Abstract
Background
Few risk factors for childhood cancer are well-established. We investigated whether advancing parental age increases childhood cancer risk.
Methods
We assessed the relationship between parental age and childhood cancer in a case-control study using pooled population-based data. Our pooling was based on linked cancer and birth registry records from New York, Washington, Minnesota, Texas, and California. Subjects included 17,672 cancer cases diagnosed at ages 0–14 years during 1980–2004 and 57,966 controls born during 1970–2004. Persons with Down syndrome were excluded. Odds ratios and 95% confidence intervals were calculated by logistic regression for the association between parental age and childhood cancer after adjustment for sex, birth weight, gestational age, birth order, plurality, maternal race, birth year, and state.
Results
Positive linear trends per 5-year maternal age increase were –observed for childhood cancers overall (odds ratio = 1.08 [95% confidence interval = 1.06–1.10]) and 7 of the 10 most frequent diagnostic groups: leukemia (1.08 [1.05–1.11]), lymphoma (1.06 [1.01–1.12]), central nervous system tumors (1.07 [1.03–1.10]), neuroblastoma (1.09 [1.04–1.15]), Wilms’ tumor (1.16 [1.09–1.22]), bone tumors (1.10 [ 1.00–1.20]), and soft tissue sarcomas (1.10 [1.04–1.17]). No maternal age effect was noted for retinoblastoma, germ cell tumors, or hepatoblastoma. Paternal age was not independently associated with most childhood cancers after adjustment for maternal age.
Conclusions
Our results suggest that older maternal age increases risk for most common childhood cancers. Investigation into possible mechanisms for this association is warranted.
Childhood cancer affects approximately one in 435 children under age 15 years.1 Established risk factors include specific genetic syndromes, prenatal exposure to ionizing radiation, demographic characteristics such as sex and race,2 and high birth weight,3–6 although even collectively these factors account for only a small fraction of cases.
Both advanced maternal age and advanced paternal age have been associated with a number of congenital syndromes, including several that predispose towards cancer.7 Since many childhood cancers may originate during or soon after pregnancy,8, 9 their relationship with parental age has been examined in many studies, with inconsistent results.10 This may be due to the small sample size of most studies, with low power to detect modest effects. Two large studies conducted in Great Britain (10,162 cases) and Sweden (7,844 cases) have provided preliminary support for an increased risk of some childhood cancers, especially leukemia, with older parental age, but their results are inconsistent with respect to an association for other cancer types.11, 12
Our objective was to determine whether advancing parental age is associated with an increased risk of childhood cancer in offspring. To our knowledge, this study is the largest to date to examine this.
METHODS
Study Population
Institutional Review Board (IRB) approvals were obtained from each state’s health department and participating institutions prior to conducting the study. Childhood cancer cases were identified in the population-based cancer incidence registries of California, Minnesota, New York (excluding New York City), Texas, and Washington. Details of each state’s eligibility criteria, matching factors, and years of inclusion have previously been reported4, 13–16 and are summarized in Table 1. Briefly, childhood cancer cases in each state’s cancer registry were linked to their respective birth certificates using sequential deterministic or probabilistic record linkage.17 These cancer registries are considered to be of high quality, with recent estimates indicating case completeness at >95%.18 Cases were classified according to the International Classification of Childhood Cancer 3rd edition (ICCC-3).19 We also obtained age at diagnosis (in months) and laterality (of Wilms’ tumor and retinoblastoma) from cancer registry records. Controls were randomly selected from each state’s birth registry in ratios to cases that varied from 1:1 to 10:1. Frequency matching was used in four states and individual matching in one (CA).
Table 1.
Characteristics of state datasets.
| State | Age at diagnosis | Years of diagnosis | Years of birth | No. cases | No. controls | Matching factors |
|---|---|---|---|---|---|---|
| California | 28 days - 4 years | 1988–1997 | 1983–1997 | 4,177 | 8,730 | Birth Year, Sex |
| Minnesota | 28 days - 14 years | 1988–2004 | 1976–2004 | 2,170 | 8,735 | Birth Year |
| New York | 28 days - 14 years | 1985–2001 | 1970–2001 | 4,357 | 12,041 | Birth Year |
| Texas | 28 days - 14 years | 1990–1998 | 1975–1998 | 4,647 | 4,732 | Birth Year, Sex |
| Washington | 28 days - 14 years | 1980–2004 | 1980–2004 | 2,321 | 23,728 | Birth Year |
We applied additional criteria prior to pooling to ensure uniformity of data. Cases were permitted to be selected as controls in Minnesota and New York; these subjects were excluded from the control group in this analysis. Although the California registry includes cases diagnosed at <28 days of age, we subsequently excluded them for consistency with other states. Subjects with reported Down syndrome (n=100) were excluded, although Down syndrome was not recorded in Texas before 1984 and Washington before 1989. The final dataset included 17,672 cases and 57,966 controls.
Variable specification
Birth records included parental age and demographic characteristics, as well as information about the infant. We used the following primary variables in this analysis: parental age, birth weight, gestational age, plurality, sex, birth order, birth year, and maternal race. Secondary analyses also included maternal education, which was collected by all states only after 1991. Four states provided gestational age calculated from both last menstrual period (LMP) and a clinical estimate, while California provided only the age based on LMP. We developed a combined gestational age variable that gave preference to the calculated (LMP) estimate when available and used the clinical estimate otherwise. Gestational lengths of <20 and >45 weeks and birth weights of <350 grams were considered implausible and treated as missing. Maternal race was classified by hospital personnel filling out birth certificates according to schema specific to each state.
Statistical analyses
Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated by unconditional logistic regression (SAS version 9.1 (Cary, NC)); individual matching in the California dataset was therefore broken. We modeled associations using continuous parental age terms with results reported as the OR per 5-year parental age increase. Dose-response between parental age and the risk of childhood cancer was evaluated using parental age categories of <20, 20–24, 25–29, 30–34, 35–39, and ≥40 years; the two youngest and oldest age categories were collapsed in some analyses where case numbers were small. Each parent’s age was examined independently and with adjustment for the other.
Covariates were chosen for inclusion in regression models based on their prior observed association with childhood cancers2; categorization of covariates is as shown in Table 2. Tabular results are given for ICCC-3 diagnostic groups as well as component diagnoses19 that had at least 200 cases, with the exception of “other soft tissue sarcomas” and “other intracranial tumors”. Results for sympathetic nervous system, renal, and hepatic tumors were not presented separately from those for neuroblastoma, Wilms’ tumor, and hepatoblastoma because each of these comprised a large majority of their respective ICCC-3 categories. Analyses were also conducted by laterality (bilateral vs. unilateral) for Wilms’ tumor and retinoblastoma, as well as by diagnosis age group for childhood cancers overall, leukemia, CNS tumors, and combined embryonal tumors (intracranial tumors, neuroblastoma, retinoblastoma, Wilms’ tumor, hepatoblastoma, rhabdomyosarcoma). Controls for each age group analysis were limited to individuals who were at risk for cancer in that age group. Evidence for heterogeneity in risk estimates by sex of child and state of birth were examined in stratified analyses. Finally, sensitivity analyses were conducted to gauge the influence of data limitations on results; these included adjustment for maternal education during the years available, consideration of missed cases of cancer in the control population, and examination of missing covariate data.
Table 2.
Characteristics of cases and controls.
| Characteristic | Cases | Controls |
|---|---|---|
| Maternal age (years) | ||
| Distribution; no. (%) | ||
| <20 | 1821 (10) | 6220 (11) |
| 20–24 | 4448 (25) | 15,268 (26) |
| 25–29 | 5682 (32) | 18,427 (32) |
| 30–34 | 3908 (22) | 12,521 (22) |
| 35–39 | 1537 (9) | 4670 (8) |
| ≥40 | 270 (2) | 270 (1) |
| Mean (SD) | 27 (6) | 27 (6) |
| Missing; no. | 6 | 21 |
| Paternal age (years) | ||
| Distribution; no. (%) | ||
| <20 | 495 (3) | 1648 (3) |
| 20–24 | 2720 (17) | 9408 (18) |
| 25–29 | 4856 (31) | 15879 (31) |
| 30–34 | 4400 (28) | 14163 (27) |
| 35–39 | 2260 (14) | 7142 (14) |
| ≥40 | 1117 (7) | 3429 (7) |
| Mean (SD) | 30 (6) | 30 (6) |
| Missing; no. | 1824 | 6297 |
| Maternal race | ||
| White | 15,243 (88) | 48,568 (85) |
| Black | 1147 (7) | 3478 (6) |
| Asian | 694 (4) | 2590 (5) |
| Other | 284 (2) | 2448 (4) |
| Missing | 304 | 882 |
| Mother's education at birth (years)a | ||
| Distribution; no. (%) | ||
| <12 | 2482 (20) | 7246 (19) |
| 12 | 4346 (35) | 13,586 (36) |
| 13–16 years | 4381 (36) | 13,815 (37) |
| 17+ | 1053 (9) | 3017 (8) |
| Mean (SD) | 12.7 (3) | 12.7 (3) |
| Missingb; no. | 5410 | 20,302 |
| Male sex | 9728 (55) | 30,573 (53) |
| Missing; no. | 2 | 10 |
| Birth weight (grams) | ||
| Distribution; no. (%) | ||
| <2500 | 998 (6) | 3153 (6) |
| 2500–4000 | 14,057 (81) | 47,105 (82) |
| >4000 | 2379 (14) | 7333 (13) |
| Mean (SD) | 3420.7 (593) | 3408.8 (573) |
| Missing; no. | 238 | 375 |
| Gestational age (weeks) | ||
| Distribution; no. (%) | ||
| <37 | 9 | 8 |
| 37–42 | 86 | 86 |
| >42 | 6 | 6 |
| Mean weeks (SD) | 39.4 (2.5) | 39.5 (2.4) |
| Missing | 704 | 2704 |
| Birth order | ||
| Distribution; no. (%) | ||
| 1 | 7186 (41) | 23,131 (41) |
| 2 | 5718 (33) | 18,383 (33) |
| 3 | 2745 (16) | 9092 (16) |
| 4 | 1076 (6) | 3454 (6) |
| ≥5 | 675 (4) | 2373 (4) |
| Mean order (SD) | 2.0 (1.2) | 2.0 (1.3) |
| Missing | 272 | 1533 |
| Plurality | ||
| Singleton | 17311 (98) | 56,626 (98) |
| Multiple | 357 (2) | 1307 (2) |
| Missing | 4 | 33 |
| Birth year quartile | ||
| Distribution; no. (%) | ||
| 1970–1985 | 4737 (27) | 17,206 (30) |
| 1986–1989 | 4635 (26) | 12,815 (22) |
| 1990–1993 | 4767 (27) | 13,588 (23) |
| 1994–2004 | 3533 (20) | 14,360 (25) |
| Mean (SD) | 1989 (6) | 1989 (6) |
| Missing | 0 | 0 |
Collected in all states beginning in 1992.
Mean excludes data from NY during 1988–1990 where education was collected as a categorical variable only
RESULTS
Cases were more likely than controls to be male and to have mothers who were white (Table 2). The mean birth weight of cases was higher than that of controls, with no appreciable difference in the mean birth order, years of maternal education, or gestational age. The percentage of subjects born to singleton vs. multiple births was similar for cases and controls.
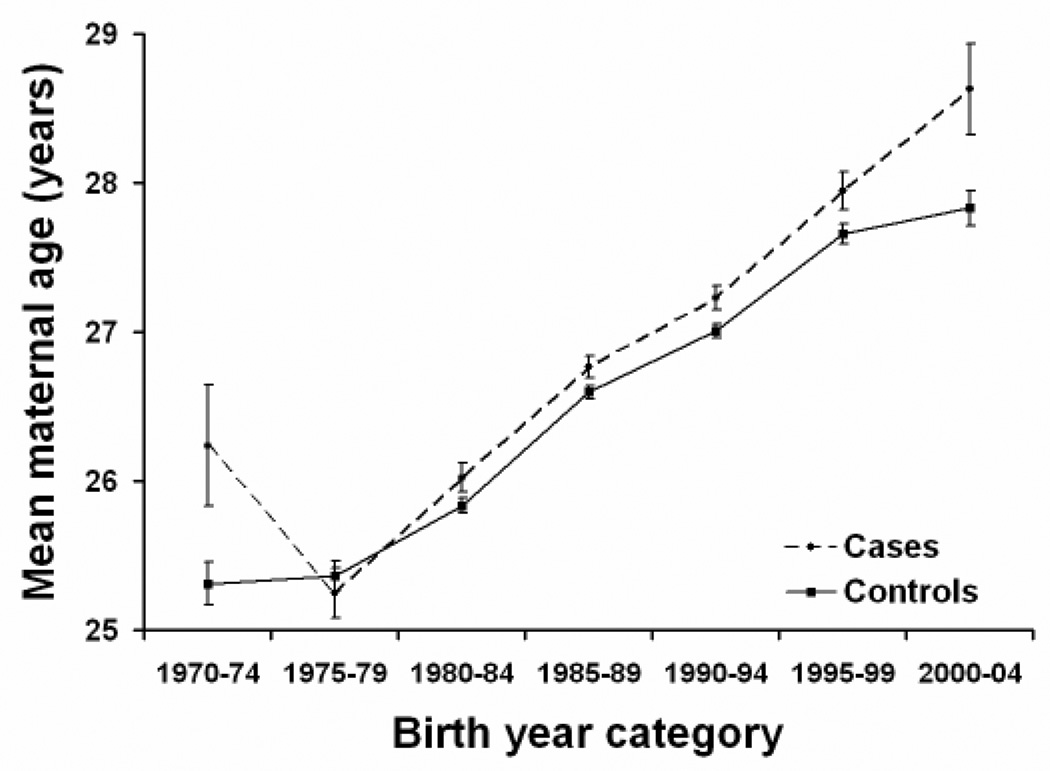
The mean age of case mothers at birth was slightly older (26.9 [SD = 5.7]) than control mothers (26.7 [5.7]) in the pooled dataset. The same pattern was also observed in each state’s dataset. Although the overall mean maternal age varied only slightly between cases and controls, it was consistently higher for cases than controls for all birth-year categories beginning with the period 1980–84 (Figure 1). Similar patterns were observed with respect to paternal age, with a slightly higher mean age at birth for fathers of cases (29.9 [6.3]) than controls (29.7 [6.3]) that was consistently higher across birth-year categories starting with 1980–1984. Pearson’s correlation coefficient between maternal and paternal age was 0.74.
Figure 1.
Mean maternal age for cases and controls by birth year. Case and control numbers, respectively, for each birth year category were as follows: 1970–1974, 177 and 1348; 1975–1979, 869 and 2726; 1980–1984, 2836 and 10,517; 1985–1989, 5487 and 15,417; 1990–1994, 5644 and 16,383; 1995–1999, 2255 and 8921; 2000–2004, 398 and 2633.
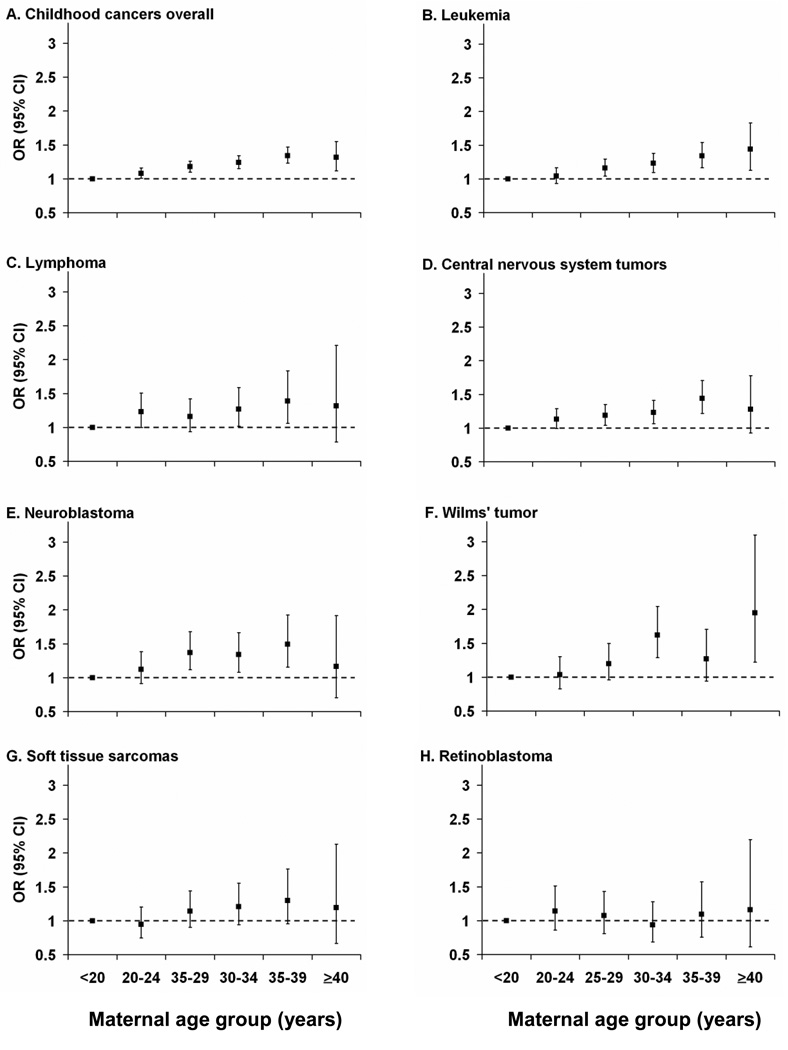
After adjustment for covariates, maternal age was associated with a linear increase in the risk of childhood cancers overall of 8% per 5-year increment (OR=1.08 [95% CI = 1.06–1.10]) (Table 3). Positive linear trends were observed for 7 of the 10 most frequent childhood cancer groups: leukemia (OR=1.08 per five-year increase [95% CI = 1.05–1.11]), lymphoma (1.06 [1.01–1.12]), central nervous system tumors (1.07 [1.00–1.10]), neuroblastoma (1.09 [1.04 – 1.15]), Wilms’ tumor (1.16 [1.09–1.22]), bone tumors (1.10 [1.00–1.20]), and soft tissue sarcomas (1.10 [1.04–1.17]). We did not find evidence for an increased risk of retinoblastoma, hepatoblastoma, or germ cell tumors with advancing maternal age. In models that adjusted for paternal age and other covariates, maternal age remained associated with childhood cancers overall, leukemia, central nervous system tumors, neuroblastoma, Wilms’ tumor, and soft tissue sarcomas (Table 3). Results from models that included maternal age as a categorical variable were generally consistent with a linear association for the most frequent childhood cancer groups (Figure 2; eTable 1, http://links.lww.com). However, OR’s were imprecisely estimated for the oldest maternal age category.
Table 3.
Risk of childhood cancer in relation to a 5-year increase in maternal age at the time of the child’s birth.
| Maternal agea | Maternal age adjusted for paternal agea | |||||
|---|---|---|---|---|---|---|
| No. casesb | OR | (95% CI) | No. casesb | OR | (95% CI) | |
| All cancers | 16,556 | 1.08 | (1.06–1.10) | 14867 | 1.07 | (1.04–1.10) |
| Leukemias | 5561 | 1.08 | (1.05–1.11) | 5053 | 1.06 | (1.01–1.10) |
| Lymphoid leukemia | 4476 | 1.08 | (1.05–1.11) | 4083 | 1.06 | (1.01–1.11) |
| Acute myeloid leukemia | 804 | 1.08 | (1.01–1.16) | 722 | 1.06 | (0.96–1.17) |
| Lymphoma | 1396 | 1.06 | (1.01–1.12) | 1265 | 1.05 | (0.97–1.14) |
| Non-Hodgkin’s lymphoma | 549 | 1.10 | (1.02–1.20) | 500 | 1.07 | (0.94–1.21) |
| Hodgkin’s lymphoma | 431 | 0.99 | (0.90–1.09) | 390 | 0.97 | (0.83–1.12) |
| Burkitt’s lymphoma | 220 | 1.05 | (0.92–1.20) | 202 | 1.02 | (0.84–1.24) |
| CNS tumors | 3561 | 1.07 | (1.03–1.11) | 3196 | 1.08 | (1.03–1.14) |
| Astrocytoma | 1559 | 1.09 | (1.03–1.14) | 1394 | 1.13 | (1.05–1.22) |
| Other gliomas | 467 | 1.08 | (0.98–1.18) | 422 | 1.00 | (0.88–1.14) |
| Ependymoma | 370 | 1.07 | (0.97–1.18) | 339 | 1.18 | (1.02–1.38) |
| Intracranial embryonal tumors | 858 | 1.01 | (0.95–1.08) | 777 | 1.01 | (0.92–1.12) |
| Neuroblastoma | 1423 | 1.09 | (1.04–1.15) | 1258 | 1.10 | (1.02–1.19) |
| Retinoblastoma | 660 | 0.97 | (0.91–1.05) | 577 | 0.93 | (0.83–1.04) |
| Wilms’ tumor | 1129 | 1.16 | (1.09–1.22) | 1008 | 1.25 | (1.14–1.36) |
| Hepatoblastoma | 262 | 0.98 | (0.88–1.10) | 227 | 1.01 | (0.85–1.20) |
| Bone tumors | 511 | 1.10 | (1.00–1.20) | 467 | 1.04 | (0.91–1.18) |
| Ewing’s sarcoma | 202 | 1.18 | (1.02–1.35) | 190 | 1.04 | (0.85–1.26) |
| Osteosarcomac | 251 | 0.99 | (0.87–1.13) | 226 | 0.97 | (0.79–1.78) |
| Soft tissue sarcomas | 1000 | 1.10 | (1.04–1.17) | 882 | 1.10 | (1.00–1.20) |
| Rhabdomyosarcoma | 556 | 1.15 | (1.06–1.25) | 484 | 1.19 | (1.05–1.34) |
| Germ cell tumors | 527 | 0.99 | (0.91–1.08) | 469 | 0.96 | (0.84–1.08) |
| Gonadal germ cell tumors | 262 | 0.94 | (0.84–1.06) | 231 | 1.00 | (0.84–1.20) |
All models were adjusted for maternal race (Asian, Black, Other, White), sex, birth weight (<2500, 2500–4000, >4000 grams), gestational age (<37, 37–42, >42 weeks), birth order (1, 2, 3, 4, >5), birth year category (1970–1985, 1986–1989, 1990–1993, 1994–2004), plurality (single, >2), and state.
The numbers of controls included in the maternal age model and maternal age adjusted for paternal age model for all cancer diagnoses except osteosarcoma were 53,728 and 47,994, respectively
California was excluded because there were no cases, bringing the total number of controls to 45,448 and 40,452 for the maternal age model and maternal age adjusted for paternal age model, respectively.
Figure 2.
Association of maternal age with childhood cancers, adjusted for factors in Table 3 footnote.
Paternal age was associated with a linear increase in the risk of childhood cancers overall (OR=1.05 [CI = 1.03–1.07]) and 5 of the 10 most frequent childhood cancer diagnostic groups (Table 4). However, when adjusted for maternal age, these results were strongly attenuated for childhood cancers overall (1.01 [0.99–1.03]) and for all diagnostic groups. ORs for paternal age modeled as a categorical variable were generally consistent with results of models that included the continuous age variable (eTable 2, http://links.lww.com).
Table 4.
Risk of childhood cancer in relation to a 5-year increase in paternal age at the time of the child’s birth.
| Paternal agea | Paternal age adjusted for maternal agea | |||||
|---|---|---|---|---|---|---|
| No. casesb | OR | (95% CI) | No. casesb | OR | (95% CI) | |
| All cancers | 14,868 | 1.05 | (1.03–1.07) | 14,867 | 1.01 | (0.99–1.03) |
| Leukemias | 5054 | 1.07 | (1.04–1.09) | 5053 | 1.03 | (1.00–1.07) |
| Lymphoid leukemia | 4083 | 1.06 | (1.03–1.09) | 4083 | 1.03 | (0.99–1.07) |
| Acute myeloid leukemia | 722 | 1.08 | (1.01–1.14) | 722 | 1.04 | (0.96–1.13) |
| Lymphoma | 1265 | 1.04 | (0.99–1.09) | 1265 | 1.01 | (0.94–1.08) |
| Non-Hodgkin’s lymphoma | 500 | 1.07 | (1.00–1.16) | 500 | 1.04 | (0.93–1.15) |
| Hodgkin’s lymphoma | 390 | 1.01 | (0.92–1.10) | 390 | 1.03 | (0.91–1.16) |
| Burkitt’s lymphoma | 202 | 1.06 | (0.94–1.19) | 202 | 1.05 | (0.89–1.23) |
| CNS tumors | 3196 | 1.04 | (1.01–1.07) | 3196 | 0.99 | (0.95–1.03) |
| Astrocytoma | 1394 | 1.04 | (0.99–1.09) | 1394 | 0.97 | (0.91–1.03) |
| Other gliomas | 422 | 1.09 | (1.01–1.18) | 422 | 1.09 | (0.98–1.22) |
| Ependymoma | 339 | 0.98 | (0.90–1.08) | 339 | 0.89 | (0.77–1.01) |
| Intracranial embryonal tumors | 777 | 1.01 | (0.95–1.07) | 777 | 1.00 | (0.92–1.09) |
| Neuroblastoma | 1258 | 1.06 | (1.01–1.11) | 1258 | 1.00 | (0.93–1.07) |
| Retinoblastoma | 577 | 0.96 | (0.90–1.03) | 577 | 1.01 | (0.92–1.11) |
| Wilms’ tumor | 1008 | 1.04 | (0.99–1.10) | 1008 | 0.91 | (0.85–0.98) |
| Hepatoblastoma | 227 | 1.06 | (0.95–1.17) | 227 | 1.05 | (0.91–1.22) |
| Bone tumors | 467 | 1.08 | (1.00–1.17) | 467 | 1.06 | (0.95–1.18) |
| Ewing’s sarcoma | 190 | 1.19 | (1.06–1.34) | 190 | 1.17 | (1.00–1.37) |
| Osteosarcomac | 226 | 0.95 | (0.84–1.07) | 226 | 0.97 | (0.82–1.14) |
| Soft tissue sarcomas | 882 | 1.08 | (1.02–1.14) | 882 | 1.03 | (0.95–1.11) |
| Rhabdomyosarcoma | 484 | 1.10 | (1.03–1.19) | 484 | 1.00 | (0.90–1.11) |
| Germ cell tumors | 469 | 1.01 | (0.94–1.09) | 469 | 1.04 | (0.94–1.15) |
| Gonadal germ cell tumors | 231 | 0.93 | (0.83–1.04) | 231 | 0.93 | (0.79–1.08) |
Adjusted for maternal race (Asian, Black, Other, White), sex, birth weight (<2500, 2500–4000, >4000 grams), gestational age (<37, 37–42, >42 weeks), birth order (1, 2, 3, 4, >5), birth year category (1970–1985, 1986–1989, 1990–1993, 1994–2004), plurality (single, >2), and state.
The numbers of controls included in the paternal age model and paternal age adjusted for maternal age model for all cancer diagnoses except osteosarcoma were 48,000 and 47,994, respectively
California was excluded because there were no cases; the total number of controls was 40,458 and 40,452, for the paternal age model and paternal age adjusted for maternal age model respectively.
We examined whether the risk of Wilms’ tumor and retinoblastoma varied by laterality, given that some bilateral tumors are known to be the result of de novo germline mutations.20 In linear models, older maternal age was more strongly associated with bilateral than with unilateral Wilms’ tumor, although there were few bilateral cases (n = 87). Neither type of retinoblastoma exhibited an association with maternal age. Adjustment for paternal age eliminated the linear association of maternal age with bilateral Wilms’ tumor, although risk estimates remained increased for the oldest age categories (eTable 3, http://links.lww.com). Paternal age was associated with an increased risk of bilateral Wilms’ tumor and was not associated with unilateral Wilms’ tumor or retinoblastoma in models including the linear paternal age term. All associations varied with adjustment for maternal age (eTable 4, http://links.lww.com).
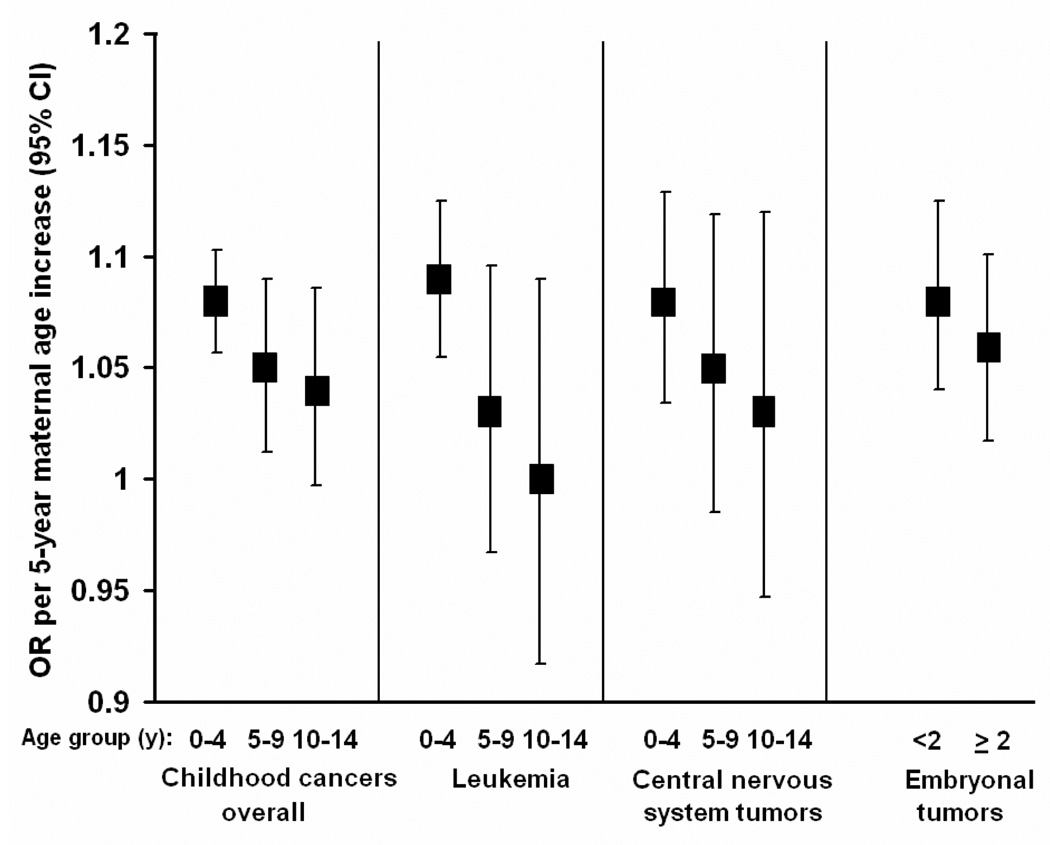
We analyzed risks by diagnosis age group for childhood cancers overall, leukemia, CNS and combined embryonal tumors (Figure 3, eTable 5, http://links.lww.com). For all cancers combined, the risks associated with maternal age declined with increasing age of diagnosis, with risks per 5-year increase in maternal age for the 0–4, 5–9, and 10–14 year age groups of 1.08 (95% CI = 1.06–1.11), 1.05 (1.01–1.09), and 1.04 (0.99–1.08), respectively; similar patterns were apparent for leukemias and CNS tumors. For embryonal tumors, which are most frequently diagnosed before 5 years of age,2 risks were slightly greater in cases diagnosed before 2 years of age (1.08 [1.04–1.13]) than among those diagnosed later (1.06 [1.02–1.10]). Results from models that adjusted for paternal age were similar except for embryonal tumors, for which there was no difference in risk by diagnosis age group (eTable 5, http://links.lww.com). Results for paternal age were less consistent than those for maternal age. Children in the 0–4 and 5–9 year age groups had a greater risk than those in the 10–14 year age group for all cancers, leukemia, and CNS tumors. Results for embryonal tumors for the younger and older age group were similar. No associations with father's age remained after maternal age adjustment, with the exception of an increased risk of 1.08 for leukemia in the 5–9 year old age group (eTable 5, http://links.lww.com).
Figure 3.
Association of maternal age with childhood cancers by age at diagnosis, adjusted for factors in Table 3 footnote. Case and control numbers for each diagnosis age group are presented in eTable 5 (http://links.lww.com).
Analyses were also conducted to determine whether the risk of childhood cancer associated with maternal age varied by sex of the child or state of birth. No sex differences in risk were observed for childhood cancers overall, or for individual diagnostic groups with maternal age modeled as a linear variable. Results for childhood cancers overall were generally consistent among the states, with estimated risks of 1.02 (MN), 1.05 (TX), 1.08 (NY), and 1.09 (CA and WA) for each 5-year increase in maternal age (data not shown).
We evaluated the potential effect of missing data. Information on maternal age was available for virtually all subjects (missing in 27), although paternal age was missing for 10% of cases (n=1,824) and 11% of controls (n = 6,297). There were 5,327 subjects excluded due to missing covariate data in maternal age models that did not include paternal age. The results from the fully-adjusted models of maternal age were unchanged when dropping covariates (one at a time) with missing values for more than 1% of subjects (birth weight, gestational age, birth order, and maternal race)(data not shown).
Discussion
The risk of childhood cancer increased slightly with advancing maternal age, especially in young children. There was also an association of paternal age with some childhood cancers, although this risk was eliminated by adjustment for maternal age. Previous large studies, with case numbers ranging from 2,437 21 to 10,16211 have detected associations of parents’ age with leukemia,11, 12 retinoblastoma,11, 12 CNS tumors,12 Wilms’ tumors,12, 21 and bone tumors.11 However, these studies are not entirely consistent with respect to the impact of maternal versus paternal age, and with the particular cancers associated with parents' age. The present study, which includes a substantially larger number of cases, confirms previous findings of a parental age effect for some childhood cancers and extends them to additional cancers. Advancing age, in particular the mother's, appears to be a risk factor across many of the common childhood cancer types.
The independent associations of maternal and paternal age on childhood cancer risk are difficult to separate, even in this large dataset, due to their strong correlation. To the extent that these results reflect an effect of advanced paternal age, the most likely mechanism would be increases in de novo germline mutations.22 Previous data have suggested a parental age effect that is likely paternal for sporadic, heritable retinoblastoma, which is mostly bilateral,23, 24 since mutations have been shown to originate predominantly from the paternal germline.22 Although the small numbers of bilateral cases in our dataset preclude firm conclusions, there appeared to be stronger linear associations of paternal age for bilateral than for unilateral retinoblastoma and Wilms’ tumor.
The mechanism through which advanced maternal age could affect childhood cancer risk is less likely to involve germline mutations. These mutations occur less frequently in oocytes compared with sperm, (presumably due to the fact that oocytes undergo far fewer cell divisions during gametogenesis).7 Other mechanisms might include differential expression of genes in cell cycle control, and DNA damage response and repair pathways in oocytes of older versus younger mothers, which have been described in recent studies of humans25 and mice.26, 27 Age-related decreases in oocyte gene expression could be due to promoter DNA methylation, which can lead to transcriptional silencing.28 We speculate that a possible mechanism for an increased risk of cancer in the offspring of older mothers could be age-related increases in de novo epimutations in oocyte genes that could be transmitted to offspring. Transgenerational inheritance of epimutations has recently been described for Lynch syndrome with transmission of an epimutation that silenced the DNA mismatch repair gene MLH1 from mother to son.29 Cancers resulting from inherited mutations (and presumably epimutations) frequently occur at a younger age than sporadic cancers. Thus, a mechanism of inherited mutation or epimutation is consistent with our finding that the maternal age effect was strongest among children diagnosed with cancer at the earliest ages.
Other mechanisms may also be relevant. Use of assisted reproductive technology increases with maternal age30 and has been considered as a possible cause of cancer in children conceived in this way.31–33 It is also possible that advancing maternal age could be a marker for other factors, such as age-related changes in hormonal levels during pregnancy,34 that could increase cancer risk in the offspring.
Mean maternal age increased over the study period in our data (Figure 1). The proportion of infants born to mothers 30 years or older in the United States rose from approximately 18% in 1970 to 37% in 2005,35, 36 suggesting that maternal age may be increasingly important as a risk factor for childhood cancer on a population level. Data from the United States Surveillance, Epidemiology, and End Results program show a modest increase in the rate of childhood cancer from the 1970s to the 1990s of about 1% per year, 2 with an apparent continuation in the decade from 1994–2004.37 Thus the secular trend in maternal age is generally consistent with the trend in incidence of childhood cancer, although such ecologic data are weak evidence of causation.
Strengths of our study include the use of high quality registry data and ascertainment of control subjects through random sampling of population-based birth registries. The large size of the study also provides statistical power to detect modest associations for most cancers. However, the fact that associations with maternal age were found only for the most common diagnoses may indicate Type II error (i.e. false negatives) in the findings regarding the least common cancers. That results of prior studies,10 even those with substantial numbers of subjects,11, 12, 21 have been inconsistent is perhaps not surprising, given the small magnitudes of the associations detected. In addition, inconsistency among prior studies could also be influenced by selection bias in case-control studies that have collected information through parental interview.10 While active participation may be related to parental age, potentially biasing results, registry-based studies are not subject to this flaw.
Differences in the results of our study and the three prior large studies11, 12, 21 may also be explained by methodologic differences in the inclusion criteria, the birth years examined, and the extent of control for covariates. For example, a large British study that included subjects born 1968 to1986 reported parental age effects for acute lymphoblastic leukemia, retinoblastoma, and bone tumors but not for other childhood tumors.11 The mean age of parents included in this study was younger than that in our study, which would reduce power to detect effects of advancing parental age. The birth cohort in a relatively large Swedish study12 (1961–2004) was similar to ours, but with fewer than half the number of cases; it is also worth noting that parental age effects observed in the Swedish study were limited to those diagnosed at 0–4 years of age, which is consistent with the stronger maternal age effect for younger children observed in our study.
To the extent we were able to examine bias in our results, it is likely to be small. Disease status may have been misclassified among controls who were sampled from birth years prior to the inception of their respective cancer registries or who moved out of state. Under the extreme assumption that all controls were lost to follow-up, we would expect about 125 cases of cancer to arise from controls, based on U.S. surveillance data.1 Relative to the total number of cases, this small number is unlikely to have biased our results to any measurable degree. When we limited the dataset to subjects with birth years coextensive with registry operation, associations were similar to those in the main analysis (not shown). Maternal education, a marker for socioeconomic status, could confound the association between maternal age and childhood cancer because SES has been reported to be associated with both variables.10 However, adjustment for maternal education (for the years it was available in all states) did not markedly change the results for any childhood cancer diagnoses. Finally, analyses addressing the influence of subject exclusions due to missing data did not indicate any substantial bias in results.
By relying on birth certificate data, some children with Down syndrome were not identified, because recording of this at birth is known to be incomplete.38 As Down syndrome predisposes mainly to leukemia, however, residual confounding would not have affected other cancer types.39 The associations observed could have also resulted from confounding by covariates we did not collect. However, exposure to putative risk factors for childhood cancer would not be expected to increase with uniformly advancing maternal age. While measurement error in birth records is a concern, most variables used in our analysis are considered accurate, with one large study estimating agreement regarding maternal age between birth records and maternal reports of more than 95%.40 Moreover, any measurement error would be nondifferential with respect to disease, which will tend to produce ORs that are underestimates.
In summary, in this large population-based study, we observed that advancing maternal age moderately increased the risk of the most frequent childhood cancers. Despite the relatively greater risk of cancer among children born to older mothers, it should be noted that the absolute risk is low for all parental ages at birth. Investigation into the mechanisms by which maternal age increases the risk of childhood cancer is warranted.
Supplementary Material
Acknowledgments
We appreciate the programming assistance of Eric Elkin, Susan Hurley, John Soler, and Bill O’Brien. We additionally thank the Washington State Department of Health, the Minnesota Cancer Surveillance System, and other collaborating institutions for allowing data access.
Funding: Children’s Cancer Research Fund, Minneapolis, MN; National Cancer Institute (N01-CN-05230 to WA, R01CA717450 to CA, R01CA92670 to TX); Fred Hutchinson Cancer Research Center; Centers for Disease Control and Prevention’s National Program of Cancer Registries by cooperative agreement (U58DP000783-01 to NY).
Footnotes
e Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com).
References
- 1.Probability of developing or dying of cancer software, Version 6.1.0. Statistical Research and Applications Branch. [Accessed September 23, 2008];National Cancer Institute. 2006 http://srab.cancer.gov/devcan.
- 2.Ries LAG, Smith MA, Gurney JG, Linet M, Young JL, Bunin GR. Cancer incidence and survival among children and adolescents: United States SEER program 1975–1995. 1999 NIH Pub. No. 99–4559. [Google Scholar]
- 3.Hjalgrim LL, Westergaard T, Rostgaard K, Schmiegelow K, Melbye M, Hjalgrim H, Engels EA. Birth weight as a risk factor for childhood leukemia: a meta-analysis of 18 epidemiologic studies. Am. J. Epidemiol. 2003;158:724–735. doi: 10.1093/aje/kwg210. [DOI] [PubMed] [Google Scholar]
- 4.Puumala SE, Soler JT, Johnson KJ, Spector LG. Birth characteristics and Wilms’ tumor in Minnesota. Int. J. Cancer. 2008;122:1368–1373. doi: 10.1002/ijc.23275. [DOI] [PubMed] [Google Scholar]
- 5.Yeazel MW, Ross JA, Buckley JD, Woods WG, Ruccione K, Robison LL. High birth weight and risk of specific childhood cancers: a report from the Children's Cancer Group. J Pediatr. 1997;131:671–677. doi: 10.1016/s0022-3476(97)70091-x. [DOI] [PubMed] [Google Scholar]
- 6.Harder T, Plagemann A, Harder A. Birth Weight and Subsequent Risk of Childhood Primary Brain Tumors: A Meta-Analysis. Am. J. Epidemiol. 2008 doi: 10.1093/aje/kwn144. [DOI] [PubMed] [Google Scholar]
- 7.Crow JF. The origins, patterns and implications of human spontaneous mutation. Nat. Rev. Genet. 2000;1:40–47. doi: 10.1038/35049558. [DOI] [PubMed] [Google Scholar]
- 8.Greaves M. In utero origins of childhood leukaemia. Early Hum. Dev. 2005;81:123–129. doi: 10.1016/j.earlhumdev.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Ross JA, Spector LG. Cancers in Children. In: Schottenfeld D, Fraumeni JF, editors. Cancer Epidemiology and Prevention. 3rd ed. Oxford; New York: Oxford University Press; 2006. pp. 1251–1268. [Google Scholar]
- 10.Little J. 149 ed. Lyon; Oxford: International Agency for Research on Cancer; Distributed by Oxford University Press; 1999. Epidemiology of childhood cancer. [Google Scholar]
- 11.Dockerty JD, Draper G, Vincent T, Rowan SD, Bunch KJ. Case-control study of parental age, parity and socioeconomic level in relation to childhood cancers. Int. J. Epidemiol. 2001;30:1428–1437. doi: 10.1093/ije/30.6.1428. [DOI] [PubMed] [Google Scholar]
- 12.Yip BH, Pawitan Y, Czene K. Parental age and risk of childhood cancers: a population-based cohort study from Sweden. Int. J. Epidemiol. 2006;35:1495–1503. doi: 10.1093/ije/dyl177. [DOI] [PubMed] [Google Scholar]
- 13.McLaughlin CC, Baptiste MS, Schymura MJ, Nasca PC, Zdeb MS. Maternal and infant birth characteristics and hepatoblastoma. Am. J. Epidemiol. 2006;163:818–828. doi: 10.1093/aje/kwj104. [DOI] [PubMed] [Google Scholar]
- 14.Podvin D, Kuehn CM, Mueller BA, Williams M. Maternal and birth characteristics in relation to childhood leukaemia. Paediatr. Perinat. Epidemiol. 2006;20:312–322. doi: 10.1111/j.1365-3016.2006.00731.x. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds P, Von Behren J, Elkin EP. Birth characteristics and leukemia in young children. Am. J. Epidemiol. 2002;155:603–613. doi: 10.1093/aje/155.7.603. [DOI] [PubMed] [Google Scholar]
- 16.Walker KM, Carozza S, Cooper S, Elgethun K. Childhood cancer in Texas counties with moderate to intense agricultural activity. J Agric Saf Health. 2007;13:9–24. doi: 10.13031/2013.22308. [DOI] [PubMed] [Google Scholar]
- 17.Jaro MA. Probabilistic linkage of large public health data files. Stat. Med. 1995;14:491–498. doi: 10.1002/sim.4780140510. [DOI] [PubMed] [Google Scholar]
- 18.Thoburn KK, German RR, Lewis M, Nichols PJ, Ahmed F, Jackson-Thompson J. Case completeness and data accuracy in the Centers for Disease Control and Prevention's National Program of Cancer Registries. Cancer. 2007;109:1607–1616. doi: 10.1002/cncr.22566. [DOI] [PubMed] [Google Scholar]
- 19.Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International Classification of Childhood Cancer. Cancer. (third edition) 2005;103:1457–1467. doi: 10.1002/cncr.20910. [DOI] [PubMed] [Google Scholar]
- 20.Pizzo PA, Poplack DG. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2002. Principles and practice of pediatric oncology. [Google Scholar]
- 21.Olson JM, Breslow NE, Beckwith JB. Wilms' tumour and parental age: a report from the National Wilms' Tumour Study. Br. J. Cancer. 1993;67:813–818. doi: 10.1038/bjc.1993.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dryja TP, Morrow JF, Rapaport JM. Quantification of the paternal allele bias for new germline mutations in the retinoblastoma gene. Hum. Genet. 1997;100:446–449. doi: 10.1007/s004390050531. [DOI] [PubMed] [Google Scholar]
- 23.Moll AC, Imhof SM, Kuik DJ, Bouter LM, Den Otter W, Bezemer PD, Koten JW, Tan KE. High parental age is associated with sporadic hereditary retinoblastoma: the Dutch retinoblastoma register 1862–1994. Hum. Genet. 1996;98:109–112. doi: 10.1007/s004390050168. [DOI] [PubMed] [Google Scholar]
- 24.Bunin GR, Meadows AT, Emanuel BS, Buckley JD, Woods WG, Hammond GD. Pre- and postconception factors associated with sporadic heritable and nonheritable retinoblastoma. Cancer Res. 1989;49:5730–5735. [PubMed] [Google Scholar]
- 25.Steuerwald NM, Bermudez MG, Wells D, Munne S, Cohen J. Maternal age-related differential global expression profiles observed in human oocytes. Reprod. Biomed. Online. 2007;14:700–708. doi: 10.1016/s1472-6483(10)60671-2. [DOI] [PubMed] [Google Scholar]
- 26.Pan H, Ma P, Zhu W, Schultz RM. Age-associated increase in aneuploidy and changes in gene expression in mouse eggs. Dev. Biol. 2008;316:397–407. doi: 10.1016/j.ydbio.2008.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamatani T, Falco G, Carter MG, Akutsu H, Stagg CA, Sharov AA, Dudekula DB, VanBuren V, Ko MS. Age-associated alteration of gene expression patterns in mouse oocytes. Hum. Mol. Genet. 2004;13:2263–2278. doi: 10.1093/hmg/ddh241. [DOI] [PubMed] [Google Scholar]
- 28.Esteller M. Epigenetics in cancer. N. Engl. J. Med. 2008;358:1148–1159. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 29.Hitchins MP, Lin VA, Buckle A, Cheong K, Halani N, Ku S, Kwok CT, Packham D, Suter CM, Meagher A, Stirzaker C, Clark S, et al. Epigenetic inactivation of a cluster of genes flanking MLH1 in microsatellite-unstable colorectal cancer. Cancer Res. 2007;67:9107–9116. doi: 10.1158/0008-5472.CAN-07-0869. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. 2005 Assisted Reproductive Technology Success Rates: National Summary and Fertility Clinic Reports. Centers for Disease Control and Prevention. [Accessed July 25, 2008];American Society for Reproductive Medicine, Society for Assisted Reproductive Technology. 2007 Available at: http://www.cdc.gov/ART/.
- 31.Schieve LA, Rasmussen SA, Buck GM, Schendel DE, Reynolds MA, Wright VC. Are children born after assisted reproductive technology at increased risk for adverse health outcomes? Obstet. Gynecol. 2004;103:1154–1163. doi: 10.1097/01.AOG.0000124571.04890.67. [DOI] [PubMed] [Google Scholar]
- 32.Jacob S, Moley KH. Gametes and embryo epigenetic reprogramming affect developmental outcome: implication for assisted reproductive technologies. Pediatr. Res. 2005;58:437–446. doi: 10.1203/01.PDR.0000179401.17161.D3. [DOI] [PubMed] [Google Scholar]
- 33.Lightfoot T, Bunch K, Ansell P, Murphy M. Ovulation induction, assisted conception and childhood cancer. Eur. J. Cancer. 2005;41:715–724. doi: 10.1016/j.ejca.2004.07.032. discussion 25-6. [DOI] [PubMed] [Google Scholar]
- 34.Troisi R, Potischman N, Roberts J, Siiteri P, Daftary A, Sims C, Hoover RN. Associations of maternal and umbilical cord hormone concentrations with maternal, gestational and neonatal factors (United States) Cancer Causes Control. 2003;14:347–355. doi: 10.1023/a:1023934518975. [DOI] [PubMed] [Google Scholar]
- 35.Martin JA, Hamilton B, Sutton PD, Ventura SJ, Menacker F, Kirmeyer, et al. “Births: Final data for 2005”. National Center for Health Statistics. 2007 Vital Health Stat Series No. 6 (56) [PubMed] [Google Scholar]
- 36.Patterson JE, Greville TNE, Armstrong RJ, Hesuer RL, Hetzel AM, Chancellor LE, et al. “Vital Statistics of the United States 1970”. National Center for Health Statistics. 1970 Vital Health Stat Series, Volume 1 Natality. [Google Scholar]
- 37.Linabery AM, Ross JA. Trends in childhood cancer incidence in the United States (1992–2004) Cancer. 2007 [Google Scholar]
- 38.Wang Y, Druschel CM, Cross PK, Hwang SA, Gensburg LJ. Problems in using birth certificate files in the capture-recapture model to estimate the completeness of case ascertainment in a population-based birth defects registry in New York State. Birt. Defects Res. A. Clin. Mol. Teratol. 2006;76:772–777. doi: 10.1002/bdra.20293. [DOI] [PubMed] [Google Scholar]
- 39.Ross JA, Spector LG, Robison LL, Olshan AF. Epidemiology of leukemia in children with Down syndrome. 2005;44:8–12. doi: 10.1002/pbc.20165. [DOI] [PubMed] [Google Scholar]
- 40.Northam S, Knapp TR. The reliability and validity of birth certificates. Journal of obstetric, gynecologic, and neonatal nursing : JOGNN / NAACOG. 2006;35:3–12. doi: 10.1111/j.1552-6909.2006.00016.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.