Abstract
Vaccinia virus, a large double-stranded DNA virus, is the prototype of the Orthopoxvirus genus, which includes several pathogenic poxviruses of humans, such as monkeypox virus and variola virus. Here, we report a comprehensive yeast two-hybrid (Y2H) screening for the protein−protein interactions between vaccinia and human proteins. A total of 109 novel vaccinia−human protein interactions were detected among 33 viral proteins. To validate subsets of those interactions, we constructed an ORFeome library of vaccinia virus strain WR using the Gateway plasmid cloning system. By co-expressing selected vaccinia and host proteins in a variety of expression systems, we found that at least 17 of the Y2H hits identified between vaccinia and human proteins can be verified by independent methods using GST pull-down assays, representing a 63% validation rate for the Y2H hits examined (17/27). Because the cloned ORFs are conveniently transferable from the entry vectors to various destination expression vectors, the vaccinia ORFeome library will be a useful resource for future high-throughput functional proteomic experiments.
Keywords: Protein−protein interaction, yeast two hybrid, vaccinia virus, ORFeome
Short abstract
A comprehensive yeast two-hybrid screen between vaccinia virus proteins and human proteins is reported. This screen identified 109 hits involving 33 vaccinia proteins. A vaccinia virus ORFeome library was constructed through Gateway cloning system and used for the validation of selected interactions. At least 17 from 27 examined vaccinia−human hits were confirmed to be positive by GST-pulldown assays. Some of the interesting interactions are discussed.
Introduction
In the postgenomic era, DNA sequencing databases are exponentially accumulating and providing a vast opportunity to construct representative libraries of cloned open reading frames (ORFs), or ‘ORFeomes’, to enable high-throughput ‘omics’ applications.(1) For larger genomes that comprise hundreds or thousands of ORFs, it is time-consuming to construct the ORFeome library through the conventional cloning method using restriction endonucleases. In contrast, the Gateway site-specific recombinational cloning system is a powerful tool, and many ORFeomes, including human,(2)Caenorhabditis elegans(3) and some bacterial,4,5 have been generated using this methodology.
Virus members from the Poxviridae family all possess a genome of double-stranded DNA containing about 150−300 ORFs.(6) For example, Variola virus from the Orthopoxvirus genus is a lethal human-specific pathogen that causes smallpox, probably the most lethal viral pathogen of man in history. Although smallpox was successfully eradicated as an extant human disease by the worldwide vaccination campaigns over 30 years ago, the concern for variola virus as a potential bioweapon, as well as the potential emergence of related viruses such as monkeypox, has renewed interest in the fundamental mechanisms of orthopoxvirus pathogenesis.7,8 However, direct studies of variola virus virulence in nonhuman primate models are exceedingly difficult,(9) and monkeypox pathogenesis studies can be very laborious and expensive.(10) Nevertheless, current proteomic technologies permit researchers to extract important clues about virus−host interaction dynamics by examination of the protein−protein interactions between a virus and its host. Such studies begin with the complete genomic database of the virus (e.g., variola virus) and its host (e.g., humans).
The number of completely sequenced poxvirus genomes now stands at over 100 (http://www.poxvirus.org/viruses.asp). Most high-throughput proteomic studies in the past have been performed with vaccinia virus, the prototype orthopoxvirus, and the vaccine strain used to eradicate smallpox. For example, 37 interactions between sets of vaccinia proteins have been identified with yeast two-hybrid (Y2H) assays.(11) In the case of the variola ORFs that are not represented in the vaccinia virus genome, several unique Y2H interaction hits with human proteins have been detected, and a subset of these (e.g., variola G1R with human NFkB1 and Skp1) have been validated recently.(12)
In addition, in vivo expression of entire poxviral proteomes has also been carried out and many vaccinia proteins have been expressed in Escherichia coli or baculovirus vectors.(13) However, a vaccinia entry ORFeome platform that is easily convertible to different destination expression vectors has not been previously reported. Poxviruses replicate extensively in the host cytoplasm and have multiple strategies to manipulate a wide spectrum of host cell pathways throughout the life cycle of the virus from the entry to the exit.(14) These targeted pathways include innate signaling circuits such as apoptosis, antiviral responses, and the ubiquitin system.14−18 Elucidation of the pathogenic mechanisms of poxviruses and specific host−pathogen interactions require functional analysis of how elements from both viruses and eukaryotic hosts intersect. Many groups have exploited Y2H screening to identify novel interactions between viral proteins and human proteins. However, most studies in the past usually focus on one viral protein at a time.19−21
Here, we report more than 100 novel interactions between proteins encoded by vaccinia virus (WR strain) and humans, as identified by directed Y2H screening. To assess how many of these Y2H hits could be validated by another independent technology, we have selected a subset of the vaccinia−human partners and expressed the tagged proteins in bacteria, transfected human cells, or in reticulocyte lysates. Then, GST-pulldown assays were utilized to assess which binding partners defined by Y2H analysis could be validated by an alternative technology. As part of this validation, we constructed a composite vaccinia ORFeome library in Gateway entry plasmid vectors and then cassetted the query viral ORFs into Gateway expression vectors suitable for expression and GST-pulldowns. This vaccinia entry library permits viral ORFs to be easily shuttled into other destination expression vectors, and should provide a useful platform for systematic analysis of the molecular mechanisms that enable vaccinia to replicate successfully within its eukaryotic host. The Y2H results provide key insights for the potential functions of vaccinia proteins to modulate host cells during infection. It will also contribute to a better understanding of host interaction dynamics of related orthopoxviruses such as monkeypox and smallpox. However, before Y2H results can be exploited, it is important to validate potential protein−protein hits in independent expression systems, such as we describe in this report.
Materials and Methods
Reagents
The vaccinia WR genomic DNA was a kind gift of the laboratory of Dr. Richard Condit. Host gene constructs are from Harvard Institute of Proteomics (HIP) or Open Biosystems. Mouse anti-HA, mouse anti-myc and rabbit anti-HA were obtained from Roche, Invitrogen, and Neomarkers.
Primer Design
All of the vaccinia ORFs were cloned as open cassettes, with the terminator codon removed so as to permit expression of C-tagged viral proteins. The primers in first round PCR were designed in the following strategy. The attB1 plus Kozak segment (5-AAAGCAGGCTCCACC-3) was added before the start codon of each forward primer, followed by ORF-specific bases (see below). The attB2 segment (5-ACAAGAAAGCTGGGTCCAA-3) was added at the 5′-end of each reverse primer, which was complementary to the end of the ORF, without the last nucleotide of the stop codon, as previously described. The size (typically 15−27 bases) of the forward and reverse primer parts that were complementary to the ORF were determined to give a similar annealing temperature during the PCR, usually in the range of 60−70 °C. The primers used in second round PCR were the universal primers GM82 (5′GGGGACCACTTTGTACAAGAAAGCTGG GTC) and GM81 (5′GGGGACAAGTTTGTACAAAAAAGCAGGCTCC). All primers were obtained from Invitrogen.
PCR Amplification of the ORFs
Each PCR reaction was performed in a 50-μL volume, with 1 unit of Platinum HiFi polymerase (Invitrogen), MgSO4 2 mM, dNTP mix (0.2 mM each), forward and reverse primers (0.125 μM each), and genomic DNA (75 ng). Thirty PCR cycles (94 °C for 45 s, 55 °C for 1 min, and 68 °C for 1 min/kb) were preceded by heating to 94 °C for 2 min, and were followed by 5-min incubation at 68 °C. Confirmation and size of the PCR products was determined by agarose gel electrophoresis.
BP Cloning Reaction of the Amplified ORFs
The BP reactions were performed in a final volume of 5 μL containing 1 μL of unpurified PCR product, 75 ng of pDONR221 or pDNOR222 plasmid (from Invitrogen), and 1 μL of BP clonase enzyme. The BP reactions were incubated for 2 h at 25 °C and used directly for bacterial transformation.
Transformation
Individual bacterial transformations were carried out using 20 μL of transformation-ready Mach One cells, 5 μL of the BP reaction products, and 200 μL of SOC medium. After the transformation reaction, E. coli transformants were transferred to LB plates containing 25 μg/mL kanamycin.
Test of the Entry Clones
To identify and confirm ORF entry clones, PCR was performed, followed by sequencing of the plasmid. The PCR was performed as described above, except that 1 μL of the plasmid preparation was used as template and universal primers GM81 and GM82 were used. The M13F and M13R primers were used for sequencing. For those ORFs longer than 2 kb, one or more internal ORF-specific primers were used for sequencing.
Yeast Two-Hybrid (Y2H) Screening
Y2H screening was carried out at Myriad Genetics (Salt Lake City, UT), similar to that described for the unique open reading frames from variola virus.(12) In brief, all vaccinia ORFs were cloned into the DNA-binding domain vector, pGBT.superB, creating an ORF for each of the vaccinia virus genes fused to the C-terminus of the GAL4 DNA-binding domain (residues 1−147). Each of the bait plasmids was introduced into Myriad’s ProNet yeast strain PNY200 (MATα ura3-52 ade2-101 trp1-901 his3-Δ200 leu2-3,112 gal4Δ gal80Δ). Expression of individual vaccinia baits was not confirmed for all constructs, and thus some potential vaccinia−human interactions may have been missed as false negatives. The bait yeast cells were allowed to mate with Myriad’s ProNet MATa yeast cells, BK100 (MATa ura3-52 trp1-901 his3-Δ200 leu2-3,112 gal4Δ gal80Δ GAL2-ADE2 LYS2::GAL1-HIS3 met2::GAL7-lacZ) containing four independent cDNA libraries (prey libraries) prepared from human tissues, cancer cell lines, or a collection of clones [human tongue/tonsil, human spleen, a combination of breast tumor and prostate tumor cell lines, and the NIH sponsored Mammalian Gene Collection (MGC, http://mgc.nci.nih.gov), respectively], fused to the C-terminus of the Gal4 activation domain (residues 768−881) of the activation domain vector, pGAD.PN2. Each library was generated from random primed, directionally cloned cDNA which was typically composed of over 5 million independent clones with an average fragment size of 600−900 base pairs. Yeast transformants positive for prey−bait interaction were selected on SD-Ade/-His/-Leu/-Trp plate, and plasmid DNA isolated from positive clones was sequenced.
GST Pulldown Assay
Purification of GST and GST fusion proteins from bacteria has been previously described.(22) Protein expression by TNT system follows the protocol recommended by the supplier (Promega). For GST-pulldown assays, all incubations were performed at 4 °C for 1 h in PBS. After the incubations, glutathione-sepharose beads (Amersham Biosciences, Piscataway, NJ) were washed three times with the incubation buffer and then analyzed by SDS-PAGE followed by Western blotting using chemiluminescence (Millipore, Billerica, MA).
Results and Discussion
Genome-Wide Yeast Two-Hybrid Screen for the Protein−Protein Interactions between Vaccinia Virus and Human Proteins
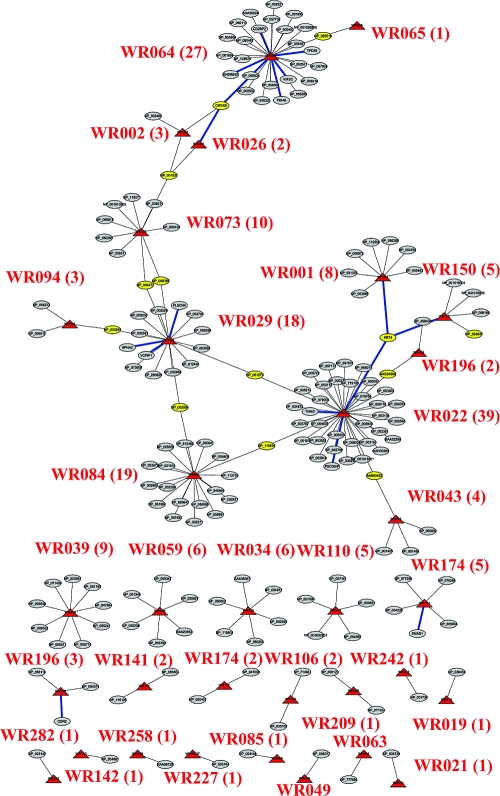
Vaccinia virus replication clearly requires a complex protein interaction network to be established between virus and host. Identification and characterization of these protein−protein interactions should provide a wealth of novel insights into the biology of poxviruses in general. To identify all possible interactions occurring between vaccinia virus and human proteins, a directed Y2H screening was carried out. These assays were conducted using four independent cDNA libraries prepared from various human tissues, cancer cell lines, or a collection of clones (human tongue/tonsil, human spleen, a combination of breast tumor and prostate tumor cell lines, and the NIH sponsored Mammalian Gene Collection (MGC, http://mgc.nci.nih.gov), respectively). Of the 284 vaccinia ORFs examined for human binding partners by Y2H screening, 33 vaccinia proteins revealed 109 new interactions with known human proteins (Figure 1). The detailed interaction data is listed in Table S1 (and is replicated at www.poxvirus.org).
Figure 1.
Y2H interaction networks between vaccinia virus and human proteins. The interactions between vaccinia and human proteins are based on Y2H screening results described in Table S1. Viral proteins are labeled by the red triangles. Human proteins are labeled in elliptical cycles. The host proteins with more than one viral binding partner are indicated in yellow. The blue lines are validated interactions in this paper. The name of vaccinia proteins are labeled in red with the numbers of binding partners inside the bracket.
For the Y2H screening, each vaccinia ORF was baited as a full-length protein, and only when it was too large to be expressed (as judged by our experience), it was divided into overlapping baits. There was no effort to remove membrane anchored tails or any other domains. The vaccinia bait constructs were all verified by sequence analysis. Since the bait protein expression was not tested in yeast, it is possible that some subclasses of interacting partners were missed. Consequently, the percentage of bait proteins that yielded no hits because they were not expressed in yeast cannot be estimated. Since each bait plasmid in the yeast was positively selected by virtue of expressing nutritional markers, we can verify that the selectable marker was expressed in each plasmid.
From these studies, 12% of Y2H-expressed vaccinia proteins revealed novel host protein−protein interactions, in which the signal/noise criteria for “hits” were defined very stringently. Among the viral proteins that exhibited human protein interactions, four vaccinia proteins WR022 (COP-C6), WR029 (COP-N2), WR064 (COP-E8), and WR084 (COP-G6) each exhibited more than 10 hits. Thirty-nine human proteins were found as the potential binding partners for one relatively unexplored viral ORF, WR022 (COP-C6), which suggests that this protein is unusually “sticky” or has particularly diverse functions in infected cells. Our validation of at least some of these interactions should provide unique clues to elucidate the role of WR022 (COP-C6).
The Y2H screening using a bait construct for WR064 (COP-E8) identified 27 candidate interacting human proteins. WR064 (COP-E8) was previously shown to be a membrane protein found in the virion and it is required for the formation of transcriptionally active virus cores.23,24 Nineteen human proteins associated with WR084 (COP-G6) in the Y2H screening. WR084 (COP-G6) belongs to NlpC/P60 superfamily and is proposed to be involved in multiple virus−host interactions.(25) Finally, we identified 18 novel candidate human proteins interacting with WR029 (COP-N2). WR029 (COP-N2) is an alpha-amanitin target but its biological function is not clear.(26)
Of the remaining vaccinia proteins with human interacting protein hits, 12 viral proteins exhibited 3−10 hits and 17 viral proteins had 1−2 hits. Note that some well-characterized vaccinia−host protein interactions were missing from the list. The Y2H technology identifies the protein−protein interactions between fusion proteins, both of which need to be faithfully expressed and capable of interaction in the yeast nucleus. Also, some proteins require specific conformation or post-translational modifications as a prerequisite to interact with their binding partners. These types of interactions would not be detected by Y2H assay (“false negatives”).
Other reasons to explain why Y2H screening would fail to identify a host interacting partner that has been identified by other means. For example, mutations can occur during the ORF cloning that can prevent or abolish the interaction between two proteins. One such example is the interaction between the human topoisomerase II and vaccinia WR176 (COP-A50), which can be prevented by mutating C11-to-Y at the N-terminus of the viral bait.(27) Second, the type of fusion construct used can affect the correct folding of the hybrid proteins. As another example of a likely false negative in this Y2H screen, the interaction between vaccinia WR040 (COP-F1) and human Bak has also been well-documented in vivo and the interaction was confirmed by co-immunoprecipitation.(28) In general, it was not possible to estimate what fraction of the recorded hits were “false positives”, nor how many vaccinia−human interactions simply could not be reproduced by the Y2H technology itself.
Nevertheless, despite these caveats, the Y2H assay is a versatile tool to detect potential virus−host protein interactions in vivo in a proteome-wide fashion. Because proteins usually assemble into complexes to perform different activities, validation and characterization of the interactions from this Y2H screening will contribute to the elucidation of which interacting partners will shed mechanist insights into poxvirus biology.
Construction of Vaccinia Virus ORFeome Library
A two-step PCR method was used in the vaccinia ORFs cloning such that the viral ORF is in the open configuration. To do so, the 3′-termination codon was removed to allow for sequence fusions with C-terminal tags in subsequent destination expression vectors. Primers for the first-round PCR were designed to generate a Gateway-compatible attB1 (forward primer) or attB2 (reverse primer) recombination site flanking the amplified ORF. Full-length attB sites at the 5′ and 3′ends were generated using universal adapter primers in the second round PCR. In cases where the production of C-terminal fusion proteins is desired, which can be expressed from appropriate destination vectors, the stop codon of each ORF was removed in the reverse primers.
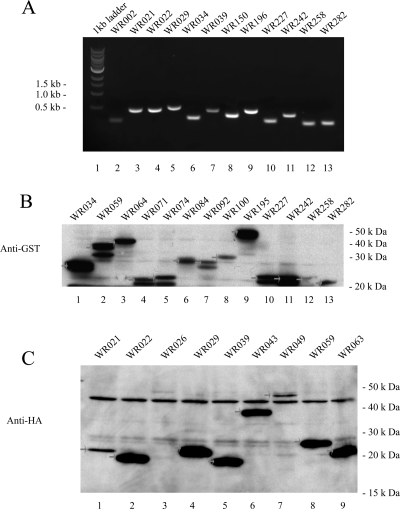
Vaccinia virus WR strain contains 284 predicted ORFs, of which 18 ORFs are duplicated within the terminal inverted repeat sequences of the viral genome. All the unique 266 ORF products could be amplified by PCR and were detected by SYBR staining. Figure 2A is a representative electrophoretic analysis of first-round PCR products. Following second-round PCR with universal primers, the products were ready for insertion into the Gateway entry vectors.
Figure 2.
Representative results of first-round PCR products and protein expression in destination vectors. (A) Electrophoretic analysis of PCR products. The first-round PCR products were analyzed on SYBR-stained gels. (B) Western analysis of viral expression in pANT7_cGST by TNT System using an antibody against GST. All GST-fusion proteins are expressed at the appropriate molecular weight (shown by an arrow). (C) Western analysis of viral expression in pANT7_n-HA by TNT System using an antibody against HA. All HA-fusion proteins except WR026 are expressed at the appropriate molecular mass (shown by an arrow).
The PCR products were next inserted into entry vectors like pDONR222 or pDONR221 by BP Clonase recombination. The products resulting from site-specific recombination were transformed into transfection-sensitized One Shot Mach One bacterial cells. A portion of the transformed cells was then plated on solid medium containing Kanamycin. Colonies from each plate were then tested in a colony-PCR with primer 81 and primer 82. The resulting PCR products were detected by gel electrophoresis and SYBR staining. Transformants that produced an insert of the expected size for the input ORF were selected and grown in liquid medium containing Kanamycin to generate bacterial glycerol stocks for long-term storage. By this approach, we successfully obtained 259 transformants carrying the anticipated vaccinia virus ORF insert within the entry plasmid. The single-colony glycerol stocks and the purified entry plasmid clones together constitute the composite ORFeome library of Vaccinia virus WR strain.
To verify that the inserted vaccinia ORFs were in the correct reading frame and indeed corresponded to the assigned identity, all the plasmids were sequenced with an M13 forward primer. For longer ORFs, M13 reverse primer or internal primers were needed to verify the ORF sequence. The summary of the vaccinia entry library is listed in Table 1. Our entry library successfully covers 97.4% of the predicted vaccinia strain WR ORFeome. A total of 202 vaccinia ORFs were found to be correctly cassettted within the entry vector. Forty-one ORFs contained a single amino acid (A.A.) change from the WR sequence reported and 16 ORFs contained 2 A.A. or more changes. Seven vaccinia ORFs (WR002, WR019, WR065, WR073, WR098, WR110 and WR176) were difficult to clone into entry vectors and remain uncloned.
Table 1. Summary of Vaccinia ORFeom Entry Vector Library.
| ORF | correct | 1 A.A. mutation | 2 A.A. and more mutations | correct and minimal mutations | difficult to clone | total |
|---|---|---|---|---|---|---|
| Number | 202 | 41 | 16 | 259 | 7 | 266 |
| percentage | 75.9% | 15.4% | 6.0% | 97.4% | 2.6% | 100% |
Expression of Recombinant Tagged Proteins from Different Expression Vectors
Different entry clones were each subjected to a recombination reaction, the LR-Reaction, which recombines the attL-sites on the entry clones with the attR-sites on the destination vectors. Entry vectors were shuttled into the pANT7_nHA vector (HIP) designed to make a fusion protein with an N-terminal HA-Tag, or with the pANT7_cGST vector (HIP), creating fusion proteins with a C-terminal GST-Tag. The resulting products were in vitro transcribed and translated with the TNT Coupled Transcription/Translation System from Promega and 2 μL of total products were analyzed by Western blot with anti-HA or anti-GST antibodies. Protein expression was detected for all GST-tagged vectors and most of the HA-tagged vectors. Representative Western blot results are shown in Figure 2B,C.
There are many valuable applications for the cloned vaccinia ORFeome library. The validation of protein−protein interactions detected from Y2H screening can be facilitated by shuttling the candidate ORFs from entry vectors into various expression vectors. For example, viral proteins with fluorescent protein tags can be generated to investigate viral proteins localization. Further application of the vaccinia ORFeome will be high-throughput recombinant proteins expression with different tags in different expression systems. The vaccinia ORFeome can also help construct DNA microarrays by amplification of the ORFs with a single pair of primers.
Validation the Protein−Protein Interactions in Vitro Using the Vaccinia Virus ORFeome
To validate the tentative protein interaction hit results obtained from Y2H screening, we primarily used pulldown assays using GST conjugated beads to precipitate GST-tagged proteins, which are generated from bacteria, transfected mammalian cells, or TNT in vitro expression system. The GST pulldown was followed by SDS-PAGE and Western blot analysis to identify the viral or host-GST partner that has a different epitope tag, such as -HA, or Myc-His.
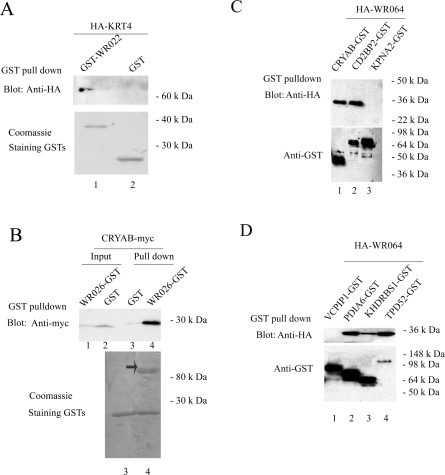
A total of 27 tentative Y2H interactions were chosen for validation, and of these, 17 proved to be positive by GST pulldown, which represents 63% of the selected Y2H hits (Table 2). The two main criteria used to select the chosen 27 Y2H interactions for validation were based on the following. First, the cloned human cDNA were readily available in Gateway vectors from HIP (Harvard Institute of Proteomics) or Open Biosystems. Second, when the levels of protein expression were determined following transfection into mammalian cells or in reticulocyte lysates, and those clones that expressed well were chosen for validation. Representative results of these validations are shown in Figure 3. Some of the more intriguing vaccinia−human protein interactions are discussed in detail below.
Table 2. Positive GST Pulldown Validation Results of Selective Y2H Hitsa.
| vaccinia WR protein (Copenhagen nomeclature) | vaccinia protein function | human protein |
|---|---|---|
| WR001 (COP-C23)* | Chemokine binding protein | KRT4* (Keratin) |
| WR022 (COP-C6)*,# | Unknown | KRT4* (Keratin 4) |
| PDCD6IP▲ (Programmed cell death 6 interacting protein) | ||
| TNNI2* (Troponin I, skeletal, fast) | ||
| WR026 (COP-C2)* | Kelch-like intracellular protein that influences the host response to VACV infection | CRYAB* (Crystallin alpha B) |
| WR029 (COP-N2)* | Unknown | KPNA2* (Karyopherin alpha 2) |
| PLSCR4* (Phospholipid scramblase 4) | ||
| VCPIP1* (Valosin containing protein (p97)/p47 complex) | ||
| WR064(COP-E8)*,▲ | Membrane protein may help wrap virosome; associates with IV/IMV and cores; F10L kinase substrate | CD2BP2* (CD2 antigen-binding protein 2) |
| CRYAB* (Crystallin alpha B) |
||
| KHDRBS1* (KH domain containing, RNA binding, signal transduction associated 1) |
||
| KIF2C* (Kinesin family member 2C) |
||
| PDIA6* (Protein disulfide isomerase-associated 6) |
||
| TPD52* (Tumor protein D52) |
||
| WR150 (COP-A27)* | IMV surface protein; roles in IMV-cell attachment, fusion, and microtubule transport | KRT4* (Keratin 4) |
| WR171 (COP-A45)* | Inactive Cu−Zn superoxide dismutase-like in virion | SMAD* [SMAD1 (MAD homologue 1)] |
| WR196 (COP-B14)* | IκB kinase inhibitor | CDR2* (Paraneoplastic cerebellar degeneration-associated antigen) |
WR, Western Reserve strain; COP, Copenhagen strain. Methods to produce recombinant proteins: *in vitro transcription/translation (TNT); ▲mammalian cell; #bacterial.
Figure 3.
Representative results of GST pulldown validation experiments. (A) WR022 (COP-C6) interacts with KRT4. WR022 (COP-C6) expressed as a GST fusion protein from bacteria was bound to beads and incubated with KRT4 expressed by TNT System for a pulldown experiment. KRT4 was detected by immunoblotting for HA, whereas GST proteins were detected by Coomassie staining. Star indicates the position of full-length GST-WR022 (COP-C6). (B) WR026 (COP-C2) interacts with CRYAB. Cell lysates from pANT7_cGST-WR026 (COP-C2) and pCDNA-CRYAB co-transfected, or from pCDNA-CRYAB transfected BSR-T7 cells were bound to beads and incubated for a pulldown experiment. CRYAB was detected by immunoblotting for myc, whereas GST proteins were detected by Coomassie staining. Arrow indicates the position of full-length GST-WR026 (COP-C2). (C) WR064 (COP-E8) interacts with CRYAB and CD2BP2. (D) WR064 (COP-E8) interacts with PDIA6, KHDRBS1 and TPD52. To generate (C) and (D), proteins were co-expressed in the TNT system as follows: plasmid pANT7_nHA-WR064, with pANT7_cGST-CRYAB, pANT7_cGST-CD2BP2, pANT7_cGST-KPNA2, pANT7_cGST-VCPIP1, pANT7_cGST-PDIA6, pANT7_cGST-KHDRBS1 or pANT7_cGST-TPD52. KPNA2 and VCPIP1were used as negative controls. On the basis of the Y2H screen, neither KPNA2 nor VCPIP1 interacts with WR064 (COP-E8). In addition, to our knowledge, there is not a report that suggests that a pair of proteins used as controls are interacting partners. Once expressed, recombinant proteins were bound to glutathione-sepharose beads for the pulldown experiment. WR064 (COP-E8) was detected by immunoblotting for HA, whereas GST fusion proteins were detected by immunoblotting for GST.
In the potential human binding partners identified in the Y2H screening, KPNA2 is noteworthy because it interacts with proteins from multiple pathogens, such as adenovirus, EBV, HIV, Papillomavirus and Sarcoma virus.(29) KPNA2 plays a key role in both the import and export of intracellular cargo across the nuclear membrane. WR029 (COP-N2), a validated Y2H partner for this cellular protein, is of unknown function, but it localizes to the host cell nucleus during VV infection.(26) Interactions between WR029 (COP-N2) and KPNA2 may provide clues as to the function of this viral protein in the nucleus. Another interesting result is the interaction between PLSC4 and WR029 (COP-N2). Phospholipid scramblases (PLSCRs) are a family of proteins involved in destroying membrane phospholipid asymmetry for diverse cellular events.(30) PLSCR4 is one of four members known in humans. Although a nuclear localization signal was identified in PLSCR4, a detailed mechanism of PLSCR4 still awaits a proper investigation. Interaction of WR029 (COP-N2) with PLSCR4 suggested that this viral protein might also have a role in the process of phospholipid translocation. Another WR029 (COP-N2) interaction partner, VCPIP1 (also named VCIP135), was identified as an essential factor for p97/p47-mediated membrane fusion, and dissociates it via p97 catalyzed ATP hydrolysis.(31) Reassembly of Golgi cisternae mediated by p97−p47 during mitotic requires the deubiquitinating activity of VCPIP1.(32) Future work needs to be done to test whether WR029 (COP-N2) actually plays a role in this process.
KRT4 was shown to interact with three different vaccinia proteins: WR001 (COP-C23), WR022 (COP-C6) and WR150 (COP-A27). KRT4 is one type of intermediate filaments and the interaction with IMV surface protein WR150 (COP-A27) may take part in IMV-cell attachment, fusion, and/or microtubule transport.
PDIA6 and CRYAB are human binding partners for WR064 (COP-E8). PDIA6 belongs to protein disulfide isomerase family, while CRYAB, small heat shock protein, has been shown to suppress the protein aggregation.(33) Considering WR064 (COP-E8) is a transmembrane protein component of the vaccinia virion, PDIA6 and CRYAB might facilitate proper protein folding for this viral protein.
TNNI2 was one binding partner identified for WR022 (COP-C6) in our validation. TNNI2 was suggested to be a novel co-activator of ERRα.(34) It will be interesting to investigate if WR022 (COP-C6) has a role in ERRα-mediated transcriptional activity.
SMAD1 is a key molecule in TGFβ/BMP signaling pathway.(35) WR171 (COP-A45) is a viral core protein and has no known role in virus replication or virulence.(36) The association of SMAD1 with WR171 (COP-A45) suggested the possibility that this vaccinia protein may be involved in TGFβ/BMP signaling pathway.
Ten Y2H interactions from our validation selection turned out to be negative by the GST-pulldown assay (Table 3). However, based on our validation methodology, we still cannot rule out the possibilities that such interactions do occur in virus-infected cells in vivo. Different reasons can justify negative results. For example, the position of the epitope tags at the N-terminus or C-terminus could be very critical in the sense that it might negatively interfere with the interaction per se. On the other hand, the TNT expression system used may not be the most efficient method to express certain viral or human proteins. Consequently, the low levels of expression or high levels of expression of particular partners can also lead to apparent negative results.
Table 3. Negative Validation Results of Selective Y2H Hitsa.
| vaccinia WR protein (Copenhagen nomenclature) | vaccinia protein function | human protein |
|---|---|---|
| WR022 (COP-C6)*,# | Unknown | AP2B1* (Adaptor-related protein complex 2, beta 1 subunit) |
| Exo7▲ (Exocyst complex component 7) | ||
| SLK* (STE20-like kinase) | ||
| WR029 (COP-N2)* | Unknown | KPNA5* (Karyopherin alpha 5) |
| PRDX4* (Peroxiredoxin 4) | ||
| WR034 (COP-K3)▲ | Interferon resistance and eIF2 alpha-like PKR inhibitor | IL32▲ (Interleukin 32) |
| WR039 (COP-K7)*,# | Suppressor of DDX3- mediated IRF activation and INFβ promoter induction | ARNTL* (Aryl hydrocarbon receptor nuclear translocator-like) |
| WR064 (COP-E8)* | Membrane protein may help wrap virosome; associates with IV/IMV and cores; F10L kinase substrate | CTNNB1* [Catenin (cadherin-associated protein), beta 1] |
| WR150 (COP-A27)* | IMV surface protein; roles in IMV-cell attachment, fusion, and microtubule transport | Unc84B* (Unc-84 homologue B) |
| BAP1* (BRCA1 associated protein 1) |
WR, Western Reserve strain; COP, Copenhagen strain. Methods to produce recombinant proteins: *in vitro transcription/translation (TNT); ▲mammalian cell; #bacterial.
Clearly, further work is needed to validate all the interactions from the Y2H screen and understand their potential physiological relevance during vaccinia virus infection.
Conclusion
We have identified over 100 protein−protein interactions between vaccinia virus and human proteins by Y2H screening. A significant number of the vaccinia proteins identified in the Y2H screen have been only partially characterized or not characterized at all. The host binding partners, when validated, will provide insightful clues for the function of those viral proteins. As well, many of the deduced mechanisms of vaccinia−host interactions can also apply to monkeypox and variola virus. The generation of nearly complete vaccinia ORFeome is an important step for introducing high-throughput approaches to orthopoxvirus research.
Acknowledgments
This work was supported by NIH contract DHHSN266200400057C to Myriad Genetics and start-up funding from University of Florida to G.M.
Supporting Information Available
Supplemental Table 1 of detailed results of yeast two-hybrid screening. This material is available free of charge via the Internet at http://pubs.acs.org.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Rual J. F.; Hill D. E.; Vidal M. ORFeome projects: gateway between genomics and omics. Curr. Opin. Chem. Biol. 2004, 8 (1), 20–25. [DOI] [PubMed] [Google Scholar]
- Rual J. F.; Hirozane-Kishikawa T.; Hao T.; Bertin N.; Li S.; Dricot A.; Li N.; Rosenberg J.; Lamesch P.; Vidalain P. O.; Clingingsmith T. R.; Hartley J. L.; Esposito D.; Cheo D.; Moore T.; Simmons B.; Sequerra R.; Bosak S.; Doucette-Stamm L.; Le Peuch C.; Vandenhaute J.; Cusick M. E.; Albala J. S.; Hill D. E.; Vidal M. Human ORFeome version 1.1: a platform for reverse proteomics. Genome Res. 2004, 14 (10B), 2128–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboul J.; Vaglio P.; Tzellas N.; Thierry-Mieg N.; Moore T.; Jackson C.; Shin-i T.; Kohara Y.; Thierry-Mieg D.; Thierry-Mieg J.; Lee H.; Hitti J.; Doucette-Stamm L.; Hartley J. L.; Temple G. F.; Brasch M. A.; Vandenhaute J.; Lamesch P. E.; Hill D. E.; Vidal M. Open-reading-frame sequence tags (OSTs) support the existence of at least 17,300 genes in C. elegans. Nat. Genet. 2001, 27 (3), 332–336. [DOI] [PubMed] [Google Scholar]
- Dricot A.; Rual J. F.; Lamesch P.; Bertin N.; Dupuy D.; Hao T.; Lambert C.; Hallez R.; Delroisse J. M.; Vandenhaute J.; Lopez-Goni I.; Moriyon I.; Garcia-Lobo J. M.; Sangari F. J.; Macmillan A. P.; Cutler S. J.; Whatmore A. M.; Bozak S.; Sequerra R.; Doucette-Stamm L.; Vidal M.; Hill D. E.; Letesson J. J.; De Bolle X. Generation of the Brucella melitensis ORFeome version 1.1. Genome Res. 2004, 14 (10B), 2201–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandner C. J.; Maier R. H.; Henderson D. S.; Hintner H.; Bauer J. W.; Onder K. The ORFeome of Staphylococcus aureus v 1.1. BMC Genomics 2008, 9, 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condit R. C.; Moussatche N.; Traktman P. In a nutshell: structure and assembly of the vaccinia virion. Adv. Virus Res. 2006, 66, 31–124. [DOI] [PubMed] [Google Scholar]
- Jahrling P. B.; Fritz E. A.; Hensley L. E. Countermeasures to the bioterrorist threat of smallpox. Curr. Mol. Med. 2005, 5 (8), 817–826. [DOI] [PubMed] [Google Scholar]
- Weaver J. R.; Isaacs S. N. Monkeypox virus and insights into its immunomodulatory proteins. Immunol. Rev. 2008, 225, 96–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubins K. H.; Hensley L. E.; Jahrling P. B.; Whitney A. R.; Geisbert T. W.; Huggins J. W.; Owen A.; Leduc J. W.; Brown P. O.; Relman D. A. The host response to smallpox: analysis of the gene expression program in peripheral blood cells in a nonhuman primate model. Proc. Natl. Acad. Sci. U.S.A. 2004, 101 (42), 15190–15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker S.; Nuara A.; Buller R. M.; Schultz D. A. Human monkeypox: an emerging zoonotic disease. Future Microbiol. 2007, 2, 17–34. [DOI] [PubMed] [Google Scholar]
- McCraith S.; Holtzman T.; Moss B.; Fields S. Genome-wide analysis of vaccinia virus protein-protein interactions. Proc. Natl. Acad. Sci. U.S.A. 2000, 97 (9), 4879–4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed M. R.; Rahman M. M.; Lanchbury J. S.; Shattuck D.; Neff C.; Dufford M.; van Buuren N.; Fagan K.; Barry M.; Smith S.; Damon I.; McFadden G. Proteomic screening of variola virus reveals a unique NF-{kappa}B inhibitor that is highly conserved among pathogenic orthopoxviruses. Proc. Natl. Acad. Sci. U.S.A. 2009, 106 (22), 9051–9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M.; Brufatto N.; Chen T.; Murley L. L.; Thalakada R.; Domagala M.; Beattie B.; Mamelak D.; Athanasopoulos V.; Johnson D.; McFadden G.; Burks C.; Frappier L. Expression profiling of herpesvirus and vaccinia virus proteins using a high-throughput baculovirus screening system. J. Proteome Res. 2005, 4 (6), 2225–2235. [DOI] [PubMed] [Google Scholar]
- Seet B. T.; Johnston J. B.; Brunetti C. R.; Barrett J. W.; Everett H.; Cameron C.; Sypula J.; Nazarian S. H.; Lucas A.; McFadden G. Poxviruses and immune evasion. Annu. Rev. Immunol. 2003, 21, 377–423. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Villa N. Y.; McFadden G. Interplay between poxviruses and the cellular ubiquitin/ubiquitin-like pathways. FEBS Lett. 2009, 583 (4), 607–614. [DOI] [PubMed] [Google Scholar]
- Gouin E.; Welch M. D.; Cossart P. Actin-based motility of intracellular pathogens. Curr. Opin. Microbiol. 2005, 8 (1), 35–45. [DOI] [PubMed] [Google Scholar]
- Barry M.; McFadden G. Apoptosis regulators from DNA viruses. Curr. Opin. Immunol. 1998, 10 (4), 422–430. [DOI] [PubMed] [Google Scholar]
- McFadden G. Poxvirus tropism. Nat. Rev. Microbiol. 2005, 3 (3), 201–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan S.; Heaphy S. The vaccinia virus E3L protein interacts with SUMO-1 and ribosomal protein L23a in a yeast two hybrid assay. Virus Genes 2000, 21 (3), 193–195. [DOI] [PubMed] [Google Scholar]
- Lin Y. C.; Li J.; Irwin C. R.; Jenkins H.; DeLange L.; Evans D. H. Vaccinia virus DNA ligase recruits cellular topoisomerase II to sites of viral replication and assembly. J. Virol. 2008, 82 (12), 5922–5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling K. M.; Schwantes A.; Schnierle B. S.; Sutter G. The highly conserved orthopoxvirus 68k ankyrin-like protein is part of a cellular SCF ubiquitin ligase complex. Virology 2008, 374 (2), 234–239. [DOI] [PubMed] [Google Scholar]
- Yang J. S.; Lee S. Y.; Spano S.; Gad H.; Zhang L.; Nie Z.; Bonazzi M.; Corda D.; Luini A.; Hsu V. W. A role for BARS at the fission step of COPI vesicle formation from Golgi membrane. EMBO J. 2005, 24 (23), 4133–4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doglio L.; De Marco A.; Schleich S.; Roos N.; Krijnse Locker J. The Vaccinia virus E8R gene product: a viral membrane protein that is made early in infection and packaged into the virions’ core. J. Virol. 2002, 76 (19), 9773–9786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S. E.; Condit R. C.; Moussatche N. The vaccinia virus E8R gene product is required for formation of transcriptionally active virions. Virology 2007, 367 (2), 398–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkevich T. G.; Wyatt L. S.; Weisberg A. S.; Koonin E. V.; Moss B. A conserved poxvirus NlpC/P60 superfamily protein contributes to vaccinia virus virulence in mice but not to replication in cell culture. Virology 2008, 374 (2), 506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamin A.; Esposito J.; Hruby D. A single nucleotide substitution in the 5′-untranslated region of the vaccinia N2L gene is responsible for both alpha-amanitin-resistant and temperature-sensitive phenotypes. Virology 1991, 182 (1), 393–396. [DOI] [PubMed] [Google Scholar]
- Lin Y.-C. J.; Li J.; Irwin C. R.; Jenkins H.,; DeLange L.; Evans D. H. Vaccinia Virus DNA Ligase Recruits Cellular Topoisomerase II to Sites of Viral Replication and Assembly. J. Virol. 2008, 82 (12), 5922–5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasilenko S. T.; Banadyga L.; Bond D.; Barry M. The vaccinia virus F1L protein interacts with the proapoptotic protein Bak and inhibits Bak activation. J. Virol. 2005, 79 (22), 14031–14043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer M. D.; Murali T. M.; Sobral B. W. The landscape of human proteins interacting with viruses and other pathogens. PLoS Pathog, 2008, 4 (2), e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu S. K.; Gummadi S. N.; Manoj N.; Aradhyam G. K. Phospholipid scramblases: an overview. Arch. Biochem. Biophys. 2007, 462 (1), 103–114. [DOI] [PubMed] [Google Scholar]
- Uchiyama K.; Jokitalo E.; Kano F.; Murata M.; Zhang X.; Canas B.; Newman R.; Rabouille C.; Pappin D.; Freemont P.; Kondo H. VCIP135, a novel essential factor for p97/p47-mediated membrane fusion, is required for Golgi and ER assembly in vivo. J. Cell Biol. 2002, 159 (5), 855–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Satoh A.; Warren G.; Meyer H. H. VCIP135 acts as a deubiquitinating enzyme during p97-p47-mediated reassembly of mitotic Golgi fragments. J. Cell Biol. 2004, 164 (7), 973–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganea E. Chaperone-like activity of alpha-crystallin and other small heat shock proteins. Curr. Protein Pept. Sci. 2001, 2 (3), 205–225. [DOI] [PubMed] [Google Scholar]
- Li Y.; Chen B.; Chen J.; Lou G.; Chen S.; Zhou D. Fast skeletal muscle troponin I is a co-activator of estrogen receptor-related receptor alpha. Biochem. Biophys. Res. Commun. 2008, 369 (4), 1034–1040. [DOI] [PubMed] [Google Scholar]
- Hoover L. L.; Kubalak S. W. Holding their own: the noncanonical roles of Smad proteins. Sci. Signal. 2008, 1 (46), pe48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almazan F.; Tscharke D. C.; Smith G. L. The vaccinia virus superoxide dismutase-like protein (A45R) is a virion component that is nonessential for virus replication. J. Virol. 2001, 75 (15), 7018–7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.