Abstract
Rationale
Although the subjective effects of caffeine abstinence, acute and chronic administration, and tolerance are well described, the corresponding neurophysiological effects are not.
Objectives
Caffeine withdrawal, acute caffeine effects, caffeine tolerance, and net beneficial effects of chronic caffeine administration were investigated using cerebral blood flow velocity, quantitative EEG and subjective effects.
Methods
Sixteen regular caffeine users participated in this double-blind, within-subject study during which they received acute caffeine and placebo challenges: 1) while maintained on 400 mg caffeine daily for ≥14 days and 2) while maintained on placebo for ≥14 days. Blood flow velocity was determined for the middle (MCA) and anterior (ACA) cerebral arteries using pulsed transcranial Doppler sonography. EEG was recorded from 16 scalp sites. Subjective effects were assessed with questionnaires.
Results
Acute caffeine abstinence (evaluated 24 hours after placebo substitution) increased mean, systolic and diastolic velocity in the MCA and ACA and decreased pulsatility index in the MCA. Acute caffeine abstinence increased EEG theta and decreased beta 2 power. Acute caffeine abstinence also increased measures of Tired, Fatigue, Sluggish and Weary and decreased ratings of Energetic, Friendly, Lively and Vigor. Acute caffeine effects were demonstrated across a wide range of measures, including cerebral blood flow, EEG and subjective effects. Tolerance and “complete” tolerance were observed on subjective but not physiological measures. Chronic caffeine effects were demonstrated only on the measure of EEG beta 2 power.
Conclusion
Acute caffeine abstinence and administration produced changes in cerebral blood flow velocity, EEG, and subjective effects. Tolerance to subjective but not physiological measures was demonstrated. There was almost no evidence for net effects of chronic caffeine administration on these measures. Overall, these findings provide the most rigorous demonstration to date of physiological effects of caffeine withdrawal.
Keywords: Cerebral blood flow velocity, EEG, Caffeine, Withdrawal, Physical dependence, Tolerance, Subjective effects, Humans
Introduction
Cessation of daily caffeine consumption produces a withdrawal syndrome comprised of subjective symptoms and functional impairment, including headache, tiredness/fatigue, decreased alertness, decreased energy and difficulty concentrating (Griffiths and Woodson 1988; Juliano and Griffiths 2004). However, while caffeine withdrawal has been well-documented, it is less clear what neurophysiological changes occur following caffeine administration and withdrawal and if these changes occur in conjunction with mood alterations. Change in cerebral blood flow is a readily measurable neurophysiological effect that may be relevant to caffeine withdrawal because acute caffeine administration decreases cerebral blood flow velocity (Cameron et al. 1990; Debrah et al. 1995). It has been widely speculated that the headache often associated with caffeine withdrawal is related to rebound dilatation of cerebral vasculature, possibly because of increased sensitivity to circulating adenosine which is a potent cerebral vasodilator (e.g., Muhonen et al. 1995). Indeed, increased cerebral blood flow has been demonstrated in several studies comparing acute caffeine abstinence with a preceding baseline condition (Mathew and Wilson 1985; Couturier et al. 1997) or with a caffeine administration condition (Field et al. 2003). These studies have also shown that the magnitude of this effect is positively correlated with daily caffeine dose before abstinence (Field et al., 2003; Mathew and Wilson 1985).
Changes in quantitative electroencephalography (EEG) are also relevant to caffeine withdrawal and can provide quantitative measures of changes in CNS electrical activity that have been correlated with drowsiness, a common symptom of caffeine withdrawal. It has been previously demonstrated that acute caffeine administration can produce a transient reduction of EEG total absolute power in healthy subjects (Saletu et al. 1987; Goldstein et al. 1963). Furthermore, two prior studies have shown caffeine abstinence to be significantly associated with increases in alpha and theta absolute power (Lader et al. 1996; Reeves et al. 1995). These results have face validity as a measure of caffeine withdrawal because increased theta and decreased beta voltages are generally correlated, respectively, with increased drowsiness and decreased alertness – both common symptoms of caffeine withdrawal.
A prior study by our group sought to directly examine the effect of caffeine withdrawal on the CNS-relevant physiological variables of cerebral blood flow velocity and quantitative EEG (Jones et al. 2000). In that double-blind, within-subject cross-over study, ten volunteers reporting daily caffeine intake (mean 333 mg/day) participated in test sessions in which they received acute caffeine and placebo administration under three conditions: 1) while consuming their usual diet (baseline period), 2) while consuming a caffeine-free diet and receiving placebo capsules and 3) while consuming a caffeine-free diet and receiving capsules containing caffeine in an amount equal to their individual average daily caffeine consumption. In that study, cerebral blood flow velocity in the four of the cerebral arteries measured was significantly greater during the placebo (caffeine withdrawal) condition than the caffeine condition. Placebo administration also significantly increased EEG theta power and produced significant decreases in several subjective effects, including ability to concentrate, energy/active, alert/attentive, vigor and friendliness. Overall, these data provided additional evidence that cessation of daily caffeine consumption produces changes in cerebral blood flow velocity and quantitative EEG that are likely related to the classic caffeine withdrawal symptoms of headache, drowsiness and decreased alertness.
While these studies suggest that cerebral blood flow and EEG may provide a physiological measure or correlate of caffeine withdrawal, all have suffered from important methodological limitations. The primary limitation is that none included a prolonged caffeine abstinence condition. Thus, the observed differences between the caffeine administration and caffeine withdrawal conditions may have been due to the direct effects of caffeine rather than to caffeine withdrawal. One aim of the present study was to evaluate the physiological bases of caffeine withdrawal and, more specifically, to extend the previous work investigating whether caffeine withdrawal affects cerebral blood flow velocity and quantitative EEG. This study used a within-subject, counterbalanced design to evaluate the effects of 1-day caffeine and placebo challenges in the context of prolonged caffeine exposure (3 weeks chronic caffeine) and prolonged placebo exposure (3 weeks chronic placebo). As with the prior study (Jones et al. 2000), Transcranial Doppler Sonography and quantitative EEG were used to assess cerebral blood flow velocity and brain electrical activity, respectively. By including a prolonged period of placebo exposure, this study enabled a more rigorous assessment of caffeine withdrawal than was possible in prior studies. Toward this end, it represents the strongest test to date of these neurophysiological changes underlying acute caffeine withdrawal.
In addition to investigating caffeine withdrawal, inclusion of the prolonged placebo condition in this study also permitted characterization of the acute and chronic effects of caffeine and the development of caffeine tolerance. With regard to acute caffeine effects, the study design allowed a test of the withdrawal-suppression hypothesis as the mechanism underlying acute caffeine effects. In particular, the validity of many studies claiming to demonstrate that acute caffeine administration increases performance and mood and decreases sleepiness have been questioned because the results have often been confounded by acute withdrawal effects (James et al. 2005; Rogers et al. 2005; James and Rogers 2005; James and Keane 2007; but also see Haskell et al. 2005 and Childs and de Wit 2006). More specifically, a conclusion of the beneficial effects of acute caffeine administration derived from a comparison of the effects of administering placebo versus caffeine in regular caffeine users may be erroneous because caffeine abstinence during the placebo condition may have resulted in withdrawal symptoms and degraded performance. The difference from the caffeine condition may be simply due to the ability of that caffeine dose to alleviate or reverse the caffeine withdrawal effects (James 1998; James and Rogers 2005). As a result, study designs that fail to sufficiently control for caffeine withdrawal may generate incorrect inferences about whether acute caffeine administration truly produces any direct positive effects or whether those effects are simply due to a reversal of acute withdrawal effects induced by acute caffeine abstinence. The rigorous methods used in the present study, including the prolonged placebo maintenance phase (i.e., 2-3 weeks) and confirmatory analyses of salivary caffeine to ensure compliance with dietary restrictions, allowed a careful test of acute caffeine effects. The design, which also permitted comparison of chronic caffeine maintenance with chronic placebo maintenance, provides information about the extent to which there are net beneficial effects of daily caffeine administration. Finally, with regard to tolerance, the study design permitted collecting novel data on complete and incomplete tolerance across a range of subjective and physiological measures.
Materials and Methods
Participants
Participants were recruited through newspaper advertisements and notices on bulletin boards in the community. Applicants were screened for eligibility by telephone and then in person. The screening included a medical, psychiatric, dietary and drug use history. Vital signs including blood pressure, heart rate, respiration and body temperature were taken. Participants were eligible for inclusion in the study if they were: 18 to 55 years old, within ± 20% of ideal body weight and consumed a minimum of 50 mg of caffeine daily (based on a seven-day food diary). Individuals were excluded if they: reported a history of a significant psychiatric disorder (e.g., bipolar disorder, psychotic disorder, schizophrenia); had abnormal blood pressure or heart rate; had a physical condition contraindicating the consumption of caffeine; reported a history of seizures or head injury with loss of consciousness; reported using illicit drugs recently or currently taking any prescription medication except for oral contraceptives; provided a urine sample at screening that tested positive for illicit drugs. Participants were told that the purpose of the study was to evaluate the effects of ingredients normally found in foods, beverages and over-the-counter medications on mood and physiology. To divert attention away from caffeine, they were told that they could receive any of the following compounds in capsules: chlorogenic acids, diterpines, caffeine, tannin, theobromine, theophylline, diphenhydramine or placebo (no drug). The research protocol was approved by the Institutional Review Boards at Johns Hopkins University School of Medicine and the National Institute on Drug Abuse (NIDA). Each participant provided written informed consent for the study.
Sixteen volunteers (12 females and 4 males) completed the study; 13 were Caucasian, 1 was African American, 1 was Asian and 1 was Hispanic. Participants were 36.3 years old (range: 24-54 years), had a mean of 16 years of education, and reported drinking a mean of 2.6 standard alcohol drinks per week (range: 0-11 drinks). They reported no recent use of illicit drugs; urine samples at screening tested negative for commonly used illicit substances. Participants reported consuming foods delivering a mean of 215 mg caffeine per day (range: 82-404 mg). One reported occasional tobacco smoking (mean = 5 cigarettes a week). This individual was required to refrain from smoking 8 hours before each challenge session.
Study Design
The 7-week outpatient study used a double-blind, within-subject cross-over design to examine the effects of acute caffeine and placebo administration under conditions of caffeine maintenance (400 mg per day) and placebo maintenance (cf. Table 1). The 400 mg caffeine dose was chosen for the present study as it is in the range of those shown to produce changes in cerebral blood flow and EEG power in prior studies (Goldstein et al. 1963; Jones et al. 2000; Saletu et al. 1987). Eight participants received the placebo maintenance phase first, after which they received the caffeine maintenance phase; eight received the caffeine maintenance phase first, after which they received the placebo maintenance phase. Each maintenance phase lasted approximately 21 days and involved twice-daily consumption of capsules containing either placebo or caffeine (described below).
Table 1.
Study Design. A schematic of the experimental design, including an example of the sequence of drug conditions for maintenance phases and challenge sessions. The sequence of maintenance and challenge conditions was mixed within and across participants.
| Sequential day number (approx) |
Number of days | Procedure | |
|---|---|---|---|
| 1-13 | 13 | Chronic placebo administration |
Placebo Maintenance Phasea |
| 14 | 1 | Challenge #1: Caffeine (plac/caff)b | |
| 15-20 | 6 | Chronic placebo administration | |
| 21 | 1 | Challenge #2: Placebo (plac/plac)b | |
| 22-34 | 14 | Chronic caffeine administration |
Caffeine Maintenance Phasea |
| 35 | 1 | Challenge #3: Caffeine (caff/caff) b | |
| 36-41 | 5 | Chronic caffeine administration | |
| 42 | 1 | Challenge #4: Placebo (caff/plac) b |
During the first 21 study days (except for challenge days 14 and 21), participants were maintained on either caffeine (N=8) or placebo (N=8). During study days 22-42 (except for challenge days 35 and 42), participants were exposed to the other maintenance phase. Each maintenance phase lasted a minimum of 14 days before the first challenge session.
Subjects received four challenge sessions (two during the placebo maintenance phase and two during the caffeine maintenance phase). Each challenge session consisted of oral administration of either caffeine (200 mg b.i.d.) or placebo (b.i.d.), followed by assessment of subjective measures, cerebral blood flow, and EEG.
Within each maintenance phase, subjects participated in two challenge sessions (a caffeine and a placebo challenge session). Order of challenge sessions was mixed within and across subjects. Participants were maintained on placebo or caffeine for a minimum of 14 days before the first challenge session. Each challenge session consisted of oral administration of either caffeine (200 mg b.i.d.) or placebo (b.i.d.), followed by collection of subjective and physiological measures (described below). Thus, there were a total of four challenge sessions from the two phases, with two challenges per phase: 1) in order to examine cerebral blood flow and EEG during chronic caffeine administration, one challenge session involved approximately two weeks of caffeine maintenance followed by a challenge day on which the participant received caffeine (caff/caff); 2) in order to examine the effects of acute caffeine withdrawal on these measures, a challenge session involved caffeine maintenance followed by a challenge day on which the participant received placebo (caff/plac); 3) in order to provide an assessment during chronic placebo administration, a challenge session involved placebo maintenance followed by a challenge day on which the participant received placebo (plac/plac) and 4) in order to examine the effects of acute caffeine administration on these measures, a fourth challenge session involved placebo maintenance followed by a challenge day on which the participant received caffeine (plac/caff).
Participants came to the laboratory three times per week throughout the study to provide saliva samples, complete subjective measures, and to take the afternoon capsule under the observation of the research staff. At each study visit, participants were required to bring with them their container of capsules so that research staff could count the remaining pills to monitor compliance with the capsule ingestion schedule. At one visit per week, participants received a new container of capsules for the upcoming week and returned the empty container. During the caffeine maintenance phase, they ingested a capsule containing 200 mg caffeine anhydrous twice daily, once between 8 and 9 AM in the morning and then again between 2 and 3 PM, for a total daily caffeine dose of 400 mg. During the placebo maintenance phase, participants ingested a placebo capsule twice daily (8-9 AM and 2-3 PM). For the eight subjects who received the caffeine maintenance phase first, caffeine content in the daily capsules was gradually reduced during the first 3 days of the subsequent placebo maintenance phase to minimize caffeine withdrawal symptoms when switching from the caffeine to placebo maintenance condition.
Capsule preparation and administration
Caffeine capsules (200 mg per capsule; size 0, opaque hard gelatin) were prepared using powdered lactose and caffeine anhydrous (USP). Identical placebo capsules were weight matched (+/− 5%) and prepared using powdered lactose and quinine sulfate (approximately 4.5 mg). The quinine was added to equate taste between caffeine and placebo capsules in the event that subjects bit or opened the capsules to taste their contents (cf. Abreu and Griffiths 1996).
Assessment of Cerebral Blood Flow and Electroencephalogram (EEG)
Cerebral blood flow velocity was measured with participants sitting in a reclined position in a quiet room. The cerebral blood flow velocity was determined using a temporal window (zygomatic arch) for four arteries (right and left middle (MCA) cerebral arteries, right and left anterior (ACA) cerebral arteries) using pulsed Transcranial Doppler Sonography (Nicolet, Model TC2000). Mean velocity (VM: cm/s), systolic velocity (VS: cm/s), diastolic velocity (VD: cm/s), and pulsatility index (PI=(VS-VD)/VM) were determined for each artery. The blood flow measurements were taken approximately 60 minutes after the afternoon capsule administration.
Following blood flow measurement, multichannel EEG was collected during a resting recording session with eyes closed. EEG was recorded starting approximately 90 minutes after the afternoon capsule administration. Participants sat in a reclining chair in a sound-attenuated, electronically shielded chamber. A 3-minute eyes-closed recording was obtained. The EEG was recorded from the following sixteen International 10/20 scalp sites: Fp1, F3, C3, P3, O1, F7, T3, T5, Fp2, F4, C4, P4, O2, F8, T4, and T6. The EEG recording used the ear tips as a reference. Eye movement was recorded from above and to the side of the left eye. Silver/silver chloride electrodes were used at all locations. The EEG was amplified with Grass (Model 7P511) amplifiers and processed with a 1 to 50 Hz half-amplitude band pass and notch filter at 60 Hz. The EEG was sampled at the rate of 104 samples per second per channel. Artifacts in the EEG were removed by computer-assisted visual inspection by an operator blind to the test day and subject. EEG spectral power was determined using the Fast Fourier Transform using 256 points per epoch (Chamberlin 1985) and then averaging the spectra over epochs. After the average spectrum was calculated, power was divided into delta (0.4-3.9 Hz), theta (4.0-7.9 Hz), alpha1 (8.0-9.9 Hz), alpha2 (10.0-13.9 Hz), beta1 (14.0-24.9 Hz) and beta2 (25.0-40.0 Hz) EEG bands. Relative power (percentage) was also calculated for each band.
Assessment of Subjective Effects
The subjective effects measured included the Profile of Mood States (POMS), a 65-item questionnaire designed to assess mood states (McNair et al. 1971) and a 33-item subjective questionnaire, a portion of which was previously reported to be sensitive in measuring caffeine withdrawal (e.g., Griffiths et al. 1990; Jones et al. 2000). Although questionnaires were completed at every study visit, only those from the 4 challenge sessions were used in data analyses. On challenge days, subjects completed the questionnaires approximately 20 minutes after the afternoon capsule and were instructed to respond based on how they felt at the time of assessment.
Dietary Restrictions
As previously described, caffeine was restricted throughout the study. The dietary restrictions were described to subjects without reference to caffeine. The only beverages allowed were milk, fruit juices and water; chocolate products were prohibited. To divert attention away from caffeine, items without caffeine were also restricted, including alcohol, coconut products, shellfish and poppy seeds. The subjects were also instructed not to take any medications.
At each study visit, subjects were asked about their dietary and medication intake since the last study visit, and a sample of saliva (6 ml) was collected to both assess and encourage volunteer compliance with dietary restrictions. Although compliance with dietary restrictions was verbally encouraged by research staff at laboratory visits, volunteers also were led to believe that any or all saliva samples would be tested for compliance. Salivary caffeine concentrations were analyzed for four samples from each participant by Gas Chromatography-Thermionic Specific Detection (Labstat Inc, Ontario, Canada) using methods previously described (Jacob et al. 1981; Griffiths and Woodson 1988). Two of the samples were from the placebo maintenance phase. Specifically, one sample from the challenge day on which participants received a placebo challenge (plac/plac) and one sample from the visit prior to that challenge session were analyzed. These two samples provided an opportunity to confirm compliance with study dietary restrictions at a timepoint when little or no caffeine should have been ingested. Median caffeine concentration for these samples was 16 ng/ml, indicating that subjects were compliant with the caffeine restrictions during the placebo period. The other two samples were from the caffeine maintenance phase.
Specifically, one sample from the challenge day on which participants received a placebo challenge during caffeine maintenance (caff/plac) and one sample from the visit immediately prior to that challenge session. These two samples provided the opportunity to observe a reduction in caffeine concentration between the two days that one would expect for this challenge session that seeks to produce caffeine withdrawal. Median caffeine concentration from the caffeine maintenance sessions was 2512 ng/ml, which decreased to 962 ng/ml following the placebo challenge. These concentrations are consistent with those from a previous report that showed a mean of 4420 ng/ml three hours after 400 mg caffeine and a mean of 350 ng/ml after overnight abstinence (Griffiths and Woodson 1988).
Data Analysis
Cerebral Blood Flow
A condition (caff/plac, caff/caff, plac/plac, plac/caff) by side (right vs. left) repeated measures analysis of variance was performed using SAS PROC MIXED on blood flow velocity measures. Because of equipment failure, cerebral blood flow data were missing in one condition from each of two subjects; however, the data from the other three conditions for these participants were included in these analyses. PROC MIXED was chosen because this analytical model is capable of modeling repeated measures designs with missing values. While there were several significant main effects of side, there were no significant condition x side interactions. Because side differences were not the primary focus of the present study, data were collapsed across side. Post-hoc comparisons (Fisher's LSD) were conducted to compare the acute caffeine withdrawal condition to the other control conditions used in the study. These post-hoc comparisons were limited to the variables showing significant effects of condition. More specifically, five post-hoc tests were conducted: 3 comparing the caff/plac (acute abstinence) condition to each of the other three conditions (caff/caff, plac/plac, plac/caff). The fourth post-hoc test compared the acute abstinence condition (caff/plac) to a combined control, in which the means of the two chronic maintenance conditions (caff/caff and plac/plac) were combined. The fifth test compared the chronic caffeine to the chronic placebo condition. Adjustments for sphericity were done using Huynh-Feldt corrections.
EEG
For each band, relative EEG power (band power/total power) was calculated. A condition (caff/plac, caff/caff, plac/plac, plac/caff) by electrode (16 scalp sites) repeated measures ANOVA was performed on relative EEG power in each band. While there were several significant main effects of electrode, electrode differences were not the primary focus of the present study and data were collapsed across electrodes. As in the analyses with cerebral blood flow data, five post-hoc tests for variables showing significant effects of condition were conducted: 3 comparing the caff/plac (acute abstinence) condition to each of the other three conditions (caff/caff, plac/plac, plac/caff). The fourth post-hoc test compared the acute abstinence condition (caff/plac) to a control combining the two chronic maintenance conditions (caff/caff and plac/plac), and the fifth compared the chronic caffeine to the chronic placebo condition. Adjustments for sphericity were done using Huynh-Feldt corrections.
Subjective Measures
A single factor (drug condition) ANOVA was performed on the raw scores of each of the subjective measures using the planned post-hoc comparisons described above. All statistical significance was indicated at P=0.05. Adjustments for sphericity were done using Huynh-Feldt corrections.
Results
The design used in the present study allowed for the analysis of withdrawal effects, acute caffeine effects, caffeine tolerance and chronic caffeine effects. It also permitted a comparison of these effects across the range of physiological and subjective measures.
Caffeine Withdrawal Effects
In interpreting the results presented below, it is helpful to consider that there are three types of comparisons between experimental conditions that can be used to characterize caffeine withdrawal. First, a significant difference between the acute abstinence (caff/plac) and chronic placebo maintenance (plac/plac) conditions provides a conservative test of caffeine withdrawal. Second, a difference between the acute abstinence (caff/plac) and chronic caffeine maintenance (caff/caff) conditions provides a test of naturalistic withdrawal. Third, a difference between the acute abstinence (caff/plac) and the combined chronic maintenance conditions (caff/caff, plac/plac) provides an overall test of withdrawal.
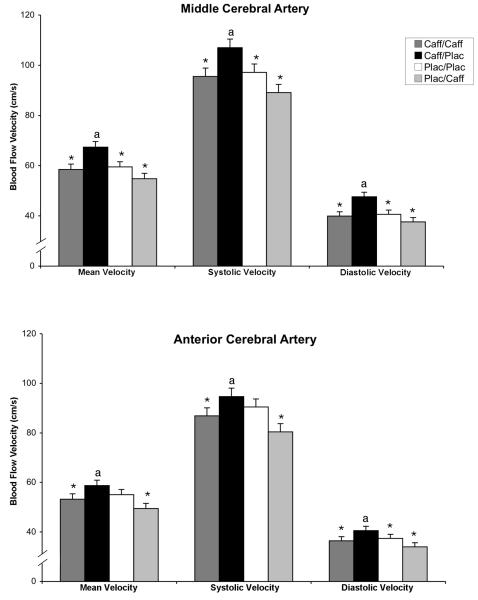
Cerebral Blood Flow Velocity Middle cerebral artery
Mean velocity (VM: cm/s), systolic velocity (VS: cm/s), diastolic velocity (VD: cm/s), and pulsatility index (PI) for the MCA were significantly altered by the experimental conditions [VM: F(1,43)=9.73, P<.0001; VS: F(1,43)=7.70, P<.0001; VD: F(1,43)=10.2, P<.0001; PI: F(1,43)=3.05, P=.04]. VM, VS and VD in the MCA were highest in the acute abstinence condition (caff/plac) (Figure 1, top panel). Post-hoc comparisons between drug conditions showed that, for VM, VS and VD, caff/plac was significantly greater (P<.005) than each of the other three conditions as well as the two chronic maintenance conditions combined (caff/caff and plac/plac). The pulsatility index (PI) was significantly decreased (P≤.05) during caff/plac relative to caff/caff and plac/plac (not shown).
Figure 1.
Effects of the four drug conditions on cerebral blood flow velocities for middle cerebral artery (top panel) and anterior cerebral artery (bottom panel). Mean, systolic and diastolic blood flow velocities, averaged over left and right sides, are presented. Dark grey bars show the caffeine maintenance condition (caff/caff); solid bars show the acute caffeine abstinence condition (caff/plac), open bars show the placebo maintenance condition (plac/plac), and light grey bars show the acute caffeine administration condition (plac/caff). The y-axis is presented on a smaller scale (40-120 cm/s) that represents a physiologically reasonable range for cerebral blood flow velocity and allows more detailed inspection of the data (normative range is 29-176 cm/s for MCA and 16-124 cm/s for ACA). Brackets indicate +1SEM. Asterisks (*) indicate that the condition was significantly different from the acute abstinence condition (caff/plac); the letter “a” indicates that the acute abstinence condition (caff/plac) was significantly different from the two chronic maintenance conditions combined (caff/caff and plac/plac).
Anterior Cerebral Artery: VM, VS and VD for the ACA were significantly altered by the experimental conditions [VM: F(1,43)=4.79, P=.006; VS: F(1,43)=4.10, P=.012; VD: F(1,43)=4.43, P=.008]. VM, VS and VD in the ACA were highest in the acute abstinence condition (caff/plac) (Figure 1, bottom panel). Post-hoc comparisons between drug conditions showed that, for VM and VS, caff/plac was significantly greater (P≤.05) than both caff/caff and plac/caff as well as the two chronic maintenance conditions combined (caff/caff and plac/plac). For VD, caff/plac was significantly greater (P≤.05) than each of the other three conditions. PI in the ACA was not significantly altered by drug condition.
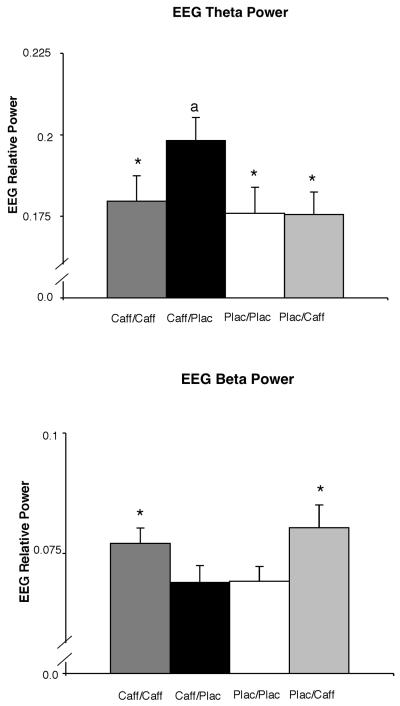
EEG Power
EEG power in two bands was significantly altered by the experimental conditions [theta: F(3,45)=5.81, P=.002; beta 2: F(3,45)=5.93, P=.002]. EEG theta power was highest in the acute abstinence condition (caff/plac) (Figure 2, top panel). Post-hoc comparisons between drug conditions showed that, for theta power, caff/plac was significantly greater (P<.01) than each of the other three conditions as well as significantly the two chronic maintenance conditions combined (caff/caff and plac/plac).
Figure 2.
Effects of the four drug conditions (caff/caff, caff/plac, plac/plac, plac/caff) on EEG relative power for theta (top panel) and beta 2 (bottom panel). The y-axis is presented on a smaller scale that represents a physiologically reasonable range for cerebral blood flow velocity and allows more detailed inspection of the data (normative range is .10-.35 for relative theta and .05-.20 for relative beta). Other details are as in Figure 1.
EEG beta 2 power was numerically lowest in the acute abstinence condition (caff/plac) relative to the other drug conditions (Figure 2, bottom panel). Post-hoc comparisons between drug conditions showed that, for beta 2 power, caff/plac was significantly lower (P≤.01) than both caff/caff and plac/caff conditions.
Subjective Measures
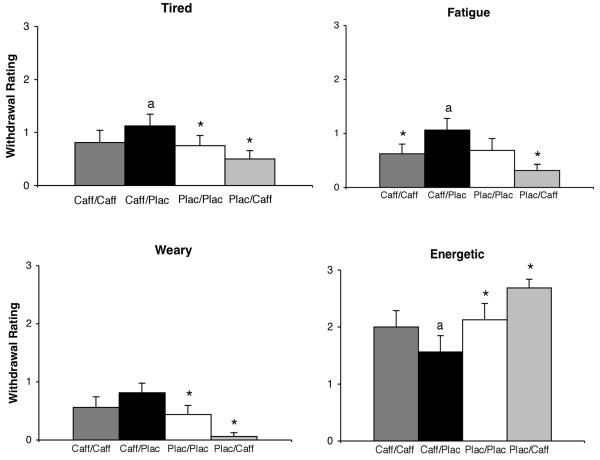
One variable on the Subjective Questionnaire was significantly altered by the experimental conditions [Tired: F(3,45)=3.78, P=.017]. Ratings of Tired were highest in the acute abstinence condition (caff/plac) (Figure 3, top left panel). Post-hoc comparisons between drug conditions showed that, for ratings of Tired, caff/plac was significantly greater (P≤.05) than both plac/plac and plac/caff as well as the two chronic maintenance conditions combined (caff/caff and plac/plac).
Figure 3.
Effects of the four drug conditions (caff/caff, caff/plac, plac/plac, plac/caff) on subjective measures. Other details are as in Figure 1.
On the POMS, six items and two subscales were also significantly altered by the experimental conditions: ratings of Fatigue (F(3,45)=4.64, P=.007), Sluggish (F(3,45)=3.34, P=.027), Weary (F(3,45)=6.50, P=.001), Energetic (F(3,45)=5.91, P=.002), Friendly (F(3,45)=3.21, P=.032), Lively (F(3,45)=2.92, P=.049), the Vigor Scale (F(3,45)=3.72, P=.018) and the Fatigue Scale (F(3,45)=3.03, P=.039). Of these, only the item Friendly showed no significant differences in post-hoc comparisons. Ratings of Fatigue and Weary were highest in the acute abstinence condition (caff/plac) (Figure 3). For ratings of Fatigue, post-hoc comparisons between drug conditions showed that caff/plac was significantly greater (P≤.05) than caff/caff and plac/caff as well as the two chronic maintenance conditions combined (caff/caff and plac/plac). For ratings of Weary, caff/plac was significantly greater (P≤.05) than plac/plac and plac/caff. Ratings of Energetic were lowest in the acute abstinence condition (caff/plac) (Figure 3), with post-hoc comparisons showing that caff/plac was significantly lower (P≤.05) than plac/plac and plac/caff as well the two chronic maintenance conditions combined (caff/caff and plac/plac).
The other POMS items (i.e., Friendly, Lively, Sluggish) and scales (i.e., Vigor, Fatigue) showed the same general pattern of effect (i.e., maximum decreases for Friendly, Lively and Vigor and maximum increases for Sluggish and Fatigue during the caff/plac condition). The only significant post-hoc difference for these measures was between the caff/plac and plac/caff conditions (P≤.03).
Cardiovascular Measures
Of the cardiovascular measures examined in the present study, only diastolic blood pressure was significantly altered by experimental conditions (F(3,45)=5.91, P=.002) (69.4, 69.4, 71.3 and 74.4 for caff/caff, caff/plac, plac/plac and plac/caff, respectively). The only significant post-hoc difference for this measure was between the caff/plac and plac/caff conditions (P=.01).
Acute Caffeine Effects, Chronic Caffeine Effects and Tolerance
In addition to caffeine withdrawal effects, the study design also provided information about the acute effects of caffeine, chronic effects of caffeine and tolerance. Comparisons between specific sets of experimental conditions can be used to characterize these effects. More specifically, a difference between the plac/caff and plac/plac conditions provides a test of acute caffeine effects, and a difference between caff/caff and plac/plac provides a test of the chronic effects of caffeine. Tolerance can be defined as a difference between the plac/caff and caff/caff conditions and, for those variables that showed tolerance (i.e., differences between the plac/caff and caff/caff conditions), “complete tolerance” can be defined by the absence of a significant difference between the caff/caff and plac/plac conditions.
These effects are presented in Table 2 that also, for comparative purposes, summarize the previously presented withdrawal data. As shown in Table 2, acute caffeine effects were demonstrated across a wide range of measures, including cerebral blood flow, EEG and subjective effects. Such effects were shown for measures that showed withdrawal effects (Table 2 and Figures 1-3) as well as those that did not show withdrawal effects (Table 2 and Figure 4). In contrast to the wide range of measures showing acute caffeine effects, the effects of chronic caffeine maintenance were evident on only one measure (i.e., EEG beta 2 power). Evidence of tolerance was limited to subjective measures, with no evidence of tolerance or “complete tolerance” to any of the physiological measures. As with acute effects, tolerance was demonstrated with measures that did and did not show withdrawal (Table 2 and Figures 3 and 4).
Table 2.
Interpretation of differences between the experimental conditions
| Measurea | Withdrawal | Acute Caffeine Effectsb |
Chronic Caffeine Effectsc |
Tolerance |
|---|---|---|---|---|
| Doppler | ||||
| Middle Cerebral Artery | ||||
| Mean Velocity | Xd, e, f | X | ||
| Systolic Velocity | Xd, e, f | X | ||
| Diastolic Velocity | Xd, e, f | |||
| Pulsatility Index | Xd, e, f | |||
| Anterior Cerebral Artery | ||||
| Mean Velocity | Xe, f | X | ||
| Systolic Velocity | Xe, f | X | ||
| Diastolic Velocity | Xe, f | X | ||
| EEG | ||||
| Relative Theta | Xd, e, f | |||
| Relative Beta2 | Xe | X | X | |
| Withdrawal Questionnaire | ||||
| Tired | Xd, f | |||
| POMS | ||||
| Vigor Scale | Xg, h | |||
| Fatigue Scale | X | |||
| Friendly | X | Xg, h | ||
| Lively | X | Xg, h | ||
| Energetic | Xd, f | X | Xg, h | |
| Fatigue | Xe, f | X | ||
| Sluggish | ||||
| Weary | Xd, f | X | Xg, h | |
| Efficient | Xg | |||
| Full of Pep | X | Xg, h | ||
| Bushed | X | Xg, h | ||
Column 1 lists dependent measures that had significant main effects in the ANOVA
Column 3 shows which measures demonstrated evidence of acute caffeine effects (significant difference between Plac/Caff and Plac/Plac)
Column 4 shows which measures demonstrated evidence of chronic caffeine effects (significant difference between Caff/Caff and Plac/Plac)
Significant difference between Caff/Plac and Plac/Plac (a conservative test of withdrawal)
Significant difference between Caff/Plac and Caff/Caff (a test of naturalistic withdrawal)
Significant difference between Caff/Plac and combined chronic maintenance conditions (Caff/Caff, Plac/Plac) (an overall test of withdrawal)
Significant difference between Plac/Caff and Caff/Caff (a test of tolerance)
Significant difference between Plac/Caff and Caff/Caff AND no significant difference between Caff/Caff and Plac/Plac (a test of “complete tolerance” development for those measures)
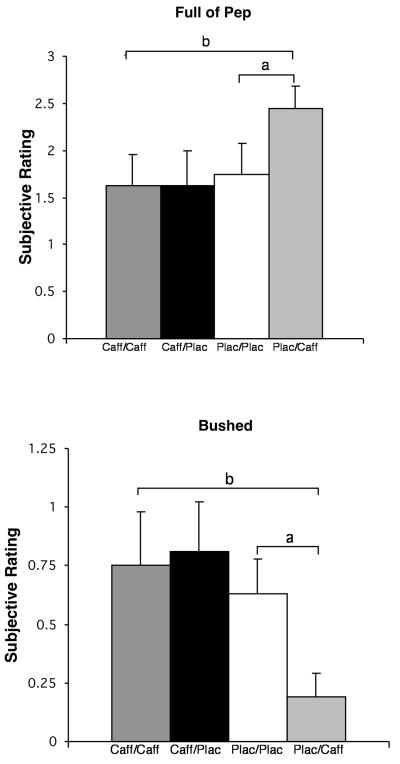
Figure 4.
Effects of the four drug conditions (caff/caff, caff/plac, plac/plac, plac/caff) on two illustrative subjective measures that did not show withdrawal effects but did show acute caffeine effects and tolerance to caffeine. Brackets indicate significant effects. Acute caffeine effects were demonstrated by a significant difference between the plac/caff and plac/plac conditions (a). Tolerance was demonstrated by a significant difference between the plac/caff and caff/caff conditions (b).
Discussion
One aim of the present study was to evaluate some of the physiological bases of caffeine withdrawal and, more specifically, to extend the previous work investigating whether caffeine withdrawal affects cerebral blood flow velocity and quantitative EEG. While numerous studies have demonstrated the subjective effects of caffeine withdrawal, less is known about the neurophysiological changes that may accompany withdrawal-related mood alterations. Data from several studies have suggested that caffeine withdrawal is associated with changes in cerebral blood flow and EEG activity (Cameron et al. 1990; Couturier et al. 1997; Mathew et al. 1983; Mathew and Wilson 1985; Reeves et al. 1995), although these studies did not include the necessary controls (including a chronic placebo condition) to permit an unambiguous determination that the effects were due to caffeine withdrawal per se. Indeed, there have been relatively few studies with experimental designs sufficient to identify an unequivocal withdrawal symptom vs. suggestive withdrawal symptom (Juliano and Griffiths 2004). The present study sought to determine the specificity of measures of EEG and cerebral blood flow in assessing caffeine withdrawal using rigorous experimental methods.
This study demonstrated physiological effects of caffeine withdrawal. Of the 21 dependent measures presented in Table 2 that had significant main effects in this study, a significant withdrawal effect was observed on 13 measures. Acute caffeine abstinence produced significant increases in blood flow velocity in both middle and anterior cerebral arteries and significant decreases in pulsatility index in the middle cerebral artery. These results are consistent with those of prior experimental studies (Mathew & Wilson 1985; Couturier et al. 1997; Jones et al. 2000; Field et al. 2003) and provide further evidence to support an underlying vascular mechanism of the common caffeine withdrawal symptom of headache. Acute caffeine abstinence also produced significant increases in EEG theta power and decreases in beta 2 power, which are associated, respectively, with the common caffeine withdrawal symptoms of increased drowsiness and decreased alertness. These physiological effects of caffeine withdrawal are consistent with those observed in prior studies (Couturier et al. 1997; Field et al. 2003; Jones et al. 2000; Lader et al. 1996; Mathew and Wilson 1985; Reeves et al. 1995) and extend those findings using a well-controlled experimental design. Finally, the changes in cerebral blood flow velocity and EEG were accompanied by significant changes in subjective effects, including increased ratings of tired, fatigue, sluggish, weary and decreased ratings of energetic, friendly, lively and vigor. These subjective effects are consistent with the previously-reported profile of subjective effects commonly associated with caffeine withdrawal which have been demonstrated under a wide range of methodological conditions (Juliano and Griffiths 2004).
In contrast to the present study that showed selective increases in EEG theta power during caffeine withdrawal, a recent well-controlled study by Keane and colleagues (2007) showed increased theta following both acute caffeine withdrawal and acute caffeine administration, leading the authors to suggest that any change in drug state, as compared to stable states of caffeine abstinence or use, may disrupt electrophysiological activity in the brain. Several differences in methodology between the two studies make interpretation of these differences challenging. For example, effects of caffeine in the Keane study were evident only during task performance and not during the sessions conducted with eyes closed, which was similar to the condition under which EEG was assessed in this study. Also, the present study used more lengthy caffeine and placebo maintenance phases than Keane et al. (≥ 2 vs. 1 week, respectively), reported changes in relative rather than absolute power. and collapsed data across electrode sites rather than evaluating data from brain regions individually.
As noted above, in the present study, three types of comparisons between experimental conditions provided different tests of caffeine withdrawal. First, a significant difference between the acute abstinence (caff/plac) and chronic placebo maintenance (plac/plac) conditions provided the most stringent test of caffeine withdrawal. This conservative comparison could be used to ensure that any difference observed was not confounded by the direct effects of caffeine (Juliano and Griffiths 2004). Of the 13 physiological and subjective effects that showed evidence of caffeine withdrawal (see Table 2), 8 met this test of caffeine withdrawal and were evident in MCA blood flow velocity, EEG theta power, and several of the subjective ratings. Second, a significant difference between the acute abstinence (caff/plac) and chronic caffeine maintenance (caff/caff) conditions provided a less conservative test of withdrawal. While a theoretically less rigorous test of caffeine withdrawal, this comparison has good ecological validity as it resembles the naturalistic pattern of caffeine consumption in the real world followed by abrupt abstinence (Juliano and Griffiths 2004). Ten of the 13 variables met this definition of naturalistic withdrawal, which were evident on all cerebral blood flow measures, both theta and beta EEG measures, and with one of the subjective ratings (i.e., fatigue). Finally, a difference between the acute abstinence (caff/plac) and the combined chronic maintenance conditions (caff/caff, plac/plac) served as an overall test of withdrawal. Only one of the 13 variables (i.e., EEG beta) failed to meet this test of withdrawal.
In the present study, we also sought to characterize physiological measures of acute and chronic caffeine administration and caffeine tolerance. In terms of acute effects, a significant difference between the plac/caff and plac/plac conditions was used as the test for acute caffeine effects. Data from this study demonstrated acute caffeine effects across a wide range of measures, including cerebral blood flow, EEG and subjective effects. Of the 21 dependent measures presented in Table 2 with significant main effects, evidence of a significant acute caffeine effect was evident on 14 measures. These effects on subjective measures are consistent with previous observations in non-tolerant individuals (Evans and Griffiths (1992). The finding that acute caffeine increases EEG beta is novel. Prior studies that did not control for caffeine tolerance and withdrawal have produced generally mixed results (cf. Introduction to Keane et al., 2007). However, the single study which did incorporate these controls, albeit with the methodological differences noted previously, did not show such effects (Keane et al., 2007). Overall, the robust effects of acute caffeine administration demonstrated in the present study are contrary to the idea that withdrawal reversal entirely accounts for the acute effects of caffeine (cf. James and Rogers, 2005).
The effects of chronic caffeine maintenance were defined as a significant difference between the caff/caff and plac/plac conditions. This effect was only observed only on the single measure of EEG relative beta 2 power. These results are generally consistent with those from a prior study by our group in which, during chronic dosing, participant ratings on mood and subjective effects did not differ significantly between chronic caffeine administration of 900 mg per day and chronic placebo administration (Evans and Griffiths, 1992). This was replicated in the present study using a daily caffeine dose (i.e., 400 mg) that more closely approximates typical dietary caffeine doses consumed habitually. Overall, the failure to demonstrate robust effects of chronic caffeine administration is consistent with previous evidence suggesting that there may be no net beneficial effects from habitual caffeine administration (Rogers and Dernoncourt, 1998; James and Rogers, 2005; James et al., 2005).
Caffeine tolerance was defined as a significant difference between the plac/caff and caff/caff conditions. Of the 21 dependent measures that had significant main effects, 8 showed evidence of tolerance. “Complete tolerance” was defined by the absence of a significant difference between the caff/caff and plac/plac conditions and was observed on 7 of these 8 measures. These data are consistent with the larger literature demonstrating the development of caffeine tolerance in humans, although a full parametric investigation of tolerance has not been done (Evans and Griffiths 1992; Griffiths and Mumford 1996). In the present study, and under rigorous conditions, we demonstrated “complete tolerance” to most of the subjective effects of caffeine. Interestingly, there was no evidence of tolerance on the physiological measures. These data suggest that, despite evidence of caffeine withdrawal, full tolerance to the physiological effects of acute caffeine administration may not occur. This finding is consistent with other studies suggesting that incomplete tolerance develops to caffeine-produced increases in blood pressure, which is another common physiological effect of caffeine (Denaro et al. 1991; James 1994; James 1996; Robertson et al. 1981). In a more recent randomized, double-blind study by Lovallo and colleagues (2004), daily caffeine consumption failed to completely eliminate the increases in blood pressure following caffeine challenge.
The differential pattern of effects of caffeine withdrawal, acute effects, and tolerance seen across the various physiological and subjective measures evaluated in this study may also be of note. Specifically, caffeine withdrawal and acute effects were generally demonstrated across cerebral blood flow, EEG and subjective measures while demonstration of tolerance was limited to the subjective measures. Looking across Table 2, these data emphasize the complexity of caffeine pharmacology profile by showing the different patterns across the various physiological and subjective effects. Considering that withdrawal and tolerance often are considered to reflect the same underlying physiological neurological adaptation, this data presents an interesting case where a number of physiological measures for which withdrawal is clearly shown exhibit no evidence of tolerance. It may be the case that there is something mechanistically different between these types of physiological and subjective measures that may result in differential response to caffeine effects. Future research efforts to determine whether this occurs with other classes of drugs and with other measures with caffeine effects are warranted.
Several limitations of this study should be noted. Because the assessments on challenge days were conducted at a single time point approximately 24 hours after the last active caffeine dose, no information was provided about the time course or peak effects. Multiple observations of each condition within each subject could have provided more power than the single observation. Furthermore, for the assessment of acute abstinence (i.e., placebo challenge during caffeine maintenance) this timing may have underestimated the magnitude of withdrawal effect because prior studies show that peak intensity can vary between 20 to 51 h after abstinence (Juliano and Griffiths, 2004).
The findings from the present study also highlight the need for additional research in several different areas. A single 400 mg (200 mg b.i.d.) dose of caffeine was used for both maintenance and challenge days. It would be important to explore whether higher and lower doses may produce different results. It has been well-established that the incidence or severity of caffeine withdrawal is proportional to the caffeine maintenance dose, so it is reasonable to expect that a higher chronic dose of caffeine may have produced more robust physiological and subjective withdrawal effects (Evans and Griffiths 1999; Juliano and Griffiths 2004). Also, the inclusion of such a prolonged placebo maintenance period (e.g., 2-3 weeks) permitted a rigorous assessment of caffeine withdrawal and helped to ensure that caffeine withdrawal had fully subsided by the time of the acute caffeine challenge was conducted in the plac/caff condition. That said, caffeine abstinence periods of longer or shorter durations may produce different effects. Finally, the duration of caffeine maintenance has been shown to be an important potential parameter in determining withdrawal effects (Evans and Griffiths 1999; Juliano and Griffiths 2004). The duration of caffeine maintenance in this study was modest (i.e., 2-3 weeks) but it would be of particular interest to evaluate the potential lower limit of the duration necessary to produce subjective and physiological withdrawal effects.
In summary, the present study demonstrated using rigorous experimental methods that acute caffeine abstinence produces orderly changes in cerebral blood flow and EEG activity. These data provide further support for the underlying neurophysiological mechanisms of the classic subjective caffeine withdrawal symptoms and provide the strongest demonstration to date of the physiological effects of caffeine withdrawal. The study also demonstrated robust acute effects of caffeine unconfounded by caffeine withdrawal, but no evidence for net beneficial effects of daily caffeine administration. Additional research will be needed to further improve our understanding of the mechanisms involved in caffeine administration and abstinence effects.
Acknowledgments
This research was supported in part by research grant R01 DA-03890 and training grant T32-DA07209 from the National Institute on Drug Abuse. The authors thank John Yingling for computer programming assistance, Allison Chausmer, Tiffany Tomlin and Krista Powell for their help with data collection and Paul Nuzzo for assistance with statistical analysis.
Footnotes
This study complies with current laws of the United States and the authors report no conflicting interests.
References
- Abreu ME, Griffths RR. Drug tasting may confound human drug discrimination studies. Psychopharmacol. 1996;125:255–257. doi: 10.1007/BF02247336. [DOI] [PubMed] [Google Scholar]
- Cameron OG, Modell JG, Hariharan M. Caffeine and human cerebral blood flow: a positron emission tomography study. Life Sciences. 1990;47:1141–1146. doi: 10.1016/0024-3205(90)90174-p. [DOI] [PubMed] [Google Scholar]
- Chamberlin H. Musical application of microprocessors. Hasbrouk Heights; NJ: Hayden: 1985. pp. 435–458. [Google Scholar]
- Childs E, de Wit H. Subjective, behavioral, and physiological effects of acute caffeine in light, nondependent caffeine users. Psychopharmacol. 2006;185:514–523. doi: 10.1007/s00213-006-0341-3. [DOI] [PubMed] [Google Scholar]
- Couturier EGM, Laman DM, van Duijn MAJ, van Duijn H. Influence of caffeine and caffeine withdrawal on headache and cerebral blood flow velocities. Cephalalgia. 1997;17:188–190. doi: 10.1046/j.1468-2982.1997.1703188.x. [DOI] [PubMed] [Google Scholar]
- Debrah K, Haigh R, Sherwin R, Murphy J, Kerr D. Effect of acute and chronic caffeine use on cerebrovascular, cardiovascular and hormonal responses to orthostasis in healthy volunteers. Clinical Science. 1995;89:475–480. doi: 10.1042/cs0890475. [DOI] [PubMed] [Google Scholar]
- Denaro CP, Brown CR, Jacob PI, Benowitz NL. Effects of caffeine with repeated dosing. Eur J Clin Pharmacol. 1991;40:273–278. doi: 10.1007/BF00315208. [DOI] [PubMed] [Google Scholar]
- Evans SM, Griffiths RR. Caffeine tolerance and choice in humans. Psychopharmacology. 1992;108:51–59. doi: 10.1007/BF02245285. [DOI] [PubMed] [Google Scholar]
- Evans SM, Griffiths RR. Caffeine withdrawal: a parametric analysis of caffeine dosing conditions. J Pharmacol Exp Ther. 1999;289:285–294. [PubMed] [Google Scholar]
- Field AS, Laurienti PJ, Yen YF, Burdette JH, Moody DM. Dietary caffeine consumption and withdrawal: confounding variables in quantitative cerebral perfusion studies? Radiology. 2003;227:129–135. doi: 10.1148/radiol.2271012173. [DOI] [PubMed] [Google Scholar]
- Goldstein L, Murphree HB, Pfeiffer CC. Quantitative electroencephalography in man as a measure of CNS stimulation. Annals New York Academy of Sciences. 1963:1045–1056. doi: 10.1111/j.1749-6632.1963.tb13348.x. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Evans SM, Heishman SJ, Preston KL, Sannerud CA, Wolf B, Woodson PP. Low-dose caffeine physical dependence in humans. J Pharmacol Exp Ther. 1990;225:1123–1132. [PubMed] [Google Scholar]
- Griffiths RR, Mumford GK. Caffeine reinforcement, discrimination, tolerance and physical dependence in laboratory animals and humans. In: Schuster CR, Gust SW, Kuhar MJ, editors. Pharmacological Aspects of Drug Dependence - Toward an Integrated Neurobehavioral Approach - Handbook of Experimental Pharmacology. Springer-Verlag; New York: 1996. pp. 315–341. [Google Scholar]
- Griffiths RR, Woodson PP. Reinforcing effects of caffeine in humans. J Pharmacol Exp Ther. 1988;246:21–29. [PubMed] [Google Scholar]
- Haskell CF, Kennedy DO, Wesnes KA, Scholey AB. Cognitive and mood improvements of caffeine in habitual consumers and habitual non-consumers of caffeine. Psychopharmacol. 2005;179:813–825. doi: 10.1007/s00213-004-2104-3. [DOI] [PubMed] [Google Scholar]
- Jacob P, III, Wilson M, Benowitz NL. Improved gas chromatographic method for the determination of nicotine and cotinine in biological fluids. J Chromatogr B. 1981;222:61–70. doi: 10.1016/s0378-4347(00)81033-6. [DOI] [PubMed] [Google Scholar]
- James JE. Chronic effects of habitual caffeine consumption on laboratory and ambulatory blood pressure levels. J Cardiovasc Risk. 1994;1:159–164. doi: 10.1177/174182679400100210. [DOI] [PubMed] [Google Scholar]
- James JE. Effects of habitual caffeine consumption on ambulatory blood pressure. Am J Cardiol. 1996;78:129. doi: 10.1016/s0002-9149(96)90323-9. [DOI] [PubMed] [Google Scholar]
- James JE. Acute and chronic effects of caffeine on performance, mood, headache, and sleep. Neuropsychobiology. 1998;38:32–41. doi: 10.1159/000026514. [DOI] [PubMed] [Google Scholar]
- James JE, Gregg ME, Kane M, Harte F. Dietary caffeine, performance and mood: enhancing and restorative effects after controlling for withdrawal relief. Neuropsychobiology. 2005;52:1–10. doi: 10.1159/000086172. [DOI] [PubMed] [Google Scholar]
- James JE, Keane MA. Caffeine, sleep and wakefulness: implications of new understanding about withdrawal reversal. Hum Psychopharmacol Clin Exp. 2007;22:1–10. doi: 10.1002/hup.881. [DOI] [PubMed] [Google Scholar]
- James JE, Rogers PJ. Effects of caffeine on performance and mood: withdrawal reversal is the most plausible explanation. Psychopharmacol. 2005;182:1–8. doi: 10.1007/s00213-005-0084-6. [DOI] [PubMed] [Google Scholar]
- Jones HE, Herning RI, Cadet JL, Griffiths RR. Caffeine withdrawal increases cerebral blood flow velocity and alters quantitative electroencephalography (EEG) activity. Psychopharmacol. 2000;147:371–377. doi: 10.1007/s002130050005. [DOI] [PubMed] [Google Scholar]
- Juliano LM, Griffiths RR. A critical review of caffeine withdrawal: empirical validation of symptoms and signs, incidence, severity and associated features. Psychopharmacol. 2004;176:1–29. doi: 10.1007/s00213-004-2000-x. [DOI] [PubMed] [Google Scholar]
- Keane MA, James JE, Hogan MJ. Effects of dietary caffeine on topographic EEG after controlling for withdrawal and withdrawal reversal. Neuropsychobiology. 2007;56:197–207. doi: 10.1159/000120625. [DOI] [PubMed] [Google Scholar]
- Lader M, Cardwell C, Shine P, Scott N. Caffeine withdrawal symptoms and rate of metabolism. J Psychopharmacol. 1996;10:110–118. doi: 10.1177/026988119601000205. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Wilson MF, Vincent AS, Sung BH, McKey BS, Whitsett TL. Blood pressure response to caffeine shows incomplete tolerance after short-term regular consumption. Hypertension. 2004;43:769–765. doi: 10.1161/01.HYP.0000120965.63962.93. [DOI] [PubMed] [Google Scholar]
- Mathew RJ, Barr DL, Weinman ML. Caffeine and cerebral blood flow. Brit J Psychiat. 1983;143:604–608. doi: 10.1192/bjp.143.6.604. [DOI] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH. Caffeine consumption, withdrawal and cerebral blood flow. Headache. 1985;25:305–309. doi: 10.1111/j.1526-4610.1985.hed2506305.x. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. EITS manual for the profile of mood states. Educational and Industrial Service; San Diego: 1971. [Google Scholar]
- Muhonen MG, Loftus CM, Heistad DD. Effects of adenosine and 2-chloroadenosine on cerebral collateral vessels. J Cerebral Blood Flow and Metabolism. 1995;15:1075–1081. doi: 10.1038/jcbfm.1995.134. [DOI] [PubMed] [Google Scholar]
- Reeves RR, Struve FA, Patrick G, Bullen JA. Topographic quantitative EEG measures of alpha and theta power changes during caffeine withdrawal: preliminary findings from normal subjects. Clin Electroencephal. 1995;26:154–162. doi: 10.1177/155005949502600306. [DOI] [PubMed] [Google Scholar]
- Robertson D, Wade D, Workman R, Woosley RL, Oates JA. Tolerance to the humoral and hemodynamic effects of caffeine in man. J Clin Invest. 1981;67:1111–1117. doi: 10.1172/JCI110124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers PJ, Heatherley SV, Hayward RC, Seers HE, Hill J, Kane M. Effects of caffeine and caffeine withdrawal on mood and cognitive performance degraded by sleep restriction. Psychopharmacol. 2005;179:742–752. doi: 10.1007/s00213-004-2097-y. [DOI] [PubMed] [Google Scholar]
- Rogers PJ, Dernoncourt C. Regular caffeine consumption: a balance of adverse and beneficial effects for mood and psychomotor performance. Pharmacol Biochem Behav. 1998;59:1039–45. doi: 10.1016/s0091-3057(97)00515-7. [DOI] [PubMed] [Google Scholar]
- Saletu B, Anderer P, Kinsperger K, Grünberger J. Topographic brain mapping of EEG in neuropsychopharmacology—Part II. Clinical applications (pharmaco EEG imaging) Methods Find Exp Clin Pharmacol. 1987;9:385–408. [PubMed] [Google Scholar]