Abstract
In previous clinical trials of childhood acute lymphoblastic leukemia (ALL), dexamethasone resulted in higher event-free survival rates than prednisone, presumably due to greater central nervous system penetration. Dexamethasone's association with long-term neurocognitive toxicity is unknown. In this multisite study, we measured neurocognitive functioning in 92 children with standard-risk ALL, 1 to 9.99 years at diagnosis, at a mean of 9.8 years after randomization to prednisone (n = 41) or dexamethasone (n = 51) on Children's Cancer Group (CCG) 1922. No significant overall differences in mean neurocognitive and academic performance scores were found between the prednisone and dexamethasone groups after adjusting for age, sex, and time since diagnosis. The exception was that patients receiving dexamethasone scored one-third of a standard deviation worse on word reading (98.8 ± 1.7 vs 104.9 ± 1.8; P = .02). There were no group differences in the distribution of test scores or the parents' report of neurologic complications, psychotropic drug use, and special education. Further analyses suggested for the dexamethasone group, older age of diagnosis was associated with worse neurocognitive functioning; for the prednisone group, younger age at diagnosis was associated with worse functioning. In conclusion, our study did not demonstrate any meaningful differences in long-term cognitive functioning of childhood ALL patients based on corticosteroid randomization. This study is registered with http://www.clinicaltrials.gov under NCT00085176.
Introduction
Corticosteroids have long been recognized as an important component of therapy for childhood acute lymphoblastic leukemia (ALL). More recently, randomized control clinical trials have established a therapeutic benefit of dexamethasone over prednisone. In the Children's Cancer Group (CCG) 1922 trial of 1060 patients, Bostrom et al concluded that patients randomized to dexamethasone had a 6-year event-free survival of 85% plus or minus 2% compared with 77% plus or minus 2% for those randomized to prednisone (P = .002).1 Patients randomized to dexamethasone had a lower rate of both isolated central nervous system (CNS) and bone marrow relapse. These results are consistent with those found by most other cooperative groups.2–4 Dexamethasone's therapeutic advantage is thought to be, in part, due to its better CNS penetration.5
The recently completed CCG 1991 trial reported event-free survival rates approaching 90% and overall survival rates of approximately 95% for standard-risk ALL patients, who were nonrandomly treated with dexamethasone.6 Therefore, the effect of different therapies on future quality of life has increasingly been considered as a critical factor in the selection of optimal treatment. Multiple previous studies of long-term survivors of childhood ALL, even those who did not receive cranial radiation,7–12 have identified deficits in neurocognitive function that might impair quality of life. Among others, investigators have consistently identified difficulties in attention, working memory, processing speed, mathematics, and visual motor integration, but the exact etiologic factors of these deficits have not yet been established.
Studies in noncancer populations suggest that exposure to corticosteroids contributes to cognitive difficulties. For example, asthmatic children demonstrate diminished verbal memory during short-term prednisone therapy13; neonates randomized to dexamethasone instead of placebo for lung disease of prematurity have lower IQ and worse visual motor integration14; and healthy male volunteers on 10 days of hydrocortisone developed impairments in visuospatial memory.15 Murine studies indicate that higher dexamethasone doses are associated with worse neurotoxicity.16 From these observations, we questioned whether dexamethasone would result in more long-term cognitive difficulties in leukemia patients given its better CNS penetration.
Sex and age may moderate the neurobehavioral outcome after brain injury. Females experience greater neurobehavioral deficits after preventive cranial irradiation for ALL17 and after stroke in sickle cell disease.18 Outcomes studies of children with cancer as well as other conditions have suggested that younger children are more vulnerable to the effects of insult on the brain,19–23 reflected by a greater magnitude of deficits and a slower rate of development than children who experience an insult at a later age.
We evaluated neurocognitive functioning in patients previously randomized to prednisone or dexamethasone on CCG 1922 study from which Bostrom et al reported improved event-free survival with dexamethasone.1 We hypothesize that (1) dexamethasone is associated with greater neurocognitive impairment, especially in processing speed, attention, memory, and visual motor integration, and (2) younger age and female sex modify the association between corticosteroid medication and neurocognitive functioning.
Methods
Study population
We conducted a cross-sectional study at limited institutions of patients previously enrolled and randomized in CCG protocol 1922, which was open between March 1993 and August 1995. This protocol consisted of a 2 × 2 factorial design in which patients with National Cancer Institute standard-risk precursor-B ALL24 were assigned randomly to (1) either prednisone or dexamethasone for the majority of therapy, and (2) either intravenous or oral 6-mercaptopurine (6-MP). Either oral prednisone 40 mg/m2 per day or oral dexamethasone 6 mg/m2 per day was given for the 28 days of induction, 2 5-day pulses during consolidation, and monthly 5-day pulses during maintenance. Maintenance duration was 20 months for girls and 32 months for boys. All patients received oral dexamethasone 10 mg/m2 per day for 21 days plus a 7-day taper during a single delayed intensification. Further treatment details about this protocol have been previously published.1
Patients were eligible for participation in the current neurocognitive follow-up study if they were diagnosed and enrolled in CCG-1922 at one of the 22 designated limited institution sites (listed in the supplemental Appendix, available on the Blood website; see the Supplemental Materials link at the top of the online article) and were in first remission. Participating institutions were chosen based on the following criteria: availability of neuropsychologic expertise, adequate research staff, and commitment to enrolling patients for this study. An effort was made to include both community and tertiary care programs from all major regions of the nation to maximize ethnic and geographic diversity. Additional eligibility requirements included no history of CNS leukemia (and thus no cranial radiation), 1 year or longer since cessation of therapy, age at evaluation of 6 to 16.99 years, no history of pre-existing developmental disorders (eg, trisomy 21, developmental delay), and no history of very low birth weight (< 1500 grams). The age restriction corresponded to the validated age range of the standardized neuropsychologic instruments used in the evaluation. In addition, individuals were excluded if they had been nonrandomly assigned to more intensive therapy because of unfavorable cytogenetic findings or a slow response after induction.
Two hundred nineteen patients were enrolled in the therapeutic study at the participating sites and were confirmed to meet the inclusion and exclusion criteria. Of these, 75 were lost to follow up and could not be traced. Of the remaining 144 patients, 52 refused and 92 consented and completed the entire evaluation. The 92 participants were similar to the 127 eligible nonparticipants in terms of age at diagnosis, elapsed time since diagnosis, sex, and therapeutic randomizations (Table 1).
Table 1.
Comparison of participants to eligible nonparticipants
| Participants, n = 92 | Eligible nonparticipants, n = 127 | P | |
|---|---|---|---|
| Mean age at diagnosis, y (SD) | 3.3 (1.2) | 3.1 (1.1) | .23 |
| Mean years between diagnosis and start of the study (SD) | 9.8 (0.6) | 9.8 (0.6) | .93 |
| Sex, no. (%) | |||
| Female | 51(55.4) | 64 (50.4) | .46 |
| Male | 41(44.6) | 63 (49.6) | |
| Corticosteroid therapy, no. (%) | |||
| Dexamethasone | 51(55.4) | 63 (49.6) | .39 |
| Prednisone | 41(44.6) | 64 (50.4) | |
| 6-Mercaptopurine therapy, no. (%) | |||
| Oral | 47 (50.5) | 69 (54.3) | .64 |
| Intravenous | 45 (49.5) | 58 (45.7) |
The institutional review board of each participating center as well as the Yale University Human Investigation Committee approved the protocol and study documents. Informed consent, and assent if indicated, was obtained from all participants in accordance with the Declaration of Helsinki.
Measures
Participants underwent a comprehensive half-day neurocognitive assessment supervised by a licensed psychologist. This evaluation was paid by research funds and was at no cost to the patient. The test battery was based on a previous CCG study, which successfully used previous editions of almost all the same tests in a longitudinal study of the neurobehavioral effects of therapy for intermediate-risk ALL.25 The neurocognitive functioning evaluation included, among others, the following tests: Wechsler Intelligence Scale for Children–Fourth Edition (WISC-IV), Wechsler Individual Achievement Test–Second Edition–Abbreviated (WIAT-II-A), Beery Developmental Test of Visual Motor Integration, the Conners' Continuous Performance Test II (CPT II), and the Children's Memory Scale (CMS). Table 2 details the subsets administered and the scores analyzed.
Table 2.
Neuropsychological evaluation
| Neuropsychological domain | Measure(s) | Normative mean (SD) |
|---|---|---|
| Intelligence | Wechsler Intelligence Scale for Children–Fourth Edition (WISC-IV): intelligence quotient | 100 (15) |
| Academic achievement | Wechsler Individual Achievement Test–Second Edition–Abbreviated (WIAT II-A): reading, spelling, math | 100 (15) |
| Processing speed | WISC-IV: processing speed index | 100 (15) |
| Visual motor integration | Beery Developmental Test of Visual Motor Integration–Fifth Edition (VMI) | 100 (15) |
| Attention-concentration | Conners' Continuous Performance Test II (CPT II): reaction time, omissions, commissions, variability | 50 (10)* |
| Memory | Children's Memory Scale (CMS): general memory index score, visual immediate index score, verbal immediate index score; Wechsler Intelligence Scale for Children–Fourth Edition (WISC-IV): working memory index | 100 (15) |
Higher scores indicate worse impairment.
Parents of subjects completed a demographic and medical history survey. Parents were asked about their marital status, education, and income. This questionnaire confirmed that the child was developing normally before the ALL diagnosis as an additional check of eligibility for this study. In addition, parents were asked about neurologic events, special education services, and psychotropic drug use during and after ALL therapy.
Data analysis
Characteristics such as age, sex, and therapy history were summarized and compared between participants and nonparticipants using the t test and the chi-square test to evaluate the potential for response bias. These characteristics were also compared between those randomized to dexamethasone versus prednisone to determine comparability of exposed groups. Multiple linear regression was used to evaluate the difference between the corticosteroid groups in standardized scores for the neurocognitive outcomes with adjustment for sex, age at diagnosis, and elapsed time since diagnosis. In addition, the proportion of patients with standard scores worse than one standard deviation below the norm was compared using chi-square test or Fisher exact test.
Subgroup analyses were also conducted to determine whether the differences in neurocognitive outcomes between treatment exposures were modified by sex and/or age at diagnosis (< 3 years vs ≥ 3 years). Adjusted least squares means, adjusted for age and sex, and standard errors as well as 95% confidence intervals for differences in means are presented. Posthoc analyses were conducted to assess the impact of the route of 6-MP on neurocognitive functioning. Data were analyzed with the SAS software package Version 9.1 (SAS Institute) with 2-sided tests at the .05 significance level. The data were analyzed by the biostatistics core of the Yale Center for Clinical Investigation.
The total number of participants was adequate to address clinically important differences as determined by sample size calculations based on the 2 independent sample t test. The sample size of 51 and 41 patients in the 2 corticosteroid groups achieved a 80% power to detect a difference of 0.6 standard deviations between the 2 group means. For example, there was adequate power to detect a difference of 9 points in full scale IQ.
Results
Participants
There were 92 subjects with neurobehavioral data available for analysis. The participants of this study were similar to the 1060 patients enrolled in CCG 1922 in terms of sex, corticosteroid randomization, and 6-MP randomization. However, the current sample was younger at diagnosis (61% were between 2-4 years and 28% were between 4-10, compared with 43% and 49%, respectively, in CCG 1922; P = .001) and slightly more likely to be white (86% vs 77% in CCG 1922; P = .02).
Table 3 displays the characteristics of participants, stratified by corticosteroid randomization. Patients in the different corticosteroid treatment groups were similar in terms of age, sex, elapsed time since diagnosis, 6-MP randomization, and race/ethnicity distribution. They also had similar socioeconomic status, as indicated by the marital status, income, and education of the primary caregiver.
Table 3.
Characteristics of participants previously randomized to either dexamethasone or prednisone
| Dexamethasone, n = 51 | Prednisone, n = 41 | P | |
|---|---|---|---|
| Age at diagnosis, y, mean (SD) | 3.4 (1.4) | 3.2 (1.0) | .70 |
| Sex, no. (%) | |||
| Female | 21 (41) | 20 (49) | .46 |
| Male | 30 (59) | 21 (51) | |
| Years between diagnosis and start of the study, mean (SD) | 9.8 (0.5) | 9.8 (0.6) | .70 |
| Race/ethnicity, no. (%) | |||
| White, non-Hispanic | 44 (88.0) | 33 (82.5) | .46 |
| Hispanic | 1 (2) | 3 (7.5) | |
| Black, non-Hispanic | 2 (4) | 1 (2.5) | |
| Mixed race and other | 1 (2) | 1 (2.5) | |
| Asian | 2 (4) | 1 (2.5) | |
| Native American | 0 (0) | 1 (2.5) | |
| Marital status of primary caregiver, no. (%) | |||
| Married | 44 (88) | 35 (89.7) | .999 |
| Unmarried | 6 (12) | 4 (10.3) | |
| Education of primary caregiver, no. (%) | |||
| High school or less | 12 (28.0) | 11 (27.5) | .93 |
| Some college | 17 (34.0) | 15 (37.5) | |
| College degree or higher | 19 (38.0) | 14 (35.0) | |
| Family income, no. (%) | |||
| Less than $50 000 | 11 (25.0) | 12 (31.6) | .67 |
| $50 000-$79 999 | 12 (31.8) | 9 (23.7) | |
| $80 000 or more | 19 (43.2) | 17 (44.7) | |
| 6-Mercaptopurine therapy, no. (%) | |||
| Oral | 23 (45.1) | 24 (58.5) | .20 |
| Intravenous | 28 (54.9) | 17 (41.5) |
Note that some participants declined to report marital status, education, and/or family income.
The 6-MP treatment groups were equally distributed between those randomized to prednisone and dexamethasone. Posthoc analyses comparing neurocognitive performance between the oral and intravenous 6-MP groups showed no differences for any of the neurocognitive domains.
Performance on neurobehavioral instruments
Table 4 displays the least squares means of neurocognitive test results among patients randomized to dexamethasone compared with prednisone, adjusted for sex, age at diagnosis, and elapsed time since diagnosis. Patients who received dexamethasone scored 6 points lower, or approximately one-third of a standard deviation, on word reading (P = .02). Otherwise, the groups performed similarly in tests of full scale IQ, attention-concentration, visual motor integration, numeric operations, spelling, and memory (including working memory).
Table 4.
Comparison of neurocognitive test scores between patients randomized to either dexamethasone or prednisone
| Domain and instrument/scale | Dexamethasone, LS means (SE)* | Prednisone, LS means (SE) | Difference† (95% CI) | P |
|---|---|---|---|---|
| Intelligence | ||||
| WISC-IV full scale IQ | 101.8 (1.7) | 101.6 (1.9) | 0.2 (−4.9-5.4) | .94 |
| Academic achievement | ||||
| WIAT II-A | ||||
| Word reading | 98.8 (1.7) | 104.9 (1.8) | −6.0 (−11.0 to −1.0) | .02‡ |
| Numeric operations | 100.6 (2.2) | 100.6 (2.5) | −0.1 (−6.8-6.6) | .99 |
| Spelling | 100.5 (1.8) | 104.5 (2.0) | −4.0 (−9.6-1.6) | .16 |
| Processing speed | ||||
| WISC-IV | ||||
| Processing speed index | 94.4 (1.8) | 92.6 (1.9) | 1.8 (−3.4-7.0) | .50 |
| Attention-concentration | ||||
| CPT-II | ||||
| Omissions | 45.4 (0.8) | 46.9 (0.9) | −1.4 (−6.3-3.1) | .25 |
| Commissions | 44.8 (1.6) | 46.4 (1.8) | −1.6 (6.3−3.1) | .50 |
| Reaction time | 47.4 (1.5) | 48.8 (1.6) | −1.4 (−5.8-3.1) | .54 |
| Variability T-score | 43.2 (1.2) | 45.4 (1.2) | −2.2 (−5.6-1.2) | .21 |
| Memory | ||||
| CMS | ||||
| Visual Immediate Index | 105.6 (1.8) | 103.0 (1.9) | 2.5 (−2.8-7.9) | .35 |
| Verbal immediate index | 106.0 (2.3) | 102.8 (2.5) | 3.2 (−3.2-10.1) | .36 |
| General memory index | 109.4 (2.1) | 106.0 (2.3) | 3.3 (−2.9-9.5) | .29 |
| Working memory | ||||
| WISC-IV | ||||
| Working memory | 98.2 (2.2) | 101.3 (2.4) | −3.0 (−9.4-3.3) | .34 |
| Visual motor integration | ||||
| Visual motor integration | 91.6 (1.7) | 95.2 (1.8) | −3.6 (−8.8-1.4) | .15 |
Data are presented as least squares means obtained from multiple linear regression, adjusted for sex, age at diagnosis, and time elapsed since diagnosis.
Least squares means (standard error).
Difference in adjusted least squares means between dexamethasone and prednisone treatment groups.
Significant difference between dexamethasone and prednisone treatment groups at P<.05.
The distribution of scores in the 2 treatment groups was then examined by comparing the proportion of patients with standardized scores at or worse than 1 standard deviation below the norm (Table 5). Children randomized to dexamethasone were not more likely to score below 1 SD from the normative mean in intelligence, most scores of academic achievement, processing speed, attention/concentration, and visual motor integration.
Table 5.
Proportion of patients with neurocognitive test scores 1 or more standard deviation worse than the normative mean, stratified by corticosteroid randomization
| Domain and instrument/scale | Dexamethasone (%) | Prednisone (%) | P* |
|---|---|---|---|
| Intelligence | |||
| WISC-IV | |||
| Full scale IQ | 4/51 (7.8) | 2/41 (4.9) | .69 |
| Academic achievement | |||
| WIAT-IIA | |||
| Word reading | 5/51 (9.8) | 2/41 (4.9) | .46 |
| Numeric operations | 8/51 (15.7) | 7/41 (17.1) | .86 |
| Spelling | 4/51 (7.8) | 2/41 (4.9) | .69 |
| Processing speed | |||
| WISC-IV | |||
| Processing speed index | 13/51 (25.5) | 7/41 (17.1) | .31 |
| Attention-concentration | |||
| CPT-II† | |||
| Omissions | 0/46 (0.0) | 0/36 (0.0) | NA |
| Commissions | 19/46 (41.3) | 9/36 (25.0) | .12 |
| Reaction time | 14/46 (30.4) | 9/36 (25.0) | .59 |
| Variability T-score | 15/46 (32.6) | 11/36 (30.6) | .84 |
| Memory | |||
| CMS† | |||
| Visual immediate index | 2/46 (4.3) | 2/38 (5.3) | .999 |
| Verbal immediate index | 4/46 (8.7) | 4/38 (10.5) | .78 |
| General memory index | 2/46 (4.3) | 3/38 (7.9) | .65 |
| Working memory | |||
| WISC-IV | |||
| Working memory | 6/51 (11.8) | 2/41 (4.9) | .29 |
| Visual motor integration | |||
| Beery | |||
| Visual motor integration | 11/51 (21.6) | 5/41 (12.0) | .24 |
Comparison between dexamethasone and prednisone groups.
Ten patients (5 in dexamethasone, 5 in prednisone) did not complete the CPT-II. Eight patients (5 in dexamethasone, 3 in prednisone) did not complete the CMS. However the rates of completion were similar between the 2 groups for each of the instruments.
Interaction between corticosteroid preparation and patient characteristics
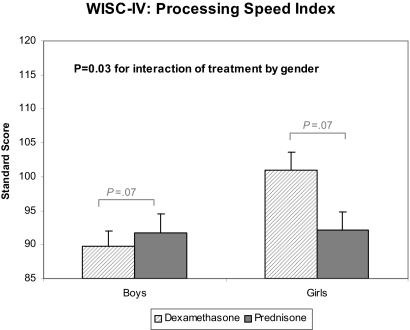
The association of treatment with several measures of cognitive functioning was modified by sex and age at diagnosis. Figures 1 and 2 display the statistically significant interactions. Figure 1 shows that the differences in processing speed between the treatment groups were dependent on sex (P = .03). Girls receiving prednisone had lower processing speed scores compared with those who received dexamethasone. No treatment difference was observed for boys. No sex differences were found in the association between steroid preparation and the other measures of neurocognitive outcomes.
Figure 1.
Sex-specific differences in processing speed between dexamethasone and prednisone. Interactions for other neurocognitive outcomes are not displayed because they were not significant at P < .05. Data are presented as least squares means (SE) from multiple linear regression adjusted for age at diagnosis and time elapsed since diagnosis. The P value for interaction represents the test of whether the magnitude of group differences was dependent on sex. Bracketed P values represent sex-specific comparisons between dexamethasone and prednisone.
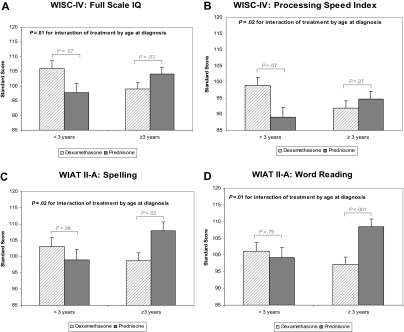
Figure 2.
Age at diagnosis-specific differences in neurocognitive outcomes between dexamethasone and prednisone. Only neurocognitive outcomes for which the interaction is significant (P < .05) are displayed: (A) WISC-IV full scale IQ, (B) WISC-IV processing speed index, (C) WIAT II-A spelling, (D) WIAT II-A word reading. Data are presented as least squares means (SE) from multiple linear regression adjusted for sex and time elapsed since diagnosis. The P values for interactions refer to tests of whether the magnitude of group differences was dependent on age at diagnosis. Bracketed P values represent age-specific comparisons between dexamethasone and prednisone.
Significant interactions with age were found for some domains of neurocognitive functioning. For full scale IQ (P = .02), processing speed index (P = .02), spelling (P = .02), and word reading (P = .01), younger age at diagnosis was associated with better performance among those who received dexamethasone. In contrast, younger age was associated with worse performance among those who received prednisone. No steroid differences were found for the other neurocognitive domains according to age at diagnosis.
Other measures of neurobehavioral status
The frequency of neurologic events, psychotropic drug use, and special education services was similar in the dexamethasone and prednisone groups both during and after ALL therapy, as reported by parents. Of note, 16% (8/50) of patients who received dexamethasone reported receiving special education after ALL therapy, compared with 5% (2/40) in the prednisone group, but the results were not statistically significant (P = .18).
Discussion
No significant overall differences in neurocognitive and academic performance were found between children with ALL treated with prednisone versus dexamethasone in this multisite, cross-sectional study. The exception was that patients who received dexamethasone scored slightly worse, approximately one-third of a standard deviation, on a test of word reading. As a further confirmation of these results, there was no difference in the parents' report of neurologic complications, psychotropic drug use, and special education services between dexamethasone and prednisone. The relationship between corticosteroid preparation and neurocognitive outcomes, however, seemed to be modified by age at diagnosis and sex. Our analyses appeared to indicate that for the dexamethasone group, older age of diagnosis is associated with worse IQ, processing speed, spelling, and reading. In contrast for the prednisone group, younger age at diagnosis is associated with worse functioning.
The major advantage of our study was that the participants of this report had been enrolled in a randomized control treatment study. Thus, any potential adjuvant therapy or patient-related confounders that could affect neurocognitive functioning would be likely equally distributed between the treatment groups. All participants were similar in terms of National Cancer Index standard-risk status and lack of cranial radiation. Chemotherapy exposures were identical except for the corticosteroid and 6-MP randomization. To verify that 6-MP did not confound the association between corticosteroid and neurocognitive functioning, we did posthoc analyses comparing neurocognitive performance between the oral and intravenous 6-MP groups. We found no differences for any of the neurocognitive domains.
Limited published data are available regarding the relative neurocognitive toxicity of dexamethasone versus prednisone. Waber et al compared patients treated on previous ALL regimens that included different corticosteroid preparations.26 They found greater neurocognitive impairment in the dexamethasone group for memory, reading comprehension, and mathematics. However, the dexamethasone group also had lower participation rates, younger age at diagnosis, higher rates of cranial radiation (70% vs 50% in the prednisone group), and lower educational attainment by the parents. Cranial radiation is associated with more severe neurocognitive impairment than that conferred by systemic and intrathecal chemotherapy.20,27–29 As previously discussed, our study overcame many of the limitations of the Waber et al study.26
Our overall results are consistent with smaller studies based on patients with ALL.7,30 Buizer et al evaluated 36 children with ALL with heterogeneous treatment and leukemia features 1 year after completion of therapy, compared with patients treated for Wilms tumor and to healthy children.7 This study found that dexamethasone treatment was not associated with greater attentional dysfunction than prednisone. Jansen et al30 followed 49 consecutive children with ALL treated with dexamethasone, not prednisone, longitudinally approximately 4 years after diagnosis. Patients had neuropsychologic outcomes similar to a healthy noncancer comparison group.
Other studies found that patients who were younger at diagnosis12,25,31 or female12,29,32 had a higher risk of neurocognitive impairments after ALL therapy. In our study, these patient subgroups were not consistently at higher risk for impairment across certain domains of functioning. Female sex was associated with worse processing speed for patients who received prednisone, but not for other areas of neurocognitive functioning. Older age, not younger age, was associated with several areas of comparative cognitive and academic deficits among those who received dexamethasone. Deficits in these specific functions may be associated with disruptions during critical periods of brain and functional development. It is possible that functions that are emerging are most at risk for disruption by a concurrent insult to the brain,34 and the implicated functions clearly have their most critical development beyond the fifth year in children. It is also possible that the interactions were significant due to chance given that multiple interactions were examined. Our study was not designed to explain reasons for differences between corticosteroid preparations in certain patient subgroups. These sex and age findings should be examined in animal models and larger clinical studies.
Our results must be interpreted in the setting of several observations. We enrolled only 92 of the eligible, traceable 127 patients at the participating institutions. However, the participants were similar to the nonparticipants in terms of age, sex, elapsed time since diagnosis, and most importantly, steroid randomization. All patients received oral dexamethasone 10 mg/m2 per day for 21 days plus a 7-day taper during the single delayed intensification, regardless of the corticosteroid randomization during induction, consolidation, and maintenance. Dexamethasone was given to all study participants because this is the state-of-the-art method of administering delayed intensification; CCG 1922 did not aim to test a delayed intensification-related hypothesis. We would expect any effect of the dexamethasone during delayed intensification to be similar in the 2 steroid randomization groups because they were administered identically. Furthermore, the corticosteroid randomization involves at least 128 additional days of therapy. We also note that our study does not include data to address neurocognitive impairment at higher dexamethasone doses than the 6 mg/m2 included in COG studies and many, but not all, other consortium groups. We are aware that other studies, such as the St Jude Total XV protocol,34 use dexamethasone pulses of 8 to 12 mg/m2. Finally, the small difference in reading in the dexamethasone group could have been due to chance since multiple comparisons were tested.
We used a cross-sectional study design so causality between treatment exposure and neurocognitive outcome can be inferred, but not known with certainty. However, longitudinal prospective studies are more expensive and difficult to complete with reasonable sample sizes. Furthermore, they are often not feasible to conduct in patients who are ill and/or receiving intensive therapy. Neuropsychologic assessments administered at diagnosis in some longitudinal studies have yielded lower than expected cognitive performance scores.31,35 Longitudinal studies also introduce the possibility of practice effect on testing in which general improvement in performance occurs due to repeated testing.36
In conclusion, our study did not show any clinically meaningful differences in cognitive functioning between patients previously randomly assigned to prednisone or dexamethasone treatment. These results provide no support for modifying steroid therapy for ALL because of differential effects of steroid preparation on neurocognitive outcome. There is substantial individual variation in neurobehavioral outcome, however, and detailed investigations of host/drug interactions must be conducted to further define the source of this variation. We identified age at diagnosis and sex as potential modifiers of outcome. As ALL leukemia survival rates progressively increase, more studies assessing the burden of therapy will be needed.
Supplementary Material
Acknowledgments
This study was supported by research funding from the American Cancer Society (J.P.N.). N.S.K.-L. was supported in part by CTSA Grant Number KL2 RR024138 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Additional support was obtained from Children's Oncology Group Chair's Grant U10 CA98543, Statistics and Data Center Grant U10 CA98413, and CCOP Grant U10 CA95861.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: N.S.K.-L. designed and performed research, analyzed data, and prepared the paper; P.B. and J.P.N. designed research, analyzed data, and prepared the paper; D.B. analyzed data, performed research, and prepared the paper; T.K. and L.C. designed research and prepared the paper; J.D., H.L., L.S., and B.B. analyzed data and prepared the paper; and M.N. performed research and prepared the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of participating institutions and investigators appears in the supplemental Appendix (available on the Blood website; see the Supplemental Materials list at the top of the online article).
Correspondence: Nina Kadan-Lottick, MSPH, 333 Cedar St LMP 2073, PO Box 208064, New Haven, CT 06520; email: nina.kadan-lottick@yale.edu.
References
- 1.Bostrom BC, Sensel MR, Sather HN, et al. Dexamethasone versus prednisone and daily oral versus weekly intravenous mercaptopurine for patients with standard-risk acute lymphoblastic leukemia: a report from the Children's Cancer Group. Blood. 2003;101(10):3809–3817. doi: 10.1182/blood-2002-08-2454. [DOI] [PubMed] [Google Scholar]
- 2.Jones B, Freeman AI, Shuster JJ, et al. Lower incidence of meningeal leukemia when prednisone is replaced by dexamethasone in the treatment of acute lymphocytic leukemia. Med Pediatr Oncol. 1991;19(4):269–275. doi: 10.1002/mpo.2950190411. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell CD, Richards SM, Kinsey SE, Lilleyman J, Vora A, Eden TO. Benefit of dexamethasone compared with prednisolone for childhood acute lymphoblastic leukaemia: results of the UK Medical Research Council ALL97 randomized trial. Br J Haematol. 2005;129(6):734–745. doi: 10.1111/j.1365-2141.2005.05509.x. [DOI] [PubMed] [Google Scholar]
- 4.Schrappe M, Reiter A, Zimmermann M, et al. Long-term results of four consecutive trials in childhood ALL performed by the ALL-BFM study group from 1981 to 1995. Berlin-Frankfurt-Munster. Leukemia. 2000;14(12):2205–2222. doi: 10.1038/sj.leu.2401973. [DOI] [PubMed] [Google Scholar]
- 5.Balis FM, Lester CM, Chrousos GP, Heideman RL, Poplack DG. Differences in cerebrospinal fluid penetration of corticosteroids: possible relationship to the prevention of meningeal leukemia. J Clin Oncol. 1987;5(2):202–207. doi: 10.1200/JCO.1987.5.2.202. [DOI] [PubMed] [Google Scholar]
- 6.Matloub Y, Angiolillo A, Bostrom B, et al. Double delayed intensification (DDI) is equivalent to single DI (SDI) in children with National Cancer Institute (NCI) standard-risk acute lymphoblastic leukemia (SR-ALL) treated on Children's Cancer Group (CCG) Clinical Trial 1991 (CCG-1991) [abstract]. 2006;108(11):146. ASH Annual Meeting Abstracts. [Google Scholar]
- 7.Buizer AI, de Sonneville LM, van den Heuvel-Eibrink MM, Veerman AJ. Chemotherapy and attentional dysfunction in survivors of childhood acute lymphoblastic leukemia: effect of treatment intensity. Pediatr Blood Cancer. 2005;45(3):281–290. doi: 10.1002/pbc.20397. [DOI] [PubMed] [Google Scholar]
- 8.Mennes M, Stiers P, Vandenbussche E, et al. Attention and information processing in survivors of childhood acute lymphoblastic leukemia treated with chemotherapy only. Pediatr Blood Cancer. 2005;44(5):478–486. doi: 10.1002/pbc.20147. [DOI] [PubMed] [Google Scholar]
- 9.Moleski M. Neuropsychological, neuroanatomical, and neurophysiological consequences of cns chemotherapy for acute lymphoblastic leukemia. Arch Clin Neuropsychol. 2000;15(7):603–630. [PubMed] [Google Scholar]
- 10.Ochs J, Mulhern R, Fairclough D, et al. Comparison of neuropsychologic functioning and clinical indicators of neurotoxicity in long-term survivors of childhood leukemia given cranial radiation or parenteral methotrexate: a prospective study. J Clin Oncol. 1991;9(1):145–151. doi: 10.1200/JCO.1991.9.1.145. [DOI] [PubMed] [Google Scholar]
- 11.Peterson CC, Johnson CE, Ramirez LY, et al. A meta-analysis of the neuropsychological sequelae of chemotherapy-only treatment for pediatric acute lymphoblastic leukemia. Pediatr Blood Cancer. 2008;51(1):99–104. doi: 10.1002/pbc.21544. [DOI] [PubMed] [Google Scholar]
- 12.von der Weid N, Mosimann I, Hirt A, et al. Intellectual outcome in children and adolescents with acute lymphoblastic leukaemia treated with chemotherapy alone: age- and sex-related differences. Eur J Cancer. 2003;39(3):359–365. doi: 10.1016/s0959-8049(02)00260-5. [DOI] [PubMed] [Google Scholar]
- 13.Bender BG, Lerner JA, Poland JE. Association between corticosteroids and psychologic change in hospitalized asthmatic children. Ann Allergy. 1991;66(5):414–419. [PubMed] [Google Scholar]
- 14.Yeh TF, Lin YJ, Lin HC, et al. Outcomes at school age after postnatal dexamethasone therapy for lung disease of prematurity. N Engl J Med. 2004;350(13):1304–1313. doi: 10.1056/NEJMoa032089. [DOI] [PubMed] [Google Scholar]
- 15.Young AH, Sahakian BJ, Robbins TW, Cowen PJ. The effects of chronic administration of hydrocortisone on cognitive function in normal male volunteers. Psychopharmacology (Berl) 1999;145(3):260–266. doi: 10.1007/s002130051057. [DOI] [PubMed] [Google Scholar]
- 16.Danilczuk Z, Ossowska G, Lupina T, Cieslik K, Zebrowska-Lupina I. Effect of NMDA receptor antagonists on behavioral impairment induced by chronic treatment with dexamethasone. Pharmacol Rep. 2005;57(1):47–54. [PubMed] [Google Scholar]
- 17.Waber DP, Tarbell NJ, Fairclough D, et al. Cognitive sequelae of treatment in childhood acute lymphoblastic leukemia: cranial radiation requires an accomplice. J Clin Oncol. 1995;13(10):2490–2496. doi: 10.1200/JCO.1995.13.10.2490. [DOI] [PubMed] [Google Scholar]
- 18.Paniucki H, Krull K, Mahoney D, Mueller B, Brouwers P. Effect of cerbrovascular complications and gender on children with sickle cell disease [abstract]. J Int Neuropsychol Soc. 2002;8(2):241. [Google Scholar]
- 19.Mulhern RK, Kepner JL, Thomas PR, Armstrong FD, Friedman HS, Kun LE. Neuropsychologic functioning of survivors of childhood medulloblastoma randomized to receive conventional or reduced-dose craniospinal irradiation: a Pediatric Oncology Group study. J Clin Oncol. 1998;16(5):1723–1728. doi: 10.1200/JCO.1998.16.5.1723. [DOI] [PubMed] [Google Scholar]
- 20.Jankovic M, Brouwers P, Valsecchi MG, et al. Association of 1800 cGy cranial irradiation with intellectual function in children with acute lymphoblastic leukaemia: ISPACC: International Study Group on Psychosocial Aspects of Childhood Cancer. Lancet. 1994;344(8917):224–227. doi: 10.1016/s0140-6736(94)92997-1. [DOI] [PubMed] [Google Scholar]
- 21.Taylor HG, Alden J. Age-related differences in outcomes following childhood brain insults: an introduction and overview. J Int Neuropsychol Soc. 1997;3(6):555–567. [PubMed] [Google Scholar]
- 22.Hack M, Breslau N, Aram D, Weissman B, Klein N, Borawski-Clark E. The effect of very low birth weight and social risk on neurocognitive abilities at school age. J Dev Behav Pediatr. 1992;13(6):412–420. [PubMed] [Google Scholar]
- 23.Kaleita TA. Central nervous system-directed therapy in the treatment of childhood acute lymphoblastic leukemia and studies of neurobehavioral outcome: Children's Cancer Group trials. Curr Oncol Rep. 2002;4(2):131–141. doi: 10.1007/s11912-002-0074-3. [DOI] [PubMed] [Google Scholar]
- 24.Smith M, Arthur D, Camitta B, et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol. 1996;14(1):18–24. doi: 10.1200/JCO.1996.14.1.18. [DOI] [PubMed] [Google Scholar]
- 25.Kaleita TA, Noll RB, Stehbens JA, et al. Age at diagnosis of acute lymphoblastic leukemia is associated with neurobehavioral outcomes independent of CNS-directed treatment with or without cranial irradiation and intensive chemotherapy: a Children's Cancer Group report [abstract]. Ped Res. 2002;51:236A. [Google Scholar]
- 26.Waber DP, Carpentieri SC, Klar N, et al. Cognitive sequelae in children treated for acute lymphoblastic leukemia with dexamethasone or prednisone. J Pediatr Hematol Oncol. 2000;22(3):206–213. doi: 10.1097/00043426-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Copeland DR, Fletcher JM, Pfefferbaum-Levine B, Jaffe N, Ried H, Maor M. Neuropsychological sequelae of childhood cancer in long-term survivors. Pediatrics. 1985;75(4):745–753. [PubMed] [Google Scholar]
- 28.Kingma A, Van Dommelen R, Mooyaart E, Wilmink J, Deelman B, Kamps W. No major cognitive impairment in young children with acute lymphoblastic leukemia using chemotherapy only: a prospective longitudinal study. J Ped Hematol Oncol. 2002;24(2):106–114. doi: 10.1097/00043426-200202000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Langer T, Martus P, Ottensmeier H, Hertzberg H, Beck JD, Meier W. CNS late-effects after ALL therapy in childhood, part III: neuropsychological performance in long-term survivors of childhood ALL: impairments of concentration, attention, and memory. Med Pediatr Oncol. 2002;38(5):320–328. doi: 10.1002/mpo.10055. [DOI] [PubMed] [Google Scholar]
- 30.Jansen NC, Kingma A, Schuitema A, Bouma A, Veerman AJ, Kamps WA. Neuropsychological outcome in chemotherapy-only-treated children with acute lymphoblastic leukemia. J Clin Oncol. 2008;26(18):3025–3030. doi: 10.1200/JCO.2007.12.4149. [DOI] [PubMed] [Google Scholar]
- 31.Jansen NC, Kingma A, Schuitema A, et al. Post-treatment intellectual functioning in children treated for acute lymphoblastic leukaemia (ALL) with chemotherapy-only: a prospective, sibling-controlled study. Eur J Cancer. 2006;42(16):2765–2772. doi: 10.1016/j.ejca.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 32.Brown RT, Madan-Swain A, Walco GA, et al. Cognitive and academic late effects among children previously treated for acute lymphocytic leukemia receiving chemotherapy as CNS prophylaxis. J Pediatr Psychol. 1998;23(5):333–340. doi: 10.1093/jpepsy/23.5.333. [DOI] [PubMed] [Google Scholar]
- 33.Brouwers P. Study of the neurobehavioral consequences of childhood cancer: entering the genomic era? J Ped Psychol. 2005;30(1):79–84. doi: 10.1093/jpepsy/jsi018. [DOI] [PubMed] [Google Scholar]
- 34.Pui C-H, Relling M, Sandlund J, Downing J, Campana D, Evans W. Rationale and design of Total Therapy Study XV for newly diagnosed childhood acute lymphoblastic leukemia. Ann Hematol. 2004;83(suppl 1):S124–S126. doi: 10.1007/s00277-004-0850-2. [DOI] [PubMed] [Google Scholar]
- 35.Wefel JS, Lenzi R, Theriault RL, Davis RN, Meyers CA. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: results of a prospective, randomized, longitudinal trial. Cancer. 2004;100(11):2292–2299. doi: 10.1002/cncr.20272. [DOI] [PubMed] [Google Scholar]
- 36.Vardy J, Wefel JS, Ahles T, Tannock IF, Schagen SB. Cancer and cancer-therapy related cognitive dysfunction: an international perspective from the Venice cognitive workshop. Ann Oncol. 2008;19(4):623–629. doi: 10.1093/annonc/mdm500. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.