Abstract
Purpose
Androgen deprivation therapy (ADT) is first-line therapy for patients with prostate cancer (PCA) who experience biochemical recurrence (BCR). However, the optimal timing of ADT initiation is uncertain, and earlier ADT initiation can cause toxicities that lower quality of life (QOL). We tested the hypothesis that elevated cancer anxiety leads to earlier ADT initiation for BCR in older men.
Patients and Methods
We conducted a prospective cohort study of older patients with BCR of PCA (n = 67). Patients completed questionnaires at presentation and each follow-up visit until initiation of ADT. PCA-specific anxiety was measured with the Memorial Anxiety Scale for Prostate Cancer (MAX-PC). Other collected data included demographics, clinical information, and general anxiety information. Treating oncologists were surveyed about their recommendations for ADT initiation. The primary outcome was the time to ADT initiation. Univariate, multivariate logistic regression, and time-to-event analyses were conducted to evaluate whether cancer anxiety was a predictor of earlier initiation of ADT.
Results
Thirty-three percent of patients initiated ADT at the first or second clinic visit. Elevated PCA anxiety (MAX-PC > 16) was the most robust predictor in multivariate analyses of early initiation (odds ratio [OR], 9.19; P = .01). PSA also independently correlated with early initiation (OR, 1.31; P = .01). PSA did not correlate with MAX-PC.
Conclusion
Cancer anxiety independently and robustly predicts earlier ADT initiation in older men with BCR. For older patients with PCA, earlier ADT initiation may not change life expectancy and can negatively impact QOL. PCA-specific anxiety is a potential target for a decision-making intervention in this setting.
INTRODUCTION
Prostate cancer (PCA) recurrence after surgery or radiation therapy for presumably localized disease is common (25% to 40% at 10 years).1 Biochemical cancer recurrence (BCR) is defined as the asymptomatic increase of prostate-specific antigen (PSA) after treatment for presumed localized disease with no evidence of disease on imaging studies. On the basis of retrospective extrapolation from studies in high-risk patients who experience BCR, androgen deprivation therapy (ADT) for BCR is an accepted standard of care.2 Nevertheless, the increase of ADT toxicity data has made immediate ADT for BCR controversial especially for older men.3
ADT-associated toxicities include decrements in quality of life,4–7 adverse bone and body composition changes,8–10 worsened physical functioning,11 and increased cardiovascular risk.12 These may be especially relevant in older patients, whose shorter life expectancies additionally limits any potential survival benefit from early ADT. The median time from BCR to bone metastases is greater than 5 years, and a cost-effectiveness analysis suggests that it is optimal to wait for bony metastasis to develop before initiation of ADT.13 However, a recent review on PCA treatment decisions indicated that men do not weigh adverse effects heavily,14 and the use of ADT is expanding.15
As in other clinical scenarios, patients who experience BCR have been characterized as high risk and low risk on the basis of disease features such as Gleason score, time from initial treatment until BCR, absolute PSA value, and PSA doubling time (PSA-DT). It has been suggested to utilize these factors in decisions to initiate ADT1; however, no professional consensus exists.16,17 Thus, other factors are likely to play a significant role in the determination of the timing of ADT. An analysis of the Surveillance, Epidemiology, and End Results–Medicare database showed that the identity of the treating urologist has a major influence on ADT timing.18 It is reasonable to hypothesize that patient psychological factors will also have an impact; however, to our knowledge, little research has been done to ascertain which patient psychological variables impact the timing of ADT for BCR.
Patient anxiety about PCA could be considered one such psychological variable. Anxiety is elevated for many men during the clinical course of PCA, including before clinical decision points.19 For example, cancer anxiety leads to earlier intervention in men who undergo active surveillance for localized disease.20 If cancer anxiety in patients has an influence on early initiation of ADT for BCR, it may cause men with asymptomatic disease to accept treatment toxicities without a life expectancy gain. Within a prospective cohort study, we tested the hypothesis that cancer-specific anxiety contributes to earlier initiation of ADT in older men who experience BCR.
PATIENTS AND METHODS
Participants
Older patients with PCA were recruited from the University of Chicago Genitourinary Oncology Clinic at the time of initial appointment for BCR. Inclusion criteria were an increasing PSA after initial therapy for localized prostate cancer and no evidence of cancer on imaging studies. BCR was defined as an increase in the PSA level on at least two successive measurements that were taken at least 2 weeks apart after definitive local therapy.21 Patients were excluded if they did not speak English, if they carried a diagnosis of dementia, or if they had previously received ADT. All provided written informed consent.
All oncology physicians (N = 20) in the Genitourinary Oncology Clinic who saw these patients, including attending physicians and oncology fellows, were invited to complete a one-time survey regarding initiation of ADT in men who experience BCR. All invited physicians participated in the survey.
Instruments and Measures
Data collection included patient surveys, physician surveys, and medical chart extraction by using a standardized data collection form. Patients completed an initial baseline survey and a shorter follow-up survey at each subsequent visit until they started on ADT. Patients completed the surveys in clinic or shortly after the clinic visit. A survey was mailed to any patients without a follow-up visit for 6 months. For psychological variables, patients were asked to answer while they considered their states of mind at the time of their previous clinic visits.
Patient demographics were collected during the initial survey. At each appointment, patients' psychological state and physical functioning were recorded by using the following validated instruments: Memorial Anxiety Scale for Prostate Cancer (MAX-PC),22–24 Hospital Anxiety and Depression Scale (HADS),25,26 Independent Activities of Daily Living (IADL), Intolerance of Uncertainty Scale (IUS),27 and the Vulnerable Elders Survey (VES-13).28 Finally, questions regarding the decision process for initiation of ADT and the patient's perception of his and the physician's relative contributions to the decision were asked.
Oncologists who cared for the participating patients with PCA in the clinic completed surveys that included sociodemographics, professional training, and preferences regarding appropriate treatment for BCR (Table 1).29 All physicians completed the survey anonymously during nonclinic times, and no data were linked to specific patients. Variables regarding the responding physicians' treatment practices and preferences were measured with multiple-choice and open-ended questions. A key portion of the survey asked respondents to rate the importance of 16 factors for deciding to initiate ADT for BCR on a 1 to 5 scale (1, not important; 2, of little importance; 3, moderately important; 4, important; 5, very important; Table 2).
Table 1.
Physician Characteristics
| Characteristic | Data by Type of Physician |
|||||
|---|---|---|---|---|---|---|
| Attending (n = 7) |
Fellow (n = 13) |
Total (N = 20) |
||||
| No. | % | No. | % | No. | % | |
| Age, years | ||||||
| Mean | 37.1 | 33.0 | 34.5 | |||
| SD | 3.3 | 4.6 | 4.6 | |||
| Years since completing residency | ||||||
| Mean | 5.9 | 1.4 | 3.0 | |||
| SD | 1.6 | 1.2 | 2.5 | |||
| Sex | ||||||
| Male | 6 | 86 | 7 | 54 | 13 | 65 |
| Female | 1 | 14 | 6 | 46 | 7 | 35 |
| Estimated No. of PCA patients seen weekly | ||||||
| 1-5 | 2 | 29 | 8 | 71 | 10 | 50 |
| 6-10 | 2 | 29 | 5 | 38 | 7 | 35 |
| 10-15 | 0 | 0 | 0 | 0 | 0 | 0 |
| 16-20 | 2 | 29 | 0 | 0 | 2 | 10 |
| > 20 | 1 | 14 | 0 | 0 | 1 | 5 |
| Estimated No. of recurrent PCA patients seen weekly | ||||||
| 1-3 | 5 | 71 | 6 | 46 | 11 | 55 |
| 4-7 | 1 | 14 | 5 | 38 | 6 | 30 |
| 7-10 | 0 | 0 | 2 | 15 | 2 | 10 |
| > 10 | 1 | 14 | 0 | 0 | 1 | 5 |
Abbreviations: SD, standard deviation; PCA, prostate cancer.
Table 2.
Treating Physicians Importance Ratings (0-5) for Initiation of ADT for BCR
| Variable | Mean Importance Rating Scores (0-5) by Physician Type |
||
|---|---|---|---|
| Attending (n = 7) | Fellow (n = 13) | Total (N = 20) | |
| PSA doubling time* | 4.7 | 4.8 | 4.8 |
| Patient preference | 4.6 | 4.4 | 4.5 |
| Time from primary treatment to PSA increase* | 4.4 | 3.9 | 4.1 |
| PSA level | 4.1 | 4.0 | 4.1 |
| Patient remaining life expectancy | 4.0 | 4.0 | 4.0 |
| Gleason grade* | 4.1 | 3.8 | 3.9 |
| Efficacy of treatment | 3.7 | 4.0 | 3.9 |
| Adverse effects of treatment | 4.0 | 3.8 | 3.9 |
| Stage at diagnosis | 3.9 | 3.8 | 3.8 |
| Geriatric comorbidities† | 3.7 | 3.7 | 3.7 |
| Previous treatment history | 3.3 | 3.9 | 3.7 |
| Medical comorbidities‡ | 3.6 | 3.5 | 3.5 |
| Partner preferences | 3.9 | 3.8 | 3.4 |
| Level of patient anxiety about cancer | 3.6 | 3.3 | 3.4 |
| PSA at diagnosis | 3.3 | 3.3 | 3.3 |
| Treatment cost | 2.4 | 2.6 | 2.6 |
Abbreviations: ADT, androgen deprivation therapy; BCR, biochemical cancer recurrence; PSA, prostate-specific antigen.
Factors found important in the literature for predicting response to ADT.
Examples of geriatric comorbidities include cognitive impairment, functional losses, osteoporosis.
Examples of medical comorbidities include cardiovascular disease, diabetes, chronic renal insufficiency.
Gleason scores, PSA values, previous treatments (surgery v radiation), treatment dates, comorbidities, and medications for each patient in the sample were extracted from the medical record. Gleason scores were recorded in three categories: 2 to 6; 7; and 8 to 10. PSA-DT was calculated as the natural log of 2 (ie, 0.693) divided by the slope of the relationship between the log of PSA and time of PSA measurement for each patient.30 For the patients who elected to start ADT, the start date was recorded.
Statistical Analyses
The primary outcome of interest was the timing of the patient-reported decision to start ADT. This decision was confirmed by physician notes, and pharmacy confirmation was provided for all but two patients. For the logistic regression analysis, we used the patient survey response as the outcome; for the time-to-event analysis, we used pharmacy confirmation. Although longitudinal patient data was collected during repeated clinic visits, the primary hypothesis was that patients with elevated PCA-specific anxiety would initiate ADT at the initial visit or at the first follow-up visit. For the primary analysis, patients who decided to start on ADT at either of these first two appointments were coded as early initiators. Others were considered late initiators and included those who started later or not at all within 2 years of enrollment.
Clinical and psychological variables were evaluated as potential predictors of early initiation of ADT. The key hypothesized predictor was PCA-specific anxiety, as measured by the MAX-PC anxiety subscale. We adapted the criterion used by the MAX-PC developers to determine a cutoff score of clinically significant, elevated PCA-specific anxiety, and the MAX-PC was used dichotomously throughout all analyses. Scores of 16 or greater, which corresponded to an approximate average score of 1.5 on the 0 to 3 scale implemented for each item in the MAX-PC, were rated as elevated.23 All hypothesized predictors were tested first in univariate relationships to initiation of ADT at first or second clinic visit (ie, early initiation) or not (late initiation) by using Spearman ρ correlation, point-biserial correlation, Kendall τ-c, or χ2 analysis, as appropriate. Multivariate analyses were conducted to account for the independent predictive contribution of multiple variables. Three logistic regression models were used to test which variables best predicted early initiation of ADT and to test the primary hypothesis that elevated PCA-specific anxiety is a significant independent predictor of early ADT. A logistic regression with simultaneous entry was performed on the five predictors that were statistically significant at the P < .05 level from the univariate analyses. A second model tested the predictors based on the clinicians' survey answers of the five most important decisional factors, with the exception of patient preferences. A third model included the MAX-PC variable with these five factors.
Finally, a Kaplan-Meier time-to-event analysis was conducted; starting on ADT was the event of interest, and the two groups for comparison were based on an elevated MAX-PC score (≥ 16) or not. For the time-to-event analysis, the event was the date on which the patient started ADT, as determined by medical records. The time between appointments was determined by usual clinical practice. Time was measured in months and started from the date that the patient was enrolled onto the study to 36 months of medical chart follow-up. For patients who did not start ADT, data was right-censored at the point at which the last clinical information was available to confirm that ADT has not been initiated. Separate survival functions were computed for the 15 elevated-anxiety patients and for the 52 nonanxious patients. The two groups compared were those with MAX-PC scores 16 or greater, which indicated high PCA-specific anxiety at presentation, and those with lower MAX-PC scores, which indicated lower levels of PCA-specific anxiety.23 All data analyses were performed by using the Statistical Package for the Social Sciences version 15 (SPSS 15.0, SPSS Inc, Chicago, IL).
RESULTS
Patients
A total of 67 qualifying patients were enrolled onto the study (Table 3); the average age was 68 years, and more than 70% indicated that their general health was very good or excellent. More than half scored 0 on the VES-13, which confirmed patients' impressions of good overall health status.
Table 3.
Patient Characteristics
| Variable | Data (%) |
|---|---|
| Age, years | |
| Mean | 67.9 |
| SD | 9.2 |
| Ethnicity | |
| White | 76 |
| African American | 24 |
| Income, $ | |
| < 35,000 | 13 |
| 35,000-50,000 | 18 |
| 50,000-100,000 | 29 |
| > 100,000 | 30 |
| Marital status | |
| Married | 73 |
| Single | 3 |
| Divorced, separated, widowed | 24 |
| Education | |
| Less than high school graduate | 10 |
| High school graduate | 9 |
| Partial college | 21 |
| College or graduate degree | 60 |
| Current health | |
| Excellent | 23 |
| Very good | 48 |
| Good | 23 |
| Fair | 5 |
| Poor | 2 |
| VES-13 | |
| 0 | 52 |
| 1 | 23 |
| 2 | 10 |
| ≥ 3 | 16 |
| Pre-appointment PSA, ng/mL | |
| < 2 | 53 |
| 2-10 | 33 |
| > 10 | 14 |
| PSA doubling time | |
| Mean | 7.6 |
| SD | 14.5 |
| Gleason grade total* | |
| Low | 57 |
| Moderate | 28 |
| High | 15 |
| Time to recurrence (months) | |
| Mean | 58.4 |
| SD | 44.1 |
| Know someone with PCA | |
| Father | 18 |
| Brother (one or more) | 16 |
| Son (one or more) | 2 |
| Other relative | 19 |
| Friend | 64 |
Abbreviations: VES-13, Vulnerable Elders Survey-13; PSA, prostate-specific antigen; PCA, prostate cancer.
Gleason grade total score ranges from 2 to 10: low is 2 to 6; moderate, 7; and high, 8 to 10.
The 11-item MAX-PC anxiety subscale exhibited excellent internal consistency reliability (Cronbachs α, 0.91). As others also have shown,22,24 the MAX-PC at presentation strongly correlated with the general anxiety score on the HADS-A, (r(67) = 0.61; P < .001), which is a well-established measure of general anxiety. The MAX-PC also correlated with the IUS (r(67) = 0.49; P < .001). By using the criterion score of ≥ 16, 22% of the sample had elevated PCA-specific anxiety at presentation.
Physician Survey
Both attending physicians and oncology fellows rated PSA-DT as the most important factor in deciding when to start a patient with BCR on ADT (Table 2). They rated other prognostic clinical factors, such as Gleason score and time from initial therapy to BCR, important as well. They rated patient anxiety as the third-least important factor. They rated patient preferences the second-most important factor.
Predicting the Decision to Start Early ADT
Univariate analyses showed that elevated MAX-PC scores at the initial appointment strongly correlated with early ADT initiation (11.7 v 7.9; χ2 [n = 67], 6.47; P = .01; Table 4). Multiple clinical variables also predicted early initiation at the univariate level, including Gleason score (χ2 [n = 67], 0.30; P = .02), time from primary therapy to BCR in months (χ2 [n = 67], −0.30; P = .02), and absolute PSA (χ2 [n = 67], 0.18; P = .04). PSA-DT was not a significant predictor of starting on ADT. The MAX-PC did not correlate with the PSA or PSA-DT at the initial visit (r [n = 64], 0.12; P = .34; r [n = 60] = −0.20; P = .12) or across all clinic visits (r [n = 125] = 0.04; P = .66; r [n = 120] = −0.16; P = .08).
Table 4.
Univariate Correlates of ADT Initiation at First or Second Visit
| Variable | Coefficient | P |
|---|---|---|
| Demographics | ||
| Age* | −0.24 | .05 |
| Marital status (partner v not)† | 3.88 | .55 |
| Ethnicity (African American v other)† | 0.60 | .44 |
| Education | 0.01 | .80 |
| Clinical characteristics | ||
| PSA* | 0.18 | .04‡ |
| PSA doubling time* | −0.21 | .11 |
| Gleason score* | 0.30 | .02‡ |
| Time to recurrence* | −0.30 | .02‡ |
| Current health* | −0.25 | .05‡ |
| VES-13* | −0.11 | .41 |
| Psychological measures | ||
| Elevated MAX-PC†§ | 5.00 | .03‡ |
| HADS anxiety* | 0.20 | .10 |
| HADS depression* | −0.09 | .43 |
| Intolerance of uncertainty* | 0.00 | .97 |
Abbreviations: ADT, androgen deprivation therapy; PSA, prostate-specific antigen; VES-13, Vulnerable Elders Survey-13; MAX-PC, Memorial Anxiety Scale for Prostate Cancer; HADS, Hospital Anxiety and Depression Scale.
Correlation coefficient.
χ2 coefficient.
Significant P value.
Elevated MAX-PC defined as ≥ 16 on 0 to 33 scale.
Logistic Regressions
Model 1 is the logistic regression of the outcome variable of early ADT initiation that included the five predictors that were significant on univariate analysis at the P < .05 level (Table 5). Elevated MAX-PC was the strongest predictor of early ADT initiation. This model correctly classified 74% of the patient cases.
Table 5.
Logistic Regression on Initiation of ADT at First or Second Clinic Visit
| Predictor Variable | Regression Models |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Significant Bivariate Predictors |
Physician Hypothesis |
||||||||
| Alone |
Plus Anxiety |
||||||||
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| Age | — | — | — | 0.96 | 0.88 to 1.04 | .31 | 0.96 | 0.88 to 1.05 | .39 |
| Current health | 0.87 | 0.40 to 1.89 | .73 | — | — | — | — | — | — |
| PSA doubling time | — | — | — | 1.18 | 0.28 to 4.92 | .82 | 1.95 | 0.39 to 9.68 | .41 |
| Time to recurrence | 0.98 | 0.96 to 1.00 | .03 | 0.98 | 0.97 to 1.00 | .14 | 0.98 | 0.96 to 1.00 | .06 |
| PSA | 1.23 | 1.03 to 1.47 | .03 | 1.22 | 1.03 to 1.46 | .02 | 1.31 | 1.07 to 1.60 | .01 |
| Gleason score ≥ 8 | 0.72 | 0.09 to 5.78 | .75 | 1.71 | 0.28 to 10.44 | .56 | 0.79 | 0.11 to 5.92 | .82 |
| Elevated MAX-PC | 7.11 | 1.33 to 37.85 | .02 | — | — | — | 9.16 | 1.63 to 51.37 | .01 |
Abbreviations: ADT, androgen deprivation therapy; OR, odds ratio; PSA, prostate-specific antigen; MAX-PC, Memorial Anxiety Scale for Prostate Cancer.
Models 2 and 3 are the theory-driven models that are based on the oncologist survey responses of the most important decision factors, with the five most-important clinical variables entered in model 2 and the MAX-PC added in model 3. In model 3, MAX-PC was a significant predictor (odds ratio, 9.10; P = .01), and its entry did not affect the significance of any physician-identified variables entered in model 2. The time from primary therapy to BCR became marginally significant in this latter model, though. Inclusion of elevated MAX-PC improved model fit, as r2 increased from 0.28 to 0.40 (χ2 (df = 1), 6.89; P = .01) and the correctly predicted patient cases increased from 71% to 78%. Of note, PSA-DT was not statistically significant in either model. Age has a listed P value of .05 in Table 4; without rounding, this value is .051. Therefore, it is excluded from the empiric regression model. It is included in other models, because the oncologists rated patient's remaining life expectancy as the fifth-most important consideration for starting ADT.
Time-to-Event Analysis
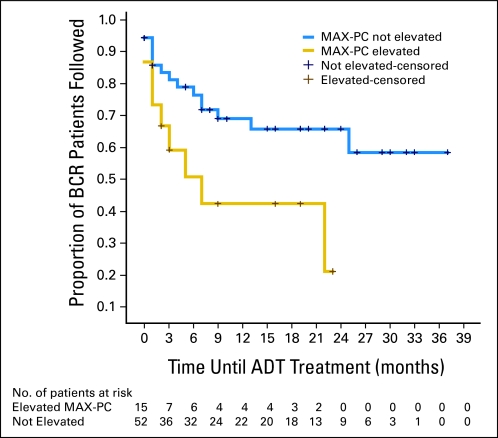
In the Kaplan-Meier time-to-event analysis, patients who had elevated PCA-specific anxiety initiated ADT earlier (Fig 1). Those with elevated MAX-PC scores started ADT, on average, 11.0 months after presentation, whereas those with lower anxiety started after 24.9 months (P = .02).
Fig 1.
Kaplan-Meier time-to-event curves by Memorial Anxiety Scale for Prostate Cancer (MAX-PC) anxiety group by months until biochemical recurrence (BCR) patients receive androgen deprivation therapy (ADT).
DISCUSSION
As hypothesized, PCA-specific anxiety predicts early ADT initiation for older men who experience BCR, even when analysis is controlled for relevant clinical factors. In fact, anxiety is the most robust predictor of early initiation of ADT. Patients with an elevated initial MAX-PC score were much more likely to start ADT by their first follow-up appointment than their nonanxious counterparts.
The predictive value of anxiety for starting ADT was disease specific: significance at the P = .05 level did not extend to a general anxiety measure (HADS), which suggests that it was anxiety about PCA that influenced behavior. Although general anxiety correlated with PCA-specific anxiety, only the PCA-specific anxiety predicted the decision to start ADT earlier.
Additionally, time-to-event data confirmed that there was a difference in the time to pharmacy-confirmed ADT initiation between elevated PCA-specific anxiety patients and their less-anxious counterparts. The anxious patients started ADT within the first year of presentation, whereas the average time to initiation in nonanxious patients was greater than 2 years. Greater than 60% of the nonanxious patients never started ADT during the duration of the study, whereas only 13% of the anxious patients did not. Therefore, patients with elevated anxiety initiated ADT, and experienced its toxicities, greater than 1 year earlier on average. This represents additional lifetime exposure to ADT, as it is the policy in our clinic (as in most places) for patients to remain on ADT for life after its initiation. However, we don't have specific information on lifetime duration in these patients.
Relevant clinical variables were univariate predictors of early ADT initiation, including PSA, Gleason score, and time to recurrence.1,31 In multivariate models, only the PSA score and time to recurrence showed continued predictive value, and PSA was significant in both empirically driven and theory-motivated models. These results support the clinical rankings from the treating physician surveys and, thus, provide face validity for the findings. It is logical that these clinical factors would be the considerations that consciously guide physicians in the decision to initiate ADT for patients who experience BCR.
The physicians rated patient anxiety as relatively low on their list of important decision factors for initiation of ADT. If physicians do not consciously weight patient anxiety when making this decision, why is it such a robust predictor? Physicians rated patient preferences as the second-most important factor in their timing decisions regarding ADT for BCR. This suggests that patient PCA anxiety might be communicated to the physician as a patient preference to start early, and the physician then starts ADT in a shared decision with the patient. This is especially interesting, because patient anxiety is not significantly correlated with the identified clinical factors. Thus, anxiety appears to lead to earlier initiation separately from the clinical factors. If this is true, then patient anxiety independently influences the timing of ADT initiation; anxiety would be a natural target of intervention for those patients in whom earlier initiation may not be beneficial.
There are limitations to our study. It is limited to one academic oncology practice, and it may not be generalizable to other clinical settings with different patient populations and providers. There was some loss to follow-up, and more of those with lower anxiety did not return to clinic, despite significant efforts to follow-up with phone calls and mailed surveys. It does, however, accurately reflect the clinical practice in an intention-to-treat setting. One potential advantage of being on ADT is the requirement of a regular 3- to 4-month follow-up to receive an injection, which ensures regular monitoring of disease status. Although this difference in follow-up is properly reflected as censoring in the time-to-event analysis, it remains a concern for patients with a recurrent cancer who need appropriate interval monitoring. Perhaps the converse of the dangers of toxicities from being anxious and starting ADT earlier is the danger of missing regular follow-up from being inadequately worried. Our sample size in this observational setting is too small to assess more subtle questions about the interplay of anxiety, clinical characteristics, physician choice, and timing of ADT initiation. After starting ADT, patients exit the sample, and a large percentage did so after the first two visits, especially from the most anxious group, which limited our ability to assess these roles over time; this assessment will require future studies.
If it is true that anxious patients who experience BCR are starting on ADT nearly 14 months earlier, for reasons unrelated to clinical factors, would this be important for their care? Patients who experience BCR are, by definition, asymptomatic. For many patients, ADT is a temporizing therapy that typically fails after about 2 years for overtly metastatic disease.32 The time until PCA becomes refractory to ADT when started strictly for BCR is not well characterized, but it is surely longer. The survival benefit of ADT in older men has not been clearly demonstrated, and initiation of ADT earlier may not improve life expectancy in these patients.3 It was shown recently that ADT, compared with watchful waiting, does not improve life expectancy for older men who start it for primary therapy.33 ADT has known toxicities, many of which are especially concerning for older men, who are vulnerable to osteoporosis, fatigue, muscle loss, and falls.11,34,35 The impact of ADT may be quite long lived, and an additional year on ADT for asymptomatic individuals is of concern, especially because anxiety could be a modifiable intervention target.
In addition to understanding how physicians weigh decision factors when making treatment decisions, it is important to collect data about what occurs during the consultation. These data suggest that the nonclinical factor of patient anxiety about cancer may be both important and overlooked in choosing when to initiate ADT for BCR. Future research should be directed towards obtaining better understanding of what information, concerns, questions, and advice are available and shared between patients and their physicians. In the meantime, it is important for physicians to discuss with patients worries and anxieties of the patient about cancer. Doing so may help prevent unnecessary early initiation of a therapy with significant toxicities and questionable impact on life expectancy in otherwise asymptomatic older men.
Footnotes
Supported in part by Paul B. Beeson Career Development Award K23 (W.D.) and by the University of Chicago Cancer Research Center and Center for Health Administration Studies.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: William Dale, Joshua Hemmerich, Kathryn Bylow, Walter M. Stadler
Financial support: William Dale
Administrative support: William Dale, Joshua Hemmerich, Walter M. Stadler
Provision of study materials or patients: William Dale, Kathryn Bylow, Supriya Mohile, Walter M. Stadler
Collection and assembly of data: William Dale, Joshua Hemmerich, Kathryn Bylow, Mary Mullaney, Walter M. Stadler
Data analysis and interpretation: William Dale, Joshua Hemmerich, Kathryn Bylow, Supriya Mohile, Walter M. Stadler
Manuscript writing: William Dale, Joshua Hemmerich, Kathryn Bylow, Supriya Mohile, Mary Mullaney, Walter M. Stadler
Final approval of manuscript: William Dale, Joshua Hemmerich, Kathryn Bylow, Supriya Mohile, Mary Mullaney, Walter M. Stadler
REFERENCES
- 1.Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer–specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–439. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 2.Ryan CJ, Small EJ. High-risk biochemical relapse and the timing of androgen deprivation therapy. J Urol. 2006;176:S61–S65. doi: 10.1016/j.juro.2006.06.072. [DOI] [PubMed] [Google Scholar]
- 3.Loblaw DA, Virgo KS, Nam R, et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2007 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2007;25:1596–1605. doi: 10.1200/JCO.2006.10.1949. [DOI] [PubMed] [Google Scholar]
- 4.Lubeck DP, Gary DG, Peter RC. The effect of androgen deprivation therapy on health-related quality of life in men with prostate cancer. Urology. 2001;58:94–99. doi: 10.1016/s0090-4295(01)01250-x. [DOI] [PubMed] [Google Scholar]
- 5.van Andel G, Kurth KH. The impact of androgen deprivation therapy on health related quality of life in asymptomatic men with lymph node positive prostate cancer. Eur Urol. 2003;44:209–214. doi: 10.1016/s0302-2838(03)00208-2. [DOI] [PubMed] [Google Scholar]
- 6.Higano CS. Side effects of androgen deprivation therapy: Monitoring and minimizing toxicity. Urology. 2003;61(suppl 1):32–38. doi: 10.1016/s0090-4295(02)02397-x. [DOI] [PubMed] [Google Scholar]
- 7.Dacal K, Sereika SM, Greenspan SL. Quality of life in prostate cancer patients taking androgen deprivation therapy. J Am Geriatr Soc. 2006;54:85–90. doi: 10.1111/j.1532-5415.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- 8.Berruti A, Dogliotti L, Terrone C, et al. Changes in bone mineral density, lean body mass and fat content as measured by dual energy x-ray absorptiometry in patients with prostate cancer without apparent bone metastases given androgen deprivation therapy. J Urol. 2002;167:2361–2367. discussion 2367. [PubMed] [Google Scholar]
- 9.Boxer RS, Kenny AM, Dowsett R, et al. The effect of 6 months of androgen deprivation therapy on muscle and fat mass in older men with localized prostate cancer. Aging Male. 2005;8:207–212. doi: 10.1080/13685530500361226. [DOI] [PubMed] [Google Scholar]
- 10.Smith M. Osteoporosis and other adverse body composition changes during androgen deprivation therapy for prostate cancer. Cancer Metastasis Rev. 2002;21:159–166. doi: 10.1023/a:1020840311573. [DOI] [PubMed] [Google Scholar]
- 11.Bylow K, Dale W, Mustian K, et al. Falls and physical performance deficits in older prostate cancer patients undergoing androgen deprivation therapy. Urology. doi: 10.1016/j.urology.2008.03.032. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai HK, D'Amico AV, Sadetsky N, et al. androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J Natl Cancer Inst. 2007;99:1516–1524. doi: 10.1093/jnci/djm168. [DOI] [PubMed] [Google Scholar]
- 13.Bayoumi AM, Brown AD, Garber AM. Cost-effectiveness of androgen suppression therapies in advanced prostate cancer. J Natl Cancer Inst. 2000;92:1731–1739. doi: 10.1093/jnci/92.21.1731. [DOI] [PubMed] [Google Scholar]
- 14.Zeliadt SB, Ramsey SD, Penson DF, et al. Why do men choose one treatment over another? A review of patient decision making for localized prostate cancer. Cancer. 2006;106:1865–1874. doi: 10.1002/cncr.21822. [DOI] [PubMed] [Google Scholar]
- 15.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate cancer survivors. N Engl J Med. 2008;358:1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 16.Loblaw DA, Mendelson DS, Talcott JA, et al. American Society of Clinical Oncology recommendations for the initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer. J Clin Oncol. 2004;22:2927–2941. doi: 10.1200/JCO.2004.04.579. [DOI] [PubMed] [Google Scholar]
- 17.Walsh PC, Deweese TL, Eisenberger MA. A structured debate: Immediate versus deferred androgen suppression in prostate cancer—Evidence for deferred treatment. J Urol. 2001;166:508–515. doi: 10.1016/s0022-5347(05)65972-1. discussion 515-516. [DOI] [PubMed] [Google Scholar]
- 18.Shahinian VB, Kuo YF, Freeman JL, et al. Determinants of androgen deprivation therapy use for prostate cancer: Role of the urologist. J Natl Cancer Inst. 2006;98:839–845. doi: 10.1093/jnci/djj230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dale W, Bilir P, Han M, Meltzer D. The role of anxiety in prostate carcinoma: A structured review of the literature. Cancer. 2005;104:467–478. doi: 10.1002/cncr.21198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Latini DM, Hart SL, Knight SJ, et al. The relationship between anxiety and time to treatment for patients with prostate cancer on surveillance. J Urol. 2007;178:826–832. doi: 10.1016/j.juro.2007.05.039. discussion 831-832. [DOI] [PubMed] [Google Scholar]
- 21.Bubley GJ, Carducci M, Dahut W, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: Recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17:3461–3467. doi: 10.1200/JCO.1999.17.11.3461. [DOI] [PubMed] [Google Scholar]
- 22.Roth AJ, Rosenfeld B, Kornblith AB, et al. The Memorial Anxiety Scale for prostate cancer: Validation of a new scale to measure anxiety in men with prostate cancer. Cancer. 2003;97:2910–2918. doi: 10.1002/cncr.11386. [DOI] [PubMed] [Google Scholar]
- 23.Roth AJ, Nelson CJ, Rosenfeld B, et al. Assessing anxiety in men with prostate cancer: Further data on the reliability and validity of the Memorial Anxiety Scale for prostate cancer (MAX-PC) Psychosomatics. 2006;47:340–347. doi: 10.1176/appi.psy.47.4.340. [DOI] [PubMed] [Google Scholar]
- 24.Dale W, Hemmerich J, Meltzer D. Extending the validity of the Memorial Anxiety Scale for Prostate Cancer (MAX-PC) at the time of prostate biopsy in a racially-mixed population. Psychooncology. 2007;16:493–498. doi: 10.1002/pon.1107. [DOI] [PubMed] [Google Scholar]
- 25.Zigmund AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavia. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 26.Bjelland I, Dahl AA, Haug TT, et al. The validity of the Hospital Anxiety and Depression Scale: An updated literature review. J Psychosomatic Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 27.Buhr K, Dugas MJ. The intolerance of uncertainty scale: Psychometric properties of the English version. Behav Res Therapy. 2002;40:931–945. doi: 10.1016/s0005-7967(01)00092-4. [DOI] [PubMed] [Google Scholar]
- 28.Mohile S, Bylow K, Dale W, et al. A pilot study of the Vulnerable Elders Survey-13 (VES-13) as compared to the Comprehensive Geriatric Assessment (CGA) for identifying disability in older prostate cancer patients receiving androgen ablation. Cancer. 2007;109:802–810. doi: 10.1002/cncr.22495. [DOI] [PubMed] [Google Scholar]
- 29.Ahmad F, Hemmerich J, Bylow K, Stadler WM, Dale W. Variance in physician attitudes toward androgen deprivation therapy initiation for rising PSA for asymptomatic recurrent prostate cancer. http://lungca.asco.org/ASCO/Abstracts+&+Virtual+Meeting/Abstracts?&vmview=abst_detail_view&confID=46&abstractID=20510.
- 30.Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after psa elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 31.D'Amico AV, Moul JW, et al. Surrogate end point for prostate cancer-specific mortality after radical prostatectomy or radiation therapy. J Natl Cancer Inst. 2003;95:1376–1383. doi: 10.1093/jnci/djg043. [DOI] [PubMed] [Google Scholar]
- 32.Eisenberger MA, Blumenstein BA, Crawford ED, et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med. 1998;339:1036–1042. doi: 10.1056/NEJM199810083391504. [DOI] [PubMed] [Google Scholar]
- 33.Lu-Yao GL, Albertsen PC, Moore DF, et al. Survival following primary androgen deprivation therapy among men with localized prostate cancer. JAMA. 2008;300:173–181. doi: 10.1001/jama.300.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bylow K, Mohile SG, Stadler WM, Dale W. Does androgen-deprivation therapy accelerate the development of frailty in older men with prostate cancer? Cancer. 2007;110:2604–2613. doi: 10.1002/cncr.23084. [DOI] [PubMed] [Google Scholar]
- 35.Smith MR. Androgen deprivation therapy for prostate cancer: New concepts and concerns. Curr Opin Endocrinol Diabetes Obes. 2007;14:247–254. doi: 10.1097/MED.0b013e32814db88c. [DOI] [PMC free article] [PubMed] [Google Scholar]