Abstract
The assembly of nucleosomes by histone chaperones is an important component of transcriptional regulation. Here, we have assessed the global roles of the HIRA histone chaperone in Schizosaccharomyces pombe. Microarray analysis indicates that inactivation of the HIRA complex results in increased expression of at least 4% of fission yeast genes. HIRA-regulated genes overlap with those which are normally repressed in vegetatively growing cells, such as targets of the Clr6 histone deacetylase and silenced genes located in subtelomeric regions. HIRA is also required for silencing of all 13 intact copies of the Tf2 long terminal repeat (LTR) retrotransposon. However, the role of HIRA is not restricted to bona fide promoters, because HIRA also suppresses noncoding transcripts from solo LTR elements and spurious antisense transcripts from cryptic promoters associated with transcribed regions. Furthermore, the HIRA complex is essential in the absence of the quality control provided by nuclear exosome-mediated degradation of illegitimate transcripts. This suggests that HIRA restricts genomic accessibility, and consistent with this, the chromosomes of cells lacking HIRA are more susceptible to genotoxic agents that cause double-strand breaks. Thus, the HIRA histone chaperone is required to maintain the protective functions of chromatin.
Chromatin restricts the access of the transcription machinery to the DNA template, and as a result, modulation of chromatin structure is central to eukaryotic transcriptional control. Two major mechanisms by which changes to chromatin structures can be achieved are posttranslational modification of histones (42) and ATP-dependent nucleosome remodeling (6). In addition, it is becoming increasingly apparent that chromatin structures are also modified by a diverse group of proteins known as histone chaperones that facilitate the assembly and, in some cases, the disassembly of nucleosomes (12).
The bulk of nucleosome assembly is coupled to DNA replication via the action of replication-dependent histone chaperones, such as CAF-1 (43). However, other DNA-dependent processes, such as transcription initiation and elongation, can result in nucleosome displacement, and so the requirement for nucleosome assembly is not restricted to S phase. Accordingly, eukaryotic cells possess a repertoire of replication-independent histone chaperones, which include an evolutionarily conserved class of WD repeat protein called HIRA (or HIR) (12). Humans have a single HIRA protein (23), whereas budding and fission yeast cells have two related proteins that function in concert (Hir1 and Hir2 in Saccharomyces cerevisiae and Hip1 and Slm9 in Schizosaccharomyces pombe) (4, 19, 44). HIRA proteins are components of multisubunit complexes and these complexes purified from a number of cell types have been shown to mediate replication-independent nucleosome deposition in vitro (15, 35, 38, 46).
Histone chaperone-dependent removal and replacement of nucleosomes have emerged as important components of transcriptional control (48), and accordingly, HIRA complexes have been linked to transcription. Indeed, S. cerevisiae Hir1 and Hir2 were originally identified as factors that repress the transcription of histone genes outside S phase (44). Subsequently, it has been demonstrated that nucleosomes assembled by the S. cerevisiae HIR complex are refractory to remodeling by the SWI/SNF complex in vitro (35), indicating that this chaperone is capable of organization of repressive forms of chromatin. Consistent with this, HIRA proteins have also been implicated in assembly of heterochromatin and silencing in a range of organisms. Loss of either HIRA protein in fission yeast leads to defective pericentric and mat locus heterochromatin (4), and mutations in S. cerevisiae HIR genes exacerbate the silencing defects associated with inactivation of CAF-1 (20, 37, 40). The assembly of senescence-associated heterochromatin in human cells is also dependent upon HIRA (49), and HIRA is required for maintenance of knox gene silencing during organogenesis in Arabidopsis (34). Despite these findings, other evidence is consistent with positive roles for HIRA in transcription. Experiments with chicken DT40 cell lines suggest that the N-terminal domain of HIRA can function as an activator (2), and HIRA is implicated in the replication-independent deposition of the histone variant H3.3, which marks actively transcribed genes (3). Furthermore, genetic interactions have also suggested that HIRA complexes are involved in transcription elongation (13).
Here, we have assessed the role of the S. pombe HIRA complex in the global regulation of transcription. We find that deletion of hip1+ or slm9+ leads to upregulation of mRNA from a large number of genes, indicating that the HIRA complex plays an important role in suppression of transcription. HIRA targets overlap with silenced genes, and consistent with this, we demonstrate that HIRA represses the expression of all intact copies of the Tf2 long terminal repeat (LTR) retrotransposable element. Furthermore, HIRA also suppresses the production of noncoding transcripts from solo LTR elements and spurious antisense transcripts originating from cryptic promoters. In addition, we also find that the HIRA complex protects cells from agents that cause double-strand breaks. Thus, the HIRA complex is required for promoter silencing, suppression of antisense transcription, and maintenance of genomic integrity.
MATERIALS AND METHODS
Plasmids and strains.
Culture of S. pombe and general genetic methods were performed as described previously (29). A strain with a lacZ reporter integrated into Tf2-1 was constructed as follows. A DNA fragment was amplified by PCR using primers Tf2REPC (5′-GCATAGGAATTCTAGTACATCGCTATTCACCAG-3′) and Tf2REPD (5′-GCATTGGGATCCAATTGCTTTGTCCGCTTGTAG-3′) and cloned into the EcoRI and BamHI sites of pSPI356 (22) to give pSPI356Tf2CD. A second DNA fragment, containing the Tf2-1 5′ LTR, was amplified using primers Tf2REPA (5′-GCATAGGGATCCTGTCAGCAATACTACACTACG-3′) and Tf2REPB (5′-GCATAGCTGCAGGGAGTAATTCTTGCCATGTAAG-3′) and cloned into the PstI and BamHI sites of pSPI356Tf2CD. Following confirmation of the sequence, the resulting plasmid was linearized with BamHI and transformed into wild-type (NT5) S. pombe cells. Integration at the correct locus was confirmed by PCR. The Tf2-10 element was tagged in the same way. The remaining Tf2 elements were tagged using the same approach, except that primer Tf2REPA was replaced with a primer complementary to a sequence located 300 to 500 bp upstream of the 5′ LTR of the appropriate Tf2 element.
A Tf2-1 lacZ reporter was also integrated into an ectopic locus on chromosome II (base pairs 1877855 to 1878381). This was achieved by amplifying two DNA fragments, the first with ChrmIIA (5′-TATGTCGAGGATCCTGTAACCGTTTAGATTGCAGC-3′) and ChrmIIB (5′-GTACTACTGCAGTTGTTACTGTTGTGTAGAGCC-3′) and the second with ChrmIIC (5′-CCTAGAGAATTCCAACTAACCGTAATACATCGG-3′) and ChrmIID (5′-CGGTTACAGGATCCTCGACATAACACTTGCAAGTG-3′). These fragments were used as the template in an overlapping PCR with primers ChrmIIC and ChrmIIB, and the resulting DNA fragment was cloned into the EcoRI and PstI sites of pSPI356 to give pSPI356ChrmIICDAB. A fragment of Tf2-1 was amplified using Tf2REPB and Tf2(A)PstI (5′-CGATAGCTGCAGGTCAGCAATACTACACTACGC-3′), cleaved with PstI, and cloned into the PstI site of pSPI356ChrmIICDAB. The resulting plasmid was linearized with BamHI and transformed into wild-type S. pombe cells. Integration at the correct locus was confirmed by PCR. A strain carrying a solo LTR-lacZ reporter was constructed using the same approach, except that the Tf2REPB primer was replaced with LTR3′del349 (5′-CGAATCCTGCAGGCATTGTAAGCTACGCAGTTTGGTA-3′). All lacZ reporters were introduced into the hip1Δ background by using standard genetic crosses. The other strains used in this study were 972 (h−), SW577 (h− hip1::ura4+), SW578 (h− slm9::ura4+), NT5 (h− ade6-M216 leu1-32 ura4-D18), SW137 (h− ade6-M210 leu1-32 ura4-D18 hip1::ura4+), SW138 (h+ ade6-M210 leu1-32 ura4-D18 hip1::ura4+), JK2246 (h− leu1-32 ura4-D18 slm9::ura4+), SW318 (h− ade6-M210 leu1-32 ura4-D18 hip3::ura4+), yYH7a (h− ade6− leu1-32 ura4-D18 rrp6::kanMX6), SW524 (h+ ade6-M216 leu1-32 ura4-D18 clr6-1), and SW611 (h− ade6-M210 leu1-32 ura4-D18 clr6-1 hip1::ura4+).
Microarray analysis.
We used DNA microarrays displaying probes for >99% of all known and predicted genes of S. pombe spotted in duplicate onto glass slides. RNA extraction, hybridization, and initial data processing and normalization were performed as previously described (24). Four independent biological experiments were performed, including two dye swaps, for each mutant. The data were visualized and analyzed using GeneSpring GX7.3 (Agilent). The significance of overlaps between different gene lists was calculated with GeneSpring by using a standard Fisher exact test, and P values were adjusted with a Bonferroni multiple-testing correction. An average mutant/wild-type ratio was calculated for each gene, and cutoff values of 1.5-fold were used for comparisons between mutants. Gene annotations were downloaded from the S. pombe GeneDB (http://www.genedb.org/genedb/pombe/). Clustering along chromosomes of genes with induced expression was analyzed using an in-house Perl script which compares clustered genes to a random distribution. P values were adjusted for multiple testing using the Benjamini-Hochberg false-discovery-rate method.
Telomere length.
Telomere length measurements were performed as previously described (10). A probe for telomere repeat sequences was made with a SacI-PstI fragment from pJCF1640 (27). A probe that was used as a loading control was made by PCR amplifying a fragment by using primers ApaIF (5′-CGTGATAGTGCATTGACGATC-3′) and ApaIR (5′-CCATCTTGCATGGCACGCTTC-3′).
RT-PCR.
RNA was purified as described for microarray analysis and subjected to reverse transcription-PCR (RT-PCR) using a one-step RT-PCR kit (Qiagen). For strand-specific RT-PCR, one primer complementary to the sense or antisense transcript was added during first-strand cDNA synthesis while the second primer was added prior to the PCR amplification steps. The primers were as previously described (30). cDNA for quantitative (real-time) RT-PCR was made using a Superscript II kit (Invitrogen). Real-time PCRs were performed using a LightCycler 2.0 PCR system (Roche) and SYBR green mixture (Molecular Probes), using the appropriate primers. Reactions were normalized using primers specific to act1+.
Pulsed-field gel electrophoresis (PFGE).
DNA plugs were prepared as described previously (36). DNA was fractionated on 1% ultrapure DNA grade agarose (Bio-Rad) gel prepared in 1× Tris-acetate-EDTA by using a CHEF-DR III system (Bio-Rad). Gels were run for 48 h at 14°C at a voltage of 2 V cm−1, with an included angle of 106° and initial and final switching times of 1,200 s and 1,800 s, respectively.
Microarray data accession number.
The microarray data set is available at Array Express (E-MTAB-130).
RESULTS
The HIRA complex is a global suppressor of transcription.
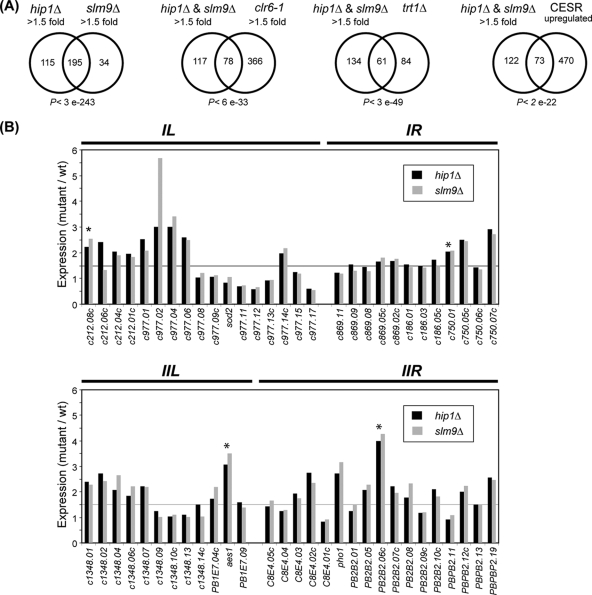
We have previously demonstrated that the S. pombe HIRA proteins Hip1 and Slm9 interact with a structurally unrelated protein called Hip3 to form a complex that is required for heterochromatic transcriptional silencing (16). In order to determine whether the HIRA complex regulates transcription in euchromatin, the RNA expression profiles of hip1Δ and slm9Δ cells were compared with those of wild-type cells by using microarrays detecting >99% of all known or predicted open reading frames and other genomic elements (24). As expected, the genes that were found to be differentially expressed in hip1Δ and slm9Δ cells were highly correlated (P < 3 × 10−243) (Fig. 1A; see also Table S1 in the supplemental material). A total of 195 mRNAs were found to be commonly upregulated ≥1.5-fold in hip1Δ and slm9Δ mutants, suggesting that the HIRA complex suppresses transcription from a large number of RNA polymerase II promoters. We observed a significant overlap (P < 6 × 10−33) between genes upregulated in hip1Δ and slm9Δ mutants and genes known to be derepressed in a clr6-1 background, which harbors a point mutation in an essential class I histone deacetylase (HDAC) (17). Clr6 functions as the catalytic core of an S. pombe HDAC-Sin3 corepressor complex, which is targeted to the promoters of numerous genes (30).
FIG. 1.
Transcriptomes of HIRA mutants. (A) Venn diagrams showing overlap between genes upregulated (≥1.5-fold) in HIRA mutants with genes upregulated under the indicated condition. The P values indicate the probability that the observed overlap happened by chance. (B) Graphs showing mutant versus wild-type (wt) expression levels of subtelomeric genes of the right (R) and left (L) arms of chromosomes I and II. Lines indicating the 1.5-fold threshold are shown. Genes whose expression was confirmed by quantitative RT-PCR are marked by asterisks.
The functional overlap with the Clr6 HDAC suggested that the HIRA complex regulates genes whose expression is repressed under normal growth conditions. Indeed, the transcriptomes of HIRA mutants were significantly enriched for mRNAs with no measurable microarray signal in vegetative cells (P < 1 × 10−8) (unpublished data) and for the 10% most lowly expressed mRNAs (P < 1 × 10−9) (21). Consistent with these data, transcripts from Tf2 LTR retrotransposons (which are largely suppressed in wild-type cells) were greatly increased in hip1Δ and slm9Δ backgrounds. Also, many of the silenced genes located in subtelomeric regions of chromosomes I and II (47) were upregulated in the absence of HIRA (Fig. 1B), and analysis of the location of HIRA-repressed genes confirmed that they are enriched for subtelomeric genes (P < 1 × 10−7). Overall, our results indicate that the HIRA complex is important for the global maintenance of transcriptional silencing.
Further examination of gene expression patterns revealed a significant overlap (P < 3 × 10−49) between genes upregulated in hip1Δ and slm9Δ mutants and those upregulated in cells deleted for telomerase (26) (Fig. 1A). Many of the genes that have been shown to be upregulated as cells undergo telomere crisis are core environmental stress response genes (9), and this is also true of those upregulated in response to loss of the HIRA complex (P < 2 × 10−22). These findings prompted us to examine whether or not the HIRA complex influences telomere length regulation, particularly as clr6-1 mutants are known to have elongated telomeres (17). However, Southern blotting revealed that loss of the HIRA complex did not result in increased telomere length; indeed, hip1Δ and slm9Δ mutants had telomeres that were slightly shorter than those of wild-type cells. Furthermore, deletion of hip1+ also partially reversed the elongated telomere phenotype that is associated with the clr6-1 allele (Fig. 2).
FIG. 2.
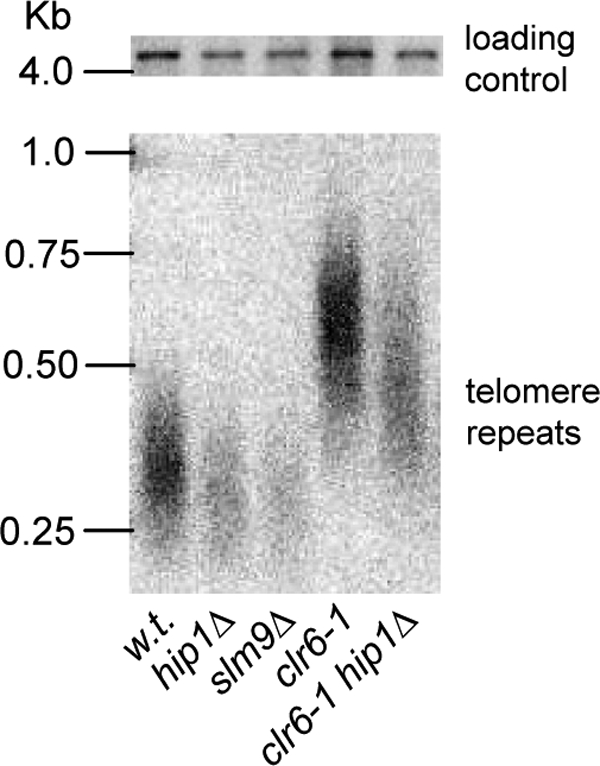
Influence of HIRA on telomere length. DNA samples purified from the indicated strains were digested with ApaI and analyzed by Southern blotting with a telomere repeat probe (lower panel) and loading control probe (upper panel) as described in Materials and Methods. w.t., wild type.
Although loss of the HIRA complex predominantly led to increased mRNA levels, the expression levels of 38 genes were reduced (≤1.5-fold) (see Table S1 in the supplemental material). Prominent among these genes were fio1+, frp1+, str1+, str3+, and fip1+, whose expression is regulated in response to intracellular iron status by the Fep1 repressor (P < 6 × 10−5) (32, 33). The expression of these genes is also downregulated in other mutants that are defective in silencing, such as clr1-5 and clr3-735, which lack the activity of the SHREC complex (17, 45). However, this effect is thought to be indirect, as in the clr1-5 and clr3-735 mutant backgrounds, downregulation of the iron regulon is correlated with increased levels of mRNA encoding the Fep1 repressor (17). In contrast, microarray and quantitative RT-PCR analyses indicated that mutation of hip1+ or slm9+ did not result in an increased abundance of fep1+ mRNA (see Table S1 in the supplemental material; also unpublished data). Furthermore, loss of HIRA did not result in increased transcript levels of components of the SWI/SNF complex, which has also recently been shown to repress the iron regulon (28) or alter intracellular iron levels (see Table S1 in the supplemental material; also unpublished data). Thus, it is possible that regulation of the iron regulon may be direct and some genes may require the HIRA complex for full expression.
HIRA represses transcription of all 13 Tf2 LTR retrotransposons.
Previous analysis has implicated the HIRA complex in the silencing of Tf2 LTR retrotransposons (16), and as outlined above, the microarray analysis confirmed that the levels of Tf2 mRNA were highly increased in the hip1Δ and slm9Δ backgrounds. The fission yeast laboratory strain (972) contains 13 full-length copies of this element (Tf2-1 to Tf2-13), and probes for nine of these elements were present on the microarray. However these elements are highly homogeneous having a pairwise identity of greater than 99% (5) and cross-hybridization between the Tf2 probes is expected. Therefore, it was not possible to determine whether the global increase in Tf2 mRNA resulted from derepression of a single element, a subset of elements, or all of the 13 Tf2 elements.
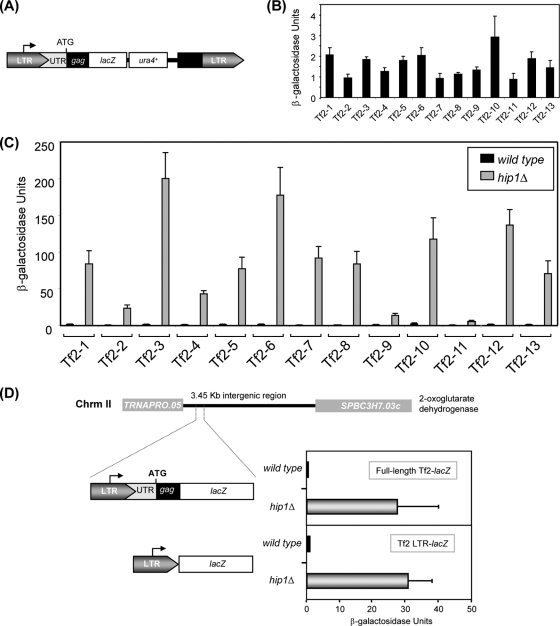
In order to address this issue, a series of 13 strains was constructed, each containing an integrated lacZ reporter gene under the control of a specific Tf2 element (Fig. 3A). β-Galactosidase assays of the resulting reporter strains indicated that all 13 elements are expressed at similar low levels, which is consistent with these elements being silenced (Fig. 3B). To determine the effect of HIRA on the expression of individual Tf2 elements, the lacZ reporter strains were then crossed into a hip1Δ background. Expression levels of all 13 Tf2-lacZ reporters were found to be increased in the absence of Hip1, indicating that the HIRA complex represses the expression of all of the Tf2 elements (Fig. 3C). Notably, the level of derepression of the Tf2-11 reporter was reproducibly lower than that of the other reporters. The Tf2-11 element has a 5′ LTR which is a hybrid of a Tf2 element and a Tf1 element, a related retrotransposon that is now extinct in the 972 genome (5). As a result, Tf2-11, unlike the other elements, is not induced in response to limitation of oxygen by the SREBP homologue Sre1 (39). Nonetheless, our results indicate that Tf2-11 is subject to HIRA-mediated repression.
FIG. 3.
HIRA silences the expression of all 13 Tf2 LTR retrotransposons. (A) Schematic of the lacZ reporter used to tag individual Tf2 elements. UTR, untranslated region. (B) Expression of Tf2-lacZ reporters in wild-type cells. Strains harboring a tagged Tf2 element were grown in rich medium (YE5S) at 30°C to mid-log phase. Cells were then harvested and processed for liquid β-galactosidase assays. Shown are the mean values of results from three experiments. Error bars indicate standard deviations. (C) Expression of Tf2-lacZ reporters in a hip1Δ background. Strains were processed as described for panel B. (D) HIRA-dependent repression is maintained at a novel site in the genome. Wild-type or hip1Δ strains harboring either the Tf2-1 or the LTR lacZ reporter integrated into chromosome II were processed as described for panel B.
We next determined whether HIRA-dependent repression is maintained when the Tf2 element is moved to a new region of the genome that does not naturally contain a retrotransposon. Therefore, we integrated a Tf2-lacZ reporter into a large intergenic region of chromosome II in between a tRNA gene (SPBTRNAPRO.05) and SPBC3H7.03c (Fig. 3D). This region was chosen as there is no evidence to suggest that HIRA complexes regulate the expression of RNA polymerase III-transcribed genes and our microarray experiments indicated that HIRA does not affect the expression of SPBC3H7.03c (see Table S1 in the supplemental material). β-Galactosidase assays revealed that the level of expression of this reporter was low in wild-type cells and was dramatically increased in a hip1Δ background, indicating that HIRA-dependent silencing is retained at a novel site in the genome. In addition to the 5′ LTR, the Tf2 reporter also contains the 5′ untranslated region and DNA encoding the first 333 bp of the Tf2 gag gene. In order to determine whether these sequences were important for HIRA-mediated repression, a reporter in which the lacZ gene is solely controlled by the 5′ LTR from the Tf2-1 element was constructed. Following integration into the ectopic locus on chromosome II, we again found that the level of expression of this reporter was low in wild-type cells and was dramatically increased in a hip1Δ background (Fig. 3D). This indicates that a Tf2 LTR element alone is a functional promoter that is repressed by the HIRA complex.
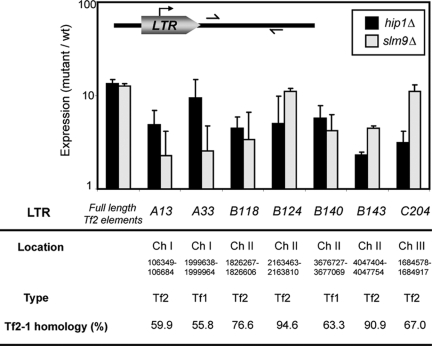
This finding is important because, in addition to the 13 full-length elements, the genome contains an extensive, but heterogeneous, population of (∼250) solo LTR elements that have arisen through LTR-LTR recombination and so mark the position of previous retrotransposon insertion events (5). In order to determine whether or not the HIRA complex is required to silence naturally occurring solo LTRs, expression from a selection of 11 of these elements from all three chromosomes was analyzed using quantitative RT-PCR. Using this approach, we were unable to detect expression from four LTRs either in wild-type or in mutant backgrounds. However, noncoding transcripts were detected for seven elements, and in all these cases, transcript levels were substantially increased in the absence of the HIRA complex (Fig. 4). Importantly, we found examples of both Tf2- and Tf1-type LTRs that were regulated in this way. This suggests that the S. pombe HIRA complex plays a widespread role in the suppression of cryptic transcripts from solo LTRs.
FIG. 4.
HIRA represses expression from Solo LTRs. RNA was prepared from wild-type (wt), hip1Δ, and slm9Δ cells. Quantitative real-time RT-PCR was then used to quantify the levels of noncoding transcripts ∼100 bp downstream of selected LTR elements. Change in expression (mutant versus wild type) is shown. Error bars indicate standard deviations. For each element, the location, type, and homology to the Tf2-1 LTR is shown.
HIRA represses spurious antisense transcription.
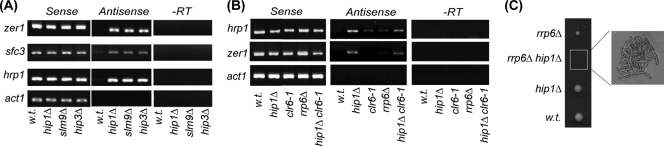
As the HIRA complex functions to limit spurious transcripts from solo LTR elements, we investigated whether it is also required to suppress other illegitimate transcripts. Recent data indicate that mutations that impair the integrity of chromatin result in the accumulation of antisense transcripts from cryptic promoters in coding regions (30). We therefore used strand-specific RT-PCR to examine several loci where defects in chromatin are known to result in increased levels of antisense transcripts. Consistent with previous findings (30), antisense transcripts originating at the zer1+ locus were not detectable in wild-type cells, but in contrast, mutation in any one of the genes encoding subunits of the HIRA complex (hip1+, slm9+, or hip3+) resulted in a large increase in the levels of these antisense transcripts (Fig. 5A). Furthermore, deletion of the HIRA complex had a similar effect on antisense transcripts from hrp1 and sfc3 loci (Fig. 5A). Thus, in addition to suppressing transcripts from numerous gene promoters and LTRs, the HIRA complex is required to suppress spurious antisense transcripts from the coding regions of RNA polymerase II-transcribed genes.
FIG. 5.
Loss of HIRA leads to the accumulation of spurious antisense transcripts. (A) RNA was purified from wild-type (w.t.), hip1Δ, slm9Δ, and hip3Δ cells and analyzed by strand-specific RT-PCR. One primer, complementary to either the forward or the reverse transcripts, was included during the reverse transcription step, and the second primer was then added during PCR amplification. Control reactions omitting the reverse transcription step (−RT) were included to demonstrate the absence of DNA. (B) RNA from wild-type, hip1Δ, rrp6Δ, clr6-1, and hip1Δ clr6-1 strains was analyzed as described for panel A. (C) The nuclear exosome is essential in the absence of HIRA. The left hand panel shows an example of a tetrad resulting from a cross between hip1Δ and rrp6Δ strains, and the right hand panel shows the terminal morphology of the hip1Δ rrp6Δ double mutant.
The HDAC Clr6 also plays a key role in the suppression of antisense transcripts (30). Clr6 is present in at least two distinct HDAC-Sin3 corepressor complexes (complexes I and II). While complex I predominantly represses transcription from gene promoters, complex II appears to be the functional equivalent of the S. cerevisiae Rpd3S complex and targets transcribed regions (30). We therefore investigated the genetic relationship between the HIRA complex and Clr6 with respect to antisense transcription. Introduction of the clr6-1 allele into the hip1Δ background did not result in further increase in antisense transcript levels at the hrp1 and zer1 loci (Fig. 5B), suggesting that the HIRA complex and Clr6 complex II function in the same pathway to suppress antisense transcription.
The nuclear exosome recognizes and degrades spurious antisense transcripts, preventing their accumulation (30). Consistent with this, combining mutations in rrp6 (which encodes a 3′ exonuclease) (18) with mutations in genes encoding components of Clr6 complex II leads to a synergistic increase in antisense transcripts (30). On the basis of this finding, we predicted that there would also be a genetic interaction between the nuclear exosome and HIRA. Indeed, we consistently failed to recover a viable double mutant from genetic crosses of hip1Δ and rrp6Δ strains. The same was also true for crosses of hip3Δ and rrp6Δ cells. Microscopic examination revealed that double mutant strains were able to germinate but failed to progress past six or seven cell divisions (Fig. 5C). Thus, the nuclear exosome is essential in the absence of the HIRA complex, suggesting that this histone chaperone plays a major role in the suppression of spurious antisense transcripts.
Sense transcription is required for antisense transcription.
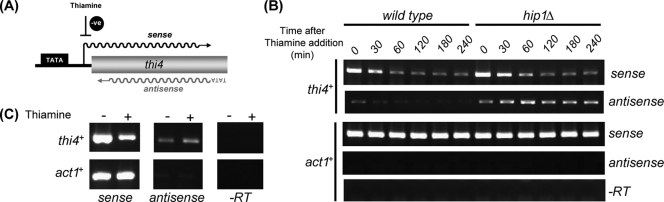
Accumulating evidence indicates that passage of RNA polymerase II can disrupt chromatin (48), suggesting that it is transcription in the sense direction that allows access to cryptic promoters. As such, sense transcription may be a prerequisite for antisense transcription. In order to examine the interdependence of sense and antisense transcriptions, we examined the thi4+ gene, whose expression is regulated in response to the availability of thiamine, being very highly expressed in its absence and repressed in its presence (50). Strand-specific RT-PCR analysis confirmed that in wild-type cells, the production of sense transcripts was reduced by the addition of thiamine to the medium (Fig. 6). Further analysis revealed that in the absence of thiamine (when high levels of sense transcription were occurring), thi4 antisense transcripts were clearly detectable. However, the addition of thiamine and the inhibition of sense transcription resulted in a concomitant suppression of antisense transcripts (Fig. 6). Thus, in wild-type cells, the levels of sense and antisense transcripts are correlated and the production of thi4 antisense transcripts is dependent upon high levels of transcription in the sense direction. Notably, the addition of thiamine to the medium also inhibited sense transcription in hip1Δ cells, but the level of antisense transcripts was not reduced by this treatment (Fig. 6). Furthermore, in a HIRA mutant background, antisense transcripts were detectable even when cells were cultured under conditions of constant thi4+ repression (Fig. 6C). Thus, in the absence of the HIRA histone chaperone, access to cryptic promoters is maintained even when sense transcription is inhibited.
FIG. 6.
Antisense transcription is correlated with sense transcription in wild-type but not HIRA mutant cells. (A) Schematic diagram showing regulation of thi4+ expression. (B) Wild-type and hip1Δ cells were grown to log phase in minimal (EMM) medium, thiamine was then added to give a final concentration of 2 μM, and samples of cells were harvested at the indicated time points. RNA, prepared from the resulting cell pellets, was analyzed by strand-specific RT-PCR as described in the legend to Fig. 5. −RT, control reactions omitting the reverse transcription step. (C) Antisense transcripts persist under conditions of constant thi4+ repression. slm9Δ cells were grown to log phase either in EMM medium or in EMM medium supplemented with 2 μM thiamine. RNA preparation and RT-PCR analysis were performed as described in the legend to Fig. 5.
HIRA is required for resistance to DNA damage.
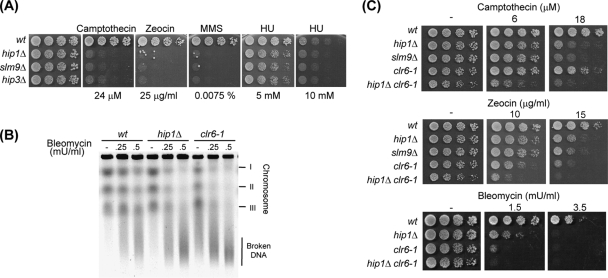
It has been proposed that increased levels of cryptic antisense transcripts are indicative of an open chromatin conformation (30). Consistent with this hypothesis, the genomes of clr6-1 mutants show an increased sensitivity to DNA-damaging agents (30). We therefore investigated the sensitivity of cells lacking the HIRA complex to a range of genotoxic agents, such as the alkylating agent methyl methanesulfonate, the radiomimetic zeocin, the topoisomerase I poison camptothecin, and hydroxyurea, which inhibits ribonucleotide reductase and causes replication fork stalling. Serial dilution assays revealed that mutation in any one of the genes encoding components of the HIRA complex (hip1Δ, slm9Δ, or hip3Δ) results in a marked increase in sensitivity to methyl methanesulfonate, zeocin, and camptothecin (Fig. 7A). In contrast, HIRA mutants exhibited only a very subtle increase in sensitivity to hydroxyurea. Thus, the HIRA complex is important for protection against agents that cause DNA double-strand breaks but not against a replication stress.
FIG. 7.
HIRA is required for protection against genotoxic agents. (A) The indicated strains were grown to log phase in YE5S medium, subjected to fivefold serial dilutions, and spotted on YE5S agar and YE5S agar supplemented with the indicated genotoxic agent. wt, wild type; HU, hydroxyurea; MMS, methyl methanesulfonate. (B) The indicated strains were treated with bleomycin at the indicated concentrations for 90 min. Chromosomal DNA was then analyzed using PFGE. (C) The indicated strains were grown to log phase and treated as described for panel A.
In order to determine whether HIRA mutants have increased susceptibility to chromosomal breakage, cells were first exposed to bleomycin and then chromosome integrity was analyzed using PFGE (Fig. 7B). As previously reported (30), the clr6-1 mutant exhibited enhanced levels of bleomycin-induced chromosome breakage as evidenced by the appearance of DNA fragments and the loss of intact chromosomes. Furthermore, the chromosomes of hip1Δ cells were also more susceptible to breakage than those of wild-type cells. This finding suggests that HIRA-mediated nucleosome assembly is required to maintain the global protective functions of chromatin. Interestingly, analysis of a clr6-1 hip1Δ double mutant indicated that this strain was more sensitive to genotoxic agents than either of the parental strains (Fig. 7C), indicating that the HIRA complex and Clr6 do not have identical roles in the response to DNA double-strand breaks.
DISCUSSION
Previous evidence has demonstrated that the HIRA histone chaperone is required for transcriptional silencing at heterochromatic loci (16). Here, we demonstrate that its function is not limited to heterochromatin, because HIRA represses transcripts from numerous promoters distributed throughout the genome, including subtelomeric genes, LTR retrotransposons, and their remnants, and it also limits the levels of cryptic antisense transcripts. Furthermore, loss of HIRA leads to increased access of genotoxic agents to the genome, indicating that HIRA is required for maintenance of the protective functions of chromatin.
Our data revealed a functional overlap between the HIRA complex and the class I HDAC Clr6 with respect to both promoter silencing and prevention of cryptic transcription. The simplest explanation of this finding is that HIRA is required to reassemble or repair nucleosomes that are subsequently modified by Clr6-Sin3 complexes. Interestingly, class I HDACs have been linked to HIRA function in vertebrate cells, as HDAC-2 stably interacts with chicken HIRA through an LXXLL motif located in the HIRA C-terminal region (1). However, neither Hip1 nor Slm9 has LXXLL motifs, and there is no evidence of a stable interaction between the fission yeast HIRA complex and Clr6. Indeed, large-scale affinity purification of Clr6-interacting subunits did not identify any of the components of the HIRA complex (30). Furthermore, a large-scale purification of Prw1, a homologue of RbAp48 that is present in all fission yeast Clr6-Sin3 complexes, also did not identify any subunits of the HIRA complex (unpublished data).
Analysis of the expression of individual Tf2 elements indicated that the high levels of Tf2 mRNA present in hip1Δ and slm9Δ cells result from increased expression levels of all 13 copies of this element. It is possible that Tf2 elements are located within regions of the genome that are associated with HIRA-dependent forms of chromatin. Arguing against this, HIRA-dependent repression of a Tf2 reporter was also maintained when it was moved to a novel site in the genome, suggesting that these elements contain sequences that limit their own expression.
Packaging of retrotransposons into repressive chromatin structures is known to occur in many cells types and is thought to limit the potentially harmful spread of these elements (25). The chromatin structures that suppress the expression of Tf2 retrotransposons appear to be dependent upon the HIRA complex. It is possible that the interaction of HIRA with these elements is transient because, as yet, we have been unable to detect binding of HIRA to Tf2 LTRs by chromatin immunoprecipitation. Global silencing of Tf2 elements is also dependent upon HDACs (Clr6, Clr3, and Hst4) and Cenp-B homologues (7, 11, 17, 45). While the influence of these factors on the expression of individual elements has not been formally investigated, chromatin immunoprecipitation analysis has indicated that Clr3 and Cenp-B proteins are located at multiple Tf2 elements (7, 45), implying that they are required for silencing of all Tf2 elements.
The involvement of HIRA proteins in the regulation of retrotransposons is not limited to fission yeast, because Hir1 and Hir2 have been implicated in the regulation of Ty1/Copia elements in S. cerevisiae. Indeed, hir mutants were found to suppress the deleterious effects of the insertion of a Ty LTR (δ element) into the HIS4 locus (41). Furthermore, mutation of HIR genes in combination with CAC genes also increases Ty1 transposition frequency, although this increased transposition was not associated with increased levels of Ty1 mRNA (37). It is important to note that the Tf2 elements of S. pombe are members of the Gypsy group of LTR retrotransposons and as such are only distantly related to the Ty1/Copia elements of S. cerevisiae (5). The finding that HIRA complexes regulate such distinct elements suggests that this chaperone regulates other classes of LTR retrotransposons in other eukaryotic organisms.
The substantial population of solo LTR elements present in the S. pombe genome have been generated by recombination between two LTRs resulting in the removal of the internal retrotransposon coding sequences. Thus, solo LTRs are the remnants of retrotransposons and mark the positions of previous insertion events. Our data, along with those of others (7, 30, 45), are consistent with these elements being assembled into silent chromatin in order to limit the production of spurious noncoding transcripts. While in many cases expression from solo LTRs would not be advantageous, there are an increasing number of examples of cells exploiting retrotransposable elements to regulate gene expression (14). Indeed, in S. pombe a set of solo LTRs that are closely related to the LTRs associated with full-length Tf2 elements are known to function as oxygen-responsive promoters (39). Some of these confer oxygen-dependent expression to neighboring genes. Our data indicate that HIRA-dependent repression is not restricted to elements that are induced in response to low oxygen levels, as all but one of the solo LTR elements examined in this work lack a consensus SRE element and we also identified Tf1-type LTR elements that were repressed by Hip1 and Slm9.
The finding that HIRA mutants have high levels of spurious antisense transcription is consistent with this histone chaperone being required to maintain the integrity of chromatin in transcribed regions. Furthermore, mutation of hip1 being synthetic lethal with rrp6Δ is consistent with HIRA having a major role in prevention of such antisense transcription in fission yeast. This result would not necessarily have been predicted from studies of S. cerevisiae where mutation of HIR genes did not result in high levels of internal initiation at the synthetic FLO8-HIS3 reporter gene, although it did increase the level of spurious sense transcripts observed in a spt2Δ background (31). Nonetheless, our analysis is the first report that HIRA proteins are required to prevent cryptic antisense transcripts at naturally occurring genes.
Passage of RNA polymerase II through chromatin has been proposed to result in partial or complete disassembly of nucleosomes (48). That antisense transcription (and by implication access to cryptic promoters) at the thi4+ gene is dependent upon high levels of sense transcription is entirely consistent with this notion. Notably, loss of the HIRA complex apparently leads to continued access to these promoters even in the absence of high levels of sense transcription. We propose that the HIRA complex restores chromatin structure in the wake of RNA polymerase II passage. Consistent with this idea, Hir1 and Hir2 have been shown to localize to transcribed regions in S. cerevisiae (31). In higher cells, HIRA is linked to deposition of variant histone H3.3 at actively transcribed genes (3), and so it will be interesting to determine whether this contributes to the prevention of spurious transcription initiation.
We also show that the HIRA complex plays an important role in protecting cells against double-strand breaks. The loss of HIRA led to an increased susceptibility to bleomycin-induced chromosomal breaks, suggesting that HIRA is required to maintain the protective functions of chromatin. This may not be the only role for HIRA in the response to DNA damage, as other histone chaperones, such as S. cerevisiae Asf1, have been shown to be required for restoration of chromatin structures following DNA repair (8). Thus, it will be important to determine whether or not HIRA is also required for removal or replacement of nucleosomes during repair of double-strand breaks.
Supplementary Material
Acknowledgments
We thank Richard Maraia and Julie Cooper for strains and plasmids and Janet Partridge, Elizabeth Veal, and Brian Morgan for comments on the manuscript.
This work was funded by a BBSRC project grant (BB/E014445/1) to S.K.W. and by a Cancer Research UK program grant (C9546/A6517) to J.B. H.E.A. was the recipient of a BBSRC DTG award.
Footnotes
Published ahead of print on 20 July 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Ahmad, A., Y. Takami, and T. Nakayama. 2004. WD dipeptide motifs and LXXLL motif of chicken HIRA are essential for interactions with the p48 subunit of chromatin assembly factor-1 and histone deacetylase-2 in vitro and in vivo. Gene 342125-136. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad, A., Y. Takami, and T. Nakayama. 2003. WD dipeptide motifs and LXXLL motif of chicken HIRA are necessary for transcription repression and the latter motif is essential for interaction with histone deacetylase-2 in vivo. Biochem. Biophys. Res. Commun. 3121266-1272. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad, K., and S. Henikoff. 2002. The histone variant H3.3 marks active chromatin by replication-independent nucleosome assembly. Mol. Cell 91191-1200. [DOI] [PubMed] [Google Scholar]
- 4.Blackwell, C., K. A. Martin, A. Greenall, A. Pidoux, R. C. Allshire, and S. K. Whitehall. 2004. The Schizosaccharomyces pombe HIRA-like protein Hip1 is required for the periodic expression of histone genes and contributes to the function of complex centromeres. Mol. Cell. Biol. 244309-4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowen, N. J., I. K. Jordan, J. A. Epstein, V. Wood, and H. L. Levin. 2003. Retrotransposons and their recognition of pol II promoters: a comprehensive survey of the transposable elements from the complete genome sequence of Schizosaccharomyces pombe. Genome Res. 131984-1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cairns, B. R. 2005. Chromatin remodeling complexes: strength in diversity, precision through specialization. Curr. Opin. Genet. Dev. 15185-190. [DOI] [PubMed] [Google Scholar]
- 7.Cam, H. P., K. Noma, H. Ebina, H. L. Levin, and S. I. Grewal. 2008. Host genome surveillance for retrotransposons by transposon-derived proteins. Nature 451431-436. [DOI] [PubMed] [Google Scholar]
- 8.Chen, C. C., J. J. Carson, J. Feser, B. Tamburini, S. Zabaronick, J. Linger, and J. K. Tyler. 2008. Acetylated lysine 56 on histone H3 drives chromatin assembly after repair and signals for the completion of repair. Cell 134231-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, D., W. M. Toone, J. Mata, R. Lyne, G. Burns, K. Kivinen, A. Brazma, N. Jones, and J. Bahler. 2003. Global transcriptional responses of fission yeast to environmental stress. Mol. Biol. Cell 14214-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper, J. P., E. R. Nimmo, R. C. Allshire, and T. R. Cech. 1997. Regulation of telomere length and function by a Myb-domain protein in fission yeast. Nature 385744-747. [DOI] [PubMed] [Google Scholar]
- 11.Durand-Dubief, M., I. Sinha, F. Fagerstrom-Billai, C. Bonilla, A. Wright, M. Grunstein, and K. Ekwall. 2007. Specific functions for the fission yeast Sirtuins Hst2 and Hst4 in gene regulation and retrotransposon silencing. EMBO J. 262477-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eitoku, M., L. Sato, T. Senda, and M. Horikoshi. 2008. Histone chaperones: 30 years from isolation to elucidation of the mechanisms of nucleosome assembly and disassembly. Cell. Mol. Life Sci. 65414-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Formosa, T., S. Ruone, M. D. Adams, A. E. Olsen, P. Eriksson, Y. Yu, A. R. Rhoades, P. D. Kaufman, and D. J. Stillman. 2002. Defects in SPT16 or POB3 (yFACT) in Saccharomyces cerevisiae cause dependence on the Hir/Hpc pathway: polymerase passage may degrade chromatin structure. Genetics 1621557-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodier, J. L., and H. H. Kazazian, Jr. 2008. Retrotransposons revisited: the restraint and rehabilitation of parasites. Cell 13523-35. [DOI] [PubMed] [Google Scholar]
- 15.Green, E. M., A. J. Antczak, A. O. Bailey, A. A. Franco, K. J. Wu, J. R. Yates III, and P. D. Kaufman. 2005. Replication-independent histone deposition by the HIR complex and Asf1. Curr. Biol. 152044-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenall, A., E. S. Williams, K. A. Martin, J. M. Palmer, J. Gray, C. Liu, and S. K. Whitehall. 2006. Hip3 interacts with the HIRA proteins Hip1 and Slm9 and is required for transcriptional silencing and accurate chromosome segregation. J. Biol. Chem. 2818732-8739. [DOI] [PubMed] [Google Scholar]
- 17.Hansen, K. R., G. Burns, J. Mata, T. A. Volpe, R. A. Martienssen, J. Bahler, and G. Thon. 2005. Global effects on gene expression in fission yeast by silencing and RNA interference machineries. Mol. Cell. Biol. 25590-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang, Y., M. A. Bayfield, R. V. Intine, and R. J. Maraia. 2006. Separate RNA-binding surfaces on the multifunctional La protein mediate distinguishable activities in tRNA maturation. Nat. Struct. Mol. Biol. 13611-618. [DOI] [PubMed] [Google Scholar]
- 19.Kanoh, J., and P. Russell. 2000. Slm9, a novel nuclear protein involved in mitotic control in fission yeast. Genetics 155623-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaufman, P. D., J. L. Cohen, and M. A. Osley. 1998. Hir proteins are required for position-dependent gene silencing in Saccharomyces cerevisiae in the absence of chromatin assembly factor I. Mol. Cell. Biol. 184793-4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lackner, D. H., T. H. Beilharz, S. Marguerat, J. Mata, S. Watt, F. Schubert, T. Preiss, and J. Bahler. 2007. A network of multiple regulatory layers shapes gene expression in fission yeast. Mol. Cell 26145-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lafuente, M. J., T. Petit, and C. Gancedo. 1997. A series of vectors to construct lacZ fusions for the study of gene expression in Schizosaccharomyces pombe. FEBS Lett. 42039-42. [DOI] [PubMed] [Google Scholar]
- 23.Lamour, V., Y. Lecluse, C. Desmaze, M. Spector, M. Bodescot, A. Aurias, M. A. Osley, and M. Lipinski. 1995. A human homolog of the S. cerevisiae HIR1 and HIR2 transcriptional repressors cloned from the DiGeorge syndrome critical region. Hum. Mol. Genet. 4791-799. [DOI] [PubMed] [Google Scholar]
- 24.Lyne, R., G. Burns, J. Mata, C. J. Penkett, G. Rustici, D. Chen, C. Langford, D. Vetrie, and J. Bahler. 2003. Whole-genome microarrays of fission yeast: characteristics, accuracy, reproducibility, and processing of array data. BMC Genomics 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maksakova, I. A., D. L. Mager, and D. Reiss. 2008. Keeping active endogenous retroviral-like elements in check: the epigenetic perspective. Cell. Mol. Life Sci. 653329-3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mandell, J. G., J. Bahler, T. A. Volpe, R. A. Martienssen, and T. R. Cech. 2005. Global expression changes resulting from loss of telomeric DNA in fission yeast. Genome Biol. 6R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller, K. M., O. Rog, and J. P. Cooper. 2006. Semi-conservative DNA replication through telomeres requires Taz1. Nature 440824-828. [DOI] [PubMed] [Google Scholar]
- 28.Monahan, B. J., J. Villen, S. Marguerat, J. Bahler, S. P. Gygi, and F. Winston. 2008. Fission yeast SWI/SNF and RSC complexes show compositional and functional differences from budding yeast. Nat. Struct. Mol. Biol. 15873-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moreno, S., A. Klar, and P. Nurse. 1991. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194795-823. [DOI] [PubMed] [Google Scholar]
- 30.Nicolas, E., T. Yamada, H. P. Cam, P. C. Fitzgerald, R. Kobayashi, and S. I. Grewal. 2007. Distinct roles of HDAC complexes in promoter silencing, antisense suppression and DNA damage protection. Nat. Struct. Mol. Biol. 14372-380. [DOI] [PubMed] [Google Scholar]
- 31.Nourani, A., F. Robert, and F. Winston. 2006. Evidence that Spt2/Sin1, an HMG-like factor, plays roles in transcription elongation, chromatin structure, and genome stability in Saccharomyces cerevisiae. Mol. Cell. Biol. 261496-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pelletier, B., J. Beaudoin, Y. Mukai, and S. Labbe. 2002. Fep1, an iron sensor regulating iron transporter gene expression in Schizosaccharomyces pombe. J. Biol. Chem. 27722950-22958. [DOI] [PubMed] [Google Scholar]
- 33.Pelletier, B., J. Beaudoin, C. C. Philpott, and S. Labbe. 2003. Fep1 represses expression of the fission yeast Schizosaccharomyces pombe siderophore-iron transport system. Nucleic Acids Res. 314332-4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phelps-Durr, T. L., J. Thomas, P. Vahab, and M. C. Timmermans. 2005. Maize rough sheath2 and its Arabidopsis orthologue ASYMMETRIC LEAVES1 interact with HIRA, a predicted histone chaperone, to maintain knox gene silencing and determinacy during organogenesis. Plant Cell 172886-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prochasson, P., L. Florens, S. K. Swanson, M. P. Washburn, and J. L. Workman. 2005. The HIR corepressor complex binds to nucleosomes generating a distinct protein/DNA complex resistant to remodeling by SWI/SNF. Genes Dev. 192534-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prudden, J., J. S. Evans, S. P. Hussey, B. Deans, P. O'Neill, J. Thacker, and T. Humphrey. 2003. Pathway utilization in response to a site-specific DNA double-strand break in fission yeast. EMBO J. 221419-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qian, Z., H. Huang, J. Y. Hong, C. L. Burck, S. D. Johnston, J. Berman, A. Carol, and S. W. Liebman. 1998. Yeast Ty1 retrotransposition is stimulated by a synergistic interaction between mutations in chromatin assembly factor I and histone regulatory proteins. Mol. Cell. Biol. 184783-4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ray-Gallet, D., J. P. Quivy, C. Scamps, E. M. Martini, M. Lipinski, and G. Almouzni. 2002. HIRA is critical for a nucleosome assembly pathway independent of DNA synthesis. Mol. Cell 91091-1100. [DOI] [PubMed] [Google Scholar]
- 39.Sehgal, A., C. Y. Lee, and P. J. Espenshade. 2007. SREBP controls oxygen-dependent mobilization of retrotransposons in fission yeast. PLoS Genet. 3e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharp, J. A., E. T. Fouts, D. C. Krawitz, and P. D. Kaufman. 2001. Yeast histone deposition protein Asf1p requires Hir proteins and PCNA for heterochromatic silencing. Curr. Biol. 11463-473. [DOI] [PubMed] [Google Scholar]
- 41.Sherwood, P. W., and M. A. Osley. 1991. Histone regulatory (hir) mutations suppress delta insertion alleles in Saccharomyces cerevisiae. Genetics 128729-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sims, R. J., III, and D. Reinberg. 2008. Is there a code embedded in proteins that is based on post-translational modifications? Nat. Rev. Mol. Cell Biol. 9815-820. [DOI] [PubMed] [Google Scholar]
- 43.Smith, S., and B. Stillman. 1989. Purification and characterization of CAF-I, a human cell factor required for chromatin assembly during DNA replication in vitro. Cell 5815-25. [DOI] [PubMed] [Google Scholar]
- 44.Spector, M. S., A. Raff, H. DeSilva, K. Lee, and M. A. Osley. 1997. Hir1p and Hir2p function as transcriptional corepressors to regulate histone gene transcription in the Saccharomyces cerevisiae cell cycle. Mol. Cell. Biol. 17545-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sugiyama, T., H. P. Cam, R. Sugiyama, K. Noma, M. Zofall, R. Kobayashi, and S. I. Grewal. 2007. SHREC, an effector complex for heterochromatic transcriptional silencing. Cell 128491-504. [DOI] [PubMed] [Google Scholar]
- 46.Tagami, H., D. Ray-Gallet, G. Almouzni, and Y. Nakatani. 2004. Histone H3.1 and H3.3 complexes mediate nucleosome assembly pathways dependent or independent of DNA synthesis. Cell 11651-61. [DOI] [PubMed] [Google Scholar]
- 47.Wang, S. W., A. L. Stevenson, S. E. Kearsey, S. Watt, and J. Bahler. 2008. Global role for polyadenylation-assisted nuclear RNA degradation in posttranscriptional gene silencing. Mol. Cell. Biol. 28656-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams, S. K., and J. K. Tyler. 2007. Transcriptional regulation by chromatin disassembly and reassembly. Curr. Opin. Genet. Dev. 1788-93. [DOI] [PubMed] [Google Scholar]
- 49.Zhang, R., M. V. Poustovoitov, X. Ye, H. A. Santos, W. Chen, S. M. Daganzo, J. P. Erzberger, I. G. Serebriiskii, A. A. Canutescu, R. L. Dunbrack, J. R. Pehrson, J. M. Berger, P. D. Kaufman, and P. D. Adams. 2005. Formation of MacroH2A-containing senescence-associated heterochromatin foci and senescence driven by ASF1a and HIRA. Dev. Cell 819-30. [DOI] [PubMed] [Google Scholar]
- 50.Zurlinden, A., and M. E. Schweingruber. 1994. Cloning, nucleotide sequence, and regulation of Schizosaccharomyces pombe thi4, a thiamine biosynthetic gene. J. Bacteriol. 1766631-6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.