Abstract
The role of the Forkhead transcription factor FOXO3a in processes that promote tumor metastasis is poorly defined. Here, we show that depletion of FOXO3a from cancer cells leads to decreased tumor size specifically due to attenuated invasive migration. During tumor progression, an increase in tumor mass is concomitant with serum deprivation prior to tumor angiogenesis. We show that nuclear retention of FOXO3a due to serum starvation results in greatly increased cancer cell invasion. Exploration of the mechanism by which FOXO3a promotes invasive migration revealed that it induces the expression of matrix metalloproteinase 9 (MMP-9) and MMP-13, both of which have been causally linked to the invasion and progression of numerous human solid tumors. Our results link Forkhead transcription factors to a previously unexplored function in cancer progression by promoting extracellular matrix degradation, allowing tumors to invade neighboring tissues and ultimately metastasize to distant organs.
The inception and progression of human cancer is a complex, multistep process in which tumor cells acquire the ability to overcome the restraints imposed by normal surrounding tissue. With increasing tumor mass, cancer cells invade neighboring tissues and the vasculature and ultimately metastasize to distant organs. Invading cells switch from a proliferative to an invasive phenotype. In this context, it has been shown that the serine/threonine kinase Akt/protein kinase B (PKB) contributes to cell proliferation but, depending on the specific Akt isoform, can either enhance or block cell invasive migration in vitro and in vivo (18, 27, 41). Processes that drive metastasis are governed by the pattern of expression of genes which provide selective advantages to overcome the adverse growth conditions (hypoxia, oxidative stress, nutrient restriction) associated with the expanding tumor mass. Equally important are genes and gene products which promote increased tumor cell motility and invasion (42). One major class of gene products which alters the migratory and invasive capacity of tumor cells is matrix metalloproteinases (MMPs). MMPs can proteolyze extracellular matrix (ECM) molecules and also cleave precursor forms of growth factors. Numerous studies in vitro and in vivo have revealed multiple crucial functions for MMPs in the progression of human cancers, such as the regulation of invasion and angiogenesis (11, 13, 37) or the induction of genomic instability (34). Especially, increased expression of the MMPs MMP-9 and MMP-13 has been causally linked to the invasion and progression of numerous human solid tumors (13). However, the precise nature of specific signaling pathways which control induction of MMPs in cancer cells and thus contribute to tumor cell invasion into neighboring tissue and eventually to metastasis has remained largely elusive.
Forkhead transcription factors have been causally linked to multiple cellular processes which are often derailed in human cancer cells. Specifically, regulation of the cell cycle and programmed cell death as well as the activation of DNA repair and reactive oxygen species detoxification pathways and regulation of longevity have all been shown to be under the control of one or more of the members of the Forkhead family (7, 15, 31, 39). The Forkhead family consists of the three members, FOXO1a/FKHR, FOXO3a/FKHRL1, and FOXO4/AFX. In proliferating cells, the transcriptional activity of FOXO1, FOXO3a, and FOXO4 is under the control of signal relay pathways initiated by growth factors, such as insulin and insulin-like growth factor 1 (IGF-1), which culminate in the phosphorylation of FOXOs (7). As an example, FOXO3a is active in cells subjected to serum deprivation (28) and is phosphorylated in response to IGF-1 by Akt and serum- and glucocorticoid-induced kinase 1 (SGK1) in a phosphoinositide 3-kinase (PI 3-K)-dependent manner (4, 6). Phosphorylation of FOXO3a by Akt in the nucleus blocks transcriptional activity by promoting nuclear export of the transcription factor. This export is mediated by 14-3-3 protein binding, which also facilitates cytoplasmic retention, thus blocking reimport into the nucleus (5). In addition to Akt, IκB kinase (IKK) has also been shown to inhibit FOXO3a activity by direct phosphorylation (17). Although FOXO transcription factors are known to be regulated by oxidative stress and serum deprivation, their role in modulating cellular responses to such stresses is incompletely understood (22, 29).
The Forkhead transcription factor FOXO3a is a suppressor of primary tumor growth and is negatively regulated by growth factors (1, 4, 6, 32, 42). However, during tumor progression, an increase in tumor mass is concomitant with serum deprivation prior to tumor angiogenesis (3, 17). Here, we show that such serum restriction leads to FOXO3a-dependent induction of MMP-9 and MMP-13 and that the expression of these genes increases the invasive potential of tumor cells. This implicates an entirely novel function for FOXO3a in modulating cancer progression by promoting tumor cell invasion.
MATERIALS AND METHODS
Cell culture, antibodies, expression plasmids, and reagents.
HeLa and MDA-MB-435 cell lines were purchased from ATCC (Manassas, VA) and maintained in high-glucose Dulbecco's modified Eagle medium (HeLa) or low-glucose Dulbecco's modified Eagle medium (MDA-MB-435), supplemented with 10% fetal bovine serum. The anti-Akt/PKB antibody was obtained from Santa Cruz (Santa Cruz, CA), anti-FOXO1, anti-FOXO4, anti-cleaved caspase 3, and anti-MMP-9 were obtained from Cell Signaling (Danvers, MA), and anti-MMP-13 for immunohistochemistry (IHC) analysis was obtained from Calbiochem (Gibbstown, NJ) or, for Western blotting, from Abcam (Cambridge, MA) and Oncogene (San Diego, CA). Anti-Ki67 was obtained from Dako (Carpinteria, CA), anti-FOXO3a for chromatin immunoprecipitation (ChIP) was obtained from Abcam (Cambridge, MA) or, for IHC analysis, from Cell Signaling, and anti-MMP-9 was obtained from EMD Biosciences (San Diego, CA). The secondary goat anti-mouse immunoglobulin G(H+L) [IgG(H+L)] Cy2-conjugated and donkey anti-rat IgG(H+L) Cy3-conjugated antibodies were obtained from Jackson Laboratories (West Grove, PA). DAPI (4′,6-diamidino-2-phenylindole) and gelatin were obtained from Sigma (St. Louis, MO). Purified recombinant MMP-9 and MMP-13 were obtained from AnaSpec (San Jose, CA). CyQUANT was obtained from Molecular Probes/Invitrogen (Carlsbad, CA). SuperFect (Qiagen, Valencia, CA), TransIT-HeLaMonster (Mirus, Madison, WI), or nucleofection (Amaxa, Gaithersburg, MD) was used for transient transfections, according to the manufacturer's instructions. The MMP-9/MMP-13 inhibitor I [N-hydroxy-1-(4-methoxyphenyl)sulfonyl-4-(4-biphenylcarbonyl)piperazine-2-carboxamide] was obtained from EMD Biosciences (Gibbstown, NJ). Amino-terminal hemagglutinin (HA)-tagged human FOXO3a, FOXO3a.TM cloned into pECE, and the Forkhead response element (FHRE) reporter have been described (4). Reporter gene plasmids were generously provided by M. Seiki (MMP-9) and R. Loeser (MMP-13). The FOXO3a binding motif (Daf-16 family member binding element [DBE]) in the MMP-13 promoter was mutated using 5′-AGTCGCCACGTAAGCATGATAACCTTCAAGTGACTAGGA-3′ and 5′-TCCTAGTCACTTGAAGGTTATCATGCTTACGTGGCGACT-3′ (motif at −123) and, in the MMP-9 promoter, using 5′-CTGACCTGGGAGGGGGTGAAGCAAAAGGCCAAGGATGGG-3′ and 5′-CCCATCCTTGGCCTTTTGCTTCACCCCCTCCCAGGTCAG-3′ (motif at −466), 5′-CCTGAGTCAGCACTTGCCTCTCAAGGAGGGGTGGGGTCA-3′ and 5′-TGACCCCACCCCTCCTTGAGAGGCAAGTGCTGACTCAGG-3′ (motif at −82), and 5′-GAGAGAGGAGGAGGTGGTGAAAGCCCTTTCTCATGCTGG-3′ and 5′-CCAGCATGAGAAAGGGCTTTCACCACCTCCTCCTCTCTC-3′ (motif at −183) as primer sequences. Mutagenesis was carried out using the QuikChange strategy (Stratagene). HistoArray slides (IMH-368 and IMH-364) were obtained from Imgenex (San Diego, CA). The FOXO3a silencing sequences cloned in pSUPER were described elsewhere (38). A second FOXO3a silencing sequence was cloned into pSUPER using the following primer sequences: 5′-GATCCCCCAAGTATACCAAGAGCCGTTTCAAGAGAACGGCTCTTGGTATACTTGTTTTTGGAAA-3′ and 5′-AGCTTTTCCAAAAACAAGTATACCAAGAGCCGTTCTCTTGAAACGGCTCTTGGTATACTTGGGG-3′. FOXO3a silent mutations for rescue experiments were obtained by site-directed mutagenesis using the following primer sequences: 5′-AGGGTTGATGATCCACCAGTTGCTCTTGCCAGTTCCCTC-3′ and 5′-GAGGGAACTGGCAAGAGCAACTGGTGGATCATCAACCCT-3′. Lentiviral short hairpin RNA (shRNA) expression constructs to silence human FOXO3a were obtained from the Mayo Clinic RNA Interference Technology Resource. The shRNA sequences used for lentiviral silencing of human FOXO3a were 5′-CCGGCTCCTTTAACAGCACGGTGTTCTCGAGAACACCGTGCTGTTAAAGGAGTTTTTG-3′ and 5′-CCGGGTCACTGCATAGTCGATTCATCTCGAGATGAATCGACTATGCAGTGACTTTTTG-3′. The lentiviral shRNA expression system we used to knock down MMP-9 and MMP-13 expression is commercially available from Sigma (Mission shRNA plasmid DNA).
3-D cell culture assays.
Three-dimensional (3-D) analysis of morphology was performed, as described previously (35). In brief, cell culture dishes (24-well plates) were precoated with undiluted, phenol red-free Matrigel (10 mg/ml). A total of 104 cells per well were suspended in a volume of 200 μl phosphate-buffered saline (PBS) and mixed with 100 μl of cold Matrigel (10 mg/ml). Cells were washed three times with PBS. The cell suspension was added dropwise over the bottom layer to cover it. After the cell layer was set completely, culture media were added on top. Media were changed every 2 days, without disturbing the cell/matrix layer. Photos were taken after 10 days using 10× magnification for an overview and 40× magnification to document the structure.
Immunoblotting.
Cells were lysed in RIPA buffer (10 mM sodium phosphate [pH 7.2], 1% NP-40, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 150 mM NaCl, 2 mM EDTA [pH 7.4], 50 mM sodium fluoride) plus the protease inhibitor cocktail (Sigma). Culture media were collected and concentrated using Centricon YM-10 obtained from Millipore (Bedford, MA). Lysates of cells and culture media were used for immunoblot analysis.
Orthotopic animal model.
Mice (female, nu/nu) were anesthetized, and breast carcinoma cell clones were injected subcutaneously into the mammary gland fat pad in the upper thorax region between the clearly visible nipples of the second and third mammary glands on the right side of each animal. For each injection, a total of 106 cells, washed three times with PBS and mixed with 30 μl of ECM (complete Matrigel without phenol red; BD Biosciences), were used. The length, width, and depth of the tumors were measured once a week (starting from week 2). At week 8, primary tumors were removed and analyzed, as indicated.
Invasion assays.
Invasion assays were performed, as previously described (19), using Transwell chambers coated with Matrigel (Fisher, Pittsburgh, PA). Briefly, cells were cotransfected with the constructs of interest and a β-galactosidase (β-Gal) reporter plasmid [pCS2-(n)β-Gal] at a ratio of 5:1. Inserts of Transwell plates were coated with Matrigel (2 μg/well), dried overnight, and rehydrated for 1 hour with 40 μl tissue culture media. A total of 24 h after transfection, cells were harvested, washed once with media containing 1% bovine serum albumin (BSA), resuspended in media containing 0.1% BSA (1 × 106/ml), and seeded on Transwells (100,000 cells). NIH 3T3-conditioned medium served as the chemoattractant in the lower chamber of the Transwell. The remaining cells were used to analyze the transfection efficiency and expression of proteins of interest. After 16 h, cells on top of the Transwell insert were removed, and cells that had migrated to the lower surface of the filters were fixed in 4% paraformaldehyde and stained with Bluo-Gal (Invitrogen). β-Gal-positive cells were counted, and the mean result from triplicates for each experimental condition was used as a percentage of invasion relative to the control.
Zymography.
Zymography was performed, as previously described (24). Briefly, 72 or 96 h after transfection, culture media were harvested and concentrated using Centricon YM-10. Samples were mixed with 2× loading buffer (50 mM Tris-HCl [pH 6.8], 10% [vol/vol] glycerol, 1% [wt/vol] SDS, 0.01% [wt/vol] bromophenol blue) and resolved on an SDS-polyacrylamide gel containing 0.12 mg/ml gelatin (porcine skin type A; Bloom 300). Gels were soaked for 1 h in 2.5% Triton X-100, then washed twice with collagenase buffer (50 mM Tris-HCl [pH 7.6], 0.2 M NaCl, 5 mM CaCl2, 0.2% Brij 35), and incubated at 37°C for 18 h. Gels were then washed with distilled water and incubated in Coomassie brilliant blue staining solution (40% methanol, 10% acetic acid/0.025% Coomassie brilliant blue R-250) at room temperature for 2 h. Gels were then washed for 24 h in distilled water and scanned using an Agfa DuoScan T1200 scanner.
Reporter gene assays.
Cells were transiently cotransfected with reporters for FOXO3a (FHRE-luc), MMP-9 [MMP-9-(−670)-luc], or MMP-13 [MMP-13-(−1600)-luc] (5 μg), 1 μg pCS2-(n)β-Gal, and the cDNA of interest (1 μg) using SuperFect (Qiagen). A total of 24 h after transfection, assays for luciferase and β-Gal activity were performed on total cell lysates and measured on a luminometer. Luciferase activity was normalized to β-Gal activity. Protein expression was controlled by immunoblot analysis.
RNA interference (RNAi). (i) Vector-based siRNA.
The sequences chosen to silence the expression of human FOXO3a for small interfering RNA (siRNA) were specific, as judged by BLASTn searches of all the GenBank, RefSeq nucleotides, EMBL, DDBJ, and Protein Data Bank sequences and of the human subset of GenBank, EMBL, and DDBJ sequences. HeLa cells were transfected with pSUPER or pSUPER.RNAi using the TransIT-HeLaMonster reagent (Mirus), and MDA-MB-435 cells were transfected using the Nucleofector kit T from Amaxa. In all experiments, the cells were transfected at 30% confluence. Transfection efficiencies (95 to 100%) were controlled using a green fluorescent protein (GFP) expression vector. For reporter gene assays, genes of interest were transfected in a second transfection using SuperFect after 24 h. Experiments were performed 48 h after the initial transfection. Reduced expression of target proteins was evaluated by reverse transcription-PCR (RT-PCR) or immunoblotting.
(ii) Lentiviral shRNA expression.
The lentiviral shRNA expression system we were using to knock down FOXO3a, MMP-9, and MMP-13 expression is commercially available from Sigma (Mission shRNA plasmid DNA). The ViraPower lentiviral expression system (Invitrogen) was used for an optimized mix of packaging plasmids, which supplies the structural and replication proteins that were required to produce lentivirus in 293FT cells.
ChIP assay.
Cells were washed twice with PBS at room temperature and resuspended (20 × 106 cells/40 ml). Formaldehyde was added to a final concentration of 1%, and samples were incubated at room temperature for 10 min. Cross-linking was terminated by adding glycine to a final concentration of 0.125 M. Cells were pelleted (12,000 rpm, 5 min) and washed once with ice-cold PBS. Cells were resuspended in 6 ml lysis buffer (5 mM PIPES [pH 8.0], 85 mM KCl, 0.5% NP-40, protease inhibitor cocktail). Crude nuclear extracts were collected by microcentrifugation (2,000 rpm, 5 min) and washed with PBS, and the pellet was resuspended in 2 ml high-salt buffer (0.5% sodium deoxycholate, 0.1% SDS, and 1% NP-40, all in PBS). Samples were sonicated on ice. Extracts were centrifuged (15 min, 10,000 rpm, 4°C) and precleared with protein A/G beads. The protein concentration of the supernatant (chromatin) was determined, and 4 mg protein and 25 μg primary antibody (i.e., anti-FOXO3a) were used for immunoprecipitations. Immunoprecipitates were analyzed by PCR using the primers 5′-TCTACCACAAACCACACTCGGGAG-3′ and 5′-GAGTCATCACTTATGGATAGGTTT-3′ to amplify a 103-bp fragment of the MMP-13 promoter corresponding to the potential FOXO3a binding site.
Immunofluorescence. (i) HistoArrays.
The human uterine cervix (IMH-368/CZA1) and human breast (IMH-364/CBA2) tissue array slides were purchased from Imgenex (San Diego, CA). Deparaffinization, hydration, and antigen retrieval (autoclave method) of tissue array slides were performed, according to the manufacturer's protocol. Slides were blocked with PBS containing 4% normal goat serum, 3% BSA, and 0.05% Tween 20 for 30 min at room temperature. For DAPI staining, cells were washed three times in DAPI buffer (100 mM NaCl, 10 mM EDTA, 10 mM Tris [pH 7.0]) and incubated for 1 h at 37°C with 0.1 μg/ml DAPI. Cells were then washed three times in DAPI buffer. The coverslips were then incubated with the primary antibody diluted in blocking solution (1:2,000 for MMP-13 [mouse]; 1:2,000 for FOXO3a [rabbit]) overnight at 4°C. Cells were then washed five times with PBS and incubated with the secondary antibody diluted in PBS-3% BSA (goat anti-rabbit IgG Cy2-conjugated antibody, 1:400; goat anti-mouse IgG Cy3-conjugated antibody, 1:400) for 2 h. After being extensively washed in PBS, coverslips were mounted in Gel Mount (Biomeda, Foster City, CA) and examined.
(ii) Cells.
Cells were transfected (5 μg DNA) and, 24 h after transfection, plated on glass coverslips at a density of 120,000 per well in a six-well plate. The next day, cells were stimulated, washed twice with PBS, and fixed in 3.5% paraformaldehyde (15 min, 37°C). Following permeabilization (0.1% Triton X-100 for 10 min), cells were blocked with PBS containing 3% BSA and 0.05% Tween 20 for 30 min at room temperature. Coverslips were then incubated with anti-FOXO3a antibody diluted in PBS-3% BSA (1:2,000) overnight at 4°C. Cells were then washed five times with PBS and incubated with the secondary antibody diluted in PBS-3% BSA (goat anti-rabbit Cy3-conjugated antibody, 1:400) for 2 h. After being extensively washed in PBS, coverslips were mounted and examined.
RT-PCR.
Cellular mRNA isolation was performed using RNA-Bee (Tel-Test, Friendswood, TX), according to the manufacturer's instructions, and was transcribed into cDNA using SuperScript II (Invitrogen, Carlsbad, CA). For the transcription reaction, 1 μg oligo (dT)18 primer (New England Biolabs, Beverly, MA) and 1 μg RNA were incubated in a total volume of 10 μl H2O at 70°C for 10 min. Buffer (5×), 40 U RNasin (Roche, Mannheim, Germany), 200 μM deoxynucleoside triphosphate (NEB), 10 mM dithiothreitol, and 300 U SuperScript II reverse transcriptase were then added to a total volume of 20 μl. The reaction was carried out at 45°C for 60 min and then heat inactivated at 95°C for 5 min. The resulting cDNA pool was subjected to PCR analysis using the following primer sequences: for human FOXO3a oligonucleotides (product size, 591 bp), 5′-TTCAAGGATAAGGGCGACAG-3′ and 5′-CAGGTCGTCCATGAGGTTTT-3′; for human MMP-13 oligonucleotides (product size, 340 bp), 5′-GTCTGGCGTTTTTGGATGTT-3′ and 5′-TAAGGAGCATGGCGACTTCT-3′; for human MMP-9 oligonucleotides (product size, 394 bp), 5′-CATCGTCATCCAGTTTGGTG-3′ and 5′-GCCTTGGAAGATGAATGGAA-3′; for human MMP-1 oligonucleotides (product size, 334 bp), 5′-GATGGGAGGCAAGTTGAAAA-3′ and 5′-CTGCTTGACCCTCAGAGACC-3′; for human MMP-2 oligonucleotides (product size, 305 bp), 5′-GTCCACTGTTGGTGGGAACT-3′ and 5′-TGATGTCATCCTGGGACAGA-3′; and for human MMP-3 oligonucleotides (product size, 415 bp), 5′-CCAGGTGTGGAGTTCCTGAT-3′ and 5′-TGAAAGAGACCCAGGGAGTG-3′. The reaction conditions used for the PCR were as follows: 1 min of annealing at 55°C and 1 min of amplification at 72°C for 30 cycles.
RESULTS AND DISCUSSION
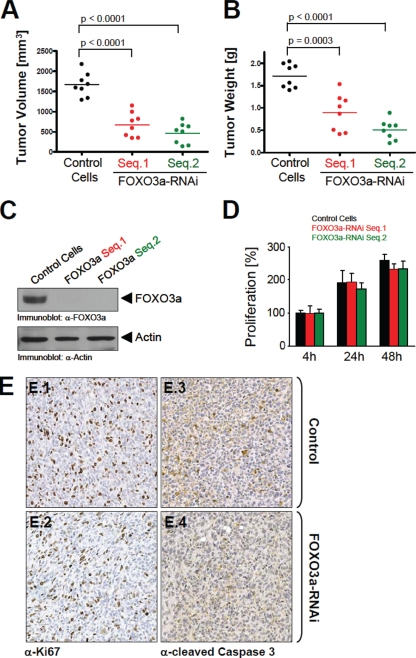
Knockdown of FOXO3a decreases tumor size.
To determine if FOXO3a functions during tumor expansion, we used an orthotopic mouse model for breast cancer that we injected with normal control MDA-MB-231 cells or MDA-MB-231 cells depleted of FOXO3a into the mammary fat pad of nude mice. The injection of two distinct cell lines containing different sequences of shRNA for FOXO3a resulted in tumors of smaller volume and weight than tumors resulting from injection of the control (scrambled shRNA) cells (Fig. 1A and B). A detailed analysis of the cells injected into the nude mice revealed that FOXO3a expression was knocked down to undetectable levels (Fig. 1C). The decrease of tumor size upon FOXO3a silencing is at odds with some of the currently published literature, which has shown that expression of FOXO3a negatively regulates tumor cell proliferation. However, silencing of FOXO3a in MDA-MB-231 cells did not alter the proliferation rate, suggesting that the effects on tumor growth are not impacted by this phenotype (Fig. 1D). Furthermore, a detailed analysis of tumors generated with control or FOXO3a-shRNA cells revealed that the knockdown of FOXO3a had no significant effect on tumor cell proliferation or apoptosis (Fig. 1E). In contrast, cells with FOXO3a shRNA appeared to be packed in with higher density within tumors.
FIG. 1.
Knockdown of FOXO3a affects tumor size. (A, B) MDA-MB-231 cells were stably transfected with control shRNA or constructs expressing two different FOXO3a-specific shRNA sequences. A total of 106 cells from each cell line were injected into the mammary fat pads of eight nude mice. Tumor growth was monitored over 8 weeks, and tumor volume and weight were determined after removal of the tumor. (C) Immunoblotting (anti-FOXO3a) was performed to demonstrate efficient knockdown of FOXO3a in the FOXO3a-RNAi cell lines. Actin expression served as a positive control. α, anti. (D) Relative proliferation of all of the FOXO3a-RNAi or control clones was determined over 48 h by using the CyQUANT reagent obtained from Molecular Probes. (E) Orthotopic tumors (control or FOXO3a RNAi) were immunohistochemically analyzed for cell proliferation (anti-Ki67) or apoptosis (anti-cleaved caspase 3).
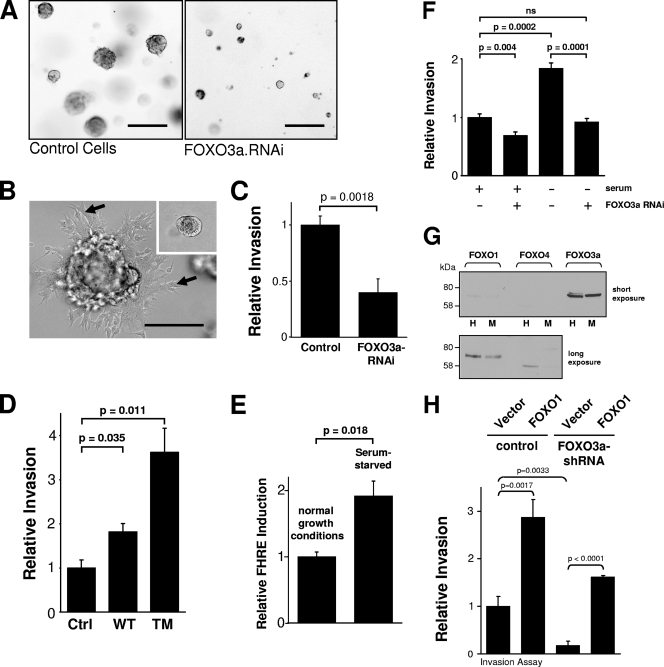
FOXO3a regulates cellular invasiveness and the ability to form multicellular spheroids.
The smaller and more-compact tumors obtained upon FOXO3a silencing suggested that cells may be compromised in their ability to degrade the ECM and invade into surrounding tissues. This was tested by comparing parental cells with FOXO3a-RNAi cells and their abilities to form multicellular spheroids when grown in three-dimensional cell culture. Interestingly, FOXO3a-RNAi cells formed only very small aggregates, whereas control cells formed approximately tenfold-larger cell aggregates (Fig. 2A). This response was even more robust after 16 days, when control cells showed stellar outgrowth of invasive cells (Fig. 2B), whereas FOXO3a-RNAi cells displayed less than a 10th of the size and no invasive phenotype (Fig. 2B, inset). These data clearly correlated with the phenotype observed in vivo, suggesting that the loss of FOXO3a leads to a reduction in cellular invasiveness and the ability to degrade the ECM.
FIG. 2.
FOXO3a regulates cancer cell invasion. (A) Stable MDA-MB-231 cell clones with either control RNAi or FOXO3a RNAi were grown in 3-D culture with Matrigel for 10 days. The bar represents 200 μm. 3-D structures of cells were analyzed using 4× magnification. (B) Stable MDA-MB-231 cell clones with either control RNAi (inset) or FOXO3a RNAi were grown in 3-D culture with Matrigel for 16 days. The bar represents 100 μm. The arrows indicate cells invading into the surrounding Matrigel. Both the picture and inset were analyzed using 10× magnification. (C) MDA-MB-435 cells were transfected with FOXO3a RNAi, and Matrigel invasion under normal growth conditions was measured. Silencing of FOXO3a was measured using RT-PCR (not shown). (D) MDA-MB-435 cells were transfected with wild-type FOXO3a (WT) or FOXO3a.TM (TM), and after 24 h, Matrigel invasion assays were performed. The expression of FOXO3a was controlled by immunoblotting using anti-HA (not shown). Ctrl, control. (E) MDA-MB-435 cells were transfected with a FOXO3a reporter gene (FHRE-luc), and FOXO3a activity under normal growth or serum-starved conditions was determined by measuring luciferase activity. (F) MDA-MB-435 cells were transfected with FOXO3a RNAi, and Matrigel invasion under normal growth or serum-starved conditions was measured using Transwell assays. ns, not significant. (G) Lysates of MDA-MB-435 (M) or HeLa (H) cells were analyzed by Western blotting for expression of FOXO1, FOXO3a, or FOXO4. The top panel is showing a short exposure (20 s) of the autoradiograph, and the bottom panel is showing a long exposure (15 min). (H) Cells were cotransfected with control or FOXO3a RNAi and vector control or FOXO1. Matrigel invasion assays were performed. Error bars shown in all experiments represent standard deviations. P values were acquired with the t test, using GraphPad software. P values indicate statistical significance. All results are typical of three independent experiments.
Tumor cells forming colonies in Matrigel are characterized by increased invasive potential and secretion of MMPs. To evaluate a potential role for FOXO3a-induced genes in tumor cell invasion, we analyzed several cell lines in standard Matrigel chemoinvasion assays. We assessed the ability of HeLa cells and MDA-MB-435 cells to invade Matrigel in a FOXO3a-dependent manner. Depletion of FOXO3a in these cells resulted in a significant decrease in invasive migration (Fig. 2C; see also Fig. S1A in the supplemental material). The specificity of FOXO3a siRNA was confirmed with a rescue approach, using transfection of a human FOXO3a allele with three silent mutations in the site targeted by the FOXO3a-specific siRNA (see Fig. S1C in the supplemental material). A FOXO3a mutant (FOXO3a.TM) mutated at three Akt/PKB phosphorylation sites is constitutively localized in the nucleus and constitutively transcriptionally active (4). The expression of this constitutively active FOXO3a.TM mutant potently increased carcinoma cell invasion in both cell lines by approximately 4- to 5-fold (Fig. 2D; see also Fig. S1B in the supplemental material). In addition to the regulation of cell invasion, FOXO3a also regulated cell migration (see Fig. S2 in the supplemental material). These results implicate FOXO3a as a critical regulator of cellular invasive migration in vitro.
Inducers of cellular stress such as oxidative stress and nutrient availability are physiological activators of FOXO3a in cancer cells (16, 28). Serum deprivation induces both types of stresses and FOXO3a activity (4) and, therefore, mimics events which occur prior to the angiogenic switch. In vitro, serum deprivation resulted in FOXO3a activation, as measured in a reporter gene assay using an FHRE linked to luciferase (Fig. 2E; see also Fig. S3 in the supplemental material). Serum deprivation also promoted increased invasion through Matrigel, and this was quantitatively blocked by silencing FOXO3a expression using siRNA in both MDA-MB-435 and HeLa cells (Fig. 2F and data not shown). These data reveal that under stress conditions which promote FOXO3a activation, FOXO3a can promote tumor cell invasion, likely through the induction of target genes specific for this transcription factor. Since FOXO3a, FOXO1, and FOXO4 were recently shown to have redundant functions (23), we analyzed the expression of both proteins in MDA-MB-435 and HeLa cells. We found that in both cell lines, FOXO3a is the predominant isoform, whereas FOXO1 and FOXO4 are expressed at very low to undetectable levels (Fig. 2G). This also prompted us to test if these transcription factors have overlapping functions in the regulation of cell invasion. To test this, we ectopically introduced FOXO1 into tumor cells and analyzed its effect on cell invasion. We found that FOXO1 increased tumor cell invasion similar to FOXO3a. A similar phenotype was observed in FOXO3a knockdown cells when FOXO1 was expressed, suggesting that FOXO1 can rescue the decreased invasion observed when cells are depleted of FOXO3a (Fig. 2H). Furthermore, expression of FOXO1 efficiently rescued the knockdown of FOXO3a (Fig. 2H, compare the first, third, and fourth bars from the right).
FOXO3a regulates the expression MMP-9 and MMP-13.
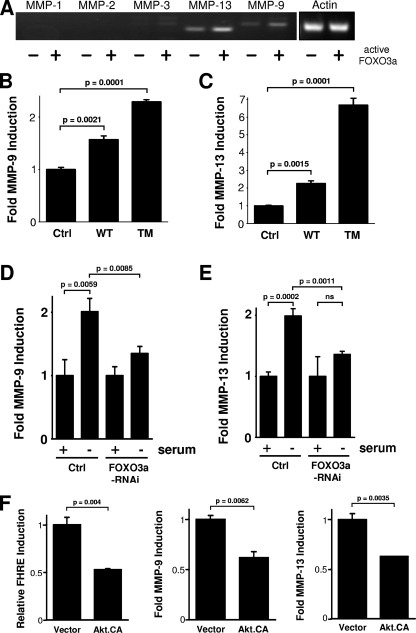
The results on the formation of multicellular spheroids suggest that the silencing of FOXO3a may be due to a defect in the degradation of the ECM. MMPs are collagenases or gelatinases and are recognized as critical mediators of ECM degradation in tumor progression (13, 37, 42). The balance of MMP activity and their inhibitors, the tissue inhibitors of metalloproteinases (TIMPs), is responsible for tissue remodeling during embryonic development, wound healing, and cell migration (9, 33). Similarly, alterations in the balance between MMPs and TIMPs are characteristic of diverse pathological conditions under which cellular migration and invasion are deregulated. This is especially evident during carcinoma invasion and subsequent metastasis. In most human cancers, MMP expression and activity are increased, correlating with invasiveness and poor prognosis (10). MMPs are expressed and secreted by both types of tumor cells as well as the surrounding tumor stroma, such as fibroblasts and infiltrating immune cells. Because MMPs are widely recognized as critical mediators of tumor cell invasion, we speculated that FOXO3a might promote cell invasion and thus tumor expansion by induction of one or more members of the collagenase family. We therefore evaluated whether FOXO3a increases carcinoma invasion through MMP induction. To test this, cells first were transfected with a constitutively active FOXO3a.TM mutant or vector control and analyzed for the expression of several MMPs by RT-PCR. Expression of active FOXO3a significantly enhanced the expression of MMP-9 and MMP-13 but not MMP-1, MMP-2, or MMP-3 (Fig. 3A). Moreover, both wild-type FOXO3a and FOXO3a.TM increased MMP-9 and MMP-13 promoter activation, as measured in reporter gene assays (Fig. 3B and C; see also Fig. S4A in the supplemental material). Similarly, serum deprivation, which leads to the activation of endogenous FOXO3a, induced MMP-9 and MMP-13 reporter gene activity, and this was blocked with FOXO3a-specific siRNA (Fig. 3D and E). It is likely that FOXO3a also regulates the expression of multiple MMPs or TIMPs. We focused on MMP-9 and MMP-13 because both have been detected in tumor cells and tissues. For example, it has been shown that MMP-13 is upregulated in injected cells of breast cancer xenografts (23). Furthermore, MMP-13 expression has been identified in several invasive neoplastic tumors, including breast carcinoma, squamous cell carcinoma of the head and neck and vulva, and primary and metastatic melanoma tumors (2). MMP-13 is primarily detected at the invading front of cell carcinomas, and this appears to correlate with the invasive and metastatic capacity of the tumor (21).
FIG. 3.
FOXO3a regulates the expression of MMP-9 and MMP-13. (A) HeLa cells were transfected with vector control or FOXO3a.TM. After 16 h, mRNA was isolated, and the expression of MMP-1, MMP-2, MMP-3, MMP-9, and MMP-13 or actin was detected by RT-PCR. (B and C) MDA-MB-435 cells were transfected with vector control (Ctrl), wild-type FOXO3a (WT), or FOXO3a.TM (TM) and MMP-9 (B) and MMP-13 (C) luciferase reporter and β-Gal reporter plasmids. Luciferase assays were performed to measure MMP-9/MMP-13 promoter and β-Gal activity. (D and E) MDA-MB-435 cells were transfected with FOXO3a RNAi or vector control. After 24 h, cells were transfected a second time with MMP-9 (D) or MMP-13 (E) and β-Gal reporter plasmids and either cultivated under normal growth conditions or serum starved. Luciferase assays were performed to measure MMP-9/MMP-13 promoter and β-Gal activity levels. (F) HeLa cells were transfected with constitutively active Akt/PKB (Akt.CA) or vector control and FHRE, MMP-9, or MMP-13 reporter plasmids, along with β-Gal. Luciferase assays were performed to measure FOXO3a, MMP-9, and MMP-13 promoter or β-Gal activity. All results are typical of three independent experiments.
Since FOXO3a is negatively regulated by Akt, we next determined if active Akt downregulates MMP-9 and MMP-13 expression. We found that a constitutively active allele of Akt inhibited the basal induction of both MMPs (Fig. 3F). In this context, Akt has been identified as a tumorigenic oncogene which increases tumor cell proliferation and survival by phosphorylating FOXO3a and other substrates. However, recent studies have also shown that whereas the Akt2 isoform is an enhancer of cell migration and invasion in vitro and in vivo, the related Akt1 isoform may function as an inhibitor of these metastatic phenotypes (18, 27, 41). Our data suggest that the Akt substrate FOXO3a may also function as an inducer of cell migration and invasion under conditions of serum starvation. Tumor cells under conditions of low nutrient or growth factor supply (mimicked by serum deprivation) may activate cell motility and inhibit proliferation.
FOXO3a regulates expression and activity of MMP-9 and MMP-13.
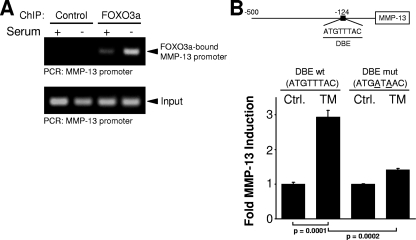
We next investigated if FOXO3a directly regulates MMP-9 and MMP-13 expression through binding to specific sites within the promoters. We analyzed the MMP-9 and MMP-13 promoters for the FOXO3a DNA binding motif TTGTTTAC (DBE), as previously described (14, 22). The MMP-9 promoter did not contain this motif, suggesting that FOXO3a leads to the expression of MMP-9 through an indirect mechanism. This indirect activation of the MMP-9 promoter could be mediated through other transcription factors, such as SMAD3/4 or NF-κB, which may serve FOXO3a as a cofactor. In contrast, ChIP analysis showed that in response to FOXO3a activation, FOXO3a directly interacts with the MMP-13 promoter (Fig. 4A). The MMP-13 promoter contains an ideal DBE motif (ATGTTTAC at position −123); and a reporter construct mutated at two bases within the DBE motif (ATGTTTAC to ATGATAAC, T to A at positions −121 and −119) resulted in complete inhibition of the induction of this promoter after FOXO3a.TM overexpression (Fig. 4B). This shows that FOXO3a regulates the MMP-13 promoter through a direct binding event. We also analyzed other MMPs and found potential DBE sites in their promoters. However, we here focused on MMP-9 and MMP-13 because both have been shown to be expressed and relevant in many cancers, including breast cancer.
FIG. 4.
FOXO3a directly activates the MMP-13 promoter. (A) Cells were cultivated in the presence or absence of serum. FOXO3a/DNA complexes were immunoprecipitated (anti-FOXO3a) after cross-linking, and precipitates were analyzed by PCR for the FOXO3a-bound MMP-13 promoter. A PCR for the MMP-13 promoter using the input DNA served as a control. (B) Cells were transfected with vector control (Ctrl.) or FOXO3a.TM (TM) and MMP-13 DBE wild-type (wt) or MMP-13 DBE-mutated (mut) luciferase reporters and β-Gal reporter plasmids. Luciferase assays were performed to measure MMP-13 promoter and β-Gal activity levels. Error bars shown represent standard deviations. P values were acquired with the t test, using GraphPad software. P values indicate statistical significance. All results are typical of three independent experiments.
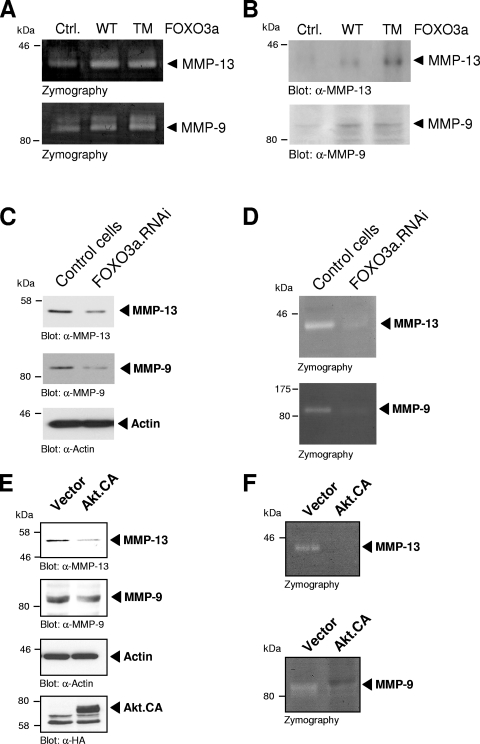
Metalloproteinase activity is regulated at the level of gene transcription via synthesis of pro-MMPs and at the protein level via activation of the proenzymes or by inhibition by TIMPs (40). MMP-9 (gelatinase B), a member of the gelatinase group, primarily proteolyzes gelatin but also collagen IV and V, whereas MMP-13 (collagenase 3), a member of the collagenase family, can cleave several collagen types (I, II, III, IV, IX, X, XIV) as well as gelatin (36). To further analyze if FOXO3a-mediated induction of MMP-9 and MMP-13 translates into release of mature and active MMP-9/MMP-13, we performed in-gel zymography. Expression of active FOXO3a increased gelatinase activity at molecular masses corresponding to MMP-9 and MMP-13 (Fig. 5A; see also Fig. S4B in the supplemental material). Immunoblot analysis of culture media collected from transfected cells also revealed that these activities were due to the secretion of active MMP-9 and MMP-13 (Fig. 5B). Similarly, the induction of FOXO3a signaling by serum deprivation led to an increase in MMP expression (see Fig. S5 in the supplemental material). We also knocked down FOXO3a using shRNA and then determined the expression and activity of MMP-9 and MMP-13. We found that depletion of FOXO3a decreased the expression of MMP-9 and MMP-13 (Fig. 5C), and this directly translated to a decreased activity of both MMPs (Fig. 5D). In addition to a reverse genetics approach to decrease FOXO3a activity, we also expressed a constitutively active Akt allele to attenuate FOXO3a activity. Under these conditions we found that activated Akt also downregulated the expression (Fig. 5E) and activity (Fig. 5F) of both MMPs.
FIG. 5.
FOXO3a induces MMP-9 and MMP-13 expression and activity. (A) MDA-MB-435 cells were transfected with vector control (Ctrl.), wild-type FOXO3a (WT), or FOXO3a.TM (TM). Culture media were collected, and zymography was performed. (B) Culture media collected from MDA-MB-435 cells transfected with vector control, wild-type FOXO3a, or FOXO3a.TM were analyzed by SDS-polyacrylamide gel electrophoresis and immunoblot analysis using anti-MMP-9 and anti-MMP-13. α, anti. (C) Cells expressing FOXO3a RNAi were compared to normal cells for MMP-9 (anti-MMP-9) or MMP-13 (anti-MMP-13) expression by Western blot analysis. Staining for actin (anti-actin) served as a loading control. (D) Culture media of control cells or cells expressing FOXO3a RNAi were collected, and zymography was performed. (E) Cells growing under serum starvation conditions and expressing control vector or constitutively active Akt were analyzed for MMP-9 (anti-MMP-9) or MMP-13 (anti-MMP-13) expression by Western blotting. Staining for actin (anti-actin) or Akt anti-HA) served as loading or expression controls. (F) Culture media of cells growing under serum starvation conditions and expressing control vector or constitutively active Akt were collected, and zymography was performed.
MMP-9 and MMP-13 contribute to cellular invasiveness.
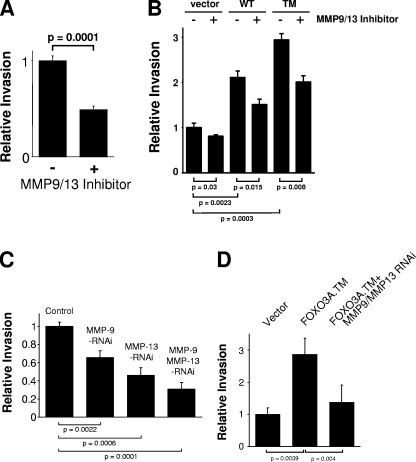
To demonstrate that MMP induction contributes to cellular invasiveness through Matrigel, cells were treated with the MMP-9/MMP-13 inhibitor [N-hydroxy-1-(4-methoxyphenyl)sulfonyl-4-(4-biphenylcarbonyl)piperazine-2-carboxamide] (8). Selective inhibition of MMP-9/MMP-13 blocked both basal as well as FOXO3a-mediated invasion of HeLa and MDA-MB-435 cells (Fig. 6A and B; see also Fig. S6 in the supplemental material). Similarly, the knockdown of MMP-9 or MMP-13 expression significantly decreased basal tumor cell invasiveness through Matrigel (Fig. 6C) as well as FOXO3a-induced invasion (Fig. 6D). Similarly, ectopic addition of recombinant MMP-9 and MMP-13 increased the invasion of FOXO3a knockdown cells (see Fig. S7 in the supplemental material). In conclusion, our results implicate that FOXO3a promotes increased carcinoma cell invasion through the MMPs MMP-9 and MMP-13.
FIG. 6.
MMP-9 and MMP-13 control cancer cell invasion through FOXO3a. (A) MDA-MB-435 cells were treated with MMP-9/MMP-13 inhibitor (5 nM). Matrigel invasion was measured under serum starvation conditions. (B) MDA-MB-435 cells were transfected with vector control, wild-type FOXO3a (WT), or FOXO3a.TM (TM) and treated with MMP-9/MMP-13 inhibitor (5 nM). Matrigel invasion was measured. FOXO3a expression was controlled by immunoblot analysis (not shown). (C) Cells were infected with lentiviral siRNA directed against MMP-9, MMP-13, or both (as indicated). Matrigel invasion was measured. (D) Cells were transfected with vector control or FOXO3a.TM and shRNA directed against MMP-9 and MMP-13. Matrigel invasion was measured. FOXO3a expression was controlled by immunoblot analysis (not shown). Error bars shown represent standard deviations. P values were acquired with the t test, using GraphPad software. P values indicate statistical significance. All results are typical of three independent experiments.
Correlation of the expression of FOXO3a and MMP in breast tumors.
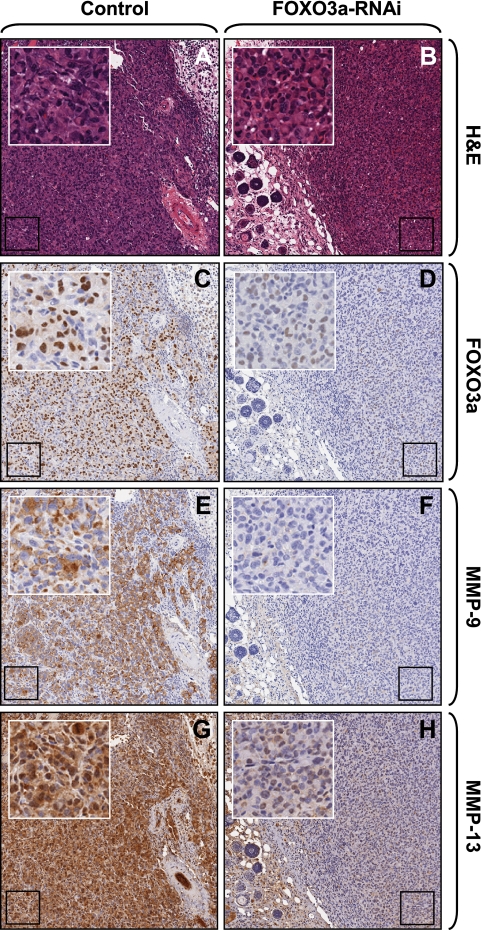
In normal tissues, MMP-9 and MMP-13 are absent or expressed at low levels (2, 26). Conversely, increased expression of both of these metalloproteinases has been detected in numerous solid tumors and has also been causally linked to tumor expansion and increased invasiveness. For example, increased MMP-9 and MMP-13 activity is associated with poor prognosis in colorectal cancer (25) and increased invasion of squamous cell carcinoma cells in vivo (2, 12). Similarly, human tongue carcinoma cell invasion and tumor expansion have been linked to MMP-9 and MMP-13 catalytic activity (30). Transgenic mice lacking MMP-9 display a decreased incidence of invasive tumors (11). FOXO3a expression was described as an in vivo marker for mammary gland neoplasms (20). Nuclear expression of FOXO3a, which suggests its activation, had been detected in approximately 70% of invasive ductal carcinomas of the breast, whereas more than 90% of the benign tumor cases showed cytosolic staining, which resembles FOXO3a in an inactive state (20). We first analyzed orthotopic mouse tumors, which were generated with MDA-MB-231 cells or MDA-MB-231 cells harboring FOXO3a shRNA for a correlation of FOXO3a and MMP expression. We found that MMP-9 and MMP-13 expression correlated with the expression of nuclear (active) FOXO3a (Fig. 7C to H).
FIG. 7.
Nuclear FOXO3a correlates with MMP-9 and MMP-13 expression in tumors. Hematoxylin and eosin (H&E) staining and immunohistochemical staining for FOXO3a (anti-FOXO3a), MMP-9 (anti-MMP-9), or MMP-13 (anti-MMP-13) of orthotopic tumors generated with either control cells or cell lines where FOXO3a was knocked down.
To correlate the expression of the direct FOXO3a target gene MMP-13 with FOXO3a in human carcinoma, we analyzed tissue microarrays from invasive breast carcinoma. Since MMPs are secreted and FOXO3a is an intracellular transcription factor, it was not possible to demonstrate direct coexpression of the proteins within cells. However, approximately 70% of the analyzed human breast cancer tissue sections (44 total samples, with 35 samples of infiltrating duct carcinoma and 9 samples of metastatic carcinoma in the lymph nodes) showed FOXO3a and MMP-13 coexpression (Table 1). Other tissues samples were predominantly negative for both markers. In all sections, tumor cells were clearly distinguishable from infiltrating immune cells or stromal cells. An apparent coexpression of nuclear FOXO3a with MMP-13 was detected in 69% of infiltrating breast duct carcinomas from various stages and in 67% of metastatic carcinoma in the lymph nodes (Table 1; see also Fig. S8 and S9 in the supplemental material). We also detected coexpression of FOXO3a and MMP-13 in other tumor types, such as squamous cell carcinomas in the uterine cervix (data not shown). The clear coexpression of MMP-13 and FOXO3a in areas of the tumor sections, together with the functional link provided by our in vitro data, supports an important role for FOXO3a in tumor cell invasion and tumor expansion. FOXO3a and MMP-13 coexpression is not dependent on the stage of infiltrating breast carcinomas, since approximately 70% of the tissue samples of infiltrating duct and metastatic carcinomas of all stages (T1 to T4) were positive for both markers (see Fig. S8 in the supplemental material). Thus, FOXO3a-mediated expression of MMPs induced by serum restriction and the increase in the invasive potential of tumor cells function at all tumor stages and may affect tumor expansion and ultimately tumor metastasis.
TABLE 1.
FOXO3a and MMP-13 expression correlate in human breast cancer tissue
| Type of human breast tissue (no. of samples)a | No. of samples (%) positive for MMP-13 and FOXO3a | No. of samples negative for MMP-13 and FOXO3a | No. of samples positive for either MMP-13 or FOXO3a |
|---|---|---|---|
| Infiltrating duct carcinoma (35) | 24 (69) | 9 | 2 |
| Metastatic carcinoma in lymph nodes (9) | 6 (67) | 3 | 0 |
| Normal tissue (5) | 0 | 3 | 2 |
A total of 44 samples of human breast cancer tissue (35 samples of infiltrating duct carcinoma and 9 samples of carcinoma in lymph nodes) as well as 5 samples of normal tissue were analyzed for FOXO3a and MMP-13 expression using IHC.
Conclusions.
A functional role for the FOXO family of transcription factors in human cancer progression has been well established by both in vitro and in vivo analyses of signaling pathways which control its transcriptional activity. The available data have suggested a model whereby in quiescent cells, the PI 3-K and Akt/PKB pathway is inactive, resulting in nuclear retention of FOXO3a, enabling the transcription of inducible genes which promote cell cycle progression and mitogenesis or alternatively induce a proapoptotic program (7). In response to factors such as IGF-1, which promote survival and proliferation, or genetic lesions in cancer cells, such as PTEN or PI 3-K mutations which hyperactivate Akt/PKB, FOXO3a is maintained in an inactive state by nuclear exclusion and cytoplasmic retention. Thus, inactivation of FOXO3a provides a prosurvival advantage in cancer cells and thus accelerates tumor growth. Consistent with this, a recent analysis of the distribution of active Akt/PKB and IKKβ in tumor sections revealed a striking correlation with cytoplasmic FOXO3a (17). However, as a solid tumor grows, serum factors such as IGF-1 become limiting. This would permit the reentry of FOXO3a into the nucleus to initiate a transcriptional program, which we propose includes the MMPs MMP-9 and MMP-13 and promotes cell invasion. Although many studies have clearly shown that FOXO3a is inactive in growing tumors, primarily due to Akt activation, our's is the first to demonstrate a proinvasion function for this transcription factor.
In summary, our data point to an important role for FOXO3a in promoting tumor expansion and metastasis by regulating MMP expression and cell invasion, suggesting that cancer cell survival and cell proliferation are not the sole cancer cell phenotypes regulated by this ubiquitous transcription factor. They also suggest that any therapies under development to inactivate FOXO3a may be effective at blocking tumor expansion and metastasis.
Supplementary Material
Acknowledgments
This work was supported by grants from the Florida Department of Health, Bankhead-Coley Program (to P.S., grant FLA07BN-08), Department of Defense (to K.J.S., grant W81XH-04-1-0360), and the National Institutes of Health (to A.T., grants NCI-R01CA075134 and R01CA122099; to J.A.C., grant NCI-R01CA104505).
This publication is a collaborative work of the laboratories of P.S. and A.T. Data shown in Fig. 2C to F, 3A to F, 5A and B, and 6A and B; Fig. S1A and B, S3, S4, S6, S8, and S9 in the supplemental material; and Table 1 were generated by P.S. in the laboratory of A.T. Data shown in Fig. 1 and 7 and Fig. S1 in the supplemental material were generated by P.S. in collaboration with K.J.S. and J.A.C. in the laboratory of P.S. Data shown in Fig. 2A and B, 2G and H, 4A and B, 5C to F, and 6C and D and Fig. S1C, S2, S5, and S7 in the supplemental material were generated by H.D. in the laboratory of P.S. The manuscript was written by P.S. and A.T.
We thank Derek C. Radisky and members of the Storz laboratory for helpful discussions. We also thank Anne Brunet and Mike Greenberg for providing the FOXO3a constructs and Richard Loeser and Motoharu Seiki for providing the MMP reporter plasmids.
Footnotes
Published ahead of print on 29 June 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Accili, D., and K. C. Arden. 2004. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 117421-426. [DOI] [PubMed] [Google Scholar]
- 2.Ala-aho, R., M. Ahonen, S. J. George, J. Heikkila, R. Grenman, M. Kallajoki, and V. M. Kahari. 2004. Targeted inhibition of human collagenase-3 (MMP-13) expression inhibits squamous cell carcinoma growth in vivo. Oncogene 235111-5123. [DOI] [PubMed] [Google Scholar]
- 3.Bergers, G., and L. E. Benjamin. 2003. Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer 3401-410. [DOI] [PubMed] [Google Scholar]
- 4.Brunet, A., A. Bonni, M. J. Zigmond, M. Z. Lin, P. Juo, L. S. Hu, M. J. Anderson, K. C. Arden, J. Blenis, and M. E. Greenberg. 1999. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96857-868. [DOI] [PubMed] [Google Scholar]
- 5.Brunet, A., F. Kanai, J. Stehn, J. Xu, D. Sarbassova, J. V. Frangioni, S. N. Dalal, J. A. DeCaprio, M. E. Greenberg, and M. B. Yaffe. 2002. 14-3-3 transits to the nucleus and participates in dynamic nucleocytoplasmic transport. J. Cell Biol. 156817-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunet, A., J. Park, H. Tran, L. S. Hu, B. A. Hemmings, and M. E. Greenberg. 2001. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a). Mol. Cell. Biol. 21952-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burgering, B. M., and G. J. Kops. 2002. Cell cycle and death control: long live Forkheads. Trends Biochem. Sci. 27352-360. [DOI] [PubMed] [Google Scholar]
- 8.Cheng, M., B. De, S. Pikul, N. G. Almstead, M. G. Natchus, M. V. Anastasio, S. J. McPhail, C. E. Snider, Y. O. Taiwo, L. Chen, C. M. Dunaway, F. Gu, M. E. Dowty, G. E. Mieling, M. J. Janusz, and S. Wang-Weigand. 2000. Design and synthesis of piperazine-based matrix metalloproteinase inhibitors. J. Med. Chem. 43369-380. [DOI] [PubMed] [Google Scholar]
- 9.Chin, J. R., and Z. Werb. 1997. Matrix metalloproteinases regulate morphogenesis, migration and remodeling of epithelium, tongue skeletal muscle and cartilage in the mandibular arch. Development 1241519-1530. [DOI] [PubMed] [Google Scholar]
- 10.Coussens, L. M., B. Fingleton, and L. M. Matrisian. 2002. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science 2952387-2392. [DOI] [PubMed] [Google Scholar]
- 11.Coussens, L. M., C. L. Tinkle, D. Hanahan, and Z. Werb. 2000. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell 103481-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Culhaci, N., K. Metin, E. Copcu, and E. Dikicioglu. 2004. Elevated expression of MMP-13 and TIMP-1 in head and neck squamous cell carcinomas may reflect increased tumor invasiveness. BMC Cancer 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egeblad, M., and Z. Werb. 2002. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2161-174. [DOI] [PubMed] [Google Scholar]
- 14.Furuyama, T., T. Nakazawa, I. Nakano, and N. Mori. 2000. Identification of the differential distribution patterns of mRNAs and consensus binding sequences for mouse DAF-16 homologues. Biochem. J. 349629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greer, E. L., and A. Brunet. 2005. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene 247410-7425. [DOI] [PubMed] [Google Scholar]
- 16.Greer, E. L., P. R. Oskoui, M. R. Banko, J. M. Maniar, M. P. Gygi, S. P. Gygi, and A. Brunet. 2007. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J. Biol. Chem. 28230107-30119. [DOI] [PubMed] [Google Scholar]
- 17.Hu, M. C., D. F. Lee, W. Xia, L. S. Golfman, F. Ou-Yang, J. Y. Yang, Y. Zou, S. Bao, N. Hanada, H. Saso, R. Kobayashi, and M. C. Hung. 2004. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell 117225-237. [DOI] [PubMed] [Google Scholar]
- 18.Irie, H. Y., R. V. Pearline, D. Grueneberg, M. Hsia, P. Ravichandran, N. Kothari, S. Natesan, and J. S. Brugge. 2005. Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelial-mesenchymal transition. J. Cell Biol. 1711023-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jauliac, S., C. Lopez-Rodriguez, L. M. Shaw, L. F. Brown, A. Rao, and A. Toker. 2002. The role of NFAT transcription factors in integrin-mediated carcinoma invasion. Nat. Cell Biol. 4540-544. [DOI] [PubMed] [Google Scholar]
- 20.Jin, G. S., E. Kondo, T. Miyake, M. Shibata, T. Takashima, Y. X. Liu, K. Hayashi, T. Akagi, and T. Yoshino. 2004. Expression and intracellular localization of FKHRL1 in mammary gland neoplasms. Acta Med. Okayama 58197-205. [DOI] [PubMed] [Google Scholar]
- 21.Johansson, N., K. Airola, R. Grenman, A. L. Kariniemi, U. Saarialho-Kere, and V. M. Kahari. 1997. Expression of collagenase-3 (matrix metalloproteinase-13) in squamous cell carcinomas of the head and neck. Am. J. Pathol. 151499-508. [PMC free article] [PubMed] [Google Scholar]
- 22.Kops, G. J., T. B. Dansen, P. E. Polderman, I. Saarloos, K. W. Wirtz, P. J. Coffer, T. T. Huang, J. L. Bos, R. H. Medema, and B. M. Burgering. 2002. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 419316-321. [DOI] [PubMed] [Google Scholar]
- 23.Lafleur, M. A., A. F. Drew, E. L. de Sousa, T. Blick, M. Bills, E. C. Walker, E. D. Williams, M. Waltham, and E. W. Thompson. 2005. Upregulation of matrix metalloproteinases (MMPs) in breast cancer xenografts: a major induction of stromal MMP-13. Int. J. Cancer 114544-554. [DOI] [PubMed] [Google Scholar]
- 24.Leber, T. M., and F. R. Balkwill. 1997. Zymography: a single-step staining method for quantitation of proteolytic activity on substrate gels. Anal. Biochem. 24924-28. [DOI] [PubMed] [Google Scholar]
- 25.Leeman, M. F., J. A. McKay, and G. I. Murray. 2002. Matrix metalloproteinase 13 activity is associated with poor prognosis in colorectal cancer. J. Clin. Pathol. 55758-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li, H., J. Liang, D. H. Castrillon, R. A. DePinho, E. N. Olson, and Z. P. Liu. 2007. FoxO4 regulates tumor necrosis factor alpha-directed smooth muscle cell migration by activating matrix metalloproteinase 9 gene transcription. Mol. Cell. Biol. 272676-2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu, H., D. C. Radisky, C. M. Nelson, H. Zhang, J. E. Fata, R. A. Roth, and M. J. Bissell. 2006. Mechanism of Akt1 inhibition of breast cancer cell invasion reveals a protumorigenic role for TSC2. Proc. Natl. Acad. Sci. USA 1034134-4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu, J. W., D. Chandra, M. D. Rudd, A. P. Butler, V. Pallotta, D. Brown, P. J. Coffer, and D. G. Tang. 2005. Induction of prosurvival molecules by apoptotic stimuli: involvement of FOXO3a and ROS. Oncogene 242020-2031. [DOI] [PubMed] [Google Scholar]
- 29.Nemoto, S., and T. Finkel. 2002. Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science 2952450-2452. [DOI] [PubMed] [Google Scholar]
- 30.Nyberg, P., P. Heikkila, T. Sorsa, J. Luostarinen, R. Heljasvaara, U. H. Stenman, T. Pihlajaniemi, and T. Salo. 2003. Endostatin inhibits human tongue carcinoma cell invasion and intravasation and blocks the activation of matrix metalloprotease-2, -9, and -13. J. Biol. Chem. 27822404-22411. [DOI] [PubMed] [Google Scholar]
- 31.Ogg, S., S. Paradis, S. Gottlieb, G. I. Patterson, L. Lee, H. A. Tissenbaum, and G. Ruvkun. 1997. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 389994-999. [DOI] [PubMed] [Google Scholar]
- 32.Paik, J. H., R. Kollipara, G. Chu, H. Ji, Y. Xiao, Z. Ding, L. Miao, Z. Tothova, J. W. Horner, D. R. Carrasco, S. Jiang, D. G. Gilliland, L. Chin, W. H. Wong, D. H. Castrillon, and R. A. DePinho. 2007. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell 128309-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pilcher, B. K., M. Wang, X. J. Qin, W. C. Parks, R. M. Senior, and H. G. Welgus. 1999. Role of matrix metalloproteinases and their inhibition in cutaneous wound healing and allergic contact hypersensitivity. Ann. N. Y. Acad. Sci. 87812-24. [DOI] [PubMed] [Google Scholar]
- 34.Radisky, D. C., D. D. Levy, L. E. Littlepage, H. Liu, C. M. Nelson, J. E. Fata, D. Leake, E. L. Godden, D. G. Albertson, M. A. Nieto, Z. Werb, and M. J. Bissell. 2005. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature 436123-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simpson, K. J., A. S. Dugan, and A. M. Mercurio. 2004. Functional analysis of the contribution of RhoA and RhoC GTPases to invasive breast carcinoma. Cancer Res. 648694-8701. [DOI] [PubMed] [Google Scholar]
- 36.Snoek-van Beurden, P. A., and J. W. Von den Hoff. 2005. Zymographic techniques for the analysis of matrix metalloproteinases and their inhibitors. BioTechniques 3873-83. [DOI] [PubMed] [Google Scholar]
- 37.Sternlicht, M. D., A. Lochter, C. J. Sympson, B. Huey, J. P. Rougier, J. W. Gray, D. Pinkel, M. J. Bissell, and Z. Werb. 1999. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell 98137-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Storz, P., H. Doppler, and A. Toker. 2005. Protein kinase D mediates mitochondrion-to-nucleus signaling and detoxification from mitochondrial reactive oxygen species. Mol. Cell. Biol. 258520-8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tran, H., A. Brunet, J. M. Grenier, S. R. Datta, A. J. Fornace, Jr., P. S. DiStefano, L. W. Chiang, and M. E. Greenberg. 2002. DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the Gadd45 protein. Science 296530-534. [DOI] [PubMed] [Google Scholar]
- 40.Visse, R., and H. Nagase. 2003. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ. Res. 92827-839. [DOI] [PubMed] [Google Scholar]
- 41.Yoeli-Lerner, M., G. K. Yiu, I. Rabinovitz, P. Erhardt, S. Jauliac, and A. Toker. 2005. Akt blocks breast cancer cell motility and invasion through the transcription factor NFAT. Mol. Cell 20539-550. [DOI] [PubMed] [Google Scholar]
- 42.Zigrino, P., S. Loffek, and C. Mauch. 2005. Tumor-stroma interactions: their role in the control of tumor cell invasion. Biochimie 87321-328. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.