Abstract
Hepcidin is the major regulator of systemic iron homeostasis in mammals. Hepcidin is produced mainly by the liver and is increased by inflammation, leading to hypoferremia. We measured serum levels of bioactive hepcidin and its effects on serum iron levels in mice infected with Borrelia burgdorferi. Bioactive hepcidin was elevated in the serum of mice resulting in hypoferremia. Infected mice produced hepcidin in both liver and spleen. Both intact and sonicated B burgdorferi induced hepcidin expression in cultured mouse bone marrrow macrophages. Hepcidin production by cultured macrophages represents a primary transcriptional response stimulated by B burgdorferi and not a secondary consequence of cytokine elaboration. Hepcidin expression induced by B burgdorferi was mediated primarily by activation of Toll-like receptor 2.
Introduction
Hepcidin is a cysteine-rich peptide produced mainly in hepatocytes and is a major regulator of cellular iron export in all vertebrates. Ferroportin, the only known cellular iron exporter, is present on the plasma membrane of hepatocytes, macrophages, enterocytes, and syncytial trophoblasts. Hepcidin binds to ferroportin leading to its internalization and degradation. The loss of cell surface ferroportin causes cellular retention of iron.1 Elevated serum hepcidin levels have been associated with persistent hypoferremia and have been causally linked to the anemia of inflammation (also known as the anemia of chronic disease). Proinflammatory cytokines, such as interleukin-6 (IL-6) and IL-1, have been shown to induce the secretion of hepcidin by hepatocytes.2–11 Hepcidin is also produced by macrophages and monocytes12,13 in response to the inflammatory mediator lipopolysaccharide (LPS) acting through Toll-like receptors (TLRs). The proportion of serum hepcidin contributed by macrophages is not known.
Lyme disease is a systemic inflammatory condition caused by the tick-borne spirochete Borrelia burgdorferi.14 A model of Lyme disease has been extensively studied in C3H and C57BL/6 mice.15,16 Although both mouse strains harbor similar numbers of spirochetes within their ankle joints, C3H mice develop severe arthritis 4 weeks after infection with B burgdorferi, whereas C57BL/6 mice display milder disease. B burgdorferi does not contain LPS, although it does contain proteins modified by a tripalmitoyl-S-glyceryl-cysteine moiety, which induces inflammatory cytokines through interaction with heterodimers of TLR2/TLR1.15,17–20 Furthermore, B burgdorferi does not have iron-containing proteins and does not use iron for survival.21 For these reasons, we thought B burgdorferi is an appropriate organism to study the relationship between systemic inflammation, serum hepcidin, and serum iron levels. We report here that persistent infection of mice with B burgdorferi leads to high levels of serum hepcidin that correlate with hypoferremia. Splenic macrophages appear to be an important contributor to serum hepcidin production, and isolated macrophages respond to B burgdorferi with induction of hepcidin transcription before serum IL-6 protein is detectable.
Methods
Animals
Animal studies were performed with the approval of the Animal Research Committee at the University of Utah. C3H/HeN (C3H) and C57BL/6 mice were obtained from Charles River Laboratories. MyD88−/− and TLR2−/− C57BL/6 mice were gifts from Dr S. Akira (Hyogo College of Medicine) and purchased from Tularik, respectively, as previously described.22 C57BL/6 TLR9−/− mice were from the MutantMouse Resource at the University of California, Davis. A total of 4 female mice were used to complete each experiment. All mice were 6 weeks of age.
Cells and media
HEK293T-ferroportin (Fpn) cells, a stable cell line in which Fpn-GFP is regulated by the ecdysone promoter, were grown as described.23 Fpn-GFP expression was induced by the addition of 10 μM Ponasterone A (AG Scientific).
B burgdorferi culture and infection
Spirochetes were cultured in Barbour-Stoenner-Kelly II medium containing 6% rabbit serum (Sigma-Aldrich) for 4 days before injection. Mice were infected by intradermal injection at 6 to 7 weeks of age with the N40 isolate of B burgdorferi (originally provided by S. Barthold, University of California, Davis). Control animals were intradermally injected with sterile Barbour-Stoenner-Kelly II containing 6% rabbit serum.
Assessment of infection status and arthritis severity
Rear ankle joint diameter was measured in mice at the time of infection and at 4 weeks later using a metric caliper. Measurements were taken of the thickest anteroposterior portion of the ankle with the joint extended. Data are reported as the ankle diameter (millimeters) at 4 weeks of infection.
Mouse tissue collection
Blood was collected by eye bleed or by cardiac puncture and kept for 1 hour at room temperature and overnight at 4°C. Serum was then obtained by centrifugation. Livers and spleens were homogenized and used for total RNA extraction using RNeasy (QIAGEN) according to the manufacturer's instructions. RNA from cultured mouse macrophages was isolated using the same procedure.
RT-PCR
Fifty nanograms of mRNA was used for reverse-transcription polymerase chain reaction (RT-PCR) One Step according to the manufacturer's instructions (Invitrogen). Mouse hepcidin, IL-6, and β-actin expression was analyzed. The relative expression in each sample was calculated using Quantity-one Software (Bio-Rad). The primer sequences used for RT-PCR are listed in Table 1.
Table 1.
The primer sequences used for RT-PCR
| Gene | Sequence |
|---|---|
| HAMP (forward) | 5′AGAGCTGCAGCCTTTGCAC-3′ |
| HAMP (reverse) | 5′GAAGATGCAGATGGGGAAGT-3′ |
| IL-6 (forward) | 5′-AGTTGCCTTCTTGGGACTGA-3′ |
| IL-6 (reverse) | 5′-TCCACGATTTCCCAGAGAAC-3′ |
| Actin (forward) | 5′-GACGGCCAAGTCATCACTATTG-3′ |
| Actin (reverse) | 5′-CCACAGGATTCCATACCCAAGA-3′ |
Mouse bone marrow macrophages isolation
Mouse bone marrow macrophages were isolated from femurs and grown in RPMI 1640 with 20% equine serum for 4 days. Adherent cells were further cultured in RPMI 1640 with 20% fetal bovine serum and 30% L cell–conditioned medium (a source of macrophage colony-stimulating factor) until experimental manipulation.
Other procedures
Serum hepcidin was measured as described previously.24 Total protein extractions from livers and spleens were obtained by homogenization in the presence of 1.0% Triton X-100, 150 mM NaCl, 10 mM ethylenediaminetetraacetic acid, and 10 mM Tris, pH 7.4, with a protease inhibitor cocktail (Roche Diagnostics). Western blot analysis was performed using rabbit antiferroportin (1:1000), rabbit antihepcidin (1:1000), mouse antitubulin (1:1000; GeneTex) followed by either peroxidase-conjugated goat anti–rabbit immunoglobulin IgG (1:10 000; Jackson ImmunoResearch Laboratories), or peroxidase-conjugated goat anti–mouse IgG (1:10 000; Jackson ImmunoResearch Laboratories).25 All Western blots were normalized for total protein concentration using the bicinchoninic acid assay (Pierce Chemical). Serum IL-6 was analyzed using Mouse IL-6 Ready-Set-Go (eBioscience) according to the manufacturer's instructions. Serum ferritin was measured as described previously.26 Transferrin saturation was assayed with a total iron-binding capacity kit and used according to the manufacturer's instructions (TECO Diagnostics). Unmethylated cytosine and guanine nucleotides separated by phosphate (CpG) were synthesized at the Core Facility of the University of Utah. LPS and peptidoglycan were purchased from Sigma-Aldrich. Polyinosine-polycytidylic acid (Poly(I-C)) was purchased from Invivogen. All experiments were performed a minimum of 3 times. Standard error (SE) bars are represented in the graphs. P values were calculated using the Student t test. P values less than .05 were considered significant.
Results
Hepcidin is elevated in B burgdorferi–infected mice
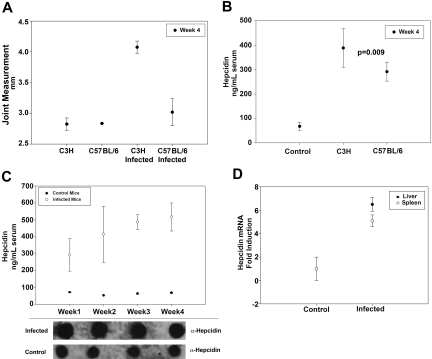
C3H- and C57BL/6-infected and uninfected control mice were sacrificed 4 weeks after inoculation with B burgdorferi. Rear ankle swelling, one measure of B burgdorferi–induced arthritis severity that correlates with the histopathologic assessment of arthritis, was used to monitor arthritis development in infected mice.18 Rear ankle measurements were greater in infected mice compared with control mice, and C3H-infected mice developed a more severe arthritis than C57BL/6 infected mice (Figure 1A). C3H and C57BL/6 mice infected with B burgdorferi had serum hepcidin levels several-fold higher than control mice, although C57BL/6-infected mice had milder arthritis. This suggests that serum hepcidin is a marker of infection but is not an indicator of arthritis severity (Figure 1B). Serum hepcidin was analyzed weekly for 4 weeks after infecting C3H mice with B burgdorferi. Serum hepcidin levels were elevated 3-fold relative to controls after one week of infection. These levels continued to increase over the following 4 weeks until serum hepcidin levels in the infected mice were 5-fold more than in control mice (Figure 1C). To determine whether hepcidin transcripts were also increased in infected mice, we performed RT-PCR on both splenic and liver tissue from C3H mice after 4 weeks of infection. Hepcidin mRNA was elevated nearly 6-fold in the livers and 5-fold in the spleens of infected C3H mice compared with controls (Figure 1D).
Figure 1.
Serum hepcidin is elevated in mice infected with B burgdorferi. (A) C3H and C57BL/6 mice were infected with B burgdorferi. Arthritis severity was determined by measuring rear ankle joint diameter in infected and control mice 4 weeks after infection. (B) C3H and C57BL/6 mice were infected with B burgdorferi as in panel A. Four weeks after infection, serum hepcidin levels were measured and compared with uninfected controls. (C) C3H mice were infected with B burgdorferi as in panel A. Serum hepcidin levels were measured at weekly intervals using a bioactive assay (top panel) and dot blot (bottom panel) for a total of 4 weeks and compared with control mice. (D) C3H mice were infected with B burgdorferi and sacrificed after 4 weeks of infection. RNA was isolated and purified from the livers and spleens. Hepcidin and actin transcripts were analyzed by RT-PCR.
B burgdorferi–infected mice with elevated serum hepcidin are hypoferremic
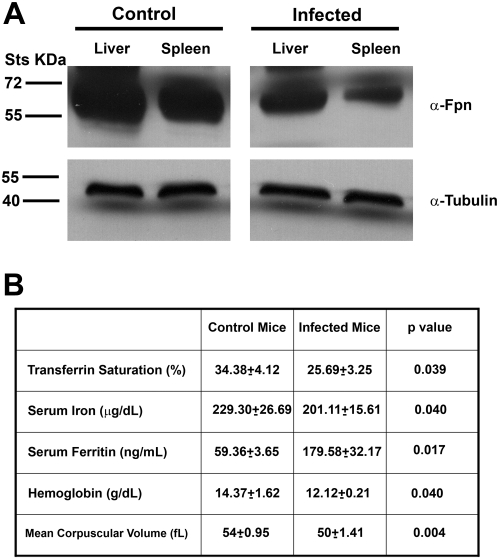
Elevated serum hepcidin levels in the infected mice were expected to lead to the internalization and degradation of plasma membrane ferroportin. We analyzed the amount of ferroportin in the liver and spleen of infected C3H mice by Western blot and compared the values with those of control mice. Ferroportin levels were lower in the livers and spleens of infected mice compared with controls (Figure 2A). Decreased levels of ferroportin were expected to affect serum iron levels. To determine whether elevated serum hepcidin levels were associated with hypoferremia (defined as low serum iron and low transferrin saturation), we analyzed serum iron concentration, transferrin saturation, and ferritin levels in infected C3H mice 4 weeks after inoculation with B burgdorferi. We compared these levels with those of control mice. Transferrin saturation was significantly decreased in infected mice compared with controls. Infected mice also had lower serum iron levels and higher levels of serum ferritin. To determine whether B burgdorferi infection had hematologic effect similar to the anemia of inflammation, we measured hemoglobin and red blood cell parameters in infected and control animals. Hemoglobin levels were significantly lower in infected mice compared with controls, and the mean corpuscular volume was also lower in infected mice compared with controls (Figure 2B). Blood smears from infected mice showed more hypochromic red blood cells and target cells consistent with iron-deficient erythropoiesis (data not shown). These results indicate that B burgdorferi infection leads to increased hepcidin levels resulting in hypoferremia and anemia of inflammation.
Figure 2.
Mice infected with B burgdorferi are hypoferremic. (A) Homogenates of livers and spleens from uninfected and infected C3H mice with B burgdorferi were analyzed using rabbit antiferroportin antibody and mouse antitubulin antibody followed by a peroxidase-conjugated goat anti–rabbit antibody or goat anti–mouse antibody. (B) Serum was isolated from infected and uninfected C3H mice 4 weeks after injection with B burgdorferi. Transferrin saturation, serum iron, serum ferritin, hemoglobin, and mean corpuscular volume were analyzed as described in “Methods.”
Hepcidin expression results from TLR2 signaling
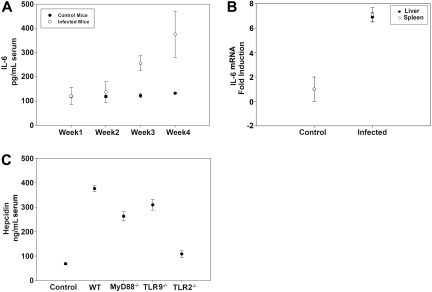
To determine whether serum IL-6 levels were associated with serum hepcidin induction, we measured IL-6 levels weekly for a total of 4 weeks in infected C3H mice. Serum IL-6 levels did not increase in infected mice for the first 2 weeks after inoculation but markedly increased 3 and 4 weeks after infection (Figure 3A). IL-6 transcripts were also elevated in the spleens and livers of infected mice 4 weeks after infection (Figure 3B). These results indicate that splenic cells, presumably macrophages, produce hepcidin and that the early hepcidin response to B burgdorferi is not induced by IL-6.
Figure 3.
Hepcidin mRNA transcripts are up-regulated in mice infected with B burgdorferi. (A) Serum was isolated from blood samples obtained weekly from B burgdorferi-infected and uninfected C3H mice over a 4-week experimental period. IL-6 levels were measured by enzyme-linked immunosorbent assay. (B) C3H mice were infected with B burgdorferi and sacrificed after 4 weeks of infection. RNA was isolated and purified from the livers and spleens, and IL-6 and actin transcripts were analyzed by RT-PCR. (C) Serum hepcidin levels were measured in uninfected WT C57BL/6 mice, infected WT C57BL/6 mice, and infected C57BL/6 mice deficient for MyD88−/−, TLR9−/−, or TLR2−/− after 4 weeks of infection.
These observations suggest that hepcidin expression might result from direct interaction of B burgdorferi with TLRs. To determine the role of TLRs in hepcidin up-regulation, we measured serum hepcidin levels in infected mice that had targeted gene deletions in specific TLRs or adapter proteins required for TLR signal transduction. We compared hepcidin levels in infected wild-type (WT) C57BL/6 mice with infected mice deficient in TLR2 (TLR2−/−), TLR9 (TLR9−/−), and MyD88 (MyD88−/−) on the same background. MyD88 is an adapter protein used by all TLRs, except TLR3.27 The levels of hepcidin in MyD88−/− and TLR9−/− mice were similar to the levels in infected WT C57BL/6 mice. In contrast, infected TLR2−/− mice had reduced hepcidin levels that were slightly above those seen in noninfected controls. Previous studies demonstrated that the number of spirochetes in tissues of TLR2−/− mice was 10- to 20-fold greater than in control mice28; thus, the absence of hepcidin was not the result of absence of bacteria in tissues. These results suggest that the interaction of B burgdorferi with TLR2 is responsible for increased hepcidin production (Figure 3C).
Macrophages produce hepcidin when exposed to B burgdorferi
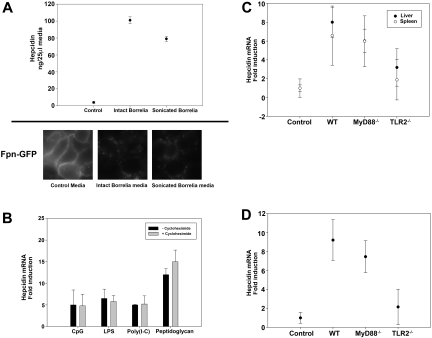
To determine whether macrophages produce hepcidin in response to B burgdorferi, bone marrow macrophages from C3H mice were cultured with either sonicated or intact B burgdorferi. Sonicated B burgdorferi is killed bacteria, and intact B burgdorferi is living bacteria. Hepcidin levels measured in the culture media 24 hours after incubation showed that macrophages responded to both sonicated and intact B burgdorferi by producing 16- to 20-fold more hepcidin than controls (Figure 4A). In the first 2 weeks after infection with B burgdorferi, increased hepcidin levels were not accompanied by increased serum levels of IL-6. IL-6 is one of the main inducers of hepcidin expression. Other cytokines might induce hepcidin expression, but we hypothesized that hepcidin expression was a direct result of TLR activation. To test this hypothesis, we examined the effect of TLR activation from TLR-specific agonists (CpG, unmethylated cytosine and guanine nucleotides separated by phosphate, a TLR9 agonist; lipopolysacchride, LPS, a TLR4 agonist; Poly(I-C), a TLR3 agonist; peptidoglycan, a TLR2 agonist) on hepcidin mRNA levels. TLR-specific agonists induced the expression of hepcidin mRNA in macrophages, and induction was not altered by the addition of cycloheximide, which inhibits the synthesis of cytokines (Figure 4B). These results indicate that hepcidin expression is a direct result of TLR activation.
Figure 4.
Hepcidin secretion by bone marrow macrophages stimulated with B burgdorferi is TLR2 dependent. (A) Bone marrow macrophages from C3H mice stimulated with either intact or sonicated B burgdorferi were analyzed for hepcidin production. Media from cultured macrophages was analyzed for hepcidin 24 hours after incubation. HEK 293T cells expressing Fpn-GFP were incubated with media used to culture WT macrophages infected with intact or sonicated B burgdorferi. Ferroportin degradation was analyzed by epifluorescence microscopy. (B) Bone marrow macrophages from C3H mice were stimulated with TLR-specific agonists (20 μg/mL CpG, TLR9 agonist; 10 ng/mL LPS, TLR4 agonist; 20 μg/mL Poly(I-C), TLR3 agonist; 20 μg/mL peptidoglycan, TLR2 agonist) in the presence or absence of cycloheximide (7.5 μg/mL). RNA was isolated and purified, and hepcidin and actin transcripts were analyzed by RT-PCR. (C) C57BL/6 uninfected (Control) and infected (WT), MyD88−/−, and TLR2−/− mice were sacrificed after 4 weeks of infection. RNA was isolated and purified from the livers and spleens, and hepcidin and actin transcripts were analyzed by RT-PCR. (D) Bone marrow macrophages from C57BL/6 control, WT, MyD88−/−, and TLR2−/− mice were stimulated with sonicated B burgdorferi. RNA was isolated and purified, and hepcidin and actin transcripts were analyzed by RT-PCR.
Peptidoglycan, a TLR2 agonist, induced the transcription of hepcidin to greater levels than the other TLR agonists that were tested. To determine whether this hepcidin response was the result of hepcidin production from liver or spleen, we measured hepcidin transcripts from the liver and spleen of infected MyD88−/− or TLR2−/− mice. TLR2−/− mice had lower levels of hepcidin transcripts than MyD88−/− or WT mice (Figure 4C). Macrophages from TLR2−/− mice, stimulated with sonicated B burgdorferi, produced lower amounts of hepcidin transcripts than did stimulated macrophages from MyD88−/− or WT mice (Figure 4D). These data show that liver and spleen increased hepcidin transcripts in response to TLR2 activation. Addition of B burgdorferi to cultured macrophages showed that hepcidin induction is a direct response to theTLR2 agonist. The data also show that the absence of TLR2 prevented hepcidin production from the spleen and from isolated macrophages. There was, however, some production of hepcidin in liver, suggesting that the increase in hepcidin mRNA (2-fold) may be the result of hepatocytes.
Discussion
Hepcidin is a systemic regulator of iron transport in all vertebrates. Hepcidin affects the ability of cells to export iron into plasma by down-regulating the cell-surface iron transporter ferroportin. Inflammation increases hepcidin expression leading to hypoferremia. If inflammation persists, the hypoferremia can result in iron-limited erythropoiesis. Hepcidin is up-regulated by proinflammatory cytokines, such as IL-6. Although expression of hepcidin occurs in hepatocytes, other cells, including macrophages, are also capable of secreting hepcidin in direct response to whole bacteria or bacterial LPS.6,12 We have shown that serum hepcidin is elevated in mice infected with B burgdorferi, a spirochete that does not express LPS but expresses surface lipoproteins.17 The consequence of elevated hepcidin is hypoferremia, which leads to anemia in the murine model of Lyme arthritis.
Serum hepcidin levels in infected mice increased after the first week of infection, before the elevations in serum IL-6, suggesting that hepcidin expression was induced by an alternative pathway. Our experiments show that TLRs, particularly TLR2, play a role in hepcidin regulation in response to B burgdorferi, an organism that does not express endotoxin but rather has tripalmitoyl-S-glyceryl-cysteine-modified cell-surface lipidated proteins.29 These lipoproteins activate TLR2/TLR1 heterodimers, which results in a vigorous inflammatory response in vitro.28 However, TLR2 is not essential for the development of Lyme arthritis in mice. Mice deficient for TLR2 (TLR2−/−) developed arthritis but minimally induced hepcidin when infected with B burgdorferi. These results suggest that a TLR2-dependent pathway is induced in the hepcidin response but not in the development of arthritis. We confirmed these findings by showing increased hepcidin levels after stimulating isolated C3H bone marrow macrophages with peptidoglycan, a TLR2 agonist. Bioactive hepcidin amounts also correlated with hepcidin mRNA levels. A previous study showed that LPS acting through TLR4 could induce hepcidin expression in response to endotoxin.6 Our study shows that agonists for TLR9 also lead to induction of hepcidin expression. These data confirm prior studies that macrophages are important producers of hepcidin.4,6,12,13 The finding that hepcidin production is dependent on TLR2 but not MyD88, as no MyD88-independent adapter has been identified for TLR2 signaling, is puzzling. One explanation could be that the extremely high levels of B burgdorferi in tissues of MyD88-deficient mice22,30 activate a parallel inflammatory pathway and circumvent the requirement for MyD88. The more modest elevation in tissue spirochetes observed in TLR2−/− mice may not reach the threshold required for activation of this parallel pathway. The proportion of total serum hepcidin contributed by macrophages compared with hepatocytes is not clear, but the finding that spleen and isolated macrophage can respond to TLR agonists shows that hepcidin production by macrophages may be more important than originally thought.
In conclusion, hepcidin levels are elevated in mice infected with B burgdorferi. Elevated levels of hepcidin are associated with hypoferremia leading to anemia. The regulation of hepcidin in chronic inflammatory conditions appears to be more complex than originally described. TLR2 appears to play an important role in the production of hepcidin in Lyme disease, and macrophages secrete hepcidin in response to infection with B burgdorferi.
Acknowledgments
This work was supported by the National Institutes of Health (grant SP30 DK072437, J.P.K.; grant DK070947, J.K.; grants AI-32223 and AR43521, J.J.W.; grant T32-AI005434) and an Arthritis Foundation Award (J.C.M.).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: C.L.K. and I.D.D. performed experiments, analyzed the data, and wrote the paper; J.C.M. performed experiments and edited the paper; J.M.N. performed experiments; D.M.W., J.P.K., J.J.W., and J.K. analyzed the data and edited the paper; and L.K.B. edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ivana De Domenico, University of Utah, Department of Internal Medicine, 30 North 1900 East, Rm 5b232 SOM, Salt Lake City, UT 84132; e-mail: ivana.dedomenico@path.utah.edu.
References
- 1.De Domenico I, McVey Ward D, Kaplan J. Regulation of iron acquisition and storage: consequences for iron-linked disorders. Nat Rev Mol Cell Biol. 2008;9(1):72–81. doi: 10.1038/nrm2295. [DOI] [PubMed] [Google Scholar]
- 2.Kemna E, Pickkers P, Nemeth E, van der Hoeven H, Swinkels D. Time-course analysis of hepcidin, serum iron, and plasma cytokine levels in humans injected with LPS. Blood. 2005;106(5):1864–1866. doi: 10.1182/blood-2005-03-1159. [DOI] [PubMed] [Google Scholar]
- 3.Lee P, Peng H, Gelbart T, Wang L, Beutler E. Regulation of hepcidin transcription by interleukin-1 and interleukin-6. Proc Natl Acad Sci U S A. 2005;102(6):1906–1910. doi: 10.1073/pnas.0409808102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu XB, Nguyen NB, Marquess KD, Yang F, Haile DJ. Regulation of hepcidin and ferroportin expression by lipopolysaccharide in splenic macrophages. Blood Cells Mol Dis. 2005;35(1):47–56. doi: 10.1016/j.bcmd.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Nemeth E, Rivera S, Gabayan V, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113(9):1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peyssonnaux C, Zinkernagel AS, Datta V, Lauth X, Johnson RS, Nizet V. TLR4-dependent hepcidin expression by myeloid cells in response to bacterial pathogens. Blood. 2006;107(9):3727–3732. doi: 10.1182/blood-2005-06-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pietrangelo A, Dierssen U, Valli L, et al. STAT3 is required for IL-6-gp130-dependent activation of hepcidin in vivo. Gastroenterology. 2007;132(1):294–300. doi: 10.1053/j.gastro.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 8.Stoian I, Manolescu B, Atanasiu V, Lupescu O, Busu C. IL-6 - STAT-3 - hepcidin: linking inflammation to the iron metabolism. Rom J Intern Med. 2007;45(3):305–309. [PubMed] [Google Scholar]
- 9.Truksa J, Lee P, Beutler E. The role of STAT, AP-1, E-box and TIEG motifs in the regulation of hepcidin by IL-6 and BMP-9: lessons from human HAMP and murine Hamp1 and Hamp2 gene promoters. Blood Cells Mol Dis. 2007;39(3):255–262. doi: 10.1016/j.bcmd.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verga Falzacappa MV, Vujic Spasic M, Kessler R, Stolte J, Hentze MW, Muckenthaler MU. STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood. 2007;109(1):353–358. doi: 10.1182/blood-2006-07-033969. [DOI] [PubMed] [Google Scholar]
- 11.Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108(9):3204–3209. doi: 10.1182/blood-2006-06-027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Theurl I, Theurl M, Seifert M, et al. Autocrine formation of hepcidin induces iron retention in human monocytes. Blood. 2008;111(4):2392–2399. doi: 10.1182/blood-2007-05-090019. [DOI] [PubMed] [Google Scholar]
- 13.Sow FB, Florence WC, Satoskar AR, Schlesinger LS, Zwilling BS, Lafuse WP. Expression and localization of hepcidin in macrophages: a role in host defense against tuberculosis. J Leukoc Biol. 2007;82(4):934–945. doi: 10.1189/jlb.0407216. [DOI] [PubMed] [Google Scholar]
- 14.Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. Lyme disease: a tick-borne spirochetosis? Science. 1982;216(4552):1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 15.Barthold SW, Beck DS, Hansen GM, Terwilliger GA, Moody KD. Lyme borreliosis in selected strains and ages of laboratory mice. J Infect Dis. 1990;162(1):133–138. doi: 10.1093/infdis/162.1.133. [DOI] [PubMed] [Google Scholar]
- 16.Barthold SW, Persing DH, Armstrong AL, Peeples RA. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am J Pathol. 1991;139(2):263–273. [PMC free article] [PubMed] [Google Scholar]
- 17.Fraser CM, Casjens S, Huang WM, et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390(6660):580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 18.Ma Y, Seiler KP, Eichwald EJ, Weis JH, Teuscher C, Weis JJ. Distinct characteristics of resistance to Borrelia burgdorferi-induced arthritis in C57BL/6N mice. Infect Immun. 1998;66(1):161–168. doi: 10.1128/iai.66.1.161-168.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weis JJ, McCracken BA, Ma Y, et al. Identification of quantitative trait loci governing arthritis severity and humoral responses in the murine model of Lyme disease. J Immunol. 1999;162(2):948–956. [PubMed] [Google Scholar]
- 20.Wooten RM, Ma Y, Yoder RA, et al. Toll-like receptor 2 plays a pivotal role in host defense and inflammatory response to Borrelia burgdorferi. Vector Borne Zoonotic Dis. 2002;2(4):275–278. doi: 10.1089/153036602321653860. [DOI] [PubMed] [Google Scholar]
- 21.Posey JE, Gherardini FC. Lack of a role for iron in the Lyme disease pathogen. Science. 2000;288(5471):1651–1653. doi: 10.1126/science.288.5471.1651. [DOI] [PubMed] [Google Scholar]
- 22.Bolz DD, Sundsbak RS, Ma Y, et al. MyD88 plays a unique role in host defense but not arthritis development in Lyme disease. J Immunol. 2004;173(3):2003–2010. doi: 10.4049/jimmunol.173.3.2003. [DOI] [PubMed] [Google Scholar]
- 23.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 24.De Domenico I, Nemeth E, Nelson JM, et al. The hepcidin-binding site on ferroportin is evolutionarily conserved. Cell Metab. 2008;8(2):146–156. doi: 10.1016/j.cmet.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Paradkar PN, De Domenico I, Durchfort N, Zohn I, Kaplan J, Ward DM. Iron depletion limits intracellular bacterial growth in macrophages. Blood. 2008;112(3):866–874. doi: 10.1182/blood-2007-12-126854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Domenico I, Vaughn MB, Li L, et al. Ferroportin-mediated mobilization of ferritin iron precedes ferritin degradation by the proteasome. EMBO J. 2006;25(22):5396–5404. doi: 10.1038/sj.emboj.7601409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16(1):3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Wooten RM, Ma Y, Yoder RA, et al. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J Immunol. 2002;168(1):348–355. doi: 10.4049/jimmunol.168.1.348. [DOI] [PubMed] [Google Scholar]
- 29.Wang G, Ma Y, Buyuk A, McClain S, Weis JJ, Schwartz I. Impaired host defense to infection and Toll-like receptor 2-independent killing of Borrelia burgdorferi clinical isolates in TLR2-deficient C3H/HeJ mice. FEMS Microbiol Lett. 2004;231(2):219–225. doi: 10.1016/S0378-1097(03)00960-1. [DOI] [PubMed] [Google Scholar]
- 30.Liu N, Montgomery RR, Barthold SW, Bockenstedt LK. Myeloid differentiation antigen 88 deficiency impairs pathogen clearance but does not alter inflammation in Borrelia burgdorferi-infected mice. Infect Immun. 2004;72(6):3195–3203. doi: 10.1128/IAI.72.6.3195-3203.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]