Abstract
Background
PINCH proteins are five LIM domain only adaptor proteins that function as key components of the integrin signaling pathway, and play crucial roles in multiple cellular processes. Two PINCH proteins, PINCH1 and PINCH2, have been described in mammals and share high homology. Both PINCH1 and PINCH2 are ubiquitously expressed in most tissues and organs, including myocardium. Cardiac specific PINCH1 knockout or global PINCH2 knockout mice exhibit no basal cardiac phenotype, which may reflect a redundant role for these two PINCH proteins in myocardium. A potential role for PINCH proteins in myocardium remains unknown.
Methods and Results
To define the role of PINCH in myocardium, we generated mice which were doubly homozygous null for PINCH1 and PINCH2 in myocardium. Resulting mutants were viable at birth, but developed dilated cardiomyopathy and died of heart failure within four weeks. Mutant hearts exhibited disruptions of intercalated discs and costameres, accompanied by fibrosis. Furthermore, multiple cell adhesion proteins exhibited reduced expression and were mislocalized. Mutant cardiomyocytes were significantly smaller and irregular in size. In addition, we observed that the absence of either PINCH1 or PINCH2 in myocardium leads to exacerbated cardiac injury and deterioration in cardiac function post myocardial infarction.
Conclusions
These results demonstrate essential roles for PINCHs in myocardial growth, maturation, remodeling and function, and highlight the importance of studying the role of PINCHs in human cardiac injury and cardiomyopathy.
Keywords: PINCH- ILK-integrin, cell-extracellular matrix interaction, intercalated discs, cardiomyopathy
Clinical impact
Cardiomyocytes communicate with their surrounding microenvironment through costameres. In both humans and animal models, dysfunction of costamere adhesion leads to cardiomyopathy and heart failure. Adhesion between cardiomaycues and the extracellular matrix at the costamere is mediated by integrinβ1 receptor and its associated complex. Engagement of integrin with extracellular matrix leads to recruitment and formation of a cytoplasmic focal adhesion complex which links integrins to the actin cytoskeleton and other intracellular pathways. This complex is composed of integrin-linked kinase, Parvin and PINCH proteins. Two PINCH proteins, PINCH1 and PINCH2, have been described in mammals and share high homology. Both PINCH1 and PINCH2 are expressed in most tissues and organs, including myocardium. However, whether PINCH is required in normal myocardium, or for post-injury healing of myocardium, has not been addressed. To explore the functional role of PINCH in cardiomyocytes, we generated several mouse lines in which PINCH1 and PINCH2 are singly or doubly ablated in myocytes. Analysis of these mouse models has demonstrated essential roles for PINCHs in myocardial growth and maturation, as well as remodeling in response to injury, and highlights the importance of studying the role of PINCHs in human cardiac remodeling and cardiomyopathy.
Introduction
Cells communicate with their microenvironment and neighbors by several specialized cell membrane associated structures. In myocardium, these include costameres and intercalated discs, which provide mechanical and electrical coupling between myocytes and enable myocardium to function as a syncytium. Dysfunction of cell adhesions in both animal models and human diseases is implicated in pathogenesis of cardiomyopathy, heart failure, and injury repair1–5.
Myocardial cell-matrix adhesion at the costamere is mediated by integrinβ1 receptor and its associated complex, and plays vital roles in morphogenesis and tissue integrity by regulating multiple cellular processes such as cell adhesion, proliferation, differentiation and survival6–10. Engagement of integrin with extracellular matrix (ECM) leads to recruitment and formation of a cytoplasmic focal adhesion complex composed of integrin-linked kinase (ILK), Parvin and PINCH (IPP), which links integrins to the actin cytoskeleton and other intracellular pathways6.
In contrast to Drosophila and C. elegans which have only one PINCH protein, two PINCH proteins have been described in mammals, including PINCH1 and PINCH2, which share high sequence and structural similarity11. PINCH1 is expressed in the blastocyst, whereas PINCH2 expression starts from embryonic (E) 14.5 onwards11,12. From E14.5 to adulthood, both of them are expressed ubiquitously in all tissues, although they may diverge within different cell types of some organs11. Furthermore, PINCH1 and PINCH2 may play redundant roles in most organs and compensate for each other under certain circumstances12–15.
PINCH proteins contain five LIM domains that are involved in mediating protein-protein interactions. The LIM1 domain of PINCH binds the N-terminal ankyrin-repeat domain of ILK and the interaction of PINCH and ILK is obligatory for targeting the IPP complex to integrin membrane sites and for the stability of each component of the complex6. Deletion of one of the components leads to the degradation of the others and impaired cell mobility and survival12–14,16.
Myocyte cell-cell interactions are mediated by intercalated discs17, which are composed of three distinct junctional components: adherens junctions, desmosomes and gap junctions. Adherens junctions are mediated by N-cadherin and its associated Catenin family proteins (α, β, γ-catenins) and serve as anchors for myofibrils at cell-cell contacts2,18. Gap junctions are formed by a family of connexins. Connexin43 is critical for electrical coupling of myocardium10,19. In addition, cross talk between cell-ECM connections and cell-cell junctions have been reported, and integrin-mediated signaling pathways have been found to regulate the formation of cell-cell junctions, and vice versa20.
Postnatal myocardial maturation and remodeling is a complex developmental process during which cardiomyocytes undergo physiological hypertrophic growth, realign cytoskeletal components and acquire a mature, adult cytoarchitecture to meet a dramatically increased hemodynamic load. One feature of postnatal myocardial remodeling and maturation is the redistribution and segregation of distinct cell-matrix and cell-cell adhesions. During embryonic development, cardiomyocytes interact with each other extensively via cadherin-mediated adherens junctions, which appear to play dominant roles in mediating myocyte adhesion and function 21. During perinatal development, the myocytes continue to elongate, and interact with each other only at the bipolar ends. Intercalated discs are disassembled from lateral cell membranes and reassembled at the bipolar ends of the cells, whereas costameres remain in the lateral cell membranes. This process occurs postnatally22,23. However, molecular mechanisms regulating the segregation and redistribution of cell adhesions during postnatal myocardium remodeling and maturation remain largely unknown.
Integrin signaling pathways have also been shown to be important for maintenance of cardiac structure and function1,4,5. Genetic studies in Drosophila and C. elegans, as well as in the mouse epiblast have confirmed that the PINCH-ILK complex plays an essential role in the integrin–actin network at cell-matrix adhesions1,4,5,24–28. Deletion of integrinβ1 in mouse cardiomyocytes leads to dilated cardiomyopathy, and cardiac dysfunction1,5. Similarly, ablation of ILK in myocardium results in disruption of the focal adhesion complex and disaggregation of cardiomyocytes, leading to cardiomyopathy and heart failure4. In addition, it has been shown that PINCH1 forms a functional complex with Thymosinβ4 and ILK which plays a role in promoting cardiomyocyte migration and survival after cardiac injury3. Whether PINCH is required in normal myocardium, or for post-injury healing of myocardium, has not been addressed.
To explore the functional role of PINCH in cardiomyocytes, we generated several mouse lines in which PINCH1 and PINCH2 are singly or doubly ablated in myocytes. Analysis of these mouse models has revealed that PINCH1 and PINCH2 play essential roles in myocardial remodeling and maturation and in maintaining myocardial integrity.
Methods
Histological Analysis
Perfused hearts fixed with 4% paraformaldehyde were processed for paraffin-embedded sections, subsequently analyzed by hematoxylin and eosin (H&E) staining12,29 and by Masson's trichrome stain according to the manufactory’s protocol (Sigma-Aldrich).
Mouse Neonatal Cardiomyocyte Isolation
Neonatal cardiomyocytes were prepared using a percoll gradient as described30.
In Situ Hybridization and Immunostaining
Whole-mount in situ hybridization and Immunostaining was performed as previously described.29. The following primary antibodies were used: α-actinin (Sigma-Aldrich), Alexa Fluor® 568 phalloidin (F-actin) (Molecular Probes),? α-E-catenin (Sigma-Aldrich), Akt (Cell Signaling), β-catenin (BIOMOL), Cadherin (Sigma-Aldrich), Connexin-43 (Zymed Lab), GAPDH (Santa Cruz Inc), integrin β1(Sigma-Aldrich), ILK (Sigma-Aldrich), Parvin, Paxillin (Abcam), phospho-Akt (serine-473) (Cell Signaling), PINCH112, Vinculin (Abcam), Alexa Fluor® 568 Wheat germ agglutinin (WGA) (Invitrogen), ZO-1 (Zymed lab). The sections were incubated with fluorescently labeled secondary antibodies except phalloidin and WGA staining and analyzed under the confocal microscope.
Northern Blotting and Western Blotting
Total RNA was isolated using TRIZOL reagent (GIBCO, BRL). Gel separation, blotting and hybridization were performed. The protein assay was performed as described31. Expression levels of proteins and RNA were quantified by Photoshop.
Myocardial Infarction (MI)
Mice are anesthetized with a mixture of ketamine and xylazine. Mice were placed in the supine position, and a midline cervical incision is made to expose the trachea and carotid arteries by microsurgical techniques. After endotracheal intubation, the cannula is connected to a volume cycled rodent ventilator. The chest cavity is entered in the second intercostal space at the left upper sternal border through a small incision. The left anterior descending branch was ligated permanently in the mouse heart. Cardiac function and infarct size were measured by echocardiography.
Electron Microscopy
Cardiac ventricles were processed for electron microscopy analysis as described30.
Statistical Analysis
Data are presented as mean ± SEM and student t test was used for 2-group comparisons. Differences were considered statistically significant at a value of p<0.05.
Results
Expression of PINCH1 and PINCH1 in mouse myocardium
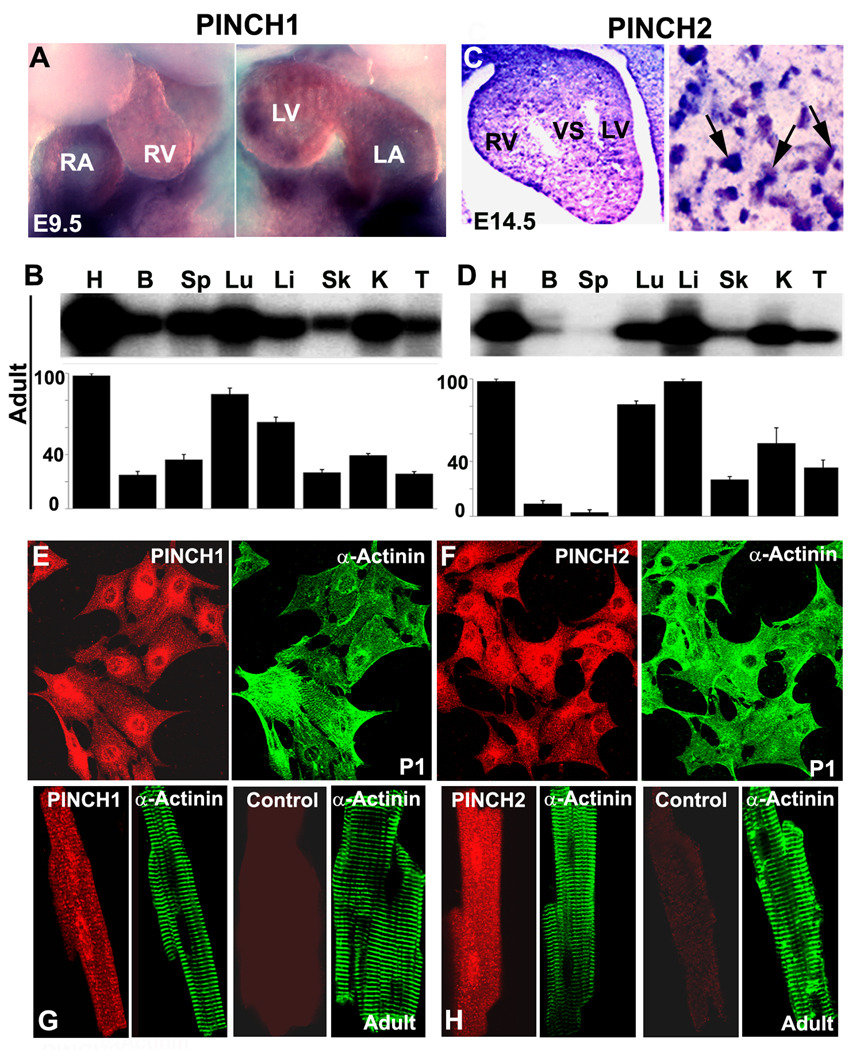
To determine the role of the two PINCHs in the heart, we first analyzed in detail their developmental expression pattern in heart. In situ hybridization revealed that PINCH1 is expressed in heart from E9.5 onward, whereas PINCH2 is not detected until E14.5 onward (Fig. 1A, C, arrows). Northern blotting revealed that, among tissues analyzed, heart showed the highest level of expression of both PINCH1 and 2 (Fig. 1B and D). To further determine whether PINCHs are expressed in cardiomyocytes, we performed immunostaining on purified cardiomyocytes from neonatal (Fig. 1E, F) and adult hearts (Fig. 1G, H) and found that both PINCHs are abundantly expressed in myocytes.
Fig. 1. The expression pattern of PINCH1 and PINCH2.
A: In Situ Hybridization detects PINCH1 in the Embryonic day (E) 9.5. B: Northern blotting (quantification shown in lower panel) reveals highest expression of PINCH1 in the adult mouse heart. C: In Situ Hybridization detects PINCH2 at E14.5. D: Northern blotting (quantification shown in lower panel) reveals highest expression of PINCH2 in the adult mouse heart. E, G: Immunstaining shows expression of PINCH1 in cardiomyocytes isolated from the new-born (E) and adult mice (G) and control section without PINCH1 antibody added shows negative staining. F, H: Immunstaining shows expression of PINCH2 in cardiomyocytes isolated from the new born (F) and adult mice (H) and control section without PINCH2 antibody added shows negative staining. α-actinin marks the myocytes. H: heart; B: brain; Sp: spleen; Lu: lung; Li: liver; Sk: skeletal muscle; K: kidney; T: thymus. RA: right atria; RV: right ventricle; LA: left atria; LV: left ventricle; VS: ventricular septum.
Ablation of PINCH1 and PINCH2 in myocardium leads to cardiomyopathy, heart failure and early postnatal lethality
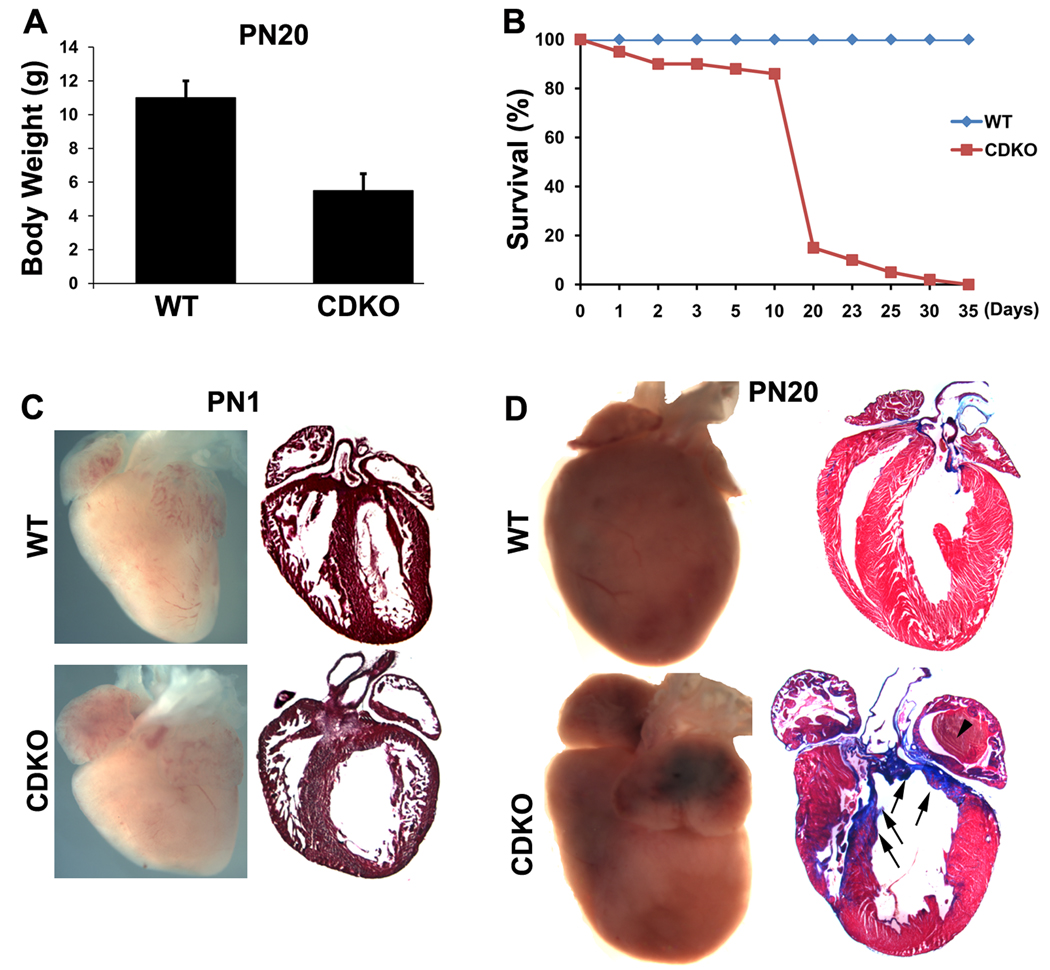
To test whether PINCH1 and PINCH2 may play a redundant role in myocardium, we generated a mouse line in which both PINCH1 and PINCH2 were ablated in cardiomyocytes. To minimize the possibility that incomplete Cre mediated excision would occur in the case of four floxed alleles of PINCH1 and PINCH2, we generated a compound PINCH1 and PINCH2 double knockout mouse line with cardiac specific PINCH1 knockout alleles and PINCH2 null alleles (CDKO: cTNT-Cre;PINCH1f/f; PINCH2−/−) (Supplementary Fig. 1B). The effective excision of PINCH1 from myocardium was confirmed by Western blot on purified cardiomyocytes (Supplementary Fig. 1C). The resulting CDKO mice were born at expected Mendelian ratios and appeared morphologically normal at birth. However, with postnatal development, CDKO pups gradually developed severe growth retardation (Fig. 2A) and exhibited progressive signs of heart failure and eventually died within four weeks (Fig. 2B). Examination of multiple organs and tissues prior to death of mutants revealed extensive edema (pulmonary edema/increased lung weight, enlarged and congested liver) and ascites, suggesting CDKO mutants died of heart failure.
Fig. 2. General analyses of PINCH CDKO.
A: Growth retardation of the double knockouts. B: The survival curve shows that the double knockouts died within one month. C: Morphological and histological analyses show a dilated heart of the new born double knockouts compared with the control littermate. D: Morphological and histological analyses show cardiomyopathy with extensive fibrosis in the double knockouts at P20 compared with the wild type littermate (WT). Arrows indicate fibrosis; arrowheads demonstrate thrombosis.
Cardiac morphology was examined in detail. At postnatal day 1, control hearts exhibited an elongated shape along the apical to basal axis and trabeculae were rearranged into an apical to basal orientation and compressed within the ventricular wall to result in a significantly increased ventricular compact myocardium (Fig. 2C). In contrast, CDKO hearts were rounder in shape, with a dilated left ventricle and largely disorganized trabeculae which failed to fuse and incorporate into compact myocardium, resulting in a significantly thinner compact myocardium (Fig. 2C). At day 20, control myocardium exhibited a matured, striated appearance with well-orientated myocardial fibers. Hearts of CDKO mutants which survived to this stage were abnormally shaped, with dilated atria and left ventricle, and a bulge near the base of the right ventricle (Fig. 2D). Histological examination showed dilated left ventricle, irregular thickness of the ventricular wall with abnormally thickened right ventricular free wall, but significantly thinner left ventricular free wall and septum when compared to control hearts (Fig. 2D). Atria of mutant hearts were dilated with abnormalities in pectinate muscles in atrial appendages. A large intracardiac thrombus was frequently found in left atria (Fig. 2D). Trichrome-staining of sections from CDKO heart at P20 revealed fibrosis in CDKO hearts, in particular in the ventricular septum and within thinned regions of ventricular myocardium (Fig. 2D).
Ultrastructural abnormalities in PINCH CDKO hearts
To investigate cell-ECM and cell-cell adhesions at the ultrastructural level, we examined ventricular myocardium of the CDKO and wild type mouse hearts by electron microscopy. At P1, intercalated disc structures remained largely similar between control and mutant samples. However in a few focal regions, gaps of intercalated discs were widened, with disarrayed sarcomeric structure and distorted Z-lines (Fig. 3A and B), which became more apparent at P10 (Fig. 3C–F, arrows). At P10, intercalated discs of control samples at were clearly observed connecting adjacent myocytes end to end (Fig. 3C, arrows). However, in CDKOs, intercalated disc structures were variably affected (Fig. 3D). In mutants, membranes of the intercalated disc were highly convoluted, and gaps of the intercalated discs were significantly widened and in some cases, filled with extracellular material (Fig. 3D, arrows). The numbers of gap junctions in CDKO mutant hearts were significantly reduced throughout the sections (Fig. 3C, D, arrowheads). Laterally, the distance between sarcolemma and sarcomeres in the CDKO myocytes was significantly increased (Fig. 3F, arrowheads), intercellular space was significantly enlarged and filled with vesicles of variable size (Fig. 3F, double-head arrows) (Fig. 3E).
Fig. 3. Ultrastructural analysis of the CDKO.
Representative electron micrographs from the ventricular myocardium of WT and CDKO at day 1 and day 10. A and B: At day 1, in a few focal regions, the gaps of intercalated discs (arrows) are observed to be widened with disarrayed sarcomeric structure and distorted Z-line. C–F: At day 10, wild type mice show preserved intercalated discs structures (arrows), and well-aligned arrays and insertions of myofibrils at Z lines (C and E); whereas CDKO mice show impaired intercalated discs (D, arrows), sarcomere and sarcolemmal connection and increased intercellular spaces (F arrowheads and double-head arrows). Bar: 2µm
Disrupted Integrin-ILK signaling in PINCH CDKO hearts
Deletion of one of the component proteins of the IPP leads to degradation of the others and impaired cell mobility and survival13,16,32. Therefore, we examined expression of a number of key proteins in the integrin pathway in PINCH CDKO mutant myocardium. Western blotting revealed a dramatic reduction in expression of ILK and Parvin in CDKO cardiomyocytes at both postnatal day 1 and 10, compared with that of controls (Fig. 4A and B, quantification shown in lower panels). Integrinβ1 was expressed initially at normal levels at P1, but significantly reduced at P10 (Fig. 4A and B). Immunostaining also confirmed that ILK in CDKO myocardium was significantly reduced compared with that of controls (Fig. 4C and D). In addition, increased and disorganized fibronectin expression was observed in mutant myocardium compared with that of controls (Fig. 4E and F). However, expression of another integrin-binding protein, Talin, was not changed (Fig. 4B).
Fig. 4. Disrupted Integrin-ILK signaling in PINCH CDKO hearts.
A and B: Deletion of PINCH1 and PINCH2 suppresses ILK and parvin expression in new-born cardiomyocytes (A) and in myocardium at day 10 (B), and reduction of integrinβ1 at day 10. Expression of Talin is unchanged (B), Quantifications of protein expression level are shown in lower panels. C and D: ILK expression is significantly reduced in the large area of CDKO myocardium (D) compared with WT (C). F-actin mainly stains the myocytes. E and F: The diffused and disorganized fibronectin expression with disarrayed F-actin is observed in the area close to aorta and pulmonary artery of myocardium in CDKO (F) compared with that of WT (E). G: Inactivation of PINCH1 and PINCH2 inhibits phosphorylation of Akt (Ser473). Quantifications are shown in right panels. H and I: Caspase 3 activation is significantly increase in myocardium of CDKO (I) compared with WT (H). Casp3: activated Caspase 3. In each case of the Western Blotting, each expression is normalized to GAPDH
Phosphorylation of Akt/PKB represents one of the key downstream events of integrin signaling, and is required for cell growth and survival. Deletion of PINCH1 and PINCH2 resulted in significant reduction in phosphorylation of PKB/Akt at Ser473 at both P1 and P10 (Fig. 4G, quantification shown in right panels), with a concomitant increase in cell death as revealed by increased caspase-3 activation (Fig. 4H and I).
Disruption of cell-cell adhesions in PINCH CDKO hearts
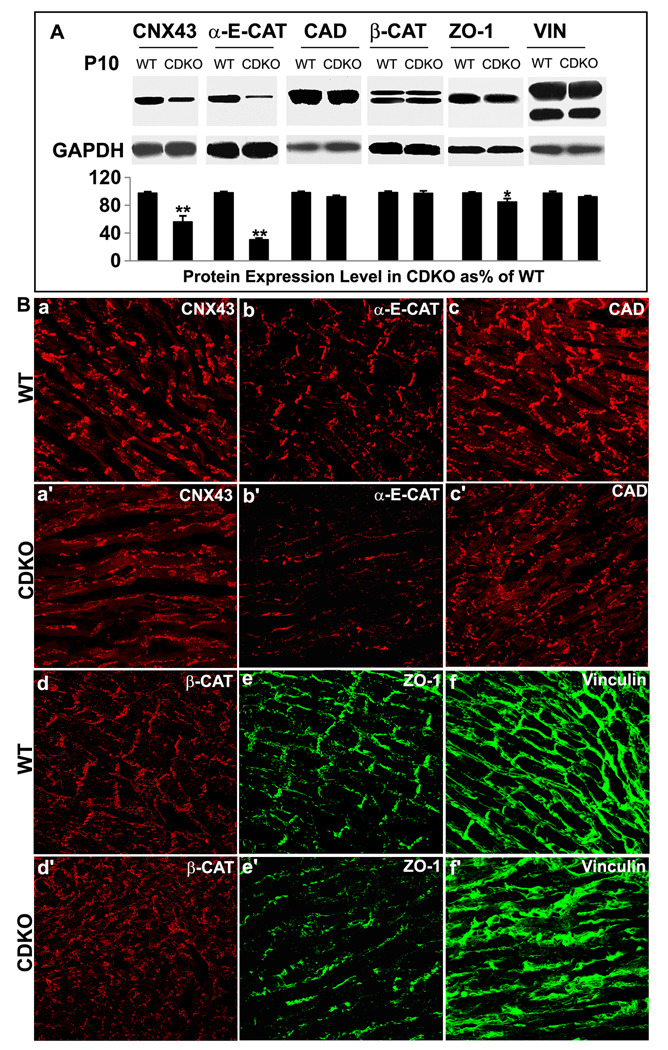
Ablation of PINCH1 and PINCH2 in myocardium resulted in disruption of intercalated disc structures. Therefore, we examined the abundance and localization of proteins within these complexes, including Cadherins, Catenins, ZO-1 and Vinculin in adherens junctions and Connexin43 in gap junctions. Western blotting of P10 hearts revealed that levels of Connexin43, α-E-catenin and ZO-1 were reduced in mutant hearts (Fig. 5A). However, levels of Cadherin,? β-catenin and Vinculin were not significantly reduced (Fig. 5A).
Fig. 5. Alteration of intercalated disc proteins in myocardium of CDKO at day 10.
A: Western blot: The expression of Connexin43 (CNX43), α-E-cadherin (α-E-CAT) and ZO-1 are reduced in myocardium of CDKO at day 10 compared with WT (**p<0.01; *p<0.05), but expression levels of Cadherin (CAD), β-catenin (β-CAT) and vinculin (VIN) are not significantly changed Each expression is normalized to GAPDH expression and quantified in lower panel. B: Immunostaining: CNX43 (a, a’) and α-E-Catenin (b, b’) are reduced and mislocalized to the lateral side of CDKO cardiomyocytes. Mislocalized Cadherin (c, c’) and β-Catenin (d, d’) expression are seen in CDKO myocardium. The ZO-1 expression is reduced and absent in the intercalated discs of CDKO (e’) compared with WT (e). The Vinculin expression is absent in the intercalated discs of CDKO (f’) compared with WT (f).
To investigate the cellular distribution of intercalated disc proteins, immunostaining was performed on cardiac samples from CDKO and control mice at P10, when most CDKO pups are still viable. This stage was chosen because fully mature and compact intercalated discs are clearly observed around P10, although gap junctions are not yet fully matured. As shown in Fig. 5Ba, gap junction protein Connexin43 in control myocardium displayed a punctate pattern in both termini and lateral sides of myocytes. In contrast, Connexin43 in PINCH CDKO myocardium was found in a dispersed pattern across the lateral cell membranes, but not in intercalated discs (Fig. 5B-a’). Similarly, adherens junctional proteins α-E-catenin, Cadherin and β-cateinin were mislocalized to the lateral cell membranes in mutant myocytes, rather than within intercalated discs as observed in control myocytes (Fig. 5B-b to d’). ZO-1 was localized to intercalated discs and along part of the lateral cell wall in control myocardium (Fig. 5B-e), and its expression in intercalated discs was not observed in CDKO myocardium (Fig. 5B-e’). Vinculin was expressed along the entire sarcolemma and within intercalated discs in control myocardium (Fig. 5B-f). In contrast, in CDKO mutant myocardium Vinculin was found only in the lateral cell membrane, with no expression observed within intercalated discs (Fig. 5B-f’). Consistent with western blotting, levels of Connexin43, α-E-catenin and ZO-1 were significantly reduced in CDKO myocardium (Fig. 5B-a’, b’ and e’).
The role of PINCH1 and PINCH2 in focal adhesion formation, spreading and growth of cardiomyocytes
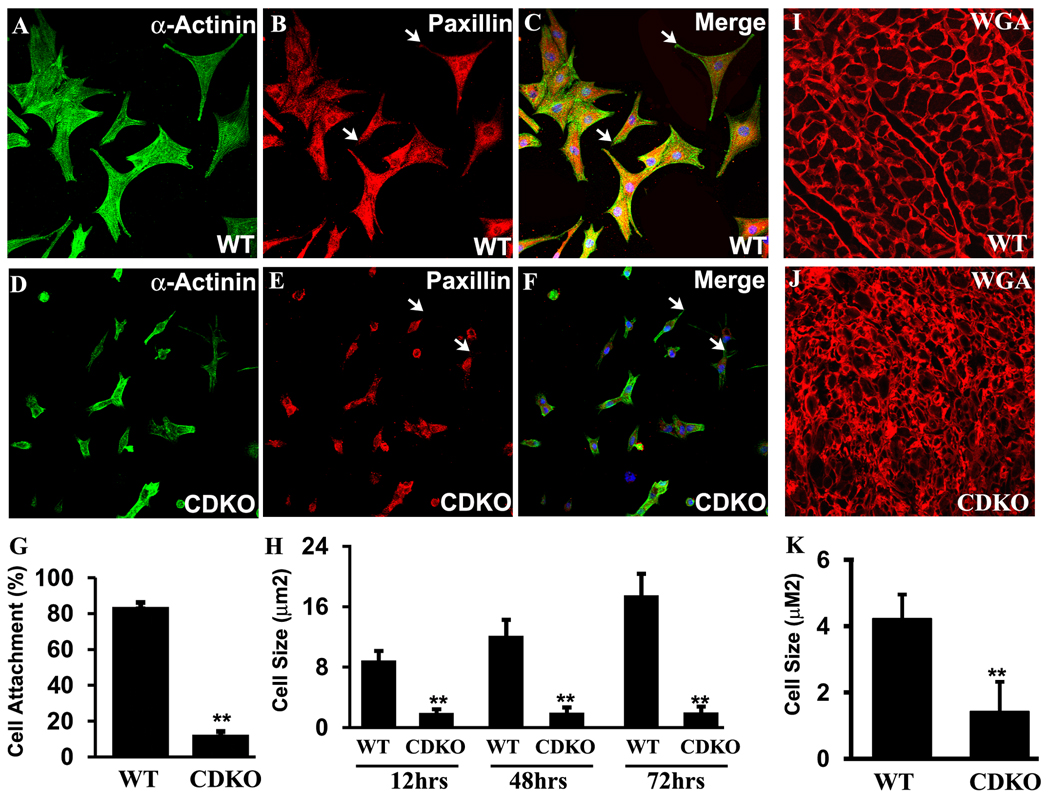
To further investigate whether PINCH1 and PINCH2 are essential for cardiomyocyte adhesion and cytoskeletal organization, we isolated neonatal cardiomyocytes and tested their ability to attach and spread on different substrates in culture, including fibronectin, laminin, and collagen. Results with all three substrates were comparable. A majority (90%) of control cardiomyocytes had attached and spread out 12–24 hours after plating. However, markedly fewer (5%) of PINCH CDKO cardiomyocytes were attached 12–24 hours after plating. Neonatal cardiomycytes were stained with antibodies to α-actinin that marks the cytoskeleton, and to Paxillin which is associated with focal adhesion complexes and the cytoskeleton33. As shown in Fig. 6A–C, control myocytes displayed a striated sarcomeric structures and punctate Paxillin staining, indicative of focal adhesion structures. However, mutant cardiomyocytes did not exhibit striated sarcomeric structures, and displayed diffuse or disorganized staining for cytoskeletal structures that were localized mainly to the cell periphery. Paxillin displayed a similar pattern as α-actinin in mutant myocytes, suggesting it remained associated with the cytoskeleton. A majority of mutant myocytes did not exhibit Paxillin staining in focal adhesions (Fig. 6D–F, arrows). The size of attached PINCH CDKO cardiomyocytes was strikingly smaller than that of control myocytes (Fig. 6A–H). Over a 72 hour period in culture, control cardiomyoctyes continued to spread out and grow, leading to a two-fold increase in cell size, whereas the size of PINCH CDKO myocytes remained the same. Consequently, the difference in cell size, which was initially about 4.5 fold, had increased to 9 fold after 72 hours in culture (Fig. 6H). Wheat germ agglutin (WGA) staining of cell membranes on cardiac sections from control and PINCH CDKO also demonstrated significantly reduced cell size and increased size variability of mutant cardiomyocytes compared with controls, which was visible at P1 (not shown), but more dramatic at P10 (Fig. 6I–K).
Fig. 6. Impaired spreading, focal adhesion and attachment in the cardiomyocytes of CDKO.
A–F: Immunostaining on isolated cardiomyocytes from WT (A–C) and CDKO (D–F) newborn hearts shows that impaired spreading and focal adhesion (indication by arrows) in the CDKO myocytes. G: Attachment of the isolated myocytes from the newborn heart of CDKO is significantly (p<0.01) reduced compared with WT. H: The size of the isolated myocytes from the newborn heart of CDKO is significantly (p<0.01) smaller compared with WT. I and J: WGA staining on myocardium of WT (I) and CDKO (J) at day 10 shows that disarrayed myocytes with reduced various sizes is observed in CDKO. K: Quantitative analysis on the WGA staining myocardium shows that the size of CDKO myocytes is significantly smaller (p<0.01).
Loss of PINCH1 or PINCH2 adversely affects infarct size and cardiac function post-infarct
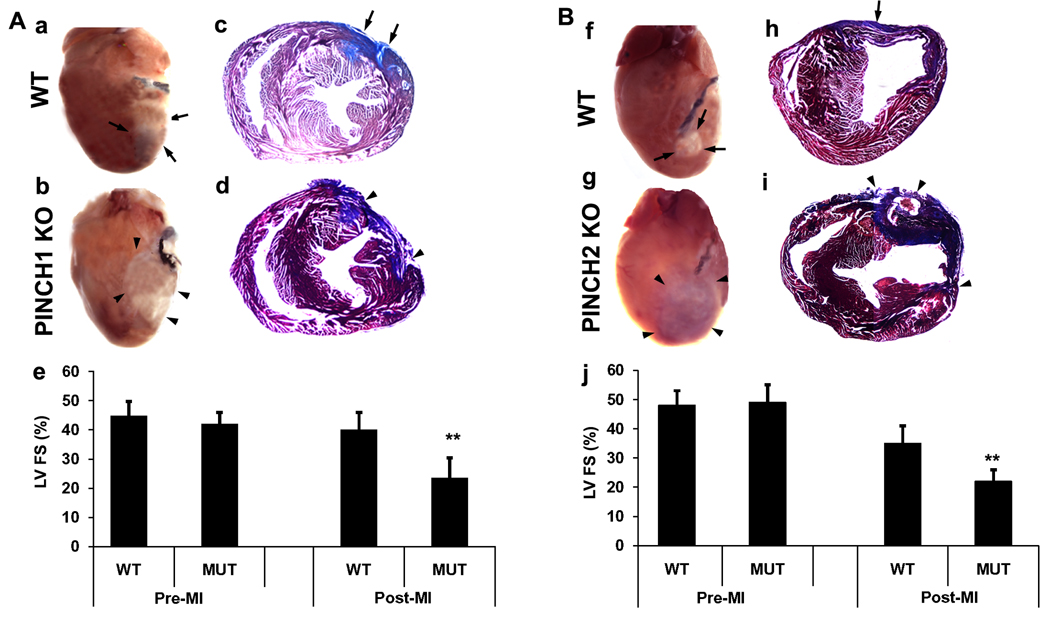
Previous studies have demonstrated that the protective function of thymosinβ4 in myocardium is dependent on activation of ILK and subsequent phosphorylation of Akt3. However, the potential involvement of PINCH in this process has not been addressed. To investigate the role of PINCH during infarct, PINCH1 cardiac specific knockout mutants and control littermates and PINCH2 null mutants and control littermates were subjected to myocardial infarction by ligation of the left anterior descending coronary artery at 20 weeks of age. Mice within each group that survived for 12 days were assessed for myocardial function and post-mortem histological analysis. No significant functional differences were observed between PINCH single mutants versus controls at 24 hours post-infarct. However, by twelve days post-infarct, echocardiographic analysis demonstrated a significant reduction in fractional shortening in PINCH1 or PINCH2 single mutants compared to controls (n=6, p<0.001) (Fig. 7). Consistent with deterioration in left ventricular function in single PINCH mutants, trichrome staining revealed a significantly larger infarct area and scar tissue with visible hemorrhage in PINCH1 or PINCH2 single mutants compared to controls (Fig. 7).
Fig. 7. Either cardiac specific PINCH1 mutants or global PINCH2 knockouts show susceptibility to injury after myocardial infarction (MI).
A: MI in cardiac specific PINCH1 mutants (PINCH1 KO) (n=6) and controls (WT) (n=6) at 20 weeks. a and b: Macroscopic appearance of representative hearts of WT (a) and MUT (b) at 12 days post-MI. c and d: Representative trichrome stain of transverse heart sections at comparable levels 12 days after MI in WT(c) and PINCH1 KO (d). e: Echocardiogram of left ventricles 12 days after MI in WT and MUT. Left ventricular fractional shortening (FS) of PINCH1 KO hearts is significantly (p<0.01) decreased compared with WT. B: MI global PINCH2 knockouts (PINCH2 KO) (n=6) and WT (n=6) at 20 weeks. f and g: Macroscopic appearance of representative mouse hearts of WT (f) and PINCH2 KO (g). h and i: Representative trichrome stain of transverse heart sections in comparable levels at 12 days after MI in WT (h) and PINCH2 KO (i). j: Echocardiogram of left ventricles 12 day after MI in WT and PINCH1 KO. Left ventricular fractional shortening (FS) of PINCH2 KO hearts is significantly decreased compared with WT (p<0.01). Arrows indicate the damaged area of the control heart; arrowheads indicate the damaged area of the PINCH1 or PINCH2 mutant heart.
Discussion
Consistent with previous reports12, we found that PINCH1 is expressed early during development, whereas PINCH2 expression in myocardium was observed from E14.5 onwards. In mice, either cardiac specific ablation of PINCH1 or germline deletion of PINCH2 did not resulted in any basal cardiac phenotype12,14 (present study), suggesting that PINCH1 and PINCH2 may play a redundant role in mouse heart. In this study, we generated mice which were doubly homozygous null for PINCH1 and PINCH2 in myocardium. Resultant PINCH CDKO mice developed cardiomyopathy and die of cardiomyopathy and heart failure within 4 weeks, suggesting that PINCH1 and PINCH2 play largely redundant yet essential roles in myocardium.
Postnatal myocardial maturation and remodeling is a complex developmental process, during which cardiomyocytes continue to grow, realign cytoskeletal components and acquire a mature, adult cytoarchitecture to meet the demands of the dramatically increased hemodynamic load. These processes lead to well aligned and striated myocardium and significantly increased thickness of the myocardial well. Results of this study suggest that both PINCHs are essential in postnatal myocardial remodeling and maturation and these processes appear to be blunted in their absence. Hearts of CDKO mutants were shaped abnormally, with dilated atria and left ventricles. Ventricular wall thickness was highly irregular with abnormal trabeculation. The upper free wall of the left ventricle, and the ventricular septum were significantly thinner and fibrotic. The upper free wall of the right ventricle was dramatically thickened, leading to narrowing of the right ventricular cavity. Approximately 10% of PINCH CDKO mutants died within 3–5 days after birth. However, those that survived this period succumbed to death within four weeks with classical signs of heart failure, including cardiac dilation, ascites and pulmonary and liver congestion.
Observed changes in myocardial architecture may result from abnormal cardiomyocyte adhesion/attachment, cell growth and cell death. In support of an essential role for PINCH in maintaining myocardial cell-matrix and cell-cell interactions in vivo, electron microscopy analysis revealed disruption of costameric structures and intercalated discs in PINCH CDKO myocardium. Consistent with this, PINCH CDKO myocytes in culture failed to attach and spread and exhibited disrupted focal adhesions. Mutant myocytes were markedly smaller and variable in size. Multiple signaling pathways have been implicated in cardiac growth and survival, many of which converge on activation of the PI3K-Akt signaling pathway. Indeed, we observed significantly decreased phosphorylation and activation of Akt and markedly increased cell death in hearts of PINCH CDKO mice. These results revealed a critical role of PINCH in cell attachment, survival and hypertrophic growth of cardiomyocytes, in part at least via ILK activation of the Akt signaling pathway.
Myocardial cell-matrix adhesion is mediated by the integrinβ1 receptor and its associated IPP complex consisting of ILK, PINCH and Parvins. We observed that cardiac deletion of PINCH1 and PINCH2 leads to markedly reduced levels of ILK and Parvin, as seen in PINCH1 null embryos and ES cells14,15,34. Reduced ILK might account for, at least in part, reduced Akt phosphorylation and increased cell death12,14. Expression of integrinβ1 was relatively spared at least during early postnatal stages. Expression levels of Talin and Vinculin, components of another complex linking integrin to actin filament20,35–37 were unchanged in PINCH CDKO hearts.
A defining feature of postnatal myocardial remodeling and maturation is the redistribution and segregation of distinct cell-cell and cell-matrix complexes. Profound changes in cell-cell adhesion in PINCH CDKO myocardium suggest that PINCH may be required for expression and distribution of cell adhesion proteins within the intercalated discs. Consistent with this, expression of proteins that function in adherens junctions and gap junctions of the intercalated discs are markedly affected in PINCH CDKO myocardium. In CDKO myocardium, Connexin43, α-E-catenin and ZO-1 were significantly reduced, and retained a disperse expression pattern throughout the lateral membrane comparable to that seen at earlier stages of development, rather than being expressed at the intercalated disc as seen in control myocardium. A similar aberrant expression pattern was observed for β-catenin and Vinculin although overall amounts of these two proteins were unchanged. These data revealed a critical requirement for PINCH in modulating the stability and polarized distribution of intercalated disc proteins during postnatal myocardial maturation and remodeling.
Previous studies have suggested that PINCH1 and ILK may play an essential role in promoting cardiomyocyte migration and survival after cardiac injury by interacting with Thymosinβ43. However, a direct involvement of PINCH in this process has not been determined. In this study, we observed that, although the presence of either PINCH1 or PINCH2 is sufficient to maintain basal myocardial structure and functional integrity, loss of either PINCH1 or PINCH2 leads to exacerbated ischemic damage and deteriorated cardiac function in this setting. Inability of PINCH1 or PINCH2 to compensate for each other in ischemic myocardium may suggest that PINCH1 and PINCH2 may play distinct roles in myocardium, or reflect a dose-dependent requirement for PINCH in response to ischemia.
Taken together, our study demonstrates that PINCH proteins play essential roles in myocardial growth, maturation, remodeling and function. In addition, the present study further highlights the importance of studying the role of PINCH proteins in human cardiac injury and cardiomyopathy.
Clinical perspective
Cardiomyocytes communicate with their microenvironment through costameres. In both humans and animal models, dysfunction of costamere adhesion leads to cardiomyopathy and heart failure. Myocardial cell-matrix adhesion at the costamere is mediated by integrinβ1 receptor and its associated complex. Engagement of integrin with extracellular matrix leads to recruitment and formation of a cytoplasmic focal adhesion complex which links integrins to the actin cytoskeleton and other intracellular pathways. This complex is composed of integrin-linked kinase, Parvin and PINCH Two PINCH proteins, PINCH1 and PINCH2, have been described in mammals and share high homology. Both PINCH1 and PINCH2 are ubiquitously expressed in most tissues and organs, including myocardium. However, whether PINCH is required in normal myocardium, or for post-injury healing of myocardium, has not been addressed. To explore the functional role of PINCH in cardiomyocytes, we generated several mouse lines in which PINCH1 and PINCH2 are singly or doubly ablated in myocytes. Analysis of these mouse models has demonstrated essential roles for PINCHs in myocardial growth, maturation, remodeling and function, and highlights the importance of studying the role of PINCHs in human cardiac injury and cardiomyopathy.
Supplementary Material
Acknowledgements
We thank Dr. Banerje for critical reading of the manuscript. Knockout mice were generated at the transgenic core facility at UCSD.
Source of Funding
The work was supported by NIH grants for JC, CW and SE.
Footnotes
Disclosures
The authors report no conflicts.
References
- 1.Shai SY, Harpf AE, Babbitt CJ, Jordan MC, Fishbein MC, Chen J, Omura M, Leil TA, Becker KD, Jiang M, Smith DJ, Cherry SR, Loftus JC, Ross RS. Cardiac myocyte-specific excision of the beta1 integrin gene results in myocardial fibrosis and cardiac failure. Circ Res. 2002;90:458–464. doi: 10.1161/hh0402.105790. [DOI] [PubMed] [Google Scholar]
- 2.Perriard JC, Hirschy A, Ehler E. Dilated cardiomyopathy: a disease of the intercalated disc? Trends Cardiovasc Med. 2003;13:30–38. doi: 10.1016/s1050-1738(02)00209-8. [DOI] [PubMed] [Google Scholar]
- 3.Bock-Marquette I, Saxena A, White MD, Dimaio JM, Srivastava D. Thymosin beta4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature. 2004;432:466–472. doi: 10.1038/nature03000. [DOI] [PubMed] [Google Scholar]
- 4.White DE, Coutu P, Shi YF, Tardif JC, Nattel S, St Arnaud R, Dedhar S, Muller WJ. Targeted ablation of ILK from the murine heart results in dilated cardiomyopathy and spontaneous heart failure. Genes Dev. 2006;20:2355–2360. doi: 10.1101/gad.1458906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ieda M, Tsuchihashi T, Ivey KN, Ross RS, Hong TT, Shaw RM, Srivastava D. Cardiac fibroblasts regulate myocardial proliferation through beta1 integrin signaling. Dev Cell. 2009;16:233–244. doi: 10.1016/j.devcel.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu C. PINCH, N(i)ck and the ILK: network wiring at cell-matrix adhesions. Trends Cell Biol. 2005;15:460–466. doi: 10.1016/j.tcb.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Fassler R, Meyer M. Consequences of lack of beta 1 integrin gene expression in mice. Genes Dev. 1995;9:1896–1908. doi: 10.1101/gad.9.15.1896. [DOI] [PubMed] [Google Scholar]
- 8.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 9.Wiesner S, Legate KR, Fassler R. Integrin-actin interactions. Cell Mol Life Sci. 2005;62:1081–1099. doi: 10.1007/s00018-005-4522-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jamora C, Fuchs E. Intercellular adhesion, signalling and the cytoskeleton. Nat Cell Biol. 2002;4:E101–E108. doi: 10.1038/ncb0402-e101. [DOI] [PubMed] [Google Scholar]
- 11.Braun A, Bordoy R, Stanchi F, Moser M, Kostka GG, Ehler E, Brandau O, Fassler R. PINCH2 is a new five LIM domain protein, homologous to PINCH and localized to focal adhesions. Exp Cell Res. 2003;284:239–250. doi: 10.1016/s0014-4827(02)00039-3. [DOI] [PubMed] [Google Scholar]
- 12.Liang X, Zhou Q, Li X, Sun Y, Lu M, Dalton N, Ross J, Jr, Chen J. PINCH1 plays an essential role in early murine embryonic development but is dispensable in ventricular cardiomyocytes. Mol Cell Biol. 2005;25:3056–3062. doi: 10.1128/MCB.25.8.3056-3062.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukuda T, Chen K, Shi X, Wu C. PINCH-1 is an obligate partner of integrin-linked kinase (ILK) functioning in cell shape modulation, motility, and survival. J Biol Chem. 2003;278:51324–51333. doi: 10.1074/jbc.M309122200. [DOI] [PubMed] [Google Scholar]
- 14.Li S, Bordoy R, Stanchi F, Moser M, Braun A, Kudlacek O, Wewer UM, Yurchenco PD, Fassler R. PINCH1 regulates cell-matrix and cell-cell adhesions, cell polarity and cell survival during the peri-implantation stage. J Cell Sci. 2005;118:2913–2921. doi: 10.1242/jcs.02422. [DOI] [PubMed] [Google Scholar]
- 15.Stanchi F, Bordoy R, Kudlacek O, Braun A, Pfeifer A, Moser M, Fassler R. Consequences of loss of PINCH2 expression in mice. J Cell Sci. 2005;118:5899–5910. doi: 10.1242/jcs.02686. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Chen K, Tu Y, Wu C. Distinct roles of two structurally closely related focal adhesion proteins, alpha-parvins and beta-parvins, in regulation of cell morphology and survival. J Biol Chem. 2004;279:41695–41705. doi: 10.1074/jbc.M401563200. [DOI] [PubMed] [Google Scholar]
- 17.Forbes MS, Sperelakis N. Intercalated discs of mammalian heart: a review of structure and function. Tissue Cell. 1985;17:605–648. doi: 10.1016/0040-8166(85)90001-1. [DOI] [PubMed] [Google Scholar]
- 18.Aberle H, Schwartz H, Kemler R. Cadherin-catenin complex: protein interactions and their implications for cadherin function. J Cell Biochem. 1996;61:514–523. doi: 10.1002/(SICI)1097-4644(19960616)61:4%3C514::AID-JCB4%3E3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 19.Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen X, Gumbiner BM. Crosstalk between different adhesion molecules. Curr Opin Cell Biol. 2006;18:572–578. doi: 10.1016/j.ceb.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Radice GL, Rayburn H, Matsunami H, Knudsen KA, Takeichi M, Hynes RO. Developmental defects in mouse embryos lacking N-cadherin. Dev Biol. 1997;181:64–78. doi: 10.1006/dbio.1996.8443. [DOI] [PubMed] [Google Scholar]
- 22.Angst BD, Khan LU, Severs NJ, Whitely K, Rothery S, Thompson RP, Magee AI, Gourdie RG. Dissociated spatial patterning of gap junctions and cell adhesion junctions during postnatal differentiation of ventricular myocardium. Circ Res. 1997;80:88–94. doi: 10.1161/01.res.80.1.88. [DOI] [PubMed] [Google Scholar]
- 23.Hirschy A, Schatzmann F, Ehler E, Perriard JC. Establishment of cardiac cytoarchitecture in the developing mouse heart. Dev Biol. 2006;289:430–441. doi: 10.1016/j.ydbio.2005.10.046. [DOI] [PubMed] [Google Scholar]
- 24.Mackinnon AC, Qadota H, Norman KR, Moerman DG, Williams BD. C. elegans PAT-4/ILK functions as an adaptor protein within integrin adhesion complexes. Curr Biol. 2002;12:787–797. doi: 10.1016/s0960-9822(02)00810-2. [DOI] [PubMed] [Google Scholar]
- 25.Sakai T, Li S, Docheva D, Grashoff C, Sakai K, Kostka G, Braun A, Pfeifer A, Yurchenco PD, Fassler R. Integrin-linked kinase (ILK) is required for polarizing the epiblast, cell adhesion, and controlling actin accumulation. Genes Dev. 2003;17:926–940. doi: 10.1101/gad.255603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hobert O, Moerman DG, Clark KA, Beckerle MC, Ruvkun G. A conserved LIM protein that affects muscular adherens junction integrity and mechanosensory function in Caenorhabditis elegans. J Cell Biol. 1999;144:45–57. doi: 10.1083/jcb.144.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacKrell AJ, Blumberg B, Haynes SR, Fessler JH. The lethal myospheroid gene of Drosophila encodes a membrane protein homologous to vertebrate integrin beta subunits. Proc Natl Acad Sci U S A. 1988;85:2633–2637. doi: 10.1073/pnas.85.8.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stephens LE, Sutherland AE, Klimanskaya IV, Andrieux A, Meneses J, Pedersen RA, Damsky CH. Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 1995;9:1883–1895. doi: 10.1101/gad.9.15.1883. [DOI] [PubMed] [Google Scholar]
- 29.Liang X, Sun Y, Schneider J, Ding JH, Cheng H, Ye M, Bhattacharya S, Rearden A, Evans S, Chen J. Pinch1 is required for normal development of cranial and cardiac neural crest-derived structures. Circ Res. 2007;100:527–535. doi: 10.1161/01.RES.0000259041.37059.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Q, Chu PH, Huang C, Cheng CF, Martone ME, Knoll G, Shelton GD, Evans S, Chen J. Ablation of Cypher, a PDZ-LIM domain Z-line protein, causes a severe form of congenital myopathy. J Cell Biol. 2001;155:605–612. doi: 10.1083/jcb.200107092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang XQ, Avraham HK, Jiang S, Avraham S. Genetic alterations of the NRP/B gene are associated with human brain tumors. Oncogene. 2004;23:5890–5900. doi: 10.1038/sj.onc.1207776. [DOI] [PubMed] [Google Scholar]
- 32.Li J, Patel VV, Radice GL. Dysregulation of cell adhesion proteins and cardiac arrhythmogenesis. Clin Med Res. 2006;4:42–52. doi: 10.3121/cmr.4.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arya R, Kedar V, Hwang JR, McDonough H, Li HH, Taylor J, Patterson C. Muscle ring finger protein-1 inhibits PKC{epsilon} activation and prevents cardiomyocyte hypertrophy. J Cell Biol. 2004;167:1147–1159. doi: 10.1083/jcb.200402033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Legate KR, Montanez E, Kudlacek O, Fassler R. ILK, PINCH and parvin: the tIPP of integrin signalling. Nat Rev Mol Cell Biol. 2006;7:20–31. doi: 10.1038/nrm1789. [DOI] [PubMed] [Google Scholar]
- 35.Calderwood DA, Shattil SJ, Ginsberg MH. Integrins and actin filaments: reciprocal regulation of cell adhesion and signaling. J Biol Chem. 2000;275:22607–22610. doi: 10.1074/jbc.R900037199. [DOI] [PubMed] [Google Scholar]
- 36.Liu S, Calderwood DA, Ginsberg MH. Integrin cytoplasmic domain-binding proteins. J Cell Sci. 2000;113(Pt 20):3563–3571. doi: 10.1242/jcs.113.20.3563. [DOI] [PubMed] [Google Scholar]
- 37.Becam IE, Tanentzapf G, Lepesant JA, Brown NH, Huynh JR. Integrin-independent repression of cadherin transcription by talin during axis formation in Drosophila. Nat Cell Biol. 2005;7:510–516. doi: 10.1038/ncb1253. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.