Abstract
The box C/D small nucleolar RNPs (snoRNPs) are essential for the processing and modification of rRNA. TIP48 and TIP49 are two related AAA+ proteins that are essential for the formation of box C/D snoRNPs. These proteins are key components of the pre-snoRNP complexes, but their exact role in box C/D snoRNP biogenesis is largely uncharacterized. Here we report that TIP48 and TIP49 interact with one another in vitro, and only the TIP48/TIP49 complex, but not the individual proteins, possesses significant ATPase activity. Loss of TIP48 and TIP49 results in a change in pre-snoRNA levels and a loss of U3 snoRNA signal in the Cajal body. We show that TIP48 and TIP49 make multiple interactions with core snoRNP proteins and biogenesis factors and that these interactions are often regulated by the presence of ATP. Furthermore, we demonstrate that TIP48 and TIP49 efficiently bridge interactions between the core box C/D proteins NOP56 or NOP58 and 15.5K. Our data imply that the snoRNP assembly factor NUFIP can regulate the interactions between TIP48 and TIP49 and the core box C/D proteins. We suggest that snoRNP assembly involves an intricate series of interactions that are mediated/regulated by bridging factors and chaperones.
In the eukaryotic nucleolus, small nucleolar RNAs (snoRNAs) are involved in the processing and modification of rRNA (1, 20, 38). The H/ACA snoRNAs and box C/D snoRNAs function as sequence-specific guides to direct the isomerization of uridine to pseudouridine and the 2′-O methylation of rRNA, (1, 20, 38). A subset of box C/D snoRNAs that includes U3, U8, and U14 box C/D snoRNAs is essential for rRNA processing. These snoRNAs base pair with specific regions of the pre-rRNA and have been proposed to function as RNA chaperones by regulating rRNA folding (36). Box C/D snoRNAs contain a conserved box C/D motif which is involved in the binding of the four core proteins, 15.5K, NOP56, NOP58, and the methyltransferase fibrillarin (10). The core proteins assemble onto the snoRNA in a stepwise manner with 15.5K first binding to the k-turn element in the box C/D motif followed by the recruitment of the remaining core proteins into the complex (41, 45).
Most box C/D snoRNAs are encoded within the introns of protein-coding genes and are processed from the spliced intron lariat (9, 19, 21, 38). In contrast, a subset of box C/D snoRNAs, including U3, U8, and U13, are independently transcribed by RNA polymerase II (9, 28, 46). The initial transcripts of these genes contain an m7G cap and a short 3′ extension. During snoRNP biogenesis the m7G cap is converted into an m3G cap and the 3′ extension is removed by exonucleases (9, 28, 46). The maturation of the snoRNA and the assembly of the snoRNP is an intricate process involving the dynamic and temporal association of numerous factors in a large multiprotein pre-snoRNP complex (29, 42, 43). These factors include proteins linked to snoRNP assembly (TIP48, TIP49, NUFIP, TAF9, NOP17, and BCD1), molecular chaperones (HSP90 and HSC70), nucleocytoplasmic transport factors (PHAX, CRM1, CBC, Nopp140, Ran, and Snurportin1), and proteins implicated in snoRNA maturation (TGS1, La, LSm proteins, and exosome) (2, 3, 29, 42, 43, 48). A complex series of interactions have been described between the components of the pre-snoRNP, and from this it was predicted that the pre-snoRNP factors NUFIP, BCD1, TIP48, and TIP49 form a scaffold which is responsible for core snoRNP protein assembly (2, 29). NUFIP bridges interactions between the core box C/D proteins and is predicted to hold these factors apart until a predefined point during assembly (2, 29). The U3 and U8 snoRNPs both undergo a stabilization event during biogenesis, suggesting that the snoRNP is remodelled or restructured during assembly (29, 42). This rearrangement is predicted to coincide with the release of any remaining pre-snoRNP factors and the formation of the mature snoRNP (29, 42).
TIP48 (also known as Rvb2p, RuvBL2, TIP49b, Reptin, TAP54b, Tih2p, or p50) and TIP49 (also known as Rvb1p, RuvBL1, TIP49a, Pontin, TAP54a, Tih1p, or p55) are two related AAA+ proteins linked to a number of important events in eukaryotes, including transcription, histone remodelling, and snoRNP assembly (6, 17, 18, 25, 26, 32, 34, 39, 42). AAA+ proteins function in a wide variety of processes in the cell and generally function in the structural remodelling and unfolding of proteins and protein complexes (7, 14). TIP48 and TIP49 are both essential for the accumulation of box C/D snoRNAs in human and yeast cells (18, 42). Both proteins contain Walker A and B motifs, the classical catalytic domains of ATPases. In the case of yeast TIP48, the Walker A and B motifs are required for the accumulation of box C/D snoRNAs (18). This suggests that ATP binding and/or hydrolysis is an important feature of box C/D snoRNP biogenesis. TIP48 and TIP49 are predicted to drive the rearrangements of the pre-snoRNP complex as well as the restructuring/remodelling of the core snoRNP that occurs during biogenesis (29, 42). Together with NOP17 (Pih1) and TAH1, TIP48 and TIP49 are proposed to function as part of the R2TP complex along with the chaperones HSC70 and HSP90 (2, 48). The precise role(s) of TIP48 and TIP49 in box C/D snoRNP biogenesis is, however, yet to be fully elucidated. There are varied and often contradictory reports on the basic ATPase, helicase, and oligomeric assembly properties of these proteins (11-13, 16, 25-27, 33, 34). TIP48 and TIP49 have been predicted to form a heterohexamer and a dimer of two homohexamers (double hexamer) (11-13, 33). A clear understanding of the biochemical properties of these proteins is required in order to fully understand their function. We therefore set out to further characterize the basic biochemical properties of TIP48 and TIP49 and to investigate their roles in snoRNP formation and localization.
MATERIALS AND METHODS
Plasmid constructs.
pET15b TIP48, pET15b TIP49, and pET28a 15.5K were kindly provided by Stuart Maxwell (22, 32). pGEX-2T NUFIP and pcDNA3-NUFIP were kindly provided by Paval Cabart (4). pGEX-6P-1 constructs for TIP48, TIP49, NOP17, NOP56, NOP58, and BCD1(1-360) were described previously (29). The coding region of fibrillarin from amino acids 83 to 322 (fibrillarin [Fib] ΔRGG) was amplified using forward (5′-GGGAAGAATGTGATGGTGGAG-3′) and reverse (5′-CTAGTTCTTCACCTTGGGGGG-3′) primers and then cloned into pET200 (Invitrogen). Plasmids encoding glutathione S-transferase (GST)-15.5K and the five GST-15.5K mutants (1 to 5) were provided by Reinhard Lührmann (35).
Protein purification.
All GST-, hexahistidine-, and thioredoxin-tagged proteins used in this study were purified as described previously (29). Hexahistidine-tagged TIP48 and TIP49 were further purified by ion exchange chromatography using a Resource Q column (GE Healthcare).
Size exclusion chromatography.
A total of 50 μl of 1 mg/ml TIP48, TIP49, or a mixture of TIP48 and TIP49 which had been preincubated for 1 h on ice was applied to a Superdex 200 PC 3.2/30 column that had been equilibrated with TIP buffer (20 mM Tris-HCl [pH 8.0], 10% [vol/vol] glycerol, 300 mM KCl, 1 mM MgCl2, 0.1% [vol/vol] Tween 20, 1 mM Tris-hydroxyphosphine [THP]). Eluting proteins were detected at 280 nm, and 50-μl fractions were collected. Fractions were then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and stained using Coomassie stain. Size exclusion analysis in the presence of nucleotide was performed using TIP buffer supplemented with 1 mM ATP.
ATPase assays.
Reactions were performed at 37°C in ATPase reaction buffer (20 mM Tris-HCl [pH 8.0], 5% [vol/vol] glycerol, 300 mM KCl, 1 mM MgCl2, 1 mM ATP, and 0.5 mM THP) and 0.005 μCi/μl of [γ-32P]ATP (Perkin-Elmer) with a final protein concentration of 2 μM. Samples from each reaction mixture were removed after 0, 5, 15, 30, and 60 min, quenched by adding EDTA to a final concentration of 100 mM, and applied to a polyethyleneimine cellulose thin-layer chromatography (TLC) plate. ATP and free phosphate were separated using 0.5 M formic acid and 0.5 M LiCl2. The TLC plates were analyzed using a PhosphorImager (Typhoon; GE Healthcare), and the released phosphate was quantified using ImageQuant software (GE Healthcare).
Protein-protein interaction studies.
Equal amounts of GST bait proteins were immobilized on glutathione-Sepharose beads. Thioredoxin (TRX) bait proteins were immobilized using anti-TRX antibodies coupled to protein A-Sepharose. Proteins were added to the immobilized bait protein and incubated at 30°C in TIP buffer or TIP buffer supplemented with 1 mM ATP. The beads were washed with the appropriate buffer, and the retained proteins were separated on a 12% polyacrylamide-SDS gel and visualized either by Coomassie staining or Western blotting using tag-specific antibodies. For the GST-15.5K interaction experiments, in vitro-translated [35S]methionine-labeled NUFIP was used. The interaction studies were then performed as above and bound proteins were separated by SDS-PAGE and analyzed by autoradiography.
To build more complicated, partial pre-snoRNP complexes, thioredoxin fusion proteins of NOP56 or NOP58 were incubated with 15.5K, the preformed TIP48/TIP49 complex, GST-NUFIP, or a combination of these proteins at 30°C in TIP buffer. Complexes were then immunoprecipitated using anti-TRX antibodies coupled to protein A-Sepharose. Bound proteins were separated by SDS-PAGE and analyzed by Western blotting using anti-His antibodies to detect NOP56, NOP58, TIP48, and TIP49 and by using anti-GST antibodies to detect GST-NUFIP.
siRNA transfection and reverse transcription-PCR (RT-PCR).
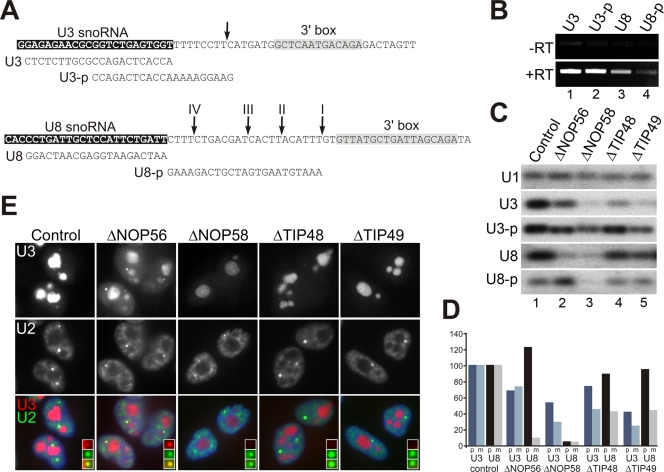
Depletion of NOP56, NOP58, TIP48 and TIP49 was performed as previously described (29, 42). The GL2 small interfering RNA (siRNA; targets the firefly luciferase gene) was used as a negative control. At 60 h after transfection, cells were either harvested and RNA was extracted or fixed and analyzed by in situ hybridization using fluorescently labeled U3 and U2 antisense probes as described previously (29). Extracted RNA was treated with Turbo DNase (Ambion) to remove contaminating DNA, and cDNAs were synthesized using avian myeloblastosis virus reverse transcriptase (Promega) in the presence of a reverse primer. PCR was then performed using GoTaq polymerase (Promega) and a specific primer pair (see below). PCR products were separated on a 2% agarose gel and analyzed by Southern blotting using probes specific for the U3 and U8 snoRNAs and the U1 snRNA. The products were then visualized and quantified by phosphorimager analysis. The linear range of amplification was determined by variation of the number of cycles used, as described previously (47). The products U3, U3-p, and U1 required 15 PCR cycles, while U8 and U8-p required 25 cycles. Levels were normalized against the U1 snRNA, which was previously shown to be unaffected by depletion of the factors used in this study (42).
The reverse primers (listed 5′ to 3′) were U3 (ACCACTCAGACCGCGTTCTCTC), U3-p (GAAGGAAAAACCACTCAGACC), U8 (AATCAGATAGGAGCAATCAGG), U8-p (AAATGTAAGTGATCGTCAGAAA), and U1 (GCCAGGGGAAAGCGCGAACGCA). The forward primers (listed 5′ to 3′) were U3 (CTCGAACGTGTAGAGCACCG), U8 (ATCGTCAGGTGGGATAATCC), and U1 (GCATACTTACCTGGCAGGGGAG).
RESULTS
TIP48 and TIP49 interact to form higher-order complexes.
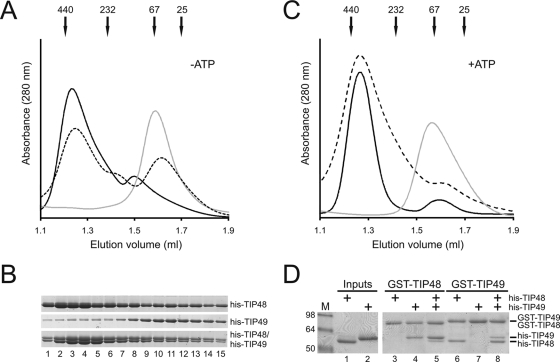
In order to investigate the function of mammalian TIP48 and TIP49 in snoRNP biogenesis it was important to first characterize the purified recombinant proteins by size exclusion chromatography. Individual hexahistidine (His)-tagged TIP48 (His-TIP48) and TIP49 (His-TIP49), or a preincubated, equimolar mixture of the two proteins, were applied to a Superdex 200 PC 3.2/30 column (Fig. 1A). The fractions were analyzed by polyacrylamide gel electrophoresis and the proteins were revealed by Coomassie staining (Fig. 1B). His-TIP48 eluted in two peaks which, when compared to the elution profiles of size exclusion standards, corresponded to complexes with molecular masses of approximately 374 kDa and 96 kDa (Fig. 1A). The predicted molecular mass of His-TIP48, based on its amino acid composition, is 52 kDa, suggesting that the larger peak corresponds to a hexameric complex. The broadness of the peak, however, suggests that it contained multiple forms of the His-TIP48 complex. The other, minor peak likely represents a His-TIP48 dimer. His-TIP49 eluted as a single well-defined peak with an estimated molecular mass of 63 kDa (Fig. 1A). Although there was some discrepancy in size, the data suggested that His-TIP49, which is predicted to be 51.1 kDa, was present as a monomer. When the equimolar mixture of His-TIP48/His-TIP49 (Fig. 1A) was applied to the column, three peaks with estimated sizes of 63 kDa, 150 kDa, and 360 kDa were observed. These peaks likely correspond to a His-TIP49 monomer, a trimer, and a larger heterogenous complex. The 150-kDa complex was consistently seen on the shoulder of the larger peak and likely corresponds to a dimer of His-TIP48 bound to a monomer of His-TIP49. The 360-kDa His-TIP48/His-TIP49 complex eluted slightly later than the corresponding peak for His-TIP48 alone. The broad natures of both the His-TIP48 and the larger His-TIP48/His-TIP49 peaks implied the presence of multiple complexes, making it difficult to conclude what larger complexes were formed. SDS-PAGE analysis of the collected fractions showed equal ratios of His-TIP48 and His-TIP49 in the large complex (Fig. 1B, fractions 2 to 6), demonstrating that the complexes present in this peak were at a 1:1 stoichiometric ratio. When His-TIP48 and His-TIP49 were mixed, peaks could be seen for smaller complexes/monomers (Fig. 1A). It was clear from the protein gel (Fig. 1B), however, that the majorities for both proteins were present in higher-order complexes.
FIG. 1.
TIP48 and TIP49 interact to form higher-order complexes. (A and C) Size exclusion analysis of TIP48 and TIP49 in the presence (C) or absence (A) of 1 mM ATP. Approximately 50 μg of purified His-TIP48 (black line), His-TIP49 (gray line), or a preformed mixture of the two proteins (dashed line) was loaded onto a Superdex 200 3.2/30 column. Protein elution from the column was monitored at 280 nm. The elution peaks of the size exclusion standards are indicated at the top. (B) Fractionated His-TIP48, His-TIP49, and the His-TIP48/His-TIP49 complex from panel A were analyzed by SDS-PAGE and Coomassie staining. Proteins loaded are indicated on the right side of the panel. (D) Recombinant GST-TIP48 or GST-TIP49 was bound to glutathione-Sepharose and then incubated with His-TIP48, His-TIP49, or both, as indicated. Retained proteins were separated by SDS-PAGE and visualized by Coomassie staining. Input was 10% of added His-TIP48 or His-TIP49.
His-TIP48 and His-TIP49 are predicted to bind and hydrolyze ATP. We therefore next performed size exclusion analysis of His-TIP48, His-TIP49, and the His-TIP48/His-TIP49 mixture, in the presence of ATP (Fig. 1C), to determine whether the addition of nucleotide would alter the size and abundance of the various complexes. Addition of 1 mM ATP did not change the migration of the His-TIP49 monomer. In the presence of nucleotide, however, His-TIP48 almost exclusively eluted in the larger, 374-kDa peak, the dimeric peak at 96 kDa was lost, and a small peak corresponding to a monomeric form of His-TIP48 was present. The preincubated His-TIP48/His-TIP49 complex produced two peaks, corresponding to 360 kDa and 65 kDa. As was seen with His-TIP48 alone, the presence of ATP resulted in a significant shift of both proteins to the larger complex. In addition, the middle peak seen in the absence of nucleotide was no longer seen in the presence of ATP. Interestingly, a very similar series of elution profiles was also produced when ADP was used (data not shown). The presence of nucleotide therefore results in His-TIP48 and the His-TIP48/His-TIP49 mixture being present predominantly in higher-order complexes. We cannot, however, determine whether the addition of nucleotide stimulates higher-order complex formation or, conversely, blocks the dissociation of the larger complexes.
To further investigate the formation of higher-order TIP48 and TIP49 complexes, purified recombinant His-TIP48 and His-TIP49 were incubated with GST-tagged TIP48 (GST-TIP48) or GST-tagged TIP49 (GST-TIP49). Complexes were then isolated using glutathione-Sepharose beads, and the bound material was analyzed by polyacrylamide gel electrophoresis followed by Coomassie staining. GST-TIP48 efficiently bound His-TIP49 but not His-TIP48 (Fig. 1D, lanes 3 and 4), and conversely, GST-TIP49 bound His-TIP48 but not His-TIP49 (Fig. 1D, lanes 6 and 7). We therefore conclude that even though His-TIP48 is present in larger, higher-order complexes, there is no rapid exchange of subunits enabling the His-tagged protein to bind to the GST-tagged form. Interestingly, GST-TIP48 did bind His-TIP48 in the presence of His-TIP49 (Fig. 1D, lane 5). This suggests that His-TIP49 either bridges the interaction or causes a structural rearrangement of the His-TIP48 complex, enabling the two forms of the protein to interact. GST-TIP49 also only bound His-TIP49 in the presence of His-TIP48, suggesting a bridging function for His-TIP48 in His-TIP49 complex formation (Fig. 1D, lane 8). Addition of either ATP or ADP, to a final concentration of 1 mM, only resulted in a slight stimulation in the formation of the GST-TIP49/His-TIP48/His-TIP49 and GST-TIP48/His-TIP49/His-TIP48 complexes (data not shown). The data indicate that both proteins are needed to form higher-order complexes. In the case of GST-TIP48, His-TIP49 is likely required to either bridge new interactions or to reorganize the preformed complexes to enable incorporation of additional molecules of His-TIP48.
The interaction between TIP48 and TIP49 is required for efficient ATP hydrolysis.
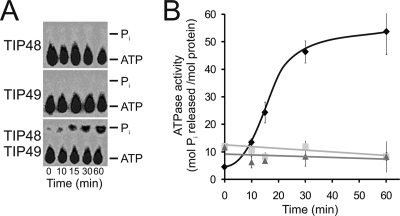
We next analyzed the ability of His-TIP48 and His-TIP49 to hydrolyze ATP. The individual proteins or an equimolar, preformed His-TIP48/His-TIP49 complex was incubated with [γ-32P]ATP. Aliquots were removed at various time points, and ATP hydrolysis was monitored by measuring inorganic phosphate release using thin-layer chromatography (Fig. 2A). Individual His-TIP48 and His-TIP49 proteins exhibited little or no detectable ATPase activity under the conditions used (Fig. 2A and B). In contrast, the preformed mixture of His-TIP48 and His-TIP49 hydrolyzed ATP (Fig. 2A and B). There was an increase in phosphate release with time, suggesting a steady rate of hydrolysis. We conclude that a cooperative interaction between His-TIP48 and His-TIP49 is required for efficient ATP hydrolysis by one or both of these proteins. The addition of single-stranded DNA, double-stranded DNA, plasmid DNA, or in vitro-transcribed box C/D snoRNA did not change the ATPase properties of the individual proteins or of the His-TIP48/His-TIP49 complex (data not shown).
FIG. 2.
The TIP48/TIP49 complex hydrolyzes ATP. (A) His-TIP48, His-TIP49, or the His-TIP48/His-TIP49 complex was incubated with 1 mM ATP that contained trace amounts of [γ-32P]ATP. The reaction mixtures were separated by TLC and visualized using a phosphorimager. The positions of ATP and released phosphate are indicated on the right of each panel. The proteins used are indicated to the left of each panel, and the sample time point is indicated at the bottom. (B) The ATPase activities (expressed as moles of phosphate released/mol of protein) of TIP48 (light gray line), TIP49 (dark gray line), and the TIP48/TIP49 complex (black line) are represented graphically. Error bars indicate the standard deviations of the means from three independent experiments.
TIP48 and TIP49 interact with all four core box C/D proteins.
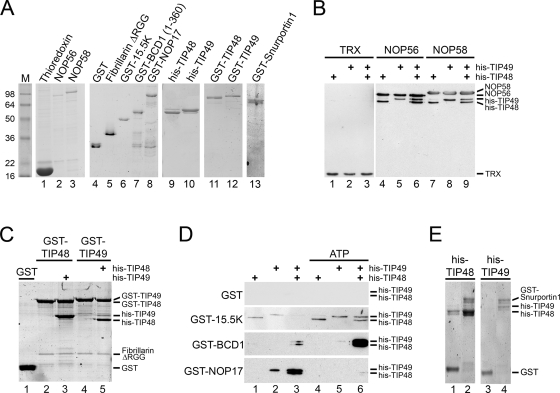
We next investigated the interaction of TIP48, TIP49, and the TIP48/TIP49 complex with the core box C/D proteins at 30°C, the temperature at which the proteins have been shown to hydrolyze ATP and to be active in box C/D snoRNP assembly in vitro (41). Thioredoxin fusion proteins of NOP56 and NOP58, which also contain a hexahistidine tag, were incubated with either the individual proteins or a preincubated complex of TIP48 and TIP49 (Fig. 3B). As a negative control, TIP48 and TIP49 were also incubated with hexahistidine-tagged TRX alone. Complexes were then immunoprecipitated using antithioredoxin antibodies. The isolated proteins were then separated using SDS-PAGE and analyzed by Western blotting using an antibody (anti-His) that recognized the hexahistidine tag present on each of the proteins. Thioredoxin-tagged NOP56 or NOP58 bound TIP48 and TIP49 when they were added individually (Fig. 3B, lanes 4, 5, 7, and 8) or as a preformed complex (lanes 6 and 9). The negative control protein, TRX, did not retain TIP48 or TIP49 under any of the conditions tested (Fig. 3B, lanes 1 to 3). The majority of protein in the TIP48/TIP49 mixture was present in higher-order complexes (Fig. 1B). This suggested that the TIP48/TIP49 complex was capable of interacting with NOP56 and NOP58. The addition of 1 mM ATP did not significantly alter the protein-protein interactions (data not shown).
FIG. 3.
TIP48 and TIP49 interact with several pre-snoRNP proteins. (A) Proteins used in protein-protein interaction studies were separated by SDS-PAGE and visualized by Coomassie staining. The protein loaded is indicated at the top of each lane. M, molecular weight marker. (B) TRX and TRX-fusion proteins of NOP56 and NOP58 were bound to anti-TRX antibody coupled to protein A-Sepharose and then incubated with His-TIP48, His-TIP49, or the His-TIP48/His-TIP49 complex. Bound proteins were separated by SDS-PAGE and analyzed by Western blotting using anti-His antibodies. The identity of the bait protein is indicated at the top and the position of the individual proteins is indicated on the right. (C) Recombinant purified GST, GST-TIP48, and GST-TIP49 were bound to glutathione-Sepharose and then incubated with the fibrillarin fragment (FibΔRGG) either alone or in the presence of His-TIP48 or His-TIP49. Bound proteins were separated by SDS-PAGE and visualized by Coomassie staining. The identity of the proteins used is indicated at the top and the position of the proteins is indicated on the right. (D) GST, GST-15.5K, GST-BCD1, and GST-NOP17 were bound to glutathione-Sepharose and then incubated with TIP48, TIP49, or the TIP48/TIP49 complex in the absence or presence of 1 mM ATP (as indicated). Bound proteins were visualized by Western blotting using anti-His antibodies. The GST-tagged bait protein used is indicated on the left of each panel. The positions of the proteins are indicated on the right. (E) GST (lanes 1 and 3) or GST-Snurportin1 (lanes 2 and 4) was immobilized on glutathione-Sepharose and then incubated with TIP48 or TIP49 (as indicated). Bound proteins were separated by SDS-PAGE and visualized by Coomassie staining. The positions of the proteins are indicated on the right.
We next analyzed whether GST-TIP48 and GST-TIP49 interacted with fibrillarin at 30°C. Full-length fibrillarin was not soluble when expressed fused to a hexahistidine tag in Escherichia coli. We therefore used N-terminally truncated His-tagged fibrillarin (FibΔRGG), from which the RGG domain was removed, which was soluble when expressed and purified from E. coli. This truncated form of the protein was incubated with GST-TIP48 or GST-TIP49, and the resultant complexes were purified using glutathione-Sepharose and analyzed by SDS-PAGE followed by Coomassie staining. GST-TIP48 and GST-TIP49 both interacted, albeit weakly, with FibΔRGG (Fig. 3C, lanes 2 and 4). This interaction was not seen with GST alone (Fig. 3C, lane 1). The use of the TIP48/TIP49 complex or the addition of 1 mM ATP did not affect the interaction with fibrillarin (Fig. 3C, lanes 3 and 5, and data not shown).
We next tested the interactions between TIP48 and TIP49 and the box C/D core protein 15.5K. Purified GST-15.5K or GST was incubated with TIP48, TIP49, or a mixture of the two proteins in the presence or absence of 1 mM ATP, and the interactions were assayed by Western blotting as described above. A weak interaction was observed between 15.5K and TIP48 and TIP49 (Fig. 3D, lanes 1 and 2) that was stimulated by the addition of 1 mM ATP (lanes 4 and 5). Interestingly, in the presence of the TIP48/TIP49 complex this interaction was only seen in the presence of 1 mM ATP (Fig. 3D, compare lanes 3 and 6). This suggested that, in the absence of ATP, there was a preferential interaction between 15.5K and the individual proteins. We suggest that the addition of ATP alters the conformation of the TIP48/TIP49 complex such that it is recognized by 15.5K.
Interactions between TIP48 and TIP49 and snoRNP biogenesis factors.
We next investigated the ability of TIP48 and TIP49 to interact with other box C/D snoRNP biogenesis factors at 30°C. Purified GST, GST-BCD1, and GST-NOP17 (Fig. 3A) were incubated with His-TIP48, His-TIP49, or the His-TIP48/His-TIP49 complex in the presence or absence of ATP. An N-terminal fragment of BCD1 (amino acids 1 to 360), was used as the full-length protein was insoluble (29). Proteins bound to the GST-fusion protein were then isolated using glutathione-Sepharose and analyzed by SDS-PAGE and Western blotting using anti-His antibodies. GST was used as a negative control, and this protein bound neither His-TIP48 nor His-TIP49 under any of the conditions tested (Fig. 3D). A clear interaction was seen between BCD1 and the His-TIP48/His-TIP49 complex (Fig. 3D, lane 3). This interaction was enhanced significantly by the presence of 1 mM ATP (lane 6). In contrast, little if any interaction was seen with the individual proteins, even in the presence of ATP (Fig. 3D, lanes 1, 2, 4, and 5). This indicated that BCD1 specifically recognizes the His-TIP48/His-TIP49 complex but not the individual proteins. GST-NOP17 bound TIP49 but only retained TIP48 in the presence of TIP49 (Fig. 3D, lanes 1 to 3). A stimulation in His-TIP49 binding to GST-NOP17 was also observed in the presence of His-TIP48 (Fig. 3D, lane 3). When 1 mM ATP was present, GST-NOP17 no longer interacted with either His-TIP49 or the His-TIP48/His-TIP49 complex (lanes 4 to 6). Taken together these results demonstrate differences in the interactions formed between either the individually added TIP48 and TIP49 proteins or preformed TIP48/TIP49 complexes with the box C/D snoRNP assembly factors. Furthermore, these interactions are modulated by the presence of 1 mM ATP.
Box C/D snoRNP biogenesis has recently been shown to involve both nuclear import and export factors that are stably bound to the pre-snoRNP complex. We therefore next tested whether import and export factors interact with His-TIP48 and His-TIP49. GST-tagged Snurportin1, overexpressed and purified from E. coli as previously described (37), was incubated with either purified His-TIP48 or His-TIP49. Complexes were then isolated as described above and analyzed by SDS-PAGE followed by Coomassie staining. GST-Snurportin1 efficiently retained both His-TIP48 and His-TIP49 at levels significantly above the background signal seen for GST alone (Fig. 3E). Note that while a background interaction was observed for His-TIP48 with GST alone in this particular experiment, the background was considerably lower than that seen between His-TIP48 and GST-Snurportin1. This interaction was also not affected by the presence of nucleotide (data not shown), and GST-Snuportin1 interacted equally well with both the individual proteins and the His-TIP48/His-TIP49 complex (data not shown). We have also tested the interaction between His-TIP48 and His-TIP49 and the RNA export factors PHAX and CRM1. No interaction was observed between the His-TIP48 and His-TIP49 proteins and the export factors (data not shown). Our data do, however, suggest that Snurportin1 makes significant contacts with TIP48 and TIP49 during snoRNP biogenesis.
TIP48 and TIP49 bridge interactions between 15.5K and both NOP56 and NOP58.
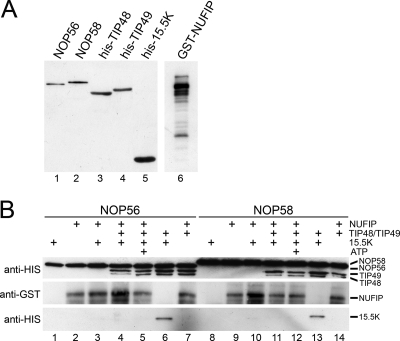
NUFIP has been shown to bring together 15.5K and both NOP56 and NOP58 in the pre-snoRNP (2, 29). Since TIP48 and TIP49 form a number of dynamic nucleotide-dependent interactions, including stable interactions with 15.5K, NOP56, and NOP58, we next sought to determine whether these AAA+ proteins could also bridge interactions between the core box C/D proteins. Thioredoxin fusion proteins of NOP56 or NOP58 were incubated with either 15.5K, NUFIP, the TIP48/TIP49 preformed complex, or various combination of these proteins at 30°C. An excess of 15.5K was used in these experiments to make sure that the protein, which can bind TIP48, TIP49, and NUFIP, was not limiting (Fig. 4A). The proteins bound to NOP56 and NOP58 were then immunoprecipitated using antithioredoxin antibodies, and the copurifying proteins were analyzed by SDS-PAGE and Western blotting.
FIG. 4.
TIP48 and TIP49 efficiently bridge interactions between 15.5K and either NOP56 or NOP58. TRX fusions of NOP56 or NOP58 were incubated with either the TIP48/TIP49 complex, 15.5K, GST-NUFIP, or various combinations of these proteins in the presence or absence of ATP. The resultant complexes were immunoprecipitated using anti-TRX antibodies. The input proteins (A) and immunoprecipitated complexes (B) were then separated by SDS-PAGE and visualized by Western blotting using anti-His (to detect NOP56, NOP58, 15.5K, TIP48, and TIP49) or anti-GST (NUFIP) antibodies (as indicated on the left). The proteins used and the inclusion of ATP are indicated above each lane. The migration of the individual proteins is indicated to the right of each panel.
As previously reported, 15.5K did not directly bind NOP56 or NOP58 (Fig. 4, lanes 1 and 8). The addition of NUFIP, which binds to 15.5K, NOP56, and NOP58 (2, 29), resulted in an extremely weak recruitment of 15.5K to either NOP56 or NOP58 that was reproducibly only visible upon longer exposures than those used in Fig. 4B (lanes 3 and 10). In contrast, the addition of the preformed TIP48/TIP49 complex together with 15.5K resulted in the efficient coprecipitation of both TIP48/TIP49 and 15.5K with either NOP56 or NOP58 (Fig. 4B, lanes 6 and 13). This result was also observed when the individual TIP48 or TIP49 proteins were used (data not shown). Surprisingly, the inclusion of both NUFIP and the TIP48/TIP49 complex did not result in the efficient coprecipitation of 15.5K with either NOP56 or NOP58 (Fig. 4B, lanes 4 and 11), even in the presence of 1 mM ATP (lanes 5 and 12). In contrast, both NUFIP and the TIP48/TIP49 complex were efficiently bound to NOP58 or NOP56. Taken together these data suggest that TIP48 and/or TIP49 is much more efficient at bridging the interaction between 15.5K and NOP56/NOP58 than NUFIP. Furthermore, NUFIP appears to limit the interaction between the core box C/D proteins, even in the presence of TIP48 and TIP49.
15.5K mutations that block snoRNP assembly specifically affect NUFIP binding.
A series of mutations were previously made in conserved regions on the surface of 15.5K, of which three (mutations 15.5K-1, -2, and -5) exhibited defects in the in vitro assembly of box C/D snoRNPs (35). These mutations likely inhibit interactions between 15.5K and key biogenesis factors, such as TIP48, TIP49, BCD1, and NUFIP. We therefore examined whether these mutations affected box C/D snoRNP assembly by inhibiting binding to TIP48 and/or TIP49. Purified GST-tagged wild-type 15.5K and the five mutants (Fig. 5A) were incubated with His-TIP48, His-TIP49, or the His-TIP48/His-TIP49 complex in the presence of 1 mM ATP. Complexes were then immobilized on glutathione-Sepharose and analyzed by SDS-PAGE followed by Western blotting using anti-His antibodies. Wild-type 15.5K and all five of the 15.5K surface mutants bound His-TIP48, His-TIP49, and the His-TIP48/His-TIP49 complex at levels significantly above the background seen with GST alone (Fig. 5B). This suggested that the defects in snoRNP assembly caused by these mutations are not due to disruption of the interaction between 15.5K and either TIP48 or TIP49.
FIG. 5.
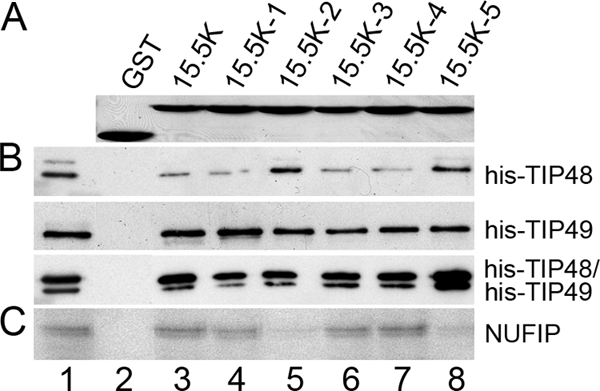
Mutations in 15.5K disrupt interactions with NUFIP but not TIP48 and TIP49. (A) SDS-PAGE analysis of recombinant GST and GST-15.5K proteins. Proteins were visualized by Coomassie staining. The protein loaded is indicated above each lane. (B) GST, GST-15.5K, and GST-15.5K (mutants 1 to 5) were immobilized on glutathione-Sepharose and then incubated with TIP48 or TIP49 in the presence of 1 mM ATP. Bound proteins were separated by SDS-PAGE and visualized by Western blotting using anti-His antibodies. Target proteins are indicated on the right of the panel. Input (lane 1), 10% of the input material. (C) GST, GST-15.5K, and the GST-15.5K mutant proteins were bound to glutathione-Sepharose and incubated with 35S-labeled NUFIP. Bound proteins were separated by SDS-PAGE and visualized by autoradiography. Input (lane 1), 10% of the input protein.
We next examined the ability of the 15.5K mutants to interact with the assembly factor NUFIP. Immobilized GST-15.5K proteins were incubated with in vitro-translated 35S-labeled NUFIP as described above, separated by SDS-PAGE, and analyzed by autoradiography. In agreement with earlier published work (2, 29), wild-type 15.5K efficiently retained 35S-labeled NUFIP (Fig. 5C, lane 3). The mutants 15.5K-1, 15.5K-3, and 15.5K-4 all retained 35S-labeled NUFIP at levels comparable to the wild type (Fig. 5C, lanes 4, 6, and 7). In contrast, the binding of 35S-labeled NUFIP to the mutants 15.5K-2 and 15.5K-5 was significantly lower than that seen with the wild-type protein (Fig. 5C, lanes 5 and 8). These results suggest that defects in box C/D assembly caused by the 15.5K-2 and 15.5K-5 mutations can be explained by a reduced affinity for NUFIP.
Differential effects of depletion of NOP56, NOP58, TIP48, and TIP49 on levels of mature and precursor U3 and U8 snoRNAs.
The importance of the core box C/D proteins and many of the snoRNP biogenesis factors in maintaining mature snoRNA levels has been documented in human cells (3, 29, 42) and yeast (23, 24, 45). It is not known, however, what effect their depletion has on human pre-snoRNA levels. We therefore next used RNA interference (RNAi) to compare the effects of depleting NOP56, NOP58, TIP48, and TIP49 on the levels of the mature and precursor forms of the U3 and U8 snoRNAs. HeLa cells were transiently transfected with siRNA duplexes targeting the mRNAs encoding the individual proteins or, as a control, the firefly luciferase mRNA. The specificity of these siRNAs and their effects on U3 and U8 snoRNA levels were documented previously (42). After 60 h RNA was extracted from the cells and analyzed by semiquantitative RT-PCR. Mature snoRNAs are over 100-fold more abundant than their precursors. Furthermore, the precursors are often only a few nucleotides longer than the mature snoRNA (3, 42, 43). After several unsuccessful attempts to analyze pre-snoRNA levels by Northern hybridization, we developed an RT-PCR approach to analyze the levels of the precursors. Primers were designed that would anneal to the mature 3′ end of U3 and U8 or specifically to the precursor transcripts (Fig. 6A). The primers directed against the mature snoRNA sequences will also amplify the pre-snoRNAs; however, due to the relatively low abundance of the pre-snoRNAs (42, 43), the vast majority of the signal seen will be derived from the mature RNAs. Due to the small differences between the different forms we could not devise primers that differentiated between the intermediate U8 snoRNA precursors (43). We therefore restricted our analysis to the longest U8 pre-snoRNA. This is believed to represent the initial transcript and has been shown to be associated with all four core box C/D proteins (43). As an internal loading control, primers were also designed to analyze the U1 snRNA. For each of the primer sets we determined the linear range of PCR amplification by varying the number of PCR cycles (see Materials and Methods). Agarose gel electrophoresis followed by Southern blotting was employed to detect the PCR products. The resulting bands were quantified by phosphorimager analysis. This experiment was repeated three times. It is important to note that with each of the samples and primer pairs no product was observed in the absence of reverse transcriptase under any conditions tested (Fig. 6B).
FIG. 6.
Differential effects of depleting TIP48 and TIP49 on U3 and U8 pre-snoRNA levels. (A) DNA sequences of the coding region (white text on black background) and the 3′ box (gray box) of the U3 and U8 snoRNA genes. The 3′ box was identified based on previous work (15, 30, 31). The 3′ ends of the human U3 and U8 snoRNA precursor transcripts are indicated by arrows at the top of the sequence. The reverse primers used in the RT-PCR analysis are shown below the sequence. (B) RNA was extracted from control HeLa cells and analyzed by RT-PCR with (+RT) our without (-RT) reverse transcriptase. The PCRs were separated by agarose gel electrophoresis and stained using SYBR Safe. The specific reverse primer used is indicated above each lane. (C) Cells were treated with siRNA duplexes targeting NOP56, NOP58, TIP48, or TIP49 or the control siRNA targeting luciferase (GL2). After 60 h of incubation, the cells were harvested and RNA extracted and analyzed by RT-PCR. The products were separated by agarose electrophoresis and detected by Southern blotting using probes specific to the U3 and U8 snoRNAs and the U1 snRNA and visualized using a phosphorimager. The protein targeted is indicated at the top and the primer used for the reverse transcriptase step indicated on the left. (D) The mature and pre-snoRNA signals revealed using the phosphorimager in panel C were quantitated and then expressed as percentages of the levels seen in the control knockdown (GL2). The mRNA targeted is indicated on the horizontal axis. (E) HeLa cells transfected with siRNAs were hybridized with fluorescent oligonucleotides complementary to the U3 snoRNA and the U2 snRNA, and images were captured by fluorescence microscopy. The RNAi-depleted protein is indicated at the top of each column of images. The first row of images from the top of the figure shows U3 snoRNA localization. The second row shows localization of the U2 snRNA. The third row shows an overlay of the U2 snRNA and U3 snoRNA images for each series of cells. The inset panels in the bottom row of images show magnified views of a single Cajal body.
Depletion of TIP48, TIP49, NOP56, and NOP58 resulted in a reduction of mature U3 and U8 levels relative to cells transfected with the GL2 control (Fig. 6C and D), consistent with previously published Northern blot analysis (42). The levels of the U1 snRNA were unaffected by the loss of these proteins. The RT-PCR approach enabled the direct comparison of the role of each protein on both precursor and mature snoRNA levels for both U3 and U8. Depletion of NOP56 resulted in a reproducible 25% and 30% reduction in the levels of the mature and precursor U3 snoRNAs, respectively. Loss of NOP56 resulted in a 10-fold decrease in mature U8 snoRNA levels and, surprisingly, a 25% increase in the levels of pre-U8 (Fig. 6C and D). This suggests that the depletion of NOP56 resulted in the slight accumulation of pre-U8 and blocked the formation of the mature U8 snoRNA. Loss of NOP58 resulted in the reduction of both pre-U3 and -U8 snoRNAs to 54% and 4% of the levels seen in the control cells, respectively. A more dramatic reduction in mature U3 and U8 levels was seen after depletion of NOP58 (reduced to 26% and 3%, respectively), suggesting that the presence of this protein was more important for the accumulation of the mature RNAs than the precursor species.
TIP48 depletion had a similar effect on the levels of the U3 and U8 snoRNA species in the cell. Protein loss resulted in a reduction of pre-U3 and pre-U8 snoRNA levels to 77% and 91%, respectively (Fig. 6C and D). Again, loss of this protein had a more significant effect on mature RNAs, with the levels of both snoRNAs reduced to about 40% of that seen in the control cells. Depletion of TIP49 resulted in a reduction of U3 and pre-U3 to 23% and 42% of that seen in the control cells, respectively. Loss of this protein had less of an effect on U8 snoRNA levels and resulted in a reduction of the pre-U8 and mature U8 snoRNAs to 95% and 45% of the levels seen in the control cells, respectively. In summary, the data suggest that the mature snoRNA levels, for both the U3 and U8 snoRNA, are generally more affected by the loss of NOP58, TIP48, and TIP49 than the precursor RNAs. Furthermore, there is clearly a difference in the requirement of individual proteins in the production of the U3 and U8 snoRNAs that may reflect differences in their biogenesis pathways (43).
TIP48 and TIP49 are essential for the localization of snoRNPs to the Cajal body.
It was previously shown that depletion of core box C/D proteins and biogenesis factors altered the subcellular distribution of the box C/D snoRNAs (29, 43). We therefore investigated the role of TIP48 and TIP49 in U3 snoRNP localization in vivo. HeLa cells were transfected with siRNAs and, after 60 h, the cells were fixed and analyzed by fluorescence in situ hybridization using probes specific for the U3 snoRNA and the spliceosomal U2 snRNA. As a comparison, cells were also transfected with siRNAs targeting NOP58, NOP56, and the control duplex targeting luciferase (GL2). The effect of depleting NOP56 and NOP58 has been recorded elsewhere, and the knockdowns were used here as a comparison (43). The signal seen for the U3 snoRNA was primarily nucleolar, with a low-level signal also seen in the Cajal bodies in control transfected cells (Fig. 6E). Depletion of NOP58 resulted in a strong reduction of nucleolar U3 snoRNA levels, while the loss of NOP56, TIP48, and TIP49 had a less severe effect (Fig. 6E). This is consistent with the RT-PCR analysis of total U3 snoRNA levels in transfected cells (Fig. 6C and D). The loss of NOP56 resulted in an increase in the U3 signals in the nucleoplasm and Cajal bodies. In contrast, depletion of NOP58, TIP48, and TIP49 resulted in the significant reduction of U3 signals in the Cajal body and no noticeable snoRNA accumulation in the nucleoplasm (Fig. 6E). As discussed previously (29), we believe that the remaining nucleolar signal seen in cells where NOP56, NOP58, TIP48, and TIP49 have been depleted represents either snoRNAs that have yet to be turned over, i.e., that were present in the cell prior to transfection, or a low level of snoRNAs that has been synthesized in the presence of reduced levels of the target protein. RNAi-mediated depletion of NOP58, NOP56, TIP48, and TIP49 did not alter the distribution of the control U2 snRNA (Fig. 6E). Taken together, the data indicate that TIP48 and TIP49 are essential for the Cajal body localization of the U3 snoRNA.
DISCUSSION
TIP48 and TIP49 complex formation stimulates ATP hydrolysis activity.
In this study, recombinant purified TIP48 and TIP49 only showed ATPase activity when both proteins were present. This is in agreement with recent publications which have shown that the individual human TIP48 and TIP49 proteins are very weak ATPases (16, 33), but it contradicts earlier work suggesting that each protein can independently hydrolyze ATP (25, 26). The rate of ATP hydrolysis for the TIP48/TIP49 complex was low when compared to many other ATPases but similar to that seen for other AAA+ proteins (7, 8). The activities of many AAA+ proteins are stimulated by adaptor proteins, and it is possible that one or more pre-snoRNP proteins, or possibly the snoRNA, may stimulate ATP hydrolysis by TIP48 and TIP49. The addition of the available recombinant snoRNP factors, or box C/D snoRNAs, to our ATPase assays did not, unfortunately, alter the activity of TIP48 and/or TIP49 (data not shown).
As AAA+ proteins typically function as large oligomeric assemblies, we examined the TIP48 and TIP49 proteins by size exclusion chromatography. In agreement with other studies, TIP49 was almost exclusively monomeric. TIP48 was, however, present as a dimer and a larger, broad peak that we believe represents a mixture of complexes that are approximately the size of a hexamer. This is in contrast to earlier work, where TIP48 was predicted to be either a monomer or a dimer/trimer complex (16, 33). These differences could reflect the different conditions used to isolate the proteins. For example, we found that the inclusion of MgCl2 in all buffers significantly enhanced the yield and solubility of both TIP48 and TIP49 expressed and purified from E. coli. In a similar study, however, TIP48, purified from E. coli using buffers containing EDTA and no MgCl2, was present as a monomer (33). A consistent element of the various studies on TIP48 and TIP49 is that when the two proteins are incubated together they combine to form higher-order complexes. TIP48 and TIP49 have been reported to form a variety of different complexes, including homomeric hexamers, a dodecameric complex, and a heterohexamer with alternating subunits (11-13, 16, 27, 33). The ability to form a variety of structures might explain the broad nature of the size exclusion peak for the large TIP48 complex(es), and alternative approaches are likely required to fully define the various TIP48/TIP49 complexes.
TIP48 and TIP49 interact with pre-snoRNP factors and core box C/D proteins.
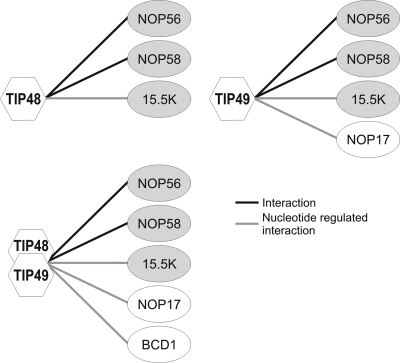
To learn more about TIP48 and TIP49 function in snoRNP biogenesis we characterized the interactions between these AAA+ proteins and a variety of pre-snoRNP factors. These experiments were performed under the approximate conditions used for in vitro assembly reactions using mammalian nuclear extracts (41, 44). Interactions with components of the pre-snoRNP, including 15.5K, fibrillarin, NOP56, NOP58, BCD1, NOP17, and the nuclear import factor Snurportin1, were observed (summarized in Fig. 7). The interactions with BCD1, 15.5K, and NOP17 were nucleotide dependent. Furthermore, BCD1 and 15.5K interacted differently with individual proteins and the TIP48/TIP49 complex.
FIG. 7.
Schematic representation of the interactions between TIP48 and TIP49 and factors involved in snoRNP assembly. The protein-protein interaction data produced during the present study are represented. Core box C/D proteins are shown in gray and snoRNP assembly factors are in white. Black lines represent interactions between individual proteins. Gray lines indicate protein-protein interactions that are altered by the nucleotide bound to either TIP48 or TIP49.
We had previously shown that, at 4°C, NUFIP bridged interactions between core box C/D proteins. Here we show that TIP48 and TIP49, either alone or as a complex, are much more efficient at bridging the interaction between NOP56/NOP58 and 15.5K at 30°C than NUFIP. Indeed, NUFIP efficiently blocks the interaction between 15.5K and the AAA+ proteins. The interaction between NUFIP and 15.5K appears functionally significant, since it is disrupted by two of the three 15.5K mutations previously documented to block box C/D snoRNP assembly. In summary, the data suggest a dynamic interplay between the snoRNP biogenesis factors and the core box C/D proteins.
Depletion of TIP48 and TIP49 results in changes in snoRNA localization and pre-snoRNA levels.
Depletion of NOP56, NOP58, TIP48, or TIP49 resulted in a reduction in the levels of the U3 and U8 snoRNAs as published earlier (43). This could either be due to destabilization of the initial pre-snoRNAs or through blocking the biogenesis pathway. Examination of the pre-snoRNA levels revealed that, with the exception of the effect of NOP56 loss resulting in a slight increase in U8 pre-snoRNA, depletion of the various factors resulted in a reduction in both U3 and U8 precursor levels. The reduction was, however, not as severe as that seen with the mature snoRNA levels with the exception of the effect of NOP56 depletion on mature and pre-U3. This suggests that the loss of these proteins results in either a block in snoRNP biogenesis or the instability/degradation of the mature form. A block in biogenesis would lead to an accumulation of pre-snoRNA, which may either be degraded or result in the inhibition of snoRNA synthesis. Many proteins bind the pre-snoRNAs (42, 43), including the RNA-binding protein La, which is often involved in stabilizing nascent transcripts. These factors may help stabilize the precursor in the absence of one of the key biogenesis factors or core box C/D proteins. Loss of a core protein may not destabilize the pre-snoRNP but could result in the production of an unstable “mature” snoRNP when the biogenesis factors dissociate. Considering that the core box C/D proteins undergo a restructuring event during biogenesis it is possible that they are not as important for the stability of the transcript as is the case for the mature complex. The loss of NOP56, NOP58, TIP48, and TIP49 resulted in a reduction in the pre-U3 snoRNA levels, but the effect was not as strong as was seen for the U8 snoRNA precursor. It is likely that the differences of the effect of protein depletion on the production of the U3 and U8 snoRNAs reflect differences in protein requirements for the assembly pathway of the snoRNP complex. Supporting this idea, clear differences in the biogenesis of the U3 and U8 snoRNAs have been observed (42, 43). In particular, there are significant differences in the size, and therefore composition, of pre-snoRNP complexes and pre-snoRNA processing pathways between these two snoRNAs.
The loss of NOP56 resulted in an increase in the level of the U3 snoRNA in both Cajal bodies and the nucleoplasm. This is consistent with the proposed localization of pre-snoRNA transcripts to the Cajal body during snoRNP biogenesis. In contrast, depletion of NOP58, TIP48, or TIP49 resulted in a loss of the U3 snoRNA signal in the Cajal body. Due to the extent of the effect of NOP58 and TIP49 depletion on pre- and mature snoRNA levels little can be concluded about the lack of Cajal body localization. Loss of TIP48 had, however, similar effects on U3 pre-snoRNA levels as did the depletion of NOP56. It is therefore possible that TIP48 is required for U3 snoRNA localization to the Cajal body. It is, however, not clear what percentage of the natural Cajal body signal is derived from mature or pre-U3 snoRNPs. Unfortunately, attempts to detect native pre-U3 snoRNA by fluorescence in situ hybridization have proved unsuccessful, and the pre-U3 has only ever been documented for transcripts derived from transfected plasmids (40).
Roles of TIP48 and TIP49 in box C/D snoRNP biogenesis.
Based on our data, we propose that TIP48 and TIP49 form many interactions within the pre-snoRNP and likely function in the formation of the pre-snoRNP. NUFIP likely plays a key role in regulating some of these interactions. In particular, NUFIP could potentially block TIP48 and TIP49 binding to the core box C/D proteins during snoRNP biogenesis. This potential chaperone-like function is similar to the proposed role for pICln in regulating the formation of the complete Sm protein complex and its association with the snRNA (5). In this case pICln binds to a subset of the Sm proteins and requires the SMN protein to remove the chaperone and catalyze snRNP formation. Interestingly, we also found that TIP48 and TIP49 both interact with the nuclear import factor Snurportin1. This implies that these proteins may play a key role, in addition to the m3G cap, in regulating the recruitment and function of Snurportin1.
It is likely that TIP48 and TIP49 also drive the recruitment and release of a number of factors in the pre-snoRNP during the dynamic biogenesis pathway. As with other AAA+ proteins, this is likely regulated by nucleotide binding and/or hydrolysis. TIP48 and TIP49, while present together in several pre-snoRNP complexes, are recruited and released at different stages during snoRNP biogenesis (42, 43). This implies that the two proteins function independently of one another at specific points during snoRNP biogenesis. We found no evidence for ATP hydrolysis by the individual proteins, although we cannot rule out the possibility that they function individually in vivo. Indeed, it is possible that we have yet to identify the adaptor protein(s) that modulates the activity of TIP48 and TIP49. The different structural organizations of TIP48/TIP49 complexes that have been reported may reflect the abilities of these proteins to form different structures in vivo. It is possible that changes in the structural organization of the TIP48/TIP49 complex may occur during snoRNP biogenesis. These changes could coincide with the reported restructuring/remodelling of the pre-snoRNP complex during biogenesis (29, 42). Indeed, we propose that the remodelling of the pre-snoRNP reflects changes in the interactions between the core box C/D proteins and NUFIP and/or the TIP48/TIP49 proteins.
Acknowledgments
We thank Reinhard Lührmann, Achim Dickmanns, Ralf Ficner, and Stuart Maxwell for generously providing plasmids. We also thank Jeremy Brown and Claudia Schneider for critically reading the manuscript.
This work was supported by grants from the BBSRC and Royal Society.
Footnotes
Published ahead of print on 20 July 2009.
REFERENCES
- 1.Bachellerie, J. P., J. Cavaille, and A. Huttenhofer. 2002. The expanding snoRNA world. Biochimie 84775-790. [DOI] [PubMed] [Google Scholar]
- 2.Boulon, S., N. Marmier-Gourrier, B. Pradet-Balade, L. Wurth, C. Verheggen, B. E. Jady, B. Rothe, C. Pescia, M. C. Robert, T. Kiss, B. Bardoni, A. Krol, C. Branlant, C. Allmang, E. Bertrand, and B. Charpentier. 2008. The Hsp90 chaperone controls the biogenesis of L7Ae RNPs through conserved machinery. J. Cell Biol. 180579-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boulon, S., C. Verheggen, B. E. Jady, C. Girard, C. Pescia, C. Paul, J. K. Ospina, T. Kiss, A. G. Matera, R. Bordonne, and E. Bertrand. 2004. PHAX and CRM1 are required sequentially to transport U3 snoRNA to nucleoli. Mol. Cell 16777-787. [DOI] [PubMed] [Google Scholar]
- 4.Cabart, P., H. K. Chew, and S. Murphy. 2004. BRCA1 cooperates with NUFIP and P-TEFb to activate transcription by RNA polymerase II. Oncogene 235316-5329. [DOI] [PubMed] [Google Scholar]
- 5.Chari, A., M. M. Golas, M. Klingenhager, N. Neuenkirchen, B. Sander, C. Englbrecht, A. Sickmann, H. Stark, and U. Fischer. 2008. An assembly chaperone collaborates with the SMN complex to generate spliceosomal snRNPs. Cell 135497-509. [DOI] [PubMed] [Google Scholar]
- 6.Conaway, R. C., and J. W. Conaway. 2009. The INO80 chromatin remodeling complex in transcription, replication and repair. Trends Biochem. Sci. 3471-77. [DOI] [PubMed] [Google Scholar]
- 7.Erzberger, J. P., and J. M. Berger. 2006. Evolutionary relationships and structural mechanisms of AAA+ proteins. Annu. Rev. Biophys. Biomol. Struct. 3593-114. [DOI] [PubMed] [Google Scholar]
- 8.Erzberger, J. P., M. L. Mott, and J. M. Berger. 2006. Structural basis for ATP-dependent DnaA assembly and replication-origin remodeling. Nat. Struct. Mol. Biol. 13676-683. [DOI] [PubMed] [Google Scholar]
- 9.Filipowicz, W., and V. Pogacic. 2002. Biogenesis of small nucleolar ribonucleoproteins. Curr. Opin. Cell Biol. 14319-327. [DOI] [PubMed] [Google Scholar]
- 10.Galardi, S., A. Fatica, A. Bachi, A. Scaloni, C. Presutti, and I. Bozzoni. 2002. Purified box C/D snoRNPs are able to reproduce site-specific 2′-O-methylation of target RNA in vitro. Mol. Cell. Biol. 226663-6668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorynia, S., P. M. Matias, T. M. Bandeiras, P. Donner, and M. A. Carrondo. 2008. Cloning, expression, purification, crystallization and preliminary X-ray analysis of the human RuvBL1-RuvBL2 complex. Acta Crystallogr. F 64840-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorynia, S., P. M. Matias, S. Goncalves, R. Coelho, G. Lopes, M. Thomaz, M. Huber, B. Haendler, P. Donner, and M. A. Carrondo. 2006. Expression, purification, crystallization and preliminary X-ray analysis of the human RuvB-like protein RuvBL1. Acta Crystallogr. F 6261-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gribun, A., K. L. Cheung, J. Huen, J. Ortega, and W. A. Houry. 2008. Yeast Rvb1 and Rvb2 are ATP-dependent DNA helicases that form a heterohexameric complex. J. Mol. Biol. 3761320-1333. [DOI] [PubMed] [Google Scholar]
- 14.Hanson, P. I., and S. W. Whiteheart. 2005. AAA+ proteins: have engine, will work. Nat. Rev. Mol. Cell Biol. 6519-529. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez, N., and A. M. Weiner. 1986. Formation of the 3′ end of U1 snRNA requires compatible snRNA promoter elements. Cell 47249-258. [DOI] [PubMed] [Google Scholar]
- 16.Ikura, T., V. V. Ogryzko, M. Grigoriev, R. Groisman, J. Wang, M. Horikoshi, R. Scully, J. Qin, and Y. Nakatani. 2000. Involvement of the TIP60 histone acetylase complex in DNA repair and apoptosis. Cell 102463-473. [DOI] [PubMed] [Google Scholar]
- 17.Jha, S., E. Shibata, and A. Dutta. 2008. Human Rvb1/Tip49 is required for the histone acetyltransferase activity of Tip60/NuA4 and for the downregulation of phosphorylation on H2AX after DNA damage. Mol. Cell. Biol. 282690-2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King, T. H., W. A. Decatur, E. Bertrand, E. S. Maxwell, and M. J. Fournier. 2001. A well-connected and conserved nucleoplasmic helicase is required for production of box C/D and H/ACA snoRNAs and localization of snoRNP proteins. Mol. Cell. Biol. 217731-7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiss, T. 2004. Biogenesis of small nuclear RNPs. J. Cell Sci. 1175949-5951. [DOI] [PubMed] [Google Scholar]
- 20.Kiss, T. 2002. Small nucleolar RNAs: an abundant group of noncoding RNAs with diverse cellular functions. Cell 109145-148. [DOI] [PubMed] [Google Scholar]
- 21.Kiss, T. 2006. SnoRNP biogenesis meets pre-mRNA splicing. Mol. Cell 23775-776. [DOI] [PubMed] [Google Scholar]
- 22.Kuhn, J. F., E. J. Tran, and E. S. Maxwell. 2002. Archaeal ribosomal protein L7 is a functional homolog of the eukaryotic 15.5kD/Snu13p snoRNP core protein. Nucleic Acids Res. 30931-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lafontaine, D. L., and D. Tollervey. 1999. Nop58p is a common component of the box C+D snoRNPs that is required for snoRNA stability. RNA 5455-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lafontaine, D. L., and D. Tollervey. 2000. Synthesis and assembly of the box C+D small nucleolar RNPs. Mol. Cell. Biol. 202650-2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makino, Y., M. Kanemaki, Y. Kurokawa, T. Koji, and T. Tamura. 1999. A rat RuvB-like protein, TIP49a, is a germ cell-enriched novel DNA helicase. J. Biol. Chem. 27415329-15335. [DOI] [PubMed] [Google Scholar]
- 26.Makino, Y., T. Mimori, C. Koike, M. Kanemaki, Y. Kurokawa, S. Inoue, T. Kishimoto, and T. Tamura. 1998. TIP49, homologous to the bacterial DNA helicase RuvB, acts as an autoantigen in human. Biochem. Biophys. Res. Commun. 245819-823. [DOI] [PubMed] [Google Scholar]
- 27.Matias, P. M., S. Gorynia, P. Donner, and M. A. Carrondo. 2006. Crystal structure of the human AAA+ protein RuvBL1. J. Biol. Chem. 28138918-38929. [DOI] [PubMed] [Google Scholar]
- 28.Maxwell, E. S., and M. J. Fournier. 1995. The small nucleolar RNAs. Annu. Rev. Biochem. 64897-934. [DOI] [PubMed] [Google Scholar]
- 29.McKeegan, K. S., C. M. Debieux, S. Boulon, E. Bertrand, and N. J. Watkins. 2007. A dynamic scaffold of pre-snoRNP factors facilitates human box C/D snoRNP assembly. Mol. Cell. Biol. 276782-6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neuman de Vegvar, H. E., and J. E. Dahlberg. 1990. Nucleocytoplasmic transport and processing of small nuclear RNA precursors. Mol. Cell. Biol. 103365-3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neuman de Vegvar, H. E., E. Lund, and J. E. Dahlberg. 1986. 3′ end formation of U1 snRNA precursors is coupled to transcription from snRNA promoters. Cell 47259-266. [DOI] [PubMed] [Google Scholar]
- 32.Newman, D. R., J. F. Kuhn, G. M. Shanab, and E. S. Maxwell. 2000. Box C/D snoRNA-associated proteins: two pairs of evolutionarily ancient proteins and possible links to replication and transcription. RNA 6861-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puri, T., P. Wendler, B. Sigala, H. Saibil, and I. R. Tsaneva. 2007. Dodecameric structure and ATPase activity of the human TIP48/TIP49 complex. J. Mol. Biol. 366179-192. [DOI] [PubMed] [Google Scholar]
- 34.Qiu, X. B., Y. L. Lin, K. C. Thome, P. Pian, B. P. Schlegel, S. Weremowicz, J. D. Parvin, and A. Dutta. 1998. An eukaryotic RuvB-like protein (RUVBL1) essential for growth. J. Biol. Chem. 27327786-27793. [DOI] [PubMed] [Google Scholar]
- 35.Schultz, A., S. Nottrott, N. J. Watkins, and R. Lührmann. 2006. Protein-protein and protein-RNA contacts both contribute to the 15.5K-mediated assembly of the U4/U6 snRNP and the box C/D snoRNPs. Mol. Cell. Biol. 265146-5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steitz, J. A., and K. T. Tycowski. 1995. Small RNA chaperones for ribosome biogenesis. Science 2701626-1627. [DOI] [PubMed] [Google Scholar]
- 37.Strasser, A., A. Dickmanns, R. Lührmann, and R. Ficner. 2005. Structural basis for m3G-cap-mediated nuclear import of spliceosomal UsnRNPs by snurportin1. EMBO J. 242235-2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Terns, M. P., and R. M. Terns. 2002. Small nucleolar RNAs: versatile trans-acting molecules of ancient evolutionary origin. Gene Expr. 1017-39. [PMC free article] [PubMed] [Google Scholar]
- 39.Venteicher, A. S., Z. Meng, P. J. Mason, T. D. Veenstra, and S. E. Artandi. 2008. Identification of ATPases pontin and reptin as telomerase components essential for holoenzyme assembly. Cell 132945-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verheggen, C., D. L. Lafontaine, D. Samarsky, J. Mouaikel, J. M. Blanchard, R. Bordonne, and E. Bertrand. 2002. Mammalian and yeast U3 snoRNPs are matured in specific and related nuclear compartments. EMBO J. 212736-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watkins, N. J., A. Dickmanns, and R. Lührmann. 2002. Conserved stem II of the box C/D motif is essential for nucleolar localization and is required, along with the 15.5K protein, for the hierarchical assembly of the box C/D snoRNP. Mol. Cell. Biol. 228342-8352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watkins, N. J., I. Lemm, D. Ingelfinger, C. Schneider, M. Hossbach, H. Urlaub, and R. Lührmann. 2004. Assembly and maturation of the U3 snoRNP in the nucleoplasm in a large dynamic multiprotein complex. Mol. Cell 16789-798. [DOI] [PubMed] [Google Scholar]
- 43.Watkins, N. J., I. Lemm, and R. Lührmann. 2007. Involvement of nuclear import and export factors in U8 box C/D snoRNP biogenesis. Mol. Cell. Biol. 277018-7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watkins, N. J., D. R. Newman, J. F. Kuhn, and E. S. Maxwell. 1998. In vitro assembly of the mouse U14 snoRNP core complex and identification of a 65-kDa box C/D-binding protein. RNA. 4582-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watkins, N. J., V. Segault, B. Charpentier, S. Nottrott, P. Fabrizio, A. Bachi, M. Wilm, M. Rosbash, C. Branlant, and R. Lührmann. 2000. A common core RNP structure shared between the small nucleoar box C/D RNPs and the spliceosomal U4 snRNP. Cell 103457-466. [DOI] [PubMed] [Google Scholar]
- 46.Weinstein, L. B., and J. A. Steitz. 1999. Guided tours: from precursor snoRNA to functional snoRNP. Curr. Opin. Cell Biol. 11378-384. [DOI] [PubMed] [Google Scholar]
- 47.Will, C. L., C. Schneider, M. Hossbach, H. Urlaub, R. Rauhut, S. Elbashir, T. Tuschl, and R. Lührmann. 2004. The human 18S U11/U12 snRNP contains a set of novel proteins not found in the U2-dependent spliceosome. RNA 10929-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao, R., Y. Kakihara, A. Gribun, J. Huen, G. Yang, M. Khanna, M. Costanzo, R. L. Brost, C. Boone, T. R. Hughes, C. M. Yip, and W. A. Houry. 2008. Molecular chaperone Hsp90 stabilizes Pih1/Nop17 to maintain R2TP complex activity that regulates snoRNA accumulation. J. Cell Biol. 180563-578. [DOI] [PMC free article] [PubMed] [Google Scholar]