antibodies specific for Raptor, Akt phosphorylation on Ser473 (p-Akt; an indicator of Akt activity), total Akt, p70S6 kinase phosphorylation on Thr389 (p-S6K1; an indicator of mTORC1 activity), total p70S6 kinase, and actin. (C) LY294002 diminished the increase in Akt activity induced by rapamycin. Tsc2−/− MEFs without treatment, in the presence of rapamycin (20 nM), or in the combined presence of rapamycin (20 nM) and LY294002 (7.5 μM) were subjected to serum deprivation for 24 h. Lysates of these cells were analyzed by immunoblotting using antibodies specific for Akt phosphorylation on Ser473 (an indicator of Akt activity), total Akt, p70S6 kinase phosphorylation on Thr389 (an indicator of mTORC1 activity), total p70S6 kinase, and actin. (D) Tsc2−/− MEFs expressing LacZ shRNA (control) without treatment, in the presence of rapamycin (20 nM), in the presence of LY294002 (7.5 μM), in the combined presence of rapamycin and LY294002 and Tsc2−/− MEFs expressing Raptor shRNA without treatment, or in the presence of LY294002 were subjected to serum deprivation. Apoptosis was quantified after 48 h of serum deprivation. ***, P < 0.001 compared to results for LacZ shRNA; ## and ###, P < 0.01 and P < 0.001, respectively, compared to results for rapamycin; ††, P < 0.01 compared to results for LY294002; ‡‡‡, P < 0.001 compared to results for Raptor shRNA; ◊, P < 0.05 compared to results for rapamycin plus LY294002. (E) Akt1 shRNA-mediated abrogation of the increase in Akt activity induced by rapamycin. Tsc2−/− MEFs expressing LacZ shRNA in the absence or presence of rapamycin (20 nM) and Tsc2−/− MEFs expressing Akt1 shRNA in the presence of rapamycin (20 nM) were subjected to serum deprivation for 14 h. Lysates of these cells were analyzed by immunoblotting using antibodies specific for Akt phosphorylation on Ser473 (an indicator of Akt activity), total Akt1, p70S6 kinase phosphorylation on Thr389 (indicator of mTORC1 activity), total p70S6 kinase, and actin. (F) Apoptosis induced by the serum deprivation of Tsc2−/− MEFs expressing LacZ shRNA in the absence or presence of rapamycin (20 nM) and Tsc2−/− MEFs expressing Akt1 shRNA in the presence of rapamycin (20 nM). The apoptosis was quantified after 48 h of serum deprivation. ** and ***, P < 0.01 and P < 0.001, respectively, compared to results for LacZ shRNA; # and ##, P < 0.05 and P < 0.01, respectively, compared to results for Akt1 shRNA. (G) Tsc2−/− MEFs were subjected to 75 μM etoposide in the presence or absence of rapamycin (20 nM). Apoptosis was measured after 48 h of serum deprivation. ***, P < 0.001 compared to results for etoposide (ETOP); #, P < 0.05 compared to results for rapamycin (RAPA).
To test this possibility, we also examined responses to the addition of the selective phosphatidylinositol 3-kinase antagonist LY294002. LY294002 completely abolished rapamycin-induced Akt activation (Fig. 3C), so Tsc2−/− MEFs also were examined for apoptotic susceptibility to serum withdrawal in the presence of rapamycin alone or in combination with LY294002. Whereas rapamycin alone only marginally, and not significantly, affected apoptosis, the combination of rapamycin and LY294002 markedly sensitized Tsc2−/− MEFs to apoptosis induced by serum withdrawal (Fig. 3C and D). Likewise, the knockdown of Raptor in combination with LY294002 markedly sensitized Tsc2−/− MEFs to apoptosis induced by serum withdrawal (Fig. 3D). To further substantiate these results, shRNAs specifically targeting Akt1, which is the predominant Akt isoform expressed in MEFs (20), or LacZ (control) were stably introduced into Tsc2−/− MEFs by lentiviral transduction (designated Tsc2−/−/Akt1 and Tsc2−/−/LacZ, respectively). Akt1 knockdown predictably and significantly decreased total Akt activity, and rapamycin failed to increase total Akt activity above the level observed in untreated control cells (Fig. 3E). When subjected to serum withdrawal in the presence of rapamycin, Tsc2−/−/Akt1 shRNA cells underwent robust apoptosis (Fig. 3F). This is consistent with the notion that rapamycin's inability to abrogate the apoptotic resistance of Tsc2−/− cells to growth factor withdrawal is mediated by Akt activation. In contrast, control Tsc2−/−/LacZ shRNA cells remained relatively resistant to apoptosis.
Given the hypersensitivity of Tsc2−/− cells to etoposide-induced apoptosis and the ability of Akt to protect against etoposide-mediated cell death, we predicted that mTORC1 inhibition in Tsc2−/− cells would increase Akt activation and hence their apoptotic resistance to etoposide. Indeed, we found that rapamycin increased Tsc2−/− cell resistance to etoposide-induced apoptosis (Fig. 3G). In summary, these results strongly suggest that mTORC1 hyperactivation can account for the resistance of Tsc2−/− cells to growth factor withdrawal. This was not, however, directly demonstrable by mTORC1 inhibition due to the resulting activation of Akt. We therefore searched for downstream effectors of mTORC1 that could mediate resistance to apoptosis induced by growth factor withdrawal.
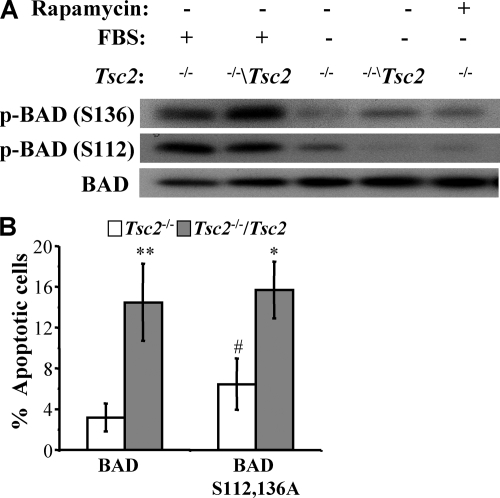
The resistance of Tsc2−/− MEFs to serum withdrawal-induced apoptosis is independent of BAD phosphorylation.
It was reported previously that the inhibition of S6K1 by the overexpression of Tsc2 activates the proapoptotic protein BAD, because S6K1 can inhibit BAD activity by the phosphorylation of Ser136 (3). In addition to phosphorylation on this site, BAD also can be inhibited by phosphorylation on Ser112 (7). We sought to examine the phosphorylation status of BAD in the Tsc2−/− and Tsc2−/−/Tsc2 MEFs. Unfortunately, the endogenous BAD could not be detected using available antibodies. Therefore, BAD was retrovirally overexpressed in the Tsc2−/− and Tsc2−/−/Tsc2 MEFs. The examination of the phosphorylation status of BAD in these cells by immunoblotting using phosphospecific antibodies revealed that in Tsc2−/− MEFs, BAD phosphorylation at the Ser136 site is reduced in the presence or absence of serum compared to that of Tsc2−/−/Tsc2 MEFs (Fig. 4A). The phosphorylation of BAD on Ser112 was similar between the Tsc2−/− and Tsc2−/−/Tsc2 MEFs cultured in the presence of serum, but upon the withdrawal of serum, the phosphorylation was maintained better in the Tsc2−/− MEFs (Fig. 4A).
FIG. 4.
Resistance of Tsc2−/− MEFs to serum withdrawal-induced apoptosis is independent of BAD phosphorylation. (A) Immunoblot showing the phosphorylation status of BAD in Tsc2−/− or Tsc2−/−/Tsc2 MEFs overexpressing BAD. The cells were cultured in either 10% serum or 0% serum and in the absence or presence of rapamycin (20 nM) for 5 h. Lysates of these cells were analyzed by immunoblotting using antibodies specific for BAD phosphorylation on Ser136 or Ser112 and total BAD. (B) Apoptosis induced by the serum deprivation of Tsc2−/− MEFs and Tsc2−/−/Tsc2 MEFs expressing either wild-type BAD or mutant BAD(Ser112,136Ala). The apoptosis was quantified after 6 h of serum deprivation. Data represent the means ± standard errors of the means from three independent experiments. * and **, P < 0.05 and P < 0.01, respectively, compared to results for Tsc2−/− MEFs; #, P < 0.05 compared to results for BAD.
To conclusively find out whether inhibitory phosphorylations of BAD determine the apoptotic response of Tsc2−/− MEFs, these cells and the Tsc2−/−/Tsc2 MEFs were infected with retroviruses expressing wild-type BAD or a mutated BAD (Ser112Ala/Ser136Ala) in which the two phosphorylated serines were mutated to alanines. The cells expressing the wild-type and mutant BAD proteins were subjected to 6 h of serum deprivation, which normally, by itself, does not cause any cell death but is sufficient to unleash the latent apoptotic potential of BAD. Compared to the Tsc2−/−/Tsc2 MEFs, the Tsc2−/− MEFs exhibit essentially the same degree of apoptotic resistance to the overexpression of both wild-type BAD and the mutant BAD(Ser112Ala/136Ala) (Fig. 4B). This suggests that the increased resistance of Tsc2−/− MEFs to serum deprivation is independent of BAD phosphorylation. These results also showed that the deficiency of Tsc2 exerts resistance to apoptosis induced by the overexpression of Bad.
The expression of both HKII and GLUT1 is induced in serum-deprived Tsc2−/− MEFs.
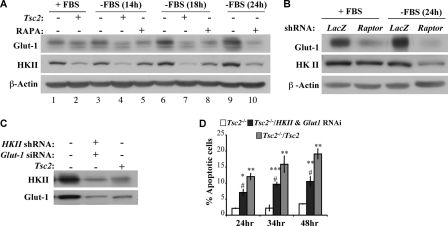
Both mitochondrial hexokinases and GLUT1 have been shown to inhibit apoptosis and to promote growth factor-mediated cell survival (5, 21). We therefore examined the levels of HKII and GLUT1. We found that, in contrast to reconstituted Tsc2−/−/Tsc2 MEFs exhibiting decreased GLUT1 abundance following serum deprivation, Tsc2−/− MEFs maintained and even increased their GLUT1 abundance after growth factor withdrawal (Fig. 5A). In addition, serum withdrawal was associated with a progressive decline in HKII abundance in Tsc2−/−/Tsc2 MEFs, whereas the corresponding HKII levels not only maintained but also increased in Tsc2−/− MEFs (Fig. 5A). The higher levels of GLUT1 and HKII in the absence of serum in Tsc2−/− MEFs are mTORC1 dependent, because both rapamycin treatment (Fig. 5A) and Raptor knockdown (Fig. 5B) diminished their expression.
FIG. 5.
Induction of GLUT1 and HKII expression by serum deprivation in Tsc2−/− MEFs inhibits apoptosis. (A) Immunoblot showing HKII and GLUT1 protein levels. Tsc2−/− MEFs (lanes 1, 3, 5, 6, 8, 9, and 10) and Tsc2−/−/Tsc2 MEFs (lanes 2, 4, and 7) were cultured in the presence (lanes 1 and 2) or absence (lanes 3 to 10) of 10% serum and in the presence (lanes 5, 8, and 10) of simultaneous treatment with rapamycin (RAPA; 20 nM) for 14, 18, or 24 h. Lysates of these cells were analyzed by immunoblotting using antibodies specific for HKII, GLUT1, and β-actin. (B) Immunoblot showing the effect of Raptor knockdown on HKII and GLUT1 protein levels. Tsc2−/− MEFs expressing either LacZ shRNA (control) or Raptor shRNA were cultured in the presence or absence of 10% serum for 24 h. Lysates of these cells were analyzed by immunoblotting using antibodies specific for HKII, GLUT1, and β-actin. (C) Immunoblot showing the knockdown of HKII and GLUT1. Tsc2−/− MEFs expressing control siRNA and shRNA, Tsc2−/− MEFs expressing GLUT1 siRNA and HKII shRNA, and Tsc2−/−/Tsc2 MEFs expressing control siRNA and shRNA were subjected to serum deprivation for 14 h. Lysates of these cells were analyzed by immunoblotting using antibodies specific for HKII, GLUT1, and β-actin. (D) Effect of HKII and GLUT1 knockdown on apoptosis induced by serum deprivation. The cells described in panel C were subjected to serum deprivation. Apoptosis was quantified after 24, 34, or 48 h of serum deprivation. Data represent the means ± standard errors of the means from three independent experiments. *, **, and ***, P < 0.05, P < 0.01, and P < 0.001, respectively, compared to results for Tsc2−/− MEFs; #, P < 0.05 compared to results for Tsc2−/−/Tsc2 MEFs.
The increased HKII and GLUT1 expression levels, observed in Tsc2−/− MEFs in the absence of serum, could contribute to their resistance to serum deprivation-induced apoptosis. To verify this possibility, HKII and GLUT1 were knocked down (Fig. 5C), and the ensuing effect on apoptosis induced by serum deprivation was evaluated (Fig. 5D). The reduction of the elevated HKII and GLUT1 protein levels in Tsc2−/− MEFs sensitized these cells to apoptosis induced by serum withdrawal. But even after the knockdown of HKII and GLUT1, the Tsc2−/− MEFs still underwent apoptosis to a lower extent than the Tsc2−/−/Tsc2 MEFs. Thus, the elevation of HKII and GLUT1 upon serum deprivation could be an important factor that determines the resistance of Tsc2−/− MEFs to apoptosis induced by serum withdrawal but may not be the only determinant of their resistance to apoptosis. The knockdown of HKII alone or GLUT1 alone were not sufficient to sensitize Tsc2−/− MEFs to serum deprivation-induced apoptosis (data not shown), indicating that in Tsc2−/− MEFs both GLUT1 and HKII are required to promote resistance to growth factor withdrawal. The elevation of GLUT1 and HKII predominantly following serum deprivation could explain why Tsc2−/− MEFs are more resistant only to apoptosis induced by the absence of serum and are not resistant to other apoptotic stimuli in the presence of serum.
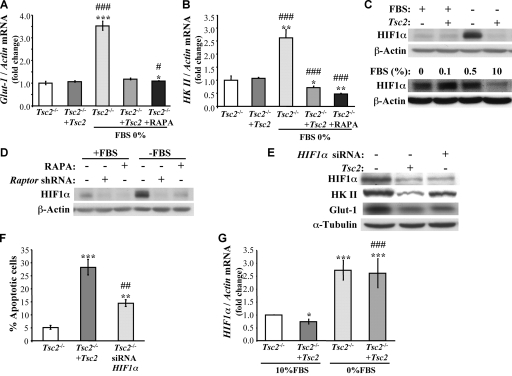
HKII and GLUT1 induction in Tsc2−/− MEFs is mediated by increased HIF1α abundance observed predominately in the absence of serum.
To determine the mechanism by which GLUT1 and HKII are elevated in Tsc2−/− MEFs in the absence of serum, we first determined GLUT1 and HKII mRNA expression. A marked increase of GLUT1 mRNA (Fig. 6A) or HKII mRNA (Fig. 6B) was observed only when Tsc2−/− MEFs were deprived of serum. These results suggest that the elevation of GLUT1 and HKII proteins is primarily through the elevation of mRNA. GLUT1 is a known target of hypoxia-inducible factor 1α (HIF1α), and HKII also was shown to be elevated by HIF1α (14). HIF1α previously was reported to be elevated in Tsc2−/− cells (2). Indeed, as shown in Fig. 6C, we found a dramatic increase in HIF1α levels in Tsc2−/− MEFs, but unlike previously reported results (2), we found that HIF1α levels are elevated in Tsc2−/− MEFs exclusively in the absence of serum or following a reduction in serum levels. In the presence of 10% serum, the levels of HIF1α protein in Tsc2−/− cells was comparable to that in control cells. In contrast, the basal level of HIF1α protein in Tsc2−/−/Tsc2 MEFs declined after serum deprivation (Fig. 6C). The elevation of HIF1α protein following serum deprivation in Tsc2−/− MEFs is dependent on mTORC1, as it is inhibited by rapamycin as well as by the knockdown of Raptor (Fig. 6D).
FIG. 6.
Elevation of HIF1α expression in serum-deprived Tsc2−/− MEFs mediates the induction of GLUT1 and HKII mRNA expression. (A and B) Real-time RT-PCR showing GLUT1 (A) and HKII (B) mRNA levels. Tsc2−/− MEFs and Tsc2−/−/Tsc2 MEFs were cultured in the presence or absence of 10% serum with or without simultaneous treatment of rapamycin (RAPA; 20 nM) for 14 h. RNA extracts from these cells were analyzed by real-time RT-PCR using primers specific for GLUT1, HKII, and actin. The data are represented as the change in GLUT1 or HKII mRNA levels that were normalized to the actin mRNA levels. *, **, and ***, P < 0.05, P < 0.01, and P < 0.001, respectively, compared to results for Tsc2−/− MEFs; # and ###, P < 0.05 and P < 0.001, respectively, compared to results for Tsc2−/−/Tsc2 MEFs. (C) Immunoblot showing HIF1α protein levels. The upper panel shows Tsc2−/− MEFs and Tsc2−/−/Tsc2 MEFs that were cultured in the presence or absence of 10% serum for 14 h. Lysates of these cells were analyzed by immunoblotting using antibodies specific for HIF1α and actin. The lower panel shows Tsc2−/− MEFs that were cultured in the presence of 10, 0.5, 0.1, or 0% serum for 14 h. Lysates of these cells were analyzed by immunoblotting using antibodies specific for HIF1α and actin. (D) Immunoblot showing the effect of mTORC1 inhibition on HIF1α expression. Tsc2−/− MEFs in the absence or presence of rapamycin (20 nM) and Tsc2−/− MEFs expressing LacZ shRNA (control) or Raptor shRNA were cultured in the presence or absence of 10% serum for 24 h. Lysates of these cells were analyzed by immunoblotting using antibodies specific for HIF1α and actin. (E) The knockdown of HIF1α reduced the expression of HKII and GLUT1 in serum-deprived Tsc2−/− MEFs. Tsc2−/− MEFs expressing either control siRNA or HIF1α siRNA and Tsc2−/−/Tsc2 MEFs expressing control siRNA were subjected to serum deprivation for 14 h. Lysates of these cells were analyzed by immunoblotting using antibodies specific for HIF1α, HKII, GLUT1, and tubulin. (F) Tsc2−/− MEFs expressing either control siRNA or HIF1α siRNA and Tsc2−/−/Tsc2 MEFs expressing control siRNA were subjected to serum deprivation for 34 h, and then apoptosis was assessed by DAPI staining. Data represent the means ± standard errors of the means from three independent experiments. ** and ***, P < 0.01 and P < 0.001 compared to results for Tsc2−/− MEFs; ##, P < 0.01 compared to results for Tsc2−/−/Tsc2 MEFs. (G) HIF1α mRNA is induced in the absence of serum. Tsc2−/− MEFs and Tsc2−/−/Tsc2 MEFs were cultured in the presence of 10 or 0% FBS for 14 h. RNA extracts from these cells were analyzed by real-time RT-PCR using primers specific for HIF1α and actin. The data are represented as the change in HIF1α mRNA levels that were normalized to the actin mRNA levels. * and ***, P < 0.05 and P < 0.001, respectively, compared to results for Tsc2−/− MEFs; ###, P < 0.001 compared to results with 10% FBS.
To assess the corresponding dependence of increased HKII and GLUT1 on HIF1α, we also knocked down HIF1α in Tsc2−/− MEFs. As shown in Fig. 6E, HIF1α knockdown markedly reduced the induction of GLUT1 and, to a lesser extent, HKII in serum-deprived Tsc2−/− MEFs. Consistently with this, the knockdown of HIF1α increased the apoptosis of serum-deprived Tsc2−/− MEFs (Fig. 6F) to an extent similar to that observed following GLUT1 and HKII knockdown (Fig. 5D). These results suggest that mTORC1-mediated HIF1α elevation is at least partly responsible for these specific increases in both HKII and GLUT1 abundance. They also raised the question of why these changes in HIF1α abundance and activity are restricted to serum-deprived Tsc2−/− MEFs.
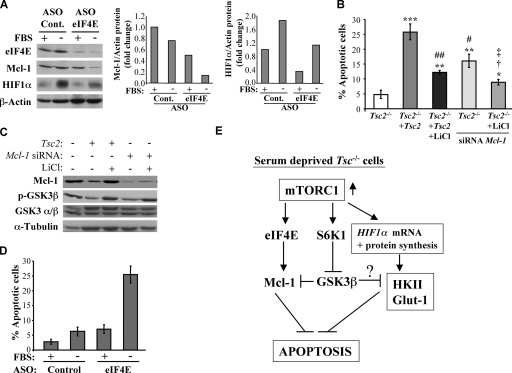
Since our results, in contrast to previous results (2), show that HIF1α levels and activity are elevated in Tsc2−/− MEFs exclusively after serum deprivation, we wanted to further verify and substantiate this observation. To identify the mechanism by which HIF1α is elevated after serum deprivation in Tsc2−/− MEFs, we first examined HIF1α mRNA levels in Tsc2−/− and Tsc2−/−/Tsc2 cells in the presence or absence of serum. As shown in Fig. 6G, mRNAs levels of HIF1α were increased to the same extent in Tsc2−/− and Tsc2−/−/Tsc2 cells following serum deprivation. However, since mRNA translation mediated by mTORC1 is decreased upon serum deprivation in Tsc2−/−/Tsc2 cells, it could not yield more HIF1α protein. In contrast, mTORC1 activity is sustained in Tsc2−/− cells following serum deprivation, yielding more HIF1α protein. Thus, the combination of the elevated HIF1α mRNA and the sustained mRNA translation in Tsc2−/− cells in the absence of serum could explain, at least in part, the elevated HIF1α protein levels in Tsc2−/− cells, particularly in reduced-serum conditions. To verify that the sustained mRNA translation in serum-deprived Tsc2−/− MEFs contributes to elevated HIF1α protein levels, we exposed the cells to eIF4E ASO, which was shown to effectively reduce the expression of eIF4E (6). Exposure to eIF4E ASO diminished the induction of HIF1α protein in serum-deprived Tsc2−/− MEFs (see Fig. 8A).
FIG. 8.
Reduced level of eIF4E diminishes Mcl-1 and HIF1α protein expression in serum-deprived Tsc2−/− MEFs and abrogates the resistance of Tsc2−/− MEFs to serum deprivation-induced apoptosis. (A) The left panel is an immunoblot showing the effect of the eIF4E ASO on Mcl-1 and HIF1α expression in the absence of FBS. Tsc2−/− MEFs were transfected with control (Cont.) or eIF4E ASOs, and then 48 h after transfection these cells were subjected to serum withdrawal for 72 h. Lysates of these cells were immunoblotted for the detection of eIF4E, Mcl-1, HIF1α, and β-actin. The panels on the right show the quantification of these immunoblotting results, represented as the changes in Mcl-1 and HIF1α protein levels normalized to the β-actin protein levels. (B) Tsc2−/− MEFs expressing control siRNA or Mcl-1 siRNA and Tsc2−/−/Tsc2 MEFs expressing control siRNA were cultured in the absence of serum and with or without simultaneous LiCl (10 mM) treatment for 34 h, and the resulting apoptosis was quantified. Data represent the means ± standard errors of the means from three independent experiments. *, **, and ***, P < 0.05, P < 0.01, and P < 0.001, respectively, compared to results for Tsc2−/− MEFs; # and ##, P < 0.05 and P < 0.01, respectively, compared to results for Tsc2−/−/Tsc2 MEFs; †, P < 0.05 compared to results for Tsc2−/−/Tsc2 MEFs plus LiCl; and ‡, P < 0.05 compared to results for Mcl-1 siRNA. (C) Immunoblot showing the knockdown of Mcl-1. Cells were treated as described for panel B for 14 h. Lysates of these cells were immunoblotted for the detection of Mcl-1, phosphorylated GSK3β (p-GSK3β), GSK3α/β, and α-tubulin. (D) Apoptosis induced by eIF4E ASO in the absence of FBS. Quantification of apoptosis in the cells described for panel A. Data represent the means ± standard errors of the means from three independent experiments. (E) Schematic illustration summarizing the sequence of events that mediate resistance to serum deprivation-induced apoptosis by hyperactive mTORC1 in Tsc-deficient cells. In the absence of serum, hyperactive mTORC1 increases the abundance of HIF1α, which in turn elevates the expression of HKII and GLUT1 to inhibit apoptosis. The increase in HIF1α expression is dependent largely on the elevated eIF4E activity in Tsc-deficient cells. In parallel, the elevation of S6K1 and eIF4E activities by the hyperactive mTORC1 maintain the expression of Mcl-1 in the absence of serum by two different mechanisms. eIF4E increases Mcl-1 protein synthesis, and the inhibition of GSK3 activity by S6K1 increases the stability of Mcl-1 protein. In addition, the inhibition of GSK3 may accelerate the ability of HKII to inhibit apoptosis.
The sustained inhibition of GSK3β in serum-deprived Tsc2−/− MEFs contributes to their resistance to serum deprivation-induced apoptosis.
In a search for an additional mechanism by which Tsc2−/− cells are rendered resistant to serum deprivation-induced apoptosis, we examined whether the constitutive inhibition of GSK3β in Tsc2−/− cells (23) contributes to their resistance. Normally, GSK3β kinase activity is inhibited by Akt through the phosphorylation of GSK3β on Ser9. In Tsc2−/− MEFs, which exhibit diminished Akt activity, hyperactive S6K1 can phosphorylate GSK3β and inhibit its activity even in the absence of serum (23). As such, hyperactive S6K1 can at least partly compensate for the reduced Akt activity observed in these cells.
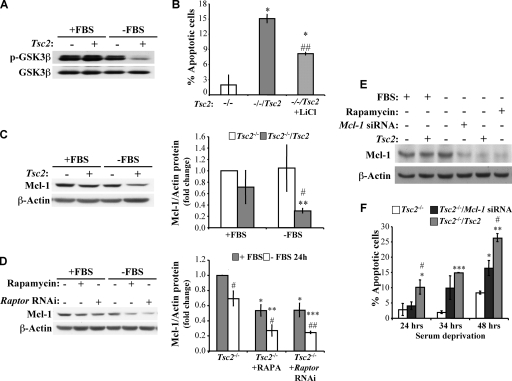
In confirmation of this prior finding, we found that in Tsc2−/− MEFs the inhibitory phosphorylation of GSK3β on Ser9 was sustained even upon serum deprivation, whereas the Tsc2-proficient MEFs rapidly lost their inhibitory phosphorylation of GSK3β when they were placed in the absence of serum (Fig. 7A). Active GSK3β can trigger apoptosis via multiple mechanisms, including the direct phosphorylation of Mcl-1, leading to its degradation (15), and voltage-dependent ion channel phosphorylation, leading to hexokinase dissociation from mitochondria (16). To confirm the contribution of GSK3β to serum deprivation-induced apoptosis, we determined the susceptibility of Tsc2−/−/Tsc2 MEFs to apoptosis in the presence or absence of LiCl, which is a widely used inhibitor of GSK3β. LiCl partially protected the Tsc2−/−/Tsc2 MEFs from apoptosis induced by serum deprivation (Fig. 7B). Thus, the constitutive inhibition of GSK3β in the absence of serum in Tsc2−/− cells could partly account for their resistance to growth factor withdrawal-induced apoptosis.
FIG. 7.
Sustained inhibition of GSK3β and the continuous expression of Mcl-1 in serum-deprived Tsc2−/− MEFs contribute to their resistance to apoptosis. (A) Immunoblot showing the inhibitory phosphorylation of GSK3β (p-GSK3β) in Tsc2−/− MEFs and Tsc2−/−/Tsc2 MEFs. The cells were cultured in the presence or absence of 10% serum for 14 h, and lysates of these cells were immunoblotted for the detection of the inhibitory phosphorylation of GSK3β on Ser9 as well as the total GSK3β levels. (B) Effect of lithium chloride (a GSK3β inhibitor) on apoptosis induced by serum deprivation. Tsc2−/− MEFs and Tsc2−/−/Tsc2 MEFs in the absence or presence of LiCl (10 mM) were subjected to serum deprivation for 34 h, and the resulting apoptosis was measured. *, P < 0.05 compared to results for Tsc2−/− MEFs; ##, P < 0.01 compared to results for Tsc2−/−/Tsc2 MEFs. (C) The panel on the left is an immunoblot showing Mcl-1 expression in Tsc2−/− and Tsc2−/−/Tsc2 MEFs. The cells were cultured in the presence or absence of 10% serum for 14 h, and lysates of these cells were immunoblotted for the detection of Mcl-1 and β-actin. The panel on the right shows the quantification of these immunoblotting results, represented as the change in Mcl-1 protein levels that were normalized to the β-actin protein levels. (D) The panel on the left is an immunoblot showing the effect of mTORC1 inhibition on Mcl-1 expression. Tsc2−/− MEFs in the absence or presence of rapamycin (RAPA; 20 nM) and Tsc2−/− MEFs expressing LacZ shRNA (control) or Raptor shRNA were cultured in the presence or absence of 10% serum for 24 h. Lysates of these cells were immunoblotted for the detection of Mcl-1 and β-actin. The panel on the right shows the quantification of these immunoblotting results, represented as the change in Mcl-1 protein levels that were normalized to the β-actin protein levels. (E) Immunoblot showing the knockdown of Mcl-1. Tsc2−/− MEFs expressing control siRNA or Mcl-1 siRNA and Tsc2−/−/Tsc2 MEFs expressing control siRNA were cultured in the presence or absence of 10% serum and with or without simultaneous rapamycin (20 nM) treatment for 14 h. Lysates of these cells were immunoblotted for the detection of Mcl-1 and β-actin. (F) Effect of Mcl-1 knockdown on apoptosis induced by serum deprivation. The cells described in panel E were subjected to serum deprivation for 24, 34, or 48 h, and the resulting apoptosis was quantified. Data represent the means ± standard errors of the means from three independent experiments. *, **, and ***, P < 0.05, P < 0.01, and P < 0.001 compared to results for Tsc2−/− MEFs; #, P < 0.05 compared to results for Tsc2−/−/Mcl-1 siRNA.
Sustained Mcl-1 abundance contributes to the resistance of Tsc2−/− MEFs to serum deprivation-induced apoptosis.
Mcl-1 is an antiapoptotic member of the Bcl2 family that is normally downregulated by serum deprivation (15). Because GSK3β inhibition increases Mcl-1 protein stability (15) and because the translational initiation factor eIF4E, which is activated by mTORC1, can elevate the translation of Mcl-1 transcripts (22), we evaluated Mcl-1 abundance in Tsc2−/− cells. Unlike reconstituted Tsc2−/−/Tsc2 MEFs in which Mcl-1 abundance is substantially reduced in the absence of serum, Mcl-1 protein levels were maintained in Tsc2−/− MEFs even in the absence of serum (Fig. 7C). The higher levels of Mcl-1 in serum-deprived Tsc2−/− MEFs reflects sustained mTORC1 activity in these cells, as both rapamycin and Raptor knockdown reduced Mcl-1 abundance in these cells (Fig. 7D). This sustained Mcl-1 expression following growth factor withdrawal also contributes to the apoptotic resistance of serum-deprived Tsc2−/− MEFs. Importantly, Mcl-1 abundance remained equivalent in both Tsc2−/− and Tsc2−/−/Tsc2 MEFs in the presence of serum. As such, Mcl-1 probably confers no antiapoptotic advantages to Tsc2−/− cells in the presence of serum. To verify the contribution of sustained mRNA translation in serum-deprived Tsc2−/− MEFs to the sustained level of Mcl-1 protein, we exposed the cells to eIF4E ASO and found that Mcl-1 protein expression was diminished (Fig. 8A). Thus, both mRNA translation and GSK3β inhibition in serum-deprived Tsc2−/− MEFs could contribute to the sustained Mcl-1 protein level.
To determine whether sustained Mcl-1 expression contributes to the apoptotic resistance of serum-deprived Tsc2−/− cells, we examined the consequences of Mcl-1 knockdown in these cells (Fig. 7E). The knockdown of Mcl-1 in Tsc2−/− MEFs reduced the levels of Mcl-1 to approximately the same levels observed in Tsc2−/−/Tsc2 MEFs in the absence of serum. As shown in Fig. 7F, the knockdown of Mcl-1 increased the sensitivity of Tsc2−/− MEFs to growth factor withdrawal-induced apoptosis, indicating that the sustained expression of Mcl-1 protein in the absence of serum in Tsc2−/− MEFs contributes to their resistance to apoptosis. The increase in apoptosis after Mcl-1 knockdown is reduced in the presence of LiCl, with a concomitant increase in Mcl-1 protein levels (Fig. 8B and C). The overexpression of GLUT1 has been reported to maintain Mcl-1 protein levels upon growth factor withdrawal. This is because increased glycolysis causes the activation of protein kinase C, which can phosphorylate and thereby inhibit GSK3β (24). However, we found the maintenance of GSK3β inhibitory phosphorylation as well as the maintenance of Mcl-1 levels in the Tsc2−/− MEFs even upon knockdown of GLUT1 and HKII (data not shown). This difference could reflect the ability of hyperactive eIF4E to maintain Mcl-1 mRNA translation and the hyperactive S6K1 to phosphorylate GSK3β in Tsc2−/− MEFs even after HKII and GLUT1 are reverted to wild-type levels. Thus, there are two independent reasons for the resistance of Tsc2−/− MEFs to serum withdrawal: increased HKII and GLUT1 abundance and the maintenance of Mcl-1 expression.
HKII and GLUT1 abundance is dependent on HIF1α. The high levels of both HIF1α and Mcl-1 proteins in serum-deprived Tsc2−/− MEFs are dependent largely on the sustained mRNA translation. Therefore, it is expected that the inhibition of the sustained mRNA translation in serum-deprived Tsc2−/− MEFs abrogates their resistance to apoptosis. Indeed, the exposure of Tsc2−/− MEFs to eIF4E ASO overcame their resistance to serum deprivation-induced apoptosis (Fig. 8D).
DISCUSSION
Akt is a major downstream effector of growth factor-mediated cell survival in mammalian cells, and its activation inhibits apoptosis induced by diverse proapoptotic stimuli. It therefore is expected that cells with reduced Akt activity will be sensitized to apoptosis. Tsc1/2-deficient cells exhibit low Akt activity due to mTORC1 hyperactivation and negative regulatory feedback to inhibit Akt activity in these cells (reviewed in reference 1). As expected, Tsc1/2-deficient cells have been shown to be hypersensitive to apoptotic stimuli such as exposure to etoposide, methyl methanesulfonate (MMS), doxorubicin, staurosporine, and glucose deprivation (4, 8, 19) (Fig. 1). However, as demonstrated herein, Tsc1/2-deficient cells are extremely resistant to growth factor deprivation-induced apoptosis, and hyperactive mTORC1 signaling is a major contributor to this phenomenon. Importantly, however, the inhibition of mTORC1 does not cause robust sensitization to growth factor deprivation-induced apoptosis because of the concomitant increase in Akt activity. Notably, the inhibition of mTORC1 by rapamycin modestly or did not sensitize cells to apoptosis, whereas Raptor knockdown actually protected the cells from apoptosis (Fig. 3A). This difference most likely is due to the fact that although both rapamycin and Raptor knockdown caused a similar inhibition of mTORC1, the knockdown of Raptor triggered a much higher increase in the Ser473 phosphorylation of Akt than did rapamycin treatment (Fig. 3B). However, the resistance to apoptosis appears to be due largely to the hyperactive eIF4E downstream of the hyperactive mTORC1 in serum-deprived Tsc2−/− cells. Consistently reduced eIF4E expression abrogates the resistance of Tsc2−/− cells to serum deprivation-induced apoptosis (Fig. 8D).
We found several mechanisms, depicted in Fig. 8E, by which the hyperactive mTORC1 in Tsc-deficient cells provides the inhibition of apoptosis in the absence of growth factors. We showed that the expression of HKII is elevated following the serum deprivation of Tsc2−/− cells, whereas in control cells its expression slightly declines. Likewise, the expression of GLUT1 also is elevated in Tsc2−/− cells upon serum deprivation. The overexpression of mitochondrial hexokinases alone (5) or in combination with GLUT1 (17) was shown to phenocopy growth factor- or Akt-mediated cell survival. Indeed, we have demonstrated that the combined elevation of HKII and GLUT1 expression in the absence of serum in Tsc2−/− cells contributes to their resistance to apoptosis. In the presence of serum, the levels of HKII and GLUT1 are similar in Tsc2−/− and control cells, and therefore under these conditions Tsc2−/− cells are more sensitive to apoptosis because of the lower Akt activity. Notably, the higher Akt activity in control cells may mediate cell survival by increasing HKII-mitochondria interaction (5).
The finding that increased HKII and GLUT1 abundance was observed exclusively in serum-deprived Tsc2−/− cells was somewhat surprising, but this can be attributed to corresponding increases in HIF1α abundance under these conditions. The fact that the observed increases in HIF1α abundance and activity also are restricted to serum-deprived Tsc2−/− MEFs also is intriguing and can be explained by the combination of HIF1α mRNA induction and constitutive mRNA translation observed in these cells. This is supported by our observation that the increase in HIF1α abundance in serum-deprived Tsc2−/− MEFs is reduced by eIF4E ASO (Fig. 8A).
An additional contributor to the apoptotic resistance of Tsc2−/− cells to growth factor withdrawal involves sustained GSK3 inhibition under these conditions. In control cells, GSK3 normally is activated upon growth factor withdrawal, reflecting decreased inhibition by Akt. However, inhibitory GSK3 phosphorylation is maintained in Tsc2−/− cells even in the absence of serum due to the phosphorylating capacity of constitutively active S6K1 in these cells (23). GSK3 can affect apoptotic susceptibility by multiple mechanisms. For example, it has been shown that interaction between HKII and VDAC at the outer mitochondrial membrane promotes cell survival (5, 12, 13). GSK3 activation has been reported to disrupt this interaction and thereby increase apoptosis (16). Thus, elevated HKII expression in serum-deprived Tsc2−/− cells also should have the preserved capacity to physically and functionally interact with mitochondria (reviewed in reference 19).
Another consequence of constitutive GSK3 inhibition in serum-deprived Tsc2−/− cells involves the maintenance of high levels of the antiapoptotic Bcl2 family member Mcl-1, which normally declines rapidly following serum withdrawal. As previously demonstrated, the phosphorylation of Mcl-1 by GSK3 targets Mcl-1 for degradation (15). As such, GSK3 inhibition could explain, in part, why Mcl-1 abundance is not diminished by serum withdrawal in these cells. However, its short half-life makes Mcl-1 abundance very sensitive to changes in mRNA translation. Indeed, it has been shown that the translation initiation factor eIF4E increases Mcl-1 abundance in the presence of serum (22). Since eIF4E also is hyperactive in Tsc2−/− cells, even in the absence of serum, this could contribute to the sustained Mcl-1 expression observed in these cells. This is supported by our observation that Mcl-1 abundance is diminished in serum-deprived Tsc2−/− MEFs after exposure to eIF4E ASO (Fig. 8A).
Although Tsc2−/− cells are highly resistant to apoptosis caused by serum deprivation, as indicated above, they are more sensitive to apoptotic stimuli like DNA damage and staurosporine. The increased sensitivity of Tsc2−/− cells to apoptotic stimuli is probably a consequence of reduced Akt activity in these cells. Serum withdrawal reduces Akt activity in both the Tsc2−/− and control cells, but unlike control cells, which lose their mTORC1 activity under these conditions, Tsc2−/− MEFs maintain their mTORC1 activity, and this is responsible for their resilience in terms of apoptosis. Notably, the increased resistance of Tsc2−/− cells to serum deprivation is not sufficient to exert increased resistance to etoposide or staurosporine under these conditions (Fig. 1I and J). Perhaps the mechanisms that promote the resistance of Tsc2−/− cells to serum deprivation cannot withstand serum deprivation combined with other apoptotic stimuli. This binary apoptotic response of Tsc2−/− MEFs also may help explain the relatively benign nature of tuberous sclerosis-associated hamartomas. Reduced Akt activity is unlikely to attenuate cell proliferation in these tumors, because we have shown previously that hyperactive mTORC1 can compensate for Akt deficiency with regard to cell proliferation and oncogenic transformation (20). We propose that the binary apoptotic response caused by Tsc1/2 inactivation is at least partly responsible for the nonmalignant nature of tuberous sclerosis-associated hamartomas. Although the resistance of Tsc2−/− cells to growth factor limitation would promote tumor progression, the marked sensitivity of these cells to other cellular stresses would serve to counteract these effects and thereby limit tumor growth in vivo. As such, our findings provide additional insights into the genesis of the hamartomas in tuberous sclerosis. They suggest that mTORC1 hyperactivity drives hamartoma formation not only through increased cell proliferation but also by promoting cell survival under conditions of growth factor limitation. Although mTORC1 inhibition failed to cause robust sensitization to growth factor deprivation-induced apoptosis because of concomitant increases in Akt activity, the combination of mTORC1 inhibition and partial Akt antagonism (to counteract the increase in Akt activity caused by mTORC1 inhibition) proved more effective. This observation has major therapeutic implications for the treatment of tuberous sclerosis, since it suggests that rapamycin analogues alone are unlikely to be efficacious in treating tuberous sclerosis unless they are combined with Akt inhibitors. In contrast, specific inhibitors of eIF4E by themselves, such as eIF4E ASO, could be effective in treating tuberous sclerosis.
Acknowledgments
This work was supported by NIH grants R01AG16927, R01AG25953, and R01CA90764 to N.H.
We thank J. Graff (Eli Lilly) for the eIF4E ASO.
Footnotes
Published ahead of print on 20 July 2009.
REFERENCES
- 1.Bhaskar, P. T., and N. Hay. 2007. The two TORCs and Akt. Dev. Cell 12487-502. [DOI] [PubMed] [Google Scholar]
- 2.Brugarolas, J. B., F. Vazquez, A. Reddy, W. R. Sellers, and W. G. Kaelin, Jr. 2003. TSC2 regulates VEGF through mTOR-dependent and -independent pathways. Cancer Cell 4147-158. [DOI] [PubMed] [Google Scholar]
- 3.Freilinger, A., M. Rosner, G. Krupitza, M. Nishino, G. Lubec, S. J. Korsmeyer, and M. Hengstschlager. 2006. Tuberin activates the proapoptotic molecule BAD. Oncogene 256467-6479. [DOI] [PubMed] [Google Scholar]
- 4.Ghosh, S., V. Tergaonkar, C. V. Rothlin, R. G. Correa, V. Bottero, P. Bist, I. M. Verma, and T. Hunter. 2006. Essential role of tuberous sclerosis genes TSC1 and TSC2 in NF-κB activation and cell survival. Cancer Cell 10215-226. [DOI] [PubMed] [Google Scholar]
- 5.Gottlob, K., N. Majewski, S. Kennedy, E. Kandel, R. B. Robey, and N. Hay. 2001. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 151406-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graff, J. R., B. W. Konicek, T. M. Vincent, R. L. Lynch, D. Monteith, S. N. Weir, P. Schwier, A. Capen, R. L. Goode, M. S. Dowless, Y. Chen, H. Zhang, S. Sissons, K. Cox, A. M. McNulty, S. H. Parsons, T. Wang, L. Sams, S. Geeganage, L. E. Douglass, B. L. Neubauer, N. M. Dean, K. Blanchard, J. Shou, L. F. Stancato, J. H. Carter, and E. G. Marcusson. 2007. Therapeutic suppression of translation initiation factor eIF4E expression reduces tumor growth without toxicity. J. Clin. Investig. 1172638-2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harada, H., B. Becknell, M. Wilm, M. Mann, L. J. Huang, S. S. Taylor, J. D. Scott, and S. J. Korsmeyer. 1999. Phosphorylation and inactivation of BAD by mitochondria-anchored protein kinase A. Mol. Cell 3413-422. [DOI] [PubMed] [Google Scholar]
- 8.Inoki, K., T. Zhu, and K. L. Guan. 2003. TSC2 mediates cellular energy response to control cell growth and survival. Cell 115577-590. [DOI] [PubMed] [Google Scholar]
- 9.Kandel, E. S., and N. Hay. 1999. The regulation and activities of the multifunctional serine/threonine kinase Akt/PKB. Exp. Cell Res. 253210-229. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy, S. G., E. S. Kandel, T. K. Cross, and N. Hay. 1999. Akt/Protein kinase B inhibits cell death by preventing the release of cytochrome c from mitochondria. Mol. Cell. Biol. 195800-5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leung, A. K., and W. L. Robson. 2007. Tuberous sclerosis complex: a review. J. Pediatr. Health Care 21108-114. [DOI] [PubMed] [Google Scholar]
- 12.Majewski, N., V. Nogueira, P. Bhaskar, P. E. Coy, J. E. Skeen, K. Gottlob, N. S. Chandel, C. B. Thompson, R. B. Robey, and N. Hay. 2004. Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol. Cell 16819-830. [DOI] [PubMed] [Google Scholar]
- 13.Majewski, N., V. Nogueira, R. B. Robey, and N. Hay. 2004. Akt inhibits apoptosis downstream of BID cleavage via a glucose-dependent mechanism involving mitochondrial hexokinases. Mol. Cell. Biol. 24730-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathupala, S. P., A. Rempel, and P. L. Pedersen. 2001. Glucose catabolism in cancer cells: identification and characterization of a marked activation response of the type II hexokinase gene to hypoxic conditions. J. Biol. Chem. 27643407-43412. [DOI] [PubMed] [Google Scholar]
- 15.Maurer, U., C. Charvet, A. S. Wagman, E. Dejardin, and D. R. Green. 2006. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol. Cell 21749-760. [DOI] [PubMed] [Google Scholar]
- 16.Pastorino, J. G., J. B. Hoek, and N. Shulga. 2005. Activation of glycogen synthase kinase 3beta disrupts the binding of hexokinase II to mitochondria by phosphorylating voltage-dependent anion channel and potentiates chemotherapy-induced cytotoxicity. Cancer Res. 6510545-10554. [DOI] [PubMed] [Google Scholar]
- 17.Rathmell, J. C., C. J. Fox, D. R. Plas, P. S. Hammerman, R. M. Cinalli, and C. B. Thompson. 2003. Akt-directed glucose metabolism can prevent Bax conformation change and promote growth factor-independent survival. Mol. Cell. Biol. 237315-7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robey, R. B., and N. Hay. 2006. Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene 254683-4696. [DOI] [PubMed] [Google Scholar]
- 19.Shah, O. J., Z. Wang, and T. Hunter. 2004. Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr. Biol. 141650-1656. [DOI] [PubMed] [Google Scholar]
- 20.Skeen, J. E., P. T. Bhaskar, C. C. Chen, W. S. Chen, X. D. Peng, V. Nogueira, A. Hahn-Windgassen, H. Kiyokawa, and N. Hay. 2006. Akt deficiency impairs normal cell proliferation and suppresses oncogenesis in a p53-independent and mTORC1-dependent manner. Cancer Cell 10269-280. [DOI] [PubMed] [Google Scholar]
- 21.Vander Heiden, M. G., D. R. Plas, J. C. Rathmell, C. J. Fox, M. H. Harris, and C. B. Thompson. 2001. Growth factors can influence cell growth and survival through effects on glucose metabolism. Mol. Cell. Biol. 215899-5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wendel, H. G., R. L. Silva, A. Malina, J. R. Mills, H. Zhu, T. Ueda, R. Watanabe-Fukunaga, R. Fukunaga, J. Teruya-Feldstein, J. Pelletier, and S. W. Lowe. 2007. Dissecting eIF4E action in tumorigenesis. Genes Dev. 213232-3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang, H. H., A. I. Lipovsky, C. C. Dibble, M. Sahin, and B. D. Manning. 2006. S6K1 regulates GSK3 under conditions of mTOR-dependent feedback inhibition of Akt. Mol. Cell 24185-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao, Y., B. J. Altman, J. L. Coloff, C. E. Herman, S. R. Jacobs, H. L. Wieman, J. A. Wofford, L. N. Dimascio, O. Ilkayeva, A. Kelekar, T. Reya, and J. C. Rathmell. 2007. Glycogen synthase kinase 3α and 3β mediate a glucose-sensitive antiapoptotic signaling pathway to stabilize Mcl-1. Mol. Cell. Biol. 274328-4339. [DOI] [PMC free article] [PubMed] [Google Scholar]