Abstract
Activated protein C (APC) has both anticoagulant activity and direct cell-signaling properties. APC has been reported to promote cancer cell migration/invasion and to inhibit apoptosis and therefore may exacerbate metastasis. Opposing these activities, APC signaling protects the vascular endothelial barrier through sphingosine-1-phosphate receptor-1 (S1P1)activation, which may counteract cancer cell extravasation. Here, we provide evidence that endogenous APC limits cancer cell extravasation, with in vivo use of monoclonal antibodies against APC. The protective effect of endogenous APC depends on its signaling properties. The MAPC1591 antibody that only blocks anticoagulant activity of APC does not affect cancer cell extravasation as opposed to MPC1609 that blocks anticoagulant and signaling properties of APC. Combined administration of anti-APC antibodies and S1P1 agonist (SEW2871) resulted in a similar number of pulmonary foci in mice in presence and absence of APC, indicating that the protective effect of APC depends on the S1P1 pathway. Moreover, endogenous APC prevents cancer cell–induced vascular leakage as assessed by the Evans Blue Dye assay, and SEW2871 treatment reversed MPC1609-dependent vascular leakage. Finally, we show that cancer cells combined with MPC1609 treatment diminished endothelial VE-cadherin expression. In conclusion, endogenous APC limits cancer cell extravasation because of S1P1-mediated VE-cadherin–dependent vascular barrier enhancement.
Introduction
Activated protein C (APC) is a natural anticoagulant serine protease that serves as one of the main modulators of the coagulation system by blocking the amplification of the coagulation cascade via inactivation of factors Va and VIIIa. The APC pathway is initiated by complex formation of thrombin, thrombomodulin, and the endothelial protein C receptor, allowing the conversion of the vitamin K–dependent zymogen protein C into its activated form.1
Cancer cells are known to activate the blood coagulation cascade, resulting in thrombin generation that plays an essential role in metastasis. Minute amounts of thrombin enhance metastasis, whereas thrombin-treated cancer cells produce more experimental metastasis.2,3 Moreover, thrombin inhibition decreases hematogenous metastasis in mice,4 and anticoagulants prolong survival of patients with cancer.5 Therefore, the ability of APC to attenuate thrombin formation may be advantageous in preventing cancer metastasis.
In addition to its anticoagulant activity, APC induces direct cellular effects that regulate the inflammatory response via its direct cell-signaling properties.6,7 Such APC-induced signal transduction promotes cancer cell migration, invasion, and angiogenesis and inhibits cancer cell apoptosis.8–10 Consequently, it has been hypothesized that APC exacerbates metastasis.11 However, APC-induced signaling enhances also the vascular endothelial barrier function through activation of endothelial protein C receptor, protease activated receptor 1, and the sphingosine-1-phosphate-receptor-1 (S1P1) pathway.12–17 This barrier protective effect of APC seems pivotal for limiting inflammatory disease and sepsis-induced mortality.18 It is thus tempting to speculate that APC-mediated vascular barrier protection may also limit metastasis by counteracting cancer cell extravasation. Indeed, vascular endothelial barrier enhancement protects against cancer cell extravasation in vivo.19–21
Overall, APC may limit metastasis by its anticoagulant and barrier protective properties, but it may stimulate metastasis by enhancing the metastatic potential of cancer cells. Therefore, we aimed to evaluate the effect of endogenous APC in cancer cell extravasation of B16F10 melanoma cells into mouse lung.
Methods
Cells and cell culture
Murine B16F10 melanoma cells were obtained from ATCC. Cells were cultured in Dulbecco modified Eagle medium (Lonza) supplemented with 10% fetal calf serum (Sigma-Aldrich), 1% penicillin-streptomycin solution, and l-glutamine at 37°C. Single cell suspensions were prepared from 0.02% EDTA-treated monolayers that were washed and diluted in phosphate-buffered saline (PBS) before counting and inoculation. Cells were stored on ice until injection.
Animals
Ten-week-old, female C57Bl/6 mice (Charles River) were maintained at the animal care facility of the Academic Medical Center, Amsterdam, The Netherlands, according to institutional guidelines. Animal procedures were carried out in compliance with Institutional Standards for Humane Care and Use of Laboratory Animals. The institutional Animal Care and Use Committee of the Academic Medical Center in Amsterdam approved all experiments.
Experimental pulmonary metastasis model
Cancer cells (3.5 × 105) suspended in 200 μL PBS were injected into the lateral tail vein as described before.22–24 After 14 days, mice were anesthetized with Domitor (Pfizer Animal Health Care; the active compound is medetomidine) and Nimatek (Eurovet Animal Health; the active compound is ketamine) and killed by vena cava puncture. Lungs were fixed directly with 4% paraformaldehyde administered through the trachea and were removed afterward. The lungs were kept in paraformaldehyde solution. Formaldehyde was substituted after 24 hours by 70% alcohol. Tumor foci on the surface of the lungs were counted macroscopically with the use o a binocular in a blinded fashion with respect to the intervention. Experiments were performed with 8 mice per group; however, results of mice with inadequate tumor cell inoculation as documented at the time of injection were not used for further analysis. Macroscopic lung pictures were acquired with a Leica MZ 9 5 stereo microscope, with occulair 10×/21B and an interchangeable objective 0.5 x PLAN. The magnification changer was at position 1.0 resulting in a 15.75 final magnification. Pictures were made with a Leica DFC 320 camera and processed with Adobe Photoshop CS Version 8.0.
Monoclonal antibodies
Endogenous APC formation was blocked with the use of the MPC1609 and MAPC1591 monoclonal antibodies to (A)PC as described previously.25 The class-matched antibody MCO1716 that is targeted against the keyhole limpet hemocyanin protein was used as negative control. Both MPC1609 and MAPC1591 inhibit APC anticoagulant activity in vivo, whereas MPC1609 also inhibits APC signaling effects. Antibodies were dialyzed with the use of the Slide-A-Lyzer (Pierce) 3.5 kDa, to remove azide. The various antibodies were injected intraperitoneally (200 μg in 0.9% NaCl) at 30 minutes before cancer cell inoculation. Antibody administration was repeated 48 and 96 hours after cancer cell inoculation. SEW2871, a selective S1P1 agonist (BIOMOL International), was administered intraperitoneally (10 mg/kg body weight)26,27 4 hours before cancer cell inoculation and was repeated once daily for 5 days.
In vivo vascular permeability assay
Vascular endothelial barrier function was assessed with the use of the Evans Blue Dye assay as described previously.28,29 In brief, Evans Blue (Sigma-Aldrich; 20 mg/kg) was injected intravenously at 3 hours before mice were killed. At time of killing, blood was sampled by vena cava puncture. Subsequently, lungs were perfused in situ with PBS through the right ventricle to remove intravascular dye from the lungs. After homogenization of the lungs, Evans Blue was extracted by the addition of 2 volumes of formamide followed by incubation for 16 hours at 60°C. After centrifugation at 5000g for 30 minutes, the absorption of Evans Blue was measured spectrophotometrically at a wavelength of 620 nm. Vascular leakage was expressed as OD620/gram of lung weight.
Hematoxylin–eosin and VE-cadherin staining
Paraffin-embedded sections of mouse lung (thickness, 3 μm) were stained with hematoxylin–eosin according to routine procedures. Immunostaining was performed on deparaffinized and rehydrated sections. Endogenous peroxidase activity was quenched with 0.03% H2O2 in methanol. After antigen retrieval (incubation for 10 minutes at 100°C in 10 mM sodium citrate, pH 6.0), sections were incubated in 10% goat serum in PBS (Dako) for 15 minutes to reduce nonspecific staining. Afterward, section were incubated with anti–mouse VE-cadherin monoclonal antibody (diluted 1:50 in PBS; Abcam) for 60 minutes at room temperature. Slides were incubated with goat anti–rabbit peroxidase-conjugated secondary antibody (Dako; diluted 1:200 in PBS) and visualized with aminoethylcarbazol as peroxidase substrate (Dako). The sections were counterstained with hematoxylin, mounted in glycerin-gelatin and analyzed with the use of a Leica DC 200 (Leica) microscope with a 40× or 100× magnification, and images were captured. Control incubations were performed in the absence of a primary antibody. All slides were analyzed by a blinded observer. Histology slides were pictured using a Leica DMLB microscope with oculars HC Plan S 10×/22. Pictures were taken with a Leica DC 200 camera and processed using Leica Qwin (1991-2002). For the HE-stained slides, pictures were acquired by magnification 40×/075 oo.0.17/D HC PL Fluotar lens. VE-cadherin pictures were acquired by magnification with 100×/1.30 oil oo/0.17/D HC PL Fluotar lens.
Statistical analysis
Statistical analysis was carried out in GraphPad Prism Version 4.03 (GraphPad Software Inc). Data are expressed as mean plus or minus SEM. For normally distributed data, significance was assessed with the Student t test. For not normally distributed data, nonparametric testing was performed with the Mann-Whitney test. Statistical significance was assumed when the P value was less than .05.
Results
Endogenous APC protects against cancer cell extravasation
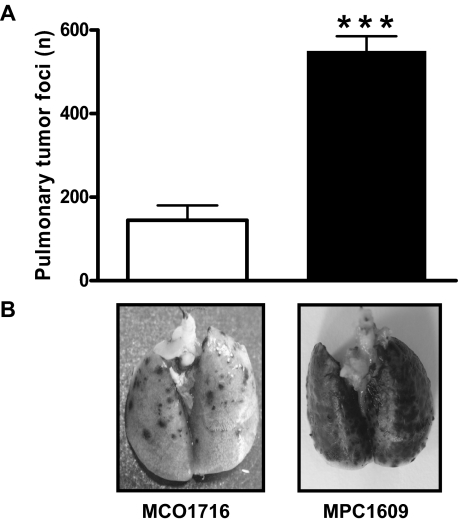
To determine the effect of endogenous APC on cancer cell extravasation, we compared the number of lung tumors in mice treated with the anti-PC antibody MPC1609 and control antibody MCO1716, respectively. As shown in Figure 1, blocking of APCs anticoagulant and signaling properties increased the number of pulmonary tumor foci 3- to 4-fold compared with control mice. Thus, endogenous APC limits the development of experimental melanoma metastasis in the lung, and endogenous APC limits cancer cell extravasation.
Figure 1.
Effect of endogenous APC on the number of B16F10 pulmonary tumor foci in C57Bl/6 mice. (A) Mice were treated intraperitoneally with 200 μg of an antibody that blocks both anticoagulant and signaling properties of APC (MPC1609; ■) or a control antibody (MCO1716; □) at 30 minutes before the administration of 3.5 × 105 B16F10 melanoma cells into the lateral tail vein. Antibody administration was repeated at 48 and 96 hours after melanoma cancer cell inoculation. Mice were killed at 14 days after cancer cell injection, and the number of tumor foci at the surface of the lungs was determined. Error bars represent means ± SEMs (n = 6-8), ***P < .001. (B) Representative lungs of mice treated with MCO1716 and MPC1609, respectively. Dark dots in the lungs represent melanoma tumors.
Endogenous APC protects against cancer cell extravasation independent of anticoagulant activity
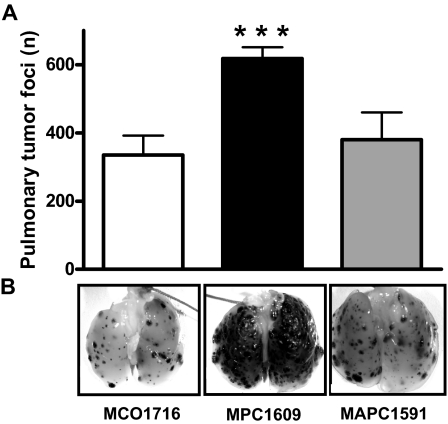
To discriminate between the anticoagulant and signaling properties of APC on cancer cell extravasation, we compared the effects of treatment with the MPC1609 antibody and the MAPC1591 antibody because this latter antibody does not affect APC-dependent signal transduction.25 Treatment with MPC1609 resulted in a highly significant increased number of pulmonary tumor foci, whereas MAPC1591 antibody treatment resulted in a similar number of pulmonary tumor foci as in the MCO1716 control group (Figure 2). These data show that endogenous APC protects against cancer cell extravasation via its cell-signaling capacity and independently of its anticoagulant activity.
Figure 2.
Differential effects of the anticoagulant and signaling properties of endogenous APC on the number of B16F10 pulmonary tumor foci in C57Bl/6 mice. (A) Mice were treated intraperitoneally with 200 μg of an antibody that blocks both anticoagulant and signaling properties of APC (MPC1609; ■), an antibody that only blocks the anticoagulant activity of APC (MAPC1591; ▩), or a control antibody (MCO1716; □) at 30 minutes before the administration of 3.5 × 105 B16F10 melanoma cells into the lateral tail vein. Antibody administration was repeated at 48 and 96 hours after cancer cell inoculation. Mice were killed at 14 days after cancer cell inoculation, and the number of tumor foci at the surface of the lungs was determined (A). Error bars represent means ± SEMs (n = 6-8), ***P < .001. (B) Representative lungs of mice treated with MCO1716, MPC1609, and MAPC1591 antibodies, respectively. Dark dots in the lungs represent melanoma tumors.
Endogenous APC signaling protects against cancer cell extravasation through S1P1-mediated barrier protection
The cell-signaling properties of APC are known to enhance vascular endothelial barrier function in inflammation models via the S1P1 pathway.12,18,30 To determine the importance of the S1P1 pathway in APC-dependent cancer cell extravasation, mice were treated with the selective S1P1 agonist SEW2871 in the presence or absence of APC. The number of pulmonary foci is similar in MPC1609- and MOC1716-treated mice when S1P1 is activated constitutively (data not shown).
To further strengthen the notion that endogenous APC signaling protects endothelial barrier function in a model of cancer cell extravasation, we assessed APC-S1P1–dependent vascular barrier function. Figure 3 shows that cancer cells induced vascular leakage 5 days after inoculation. MPC1609 treatment markedly increased cancer cell–induced vascular leakage. Coadministration of the selective S1P1 receptor agonist SEW2871 with cancer cells completely abrogated MPC1609-induced vascular leakage. These data suggest that endogenous APC limits cancer cell extravasation through activation of the S1P1 pathway, thereby enhancing vascular barrier function.
Figure 3.
Endogenous APC protects against cancer cell–induced vascular leakage in the lung through S1P1. Vascular leakage in the lungs was assessed in C56Bl/6 mice treated intraperitoneally with 200 μg of an antibody that both blocks anticoagulant and signaling properties (MPC1609; ■) and a control antibody (MCO1716; □) at 30 minutes before the administration of 1.75 × 105 B16F10 into the lateral tail vein in the absence or presence of the S1P1 agonist SEW2871 (10 mg/kg), using the Evans Blue Dye assay. Antibody administration was repeated at 48 and 96 hours after cancer cell inoculation. Mice were killed at 5 days after cancer cell inoculation. Evans Blue Dye was injected intravenously (20 mg/kg) at 3 hours before mice were killed and lungs were harvested, homogenized, and incubated at 60°C in formamide for 16 hours to extract the Evans Blue dye. Vascular leakage was subsequently assessed spectrophotometrically at a wavelength of 620 nm and is indicated as OD620 per gram of lung tissue. Error bars represent means ± SEMs (n = 7-8), ***P < .001; **P < .01.
APC signaling prevents cancer cell extravasation through VE-cadherin expression
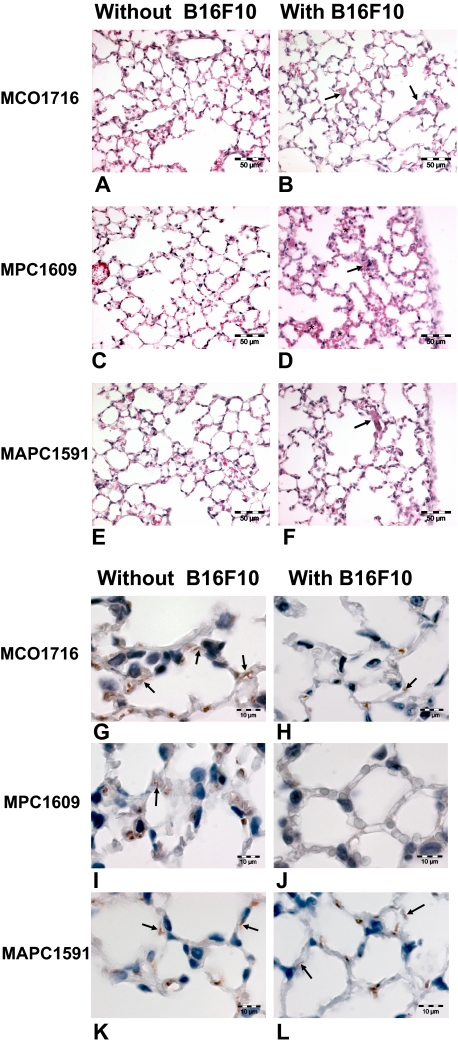
S1P1 is known to enhance barrier function by stabilizing VE-cadherin–dependent cell-cell junctions. The APC blocking antibodies did not affect lung morphology in the absence of cancer cells (Figure 4A,C,E). However, treatment of MPC1609 and cancer cell inoculation resulted in alveolar edema and focal erythrocyte extravasation (Figure 4D). In contrast, MAPC1591 and MCO1716 treatment in combination with cancer cell inoculation did not induce edema formation or erythrocyte extravasation (Figure 4B,F). To substantiate these findings, we performed immunohistologic VE-cadherin staining. As shown in Figure 4 (G-L), cancer cell administration down-regulated VE-cadherin expression on the vascular endothelium. MPC1609 treatment and cancer cell inoculation (almost) completely abolished VE-cadherin expression, whereas MAPC1591 treatment resulted in comparable VE-cadherin expression levels (Figure 4L) to the treatment of cancer cells alone (Figure 4H). Overall, these data suggest that APC targets the S1P1 pathway, thereby limiting cancer cell–induced disruption of VE-cadherin–mediated cell-cell junctions.
Figure 4.
Endogenous APC protects the endothelial barrier by inducing VE-cadherin expression. Lung histology was assessed in C56Bl/6 mice treated with 200 μg control MCO1716 antibody (A,B,G,H) or APC blocking antibodies MPC1609 (C,D,I,J) or MAPC1591 (E,F,K,L) in the presence (B,D,F,H,J,L) or absence (A,C,E,G,I,J) of B16F10 cancer cells. (A-F) hematoxylin–eosin staining section showing normal histology of lungs treated with antibody alone (A,C,E) and virtual normal histology of lungs containing B16F10 cancer cell ( ) and treated with MCO1716 (B) and MAPC 1591 antibody (F). Treatment with cancer cells at 1 hour after inoculation and MPC1609 antibody caused accumulation of erythrocytes in the alveolar walls (*), indicating extravasation (D). Bar = 50 μm. (G-L) Immunohistochemical staining of VE-cadherin showing endothelial cell adherens junctions (
) and treated with MCO1716 (B) and MAPC 1591 antibody (F). Treatment with cancer cells at 1 hour after inoculation and MPC1609 antibody caused accumulation of erythrocytes in the alveolar walls (*), indicating extravasation (D). Bar = 50 μm. (G-L) Immunohistochemical staining of VE-cadherin showing endothelial cell adherens junctions ( ) in lungs treated with antibody alone (G,I,K). Adherens junctions in lungs at 1 hour after cancer cell inoculation and treatment with MCO1716 and MAPC 1591 antibody was reduced in comparison to lungs that did not receive cancer cells. Adherens junctional staining is completely lost after treatment with cancer cells and MPC1609 antibody (J). In this case the alveolar walls were swollen. Erythrocytes were also stained positive for VE-cadherin. Bar = 10 μm.
) in lungs treated with antibody alone (G,I,K). Adherens junctions in lungs at 1 hour after cancer cell inoculation and treatment with MCO1716 and MAPC 1591 antibody was reduced in comparison to lungs that did not receive cancer cells. Adherens junctional staining is completely lost after treatment with cancer cells and MPC1609 antibody (J). In this case the alveolar walls were swollen. Erythrocytes were also stained positive for VE-cadherin. Bar = 10 μm.
Discussion
We aimed to elucidate the role of the anticoagulant and cell-signaling effects of endogenous APC on cancer cell extravasation in vivo. Our data indicate that endogenous APC limits cancer cell extravasation through its signaling properties independent of its anticoagulant activity. Moreover, endogenous APC diminishes cancer cell extravasation because of endothelial barrier protection via the S1P1 axis; APC signaling targets VE-cadherin expression, thereby preserving stability of endothelial cell-cell junctions. This implicates that endogenous APC signaling plays a key role in vivo to inhibit cancer cell extravasation.
Our data suggest that the availability of endogenous APC may be an important clinical and pharmacologic parameter in patients with cancer. Interestingly, APC deficiency is observed in patients with cancer, especially in patients using certain types of chemotherapy.31,32 Moreover, anticoagulant treatment, which is regularly prescribed to patients with cancer with thrombotic complications, may significantly affect endogenous APC generation.33 Consequently, preservation or restoration of endogenous APC generation or both might thus be an interesting target for limiting cancer progression.
Our findings that endogenous APC limits metastasis by signal transduction–mediated vascular barrier enhancement through S1P1 signaling are in agreement with studies in patients with systemic inflammation. The PROWESS trial showed that APC treatment significantly reduced mortality in patients with severe sepsis.34 Subsequent experimental animal models that used APC mutants with diminished anticoagulant activity but normal cytoprotective properties showed that the protective effect of APC in systemic inflammation depends on its cytoprotective activity.6 More insight into the protective role of APC in inflammatory disease was obtained by animal studies, showing that APC protects the endothelial barrier through S1P1 cross-activation.12,14 The relevance of this latter observation was recently established by showing that APC-S1P1–dependent signaling prevents inflammation-induced vascular leakage and lethality in mice.18
The anticoagulant capacity of APC does not seem to affect lung tumor colony formation in our experiments. This is surprising because thrombin plays an essential role in metastasis.2,3 Interventions with direct or indirect thrombin inhibitors, ie, hirudin and low molecular weight heparin, respectively, inhibit experimental metastasis.35–38 Besides, congenital susceptibility to either bleeding or thrombosis modifies the metastatic capacity of some types of cancer cells23 but not others39 in the blood stream. Hemophilic (factor VIII deficient) mice were protected against melanoma lung metastasis, whereas thrombophilic factor V Leiden mice developed more metastases than did wild-type littermates.23 At first glance there may be discrepancies between our data and this previous report on factor V Leiden in cancer biology. Both factor V Leiden mice and mice treated with MAPC1591 are thrombophilic, and the cytoprotective effects of APC are preserved. We do not have a proper explanation for this potential discrepancy yet. However, it should be realized that factor V Leiden mice do not necessarily have the same properties as mice in which the anticoagulant function of APC is blocked selectively. For instance, factor V activation products that are lacking in factor V Leiden mice may induce specific effects. In addition, life-long thrombophilia in factor V Leiden mice may induce compensatory mechanisms affecting cancer cell extravasation that are not induced in APC antibody-treated mice.
Because endogenous APC limits cancer cell extravasation may suggest that exogenous APC administration would be a potential novel therapeutic avenue to fight cancer metastasis. However, administration of APC may not be as straightforward as expected in patients with cancer. Most importantly, APC has a short half-life of approximately 15 minutes and therefore requires continuous infusion for optimal effect. Such a treatment strategy seems feasible for severe sepsis, where optimal timing of short-term treatment is evident (ie, immediately after admission), but it seems unrealistic for patients with cancer. The timing of APC administration in patients with cancer would be difficult to establish, and APC treatment would probably require long-term treatment. Moreover, the clinically relevant complications of APC treatment, such as bleeding, suggest that long-term treatment may be deleterious.40 One should realize however that these bleeding complications occur with high-dose APC, whereas we show that endogenous levels appear adequate when bleeding should be much less of a complication. Moreover, high doses of APC are barrier disruptive,12 suggesting that adequate dosing of APC is highly relevant. Indeed, a case report of a patient with colorectal cancer and sepsis showed massive symptomatic bone marrow infiltration after APC administration.41 Although this case report may show a barrier disruptive effect of APC, one cannot rule out the role of sepsis in this particular patient.
Despite these potential objections to exogenous APC administration, Bezuhly et al42 recently showed that repeated administration of exogenous human APC reduced the number of experimental metastasis in mice. Our results extend these interesting observations on the effects of exogenous APC in cancer metastasis. In that study, the endogenous protein C pathway remained intact, leaving the question open whether the signaling or anticoagulant functions of the endogenous protein C pathway were important. Here, we pinpoint the important effect of endogenous APC signaling on vascular endothelial barrier integrity in limiting cancer cell extravasation.
The experimental metastasis model, although routinely used,23,24 mimics the pathophysiology of the metastatic process only partially because cancer cells are inoculated directly into the bloodstream and are not primary tumor derived. However, this particular model selectively assesses the extravasation step of cancer metastasis43 and allows studies on cancer cell extravasation without confounding treatment effects on the primary tumor. Importantly, B16F10 cells specifically extravasate in the lung vasculature,43 mimicking the clinical setting in which melanoma preferentially metastasize to the lung. Thus, although our model is appropriate to study cancer cell extravasation, subsequent experiments should validate our findings in spontaneous metastasis models to determine the effect of endogenous APC on primary tumor growth and angiogenesis, thereby establishing the net effect of endogenous APC on cancer progression and metastasis.
A final important finding of our study is that coadministration of SEW2871 abolishes the aggravating effect of MPC1609 on cancer cell extravasation. The S1P1 selective agonist on itself, however, resulted in more pulmonary tumor foci. This is in line with the notion that S1P1 is known to impair immune responses and to affect cell survival and angiogenesis.44–46
In conclusion, our data show that (1) endogenous APC limits cancer cell extravasation, (2) APC limits cancer cell extravasation via its signaling properties, (3) cancer cell extravasation is diminished because of endothelial barrier protection via the S1P axis, and (4) APC signaling targets VE-cadherin expression, thereby preserving stability of endothelial cell-cell junctions. Overall, the APC pathway provides us with a potential clinically relevant finding in cancer progression and metastasis.
Acknowledgments
We thank Joost Daalhuisen and Marieke Ten Brink of the Center for Experimental and Molecular Medicine of the Academic Medical Center in Amsterdam for the carefully performed animal experiments.
This work was supported by the Leducq Foundation (C.T.E.). C.T.E. is an investigator of the Howard Hughes Medical Institute.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: G.L.V.S. designed and performed the research, collected, analyzed, and interpreted data, performed statistical analysis, and wrote the manuscript; T.M.H.N. designed the research and contributed to writing the manuscript; C.T.E. contributed vital new reagents and performed critical reading; W.T. collected, analyzed, and interpreted data, and performed the analysis of histochemical stainings; D.J.R. and H.R.B. designed the research and contributed to writing the manuscript; C.J.F.V.N. designed the research, collected, analyzed, and interpreted data, performed the analysis of histochemical stainings; and contributed to writing the manuscript; and C.A.S. designed and performed the research, collected, analyzed, and interpreted data, performed statistical analysis, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: G. L. Van Sluis, Department of Vascular Medicine, Academic Medical Center, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; e-mail: g.l.vansluis@amc.uva.nl.
References
- 1.Esmon CT. The protein C pathway. Chest. 2003;124(90030):26S–32S. doi: 10.1378/chest.124.3_suppl.26s. [DOI] [PubMed] [Google Scholar]
- 2.Nierodzik ML, Plotkin A, Kajumo F, Karpatkin S. Thrombin stimulates tumor-platelet adhesion in vitro and metastasis in vivo. J Clin Invest. 1991;87(1):229–236. doi: 10.1172/JCI114976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nierodzik ML, Kajumo F, Karpatkin S. Effect of thrombin treatment of tumor cells on adhesion of tumor cells to platelets in vitro and tumor metastasis in vivo. Cancer Res. 1992;52(12):3267–3272. [PubMed] [Google Scholar]
- 4.Hu L, Lee M, Campbell W, Perez-Soler R, Karpatkin S. Role of endogenous thrombin in tumor implantation, seeding, and spontaneous metastasis. Blood. 2004;104(9):2746–2751. doi: 10.1182/blood-2004-03-1047. [DOI] [PubMed] [Google Scholar]
- 5.Akl EA, van Doormaal FF, Barba M, et al. Parenteral anticoagulation for prolonging survival in patients with cancer who have no other indication for anticoagulation. Cochrane Database Syst Rev. 2007;(3):CD006652. doi: 10.1002/14651858.CD006652. [DOI] [PubMed] [Google Scholar]
- 6.Kerschen EJ, Fernandez JA, Cooley BC, et al. Endotoxemia and sepsis mortality reduction by non-anticoagulant activated protein C. J Exp Med. 2007;204(10):2439–2448. doi: 10.1084/jem.20070404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mosnier LO, Yang XV, Griffin JH. Activated protein C mutant with minimal anticoagulant activity, normal cytoprotective activity, and preservation of thrombin activable fibrinolysis inhibitor-dependent cytoprotective functions. J Biol Chem. 2007;282(45):33022–33033. doi: 10.1074/jbc.M705824200. [DOI] [PubMed] [Google Scholar]
- 8.Ruf W. PAR1 signaling: more good than harm? Nat Med. 2003;9(3):258–260. doi: 10.1038/nm0303-258. [DOI] [PubMed] [Google Scholar]
- 9.Beaulieu LM, Church FC. Activated protein C promotes breast cancer cell migration through interactions with EPCR and PAR-1. Exp Cell Res. 2007;313(4):677–687. doi: 10.1016/j.yexcr.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uchiba M, Okajima K, Oike Y, et al. Activated protein C induces endothelial cell proliferation by mitogen-activated protein kinase activation in vitro and angiogenesis in vivo. Circ Res. 2004;95(1):34–41. doi: 10.1161/01.RES.0000133680.87668.FA. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi H, Moniwa N, Gotoh J, Sugimura M, Terao T. Role of activated protein C in facilitating basement membrane invasion by tumor cells. Cancer Res. 54(1):261–267. [PubMed] [Google Scholar]
- 12.Feistritzer C, Riewald M. Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood. 2005;105(8):3178–3184. doi: 10.1182/blood-2004-10-3985. [DOI] [PubMed] [Google Scholar]
- 13.Riewald M, Petrovan RJ, Donner A, Ruf W. Activated protein C signals through the thrombin receptor PAR1 in endothelial cells. J Endotoxin Res. 2003;9:317–321. doi: 10.1179/096805103225002584. [DOI] [PubMed] [Google Scholar]
- 14.Finigan JH, Dudek SM, Singleton PA, et al. Activated protein C mediates novel lung endothelial barrier enhancement: role of sphingosine 1-phoshate receptor transactivation. J Biol Chem. 2005;280(17):17286–17293. doi: 10.1074/jbc.M412427200. [DOI] [PubMed] [Google Scholar]
- 15.Bae JS, Yang L, Rezaie AR. Receptors of the protein C activation and activated protein C signaling pathways are colocalized in lipid rafts of endothelial cells. Proc Natl Acad Sci U S A. 2007;104(8):2867–2872. doi: 10.1073/pnas.0611493104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JF, Zeng Q, Ozaki H, et al. Dual roles of tight junction-associated protein, zonula occludens-1, in sphingosine 1-phosphate-mediated endothelial chemotaxis and barrier integrity. J Biol Chem. 2006;281(39):29190–29200. doi: 10.1074/jbc.M604310200. [DOI] [PubMed] [Google Scholar]
- 17.Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther. 2007;115(1):84–105. doi: 10.1016/j.pharmthera.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Niessen F, Furlan-Freguia C, Fernandez JA, et al. Endogenous EPCR/aPC-PAR1 signaling prevents inflammation-induced vascular leakage and lethality. Blood. 2009;113(12):2859–2866. doi: 10.1182/blood-2008-12-192385. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Galaup A, Cazes A, Le Jan S, et al. Angiopoietin-like 4 prevents metastasis through inhibition of vascular permeability and tumor cell motility and invasiveness. Proc Natl Acad Sci U S A. 2006;103(49):18721–18736. doi: 10.1073/pnas.0609025103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Nieuw Amerongen GP, Beckers CML, Achekar ID, Zeeman S, Musters RJP, van Hinsbergh VWM. Involvement of Rho kinase in endothelial barrier maintenance. Arterioscler Thromb Vasc Biol. 2007;27(11):2332–2339. doi: 10.1161/ATVBAHA.107.152322. [DOI] [PubMed] [Google Scholar]
- 21.Criscuoli ML, Nguyen M, Eliceiri BP. Tumor metastasis but not tumor growth is dependent on Src-mediated vascular permeability. Blood. 2005;105(4):1508–1514. doi: 10.1182/blood-2004-06-2246. [DOI] [PubMed] [Google Scholar]
- 22.Brüggemann LW, Versteeg HH, Reitsma PH, Spek CA. High factor VIIa levels do not promote tumor metastasis. Thromb Haemost. 2008;99(4):787–788. doi: 10.1160/TH07-11-0702. [DOI] [PubMed] [Google Scholar]
- 23.Brüggemann LW, Versteeg HH, Niers TM, Reitsma PH, Spek CA. Experimental melanoma metastasis in lungs of mice with congenital coagulation disorders. J Cell Mol Med. 2008;12(6B):2622–2627. doi: 10.1111/j.1582-4934.2008.00316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3(6):453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 25.Xu J, Ji I, Zhang X, Drake M, Esmon CT. Endogenous activated protein C signaling is critical to protection of mice from lipopolysaccaride induced septic shock. J Thromb Haemost. 2009;7(5):851–856. doi: 10.1111/j.1538-7836.2009.03333.x. [DOI] [PubMed] [Google Scholar]
- 26.Tager AM, LaCamera P, Shea BS, et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14(1):45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- 27.Awad AS, Ye H, Huang L, et al. Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol. 2006;290(6):F1516–F1524. doi: 10.1152/ajprenal.00311.2005. [DOI] [PubMed] [Google Scholar]
- 28.Cavriani G, Oliveira-Filho RM, Trezena AG, et al. Lung microvascular permeability and neutrophil recruitment are differently regulated by nitric oxide in a rat model of intestinal ischemia-reperfusion. Eur J Pharmacol. 2004;494(2-3):241–249. doi: 10.1016/j.ejphar.2004.04.048. [DOI] [PubMed] [Google Scholar]
- 29.Sanna MG, Wang SK, Gonzalez-Cabrera PJ, et al. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nat Chem Biol. 2006;2(8):434–441. doi: 10.1038/nchembio804. [DOI] [PubMed] [Google Scholar]
- 30.Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002;296(5574):1880–1882. doi: 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]
- 31.Mewhort-Buist TA, Liaw PC, Patel S, Atkinson HM, Berry LR, Chan AKC. Treatment of endothelium with the chemotherapy agent vincristine affects activated protein C generation to a greater degree in newborn plasma than in adult plasma. Thromb Res. 2008;122(3):418–426. doi: 10.1016/j.thromres.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Woodley-Cook J, Shin LYY, Swystun L, Caruso S, Beaudin S, Liaw PC. Effects of the chemotherapeutic agent doxorubicin on the protein C anticoagulant pathway. Mol Cancer Ther. 2006;5(12):3303–3311. doi: 10.1158/1535-7163.MCT-06-0154. [DOI] [PubMed] [Google Scholar]
- 33.Warkentin TE. Venous limb gangrene during warfarin treatment of cancer-associated deep venous thrombosis. Ann Intern Med. 2001;135(8 Pt 1):589–593. doi: 10.7326/0003-4819-135-8_part_1-200110160-00009. [DOI] [PubMed] [Google Scholar]
- 34.Bernard GR, Vincent JL, Laterre PF, et al. The recombinant human activated protein. efficacy and safety of recombinant human activated protein c for severe Sepsis. N Engl J Med. 2001;344(10):699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 35.Esumi N, Fan D, Fidler IJ. Inhibition of murine melanoma experimental metastasis by recombinant desulfatohirudin, a highly specific thrombin inhibitor. Cancer Res. 1991;51(17):4549–4556. [PubMed] [Google Scholar]
- 36.Niers TMH, Klerk CPW, DiNisio M, et al. Mechanisms of heparin induced anti-cancer activity in experimental cancer models. Crit Rev Oncol Hematol. 2007;61(3):195–207. doi: 10.1016/j.critrevonc.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Mousa SA, Linhardt R, Francis JL, Amirkhosravi A. Anti-metastatic effect of a non-anticoagulant low-molecular-weight heparin versus the standard low-molecular-weight heparin, enoxaparin. Thromb Haemost. 2006;96(6):816–821. doi: 10.1160/th06-05-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smorenburg SM, Van Noorden CJF. The complex effects of heparins on cancer progression and metastasis in experimental studies. Pharmacol Rev. 2001;53(1):93–106. [PubMed] [Google Scholar]
- 39.Klerk CP, Smorenburg SM, Spek CA, Van Noorden CJ. Colon cancer metastasis in mouse liver is not affected by hypercoagulability due to factor V Leiden mutation. J Cell Mol Med. 2007;11(3):561–568. doi: 10.1111/j.1582-4934.2007.00046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levi M. Activated protein C in sepsis: a critical review. Curr Opin Hematol. 2008;15(5):481–486. doi: 10.1097/MOH.0b013e328304b3e3. [DOI] [PubMed] [Google Scholar]
- 41.Pleyer L, Went P, Russ G, et al. Massive infiltration of bone marrow in colon carcinoma after treatment with activated protein C. Wiener Klinische Wochenschrift. 2007;119(7):254–258. doi: 10.1007/s00508-007-0774-7. [DOI] [PubMed] [Google Scholar]
- 42.Bezuhly M, Cullen R, Esmon CT, et al. Role of activated protein C and its receptor in inhibition of tumor metastasis. Blood. 2009;113(14):3371–3374. doi: 10.1182/blood-2008-05-159434. [DOI] [PubMed] [Google Scholar]
- 43.Crissman JD, Hatfield J, Schaldenbrand M, Sloane BF, Honn KV. Arrest and extravasation of B16 amelanotic melanoma in murine lungs. A light and electron microscopic study. Lab Invest. 1985;53(4):470–478. [PubMed] [Google Scholar]
- 44.Yamaguchi H, Kitayama J, Takuwa N, et al. Sphingosine-1-phosphate receptor subtype-specific positive and negative regulation of Rac and haematogenous metastasis of melanoma cells. Biochem J. 2003;374(3):715–722. doi: 10.1042/BJ20030381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol. 2005;5(7):560–570. doi: 10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- 46.Schmid G, Guba M, Ischenko I, et al. The immunosuppressant FTY720 inhibits tumor angiogenesis via the sphingosine 1-phosphate receptor 1. J Cell Biochem. 2007;101(1):259–270. doi: 10.1002/jcb.21181. [DOI] [PubMed] [Google Scholar]