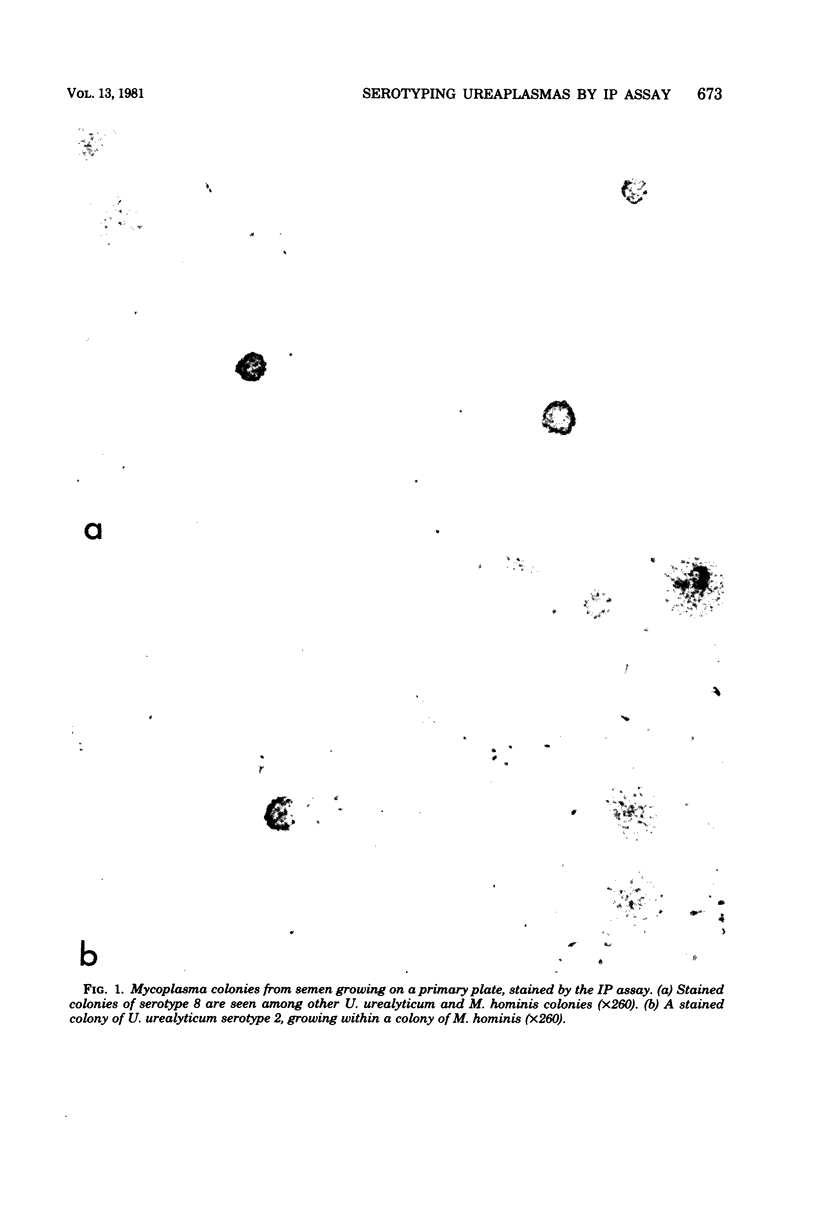
Abstract
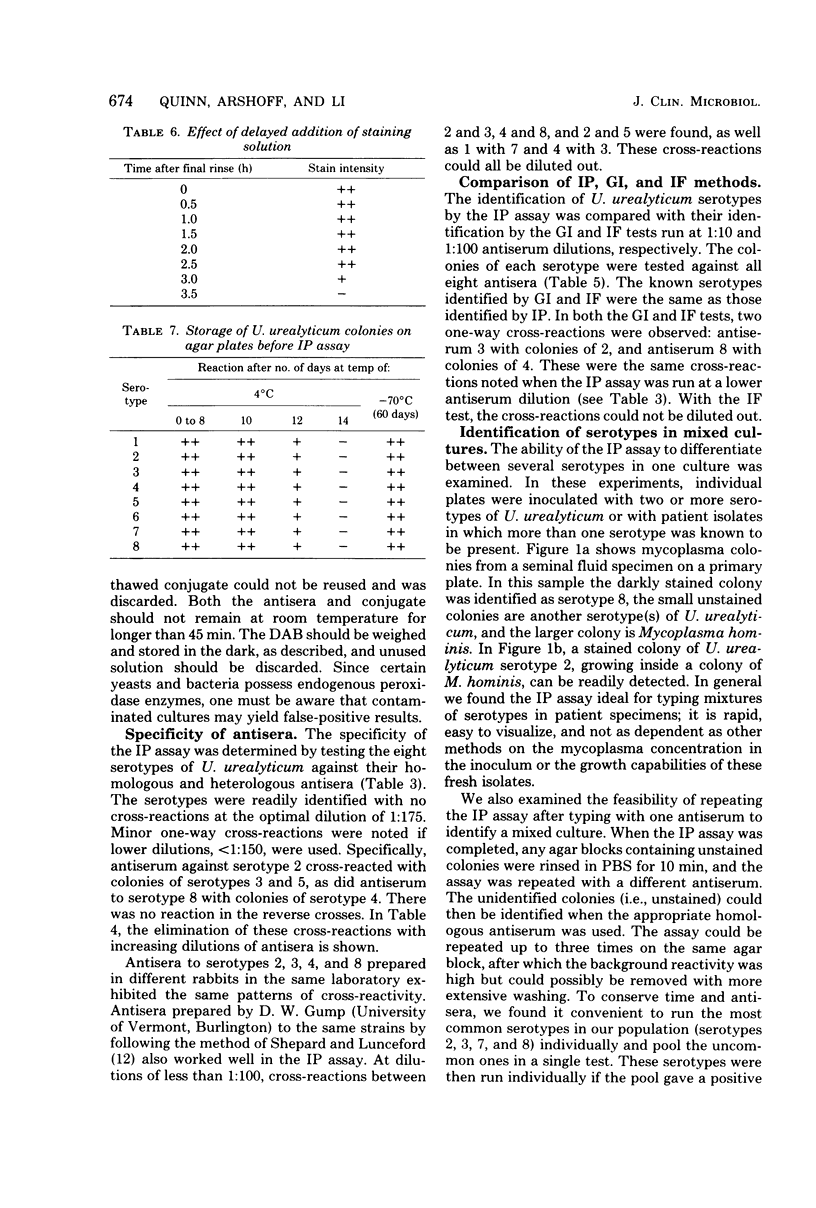
The immunoperoxidase method was applied to the identification of Urea-plasma urealyticum serotypes. The assay used highly diluted antisera and could be run directly on primary plate isolates. It was ideal for detecting and identifying mixed serotypes because stained and unstained colonies could be visualized simultaneously by conventional light microscopy. Antisera run against eight serotypes revealed one-way cross-reactions between serotypes 3 and 5 and antiserum to 2, and between serotype 4 and antiserum to 8, at dilutions of less than 1:150. This cross-reactivity could be diluted out at the optimal antiserum dilution for the immunoperoxidase assay, but not for the growth inhibition assay or immunofluorescence test. The immunoperoxidase assay therefore proved ideal for serotyping U. urealyticum.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black F. T., Krogsgaard-Jensen A. Application of indirect immunofluorescence, indirect haemagglutination and polyacrylamide-gel electrophoresis to human T-mycoplasmas. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Jun;82(3):345–353. doi: 10.1111/j.1699-0463.1974.tb02336.x. [DOI] [PubMed] [Google Scholar]
- Black F. T. Modifications of the growth inhibition test and its application to human T-mycoplasmas. Appl Microbiol. 1973 Apr;25(4):528–533. doi: 10.1128/am.25.4.528-533.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dische M. R., Quinn P. A., Czegledy-Nagy E., Sturgess J. M. Genital mycoplasma infection. Intrauterine infection: pathologic study of the fetus and placenta. Am J Clin Pathol. 1979 Aug;72(2):167–174. doi: 10.1093/ajcp/72.2.167. [DOI] [PubMed] [Google Scholar]
- Lin J. S., Kendrick M. I., Kass E. H. Serologic typing of human genital T-mycoplasmas by a complement-dependent mycoplasmacidal test. J Infect Dis. 1972 Dec;126(6):658–663. doi: 10.1093/infdis/126.6.658. [DOI] [PubMed] [Google Scholar]
- Piot P. Comparison of growth inhibition and immunofluorescence tests in serotyping clinical isolates of Ureaplasma urealyticum. Br J Vener Dis. 1977 Jun;53(3):186–189. doi: 10.1136/sti.53.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piot P. Distribution of eight serotypes of Ureaplasma urealyticum in cases of non-gonococcal urethritis and of gonorrhoea, and in healthy persons. Br J Vener Dis. 1976 Aug;52(4):266–268. doi: 10.1136/sti.52.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak-Vogelzang A. A., Hagenaars R., Nagel J. Evaluation of an indirect immunoperoxidase test for identification of acholeplasma and mycoplasma. J Gen Microbiol. 1978 Jun;106(2):241–249. doi: 10.1099/00221287-106-2-241. [DOI] [PubMed] [Google Scholar]
- Robertson J. A., Stemke G. W. Modified metabolic inhibition test for serotyping strains of Ureaplasma urealyticum (T-strain Mycoplasma). J Clin Microbiol. 1979 Jun;9(6):673–676. doi: 10.1128/jcm.9.6.673-676.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosendal S., Black F. T. Direct and indirect immunofluorescence of unfixed and fixed Mycoplasma colonies. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(4):615–622. doi: 10.1111/j.1699-0463.1972.tb00186.x. [DOI] [PubMed] [Google Scholar]
- Shepard M. C., Lunceford C. D. Differential agar medium (A7) for identification of Ureaplasma urealyticum (human T mycoplasmas) in primary cultures of clinical material. J Clin Microbiol. 1976 Jun;3(6):613–625. doi: 10.1128/jcm.3.6.613-625.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard M. C., Lunceford C. D. Serological typing of Ureaplasma urealyticum isolates from urethritis patients by an agar growth inhibition method. J Clin Microbiol. 1978 Nov;8(5):566–574. doi: 10.1128/jcm.8.5.566-574.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]