Abstract
The small GTPase Rap1 is implicated in a variety of cellar functions. In this study, we investigated the effect of prostaglandin E2 (PGE2) on Rap1 activation in rheumatoid synovial fibroblasts (RSF). Rap1 was expressed in RSF, and GTP-bound active Rap1 (GTP-Rap1) was rapidly increased by PGE2. The effect of PGE2 was mimicked by an EP2 receptor agonist, an EP4 agonist and a cAMP elevating agent forskolin with association to increase of cAMP, but not by an EP1 or an EP3 agonist. RSF expressed the downstream signaling partners of cAMP, exchange protein directly activated by cAMP (Epac1) and protein kinase A (PKA). Both 8-pCPT-2-O-Me-cAMP (an Epac-specific cAMP analog) and 6-Bnz-cAMP (a PKA-specific cAMP analog) activated Rap1 in RSF. Activation of Rap1 by PGE2 via cAMP signaling may play an important role in the articular pathology of rheumatoid arthritis (RA).
1. Introduction
Rheumatoid arthritis (RA) is an autoimmune disease characterized by chronic inflammation of synovial tissues associated with loss of cartilage, erosion of juxtarticular bone and joint destruction. In RA, the synovial membrane is transformed to a hypertrophic inflammatory tissue with changes of phenotype of resident synovial fibroblasts and endothelial cells in addition of infiltration of macrophages, neutrophils, T and B cells. Rheumatoid synovial tissue characteristically overgrows and destroys articular cartilage and erodes juxtaarticular bone 1. High concentrations of PGE2 have been detected in the synovial fluid of patients with rheumatoid arthritis (RA), and cytokine-activated synovial cells are a primary source of PGE2 in affected joints 2. Production of PGE2 is preferentially increased in RA and other inflammatory conditions as a result of the sequential activation of two cytokine-inducible enzymes, cyclooxygenase-2 (COX-2; also known as PGH synthase-2) and microsomal prostaglandin E synthase-1 (mPGES-1) 3–8. COX-2 and mPGES-1 are highly expressed in inflamed synovial tissues in RA 5, 9, 10. Recent studies using mPGES-1 null mice showed a significant reduction of collagen-induced arthritis 11, 12 and collagen antibody induced arthritis 13. Furthermore, PGE2 is specifically implicated in the symptoms of arthritis because neutralizing antibody against PGE2 is able to inhibit acute and chronic inflammation in the rat adjuvant arthritis model 14. Taken together, these previous findings suggest that PGE2 is a critically important proinflammatory mediator in arthritis 5, 15–18.
PGE2 exerts its effects through a family of 4 different G protein-coupled receptors, EP1-4 19. Among these, the EP2 and EP4 receptors increase cAMP via activation of adenylate cyclase 20. To date, most cAMP-mediated effects of PGE2 via EP2/4 have been explained by the classic downstream target, protein kinase A (PKA), which phosphorylates downstream targets, such as the cAMP response element binding protein (CREB), in a variety of mammalian cells. However, PKA-independent actions of cAMP have been recognized in various experimental systems 21, 22.
Recently, exchange protein directly activated by cAMP (Epac, also designated cAMP-GEFs) have been identified as novel targets for cAMP 23–25. At least two forms of Epac, Epac1 and Epac2, have been identified and characterized. The Epac proteins contain a guanine nucleotide-exchange factor (GEF) domain which activates the small GTPase Rap1 (a member of the small GTPase Ras family) by directly converting GDP-bound inactive form to GTP-bound active form in response to increases of intracellar cAMP. Several important biological effects on various cellar functions including adhesion, phagocytosis, secretion, proliferation, differentiation, morphogenesis, migration and spreading in mammalian cells have been attributed to Rap1 26–30. Rap1 has been reported to be activated by Epac in a cAMP-dependent but PKA-independent manner 31. However, another recent study demonstrated cAMP-dependent Rap1 activation by PKA signaling via C3G (Crk SH3 domain GEF) 32, 33, the first identified Rap1-specific GEF 34.
In the present study, we demonstrate for the first time that Rap1 is functionally expressed in RSF and it is activated by PGE2 via EP2 and EP4 receptors in a cAMP-dependent manner. We also demonstrate that Rap1 is activated via both Epac1- and PKA-mediated signaling in RSF. Our findings provide further insight into the role of PGE2 in RA.
2. Materials and Methods
2.1. Materials
PGE2 and an enzyme-linked immunosorbent assay (ELISA) kit for cAMP were purchased from Cayman Chemical Co. (Ann Arbor, MI). Rabbit anti-human EP2 and EP4 receptor polyclonal antibodies were kindly gifted from Cayman Chemical Co. EZ-Detect™ Rap1 Activation Kit for Rap1 pull down assay and bicinchoninic acid (BCA) protein assay reagent were purchased from Pierce Biotechnology Inc (Rockford, IL). Rabbit anti-human Epac1 polyclonal antibody and normal mouse IgG were purchased from Upstate (Chicago, IL). Goat anti-human Epac2 polyclonal antibody, rabbit anti-human MAP1A polyclonal antibody, rabbit anti-human PKAα cat polyclonal antibody, rabbit anti-human C3G polyclonal antibody, rat cerebellum extract and lysates of human renal adenocarcinoma Caki-1 cells and neuroglioma H4 cells were purchased from Santa Cruz (Santa Cruz, CA). Rabbit anti-human Phospho-CREB (Ser133) polyclonal antibody and rabbit anti human CREB polyclonal antibody were purchased from Cell Signaling Technology, Inc. (Danvers, MA). Horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG, HRP-conjugated goat anti-mouse IgG and HRP-conjugated rabbit anti-goat IgG were purchased from Jackson ImmunoResearch (West Grove, PA). The PKA-specific cAMP analog, 6-Bnz-cAMP (N6-Benzoyladenosine-3′,5′-cyclic monophosphate), and Epac-specific cAMP analog, 8-pCPT-2-O-Me-cAMP (8-(4-chlorophenylthio)-2′-O-methyladenosine-3′,5′-cyclic monophosphate), were purchased from Biolog Life Science Institute (Hayward CA). Forskolin and 3-isobutyl-1-methylxanthine (IBMX) were purchased from Calbiochem (San Diego, CA). A selective EP1 receptor agonist (ONO-DI-004, (17S)-2,5-ethano-6-oxo-17,20-dimethylPGE1), an EP2 receptor agonist (ONO-AE1-259, (16)-9-deoxy-9β-chloro-15-deoxy-16-hydroxy-17,17-trimethylene-19,20-didehydro PGE1), an EP3 receptor agonist (ONO-AE-248, 11,15-O-dimethyl PGE2), and an EP4 receptor agonist (ONO-AE1-329, 16-(3-methoxymethyl)phenyl-ω-tetranor-3,7-dithia PGE1) were provided by Ono Pharmaceutical Co., Ltd. (Osaka, Japan). The polyvinylidene difluoride (PVDF) membrane and enhanced chemiluminescence (ECL) reagent were purchased from Amersham Pharmacia Biotech (Piscataway, NJ). SuperScriptR First-Strand Synthesis System for reverse transcription-polymerase chain reaction (RT-PCR), Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased from Invitrogen (Carlsbad, CA). TRIpure reagent (Roche Applied Science, Indianapolis, IN) and HotStarTaq polymerase (Qiagen, Valencia, CA) were purchased from the indicated companies.
2.2. Preparation of RSF from patients with RA
Synovial tissues were obtained at the time of joint replacement surgery from patients with RA who fulfilled the revised American Rheumatism Association criteria for this disease 35. Experiments were carried out according to a protocol that was approved by the institutional review board of the University of Kentucky. RSF were prepared by a previously described method 3. Briefly, minced synovial tissues were digested overnight with 1 mg/ml collagenase (Type I, Sigma, St. Louis, MO) in DMEM in a humidified 5% CO2 incubator at 37°C and the isolated cells were cultured in 175 cm2 culture flasks in DMEM supplemented with 20% FBS, L-glutamine (2 mM), penicillin (100 units/ml) and streptomycin (100 μg/ml). At greater than 95% confluency, the adherent RSF were passaged by digestion with 0.05% trypsin/EDTA and used between passages 4 and 6 for all experiments. Cells were plated into 6-well plates (3 × 105 cells/well) or 75 cm2 culture flasks (2 × 106 cells/flask) in DMEM containing 20% FBS. Cells were starved for 72 hrs in serum-free DMEM and then incubated with or without various stimuli.
2.3. Detection of GTP-Rap1 by Rap1 Pull-down assay
Rap1 pull-down assay was performed by using EZ-Detect™ Rap1 Activation Kit according to the manufacturer’s recommendation. Briefly, after RSF were made quiescent by culturing in serum-free DMEM for 72 hrs, cells were incubated with various stimuli. After washing with ice-cold PBS, cells were lysed in the provided lysis/wash/buffer with protease cocktail inhibitor and then lysates were centrifuged at 16,000 × g for 15 min at 4°C. The protein content of the supernatant was determined using the BCA protein assay reagent with bovine serum albumin as standard. Equal amounts of protein (750 μg protein) were subjected to affinity precipitation of GTP-Rap1 using the EZ-Detect™ Rap1 Activation Kit, following by Western blotting with provided antibody (1:1000 dilution). To assess the levels of total Rap1, cell lysates adjusted for protein concentration were directly applied to Western blotting without pull-down assay.
2.4. Measurement of intracellular cAMP concentration
Intracellular cAMP concentration was measured with an ELISA kit according to the manufacturer’s recommendation by a previously described method 36. Briefly, after starved in serum-free DMEM for 72 hrs, cells were stimulated under various conditions. Next, cells were lysed in 0.1 N HCl by incubation for 20 min at room temperature. After centrifugation of the lysate at 1000 ×g for 10 min at 4°C, cAMP concentration in supernatants was measured with an ELISA. The protein amount was determined using the BCA protein assay reagent.
2.5. RT-PCR
RNA from the cells was extracted with TRIpure reagent according to the manufacturer’s instructions. Reverse transcription was performed according to the manufacturer’s instructions using a SuperScript preamplification system with 1 μg of total RNA from the cells as a template. Subsequent amplifications of the cDNA fragments by PCR with HotStarTaq polymerase were performed using 0.5 μl of the reverse-transcribed mixture as a template with specific oligonucleotide primers for EP receptor subtype 37, 38, Epac 39, and MAP1A (light chain 2, LC2) and cycle number as follows: human EP1 receptor primers (34 cycles, product size 519 bp), sense 5′-CTC GCC GCG CTG GTG TGC AAC ACG C-3′ and antisense 5′-GGC CTC CCA GGC GCT CGG TGT TAG G-3′; human EP2 primers (34 cycles, product size 510 bp), sense 5′-TTC ATC CGG CAC GGG CGG ACC GC-3′ and antisense 5′-GTC AGC CTG TTT ACT GGC ATC TG-3′; human EP3 primers (34 cycles, product size 398 bp), sense 5′-TGT GTC GCG CAG CTA CCG GCG-3′ and antisense 5′-CGG GCC ACT GGA CGG TGT ACT-3′; human EP4 receptor primers (34 cycles, product size 366 bp), sense 5′-CCT CCT GAG AAA GAC AGT GCT-3′ and antisense 5′-AAG ACA CTC TCT GAG TCC T-3′; human Epac1 primers (40 cycles, product size 304 bp), sense 5′-GCT TCC TCC ACA AAC TCT CA-3′ and antisense 5′-AAC GCT GCC ATC ACC TCT CT-3′; human Epac2 primers (30 cycles, product size 333 bp), sense 5′-AGC CTT ATC CCA TCT TTC TA-3′ and antisense 5′-CTG ACT GTA TTC GCC TCC AC-3′; human MAP1A (LC2) primers (30 cycles, product size 392 bp), sense 5′-AAG GTT CAG GGG CGA GTA G-3′ and antisense 5′-CCA TTG GCA GGG TCA TTC C-3′. After initial denaturation at 95°C for 15 min, PCR involved amplification cycles of 30 sec at 95°C, 30 sec at 56°C (for EP1-4, Epac2 and MAP1A) or 52°C (for Epac1), and 45 sec at 72°C, followed by elongation for 5 min at 72°C. The amplified cDNA fragments were resolved by electrophoresis on 2% (w/v) agarose gel and were visualized under UV light using a BioRad Chemidoc Apparatus (Hercules, CA) after staining of the gel with ethidium bromide.
2.6. Western blot analysis
Cells were lysed in Tris-buffered saline (TBS) containing 0.1 % sodium dodecyl sulfate (SDS). After determined protein content by BCA protein assay reagent with bovine serum albumin as standard, cell lysates adjusted to equal equivalents of protein were applied to SDS-polyacrylamide gel electrophoresis (SDS-PAGE) for electrophoresis and then the proteins were electroblotted onto PVDF membrane. After blocking the membranes in 10 mM TBS containing 0.1 % Tween-20 (TBS-T) containing 5 % skim milk, the membranes were probed with the respective antibodies (1:200 for Epac1; 1:500 for EP2, EP4, Epac2, MAP1A, PKAα cat and C3G) in TBS-T. After washing the membranes with TBS-T, the membranes were incubated with HRP-conjugated secondary antibody (1:10,000 in TBS-T containing 5% skim milk) for overnight at 4°C. After further washing with TBS-T, protein bands were visualized with an ECL Western blot analysis system using a BioRad Chemidoc Apparatus (BioRad, Hercules, CA). For detection of phospho- and total CREB, cells were lysed in buffer (50 mM HEPES [pH 7.4], 150 mM NaCl, 1 mM EGTA, 10 mM Na4P2O7, 100 mM NaF, 1% Triton X-100, 10% glycerol, 0.5% deoxycholic acid, 0.1% SDS, 50 mM glycerophosphate, 3 mM Na3VO4 and 10 μg/ml aprotinin). Cell lysates adjusted to equal equivalents of protein were applied to SDS-PAGE and followed by Western blotting with anti-phospho-CREB antibody (1:500) for phosphorylated CREB or anti-CREB antibody (1:500) for total CREB.
2.7. Statistical analysis
All data are expressed as mean ± SEM. Statistical analysis was performed using Student’s t-test. P < 0.05 was considered statistically significant.
3. Results
3.1. Expression of Rap1 and its activation by PGE2 in RSF
To elucidate whether Rap1 exists and can be activated in RSF, we first examined the activation of Rap1 in the presence of GTPγS (a substrate for GTP) or GDP in vitro with lysates isolated from RSF. As shown in Figure 1A, the levels of GTP-Rap1 (GTP-bound active form of Rap1) were increased by incubation with GTPγS and decreased in the presence of GDP, indicating that Rap1 is functionally expressed in RSF. We next examined the effect of PGE2 on activation of Rap1 in cultured RSF. As shown in Figure 1B, the levels of GTP-Rap1 were rapidly increased from 30 to 120 min after PGE2 stimulation and were decreased at 240 min, without changes in the levels of total Rap1. GTP-Rap1 was increased by PGE2 in a concentration dependent manner (Figure 1C).
Fig. 1. Expression of Rap1 and its activation by PGE2 in RSF.
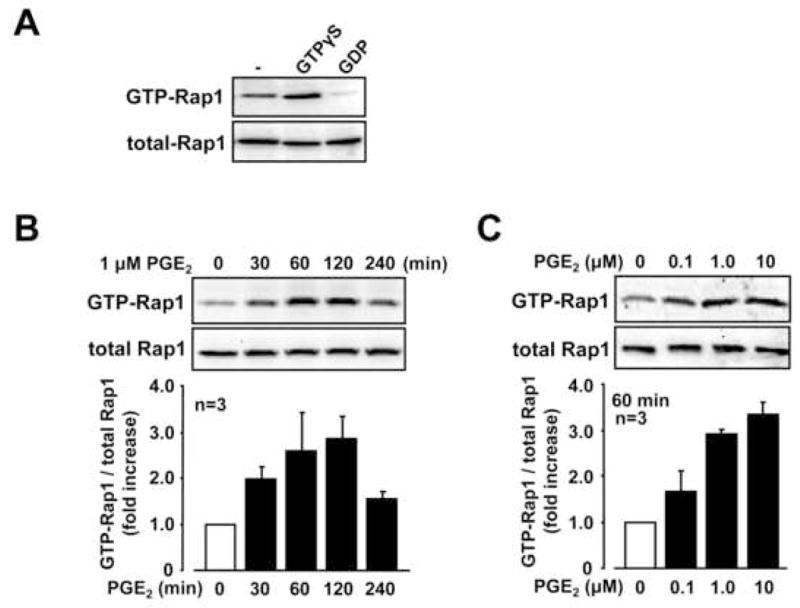
A, Cell lysates from RSF were treated in vitro with GTPγS (10 μM) or GDP (100 μM) for 30 min at 30°C, according to the EZ-Detect™ Rap1 Activation Kit manufacturer’s protocol. GTP-Rap1 was detected by Rap1 pull down assay followed by Western blotting as descried in Materials and Methods. Total Rap1 levels are also shown. B and C, Serum-starved RSF were stimulated with or without PGE2 (0.1–10 μM) for the time period as indicated and cell lysates were subjected to Rap1 pull down assay. Rap1 activation indicates the ratio of GTP-Rap1 against total Rap1 and a value of “1” was assigned to the value of 0 min (B) or value of non-stimulation (C). Blots are representative data from the indicated number of patients with RA in each panel.
3.2. Contribution of PGE2 receptor subtypes in Rap1 activation in RSF
Since PGE2 stimulated GTP-Rap1 in RSF, we next determined the PGE2 receptor subtypes, EP1-4, in activation of Rap1. As shown in Figure 2A, mRNA for the EP2 and EP4 receptors was co-expressed in RSF, whereas mRNA for the EP1 and EP3 receptors was not detected. While, EP1 and EP3 mRNA was detectable in some lines of cultured chondrocytes from patients with osteoarthritis (data not shown). In addition, we detected immunoreactive bands of the expected size for EP2 (52 kDa) and EP4 (65 kDa) in RSF by Western blotting (Figure 2B). We next determined the effect of specific EP receptor agonists on Rap1 activation. The specificity of each EP receptor agonist used in this study was confirmed by Suzawa et al. using a binding assay for the respective receptor subtypes expressed in CHO cells 40. As shown in Figure 2C, an EP2 receptor agonist (ONO-AE1-259) and an EP4 receptor agonist (ONO-AE1-329), as well as PGE2, up-regulated the levels of GTP-Rap1 in RSF. However, an EP1 receptor agonist (ONO-DI-004) and an EP3 receptor agonist (ONO-AE-248) did not. Increased levels of cAMP were also observed under the stimulation by EP2 agonist and EP4 agonist but not by EP1 agonist and EP3 agonist (Figure 2D). These data suggested that PGE2 activates Rap1 via the EP2 and EP4 receptor subtypes associated with an increase in cAMP.
Fig. 2. Contribution of PGE2 receptor subtypes in Rap1 activation in RSF.
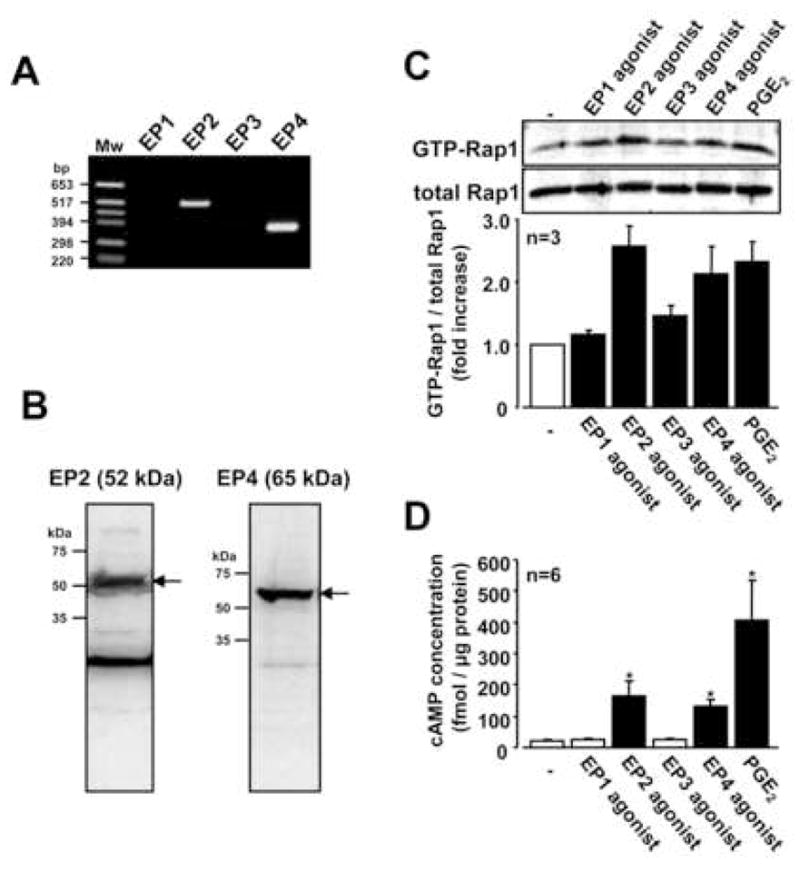
A, mRNA expression of EP1, EP2, EP3 and EP4 receptors in RSF were evaluated by RT-PCR. Only EP2 and EP4 mRNA was detected. Results are representative examples from 3 patients with RA. B, Protein expression of EP2 and EP4 receptors in RSF were analyzed by Western blotting. Blots are representative data from 3 patients with RA. C, Serum-starved RSF were incubated for 60 min with or without PGE2 (1 μM) or EP receptor agonists (10 μM). Cell lysates were subjected to Rap1 pull down assay. Blots are representative data and values are expressed as means ± standard errors from the number of patients with RA. D, RSF were incubated for 30 min with or without PGE2 (1 μM) or EP receptor agonists (EP1-4, 10 μM). Levels of cAMP were measured by ELISA. Values are expressed as means ± standard errors from the number of patients with RA. A significant difference from the value of non-stimulation determined by Student’s t-test is indicated with a single asterisk (P < 0.05).
3.3. Effect of cAMP-elevating agents on Rap1 activation in RSF
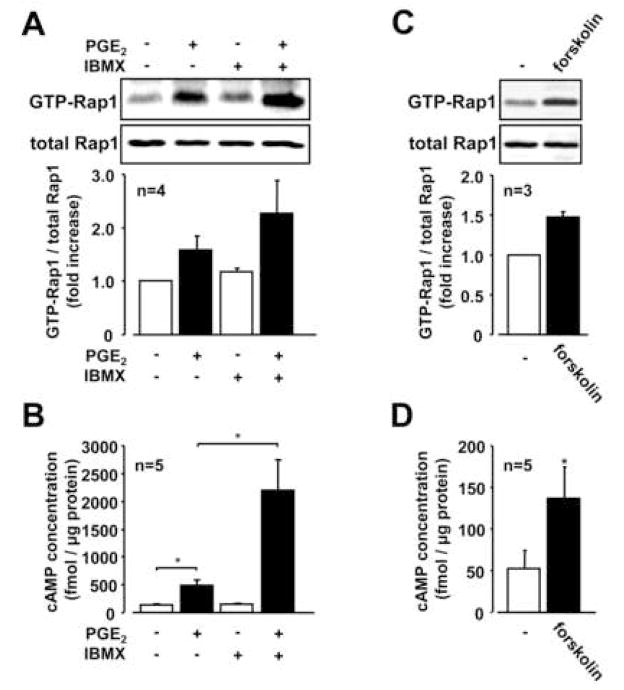
PGE2 caused a rapid increase in the levels of intracellar cAMP with maximal levels at 15 min after PGE2 stimulation (Figure 3A), and we observed a concentration-dependent effect of PGE2 on the increase the elevation of cAMP in RSF (Figure 3B). The PGE2-mediated change in cAMP mirrored the time and concentration change to Rap-1 following treatment with PGE2 as shown in Figure 1. To further confirm the importance of cAMP in mediating the effects of PGE2 on activation of Rap1, we examined the effect of 3-isobutyl-1-methylxanthine (IBMX, a phosphodiesterase inhibitor) on PGE2-induced Rap1 activation. As shown in Figure 4A, increased levels of GTP-Rap1 by PGE2 were enhanced in the presence of IBMX, which was correlated with further increased levels of cAMP (Figure 4B). To determine whether other cAMP-elevating agent induced the same biological effects as PGE2 on Rap1 activation, we examined the effect of forskolin (a direct activator of adenylate cyclase). Forskolin also increased GTP-Rap1 concomitant with increasing cAMP levels (Figure 4C and 4D). These data indicate that cAMP is involved in the PGE2-induced Rap1 as a second messenger in RSF.
Fig. 3. Increase of cAMP by PGE2 in RSF.
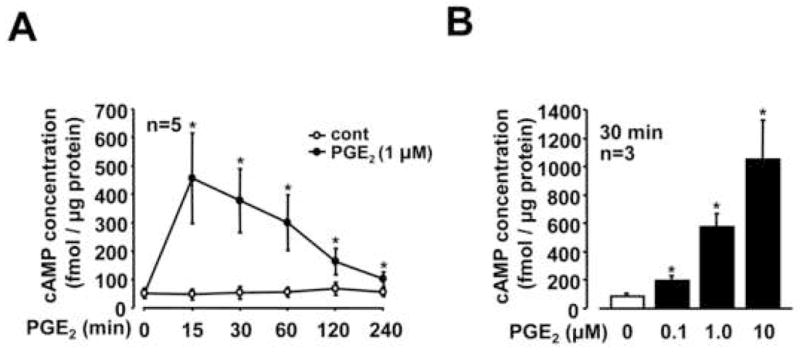
Serum-starved RSF were stimulated with or without PGE2 (0.1–10 μM) for the time period as indicated. Levels of cAMP were measured by ELISA. A significant difference from the value of 0 min (A) or non-stimulation (B) determined by Student’s t-test is indicated with a single asterisk (P < 0.05). Values are expressed as means ± standard errors from the number of patients with RA indicated in each panel.
Fig. 4. Effect of cAMP-elevating agents on Rap1 activation in RSF.
A, Serum-starved RSF were stimulated with or without PGE2 (1 μM) for 60 min in the presence or absence of 3-Isobutyl-1-methylxanthine (IBMX, 100 μM). Lysates from the cells were subjected to Rap1 pull down assay. B, cAMP levels in cell lysates from RSF stimulated with or without PGE2 (1 μM) for 30 min in the presence or absence of 3-Isobutyl-1-methylxanthine (IBMX, 100 μM) were analyzed by ELISA. C, Rap1 activation was analyzed by detecting GTP-Rap1 with lysates from RSF stimulated with forskolin (10 μM) for 60 min. D, cAMP levels in cell lysates from RSF stimulated with or without forskolin (10 μM) for 30 min were analyzed by ELISA. Blots are representative data and values are expressed as means ± standard errors from the number of patients with RA indicated in each panel. A significant difference between two groups (B & D) determined by Student’s t-test is indicated with a single asterisk (P < 0.05).
3.4. Expression profile of cAMP-dependent Rap1 signaling molecules in RSF
We next investigated further the downstream targets of cAMP signaling responsible for activation of Rap1 in PGE2-stimulated RSF. As shown in Figure 5A and 5B, Epac1 mRNA and protein were expressed in RSF, while Epac2 expression was absent. MAP1A, an Epac1-associated microtubular protein, was also detected (Figure 56A and 5B). Interestingly, Epac1 is represented by double bands at both the protein and mRNA levels. Kooistra et al. previously reported the double bands of Epac1 protein in HUVEC (human umbilical vein endothelial cells) and Ovcar3 cells (ovarian carcinoma cell line) 41. They also reported that both bands were abolished by Epac1 siRNA, suggesting the existence of two variants of Epac1. We also detected protein expression of PKAα cat (a catalytic subunit of PKA) and C3G, also known as a Rap1-specific GEF, in RSF (Figure 5C).
Fig. 5. Expression profile of cAMP-dependent Rap1 signaling molecules in RSF.
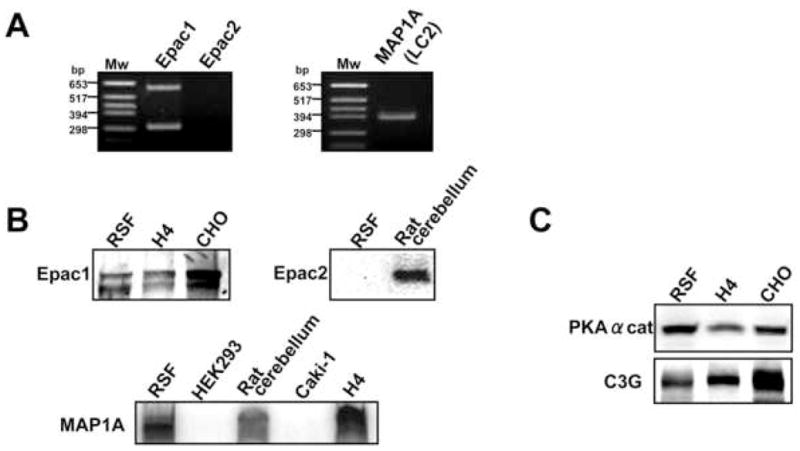
A, mRNA expressions of Epac1, Epac2 and MAP1A in RSF were detected by RT-PCR. B, Protein expression of Epac1, Epac2, and MAP1A were determined by Western blotting. C, Protein expression of PKAα cat and C3G were determined by Western blotting. Results are representative examples from 2–4 patients with RA. H4 cells, CHO cells, rat cerebellum extract were used as positive controls. HEK293 cells and Caki-1 cells were used as negative controls.
3.5. Effect of Epac-specific and PKA-specific cAMP analog on activation of Rap1 and CREB in RSF
Distinct roles for Epac and PKA in Rap1 signaling have been previously described by using highly specific cAMP analogs, Epac-specific 8-CPT-2Me-cAMP and PKA-specific 6-bnz-cAMP 42. Thus, to assess whether Rap1 activation in RSF is linked with Epac-and/or PKA-mediated signaling pathway in RSF, we examined the effect of these analogues on Rap1 activation. Since it is well known that CREB phosphorylation is mediated by PKA signaling but not by Epac signaling in various cell types, we also evaluated analogue specificity by examining the effect of these agonists on phosphorylation of CREB. The Epac-specific cAMP analog increased the levels of GTP-Rap1 (Figure 6A), but had no effect on the phosphorylation of CREB (Figure 6B). On the other hand, the PKA-specific cAMP analog increased both the levels of phospholyrated CREB (Figure 6B) and GTP-Rap1 (Figure 6A). These results suggest that cAMP-dependent activation of Rap1 is mediated by Epac signaling, but also by PKA signaling, in RSF.
Fig. 6. Effect of Epac-specific and PKA-specific cAMP analog on activation of Rap1 and CREB in RSF.
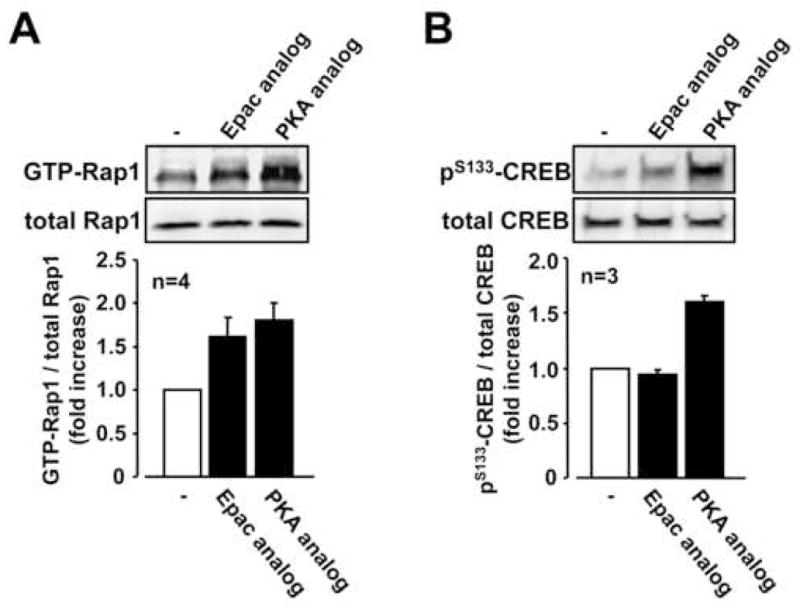
A, Serum-starved RSF were stimulated with 8-CPT-2-O-Me-cAMP (Epac-specific cAMP analog, 500 μM) and 6-Bnz-cAMP (PKA-specific cAMP analog, 500 μM) for 45 min. Lysates from the cells were subjected to Rap1 pull down assay. B, Serum-starved RSF were stimulated with 8-CPT-2-O-Me-cAMP (Epac-specific cAMP analog, 500 μM) and 6-Bnz-cAMP (PKA -specific cAMP analog, 500 μM) for 10 min. Levels of phospho-CREB and total CREB were analyzed by Western blotting. Blots are representative data and values are expressed as means ± standard errors from the indicated number of patients with RA in each panel.
4. Discussion
The novel findings of this study are that Rap1 is functionally expressed in RSF and is activated by PGE2 due to increased cAMP via the Gαs-coupled EP2 and EP4 receptors. In addition, cAMP-dependent Rap1 activation is mediated by both Epac1 and PKA in RSF.
Rap1 is strongly implicated in the regulation of cytoskeletal structure and cell-cell adhesion in mammalian cells 26, 27, 43. In RA, cell-cell adhesion mediated by cadherin-11, a member of the cadherin superfamily, is thought to be essential for synovial tissue organization 44, 45 and cadherin-11 plays an important role in the response to experimental arthritis 46. In addition, cell-cell contact between T-cells and adherent RA synovial cells mediates Rap1 inactivation and regulates production of reactive oxygen species in T lymphocytes after exposure to inflammatory cytokines 47.
We clearly demonstrate a role for cAMP signaling including its downstream partners, Epac and PKA, in Rap1 activation in RSF. RSF expressed Epac1, but not Epac2, and an Epac-specific cAMP analog led to activation of Rap1 in RSF. However, we also found that Rap1 was also activated by a PKA-specific cAMP analog. These results demonstrate that cAMP-dependent Rap1 activation is mediated not only by Epac1, but also by PKA in RSF. Most reports have implicated Epac as responsible for cAMP-dependent Rap1 activation in other cell types. However, a recent study also proposed that Rap1 could be activated by PKA signaling via C3G 32, 33. Indeed, we observed C3G protein expression in RSF in the present study. Wang et al. 33 demonstrated the existence of distinct pools of Rap1 that can be selectively activated by either PKA or Epac, which may differentially distinguish downstream effects of Rap1. In fact, their report clearly showed that Epac1 activated a perinuclear pool of Rap1 and this did not result in ERK activation; however, the addition of a membrane-targeting motif to Epac1 resulted in relocation of Epac1 from its normal perinuclear localization to the plasma membrane and revealed an ability to activate ERK in a cAMP/Rap1-dependent manner. Further assessment of the subcellular localization of Epac1 and/or other downstream effectors associated with Epac1/Rap1 in RSF may provide further understanding of independent Epac and PKA effects.
A recent report suggested that the interaction of the Epac agonist, 8-CPT-2-O-Me-cAMP, with phosphodiesterases (PDEs) may inhibit the hydrolysis of endogenous cAMP, thereby increasing the levels of this second messenger 48. If this were the case in RSF, the expected outcome might be the indirect activation of PKA by 8-CPT-2-O-Me-cAMP. However, we detected Rap1 activation but not PKA activation by 8-CPT-2-O-Me-cAMP, suggesting the Rap1 activation by agonistic effect on Epac rather than PDE inhibition by the agonist in this study..
The present study suggests that the coordinated action of Epac1 and PKA are involved in the Rap1 activation in response to elevation of cAMP after exposure to PGE2 in RSF. Enhancing properties of Epac signaling to PKA signaling have been recognized in several cellular events. For example, cAMP analogs selectively activating Epac synergize strongly with PKA-specific cAMP analog to induce neurite outgrowth in rat neuronal pheochromocytoma PC-12 cells 42. In addition, Epac and PKA cooperatively enhance functional gap junction neoformation in cardiomyocytes 49.
Since cAMP is the key mediator in the activation of Rap-1 by PGE2, we expected the receptors linked to stimulation of cAMP, EP2 and EP4, would be involved. This was indeed the case. Actions of PGE2 via EP2 and EP4 have been implicated in a number of critical mechanisms critical to arthritis. We have previously reported that PGE2 positively auto-regulates the expression of mPGES-1 which is a terminal enzyme for PGE2 biosynthesis, via activation of EP2 and EP4 receptors in RSF under interleukin (IL)-1β-stimulated condition 38. PGE2 also regulates the production of cytokine and growth factor such as IL-6, vascular endothelial growth factor, parathyroid hormone-related peptide, and macrophage colony stimulating factor though the activation of EP2 and EP4 receptors in IL-1-stimulated synovial fibroblasts 50, 51. Furthermore, previous in vivo studies have been demonstrated that EP2 and/or EP4 null mice are resistant to chronic inflammation of joints in the experimental arthritis models including collagen antibody induced arthritis 52 and collagen induced arthritis 53.
It is likely that PGE2 exerts its effects on inflammation and on synovial morphology through activation of Rap-1 and perhaps other small GTPases that regulate cytoskeletal organization, migration, and activation status via mitogen activated protein kinases, known to be highly expressed in RA synovial tissue 54. The PGE2-induced changes in RSF morphology and cytoskeleton organization could have significant consequences 55, 56. PGE2 has been previously shown to regulate migration of variety of cells associated with changes of cell morphology and cytoskeleton organization via cAMP signaling and small GTPases 57–61. In a separate study, we showed morphologic changes, redistribution of cadherin-11 on the plasma membrane, and dissociation of the actin cytoskeleton after treatment with PGE2 or a combination of PKA and Epac agonists (data not shown). Since cadherin-11 deficiency is associated with altered response to an inflammatory insult, PGE2 could mediate some of its pro-inflammatory effects by directly altering the architecture and function of synovial tissue via Rap1. Further understanding of these key pathways may yield new therapeutic targets in patients with RA.
Acknowledgments
We thank Ono Pharmaceutical Co. Ltd. (Osaka Japan) for providing us with PGE2 receptor subtype agonists (ONO-DI-004, ONO-AE1-259, ONO-AE-248, and ONO-AE1-329), and Cayman Chemical Co. (Ann Arbor, MI) for providing us with rabbit anti-human EP2 and EP4 receptor polyclonal antibodies.
This work was supported by the NIH/NIAMS R01 AR049010, the Arthritis Foundation Biomedical Sciences Grant and Travel Award from the Japanese Society of Clinical Pharmacology and Therapeutics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ospelt C, Gay S. The role of resident synovial cells in destructive arthritis. Best Pract Res Clin Rheumatol. 2008;22(2):239–52. doi: 10.1016/j.berh.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Egg D, Gunther R, Herold M, Kerschbaumer F. Prostaglandins E2 and F2 alpha concentrations in the synovial fluid in rheumatoid and traumatic knee joint diseases. Z Rheumatol. 1980;39(5–6):170–5. [PubMed] [Google Scholar]
- 3.Kojima F, Naraba H, Sasaki Y, Okamoto R, Koshino T, Kawai S. Coexpression of microsomal prostaglandin E synthase with cyclooxygenase-2 in human rheumatoid synovial cells. J Rheumatol. 2002;29(9):1836–42. [PubMed] [Google Scholar]
- 4.Stichtenoth DO, Thoren S, Bian H, Peters-Golden M, Jakobsson PJ, Crofford LJ. Microsomal prostaglandin E synthase is regulated by proinflammatory cytokines and glucocorticoids in primary rheumatoid synovial cells. J Immunol. 2001;167(1):469–74. doi: 10.4049/jimmunol.167.1.469. [DOI] [PubMed] [Google Scholar]
- 5.Kojima F, Kato S, Kawai S. Prostaglandin E synthase in the pathophysiology of arthritis. Fundam Clin Pharmacol. 2005;19(3):255–61. doi: 10.1111/j.1472-8206.2005.00316.x. [DOI] [PubMed] [Google Scholar]
- 6.Jakobsson PJ, Thoren S, Morgenstern R, Samuelsson B. Identification of human prostaglandin E synthase: a microsomal, glutathione-dependent, inducible enzyme, constituting a potential novel drug target. Proc Natl Acad Sci U S A. 1999;96(13):7220–5. doi: 10.1073/pnas.96.13.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kapoor M, Kojima F, Qian M, Yang L, Crofford LJ. Shunting of prostanoid biosynthesis in microsomal prostaglandin E synthase-1 null embryo fibroblasts: regulatory effects on inducible nitric oxide synthase expression and nitrite synthesis. Faseb J. 2006;20(13):2387–9. doi: 10.1096/fj.06-6366fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murakami M, Naraba H, Tanioka T, et al. Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthase that acts in concert with cyclooxygenase-2. J Biol Chem. 2000;275(42):32783–92. doi: 10.1074/jbc.M003505200. [DOI] [PubMed] [Google Scholar]
- 9.Westman M, Korotkova M, af Klint E, et al. Expression of microsomal prostaglandin E synthase 1 in rheumatoid arthritis synovium. Arthritis Rheum. 2004;50(6):1774–80. doi: 10.1002/art.20286. [DOI] [PubMed] [Google Scholar]
- 10.Murakami M, Nakashima K, Kamei D, et al. Cellular prostaglandin E2 production by membrane-bound prostaglandin E synthase-2 via both cyclooxygenases-1 and -2. J Biol Chem. 2003;278(39):37937–47. doi: 10.1074/jbc.M305108200. [DOI] [PubMed] [Google Scholar]
- 11.Trebino CE, Stock JL, Gibbons CP, et al. Impaired inflammatory and pain responses in mice lacking an inducible prostaglandin E synthase. Proc Natl Acad Sci U S A. 2003;100(15):9044–9. doi: 10.1073/pnas.1332766100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kojima F, Kapoor M, Yang L, et al. Defective generation of a humoral immune response is associated with a reduced incidence and severity of collagen-induced arthritis in microsomal prostaglandin E synthase-1 null mice. J Immunol. 2008;180(12):8361–8. doi: 10.4049/jimmunol.180.12.8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamei D, Yamakawa K, Takegoshi Y, et al. Reduced pain hypersensitivity and inflammation in mice lacking microsomal prostaglandin e synthase-1. J Biol Chem. 2004;279(32):33684–95. doi: 10.1074/jbc.M400199200. [DOI] [PubMed] [Google Scholar]
- 14.Portanova JP, Zhang Y, Anderson GD, et al. Selective neutralization of prostaglandin E2 blocks inflammation, hyperalgesia, and interleukin 6 production in vivo. J Exp Med. 1996;184(3):883–91. doi: 10.1084/jem.184.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kojima F, Kapoor M, Kawai S, Crofford LJ. New insights into eicosanoid biosynthetic pathways: Implications for arthritis. Expert Rev Clin Immunol. 2006;2:277–91. doi: 10.1586/1744666X.2.2.277. [DOI] [PubMed] [Google Scholar]
- 16.Kapoor M, Kojima F, Crofford LJ. Arachidonic acid-derived eicosanoids in rheumatoid arthritis: implications and future targets. Future Rheumatology. 2006;1(3):323–30. [Google Scholar]
- 17.Crofford LJ. COX-2 in synovial tissues. Osteoarthritis Cartilage. 1999;7(4):406–8. doi: 10.1053/joca.1999.0226. [DOI] [PubMed] [Google Scholar]
- 18.Sampey AV, Monrad S, Crofford LJ. Microsomal prostaglandin E synthase-1: the inducible synthase for prostaglandin E2. Arthritis Res Ther. 2005;7(3):114–7. doi: 10.1186/ar1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79(4):1193–226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- 20.Regan JW. EP2 and EP4 prostanoid receptor signaling. Life Sci. 2003;74(2–3):143–53. doi: 10.1016/j.lfs.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 21.Aronoff DM, Carstens JK, Chen GH, Toews GB, Peters-Golden M. Short communication: differences between macrophages and dendritic cells in the cyclic AMP-dependent regulation of lipopolysaccharide-induced cytokine and chemokine synthesis. J Interferon Cytokine Res. 2006;26(11):827–33. doi: 10.1089/jir.2006.26.827. [DOI] [PubMed] [Google Scholar]
- 22.Aronoff DM, Canetti C, Serezani CH, Luo M, Peters-Golden M. Cutting edge: macrophage inhibition by cyclic AMP (cAMP): differential roles of protein kinase A and exchange protein directly activated by cAMP-1. J Immunol. 2005;174(2):595–9. doi: 10.4049/jimmunol.174.2.595. [DOI] [PubMed] [Google Scholar]
- 23.de Rooij J, Zwartkruis FJ, Verheijen MH, et al. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396(6710):474–7. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 24.Kawasaki H, Springett GM, Mochizuki N, et al. A family of cAMP-binding proteins that directly activate Rap1. Science. 1998;282(5397):2275–9. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- 25.Ueno H, Shibasaki T, Iwanaga T, et al. Characterization of the gene EPAC2: structure, chromosomal localization, tissue expression, and identification of the liver-specific isoform. Genomics. 2001;78(1–2):91–8. doi: 10.1006/geno.2001.6641. [DOI] [PubMed] [Google Scholar]
- 26.Bos JL, de Rooij J, Reedquist KA. Rap1 signalling: adhering to new models. Nat Rev Mol Cell Biol. 2001;2(5):369–77. doi: 10.1038/35073073. [DOI] [PubMed] [Google Scholar]
- 27.Bos JL. Linking Rap to cell adhesion. Curr Opin Cell Biol. 2005;17(2):123–8. doi: 10.1016/j.ceb.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Caron E. Cellular functions of the Rap1 GTP-binding protein: a pattern emerges. J Cell Sci. 2003;116(Pt 3):435–40. doi: 10.1242/jcs.00238. [DOI] [PubMed] [Google Scholar]
- 29.Hattori M, Minato N. Rap1 GTPase: functions, regulation, and malignancy. J Biochem (Tokyo) 2003;134(4):479–84. doi: 10.1093/jb/mvg180. [DOI] [PubMed] [Google Scholar]
- 30.Huang SK, Wettlaufer SH, Chung J, Peters-Golden M. Prostaglandin E2 inhibits specific lung fibroblast functions via selective actions of PKA and Epac-1. Am J Respir Cell Mol Biol. 2008;39(4):482–9. doi: 10.1165/rcmb.2008-0080OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Enserink JM, Christensen AE, de Rooij J, et al. A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol. 2002;4(11):901–6. doi: 10.1038/ncb874. [DOI] [PubMed] [Google Scholar]
- 32.Schmitt JM, Stork PJ. PKA phosphorylation of Src mediates cAMP’s inhibition of cell growth via Rap1. Mol Cell. 2002;9(1):85–94. doi: 10.1016/s1097-2765(01)00432-4. [DOI] [PubMed] [Google Scholar]
- 33.Wang Z, Dillon TJ, Pokala V, et al. Rap1-mediated activation of extracellular signal-regulated kinases by cyclic AMP is dependent on the mode of Rap1 activation. Mol Cell Biol. 2006;26(6):2130–45. doi: 10.1128/MCB.26.6.2130-2145.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gotoh T, Hattori S, Nakamura S, et al. Identification of Rap1 as a target for the Crk SH3 domain-binding guanine nucleotide-releasing factor C3G. Mol Cell Biol. 1995;15(12):6746–53. doi: 10.1128/mcb.15.12.6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 36.Kapoor M, Kojima F, Qian M, Yang L, Crofford LJ. Microsomal prostaglandin E synthase-1 deficiency is associated with elevated peroxisome proliferator activated receptor gamma: Regulation by prostaglandin E2 via the PI3 kinase and AKT pathway. J Biol Chem. 2006 doi: 10.1074/jbc.M610153200. [DOI] [PubMed] [Google Scholar]
- 37.Kojima F, Naraba H, Miyamoto S, Beppu M, Aoki H, Kawai S. Membrane-associated prostaglandin E synthase-1 is upregulated by proinflammatory cytokines in chondrocytes from patients with osteoarthritis. Arthritis Res Ther. 2004;6(4):R355–65. doi: 10.1186/ar1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kojima F, Naraba H, Sasaki Y, Beppu M, Aoki H, Kawai S. Prostaglandin E2 is an enhancer of interleukin-1beta-induced expression of membrane-associated prostaglandin E synthase in rheumatoid synovial fibroblasts. Arthritis Rheum. 2003;48(10):2819–28. doi: 10.1002/art.11261. [DOI] [PubMed] [Google Scholar]
- 39.Fujita T, Meguro T, Fukuyama R, Nakamuta H, Koida M. New signaling pathway for parathyroid hormone and cyclic AMP action on extracellular-regulated kinase and cell proliferation in bone cells. Checkpoint of modulation by cyclic AMP. J Biol Chem. 2002;277(25):22191–200. doi: 10.1074/jbc.M110364200. [DOI] [PubMed] [Google Scholar]
- 40.Suzawa T, Miyaura C, Inada M, et al. The role of prostaglandin E receptor subtypes (EP1, EP2, EP3, and EP4) in bone resorption: an analysis using specific agonists for the respective EPs. Endocrinology. 2000;141(4):1554–9. doi: 10.1210/endo.141.4.7405. [DOI] [PubMed] [Google Scholar]
- 41.Kooistra MR, Corada M, Dejana E, Bos JL. Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS Lett. 2005;579(22):4966–72. doi: 10.1016/j.febslet.2005.07.080. [DOI] [PubMed] [Google Scholar]
- 42.Christensen AE, Selheim F, de Rooij J, et al. cAMP analog mapping of Epac1 and cAMP kinase. Discriminating analogs demonstrate that Epac and cAMP kinase act synergistically to promote PC-12 cell neurite extension. J Biol Chem. 2003;278(37):35394–402. doi: 10.1074/jbc.M302179200. [DOI] [PubMed] [Google Scholar]
- 43.Kooistra MR, Dube N, Bos JL. Rap1: a key regulator in cell-cell junction formation. J Cell Sci. 2007;120(Pt 1):17–22. doi: 10.1242/jcs.03306. [DOI] [PubMed] [Google Scholar]
- 44.Valencia X, Higgins JM, Kiener HP, et al. Cadherin-11 provides specific cellular adhesion between fibroblast-like synoviocytes. J Exp Med. 2004;200(12):1673–9. doi: 10.1084/jem.20041545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kiener HP, Lee DM, Agarwal SK, Brenner MB. Cadherin-11 induces rheumatoid arthritis fibroblast-like synoviocytes to form lining layers in vitro. Am J Pathol. 2006;168(5):1486–99. doi: 10.2353/ajpath.2006.050999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee DM, Kiener HP, Agarwal SK, et al. Cadherin-11 in Synovial Lining Formation and Pathology in Arthritis. Science. 2007 doi: 10.1126/science.1137306. [DOI] [PubMed] [Google Scholar]
- 47.Remans PH, Gringhuis SI, van Laar JM, et al. Rap1 signaling is required for suppression of Ras-generated reactive oxygen species and protection against oxidative stress in T lymphocytes. J Immunol. 2004;173(2):920–31. doi: 10.4049/jimmunol.173.2.920. [DOI] [PubMed] [Google Scholar]
- 48.Laxman S, Riechers A, Sadilek M, Schwede F, Beavo JA. Hydrolysis products of cAMP analogs cause transformation of Trypanosoma brucei from slender to stumpy-like forms. Proc Natl Acad Sci U S A. 2006;103(50):19194–9. doi: 10.1073/pnas.0608971103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Somekawa S, Fukuhara S, Nakaoka Y, Fujita H, Saito Y, Mochizuki N. Enhanced functional gap junction neoformation by protein kinase A-dependent and Epac-dependent signals downstream of cAMP in cardiac myocytes. Circ Res. 2005;97(7):655–62. doi: 10.1161/01.RES.0000183880.49270.f9. [DOI] [PubMed] [Google Scholar]
- 50.Inoue H, Takamori M, Shimoyama Y, Ishibashi H, Yamamoto S, Koshihara Y. Regulation by PGE2 of the production of interleukin-6, macrophage colony stimulating factor, and vascular endothelial growth factor in human synovial fibroblasts. Br J Pharmacol. 2002;136(2):287–95. doi: 10.1038/sj.bjp.0704705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoshida T, Sakamoto H, Horiuchi T, et al. Involvement of prostaglandin E(2) in interleukin-1alpha-induced parathyroid hormone-related peptide production in synovial fibroblasts of patients with rheumatoid arthritis. J Clin Endocrinol Metab. 2001;86(7):3272–8. doi: 10.1210/jcem.86.7.7687. [DOI] [PubMed] [Google Scholar]
- 52.McCoy JM, Wicks JR, Audoly LP. The role of prostaglandin E2 receptors in the pathogenesis of rheumatoid arthritis. J Clin Invest. 2002;110(5):651–8. doi: 10.1172/JCI15528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Honda T, Segi-Nishida E, Miyachi Y, Narumiya S. Prostacyclin-IP signaling and prostaglandin E2-EP2/EP4 signaling both mediate joint inflammation in mouse collagen-induced arthritis. J Exp Med. 2006;203(2):325–35. doi: 10.1084/jem.20051310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81(1):53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 55.Gadher SJ, Woolley DE. Comparative studies of adherent rheumatoid synovial cells in primary culture: characterisation of the dendritic (stellate) cell. Rheumatol Int. 1987;7(1):13–22. doi: 10.1007/BF00267337. [DOI] [PubMed] [Google Scholar]
- 56.Goto M, Sasano M, Yamanaka H, et al. Spontaneous production of an interleukin 1-like factor by cloned rheumatoid synovial cells in long-term culture. J Clin Invest. 1987;80(3):786–96. doi: 10.1172/JCI113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sandulache VC, Parekh A, Li-Korotky HS, Dohar JE, Hebda PA. Prostaglandin E2 differentially modulates human fetal and adult dermal fibroblast migration and contraction: implication for wound healing. Wound Repair Regen. 2006;14(5):633–43. doi: 10.1111/j.1743-6109.2006.00156.x. [DOI] [PubMed] [Google Scholar]
- 58.Sandulache VC, Parekh A, Li-Korotky H, Dohar JE, Hebda PA. Prostaglandin E2 inhibition of keloid fibroblast migration, contraction, and transforming growth factor (TGF)-beta1-induced collagen synthesis. Wound Repair Regen. 2007;15(1):122–33. doi: 10.1111/j.1524-475X.2006.00193.x. [DOI] [PubMed] [Google Scholar]
- 59.Kohyama T, Ertl RF, Valenti V, et al. Prostaglandin E(2) inhibits fibroblast chemotaxis. Am J Physiol Lung Cell Mol Physiol. 2001;281(5):L1257–63. doi: 10.1152/ajplung.2001.281.5.L1257. [DOI] [PubMed] [Google Scholar]
- 60.Nicola C, Lala PK, Chakraborty C. Prostaglandin E2-mediated migration of human trophoblast requires RAC1 and CDC42. Biol Reprod. 2008;78(6):976–82. doi: 10.1095/biolreprod.107.065433. [DOI] [PubMed] [Google Scholar]
- 61.Nicola C, Chirpac A, Lala PK, Chakraborty C. Roles of Rho guanosine 5′-triphosphatase A, Rho kinases, and extracellular signal regulated kinase (1/2) in prostaglandin E2-mediated migration of first-trimester human extravillous trophoblast. Endocrinology. 2008;149(3):1243–51. doi: 10.1210/en.2007-1136. [DOI] [PubMed] [Google Scholar]