Abstract
Angiostatin, a proteolytic fragment of plasminogen, is a potent endogenous antiangiogenic agent. The molecular mechanisms governing angiostatin's antiangiogenic and antitumor effects are not well understood. Here, we report the identification of mitochondrial compartment as the ultimate target of angiostatin. After internalization of angiostatin into the cell, at least 2 proteins within the mitochondria bind this molecule: malate dehydrogenase, a member of Krebs cycle, and adenosine triphosphate synthase. In vitro and in vivo studies revealed differential regulation of key prosurvival and angiogenesis-related proteins in angiostatin-treated tumors and tumor-endothelium. Angiostatin induced apoptosis via down-regulation of mitochondrial BCL-2. Angiostatin treatment led to down-regulation of c-Myc and elevated levels of another key antiangiogenic protein, thrombospondin-1, reinforcing its antitumor and antiangiogenic effects. Further evidence is provided for reduced recruitment and infiltration of bone marrow–derived macrophages in angiostatin-treated tumors. The observed effects of angiostatin were restricted to the tumor site and were not observed in other major organs of the mice, indicating unique tumor specific bioavailability. Together, our data suggest mitochondria as a novel target for antiangiogenic therapy and provide mechanistic insights to the antiangiogenic and antitumor effects of angiostatin.
Introduction
Human plasminogen is an abundant protein in blood circulation. Its plasma concentration is approximately 200 μg/L. It is a glycoprotein with a molecular mass of 92 kDa, containing 2% carbohydrate and consisting of 5 kringles with a total of 24 disulfide bonds.1
In 1994, it was reported that a proteolytic fragment of this protein, generated endogenously, demonstrated potent antiangiogenic activity in mice.2 It was called angiostatin and consisted of kringles 1 to 4 of plasminogen. It is reasonable to assume that angiostatin has properties distinct from plasminogen. Presumably, removal of a segment of plasminogen introduces a conformational change in the molecule that confers unique binding properties compared with the plasminogen.
To understand the mechanism of action of angiostatin, a search for receptors and binding proteins was initiated by several laboratories. Annexin,3 angiomotin,4 integrin αvβ3,5 and c-met6 have been identified as some of the prominent candidates on the cell surface for binding angiostatin. However, some of these proteins appear also to bind plasminogen, thus failing to describe the distinct properties of angiostatin compared with its abundant precursor protein, plasminogen.7
F1F0 adenosine triphosphate (ATP) synthase has been reported to be a surface-binding receptor on endothelial cells that selectively binds angiostatin but not plasminogen.8–11 ATP synthase is a multicomponent enzyme with mechanochemical properties.12 It couples ATP synthesis to rotation of the molecule, generating a pumping mechanism for protons. Depending on the clockwise or counterclockwise rotation of the molecule, ATP and adenosine diphosphate are interconverted, and protons are pumped in or out. Despite the controversies on localization of the ATP synthase on endothelial cell surface, in addition to mitochondria, evidence from other laboratories confirmed this observation.13,14
To investigate angiostatin's mechanism of action, we used protein affinity purification to identify potential angiostatin-binding partner. We have now discovered mitochondrial malate dehydrogenase (MDH2), a member of Krebs cycle, as an angiostatin-binding protein.
Energy production in the cells depends on 2 pathways: one is glycolysis, which originally in evolution was an anaerobic process. The second pathway is the more efficient oxidative phosphorylation (Krebs cycle). The glycolytic pathway, although less efficient in terms of overall ATP production, is used by a large number of tumor cells for processing glucose to ATP and lactate. On the other hand, malignant cells produce large amounts of lactate dehydrogenase A (LDH-A). It has been shown that the ability of tumor cells to metabolize glucose was compromised by reducing LDH, limiting their proliferation under hypoxia conditions and stimulating mitochondrial respiration.15
More recently, genetic analysis in patients with brain tumor (glioblastoma multiforme) identified a mutation in the active site of the enzyme isocitrate dehydrogenase, a member of Krebs cycle.16
We identified cell type–specific actions of angiostatin by demonstrating selective inhibition of c-Myc, whereas a key antiangiogenic protein thrombospondin was up-regulated in endothelial cells. We confirmed these data in vivo in a human melanoma tumor xenograft model. We found reduced levels of mitochondrial antiapoptotic protein BCL-2 and increased apoptosis in angiostatin-treated tumors. Further, decreased c-Myc protein levels and elevated thrombospondin-1 expression correlated with impaired angiogenesis and reduced infiltration of bone marrow-derived macrophages in angiostatin-treated tumors.
Methods
Cell lines and cell culture
Human tumor cell lines A2058 (melanoma) and BxPC-3 (pancreatic) were cultured in Dulbecco modified Eagle medium or RPMI 1640 with l-glutamine, respectively, and supplemented with 10% fetal calf serum and antibiotics. Human umbilical vein endothelial cells (HUVECs) and human dermal microvascular endothelial cells (HMVECs; Lonza Walkersville) were maintained in EGM endothelial growth media and EGM Bullet Kit or EGM-2MV microvascular growth media (Lonza Walkersville) with antibiotics.
Human FcAS and mouse FcAS expression and purification
Construction, expression, and purification of human Fc-angiostatin (hFcAS) and mouse Fc-angiostatin (mFcAS) have been described previously.17 The recombinant constructs were prepared by placing the Fc regions at the N terminus of angiostatin. Stable cell lines of these constructs were produced in NS/0 murine myeloma cells. The proteins were expressed and secreted into the media. Protein A was used for purification of the recombinant proteins (at least 90% purity). We obtained approximately 50 mg/L of Fc-AS using fermentors of 10- to 18-L capacity.
Animal and tumor models
All animal procedures were carried out in compliance with Children's Hospital Boston guidelines. Protocols were approved by the Institutional Animal Care and Use Committee. Eight-week-old male (24-27 g) severe combined immunodeficiency (SCID) mice (Massachusetts General Hospital, Boston, MA) were used. Mice were acclimated, caged in groups of 5 in a barrier care facility, and fed animal chow and water ad libitum. Animals were killed by CO2 inhalation. Human melanoma cell line A2058 was used for animal studies. Mice were shaved, and the dorsal skin was cleaned with ethanol before cell injection. A suspension of 5 × 106 tumor cells in 0.1 mL of phosphate-buffered saline (PBS) was injected subcutaneously into the dorsa of mice at the proximal midline. Mice were weighed and tumors were monitored twice a week in 2 diameters with digital calipers. Tumor volumes were determined using a2 × b × 0.52 (where a is the shortest and b is the longest diameter). Tumors were allowed to grow to approximately 100 mm3, and mice were randomized. Treatment was by bolus subcutaneous injections. Concentrations of hFcAS were corrected for the Fc contribution. Consequently, all indicated concentrations refer to concentrations of angiostatin in Fc-angiostatin. After experiments were completed, tumors and organs were excised and fixed in either 4% paraformaldehyde or were snap frozen. Ten mice were treated with each dose of angiostatin. Unless specified otherwise, antitumor studies were performed with injection doses delivered every 6 days. The dose amounts were converted into mg/kg/day to compare with previously published data for angiostatin alone (without Fc).2
ELISA determination of hFcAS
Mouse serum samples were obtained by retro-orbital puncture with nonheparinized capillary tubes under anesthesia. Serum was collected after centrifugation at 10 000g for 10 minutes, and human IgG concentrations were determined by sandwich enzyme-linked immunosorbent assay (ELISA; GenWay) following the manufacturer's protocol.
TUNEL assay
Apoptosis was examined by use of the terminal deoxynucleotidyltransferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) assay18 through the manufacturer's protocol (Promega).
Western blotting analysis
In vivo, tumor masses were homogenized with RIPA buffer (Boston Bioproducts) containing a mixture of protease inhibitors (Roche). In vitro, cells were starved overnight and treated with hFcAS (10 μg/mL) or hFc in starvation medium for 6, 16 hours and lysed by the same buffer. Equal amount of total protein was fractionated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and then blotted onto nitrocellulose membrane. The protein blot was hybridized with thrombospondin-1 (TSP-1), c-Myc, BCL-2, Bax, and β-actin antibodies and then with secondary antibody, followed by detection with Renaissance chemiluminescence reagent (Thermo Fisher).
Flow cytometric analysis of hFcAS binding on cell-surface
All operations were performed at 4°C. Cells were trypsinized and resuspended in PBS (2% bovine serum albumin) for 30 minutes followed by 1 hour of incubation with 1 and 10 μg/mL hFcAS or hFc. Cells were centrifuged and washed by cold PBS and then incubated with fluorescein isothiocyanate-labeled secondary antibodies (Sigma-Aldrich) against human Fc fragment and analyzed by BD Biosciences FACS Calibur flow cytometer.
Immunocytochemistry
HUVECs and A2058 cells were plated on cover slips and fixed by 4% paraformaldehyde for surface staining (nonpermeabilized) or methanol, acetone mixture buffer (1:1 ratio) for intracellular staining (permeabilized). Cells were incubated with hFcAS for 2 hours and detected by Alexa 488 anti–human IgG. The cells were double-stained by anti-ATP synthase β subunit antibody, mitochondria marker (Mito-tracker), or actin staining (phalloidin) and detected by AlexaFluor 594 antibody and imaged by confocal microscopy (model DM IRE2; Leica). 4,6-Diamidino-2-phenylindole (DAPI) was used for counterstaining of nuclei.
Tumors and organs were embedded in OCT medium. Sections were rinsed by cold PBS and fixed with 4% paraformaldehyde for 10 minutes with before staining. Human Fc-angiostatin was detected by Alexa 488 antihuman IgG. Antibodies toTSP-1 (Thermo Fisher), CD31 (BD Biosciences PharMingen), and von Willebrand factor (Dako) were used for staining. Antibodies to CD11b (AbD Serotec), CD45 (BD Biosciences PharMingen), and F4/80 (Abcam) were used to detect host leukocytes, including monocytes and macrophages. The primary antibodies were detected by Alexa 488-, 568-, 594-, and 647-labeled secondary antibodies (Invitrogen). The sections were imaged by confocal microscopy (model DM IRE2; Leica). An F4/80 marker was considered positive if the fluorescent pixel was present on or around it.
RNA interference and MDH2 transfection
HUVECs or A2058 cells were seeded in 6-cm dishes at a density of 1.5 × 105 per dish and transfected at 50% confluence with 20 nM control nonspecific siRNA or ATP synthase α,β–specific siRNA (Dharmacon RNA Technologies) using Silentfect (Bio-Rad) according to the manufacturer's instructions. Seventy-two hours after transfection, cells were fixed or lysed for immunostaining or immunoblotting. The MDH2 clone was provided by Harvard Plasmid Repository (http://plasmid.med.harvard.edu/PLASMID). The MDH2 insert was cloned into a GFP destination vector (pcDNA-DEST47, Invitrogen). The vector expresses MDH2-GFP fusion protein under cytomegalovirus promoter and provides ampicillin and neomycin resistance for selection. The cloning and plasmid preparation procedure were performed by recombination according to the manufacturer's protocol (Invitrogen). The integrity of the MDH2 insert was confirmed by sequence analysis (GATC); 1.6 μg GFP-MDH2 plasmid was used for transfection assay through the protocol of the manufacturer.
Internalization assay
HUVECs were plated and grown to approximately 70% confluence on cover slips. Cells were incubated with 10 μg/mL hFcAS, hFc, or plasminogen for 30 minutes or 120 minutes at 37°C and then fixed by permeable condition. The slips were incubated in the blocking buffer (2% bovine serum albumin PBS) for 30 minutes and then incubated with Alexa 488 anti–human IgG and imaged by confocal microscopy.
Isolation of MDH2 by affinity chromatography
Mouse livers were homogenized in PBS + 50 mM octyl β-D glucoside followed by centrifugation and filtration. It was passed over a 10-mL column of Fc-Sepharose. The flow-through was subjected to a second affinity column containing Fc-angiostatin-Sepharose. The affinity columns were prepared according to the protocol provided by Pharmacia (GE Healthcare) using activated-CNBr Sepharose and purified mFc and mFcAS. Before application of the samples, both columns were equilibrated with 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 150 mM NaCl, 2 mM Mg, 2 mM Ca, pH 7.4. For elution, 90 mM citrate, 2 M NaCl, pH 3, was used. After dialysis overnight, the sample was concentrated.
ATP generation by bioluminescent luciferase assay
Confluence HUVECs and A2058 cells in 48-well plates were incubated with 10 or 50 μg/mL hFcAS or hFc with fresh medium for 30, 120, and 360 minutes in 37°C. Aliquots of cellular supernatants from cell surface ATP and cell lysis buffer (100 mM Tris, 4 mM ethylenediaminetetraacetic acid, pH 7.75) from intracellular ATP were analyzed using the ATP bioluminescence assay kit (Roche).
Statistical methods
Data are expressed as mean plus or minus SD. Statistical significance was assessed using the Student t test.
Results
In vivo efficacy and bioavailability of angiostatin
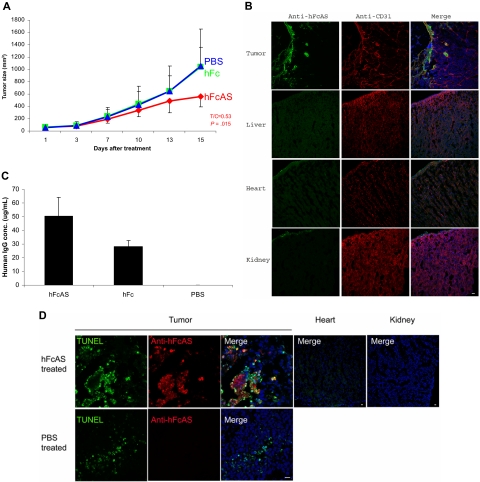
To examine the antitumor properties of angiostatin, SCID mice received subcutaneous injections of cultured tumor cells (human melanoma A2058). Animals were given a dose of 100 μg of hFcAS/mouse once every 6 days (subcutaneously). An approximate 50% reduction of tumor volume was observed as a result of treatment with angiostatin (Figure 1A). The distribution of exogenous angiostatin, using antibody to Fc, is shown in Figure 1B. The concentration of exogenous angiostatin in the liver, heart, and kidney was found to be below the detection level in treated mice. In contrast, angiostatin was highly enriched in tumor, in particular in surrounding tumor vessels. This phenomenon is probably the result of leaky tumor vasculature.19
Figure 1.
Treatment of mouse bearing human melanoma cancer cells (A2058) with hFc-angiostatin. (A) Ten tumor-bearing SCID mice in each group were subcutaneously treated with hFcAS, hFc (100 μg/mouse once every 6 days), or PBS. Treatment was stopped before the development of necrosis. Sites of injection were away from tumors. Tumor sizes and the ratio of treated/control (T/C) and P values are shown for the hFcAS-treated group. (B) Immunostaining shows that treated angiostatin (green) only bound in tumor masses but not in normal organs, such as liver, heart, and kidney. CD31 staining for vessels (red) and DAPI for nuclei (blue; original magnification ×20) is also presented. (C) ELISAs of circulating human IgG concentrations are shown 4 days after the last injection. (D) TUNEL assay shows more apoptosis cells in tumor area and no TUNEL-positive cells in normal organs after the treatment. Bar represents 20 μm.
We also determined the concentration of exogenous angiostatin in the plasma of SCID mice. Because endogenous plasminogen interferes with a direct assay, we used the human Fc of the exogenous angiostatin (hFcAS) as a tag and performed ELISA to measure the protein concentration. The circulating angiostatin (FcAS) concentration was found to be approximately 50 μg/mL (at the stated dose; Figure 1C). The level for control mice treated with the same amount of Fc was 25 μg/mL.
Angiostatin induces apoptosis via down-regulation of BCL-2 protein
To detect the extent of apoptosis in angiostatin-treated tumors, TUNEL assay was performed.18 TUNEL-positive apoptotic cells were increased as a result of angiostatin treatment (Figure 1D). Histologic sections of tumors revealed the mean percentage of apoptotic tumor cells to be 34.46% plus or minus 4.31% with 10.16% plus or minus 2.22% in PBS-treated tumor sections.
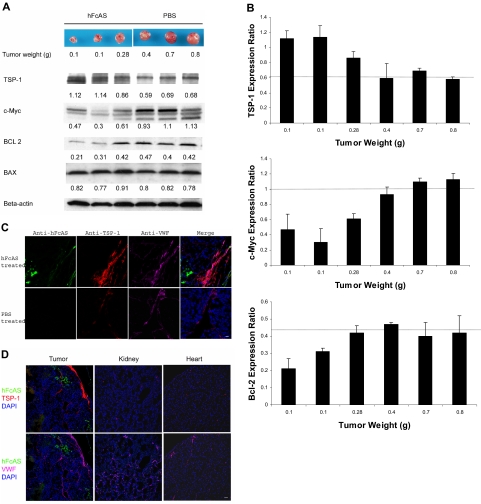
The family of BCL-related proteins is located in the mitochondria of mammalian cells and considered key regulators of the programmed cell death.20 Two members of this family, called BAX and BAD, are proapoptotic, whereas BCL-2 exerts antiapoptotic effects. We examined the expression levels of these proteins in tumor samples by Western blot analysis (Figure 2A). We found no differences in BAX expression after angiostatin treatment. Likewise, BAD expression level remains unchanged after angiostatin therapy (data not shown). In contrast, the amount of the antiapoptotic BCL-2 protein was reduced in the angiostatin-treated tumors compared with sham-treated control tumors. Thus, inhibition of BCL-2 survival signaling might be a mechanism by which angiostatin induced apoptosis in our tumor model in vivo.
Figure 2.
Angiostatin treatment of tumor-bearing mice affects the expression of several proteins. (A) Tumors reaching the sizes and weights of 0.1 to 0.8 g after treatment with angiostatin or PBS. Equal amounts of protein extracts from tumors were subjected to Western blot analysis with antibodies against TSP-1, c-MYC, BCL-2, BAX, and β-actin. TSP-1 levels shown increased and c-Myc expressions decreased after angiostatin treatment. Relative amounts are indicated by the ratio of each protein to the corresponding level of β-actin (B). The intensity of the protein bands was quantitated by densitometry and normalized against that of β-actin. (C) Immunostaining of the aforementioned tumor sections after incubation with antibodies directed to TSP-1 and VWF, followed by Alexa 568 anti–rat and Alexa 647 anti–rabbit IgG. The TSP-1 (red) was overexpressed in tumor vessels (pink). hFcAS (green) could be detected by Alexa 488 anti–human IgG. Bar represents 20 μm. (D) Large-scale sections showed that after treatment TSP-1 is overexpressed only in tumor site but not in normal organs. Bar represents 100 μm.
Angiostatin up-regulates thrombospondin-1 protein and down-regulates c-Myc
Thrombospondin is a suppressor of angiogenesis. Down-regulation of thrombospondin follows activation of oncogenes.21 TSPs reduce tumor growth by several mechanisms, including interactions with VEGF, inhibition of MMP-9, and inhibition of endothelial cell migration.22 On the other hand, c-Myc has been identified as a major promoter of tumor growth.23 Western blot analysis for the expression of TSP-1 and c-Myc is shown in Figure 2A. Angiostatin treatment increased TSP-1 level by 1.6-fold and reduced c-Myc expression by 2.3-fold (Figure 2B).
A dramatic increase in TSP-1 expression was observed in tumor vessels after treatment with angiostatin (Figure 2C), a novel observation for angiostatin. Increase in TSP-1 expression after treatment with angiostatin is consistent with several previous reports, demonstrating up-regulation of TSP-1 expression in tumor vessels as an antiangiogenic response.24,25 Similar immunofluorescence (IF) data are shown for different mouse organs (Figure 2D). The increase in TSP-1 is mainly confined to the tumor vessels.
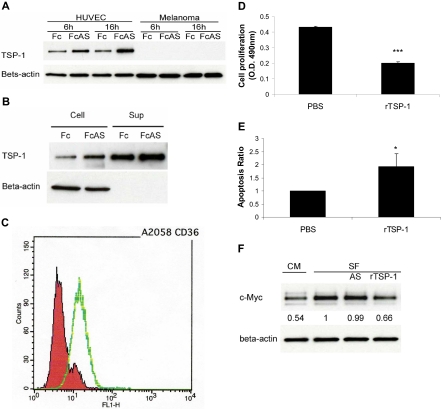
In vitro assay using tumor cells and HUVECs provides strong evidence that, after treatment of these cells by angiostatin, TSP-1 is up-regulated in endothelial cells and not in the tumor cells (Figure 3A). TSP-1 was secreted into cell culture medium (Figure 3B). The major receptor of TSP-1, CD36, was expressed on A2058 melanoma tumor cell surface (Figure 3C). Melanoma cells showed lower proliferation and higher apoptosis rates after treatment with recombinant TSP-1 (rTSP-1; Figure 3D-E). c-Myc protein level of tumor cells decreased after treatment with TSP-1-rich HUVEC culture conditional medium (CM) or serum-free medium (SF) plus rTSP-1 (Figure 3F). No change in c-Myc was observed when cells were treated with angiostatin in SF (Figure 3F). We conclude that certain factors present in CM contribute to the increase of TSP in media. Overall, these data indicate that an increase in TSP-1 is associated with a decrease of c-Myc in tumors, consistent with previous published reports26,27 (Figure 2A).
Figure 3.
TSP-1 overexpressed, induced by angiostatin, inhibits melanoma tumor cell growth. (A) HUVECs overexpressed TSP-1 by angiostatin treatment, whereas tumor cells do not. (B) HUVECs secreted TSP-1 into cell culture medium (Sup). (C) Fluorescence-activated cell sorter (FACS) analysis showed that A2058 melanoma cells express CD36 on the surface. (D) Melanoma tumor cell proliferation was inhibited by 10 μg/mL recombinant TSP-1 treatment. (E) TUNEL assay showed more apoptosis of tumor cells after rTSP-1 treatment. (F) c-Myc protein level of tumor cells was decreased after treatment with TSP-1–rich HUVEC culture conditional medium (CM) or serum-free medium (SF).
Angiostatin inhibits migration of host macrophages to the tumor
Bone marrow–derived cells that infiltrate tumors and differentiate into macrophages are called tumor-associated macrophages. Migration and infiltration of macrophages are hallmarks of tumor growth.28–30 Tumor-associated macrophages release several angiogenic factors, including MMP-9, VEGF, and IL-8.
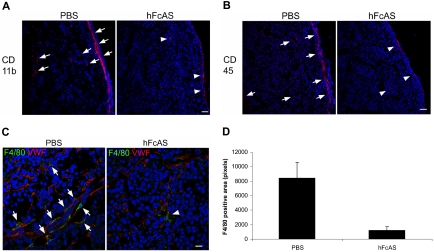
Our data for the presence of macrophages and their infiltration into the tumors are shown in Figure 4A through C. We used 3 markers for identification of macrophages: CD11b, CD 45, and F4/80. Immunohistochemical analysis of mouse tumors showed reduced macrophage infiltration in angiostatin-treated mice relative to sham-treated controls (Figure 4D).
Figure 4.
Angiostatin inhibits host macrophage recruitment to the tumors. Markers CD11b, CD45, and F4/80 (A-C). Infiltration of the host bone marrow–derived cells decreased after hFcAS treatment. (D) Quantification of F4/80 positive area was counted by pixel numbers (P < .05). Bar represents 100 μm.
Flow cytometry and IF studies demonstrate that angiostatin targets mitochondria
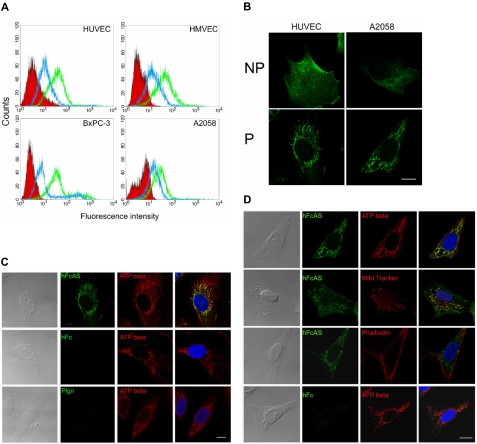
Flow cytometry was used to monitor the binding of angiostatin to the cell surfaces of HUVECs, HMVECs, and 2 tumor cell lines (pancreatic BxPC-3 and melanoma A2058; Figure 5A). Two concentrations of angiostatin were used, and angiostatin was shown to bind to the cell surface.
Figure 5.
Angiostatin binding activity and colocalization with ATP synthase in mitochondria. (A) Endothelial cell lines HUVECs and HMVECs and tumor cell lines AsPC-1 and A2058 were subjected to FACS analysis using angiostatin concentrations of 1 (blue) and 10 μg/mL (green). The red area represents the control Fc binding. (B) IF analysis of HUVEC and A2058 under permeable (P) and nonpermeable (NP) conditions. No staining was observed with control Fc. (C) HUVECs and (D) A2058 cells were fixed under permeabilized conditions and incubated with hFcAS (50 ng/mL) for 2 hours and detected by AlexaFluor 488 anti–human IgG (green). Those cells were also double stained by anti-ATP synthase β subunit antibody, mitochondria marker (MitoTracker), or actin staining phalloidin and detected by Alexa Fluor 594 antibody (red). In merged (last column), hFcAS and ATP synthase β subunit or MitoTracker showed colocalization (yellow) in both endothelial and tumor cells by confocal microscopy. DAPI staining of nuclei is shown in blue. Bar represents 20 μm.
IF analysis of some of these cell lines under permeable and nonpermeable conditions is presented in Figure 5B. Angiostatin appears to be clustered on the cell surface under nonpermeabilized conditions. Under permeable conditions, angiostatin location resembles mitochondria (Figure 5C). ATP synthase, in addition to being a mitochondrial protein, has been reported to bind angiostatin on endothelial cell surface.8,9 We used a monoclonal antibody directed to the β subunit of ATP synthase. Colocalization of angiostatin and ATP synthase in mitochondria are observed (Figure 5C first row). Confocal 3-dimensional image showed the distributions of angiostatin and ATP synthase in mitochondria of the endothelial cell (supplemental data, available on the Blood website; see the Supplemental Materials link at the top of the online article). Both human Fc and plasminogen failed to be located in mitochondria. Images in the fourth column in Figure 4D were obtained by merging of the previous 2 columns.
MitoTracker, a small molecule that passively diffuses across plasma membrane and accumulates in active mitochondria and phalloidin (directed to actin) probes, was also used. The data are shown in Figure 5D. MitoTracker and angiostatin have similar patterns and overlap (Figure 5D second row). In contrast, phalloidin, which binds actin, has a distinct distribution compared with angiostatin (Figure 5D third row).
Immunostaining of angiostatin-treated tumors showed localization of angiostatin to mitochondria in both endothelial cells and tumor cells in vivo (supplemental data).
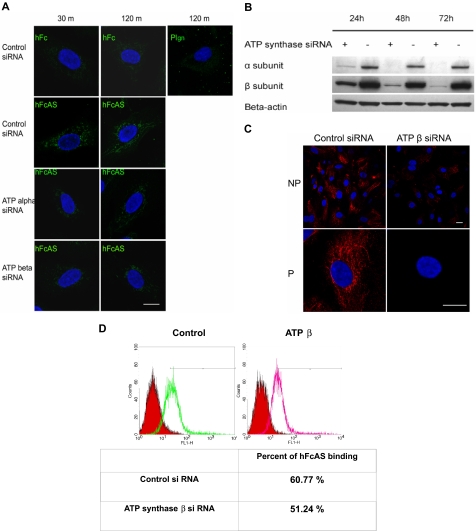
Angiostatin targeting to mitochondria is achieved by internalization
Endothelial cells were incubated in the presence of angiostatin at different intervals, and IF measurements were carried out. After internalization of angiostatin by the cell, angiostatin was detected in the mitochondria (Figure 6A). Similar results were obtained by removing Fc and treating the cells with angiostatin alone (data not shown). No internalization of plasminogen was observed.
Figure 6.
Angiostatin internalization and its migration to mitochondria. (A) Endothelial cells were incubated with hFcAS (10 μg/mL) for 30 minutes and 120 minutes, then fixed and permeabilized, and probed by Alexa 488 anti–human IgG. hFcAS, not plasminogen or hFc, could be internalized. Internalization was achieved at the indicated intervals after siRNA treatment for 72 hours. Internalization of hFcAS was decreased in ATP synthase α or β subunit–interfering RNA-treated cells. Confirming that ATP synthase expression has been decreased after siRNA-treated cells, (B) Western blot and (C) immunostaining of β subunit after 72-hour treatment with siRNA. Bar represents 20 μm. (D) HUVECs were subjected to FACS analysis using angiostatin concentration of 10 μg/mL and probed by Alexa 488 anti–human IgG. The red area represents the control Fc binding.
Expression of ATP synthase α and β subunits was diminished by treatment with interfering RNA (Figure 6B). Inhibition of either subunit by siRNA reduced the presence of angiostatin in mitochondria (Figure 6A). Thus, reduced angiostatin staining in mitochondria may be the result of ATP synthase knockdown on the cell surface and/or in the inner mitochondrial membrane. IF analysis of HUVECs under nonpermeable and permeable conditions, after siRNA treatment of the β subunit, is shown in Figure 6C. A marked reduction in expression of the β subunit is observed on the surface and in the mitochondria. Flow cytometric data show that ATP synthase accounts for approximately 9% of total binding of angiostatin to the cell surface, consistent with the observation that angiostatin binds to several cell surface proteins, including ATP synthase (Figure 6D).
Angiostatin binds to malate dehydrogenase in mitochondria
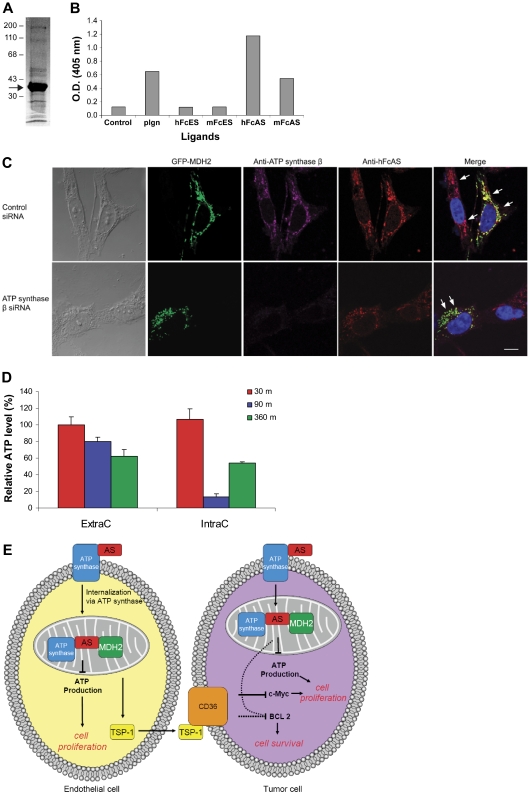
To identify the cellular proteins binding to angiostatin, we adopted a simple affinity purification protocol of the mouse liver homogenate preparation. Two affinity columns were used, and the liver homogenate was first passed through mFc-Sepharose. The flow-through was subsequently applied to a mouse Fc-angiostatin affinity column (mouse Fc and mouse angiostatin). Proteins bound to the second column were eluted and sequenced (Figure 7A). The most intense band was identified as mitochondria malate dehydrogenase (mt MDH or MDH2), based on the sequence results (data not shown). Mitochondrial MDH, a member of the Krebs cycle, is a dimer composed of 2 identical subunits each with a molecular mass of 34 kDa.31 Subsequently, binding assay (ELISA) of the 2 types of MDH (cytoplasmic and mitochondrial) using a small number of ligands was undertaken. Only MDH2 bound angiostatin (Figure 7B). No binding was observed between angiostatin and cytoplasmic MDH (data not shown).
Figure 7.
Identification of MDH2 as a binding protein to angiostatin. Mouse liver homogenate was first passed over an mFc-Sepharose column followed by mFc-angiostatin-Sepharose step. PAGE analysis of the eluant is shown in panel A (arrow points to the major band identified as MDH2) using Coomassie stain. ELISA (B) was performed by coating the plate with porcine heart mitochondria MDH (1 μg/mL). The ligands are indicated at the bottom (1 μg/mL). (C) Either ATP synthase knocked down or wild-type tumor cells were transfected with GFP-MDH2 plasmid (transfection efficiency was ∼ 40%). Cells were permeabilized and incubated with hFcAS and anti-ATP synthase β monoclonal antibody. Alexa 568 antihuman and 647 antimouse antibodies were used for detection. HFcAS (red) showed colocalization with ATP synthase (pink) in ATP synthase wild-type cells and also with MDH2 (green) in ATP synthase knocked down cells. Bar represents 20 μm. (D) ATP production in HUVECs was inhibited by hFcAS in both extracellular (ExtraC) and intracellular (IntraC) regions. The incubation times were 30, 90, and 360 minutes. (E) A diagram summarizing the identified pathways after angiostatin treatments in vivo and in vitro.
Both angiostatin and plasminogen bind MDH2 at the indicated concentrations (Figure 7B). However, as we showed earlier, angiostatin is capable of being internalized by the cell, whereas plasminogen entry is blocked (Figure 6A). Furthermore, the binding of plasminogen to mitochondria is reduced considerably compared with angiostatin in immunostaining using the same concentration of both proteins (Figure 5C).
Initially, we tested commercial monoclonal antibody and polyclonal antibodies against MDH2 in IF. The monoclonal antibody showed very weak staining in IF. The results for polyclonal antibodies were not highly specific. To overcome this problem, a GFP-MDH2 DNA plasmid was constructed (“RNA interference and MDH2 transfection”). A2058 tumor cells were transfected with this plasmid and IF was undertaken (Figure 7C). The efficiency of transfection was approximately 40%. Transfection of endothelial cells with GFP-MDH DNA construct caused toxicity (data not shown).
Staining with antibodies to ATP synthase β and angiostatin is presented for cells after treatment with Fc-angiostatin (Figure 7C top panel). Three proteins, namely, ATP synthase, MDH2, and angiostatin, show colocalization. On treatment of the cells with ATP synthase β siRNA, colocalization of angiostatin and MDH2 remains intact, even though the signal for ATP synthase β has disappeared (Figure 7C bottom panel).
ATP generation of cells is reduced after treatment with angiostatin
We hypothesized that angiostatin inhibits production of ATP by targeting 2 critical mitochondrial proteins MDH2 and ATP synthase. To answer this question, we measured intracellular and extracellular ATP in HUVECs using a bioluminescence assay (Figure 7D). ATP concentration was reduced by approximately 20% (extracellular) and 80% (intracellular) after 90-minute incubation with 10 μg/mL angiostatin. Similar data were obtained with A2058 tumor cells (data not shown). Analogous ATP inhibition in HUVECs was previously reported on treatment with angiostatin.9
In a separate experiment, HUVECs were treated with siRNAs of ATP synthase and MDH2 for 72 hours. Approximately, 20% reduction of ATP was observed (data not shown). Apparently, the cells may compensate for ATP reduction by other mechanisms, after a period of time, consistent with the data of Figure 7D where IntraC level of ATP increases after 6 hours.
Discussion
The antitumor mechanism of angiostatin is poorly understood. There is a general consensus about the following properties of angiostatin: (1) Angiostatin binds to a variety of cell surface proteins. Some of these binding proteins are shared by plasminogen. It is not clear which of these sites play a major role in determining its antitumor activity.7 (2) The antitumor activity of angiostatin has been demonstrated to reside in kringles 1 to 3, kringles 1 to 4, kringles 1 to 4.5, and kringle 5.7,32 (3) Several mitochondrial inner membrane proteins are also present on the endothelial cell plasma membrane, a phenomenon requiring more investigation.7
Here, we present strong evidence that angiostatin targets mitochondria and at least binds to 2 mitochondrial proteins: ATP synthase and MDH2. It has been reported that ATP synthase on endothelial cell surface is a selective receptor for angiostatin.8,9 However, the same group of investigators have also reported that angiostatin blocks the binding of plasminogen to CD26 in human prostate tumor cells.33 We show that knockdown of α or β subunits of ATP synthase results in reduced angiostatin signals in mitochondria. We also show that the binding of angiostatin to cell surface is reduced only by 9% as a result of knocking out the ATP synthase (Figure 6D). Thus, ATP synthase seems to be at least in part involved in angiostatin internalization. However, other cell surface receptors and mechanisms for angiostatin internalization could not be excluded. Clearly, angiostatin is capable of binding to a number cell surface proteins, and the contribution of these proteins in facilitating angiostatin internalization remains to be elucidated.
Glycolysis, the main pathway for using glucose by tumor cells, has been extensively studied for decades. Angiostatin appears to impact Krebs cycle. Both cycles use glucose under aerobic conditions to produce ATP. Comparing the 2 pathways, glycolysis is more efficient in terms of ATP production in a short time but less efficient in overall ATP production. Tumor cells use more glucose through glycolysis to generate ATP.
Lactate dehydrogenase has been shown to play an important role in regulating glycolysis in tumor growth (Warburg effect).15,34 Interestingly, it has been found that MDH from the extremely halophilic archaebacterium Haloarcula marismortui has more sequence homology to LDH than to other MDHs.35 Apparently, there has been an overlap between the 2 families of the enzymes in early evolution before divergence to later MDHs.
Involvement of enzymes of Krebs cycle in tumorigenesis is not unprecedented. Krebs cycle–associated metabolites released from tissues, in the presence of insufficient oxygen, have been shown to be proangiogenic.36 Under these conditions, Krebs cycle intermediates accumulate in a process independent of hypoxia-inducible factor 1α (HIF-1α).37
On the other hand, succinate dehydrogenase and fumarase have been shown to be tumor suppressors because mutations in the genes of these enzymes have been shown to promote cancer growth.38–40 Apparently, as a result of mutations in these genes, degradation of HIF-1α is inhibited. Evidence has been presented showing that stabilization of HIF-1α results in up-regulation of important angiogenesis genes, such as VEGF and MMP-9.41 We have not observed any changes in HIF expression after analysis of the angiostatin-treated tumors discussed in this paper (data not shown).
Genetic analysis of patients with glioblastoma revealed the presence of a single mutation in the active site of isocitrate dehydrogenase, one of the major enzymes in the Krebs cycle.16 These data clearly imply the importance of targeting anticancer drugs to mitochondria.
We have analyzed protein levels of different important markers in tumors obtained from PBS and angiostatin-treated mice. A decrease in BCL-2 expression is consistent with observed higher apoptosis. However, as previously pointed out, we did not detect changes in protein levels for BAK and BAD, 2 proapoptotic proteins belonging to the BCL-2 family. It is possible that the observed decrease in BCL-2 correlates with tumor size and does not relate to interactions of angiostatin with mitochondria.
An important finding in this paper relates to the increase in TSP-1 expression observed in angiostatin-treated mice. The role of microenvironment in cancer has been a major focus of researchers since the mid-1990s.42 The extracellular matrix embodies a large number of factors, which play an important role in tumor growth and angiogenesis. Migration of macrophages to tumor sites is associated with providing a series of enzymes required for tumor survival and formation of blood vessels. Angiostatin has been reported to be an anti-inflammatory factor by inhibiting leukocyte recruitment.43 It has been shown that inhibition of plaque neovascularization by angiostatin reduces macrophage accumulation and progression of advanced atherosclerosis.44 Angiostatin inhibits migration of macrophages by disruption of their actin cytoskeleton.45 Macrophages secrete metalloelastase, which is responsible for generation of angiostatin in Lewis lung carcinoma.46
We have observed 3 novel features in angiostatin-treated SCID mice, which are known to be important components of tumor regulation: a decrease in c-Myc, an increase in TSP-1, and a decrease in macrophage recruitment. Available evidence indicates that the mechanisms of action of these 3 are related. On c-Myc inactivation, an increase of either p53 or TSP-1 is required to maintain tumor regression.26,27,47 Our data indicate that TSP-1 is secreted by endothelial cells and not tumor cells. On the other hand, we have observed an elevated level of TSP-1 in tumor cells of treated mice while showing a decline in infiltrating macrophages. The role of c-Myc is not limited to an increase in TSP-1 or p53. It has been shown to transactivate LDH, an important enzyme in glycolysis.48
The fact that antitumor properties of angiostatin are independent of the length of the kringles raises the possibility that any one kringle is capable of eliciting antitumor response. We propose that a single disulfide-bonded kringle is an important component for binding to the diverse cell surface proteins reported for angiostatin. Additional support for this hypothesis comes from binding of angiostatin to c-met, a receptor for HGF, another kringle-shaped ligand.6 Furthermore, for a kringle to be active, it may require having a basic isoelectric point. Examination of all 5 kringles of plasminogen confirms that indeed all 5 kringles are positively charged. Therefore, it is interesting to point out to a report from Fantin et al in which a small positively charged molecule, called F16, accumulated in mitochondria and showed inhibition of tumor growth.49
We have attempted to include the data addressed in this publication in a diagram containing both endothelial cells and tumor cells. Angiostatin targets mitochondria in endothelial cells. At least 2 proteins, ATP synthase and MDH2 in mitochondria, bind angiostatin. ATP production is reduced, and TSP-1 is increased. TSP-1 is secreted and binds to corresponding receptors on the tumor cells (eg, CD36). Consequently, BCL-2 and c-Myc are down-regulated (Figure 7E).
Finally, an interesting finding reported in this paper is the selective tumor uptake of angiostatin, whereas most examined organs were spared from angiostatin treatment. This phenomenon has been reported to be a consequence of enhanced permeability and retention.19 Previously, other investigators in our laboratory reported a similar observation with TNP-40, an antiangiogenic drug, conjugated to hydroxypropylmethacrylate polymer.50 If this specificity for angiostatin is confirmed in other tumor models, angiostatin has the potential for use in the conjugation of small molecular size drugs directed to tumors.51
Supplementary Material
Acknowledgments
This article is dedicated to the memory of Dr Judah Folkman, a great mentor and the leading pioneer of the field of angiogenesis research.
The authors thank Professors Yussef Hatefi, Douglas Hanahan, Lena Claesson-Welsh, and Bruce Zetter for reading the manuscript and making helpful suggestions; Sandra Ryeom, Taturo Udagawa, Diane Bielenberg, Randy Watnik, Carmen Barnes, Sarah Short, and Han-Chung Wu for their comments on the manuscript; Dr Kim-Ming Lo of Merck Serono for constructing Fc-angiostatin; Pauline Breen and Melissa Herman for proofreading the manuscript; and Kristin Johnson for preparing the figures.
This work was supported by the National Institutes of Health (grants R01-CA064481 and P01-CA45548, J.F.), NASA (grant NNJ04HJ12G, A.A., J.F.), Deutsche Forschungsgemeinschaft (DKG, German Research Foundation; grant SPP1190, A.A.), Department of Defense (grant W81XWH-05-1-0115, J.F.), and the Breast Cancer Research Foundation (J.F.).
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: T.-Y.L., J.F., A.A., and K.J. designed and analyzed the experiments and prepared the manuscript; and T.-Y.L., S.M., E.A.P., and K.J. performed the experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kashi Javaherian, 1 Blackfan Cir, Karp Research Bldg, Boston, MA 02115; e-mail: Kashi.Javaherian@childrens.harvard.edu.
References
- 1.Collen D, Lijnen HR, Verstraete M. In: Blood Principles and Practice of Hematology. Hardin RI, Lux S, Stossel TP, editors. Philadelphia, PA: Lippincott; 1995. pp. 1261–1288. [Google Scholar]
- 2.O'Reilly MS, Holmgren L, Shing Y, et al. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 3.Tuszynski GP, Sharma MR, Rothman VL, Sharma MC. Angiostatin binds to tyrosine kinase substrate annexin II through the lysine-binding domain in endothelial cells. Microvasc Res. 2002;64:448–462. doi: 10.1006/mvre.2002.2444. [DOI] [PubMed] [Google Scholar]
- 4.Troyanovsky B, Levchenko T, Mansson G, Matvijenko O, Holmgren L. Angiomotin: an angiostatin binding protein that regulates endothelial cell migration and tube formation. J Cell Biol. 2001;152:1247–1254. doi: 10.1083/jcb.152.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tarui T, Miles LA, Takada Y. Specific interaction of angiostatin with integrin αvb3 in endothelial cells. J Biol Chem. 2001;276:39562–39568. doi: 10.1074/jbc.M101815200. [DOI] [PubMed] [Google Scholar]
- 6.Wajih N, Sane DC. Angiostatin selectively inhibits signaling by hepatocyte growth factor in endothelial and smooth muscle cells. Blood. 2003;101:1857–1862. doi: 10.1182/blood-2002-02-0582. [DOI] [PubMed] [Google Scholar]
- 7.Wahl ML, Kenan DJ, Gonzalez-Gronow M, Pizzo SV. Angiostatin's molecular mechanism: aspects of specificity and regulation elucidated. J Cell Biochem. 2005;96:242–261. doi: 10.1002/jcb.20480. [DOI] [PubMed] [Google Scholar]
- 8.Moser TL, Stack M, Asplin I, et al. Angiostatin binds ATP synthase on the surface of human endothelial cells. Proc Natl Acad Sci U S A. 1999;96:2811–2816. doi: 10.1073/pnas.96.6.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moser TL, Kenan DJ, Ashley TA, et al. Endothelial cell surface F1-Fo ATP synthase is active in ATP synthesis and is inhibited by angiostatin. Proc Natl Acad Sci U S A. 2001;98:6656–6661. doi: 10.1073/pnas.131067798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wahl ML, Grant DS. Effects of microenvironmental extracellular pH and extracellular matrix proteins on angiostatin activity and on intracellular pH. J Gen Pharm (Vascular) 2002;35:1–10. doi: 10.1016/s0306-3623(01)00115-x. [DOI] [PubMed] [Google Scholar]
- 11.Arakaki N, Nagao T, Niki R, et al. Possible role of cell surface H+ATP synthase in the extracellular ATP synthase and proliferation of human umbilical vein endothelial cells (HUVECs). Mol Cancer Res. 2003;1:931–939. [PubMed] [Google Scholar]
- 12.Boyer PD. The ATP synthase: a splendid molecular machine. Annu Rev Biochem. 1997;66:717–749. doi: 10.1146/annurev.biochem.66.1.717. [DOI] [PubMed] [Google Scholar]
- 13.Das B, Mondragon MO, Sadeghian M, Hatcher VB, Norin AJ. A novel ligand in lymphocyte-mediated cytotoxicity: expression on the subunit of H+ transporting ATP synthase on the surface of tumor cell lines. J Exp Med. 1994;180:273–281. doi: 10.1084/jem.180.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soltys BJ, Gupta RS. Mitochondrial-matrix proteins at unexpected locations: are they exported? Trends Biochem Sci. 1999;24:174–177. doi: 10.1016/s0968-0004(99)01390-0. [DOI] [PubMed] [Google Scholar]
- 15.Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425–434. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 16.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergers G, Javaherian K, Lo K-M, Folkman J, Hanahan D. Effects of angiogenesis inhibitors on multistage carcinogenesis in mice. Science. 1999;284:808–812. doi: 10.1126/science.284.5415.808. [DOI] [PubMed] [Google Scholar]
- 18.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dvorak HF, Nagy JA, Dvorak JT, Dvorak AM. Identification and characterization of the blood vessels of solid tumors that are leaky to circulating macromolecules. Am J Pathol. 1998;133:95–109. [PMC free article] [PubMed] [Google Scholar]
- 20.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 21.Lawler J, Detmar M. Tumor progression: the effects of thrombosondin-1 and -2. Int J Biochem Cell Biol. 2004;36:1038–1045. doi: 10.1016/j.biocel.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Rodríguez-Manzaneque JC, Lane TF, Ortega MA, Hynes RO, Lawler J, Iruela-Arispe ML. Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor. Proc Natl Acad Sci U S A. 2001;98:12485–12490. doi: 10.1073/pnas.171460498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hahn WC, Weinberg RA. Rules for making human tumor cells. N Engl J Med. 2002;347:1593–1604. doi: 10.1056/NEJMra021902. [DOI] [PubMed] [Google Scholar]
- 24.Dameron KM, Volpert OV, Tainsky MA, Bouck N. Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin-1. Science. 1994;265:1582–1584. doi: 10.1126/science.7521539. [DOI] [PubMed] [Google Scholar]
- 25.Lawler J. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. J Cell Mol Med. 2002;6:1–12. doi: 10.1111/j.1582-4934.2002.tb00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tikhonenko AT, Black DJ, Linial ML. Viral Myc oncoproteins in infected fibroblasts down-modulate thrombospondin-1, a possible tumor suppressor gene. J Biol Chem. 1996;271:30741–30747. doi: 10.1074/jbc.271.48.30741. [DOI] [PubMed] [Google Scholar]
- 27.Watnick RS, Cheng YN, Rangarjan A, Ince TA, Weinberg RA. Ras modulates Myc activity to repress thrombospondin-1 expression and increase tumor angiogenesis. Cancer Cell. 2003;3:219–231. doi: 10.1016/s1535-6108(03)00030-8. [DOI] [PubMed] [Google Scholar]
- 28.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 29.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 30.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Musrati RA, Kollárová M, Mernik N, Mikulášová D. Malate dehydrogenase: distribution, function and properties. Gen Physiol Biophys. 1998;17:193–210. [PubMed] [Google Scholar]
- 32.Chen Y-H, Wu H-L, Li C, et al. Anti-angiogenesis mediated by angiostatin K1-3, K1-4 and K1-4.5. Thromb Haemost. 2006;95:668–677. [PubMed] [Google Scholar]
- 33.Gonzalez-Gronow M, Grenett HE, Gawdi G, Pizzo SV. Angiostatin directly inhibits human prostate tumor cell invasion by blocking plasminogen binding to its cellular receptor, CD26. Exp Cell Res. 2005;303:22–31. doi: 10.1016/j.yexcr.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Christofk HR, Vander Heiden KG, Harris MH, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumor growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 35.Cendrin F, Chroboczek J, Zaccai G, Eisenberg H, Mevarech M. Cloning, sequencing, and expression in E. coli of the gene coding for malate dehydrogenase of the extremely halophilic archaebacterium Haloarcula marismortui. Biochemistry. 1993;32:4308–4313. doi: 10.1021/bi00067a020. [DOI] [PubMed] [Google Scholar]
- 36.Murray B, Wilson DJ. A study of metabolites as intermediate effectors in angiogenesis. Angiogenesis. 2001;4:71–77. doi: 10.1023/a:1016792319207. [DOI] [PubMed] [Google Scholar]
- 37.Sapieha P, Sirinyan M, Hamel D, et al. The succinate receptor GPR91 in neurons has a major role in retinal angiogenesis. Nat Med. 2008;14:1067–1076. doi: 10.1038/nm.1873. [DOI] [PubMed] [Google Scholar]
- 38.Selak MA, Armour SM, MacKenzie ED, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-1α prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 39.Gottlieb E, Tomlinson IPM. Mitochondrial tumor suppressors: a genetic and biochemical update. Nat Rev Cancer. 2005;5:857–865. doi: 10.1038/nrc1737. [DOI] [PubMed] [Google Scholar]
- 40.Pollard PJ, Spencer-Dene B, Shukla D, et al. Targeted inactivation of Fh1 causes proliferative renal cyst development and activation of the hypoxia pathway. Cancer Cell. 2007;11:311–319. doi: 10.1016/j.ccr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Du R, Lu KV, Petritsch C, et al. HIF1α induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marx J. All in the stroma: cancer's Cosa Nostra. Science. 2008;320:38–41. doi: 10.1126/science.320.5872.38. [DOI] [PubMed] [Google Scholar]
- 43.Chavakis T, Athanasopoulos A, Rhee J-S, et al. Angiostatin is a novel anti-inflammatory factor by inhibiting leukocyte recruitment. Blood. 2005;105:1036–1043. doi: 10.1182/blood-2004-01-0166. [DOI] [PubMed] [Google Scholar]
- 44.Moulton K, Vakili K, Zurakowski D, et al. Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atherosclerosis. Proc Natl Acad Sci U S A. 2003;100:4736–4741. doi: 10.1073/pnas.0730843100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perri SR, Annabi B, Galipeau J. Angiostatin inhibits monocyte/macrophage migration via disruption of actin cytoskeleton. FASEB J. 2007;21:3928–3936. doi: 10.1096/fj.07-8158com. [DOI] [PubMed] [Google Scholar]
- 46.Dong Z, Kumar R, Yang X, Fidler I. Macrophage-derived metalloelastase is responsible for the generation of angiostatin in Lewis lung carcinoma. Cell. 1997;88:801–810. doi: 10.1016/s0092-8674(00)81926-1. [DOI] [PubMed] [Google Scholar]
- 47.Giuriato S, Ryeom S, Fan AC, et al. Sustained regression of tumors upon MYC inactivation requires p53 or thrombospondin-1 to reverse the angiogenic switch. Proc Natl Acad Sci U S A. 2006;103:16266–16271. doi: 10.1073/pnas.0608017103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shim H, Dolde C, Lewis BC, et al. c-Myc transactiavation of LDH-A: implications for tumor metabolism and growth. Proc Natl Acad Sci U S A. 1997;94:6658–6663. doi: 10.1073/pnas.94.13.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fantin VR, Berardi MJ, Scorrano L, Korsmeyer SJ, Leder P. A novel mitochondriotoxic small molecule that selectively inhibits tumor cell growth. Cancer Cell. 2002;2:29–42. doi: 10.1016/s1535-6108(02)00082-x. [DOI] [PubMed] [Google Scholar]
- 50.Ronit-Satchi F, Puder M, Davies JW, et al. Targeting angiogenesis with a conjugate HPMA copolymer and TNP-470. Nat Med. 2004;10:255–261. doi: 10.1038/nm1002. [DOI] [PubMed] [Google Scholar]
- 51.Lee TY, Lin CT, Kuo SY, Chang D-K, Wu HC. Peptide-mediated targeting to tumor blood vessels of lung cancer for drug delivery. Cancer Res. 2007;67:10958–10965. doi: 10.1158/0008-5472.CAN-07-2233. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.