Abstract
The partial volume effect (PVE) significantly restricts the absolute quantification of regional myocardial uptake and thereby limits the accuracy of absolute measurement of blood flow and coronary flow reserve by SPECT. The template-projection-reconstruction method has been previously developed for PVE compensation. This method assumes the availability of coregistered high-spatial resolution anatomical information as is now becoming available with commercial dual-modality imaging systems such as SPECT∕CTs. The objective of this investigation was to determine the extent to which the impact of the PVE on cardiac perfusion SPECT imaging can be diminished if coregistered high-spatial resolution anatomical information is available. For this investigation the authors introduced an additional parameter into the template-projection-reconstruction compensation equation called the voxel filling fraction (F). This parameter specifies the extent to which structure edge voxels in the emission reconstruction are filled by the structure in question as determined by the higher spatial-resolution imaging modality and the fractional presence of the structure at different states of physiological motion as in combining phases of cardiac motion. During correction the removal of spillover to the cardiac region from the surrounding structures is performed first by using reconstructed templates of neighboring structures (liver, blood pool, lungs) to calculate spillover fractions. This is followed by determining recovery coefficients for all voxels within the heart wall from the reconstruction of the template projections of the left and right ventricles (LV and RV). The emission data are subsequently divided by these recovery coefficients taking into account the filling fraction F. The mathematical cardiac torso phantom was used for investigation correction of PVE for a normal LV distribution, a defect in the inferior wall, and a defect in the anterior wall. PVE correction resulted in a dramatic visual reduction in the impact of extracardiac activity, improved the uniformity of the normally perfused heart wall, and enhanced defect visibility without undue noise amplification. No significant artifacts were seen with PVE correction in the presence of mild (one voxel) misregistration. A statistically significant improvement in the accuracy of the count levels within the normal heart wall was also noted. However, residual spillover of counts from within the myocardium creates a bias in regions of decreased wall counts (perfusion defects∕abnormal wall motion) when the anatomical imaging modality does not allow definition of templates for defects present in the heart during emission imaging.
Keywords: cardiac SPECT, partial volume effect, spillover
INTRODUCTION
SPECT and PET imaging are hampered by the finite spatial resolution of the currently available imaging systems. As a result, detected photons originating at locations within the heart wall are blurred into surrounding locations such as the blood pool, lungs, and liver. The flip side of this process is that photons emitted in the blood pool, lungs, and liver in close proximity to the heart walls also blur into the myocardium during data acquisition. Filtering to control noise adds significantly to this blurring process. As long as there are uniform activity concentrations in structures larger than 2–3 times the system spatial resolution, there is an equilibrium between the blurring of counts into and out of central locations within the structure such that the measured concentration is not distorted.1, 2 As one moves towards the edge of large structures and throughout structures smaller than 2–3 times the system spatial resolution, equilibrium of the count distribution by blurring will not exist and the apparent concentration will be distorted. We term this process which is a reflection of the limited spatial resolution of imaging as the partial volume effect (PVE).1, 2, 3 When we wish to focus on the portion of the PVE relating to the spill-in of counts from neighboring structures we will call this spillover.
The PVE impacts both the apparent concentration and absolute quantification of activity within structures as illustrated in count profiles plotted in Fig. 1. In Fig. 1 the walls of the left ventricle (LV) (labeled X1 and X2) are set to be 3 voxels thick (∼1.4 cm) for simplicity. The structure labeled Y represents the liver which is 26 voxels or ∼12.1 cm thick and is in close proximity to the heart. The rectangular representations shown as solid lines are profiles through the original source distribution with activity levels in the LV and liver set to the same value. The dashed lines represent the profiles of the individual structures after analytically simulated acquisition which includes the distance-dependent spatial-resolution of the imaging system with a low-energy high-resolution collimator, reconstruction, and low-pass filtering. Finally, the solid circles represent the summed counts of all the structures taking into consideration the combined effects of the loss of counts to outside the structure and spillover from adjacent structures. As observed, the impact on the apparent concentration (height) is more significant for the heart walls as they are much thinner than the 2–3 times the system spatial resolution (FWHM) needed to avoid significant influence of the PVE on the maximum counts within the structure.1, 2, 4 Notice also the significant spillover from the liver to the heart wall nearby.
Figure 1.
Plot of one-dimensional curves illustrating the concepts of the PVE. The rectangular profiles drawn with solid lines represent the true superior (X1) and inferior (X2) myocardium of the left-ventricle and the liver (Y) with the height indicating similar concentrations of activity in the structures. The dashed line profiles are the result of analytical simulation of the imaging of the structures represented by the solid lines and show the influence of the PVE on individual structures. The solid circles represent the combined counts from the three structures. The horizontally and vertically shaded areas are spillover from the liver and heart, respectively.
In cardiac perfusion imaging the PVE significantly restricts the absolute quantification of regional myocardial uptake of perfusion agents and thereby limits the accuracy of absolute measurement of blood flow and coronary flow reserve by SPECT.5, 6, 7 The PVE can also lead to an apparent nonuniformity of perfusion of the myocardium due to variations in wall thickness8, 9, 10, 11 resulting in false positive perfusion scans. Increased counts (compared to other regions) at the joining of the right ventricle to the LV and the location of the papillary muscles can be seen due to these structures causing an increase in wall thickness.11, 12 Also, apparent decreases in perfusion occur at the site of the apical thin point.8, 9, 10, 11 Spillover from significant concentrations of extracardiac activity can have a substantial impact on the appearance of the nearby myocardium.13 Improving the absolute quantification of and eliminating known artifacts in cardiac perfusion imaging should improve patient management.
Cardiac perfusion imaging can therefore benefit from PVE compensation, and several methods have already been proposed to diminish the impact.2, 7, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 Nuyts et al.21 investigated modeling ROIs for the LV wall and blood pool which were convolved with an estimated PET camera response function to determine recovery and spillover coefficients. Hasegawa’s group7, 16 developed a similar method for quantifying the regional myocardial uptake of SPECT perfusion agents called template-projection reconstruction. This method uses three-dimensional regions-of-interest or templates formed from high-resolution anatomical images to represent structures of interest in emission images. In their studies Hasegawa’s group used CT defined cardiac anatomy to determine voxel-by-voxel recovery coefficients (R) within the myocardium through projecting a template of the heart wall and reconstructing these template projections in the same way the SPECT acquisitions were reconstructed. The counts in voxels within the myocardium of the SPECT reconstruction are then divided by these recovery coefficients and summed over the region of interest to obtain the PVE corrected estimate of activity.
The earliest form of template-projection reconstruction was introduced by Gärtner et al.15 when they determined gray matter recovery coefficients by using high-resolution MRI defined regions convolved with the measured PET response functions to correct for the effects of partial volume. Rousset et al.22 endeavored to take partial volume compensation a step further by employing registered MRI studies and used the geometric transfer matrix (GTM) method to invert a matrix representing the fraction of activity in each region to be PVE corrected. The GTM is a matrix of weighting factors built by integrating with normalization the system point spread function in regions with identifiable activity distributions. The template-projection-reconstruction method is therefore a simplified version of the GTM method22 with one activity region and is similar to the work of Müller–Gärtner et al.15 and Nuyts et al.21 in PET.
Herein, we investigate an adaptation of Hasegawa’s group’s template-projection PVE correction method by adding an additional parameter, the voxel filling fraction (F), and employing correction for multiple neighboring regions. We explore the impact of correction on the visualization and accuracy of recovering the normal myocardial wall distribution and perfusion defects whose presence is not included in the myocardial wall template. The goal of our application of PVE correction is to improve not only quantitative accuracy of cardiac activity estimation but also to move towards improving the visual fidelity of cardiac perfusion SPECT images with the ultimate goal of improving the diagnostic accuracy of the reconstructions. With one exception where we explicitly investigate the impact of misregistration, the studies performed herein assume ideal registration between the high-resolution imaging modality and the emission slices, and ideal segmentation of the templates from the slices of the high-resolution imaging modality. Thus our studies were designed to investigate and illustrate the upper limit of performance of our correction methodology.
THEORY
In a clinical environment, it is envisioned implementing PVE correction by segmenting coregistered high-resolution finely sampled contrast enhanced gated CT or MRI slices to obtain binary templates of relevant structures such as the heart, liver, lungs, and blood pool. These binary templates will be forward projected using a model of the distance-dependent spatial resolution of the gamma camera and attenuation maps also derived from the CT or MRI slices. After reconstruction and filtering, the reconstructed template of the heart wall and other structures provide voxel and structure specific sets of recovery coefficients (R). The recovery corrected count (RCVX) for a voxel (V) in structure X (for example myocardium, see Fig. 1) can then be defined as the count in V from the SPECT reconstruction (CV) times the fraction of X in V (FVX) divided by the R specific to that V (RVX), or as an equation
| (1) |
FVX (or the voxel filling fraction) has a value of 1.0 when V is completely in X and less than 1.0 when V is partially in X. FVX is determined from a template which includes sub-SPECT-voxel sampling of the structure created by folding down to the resolution of SPECT the coregistered high-resolution template originally segmented from the anatomical slices. F can also be used to correct for the fractional presence of a structure due to physiological motion. Herein, PVE correction is made to the emission data in the absence of cardiac gating. Thus, F also corrects for the fractional presence of the structure at each stage of the cardiac cycle. It controls overcompensation at the edges of structures and also accurately scales activity when the structure is smaller than the SPECT voxel size as can happen with apical thinning.
Equation 1 is only applicable when no activity other than the distribution X (herein myocardium) is present. With the liver (Y in Fig. 1) and other structures close to the myocardium (lungs, blood pool) accumulating activity, a spillover correction for their contribution to the voxel also needs to be implemented. Following the lead of Nuyts et al.22 we approximate the spillover counts (SCVY) at voxel V from activity in some structure Y as being the spillover coefficient (SVY) times the average count per voxel in the structure (AY) assuming uniform uptake, or as an equation
| (2) |
As with R, S is obtained by forward projecting and reconstructing a CT segmented binary structure Y. AY for large structures should be assessed with regions-of-interest (ROIs) defined to avoid regions where counts are diminished (i.e., defined where R=1.0). Spill-out of activity from the heart wall and blood pool can also be addressed in this way, but only after recovery compensation is applied to them. In these cases (R<1.0), compensation is necessary to accurately determine the maximum count level for an accurate estimate of AY. One could use an iterative process to refine the estimates of AY; however, we have not done so herein. The combined PVE corrected slices will be formed voxel by voxel based on a combination of Eqs. 1, 2. The PVE corrected count at a voxel V in structure X (PCVX) will be calculated as
| (3) |
where TSCVY is the total spillover count correction from all structures Y (liver, lungs, background, blood pool, etc.) contributing to V in structure X.
MATERIALS AND METHODS
Two versions of the mathematical cardiac torso (MCAT) phantom27, 28 were used to investigate the application of PVE correction under controlled conditions for Tc-99m Sestamibi perfusion SPECT. Emission projections for a normal heart and hearts with decreased activity simulating small defects (20 deg radial and 1.8 cm axial extent at the center) in the inferior and anterior LV walls respectively were created.29 The decrease was varied between 0% and 100% in steps of 20% to study the accuracy with which the activity in the defect could be reproduced. Besides a decrease in activity, one rendition of the inferior and anterior defects also included a slight narrowing of the heart wall and change in contraction as might occur in the cases of myocardial stunning and hibernation30 during stress. The narrowing and change in contraction were not included in the version used to represent the CT maps since these maps were created to represent CT imaging at rest. Thus these changes would not be reflected in the heart wall templates defined from the CT slices. The beating motion of the heart was simulated for 16 stages evenly spaced throughout the cardiac cycle and combined into ungated data sets for both the SPECT and CT data. The liver was elevated to lie within the slices containing the LV at 0.6 cm at its closest approach to the myocardium making the inferior wall defects a challenge for the imaging system. In addition, lungs, blood pool, and tissue background were included in the simulation. All the different organs (activity distributions) were generated separately with unit count levels for flexible combination after Monte Carlo simulation.
The two versions of the MCAT phantom varied in spatial and voxel sampling (Table 1). The first version of the phantom was used as the source and attenuation distributions to generate the emission acquisitions. These phantoms (normal, anterior defect, inferior defect, and wall narrowing and change in contraction of defect) were generated with 3×3×3 subsampling and consisted of 256×256×256 voxel arrays with an isotropic voxel width of 0.2335 cm to minimize aliasing during the simulated acquisitions described below. No subsampling (voxels were either fully inside or fully outside a structure) was employed in the second version of the phantom which simulated a segmented high-resolution set of CT slices with an in plane voxel width of 0.058375 cm and an axial voxel width 0.11675 cm (four and two times smaller than the emission phantom, respectively). Due to the coarser axial sampling typically employed in CT (compared to in plane sampling), the second version of the phantoms consisted of the centrally located 128 axial slices with a 512×512 voxel dimension for each slice. From this phantom the templates used for calculating the correction factors were generated, paralleling the use of segmentation of the templates from CT slices registered with the emission data for this purpose clinically. The templates were downsampled to the desired reconstructed voxel dimension of the emission reconstructions keeping track of the fractional contribution of each voxel of the structures at the original “CT” resolution to the voxels at the emission sampling. This is the factor FVX of Eqs. 1, 3. Note that it corrects for both downsampling and the fractional presence of the activity in slices summed over the cardiac cycle.
Table 1.
Summary of the sampling used to generate the SPECT source, attenuation distributions, and CT derived templates.
| SPECT phantom | CT phantom | |
|---|---|---|
| Dimensions | 256×256×256 | 512×512×128 |
| In-plane sampling (cm) | 0.2335 | 0.058375 |
| Axial sampling (cm) | 0.2335 | 0.11675 |
Cardiac perfusion projections of the first version of the MCAT phantom were simulated using the SIMIND Monte Carlo package.31 Projections of the templates were made using a ray-driven analytical projector32 which modeled the distance-dependent spatial resolution of the SPECT system simulated by SIMIND and nonuniform attenuation using the attenuation maps provided to SIMIND. The maps were downsampled to match the template sampling. Simulated projection data were acquired through 360 deg into 120 projections with voxel sizes of 0.317, 0.467, and 0.634 cm, respectively. For the first two voxel sizes 128×128 matrices were employed while for the 0.634 cm voxel size a 64×64 matrix was used. Simulating different voxel sizes enabled us to study the effect of SPECT voxel sampling on PVE compensation as well as the interpolation problem when noncubic voxels (as present in CT) need to be converted to arbitrary voxel sizes (not multiples of original dimension). In both SIMIND and analytical modeling the imaging properties of an IRIX SPECT gamma camera (Philips Medical, Cleveland, OH) fitted with low-energy high-resolution parallel-hole collimators was simulated. In SIMIND, primary photons (assuming perfect scatter rejection) and scattered photons were acquired separately for the same 15% Tc-99m photopeak window (129.93–151.38 keV). In addition, an 8% energy window below the Tc-99m photopeak (118.08–127.92 keV) was simulated for triple-energy-window (TEW) scatter compensation.33 That is, we employ the version of TEW for Tc-99m which makes use of solely a window below the photopeak. With SIMIND separate near noise-free projection sets of the heart, liver, lungs, blood pool, and tissue background were generated, combined, and Poisson noise added, guided by count levels seen in our clinic. When combined the true concentration ratio (without attenuation, scatter, and resolution degradation) relative to heart for liver was 1.0, for blood pool was 0.1, for lungs was 0.05, and for background was 0.1.27 A total of ∼14.2 million counts were present in the projection image sets of which only ∼0.68 million originated from the heart. All slices and plots from the slices included in the figures of the manuscript include the impact of this noise level. Analytical projections of the same structures mentioned, except the background, were also produced from the “CT” templates. Although easy to distinguish in the MCAT phantom, the background was not included as a template because of the complexity of segmenting a wide variety of background structures in real CT that might contain radioactivity.
SIMIND projection data were reconstructed using five iterations of an ordered-subset rescaled-block-iterative34 (RBI) algorithm with four angles per subset. Five iterations were found to be optimal in terms of detection accuracy in a clinical cardiac perfusion study using our reconstruction software.11 Scatter compensation (SC) employing the TEW method33 was implemented by adding the TEW estimate of scatter to the projection of the current voxel values prior to dividing this sum into the photopeak voxel values during iteration of the algorithm.35 During reconstruction, data were also left uncorrected for any physical degradation (NC), corrected for nonuniform attenuation (AC), and distance-dependent resolution compensation (RC) by the Gaussian diffusion algorithm.36 Postreconstruction 3D Gaussian filtering with sigma’s [sigma (σ)=standard deviation] accounting for the different voxel sizes was applied to produce similar noise suppression as in our clinical studies. That is, sigma’s of 1.5, 1.0, and 0.75 voxels were used respectively for the 0.317, 0.467, and 0.634 cm voxel sizes, with the latter voxel size the same as the clinical study.11
The analytical projections of the CT templates of the heart, liver, blood pool, and lung were reconstructed similar to that of the SPECT slices including the postreconstruction filtering step, but excluding scatter compensation. Because the heart wall is the focus of cardiac perfusion imaging, PVE compensation was performed solely on the myocardium of all data sets, and compared with an implementation without the F, similar to that proposed by Hasegawa’s group.7 Filtered backprojection (FBP) reconstruction of the emission projections using the best 180 deg (right-anterior oblique to left-posterior oblique) without any form of correction was also included as a baseline. The FBP slices were filtered using the Butterworth filter on our clinical SPECT system with an order of 5 and cutoffs of 0.25 voxel−1 for 0.634 cm voxels, 0.185 voxel−1 for 0.467 cm voxels, and 0.125 voxel−1 for 0.317 cm voxels, respectively. We previously determined these parameters to be optimal for our clinical cardiac perfusion studies based on a human-observer ROC study.11 We opted to use FBP rather than RBI without any corrections as the base line because it is still the standard at most clinical sites.
There is the distinct possibility that by reconstructing the Monte Carlo projections for activity in all organs and the different template projections separately, the solutions of these reconstructed data sets will be at different points of convergence at a given iteration number.37, 38, 39 To study the influence of possible differences in convergence rates on PVE correction, we added a fraction of the template projections to the Monte Carlo projections. The added fraction for each template projection data set was 10% of the total counts in the Monte Carlo projections belonging to the template structure. After reconstruction using the same strategies as before, the reconstructed data without the fractional template present were subtracted from the reconstructions with the fractional template present leaving only the fraction of the template added in the first place. The results were scaled back to the original template count levels and filtered employing the same filter parameters described previously. This methodology is described in greater detail in a study we conducted of the impact of PVE correction on lesion activity quantification and visualization40 and in the work of Duet al.23 where they investigated its application to account for possible differences in convergence rates in SPECT brain imaging.
All SPECT data including the source distribution were reoriented sequentially using the same angular rotations to ensure fair comparison. The PVE correction method was evaluated qualitatively using visual inspection of short axis slices. Fidelity was evaluated using circumferential profiles normalized employing the expected counts in the heart wall. Expected counts are derived from knowledge of the scaling performed within the SIMIND Monte Carlo simulation31 and the level of noise added. The expected counts are a measure of absolute quantification and similar to using a measure of collimator sensitivity to convert counts per voxel to MBq per gram of tissue. The profiles were obtained from averaging three short-axis slices selected to coincide with the center of the defects. A ROI was drawn on the center slice and used as a mask to eliminate any liver interference from outside the LV boundary. The center of counts in the ROI served as the starting point to determine 120 samples through 360 deg around the heart wall by searching for the maximums. The profiles were generated beginning at the anterior wall proceeding clockwise to the lateral, inferior and septal walls, back to the anterior wall.
Mismatch between the template and emission data whether through a misalignment in the CT and emission data, or an error in segmentation of the CT data presents a significant source of potential difficulty with the methodology presented herein when applied clinically. To provide an initial sense of the magnitude of the problem of misalignment we investigated the impact of axial shifts in the cranial direction of the anatomical templates and attenuation maps of 1 and 2 voxels (3.17 and 6.34 mm, respectively) with application of PVE correction to our simulated 128×128 acquisitions. Axial motion was selected as it is the major component of respiratory motion.41 The cranial direction of the shift as opposed to caudal was to avoid the problems with AC encountered when the emission data are mistakenly aligned such the heart is corrected as if it were positioned partially within the lungs.
We also investigated the influence of segmentation errors. To accomplish this task, we started with the finely sampled average-gated MCAT heart and created templates by forcing all voxels above thresholds of 1%, 25%, 50%, 70%, and 90% of the maximum to 1.0. These resulting templates vary in wall thickness between ∼1.46 and 0.82 cm, the first approaching the end-systolic thickness (1.59 cm) and the latter being thinner than the end-diastolic thickness (1.1 cm). In the worst case (1% threshold) the segmentation error added approximately 10 additional voxels to the left ventricle and all averaging information from summing the segmented sum of all phases of the cardiac cycle was lost. The templates were down sampled to the 0.317 cm voxel size and used for PVE compensation.
RESULTS
Visual inspection of Figs. 234 clearly shows the dramatic improvement in wall uniformity with PVE compensation [PCVX in Eq. 3 and abbreviated to PC henceforth] in a normal perfusion study. In these figures comparison are made between the use of a combination of AC, SC, RC, and PC (AC+SC+RC+PC), a combination of AC, SC, and RC (AC+SC+RC), a combination of AC, and SC (AC+SC), and without corrections (NC) in iterative reconstruction, and FBP with no added correction. The true source distribution is also given. Three different voxel sizes are presented. The slices are displayed from apical to the basal proceeding left to right. The short-axis slices with PC were corrected using the reconstructed templates generated by adding a fraction of the template projections to the Monte Carlo acquired projections as described in Sec. 3.23, 40 This will be true in all following figures unless otherwise specified. The decrease in counts in the inferior wall (already noticeable in RBI NC) of the slices reconstructed using FBP [Figs. 2e, 3e, 4e] is due to the close proximity of the hot liver combined with the effects of the negative frequency response of Butterworth filtering at organ edges.42, 43 Such a decrease could be interpreted as a defect and its absence in the iterative methods illustrates the value of AC, SC, and RC. It is clear from Figs. 234 that adding PC better separates the inferior myocardial wall from the liver, and correctly scales the counts∕voxel in the myocardium to be similar to the larger liver which, because of its size, does not have a significant PVE at its center. From the figures it is also obvious that noise is not overly enhanced. Voxel size, however, influences PVE compensation with the 0.634 cm sampling [Fig. 4b] clearly not as able to improve the visual representation of the heart to the same degree as the smaller sized voxels. Henceforth, we will only give results for the 0.317 cm voxel size.
Figure 2.
A selected set of short-axis slices from the normal MCAT heart at a 0.317 cm voxel size. Starting at the top we have (a) the true source distribution, (b) AC+SC+RC+PC, (c) AC+SC+RC, (d) AC+SC, (e) NC, and (f) FBP. No thresholding of slices was employed and each row is displayed relative to its own maximum.
Figure 3.
A selected set of short-axis slices from the normal MCAT heart at a 0.467 cm voxel size. Starting at the top we have (a) the true source distribution, (b) AC+SC+RC+PC, (c) AC+SC+RC, (d) AC+SC, (e) NC, and (f) FBP. No thresholding of slices was employed and each row is displayed relative to its own maximum.
Figure 4.
A selected set of short-axis slices from the normal MCAT heart at a 0.634 cm voxel size. Starting at the top we have (a) the true source distribution, (b) AC+SC+RC+PC, (c) AC+SC+RC, (d) AC+SC, (e) NC, and (f) FBP. No thresholding of slices was employed and each row is displayed relative to its own maximum.
Figure 5 gives the results for the MCAT heart with a 40% defect with abnormal motion and thinning present in the inferior wall for the 0.317 cm voxel size. The MCAT heart with a 40% defect and normal motion gave similar visual results. Starting at the top in Fig. 5 we have the (a) MCAT truth, (b) AC+SC+RC+PC, (c) AC+SC+RC, (d) AC+SC, (e) NC, and (f) FBP for comparison. The MCAT truth shows a resampled version of the true location, size, and contrast of the defect in the inferior wall generated originally with a spatial sampling of 0.2335 cm. The slices are displayed in the same way as Figs. 234 (apical side to the basal side) and are the same slices displayed in Figs. 2a, 2b, 2c, 2d, 2e for the normal myocardium. From the figure it is evident that the defect is more accurately visualized in all aspects (size, location, and contrast) when PVE compensation (PC) is included. Although FBP visualizes the defect quite dramatically, this is an overestimate due to the presence of the liver which was seen in Figs. 234 to already cause an apparent defect in the inferior wall of the normal heart.42, 43
Figure 5.
A selected set of short-axis slices from the MCAT heart with a small defect (40% decrease in activity) in the inferior wall for the 0.317 cm voxel acquisition. Starting at the top we have as rows the (a) MCAT truth (b) AC+SC+RC+PC, (c) AC+SC+RC, (d) AC+SC, (e) NC, and (f) FBP. No thresholding of slices was employed and each row is displayed relative to its own maximum.
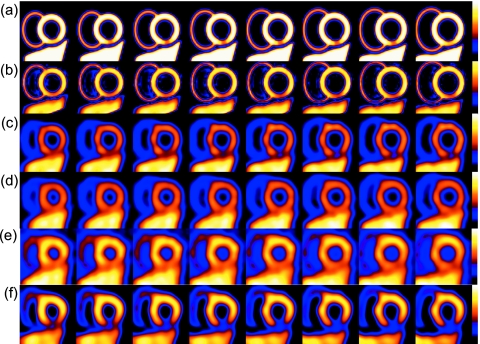
Figure 6 demonstrates the effect of F, the filling fraction, for the 0.317 cm voxel size. Figure 6a represent the 40% inferior wall defect (with abnormal motion and thinning) compensated using our proposed method (including F). Figures 6b, 6c, 6d, 6e show slices corrected with the template-projection-reconstruction algorithm7 without direct use of F. The latter method was implemented by using templates and template projection reconstructions with heart-wall voxels above a selected threshold, mimicking different manually segmented heart boundaries without the added benefit of subsampling as in the case when folding the finely sampled CT templates down to the SPECT voxel size. This scenario is similar to not having the finely sampled CT data available for segmentation. Thresholds of 1%, 25%, 50%, 70%, and 90% are illustrated. Compared to the liver (most accurate representation of true counts) it is clear that the previously proposed method7 does a fairly good job in scaling the heart counts to its expected value when the template projection reconstruction was done with a 50% threshold, but over emphasized voxels that are not fully occupied by the heart, influencing the apparent thickness of the myocardium. With thresholds lower than 50% the apparent thickness increased while the higher thresholds (70% and 90%) are closer to the true thickness. In each of these cases, the presence of the filling fraction could have improved the visual appearance of the heart.
Figure 6.
A selected set of short-axis slices from the MCAT heart with the inferior wall defect for the 0.317 cm voxel acquisition demonstrating the impact of the voxel filling fraction F. Starting at the top rows are (a) AC+SC+RC+PC including F. All the other rows (b)–(f) show AC+SC+RC+PC excluding F and using heart templates and template projection reconstructions when the threshold is (b) 1%, (c) 25%, (d) 50%, (e) 70%, and (f) 90%. The liver intensity can be used to judge the accuracy of the compensation.
In Figs. 78 we show PVE corrected short-axis slices when a misalignment between the template derived from the high resolution CT and the emission slices exist. The mismatch was created by simulating rigid-body motion of 1 or 2 voxels representing 3.17 mm [Fig. 7d], and 6.34 mm [Fig. 7e] displacements in the cranial direction of the CT acquisition, and hence, derived templates and attenuation maps. The PVE corrected short-axis slices without mismatch are shown in Fig. 7c. For comparison the MCAT simulated source distribution (truth) is given in Fig. 7a. Figure 7b shows the reconstructed short axis slices with AC+SC+RC before PVE compensation. The decrease in activity in the inferior wall in this example is 40% with normal wall motion and no thinning simulated. With mismatch no gross artifacts are visible even with the 2 voxel shift. The major change is that of an apparent mild increase in the size of the defect in the inferior wall for the 1 voxel displacement, and a further change in its apparent size for the 2 voxel shift. Also with the 2 voxel shift, the uniformity of the cardiac wall is seen to be mildly degraded. This lack of a significant deterioration of PVE correction with a 1 voxel shift is in line with our results of a more detailed study of the impact of mismatch on PVE correction we have previously reported.44
Figure 7.
A selected set of short-axis slices from the MCAT heart with inferior wall defect for the 0.317 cm acquisition demonstrating the effects of mild mismatches between the projection templates derived from the simulated CT and emission reconstructions. The original MCAT source distribution is given in (a) while (b) represents the reconstructed slices with AC+SC+RC but no PVE correction. The PVE corrected data are given in (c), (d), and (e) for axial rigid-body motion between high resolution CT and emission acquisitions of 0, 3.17, and 6.34 mm, respectively.
Figure 8.
Circumferential profiles depicting the quantitative influence of a misalignment of 1 and 2 voxels. The truth and correctly aligned PVE compensated profiles are given for comparison.
The quantitative assessment of the influence of mismatched CT templates on the size and severity of the 40% defect with normal motion are given in Fig. 8. PVE compensation underestimates the defect contrast without and with mismatched templates to a similar degree. Defect contrasts expressed as a percentage appeared to be 33.6%, 33.4%, and 32.6% compared to the true maximum counts for no, 1, and 2 voxels shifts, respectively. The apparent size of the defect increased with increasing mismatch as was already evident in the visual evaluation of Fig. 7.
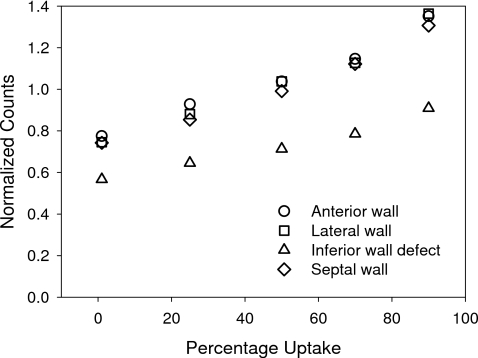
Results due to segmentation errors are presented in Figs. 910. The visual effects (Fig. 9) are an apparent thickening of the heart wall when erroneously voxels included in the segmentation, and an apparent thinning when erroneously excluding voxels. In all cases use of the filling fraction resulted in a smooth transition of the LV wall boundaries compared without using it as depicted in Fig. 6. Quantitatively, (Fig. 10) the segmentation errors slowly deteriorate the results of our proposed PVE compensation technique by either under or over estimating maximum heart wall counts due to the change in partial volume of the reconstructed template projections. The error varies between 0.75 and 1.31 and appears to be the same for locations outside the defect. A value of 1.0 indicates the correct level of activity in the heart wall.
Figure 9.
Short axis slices of the heart wall showing the visual influence of errors in segmentation heart-template boundaries on PVE compensation. The results of an accurate segmentation in (a) is followed by including all voxels in the finely sampled gated average MCAT larger than (b) 1%, (c) 25%, (d) 50%, (e) 70%, and (f) 90% of the maximum count.
Figure 10.
A graph depicting the variation in maximum wall counts as a function of the segmentation error. Note the 1.0 is the correct normalized uptake in the normal wall and 0.6 is the ideal value for the defect in the inferior wall.
To quantitatively investigate the impact of the various combinations of compensation strategies on the accurate reproduction of defect count levels in the slices, average circumferential profiles for three short-axis slices spanning the 40% defect were generated and plotted in Fig. 11 for the 0.317 cm voxel size. The circumferential profiles were scaled using the expected count described previously. A value closer to 1.0 is preferred in the profile as it indicates a more accurate quantitative method. Results for AC+SC, AC+SC+RC, and AC+SC+RC+PC using projections of the template reconstructed alone and as a fractional addition to the emission data23, 40 are presented. As can be seen in this figure the addition of RC to AC+SC is needed to make the defect visible, in line with our clinical experience.11 After PC, the defect is clearly visible and the maximum signal to background ratio (contrast) in the defect location seems to approach the original 40% decrease in activity. AC+SC+RC+PC overestimated the size of the defect slightly while the FBP profile confirms the visual impression of Fig. 5 that FBP overestimated the size as well as the contrast (∼62%) of the defect due to the nearby liver as already seen in Figs. 234. Also, FBP, as expected, is not quantitative (no compensation for physical degradations), and although AC+SC and AC+SC+RC are an improvement quantitatively, they still fall short of being accurate. Also, note the difference in defect contrast in PC when using the template and fractional template. The maximum percent difference in defect contrast for the 0.317 cm voxel data between PC using the template projections reconstructed separately and adding a fraction of the template to the emission data before reconstruction to estimate the recovery coefficients are 22.6%±6.7% with normal and 30.6%±7.2% with abnormal motion for the 100% inferior wall defects. These values decreased to 5.9%±4.3% and 8.3%±5.0% for 40% inferior wall defects, and to even smaller values for the lesser defects.
Figure 11.
Circumferential profiles of selected short axis slices for the simulated distribution including a 40% decrease with abnormal wall motion and thickening reconstructed using the 0.317 voxel size comparing PVE compensation using the reconstructed template projections, and a fraction of the template projections added to the emission projection before reconstruction. The true source distribution profiles, profiles without PVE compensation (AC+SC+RC and AC+SC), and FBP are given for comparison.
Figure 12 summarizes the results of the accuracy with which PC can reproduce perfusion defects of different maximal severities. Five different noise realizations (represented by the error bars) were generated to illustrate the variability in correction with noise for each case shown. Figures 12a, 12b show the results for the inferior defect (a) without and (b) with regional contractual motion and thickening abnormalities. Figures 12c, 12d are the equivalent results for the anterior defects. Note that the presence of a regional decrease in contraction and thickening in addition to the perfusion defect causes an additional decrease in the apparent severity of the perfusion defect. Also note that the severity of the defects (maximal depth) is generally underestimated. The exception to this is for defects of 40% or less in the inferior wall for the case of the abnormal regional contraction and thickening [Fig. 12b]. This lack of under estimation is explained by the depth of the defects in the inferior wall being enhanced by correlative noise generated during scatter compensation introducing an artifactual decrease in counts in the defect location which is quite near the liver. This artifactual decrease is seen as a slight reduction in counts visible when no defect and no wall motion abnormality are present [Fig. 12a], and a larger reduction when the wall motion abnormality (less thickening) is present [Fig. 12b]. This observation was confirmed by reconstructing only the primary photons. Also, the circumferential profiles of the anterior wall defects do not show the same biases [Figs. 12c, 12d]. They show only some increased variation between noise realizations in the inferior wall portion of the circumferential profiles.
Figure 12.
Circumferential profiles representing the inferior [(a), (b)] and anterior [(c), (d)] defects where (a) and (c) are without, and (b) and (d) with motion abnormalities, respectively.
DISCUSSION
Our results have shown both visually and quantitatively that very good recovery of the simulated count levels can be achieved with PVE correction in structures containing uniform counts, and for which templates can be defined. However, in areas of impaired blood flow (decreased activity), PVE compensation failed to remove the bias created by the spillover of activity from within the myocardial wall (Fig. 12). That is, PVE correction cannot clean up regions (defects for example) for which they are not provided a unique template. We believe that filtering to control noise is also responsible for a portion of this bias. In comparing the circumferential profiles of the inferior defects with that of the anterior defects, it is obvious that imperfect scatter compensation adds to the bias in the more challenging case where large amounts of extra cardiac activity are present. The variation (standard deviation) with noise realization in Figs. 12a, 12b makes it obvious that we can distinguish a 20% reduction in blood flow when large amounts of extracardiac activity abut the inferior wall. Data not shown suggest that it is more difficult to distinguish a 10% reduction in blood flow in the inferior wall, but not so in the anterior wall.
It is evident from Fig. 11 there are quantitative differences when the recovery coefficients are estimated using a fraction of the heart template projections added to the emission projections compared to when the template projections are reconstructed separately. The reason for these differences is twofold. First, the difference in the distribution of counts in the emission and template maps are to blame. The templates by definition have uniform counts in the heart wall while the diseased hearts have decreased counts in the affected area. Reconstructing a combination of these two sets of projections leads to the template reconstructions taking some of the properties of the emission reconstructions. We observed a decrease in counts in the template reconstructions that becomes more prominent with increasing defect contrast. Second, a smaller contributing factor is the mismatch in wall thickness in the defect area between the template and emission data as is noticeable in the differences in the values for normal and abnormal motion. A limitation of the fractional template approach is that scatter compensation methods using the emission data to estimate scatter will have a bias due to the fraction of the template added. Some scaling strategy might remove such a bias.
For the majority of data in this study we assumed perfect registration between segmented anatomy (template) and reconstructed emission data with only averaging or re-sampling of the template voxels down to the emission voxel sizes using linear interpolation. However, the myocardial wall in the template differed in thickness and contraction pattern at the location of the inferior and anterior wall defects in some cases, making it more realistic. The amplitude of respiratory motion41 in patients and normal volunteers while lying in the imaging position was determined to be on the order of at most 10 mm in the axial direction which is less significant than previously thought. We also showed that the effect of respiratory motion presents itself as a slight decrease in activity (cooling) in the inferior and anterior portions of the myocardium and that it is more evident with finer sampling of the emission images.45 These decreases in activity are due to position averaging of the myocardium when respiratory motion is of similar amplitude as the myocardium thickness. PVE compensation will not be able to undo these averaging effects, however, correction methods for cardiac respiratory motion46 are under development which may be able to decrease this cause of mismatch. Mismatches due to poor registration or body motion (rigid or nonrigid) between the CT and emission portion of a SPECT∕CT study will have a greater impact on the qualitative and quantitative assessment of myocardium than the position averaging of respiratory motion (illustrated by the results of Figs. 78). In this case the misalignment could be due to a discrepancy in the respiratory stage of breath hold CT and the average respiration in SPECT. We would expect a 10 mm mismatch to present a significant challenge to our methods if not corrected. Errors in cardiac PET∕CT due to misregistration47 are receiving more attention and that can be used to guide correction of such errors in SPECT∕CT.
Another possible source of error could be the segmentation of the templates from the high-resolution contrast-enhanced gated-CT data. In our simulated case, an error of a voxel either way at the emission sampling (0.317 cm), would mean including or excluding 6 voxels at the in-plane sampling (0.058 cm) and 2.5 voxels at the cross-plain sampling (0.117 cm) of the CT, respectively. We already showed in another study48 that it is possible to get a fairly accurate segmentation of the contrast enhanced gated CT myocardium and separation from the liver using a combination of the gray scale histogram method, thresholding and region growing. From Fig. 10 it is obvious that segmentation errors slowly deteriorate the results of our proposed PVE compensation technique. The heart wall also appears thicker or thinner as in the case when not employing the filling fraction, but less severe and with smoother edges.
CONCLUSIONS
In this work we have described an improved template-projection-reconstruction compensation method7 for PVE by incorporating a filling fraction (F). We also evaluated the addition of fractional template projections to the emission data to balance the template convergence with that of the emission reconstruction, and endeavor to move toward improving image quality as well as image quantification.
The enhanced template-projection-reconstruction compensation method was seen to dramatically improve the fidelity of the rendering of the myocardial wall in the case of normal studies beyond that obtainable with AC, SC, and RC alone. The maximums in the cardiac wall were recovered and the impact of the nearby liver was dramatically reduced. This recovery was remarkable given the clinically realistic cardiac wall count level in the simulated projection data. Thus, PVE compensation is an excellent method to accurately correct uniform myocardial activity and so improve absolute quantification of blood flow and coronary flow reserve by SPECT.5, 6, 7 However, PVE compensation as implemented in this work [Eq. 3] is in essence a scaling technique based on prior knowledge of the myocardium. Without prior knowledge of the size and shape of the perfusion defect, spillover (due to residual blurring and filter effects) from the adjacent normal myocardium stays uncorrected. This is illustrated in our study by a decrease in the heart wall contrast and an overestimate of absolute counts in the defect (Fig. 12). For a lesion with increased activity, with size and shape unknown, and embedded in some background, the improvement in accuracy will also depend on the size of the larger background structure for which prior knowledge is available.
ACKNOWLEDGMENTS
This work was supported by the National Heart, Lung and Blood Grant No. HL 50349 and a grant from Philips Medical Systems. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or Philips Medical Systems.
References
- Hoffman E. J., Huang G., and Phelps M. E., “Quantitation in positron emission computed tomography: 1. Effect of object size,” J. Comput. Assist. Tomogr. 3, 299–308 (1979). [DOI] [PubMed] [Google Scholar]
- Kessler R. M., J. R.Ellis, Jr., and Eden M., “Analysis of emission tomographic scan data: limitations imposed by resolution and background,” J. Comput. Assist. Tomogr. 10.1097/00004728-198406000-00028 8, 514–522 (1984). [DOI] [PubMed] [Google Scholar]
- Cherry S. R., Sorenson J. A., and Phelps M. E., Physics in Nuclear Medicine, 3rd ed. (Saunders, Philadelphia, 2003). [Google Scholar]
- Galt J. R., Garcia E. V., and Robbins W. L., “Effects of myocardial wall thickness on SPECT quantification,” IEEE Trans. Med. Imaging 10.1109/42.56338 9, 144–150 (1990). [DOI] [PubMed] [Google Scholar]
- Zaidi H. and Hasegawa B. H., “Determination of the attenuation map in emission tomography,” J. Nucl. Med. 44, 291–315 (2003). [PubMed] [Google Scholar]
- Iida H. and Eberl S., “Quantitative assessment of regional myocardial blood flow with thallium-201 and SPECT,” J. Nucl. Cardiol. 5, 313–331 (1998). [DOI] [PubMed] [Google Scholar]
- Da Silva A. J. et al. , “Absolute quantification of regional myocardial uptake of 99mTc-sestamibi with SPECT: Experimental validation in a porcupine model,” J. Nucl. Med. 42, 772–779 (2001). [PubMed] [Google Scholar]
- Cook D. J. et al. , “Thallium-201 for myocardial imaging: appearance of the normal heart,” J. Nucl. Med. 17, 583–589 (1976). [PubMed] [Google Scholar]
- Clausen M. et al. , “Circumferential wall thickness measurements of the human left ventricle: reference data for thallium-201 single-photon emission computed tomography,” Am. J. Cardiol. 58, 827–831 (1986). [DOI] [PubMed] [Google Scholar]
- Gewirtz H. et al. , “The influence of left ventricular volume and wall motion in myocardial images,” Circulation 59, 1172–1177 (1979). [DOI] [PubMed] [Google Scholar]
- Narayanan M. V. et al. , “Human observer ROC evaluation of attenuation, scatter and resolution compensation strategies for Tc-99m myocardial perfusion imaging,” J. Nucl. Med. 44, 1725–1734 (2003). [PubMed] [Google Scholar]
- Pretorius P. H. et al. , “A study of the influence of local variations in myocardial thickness on SPECT perfusion imaging,” IEEE Trans. Nucl. Sci. 10.1109/TNS.2002.803809 49, 2304–2308 (2002). [DOI] [Google Scholar]
- DePuey E. G. and Garcia E. V., “Optimal specificity of thallium-201 SPECT through recognition of imaging artifacts,” J. Nucl. Med. 30, 441–449 (1989). [PubMed] [Google Scholar]
- Pretorius P. H. et al. , “Reducing the influence of the partial volume effect on SPECT activity quantitation with 3D modelling of spatial resolution in iterative reconstruction,” Phys. Med. Biol. 10.1088/0031-9155/43/2/014 43, 407–420 (1998). [DOI] [PubMed] [Google Scholar]
- Muller-Gärtner H. W. et al. , “Measurement of radiotracer concentration in brain gray matter using positron emission tomography: MRI-based correction for partial volume effects,” J. Cereb. Blood Flow Metab. 12, 571–583 (1992). [DOI] [PubMed] [Google Scholar]
- Tang H. R., Brown J. K., and Hasegawa B. H., “Use of x-ray CT-defined regions of interest for the determination of SPECT recovery coefficients,” IEEE Trans. Nucl. Sci. 44, 1594–1599 (1997). [Google Scholar]
- Muller S. P. et al. , “Maximum-likelihood estimation: A mathematical model for quantitation in nuclear medicine,” J. Nucl. Med. 31, 1693–1701 (1990). [PubMed] [Google Scholar]
- Hutton B. F. and Osiecki A., “Correction of partial volume effects in myocardial SPECT,” J. Nucl. Cardiol. 5, 402–413 (1998). [DOI] [PubMed] [Google Scholar]
- Hutchins G. D., Caraher J. M., and Raylman R. R., “A region of interest strategy for minimizing resolution distortions in quantitative myocardial PET studies,” J. Nucl. Med. 33, 1243–1250 (1992). [PubMed] [Google Scholar]
- Muzik O. et al. , “Validation of nitrogen-13-ammonia tracer kinetic model for quantitation of myocardial blood flow using PET,” J. Nucl. Med. 34, 83–91 (1993). [PubMed] [Google Scholar]
- Nuyts J. et al. , “Three-dimensional correction for spillover and recovery of myocardial PET images,” J. Nucl. Med. 37, 767–774 (1996). [PubMed] [Google Scholar]
- Rousset O. G., Ma Y., and Evans A. C., “Correction for partial volume effects in PET: Principle and validation,” J. Nucl. Med. 39, 904–911 (1998). [PubMed] [Google Scholar]
- Du Y., Tsui B. M. W., and Frey E. C., “Partial volume effect compensation for quantitative brain SPECT imaging,” IEEE Trans. Med. Imaging 10.1109/TMI.2005.850547 24, 969–976 (2005). [DOI] [PubMed] [Google Scholar]
- Soret M., Bacharach S. L., and Buvat I., “Partial-volume effect in PET tumor imaging,” J. Nucl. Med. 10.2967/jnumed.106.035774 48, 932–945 (2007). [DOI] [PubMed] [Google Scholar]
- Teo B.-K. et al. , “Partial-volume correction in PET: Validation of an iterative postreconstruction method with phantom and patient data,” J. Nucl. Med. 48, 802–810 (2007). [DOI] [PubMed] [Google Scholar]
- Boussion N. et al. , “A multiresolution image based approach for correction of partial volume effects in emission tomography,” Phys. Med. Biol. 10.1088/0031-9155/51/7/016 51, 1857–1876 (2006). [DOI] [PubMed] [Google Scholar]
- Tsui B. M. W. et al. , “Quantitative cardiac SPECT reconstruction with reduced image degradation due to patient anatomy,” IEEE Trans. Nucl. Sci. 10.1109/23.340655 41, 2838–2844 (1994). [DOI] [Google Scholar]
- Pretorius P. H. et al. , “A mathematical model of motion of the heart for use in generating source and attenuation maps for simulating emission imaging,” Med. Phys. 10.1118/1.598746 26, 2323–2332 (1999). [DOI] [PubMed] [Google Scholar]
- Pretorius P. H., King M. A., and Gifford H. C., “A 5-dimensional mathematical model for regional and global changes in cardiac uptake and motion,” IEEE Trans. Nucl. Sci. 10.1109/TNS.2004.835764 51, 2634–2640 (2004). [DOI] [Google Scholar]
- Gowda R. M. et al. , “Reversible myocardial dysfunction: basics and evaluation,” Int. J. Cardiol. 97, 349–353 (2004). [DOI] [PubMed] [Google Scholar]
- Ljungberg M. and Strand S. E., “A Monte Carlo program for the simulation of scintillation camera characteristics,” Comput. Methods Programs Biomed. 10.1016/0169-2607(89)90111-9 29, 257–272 (1989). [DOI] [PubMed] [Google Scholar]
- Pan T.-S., Luo D.-S., and King M. A., “Design of An efficient 3-D projector and backprojector pair for SPECT,” Proceedings of fully 3-D Image Reconstruction in Radiology and Nuclear Medicine, France, 1995.
- Ichihara T. et al. , “Compton scatter compensation using the triple-energy window method for single- and dual-isotope SPECT,” J. Nucl. Med. 34, 2216–2221 (1993). [PubMed] [Google Scholar]
- Byrne C. L., “Block-iterative methods for image reconstruction from projections,” IEEE Trans. Image Process. 10.1109/83.499919 5, 792–794 (1996). [DOI] [PubMed] [Google Scholar]
- King M. A. et al. , “An investigation of the filtering of TEW scatter estimates used to compensate for scatter with ordered subset reconstructions,” IEEE Trans. Nucl. Sci. 10.1109/23.596978 44, 1140–1145 (1997). [DOI] [Google Scholar]
- McCarthy A. W. and Miller M. I., “Maximum likelihood SPECT in clinical computation times using mesh-connected parallel computers,” IEEE Trans. Med. Imaging 10.1109/42.97593 10, 426–436 (1991). [DOI] [PubMed] [Google Scholar]
- Liow J.-S. and Strother S. C., “The convergence of object dependent resolution in maximum likelihood based tomographic image reconstruction,” Phys. Med. Biol. 38, 66–70 (1993). [DOI] [PubMed] [Google Scholar]
- Fessler J. A. and Rogers M. L., “Spatial resolution properties of penalized-likelihood image reconstruction: space-invariant tomographs,” IEEE Trans. Image Process. 10.1109/83.535846 5, 1346–1358 (1996). [DOI] [PubMed] [Google Scholar]
- Pan T.-S., Luo D.-S., and King M. A., “Influence of OSEM, elliptical orbits and background activity on SPECT 3D resolution recovery,” Phys. Med. Biol. 43, 2517–2529 (1997). [DOI] [PubMed] [Google Scholar]
- Boening G., Pretorius P. H., and King M. A., “Study of relative quantification of Tc-99m with partial volume effect and spillover correction for SPECT oncology imaging,” IEEE Trans. Nucl. Sci. 53, 1205–1212 (2006). [Google Scholar]
- McLeish K. et al. , “A study of the motion and deformation of the heart due to respiration,” IEEE Trans. Med. Imaging 10.1109/TMI.2002.804427 21, 1142–1150 (2002). [DOI] [PubMed] [Google Scholar]
- Nuyts J. et al. , “A study of the liver-heart artifact in emission tomography,” J. Nucl. Med. 36, 133–139 (1995). [PubMed] [Google Scholar]
- King M. A. et al. , “A Monte Carlo investigation of artifacts caused by liver uptake in single-photon emission computed tomography perfusion imaging with technetium 99m-labeled agents,” J. Nucl. Cardiol. 3, 18–29 (1996). [DOI] [PubMed] [Google Scholar]
- Pretorius P. H., King M. A., and Bruyant P. P., “Influence of mismatched CT anatomy on the accuracy of partial volume compensation in cardiac SPECT perfusion imaging,” Proceedings of 2004 IEEE Medical Imaging Conference, Rome.
- Pretorius P. H. and King M. A., “A study of possible causes of artifactual decreases in the left ventricular apex with SPECT cardiac perfusion imaging,” IEEE Trans. Nucl. Sci. 10.1109/23.790815 46, 1016–1023 (1999). [DOI] [Google Scholar]
- Kovalski G., Israel O., Keidar Z. Frenkel A., Sachs J., and Azhari H., “Correction of heart motion due to respiration in clinical myocardial perfusion SPECT scans using respiration gating,” J. Nucl. Med. 48, 630–636 (2007). [DOI] [PubMed] [Google Scholar]
- Gould K. L. et al. , “Frequent diagnostic errors in cardiac PET∕CT due to misregistration of CT attenuation and emission PET images: A definitive analysis of causes, consequences, and corrections,” J. Nucl. Med. 10.2967/jnumed.107.039792 48, 1112–1121 (2007). [DOI] [PubMed] [Google Scholar]
- Pretorius P. H., Pan T.-S., Narayanan M. V., and King M. A., “A study of the influence of local variations in myocardial thickness on SPECT perfusion imaging,” IEEE Trans. Nucl. Sci. 10.1109/TNS.2002.803809 49, 2304–2308 (2002). [DOI] [Google Scholar]