Abstract
Purpose
Optimal therapy of follicular lymphoma (FL) is not defined. We analyzed a large prospective cohort study to identify current demographics and patterns of care of FL in the United States.
Patients and Methods
The National LymphoCare Study is a multicenter, longitudinal, observational study designed to collect information on treatment regimens and outcomes for patients with newly diagnosed FL in the United States. Patients were enrolled between 2004 and 2007. There is no study-specific prescribed treatment regimen or intervention.
Results
Two thousand seven hundred twenty-eight subjects were enrolled at 265 sites, including the 80% of patients enrolled from nonacademic sites. Using the Follicular Lymphoma International Prognostic Index (FLIPI), three distinct groups independent of histologic grade could be defined. Initial therapeutic strategy was: observation, 17.7%; rituximab monotherapy, 13.9%; clinical trial 6.1%; radiation therapy, 5.6%; chemotherapy only, 3.2%; chemotherapy plus rituximab, 51.9%. Chemotherapy plus rituximab regimens were: rituximab plus cyclophosphamide, doxorubicin, vincristine, prednisone, 55.0%; rituximab plus cyclophosphamide, vincristine, and prednisone, 23.1%; rituximab plus fludarabine based, 15.5%; other, 6.4%. The choice to initiate therapy rather than observe was associated with age, FLIPI, stage, and grade (P < .01). Significant differences in treatment (P < .01) across regions of the United States were noted. Contrary to practice guidelines, treatment of stage I FL frequently omits radiation therapy.
Conclusion
Widely disparate therapeutic approaches are utilized for FL. Initial therapy is deferred in a small subset of patients. There is no single standard of care for the treatment of de novo FL, although antibody use is ubiquitous when therapy is initiated. These disparate approaches to the initial care of patients with FL render a heterogeneous group of patients at relapse.
INTRODUCTION
Follicular lymphoma (FL) is the second most common lymphoma in the United States, representing nearly 14,000 patients diagnosed annually.1,2 No consensus exists on the optimal management of FL or even on appropriate short term goals of management. Debate encompasses many topics including: the value of early use of anthracyclines; identifying patients for whom FL will not cause morbidity or mortality and thus do not require therapy; the value of extended dosing maintenance therapy with monoclonal antibodies compared to intermittent redosing; the role of radiation therapy with curative intent in early stage disease; and the optimal role and timing of radioimmunotherapy or stem cell transplantation.
New therapeutic options have likely contributed to the improved outcome of patients with FL in recent years as described by several authors.3–5 However, the rate of new therapy development has rendered systematic comparisons difficult. Many well-constructed phase II trials have restrictive enrollment criteria, and utilize comparisons to historical controls. Even phase III trials that have established standards for patient management have relatively short follow-up for a disease with a prolonged natural history.6,7 Studies with long-term follow-up often include retrospective analyses from referral institutions, potentially biased by unintended patient selection leading to ‘limited generalizability.8,9 Thus, as a practice community, we have a plethora of knowledge about short-term outcomes in FL but less understanding of long-term outcomes for combinations of sequential therapies.
FL has a heterogeneous pattern of clinical presentations and clinical outcomes. Predictors of survival and treatment response include histologic grade, and clinical factors such as the International Prognostic Index,10 or the Follicular Lymphoma International Prognostic Index (FLIPI).11
We report here the first analysis of a large national prospective observational study of newly diagnosed FL patients designed to collect data on representative patterns of clinical presentation, management, and outcome. This analysis describes the relationship between histology grading and FLIPI scores at presentation, and the patterns of initial management for FL among United States practitioners in the monoclonal antibody therapy era.
PATIENTS and METHODS
The National LymphoCare study (NLCS) is a joint venture operated by Genentech Inc (South San Francisco, CA) and Biogen Idee (Cambridge, MA). The authors served as the advisory board for this study, and participated in all phases of the study, including initial protocol design, prospective determination of data to be collected, and consideration of participating sites. The advisory board met quarterly, had full access to data listings, and collaborated with investigators and the sponsor regarding interpretation and publication of the data. This article was written de novo by members of the advisory board.
Final selection of academic and community sites was determined by study sponsors based on responses to a survey assessing capability to participate in an observational study of FL. Questions included number of newly diagnosed FL patients seen annually, logistics and support for clinical research, and previous experience with sponsored clinical research.
All patients signed informed consent before participation, and the protocol was approved by an institutional review board. Patients were recruited between March 2004 and March 2007. All patients newly diagnosed (within 6 months) with FL and no prior history of lymphoma were eligible. There was no central pathology review; the local pathology report defined FL diagnosis after investigator education on WHO definitions of FL. Grade and evidence for concurrent second lymphoma were evaluated when available. Patients were evaluated according to the treating physician's standard practice without study-specific visits or evaluations required for this study either at baseline or during the course of the study. Collected data include: demographics; clinical data (including performance status, stage, number of nodal and extra-nodal sites); routine labs including lactate dehydrogenase when available; serial management strategies; response to treatment; and events including relapses and death. After 12 months of data, the advisory board encouraged investigators to include lactate dehydrogenase as part of initial evaluation. Patients were assigned highest documented stage when staging was incomplete. There is no study-specific prescribed treatment regimen or intervention. Regular investigator newsletters and three in-person investigator meetings focused on data acquisition and study findings, but did not discuss clinical aspects of lymphoma management.
All treatment strategies (including observation) for patients were recorded. Patients who did not receive therapy within 90 days of diagnosis date were considered to be in the observation cohort. Treatment and outcomes (including response, time to progression, and survival) were collected quarterly. Follow-up data were actively solicited from providers at time of clinical follow-up. Enrolled patients are to be observed for up to 10 years from enrollment or until death, withdrawal of consent, or loss to follow-up. Enrollment sites were categorized as academic (affiliated with an academic institution) or community based on self report.
Statistical Methods
Demographic and baseline disease characteristics, as well as initial treatment strategy, were summarized using descriptive statistics (median and range for continuous variables and frequencies for categoric variables). Univariate associations among and between demographic and baseline disease characteristics, as well as initial treatment strategy, were tested using a standard χ2 test.
RESULTS
Patient Cohort Defined
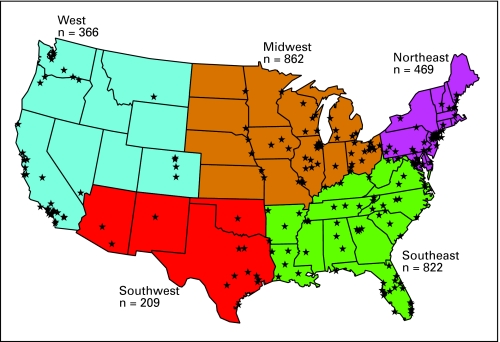
Two thousand seven hundred twenty eight subjects were enrolled at 265 sites from March 2004 through March 2007. Rate of enrollment was consistent across the enrollment period with sequential 12-month enrollment totals of 879, 971, and 878. Two hundred twenty-seven community sites enrolled 2,193 subjects (80%) and 38 academic sites enrolled 535 subjects (20%). Figure 1 depicts the location of sites and enrollment by region.
Fig 1.
Sites and enrollment by region.
FL patients enrolled in NLCS were similar to those in the Surveillance, Epidemiology, and End Results (SEER) database, and should be representative of the United States population. Patients in the NLCS are comparable with FL patients diagnosed in 2004 in the national SEER registry12 in age (median, 61 years), sex (female, 52%), ethnicity (white, 90%), and grade distribution (grade 1, 43%; grade 2, 29%; grade 3, 19%; NOS, 10%), but had a stage distribution skewed toward higher Ann Arbor stage (I, 17%; II, 15%; III, 29%; IV, 37%; unknown, 1%) although direct comparisons are hampered by higher rates of missing grade and stage in the SEER data set. Patients enrolled at community and academic sites differed in age, grade, and stage at diagnosis (Table 1), although minor differences in grade distribution may not be meaningful in the absence of central pathology review. Bone marrow biopsies were reported in 64% of patients at academic sites and 52% at community sites which may contribute to slightly higher stage at academic sites.
Table 1.
Patient Demographics and Baseline Disease Characteristics: Overall and by Center Type
| Characteristic | % |
P† | |||
|---|---|---|---|---|---|
| NLCS 2004-07 (n = 2,728) | SEER 2004 (n = 2,756) | NLCS Community Sites | NLCS Academic Sites | ||
| Median age, years | 61 | 63 | 62 | 59 | < .01 |
| Sex, female | 51.8 | 50.6 | 51.5 | 52.9 | .56 |
| Ethnicity | .27 | ||||
| White | 90.4 | 89.6 | 90.9 | 88.6 | |
| Black | 3.4 | 4.1 | 3.3 | 4.1 | |
| Other | 6.1 | 6.3 | 5.8 | 7.3 | |
| Histology, grade* | < .01 | ||||
| 1 | 42.9 | 27.0 | 41.9 | 47.0 | |
| 2 | 28.6 | 22.8 | 28.1 | 30.6 | |
| 3 | 18.7 | 17.7 | 19.2 | 16.6 | |
| Unknown/not done | 9.8 | 32.5 | 10.8 | 5.8 | |
| Stage | < .01 | ||||
| I | 17.4 | 28.6 | 17.7 | 16.1 | |
| II | 15.3 | 16.4 | 16.3 | 11.0 | |
| III | 29.4 | 22.0 | 28.5 | 33.1 | |
| IV | 37.1 | 25.8 | 36.5 | 39.6 | |
| Unknown/not done | 0.8 | 7.1 | 1.0 | 0.2 | |
Abbreviations: NLCS, National LymphoCare study; SEER, Surveillance, Epidemiology, and End Results.
Excludes 52 patients with mixed histology.
Comparison of community and academic sites.
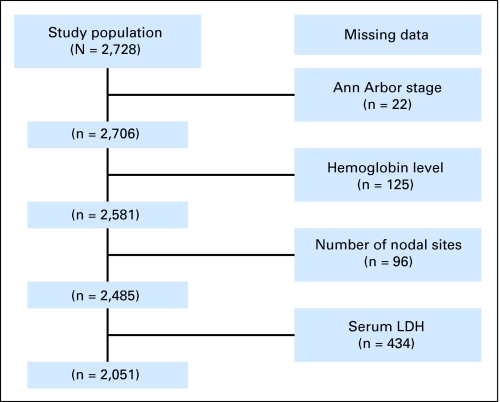
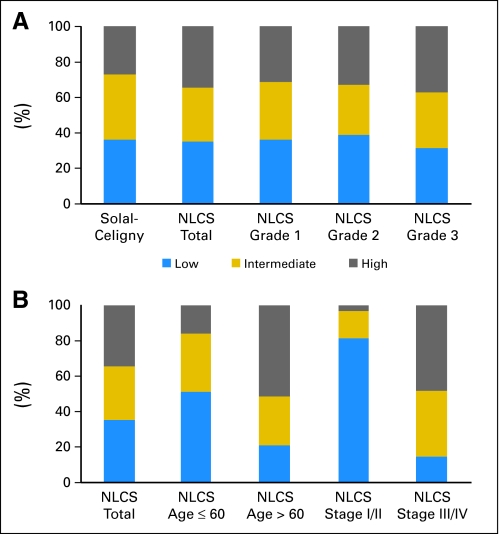
FLIPI continued to define three distinct groups in the NLCS and was independent of histologic grade. As shown in Figure 2, 2,234 (82%) of 2,728 patients had calculable FLIPI scores based on initial investigator work-up. Two thousand fifty one of 2,728 had data for all FLIPI components. FLIPI was calculable for an additional 183 patients because missing data would not change the FLIPI category. Lactate dehydrogenase was collected by investigators in 79% of patients. Geography and academic/community sites were significantly associated with collection of lactate dehydrogenase: 88% in academic sites versus 76% in community sites (P < .01). The distribution of FLIPI scores among 2,234 patients with calculable FLIPI scores are shown in Figure 3. There was remarkable consistency across grades 1 and 2 with high to low category (H/L) ratios of 0.86:0.85, and a suggestion of correlation with grade 3 histology and higher FLIPI (H/L ratio 1.19; Fig 3A). Stage and age, as might be expected, were dominant predictors of FLIPI score (Fig 3B). The distribution of patients among FLIPI categories was similar to those in the validation study.11
Fig 2.
Missing elements in Follicular Lymphoma International Prognostic Index scores for entire cohort. LDH, lactate dehydrogenase.
Fig 3.
Follicular Lymphoma International Prognostic Index (FLIPI) distribution, (A) overall, and (B) stratified by grade, age, and stage. NLCS, National LymphoCare study.
Initial Therapy of FL in the United States, 2004 to 2007
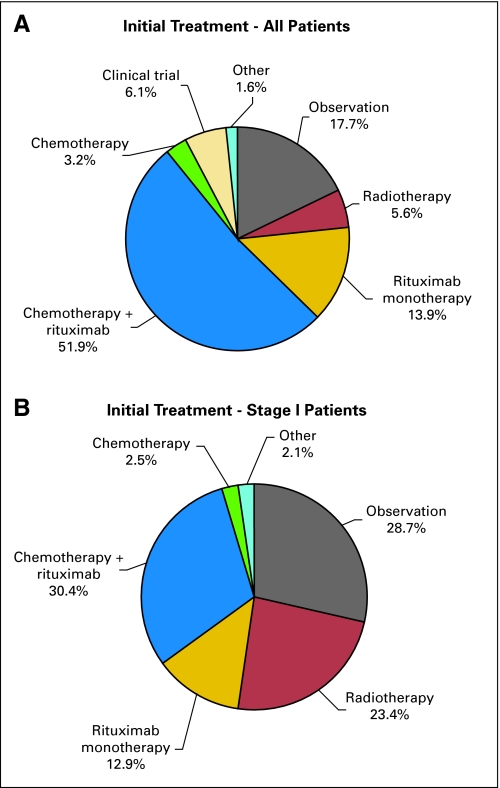
Collectively, the initial therapeutic strategy for FL was: observation, 17.7%; rituximab monotherapy, 13.9%; chemotherapy plus rituximab (R), 51.9%; external-beam radiotherapy (XRT), 5.6%; chemotherapy only, 3.2% clinical trials 6.1% (Fig 4A). R plus chemotherapy regimens were: rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), 55.0%; rituximab, cyclophosphamide, vincristine, and prednisone (R-CVP), 23.1%; R plus fludarabine based, 15.5%; other, 6.4%. Single-agent alkylator therapy was chosen in only 0.7%. Among patients receiving active treatment as an initial management strategy, the use of anthracyclines was associated (P < .01) with age, stage of disease, and histology (Table 2). FLIPI was not associated with the use of an anthracycline.
Fig 4.
Initial treatment, (A) all, and (B) stage I patients.
Table 2.
Demographic and Baseline Disease Characteristics for Patients Initially Treated With or Without an Anthracycline Among Patients Initially Managed With an Active Treatment Regimen (N = 2,244)
| Characteristic | Anthracycline (n = 945) |
Non-Anthracycline (n = 1,299) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age, years | < .01 | ||||
| < 45 | 110 | 45.3 | 133 | 54.7 | |
| 45-59 | 393 | 50.4 | 386 | 49.6 | |
| 60-74 | 353 | 43.2 | 464 | 56.8 | |
| ≥ 75 | 89 | 22.0 | 316 | 78.0 | |
| Stage | < .01 | ||||
| I | 108 | 32.0 | 230 | 68.0 | |
| II | 132 | 39.6 | 201 | 60.4 | |
| III | 300 | 45.9 | 354 | 54.1 | |
| IV | 394 | 43.9 | 504 | 56.1 | |
| Unknown/not done | 11 | 52.4 | 10 | 47.6 | |
| Histology by grade | < .01 | ||||
| I | 230 | 26.6 | 635 | 73.4 | |
| II | 262 | 40.2 | 389 | 59.8 | |
| III | 305 | 67.2 | 149 | 32.8 | |
| Mixed | 37 | 74.0 | 13 | 26.0 | |
| Unknown/not done | 111 | 49.6 | 113 | 50.4 | |
| FLIPI | .41 | ||||
| Low, 0-1 | 250 | 40.4 | 369 | 59.6 | |
| Intermediate, 2 | 226 | 41.9 | 314 | 58.1 | |
| High, 3–5 | 307 | 44.0 | 391 | 56.0 | |
Abbreviation: FLIPI, Follicular Lymphoma International Prognostic Index.
The choice to initiate therapy rather than observe patients was also associated with age, FLIPI, stage, and grade (P < .01). Significant differences of treatment (P < .01) across regions of the United States were noted: for example, observation was used in 13.3% of patients in Southeast and 29.0% in Northeast; fludarabine-based R plus chemotherapy was used in 11.5% of patients in Southwest and only 4.5% in Northeast. Academic sites were more likely than community sites to treat patients on clinical trials, 13.1% versus 4.4% (P < .01). In patients treated with rituximab monotherapy or R plus chemotherapy, a higher FLIPI was not associated with decision to utilize chemotherapy. Initial management strategy by baseline disease and demographic characteristics is detailed in the online-only Appendix Table A1.
Twenty-two percent of patients initially observed received active therapy within 12 months and 31.2% within 24 months. Among the 148 patients initially observed who subsequently received an active treatment, 27.0% received rituximab monotherapy, 35.8% received R plus chemotherapy, 6.8% received chemotherapy alone, 6.1% received investigational therapy, 7.4% received radiotherapy, with the remainder unspecified.
Stage I FL
Four hundred seventy-four patients (17.4%) had stage I disease at diagnosis. Of these patients, 136 were observed (28.7%), 61 were treated with rituximab alone (12.9%), 144 were treated with rituximab and chemotherapy (30.4%), and 111 received XRT (23.4%; Fig 4B). Among stage I patients treated at academic centers, 25 were observed (29.1%), six were treated with rituximab alone (7.0%), and 25 received XRT (29.1%). Among stage I patients treated at community centers, 111 were observed (28.6%), 55 were treated with rituximab alone (14.2%), and 86 received XRT (22.2%). Further examination of patients with stage I disease, found XRT use in 20.9% of patients younger than 45 years at diagnosis, 25.9% 45 to 59 years, 24.3% 60 to 74 years, and 20.0% ≥75 years of age at diagnosis. Among stage I patients not receiving radiation as initial treatment and with at least 90 days of follow-up after stopping initial treatment, 50 (20.7%) of 242 received radiation as a second treatment within 90 days of completing the initial treatment, suggesting a planned combined modality approach.
DISCUSSION
The NLCS is the largest prospective database in FL in the United States. All patients have been enrolled since 2004, with access to modern treatment regimens. As with any observational study, our results are susceptible to unintentional bias resulting from subject selection. Possible biases include positive selection of physicians with a self-declared interest in lymphoma or physicians with a bias in favor of rituximab use—a product of the study sponsors. We have demonstrated that this registry includes patients with similar demographics to the SEER registry.12 Eighty percent of patients in the NLCS were enrolled at community (not academic) practices, and all regions of the continental United States are represented in the 265 enrolling sites. We feel, therefore, that observations made from this registry may be generalized, and truly reflect the current practice patterns in the United States.
The FLIPI defines three risk groups of patients with FL, with outcomes ranging from 52% to 90% survival at 5 years.11 In this index, the risk groups each compromised approximately one third of patients, unlike the standard International Prognostic Index, which, when utilized for FL, has very few patients in the high-risk group.13,14 Our data support the notion that FLIPI divides patients into three relatively equal sized groups. FLIPI scores could not be calculated in 18% of patients in our series, most often because no serum lactate dehydrogenase was measured at diagnosis. Recent data suggest this index provides valuable prognostic data in patients treated with monoclonal antibody containing chemotherapy regimens.7,37 We strongly advocate that all patients with a new diagnosis of FL should be scored according to FLIPI, and thus should have all elements, including serum lactate dehydrogenase, obtained at diagnosis.
The observed variation of practice patterns emphasizes that there is no current standard of care in the United States for the treatment of de novo FL. As expected, observation rather than active therapy was utilized more frequently in low-risk FLIPI patients than in patients with higher risk disease. However, the vast majority of patients even with low-risk disease were treated at diagnosis. Fourteen percent of patients were treated with rituximab monotherapy, despite the lack of a supporting US Food and Drug Administration–labeled indication. Among treated patients, FLIPI was not associated with the choice to add chemotherapy to R. The use of anthracyclines was influenced by region of the country, grade of tumor, FLIPI score, and other patient-associated factors. Although there was a statistical association between grade of tumor and anthracycline use, 33% of patients with grade 3 histology did not receive anthracycline-based therapy, despite National Comprehensive Cancer Network guidelines and studies advocating this use.15 Regional differences in patterns of care have also been observed in an analysis of the SEER data in elderly patients with non-Hodgkin's lymphoma.16
Such disparate approaches to the initial care of patients with FL render a very heterogeneous group of patients at relapse in the United States as contrasted with European countries like Germany where anthracycline use is a more consistent feature of treatment.17 This has important implications in the interpretation of future clinical trials enrolling patients with relapsed FL. Historically, clinical prognostic factors of FL at relapse were similar to those at diagnosis.18 However, patients who relapse late after first therapy with single-agent rituximab are likely different from patients who relapse early after anthracycline-containing chemotherapy as first therapy. It is imperative that studies in relapsed disease detail prior therapeutic regimens, and evaluate response outcomes in light of these prior therapies. Phase II trials in relapsed FL in the United States that utilize comparisons to historical controls are therefore very difficult to interpret at the present time.
In 1984, Horning and Rosenberg8 published a study of untreated patients with FL, demonstrating no apparent increased risk of transformation when deferring first-line therapy, and a 30% rate of spontaneous remissions. Observation rather than active therapy remains a recommended approach in asymptomatic patients,19 and continues to be practiced widely in the United States—21% of patients with low-risk FLIPI scores were observed rather than placed on immediate therapy in our series. The role of observation has become controversial in view of recently observed aforementioned survival improvements in FL, and the suggestion that early therapy in the modern era may decrease the risk, and associated morbidity and mortality, of histologic transformation.20 Historically, the median time to therapy of patients initially observed was 2.6 years in a randomized trial from the British National Lymphoma Investigation,21 and over 3 years in the Horning series.8 Despite the availability of R as a low morbidity therapeutic option we do not detect an apparent change in the threshold for initiating therapy in this setting, with 22% of patients in our series who were initially observed receiving therapy within 1 year of observation and less than one third after 2 years.
Of interest, 17% of patients in our registry had stage I FL. XRT is a recommended therapeutic approach for this group of patients based on results of studies suggesting a substantial minority of patients enjoy very prolonged disease-free intervals after XRT.22–24 Only 23% of patients in our series with stage I FL received XRT at diagnosis; an additional 8% of patients had XRT immediately after chemotherapy, suggesting a combined modality approach.25 In the SEER database, only 31% of patients with stage I FL diagnosed in 2004 were treated with XRT within 4 months of diagnosis. Both the National Comprehensive Cancer Network and European Society for Medical Oncology have published guidelines recommending primary XRT for early stage FL.26,27 Observation may be an option for carefully selected patients based on recently published series from Stanford of 43 patients with early stage FL who were not treated, and had comparable outcome to patients historically treated with XRT.28 However, 43% of patients in our registry who had stage I disease were treated with R or chemotherapy combinations containing R as initial treatment. Although size and location of early-stage disease may impact choices of therapy based on toxicity, few data have been published supporting systemic therapy alone as initial treatment for this group of patients. It has been suggested that FLIPI may help to define a high-risk subgroup of patients with early-stage disease who may benefit from more aggressive therapeutic options.29 Our findings suggest published guidelines advocating primary XRT have not had a substantial impact in treatment patterns for this subset of patients.
Given the incurable nature of FL with standard therapeutic approaches, the low clinical trial enrollment (6.1% of patients) was disappointing. At least two National Cancer Institute–sponsored cooperative group phase III trials in de novo FL were accruing patients during this time period, and open in most regions of the country either through group affiliates or the Cancer Trials Support Unit. A complete discussion of barriers to cancer clinical trial accrual is beyond the scope of our study, and have been published elsewhere.30,31 Individual physician factors appear to be most important in determining successful enrollment of patients onto clinical trials.3233 Through participation in the registry, our investigators have demonstrated interest in clinical research, and the ability to oversee data collection. This interest did not lead to active enrollment of patients on therapeutic trials for the vast majority of participants. Perceptions of access problems, inadequate reimbursements, lack of time, logistical complexity, and patient refusal have all been cited as reasons why clinical trial participation is low.3435 Clearly, it is critical to address these issues in the development and conduct of future national clinical trials in FL to improve clinical trial enrollment.
The choice of first-line therapy for an incurable disease like FL should consider not only response rates, and time to progression, but also the quality of life, potential impact on future therapies, including transplantation, and risk of secondary malignancies.19,36 With the recent success of improving short-term outcome for these patients with monoclonal antibodies and novel agents comes a responsibility to ensure that we do not compromise future survival due to late toxic effects of treatment. It is imperative that all current clinical trials in de novo indolent non-Hodgkin's lymphoma observe patients for late effects. We hope the National LymphoCare Study, in light of its representative demographics and treatment patterns, along with other emerging registries,37 will substantially contribute to this critical responsibility.
Appendix
Table A1.
Initial Management Strategy by Baseline Disease and Patient Demographics Characteristics
| Characteristic | Observation |
Rituximab Monotherapy |
Chemotherapy |
Rituximab Chemotherapy |
XRT |
Clinical Trial |
Other |
Total |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| No. of patients | 484 | 378 | 86 | 1,415 | 154 | 167 | 44 | 2,728 | ||||||||
| FLIPI | ||||||||||||||||
| Low, 0–1 | 156 | 21.3 | 89 | 12.1 | 19 | 2.6 | 334 | 45.6 | 92 | 12.6 | 33 | 4.5 | 10 | 1.4 | 733 | 100 |
| Intermediate, 2 | 135 | 20.0 | 92 | 13.6 | 25 | 3.7 | 340 | 50.4 | 10 | 1.5 | 64 | 9.5 | 9 | 1.3 | 675 | 100 |
| High, 3–5 | 59 | 9.2 | 90 | 14.0 | 22 | 3.4 | 404 | 62.8 | 5 | 0.8 | 52 | 8.1 | 11 | 1.7 | 643 | 100 |
| Unknown | 134 | 19.8 | 107 | 15.8 | 20 | 3.0 | 337 | 49.8 | 47 | 6.9 | 18 | 2.7 | 14 | 2.1 | 677 | 100 |
| Histology by grade | ||||||||||||||||
| 1 | 282 | 24.6 | 193 | 16.8 | 34 | 3.0 | 459 | 40.0 | 76 | 6.6 | 82 | 7.1 | 21 | 1.8 | 1,147 | 100 |
| 2 | 115 | 15.0 | 108 | 14.1 | 25 | 3.3 | 404 | 52.7 | 46 | 6.0 | 54 | 7.0 | 14 | 1.8 | 766 | 100 |
| 3 | 47 | 9.4 | 41 | 8.2 | 17 | 3.4 | 355 | 70.9 | 13 | 2.6 | 24 | 4.8 | 4 | 0.8 | 501 | 100 |
| Multiple histology | 2 | 3.8 | 3 | 5.8 | 2 | 3.8 | 44 | 84.6 | 0 | 0.0 | 1 | 1.9 | 0 | 0.0 | 52 | 100 |
| Unknown | 38 | 14.5 | 33 | 12.6 | 8 | 3.1 | 153 | 58.4 | 19 | 7.3 | 6 | 2.3 | 5 | 1.9 | 262 | 100 |
| Stage | ||||||||||||||||
| I | 136 | 28.7 | 61 | 12.9 | 12 | 2.5 | 144 | 30.4 | 111 | 23.4 | 0 | 0.0 | 10 | 2.1 | 474 | 100 |
| II | 84 | 20.1 | 70 | 16.8 | 16 | 3.8 | 209 | 50.1 | 24 | 5.8 | 6 | 1.4 | 8 | 1.9 | 417 | 100 |
| III | 148 | 18.5 | 121 | 15.1 | 29 | 3.6 | 430 | 53.6 | 4 | 0.5 | 59 | 7.4 | 11 | 1.4 | 802 | 100 |
| IV | 115 | 11.4 | 123 | 12.1 | 28 | 2.8 | 620 | 61.2 | 12 | 1.2 | 101 | 10.0 | 14 | 1.4 | 1,013 | 100 |
| Unknown | 1 | 4.5 | 3 | 13.6 | 1 | 4.5 | 12 | 54.5 | 3 | 13.6 | 1 | 4.5 | 1 | 4.5 | 22 | 100 |
| Region | ||||||||||||||||
| Midwest | 149 | 17.3 | 118 | 13.7 | 18 | 2.1 | 435 | 50.5 | 59 | 6.8 | 69 | 8.0 | 14 | 1.6 | 862 | 100 |
| Northeast | 136 | 29.0 | 63 | 13.4 | 15 | 3.2 | 182 | 38.8 | 28 | 6.0 | 40 | 8.5 | 5 | 1.1 | 469 | 100 |
| Southeast | 109 | 13.3 | 109 | 13.3 | 40 | 4.9 | 474 | 57.7 | 42 | 5.1 | 33 | 4.0 | 15 | 1.8 | 822 | 100 |
| Southwest | 35 | 16.7 | 18 | 8.6 | 6 | 2.9 | 131 | 62.7 | 9 | 4.3 | 4 | 1.9 | 6 | 2.9 | 209 | 100 |
| West | 55 | 15.0 | 70 | 19.1 | 7 | 1.9 | 193 | 52.7 | 16 | 4.4 | 21 | 5.7 | 4 | 1.1 | 366 | 100 |
| Site type | ||||||||||||||||
| Academic | 112 | 20.9 | 61 | 11.4 | 18 | 3.4 | 238 | 44.5 | 30 | 5.6 | 70 | 13.1 | 6 | 1.1 | 535 | 100 |
| Community | 372 | 17.0 | 317 | 14.5 | 68 | 3.1 | 1,177 | 53.7 | 124 | 5.7 | 97 | 4.4 | 38 | 1.7 | 2,193 | 100 |
Abbreviations: FLIPI, Follicular Lymphoma International Prognostic Index; XRT, external-beam radiotherapy.
Footnotes
Supported in part by a Career Development Award from the National Cancer Institute (CA-102216 to J.W.F.); the National LymphoCare Study is funded by Genentech Inc and Biogen Idec.
Presented in part in abstract format at the 47th Annual Meeting of the American Society of Hematology, Atlanta, December 10–13, 2005; Atlanta, GA; 42nd Annual Meeting of the American Society of Clinical Oncology, June 2–6, 2006, Atlanta, GA; and 49th Annual Meeting of the American Society of Hematology, December 8–11, 2007, Atlanta, GA.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00097565.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Michael D. Taylor, Genentech (C); Elaine K. Wong, Genentech (C) Consultant or Advisory Role: Jonathan W. Friedberg, Genentech (C); James R. Cerhan, Genentech, (C); Christopher R. Flowers, Genentech, Biogen Idec (U), Genentech, Biogen Idec (C); Eric S. Rogers, Genentech, (C); John D. Hainsworth, Genentech, (C); Andrew D. Zelenetz, GlaxoSmithKline (C), Genentech (C), Favrille (C); Brian K. Link, Genentech (C), Biogen Idec (C), Millennium Pharmaceuticals (C) Stock Ownership: Michael D. Taylor, Genentech; Elaine K. Wong, Genentech Honoraria: James R. Cerhan, Genentech; Andrew D. Zelenetz, Center for Biomedical Continuing Education, Physicians' Education Resource Research Funding: Christopher R. Flowers, Biovest, Millennium Pharmaceuticals, Bayer Pharmaceuticals, Johnson & Johnson; John D. Hainsworth, Genentech; Andrew D. Zelenetz, GlaxoSmithKline, Genentech, Biogen Idec, Amgen; Brian K. Link, Genentech, Biogen Idec, Millennium Pharmaceuticals, GlaxoSmithKline Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Jonathan W. Friedberg, James R. Cerhan, Hildy Dillon, Charles M. Farber, John D. Hainsworth, Elaine K. Wong, Julie M. Vose, Andrew D. Zelenetz, Brian K. Link
Provision of study materials or patients: Jonathan W. Friedberg, Charles M. Farber, Julie M. Vose, Brian K. Link
Collection and assembly of data: Michael D. Taylor, Eric S. Rogers, Elaine K. Wong, Julie M. Vose, Brian K. Link
Data analysis and interpretation: Jonathan W. Friedberg, Michael D. Taylor, James R. Cerhan, Christopher R. Flowers, Charles M. Farber, Elaine K. Wong, Julie M. Vose, Brian K. Link
Manuscript writing: Jonathan W. Friedberg, Michael D. Taylor, James R. Cerhan, Christopher R. Flowers, Elaine K. Wong, Julie M. Vose, Andrew D. Zelenetz, Brian K. Link
Final approval of manuscript: Jonathan W. Friedberg, Michael D. Taylor, James R. Cerhan, Christopher R. Flowers, Hildy Dillon, Charles M. Farber, Eric S. Rogers, John D. Hainsworth, Elaine K. Wong, Julie M. Vose, Andrew D. Zelenetz, Brian K. Link
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JR, Armitage JO, Weisenburger DD. Epidemiology of the non-Hodgkin's lymphomas: Distributions of the major subtypes differ by geographic locations. Non-Hodgkin's Lymphoma Classification Project. Ann Oncol. 1998;9:717–720. doi: 10.1023/a:1008265532487. [DOI] [PubMed] [Google Scholar]
- 3.Swenson WT, Wooldridge JE, Lynch CF, et al. Improved survival of follicular lymphoma patients in the United States. J Clin Oncol. 2005;23:5019–5026. doi: 10.1200/JCO.2005.04.503. [DOI] [PubMed] [Google Scholar]
- 4.Fisher RI, LeBlanc M, Press OW, et al. New treatment options have changed the survival of patients with follicular lymphoma. J Clin Oncol. 2005;23:8447–8452. doi: 10.1200/JCO.2005.03.1674. [DOI] [PubMed] [Google Scholar]
- 5.Liu Q, Fayad L, Cabanillas F, et al. Improvement of overall and failure-free survival in stage IV follicular lymphoma: 25 years of treatment experience at The University of Texas M.D. Anderson Cancer Center. J Clin Oncol. 2006;24:1582–1589. doi: 10.1200/JCO.2005.03.3696. [DOI] [PubMed] [Google Scholar]
- 6.Marcus R, Imrie K, Belch A, et al. CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood. 2005;105:1417–1423. doi: 10.1182/blood-2004-08-3175. [DOI] [PubMed] [Google Scholar]
- 7.Buske C, Hoster E, Dreyling M, et al. The Follicular Lymphoma International Prognostic Index (FLIPI) separates high-risk from intermediate- or low-risk patients with advanced-stage follicular lymphoma treated front-line with rituximab and the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) with respect to treatment outcome. Blood. 2006;108:1504–1508. doi: 10.1182/blood-2006-01-013367. [DOI] [PubMed] [Google Scholar]
- 8.Horning SJ, Rosenberg SA. The natural history of initially untreated low-grade non-Hodgkin's lymphomas. N Engl J Med. 1984;311:1471–1475. doi: 10.1056/NEJM198412063112303. [DOI] [PubMed] [Google Scholar]
- 9.Rohatiner AZ, Nadler L, Davies AJ, et al. Myeloablative therapy with autologous bone marrow transplantation for follicular lymphoma at the time of second or subsequent remission: Long-term follow-up. J Clin Oncol. 2007;25:2554–2559. doi: 10.1200/JCO.2006.09.8327. [DOI] [PubMed] [Google Scholar]
- 10.A predictive model for aggressive non-Hodgkin's lymphoma: The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 11.Solal-Celigny P, Roy P, Colombat P, et al. Follicular lymphoma international prognostic index. Blood. 2004;104:1258–1265. doi: 10.1182/blood-2003-12-4434. [DOI] [PubMed] [Google Scholar]
- 12.Morton LM, Wang SS, Devesa SS, et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006;107:265–276. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bastion Y, Coiffier B. Is the International Prognostic Index for aggressive lymphoma patients useful for follicular lymphoma patients? J Clin Oncol. 1994;12:1340–1342. doi: 10.1200/JCO.1994.12.7.1340. [DOI] [PubMed] [Google Scholar]
- 14.Etto LY, Silva VC, Inaoka RJ, et al. Is the follicular lymphoma international prognostic index better than the international prognostic index to identify high-risk follicular lymphoma patients? Leuk Lymphoma. 2007;48:526–530. doi: 10.1080/10428190601113576. [DOI] [PubMed] [Google Scholar]
- 15.Ganti AK, Weisenburger DD, Smith LM, et al. Patients with grade 3 follicular lymphoma have prolonged relapse-free survival following anthracycline-based chemotherapy: The Nebraska Lymphoma Study Group experience. Ann Oncol. 2006;17:920–927. doi: 10.1093/annonc/mdl039. [DOI] [PubMed] [Google Scholar]
- 16.Berrios-Rivera JP, Fang S, Cabanillas ME, et al. Variations in chemotherapy and radiation therapy in a large nationwide and community-based cohort of elderly patients with non-Hodgkin lymphoma. Am J Clin Oncol. 2007;30:163–171. doi: 10.1097/01.coc.0000251356.63237.4f. [DOI] [PubMed] [Google Scholar]
- 17.Dreyling M, Fetscher S, Kornek P, et al. Treatment of indolent non-Hodgkin's lymphoma in Germany: Results of a representative population-based survey. Blood. 2007;110:407a. doi: 10.1016/j.clml.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Montoto S, Lopez-Guillermo A, Ferrer A, et al. Survival after progression in patients with follicular lymphoma: Analysis of prognostic factors. Ann Oncol. 2002;13:523–530. doi: 10.1093/annonc/mdf119. [DOI] [PubMed] [Google Scholar]
- 19.Gribben JG. How I treat indolent lymphoma. Blood. 2007;109:4617–4626. doi: 10.1182/blood-2006-10-041863. [DOI] [PubMed] [Google Scholar]
- 20.Montoto S, Davies AJ, Matthews J, et al. Risk and clinical implications of transformation of follicular lymphoma to diffuse large B-cell lymphoma. J Clin Oncol. 2007;25:2426–2433. doi: 10.1200/JCO.2006.09.3260. [DOI] [PubMed] [Google Scholar]
- 21.Ardeshna KM, Smith P, Norton A, et al. Long-term effect of a watch and wait policy versus immediate systemic treatment for asymptomatic advanced-stage non-Hodgkin lymphoma: A randomised controlled trial. Lancet. 2003;362:516–522. doi: 10.1016/s0140-6736(03)14110-4. [DOI] [PubMed] [Google Scholar]
- 22.Mauch P. Follicular non-Hodgkin's lymphoma: The role of radiation therapy. Ann Hematol. 2001;80(suppl 3):B63–B65. doi: 10.1007/pl00022793. [DOI] [PubMed] [Google Scholar]
- 23.Tsang RW, Gospodarowicz MK. Radiation therapy for localized low-grade non-Hodgkin's lymphomas. Hematol Oncol. 2005;23:10–17. doi: 10.1002/hon.743. [DOI] [PubMed] [Google Scholar]
- 24.Guadagnolo BA, Li S, Neuberg D, et al. Long-term outcome and mortality trends in early-stage, grade 1-2 follicular lymphoma treated with radiation therapy. Int J Radiat Oncol Biol Phys. 2006;64:928–934. doi: 10.1016/j.ijrobp.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Besa PC, McLaughlin PW, Cox JD, et al. Long term assessment of patterns of treatment failure and survival in patients with stage I or II follicular lymphoma. Cancer. 1995;75:2361–2367. doi: 10.1002/1097-0142(19950501)75:9<2361::aid-cncr2820750928>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 26.Zelenetz AD, Advani RH, Buadi F, et al. Non-Hodgkin's lymphoma: Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2006;4:258–310. doi: 10.6004/jnccn.2006.0025. [DOI] [PubMed] [Google Scholar]
- 27.Hiddemann W, Dreyling M, Stahel RA. Minimum clinical recommendations for diagnosis, treatment and follow-up of newly diagnosed follicular lymphoma. Ann Oncol. 2005;16(suppl 1):i56–i57. doi: 10.1093/annonc/mdi819. [DOI] [PubMed] [Google Scholar]
- 28.Advani R, Rosenberg SA, Horning SJ. Stage I and II follicular non-Hodgkin's lymphoma: Long-term follow-up of no initial therapy. J Clin Oncol. 2004;22:1454–1459. doi: 10.1200/JCO.2004.10.086. [DOI] [PubMed] [Google Scholar]
- 29.Plancarte F, Lopez-Guillermo A, Arenillas L, et al. Follicular lymphoma in early stages: High risk of relapse and usefulness of the Follicular Lymphoma International Prognostic Index to predict the outcome of patients. Eur J Haematol. 2006;76:58–63. doi: 10.1111/j.1600-0609.2005.00564.x. [DOI] [PubMed] [Google Scholar]
- 30.Lara PN, Jr, Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: Identifying potential barriers to enrollment. J Clin Oncol. 2001;19:1728–1733. doi: 10.1200/JCO.2001.19.6.1728. [DOI] [PubMed] [Google Scholar]
- 31.Simon MS, Du W, Flaherty L, et al. Factors associated with breast cancer clinical trials participation and enrollment at a large academic medical center. J Clin Oncol. 2004;22:2046–2052. doi: 10.1200/JCO.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Mannel RS, Walker JL, Gould N, et al. Impact of individual physicians on enrollment of patients into clinical trials. Am J Clin Oncol. 2003;26:171–173. doi: 10.1097/00000421-200304000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Finn R. Oncologist's role critical to clinical trial enrollment. J Natl Cancer Inst. 2000;92:1632–1634. doi: 10.1093/jnci/92.20.1632. [DOI] [PubMed] [Google Scholar]
- 34.Paskett ED, Cooper MR, Stark N, et al. Clinical trial enrollment of rural patients with cancer. Cancer Pract. 2002;10:28–35. doi: 10.1046/j.1523-5394.2002.101006.x. [DOI] [PubMed] [Google Scholar]
- 35.Hillner BE. Barriers to clinical trial enrollment: Are state mandates the solution? J Natl Cancer Inst. 2004;96:1048–1049. doi: 10.1093/jnci/djh225. [DOI] [PubMed] [Google Scholar]
- 36.Friedberg JW. Potential long-term toxicities should influence the choice of therapy for indolent non-Hodgkin's lymphoma. Haematologica. 2006;91:1453–1455. [PubMed] [Google Scholar]
- 37.Federico M, Bellei M, Marcheselli L, et al. F2 prognostic index. Ann Oncol. 2008;19(suppl 4):iv101. [Google Scholar]