Abstract
GATA-2 is an essential transcription factor that regulates multiple aspects of hematopoiesis. Dysregulation of GATA-2 is a hallmark of acute megakaryoblastic leukemia in children with Down syndrome, a malignancy that is defined by the combination of trisomy 21 and a GATA1 mutation. Here, we show that GATA-2 is required for normal megakaryocyte development as well as aberrant megakaryopoiesis in Gata1 mutant cells. Furthermore, we demonstrate that GATA-2 indirectly controls cell cycle progression in GATA-1-deficient megakaryocytes. Genome-wide microarray analysis and chromatin immunoprecipitation studies revealed that GATA-2 regulates a wide set of genes, including cell cycle regulators and megakaryocyte-specific genes. Surprisingly, GATA-2 also negatively regulates the expression of crucial myeloid transcription factors, such as Sfpi1 and Cebpa. In the absence of GATA-1, GATA-2 prevents induction of a latent myeloid gene expression program. Thus, GATA-2 contributes to cell cycle progression and the maintenance of megakaryocyte identity of GATA-1-deficient cells, including GATA-1s-expressing fetal megakaryocyte progenitors. Moreover, our data reveal that overexpression of GATA-2 facilitates aberrant megakaryopoiesis.
The development of mature blood cells is tightly regulated by transcription factors that coordinate the expression of multiple classes of downstream genes, including cell cycle regulators and lineage-specific genes. GATA-1 and GATA-2 are related essential transcription factors that govern the development of multiple hematopoietic cells, including erythrocytes and megakaryocytes (12). Mutations in GATA1 are associated with several human hematologic disorders, including X-linked dyserythropoietic anemia and thrombocytopenia, X-linked thrombocytopenia, and beta-thalassemia, and a form of acute megakaryocytic leukemia that is relatively common in children with Down syndrome (DS-AMKL) (3). The GATA1 mutations in DS-AMKL patients prevent translation of full-length GATA-1 but allow for expression of GATA-1s, an N-terminal-truncated isoform (41). Studies with mouse models have shed light on the specific functions of GATA-1 in hematopoiesis. Gata1-null mouse embryonic stem cells have the ability to contribute to nonhematopoietic lineages as well as myeloid and lymphoid cells but do not support erythroid cell development (10, 27, 28). Furthermore, Gata1 knockdown (G1KD) mice, which fail to express GATA-1 specifically in megakaryocytes, display a persistent thrombocytopenia and accumulation of immature megakaryocytes within their bone marrow and spleen that ultimately leads to myelofibrosis (25, 31, 38, 39). In contrast, knock-in mice that express GATA-1s in place of full-length GATA-1 (G1SKI mice), engineered to model the mutations in human DS-AMKL patients, show a dramatic, but transient, expansion in megakaryopoiesis during embryonic development (20). Loss of full-length GATA-1 likely contributes to abnormal megakaryopoiesis by altering the expression of critical target genes that drive the proliferation or differentiation of megakaryocytes. In support of this model, studies have revealed that GATA-1 target genes, such as GATA2, c-kit, c-myc, and Sfpi1, are aberrantly expressed in Gata1-deficient mice and in human DS-AMKL blasts (1, 20, 25). How dysregulation of these genes contributes to abnormal megakaryopoiesis in the absence of wild-type GATA-1 remains unclear.
During normal hematopoiesis, GATA-2 plays essential roles in hematopoietic stem and progenitor cell compartments. Gata2-deficient mouse embryos fail to survive beyond the primitive hematopoiesis stage, while Gata2-null embryonic stem cells do not contribute to any hematopoietic tissues in chimeric mice (36). Furthermore, loss of GATA-2 causes a proliferation defect in early hematopoietic cells and a block in mast cell formation, even though it was found to be dispensable for terminal maturation of erythroid and myeloid lineages (37). Interestingly, haploinsufficiency of GATA-2 is associated with a decrease in hematopoietic stem cell (HSC) numbers in the bone marrow and an increase in the quiescent and apoptotic fractions (29). GATA-2 haploinsufficiency also leads to a reduction in the number of HSCs in the aorta-gonad-mesonephros (AGM) region of embryos, as well as defects in hematopoietic stem cells at all stages, including the yolk sac, AGM, and bone marrow (22). Thus, GATA-2 appears to play functionally distinct roles during ontogeny of HSCs, including the production and expansion of HSCs in AGM and the proliferation and survival of HSCs in the adult bone marrow.
The effect of overexpression of GATA-2 is less clear. Ectopic expression of GATA-2 promotes erythroblast proliferation while it blocks erythroid terminal differentiation (2). It also inhibits hematopoietic progenitor cell (HPC) expansion through upregulation of p21 and p27, which regulate HSC and HPC quiescence or expansion (8, 9). Consistent with a negative effect of ectopic GATA-2 on proliferation, enforced expression of GATA-2 was found to disrupt normal hematopoiesis and lead to pancytopenia in transplant recipients (26). These observations highlight the dose-dependent effect of GATA-2 on hematopoietic progenitor cells.
Given that GATA-2 plays essential roles in the proliferation and survival of hematopoietic stem and progenitor cells and that its sustained expression in erythroblasts interferes with differentiation (2, 8, 22, 29, 36, 37), we hypothesized that overexpression of GATA-2 contributes to abnormal megakaryopoiesis in Gata1-deficient mice and DS-AMKL. Here we dissect the function of GATA-2 in normal and aberrant megakaryopoiesis. We show that GATA-2 promotes megakaryopoiesis by driving progenitor cell proliferation and megakaryocytic lineage gene expression while suppressing myeloid gene expression. Furthermore, by using a mouse model of human GATA1 DS-AMKL mutations, we reveal that GATA-2 overexpression contributes to dysregulated megakaryocyte proliferation in the absence of full-length GATA-1. By using a genome-wide microarray analysis coupled with chromatin immunoprecipitation, we identify a set of GATA-2 target genes, including Sfpi1 and Cebpa. Notably, downregulation of Sfpi1 or Cebpa partially restores the cell cycle arrest caused by a GATA-2 deficiency. Together, our findings reveal that GATA-2 is a critical transcription factor that coordinates cellular proliferation and maintains megakaryocyte identity in GATA-1-deficient or mutant cells.
MATERIALS AND METHODS
Megakaryocyte cultures, overexpression, and knockdown studies.
G1ME cells were maintained in α minimal essential medium supplemented with 20% fetal bovine seurm and 1% thrombopoietin (TPO) conditional medium as previously described (33). Liquid culture studies were performed as described previously (25). For knockdown of GATA-2 in primary cultures, cells were infected with control retrovirus (pSM2; Open Biosystems) or a virus that contained a short-hairpin RNA against GATA-2 (pSM2-shRNA GATA-2; Open Biosystems; oligo ID V2MM_75808) and selected with puromycin during the expansion and differentiation phases prior to analysis. For knockdown of GATA-2 in G1ME cells, cells were infected with Banshee retroviruses harboring green fluorescent protein (GFP) alone or a modified version that contained GFP and shRNA against GATA-2, which were subcloned from the pSM2 vector. For GATA2 overexpression studies, primary cells were infected by spinoculation with murine stem cell virus-puro control or murine stem cell virus-GATA2 during the expansion phase. To enrich for infected cells, puromycin (1 μg/ml) was included in both expansion and differentiation media. Overexpression of Hhex, PU.1, and C/EBPα was established by infecting G1ME cells with MIGR1 harboring these genes. For knockdown of Hhex, Pu.1, or C/EBPα in G1ME cells, lentiviral constructs (pLKO.1) harboring specific short hairpin RNA were purchased form Open Biosystems (PU.1, RMM4534-NM_011355; C/EBPα, RMM4534-NM_007678; Hhex, RMM4534-NM_008245).
CFU assay.
For the megakaryocyte CFU assay, transduced lineage-negative (lin−) bone marrow or fetal liver cells were selected with puromycin (1 μg/ml) for 2 days after spinoculation and then 2,000 cells were plated in Methocult medium for evaluation of total colony numbers (CFU total), while 1 × 104 cells were seeded in Megacult-C medium for the analysis of pure megakaryocyte colonies (CFU-MK). Methylcellulose was supplemented with 50 ng/ml of stem cell factor, 10 ng/ml of interleukin-3 (IL-3), 10 ng/ml of IL-6, 10 ng/ml of IL-11, 5 ng/ml of TPO, and 1 μg/ml of puromycin and cultured for 7 days. Total numbers of colonies on the Methocult plates were enumerated to generate the CFU total values. For determination of the numbers of CFU-MK colonies, slides of Megacult cultures were stained with acetylcholinesterase according to the manufacturer's instructions, and only stained colonies were counted. For CFU erythroid or bursting-forming unit erythroid (CFU-E or BFU-E, respectively) assays, GFP+ transduced cells were first purified by sorting and then 1 × 104 (BFU-E) or 1 × 103 (CFU-E) GFP+ cells were seeded in Methocult medium supplemented with 20 ng/ml of stem cell factor, 10 ng/ml of IL-3, and 10 U/ml of erythropoietin (EPO). CFU-E and BFU-E colonies were enumerated on days 3 and 7, respectively.
Animals.
Wild-type C57BL/6 and G1SKI (Gata1Δex2) (20) mice were maintained in microisolator housing within a barrier facility. All animal studies were approved by the Northwestern University Animal Care and Use Committee.
Flow cytometry.
Megakaryopoiesis was analyzed by staining cells with anti-CD41 or anti-CD42 antibodies and 4′,6-diamidinio-2-phenylindole (DAPI) as previously described (25). A gate was set for CD41+ cells for analysis of polyploidy. For annexin V staining, the transduced cells were incubated with an annexin V antibody (BioVision) in staining buffer (10 mM HEPES, 140 mM NaCl, 2.5 mM CaCl2, pH 7.4) for 15 min and then assayed for apoptosis by flow cytometry. Flow cytometry was performed on an LSRII apparatus (BD), and data were analyzed with FlowJo software (Tree Star, Ashland, OR). For the G1ME microarray assay GFP+ cells were purified with a MoFlo high-speed sorter (DakoCytomation, Fort Collins, CO).
RNA preparation and microarray assays.
The Banshee control vector or Banshee-shGATA-2-transduced cells were collected by fluorescence-activated cell sorting 24 h after spinoculation. RNA was extracted from three independent sorted G1ME populations with the RNeasy kit (Qiagen) and processed in duplicate for hybridization to Illumina mouse ref-6 arrays. Gene expression data were preprocessed by using the Bioconductor Illumina package with default settings (6, 21). Probes with all samples “absent” (lower than background levels) were removed from further analysis. To identify differentially expressed genes, we applied routines implemented in the Ilumina package (32) to fit linear models to the normalized expression values. The variance used in the t score calculation was corrected by an empirical Bayesian method (32) for better estimation under small sample size. Genes identified in the comparison of experimental samples to baseline had changes of >1.5-fold and a P value (false discovery rate [FDR] adjusted) of <0.01 for the differences to be considered significant.
qRT-PCR.
G1ME cells transduced with the Banshee control vector or Banshee-shGATA-2 were purified by fluorescence-activated cell sorting and RNA was isolated from the GFP+ cells. After preparation of cDNA by standard protocols, quantitative reverse transcription-PCR (qRT-PCR) was performed under the following conditions: hot start at 95°C for 15 min followed by 95°C for 30 s, 57°C for 30 s, and 72°C for 30 s for 40 cycles. The relative quantities of real-time PCR products were determined using the comparative ΔΔCT method (as described in ABI Prism 7700 sequence detection system user bulletin no. 2). Primer sequences are available upon request.
BrdU staining.
Cells were labeled with 30 μg/ml bromodeoxyuridine (BrdU) for 30 min, fixed with 2% paraformaldehyde (PFA) for 10 min at room temperature, permeabilized with ethanol (400 μl of 150 mM NaCl, 850 μl of 100% ethanol) for 30 min on ice, and fixed (1% PFA and 0.1% Tween 20 in Hanks balanced salt solution) overnight at 4°C. Finally, cells were stained with Alexa 647-labeled anti-BrdU antibody for 1 h at room temperature and analyzed by flow cytometry.
ChIP assays.
Chromatin immunoprecipitation (ChIP) assays were performed as described previously, using antibody against GATA2 (H116; sc-9008) or purified rabbit immunoglobulin G (11). Relative occupancy was determined by comparing the signals to a dilution series of the input sample amplified with the same primers. Cd3ɛ and Gapdh served as negative control genes that were not regulated by GATA-2. To identify potential GATA-2 binding sites in or near genes of interest, we performed qPCR on ChIP DNA at regions of high regulatory potential (7) containing conserved consensus GATA binding motifs as previously described (40). Primer sequences are available upon request.
Statistics analysis.
All statistical analyses were performed using Student's t test (two-tailed, unpaired). A P value of 0.05 or less was considered significant.
Gene array accession number.
Gene array data for the comparison between control and GATA-2 knockdown G1ME cells have been deposited in the Gene Expression Omnibus database (accession number GSE16521).
RESULTS
Downregulation of GATA-2 impairs megakaryopoiesis from WT hematopoietic progenitors.
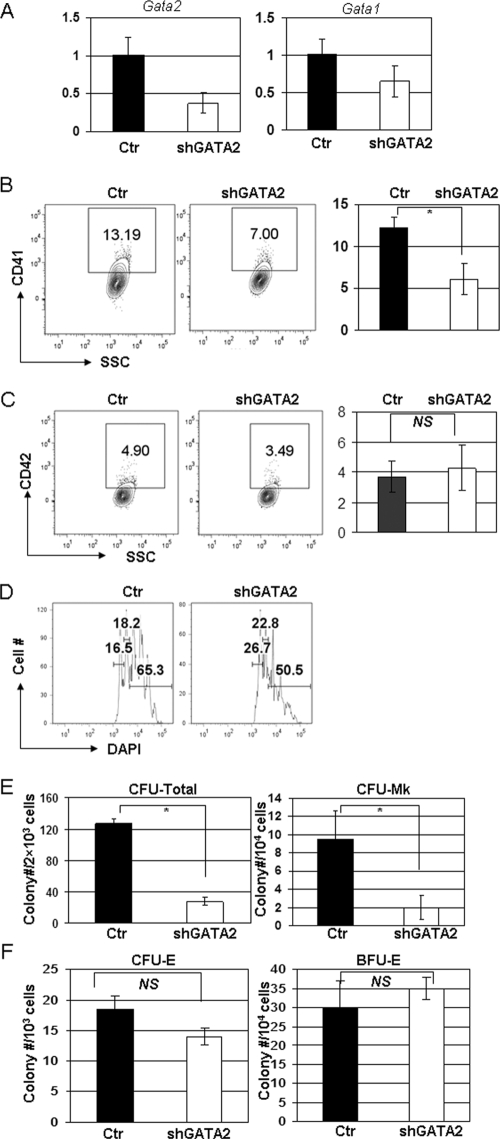
To investigate the function of GATA-2 in megakaryocyte development, we first knocked down its expression in wild-type (WT) bone marrow progenitors with an anti-GATA-2 shRNA and then cultured the infected cells in either liquid or semisolid medium. The short 3-day liquid culture experiment measures the ability of committed megakaryocytes to differentiate, whereas the 7-day methylcellulose colony-forming assay evaluates the proliferation or survival of hematopoietic progenitors. We achieved a 65% knockdown of GATA-2, which was accompanied by a 40% reduction in GATA-1 expression (Fig. 1A; see also Table S1 in the supplemental material). This knockdown led to a significant decrease in the generation of CD41+ cells (6.2% ± 1.9% compared to 12.2% ± 1.3% for control [means ± standard deviations]; P < 0.05), reduced polyploidization (50.5% compared to 65.3% for control), and markedly diminished colony-forming activity (27.8 ± 4.8 compared to 127.3 ± 5.3 for control for CFU total and 2 ± 1.3 compared to 9.5 ± 3.1 for control for CFU-MK; both P < 0.05) (Fig. 1B to E). Furthermore, megakaryocyte colonies generated from the GATA-2 knockdown progenitors were smaller than those derived from control progenitors. Given that decreased expression of GATA-1 is associated with enhanced megakaryocyte proliferation (25, 31, 38, 39), we surmise that the cause of the reduced megakaryopoiesis afforded by the GATA-2 shRNA is the profound decrease in GATA-2 expression. In contrast, knockdown of GATA-2 did not significantly affect erythroid colony formation (Fig. 1F). These studies demonstrate that megakaryocytes and their specific progenitors have a more stringent requirement for proper levels of GATA-2 than the erythroid lineage. Moreover, the results indicate that the defect in the megakaryocyte lineage occurs at the level of the megakaryocyte progenitor rather than at the megakaryocyte-erythroid progenitor (MEP).
FIG. 1.
Downregulation of GATA-2 impairs megakaryopoiesis in WT bone marrow cells (A) GATA-1 and GATA-2 expression in WT lin− bone marrow cells transduced with a retroviral vector harboring a short hairpin RNA against the mouse Gata2 gene (shGATA-2) or the control vector (Ctr) were detected by qRT-PCR. Results were normalized to those for control WT cultures. (B and C) Percentages of CD41-positive (B) and CD42-positive (C) cells in control or shGATA-2 cultures were determined by flow cytometry. Means ± standard deviations for three experiments are shown to the right of the representative flow plots. (D) DNA content of the infected cells was evaluated by DAPI staining. Percentages of 2N, 4N, and ≥8N populations are shown. Data are representative of three independent experiments. (E) Numbers of total colonies (CFU total) and pure megakaryocyte colonies (CFU-MK) formed after 7 days in methylcellulose cultures are shown. Means ± standard deviations for two independent experiments performed in triplicate are depicted. (F) Numbers of CFU-E and BFU-E colonies after knockdown of GATA-2. Means ± standard deviations for two independent experiments performed in triplicate are depicted. *, P < 0.05; NS, not significant.
Downregulation of GATA-2 impairs megakaryopoiesis from GATA-1s knock-in hematopoietic progenitors.
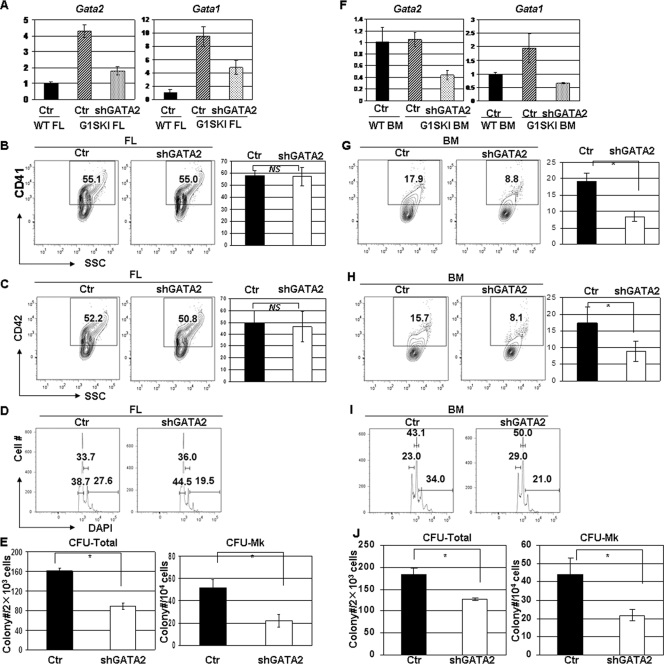
G1SKI mice, which model the GATA1 mutations seen in DS-AMKL by expressing GATA-1s in place of full-length GATA-1, display a robust, transient expansion of megakaryopoiesis within the fetal liver but no abnormalities within the bone marrow (20). To investigate whether GATA-2 levels correlate with this transient megakaryocyte phenotype, we measured GATA-1 and GATA-2 expression in lineage-depleted (lin−) fetal liver and bone marrow cells isolated from G1SKI and wild-type mice. In comparison to their wild-type counterparts, we found that GATA-2 expression was elevated fourfold in G1SKI fetal liver cells but unchanged in G1SKI bone marrow cells (Fig. 2A; see also Table S1 in the supplemental material). Similarly, GATA-1 expression, which leads to production of GATA-1s in place of full-length GATA-1, was elevated 10-fold in G1SKI fetal liver cells but only 2-fold higher in G1SKI bone marrow cells. These results show that increased GATA-1 and GATA-2 expression are associated with the hyperproliferative phenotype and suggest that the increased dosages of these GATA factors may contribute to the transient phenotype.
FIG. 2.
Downregulation of GATA-2 impairs megakaryopoiesis in G1SKI fetal liver and bone marrow cells (A) GATA-1 and GATA-2 expression levels in G1SKI fetal liver (FL) progenitor cells infected with control or shGATA-2 retroviruses were measured by qRT-PCR and normalized to control WT FL cells (Ctr WT FL). (B and C) Percentages of CD41+ (B) and CD42+ (C) cells in control or shGATA-2 cultures were determined by flow cytometry. Means ± standard deviations for three experiments are shown to the right of the representative flow plots. (D) DNA content of the infected CD41+ cells was evaluated by DAPI staining. Percentages of 2N, 4N, and ≥8N populations are shown. Data are representative of three independent experiments. (E) The numbers of total (CFU total) and pure megakaryocyte colonies (CFU-MK) formed after 7 days in methylcellulose cultures are shown. Means ± standard deviations for three independent experiments performed in duplicate are depicted. (F) GATA-1 and GATA-2 expression levels in G1SKI bone marrow (BM) progenitor cells infected with control or shGATA-2 retroviruses were measured by qRT-PCR and normalized to control WT BM cells. (G and H) Percentages of CD41+ (G) and CD42+ (H) cells in control or shGATA-2 cultures were determined by flow cytometry. Means ± standard deviations for three experiments are shown to the right of the representative flow plots. (I) DNA content of the infected CD41+ cells was evaluated by DAPI staining. Percentages of 2N, 4N, and ≥8N populations are shown. Data are representative of three independent experiments. (J) The numbers of total colonies (CFU total) and pure megakaryocyte colonies (CFU-MK) formed after 7 days in methylcellulose cultures are shown. Means ± standard deviations for three independent experiments performed in duplicate are depicted. *, P < 0.05; NS, not significant.
To determine whether elevated GATA-2 expression contributes to the fetal megakaryocyte hyperproliferation in G1SKI mice, we introduced the anti-GATA-2 shRNA into G1SKI fetal liver cells. This strategy led to a 65% decrease in GATA-2 expression that was accompanied by a 50% decrease in GATA-1 expression (Fig. 2A; see also Table S1 in the supplemental material). Despite these reductions in GATA factor expression, we observed a continued robust megakaryocyte expansion from fetal liver progenitors in liquid culture, as evidenced by the high proportion of CD41+ (57.7% ± 4.3% compared to 57.6% ± 7.6% for control; P > 0.05) and CD42+ cells (49.8% ± 10.0% compared to 46.2% ± 12.8% for control; P > 0.05) (Fig. 2B and C). However, the normalization of GATA-2 expression did affect other aspects of G1SKI cells. First, polyploidization was modestly reduced in the GATA-2 shRNA-expressing G1SKI fetal liver megakaryocytes (19.5% compared to 27.6% for control) (Fig. 2D). Second, downregulation of GATA-2 in G1SKI fetal liver progenitors led to significant reductions in the numbers of CFU-MK (22 ± 5.6 compared to 52 ± 6.9 for control; P < 0.05) and CFU total (88.7 ± 7.1 compared to 161 ± 6.3; P < 0.05) (Fig. 2E). These findings suggest that the increased expression of GATA-2 and GATA-1s in G1SKI fetal liver progenitors contributes substantially to the expansion of megakaryocyte progenitors but that their overexpression is not necessary for megakaryocyte development from the committed progenitor.
We next investigated the consequences of knocking down GATA-2 in G1SKI bone marrow progenitors, which do not overexpress GATA-2 or show a hyperproliferative phenotype. Using the shRNA, we achieved a 60% reduction of GATA-2 expression, which was accompanied by a nearly threefold decrease in GATA-1 expression (Fig. 2F). Knockdown of GATA-2 led to dramatic and significant reductions in CD41 (8.5% ± 1.5% compared to 19.5% ± 2.3% for control; P < 0.05) and CD42 (8.9% ± 3.0% compared to 17.4% ± 4.8% for control; P < 0.05) positivity in liquid culture, reduced polyploidization (21% compared to 34% for control), and decreased colony-forming activity (127.3 ± 2.5 compared to 183.3 ± 13.8 for control for CFU total and 21 ± 3.4 compared to 44 ± 9.0 for CFU-MK; both P < 0.05) (Fig. 2G to J). In contrast to fetal liver cells, therefore, GATA-2 knockdown in G1SKI bone marrow impaired every aspect of megakaryopoiesis. This is reminiscent of the requirement for GATA-2 in WT progenitors and is likely a consequence of sub-WT levels of GATA-2 expression.
Overexpression of GATA-2 causes aberrant megakaryopoiesis in WT and G1SKI hematopoietic progenitors.
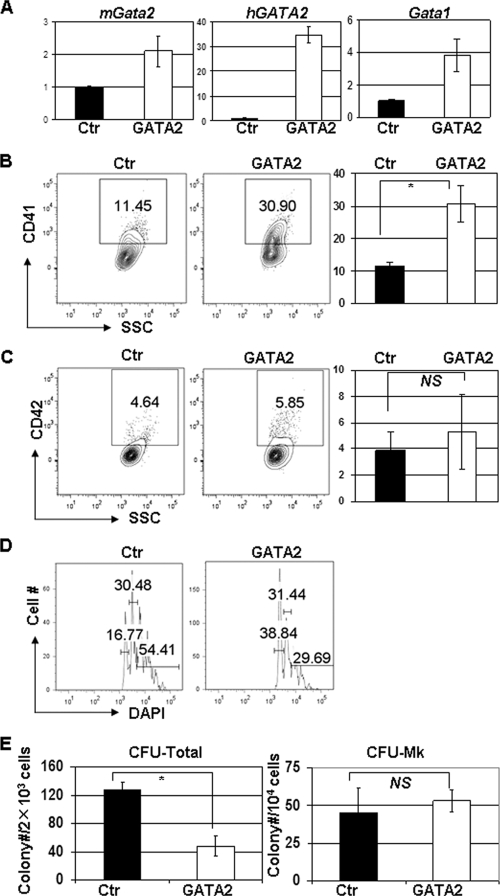
To determine whether increased expression of GATA-2 alone is sufficient to promote megakaryocyte hyperproliferation, we overexpressed GATA-2 in WT murine bone marrow progenitors (Fig. 3A). Overexpression of a human GATA-2 cDNA was accompanied by a twofold increase in endogenous murine GATA-2 and a fourfold increase in GATA-1. Forced overexpression of GATA-2 led to an increased proportion of CD41+ cells (30.7% ± 5.6% compared to 11.6% ± 1.0%; P < 0.05) and reduced polyploidization (29.69% compared to 54.41% for control) but did not affect CFU-MK activity (53 ± 7.4 compared to 45.4 ± 15.4; P > 0.05) (Fig. 3B to E). These findings suggest that the overexpression of GATA-2 may contribute to the expansion of megakaryocytes seen in Gata1-mutant progenitors.
FIG. 3.
GATA2 overexpression enhances megakaryocyte development in WT bone marrow cells. (A) Expression levels of GATA1 and GATA2 (hGATA2, human GATA2; mGata2, mouse GATA2) were measured in pBabe-GATA-2-transduced wild-type cells by qRT-PCR and normalized to the level found in pBabe-infected WT bone marrow cells (Ctr). (B and C) Percentages of CD41+ (B) and CD42+ (C) cells in cultures of pBabe-puro- or pBabe-GATA2-infected wild-type progenitors were determined by flow cytometry. Means ± standard deviations for three experiments are shown to the right of the representative flow plots. (D) DNA content of the infected cells was evaluated by DAPI staining. Percentages of 2N, 4N, and ≥8N populations are shown. Data are representative of three independent experiments. (E) The numbers of total colonies (CFU Total) and pure megakaryocyte colonies (CFU-MK) formed after 7 days in methylcellulose cultures are shown. Means ± standard deviations from two independent experiments performed in triplicate are depicted. *, P < 0.05; NS, not significant.
We next overexpressed GATA-2 in G1SKI bone marrow progenitors, which do not show a dramatic megakaryocyte phenotype. In this setting, overexpression of GATA-2 did not significantly affect GATA-1 expression (Fig. 4A). Overexpression of GATA-2 was sufficient to drive a marked expansion of megakaryocytes in liquid culture, as evidenced by an increase in the proportions of CD41+ (40.4% ± 4.0% compared to 18.5% ± 2.1% for control; P < 0.05) and CD42+ cells (14.9% ± 2.4% compared to 6.3% ± 0.8% for control; P < 0.05) (Fig. 4B and C). Given that GATA-1 levels were only marginally increased in these cells, these observations reveal that elevated GATA-2 expression directly contributes to the megakaryocyte expansion that is characteristic of G1SKI fetal liver cells. Furthermore we noticed that overexpression of GATA-2 in both WT and G1SKI progenitors led to significant decreases in CFU total colonies (47.7 ± 15.3 compared to 127.5 ± 10.7 for G1SKI FL and 60.5 ± 10.6 compared to 95.3 ± 8.7 for G1SKI BM; both P < 0.05), which were primarily comprised of myeloid colonies and consistent with previous findings (26). The enrichment in megakaryocytes in liquid culture is likely partially due to this decrease in expansion of myeloid cells. Taken together, these observations suggest that GATA-2 overexpression contributes to distinct aspects of megakaryocyte development, including commitment, survival, and/or terminal differentiation.
FIG. 4.
GATA2 overexpression promotes megakaryocyte development in G1SKI bone marrow cells (A) Expression levels of GATA1 and GATA2 were measured in pBabe-GATA-2-transduced bone marrow cells by qRT-PCR and normalized to the level of pBabe-infected G1SKI bone marrow cells (Ctr). (B and C) Percentages of CD41+ (B) and CD42+ (C) cells in megakaryocyte liquid cultures were measured by staining and analyzed by flow cytometry. Means ± standard deviations for two experiments performed in duplicate are shown to the right of the representative flow plots. (D) DNA content of the infected cells was evaluated by DAPI staining. Percentages of 2N, 4N, and ≥8N populations are shown. Data are representative of three independent experiments. (E) The numbers of total colonnes (CFU total) and pure megakaryocyte colonies (CFU-MK) formed after 7 days in methylcellulose cultures are shown. Means ± standard deviations from two independent experiments performed in duplicates are depicted. *, P < 0.05; NS, not significant.
GATA-2 expression is required for megakaryocytic cell proliferation.
As shown above, overexpression or knockdown of GATA-2 resulted in changes in expression of GATA-1. In order to probe the requirements of GATA-2 without the confounding effect of altered GATA-1 expression, we overexpressed and knocked down GATA-2 in G1ME cells, which were derived from murine Gata1− embryonic stem cells by in vitro differentiation (33). G1ME cells express CD41 and proliferate as TPO-dependent, undifferentiated blasts. Reconstitution with full-length GATA-1 induces erythroid and megakaryocytic differentiation in the presence of EPO or TPO, respectively. Importantly, G1ME cells do not differentiate along the myeloid lineage even when cultured with myeloid cytokines (33). Thus, G1ME cells approximate a bipotential MEP whose development is arrested by loss of GATA-1.
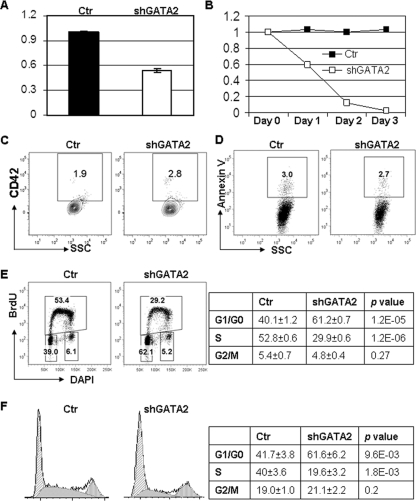
To study the requirements for GATA-2 in G1ME cell proliferation and viability, we infected cells with a retrovirus that encodes GFP and a short hairpin against GATA-2. After transduction, we monitored proliferation, differentiation, and viability by flow cytometry. The shRNA led to a 50% reduction in GATA-2 expression that was accompanied by a rapid loss of GFP+ transduced cells (Fig. 5A and B): the relative percentage of shGATA-2-transduced cells dropped to 5% 3 days postinfection. We did not detect any significant changes in cell surface expression of annexin V, CD41, CD42, Gr-1, or Mac-1, indicating that the loss of the GATA-2 shRNA-expressing GFP+ cells was not due to apoptosis or differentiation (Fig. 5C and D and data not shown). Instead, we detected a dramatic decrease in BrdU-positive S-phase cells and a concomitant increase in cells within G1 phase in the shGATA-2-transduced G1ME cells (29.9% ± 0.6% compared to 52.8% ± 0.6% for control; P < 0.05) (Fig. 5E). Cell cycle analysis of DAPI-stained G1ME cells confirmed that knockdown of GATA-2 led to a significant increase of cells in G1 and a decrease in S phase cells (Fig. 5F). These data reveal that downregulation of GATA-2 leads to a rapid cessation of proliferation. Therefore, in megakaryocytes that lack GATA-1, GATA-2 controls the progression of cells from G1 to S phase.
FIG. 5.
GATA-2 is required for G1ME cell proliferation. (A) Downregulation of GATA-2 expression in GFP+ G1ME cells transduced with retroviruses harboring shGATA-2 or control vector was confirmed by qRT-PCR. (B) The percentage of transduced GFP+ cells was monitored by flow cytometry over 4 days and normalized to the day 0 value, which corresponded to the day of the last infection. (C and D) Day 2 transduced cells were evaluated for CD42 or annexin V staining by flow cytometry. Representative flow plots are shown. (E) Transduced cells were labeled with BrdU, stained with an anti-BrdU antibody, and analyzed by flow cytometry. Plots are representative of three experiments, with the means ± standard deviations displayed in the table (n = 3). (F) Transduced cells were fixed, permeabilized, and stained with DAPI, and the stages of the cell cycle were determined by the Dean-Jett-Fox model. The means ± standard deviations of the percentages of cells in the various stages of the cell cycle are depicted in the table (n = 3).
Separately, we overexpressed GATA-2 in G1ME cells. Ectopic expression of GATA-2 promoted some features of megakaryocytic differentiation, as evidenced by increased polyploidization and CD42 expression over 6 days (see Fig. S1 in the supplemental material). GATA-2, however, was far less potent than either GATA-1 or GATA-1s in inducing differentiation, as we observed a much slower decrease of GFP+ cells in the culture, decreased apoptosis, reduced CD42 expression, and a lower percentage of polyploid cells in GATA-2-tranduced cells compared to the GATA-1- and GATA-1s-transduced groups. These data show that GATA-2 fails to activate the megakaryocyte terminal maturation program in the absence of GATA-1. To address the differential abilities of GATA-1 and GATA-2 to induce megakaryocytic differentiation, we swapped the N-terminal and the C-terminal halves of the two proteins to create mosaic molecules termed NG1G2C and NG2G1C. NG1G2C contains the N-terminal half of GATA-1 fused to the C-terminal half of GATA-2, whereas NG2G1C contains the N-terminal half of GATA-2 fused to the C-terminal half of GATA-1 (details provided in the methods section of the supplemental material). These chimeric molecules induced cell cycle arrest (as measured by loss of GFP+ cells), CD42 expression, and polyploidization at levels between those of wild-type GATA-1 and GATA-2 (see Fig. S1A to D in the supplemental material). Thus, these results reveal that optimal megakaryocytic differentiation requires both the C- and N-halves of GATA-1.
To further address the different activities of GATA-1 and GATA-2 on megakaryoycte proliferation, we separately overexpressed GATA-1 and GATA-2 in WT bone marrow progenitors and compared CFU-MK formation. GATA-1 overexpression reduced megakaryocyte colonies to nearly one-third of control (14.8 ± 3.6 compared to 40 ± 4.2 for vector control; P < 0.05), whereas GATA-2 expression did not significantly reduce megakaryocyte colony numbers (30.3 ± 8.2 compared to 40 ± 4.2 for vector control; P > 0.05) (see Fig. S1E in the supplemental material). We also observed increased CFU-E but reduced BFU-E colony numbers in GATA-1-overexpressing cells, whereas GATA-2 overexpression inhibited both colony types (see Fig. S1E in the supplemental material).
Analysis of GATA-2 target genes in megakaryocytes.
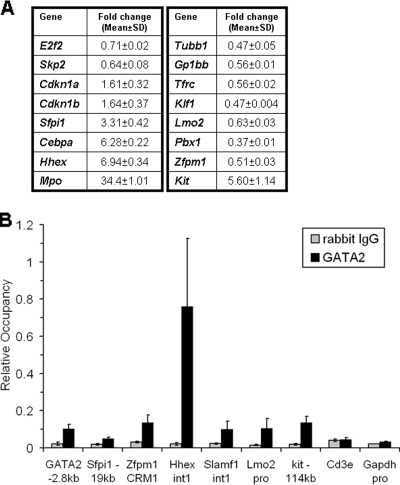
To identify GATA-2 target genes that control the proliferation and differentiation of megakaryocytes, we performed a genome-wide microarray comparison of shGATA-2-transduced G1ME cells versus control-infected G1ME cells. We identified 191 probes, representing 146 genes, whose signal changed more than 1.5-fold with a P value of <0.01 (after FDR adjustment) (see Table S2 in the supplemental material). Of these 146 genes, 39 genes were downregulated and 107 were upregulated in shGATA-2-transduced cells. Gene ontology analysis revealed that these genes fell into multiple classes, including cell cycle regulators, cell proliferation, transcription factors, and markers of terminal differentiation of myeloid cells, erythrocytes, and megakaryocytes. We validated the changes observed in the microarray analysis for 16 genes by qRT-PCR (Fig. 6A). Consistent with a potential role for GATA-2 in the transition from G1 to S phase, we observed reduced expression of E2f2 and Skp2 and increased expression of the cell cycle regulators Cdkn1a (p21) and Cdkn1b (p27) in the shGATA-2-infected cells. In addition, we found that expression levels of genes implicated in granulocyte/monocyte development, including Mpo, Sfpi1, Hhex, and Cebpa, were robustly induced in the shGATA-2 group. On the other hand, erythrocyte/megakaryocyte lineage genes such as Tubb1, Gp1bb, Klf1, and Trfc were downregulated. Furthermore, we found that GATA-2 was also required for proper expression of genes that have been implicated in hematopoietic progenitors, including Lmo2, Zfpm1, and c-kit. Thus, GATA-2 appears to play multiple roles in megakaryocytes by regulating genes that control progenitor cell proliferation, lineage commitment, and differentiation.
FIG. 6.
Validation of GATA-2 target genes by qRT-PCR and ChIP. (A) qRT-PCR was used to confirm changes in expression upon downregulation of GATA-2. (B) Enrichment of GATA-2 on various conserved regulatory elements was determined by qPCR of chromatin from G1ME cells. Binding is depicted as mean ± standard deviation values for at least two qPCR reactions from three independent ChIP experiments.
To determine which of the genes identified in our microarray study are most likely to be direct targets of GATA-2, we performed ChIP assays using chromatin purified from proliferating G1ME cells. As expected, we detected enrichment in the GATA2, c-kit, and Zfpm1 loci, which are known to harbor conserved GATA motifs (Fig. 6B). Interestingly, enrichment was also observed at conserved elements within intron 1 of Hhex, the loci of Slamf1, Sfpi1, and Lmo2, suggesting that these genes are four novel and direct targets of GATA-2.
Overexpression of myeloid genes causes cell cycle arrest accompanied by apoptosis and myeloid differentiation.
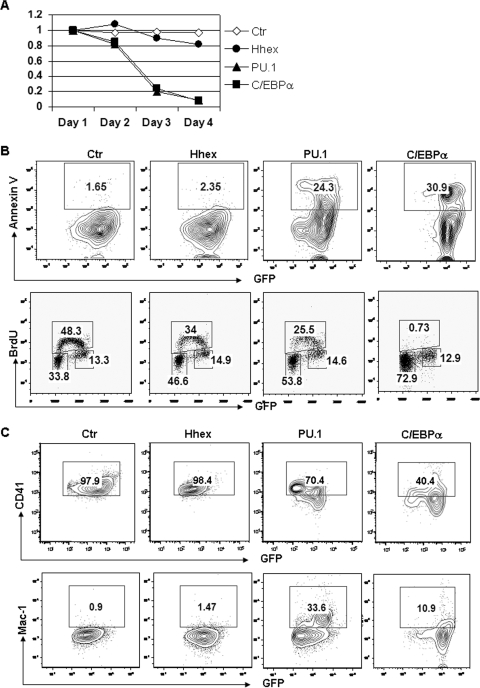
Given that decreased expression of GATA-2 was associated with pronounced upregulation of the myeloid genes Hhex, Sfpi1, and Cebpa, we predicted that cell cycle arrest might be indicative of myeloid differentiation. To determine whether upregulation of these myeloid genes could induce cell cycle arrest of G1ME cells, we overexpressed individual genes in G1ME cells by retroviral transduction. Overexpression of each gene caused cell cycle arrest as evidenced by decreased percentages of GFP+ cells and BrdU-positive S-phase cells (34%, 25.5%, and 0.7% for Hhex, PU.1, and C/EBPα, respectively, compared to 48.3% for the GFP control) (Fig. 7A and B). Ectopic expression of PU.1 or C/EBPα also caused profound apoptosis and myeloid differentiation (Fig. 7B and C). We observed downregulation of CD41 (70.4% and 40.4% for PU.1 and C/EBPα, respectively, compared to 97.9% for the GFP control) and upregulation of Mac-1 (33.6% and 10.9% for PU.1 and C/EBPα, respectively, compared to 0.9% for the GFP control) in PU.1- or C/EBPα-transduced G1ME cells (Fig. 7B and C). On the other hand, Hhex only had a minimal effect on cell proliferation. These observations suggest that upregulation of myeloid genes due to loss of repression by GATA2 may cause cell cycle arrest.
FIG. 7.
Overexpression of Hhex, PU.1, or C/EBPα causes cell cycle arrest in G1ME cells. (A) G1ME cells were transduced by MIGR1, Hhex, PU.1, or C/EBPα. The percentages of transduced cells were measured through detecting GFP+ cells by flow cytometry and normalized to the day 1 value. (B) The apoptotic cells were measured by detecting annexin V and analyzed by flow cytometry. The transduced cells were further labeled with BrdU. The labeled cells were measured by staining for BrdU. The cell cycle profile was analyzed by flow cytometry. (C) The expression levels of CD41 and Mac-1 in transduced cells were measured by surface staining with specific antibodies and analysis by flow cytometry.
Downregulation of PU.1 or C/EBPα partially restores the phenotype in GATA-2 knockdown G1ME cells.
Since Skp2 and E2f2 are known to play important roles in promoting S-phase entry and were downregulated in GATA-2 knockdown G1ME cells, we first assayed whether their restoration could rescue the cell cycle phenotype. We overexpressed Skp2 or E2f2 in G1ME cells and then infected these cells with shGATA-2 retroviruses. Overexpression of Skp2 or E2f2 failed to rescue the cell cycle arrest caused by ectopic expression of GATA-2 hairpin (see Fig. S2 in the supplemental material). Moreover, we could not detect binding of GATA-2 to Skp2, p21, or p27 loci (data not shown). These results suggest that GATA-2 regulates megakaryocyte cell cycle progression through an indirect mechanism.
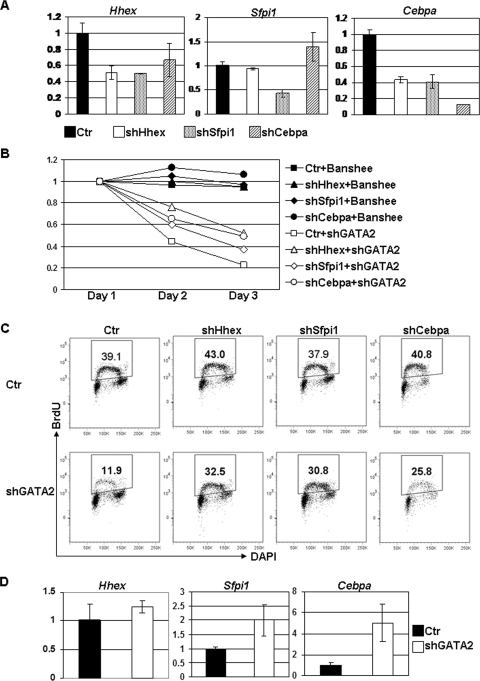
Overexpression of myeloid genes that were upregulated in GATA-2-knocked down G1ME cells caused cell cycle arrest accompanied by myeloid differentiation and apoptosis. These observations raise the possibility that GATA-2 may indirectly control the cell cycle by repressing these myeloid genes. To directly test whether upregulation of targets of GATA-2 repression such as Hhex, Spfi1, or Cebpa are the cause of the cell cycle arrest in GATA-2 knockdown cells, we transduced G1ME cells individually with shRNAs specific to these target genes (Fig. 8A). Downregulation of each gene did not cause a significant change in cell cycle or cell differentiation (data not shown). Next, we introduced the anti-GATA-2 hairpin into these cells and monitored cell cycle progression. We found that knockdown of PU.1 or C/EBPα partially reversed the proliferative defect, as evidenced by a delayed disappearance of GFP+ cells and an increased percentage of BrdU-stained cells in S phase (30.8% for PU.1 and 25.8% for C/EBPα compared to 11.9% for control) (Fig. 8B and C). Finally, to show that GATA-2 similarly contributes to the reinforcement of megakaryocyte development in vivo, we assayed for expression of myeloid genes in G1SKI fetal liver cells. Both Sfpi1 and Cebpa were significantly upregulated (two- to fivefold; P < 0.05) in these cells upon knockdown of GATA-2 (Fig. 8D). These observations show that GATA-2 reinforces megakaryopoiesis by antagonizing the expression of the key myeloid differentiation factors PU.1 and C/EBPα.
FIG. 8.
Restoration of cell cycle arrest by downregulation of Hhex, PU.1, and C/EBPα in G1ME cells. (A) G1ME cells were transduced with control lentivirus (Ctr) or lentivirus expressing short hairpin specific to Hhex (shHhex), PU.1 (shSfpi1), or C/EBPα (shCebpa) and selected with puromycin. The downregulation of each gene were confirmed by qPCR. (B) The transduced cells were further infected by control retrovirus expressing GFP alone (Banshee) or retrovirus expressing both GFP and shGATA-2. The percentages of infected cells were monitored by flow cytometry during the following 3 days and normalized to values for day 1. (C) The double-transduced cells were labeled with BrdU on day 2. The labeled cells were measured by staining with antibody specific for BrdU and analyzed by flow cytometry. A gate was set up for GFP+ cells. (D) Progenitor cells from G1SKI fetal liver were transduced with shGATA-2 and selected by puromycin. The expression levels of Hhex, Sfpi1, and Cebpa were confirmed by qPCR. Data are representative of two independent experiments with similar results.
DISCUSSION
DS-AMKL initiates in the fetal liver and requires the presence of both trisomy 21 and a mutation in GATA1, which leads to the exclusive expression of the N-terminal-truncated isoform GATA-1s (24). Fetal liver megakaryocyte progenitors from G1SKI mice, which express only GATA-1s, and human DS-AMKL blasts show aberrant proliferation as well as a significant increase in GATA-2 expression, consistent with the hypothesis that overexpression of GATA-2 contributes to altered gene expression and hyperproliferation of megakaryoblasts. Here we show that the dosage of GATA-2 affects megakaryocyte development in multiple ways. First, when overexpressed, megakaryocyte progenitors expand dramatically and their maturation is not blocked, as has been reported for other cell types (23). Second, when GATA-2 levels are restored to wild-type levels in G1SKI fetal liver progenitors, megakaryocyte maturation is only marginally affected but CFU-MK activity is significantly reduced. Third, when GATA-2 expression drops to 50% of wild-type levels, megakaryocyte proliferation is severely diminished but myeloid genes are induced. Taken together, these results show that GATA-2 is a key mediator of normal and malignant megakaryopoiesis.
Interplay between GATA-1 and GATA-2.
GATA-2 is highly expressed in proliferating erythroid progenitors. Upon differentiation, GATA-1 expression increases whereas GATA-2 expression drops precipitously. Elegant studies have shown that this occurs via the GATA switch, which involves exchange of one GATA factor for another on a gene regulatory element (11). Similarly, GATA-1 displaces GATA-2 from an upstream enhancer of c-Kit during erythroid differentiation (15). Although it is tempting to speculate that a similar GATA switch operates during megakaryocyte maturation, several lines of evidence suggest that the situation is more complex. First, GATA-2 expression is not rapidly downregulated during maturation of human megakaryocytes (18, 35). Second, whereas overexpression of GATA-2 interferes with erythroid maturation, ectopic expression of GATA-2 promotes megakaryopoiesis (reference 13 and this report). Third, although GATA-2 occupancy is associated with active c-Kit expression, levels of c-Kit were dramatically increased in G1ME cells harboring the shRNA against GATA-2. This latter observation suggests that GATA-2 normally restrains, rather than activates, c-kit transcription in megakaryocytes. Further studies to evaluate the interplay between GATA-1 and GATA-2 are necessary to fully understand how GATA-2 controls normal development and how the loss of full-length GATA-1 drives AMKL.
The complexity of the interplay between GATA-1 and GATA-2 is underscored by our observations that expression of GATA-1 was affected by knockdown or overexpression of GATA-2 (summarized in Table S1 of the supplemental material). Upon overexpression of GATA-2 in wild-type or G1SKI bone marrow, we detected 4-fold and 1.5-fold increases in GATA-1 expression, respectively. Similarly, when GATA-2 was downregulated in wild-type or G1SKI progenitors, we witnessed concomitant drops in GATA-1 expression. These changes in GATA-1 expression are most likely the result of transcriptional feedback between GATA-1 and GATA-2 regulatory elements rather than a direct effect of the shRNA on mRNA, because GATA-2 overexpression also affected GATA-1 expression.
Despite the confounding effect of variations in GATA-1 expression, two important conclusions can be drawn from our knockdown experiments. First, decreases in GATA-2 expression were always associated with diminished CFU-Mk activity, even when overall GATA-2 levels remained above the level in WT bone marrow. Although GATA-1 levels were also decreased in GATA-2 shRNA-infected cells, the fact that reductions in GATA-1 activity were associated with increased CFU-MK activity argues that the reduction in colony formation is the direct result of downregulation of GATA-2. Second, in contrast to the consistent effect of GATA-2 downregulation on CFU-MK activity, reductions in GATA-2 expression led to diminished expansion of megakaryocytes in liquid culture only when the level of GATA-2 expression was brought below those seen in WT counterparts. These results indicate that megakaryocyte CFU progenitors are more reliant on GATA-2 expression compared to the more mature megakaryocytes evaluated in liquid culture assays.
GATA-2 reinforces megakaryocyte identity.
A notable phenotype that we observed in our studies was that the GATA-2 knockdown impaired megakaryopoiesis of both WT and G1SKI cells. A reduction in GATA-2 expression significantly impaired colony-forming activity of primary cells and caused proliferation arrest of megakaryocytes. On the other hand, ectopic expression of GATA-2 facilitated megakaryocyte differentiation. These findings provide evidence that overexpression of GATA-2 likely contributes to expansion of immature megakaryocytes in the G1SKI fetal liver as well as the proliferation of megakaryoblasts in DS-AMKL. GATA-2 may accomplish this, in part, by antagonizing the adverse effect of other lineage transcription factors, as evidenced by an upregulation of granulocyte/monocyte genes, including Cebpa, Sfpi1, and Hhex, in the GATA-2 knockdown population. Consistent with this gene expression profile, we observed a decreased number of CFU total (Fig. 3E and 4E), likely due to the compromised myelopoiesis caused by ectopic expression of GATA-2. Such an inhibitory effect of GATA-2 on myelopoiesis has been previously suggested (26). Further evidence to link GATA-2 overexpression with excessive proliferation of megakaryocytes comes from our discovery that G1SKI mice did not show elevated levels of GATA-2 in the bone marrow, consistent with the absence of hyperproliferation at that stage of development. The cause of the difference in GATA-2 expression between fetal liver and bone marrow progenitors of G1KI mice is unclear. A recent report comparing gene expression between these two types of megakaryocyte progenitors identified a striking upregulation of alpha interferon signaling in bone marrow relative to fetal liver megakaryocyte progenitors (42). It is interesting to speculate that this difference may contribute to altered regulation of the GATA-2 gene.
To date, only a few direct GATA-2 target genes have been described, with the most prominent of these being GATA2 itself. Apart from the GATA2 gene, our ChIP studies revealed that GATA-2 binds to conserved regulatory regions of Zfpm1, Sfpi1, Slamf1, and Hhex. Although direct regulation of Zfpm1 by GATA-2 has not been reported, the decrease in Zfpm1 expression seen in GATA-2 knockdown cells is consistent with an activating role for GATA-2 on this gene. In the megakaryocyte lineage, FOG-1 plays a key role in early development as well as in terminal differentiation through its association with both GATA-1 and GATA-2 (5). In contrast, FOG1 antagonizes the development of mast cells (4, 34), which depend upon GATA-2 for their development. Thus, as for GATA-2 in erythroid/megakaryocyte lineages, the correlation between chromatin binding and transcription is cell type dependent. Another class of genes that were affected by the reduction in GATA-2 expression included the myeloid genes Cebpa, Sfpi1, and Mpo. These observations are consistent with previous findings that GATA-2 inhibits myelopoiesis (26). Finally, we identified Hhex, Slamf1, and Lmo2 as novel, direct GATA-2 target genes. Hhex has been implicated in the early stages of hematopoiesis and monocyte differentiation (16, 19). Dysregulation of Hhex due to translocation has also been identified in leukemogenesis (14). Slamf1 has been associated with hematopoietic stem cells with unknown function in HSCs (17). Although the roles of these genes in megakaryopoiesis remain unclear, future studies on their function will shed light on how GATA-2 coordinates proliferation and differentiation of this lineage. Surprisingly, we failed to detect a GATA-2 binding site in a conserved GATA-binding region in the Cebpa gene locus, although downregulation of Cebpa partially reversed the proliferative defect of GATA-2 knockdown. Future studies will investigate the specific mechanisms by which GATA-2 controls expression of Cebpa.
GATA-2 indirectly regulates megakaryocyte progenitor proliferation.
Although studies have shown that transcription factors regulate expression of lineage-specific genes during hematopoietic cell differentiation, there are few examples of such factors directly affecting cell cycle progress. One recent study showed that the ETS protein GABPα directly regulates expression of Skp2, a key mediator of the G1-to-S transition (43). Ectopic expression of its target gene Skp2 partially rescued the S-phase defect of Gabpa-deficient fibroblasts. Due to the similarity between the cell cycle phenotypes in the GATA-2 knockdown G1ME cells and Gabpa-deficient mouse embryonic fibroblasts, we predicted that ectopic Skp2 expression would rescue the GATA-2 proliferation defect. However, overexpression of Skp2 failed to rescue this deficiency in GATA-2 knockdown cells (see Fig. S1 in the supplemental material). We also failed to detect enrichment of GATA-2 on conserved regulatory regions of the Skp2 gene by ChIP and could not identify conserved GATA-binding motifs within the loci of p21 and p27, two genes whose expression was also altered by the GATA-2 knockdown (data not shown).
An alternative hypothesis is that GATA-2 indirectly regulates cell cycle progression, such as by repressing expression of other lineage-specific transcription factors. Indeed, we found that knockdown of GATA-2 resulted in a striking upregulation of myeloid transcription factors PU.1 and C/EBPα. Consistent with this hypothesis, C/EBPα is known to control cell cycle progression through several mechanisms, including stabilization of p21 (30). Thus, loss of GATA-2 results in a partial myeloid gene expression program and cell cycle arrest. We confirmed that this is the likely mechanism by showing that ectopic expression of PU.1 or C/EBPα in G1ME cells led to cell cycle arrest and expression of myeloid-specific genes, while knockdown of PU.1 or C/EBPα partially reversed the phenotype of GATA-2 knockdown. Taken together these findings suggest that GATA-2 regulates S-phase progression indirectly through inhibiting C/EBPα and PU.1.
Supplementary Material
Acknowledgments
We thank the Robert H. Lurie Comprehensive Cancer Center Flow Cytometry and Genomics Core Facilities for technical support, Mitchell Weiss for reviewing the manuscript, and Alex Minella for helpful discussions. We also thank John Rossi for the Banshee vector.
This research was funded by a grant from the National Cancer Institute (R01 CA101774), by the Chicago Center for Systems Biology (P50 GM081892), and by the Chicago Biomedical Consortium with support from the Searle Funds at the Chicago Community Trust. J.D.C. is a Scholar of the Leukemia and Lymphoma Society.
Footnotes
Published ahead of print on 20 July 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Bourquin, J. P., A. Subramanian, C. Langebrake, D. Reinhardt, O. Bernard, P. Ballerini, A. Baruchel, H. Cave, N. Dastugue, H. Hasle, G. L. Kaspers, M. Lessard, L. Michaux, P. Vyas, E. van Wering, C. M. Zwaan, T. R. Golub, and S. H. Orkin. 2006. Identification of distinct molecular phenotypes in acute megakaryoblastic leukemia by gene expression profiling. Proc. Natl. Acad. Sci. USA 1033339-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Briegel, K., K. C. Lim, C. Plank, H. Beug, J. D. Engel, and M. Zenke. 1993. Ectopic expression of a conditional GATA-2/estrogen receptor chimera arrests erythroid differentiation in a hormone-dependent manner. Genes Dev. 71097-1109. [DOI] [PubMed] [Google Scholar]
- 3.Cantor, A. B. 2005. GATA transcription factors in hematologic disease. Int. J. Hematol. 81378-384. [DOI] [PubMed] [Google Scholar]
- 4.Cantor, A. B., H. Iwasaki, Y. Arinobu, T. B. Moran, H. Shigematsu, M. R. Sullivan, K. Akashi, and S. H. Orkin. 2008. Antagonism of FOG-1 and GATA factors in fate choice for the mast cell lineage. J. Exp. Med. 205611-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, A. N., A. B. Cantor, Y. Fujiwara, M. B. Lodish, S. Droho, J. D. Crispino, and S. H. Orkin. 2002. GATA-factor dependence of the multitype zinc-finger protein FOG-1 for its essential role in megakaryopoiesis. Proc. Natl. Acad. Sci. USA 999237-9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du, P., W. A. Kibbe, and S. M. Lin. 2007. nuID: a universal naming scheme of oligonucleotides for Illumina, Affymetrix, and other microarrays. Biol. Direct 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elnitski, L., R. C. Hardison, J. Li, S. Yang, D. Kolbe, P. Eswara, M. J. O'Connor, S. Schwartz, W. Miller, and F. Chiaromonte. 2003. Distinguishing regulatory DNA from neutral sites. Genome Res. 1364-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ezoe, S., I. Matsumura, S. Nakata, K. Gale, K. Ishihara, N. Minegishi, T. Machii, T. Kitamura, M. Yamamoto, T. Enver, and Y. Kanakura. 2002. GATA-2/estrogen receptor chimera regulates cytokine-dependent growth of hematopoietic cells through accumulation of p21WAF1 and p27Kip1 proteins. Blood 1003512-3520. [DOI] [PubMed] [Google Scholar]
- 9.Ezoe, S., I. Matsumura, Y. Satoh, H. Tanaka, and Y. Kanakura. 2004. Cell cycle regulation in hematopoietic stem/progenitor cells. Cell Cycle 3314-318. [PubMed] [Google Scholar]
- 10.Fujiwara, Y., C. P. Browne, K. Cunniff, S. C. Goff, and S. H. Orkin. 1996. Arrested development of embryonic red cell precursors in mouse embryos lacking transcription factor GATA-1. Proc. Natl. Acad. Sci. USA 9312355-12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grass, J. A., M. E. Boyer, S. Pal, J. Wu, M. J. Weiss, and E. H. Bresnick. 2003. GATA-1-dependent transcriptional repression of GATA-2 via disruption of positive autoregulation and domain-wide chromatin remodeling. Proc. Natl. Acad. Sci. USA 1008811-8816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harigae, H. 2006. GATA transcription factors and hematological diseases. Tohoku J. Exp. Med. 2101-9. [DOI] [PubMed] [Google Scholar]
- 13.Ikonomi, P., C. E. Rivera, M. Riordan, G. Washington, A. N. Schechter, and C. T. Noguchi. 2000. Overexpression of GATA-2 inhibits erythroid and promotes megakaryocyte differentiation. Exp. Hematol. 281423-1431. [DOI] [PubMed] [Google Scholar]
- 14.Jankovic, D., P. Gorello, T. Liu, S. Ehret, R. La Starza, C. Desjobert, F. Baty, M. Brutsche, P. S. Jayaraman, A. Santoro, C. Mecucci, and J. Schwaller. 2008. Leukemogenic mechanisms and targets of a NUP98/HHEX fusion in acute myeloid leukemia. Blood 1115672-5682. [DOI] [PubMed] [Google Scholar]
- 15.Jing, H., C. R. Vakoc, L. Ying, S. Mandat, H. Wang, X. Zheng, and G. A. Blobel. 2008. Exchange of GATA factors mediates transitions in looped chromatin organization at a developmentally regulated gene locus. Mol. Cell 29232-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keng, V. W., H. Yagi, M. Ikawa, T. Nagano, Z. Myint, K. Yamada, T. Tanaka, A. Sato, I. Muramatsu, M. Okabe, M. Sato, and T. Noguchi. 2000. Homeobox gene Hex is essential for onset of mouse embryonic liver development and differentiation of the monocyte lineage. Biochem. Biophys. Res. Commun. 2761155-1161. [DOI] [PubMed] [Google Scholar]
- 17.Kiel, M. J., O. H. Yilmaz, T. Iwashita, O. H. Yilmaz, C. Terhorst, and S. J. Morrison. 2005. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 1211109-1121. [DOI] [PubMed] [Google Scholar]
- 18.Kitajima, K., M. Tanaka, J. Zheng, H. Yen, A. Sato, D. Sugiyama, H. Umehara, E. Sakai, and T. Nakano. 2006. Redirecting differentiation of hematopoietic progenitors by a transcription factor, GATA-2. Blood 1071857-1863. [DOI] [PubMed] [Google Scholar]
- 19.Kubo, A., V. Chen, M. Kennedy, E. Zahradka, G. Q. Daley, and G. Keller. 2005. The homeobox gene HEX regulates proliferation and differentiation of hemangioblasts and endothelial cells during ES cell differentiation. Blood 1054590-4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li, Z., F. J. Godinho, J. H. Klusmann, M. Garriga-Canut, C. Yu, and S. H. Orkin. 2005. Developmental stage-selective effect of somatically mutated leukemogenic transcription factor GATA1. Nat. Genet. 37613-619. [DOI] [PubMed] [Google Scholar]
- 21.Lin, S. M., P. Du, W. Huber, and W. A. Kibbe. 2008. Model-based variance-stabilizing transformation for Illumina microarray data. Nucleic Acids Res. 36e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ling, K. W., K. Ottersbach, J. P. van Hamburg, A. Oziemlak, F. Y. Tsai, S. H. Orkin, R. Ploemacher, R. W. Hendriks, and E. Dzierzak. 2004. GATA-2 plays two functionally distinct roles during the ontogeny of hematopoietic stem cells. J. Exp. Med. 200871-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lulli, V., P. Romania, O. Morsilli, M. Gabbianelli, A. Pagliuca, S. Mazzeo, U. Testa, C. Peschle, and G. Marziali. 2006. Overexpression of Ets-1 in human hematopoietic progenitor cells blocks erythroid and promotes megakaryocytic differentiation. Cell Death Differ. 131064-1074. [DOI] [PubMed] [Google Scholar]
- 24.Mundschau, G., S. Gurbuxani, A. S. Gamis, M. E. Greene, R. J. Arceci, and J. D. Crispino. 2003. Mutagenesis of GATA1 is an initiating event in Down syndrome leukemogenesis. Blood 1014298-4300. [DOI] [PubMed] [Google Scholar]
- 25.Muntean, A. G., and J. D. Crispino. 2005. Differential requirements for the activation domain and FOG-interaction surface of GATA-1 in megakaryocyte gene expression and development. Blood 1061223-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Persons, D. A., J. A. Allay, E. R. Allay, R. A. Ashmun, D. Orlic, S. M. Jane, J. M. Cunningham, and A. W. Nienhuis. 1999. Enforced expression of the GATA-2 transcription factor blocks normal hematopoiesis. Blood 93488-499. [PubMed] [Google Scholar]
- 27.Pevny, L., C. S. Lin, V. D'Agati, M. C. Simon, S. H. Orkin, and F. Costantini. 1995. Development of hematopoietic cells lacking transcription factor GATA-1. Development 121163-172. [DOI] [PubMed] [Google Scholar]
- 28.Pevny, L., M. C. Simon, E. Robertson, W. H. Klein, S. F. Tsai, V. D'Agati, S. H. Orkin, and F. Costantini. 1991. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature 349257-260. [DOI] [PubMed] [Google Scholar]
- 29.Rodrigues, N. P., V. Janzen, R. Forkert, D. M. Dombkowski, A. S. Boyd, S. H. Orkin, T. Enver, P. Vyas, and D. T. Scadden. 2005. Haploinsufficiency of GATA-2 perturbs adult hematopoietic stem-cell homeostasis. Blood 106477-484. [DOI] [PubMed] [Google Scholar]
- 30.Schuster, M. B., and B. T. Porse. 2006. C/EBPα: a tumour suppressor in multiple tissues? Biochim. Biophys. Acta 176688-103. [DOI] [PubMed] [Google Scholar]
- 31.Shivdasani, R. A., Y. Fujiwara, M. A. McDevitt, and S. H. Orkin. 1997. A lineage-selective knockout establishes the critical role of transcription factor GATA-1 in megakaryocyte growth and platelet development. EMBO J. 163965-3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smyth, G. K. 2004. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3Article3. [DOI] [PubMed] [Google Scholar]
- 33.Stachura, D. L., S. T. Chou, and M. J. Weiss. 2006. Early block to erythromegakaryocytic development conferred by loss of transcription factor GATA-1. Blood 10787-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugiyama, D., M. Tanaka, K. Kitajima, J. Zheng, H. Yen, T. Murotani, A. Yamatodani, and T. Nakano. 2008. Differential context-dependent effects of friend of GATA-1 (FOG-1) on mast-cell development and differentiation. Blood 1111924-1932. [DOI] [PubMed] [Google Scholar]
- 35.Terui, K., Y. Takahashi, J. Kitazawa, T. Toki, M. Yokoyama, and E. Ito. 2000. Expression of transcription factors during megakaryocytic differentiation of CD34+ cells from human cord blood induced by thrombopoietin. Tohoku J. Exp. Med. 192259-273. [DOI] [PubMed] [Google Scholar]
- 36.Tsai, F. Y., G. Keller, F. C. Kuo, M. Weiss, J. Chen, M. Rosenblatt, F. W. Alt, and S. H. Orkin. 1994. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature 371221-226. [DOI] [PubMed] [Google Scholar]
- 37.Tsai, F. Y., and S. H. Orkin. 1997. Transcription factor GATA-2 is required for proliferation/survival of early hematopoietic cells and mast cell formation, but not for erythroid and myeloid terminal differentiation. Blood 893636-3643. [PubMed] [Google Scholar]
- 38.Vannucchi, A. M., L. Bianchi, C. Cellai, F. Paoletti, R. A. Rana, R. Lorenzini, G. Migliaccio, and A. R. Migliaccio. 2002. Development of myelofibrosis in mice genetically impaired for GATA-1 expression (GATA-1low mice). Blood 1001123-1132. [DOI] [PubMed] [Google Scholar]
- 39.Vyas, P., K. Ault, C. W. Jackson, S. H. Orkin, and R. A. Shivdasani. 1999. Consequences of GATA-1 deficiency in megakaryocytes and platelets. Blood 932867-2875. [PubMed] [Google Scholar]
- 40.Wang, H., Y. Zhang, Y. Cheng, Y. Zhou, D. C. King, J. Taylor, F. Chiaromonte, J. Kasturi, H. Petrykowska, B. Gibb, C. Dorman, W. Miller, L. C. Dore, J. Welch, M. J. Weiss, and R. C. Hardison. 2006. Experimental validation of predicted mammalian erythroid cis-regulatory modules. Genome Res. 161480-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wechsler, J., M. Greene, M. A. McDevitt, J. Anastasi, J. E. Karp, M. M. Le Beau, and J. D. Crispino. 2002. Acquired mutations in GATA1 in the megakaryoblastic leukemia of Down syndrome. Nat. Genet. 32148-152. [DOI] [PubMed] [Google Scholar]
- 42.Wieland, K., and A. B. Cantor. 2008. Gene expression differences between fetal liver-derived and adult bone marrow-derived murine megakaryocyte progenitors: implications for DS-TMD. Blood 112440. [Google Scholar]
- 43.Yang, Z. F., S. Mott, and A. G. Rosmarin. 2007. The Ets transcription factor GABP is required for cell-cycle progression. Nat. Cell Biol. 9339-346. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.